Abstract
Introduction
“Re-infection” with COVID-19 is a growing concern; re-infection cases have reported worldwide. However, the clinical characteristics of SARS-CoV-2 re-infection, including the levels and role of anti-SARS-CoV-2 Spike protein IgG antibodies and the half-maximal concentration (IC50) of neutralizing antibodies remain unknown.
Methods
Both the epidemiological and clinical information has been collected during two episodes of COVID-19 in a patient. Laboratory results, including RT-PCR, Ct values, anti-SARS-CoV-2 Spike protein IgG antibodies, and the IC50 of neutralizing antibodies levels were analyzed on the patient.
Results
The patient was a 58-year-old man who developed moderate COVID-19 pneumonia with oxygen demand (cannula 2 L/min) in the first episode. By day 30, he recuperated and was discharged after testing negative for SARS-CoV-2. After two and a half months, his three family members showed COVID-19 symptoms and tested positive for SARS-CoV-2. He tested positive for SARS-CoV-2 once again and was asymptomatic (the second episode). The IC50 of neutralizing antibodies against SARS-CoV-2 greatly increased from 50.0 μg/mL (after the first episode) to 14.8 μg/mL (after the second episode), and remained strongly reactive (20.1 μl/mL) after 47 days of the second episode.
Conclusions
Epidemiological, clinical, and serological analyses confirmed that the patient had re-infection instead of persistent viral shedding from first infection. Our results suggest that SARS-CoV-2 re-infection may manifest as asymptomatic with increased neutralizing antibody levels. Further studies such as the virus characteristics, immunology, and epidemiology on SARS-CoV-2 re-infection are needed.
Keywords: COVID-19, SARS-CoV-2, Re-infection, Anti-Spike protein IgG antibody, Neutralizing antibody
1. Introduction
Since December 2019, the novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide; more than 120 million cases including over 2.6 million deaths have been detected [1].
Over 25 million patients have recovered from COVID-19, but “re-infection” cases are a major concern [[2], [3], [4]]. As IgG antibody titers and neutralizing activity are elevated initially and decline after 1–2 months of acute infection, re-infection is a theoretical possibility [5,6]. Although some re-infection cases with differences in viral genome sequences have been reported [[2], [3], [4]], the clinical characteristics of SARS-CoV-2 re-infection including the levels and role of anti-Spike protein neutralizing antibodies and the half-maximal concentration (IC50) of neutralizing antibodies remain unknown.
Here, we evaluate the epidemiological, clinical, and serological data from an asymptomatic patient re-infected with SARS-CoV-2105 days after the first episode.
2. Patients and methods
Ethical approval
This study was approved by the ethics committee of the NCGM (approval no: NCGM-G-003536-03) and was conducted in accordance with the Declaration of Helsinki.
2.1. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
COVID-19 was diagnosed based on SARS-CoV-2 RNA detection in nasopharyngeal swab-samples using qRT-PCR [7].
2.2. Enzyme-linked immunosorbent assay (ELISA)
Recombinant SARS-CoV-2 Spike protein was prepared from cells transfected with a plasmid bearing the DNA encoding the full-length protein using the Expi293 expression system (Thermo Fisher Scientific, Waltham, MA) [8]. The purified protein was coated onto a MaxiSorp 96-well ELISA plate (Thermo Fisher Scientific) overnight at 4 °C. Coated wells were blocked with 1% BlockAce (KAC, Kyoto, Japan) for 1 h at 37 °C and washed six times with phosphate-buffered saline (PBS). The 1/800-diluted patient serum/plasma samples were added and incubated at 37 °C for 1 h. The plate was washed with PBS-T (PBS containing 0.2% Tween 20) and incubated with horseradish peroxidase-conjugated anti-human IgG (GeneTex, Irvine, CA) at 37 °C for 30min. After washing with PBS-T, the captured anti-Spike antibodies were detected with 3,3′,5,5′-tetramethylbenzidine substrate (Nacalai Tesque, Kyoto, Japan). Finally, absorbance at 450 nm (OD450) was measured using a microplate reader (Bio-Rad, Irvine, CA). Samples from healthy volunteer without SARS-CoV-2 were used as negative controls, whereas those from infected patients with high levels of anti-Spike antibodies were used as positive controls. Each sample was assayed in triplicate. Each antibody titer (OD ratio) was calculated by dividing each sample OD value by the mean plus 6 standard deviation of the negative control OD values.
