Abstract
Objective: To evaluate the long-term cost-effectiveness of ginkgolide plus aspirin compared with placebo plus aspirin treatment of ischemic stroke.
Background: Stroke is the leading cause of death and long-term disability in China, with high incidence, high mortality, and heavy disease burden. In addition to Western medicines, Chinese clinical guidelines for diagnosis and treatment of acute ischemic stroke recommend application of Chinese patent medicines. Ginkgolide injection is commonly used in the clinical treatment of stroke in China to promote blood circulation and remove blood stasis. The economy of ginkgolide injection needs to be evaluated.
Methods: A Markov model was constructed consisting of four disease states: no significant disability, disability, stroke recurrence, and death. Therapeutic data were taken from the Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis (GISAA) study. Utilities and transition probabilities were extracted from the literature. Cost data were obtained from the China Health Statistics Yearbook and hospital record survey. Expected costs and quality-adjusted life-years (QALYs) of 13 years of cycles (calculated by average age of subjects and Chinese life expectancy) were calculated through TreeAge Pro11 software. The willingness-to-pay (WTP) threshold was set as the Chinese per capita Gross Domestic Product (GDP) in 2019, CN¥70,892/QALY. The results were analyzed by single factor and probability sensitivity analyses.
Results: Ginkgolide plus aspirin had a higher expected per-patient cost than placebo plus aspirin but a higher QALYs. Compared with placebo plus aspirin, ginkgolide plus aspirin produced an incremental cost-effectiveness ratio of CN¥14,866.06/QALY, which is below the WTP threshold. Probabilistic sensitivity analysis suggested the acceptability of ginkgolide plus aspirin was higher than that of placebo plus aspirin.
Conclusions: The present cost-effectiveness analysis showed that addition of ginkgolides to conventional treatment is cost-effective at a threshold the Chinese per capita GDP.
Keywords: stroke, cost-effectiveness analysis, ginkgolides, quality-adjusted life-years, Markov model
Introduction
Ischemic heart disease and stroke are the leading cause of global mortality. In China, stroke is the primary cause of death and disability in adult populations, with a high incidence, mortality, and disability rate.1 In a study that surveyed 480,687 adults in 31 provinces in China, the annual rate of age-standardized prevalence, incidence, and mortality were 1114.8, 246.8, and 114.8/100,000, respectively.2
Stroke includes both hemorrhagic and ischemic types. According to the 2019 update of Heart Disease and Stroke Statistics by the American Heart Association, ischemic stroke is a leading cause of death and serious long-term disability worldwide.3 Ischemic stroke carries a huge disease burden. According to Global Burden of Disease research data, the disability-adjusted life-years of ischemic stroke in China was 891.41/100,000 in 2015.1 According to the 2018 China Health and Family Planning statistical yearbook, the number of discharged patients with cerebral ischemic stroke in 2015 was 2,365,110, and the average cost of hospitalization was CN¥9174.20; both statistics increased by 94.2% and 28.4%, respectively, from 2010, which brought heavy economic burden to individuals and society.1 Under the current treatment conditions, 20% of stroke patients still need to enter rehabilitation institutions for treatment after leaving hospital, whereas 30% have a lifetime disability.4
At present, intravenous thrombolysis and intra-arterial thrombectomy are widely recognized as standard clinical treatment strategies for patients with acute ischemic stroke.5 However, these treatments have a sharp treatment time window and a severe hemorrhagic risk, and only a small number of patients benefit.6–8 Drug therapy in the acute and convalescence stages plays an important role. At present, American Heart Association/American Society of Anesthesiologists guidelines recommend statin treatment and neuroprotection for ischemic stroke to improve cerebral circulation.9 In addition, 2018 Chinese clinical guidelines for diagnosis and treatment of acute ischemic stroke uses Chinese patent medicine as a level III recommendation, level C evidence, and points out that “its efficacy needs to be further confirmed by more high-quality randomized controlled trial (RCT).”10
In China, Traditional Chinese Medicine (TCM), especially compound TCM preparations, has been widely used for the treatment of stroke for thousands of years.11 Ginkgo is an effective complementary drug for stroke and cerebral ischemia.
Previous studies have revealed that ginkgo biloba extracts can inhibit platelet aggregation,5,12,13 and ginkgo biloba is the most powerful platelet-activating factor antagonist found in nature. Ginkgolides also exert neuroprotective and anti-inflammatory effects as they can prevent elevation of intracellular calcium concentrations, regulate glucocorticoid biosynthesis, and reduce levels of inflammatory factors.13–16 Ginkgo biloba extracts have been shown to alleviate cognitive and neurological deficits after acute ischemic stroke without increasing incidence of vascular events.17 Ginkgo biloba extracts combined with aspirin increase regeneration of neuroblastoma cells injured by hypoxia and reperfusion.18 As an extract of ginkgo biloba leaves, ginkgolide injection has been used in the treatment of acute ischemic stroke in China since it was approved by the China Food and Drug Administration in 2011. A systematic review of treatment of patients with ischemic stroke showed ginkgolide injection plus conventional therapy was superior to the control in improving clinical curative effect and neurological severity scores.19
To comprehensively evaluate the efficacy and safety of ginkgolide injection in the treatment of ischemic stroke, the Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis (GISAA) randomized clinical trial was launched in April 2016.20 The study began in October 2016, and all follow-up was completed in August 2018, with 61 hospitals from 8 cities in China participating, and 949 patients in the group.
