Abstract
The surface of articular cartilage is integral to smooth, low-friction joint articulation. However, the majority of cartilage literature rarely includes measurements of surface characteristics and function. This may, in part, be due to a shortage of or unfamiliarity with fast, nondestructive, and, preferably, noncontact methods that can be applied to large cartilage surfaces for evaluating cartilage surface characteristics. A comprehensive methodology for characterizing cartilage surfaces is useful in determining changes in tissue function, as for example, in cases where the quality of cartilage grafts needs to be assessed. With cartilage storage conditions being an area of ongoing and active research, this study used interferometry and tribology methods as efficient and nondestructive ways of evaluating changes in cartilage surface topography, roughness, and coefficient of friction (CoF) resulting from various storage temperatures and durations. Standard, destructive testing for bulk mechanical and biochemical properties, as well as immunohistochemistry, were also performed. For the first time, interferometry was used to show cartilage topographical anisotropy through an anterior–posterior striated pattern in the same direction as joint articulation. Another novel observation enabled by tribology was frictional anisotropy, illustrated by a 53% increase in CoF in the medial–lateral direction compared to the anterior–posterior direction. Of the storage conditions examined, 37°C, 4°C, −20°C, and −80°C for 1 day, 1 week, and 1 month, a 49% decrease in CoF was observed at 1 week in −80°C. Interestingly, prolonged storage at 37°C resulted in up to an 83% increase in the compressive aggregate modulus by 1 month, with a corresponding increase in the glycosaminoglycan (GAG) bulk content. This study illustrates the differential effects of storage conditions on cartilage: freezing tends to target surface properties, while nonfreezing storage impacts the tissue bulk. These data show that a bulk-only analysis of cartilage function is not sufficient or representative. The nondestructive surface characterization assays described here enable improvement in cartilage functionality assessment by considering both surface and bulk cartilage properties; this methodology may thus provide a new angle to explore in future cartilage research and tissue engineering endeavors.
Impact statement
Cartilage's major functions are load distribution and low-friction articulation. These functions are primarily carried out by the cartilage bulk tissue and surface layer, respectively. Although cartilage bulk properties are frequently assessed, surface characterization is often overlooked despite its importance to proper cartilage function. Toward closing this gap, this study presents cartilage evaluation that uses interferometry, tribology, and lubricin immunohistochemistry for comprehensive surface characterization. In the context of cartilage function preservation after storage, the conditions that alter bulk versus surface characteristics were found to differ, highlighting the importance of assessing surface function for both clinical and tissue engineering applications.
Keywords: articular cartilage, surface characterization, tribology, anisotropy, osteochondral allografts, storage
Introduction
Cartilage biomechanical function hinges on the tissue's ability to promote both load distribution and low-friction articulation. Preservation of cartilage functionality is of great clinical value, particularly with regard to the use of osteochondral allografts.1 Although allograft functionality is primarily determined by cell viability,2 assessments of biomechanical properties can provide important insights into the success or failure of transplanted tissue.
Currently, assessments of articular cartilage mechanical function provide an exceptional amount of information on the bulk tissue properties that confer cartilage's load-transferring capability.3 These properties are tested using a wide variety of loading paradigms, including compression, tension, and shear.4 However, few of these protocols include evaluations of the cartilage surface, thus, ignoring the surface's crucial role in smooth joint articulation.5 The lack of surface characterization data is illustrated in a recent review showing that only about 10% of studies on “articular cartilage mechanical testing” from 2009 to 2019 provided surface lubrication data along with bulk mechanical measures.3 Increased investigation in this area will improve the field's current understanding of cartilage function and would be facilitated by the development of a cartilage functionality assessment protocol that includes both bulk and surface characterization.
Articular cartilage's surface function heavily depends on friction, topography, and lubrication. Tribology is often performed to measure the coefficient of friction (CoF) between cartilage and a testing substrate material, such as polished glass or metals commonly used for implants.6,7 The CoF broadly describes the interaction between cartilage and other surfaces, but does not provide mechanistic information. To further elucidate a surface-specific structure–function relationship, elements that contribute to the CoF, such as topography and lubrication, must also be considered.
White-light interferometry is a noncontact imaging modality that can quickly capture surface topography; however, interferometry has been underused in cartilage research.8 While atomic force microscopy excels at providing nanoscale detail,9 interferometry functions at a larger scale that may be more relevant to assessing overall cartilage function. The impact of topographical imperfections is lessened with increased lubrication. Products of the proteoglycan 4 (PRG4) gene, such as lubricin and surface zone protein (SZP), are well known to enhance boundary lubrication at the cartilage surface and can be localized using immunohistochemistry (IHC).5 Inclusion of the three surface-related metrics, friction, topography, and lubrication, is rarely found in the literature, but would greatly enhance evaluations of cartilage function.
Despite decades of study, the preservation of articular cartilage function after long-term storage remains a topic of great interest to both clinicians and basic scientists. The increasing demand for osteochondral allografts10 and the required 14-day donor screening period1 have resulted in a need for transplant storage. Many preservation studies have focused on cell viability, finding that refrigeration best maintains viability above the 70% threshold generally accepted for allograft use.2,10 It is also known that cell viability is greatly reduced with freezing.11 Since viability has been extensively studied, the present work is instead focused on the role of surface and bulk tissue properties. The maintenance of these properties is also important in the context of the basic science laboratory. Lengthy experimental protocols commonly used in cartilage research often require tissue storage for long-term functional maintenance. The current understanding among most researchers is that cartilage bulk mechanical properties remain intact with freezing,11 but deteriorate with extended refrigeration of 3 weeks or more.12,13 Although the cartilage preservation literature is expansive, minimal attention has been given to surface changes due to storage.
Surprisingly, little is known about the effect of storage on cartilage surface properties considering how sliding and lubrication are key to joint function. Prior work has shown that storage-associated cell death is predominantly observed in the superficial zone,14 suggesting that tissue surface properties may be particularly sensitive to storage. Conflicting data from other surface-related assays,15,16 however, motivate additional research. For example, there was no difference in CoFs between samples stored at 2°C and −80°C,15 but they were not compared to a prestorage control. In contrast, another study showed that freezing at −70°C had a detrimental effect on PRG4 secretion compared to fresh samples stored at 4°C.16 Clearly, additional work is necessary to understand how storage conditions impact different aspects of cartilage surface function.
