Abstract
Objectives
The patients with hematological malignancies are a vulnerable group to COVID-19, due to the immunodeficiency resulting from the underlying disease and oncological treatment that significantly impair cellular and humoral immunity. Here we report on a beneficial impact of a passive immunotherapy with convalescent plasma to treat a prolonged, active COVID-19 infection in a patient with a history of nasopharyngeal diffuse large B-cell lymphoma treated with the therapy inducing substantial impairment of particularly humoral arm of immune system. The specific aim was to quantify SARS-CoV2 neutralizing antibodies in a patient plasma during the course of therapy.
Materials and methods
Besides the standard of care treatment and monitoring, neutralizing antibody titers in patient's serum samples, calibrated according to the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (human), were quantified in a time-dependent manner. During the immunotherapy period peripheral blood flow cytometry immunophenotyping was conducted to characterize lymphocyte subpopulations.
Results
The waves of clinical improvements and worsening coincided with transfused neutralizing antibodies rises and drops in the patient's systemic circulation, proving their contribution in controlling the disease progress. Besides the patient's lack of own humoral immune system, immunophenotyping analysis revealed also the reduced level of helper T-lymphocytes and immune exhaustion of monocytes.
Conclusion
Therapeutic approach based on convalescent plasma transfusion transformed a prolonged, active COVID-19 infection into a manageable chronic disease.
Keywords: Convalescent plasma, COVID-19, Immunodeficiency, Neutralizing antibody, Infective virus-neutralization assay, SARS-CoV-2
1. Introduction
Since the first described case in December 2019, the coronavirus disease 2019 (COVID-19) has been one of the most significant challenges of our time [1]. The clinical spectrum ranges from asymptomatic to critical forms with acute respiratory distress syndrome and death. The most endangered patients are the adults older than 60 years and the patients with comorbidities, especially immunodeficiencies [2]. Although clinical presentation and radiological findings are relatively familiar, the pathogenesis is complex and not completely clarified, which hinders the development of effective targeted therapy [3]. There are 4805 ongoing COVID-19 clinical trials, but only antiviral drug remdesivir has been approved by the Food and Drug Administration (FDA) [4].
One of the oldest and most intriguing treatment options, when dealing with diseases that require quick and efficient pathogen elimination, is transfusion of convalescent plasma enriched with specific antibodies and non-specific humoral innate immunity factors [5], [6], [7], [8]. In viral infections, antibodies act by neutralization, complement activation, opsonization and antibody-dependent cellular cytotoxicity mediation [9]. The essential and the most effective components of COVID-19 convalescent plasma are virus-specific neutralizing antibodies that target multiple epitopes of SARS-CoV-2 surface (glyco)proteins, including S protein receptor-binding domain, which they block, preventing the virus from entering the host cells. Additionally, it contains other proteins such as defensins, pentraxins, anti-inflammatory cytokines, clotting factors, that have an immunomodulatory effect, reduce exaggerated immune processes and lessen disease severity [10]. Infective virus-neutralization assay provides the most accurate answer to whether or not an individual has antibodies that can neutralize the infectivity of a specific virus strain [11].
The valid proof of the effectiveness of convalescent plasma treatment against COVID-19 from the properly conducted clinical trials is still lacking. After all, this is the therapeutic principle that is most widely used only in the early period of encountering with the novel infective pathogen, when the methods of antibody assessment, as well as other relevant methodology for the disease diagnostics and monitoring, have not been properly developed and validated yet. That is precisely the situation nowadays in COVID-19 pandemics [12]. In other words, trend of convalescent plasma usage usually has short lifetime, since in practice it is replaced by better characterized and less complex and variable medicines, as soon as those become available. Consequently, the reports on case studies and series are the major source of data on the positive experiences associated with implementation of convalescent plasma treatment into clinics.
According to the current literature, patients with impaired cellular and/or humoral immunity might be at higher risk of COVID-19 [13]. For this group of patients there is a growing evidence that COVID-19 convalescent plasma therapy might be an effective treatment [13], [14], [15], [16], [17], [18], [19], [20], although exact proof is missing since they are mostly excluded from clinical trials. Numerous case reports dealing with the usage of convalescent plasma in immunocompromised patients suffer from the lack of proper quantification of neutralizing antibodies in donated plasma samples, as well as in patients’ serum upon transfusion. Also, there are no standardized guidelines on recommended quality of donated COVID-19 convalescent plasma.
Here we aim to contribute to the topic by reporting the case of COVID-19 convalescent plasma therapy of immunocompromised patient with special emphasis on monitoring of post-transfusion neutralizing antibodies’ dynamics, which enables estimation of the longevity of passive humoral immunity. Also, in addition to the clinical presentation, it supports effectiveness of such therapeutic principle.
