Abstract
Background
Trastuzumab emtansine (T-DM1) treatment for human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer after taxane with trastuzumab and pertuzumab is standard therapy. However, treatment strategies beyond T-DM1 are still in development with insufficient evidence of their effectiveness. Here, we aimed to evaluate real-world treatment choice and efficacy of treatments after T-DM1 for HER2-positive metastatic breast cancer.
Methods
In this multi-centre retrospective cohort study involving 17 hospitals, 325 female HER2-positive metastatic breast cancer patients whose post-T-DM1 treatment began between April 15, 2014 and December 31, 2018 were enrolled. The primary end point was the objective response rate (ORR) of post-T-DM1 treatments. Secondary end points included disease control rate (DCR), progression-free survival (PFS), time to treatment failure (TTF), and overall survival (OS).
Results
The median number of prior treatments of post-T-DM1 treatment was four. The types of post-T-DM1 treatments included (1) chemotherapy in combination with trastuzumab and pertuzumab (n = 102; 31.4%), (2) chemotherapy concomitant with trastuzumab (n = 78; 24.0%), (3), lapatinib with capecitabine (n = 63; 19.4%), and (4) others (n = 82; 25.2%). ORR was 22.8% [95% confidence interval (CI): 18.1–28.0], DCR = 66.6% (95% CI 60.8–72.0), median PFS = 6.1 months (95% CI 5.3–6.7), median TTF = 5.1 months (95% CI 4.4–5.6), and median OS = 23.7 months (95% CI 20.7–27.4).
Conclusion
The benefits of treatments after T-DM1 are limited. Further investigation of new treatment strategies beyond T-DM1 is awaited for HER2-positive metastatic breast cancer patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12282-020-01192-y.
Keywords: Anti-HER2, Metastatic, Trastuzumab emtansine, Real world
Introduction
Recent developments in diagnostic accuracy and drug therapies have improved the outcomes of early-stage breast cancer patients [1, 2]. However, ~ 20% of breast cancer patients have a poor prognosis due to metastasis [3–5]. Metastatic spread has been estimated to be the primary cause of 90% of cancer-related deaths [6]. In routine clinical practice, the treatment regimens for metastatic breast cancer are determined based on a combination of immunohistochemical analyses of oestrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor 2 (HER2) expression [7–9]. HER2-positive metastatic breast cancer was previously associated with a worse prognosis due to aggressive tumour progression and shorter patient survival [10]. However, the treatment strategy of HER2-overexpressing metastatic breast cancer changed drastically after trastuzumab was approved by the Food and Drug Administration (FDA) in 1998 [11–13]. Following this, additional HER2-targeted agents, such as lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1), were approved for the treatment of HER2-positive metastatic breast cancers [14]. In 2019–2020, FDA also approved trastuzumab deruxtecan (T-Dxd), tucatinib, and neratinib for HER2-positive metastatic breast cancer.
T-DM1 is an antibody–drug conjugate that incorporates the effectiveness of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent emtansine (DM1). T-DM1 inhibits the HER2 signalling pathway, resulting in cessation of cancer cell proliferation. Moreover, the use of T-DM1 avoids the exposure of chemotherapy to normal tissues by targeting chemotherapy delivery, specifically to HER2-overexpressing cancer cells. Recent clinical trials indicate that T-DM1 is useful for the treatment of HER2-positive metastatic breast cancer patients. In the EMILIA trial that assessed the efficacy of T-DM1 in HER2-positive metastatic breast cancer, T-DM1 significantly prolonged progression-free and overall survival with less toxicity compared with lapatinib plus capecitabine in patients previously treated with trastuzumab and a taxane [15]. Results from the MARIANNE study involving patients with HER2-positive metastatic breast cancer showed that the median response duration was 12.5 months in patients who were treated with trastuzumab plus taxane, 20.7 months in those treated with T-DM1, and 21.2 months in patients treated with T-DM1 plus pertuzumab [16].
Since T-DM1 was approved as a new agent based on these clinical studies in 2014, the use of T-DM1 for HER2-positive metastatic breast cancer after taxane with trastuzumab and pertuzumab has been established as standard therapy. However, treatment strategies beyond T-DM1 are still in development as there is insufficient evidence regarding their current status in this setting. In this multi-centre cohort study, we aimed to clarify the real-world treatment choice and efficacy of treatments after T-DM1 for HER2-positive metastatic breast cancer.
Patients and methods
Study design
This study was an observational retrospective multi-centre cohort study involving 17 hospitals which participated in the West Japan Oncology Group (WJOG). The study was registered in the UMIN Clinical Trial Registry (UMIN000037747) and approved by the ethics committee in all 17 sites. Data from each facility were collected by the data centre at WJOG using an electronic data capture system.