To evaluate the economy of ginkgolide plus aspirin injection in the treatment of ischemic stroke, this study evaluated the long-term cost-effectiveness of aspirin plus ginkgolide injection compared with aspirin (plus placebo) in the treatment of atherosclerotic ischemic stroke based on GISAA data to provide sound pharmacoeconomic evidence as a framework for decision and policy making in stroke treatment.
Materials and Methods
Clinical trials
A 28-day multicenter, double-blind, placebo parallel-controlled clinical trial GISAA was performed. The study has prospectively registered on www.chictr.org.cn (ChiCTR-IPR-17012310). The GISAA RCT study evaluated the efficacy and safety of treating initial onset of acute cerebral ischemic stroke for 14 days with 10 mL q.i.d., intravenously guttae (IVGTT) ginkgolide injection plus 100 mg q.i.d., PO aspirin (experimental group, n = 471 cases) or 10 mL q.i.d., IVGTT sterile water (placebo) plus 100 mg q.i.d., PO aspirin (control group, n = 478 cases). Patients within the first 72 h of an ischemic stroke were eligible (Supplementary Table S1). Baseline characteristics of the patients are given in Supplementary Table S2. There was no significant difference in death and recurrence rate between the two groups on day 28 (Table 1). Patients were measured the degree of disability in the daily activities by modified Rankin Scale (mRS) and qualified the stroke impairment by The National Institutes of Health Stroke Scale (NIHSS). mRS score ranges from 0 to 6. The higher the score, the more serious the situation, and a score of 6 was death. NIHSS score ranges from 0 to 42. The higher the score, the more serious the situation. The proportion of subjects with mRS ≤2 was 87.95% in the experimental group and 83.03% in the control group (relative risk [RR] = 1.05, 95% confidence interval [CI] = 1.00–1.12, p = 0.0418). The NIHSS score (total score) of the two groups decreased by 3.7 and 3.4 points, respectively, in the experimental group and the control group, and the difference between the two groups was statistically significant (p = 0.0157) (Table 2).21
Table 1.
The Death and Recurrence of the Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis on the 28th Day
| Variable | EG (N = 463) | CG (N = 473) | p |
|---|---|---|---|
| Death and mortality, n (%) | |||
| Death | 0 (0) | 2 (0.4) | 0.4995 |
| Recurrence | 0 (0) | 3 (0.6) | 0.2493 |
| Death+recurrence | 0 (0.0) | 5 (1.1) | 0.0619 |
CG, control group; EG, experiment group.
Table 2.
The Modified Rankin Scale Score and National Institutes of Health Stroke Scale Score of the Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis on Day 28
| Variable | EG (N = 415) | CG (N = 436) | p |
|---|---|---|---|
| mRS score, n (%) | |||
| mRS ≤2 | 365 (87.95) | 362 (83.03) | 0.0418 |
| mRS >2 | 50 (12.05) | 74 (16.97) | |
| NIHSS scorea | |||
| Mean | 3.7 | 3.4 | 0.0157 |
One case of CG lost to follow-up.
CG, control group; EG, experiment group; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Markov model structure
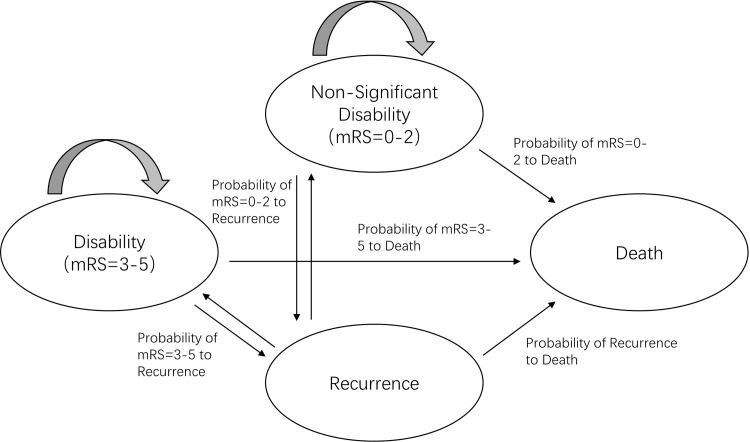
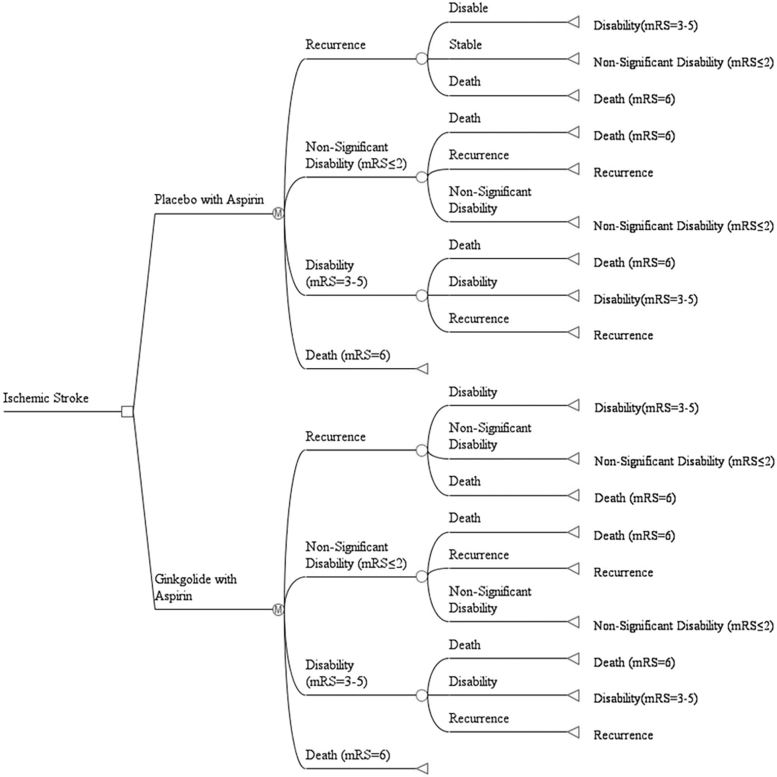