The objective of this study was to develop a comprehensive methodology to not only characterize bulk properties but also surface characteristics. Following the establishment of this methodology, we wanted to apply it and determine the effect of storage temperature and duration on surface and bulk cartilage functionality. In addition to a standard array of bulk tissue assays, surface properties using interferometry, tribology, and lubricin IHC were examined. These data will be used to test the hypothesis that surface characteristics significantly change after storage. The results from this work will not only elucidate the impact that frequently used storage protocols have on cartilage surface properties but also serve as a model for incorporating surface characterization in future cartilage-related studies.
Materials and Methods
The Method consists of a set of three surface characterization assays: interferometry, tribology, and lubricin IHC. Each of these contributes complementary information regarding the state of the cartilage surface. The Method will then be applied to the Experiment below.
Interferometry
Surface topography was measured using a 20 × Mirau objective on an MSA-500 Micro System Analyzer (Polytec, Waldbronn, Germany). Cartilage punches were placed on the stage and lightly blotted to remove excess moisture. When necessary, the stage was tilted to ensure that the sample surface was perpendicular to the objective. The system's focus was adjusted until an interference fringe pattern was clearly observed. Measurement start point was defined as the point where the fringe pattern was first seen, and the endpoint was defined as when the fringe pattern is no longer visible, and topographical data were collected. The topographical data were rendered into images that demonstrate the sample's surface features. Scan times ranged from 45 s to 1.5 min per sample.
The TMS 3.8 software (Polytec) was used for postprocessing and roughness quantification. A linear regression was applied to the topographical data to correct for any remaining physical tilt. This was followed by a Gaussian low pass filter set to a 1.5 μm cutoff wavelength. Average surface roughness (Sa) was then determined for each sample's processed topographical data. Images exported from the Polytec software were further analyzed using the Directionality plugin in ImageJ (National Institutes of Health) and the Circular Statistics toolbox in Matlab17 to determine anisotropy. The degree of alignment for the observed surface features is defined such that 1 denotes perfect alignment, that is, all features are aligned in the same direction.18 Greater alignment denotes greater anisotropy.
Tribology
Separate from the samples described in the Experiment, additional, fresh cartilage samples (n = 6) were harvested to assess the tribological dependence on measurement direction. Harvested samples were marked for their anatomical directional orientation using India ink. Tribology was performed using a custom-made pin-on-plate tribometer under boundary lubrication conditions,7 with velocity set to 1 mm/s and a compressive normal force applied by a 300 g weight. The samples were allowed to equilibrate for 2 min and then sheared against the test surface for 5 min. The measured CoF describes the interaction between the articular cartilage sample and the underlying glass plate immersed in phosphate-buffered saline (PBS). Each sample was measured along the anterior–posterior and medial–lateral directions.
Immunohistochemistry
IHC was used to detect lubricin on the articular surface. Samples were fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at a thickness of 6 μm. Sections on silanized slides were baked at 60°C in 2 mL of formalin for 1 h. Sections were quenched of endogenous peroxidase activity using a 3% hydrogen peroxide in methanol solution. Antigen retrieval was performed in citrate buffer at 90–100°C for 30 min. After blocking, the slides were incubated overnight with a 1:500 dilution of the mouse antilubricin antibody, clone 9G3 (Sigma) at 4°C. Secondary antibody incubation and color development were performed using the mouse IgG VECTASTAIN ABC kit and DAB (Vector Laboratories).
Experiment
The Method described above was applied here to discern surface changes due to the storage of cartilage. Five bovine knee joints from 2- to 4-week-old calves were obtained 48 h postmortem (Research 87). Seventy-two articular cartilage explants from the medial and lateral condyles were harvested using 7 mm biopsy punches. The punches were marked to indicate their orientation on the condyle by placing sterile-filtered India ink at the punch's most anterior point. The punches were stored at various storage conditions, detailed below.
Storage conditions
This study used a full-factorial design of storage temperature (37°C, 4°C, −20°C, and −80°C) and duration (1 day, 1 week, and 1 month) for a total of 12 groups. In this study, 1 month is defined as 4 weeks. Explants were randomly assigned to groups to avoid variation due to the topographic location of the sample. For the 37°C and 4°C conditions, explants were kept in Dulbecco's modified Eagle medium containing 4.5 g/L glucose and GlutaMAX (Gibco), 1% (v/v) penicillin/streptomycin/fungizone (Lonza), and 25 mM HEPES (4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid) buffer. Samples stored at −20°C and −80°C were placed in individual microcentrifuge tubes. Samples stored at −80°C were snap frozen in liquid nitrogen.
Surface characterization
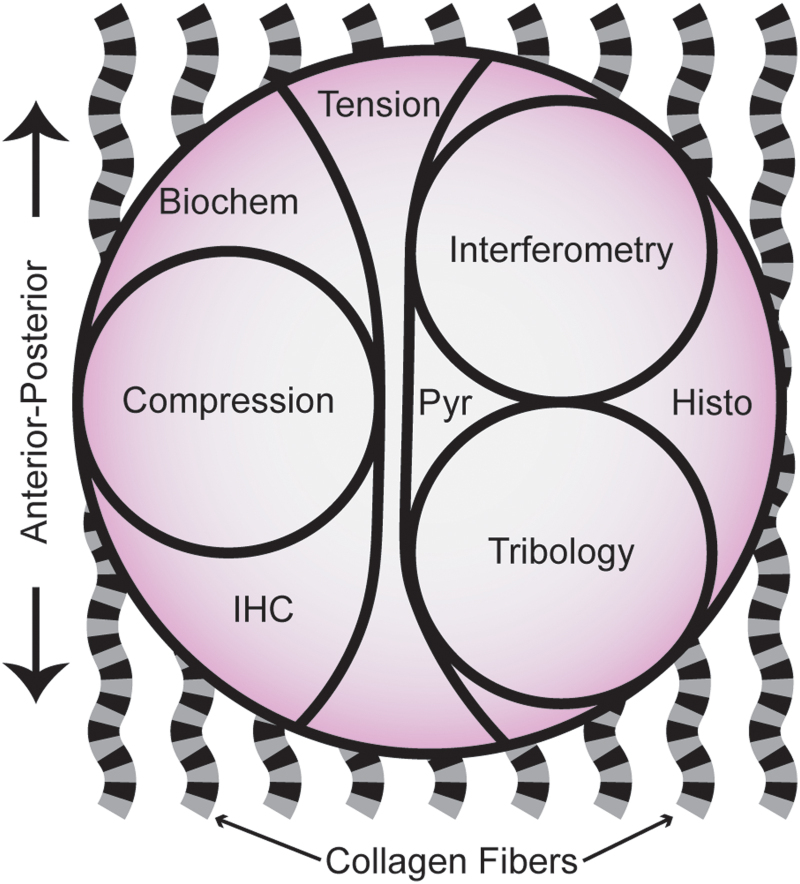
After storage, explants were trimmed to a 1 mm thickness inclusive of the surface zone to ensure a consistent thickness across all samples. Maintaining the orientation, three 3 mm punches were obtained from each explant for tribology and interferometry (Fig. 1) as described above. Tribological data were only collected in the anterior–posterior direction. IHC was also performed from sections of the samples as described above. The remaining tissue was used for mechanical testing (creep indentation and uniaxial tensile testing), biochemical assays, and histology.