HER2-positive metastatic breast cancer patients who had received T-DM1 were included in this trial. We defined the treatments after treatment with T-DM1 as “post-T-DM1 treatments.” The main eligibility criteria were as follows: (1) metastatic breast cancer, (2) HER2-positive breast cancer, and (3) patients whose post-T-DM1 treatments initiated between January 1, 2014 and December 31, 2018. Patients who received investigational drugs as the post-T-DM1 treatment were excluded from the current study. ER and PgR are considered positive if 1% or more of the tumour cells demonstrate positive nuclear staining by immunohistochemistry. The definition of HER2 positive is “HER2 3 + ” or “HER2 ISH positive”.
Outcomes of interest
The analysis was designed to evaluate clinical backgrounds and the effectiveness of post-T-DM1 treatments in HER2-positive metastatic breast cancer patients in practice in Japan. The primary end point was objective response rate (ORR), and secondary end points included disease control rate (DCR), progression-free survival (PFS), time to treatment failure (TTF), and overall survival (OS).
The ORR is the percentage of patients with a complete response (CR) or partial response (PR) in the best overall response. The DCR is the proportion of patients with CR, PR, or stable disease (SD) in the best overall response. PFS was calculated starting from the start date of the treatment and ending with the earliest event of disease progression or death. Deterioration of the disease was defined as the day clinically judged as progressive disease (PD), regardless of imaging. The therapeutic effect was mainly calculated following the response evaluation criteria in solid tumours (RECIST), with an additional aid by the attending physician. TTF was calculated starting from the start date of the target treatment and ending with the earliest event of disease progression or treatment discontinuation. OS was defined as the time from the date of diagnosis until the last date for which survival status data were available (last follow-up date: June 31st, 2019). All deaths were considered events regardless of the reason.
The number of prior treatments was determined as follows. Single use of anti-HER2 drugs was not counted as a treatment line, whereas single use of endocrine therapy or chemotherapy was counted as one treatment line. T-DM1 was counted as one line of chemotherapy. Pre- and post-operative chemotherapies and endocrine therapies were not included.
Statistical analysis
For patient backgrounds, frequencies and percentages were calculated for categorical data, and descriptive statistics values were calculated for quantitative data.
ORR and DCR were calculated for populations with target lesions, and 95% confidential intervals (CIs) were calculated based on the Clopper–Pearson method. PFS, TTF, and OS were calculated for the overall population, and the survival curves were estimated using the Kaplan–Meier method. The CIs for median survival and survival rates were calculated according to the Brookmeyer and Crowley method and Greenwood’s formula, respectively. The date of disease progression was defined as the date clinically determined as PD.
The subgroup analyses were pre-planned with the following parameters: T-DM1 resistant or refractory, Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1, the presence or absence of brain metastases, age under 65 or 65 and up, best overall response for T-DM1 CR/PR/SD or PD, prior therapy with or without pertuzumab, positive or negative ER and PgR status, the presence or absence of visceral metastases at the start of post-T-DM1 treatment, and the number of treatment lines before T-DM1 of zero and one or two or more. “Resistant” was defined as cases that once showed SD, PR, or CR but eventually resulted in PD. The category “refractory” was defined as cases in which the response of T-DM1 treatment was PD from the beginning. Visceral metastases refer to liver or lung/pleural metastases.
Statistical significance was defined as a p value less than 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, North Carolina, USA).
Results
Patient characteristics
A flow diagram of the included patients is shown in Fig. 1. Of the 328 enrolled patients, 325 patients were included in the final analysis. Of all cases analysed, 290 patients had a target lesion (89.2%).
Fig. 1.
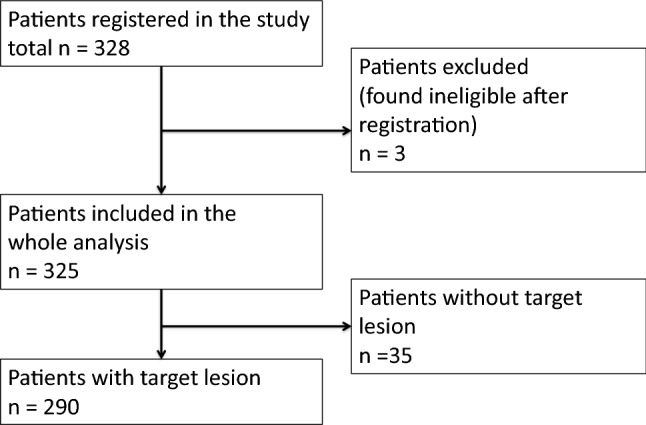
Flow diagram of the patient population
A summary of patient characteristics is shown in Table 1 and Supplemental Table 1. All patients were female, and the median age was 59 years (range 25–96). Of the 325 patients, there were 182 (56%) ER-positive cases. The number of patients with de novo stage IV was 124 (38.2%), and 201 (61.8%) experienced recurrence. There were 273 (84%) patients with an ECOG PS of 0 or 1 at the start of post-T-DM1 therapy. Brain metastases occurred in 61 cases (18.8%). The number of patients previously treated with pertuzumab, taxane, or anthracycline was 217 (66.8%), 279 (85.8%), and 102 (31.4%), respectively. The median line of post-T-DM1 treatment was 4 (range 2 to more than 10).