The state division and transfer path of Markov model is the simulation of the occurrence and development of stroke patients after treatment. We constructed a Markov model using TreeAge Pro 2011 (TreeAge Software, Williamstown, MA) from a society perspective. Based on the mRS score on day 28 in the GISAA clinical trial, we set up a Markov model with four disease states: (1) nonsignificant disability, with the mRS score of 0–2 after treatment, which indicates that the prognosis is good after treatment, the patient able to look after own affairs without assistance or carry out all usual activities; (2) disability, with the mRS score of 3–5 after treatment, which indicates that the prognosis is poor and the patient has moderate or severe disability22; (3) recurrence; and (4) death (Fig. 1). The study cycle was set as 1 year. The average life expectancy in China is 77 years, and the average age of subjects in the GISAA trial was 64 years. Therefore, the current assessment was set to 13 cycles in total to simulate the long-term cost-effectiveness of patients 13 years after treatment, with a half cycle correction (Fig. 2).
FIG. 1.
Health state structure for the Markov models. The Markov model contains four-state, nonsignificant disability, disability, recurrence, and death. mRS, Modified Rankin Scale.
FIG. 2.
Markov model structure. Schematic representation of the Markov model illustrates that all patients start at 64 years old with acute cerebral ischemic stroke. All patients receive a 28-day treatment and cycle between health states until death occurs, or the 13-year model time-horizon is reached. Depicted in the diagram is the decision node (square), chance nodes (empty circles) directed by transition probabilities, Markov nodes (circles with “M”), and terminal nodes (triangles). mRS, Modified Rankin Scale.
Model assumptions
Considering the availability of data, our model makes the following assumptions: (1) In the first period of model simulation, all patients were treated in the first experimental or control group and entered the stroke simulation period. (2) All patients were assumed to receive standardized treatment. (3) It is assumed that the transition probability between the recurrence and death of stroke patients in this study was the same as that of the whole population of stroke patients. (4) As the absorption state, the utility and cost of death state were both zero.
Transition probability
The initial distribution probability of the states in the experimental and control groups, which is the transition probability of the first 28-day treatment, were obtained based on the GISAA study. In the GISAA test, the proportion of subjects with an mRS ≤2 in the experimental and the control groups was 87.95% and 83.03%, respectively (RR = 1.05, 95% CI = 1.00–1.12, p = 0.0418). The long-term probability of state transition mainly refers to a large-scale clinical study of stroke and related economic literature23,24 (Table 3).
Table 3.
Base Case and Plausible Ranges of Model Transition Probability
| Transition probability | Base case | Upper limit | Lower limit | Source | Range (%) |
|---|---|---|---|---|---|
| INITIAL PROBABILITY | |||||
| EG mRS 0–2 | 0.88 | 0.97 | 0.79 | GISAA | ±10 |
| CG mRS 0–2 | 0.83 | 0.90 | 0.74 | GISAA | ±10 |
| EG mRS 3–5 | 0.12 | 0.13 | 0.11 | GISAA | ±10 |
| CG mRS 3–5 | 0.17 | 0.18 | 0.15 | GISAA | ±10 |
| EG recurrence | 0.00 | 0.00 | 0.00 | GISAA | ±10 |
| CG recurrence | 0.01 | 0.01 | 0.01 | GISAA | ±10 |
| EG death | 0.00 | 0.00 | 0.00 | GISAA | ±10 |
| CG death | 0.00 | 0.00 | 0.00 | GISAA | ±10 |
| Transition probability | |||||
| mRS 0–2 to recurrence | 0.10 | 0.11 | 0.09 | Pan et al.23 | ±10 |
| mRS 3–5 to recurrence | 0.14 | 0.16 | 0.13 | Pan et al.23 | ±10 |
| Recurrence to mRS 0–2 | 0.40 | 0.43 | 0.36 | Wang et al.24 | ±10 |
| Recurrence to mRS 3–5 | 0.40 | 0.43 | 0.36 | Wang et al.24 | ±10 |
| Recurrence to death | 0.21 | 0.23 | 0.19 | Pan et al.23 | ±10 |
| mRS 0–2 to death | 0.01 | 0.01 | 0.01 | Pan et al.23 | ±10 |
| mRS 3–5 to death | 0.02 | 0.02 | 0.01 | Pan et al.23 | ±10 |
mRS 0–2 is the state of nonsignificant disability; mRS 3–5 is the state of disability.