2.3. Levels of neutralizing antibodies
IgG fractions were obtained from convalescent patients' plasma/serum to detect neutralizing antibodies. We used TMPRSS2-overexpressing VeroE6 (VeroE6TMPRSS2) cells and SARS-CoV-205−2N (isolated from a patient with COVID-19- [CoV-05]) treated at the NCGM hospital. IgG fractions were purified using a spin column-based antibody purification kit (Cosmo Bio, Tokyo, Japan) according to the manufacturer's instructions. For the antiviral assay, VeroE6TMPRSS2 cells were seeded overnight in 96-well plates (1 × 104 cells/well). SARS-CoV-205−2N was then mixed with each of the purified IgG fractions, incubated for 20 min at 37 °C, and inoculated into the VeroE6TMPRSS2 cells. After culturing for 3 days, cytopathic effects in SARS-CoV-2-exposed cells were determined using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).
3. Results
3.1. Epidemiological and clinical course of the patient
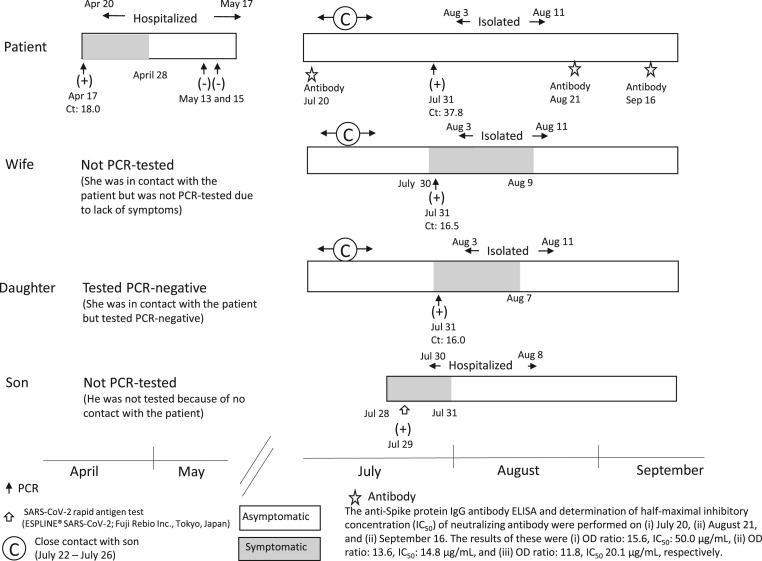
A 58-year-old Japanese man, with a history of mild dyslipidemia, underwent nasopharyngeal testing for SARS-CoV-2 RNA and presented with fever on April 17, 2020. After two days, qRT-PCR revealed a positive readout (cycle threshold [Ct] values of 18.1), and the patient was hospitalized. He works as a branch manager of a company, and his driver, who drives him to and from the company for 1 h one way, was diagnosed with severe COVID-19 a few days before. His daughter who had contact with him developed cough and tested negative for SARS-CoV-2 RNA, whereas his wife was asymptomatic and did not undergo testing. His son had no contact with the patient and did not require testing. Upon admission, his body temperature was 37.5 °C. Chest computed tomography showed multiple bilateral patchy ground glass opacities. Blood test results showed C-reactive protein levels of 7.1 mg/dL and leukocyte counts of 3700 cells/μL. On April 21, favipiravir was administered due to high oxygen demand (cannula 2 L/min) to treat moderate COVID-19 pneumonia. His fever broke on April 28. Favipiravir was administered until May 4. On May 17, his symptoms improved, two sequential nasopharyngeal qRT-PCR tests were negative, and he was discharged. There was no relapse of symptoms after discharge (the first episode).
On July 31, the patient, his wife, and daughter underwent a nasopharyngeal test for SARS-CoV-2 RNA because they had close contact with his son, who was febrile and diagnosed with COVID-19 because of positive result of SARS-CoV-2 antigen test on July 29. qRT-PCR readouts were positive for the patient, his wife, and daughter with Ct values of 37.8, 16.5, and 16.0, respectively. Although the patient was asymptomatic, his wife and daughter had mild fever and dysosmia for several days. They returned home after 10 days of hotel isolation (the second episode) (Fig. 1 ).
Fig. 1.
Timeline of SARS-CoV-2 infection.