2. Methods
2.1. Virus detection in clinical samples
2.1.1. Molecular detection of viral RNA
Viral RNA was detected in nasopharyngeal swabs and serum on an automated Roche Cobas 6800 system (Roche, Mannheim, Germany) with the Roche cobas® SARS-CoV-2 Test (Roche, Mannheim, Germany).
To confirm the presence of SARS-CoV-2 in the cell culture supernatant, RNA was isolated from 100 μL of the supernatant which was added to 300 μL of a TriPure Isolation Reagent (Roche, Mannheim, Germany). After the addition of 95–100% ethanol (100 μL), the samples were incubated at room temperature for 5 min and then centrifuged through the QIAamp Mini Spin Columns (Qiagen, Hilden, Germany) for 1 min at 6000 × g. Further isolation was performed following a Qiagen QIAamp® Viral RNA Mini Kit (Qiagen, Hilden, Germany) protocol. Viral RNA was detected by the real-time RT-PCR [21] from 5 μL of the eluate using a TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher Scientific, Waltham, MA USA) in a 20 μL reaction mixture on an Applied Biosystems StepOnePlus thermocycler (Thermo Fisher Scientific, Waltham, MA USA).
2.1.2. Infective virus isolation from nasopharyngeal swabs
Vero E6 cells acquired from the American Type Culture Collection (ATCC–CRL 1587) were maintained in MEM with stable L-glutamine (PAN-Biotech, Aidenbach, Germany) supplemented with 10% FBS (PAN-Biotech, Aidenbach, Germany), penicillin (100 IU/mL), streptomycin (100 μg/mL) and non-essential amino acids (PAN-Biotech, Aidenbach, Germany). For virus isolation, Vero E6 cells were seeded at 300 000 cells per well in a 6-well cell culture plate and left to adhere overnight. The following day, nasopharyngeal swabs were filtered through a 0.22 μm filter and diluted 1:1 with the addition of MEM with stable L-glutamine supplemented with penicillin (200 IU/mL) and streptomycin (200 μg/mL). Further dilutions to final 1:10, 1:40, and 1:100 were prepared in MEM with stable L-glutamine supplemented with 2% FBS (PAN-Biotech, Aidenbach, Germany), penicillin (100 IU/mL), streptomycin (100 μg/mL) and non-essential amino acids. After 1 h long incubation in a 5% CO2 environment at 37 °C, the inoculum was removed and DMEM with 4.5 g/L glucose and stable L-glutamine (PAN-Biotech, Aidenbach, Germany), supplemented with 2% FBS, penicillin (100 IU/mL) and streptomycin (100 μg/mL), in a volume of 2 mL was added. Cell culture was checked daily for cytopathic effect until day 5 post-infection when the supernatant was collected from the positive cultures.
2.2. Convalescent plasma donation and preparation
COVID-19 convalescent plasma was collected by apheresis procedure from recovered and healthy donors with a documented history of laboratory-confirmed SARS-CoV-2 infection based on a positive RT-PCR test result and being asymptomatic for ≥ 28 days. Donors fulfilled all selection criteria required by the law of the Republic of Croatia, including the results of obligatory tests performed on each donation. Sera of eligible plasma donors were analyzed by the infective virus-neutralization assay for the quantification of SARS-CoV-2 neutralizing antibodies titers (as described in section 2.3.2.). The neutralizing potencies of plasma units used for therapy were in the range between 108 and 890 IU/mL, as calibrated according to the WHO International Standard. The patient was transfused with eight ABO-compatible convalescent plasma units. Each unit of approximately 200 mL was infused over one to two hours. The dynamics of blood sampling for estimation of neutralizing potencies is indicated in the Results section.
2.3. Quantification of SARS-CoV-2 neutralizing antibodies
2.3.1. Cell and virus culture
Vero E6 cells were maintained in MEM (GIBCO, Thermo Fisher Scientific), supplemented with 10% FBS (PAN-Biotech, Aidenbach, Germany) previously inactivated at 56̊C for 60 min, penicillin (100 IU/mL), streptomycin (100 μg/mL) and L-glutamine (2 mM) (Capricorn Scientific), in a 5% CO2 environment at 37̊C, and passaged every 3–4 days. Vero E6 cells were routinely tested for mycoplasma and found to be mycoplasma-free.
SARS-CoV-2 297/20 Zagreb isolate was derived from a positively tested nasopharyngeal swab in Zagreb, Croatia, and was propagated in Vero E6 cells to obtain a Laboratory working stock and reference for the virus titer determination using 50% cell culture infective dose (CCID50) assay in a 96-well format. Three-fold serial dilutions of virus suspension, each in octaplicate (100 μL/well), were incubated with Vero E6 cell suspension (3 × 105/mL; 100 μL/well). After four days of incubation (37̊C, 5% CO2) wells with cytopathic effect were counted and CCID50/mL was calculated using the Spearman-Kerber method.