CG, control group; EG, experiment group; GISAA, Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis; mRS, modified Rankin Scale.
Quality-of-life and utility values
Health output is utility, which is measured by quality-adjusted life-years (QALYs). Since the QALYs of patients were not measured in the GISAA trial, the utility of different states, which were set based on the mRS score, referred to that presented in previous literature (Table 4).22,25 Here, 5% was used as the discount rate and 0%–8% as the upper and lower limit in sensitivity analysis.
Table 4.
Base Case and Plausible Ranges of Model Utility
| Utility | Base case | Upper limit | Lower limit | Source | Range |
|---|---|---|---|---|---|
| mRS 0–2 | 0.76 | 0.91 | 0.61 | Yi-Long (2015) | 95% CI |
| mRS 3–5 | 0.21 | 0.26 | 0.17 | Yi-Long (2015) | 95% CI |
| Death | 0 | 0 | 0 | Yi-Long (2015) | |
| Recurrence | 0.34 | 0.36 | 0.32 | Ganesalingam (2015) | 95% CI |
The utility mRS 0–2 is the utility of the nonsignificant disability state; the utility mRS 3–5 is the utility of the n disability state.
CI, confidence interval; mRS, modified Rankin Scale.
Resources use
All costs and resource use inputs were obtained from GISAA trail, publicly available sources, published health economic literature, and/or expert opinion.23,24,26,27 Treatment algorithms were obtained from the published literature, including drug use, examination test, sickbed, transportation, nursing, recurrent treatment. All costs were converted to 2019 Chinese Yuan by using the consumer price index28; CN¥ was converted to U.S.$ by dividing by 6.8985, the average exchange rate in 2019.
Costs
From the perspective of the whole society, this study considered direct and indirect cost. Within 28 days of treatment, the study considered drug cost, examination cost, bed cost, transportation cost, and care cost. In the long-term simulation, the study considered nursing cost and recurrent treatment cost. The drug cost of the experimental group and the control group was from the real drug data of patients in the GISAA trial. The average drug consumption of the two groups is selected as the drug dosage.26 The unit price is selected from the bid-winning price in China in 2019. Examination cost, bed cost, and nursing cost after discharge all came from the previous literatures of stroke pharmacoeconomics.23,24,27 Other treatment-related costs, care costs, and transportation costs of the experimental and the control groups were collected from the GISAA trial. Since the average life span of the study population has reached 64 years, which is close to the retirement age in China, the study did not calculate the indirect cost of work delay. The discount rate of the utility is equal to that of the utility value. The costs involved in this study and their sources, determination methods, and upper and lower limit interval settings are given in Table 5.
Table 5.
Base Case and Plausible Ranges of Model Cost
| Parameter | Base case | Upper limit | Lower limit | Resource |
|---|---|---|---|---|
| Direct medical costs | ||||
| Drug costs (EG) | 1,310.49 | 1,441.54 | 1,179.44 | GISAA |
| Drug costs (CG) | 59.20 | 65.12 | 53.28 | GISAA |
| Examination costs | 1,634.70 | 1,798.17 | 1,471.23 | China Health Statistics Yearbook |
| Bed costs | 515.00 | 566.50 | 463.50 | China Health Statistics Yearbook |
| Other treatment costs (EG) | 12,265.00 | 13,491.5 | 11,038.5 | GISAA |
| Other treatment costs (CG) | 10,102.00 | 11,112.2 | 9,091.8 | GISAA |
| Direct nonmedical costs | ||||
| Care costs (EG) | 1,228.57 | 1351.424 | 1,105.71 | GISAA |
| Care costs (CG) | 1,226.58 | 1349.2396 | 1,103.92 | GISAA |
| Transportation costs (EG) | 1,140.50 | 1,235.30 | 1,045.80 | GISAA |
| Transportation costs (CG) | 1,198.80 | 1,303.70 | 1,093.80 | GISAA |
| Nursing costs (after discharge) | ||||
| mRS 0–2 | 8,316.20 | 9,147.815 | 7,484.58 | Pan et al.23 |
| mRS 3–5 | 12,781.15 | 14,059.27 | 11,503.04 | Pan et al.23 |
| Recurrent treatment costs | 28,987.05 | 31,885.76 | 26,088.35 | Pan et al.,23 Wang et al.24 |
CG, control group; EG, experiment group; GISAA, Ginkgolide in Ischemic Stroke Patients with Large Artery Atherosclerosis; mRS, modified Rankin Scale.