The patient was admitted to the hospital after presenting symptoms related to moderate COVID-19 pneumonia between April 20 and May 17, 2020. He showed no relapse of symptoms after discharge (the first episode). On July 31, the patient, his wife, and daughter underwent a nasopharyngeal test for SARS-CoV-2 RNA after coming in close contact with his son, who was febrile and was diagnosed with COVID-19 on July 29. Quantitative reverse transcription-polymerase chain reaction showed a positive readout for SARS-CoV-2, and they were isolated in a hotel for 10 days (the second episode). The half-maximal inhibitory concentration (IC50) of neutralizing antibodies was 50.0 μg/mL after first episode (July 20). However, the IC50 of neutralizing antibodies after the second episode (August 21) was greater than that after the first episode (14.8 μg/mL), and that at 152 days after the onset of the first infection (September 16) remained strongly positive at 20.1 μg/mL.
3.2. Anti-spike protein IgG antibodies and neutralizing antibodies against SARS-CoV-2
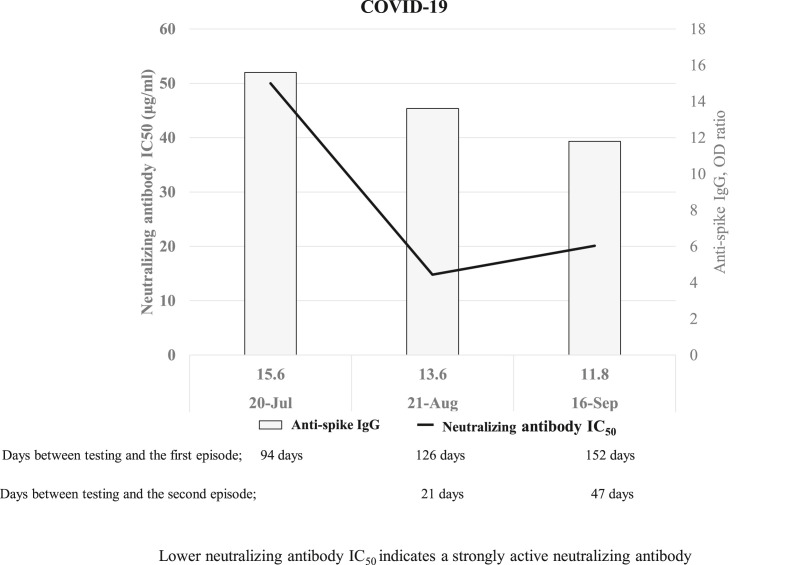
On July 20, the patient visited our hospital to take screening as a candidate for convalescent plasma donor of COVID-19, and then he received the antibody titer test before the second episode. The anti-SARS-CoV-2 Spike protein IgG antibodies levels on July 20 (94 days after the first episode), August 21 (126 days after the first and 21 days after the second episode), September 16 (152 days after the first and 47 days after the second episode) remained high (OD ratios were 15.6, 13.6, and 11.8, respectively). Although the IC50 of neutralizing antibodies on July 20 was high (50 μg/mL), that on August 21 was lower, suggesting a strong reactivity (14.8 μg/mL), that was maintained on September 16 (20.1 μg/mL) (Figs. 1 and 2 ).
Fig. 2.
The results of anti-spike IgG and neutralizing antibody IC50upon re-infection with COVID-19.
Lower the half-maximal inhibitory concentration (IC50) of neutralizing antibodies indicates a strongly active neutralizing antibody. The IC50 of neutralizing antibodies was 50.0 μg/mL after first episode (July 20). However, the IC50 of neutralizing antibodies after the second episode (August 21) was greater than that after the first episode (14.8 μg/mL), and that at 152 days after the onset of the first infection (September 16) remained strongly positive at 20.1 μg/mL.
4. Discussion
We evaluate the results of elevated IC50 of neutralizing antibodies before and after the second episode of an asymptomatic case of COVID-19 re-infection. In the previously reported cases, two genetically different SARS-CoV-2 strains were responsible for re-infection, as per whole-genome sequencing results [[2], [3], [4]]. Although we could not isolate the virus, the patient was diagnosed as re-infected for the following reasons. First, the interval between the first and second episodes was 105 days. In a previous case report, a pregnant woman (immunocompromised) showed prolonged SARS-CoV-2 RNA shedding for 104 days [9]. Among most COVID-19 patients, SARS-CoV-2 RNA was reduced to undetectable levels one month after the onset of symptoms [5,10,11]. Concurrently, our patient tested negative for SARS-CoV-2 one month after the onset of symptoms. Second, the patient had close contact with a newly infected family member, and intra-family discrepancy was found in Ct values and clinical symptoms. Third, the IC50 of neutralizing antibodies declined from weakly reactive; 50 μg/mL (after the first episode) to strongly reactive; 14.8 μg/mL (after the second episode), and at 47 days from the second episode they remained strongly positive as 20.1 μg/mL (Fig. 2), despite declined neutralizing antibody levels after 1–2 months of acute infection [3].
