Abstract
Aims
The proportion of UK oncology healthcare professionals (HCPs) infected with SARS-CoV-2 during the COVID-19 pandemic's first wave is unknown. The primary aim of this study was to determine the SARS-CoV-2 infection and seroprevalence rates among HCPs.
Materials and methods
Patient-facing oncology HCPs working at three large UK hospitals during the COVID-19 pandemic's first wave underwent polymerase chain reaction (PCR) and antibody testing [Luminex and point-of-care (POC) tests] on two occasions 28 days apart (June–July 2020).
Results
In total, 434 HCPs were recruited: nurses (58.3%), doctors (21.2%), radiographers (10.4%), administrators (10.1%); 26.3% reported prior symptoms suggestive of SARS-CoV-2. All participants were PCR negative during the study, but 18.4% were Luminex seropositive on day 1, of whom 42.5% were POC seropositive. Nurses had the highest seropositive prevalence trend (21.3%, P = 0.2). Thirty-eight per cent of seropositive HCPs reported previous SARS-CoV-2 symptoms: 1.9 times higher odds than seronegative HCPs (P = 0.01). Of 400 participants retested on day 28, 13.3% were Luminex seropositive (92.5% previously, 7.5% newly). Thirty-two per cent of initially seropositive HCPs were seronegative on day 28.
Conclusion
In this large cohort of PCR-negative patient-facing oncology HCPs, almost one in five were SARS-CoV-2 antibody positive at the start of the pandemic's first wave. Our findings that one in three seropositive HCPs retested 28 days later became seronegative support regular SARS-CoV-2 PCR and antibody testing until widespread immunity is achieved by effective vaccination.
Key words: Antibody, COVID-19, healthcare professionals, PCR, SARS-CoV-2, serology
Introduction
The global SARS-CoV-2 (COVID-19) pandemic has caused substantial morbidity and economic turmoil across the world. Since the start of the pandemic, various populations in multiple countries have been tested for SARS-CoV-2 infection by polymerase chain reaction (PCR), with a wide range of reported asymptomatic positive carrier rates (4–80%) [[1], [2], [3], [4], [5]]. Antibody tests are the gold standard for providing evidence of exposure to pathogens following an adaptive immune response, including SARS-CoV-2 infection [6,7]. Rates of antibody seroconversion after SARS-CoV-2 infection seem to vary but have been consistently high. Examples during the first wave of the pandemic include the first large Chinese series (n = 285) of hospitalised SARS-CoV-2 PCR-positive patients, where 100% developed SARS-CoV-2 IgG within 19 days of symptom onset [8]; a study of 624 SARS-CoV-2 PCR-positive outpatients, where 82% developed antibodies 10–14 days after symptom onset [9]; and a study of 14 SARS-CoV-2 PCR-positive healthcare professionals (HCPs), where 71% developed antibodies 15 days after PCR testing [10]. The significance of antibody generation in terms of subsequent disease protection is likely to be one of multiple mechanisms of SARS-CoV-2 immunity [11].
Most HCPs do not work directly with SARS-CoV-2-infected hospital inpatients. HCP seropositivity data collected during the first pandemic wave show a range of reported rates. A French HCP study reported three (1.3%) of 230 asymptomatic PCR-naïve HCPs to be SARS-CoV-2 IgG positive. All three HCPs were PCR negative at the time of testing, suggesting past exposure with successful eradication of the virus [10]. A Spanish HCP study reported an asymptomatic HCP SARS-CoV-2 seropositive rate of 7.6% (44/578) [12], and a Swedish study reported an overall HCP seroprevalence of 19.1% (410/2149), of which 1.2% (37/2149) were asymptomatic [13]. A UK study reported an asymptomatic IgG seroprevalence of 1% (4/400) and a symptomatic IgG seroprevalence of 3% (12/400) [14], whereas another UK study reported an asymptomatic IgG seroprevalence of 17.1% and a symptomatic IgG seroprevalence of 37.5% [15]. Data from screening asymptomatic HCPs for active SARS-CoV-2 viral infection during the first pandemic wave show similar results. In the UK, one study showed a peak asymptomatic HCP infection rate of 7.1% in late March 2020, decreasing to 1.1% 5 weeks later [16], whereas another study reported that 30 (3%) of 1032 asymptomatic hospital HCPs tested in April 2020 were SARS-CoV-2 PCR positive [17].
