Abstract
Background
The macroalgal flora of the Island of São Miguel (eastern group of the Azores Archipelago) has attracted the interest of many researchers in the past, the first publications going back to the nineteenth century. Initial studies were mainly taxonomic, resulting in the publication of a checklist of the Azorean benthic marine algae. Later, the establishment of the University of the Azores on the Island permitted the logistic conditions to develop both temporal studies and long-term research and this resulted in a significant increase on research directed at the benthic marine algae and littoral communities of the Island and consequent publications.
Prior to the present paper, the known macroalgal flora of São Miguel Island comprised around 260 species. Despite this richness, a significant amount of the research was never made public, notably Masters and PhD theses encompassing information regarding presence data recorded at littoral and sublittoral levels down to a depth of approximately 40 m around the Island and the many collections made, which resulted in vouchers deposited in the AZB Herbarium Ruy Telles Palhinha and the LSM- Molecular Systematics Laboratory at the Faculty of Sciences and Technology of the University of the Azores.
The present publication lists the macroalgal taxonomic records, together with information on their ecology and occurrence around São Miguel Island, improving the knowledge of the Azorean macroalgal flora at local and regional scales.
New information
A total of 12,781 specimens (including some identified only to genus) belonging to 431 taxa of macroalgae are registered, comprising 284 Rhodophyta, 59 Chlorophyta and 88 Ochrophyta (Phaeophyceae). Of these, 323 were identified to species level (212 Rhodophyta, 48 Chlorophyta and 63 Ochrophyta), of which 61 are new records for the Island (42 Rhodophyta, 9 Chlorophyta and 10 Ochrophyta), one an Azorean endemic (Predaea feldmannii subsp. azorica Gabriel), five are Macaronesian endemisms (the red algae Botryocladia macaronesica Afonso-Carrillo, Sobrino, Tittley & Neto, Laurencia viridis Gil-Rodríguez & Haroun, Millerella tinerfensis (Seoane-Camba) S.M.Boo & J.M.Rico, Phyllophora gelidioides P.Crouan & H.Crouan ex Karsakoff and the green alga Codium elisabethiae O.C.Schmidt), 19 are introduced species (15 Rhodophyta, two Chlorophyta and two Ochrophyta) and 32 are of uncertain status (21 Rhodophyta, five Chlorophyta and six Ochrophyta).
Keywords: macroalgae, Azores, São Miguel Island, new records, endemism, native, uncertain, introduced, occurrence data, ecology
Introduction
Research on the marine algae from the Azores started in the mid-nineteenth century (1838) when Guthnick and the two Hochstetters, father and son, visited the Archipelago (Neto 1994). Since then, many other researchers and naturalists have visited the Archipelago, resulting in several publications on the marine algal flora of this region (see summary in Neto 1994, Neto 1997). Most initial studies were taxonomic, focusing on the production of species lists. Almost a century later, the German botanist Otto Christian Schmidt visited several islands, including São Miguel and initiated a more comprehensive ecological approach describing species associations and their spatial organisation (Schmidt 1931). Ever since the first half of the last century, several studies have focused more widely on intertidal and shallow subtidal communities providing information on the vertical distribution of macroalgae and invertebrates and their trophic relations (see Neto 1992, Neto 2000, Neto 2001 for a review on this subject). Taxonomic investigations have continued and the first checklist of the Azorean benthic marine algae published by Neto (1994) brought together the existing published information, provided distributional records within the Archipelago and reported 307 species, indicating a moderately rich flora given its isolated mid-Atlantic position. A revision of this first checklist was made by Parente (2010), increasing the number of algae species to 327, but without providing their distributional information on the Archipelago. Later, Rosas-Alquicira et al. (2011) published a catalogue of non-fossil geniculate coralline red algae (Corallinales, Rhodophyta) of the Macaronesia, in which they made both a critical review of species and infraspecific taxa, as well as an assessment of species diversity in the region. Research by local teams was also dedicated to the Azorean littoral communities and biota conservation (see, for example, Abecasis et al. 2015, Amorim et al. 2015, Chainho et al. 2015). Taxonomic, ecological and biotechnological investigations have continued generating knowledge on the Azorean macroalgae flora, its biotechnological potential and also on the structure and functioning of littoral communities (see revisions on Neto et al. 2014, Haroun et al. 2019 and Haroun et al. 2019). Recently, several additional studies have been published with important information on the Azorean algae biodiversity, biogeography, conservation, ecology and taxonomy (see, for example, Bruno de Sousa et al. 2019, Cacabelos et al. 2019, Cacabelos et al. 2020, Freitas et al. 2019, Kellaris et al. 2019, Martins et al. 2019, Parente et al. 2019, Parente et al. 2020, Patarra et al. 2017, Patarra et al. 2019, Patarra et al. 2020, Sousa et al. 2019, Faria et al. 2020a, Faria et al. 2020b, Vieira et al. 2020).
The paper by Freitas et al. (2019) increased the number of macroalgae species occurring in the Azores to 405 and reported that, amongst the mid-Atlantic archipelagos, the Azores is second in species richness after the Canary Islands, with 689 species, followed by Madeira (396), Cabo Verde (333) and Selvagens (295 species). For some species, the Azores Archipelago forms a boundary in their distribution. Codium effusum (Rafinesque) Delle Chiaje, for example, is as its western distribution limit in the Archipelago (Leon-Cisneros et al. 2012), whereas for Dudresnaya crassa M.Howe, a western Atlantic warm-water species, the Azores extends its known distributional range to the east. Some northern species such as the red alga Schizymenia dubyi (Chauvin ex Duby) J.Agardh and Lomentaria orcadensis (Harvey) Collins come close to their southern limit of distribution in the Azores, while some southern warm-water species, such as green alga Anadyomene stellata (Wulfen) C.Agardh and the red alga Sebdenia rodrigueziana (Feldmann) Codomier ex Athanasiadis, reach their Atlantic northern limit of distribution on the Islands (Neto et al. 2005, Leon-Cisneros et al. 2012). Some species, relatively common in the region a few years ago, have become uncommon or even very rare, for example, Scytosiphon lomentaria (Lyngbye) Link, Schimmelmannia schousboei (J.Agardh) J.Agardh. In contrast, there has been an increase in unexpected macroalgae in the Azores, with the arrival and establishment of several non-native species (see Cardigos et al. 2006, Micael et al. 2014, Vaz-Pinto et al. 2014, Parente et al. 2019, Cacabelos et al. 2019, Cacabelos et al. 2020, Martins et al. 2019).