FIG. 1.
Schematic diagram of the cartilage explants obtained from juvenile bovine femoral condyles and the subdivisions used for mechanical, biochemical, and histological assays. Wavy, banded lines represent collagen fibers. This schematic illustrates the wide range of assays used to comprehensively examine the effects of storage. Histo, histology; IHC, immunohistochemistry; Pyr, pyridinoline crosslinks analysis. Color images are available online.
Mechanical testing
Compressive properties of the explants were determined by creep indentation testing. In brief, 3 mm punches obtained from each explant were submerged in PBS and indented with a flat porous 1 mm diameter tip perpendicular to the surface of the sample. A tare weight of 0.5 g was applied until equilibrium was achieved. Then, a test weight of 7.5–10 g was applied, which corresponded to ∼10% strain. The aggregate and shear modulus (SM) values were obtained using a biphasic model and finite-element optimization.19
Tensile properties were determined using uniaxial tension in an Instron model 5565 (Instron, Canton, MA, USA). Dogbone-shaped samples were obtained from each explant along the anterior–posterior direction (Fig. 1). The dogbones were photographed to measure thickness and width in ImageJ. The ends of the dogbone were fixed to paper with cyanoacrylate. All samples had a gauge length of 1.55 mm. A strain rate of 1% of the gauge length per second was used until failure. The Young's modulus was obtained from the linear region of the stress-strain curve, and the ultimate tensile strength (UTS) was defined as the maximum stress obtained.
Biochemical assays
Cartilage samples were weighed to obtain wet weight, lyophilized, and weighed again to obtain dry weight (DW). Lyophilized samples were digested in 125 μg/mL papain (Sigma) +5 mM N-acetyl-l-cysteine +5 mM EDTA (ethylenediaminetetraacetic acid) in phosphate buffer pH 6.5 for 18 h at 60°C. Glycosaminoglycan (GAG) content was quantified using a Blyscan Glycosaminoglycan Assay kit (Biocolor, Newtownabbey, Northern Ireland). Total collagen content was quantified using a modified chloramine-T hydroxyproline assay20 using Sircol 0.5 mg/mL acid-soluble bovine collagen as a standard (Biocolor). DNA content was quantified with a PicoGreen assay (ThermoFisher Scientific).
For the quantification of pyridinoline crosslinks, samples were weighed, lyophilized, and acid-digested for 12 h in 6N HCl at 105°C. After evaporation, each dried hydrolysate was resuspended in a 75%/25% (v/v) solution of 0.1% formic acid and acetonitrile, centrifuged at 15,000 g for 5 min, and the supernatant was transferred to a LCMS autosampler vial. Samples were measured via liquid chromatography mass spectrometry using a Cogent diamond hydride high-performance liquid chromatography (HPLC) column (2.1 × 150 mm, particle size 2.2 μm, pore size 120Å; MicroSolv) and a pyridinoline standard (BOC Sciences) as previously described.21
Histology
Samples were fixed, dehydrated, embedded, and sectioned as described for IHC above. Sections were subsequently processed and stained with hematoxylin and eosin, safranin-O and fast green, and picrosirius red using standard protocols.22
Determination of functionality indices
Previously, a functionality index (FI) was developed to describe, in one value, the bulk characteristics of a sample compared to a reference sample.23 To differentiate surface and bulk characteristics, the subscripts “s” and “b” are used; that is, a modified FI to represent surface characteristics, or FIs [Eq. (1a)], uses values of the CoF and roughness (Sa). The bulk functionality, or FIb [Eq. (1b)], includes Young's modulus (E), UTS, aggregate modulus (HA), SM, collagen/DW (Col), pyridinoline/DW (Pyr), and GAG/DW (GAG). Differences in cartilage function between each storage condition test group (denoted by superscript “t”) and the 37°C at 1-day control group (denoted by superscript “c”) were quantified using these indices.
In addition, a combined FI [Eq. (1c)] was calculated. In the combined FI, equal weight was apportioned to each assay. Previous iterations of the FI have considered any deviation from the “gold standard” control value a flaw, through the use of absolute values. In the current form of the FI, the possibility for improvement beyond the control is available. For example, an explant may have improved mechanical properties after culturing compared to the unstored control. An FI of 1 indicates that there is no difference with respect to the control group. Improvements will potentially increase the FI to greater than 1. Conversely, degradation of properties will reduce the FI. For the purposes of the present study, decreases in surface properties and increases in bulk properties are deemed as improvements. These assumptions are based on the need for smooth joint articulation and increased mechanical strength in allografts.
| (1a) |
| (1b) |
| (1c) |
Statistical analysis
All quantitative data are presented as mean ± standard deviation. A paired Student's t-test was used to analyze the directional tribology data. Experimental results were analyzed using two-way analysis of variance (ANOVA) in Prism 8.1 (GraphPad Software) with a sample size of six per group. A Fisher's least significant difference (LSD) post hoc test was used to identify significant differences between each storage condition and the control condition, defined as 37°C at 1 day. Finally, one-way ANOVAs with a Fisher's LSD post hoc test were used to compare functionality indices within temperature groups.
Results
Topographical and frictional anisotropy
Before examining storage effects, interferometry performed on the condylar surface revealed a well-defined striated pattern in the cartilage surface corresponding to the anterior–posterior direction (Fig. 2A). Although this topographical anisotropy was not always observed, likely due to differences in tissue hydration, this is the first time that interferometry was used to show the striated pattern of the cartilage surface.