People with cancer may be more likely to contract SARS-CoV-2 infection [18,19]. This risk is amplified by multiple hospital attendances required for diagnosis, treatment and follow-up. Although recent results suggest that systemic anti-cancer treatment (SACT) does not increase mortality in infected cancer patients [20], it may increase the risk of serious complications following infection [18,19]. Although guidelines advise SARS-CoV-2 testing for cancer patients prior to and during SACT, the risks posed from and to HCPs working in oncology departments is not known. The COVID-19 Serology in Oncology Staff (CSOS) study is a multicentre UK study designed to determine SARS-CoV-2 infection and antibody seroprevalence rates among the main groups of HCPs coming into contact with cancer patients attending hospital for SACT during the peak of the first wave of the UK COVID-19 pandemic, with sample collection at multiple time points [21]. The primary objective was to determine the SARS-CoV-2 infection and seroprevalence rates among HCPs. Secondary outcomes included the proportion of previously symptomatic and asymptomatic SARS-CoV-2-seropositive HCPs, how long SARS-CoV-2 seropositivity lasted, the rate of persistent asymptomatic PCR positivity over time and the proportion of HCPs who did not become seropositive following a positive PCR result.
Materials and Methods
HCPs involved in treating cancer patients in non-surgical oncology departments at three UK hospitals in the Eastern Region were recruited (see Supplementary Material for details). HCPs were excluded if they had not been working during the pandemic peak from March to June 2020 or were not patient facing. HCPs (which included patient-facing administrative staff, such as clinic receptionists, healthcare assistants and ward clerks) had to be working within the oncology department ward or outpatient setting and not primarily within a dedicated SARS-CoV-2 inpatient ward. HCPs who returned to work after self-isolating due to SARS-CoV-2 symptoms or exposure to a potentially affected household member (as per UK Government rules) were eligible to participate. Following consent, samples were collected during June 2020 (day 1 samples) and July 2020 (day 28 samples). Samples collected were a nasopharyngeal swab for SARS-CoV-2 PCR testing and blood for SARS-CoV-2 antibody testing. Anonymised participant characteristics were collected: age, sex, job role, smoking status, history of any underlying health conditions, details of any suspected SARS-CoV-2 illness/exposure (high temperature, new cough, alterations in taste/smell) and leave taken, dates of start to resolution of presumed or confirmed SARS-CoV-2 illness, date of any prior SARS-CoV-2 tests and results. Regulatory approval for the study was granted by the UK Health Research Authority (IRAS: 284231; 26 May 2020). Data were analysed using Prism 8 (Graphpad Software) and R. One-way ANOVA was used to compare multiple means; Fisher's exact test to compare groups and categorical variables; and the Mann–Whitney U test to compare day 1 antibody-positive outcomes at day 28.
SARS-CoV-2 Polymerase Chain Reaction Assay
RNA extraction was undertaken using the Zymo (Cambridge, UK) Quick-RNA 96 kit (R1053). SARS-CoV-2 PCR detection was carried out using Primerdesign's (Eastleigh, UK) Coronavirus COVID-19 Genesig RT-PCR assay (Z-Path-COVID-19-CE) and a Roche LightCycler 480 in a 96-well format. All kits were used according to manufacturer's guidelines.
SARS-CoV-2 Serology Assays
Two different antibody assays were used to detect SARS-CoV-2 antibodies: a rapid point-of-care (POC) finger-prick assay and a laboratory-based assay.
The POC test was the Abbexa (Cambridge, UK) COVID-19 IgG/IgM Rapid Test Kit (abx294171), which detects antibodies against the SARS-CoV-2 nucleocapsid (N) and spike (S) antigens. The manufacturer claimed no cross-reactivity with antibodies against other coronaviruses (HKU1, OC43, NL63, 229E) and a sensitivity of 98.5% and a specificity of 97.94%. Results from a finger-prick blood sample were read 10–15 min after assaying.
The laboratory-based assay was a SARS-CoV-2 multiplex particle-based flow cytometry (Luminex) assay developed at Addenbrooke's Hospital (Cambridge UK), which detects IgG antibodies against the SARS-CoV-2 N and full-length S antigens [22] and was run as described previously [22,23]. The test requires 4 ml blood, collected in a serum tube, and results were generally available within 2–3 days of sample collection. Sensitivity and specificity were reported to be 84% and 100%, respectively, based on a cohort of pre-pandemic healthy controls versus a cohort of unselected SARS-CoV-2 PCR-positive patients [19]. At the time of writing, testing for cross-reactivity against other coronaviruses had not been carried out for this semi-quantitative assay. A level of detection comparison was carried out between the abovementioned SARS-CoV-2 IgG assays using sequentially diluted SARS-CoV-2 IgG-positive and -negative sera.
