Abstract
Purpose: Rates of obesity and obesity-related health consequences, including type 2 diabetes (T2D) and cancer, continue to rise. While cancer patients are at an increased risk of developing T2D, the prevalence of T2D and insulin prescription among young patients with cancer remains unknown.
Methods: Using the Total Cancer Care Study cohort at Huntsman Cancer Institute (Salt Lake City, UT), we identified individuals age 18–39 years at cancer diagnosis between 2009 and 2019. Multivariable logistic regression was used to investigate associations between body mass index (BMI) with insulin prescription within 1 year of cancer diagnosis.
Results: In total, 344 adolescents and young adults (AYAs) were diagnosed with primary invasive cancer. Within this cohort, 19 patients (5.5%) were ever diagnosed with T2D, 48 AYAs ever received an insulin prescription (14.0%), and 197 were overweight or obese (BMI: 25+ kg/m2) at cancer diagnosis. Each kg/m2 unit increase in BMI was associated with 6% increased odds of first insulin prescription within 1 year of cancer diagnosis among AYAs, even after adjustment for age, sex, smoking history, marital status, glucocorticoid prescription, and cancer treatments (odds ratio = 1.06, 95% confidence interval 1.02–1.11; p = 0.005).
Conclusion: One in every 18 AYAs with cancer ever had T2D, 1 in 7 AYA patients with cancer ever received an insulin prescription, and higher BMI was associated with increased risk of insulin prescription within a year of cancer diagnosis among AYAs. Understanding the incidence of T2D and insulin prescription/use is critical for short-term and long-term clinical management of AYAs with cancer.
Keywords: cancer, young-onset, early-onset, obesity, overweight, BMI, body fatness, insulin, insulin resistance, glucocorticoids, diabetes, type 2 diabetes
Introduction
Obesity rates have increased substantially over the last 40 years, and a plethora of obesity-related health consequences, including cancer and type 2 diabetes (T2D), have increased in tandem. It is estimated that ∼40% of cancer cases diagnosed annually in the United States are associated with overweight and obesity,1 and elevated body mass index (BMI) after cancer diagnosis contributes to worsening disease prognosis.2 Moreover, nearly half of all cancers among individuals younger than age 65 years are associated with overweight and obesity3—such that it is estimated that the annual age-adjusted rate of overweight- and obesity-related invasive cancer cases among individuals between ages 20–49 years at diagnosis is 47.2 per 100,000 population.1 Given these trends and the high prevalence of overweight/obesity among adolescents and young adults (AYAs), the burden of overweight- and obesity-related cancers among AYAs4 may continue to increase and warrants additional investigation.
Complications related to excess adiposity also include diabetes mellitus, as ∼90% of individuals with T2D have a BMI classified as overweight or obese (25+ kg/m2).5 Emerging evidence also suggests that cancer patients are at increased risk of developing or worsening T2D when compared with age- and BMI-matched cancer-free adults, and this risk may be greatest in the year after a cancer diagnosis.6,7 In addition to promoting healthy lifestyle changes for weight loss,8 antihyperglycemic medication management is a critical component of treating patients with T2D and obesity. However, comprehensive evidence-based guidelines for antihyperglycemic medication use among patients diagnosed with cancer are lacking.
The use of exogenous insulin for glycemic control among cancer patients remains controversial due to its potential role as a growth factor and negative impact on quality of life and disease outcomes.9,10 Insulin enhances tumor growth in a variety of cancer models,11,12 and compensatory hyperinsulinemia induced by insulin resistance may underlie the link between obesity and survival among cancer patients.13,14 Previous studies have begun to evaluate associations between body fatness, T2D, and cancer among all-comers and survivors of childhood cancer.8,10,11,14 Yet the increasing epidemic of early-onset cancers, including cancers of the colorectum, appendix, and noncardia gastric cancer,15–19 suggests a unique need to better understand the intricate link between body fatness, T2D, and cancer within the population of AYAs with cancer.
In addition to the potentially deleterious effect of insulin on cancer prognosis and the challenges associated with insulin-based regulation of glycemic control, the rising cost of insulin—together with the significance and persistent societal and economic effects associated with a cancer diagnosis at a young age—present unique challenges for this underserved age group.4 While AYA survivors of cancer are at elevated risk of cardiovascular disease as a comorbidity,20 the relationships between body composition, T2D, and insulin prescription in the first year after cancer diagnosis among AYAs remain unknown. The study of AYA patients with cancer offers an opportunity to examine the prevalence of overweight and obesity, T2D, and insulin prescription within the first year of cancer diagnosis, minimizing the potential impact that comorbidities among individuals aged 40 years and older might have on obesity-related health consequences.
The purpose of this study was to investigate associations between body fatness, T2D, and insulin prescription among individuals aged 18–39 years diagnosed with cancer.