2.3.2. Infective virus-neutralization assay
Infective virus-neutralization assay followed the general principles already described for other viruses [22], [23], but was adapted specifically to the SARS-CoV-2 virus, as follows. Two-fold serial dilutions of patient's serum samples and/or COVID-19 convalescent plasma donations’ samples, each in octaplicate (50 μL/well), were preincubated with approximately 20 CCID50 (50 μL/well) of the Laboratory working stock preparation of SARS-CoV-2 297/20 Zagreb isolate at 37̊C and 5% CO2 for 90 minutes. After the addition of Vero E6 cells (3 × 105/mL; 100 μL/well), the plates were incubated at 37 °C and 5% CO2. Wells with pure cell suspension in the first row served as cell growth control. After four days of incubation, plates were inspected by an inverted optical microscope, and the wells with cytopathic effect counted. The effective dose 50 (ED50), the amount of undiluted serum that inhibits the cytopathic effect in 50% of infected wells, was calculated according to the Spearman-Karber method. Neutralizing titer of each serum sample was expressed as the number of ED50 doses in 1 mL. The sample of known neutralizing capacity (internal reference) was included in every experiment and used for correction of tested samples’ results for the value of reference deviation from its nominal value. The internal reference was calibrated according to the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (human) (NIBSC code 20/136, NIBSC, Potters bar, Hertfordshire, UK), so the results of this assay are finally expressed in IU/mL.
2.4. Peripheral blood immunophenotyping
Peripheral blood flow cytometry immunophenotyping was conducted by using two six-color antibody panels for HLA-DR, CD3, CD4, CD8, CD16, CD19, CD27, CD45, CD56 surface markers detection, to analyze T cells, B cells, and NK cells (Table 1 ) [24]. The lyse + fix/wash protocol was used for sample preparation. An additional sample was stained with IOTest Myeloid Activation CD169-PE/HLA-DR-APC/CD64-PB Antibody Cocktail (Beckman Coulter, USA) [25]. All samples were collected by using the Navios™ flow cytometer (Beckman Coulter, USA). FCS data files were analyzed using the Kaluza™ software (Beckman Coulter, USA). Expression of CD279 and CD38 on T cells was analyzed using a third antibody panel and lyse + fix/wash protocol. Samples were collected by using the BD LSR II flow cytometer (Becton Dickinson, USA). FCS data files were analyzed using the FlowJo™ software (BD FlowJo, USA).
Table 1.
Antibody panels.
| Antibody | # Ref | Manufacturer |
|---|---|---|
| Panel 1 | ||
| HLA-DR FITC | 347400 | BD Biosciences |
| CD4 PE | 555347 | BD Biosciences |
| CD3 APC | 17-0037-42 | eBioscience |
| CD8 APC-H7 | 560179 | BD Biosciences |
| CD16 eF450 | 48-0168-42 | eBioscience |
| CD45 KO | B36294 | Beckman Coulter |
| Panel 2 | ||
| CD56 FITC | 11-0566-42 | eBioscience |
| CD27 PE | 555441 | BD Biosciences |
| CD3 APC | 17-0037-42 | eBioscience |
| CD19 APC-H7 | 560177 | BD Biosciences |
| CD16 eF450 | 48-0168-42 | eBioscience |
| CD45 KO | B36294 | Beckman Coulter |
| Panel 3 | ||
| CD45 FITC | 347463 | BD Biosciences |
| CD3 PE | 345765 | BD Biosciences |
| CD4 PB | 317424 | Biolegend |
| CD38 PE-Cy5 | 555461 | BD Biosciences |
| CD279 PE-Cy7 | 329918 | Biolegend |
| CD14 APC | 555399 | BD Biosciences |
3. Results
3.1. Clinical course
A 53-year old man was diagnosed with nasopharyngeal diffuse large B-cell lymphoma in 2018. Chemotherapy with R-CHOP/R-DHAP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone/rituximab, dexamethasone, cytarabine, cisplatin) was administered, following autologous stem cell transplantation and radiotherapy of Waldeyer's ring. Maintenance therapy with an anti-CD20 monoclonal antibody (MoAb) rituximab was continued every three months in the next two years.