Results
Baseline Characteristics
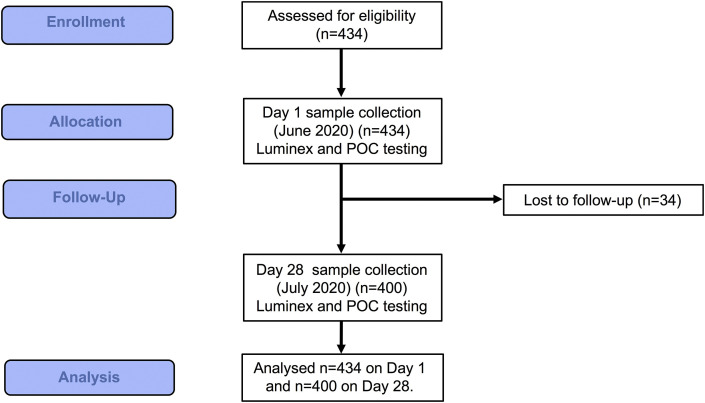
The characteristics of 434 recruited HCPs (see Figure 1 ) are summarised in Table 1 (aggregated) and Supplementary Table S1 (by hospital site). Data from the pilot study (n = 70) were also included [21]. Most of the participants were nurses (253/434; 58.3%) and doctors (92/434; 21.2%), with smaller proportions of radiographers (45/434; 10.4%) and administrative staff (44/434; 10.1%). Physiotherapists (n = 8) were grouped with the nurses as their job role involved similar close physical patient contact. Most of the participants were female (356/434; 82%) (P < 0.001); the median participant age was 41 years (range 19–66). Administrators had the highest median age (median 47.5 years) followed by doctors (42 years), nurses (39 years) and radiographers (33 years) (P < 0.001). Chronic underlying health conditions were reported in 103/434 (23.7%) of all participants (Table 1), with asthma and hypertension being the most common in all groups (see Supplementary Table S2).
Fig 1.
CONSORT diagram: study outline.
Table 1.
Participant characteristics
| Total | Doctors | Nurses | Radiographers | Administrators | |
|---|---|---|---|---|---|
| Number | 434 | 92 | 253 | 45 | 44 |
| Median age, years (range) | 40 (19–66) | 42 (25–63) | 39 (21–65) | 33 (19–57) | 47.5 (24–66) |
| Female | 356 (82%) | 49 (53.3%) | 227 (89.7%) | 39 (86.7%) | 41 (93.2%) |
| Male | 78 (18%) | 43 (46.7%) | 26 (10.3%) | 6 (13.3%) | 3 (6.8%) |
| Smoker | 37 (8.5%) | 2 (2.1%) | 22 (8.7%) | 2 (4.4%) | 11 (25%) |
| Underlying health condition | 103 (23.7%) | 15 (16.3%) | 60 (23.7%) | 14 (31.1%) | 14 (31.8%) |
| Previously symptomatic healthcare professionals | |||||
| Prior symptoms suggestive of COVID-19 | 114/434 (26.3%) | 31/92 (33.7%) | 65/253 (25.7%) | 8/45 (17.8%) | 10/44 (22.7%) |
| Median symptom duration in days (range) | 7.5 (1–61) | 7 (1–61) | 8 (1–48) | 7 (2–20) | 8 (1–37) |
| Median time (days) from symptom resolution to sample collection (range) | 67 (1–172) | 70 (1–169) | 67 (8–172) | 63 (28–139) | 69 (12–94) |
| Pre-study SARS-CoV-2 PCR test | 178/434 (41%) | 46/92 (50%) | 108/253 (42.7%) | 15/45 (33.3%) | 9/44 (20.5%) |
| Pre-study positive PCR test | 6/178 (3.4%) | 3/46 (6.5%) | 3/108 (2.7%) | 0/15 | 0/9 |
| Previously asymptomatic healthcare professionals | |||||
| Asymptomatic during pandemic | 320/434 (73.7%) | 61/92 (66.3%) | 188/253 (74.3%) | 37/45 (82.2%) | 34/44 (77.3%) |
| Asymptomatic, but household member possibly symptomatic | 47/320 (14.7%) | 11/61 (18%) | 27/188 (14.4%) | 3/37 (8.1%) | 6/34 (17.6%) |
PCR, polymerase chain reaction.
In total, 114 (26.3%) participants reported symptoms suggestive of SARS-CoV-2 infection prior to study entry (Table 1); 33.7% of doctors, 25.7% of nurses, 22.7% of administrators and 17.8% of radiographers reported symptoms. The median duration of reported symptoms was 7.5 (range 1–61) days and was similar for all staff groups (P = 0.1). The median time from symptom resolution to the first study sample collection date was 67 days (range 1–172), with no differences between staff groups (P = 0.8). In total, 178 (41%) participants underwent nasopharyngeal SARS-CoV-2 PCR testing between March and June 2020 prior to the initiation of this study (doctors had the highest frequency of testing, P = 0.006). Seventy of those previously tested had experienced symptoms, 108 were asymptomatic and were tested as part of hospital screening programmes. Six of 70 (12.9%) of the previously tested symptomatic population were SARS-CoV-2 PCR positive (three doctors and three nurses), whereas the previously tested asymptomatic group were all PCR negative. Most participants (320; 73.7%) reported no prior symptoms. Of these, 47 (14.7%) reported a household member having symptoms suggestive of SARS-CoV-2 infection.