Table 1.
Patient characteristics of eligible cohort (n = 325)
| Characteristics | Number of cases (n = 325) | % |
|---|---|---|
| Sex | ||
| Male | 0 | 0.0% |
| Female | 325 | 100.0% |
| Age | Median (min—max) | 59 (25–96) |
| < 40 | 19 | 5.8% |
| 40–64 | 200 | 61.5% |
| 65–74 | 74 | 22.8% |
| 75 or more | 32 | 9.8% |
| Menopause | ||
| Pre | 39 | 12.0% |
| Post | 230 | 70.8% |
| Unknown | 56 | 17.2% |
| Stage | ||
| De novo Stage IV | 124 | 38.2% |
| Recurrence | 201 | 61.0% |
| Operation | ||
| Yes | 230 | 70.8% |
| No | 95 | 29.2% |
| ER | ||
| Positive | 182 | 56.0% |
| Negative | 143 | 44.0% |
| Unknown | 0 | 0.0% |
| PgR | ||
| Positive | 114 | 35.1% |
| Negative | 209 | 64.3% |
| Unknown | 2 | 0.6% |
| HER2 score | ||
| 0 | 0 | 0.0% |
| 1 + | 1 | 0.3% |
| 2 + | 77 | 23.7% |
| 3 + | 246 | 75.7% |
| Missing value | 1 | 0.3% |
| HER2 ISH | ||
| − | 5 | 1.5% |
| + | 87 | 26.8% |
| Unknown | 233 | 71.7% |
| Pertuzumab use in treatment before T-DM1 | ||
| Yes | 217 | 66.8% |
| No | 108 | 33.2% |
| Taxane use in treatment before T-DM1 | ||
| Yes | 279 | 85.8% |
| No | 46 | 14.2% |
| Anthracycline use in treatment before T-DM1 | ||
| Yes | 102 | 31.4% |
| No | 223 | 68.6% |
| Number of treatment before T-DM1 | median (min–max) | 2 (0–≥ 10) |
| 0 or 1 | 120 | 36.9% |
| 2 or more | 205 | 63.1% |
| PS at starting post-T-DM1 treatment | ||
| 0 | 102 | 31.4% |
| 1 | 171 | 52.6% |
| 2 or more | 18 | 5.5% |
| Unknown | 34 | 10.5% |
| Presence of metastasis | ||
| Yes | 314 | 96.6% |
| No | 11 | 3.4% |
| Metastatic cites (multiple selection allowed) | ||
| Liver | 110 | 33.8% |
| Lung or pleural | 162 | 49.8% |
| Brain | 61 | 18.8% |
| Others | 224 | 68.9% |
| Presence of target lesion | ||
| Yes | 290 | 89.2% |
| No | 35 | 10.8% |
Clinical benefit of post-T-DM1 treatment
We analysed ORR and DCR in 290 cases with a target lesion (Table 2). The ORR was 22.8% (95% CI 18.1–28.0%), and DCR was 66.6% (60.8–72.0%). CR occurred in 0.7% of patients.
Table 2.
ORR and DCR of post T-DM1 (n = 290)
| Number of cases (n = 290) | % | 95% CI | |
|---|---|---|---|
| CR | 2 | 0.7 | |
| PR | 64 | 22.1 | |
| SD | 127 | 43.8 | |
| PD | 81 | 27.9 | |
| NE | 16 | 5.5 | |
| ORR | 66 | 22.8 | (18.1–28.0) |
| DCR | 193 | 66.6 | (60.8–72.0) |
Treatment line: median 4 (2–≥ 10)
CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable, ORR overall response rate, DCR disease control rate, 95% CI 95% confidence interval
Survival was analysed in the entire population (n = 325) with a median follow-up time of 16.3 months (95% CI 14.3–18.2). Median PFS was 6.1 months (95% CI 5.3–6.7) with a 1-year PFS rate of 24.8% (Fig. 2a). The median TTF was 5.1 months (95% CI 4.4–5.6) with a 1-year TTF rate of 19.8% (Fig. 2b). Median OS was 23.7 months (95% CI 20.7–27.4) with a 1-year OS rate of 75.4% and 3-year OS rate of 33.4% (Fig. 2c).
Fig. 2.