Five weeks after the last rituximab administration, the patient complained of fatigue, nausea, and fever (up to 39 °C) caused by SARS-CoV-2 infection which was confirmed by RT-PCR test of a nasopharyngeal swab. Initially, home care was recommended. Further, five days later, the patient came back to the emergency department because of persistent pyrexia and malaise. A blood test showed lymphopenia (6.7%, 0.7 × 109/L) and elevated inflammatory marker, C reactive protein (CRP) (100.2 mg/L). Chest roentgenogram (RTG) revealed bilateral interstitial lung infiltrates (Fig. 1 A) with respiratory insufficiency. The patient was hospitalized and oxygen therapy with prophylactic low-molecular-weight heparin (LMWH), dexamethasone and proton-pump inhibitor was initiated, in accordance with the guidelines given by the Croatian Ministry of Health [26]. During the hospitalization, the patient was constantly febrile with a gradual worsening of dyspnea and clinical deterioration. The control tests showed the progression of bilateral pneumonia in chest RTG and an increase in CRP (178 mg/L) with neutropenia (26.3%, 0.2 × 109/L). All blood cultures were sterile, bacterial superinfection was not proven, but because of the clinical deterioration, empirical antibiotic therapy (piperacillin-tazobactam), filgrastim and a therapeutic dose of LMWH were administered. Head, neck, chest, abdomen and pelvic computed tomography did not show any signs of lymphoma relapse.
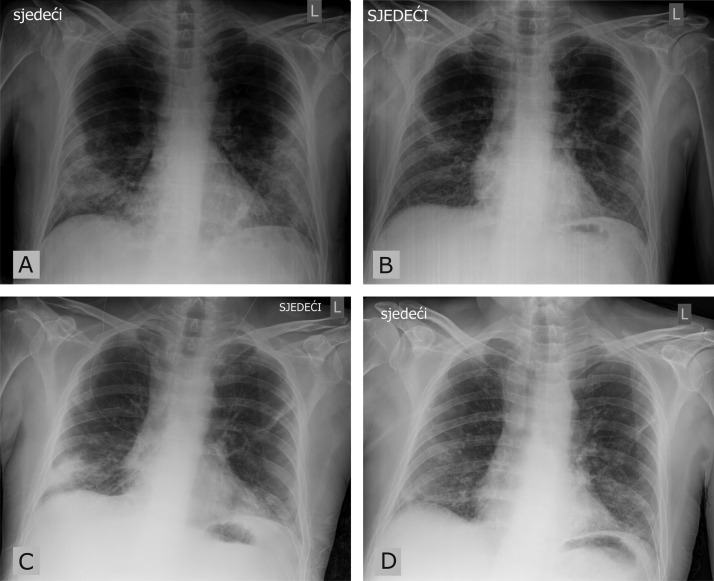
Fig. 1.
Chest roentgenogram. A) admission to the hospital, day 13 of the disease; B) after the first cycle of convalescent plasma therapy, day 58 of the disease; C) upon COVID-19 symptoms recurrence and second admission to hospital, day 98 of the disease; D) after the second cycle of convalescent plasma therapy, day 115 of the disease.
In serology testing, specific SARS-CoV-2 antibodies were not detected, SARS-CoV-2 RT-PCR test of the nasopharyngeal swab on day 45 was still positive and an infective virus was detected in nasopharyngeal culture.
3.2. COVID-19 convalescent plasma therapy outcomes
Considering all the above-described and still active COVID-19 even after 45 days from the onset of symptoms, therapy with ABO-compatible COVID-19 convalescent plasma was applied. The patient's consent was obtained before the transfusion. A total of six units of COVID-19 convalescent plasma (200 mL each) were administered on the 48th, 49th, 54th, 55th, 56th and 57th day of the disease. The therapy with remdesivir was administered for five days, starting from the 55th day of the disease. Following COVID-19 convalescent plasma therapy, the patient was afebrile, with a decline in CRP (9.8 mg/L). A chest RTG showed the regression of lung infiltrates (Fig. 1B) and oxygen therapy was no longer needed. The patient was discharged to home isolation. On the 67th day of the disease, the patient complained about chilblain-like lesions with acral purpuric and necrotic finger skin changes. Color doppler ultrasound revealed normal arterial circulation on both hands, and acetylsalicylic acid was added to the therapy, resulting in partial regression of the skin changes. On the 98th day of the disease, the patient was presented with a fever (up to 38.5˚C), dyspnea and yellow sputum expectoration. A chest RTG showed bilateral pneumonia progression (Fig. 1C), while CRP was elevated (138 mg/L). The patient was hospitalized, there were no specific SARS-CoV-2 antibodies in control tests, while SARS-CoV-2 was detected by RT-PCR test of blood, nasopharyngeal swab and culture. Due to a still active COVID-19, after more than 100 days from the onset of symptoms, two units of COVID-19 convalescent plasma were administered (200 mL each, on 105th and 109th day of the disease), along with the antibiotic therapy following the sputum antibiogram. After the convalescent therapy, oxygen therapy was reduced, CRP level decreased (52.7 mg/L) and the patient was afebrile with regression of pneumonia in chest RTG (Fig. 1D). Although the SARS-CoV-2 RT-PCR serum test was positive on the 129th day of the disease, virus isolation from the serum samples was unsuccessful, possibly indicating the absence of infective virus in the blood.