Seroprevalence of Antibodies Against SARS-CoV-2
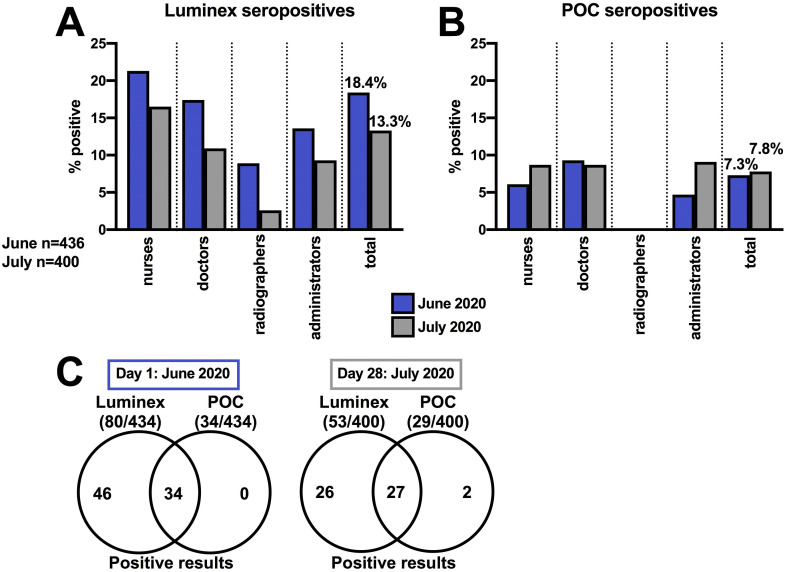
All participants tested at day 1 (June 2020) were nasopharyngeal swab PCR negative for SARS-CoV-2 (Table 2 and Supplementary Table S3). SARS-CoV-2 IgG was detected at day 1 in 80 (18.4%) participants using the Luminex test and in 34 (7.8%) participants using the POC test (Figure 2 A,B; Table 2). All seropositive participants using the POC test were also seropositive using the Luminex test (Figure 2C). The Luminex test was able to detect SARS-CoV-2 IgG at 1:100 dilution, whereas the POC test was less sensitive (Supplementary Table S4), so results from the Luminex assay were subsequently used to explore participant serology status (seropositive or seronegative).
Table 2.
SARS-CoV-2 polymerase chain reaction (PCR) and antibody [Luminex and point-of-care (POC)] test results at day 1 and day 28
| Total | Doctors | Nurses | Radiographers | Administrators | |
|---|---|---|---|---|---|
| Day 1 |
n = 434 |
n = 92 |
n = 253 |
n = 45 |
n = 44 |
| Positive PCR test | 0/434 | 0/92 | 0/253 | 0/45 | 0/44 |
| Positive Luminex antibody test | 80/434 (18.4%) | 16/92 (17.4%) | 54/253 (21.3%) | 4/45 (8.9%) | 6/44 (13.6%) |
| Seropositive for antibodies to both N and S antigens | 38/80 (47.5%) | 8/16 (50%) | 28/54 (51.9%) | 0/4 | 2/6 (33.3%) |
| Seropositive for antibodies to only N antigen | 32/80 (40%) | 7/16 (43.8%) | 18/54 (33.3%) | 3/4 (75%) | 4/6 (66.7%) |
| Seropositive for antibodies to only S antigen | 10/80 (12.5%) | 1/16 (6.3%) | 8/54 (14.8%) | 1/4 (25%) | 0/6 |
| Seropositive after pre-study positive PCR | 6/6 (100%) | 3/3 (100%) | 3/3 (100%) | NA | NA |
| Seropositive, previously symptomatic | 30/114 (26.3%) | 9/31 (29%) | 19/65 (29.2%) | 0/8 (0%) | 2/10 (20%) |
| Seropositive, previously asymptomatic | 50/320 (15.6%) | 7/61 (11.5%) | 35/188 (18.6%) | 4/37 (10.8%) | 4/34 (11.7%) |
| Seropositive, previously asymptomatic with possible household contact | 12/47 (25.5%) | 2/11 (18.2%) | 10/27 (37%) | 0/3 (0%) | 0/6 (0%) |
| Seropositive, with chronic underlying health condition | 18/103 (17.5%) | 4/15 (26.7%) | 12/60 (20%) | 2/14 (14.3%) | 0/14 (0%) |
| Seropositive, smokers | 9/37 (24.3%) | 1/2 (50%) | 6/22 (27.3%) | 0/2 (0%) | 2/11 (18.2%) |
| Positive POC test |
34/434 (7.8%) |
8/92 (8.7%) |
22/253 (8.7%) |
0/45 |
4/44 (9.1%) |
| Day 28 |
n = 400 |
n = 82 |
n = 237 |
n = 38 |
n = 43 |
| Positive PCR test | 0/400 | 0/82 | 0/237 | 0/38 | 0/43 |
| Positive Luminex antibody test | 53/400 (13.3%) | 9/82 (10.9%) | 39/237 (16.5%) | 1/38 (2.6%) | 4/43 (9.3%) |
| Seropositive for antibodies to both N and S antigens | 34/53 (64.2%) | 7/9 (77.8%) | 25/39 (64.1%) | 0/1 (0%) | 2/4 (50%) |
| Seropositive for antibodies to only N antigen | 9/53 (16.9%) | 0/9 (0%) | 7/39 (17.9%) | 0/1 (0%) | 2/4 (50%) |
| Seropositive for antibodies to only S antigen | 10/53 (18.9%) | 2/9 (22.2%) | 7/39 (17.9%) | 1/1 (100%) | 0/4 (0%) |
| Seropositive on days 1 and 28 | 49/53 (92.5%) | 9/9 (100%) | 35/39 (89.7%) | 1/1 (100%) | 4/4 (100%) |
| Seronegative on day 1, seropositive on day 28 | 4/53 (7.5%) | 0/9 (0%) | 4/39 (10.3%) | 0/1 (0%) | 0/4 (0%) |
| Seropositive on day 1, seronegative on day 28 | 26/80 (32.5%) | 5/16 (29.4%) | 17/54 (31.2%) | 2/4 (50%) | 2/6 (33.3%) |