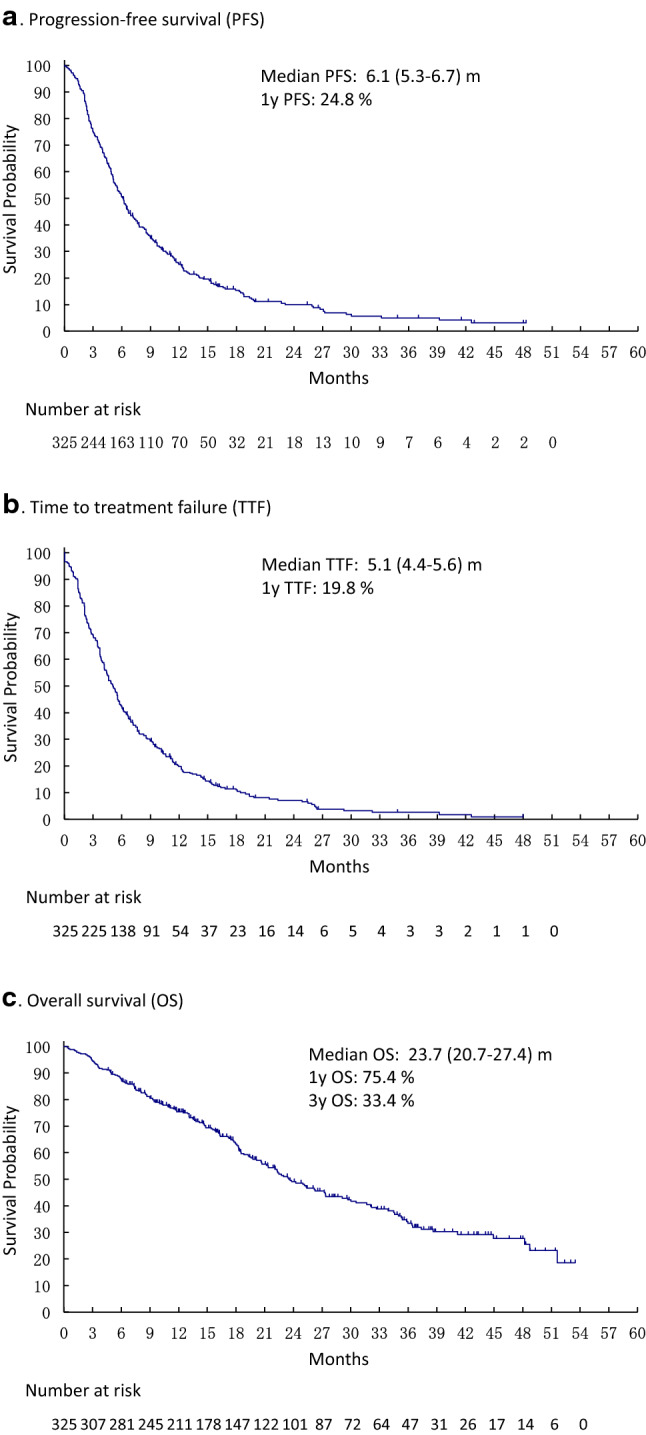
Survival curves of post-T-DM1 treatments (n = 325). a Progression-free survival (PFS) b Time to treatment failure (TTF). c Overall survival (OS)
The selected post-T-DM1 treatment was as follows (Table 3 and Supplemental Table 2): (1) chemotherapy in combination with trastuzumab and pertuzumab (n = 102; 31.4%), (2) chemotherapy concomitant with trastuzumab only (n = 78; 24.0%), (3) lapatinib with capecitabine (n = 63; 19.4%), and (4) others (n = 82; 25.2%). Chemotherapy used with trastuzumab and pertuzumab in descending order were taxane, eribulin, and vinorelbine, while chemotherapies used with trastuzumab only included gemcitabine and capecitabine. Anthracycline was selected in 17 cases (5.2%). Of 217 cases with previous pertuzumab use, pertuzumab was administered again in 67 cases as post-T-DM1 treatment.
Table 3.