3.3. Laboratory investigations
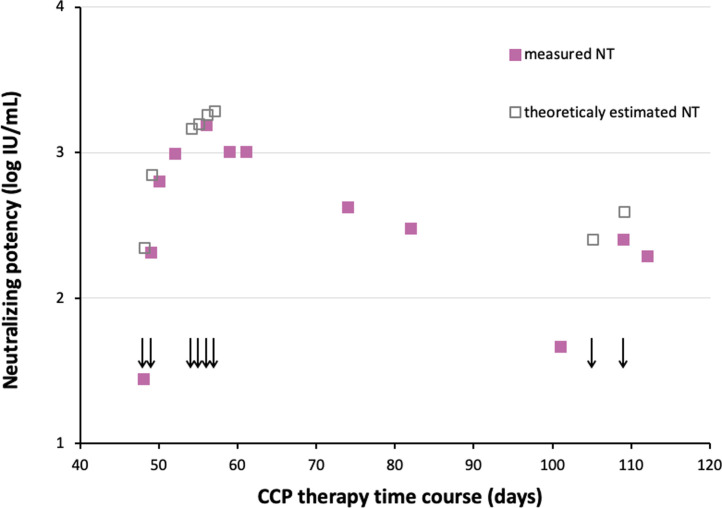
Neutralizing titers (NTs) of antibodies in the patient's serum samples were continuously quantified (full squares in Fig. 2 ). Knowing the volume and NTs of infused COVID-19 convalescent plasma donations, and assuming that blood volume comprises 8% of the patient's body weight, the theoretically expected NTs in serum were calculated by summing up values of all infused plasma units (empty squares in Fig. 2) and compared to the measured neutralizing activity in the patient's serum. The theoretical and real values nicely overlapped at the beginning of the therapy, followed by the slow decrease of real NTs, due to the exhaustion of available antibodies for virus neutralization and/or natural clearance of immunoglobulins from the serum. Such antibody dynamics was in harmony with the improvement of the clinical presentation. Subsequent plasma administration on 105th and 109th day from COVID-19 onset showed the outcome which was consistent with the preceding administration, as shown in Fig. 2.
Fig. 2.
Timeline of neutralizing antibodies titers measured in the serum samples of the presented immunodeficient patient during the therapy with SARS-CoV-2-specific convalescent plasma (CCP), together with their theoretical estimates. CCP infusions are denoted by arrows.
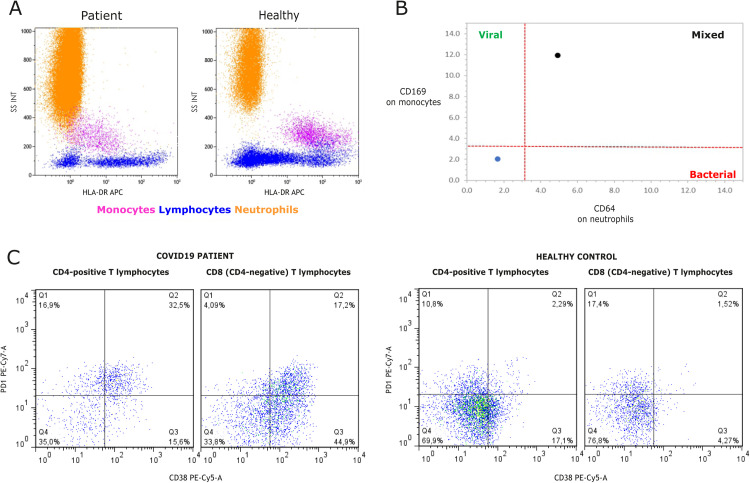
In line with a low total lymphocyte count (0.7 × 109/L), the peripheral blood immunophenotyping, performed by flow cytometry before the second cycle of COVID-19 convalescent plasma administration, showed the predominance of CD3+ T-lymphocytes (92.33%), the normal value of NK cells (7.64%), the absolute lack of CD19+ B-lymphocytes (0%) and a reduced CD4+/CD8+ ratio (0.33), due to the low level of helper T-lymphocytes (0.09 × 109 cell/L), while the level of cytotoxic T-lymphocytes (0.27 × 109/L) remained normal. The additional analysis revealed immune exhausted monocytes with a reduced HLA-DR expression and an increased CD169 expression accompanied by the increased CD64 expression on neutrophils (Fig. 3 ). Moreover, a high level of HLA-DR-positive NK cells (60% out of total NK cells) and also programmed cell death protein-1 (PD-1) expression on CD4 (49%) and CD8 (21%) lymphocytes were found.