| Positive POC antibody test | 29/400 (7.3%) | 5/82 (6.1%) | 22/237 (9.3%) | 0/38 (0%) | 2/43 (4.7%) |
Fig 2.
(A) Proportion of healthcare professionals who were Luminex seropositive and (B) point-of-care (POC) seropositive on day 1 (June 2020) and day 28 (July 2020). (C) Comparison of participant seropositivity by method at each time point.
At day 1, nurses were the HCPs with the highest proportion of SARS-CoV-2 seropositivity (54/253; 21.3%), followed by doctors (16/92; 17.4%), administrators (6/44; 13.6%) and radiographers (4/45; 8.9%), although these differences were not statistically significant (P = 0.2) (Figure 1A, Table 2). Of the 80 seropositive HCPs, 38 (47.5%) had antibodies to both the N and S SARS-CoV-2 antigens, 32 (40%) had antibodies only to the N antigen and 10 (12.5%) had antibodies only to the S antigen (Table 2). All participants with a previous positive PCR result tested seropositive (6/6; 100%), with detectable antibodies to both N and S SARS-CoV-2 antigens.
Thirty-eight per cent (30/80) of HCPs who were seropositive on day 1 reported previous symptoms consistent with SARS-CoV-2 infection: a 1.9 times higher odds than seronegative participants (84/354) (P = 0.01) (Table 2). Of the total number of participants reporting previous symptoms, 50/320 (15.6%) had detectable SARS-CoV-2 antibodies at day 1, which was similar across staff groups (P = 0.4). Of 47 participants who had no prior symptoms, but had been exposed to a suspected infected household member, 12 (25.5%) had positive antibodies (Table 2). There was no significant difference in seropositivity between those with (18/103; 17.5%) or without (62/331; 18.7%) underlying health conditions (P = 0.2), or between smokers (9/37; 24.3%) and non-smokers (71/397; 17.9%) (P = 0.6) (Table 2).
Seroprevalence Repeat Testing After 28 Days
In total, 400 (92.2%) participants were retested on day 28 during July 2020 (Table 2). All participants remained PCR negative for SARS-CoV-2 on retesting. Positive SARS-CoV-2 serology was detected in 53/400 (13.3%) participants using the Luminex test and in 29/400 (7.3%) using the POC test (Figure 2A,B). Of the 29 seropositive HCPs detected by the POC test at day 28, 27 were also positive with the Luminex test, but two were not (Figure 2C). The two discordant results were previously negative using both assays at day 1 (Table 2 and Supplementary Table S3) with both participants denying any intervening symptoms or virus exposure between testing dates.
Nurses remained the staff group with the highest rate of Luminex test seropositivity (39/237; 16.5%), followed by doctors (9/82; 10.9%), administrators (4/43; 9.3%) and radiographers (1/38; 2.6%), although the trend did not reach statistical significance (P = 0.07) (Figure 2A).
Thirty-two per cent (26/80) of those previously seropositive at day 1 with the Luminex test were seronegative on day 28 (Table 2). Of those HCPs who were seropositive on day 28, 49/53 (92.5%) were persistently positive on both day 1 and day 28, and 4/53 (7.5%) were new seroconversions, with no reported symptoms. All those with a positive SARS-CoV-2 PCR test prior to study entry remained SARS-CoV-2 seropositive using the Luminex test (6/6; 100%).