FIG. 2.
Surface topography impacts tribology measurements. (A) A topographical image of a representative sample's surface taken by interferometry reveals a striated pattern in the direction of articulation. (B) Tribology measurements performed in the A–P and M–L directions indicate that the M–L CoF is 53% greater than in the A–P direction, providing further support for the anisotropy of articular cartilage surfaces. The asterisks denotes significant differences compared to the control condition based on Fisher's LSD post hoc test (* denotes p < 0.05). A–P, anterior–posterior; CoF, coefficient of friction; M–L, medial–lateral. Color images are available online.
Given the observed topographical anisotropy, it was of interest to determine if the functional properties of the articular cartilage surface also vary with the direction of motion. Tribological measurements revealed that a significant difference existed between the CoF obtained along the anterior–posterior and the medial–lateral orientations. When the test was performed in the medial–lateral direction, the CoF was 53% higher (Fig. 2B). The impact of this novel finding is that it is crucial to control for this topographical and frictional anisotropy for an accurate evaluation of surface-level changes due to storage; the storage data presented below accounted for this anisotropy.
Storage effects on the surface characteristics of cartilage
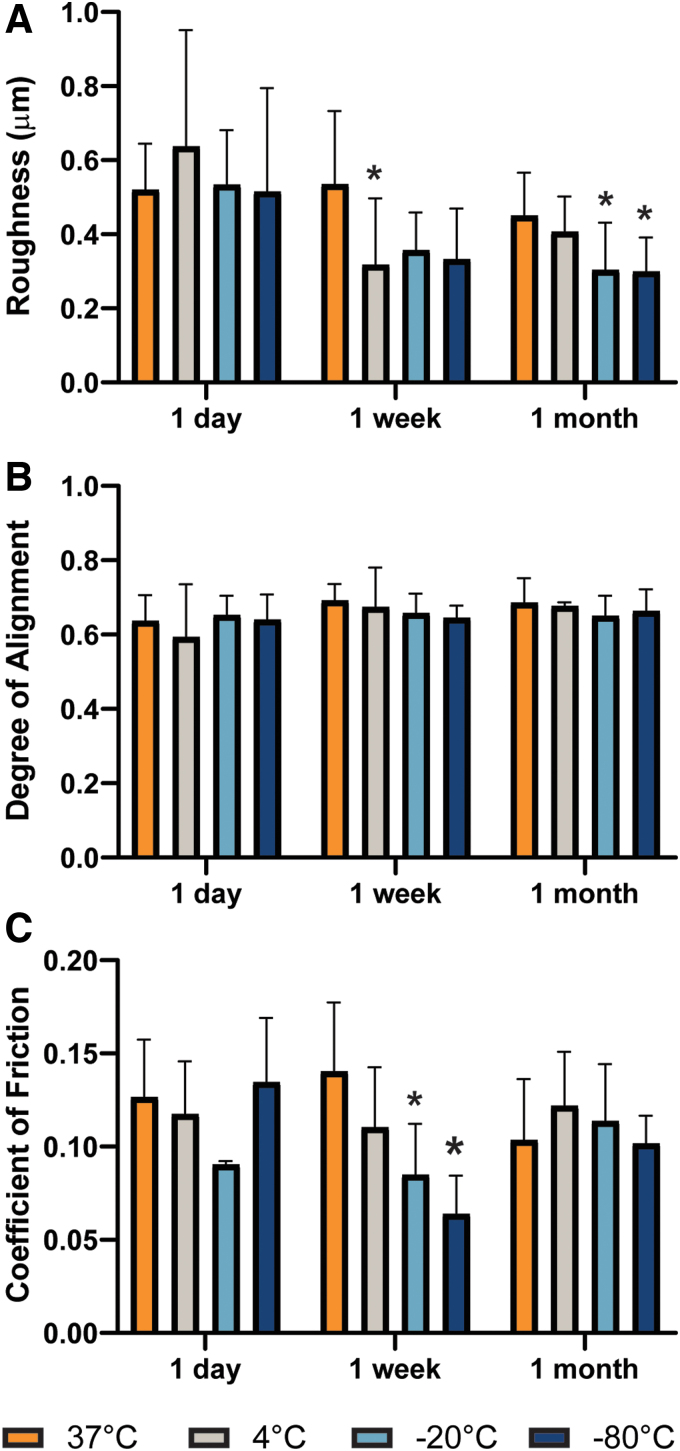
Storage conditions were found to minimally alter individual surface properties of cartilage. Average surface roughness, determined through interferometry, was significantly reduced at various refrigerated or frozen conditions, starting as early as 1 week (Fig. 3A). Despite the changes in roughness, quantification of the surface striation alignment did not reveal changes due to storage time or temperature and averaged at 0.66 ± 0.07 (Fig. 3B). The measurements of CoF were generally lower at frozen temperatures (Fig. 3C). Samples stored at −20°C and −80°C for 1 week showed a 33% and 49% reduction in CoF, respectively, compared to controls (CoFcontrol = 0.13 ± 0.03), respectively. Our results show that the cartilage surface is unaffected by physiological temperature, but lower temperatures may give rise to surface-level changes.
FIG. 3.
Surface properties are unaffected by storage at nonfreezing temperatures. Using interferometry measurements, significant differences in (A) roughness were detected at subphysiological temperatures. No significant differences were observed in (B) alignment compared to the control samples (37°C at 1 day). However, tribological analysis showed that (C) CoF is significantly reduced at both −20°C and −80°C at 1 week. The asterisks denotes significant differences compared to the control condition based on Fisher's LSD post hoc test (* denotes p < 0.05). Color images are available online.
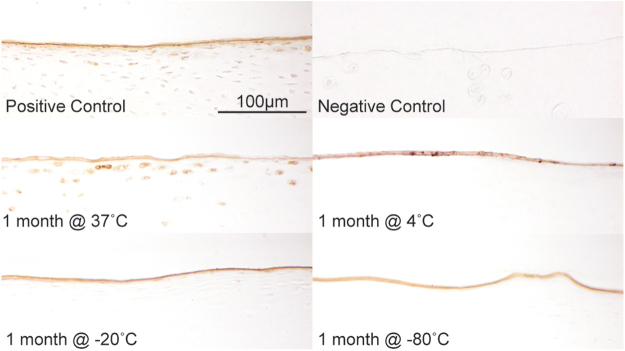
Localization of lubricin was determined through IHC (Fig. 4). As expected and in accordance to its function, coloration against the antilubricin antibody revealed the presence of the protein predominantly at the surface, after which it rapidly decreases and was only colocalized with lacunae and chondrocytes. No lubricin was found in the deeper zones. Lubricin at the articular surface was not impacted by storage conditions even after a month of storage. However, it is important to note that lubricin cellular localization disappeared at nonphysiological temperatures.