Fig. 3.
Immunophenotyping of the peripheral blood leukocytes by flow cytometry: A) Side scatter (SSC) and HLA-DR expression on leukocytes from the patient (left panel) and a healthy control (right panel). B) CD64 expression on neutrophils and CD169 expression on monocytes from the patient (black dot) and a healthy control (blue dot). C) Increased percentages of exhaustion marker PD-1 positive peripheral blood CD4+ and CD8+ T-lymphocytes of COVID-19 patient (left panel) compared to age and sex-matched healthy control (right panel). Interestingly and unlikely for healthy control, in COVID-19 patient most PD-1 positive T-cells express activation marker CD38 that has been associated with the inability to control prolonged/chronic viral infections.
4. Discussion
The patients with hematological malignancies are a vulnerable group to COVID-19, due to the immunodeficiency resulting from the underlying disease and oncological treatment that can impair cellular and humoral immunity [27].
Our case is the example of a prolonged COVID-19 in the patient with lymphoma in which the persistence of positive RT-PCR test for SARS-CoV-2 and the absence of virus-specific antibodies are probably the consequence of anti-CD20 MoAb rituximab therapy [18], resulting in an active viral replication lasting for 129 days. Rituximab, frequently used as a treatment for hematological malignancy and autoimmune disease, generally is well-tolerated but can cause secondary humoral deficiency. Rituximab has a divergent cytotoxic mechanism toward CD20+ cells–it inhibits cell proliferation either by directly binding to B-cells receptors or by complement-mediated and antibody-dependent cell-mediated cytotoxicity [18]. The results of B-cell depletion are reduced antibody production and transient hypogammaglobulinemia [28]. Most studies have shown that B-lymphocytes usually recover from naive cells in bone marrow usually six months after rituximab therapy [28]. Additionally, T-lymphocytes have an important function in viral clearance but prolonged exposure to the virus can cause T-cells’ functional impairment [29], [30]. Even the patients without comorbidities develop lymphopenia in COVID-19, more often in severe forms of the disease [31], [32]. Lymphopenia is the primary result of a reduced number of CD4+ and CD8+ lymphocytes, but the mechanism is complex and not fully understood [31].
The immunophenotyping tests have shown that our patient also has impaired cellular immunity. (Fig. 3). Commonly, immune check receptor PD-1 is overexpressed on CD4+ and CD8+ T-cells of COVID-19 patients and seems to be associated with the alterations in cytokines release due to T-lymphocytes depletion and exhaustion, as PD-1 regulates lymphocyte function and apoptosis [33], [34]. Suppressed activity of T-lymphocytes and their reduced number lead to the impaired viral control and clearance [34], particularly in more severe cases. A prolonged infection correlates with higher PD-1 expression, while the infection resolution is usually followed by PD-1 reduction and recovery of T-lymphocytes [33]. Another factor of a poor prognosis in viral infection and bacterial sepsis is a reduced HLA-DR on monocytes, a sign of monocyte exhaustion, which leads to their impaired antigen-presentation activity [35].
The results of the current COVID-19 convalescent plasma studies are diverse and generally inconclusive. Based on the results of the largest trial, Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19 program, the FDA released the emergency authorization for the COVID-19 convalescent plasma use which was revised in February 2021, limiting it to the hospitalized patients with the impaired humoral immunity in the early stages of the disease [36]. However, the definition of an “early stage of COVID-19” and the exact algorithm of convalescent plasma treatment for COVID-19 patients have not been defined yet [37].
5. Conclusion
We suggest that for some patients with impaired cellular and humoral immunity, it would be reasonable to frequently analyze neutralizing antibody titers and periodically administer COVID-19 convalescent plasma, or purified polyclonal SARS-CoV-2-specific antibodies, to maintain effective antibody blood levels. Also, it would be useful to track T- and B-cell recovery with immunophenotyping tests. Taking into consideration all the above mentioned, COVID-19 convalescent plasma or preferably anti-SARS-CoV-2 immunoglobulins [38] (to reduce the chance of unwanted coagulation disturbances appearance) could be a long-term, “chronic” therapy against COVID-19 for this group of patients, especially for bridging the period when the immune system cannot produce by itself the antibodies needed for viral clearance. In this way, we could achieve a partial control of prolonged, active COVID-19, and convert it into a chronic infection. For the immunodeficient patients as in the presented case, this could reduce mortality and maybe “buy precious time” for the immune system to recover. Further research is needed, especially in the area of immunodeficient patients’ participation in the clinical trials for COVID-19 treatment.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
This research was funded by Croatian Science Foundation (grant IP-CORONA-04-2053 to BH) and by European Regional Development Fund, grant number KK.01.1.1.01.0006, “Strengthening the capacity of CerVirVac for research in virus immunology and vaccinology”.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., et al. Author correction: a new coronavirus associated with human respiratory disease in China. Nature [Internet] 2020;580:E7. doi: 10.1038/s41586-020-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease 2019 (COVID19) Treatment Guidlines. National Instututes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 28.02.2021. Coronavirus Disease 2019 (COVID19) Treatment Guidlines. National Instututes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed.[28.02.2021.].