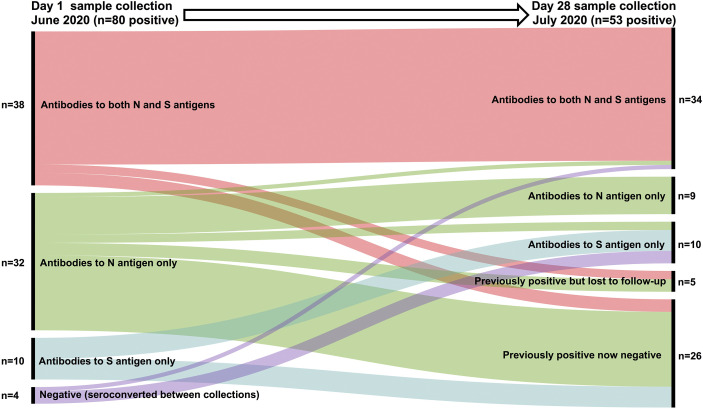
Of the 53 seropositive participants identified at day 28 with the Luminex test, 34 (64.2%) had IgG antibodies to both the N and S SARS-CoV-2 antigens, whereas nine (16.9%) had IgG antibodies to only the N antigen and 10/53 (18.9%) to only the S antigen (Table 2). Loss of seropositivity affected all antigen target groups, with the largest decrease affecting those who were IgG N antigen positive on day 1 (P < 0.0001) (Table 2, Table 3 ). At day 28, 18 of the 33 participants originally positive on day 1 with IgG antibodies reactive to the N antigen alone became seronegative, whereas two lost their N antigen antibodies and developed S antigen antibodies (Table 3). This loss only affected those who were previously asymptomatic. In those who were retested on day 28, there was no loss of positivity in the previously symptomatic group who had antibodies reactive against both N and S antigen. In the asymptomatic group, loss of positivity by day 28 was lowest in those with antibodies reactive to both N and S antigens, compared with participants reactive to only one antigen (Table 3). These findings are visualised in Figure 3 .
Table 3.
Antigenic targets in SARS-CoV-2 IgG antibody-positive participants (as determined by Luminex testing) contrasted by presence or absence of reported symptoms
| Antibody epitope |
Day 1 (positive) | Loss of positivity | Lost to follow-up | New sero-conversions | Epitope changes | Day 28 (positive) | |
|---|---|---|---|---|---|---|---|
| Previously reported symptoms | N and S antigens | 24 | 0 | 2 | 0 | 22 | |
| N antigen only | 3 | 0 | 1 | 0 | 1x N->S swap | 1 | |
| S antigen only | 3 | 1 | 0 | 1 | 4 | ||
| Previously asymptomatic | N and S antigens | 14 | 3 | 0 | 1 | 12 | |
| N antigen only | 29 | 18 | 2 | 0 | 1x N->S swap | 8 | |
| S antigen only | 7 | 4 | 0 | 2 | 6 | ||
| Total | 80 | –26 | –5 | +4 | 53 |
N, nucleocapsid; S, spike.
Fig 3.
Summary of relationship between day 1 and day 28 positive IgG antibody results by SARS-CoV-2 antigen target. All participants were nasopharyngeal swab SARS-CoV-2 polymerase chain reaction negative at the time of SARS-CoV-2 antibody testing.
Discussion
The CSOS study is the first report of SARS-CoV-2 exposure in patient-facing oncology HCPs who worked during the first COVID-19 pandemic peak between March and June 2020 within non-surgical oncology departments in three hospitals of differing size and structure in the Eastern Region of the UK. In this large cohort of PCR-negative HCPs, almost one in five were SARS-CoV-2 antibody positive at the start of June 2020, suggesting significant prior SARS-CoV-2 infection. Seropositivity was, not surprisingly, higher in those HCPs who described previous symptoms compared with those who did not. Nearly one-third of seropositive HCPs became seronegative after 28 days. Comparing HCP subgroups, nurses had the highest seroprevalence rates at both time points.
We used two different antibody detection methods to compare and contrast their potential clinical utility for measuring SARS-CoV-2 serology. The POC test, with the advantage of immediate read-out within 15 min, was reported by the manufacturer to have high sensitivity and specificity and to not cross-react with the four main other coronavirus types. Of note, a study of 11 POC tests (not including the POC test used in our study) reported sensitivity and specificities lower than manufacturer claims [24], which fits with our findings. The Luminex test, which had a processing time of 2–3 h, but real-time results returned between 2 and 4 days, was able to detect antibodies at a much lower concentration level compared with the POC test. Although already being used for routine clinical practice at one of the study hospitals (Addenbrooke's Hospital, Cambridge, UK), at the time of writing the Luminex test had not been investigated for cross-reactivity against other coronaviruses, so we cannot exclude the possibility of false positives and that participants may have been exposed to other coronaviruses rather than SARS-CoV-2 infection. Although the risk of false positives should be acknowledged as a risk to the validity of this study, it is reassuring that a recent study found no cross-reactivity between antibodies against SARS-CoV-2's N antigen and antigens of endemic coronaviruses [25]. In order to ensure maximal validity, we used both tests. If we had only used the POC test, we would have missed over half the cases with lower levels of seropositivity.