Types of post T-DM1 treatment (n = 325)
| Number of cases (n = 325) | % | |
|---|---|---|
| Lapatinib + capecitabine | 63 | 19.4% |
| Trastuzumab + pertuzumab + CTx | 102 | 31.4% |
| Trastuzumab + pertuzumab + HTx | 3 | 0.9% |
| Trastuzumab + pertuzumab | 2 | 0.6% |
| Trastuzumab + CTx | 78 | 24.0% |
| Trastuzumab + HTx | 10 | 3.1% |
| Trastuzumab | 11 | 3.4% |
| Anthracycline | 17 | 5.2% |
| other CTx | 24 | 7.4% |
| HTx | 6 | 1.8% |
| Others | 9 | 2.8% |
CTx chemotherapy, HTx hormonal therapy
To determine the effectiveness of each treatment, we evaluated the following three groups individually: chemotherapy concomitant with trastuzumab and pertuzumab, chemotherapy concomitant with trastuzumab only, and lapatinib with capecitabine. Patients treated with chemotherapy in combination with trastuzumab and pertuzumab showed the highest ORR of 27.1% (95% CI 18.5–37.1), while ORR of chemotherapy in combination with trastuzumab, and lapatinib with capecitabine were 17.9% (95% CI 9.6–29.2) and 20.0% (95% CI 10.4–33.0), respectively. Those treated with lapatinib with capecitabine showed the highest DCR of 74.5% (95% CI 60.1–85.3) due to the percentage of the SD population accounting for more than 50%, whereas DCR of chemotherapy in combination with trastuzumab and pertuzumab, and chemotherapy in combination with trastuzumab were 67.7% (95% CI 57.4.6–76.9) and 58.2% (95% CI 45.5–70.2), respectively. The survival curves of each treatment were almost identical (Supplemental Fig.1). Furthermore, the OS survival curves were slightly worse in the lapatinib with capecitabine group. Detailed patient backgrounds showed that patients with T-DM1 best response PD were 39.7% in lapatinib with capecitabine, 31.4% in chemotherapy in combination with trastuzumab and pertuzumab, and 30.8% in chemotherapy in combination with trastuzumab. As for patients with brain metastasis, these were 31.7%, 14.7%, and 15.4%, respectively (Data not shown).
Use of post-T-DM1 treatment in subgroup analysis
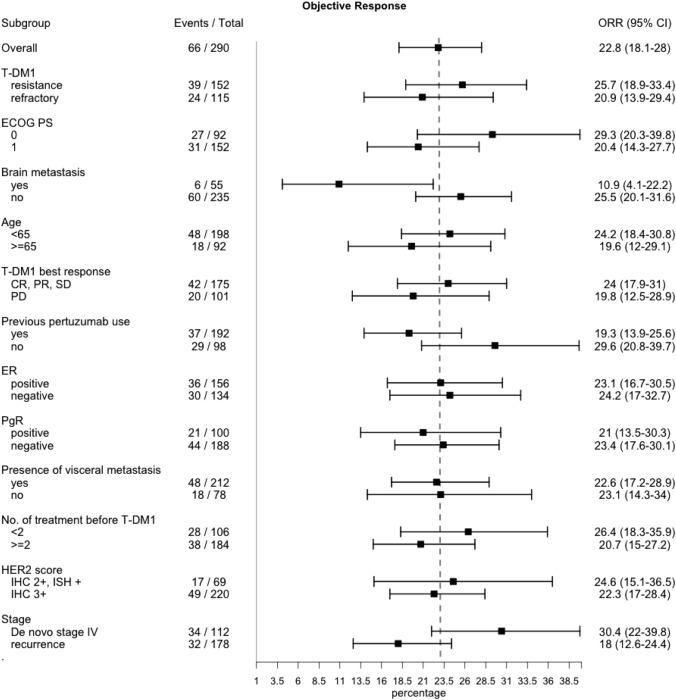
Prespecified subgroup analyses of ORR showed consistent results across subgroups as follows (Fig. 3): age (younger than 65 = 24.2%, 65 or older = 19.6%), response to T-DM1 (CR/PR/SD = 24.0%, PD = 19.8%), T-DM1 best response (resistance = 25.7%, refractory = 20.9%), ER status (positive = 23.1%, negative = 24.2%), PgR status (positive = 21%, negative = 23.4%), the presence of visceral metastasis (with metastasis = 22.6%, without metastasis = 23.1%), and treatment line before T-DM1 (fewer than two = 26.4%, two or more = 20.7%). Subgroup analyses of TTF and PFS also showed consistent results across all subgroups (Supplemental Fig. 3 and 4). Patients with previous pertuzumab use showed similar results (ORR = 19.3%, DCR = 64.1%, PFS = 5.9 months, TTF = 4.6 months, and OS = 23 months) with those without previous pertuzumab use (ORR = 29.6%, DCR = 71.4%, PFS = 6.5 months, TTF = 5.6 months, and OS = 24.2 months). Subgroup analyses of pertuzumab rechallenge consisting of 217 cases with previous pertuzumab use showed the following results: the pertuzumab rechallenge group (ORR = 19%, DCR = 63.5%, PFS = 5.5 months, TTF = 4.6 months, and OS = 29.7 months) and the pertuzumab non-rechallenge group (ORR = 19.4%, DCR = 64.3%, PFS = 4.5 months, TTF = 6.0 months, and OS = 22.1 months) (Table 4).
Fig. 3.
Subgroup analysis of ORR (n = 290)
Table 4.