Methods
Data sources and patient selection
Patients in this study derive from the Total Cancer Care® (TCC)21 longitudinal study cohort at Huntsman Cancer Institute. The TCC cohort includes men and women aged 18 years and older with a cancer diagnosis as defined by International Classification of Diseases for Oncology (ICD-O) codes. Patients diagnosed with cancer between 2015 and 2019 were prospectively recruited, and patients diagnosed between 2009 and 2015 were approached and retrospectively consented to the TCC study. Data on clinical and demographic features, including BMI, age, sex, marital status, history of smoking, insulin and glucocorticoid prescriptions, diabetes ICD/diagnosis codes, cancer treatment regimens, disease stage/site, and follow-up were captured using electronic medical charts, including pathological reports and health records, obtained from the Huntsman Cancer Registry and the University of Utah Health Enterprise Data Warehouse. All patients provided informed consent and this study site protocol was approved by the University of Utah Institutional Review Board.
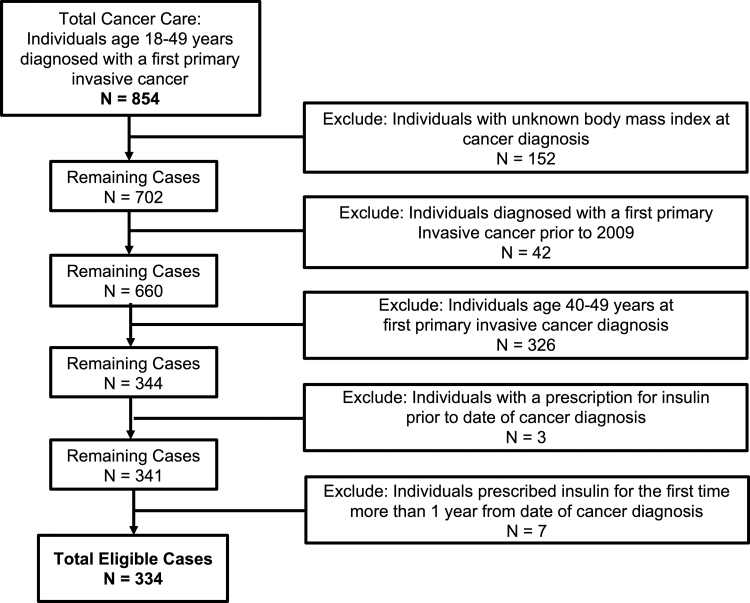
A total of 854 individuals, recruited as part of the TCC study for a first primary invasive cancer diagnosis between the ages of 18–49 years at local sites in Salt Lake City, Utah, had a confirmed cancer diagnosis by ICD-O code (Fig. 1). Patients were excluded if they were diagnosed with a first primary invasive cancer: with an unknown BMI ±90 days of cancer diagnosis (n = 152), before 2009 (n = 42), between the ages of 40–49 years (n = 326). Our final cohort consisted of 344 AYAs diagnosed with cancer between 2009 and 2019.
FIG. 1.
Composition of study population with exclusion criteria.
ICD-O codes were used to group tumor location by primary site and histology according to standard SEER recodes. Other variables of interest included date of cancer diagnosis, date of first insulin prescription, date of first glucocorticoid prescription, patient sex, age at cancer diagnosis, BMI at cancer diagnosis (kg/m2, continuous; BMI category [underweight/normoweight: <25 kg/m2; overweight: 25–29.99 kg/m2; obese: 30+ kg/m2]), race, smoking history (never; ever [current/former] smoker), marital status, American Joint Committee on Cancer (AJCC) clinical stage, cancer treatment regimen (receipt of chemotherapy, surgery, radiation therapy, hormone therapy, and/or immunotherapy), and state of residence.
Statistical analysis
Differences in the distribution of baseline characteristics by insulin prescription within 1 year of cancer diagnosis were summarized by frequency and compared using chi-square tests and t-tests for categorical and quantitative variables, respectively. Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) to quantify associations among BMI, clinical/demographic features, and insulin prescription within 1 year of cancer diagnosis. Associations between BMI and insulin prescription were assessed in unadjusted and adjusted models. The adjusted model included age at diagnosis (years, continuous), sex, BMI (kg/m2, continuous), smoking history, marital status, glucocorticoid prescription (first glucocorticoid prescription 1+ days after cancer diagnosis), and the uptake of surgical resection, hormone therapy, radiation therapy, immunotherapy, and/or chemotherapy. Sensitivity analyses were also performed for individuals with AJCC clinical staging at cancer diagnosis. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC). All tests were two-sided, and a p-value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics
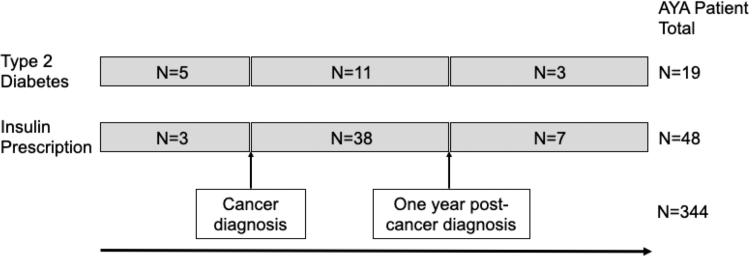
A total of 344 cases of a first primary invasive cancer diagnosed in individuals aged 18–39 years comprised the study cohort (Fig. 1). Nearly 60% of AYAs had a BMI classified as overweight or obese (25+ kg/m2) at cancer diagnosis (197 of 344, 57.3%; mean BMI 28.0 kg/m2; standard deviation, 7.6 kg/m2; data not shown). Approximately one out of every 18 AYA patients ever had T2D (19 of 344, 5.5%), and one out of every seven AYA patients (48 of 344, 14.0%) ever received a prescription for insulin. Over 90% of AYAs with a history of insulin prescription (45 of 48, 93.8%) were prescribed insulin at or after a first primary invasive cancer diagnosis (Figs. 1 and 2). Further, among AYAs with an insulin prescription at or after cancer diagnosis, nearly 85% of patients (38 of 45, 84.4%) were prescribed insulin within 1 year of cancer diagnosis (Fig. 2).