SARS-CoV-2 expresses spike protein on its surface and its receptor binding domain (RBD) attaches to angiotensin converting enzyme 2 (ACE2) of host cell. Previous report showed spike protein has multiple epitopes including RBD, and some anti-RBD antibodies prevent RBD-ACE2 binding and function as neutralizing antibodies [12]. There is correlation between the overall levels of anti-spike or anti-RBD IgG antibodies and neutralizing activity [13], while non-anti-spike proteins may have neutralizing activity as shown in SARS-CoV, 2002 [14]. Our study may demonstrate the role of anti-Spike protein and neutralizing antibodies in SARS-CoV-2 re-infection. Anti-Spike protein IgG was present ∼3 months after the first infection, but was weakly reactive. However, at 21 days after re-infection, neutralizing antibody levels increased, suggesting that re-infection induced neutralizing antibody production.
Clinical significance of neutralizing antibodies against SARS-CoV-2 has not been well understood, but our study and several reports may indicate the clinical significance. Regarding first episode of infection, previous reports revealed that anti-spike antibody titers, anti-RBD antibody titer, and neutralizing antibody activities tended to be higher in patients with severe disease than in those with mild or moderate disease [6,13,15]. Although the severity of COVID-19 remains unknown in re-infection cases [2,16,17], this patient was asymptomatic. We think that high levels of neutralizing antibodies may reduce disease onset and severity in reinfection episode. First, SARS-CoV-2 mRNA vaccination successfully induced neutralizing antibody and showed efficacy for protection [18,19]. Second, an asymptomatic re-infection patient will be rarely diagnosed because such patients do not seek for medical care and diagnostic test. For these reasons, there may be much more asymptomatic or mild symptomatic re-infection patients like our patient worldwide. This suggests that re-infection episode will be too mild or even asymptomatic to be observed due to neutralizing antibodies and other acquired immune response.
While our patient is not incredibly old, nor does he have the obvious immunodeficiency (only dyslipidemia), there are a few reports suggesting the role of neutralizing antibody in immunocompromised host with persistent COVID-19 [20,21]. One is a case of a woman in her 60s with history of non-Hodgkin lymphoma in remission while on maintenance therapy with obinutuzumab, anti-CD20 monoclonal antibody, who has persistent symptoms and positive nasopharyngeal PCR test over 12 weeks, and her IgG antibodies for SARS-CoV-2 were undetectable. The patient's symptoms resolved after convalescent plasma administration [20]. Another report described a case of a man in his 70s with history of marginal B cell lymphoma with previous chemotherapy including vincristine, prednisolone, cyclophosphamide, and rituximab. Despite repeated administration of convalescent plasma and his own anti-RBD of spike antibody production, SARS-CoV-2 produced genomic variants during the course, and he deceased on day 102 [21]. Both cases show persistent infection may occur in immunocompromised host. Convalescent plasma or neutralizing antibody could protect against infection, but it would not be sufficient depending on the type and degree of host immunodeficiency or viral mutation.
This study has some limitations. First, this is about the evaluation of anti-spike antibodies and neutralizing antibodies in a single case of suspected re-infection. Second, we could not isolate the virus and compare differences in genome-wide sequences. However, increased neutralizing antibody levels suggest re-infection. Despite these limitations, to best our knowledge, this case is the first possible SARS-CoV-2 re-infection case in Japan.
In conclusion, SARS-CoV-2 re-infection may manifest as asymptomatic with increased neutralizing antibody levels. Further studies such as the virus characteristics, immunology, and epidemiology on SARS-CoV-2 re-infection are needed.
5. ICMJE statement
All authors meet the ICMJE authorship criteria.
Author contributions are as described below.
Conceptualization: Makoto Inada, Masahiro Ishikane, Mari Terada.
Data curation: Makoto Inada, Masahiro Ishikane, Mari Terada, Kenji Miura, Yu Sairenji.
