Abstract
Objectives
Disinfection effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on human skin remains unclear because of the hazards of viral exposure. An evaluation model, which has been previously generated using human skin obtained from forensic autopsy samples, accurately mimics in vivo skin conditions for evaluating the effectiveness of disinfection against the virus. Using this model, we evaluated disinfection effectiveness against viruses on human skin.
Methods
Ethanol (EA), isopropanol (IPA), chlorhexidine gluconate (CHG) and benzalkonium chloride (BAC) were used as target disinfectants. First, disinfectant effectiveness against SARS-CoV-2 and influenza A virus (IAV) was evaluated in vitro. Disinfectant effectiveness against SARS-CoV-2 and IAV on human skin was then evaluated by titrating viruses present on the skin after applying each disinfectant on the skin for 5–60 seconds.
Results
Both, SARS-CoV-2 and IAV on human skin were completely inactivated within 5 seconds by 40%–80% EA and 70% IPA (log reduction values (LRVs) were >4). However, SARS-CoV-2 and IAV were barely inactivated by 20% EA (LRVs were <1). In vitro evaluation showed that, compared with EA and IPA, CHG and BAC were significantly inferior in terms of disinfection effectiveness. Conversely, the disinfection effectiveness of CHG and BAC against SARS-CoV-2 was higher on human skin than in vitro, and increased with increases in their concentration and reaction time (LRVs of 0.2% CHG/0.05% BAC were >2, and LRVs of 1.0% CHG/0.2% BAC were >2.5).
Conclusions
Proper hand hygiene practices using alcohol-based disinfectants such as EA/IPA effectively inactivate SARS-CoV-2 and IAV on human skin.
Keywords: Disinfectant, Hand hygiene, Human skin, Influenza A virus, Severe acute respiratory syndrome coronavirus 2
Introduction
Contact transmission may be one of the factors responsible for spreading the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019. A previous study has reported that proper hand hygiene is important in preventing contact transmission of SARS-CoV-2, because of the long-term survival of SARS-CoV-2 on the human skin surface [[1], [2], [3], [4]]. Therefore, knowledge regarding disinfection effectiveness against SARS-CoV-2 on human skin is very important for preventing transmission. Several previous studies have performed the in vitro evaluation of disinfection effectiveness against SARS-CoV-2 [2,3,[5], [6], [7], [8]]. These studies have suggested that alcohol-based disinfectants such as ethanol (EA) and isopropanol (IPA) are effective against SARS-CoV-2 [[5], [6], [7]]. Conversely, the disinfection effectiveness of low-level disinfectants, such as chlorhexidine gluconate (CHG) and benzalkonium chloride (BAC), is unclear [3].
Only in vitro evaluations were performed in these studies, so clinical studies are needed to evaluate these disinfectants' effectiveness for hand hygiene under conditions close to actual use. However, it is dangerous to apply highly pathogenic and infectious agents, including SARS-CoV-2, directly to human skin for disinfection effectiveness evaluation. Consequently, it is extremely difficult to conduct clinical studies, and the disinfection effectiveness of these disinfectants against SARS-CoV-2 on human skin remains unclear.
A model to evaluate pathogen stability on human skin obtained from forensic autopsy specimens has been previously generated. Additionally, we confirmed that the model could accurately replicate in vivo skin conditions, and could also be applied to evaluate disinfection effectiveness against viruses on human skin [1,4]. Hence, we evaluated the disinfection effectiveness of various disinfectants used for hand hygiene against SARS-CoV-2 on the human skin surface using the model. We compared them with in vitro evaluation results. Moreover, we compared the disinfection effectiveness against SARS-CoV-2 and influenza A virus (IAV), a common virus transmitted through droplets and contact worldwide.
Materials and methods
For evaluating the target disinfectants, we used 80%, 60%, 40% and 20% (weight (w)/w) EA (Nacalai Tesque, Kyoto, Japan), 70% (w/w) IPA (Nacalai Tesque), 0.2% and 1.0% (w/volume (v)) CHG (Saraya, Osaka, Japan) and 0.05% and 0.2% (w/v) BAC (Yakuhan Pharmaceutical, Hokkaido, Japan).