Threshold
The incremental cost-effectiveness ratio (ICER) was used for the economic evaluation of two treatments. ICER is calculated as “cost difference/health output difference,” which represents the additional cost for each additional unit of health output between the study group and the control group. If the ICER is below the willingness-to-pay (WTP) threshold, the treatment is cost-effective. In this study, the per capita Gross Domestic Product (GDP) of China in 2019 was used as the WTP threshold. According to the website of the National Bureau of Statistics, the per capita GDP of China in 2019 is CN¥70,892,28 so the threshold value set in this study was CN¥70,892 per QALY gained.
Sensitivity analyses
One-way sensitivity analyses
One-way sensitivity analyses were conducted to test alternative modeling assumptions and alternative values for key model parameters. Depending on data availability, the ranges considered in one-way sensitivity analyses included 95% CIs or ±10% of the base case values. The main indicators of one-way sensitivity analysis were the price of the two drugs, clinical outcome, probability of each transfer, and utility value of the two drugs after treatment. The results from one-way sensitivity analyses were presented in a Tornado diagram, showing sequentially the variables with the largest impact on cost-effectiveness results.
Probabilistic sensitivity analyses
The overall impact of uncertainty in the model was assessed with probabilistic sensitivity analyses (PSA) by defining distributions for key parameters in the model. The key parameters included in the PSA were clinical outcome, unit cost, and utility variables. Normal distribution is applied to clinical data, γ distribution is applied to cost data, and β distribution is applied to utility data (Table 6). The PSA was run for 1000 iterations (simulations), and the results were plotted on the cost-effectiveness plane as scatterplots and cost-effectiveness acceptability curves to evaluate the pharmacoeconomic value of the two treatments.
Table 6.
Summary of Probabilistic Distributions Applied in the Model
| Parameter cluster | Parameters | Distribution |
|---|---|---|
| Clinical data | Clinical effect after acute treatment, transition probability | Normal distribution |
| Cost data | Direct medical, direct nonmedical, indirect costs; nursing, recurrent treatment costs | γ distribution |
| Utility data | Utility weights assigned to each states | β distribution |
Results
Base case results for estimating the effectiveness of ginkgolide plus aspirin (experiment group) and placebo plus aspirin (control) are given in Table 7. Ginkgolide plus aspirin had a higher total cost and also higher utility. Incremental costs were CN¥2,889.83, and incremental utility was 0.19 QALYs. The ICER of ginkgolide plus aspirin versus placebo plus aspirin was CN¥16,353 per QALY gained, which is below the WTP threshold.
Table 7.
Cost-Effectiveness Results for Ginkgolide plus Aspirin Injection vs. Placebo plus Aspirin
| Name | Cost (CN¥) | QALYs | Incremental cost (CN¥) | Incremental QALYs | Incremental cost per QALY gained (CN¥) |
|---|---|---|---|---|---|
| Placebo with aspirin | 106,382.30 | 4.87 | 0 | 0 | 0 |
| Ginkgolide with aspirin | 109,272.13 | 5.05 | 2,889.83 | 0.18 | 16,353.80 |
QALY, quality-adjusted life-year.
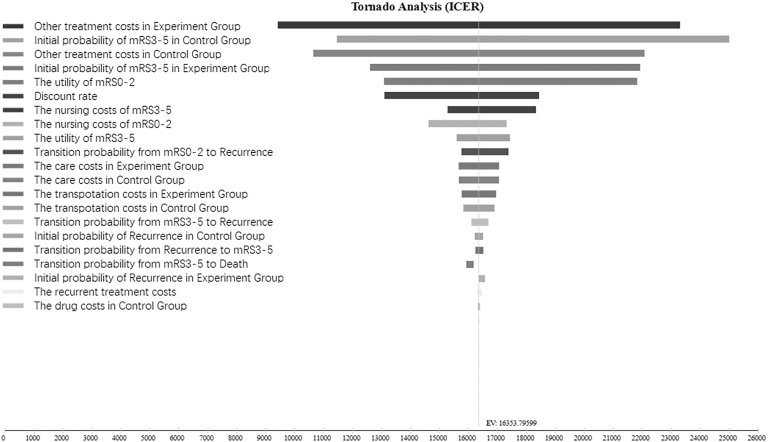
Single factor sensitivity analysis of the ICER was carried out within the range of all indicator changes (Fig. 3). According to the Tornado diagram of the single factor sensitivity analysis, the top five factors that had great influence on ICER were the other treatment costs of the experimental group, the initial number of patients with an mRS = 3–5 in the control group, other treatment costs of the control group, the initial probability of mRS = 3–5 in the experimental group, and the utility of mRS = 0–2.
FIG. 3.
Tornado diagram of one-way sensitivity analyses results for ginkgolide plus aspirin injection compared with placebo plus aspirin. Each horizontal bar in the tornado diagram represents cost-effectiveness values expected from a range of values evaluated for each variable. The vertical black line represents a change in the preferred treatment for a given variable being analyzed. ICER, incremental cost-effectiveness ratio.