FIG. 2.
Timeline of cancer diagnosis, type 2 diabetes diagnoses and first insulin prescription among AYAs with cancer. AYA, adolescents and young adult.
Insulin prescription after cancer diagnosis among AYAs with cancer
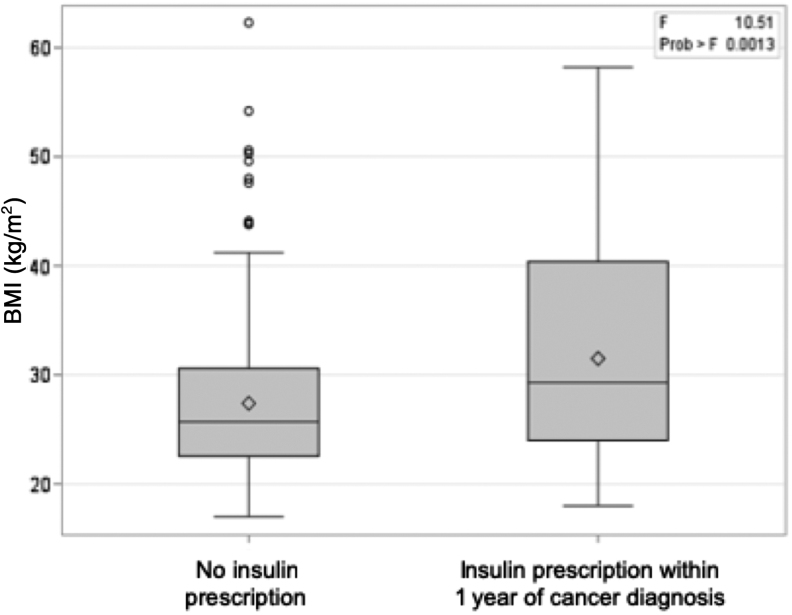
Our subsequent analyses focused on the subset of this AYA population (n = 334) who either received their first prescription for insulin within 1 year at or after cancer diagnosis (n = 38), or those with a negative history for insulin prescription (n = 296) (Figs. 1 and 2, and Table 1). Similar to the entire cohort of AYAs, mean BMI at cancer diagnosis of this population was classified as overweight (27.9 kg/m2; standard deviation, 7.4 kg/m2) (Table 1). BMI differed by insulin status, as patients who were prescribed insulin within 1 year of cancer diagnosis had a mean BMI classified as obese (31.5 kg/m2) versus individuals with a negative history for insulin prescription with a mean BMI classified as overweight (27.4 kg/m2; p = 0.001) (Table 1 and Fig. 3). One in every three AYAs with cancer in our cohort were younger than age 30 years at diagnosis (107 of 334, 32.0%). Mean age at cancer diagnosis was 31.4 years and did not differ by insulin status (Table 1). As expected, patients with T2D were more likely to have been prescribed insulin within 1 year of cancer diagnosis compared to those with no history of diabetes (18.4% vs. 2.7%, p < 0.001). Approximately one-quarter of AYAs with cancer were ever smokers (86 of 334, 25.7%). While men comprised a higher proportion of AYAs prescribed insulin within 1 year of cancer diagnosis compared with patients with a negative history for insulin prescription (62.5% vs. 50.0%), no differences in marital status, AJCC clinical stage, cancer sequence, site, glucocorticoid prescription after cancer diagnosis, or receipt of chemotherapy, surgery, hormone therapy, or immunotherapy were observed by insulin status among AYA patients.
Table 1.