FIG. 4.
IHC of explants from the femoral condyle. Antilubricin shows expression of the protein predominantly in the superficial zone of cartilage in the positive control (1 day at 37°C). No lubricin is detected in nonarticulating costal cartilage surfaces (negative control). Even after a month of storage, the surface layer of lubricin is unaffected by temperature, however, cellular expression of lubricin is only maintained at 37°C. Color images are available online.
Storage effects on the bulk properties of cartilage
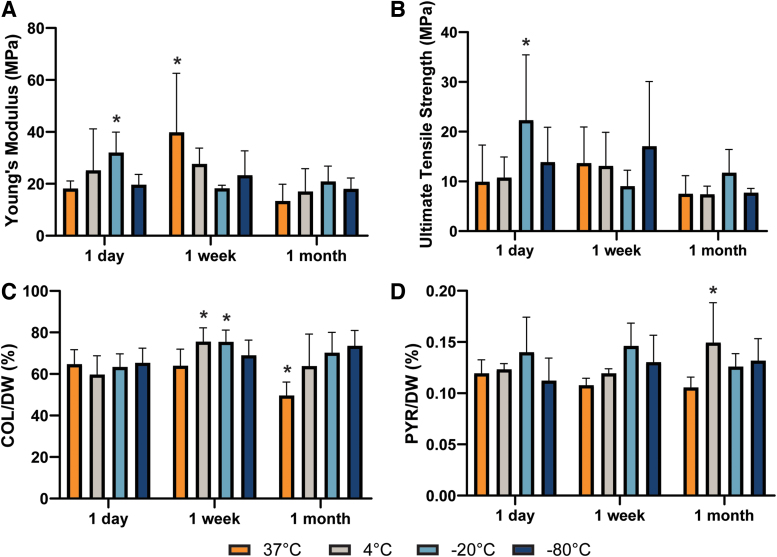
Healthy articular cartilage has high biomechanical stability with little to no turnover. However, the tensile mechanical properties of samples stored under different conditions were quite variable. Although the Young's modulus and UTS (Fig. 5A, B) of a few groups significantly differed from the control values (18.2 ± 2.9 and 9.9 ± 7.3 MPa, respectively), there was no discernable trend in these changes. Biochemical components associated with the tensile properties of cartilage, mainly collagen and pyridinoline crosslinks, were measured at 64.7% ± 7.0% and 0.12% ± 0.01% per DW, respectively, for the control group. Their contributions to cartilage content after storage were also variable (Fig. 5C, D and Supplementary Table S1).
FIG. 5.
The (A) Young's modulus and (B) ultimate tensile strength values significantly differed for a few groups compared to controls, but there is no clear trend to the changes. (C) Collagen per DW and (D) pyridinoline crosslinks per DW, both of which are associated with the tensile properties of cartilage, similarly lacked any trends, despite the presence of a few significantly different groups. The asterisks denotes significant differences compared to the control condition based on Fisher's LSD post hoc test (* denotes p < 0.05). DW, dry weight. Color images are available online.
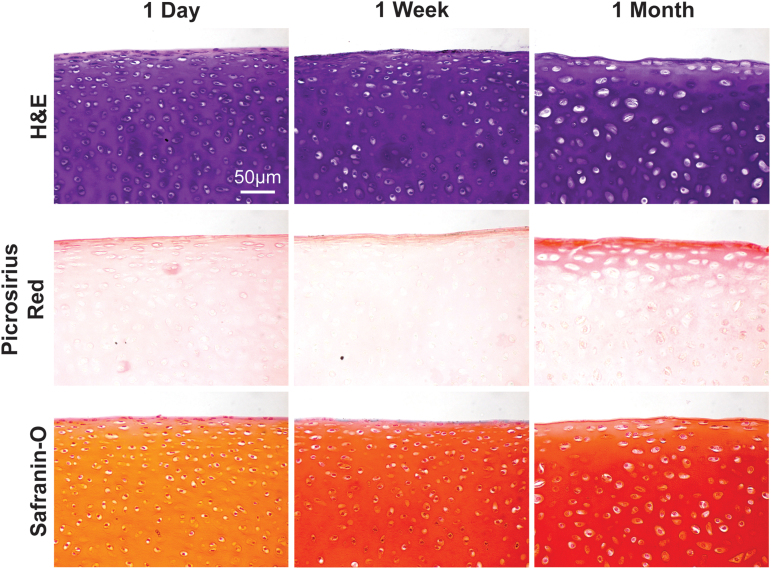
While no significant differences in compressive properties were found in explants stored at 4°C, −20°C, and −80°C, prolonged storage at 37°C resulted in 67% and 83% increases in the compressive aggregate modulus value at 1 week and 1 month, respectively, compared to the 395.4 ± 53.9 kPa value for the control group (Fig. 6A). A significant increase was also observed for the SM value (Fig. 6B). Correspondingly, the GAG content, associated with the compressive properties of articular cartilage, was significantly increased by 33% in the same group after 1 month (Fig. 6C). While the histological appearance of all other groups besides those stored at 37°C were unremarkable compared to controls, the increase in GAG quantified biochemically was corroborated by histological staining of samples stored at 37°C (Fig. 7C).
FIG. 6.

Compressive properties and GAG content increased in samples stored at physiological temperature. A significant increase was found in the (A) aggregate modulus and (B) SM values of samples stored at 37°C beginning after 1 week. (C) GAG normalized by DW also increased after a month in samples stored at 37°C. The compressive properties and GAG content were not adversely affected by storage at 4°C or lower. The asterisks denotes significant differences compared to the control condition based on Fisher's LSD post hoc test (* denotes p < 0.05). GAG, glycosaminoglycan; SM, shear modulus. Color images are available online.
FIG. 7.
H&E, picrosirius red, and safranin-O stains of samples stored at 37°C. Safranin-O histological stains indicated that the GAG content of cultured cartilage explants increased over time. H&E, hematoxylin and eosin. Color images are available online.