Effects of pertuzumab rechallenge in post T-DM1 treatment (ORR and DCR, n = 192, TTF, PFS, and OS, n = 217)
| Response | Pertuzumab rechallenge (n = 63) | Pertuzumab non-rechallenge (n = 129) | ||||
|---|---|---|---|---|---|---|
| Events/total | % | 95% CI | Events/total | % | 95% CI | |
| ORR | 12/63 | 19 | (10.2–30.9) | 25/129 | 19.4 | (13.0–27.3) |
| DCR | 40/63 | 63.5 | (50.4–75.3) | 83/129 | 64.3 | (55.4–72.6) |
| Survival | Pertuzumab rechallenge (n = 67) | Pertuzumab non-rechallenge (n = 150) | ||||
|---|---|---|---|---|---|---|
| Events/total | Months | 95% CI | Events/total | Months | 95% CI | |
| TTF | 56/67 | 4.6 | (3.0–7.8) | 141/150 | 4.5 | (3.7–5.8) |
| PFS | 54/67 | 5.5 | (4.4–10.2) | 133/150 | 6 | (4.9–6.7) |
| OS | 27/67 | 29.7 | (18.8–51.6) | 82/150 | 22.1 | (18.3–26.3) |
ORR overall response rate, DCR disease control rate, TTF time to treatment failure, PFS progressive-free survival, OS overall survival, 95% CI 95% confidence interval. ORR and DCR were calculated for populations with target lesions, and 95% confidential intervals (CIs) were calculated based on the Clopper–Pearson method. PFS, TTF, and OS were calculated for the overall population, and the survival curves were estimated using the Kaplan–Meier method. The CIs for median survival and survival rates were calculated according to the Brookmeyer and Crowley method and Greenwood’s formula, respectively
Some trends were observed in subgroup analyses. Regarding ORR, patients without brain metastasis showed ORR of 25.5% (95% CI 20.1–31.6), as compared with 10.9% (95% CI 4.1–22.2) in cases with brain metastasis. Patients with de novo stage IV breast cancer showed ORR of 30.4% (95%CI: 22–39.8), while those with recurrence showed 18.0% (95% CI 12.6–24.4) (Fig. 3). Subgroup analyses of DCR, TTF, PFS, and OS are shown in Supplemental Fig. 2–5.
Discussion
In this study, we evaluated the treatment choice and efficacy of post-T-DM1 treatments in real-world practice. We found that chemotherapy in combination with trastuzumab and pertuzumab was the most commonly selected post-T-DM1 treatment, and the efficacy of post-T-DM1 treatment was relatively limited (ORR = 22.8%, PFS = 6.1 months, and OS = 23.7 months). This study results provided insights into understanding the current practice patterns for patients who had prior treatment with T-DM1.
The efficacy of post-T-DM1 treatment in this study is lower than that of T-DM1 as the second-line therapy for HER2-positive metastatic breast cancer in the EMILIA study (ORR = 43.6%, PFS = 9.6 months, OS = 30.9 month). With a very limited effect of PFS at 6 months, third-line treatment for HER2-positive breast cancer requires further improvement. As will be described later, the development of new drugs is being carried out for this purpose, and promising drugs are appearing. Second, regarding the subgroup analysis, some trends seen in the results of this study are consistent with the results of other HER2-positive metastatic breast cancer trials. Greater ORR can be expected with patients without brain metastasis, and patients with de novo stage IV. Greater DCR can be expected when the prior T-DM1 resulted in a good response. With better PS and response to prior T-DM1 therapy, patients may likely achieve longer overall survival. Although the effect of third-line treatment is limited, it is expected that the current treatment is to some extent efficient on patients in these subgroups. Third, the patients in the group of laptainib with capecitabine as post-T-DM1 treatment tended to have inferior OS. Based on the patient background, it is surmised that this is due to two factors: that there were many cases with (1) low response to prior T-DM1, and (2) brain metastasis.
At least two implications from the subgroup analyses can be pointed out. First, previous pertuzumab use may not affect the effect of post-T-DM1. In our study, previous pertuzumab use did not affect ORR, DCR, PFS, TTF and OS. In post hoc analysis of T-DXd, previous pertuzumab use did not affect the results either [17]. But the possibility that treatment after pertuzumab can be less effective has been suggested [18–20], though it was based on small and observational studies examining the efficacy of T-DM1 in patients with previous pertuzumab. Thus, further investigation is warranted to evaluate the influence of previous pertuzumab use over post-T-DM1 treatment.
Second, we examined the effect of pertuzumab rechallenge. At this time, there is no evidence of the rechallenge effect of pertuzumab, and the guidelines do not recommend rechallenge. All results of the subgroup analyses were consistent between the pertuzumab rechallenge group and non-rechallenge group. OS tends to be better in pertuzumab rechallenge group, though evaluation in a prospective study is necessary. The results of an ongoing multicentre randomized phase-three study examining the effect of pertuzumab rechallenge are awaited (PRECIOUS study: UMIN000018202) [21]. For the above two points, we are planning additional post hoc analysis using the propensity score matching method.