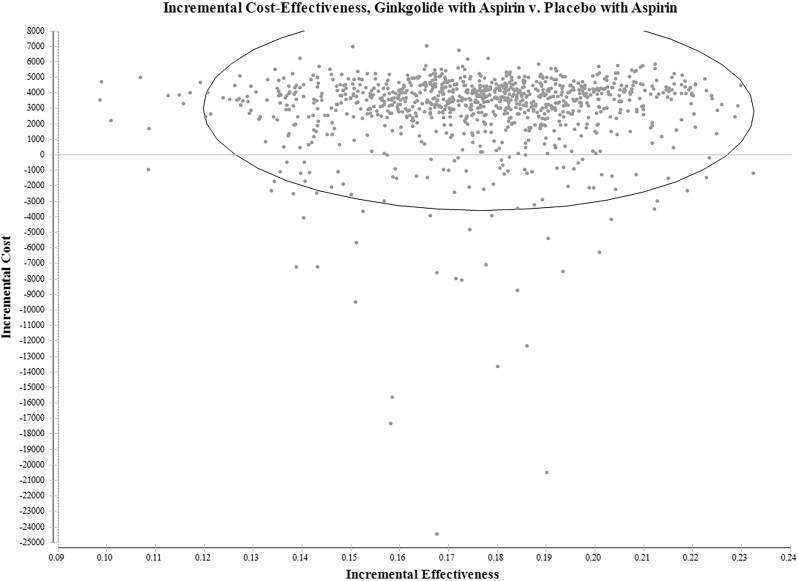
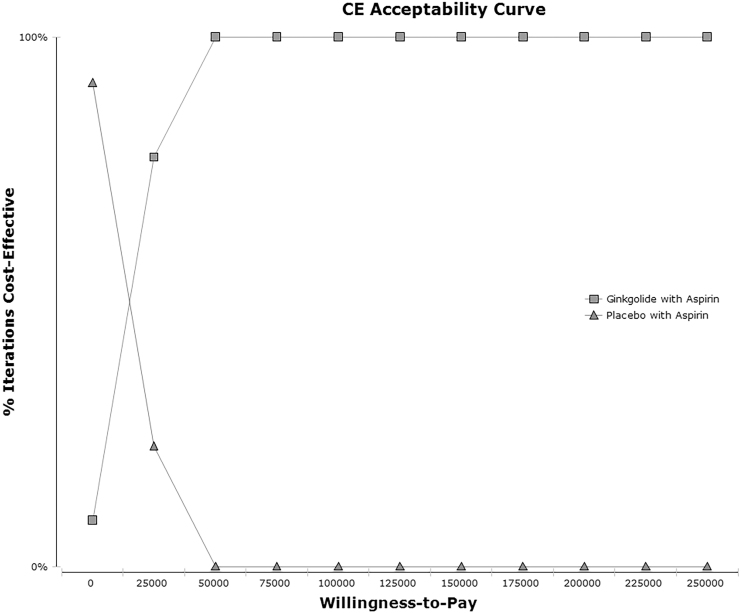
The probability sensitivity analysis of the ICER was carried out, and Monte Carlo simulation was conducted 1000 times to reflect the influence of each parameter on the model. The sensitivity analysis results compare the pharmacoeconomic value of different schemes by drawing the scatter diagram of the ICE. Figure 4 shows the average ICER scatter diagram of the 2 strategies for 1000 simulations, and Figure 5 shows the cost-effectiveness acceptability curve of the 2 strategies. The results showed that ginkgolide plus aspirin was more cost-effective than placebo plus aspirin in 100% of the simulation.
FIG. 4.
Cost-effectiveness scatterplot—ginkgolide plus aspirin injection versus placebo plus aspirin. Each point in the scatterplot diagram represents one simulation of sensitive analysis. The circle represents the 95% confidence interval.
FIG. 5.
Cost-effectiveness acceptability curve—ginkgolide plus aspirin injection versus placebo plus aspirin. This cost-effectiveness acceptability curve illustrates the probability that a treatment will be cost-effective (percentage of iterations for which the treatment was cost-effective is indicated along the y axis) at varying willingness-to-pay thresholds (shown along the x axis as the amount, in dollars, a decision maker is willing-to-pay to achieve an additional quality-adjusted life-year) for a patient. CE, cost-effectiveness.
Discussions
TCM is playing a more and more important role in the treatment of diseases. During COVID-19, Chinese medicine (including TCM injection) was regarded as an effective method in the diagnosis and treatment specifications formulated by the National Health Commission of the People's Republic of China.29 Moreover, pharmacological experiments and real-world data have proven the efficiency of TCM for various diseases.29–31
Previous studies have revealed that ginkgolide A, ginkgolide B, and ginkgolide C can inhibit platelet aggregation.5,12,13 Ginkgolide B is considered to be the most powerful platelet-activating factor antagonist found in nature. Ginkgolides also exert neuroprotective and anti-inflammatory effects as they can prevent elevation of intracellular calcium concentrations, regulate glucocorticoid biosynthesis, and reduce levels of inflammatory factors.13–16 The GISAA study confirmed the clinical effectiveness of ginkgolide injection. From an economic perspective, this study showed that treatment of stroke with ginkgolide injection is cost-effective. This study used published clinical trial data to build a decision model, and our analyses indicated that for patients ≥64 years old with first onset of an acute cerebral ischemic stroke, ginkgolide injection may be a cost-effective substitute for aspirin in the prevention of disability (WTP threshold was CN¥70,892 per QALY gained). This finding is in line with recently published cost-effectiveness studies, including decision models and real-world research of ginkgolide injection conducted in China.4,32
Published economic literature about stroke to date has solely focused on the use of chemical drugs. Globally, few studies assessing the pharmacoeconomics of Chinese medicine have been reported. Wang performed a cost-effectiveness analysis of TCM using a Markov model.33 Sun analyzed the cost-effectiveness of JinQi Jiangtang tablets for the treatment of prediabetes.34 Zheng evaluated the economics of a Yuxingcao Qinlan mixture and LanQin oral liquid in treating upper respiratory tract infections.35 However, these studies only carried out short-term economic evaluations, without long-term cost-effectiveness analysis.