Functionality indices
Storage-related changes to functionality impacted the cartilage surface and bulk properties differently. On the one hand, FIs, which accounts for surface roughness and CoF, was significantly different in the −80°C at 1 week and 1 month groups (FIs = 1.43 ± 0.19 and 1.31 ± 0.11, respectively). On the other hand, significant changes in FIb were observed only after a month at nonphysiological temperatures, reaching as low as 0.81. Surface and bulk functionality changes were captured in the combined FI scores (Table 1).
Table 1.
Storage Conditions Affect Surface and Bulk Functionality (Functionality Index) Differently
| Surface FI | Bulk FI | Combined FI | |
|---|---|---|---|
| 1 Day | |||
| 37°C | 1.00 ± 0.13 | 1.06 ± 0.16 | 1.04 ± 0.14 |
| 4°C | 0.84 ± 0.51 | 1.10 ± 0.14 | 1.05 ± 0.16 |
| −20°C | 1.14 ± 0.17 | 1.14 ± 0.24 | 1.13 ± 0.17 |
| −80°C | 0.97 ± 0.27 | 1.07 ± 0.12 | 1.04 ± 0.09 |
| 1 Week | |||
| 37°C | 0.93 ± 0.24 | 1.15 ± 0.20 | 1.09 ± 0.14 |
| 4°C | 1.26 ± 0.24 | 1.08 ± 0.11 | 1.12 ± 0.13 |
| −20°C | 1.31 ± 0.20 | 1.12 ± 0.17 | 1.16 ± 0.13 |
| −80°C | 1.43 ± 0.19* | 1.18 ± 0.20 | 1.23 ± 0.16* |
| 1 Month | |||
| 37°C | 1.15 ± 0.18 | 1.01 ± 0.17 | 1.04 ± 0.11 |
| 4°C | 1.12 ± 0.15 | 0.83 ± 0.09* | 0.90 ± 0.07* |
| −20°C | 1.26 ± 0.15 | 0.92 ± 0.06 | 0.99 ± 0.07 |
| −80°C | 1.31 ± 0.11* | 0.81 ± 0.11* | 0.92 ± 0.10 |
The combined FI incorporates all changes to function. Groups marked with an asterisk (*) are considered significantly different compared to their respective control. For these groups, all surface-related changes are highlighted in bold, while bulk-related changes are in italics and may be carried forward to the combined FI.
FI, functionality index.
Discussion
The role of cartilage surface properties on joint function has been historically understudied in comparison to the tissue's bulk properties. To address this disparity, the first objective of this work was to develop a new methodology that incorporates surface characterization alongside the bulk assessments typically used for cartilage. The second objective was to then apply the methodology to an active area of research: cartilage explant preservation. Both objectives were accomplished, thus providing the field with both a vetted protocol and an example of the impact surface properties may have on cartilage function.
This methodology and subsequent experiment used primarily nondestructive surface characterization techniques that relate to roughness, friction, and lubrication. While compressive and tensile properties12,24 are routinely measured to assess the state of cartilage tissue, the surface properties listed above are infrequently evaluated. This is true as well of most studies on cartilage storage and preservation for clinical use, although a functional therapeutic would require a smooth cartilage surface exhibiting low friction. To-date, little attention has been given to the effect of storage conditions on surface characteristics. Except for brief reports comparing two temperatures,15,16,25 no studies have been conducted with the main objective of examining how storage conditions affect the cartilage surface.
The field currently operates under the assumption that cartilage surface characteristics are either insensitive to storage or respond similar to the often-examined bulk characteristics. By applying the tribology and interferometry methods described here, this assumption was tested based on the null hypothesis that no differences would be found among surface characteristics for cartilages stored under various temperatures and durations. The null hypothesis was rejected due to observing significant surface and bulk changes. In general, changes to cartilage surface function were greatest at freezing temperatures after at least a week of storage. Interestingly, bulk properties were well maintained in storage at 37°C,14,26,27 which is akin to culturing. In contrast, there was a significant reduction in bulk function after a month of refrigeration, often considered the gold standard for cartilage storage.
The use of surface characterization methods offers new information to the field and is an important complement to the bulk data in this study. These assays also paved the way to novel observations, such as determining topographical anisotropy using interferometry and finding frictional anisotropy through tribology. Further use of roughness and CoF values to assess the quality of cartilage may hold significant relevance to many, ranging from the tissue engineer creating biomimetic neocartilage to the surgeon implanting an allograft at a given orientation. Together, these findings not only elucidate important cartilage surface characteristics but also make a compelling case for their inclusion in any comprehensive study of cartilage function.
Interferometry and tribology were used to quantify storage-related surface-level changes. Interferometry proved to be a fast, noncontact imaging modality well-suited for assessing cartilage surface roughness and topography. Although interferometry has been previously used to image individual chondrocytes28 and articular cartilage,8 this technology has not become a standard tool in cartilage research. Our work with interferometry revealed topographical anisotropy in the form of striations along the anterior–posterior direction. Tribological testing was exceptionally consistent: each individual sample displayed frictional anisotropy. These data conclusively demonstrated that the friction-related function of articular cartilage was anisotropic.
With regard to storage, significant changes in both roughness and CoF were included in the calculations of surface function, FIs. When roughness and CoF are considered together, surface functionality appears to be most affected by storage at −80°C and as early as 1 week. While not quantitively measured here, lubricin also plays an important role in joint articulation. Previous work has shown topographical differences in both SZP and CoF across the cartilages of the knee joint.7 In this study, we only used explants from the femoral condyle to mitigate these regional differences. Lubricin localization at the surface was generally unaffected by storage, even up to a month.
Our results showed that cartilage roughness and CoF are most impacted by freezing temperatures, which is accurately reflected in our quantification of surface functionality. Although a reduction in roughness or CoF may be interpreted as an improvement in function, it is also possible that surface-level changes are due to tissue damage caused by ice crystal formation and warrants further investigation.29 Thus, the reduction in these two parameters may potentially be detrimental to surface function. For example, reduced roughness and CoF could indicate a loss of surface anisotropy and impact joint articulation. Although there is no significant difference in surface alignment along the direction of articulation from this study's interferometry data, the observed surface striations may be diminished in magnitude and, therefore, have a reduced influence on articulation. As further improvements to cartilage storage protocols are developed, it is in the best interest of the field to include surface characterization in functional assessments of potential osteochondral grafts.