First, in vitro evaluation of disinfectant effectiveness against SARS-CoV-2 and IAV was performed [9]. Disinfectant effectiveness against SARS-CoV-2 and IAV on human skin was then evaluated using the constructed model [1]. Specifically, disinfectant effectiveness was evaluated by titrating SARS-CoV-2 and IAV present on the skin after applying each disinfectant on the skin for 5, 15 and 60 seconds. The measurement limits of the titres of IAV and SARS-CoV-2 were 101 focus-forming units/mL and 100.5 50% tissue culture infectious dose (TCID50)/mL, respectively. The log reduction value was calculated using the virus titres with phosphate-buffered saline instead of disinfectants as a control to evaluate the disinfectant effectiveness under each condition. The study protocol, including the sample collection procedures, was reviewed/approved by the Institutional Review Board of the Kyoto Prefectural University of Medicine (ERB-C-1593). The detailed method of each evaluation is described in the Supplementary materials (Appendix S1 and Fig. S1).
Results
First, the disinfection effectiveness was evaluated in vitro (Table 1 and Fig. 1 ). Although both SARS-CoV-2 and IAV were completely inactivated within 5 seconds by EA of 40%, 60% and 80% concentrations, and by 70% IPA (log reduction values were >4), SARS-CoV-2 and IAV were barely inactivated by 20% EA (log reduction values were <1). Conversely, the in vitro evaluation of disinfectant effectiveness showed that CHG and BAC were significantly inferior to EA and IPA. The disinfection effectiveness of CHG on SARS-CoV-2 was slightly higher than that on IAV; however, that of CHG was generally low under all conditions, with the log reduction values of 0.2% and 1.0% CHG being <1 and <2, respectively. Moreover, BAC had higher disinfection effectiveness against both viruses than CHG; its disinfection effectiveness increased with increasing concentrations and reaction time (the log reduction values were >3.0 for 0.2% BAC reaction for 60 seconds).
Table 1.
Evaluation of disinfectant effectiveness against SARS-CoV-2 and IAV
| Log reduction (SARS-CoV-2) |
Log reduction (IAV) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
in vitro |
on skin |
in vitro |
on skin |
|||||||||
| 5 s | 15 s | 60 s | 5 s | 15 s | 60 s | 5 s | 15 s | 60 s | 5 s | 15 s | 60 s | |
| 80% EA | >4.50 | >4.50 | >4.50 | >4.19 | >4.17 | >4.14 | >4.10 | >4.11 | >4.07 | >4.12 | >4.16 | >4.16 |
| 60% EA | >4.50 | >4.50 | >4.50 | >4.19 | >4.17 | >4.14 | >4.10 | >4.11 | >4.07 | >4.12 | >4.16 | >4.16 |
| 40% EA | >4.50 | >4.50 | >4.50 | >4.19 | >4.17 | >4.14 | >4.10 | >4.11 | >4.07 | >4.12 | >4.16 | >4.16 |
| 20% EA | 0.08 ± 0.14 | 0.25 ± 0.25 | 0.33 ± 0.14 | 0.53 ± 0.23 | 0.61 ± 0.22 | 0.81 ± 0.27 | 0.09 ± 0.07 | 0.07 ± 0.05 | 0.06 ± 0.07 | 0.73 ± 0.08 | 0.85 ± 0.24 | 0.88 ± 0.28 |
| 70% IPA | >4.50 | >4.50 | >4.50 | >4.19 | >4.17 | >4.14 | >4.10 | >4.11 | >4.07 | >4.12 | >4.16 | >4.16 |
| 0.2% CHG | 0.33 ± 0.14 | 0.42 ± 0.14 | 0.58 ± 0.14 | 2.19 ± 0.17 | 2.31 ± 0.21 | 2.42 ± 0.18 | 0.08 ± 0.05 | 0.17 ± 0.04 | 0.19 ± 0.07 | 0.74 ± 0.16 | 0.95 ± 0.14 | 1.02 ± 0.14 |
| 1.0% CHG | 1.00 ± 0.25 | 1.42 ± 0.14 | 1.83 ± 0.29 | 2.64 ± 0.18 | 2.94 ± 0.30 | 3.17 ± 0.33 | 0.23 ± 0.01 | 0.24 ± 0.04 | 0.40 ± 0.06 | 2.85 ± 0.71 | 3.25 ± 0.69 | 3.39 ± 0.55 |
| 0.05% BAC | 1.33 ± 0.52 | 1.75 ± 0.43 | 2.17 ± 0.29 | 2.03 ± 0.51 | 2.19 ± 0.50 | 2.36 ± 0.38 | 0.69 ± 0.13 | 1.78 ± 0.07 | 2.71 ± 0.09 | 0.78 ± 0.20 | 1.04 ± 0.27 | 1.23 ± 0.60 |
| 0.2% BAC | 1.83 ± 0.38 | 2.42 ± 0.58 | 3.00 ± 0.43 | 2.72 ± 0.15 | 2.97 ± 0.20 | 3.19 ± 0.21 | 1.43 ± 0.23 | 2.34 ± 0.04 | >4.07 | 1.64 ± 0.41 | 2.85 ± 0.45 | 3.24 ± 0.81 |
Abbreviations: BAC, benzalkonium chloride; CHG, chlorhexidine gluconate; EA, ethanol; IAV, influenza A virus; IPA, isopropanol; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The log reduction value was calculated to evaluate disinfectant effectiveness under each condition and was expressed as mean ± standard deviation. Additionally, the log reduction value of the condition wherein the virus was inactivated below the measurement limit was 4 or more and was expressed as ‘>4.xx’.See Fig. 1 for the evaluation results of disinfection effectiveness against SARS-CoV-2 on each human skin sample.