According to the present sensitivity analysis, the top factors with great influence on ICER were other treatment costs and the initial number of patients with an mRS = 3–5. The biggest burden of stroke is resultant disability, so a treatment strategy that can improve the disability rate after acute treatment will be more cost-effective. The cost of conventional therapy (aspirin) plus other drugs is based on more than just a single use. According to the WHO's suggestion, the ICER below threshold of three-times GDP is cost-effective. However, according to China's medical insurance negotiation policy in recent years, the decision-making departments tend to take one-time GDP and about as the cost-effectiveness criteria, so we use CN¥70,892 as threshold. Only when the threshold is about CN¥20,000 does conventional therapy begin to be an acceptable strategy in terms of cost.
This study has some limitations. The data used were obtained from the GISAA RCT, and the extrapolation remains to be verified. Due to the short observation time in the RCT, mortality and recurrence data are limited. Thus, the results need to be verified by long-term, real-world data. Some cost data (such as nursing costs) were obtained from previous literature, and some state utility was taken from non-Asian/Chinese populations, which may be biased. Part of the cost data used were collected before 2019, so the consumer price index indicator was used for discount conversion. However, the consumer price indicator of medical service may be different from the average consumer price indicator of the society. The utility data in this study were obtained by literature and not the GISAA study, which is from non-Asian/Chinese population. At present, there are few studies on utility value in China mainland. This may be a challenge for Chinese pharmacoeconomics evaluation. There is not much published information regarding the adverse reactions of ginkgolide injection, and such symptoms can be alleviated by stopping the drug or reducing the dropping rate, so the cost and effect of adverse reactions were not considered in this study.
Conclusions
This study demonstrated that ginkgolide injection plus aspirin treatment for acute cerebral ischemic stroke is cost-effective in the long term for patients with first paroxysm. The experimental and control schemes herein were based on conventional aspirin treatment and reflect the efficacy and economy of combination aspirin plus ginkgolide injection treatment versus aspirin alone in clinical practice. These results provide important practical information for clinical practice decision-making.
Supplementary Material
Authors' Contributions
Y.X. performed the statistical analysis and drafted the article. N.Y. handled funding and drafted the article. Z.G. and L.Z. acquired and interpreted the data. J.J.G. provided technology support and made critical revision to the article. M.H. conceived the overall study, acquired the data, and made critical revision to the article. Y.X. and N.Y. contributed equally to this article. All authors have reviewed and approved the article and agree with submission to the Journal of Alternative and Complementary Medicine.
Author Disclosure Statement
There are no competing interests for any author.
Funding Information
This research has been supported by National Key R&D Program of China (Grant No. 2019YFC1709804), Sichuan Administration of Traditional Chinese Medicine (Project No. 2018ZC004), and Innovation and Talent Recruiting Program for Higher Education (The 111 Project; Project No. B18035).
Supplementary Material
References
- 1. Wang L, Liu J, Yang Y, et al. Summary of China Stroke Prevention report 2017. Chin J Cerebrovasc Dis 2018;15:611–617 [Google Scholar]
- 2. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China. Circulation 2017;135:759. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Bittencourt MS. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 4. Ji C, He C, Li H. Multicenter evaluation on pharmacoeconomics of Ginkgolide Injection in treatment of severe ischemic stroke. Drug Eval Res 2018;41:29–33 [Google Scholar]
- 5. Fan Y, Liao X, Pan Y, et al. Intravenous thrombolysis is safe and effective for the cryptogenic stroke in China: Data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China). J Stroke Cerebrovasc Dis 2019;28:220–226 [DOI] [PubMed] [Google Scholar]
- 6. Muchada M, Rodriguez-Luna D, Pagola J, et al. Impact of time to treatment on tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 2014;45:2734–2738 [DOI] [PubMed] [Google Scholar]
- 7. Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet 2012;379:2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen PM, Lehmann B, Meyer BC, et al. Timing of symptomatic intracerebral hemorrhage after rt-PA treatment in ischemic stroke. Neurol Clin Pract 2019;9:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 10. Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol 2018;51:666–682 [Google Scholar]
- 11. Wang Y, Xiao G, He S, et al. Protection against acute cerebral ischemia/reperfusion injury by QiShenYiQi via neuroinflammatory network mobilization. Biomed Pharmacother 2020;125:109945. [DOI] [PubMed] [Google Scholar]