Using the Luminex test, almost one in five staff members were SARS-CoV-2 seropositive despite being PCR negative at the time of sampling, which suggests a substantial past exposure and infection rate, especially considering that most participants were not working within dedicated SARS-CoV-2 wards or participating in high-risk aerosol-generating procedures in known SARS-CoV-2 patients. Our finding of 18.4% seroprevalence is higher than large-scale community seroprevalence studies during the pandemic's first wave, which have reported seropositive rates ranging from 5% (in 51 958 Spanish participants) [26] to 6% (in 109 076 UK participants) [27] and is on the higher end of non-frontline HCP seroprevalence studies, ranging from 5.4% (in 244 French HCPs) [10], 9.3% (in 578 Spanish HCPs) [28] to 19.1% (410/2149 Swedish HCPs) [13]. Our finding that symptomatic individuals had a higher odds of being seropositive than asymptomatic individuals fits with previous reports [11]. We hypothesise that most staff became infected at work rather than in the community, due to the nature of the hospital admission process and general patient care, compounded by the issue of personal protective equipment being less readily available within the National Health Service in the early stages of the pandemic.
This hypothesis is further substantiated when considering outcomes among staff groups. Nurses seem to be the most affected group, followed by doctors, administrators (all of whom were patient facing, with roles such as receptionists, healthcare assistants or ward clerks) and radiographers: although nurses represented 58% of total participants, they also accounted for 67% of total seropositive cases on day 1 and 74% of total seropositive cases on day 28. The seroprevalence rate of 21.3% of nurses tested on day 1 and 16.5% tested on day 28 was the highest of all the four staff groups. Although not statistically significant compared with other staff groups (due to the smaller numbers of other staff groups recruited), the daily interactions of nurses with multiple patients at close quarters will undoubtedly have contributed to these stark statistics.
Our finding that 32.5% of seropositive participants became seronegative during our 4-week study interval is consistent with other reports showing declining seropositivity over time [[29], [30], [31]]. That asymptomatic participants were the majority of those who became seronegative confirms a previous report suggesting that SARS-CoV-2 antibody response may be dependent on disease severity [29].
We also noted that two previously asymptomatic seropositive participants lost their previously detectable IgG antibodies against the N antigen while developing detectable IgG antibodies against the S antigen. This may suggest an evolving humoral immune response in both participants, but the possibility of artefact cannot be excluded. Although there are insufficient data available to clarify the implications of having either N or S antigen IgG antibodies, there is evidence that IgG antibodies targeting the SARS-CoV-2 S antigen can elicit both neutralising responses (viral eradication) [32,33] and non-neutralising responses (no viral eradication) [34] and that detectable N antigen antibodies develop earlier than S antigen antibodies [35]. This opens interesting avenues for further research.
This study's findings add to the growing literature, enhancing our understanding of SARS-CoV-2, which will inform future policy decisions during this ongoing, as well as future, pandemics. Participants continue to be followed in this study, with additional samples being collected over time. This will enable assessment of the durability of SARS-CoV-2 antibody responses in previously symptomatic and asymptomatic individuals, as well as the impact of any future surges in infection rates. Uncertainty exists regarding the Luminex test cross-reactivity against endemic coronaviruses, while the rapid turnaround speed of the POC test result was offset with loss in test sensitivity.