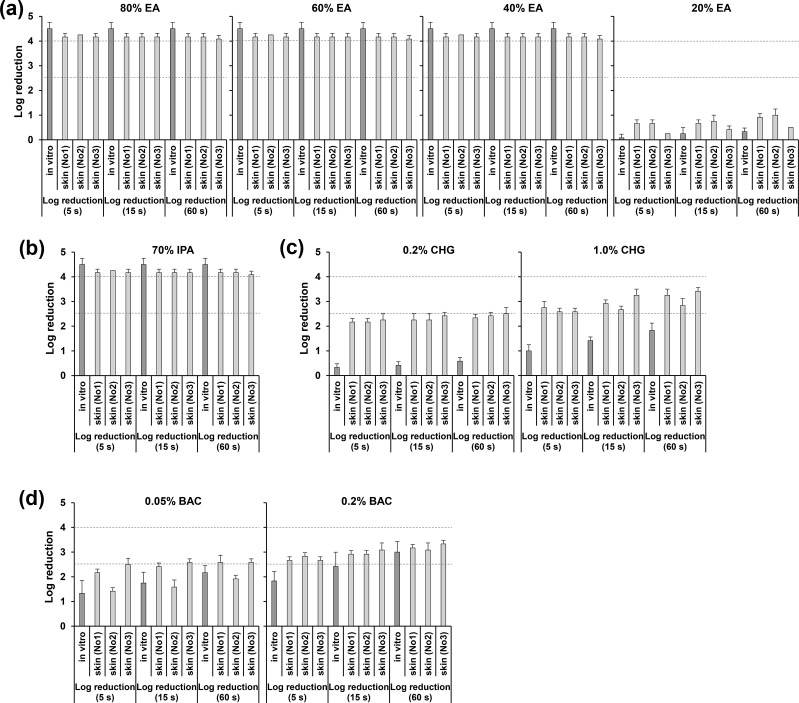
Fig. 1.
Evaluation of disinfection effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on each human skin sample (sample nos 1, 2 and 3) and of in vitro disinfection effectiveness. The log reduction value was calculated using the virus titres under conditions where phosphate-buffered saline was used instead of disinfectant as a control to evaluate the disinfectant effectiveness under each condition. Target disinfectants used included 80%, 60%, 40% and 20% (w/w) ethanol (a), 70% (w/w) isopropanol (b); 0.2% and 1.0% (w/v) chlorhexidine gluconate (c); and 0.05% and 0.2% (w/v) benzalkonium chloride (d). Data are expressed as the mean ± standard deviation of more than three independent experiments (error bars represent the standard deviation). Abbreviations: BAC, benzalkonium chloride; CHG, chlorhexidine gluconate; EA, ethanol; IPA, isopropanol.
Table 1 and Fig. 1 show that, similar to the in vitro disinfection effectiveness evaluation results, SARS-CoV-2 and IAV on human skin were both completely inactivated within 5 seconds by EA at 40%, 60% and 80% concentrations, and by 70% IPA (log reduction values for 40%–80% EA or 70% IPA applied to the skin for 5–60 seconds were all >4). In contrast, 20% EA barely inactivated SARS-CoV-2 and IAV (log reduction values were <1). Moreover, the disinfection effectiveness of CHG against SARS-CoV-2 and IAV on human skin was higher than that in vitro and increased with increased CHG concentrations and reaction time. In particular, the log reduction values on application of 1.0% CHG for 5seconds were >2.5, and those on application of 1.0% CHG for 60 seconds were >3.0. The disinfection effectiveness of BAC on SARS-CoV-2 and IAV was also relatively high and followed a similar trend to that observed in the in vitro evaluation results. Log reduction values when 0.2% BAC reacted for 15 seconds were >2.5, and those when reacted for 60 seconds were >3.0.