Study Population of Adolescents and Young Adults with a First Primary Invasive Cancer by Insulin Status Within 1 Year of Cancer Diagnosis: 2009–2019
| Characteristic | Study Population |
Insulin Status |
p | ||||
|---|---|---|---|---|---|---|---|
| No insulin prescription |
Insulin prescription within 1 year of cancer diagnosis |
||||||
| n | % | n | % | n | % | ||
| Total | 334 | 296 | 88.6 | 38 | 11.4 | ||
| Age at cancer diagnosis (years) | 0.23 | ||||||
| 18–24 | 55 | 16.5 | 48 | 16.2 | 7 | 18.4 | |
| 25–29 | 52 | 15.6 | 50 | 16.9 | 2 | 5.3 | |
| 30–34 | 103 | 30.8 | 92 | 31.1 | 11 | 28.9 | |
| 35–39 | 124 | 37.1 | 106 | 35.8 | 18 | 47.4 | |
| Mean (SD) | 31.4 | (5.7) | 31.3 | (5.7) | 32.2 | (5.9) | 0.40 |
| BMI | 0.18 | ||||||
| Underweight/normoweight (<25 kg/m2) | 143 | 42.8 | 131 | 44.3 | 12 | 31.6 | |
| Overweight (25–29.99 kg/m2) | 91 | 27.2 | 81 | 27.4 | 10 | 26.3 | |
| Obese (30+ kg/m2) | 100 | 29.9 | 84 | 28.4 | 16 | 42.1 | |
| Mean (SD), kg/m2 | 27.9 | (7.4) | 27.4 | (7.0) | 31.5 | (9.8) | 0.001 |
| Sex | 0.14 | ||||||
| Female | 204 | 61.1 | 185 | 62.5 | 19 | 50.0 | |
| Male | 130 | 38.9 | 111 | 37.5 | 19 | 50.0 | |
| Race | — | ||||||
| White | 306 | 91.6 | 270 | 91.2 | 36 | 94.7 | |
| Other/unknown | 28 | 8.4 | 26 | 8.8 | 2 | 5.3 | |
| Marital status | 0.23 | ||||||
| Married | 207 | 62.0 | 186 | 62.8 | 21 | 55.3 | |
| Unmarried | 116 | 34.7 | 99 | 33.4 | 17 | 44.7 | |
| Unknown | 11 | 3.3 | 11 | 3.7 | 0 | 0.0 | |
| Smoking history | 0.64 | ||||||
| Never smoker | 247 | 74.0 | 220 | 74.3 | 27 | 71.1 | |
| Ever smokera | 86 | 25.7 | 75 | 25.3 | 11 | 28.9 | |
| Unknown | 1 | 0.3 | 1 | 0.3 | 0 | 0.0 | |
| State of residence | 0.46 | ||||||
| Utah | 242 | 72.5 | 213 | 72.0 | 29 | 76.3 | |
| Idaho | 40 | 12.0 | 36 | 12.2 | 4 | 10.5 | |
| Wyoming | 19 | 5.7 | 18 | 6.1 | 1 | 2.6 | |
| Nevada | 21 | 6.3 | 17 | 5.7 | 4 | 10.5 | |
| Other | 12 | 3.6 | 12 | 4.1 | 0 | 0.0 | |
| Type 2 diabetes | <0.001 | ||||||
| None | 319 | 95.5 | 288 | 97.3 | 31 | 81.6 | |
| Yes | 15 | 4.5 | 8 | 2.7 | 7 | 18.4 | |
| Glucocorticoid prescriptionb | 0.48 | ||||||
| None | 98 | 29.3 | 85 | 28.7 | 13 | 34.2 | |
| Yes | 236 | 70.7 | 211 | 71.3 | 25 | 65.8 | |
| AJCC clinical stage | 0.93 | ||||||
| I | 79 | 23.7 | 74 | 25.0 | 5 | 13.2 | |
| II | 43 | 12.9 | 39 | 13.2 | 4 | 10.5 | |
| III | 30 | 9.0 | 28 | 9.5 | 2 | 5.3 | |
| IV | 22 | 6.6 | 20 | 6.8 | 2 | 5.3 | |
| Not specified/Not applicablec | 160 | 47.9 | 135 | 45.6 | 25 | 65.8 | |
| Cancer sequence | 0.86 | ||||||
| Only 1 primary | 310 | 92.8 | 275 | 92.9 | 35 | 92.1 | |
| First of 2+ primaries | 24 | 7.2 | 21 | 7.1 | 3 | 7.9 | |
| Chemotherapy | 0.59 | ||||||
| None/unknown | 172 | 51.5 | 154 | 52.0 | 18 | 47.4 | |
| Yes | 162 | 48.5 | 142 | 48.0 | 20 | 52.6 | |
| Surgery | 0.96 | ||||||
| None/unknown | 27 | 8.1 | 24 | 8.1 | 3 | 7.9 | |
| Yes | 307 | 91.9 | 272 | 91.9 | 35 | 92.1 | |
| Hormone therapy | 0.18 | ||||||
| None/unknown | 262 | 78.4 | 229 | 77.4 | 33 | 86.8 | |
| Yes | 72 | 21.6 | 67 | 22.6 | 5 | 13.2 | |
| Immunotherapy | 0.10 | ||||||
| None/unknown | 300 | 89.8 | 263 | 88.9 | 37 | 97.4 | |
| Yes | 34 | 10.2 | 33 | 11.1 | 1 | 2.6 | |
| Radiation therapy | 0.17 | ||||||
| None/unknown | 218 | 65.3 | 197 | 66.6 | 21 | 55.3 | |
| Yes | 116 | 34.7 | 99 | 33.4 | 17 | 44.7 | |
p values do not include unknown or not applicable values.
