To the Editor:
Ding et al. recently published a retrospective analysis of potential predictors for mortality in 2,073 Chinese patients hospitalized with COVID-19.1 As their main findings, they reported that increased liver parameters as well as liver injury were predictive for 28-day mortality, and proposed a nomogram that estimates the mortality risk of hospitalized patients with COVID-19 disease. However, as the understanding of COVID-19 disease improves, it becomes evident that the disease may present differently in different regions. Liver injury, as well as other gastrointestinal symptoms were associated with worse outcome in some, but not all studies.[2], [3], [4], [5], [6], [7] Therefore, we set out to validate the main findings in an Austrian cohort of 405 hospitalized patients with COVID-19 disease.
We retrospectively collected demographic and laboratory data as well as in-hospital mortality from all patients with a positive SARS-Cov-2 PCR test hospitalized at either the University Hospital Graz or the State Hospital Graz II between February 28th, 2020 and May 30th, 2020. Due to availability, the nomogram parameters direct bilirubin and troponin I were substituted with total bilirubin and troponin T, respectively. Cox-Regression was used to estimate hazard ratios for 7-, 14-, 21- and 28-day in-hospital mortality; p values below 0.05 were considered significant. Monte-Carlo simulation were run to define cut-offs with the highest overall accuracy.
Firstly, we analysed whether elevated liver enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT]) or liver injury are predictive of 28-day mortality, as proposed in the original article. AST levels above the upper limit of normal were found in 189 of 305 (61,4%) patients, elevated ALT levels in 85 of 310 (27.4%) patients; 21 of 243 (8.6%) patients fulfilled the criteria for liver injury. We found that neither elevated AST (hazard ratio [HR] 1.23; 95% CI 0.77–1.96; p = 0.4), elevated ALT (HR 0.61; 95% CI 0.35–1.07; p = 0.09) nor liver injury (HR 0.74; 95% CI 0.27–2.05; p = 0.6) could predict hospital mortality in COVID-19 patients in Graz, Austria. Similar, non-predictive results were obtained for 7-, 14-, or 21-day mortality. GI symptoms in general, as well as diarrhoea in particular, were not associated with 28-day mortality (HR 0.58; 95% CI 0.15–2.16; p = 0.4 and HR 0.42; 95% CI 0.04–4.00; p = 0.5, respectively).
Secondly, we tested whether the proposed nomogram, including age, severe pneumonia, lymphocyte count, platelet count, C-reactive protein, D-dimer, creatinine, troponin, AST and bilirubin, could predict 28-day mortality in the Austrian cohort. A complete data set could be obtained from 151 patients, 37 (24.5%) of whom died within 28 days of hospitalization. The score derived from the nomogram was significantly associated with 28-day in-hospital mortality (HR 1.009; 95% CI 1.005–1.013; p <0.001) and showed a high predictive accuracy (AUROC 0.80; 95% CI 0.71–0.88; p <0.001). Similar results were obtained for 7-, 14-, or 21-day mortality. However, not all factors of the nomogram could predict in-hospital mortality individually. While age points (i.e. age∗1.11-1.11), severe pneumonia, C-reactive protein over 10 mg/L, D-dimer over 1.3 mg/L (adjusted cut-off), creatinine over 84 μmol/L and troponin T over 15.6 ng/ml were predictive, lymphocyte count, platelet count, AST and bilirubin were not.
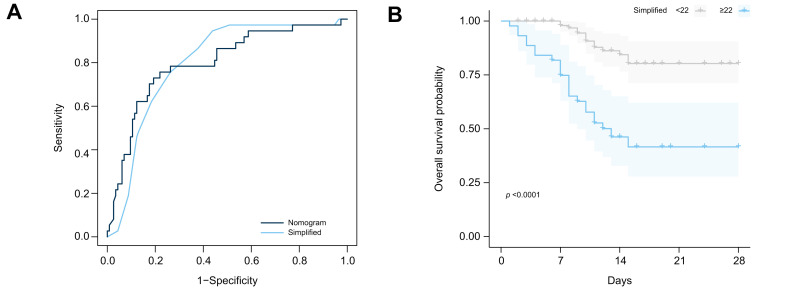
Although the proposed nomogram is performing well, it is rather complicated to calculate which could be a considerable disadvantage in well-frequented COVID-19 wards. We propose a simplified version based on the predictors that we could reliably reproduce in the Austrian cohort. The simplified score is composed of the sum of the following: age decade, 4 points for severe pneumonia, 8 points for C-reactive protein over 10 mg/L, 4 points for creatinine over 84 μmol/L. This simplified version can still predict 28-day mortality (HR 1.24; 95% CI 1.16–1.34; p <0.001) and shows comparable accuracy to the full version of the nomogram (AUC difference: -0.001; 95% CI -0.05 to 0.05; p = 0.9). A score of 22 or higher best identified patients at risk of 28-day in-hospital mortality (Fig. 1 ).
Fig. 1.
Prediction of in-hospital mortality.
(A) AUROC for the proposed nomogram and the simplified version. (B) Kaplan-Meier curves according to the simplified nomogram (p <0.0001, Log-rank test).
In conclusion, we validated and simplified the nomogram proposed by Ding et al. 1 although liver injury itself was not predictive in our cohort. The divergence in liver function-related findings is not unexpected. Several meta-analyses in the past 6 months showed that a certain fraction of individual studies indicated no association between liver function abnormalities and outcome, although, overall, they were associated with disease severity and outcome.3 , 5 , 6 Our retrospective study design is not suited to address the source of this discrepancy. However, direct comparison of the 2 study populations showed that patients in our cohort were 74 (±14) years old and therefore 12 years older on average compared to Ding et al., which might explain the considerably higher mortality rate as well as the higher prevalence of liver function abnormalities (74% vs. 61%, respectively) in our cohort. Our cohort is limited by the smaller sample size compared to Ding et al. and the unavailability of direct bilirubin which might also contribute to the observed discrepancies. Despite these limitations we could validate the nomogram proposed by Ding et al. and call for validation of the full1 as well as our simplified version of the nomogram in other cohorts across the globe to improve the risk stratification of COVID-19 patients.
Financial support
No financial support was given in order to complete the study or write the manuscript.
Authors’ contributions
TL, NF, HW collected data, AH, VS analysed the data and wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.04.024.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Ding Z.Y., Li G.X., Chen L., Shu C., Song J., Wang W., et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021 Jun;74(6):1295–1302. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Qiu P., Liu J., Wang F., Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44:653–661. doi: 10.1016/j.clinre.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni A.V., Kumar P., Tevethia H.V., Premkumar M., Arab J.P., Candia R., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y., Li H., Guo X., Yoshida E.M., Mendez-Sanchez N., Levi Sandri G.B., et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z.H., Yang D.L. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Zompo F., De Siena M., Ianiro G., Gasbarrini A., Pompili M., Ponziani F.R. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072–13088. doi: 10.26355/eurrev_202012_24215. [DOI] [PubMed] [Google Scholar]
- 7.Tariq R., Saha S., Furqan F., Hassett L., Pardi D., Khanna S. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95:1632–1648. doi: 10.1016/j.mayocp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.