Formal analysis: Akihiro Matsunaga, Kenji Maeda, Kiyoto Tsuchiya, Yukihito Ishizaka, Hiroaki Mitsuya.
Supervision: Noriko Kinoshita, Mugen Ujiie, Satoshi Kutsuna, Yukihito Ishizaka, Hiroaki Mitsuya, Norio Ohmagari.
All authors contributed to the writing of the final manuscript.
Funding
This work was supported by the Health, Labor and Welfare Policy Research Grants, Research on Emerging and Reemerging Infectious Diseases and Immunization [grant number 20HA1006], Japan Agency for Medical Research and Development [grant numbers JP19fk0108163, JP20fk0108160, and JP20fk108262], and the NCGM Intramural Research Fund [grant number 20A2003D].
Ethical approval
This study was approved by the ethics committee of the NCGM (approval no: NCGM-G-003536-03) and was conducted in accordance with the principles embodied in the Declaration of Helsinki.
Declaration of competing interest
All authors declare no conflicts of interest regarding this study.
Acknowledgments
We thank Drs. Rei Inoue and Hiroko Aiko (Department of Respiratory Medicine, Yokohama Sakae Kyosai Hospital) for providing patient care, Drs. Okba N.M.A. and Haagmans B.L. (Dept. Vioscience, Erasmus Medical Center, NL) for providing the plasmid encoding full-length SARS-CoV-2 Spike protein, Drs. Teratake Y and Ueno M (Dept. Intractable Diseases, NCGM) for preparing the Spike protein, and the City of Yokohama Public Health Center and Yokohama City Institute of Public Health for close contact investigation and nasopharyngeal testing for SARS-CoV-2 RNA.
References
- 1.WHO, World Health Organization Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation- reports (Accessed March 19, 2021)
- 2.To K.K., Hung I.F., Ip J.D., Chu A.W., Chan W.M., Tam A.R., et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020;25 doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta V., Bhoyar R.C., Jain A., Srivastava S., Upadhayay R., Imran M., et al. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Elslande J., Vermeersch P., Vandervoort K., Wawina-Bokalanga T., Vanmechelen B., Wollants E., et al. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutsuna S., Asai Y., Matsunaga A. Loss of anti-SARS-CoV-2 antibodies in mild covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 7.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 8.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina L.P., Chow S.-K., Nickel A., Love J.E. Prolonged detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in an obstetric patient with antibody seroconversion. Obstet Gynecol. 2020;136(4):838–841. doi: 10.1097/AOG.0000000000004086. [DOI] [PubMed] [Google Scholar]
- 10.KCDC, Korea Centers for Disease Control and Prevention Findings from investigation and analysis of re-positive cases Korean centers for disease control & prevention. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&l%20ist_no=367267&nPage=1
- 11.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emilie S., Leah J.H., Anna J.M., Rachael P.K., Nicholas K.H., Madeleine F.J., et al. Analysis of a SARS-CoV-2-infected individual reveals development of potent neutralizing antibodies with limited somatic mutation immunity. 2020 Jul 14;53(1):98–105. doi: 10.1016/j.immuni.2020.06.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davide F.R., Christian G., Frauke M., Julio C.C.L., Zijun W., Alice C., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 Aug;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph A.T., Lia M.H., Deborah M., Barbara A., Azaibi T., Brian H.H., et al. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J Virol Methods. 2005 Sep;128(1–2):21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christian G., Zijun W., Julio C.C.L., Frauke M., Shlomo F., Minami T., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021 Jan 18 doi: 10.1038/s41586-021-03207-w. [DOI] [Google Scholar]
- 16.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BNO News COVID-19 reinfection tracker. https://bnonews.com/index.php/2020/08/covid-19-reinfection-tracker/
- 18.Edward E.W., Robert W.F., Ann R.F., Nicholas K., Judith A., Alejandra G., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020 Dec 17;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernando P.P., Stephen J.T., Nicholas K., Judith A., Alejandra G., Stephen L., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joanna L.M., Pavan V.G., Caroline P.K., Booth W. A 63-year-old woman with a history of non- Hodgkin lymphoma with persistent SARS-CoV-2 infection who was seronegative and treated with convalescent plasma. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steven A.K., Dami A.C., Rawlings P.D., Isabella A.T.M.F., Salma G., Aminu J., et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021 Feb 5 doi: 10.1038/s41586-021-03291-y. [DOI] [Google Scholar]