Changes to the bulk extracellular matrix (ECM) and mechanical properties of articular cartilage due to prolonged storage at various temperatures are still not well understood. At freezing temperatures, decreases in collagen fibril diameter and increases in interfibrillar space have been observed in highly collagenous tissue, but statistical significance is not always achieved.30–32 Similarly, there are several conflicting reports on changes in GAG content due to freezing or refrigeration.12,33 Although many of these articles note changes to the ECM ultrastructure, few provide corresponding mechanical data. When mechanical function is assessed, freezing does not typically result in significant changes, while long-term refrigeration seems to result in degradation of mechanical properties.12,13,24
The data in this study provide a broader picture of both the biochemical and biomechanical state of stored articular cartilage. These data were then used to determine bulk functionality, FIb. Given the importance of collagen to cartilage mechanics, pyridinoline crosslinks across each of the examined groups were assayed and were also included in FIb calculations. Tensile properties, which are often overlooked but inherently related to collagen structure and content, were also measured. These data support previous work showing that collagen-related content and tensile mechanics minimally change with freezing,12,13 but additionally show that tensile properties may have a variable response to long-term refrigeration.
Many previous studies on cartilage preservation exclude storage at physiological temperature. At 37°C, the data show a marked increase in GAG content and a concomitant increase in aggregate and SM values. This is contrary to the response seen in explants from adult bovine elbows that were cultured for 4 weeks.34 A key difference between this prior study and the present work is the use of fetal bovine serum (FBS) in the prior study. In cartilage explants cultured at 37°C, increased FBS concentration reduced chondrocyte viability.14 The present study stands in agreement with the work of others that have shown the benefits of cartilage tissue culture with serum-free media.14,26,27
These enhancements to compressive mechanics and GAG content countered the tensile and collagen-related data, thus maintaining the FIb stable with culturing. Our safranin-O staining further supported the positive effects of storage at physiological temperature on GAG content. In addition, the 37°C, 1 month group also appears to display an increase in lacuna size and, in the frequency of cell division, reminiscent of cartilage developmental processes.35 Taking into account the immature state of the chondrocytes used in this study and that aging chondrocytes show a decrease in biosynthesis of protein and GAG, it is likely that this effect will be less noticeable if more mature tissues are used (e.g., osteochondral allografts from middle-aged donors).36
For the purposes of this investigation, where differences in function were used to interrogate the proposed methodology, the use of immature chondrocytes was appropriate. While this study is specific to immature cartilage, our quantitative measures of composition and mechanics suggest that storage at physiological temperature not only preserves cartilage but may also promote further growth and compressive function in immature tissue.
This study not only shows a comprehensive picture of the effect of storage on cartilage bulk properties, but it is also the first to significantly include effects on surface function using interferometry, tribology, and lubricin staining. While this study focused on storage temperature and duration, there are many other factors that should also be considered. One such factor is medium composition, which can dramatically influence explant biochemical content and mechanics.27 Distributors of fresh allografts in the United States all use different formulations, with some even including FBS.1 Our results with a serum-free medium suggest that these differences may be impactful and warrant standardization of storage media.
Similarly, thawing protocols should also be examined as these may be as critical to cartilage preservation as storage conditions. In addition to studying storage effects on native tissue, similar studies should be conducted on tissue engineered cartilage. These studies would all benefit from the use of surface- and bulk-specific FIs to allow for a more focused interpretation of functional changes. The combined FI then ensures that cartilage function is evaluated holistically, without ignoring the important role of surface function.
Recommendations on cartilage function preservation depend on cell viability, bulk mechanics, and surface articulation, all of which may be included in the calculation of an FI. This study did not revisit the effects of storage on chondrocyte viability because this is already well established for the storage conditions examined here.11 However, viability can be easily added to an FI should this be of interest, such as in clinical applications. If maintaining cell viability is of interest, storage at freezing temperatures should be avoided.
In this study, assumptions were made on whether an observed change in bulk or surface function was beneficial or detrimental to a tissue's use as an implant; however, other studies may value parameters differently. While this allows researchers flexibility to optimize the FI score for specific studies, we recommend that value judgments on parameter changes be made based on each study's particular hypothesis, design, or desired outcomes. Given the assumptions used here, freezing specifically impacts surface function, but it is currently unclear if this is beneficial. In addition, bulk properties appear to be negatively affected after a month of storage at subphysiological temperature. Therefore, for the purpose of graft transplantation, we encourage that culturing at physiological temperature be further investigated and strongly considered, particularly in light of the reduced FI observed with long-term refrigeration.
Conclusion
Although the cartilage surface plays a critical role in proper joint articulation, it is often ignored in assessments of cartilage tissue and function. Tribology, interferometry, and lubricin staining each provide unique and important information about the cartilage surface. In addition to obtaining baseline surface characteristics, these measurements also revealed topographical and frictional anisotropy. Quantitative surface metrics, such as roughness and CoF, were used to identify groups with significant changes in surface functionality due to storage. Interestingly, these did not necessarily coincide with the groups displaying significant changes in bulk functionality, suggesting that bulk tissue behavior alone is not representative of cartilage function. These findings most immediately convey the importance of surface characterization in determining cartilage storage conditions for laboratory and clinical use, but may also have far-reaching applications. Surface functionality measures of cartilage tissue are not only sorely needed in native tissue studies but are also instrumental for developing design criteria for functional tissue engineered cartilage.
Supplementary Material
Authors' Contributions
M.G.E., G.A.O., J.C.H., and K.A.A. conceived and designed the experiments. M.G.E. and G.A.O. performed the experiments, analyzed the data, and wrote the article. J.C.H. and K.A.A. edited the article.
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors acknowledge support from the National Institutes of Health (R01 DE015038 and R01 AR067821), National Institutes of Health Diversity Supplement (for M.G.E.), Fulbright Chile scholarship (for G.A.O.), and Becas Chile scholarship (for G.A.O.).