In terms of clinical application, earlier guidance from the UK [36] and from the European Society for Medical Oncology [37] recommends that all patients receiving SACT should undergo SARS-CoV-2 PCR testing prior to starting treatment and that consideration should be given to subsequent testing at intervals during treatment. The guidance advises testing HCPs in the broadest sense. Our study has identified the first pandemic wave rates of SARS-CoV-2 seropositivity in oncology HCPs in the East of England, but these rates are likely to fluctuate significantly over time [29,31,38]. Learning from the past pandemic year has taught us that HCPs are at high risk of viral infection and that they themselves are a source of infection risk to the patients they are caring for. Although our study was not designed to conclude on ideal mitigation strategies, it adds weight to supporting routine regular testing of oncology HCPs (especially nurses) for viral antigen and antibodies during both the current and future pandemics. The increasing availability of lower cost, high sensitivity and specificity SARS-CoV-2 testing methods should make this targeted approach viable, which would help protect patients and staff by enabling containment and large-scale tracking of new asymptomatic infections until there is high-prevalence durable immunity following the ongoing SARS-CoV-2 vaccination programme.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
This study was funded by the Oncology Department Charity Fund at the Queen Elizabeth Hospital NHS Foundation Trust, the Oncology Department Research Fund at Peterborough City Hospital, North West Anglia NHS Foundation Trust, and the Addenbrooke's Charitable Trust. Funders had no role in the study’s design and analysis or in the writing of this report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2021.04.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou X., Li Y., Li T., Zhang W. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(7):957–959. doi: 10.1016/j.cmi.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368:m1165. doi: 10.1136/bmj.m1165. [DOI] [PubMed] [Google Scholar]
- 4.Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation . 2020. Q&A: similarities and differences – COVID-19 and influenza. [Google Scholar]
- 6.Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 9.Wajnberg A., Mansour M., Leven E., Bouvier N.M., Patel G., Firpo A. Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region. medRxiv. 2020 04.30.20085613. [Google Scholar]
- 10.Solodky M.L., Galvez C., Russias B., Detourbet P., N’Guyen-Bonin V., Herr A.L. Lower detection rates of SARS-COV2 antibodies in cancer patients vs healthcare workers after symptomatic COVID-19. Ann Oncol. 2020;31(8):1087–1088. doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudberg A.S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augusto J.B., Menacho K., Andiapen M., Bowles R., Burton M., Welch S. Healthcare Workers Bioresource: Study outline and baseline characteristics of a prospective healthcare worker cohort to study immune protection and pathogenesis in COVID-19. Wellcome Open Res. 2020;5:179. doi: 10.12688/wellcomeopenres.16051.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treibel T.A., Manisty C., Burton M., McKnight Á., Lambourne J., Augusto J.B. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favara D.M., Cooke A., Doffinger R., McAdam K., Corrie P., Ainsworth N.L. COVID-19 serology in oncology staff study: understanding SARS-CoV-2 in the oncology workforce. Clin Oncol. 2021;33(1):e61–e63. doi: 10.1016/j.clon.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong X., Qu K., Ciazynska K.A., Hosmillo M., Carter A.P., Ebrahimi S. A thermostable, closed SARS-CoV-2 spike protein trimer. Nat Struct Mol Biol. 2020;27(10):934–941. doi: 10.1038/s41594-020-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favara D.M., Ceron-Gutierrez M.L., Carnell G.W., Heeney J.L., Corrie P., Doffinger R. Detection of breastmilk antibodies targeting SARS-CoV-2 nucleocapsid, spike and receptor-binding-domain antigens. Emerg Microbe Infect. 2020 Dec;9(1):2728–2731. doi: 10.1080/22221751.2020.1858699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tollånes M.C., Bakken Kran A.M., Abildsnes E., Jenum P.A., Breivik A.C., Sandberg S. Evaluation of eleven rapid tests for detection of antibodies against SARS-CoV-2. Clin Chem Lab Med. 2020;58:1595–1600. doi: 10.1515/cclm-2020-0628. [DOI] [PubMed] [Google Scholar]
- 25.de Assis R.R., Jain A., Nakajima R., Jasinskas A., Felgner J., Obiero J.M. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent plasma using a coronavirus antigen microarray. bioRxiv. 2020 doi: 10.1038/s41467-020-20095-2. 04.15.043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward H., Atchison C.J., Whitaker M., Ainslie K.E.C., Elliott J., Okell L.C. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv. 2020 08.12.20173690. [Google Scholar]
- 28.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. medRxiv. 2020 doi: 10.1038/s41467-020-17318-x. 04.27.20082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 30.Seow J., Graham C., Merrick B., Acors S., Steel K.J.A., Hemmings O. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv. 2020 07.09.20148429. [Google Scholar]
- 31.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 33.Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A.J. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachim A., Kavian N., Cohen C.A., Chin A.W.H., Chu D.K.W., Mok C.K.P. ORF8 and ORF3b antibodies are accurate serological markers of early and late SARS-CoV-2 infection. Nat Immunol. 2020;21(10):1293–1301. doi: 10.1038/s41590-020-0773-7. [DOI] [PubMed] [Google Scholar]
- 35.Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S. Detection of nucleocapsid antibody to SARS-CoV-2 is more sensitive than antibody to spike protein in COVID-19 patients. medRxiv. 2020 04.20.20071423. [Google Scholar]
- 36.Roques T., Board R. Royal College of Radiologists; London: 2020. Guidance on SARS-CoV-2 antigen testing for asymptomatic healthcare workers (HCW) and patients in non-surgical oncology in the UK. [Google Scholar]
- 37.Curigliano G., Banerjee S., Cervantes A., Garassino M.C., Garrido P., Girard N. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31:1320–1335. doi: 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward H., Cooke G., Atchison C., Whitaker M., Elliott J., Moshe M. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020 doi: 10.1016/j.lanepe.2021.100098. 10.26.20219725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.