Ever smoker includes current and former smokers.
First glucocorticoid prescription 1+ days after cancer diagnosis.
Brain and other nervous system cancers do not have applicable staging information.
AJCC, American Joint Committee on Cancer; BMI, body mass index, SD, standard deviation.
FIG. 3.
Distribution of BMI among AYAs by insulin status within 1 year of first primary invasive cancer diagnosis. The bold line within each boxplot represents the median BMI, and the upper and lower bounds of the boxplot represent the 75th and 25th percentiles, respectively. Outer edges reflect the 10th (lower) and 90th (upper) percentiles, respectively, mean BMI is denoted by the diamond, and outliers are denoted in circles. BMI, body mass index.
The most commonly diagnosed cancers were brain and nervous system cancers, accounting for one-fifth (21.0%) of all cancer cases in this AYA population (Table 1 and Supplementary Table S1). By insulin status, brain and other nervous system cancers accounted for one-third of all AYAs who received an insulin prescription within 1 year of cancer diagnosis compared with 18.9% of the AYA population with no history of insulin prescription (Supplementary Table S1). While 7.4% of AYAs with no insulin prescription were diagnosed with cancers of the digestive system, nearly one in every six cases received an insulin prescription within the first year of gastrointestinal cancer diagnosis (15.8%; p = 0.07). Moreover, breast cancers comprised 14.9% of cases with no history of insulin prescription among AYAs whereas no cases who received an insulin prescription within 1 year of cancer diagnosis were diagnosed with breast malignancies.
Association between BMI and insulin status among AYAs with cancer
We next quantified the association between BMI and insulin status in both unadjusted and adjusted multivariable logistic regression models (Table 2). In the unadjusted model, each kg/m2 unit increase in BMI was associated with a 6% increased likelihood to be prescribed insulin within 1 year of cancer diagnosis among AYAs (OR: 1.06, 95% CI: 1.02–1.11; p = 0.002). This association remained after adjusting for age at cancer diagnosis, sex, marital status, smoking history, glucocorticoid prescription, and receipt of chemotherapy, surgery, hormone therapy, immunotherapy, and radiation therapy; each unit increase in BMI was associated with a 6% increased risk of being prescribed insulin within the first year of cancer diagnosis (OR: 1.06, 95% CI: 1.02–1.11; p = 0.005). No association was found between insulin status and age at diagnosis, patient sex, marital status, smoking history, glucocorticoid prescription post cancer diagnosis, or receipt of surgery, hormone therapy, immunotherapy, radiation therapy, or chemotherapy in adjusted models.
Table 2.
Logistic Regression for Odds of Insulin Prescription Within 1 Year of First Primary Invasive Cancer Diagnosis Among Adolescents and Young Adults: 2009–2019
| Characteristic | Adjusted model |
|
|---|---|---|
| Insulin prescription within 1 year of cancer diagnosis vs. no history of insulin prescription | ||
| OR (95% CI) | p | |
| BMI | ||
| kg/m2, continuous | 1.06 (1.02–1.11) | 0.005 |
| Age at cancer diagnosis | ||
| Years, continuous | 1.03 (0.96–1.11) | 0.38 |
| Sex | ||
| Female | 1.00 (—) | |
| Male | 1.78 (0.86–3.70) | 0.12 |
| Marital status | ||
| Unmarried | 1.00 (—) | |
| Married | 0.64 (0.30–1.40) | 0.26 |
| Smoking history | ||
| Never smoker | 1.00 (—) | |
| Ever smoker* | 0.89 (0.39–2.02) | 0.78 |
| Glucocorticoid prescription** | ||
| No | 1.00 (—) | |
| Yes | 1.00 (0.46–2.17) | 0.99 |
| Chemotherapy | ||
| None/unknown | 1.00 (—) | |
| Yes | 1.13 (0.52–2.48) | 0.76 |
| Surgery | ||
| None/unknown | 1.00 (—) | |
| Yes | 0.90 (0.24–3.38) | 0.88 |
| Hormone therapy | ||
| None/unknown | 1.00 (—) | |
| Yes | 0.54 (0.18–1.60) | 0.26 |
| Immunotherapy | ||
| None/unknown | 1.00 (—) | |
| Yes | 0.20 (0.03–1.55) | 0.12 |
| Radiation therapy | ||
| None/unknown | 1.00 (—) | |
| Yes | 1.80 (0.81–4.04) | 0.15 |
Ever smoker includes current and former smokers.