- 3.Jin H., Reed J.C., Liu S.T.H., Ho H.-E., Lopes J.P., Ramsey N.B., et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol In practice [Internet] 2020;8:3594–3596. doi: 10.1016/j.jaip.2020.08.059. [e3. Available from: https://pubmed.ncbi.nlm.nih.gov/32947026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velkury (remdesivir) EUA Letter of Approval. FDA O 2020. No Title [Internet]. Available from: https://www.fda.gov/media/137564/download.
- 5.Yiğenoğlu T.N., Hacıbekiroğlu T., Berber İ., Dal M.S., Baştürk A., Namdaroğlu S., et al. Convalescent plasma therapy in patients with COVID-19. J Clin Apher Journal [Internet] 2020;35:367–373. doi: 10.1002/jca.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown B.L., McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci [Internet] 2020;59:102790. doi: 10.1016/j.transci.2020.102790. Available from: https://pubmed.ncbi.nlm.nih.gov/32345485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garraud O., Lacombe K., Tiberghien P. 2020. A look-back at convalescent plasma to treat COVID-19; pp. 19–22. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garraud O., Heshmati F., Pozzetto B., Lefrere F., Girot R., Saillol A., et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23:39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morabito C.J., Gangadharan B. Active therapy with passive immunotherapy may be effective in the fight against COVID-19. Clin Translational Sci [Internet] 2020;13:835–837. doi: 10.1111/cts.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev [Internet] 2020;19 doi: 10.1016/j.autrev.2020.102554. 102554. Available from: https://pubmed.ncbi.nlm.nih.gov/32380316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Algaissi A., Hashem A.M. Evaluation of MERS-CoV neutralizing antibodies in sera using live virus microneutralization assay. Methods Mol Biol [Internet] 2020;2099:107–116. doi: 10.1007/978-1-0716-0211-9_9. Available from: https://pubmed.ncbi.nlm.nih.gov/31883091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbarao K., Mordant F., Rudraraju R. Convalescent plasma treatment for COVID-19: tempering expectations with the influenza experience. Eur J Immunol [Internet] 2020;50:1447–1453. doi: 10.1002/eji.202048723. [DOI] [PubMed] [Google Scholar]
- 13.Hueso T., Pouderoux C., Péré H., Beaumont A.-L., Raillon L.-A., Ader F., et al. Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19. Blood [Internet] 2020;136:2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell [Internet] 2020;183:1901–1912. doi: 10.1016/j.cell.2020.10.049. e9. Available from: https://www.sciencedirect.com/science/article/pii/S0092867420314562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baang J.H., Smith C., Mirabelli C., Valesano A.L., Manthei D.M., Bachman M.A., et al. Prolonged severe acute respiratory syndrome Coronavirus 2 replication in an immunocompromised patient. J Infect Dis [Internet] 2021;223:23–27. doi: 10.1093/infdis/jiaa666. Available from: https://pubmed.ncbi.nlm.nih.gov/33089317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honjo K., Russell R.M., Li R., Liu W., Stoltz R., Tabengwa E.M., et al. Convalescent plasma-mediated resolution of COVID-19 in a patient with humoral immunodeficiency. Cell Reports Medicine [Internet] 2021;2:100164. doi: 10.1016/j.xcrm.2020.100164. Available from: https://www.sciencedirect.com/science/article/pii/S2666379120302111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S., Caprioli C., Weber A., Rambaldi A., Lussana F. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk Lymphoma [Internet] 2021:1–9. doi: 10.1080/10428194.2021.1872070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore J.L., Ganapathiraju P.V., Kurtz C.P., Wainscoat B. A 63-year-old woman with a history of nonhodgkin lymphoma with persistent sars-cov-2 infection who was seronegative and treated with convalescent plasma. Am J Case Reports. 2020;21:1–5. doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay D., Seah C., Schneider T., Bhalla S., Feld J., Naymagon L., et al. Convalescent plasma for the treatment of severe COVID-19 infection in cancer patients. C Med. 2020;9:8571–8578. doi: 10.1002/cam4.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.London J., Boutboul D., Lacombe K., Pirenne F., Heym B., Zeller V., et al. Severe COVID-19 in Patients with B Cell Alymphocytosis and Response to Convalescent Plasma Therapy. J Clin Immunol. 2021;41:356–361. doi: 10.1007/s10875-020-00904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brgles M., Kurtović T., Lang Balija M., Hećimović A., Mušlin T., Halassy B. Impact of complement and difference of cell-based assay and ELISA in determination of neutralization capacity against mumps and measles virus. J Immunol Methods [Internet] 2021;490:112957. doi: 10.1016/j.jim.2021.112957. Available from: https://www.sciencedirect.com/science/article/pii/S0022175921000028. [DOI] [PubMed] [Google Scholar]