Supplementary Material
References
- 1. Goodfriend, B., Essilfie, A.A., Jones, I.A., and Thomas Vangsness, C.. Fresh osteochondral grafting in the United States: the current status of tissue banking processing. Cell Tissue Bank 20, 331, 2019 [DOI] [PubMed] [Google Scholar]
- 2. Cook, J.L., Stannard, J.P., Stoker, A.M., et al. Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med 44, 1260, 2016 [DOI] [PubMed] [Google Scholar]
- 3. Patel, J.M., Wise, B.C., Bonnevie, E.D., and Mauck, R.L.. A systematic review and guide to mechanical testing for articular cartilage tissue engineering. Tissue Eng Part C Methods 25, 593, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salinas, E.Y., Hu, J.C., and Athanasiou, K.. A guide for using mechanical stimulation to enhance tissue-engineered articular cartilage properties. Tissue Eng Part B Rev 24, 345, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Link, J.M., Salinas, E.Y., Hu, J.C., and Athanasiou, K.A.. The tribology of cartilage: mechanisms, experimental techniques, and relevance to translational tissue engineering. Clin Biomech (Bristol, Avon) 79, 104880, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oungoulian, S.R., Durney, K.M., Jones, B.K., Ahmad, C.S., Hung, C.T., and Ateshian, G.A.. Wear and damage of articular cartilage with friction against orthopedic implant materials. J Biomech 48, 1957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng, G., McNary, S.M., Athanasiou, K.A., and Reddi, A.H.. The distribution of superficial zone protein (SZP)/lubricin/PRG4 and boundary mode frictional properties of the bovine diarthrodial joint. J Biomech 48, 3406, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shekhawat, V., Laurent, M., Muehleman, C., and Wimmer, M.. Surface topography of viable articular cartilage measured with scanning white light interferometry. Osteoarthritis Cartilage 17, 1197, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Achanta, S., and Celis, J.-P.. On the scale dependence of coefficient of friction in unlubricated sliding contacts. Wear 269, 435, 2010 [Google Scholar]
- 10. Torrie, A.M., Kesler, W.W., Elkin, J., and Gallo, R.A.. Osteochondral allograft. Curr Rev Musculoskelet Med 8, 413, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pisanu, G., Cottino, U., Rosso, F., et al. Large osteochondral allografts of the knee: surgical technique and indications. Joints 6, 42, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szarko, M., Muldrew, K., and Bertram, J.E.. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet Disord 11, 231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams, S.K., Amiel, D., Ball, S.T., et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am 85, 2111, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Pallante, A.L., Bae, W.C., Chen, A.C., Görtz, S., Bugbee, W.D., and Sah, R.L.. Chondrocyte viability is higher after prolonged storage at 37°C than at 4°C for osteochondral grafts. Am J Sports Med 37, 24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore, A.C., and Burris, D.L.. Tribological and material properties for cartilage of and throughout the bovine stifle: support for the altered joint kinematics hypothesis of osteoarthritis. Osteoarthritis Cartilage 23, 161, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pallante-Kichura, A.L., Chen, A.C., Temple-Wong, M.M., Bugbee, W.D., and Sah, R.L.. In vivo efficacy of fresh versus frozen osteochondral allografts in the goat at 6 months is associated with PRG4 secretion. J Orthop Res 31, 880, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berens, P. CircStat: A MATLAB toolbox for circular statistics. J Stat Softw 31, 21, 2009 [Google Scholar]
- 18. Espinosa, M.G., Taber, L.A., and Wagenseil, J.E.. Reduced embryonic blood flow impacts extracellular matrix deposition in the maturing aorta. Dev Dyn 247, 914, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athanasiou, K., Niederauer, G., and Schenck, R.. Biomechanical topography of human ankle cartilage. Ann Biomed Eng 23, 697, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Cissell, D.D., Link, J.M., Hu, J.C., and Athanasiou, K.A.. A modified hydroxyproline assay based on hydrochloric acid in Ehrlich's solution accurately measures tissue collagen content. Tissue Eng Part C Methods 23, 243, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez-Leon, E.A., Bielajew, B.J., Hu, J.C., and Athanasiou, K.A.. Engineering self-assembled neomenisci through combination of matrix augmentation and directional remodeling. Acta Biomater 109, 73, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carson, F.L., and Hladik, C.. Histotechnology: A Self-Instructional Text. Chicago, IL: ASCP Press, 1997 [Google Scholar]
- 23. Huwe, L.W., Brown, W.E., Hu, J.C., and Athanasiou, K.A.. Characterization of costal cartilage and its suitability as a cell source for articular cartilage tissue engineering. J Tissue Eng Regen Med 12, 1163, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Changoor, A., Fereydoonzad, L., Yaroshinsky, A., and Buschmann, M.D.. Effects of refrigeration and freezing on the electromechanical and biomechanical properties of articular cartilage. J Biomech Eng 132, 064502, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Gleghorn, J.P., and Bonassar, L.J.. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech 41, 1910, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Bian, L., Lima, E.G., Angione, S.L., et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech 41, 1153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garrity, J.T., Stoker, A.M., Sims, H.J., and Cook, J.L.. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med 40, 2542, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Scott, C.C., Luttge, A., and Athanasiou, K.A.. Development and validation of vertical scanning interferometry as a novel method for acquiring chondrocyte geometry. J Biomed Mater Res A 72, 83, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Pegg, D.E. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 60, S36, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Gelber, P.E., Gonzalez, G., Torres, R., Garcia Giralt, N., Caceres, E., and Monllau, J.C.. Cryopreservation does not alter the ultrastructure of the meniscus. Knee Surg Sports Traumatol Arthrosc 17, 639, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Jacquet, C., Erivan, R., Argenson, J.-N., Parratte, S., and Ollivier, M.. Effect of 3 preservation methods (freezing, cryopreservation, and freezing + irradiation) on human Menisci ultrastructure: an ex vivo comparative study with fresh tissue as a gold standard. Am J Sports Med 46, 2899, 2018 [DOI] [PubMed] [Google Scholar]
- 32. Ozcelikkale, A., and Han, B.. Thermal destabilization of collagen matrix hierarchical structure by freeze/thaw. PLoS One 11, e0146660, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng, S., Xia, Y., Bidthanapally, A., Badar, F., Ilsar, I., and Duvoisin, N.. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging 27, 648, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Natoli, R.M., Scott, C.C., and Athanasiou, K.A.. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng 36, 780, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Pavasant, P., Shizari, T., and Underhill, C.B.. Hyaluronan contributes to the enlargement of hypertrophic lacunae in the growth plate. J Cell Sci 109, 327, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Sandy, J., Barrach, H., Flannery, C., and Plaas, A.. The biosynthetic response of the mature chondrocyte in early osteoarthritis. J Rheumatol 14, 16, 1987 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.