First glucocorticoid prescription 1+ days after cancer diagnosis.
CI, confidence interval; OR, odds ratio.
Finally, due to the large proportion of AYA cancers occurring in the brain and nervous system that are unable to be clinically staged, we also assessed the influence of patient BMI on insulin status among those 174 AYA patients with complete clinical staging information at cancer diagnosis. Similar to the entire AYA cohort, each unit increase in BMI was associated with an increased risk of being prescribed insulin within the first year of cancer diagnosis in the unadjusted model (OR: 1.16, 95% CI: 1.08–1.25; p < 0.001) (Supplementary Table S2). After adjusting for cancer stage, age at cancer diagnosis, sex, marital status, glucocorticoid prescription, AJCC clinical stage, and receipt of chemotherapy, surgery, hormone therapy, immunotherapy, and radiation therapy, each unit increase in BMI was associated with a 28% increased risk of being prescribed insulin within the first year of cancer diagnosis (OR: 1.28, 95% CI: 1.12–1.47; p < 0.001). In the multivariable model, marital status was an independent predictor of being prescribed insulin within 1 year of cancer diagnosis among AYA patients. AYAs who were married were 83% less likely to be prescribed insulin within the first year after cancer diagnosis compared with unmarried individuals (OR: 0.17, 95% CI: 0.03–0.94; p = 0.04). Of note, statistical power to adequately detect differences in this sensitivity analysis was limited by sample size such that these findings should be considered exploratory.
Discussion
In this cohort study of AYAs diagnosed with a first primary invasive cancer, ∼1 in every 18 AYA patients also had a diagnosis of T2D. One out of every 7 AYA patients received a prescription for insulin—with over 90% of these AYA patients prescribed insulin at or after first primary invasive cancer diagnosis. Approximately 60% of patients were overweight or obese at cancer diagnosis, and higher BMI was associated with increased risk of insulin prescription within the first year of cancer diagnosis among AYAs. These findings are novel as our study is the first to evaluate T2D, body fatness, and insulin prescription among AYAs diagnosed with cancer, and the first to characterize clinicodemographic features by receipt of insulin prescription within the population of individuals aged 18–39 years at first primary invasive cancer diagnosis.
In the United States, one in every three AYAs (individuals age 20–39 years) is obese.22 However, among AYAs diagnosed with cancer, here we find that nearly two in every three patients were overweight or obese. Excess adiposity predominantly results from energy imbalance, although changes in metabolism and hereditary factors can also contribute to body fatness.2,23,24 As the obesity epidemic continues to rise, the burden of overweight- and obesity-related cancers among AYAs is also growing—as cancer incidence rates, including sporadic colorectal cancers25 and noncardia gastric cancers,26 continue to increase within this population. Yet compared to older-onset cancer cases, emerging evidence also suggests that AYAs harbor a distinct biology of malignancies.27–29 Recent findings have highlighted that sporadic colorectal cancers among young patients have a unique metabolic imbalance compared to older-onset cases.29 This difference in age of disease-onset between cancer cases may be attributed to a combination of factors—including genetic predisposition, race/ethnicity, lifestyle changes, and environmental exposures.15–17 Given the higher proportion of AYAs who were overweight or obese at cancer diagnosis compared to the general population, a better understanding of the distinct biological mechanisms connecting obesity and carcinogenesis is urgently needed to curb the obesity epidemic and develop cancer prevention and intervention strategies for AYAs.
Several studies have identified obesity-induced insulin resistance and the subsequent increase in circulating insulin as one potential mechanism linking excess body fatness and cancer development.2,30 While the primary glucoregulatory role of insulin is to restrain hepatic glucose production and induce peripheral glucose uptake, insulin also stimulates cellular growth, including the growth of several cancers.11,12,31 In this study, we found that ∼1 in every 18 AYA patients with cancer also had a diagnosis of T2D and 1 out of every 7 AYA patients ever received a prescription for insulin—with the majority of AYAs being prescribed insulin at or after cancer diagnosis. Moreover, higher BMI was independently associated with increased risk of insulin prescription within the first year of cancer diagnosis among AYAs. Therefore, it is possible that elevated insulin concentrations, whether endogenous or exogenous, observed in overweight/obese patients with cancer could increase risk of disease recurrence or a second primary cancer diagnosis and/or lead to poor cancer outcomes, independent of diabetes status.32 As AYAs with cancer harbor an excess risk of subsequent primary neoplasm that rises with increasing years from diagnosis33—together with the unique needs in clinical care for AYA patients with potential late effects of disease4—additional studies are warranted to investigate T2D among AYAs with cancer, and the link between hyperinsulinemia and prognostic outcomes within the population of AYAs diagnosed with cancer.