- 23.Halassy B., Kurtović T., Brgles M., Lang Balija M., Forčić D. Factors influencing preclinical in vivo evaluation of mumps vaccine strain immunogenicity. Hum vaccin immunotherapeutics [Internet] 2015;11:2446–2454. doi: 10.1080/21645515.2015.1062191. Available from: https://pubmed.ncbi.nlm.nih.gov/26376015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streitz M., Miloud T., Kapinsky M., Reed M.R., Magari R., Geissler E.K., et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplantation research [Internet] 2013;2:17. doi: 10.1186/2047-1440-2-17. Available from: https://pubmed.ncbi.nlm.nih.gov/24160259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourgoin P., Soliveres T., Barbaresi A., Loundou A., Belkacem I.A., Arnoux I., et al. CD169 and CD64 could help differentiate bacterial from CoVID-19 or other viral infections in the Emergency Department. Cytometry Part A. 2021 doi: 10.1002/cyto.a.24314. n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidelines for COVID-19 treatment. Croatian Ministry of health N 2020. No Title [Internet]. Available from: https://zdravlje.gov.hr/UserDocsImages//2020 CORONAVIRUS//Smjernice za liječenje oboljelih od koronavirusne bolesti 2019 (COVID-19), verzija 2 od 19. studenoga 2020.pdf?.
- 27.Clark E., Guilpain P., Filip I.L., Pansu N., le Bihan C., Cartron G., et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol [Internet] 2020;190:e154–e156. doi: 10.1111/bjh.16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts D.M., Jones R.B., Smith R.M., Alberici F., Kumaratne D.S., Burns S., et al. Rituximab-associated hypogammaglobulinemia: Incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–65. doi: 10.1016/j.jaut.2014.11.009. Available from: https://www.sciencedirect.com/science/article/pii/S0896841114001735. [DOI] [PubMed] [Google Scholar]
- 29.Sattler A., Angermair S., Stockmann H., Heim K.M., Khadzhynov D., Treskatsch S., et al. SARS–CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest [Internet] 2020;130:6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T Cells in patients with Coronavirus Disease 2019 (COVID-19) Frontiers in immunology [Internet] 2020;11:827. doi: 10.3389/fimmu.2020.00827. Available from: https://pubmed.ncbi.nlm.nih.gov/32425950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z., Long W., Tu M., Chen S., Huang Y., Wang S., et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J infect [Internet] 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.054. Available from: https://pubmed.ncbi.nlm.nih.gov/32283159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghbash P.S., Eslami N., Shamekh A., Entezari-Maleki T., Baghi H.B. SARS-CoV-2 infection: The role of PD-1/PD-L1 and CTLA-4 axis. Life sciences [Internet] 2021;270:119124. doi: 10.1016/j.lfs.2021.119124. Available from: https://pubmed.ncbi.nlm.nih.gov/33508291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunology letters [Internet] 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. Available from: https://pubmed.ncbi.nlm.nih.gov/32569607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benlyamani I., Venet F., Coudereau R., Gossez M., Monneret G. Monocyte HLA-DR measurement by flow cytometry in COVID-19 patients: an interim review. Cytometry Part A [Internet] 2020;97:1217–1221. doi: 10.1002/cyto.a.24249. [DOI] [PubMed] [Google Scholar]
- 36.Coronavirus (COVID-19) Update: February 5, 2021. FDA F 2021. (https://www. fda. gov/news-events/press-announcements/coronavirus-covid-19-update-february-5-2021). No Title.
- 37.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384 doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali S., Uddin S.M., Ali A., Anjum F., Ali R., Shalim E., et al. Production of hyperimmune anti-SARS-CoV-2 intravenous immunoglobulin from pooled COVID-19 convalescent plasma. Immunotherapy [Internet] 2021;13:397–407. doi: 10.2217/imt-2020-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]