Discussion
The skin model used in this study for evaluating disinfection effectiveness mimics the human skin surface from disinfectant exposure to natural drying [1]. Three skin samples were prepared to evaluate disinfection effectiveness under each condition in this study, and the evaluation results using each skin sample showed the same tendency (see Fig. 1). Levels of SARS-CoV-2 on the surface of human skin were inactivated to below the measurement limit by applying more than 40% (w/w) EA for 5 seconds or longer. Most EA-base disinfectants are mainly composed of 52%–75% (w/w) EA and the disinfectant rubbing time in general hand hygiene practice is approximately 15 seconds, so EA-based disinfectants were shown to be suitable for use in hand hygiene targeting SARS-CoV-2 on skin. Additionally, IPA was shown to be as effective as EA. The above results strongly support previous research and recommendations that hand hygiene using alcohol-based disinfectants effectively controls SARS-CoV-2 transmission [[5], [6], [7],10].
The effectiveness of low-level disinfectants such as CHG and BAC against SARS-CoV-2 has been unclear. The in vitro disinfection effectiveness evaluation showed that CHG and BAC were significantly inferior in disinfection effectiveness to alcohol-based disinfectants. However, the disinfection effectiveness of CHG and BAC increased during evaluation using this skin model; this suggested that high concentrations of low-level disinfectants such as 0.2% BAC and 1.0% CHG may be relatively effective against SARS-CoV-2 on the skin. As the disinfectant effect of CHG and BAC can last after the application, unlike EA and IPA, the effective disinfectant duration when applied on the skin is expected to be longer than the disinfectant application time. The above results suggest the potential effectiveness of BAC and CHG. However, as our skin model still has scope for improvement and neutralization of BAC/CHG is considerably difficult, the disinfection effectiveness of BAC and CHG on SARS-CoV-2 may have been overestimated. This is a limitation of our study, and it needs to be addressed in future studies; the use of BAC/CHG cannot be recommended based on these findings.
This study showed that proper hand hygiene practices using alcohol-based disinfectants effectively prevent SARS-CoV-2 from adhering to human skin. Our disinfection effectiveness evaluation model can also be applied to disinfection effectiveness evaluation against other highly pathogenic and infectious microbes on human skin other than SARS-CoV-2.
Author contributions
RH produced the study concept and design, drafted the manuscript, performed the statistical analysis and secured the funding. Data acquisition was by RH, HI, NW, TY, RB and TD; data analysis and interpretation was by RH, RB, YN, YI and TN; administrative/technical/material support was given by RH, RB and HI, and the study was supervised by RH and TN.
Transparency declaration
All authors have disclosed no financial relationships relevant to this publication.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. This research was supported by AMED (grant number JP19fk0108077 and JP20fk0108270) and JSPS KAKENHI (grant number JP18K16183 and JP18H03040). This research was also supported by grants from the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Uehara Memorial Foundation, the Ichiro Kanehara Foundation, the Takeda Science Foundation, the Mitsubishi Foundation and the Daiwa Securities Health Foundation.
Editor: E.J. Kuipers
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.04.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hirose R., Ikegaya H., Naito Y., Watanabe N., Yoshida T., Bandou R., et al. Survival of SARS-CoV-2 and influenza virus on the human skin: importance of hand hygiene in COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1517. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan K.H., Sridhar S., Zhang R.R., Chu H., Fung A.Y., Chan G., et al. Factors affecting stability and infectivity of SARS-CoV-2. J Hosp Infect. 2020;106:226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirose R., Ikegaya H., Naito Y., Watanabe N., Yoshida T., Bandou R., et al. Reply to Gracely. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab023. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee S., Vincent C.K., Jayasekera H.W., Yekhe A.S. Antiviral efficacy of personal care formulations against severe acute respiratory syndrome coronavirus 2. Infect Dis Health. 2021;26:63–66. doi: 10.1016/j.idh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leslie R.A., Zhou S.S., Macinga D.R. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am J Infect Contr. 2021;49:401–402. doi: 10.1016/j.ajic.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kratzel A., Todt D., V'Kovski P., Steiner S., Gultom M., Thao T.T.N., et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golin A.P., Choi D., Ghahary A. Hand sanitizers: a review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am J Infect Contr. 2020;48:1062–1067. doi: 10.1016/j.ajic.2020.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose R., Nakaya T., Naito Y., Daidoji T., Bandou R., Inoue K., et al. Situations leading to reduced effectiveness of current hand hygiene against infectious mucus from influenza virus-infected patients. mSphere. 2019;4 doi: 10.1128/mSphere.00474-19. e00474–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevention and control recommendations for patients with suspected or confirmed Coronavirus Disease 2019 (COVID-19) in healthcare settings. US Centers Dis Contr Prev. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.