There has recently been remarkable progress in the development of new drugs for HER2-positive metastatic breast cancer [22]. These include tyrosine kinase inhibitors (neratinib and tucatinib), novel antibody–drug conjugates (trastuzumab-deruxtecan; T-DXd), novel anti-HER2 antibodies (margetuximab), and combination therapies of immune checkpoint inhibitors and anti-HER2 drugs. Some of these agents are primarily being developed for the treatment after T-DM1. DESTINY-Breast01 [23], HER2CLIMB [24], NALA [25], and SOPHIA [26] clinical trials of T-DXd, tucatinib, neratinib, and margetuximab, respectively, have recently reported promising results. By offering real-world evidence on the current use of post-T-DM1 therapies, our results provide an opportunity for the reinterpretation of studies and implications for the understanding of these four studies.
The results of the current study complement the DESTINY-Breast01 trial, which lacked a control arm. The patients enrolled in the DESTINY trial had prior T-DM1 and had median of six lines of previous treatments. Regardless of the heavily treated population, the study showed markedly positive results, including an ORR of 60.9%, DCR of 97.3%, and PFS of 16.4 months. Although a direct comparison is not readily applicable, higher response rate and longer PFS have been shown despite the heavily treated and less treatable population background compared with our study; thus the advantage of T-DXd has been more evident. T-DXd is a highly promising treatment, even though careful monitoring and management are still required for adverse events, such as interstitial pneumonia.
The patients in the intervention group in HER2CLIMB received tucatinib with trastuzumab and capecitabine. Adding tucatinib to trastuzumab and capecitabine resulted in better PFS and OS outcomes than adding a placebo. Specifically, the ORR was 41% vs. 23%, PFS was 7.8 vs. 5.6 months, and OS was 21.9 vs. 17.4 months at a median of three previous treatments. PFS of the control arm in HER2CLIMB was shorter than that of our study, which may be attributed to the large number of cases of brain metastasis included in this trial (18.8% in our study and ~ 46% in HER2CLIMB). Taking this into account, the ORR and PFS of HER2CLIMB are considered to be very high.
The NALA trial compared neratinib (an irreversible pan-HER tyrosine kinase inhibitor) plus capecitabine with lapatinib plus capecitabine in patients with stage IV HER2-positive metastatic breast cancer who had received two or more previous HER2-directed therapies. The ORR was 32.8% vs. 26.7%, and the median PFS and OS were 5.6 months vs. 5.5 and 21 months vs. 18.7, respectively. In the NALA trial, the number of patients who received T-DM1 and pertuzumab as prior anti-HER2 therapy was relatively small at 54% and 42%, respectively, which may account for the shorter prognosis in this trial.
The SOPHIA trial compared margetuximab plus chemotherapy with trastuzumab plus chemotherapy in HER2-positive metastatic breast cancer patients after one to three prior anti-HER2 therapies. Margetuximab is an Fc-modified anti-HER2 monoclonal antibody that showed enhanced antibody-dependent cell-mediated cytotoxicity compared with trastuzumab in in vitro studies. Margetuximab prolonged PFS (6.9 vs. 5.1 months) and showed a higher ORR (22% vs. 16%) compared with trastuzumab. Similar to trastuzumab, its safety was acceptable. Infusion-related reactions were more common with margetuximab, but these were manageable with premedication. Our studies differ in that all patients received pertuzumab as a pre-treatment in SOPHIA. The publication of this paper is highly anticipated, preferably with the details of other patient backgrounds.
Our results are consistent with the results of these trials in that all conventional treatments have low efficacy in terms of ORR, PFS. The current study adds to a positive perspective that new drugs are expected to prolong overall survival. Furthermore, our real-world evidence on the current use of post-T-DM1 treatments contributes to an in-depth understanding and renewed evaluation of preceding trials on promising drugs under development.
We recognized several limitations of this study. First, due to the retrospective nature of this study, heterogeneity in the patient population and differences in treatment choice are inevitable. Because of the absence of a comparative treatment group, only limited comparisons with prior clinical trial results are possible. Second, because this study collected clinical data from daily practice, there may have been differences in timing and tumour evaluation methods, which may have affected our results. Third, as for selection bias, countermeasures were taken to ensure that the patients were registered not only from cancer specialized institutions, but also from other non-specialized institutions, thereby reducing referral filter bias. Additionally, selection bias within institutions was reduced because all eligible cases were registered impartially. Fourth, no information on adverse events was collected.
In conclusion, we found that post-T-DM1 treatments showed modest anti-tumour activity. Further investigation of new treatment strategy beyond T-DM1 is awaited for HER2-positive metastatic breast cancer patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We give special thanks to the support members of the West Japan Oncology Group, specifically Dr. Shinichiro Nakamura, M.D. and Kaori Mori, the data managing staffs. The authors also thank all patients and investigators who participated in this study.