Physiologically, studies have found that marriage confers health benefits—including improvements in endocrine and immune function.34 Yet cohabitation and marriage can also lead to weight gain in men and women35—which is correlated to increased cancer risk and a higher likelihood of developing obesity-related comorbidities. Aligned with the health benefits of marriage, we found that among patients with known clinical stage of disease, individuals who were married were less likely to be prescribed insulin within the first year of cancer diagnosis. Indeed, a large population-based study among patients who are married at cancer diagnosis across all age groups found that married individuals display less depression, anxiety, and distress compared with unmarried individuals.36 As unmarried patients with cancer represent an at-risk population across all ages that may benefit from support-based interventions,37 additional studies into the role of unique supportive measures and palliative care needs specifically among AYA patients diagnosed with cancer are urgently needed to optimize population-based strategies to improve support mechanisms among young patients nationwide.
Although our findings suggest that higher BMI is associated with an increased risk of insulin prescription in the first year after cancer diagnosis among young patients, we acknowledge the limitations of our study. Our study was unique in the ability to longitudinally evaluate the onset of insulin prescription in cancer patients, however, we were unable to ascertain the rates and patterns of individual insulin usage, and lacked consistent data on reliable testing criterion for T2D (e.g., oral glucose tolerance test, fasting blood glucose, HbA1c), in this AYA cohort. In addition, the persistence of insulin prescription, escalation or reduction in use after the cessation of cancer treatment, and the influence of potential external factors (e.g., a patient's ability to adapt to nutritional advice) on insulin prescription was not quantifiable. Given the rising costs of insulin,38 prior health care resource utilization, and type of initiated insulin,39 coupled with a cancer diagnosis and the financial toxicity and distress of cancer treatment,40 early discontinuation of insulin treatment may be disproportionately higher in the population of AYAs with cancer compared with the general population.
We also acknowledge that two-thirds of AYAs with cancer in our cohort were aged 30 years and older at diagnosis, such that further studies centered upon adolescents with cancer are warranted to better understand age-related differences in T2D, body fatness, and insulin prescription. We were unable to examine racial or ethnic differences in receipt of insulin prescription within our cohort or to examine differences in T2D and receipt of insulin prescription in a noncancer control group with matched BMI. Although similar patterns of obesity are observed among adults and youth, racial/ethnic patterns persist across cancers15–17 and in the prevalence of obesity22 among AYAs—as nearly half of all non-Hispanic black and Hispanic adults are obese compared with <40% of non-Hispanic whites. Notwithstanding these limitations, our findings yield key clinical implications. With obesity now ranked as the second leading risk factor for cancer,2 and with the relative annual increase in T2D incidence of 4.8% among youths,41 further investigations into the energy balance-insulin-cancer link remains critical to the optimization of cancer prevention and intervention strategies among AYAs.
To our knowledge, this study represents the most complete reporting of patterns of T2D, body fatness, and insulin prescription among AYAs diagnosed with cancer. We observed that approximately 60% of individuals aged 18–39 years at cancer diagnosis are overweight or obese, one-quarter of patients were ever smokers, and that T2D was diagnosed among one in every 18 young patients with cancer. Further, there was an association between increasing BMI and risk of insulin prescription among AYAs within the first year of cancer diagnosis, independent of glucocorticoid prescription. Future studies of the clinical and genetic characteristics of AYA cancers are needed to explore potential body fatness/T2D/tumor interactions associated with patient outcomes and to guide the development of obesity, diabetes, and cancer prevention programs targeting young individuals.
Supplementary Material
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors declare no conflicts of interest with this work. C.M.U. has as cancer center director oversight over research funded by several pharmaceutical companies but has not received funding directly herself.
Funding Information
A.N.H. was supported by the Vanderbilt University Medical Center and the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. This work was also supported by grants from the National Institutes of Health/National Cancer Institute (R01 CA189184 and R01 CA207371 to C.M.U.; U01 CA206110 to C.M.U.), the Huntsman Cancer Foundation, the ACSM Paffenbarger-Blair Fund for Physical Activity Epidemiology, and the “Driving out Diabetes: A Larry H. Miller Family Wellness Initiative.” The research reported in this publication utilized the Research Informatics and Cancer Biostatistics Shared Resources at Huntsman Cancer Institute and the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA042014. The Total Cancer Care protocol at Huntsman Cancer Institute is in part supported by a collaboration with the Oncology Research Information and Exchange Network (ORIEN).