- 12. Kubota Y, Tanaka N, Kagota S, et al. Effects of Ginkgo biloba extract on blood pressure and vascular endothelial response by acetylcholine in spontaneously hypertensive rats. J Pharm Pharmacol 2010;58:243–249 [DOI] [PubMed] [Google Scholar]
- 13. Wang S, Ouyang B, Aa J, et al. Pharmacokinetics and tissue distribution of ginkgolide A, ginkgolide B, and ginkgolide K after intravenous infusion of ginkgo diterpene lactones in a rat model. J Pharm Biomed Anal 2016;126:109–116 [DOI] [PubMed] [Google Scholar]
- 14. Xiaoyan L, Li C, Liu T, et al. Chemical analysis, pharmacological activity and process optimization of the proportion of bilobalide and ginkgolides in Ginkgo biloba extract. J Pharm Biomed Anal 2018;160:46–54 [DOI] [PubMed] [Google Scholar]
- 15. Chen Z, Bai S, Hu Q, et al. Ginkgo biloba extract and its diterpene ginkgolide constituents ameliorate the metabolic disturbances caused by recombinant tissue plasminogen activator in rat prefrontal cortex. Neuropsychiatr Dis Treat 2018;14:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen M, Zou W, Chen M, et al. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur J Pharmacol 2018;833:221–229 [DOI] [PubMed] [Google Scholar]
- 17. Li S, Zhang X, Fang Q, et al. Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: A randomised controlled trial. Stroke Vasc Neurol 2017;2:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moon SH, Lee YJ, Park SY, et al. The combined effects of Ginkgo biloba extracts and aspirin on viability of SK-N-MC, neuroblastoma cell line in hypoxia and reperfusion condition. J Korean Neurosurg Soc 2011;49:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao H, Guo Q, Ning J, et al. Effectiveness and safety of types of bilobalide injections for the treatment of patients with ischemic stroke: A systematic review. Drug Eval Res 2018;41:1196–1202 [Google Scholar]
- 20. Dong Q. Ginkgolide in Ischmic Stroke Patients with Large Artery Atherosclerosis (GISAA): A randomized, double-blinded, multicenter, placebo-controlled study. Br J Heart Dis 2019;2:119–124 [Google Scholar]
- 21. Dong Q. GISAA—New progress in clinical research of large artery atherosclerotic ischemic stroke. In: The 19th Congress of Chinese Cerebrovascular Diseases, Nanjing, 2019 [Google Scholar]
- 22. Yi-Long W, Yue-Song P, Xing-Quan Z, et al. Recurrent stroke was associated with poor quality of life in patients with transient ischemic attack or minor stroke: Finding from the CHANCE trial. CNS Neurosci Ther 2015;20:1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan Y, Cai X, Huo X, et al. Cost-effectiveness of mechanical thrombectomy within 6 hours of acute ischaemic stroke in China. BMJ Open 2018;8:e018951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang F, Tao L, Jia S, et al. Health economic evaluation of intravenous thrombolysis with mechanical thrombectomy compared with intravenous thrombolysis alone on treatment of acute ischemic stroke. China Health Insurance 2019;3 [Google Scholar]
- 25. Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke 2015;46:2591–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. YaoZH. Drug bid winning information database. 2019. Online document at: https://db.yaozh.com/yaopinzhongbiao, accessed November9, 2019
- 27. Wu J, Wang X, Liu G. Pharmacoeconomic evaluation of Ginkgolides meglumine injection treating ischemic stroke. China J Pharm Econ 2017;12:21–26 [Google Scholar]
- 28. China National Bureau of Statistics (CNBS). Indicators of national accounts. 2018. Online document at: http://data.stats.gov.cn/tablequery.htm?code=AD02, accessed October2, 2020
- 29. Zhifei W, Yanping W, Huamin Z, et al. Thinking on Clinical rational use of TCM injection in the treatment of novel coronavirus pneumonia (COVID-19). Natl Med J China 2020;100:E016. [DOI] [PubMed] [Google Scholar]
- 30. Ren X, Shao X-X, Li X-X, et al. Identifying potential treatments of COVID-19 from Traditional Chinese Medicine (TCM) by using a data-driven approach. J Ethnopharmacol 2020;258:112932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng W, Xu Y, Kong Q, et al. Therapeutic efficacy of Pudilan Xiaoyan Oral Liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct Target Ther 2020;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Ji C. Multicenter evaluation on pharmacoeconomics of Ginkgolide Injection in treatment of ischemic stroke. Drug Eval Res 2017;40:759–763 [Google Scholar]
- 33. Wang N, Zhang ZJ, Chang D. Using Markov model to cost-effectiveness analysis of traditional Chinese medicine interference. China J Chin Mater Med 2012;37:2698. [PubMed] [Google Scholar]
- 34. Xiao S. The cost-effectiveness analysis of JinQi Jiangtang tablets for the treatment on prediabetes: A randomized, double-blind, placebo-controlled, multicenter design. Trials 2015;16:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng M, Tian L, Huang H-L, et al. Cost-effectiveness analysis of traditional Chinese medicine for the treatment of upper respiratory tract infections. Eur J Integr Med 2017;9:97–102 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.