Author contributions
All authors contributed to the study conception and design. Data collection was performed by all authors. Analysis was performed by EPS Corporation (Tokyo, Japan). The analysed data were interpreted by Takamichi Yokoe, Sasagu Kurozumi, Kazuki Nozawa, Yukinori Ozaki, Mototsugu Shimokawa, and Toshimi Takano. The first draft of the manuscript was written by Takamichi Yokoe, and was reviewed and revised by Sasagu Kurozumi, Kazuki Nozawa, Yukinori Ozaki, and Toshimi Takano. All authors commented on the previous versions of the manuscript and approved the final submitted version.
Funding
This work was financially supported by Daiichi-Sankyo.
Compliance with ethical standards
Conflict of interest
Takamichi Yokoe has received lecture honoraria from Kyowa Kirin, Pfizer, and Eisai. Yukinori Ozaki has received honoraria from Chugai. Takuma Onoe has received lecture honoraria from Bristol-Myers Squibb. Mototsugu Shimokawa has received a consulting fee from Sysmex. Toshimi Takano received honoraria from Daiichi-Sankyo, Chugai, Kyowa Kirin, Eisai, Pfizer, Eli Lilly, and Celltrion, and research funding from Daiichi-Sankyo, Chugai, Kyowa Kirin, Eisai, Ono, Bristol-Myers Squibb, MSD, Merck Serono, Taiho, and Novartis. All remaining authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. Ethics approval and consent were obtained from the review board in each institution.
Consent for publication
Patient approval and consent were obtained through opt-out.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Autier P, Boniol M, La Vecchia C, Vatten L, Gavin A, Hery C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ. 2010;341:c3620. doi: 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 5.Lee ES, Jung SY, Kim JY, Kim JJ, Yoo TK, Kim YG, et al. Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann Oncol. 2016;27:82833. doi: 10.1093/annonc/mdw036. [DOI] [PubMed] [Google Scholar]
- 6.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Van Poznak C, Somerfield MR, Bast RC, Cristofanilli M, Goetz MP, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society Of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33:2695–2704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 13.Valachis A, Mauri D, Polyzos NP, Chlouverakis G, Mavroudis D, Georgoulias V. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast. 2011;20:485–490. doi: 10.1016/j.breast.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Paracha N, Reyes A, Dieras V, Krop I, Pivot X, Urruticoechea A. Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis. Breast Cancer Res Treat. 2020;180:597–609. doi: 10.1007/s10549-020-05577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Tsurutani J, Takahashi S, Iwata H, Krop IE, Redfern C, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 18.Noda-Narita S, Shimomura A, Kawachi A, Sumiyoshi-Okuma H, Sudo K, Shimoi T, et al. Comparison of the efficacy of trastuzumab emtansine between patients with metastatic human epidermal growth factor receptor 2-positive breast cancers previously treated with combination trastuzumab and pertuzumab and with trastuzumab only in Japanese population. Breast Cancer. 2019;26:492–498. doi: 10.1007/s12282-019-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzimitrowicz H, Berger M, Vargo C, Hood A, Abdelghany O, Raghavendra AS, et al. T-DM1 activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with Trastuzumab and Pertuzumab. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(29):3511–3517. doi: 10.1200/JCO.2016.67.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabi A, Giannarelli D, Moscetti L, Santini D, Zambelli A, Laurentiis M, et al. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol. 2017;13(30):2791–2797. doi: 10.2217/fon-2017-0336. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Iwata H, Ueno T, Taira N, Kashiwaba M, Takahashi M, et al. A randomized, open-label, Phase III trial of pertuzumab retreatment in HER2-positive locally advanced/metastatic breast cancer patients previously treated with pertuzumab, trastuzumab and chemotherapy: the Japan Breast Cancer Research Group-M05 PRECIOUS study. Jpn J Clin Oncol. 2018;48:855–859. doi: 10.1093/jjco/hyy097. [DOI] [PubMed] [Google Scholar]
- 22.Ponde N, Brandao M, El-Hachem G, Werbrouck E, Piccart M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2018;67:10–20. doi: 10.1016/j.ctrv.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 25.Cristina S, Mafalda O, Yin-Hsun F, Ming-Shen D, Sara AH, Sung-Bae K, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J Clin Oncol. 2019;37:1002. doi: 10.1200/JCO.2019.37.15_suppl.1002. [DOI] [Google Scholar]
- 26.Hope SR, Seock-Ah I, Gail LSW, Santiago ER, De Michelino L, Javier C, et al. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) J Clin Oncol. 2019;37:1000. doi: 10.1200/JCO.2019.37.15_suppl.1000. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.