Supplementary Material
References
- 1. Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol. 2018;15(11):683–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massetti GM, Dietz WH, Richardson LC. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA. 2017;318(20):1975–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fidler MM, Gupta S, Soerjomataram I, et al. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–89 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Obesity and overweight fact sheet [Internet]. Accessed November2019 from: www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf
- 6. Singh S, Earle CC, Bae SJ, et al. Incidence of diabetes in colorectal cancer survivors. J Natl Cancer Inst. 2016;108(6):djv402. [DOI] [PubMed] [Google Scholar]
- 7. Hwangbo Y, Kang D, Kang M, et al. Incidence of diabetes after cancer development: a Korean National Cohort Study. JAMA Oncol. 2018;4(8):1099–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bramante CT, Lee CJ, Gudzune KA. Treatment of obesity in patients with diabetes. Diabetes Spectr. 2017;30(4):237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Chubak J, Boudreau DM, et al. Diabetes treatments and risks of adverse breast cancer outcomes among early-stage breast cancer patients: a SEER-medicare analysis. Cancer Res. 2017;77(21):6033–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–8 [DOI] [PubMed] [Google Scholar]
- 11. Gallagher EJ, Alikhani N, Tobin-Hess A, et al. Insulin receptor phosphorylation by endogenous insulin or the insulin analog AspB10 promotes mammary tumor growth independent of the IGF-I receptor. Diabetes. 2013;62(10):3553–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ter Braak B, Siezen C, Speksnijder EN, et al. Mammary gland tumor promotion by chronic administration of IGF1 and the insulin analogue AspB10 in the p53R270H/(+)WAPCre mouse model. Breast Cancer Res. 2015;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verheus M, Peeters PH, Rinaldi S, et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006;119(3):659–67 [DOI] [PubMed] [Google Scholar]
- 14. Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holowatyj AN, Lewis MA, Pannier ST, et al. Clinicopathologic and racial/ethnic differences of colorectal cancer among adolescents and young adults. Clin Transl Gastroenterol. 2019;10(7):e00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J Clin Oncol. 2016;34(18):2148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holowatyj AN, Ulrich CM, Lewis MA. Racial/ethnic patterns of young-onset noncardia gastric cancer. Cancer Prev Res (Phila). 2019;12(11):771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holowatyj AN, Washington MK, Salaria SN, et al. Early-onset appendiceal cancer survival by race or ethnicity in the United States. Gastroenterology. 2020. [Epub ahead of print]; DOI: 10.1053/j.gastro.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers CR, Moore JX, Qeadan F, et al. Examining factors underlying geographic disparities in early-onset colorectal cancer survival among men in the United States. Am J Cancer Res. 2020;10(5):1592–607 [PMC free article] [PubMed] [Google Scholar]
- 20. Chao C, Xu L, Bhatia S, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA cancer survivors study. J Clin Oncol. 2016;34(14):1626–33 [DOI] [PubMed] [Google Scholar]
- 21. Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer J. 2011;17(6):528–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017(288):1–8 [PubMed] [Google Scholar]
- 23. Haffa M, Holowatyj AN, Kratz M, et al. Transcriptome profiling of adipose tissue reveals depot-specific metabolic alterations among patients with colorectal cancer. J Clin Endocrinol Metab. 2019;104(11):5225–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ose J, Holowatyj AN, Nattenmüller J, et al. Metabolomics profiling of visceral and abdominal subcutaneous adipose tissue in colorectal cancer patients: results from the ColoCare study. Cancer Causes Control. 2020;31(8):723–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109(8):djx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110(6):608–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–98 [DOI] [PubMed] [Google Scholar]
- 28. Archambault AN, Su YR, Jeon J, et al. Cumulative burden of colorectal cancer-associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology. 2020;158(5):1274..e12–86.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holowatyj AN, Gigic B, Herpel E, et al. Distinct molecular phenotype of sporadic colorectal cancers among young patients based on multiomics analysis. Gastroenterology. 2020;158(4):1155..e2–58.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Draznin B. Mitogenic action of insulin: friend, foe or ‘frenemy’? Diabetologia. 2010;53(2):229–33 [DOI] [PubMed] [Google Scholar]
- 32. de Kort S, Masclee AAM, Sanduleanu S, et al. Higher risk of colorectal cancer in patients with newly diagnosed diabetes mellitus before the age of colorectal cancer screening initiation. Sci Rep. 2017;7:46527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bright CJ, Reulen RC, Winter DL, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20(4):531–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gallo LC, Troxel WM, Matthews KA, Kuller LH. Marital status and quality in middle-aged women: associations with levels and trajectories of cardiovascular risk factors. Health Psychol. 2003;22(5):453–63 [DOI] [PubMed] [Google Scholar]
- 35. Mata J, Richter D, Schneider T, Hertwig R. How cohabitation, marriage, separation, and divorce influence BMI: a prospective panel study. Health Psychol. 2018;37(10):948–58 [DOI] [PubMed] [Google Scholar]
- 36. Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21(4):877–83 [DOI] [PubMed] [Google Scholar]
- 37. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fralick M, Kesselheim AS. The U.S. insulin crisis—Rationing a lifesaving medication discovered in the 1920s. N Engl J Med. 2019;381(19):1793–95 [DOI] [PubMed] [Google Scholar]
- 39. Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.