Within the spread of the Archipelago, there are no marked differences between floras of individual Islands or Island groups and, biogeographically, the Azores algal flora reveals itself to have a mixed nature, with species shared with Macaronesia, North Africa, the Mediterranean Sea, Atlantic Europe and America (Tittley and Neto 1995, Tittley and Neto 2005, Tittley and Neto 2006, Tittley 2003, Wallenstein et al. 2009b). This nature of the Azorean marine algal flora was reinforced by the work of Freitas et al. (2019), who, using an extensive analysis encompassing data on coastal fishes, brachyurans, polychaetes, gastropods echinoderms and macroalgae, suggested that the Azores should be a biogeographical entity on its own and proposed a re-definition of the Lusitanian biogeographical province, in which they included four ecoregions: the South European Atlantic Shelf, the Saharan Upwelling area, the Azores ecoregion and a new ecoregion they named Webbnesia, which comprises the archipelagos of Madeira, Selvagens and the Canary Islands.
Not all the Azorean Islands have received the same attention regarding the studies on macroalgae. Furthermore, many species may have been overlooked due to their small size, opportunistic nature or ephemeral life span.
To overcome this and gain a better and up-to-date knowledge of the Archipelago’s macroalgae flora, an effort was made by resident teams to undertake a considerable amount of research over the past three decades on several Islands. The present paper is the last one of a series and presents physical, occurrence data and information gathered from macroalgal surveys undertaken on São Miguel Island between 1989 and 2019 mainly by the Island Aquatic Research Group of the Azorean Biodiversity Centre of the University of the Azores (Link: https://ce3c.ciencias.ulisboa.pt/sub-team/island-aquatic-ecology), the BIOISLE, Biodiversity and Islands Research Group of CIBIO-Açores at the University of the Azores (Link: https://cibio.up.pt/research-groups-1/details/bioisle) and the OKEANOS Centre of the University of the Azores (Link: http://www.okeanos.uac.pt). In these surveys, particular attention was given to the small filamentous and thin sheet-like forms that are often short-lived and fast-growing and usually very difficult to identify in the field, without the aid of a microscope and specialised literature in the laboratory.
This paper aims to provide a valuable marine biological tool to aid research on the systematics, diversity and conservation, biological monitoring, climate change, ecology and more applied studies, such as biotechnological applications, which will be of assistance to a wide range of focal groups including academics, students, governments, private organisations and the general public.
General description
Purpose
This paper presents taxonomic records of macroalgae for São Miguel Island and provides general information on their occurrence and distribution. By doing this, it will contribute to address several biodiversity shortfalls (see Cardoso et al. 2011, Hortal et al. 2015), namely the need to catalogue the Azorean macroalgae (Linnean shortfall) to improve current information on their local and regional geographic distribution (Wallacean shortfall), as well as to provide a better understanding of species abundance and dynamics in space (Prestonian shortfall).
Project description
Title
Marine algal flora of São Miguel Island, Azores
Personnel
Collections were conducted and occurrence data recorded over several years (1989 - 2019). The main collectors were Adriá Pajares, Afonso C. L. Prestes, Alexandra Pacheco, Amine Sebti, Ana Bettencourt, Ana Carreiro, Ana Cristina Costa, Ana F. Ferreira, Ana Filipa Sousa, Ana I. Neto, Ana Leonado, Ana Rita Carreiro, Ana Rodriguez, André Amaral, André Gillon, Andrea Salamanca, Andrea Z. Botelho, Andreia Levi, Andreia Tracana, Anna Lloveras, Antalova Janouchová, Artur Oliveira, Brigida Garcia, Bruno Magalhães, Bruno Sérgio, Camille Fontaine, Carlos Campos, Carlos Mir, Carlos Rius, Carolina Moreira, Catarina Santos, Célia Albuquerque, Clara Gaspar, Cláudia Hipólito, Cristiana Figueredo, Cristina Seijo, Dálida Pereira, Daniel Torrão, Daniela Gabriel, David Milla-Figueras, Délia Cravo, Dinis Geraldes, Dolores Campos, Edgar F. Rosas-Alquicira, Emanuel Xavier, Enric Ballesteros, Eunice Nogueira, Eva Cacabelos, Fernando Feiteira, Filipe Parreira, Flávio Rodrigues, Francisco Wallenstein, Gloria Cantos, Gustavo Martins, Heather Baldwin, Helena Abreu, Hélio Dias, Hugo Lopes, Ian Tittley, Ignacio Moreu, Isadora Moniz, Joana Duarte, Joana Matzen, Jana Verdura, Joana Michael, João Brum, João Faria, João Feijó, José M. N. Azevedo, José Medeiros, Juan Garcia Marino, Juan Izaguirre, Juliana Dal Molin, Juliane Bernardi, Karla León-Cisneros, Laura Rovira, M. Canto, Marco Enoch, Margarida Leonardo, Manuela I. Parente, Marc Balcells, Marc Fernandez, Marco Henrique, Marco Santos, Maria Ana Dionísio, Maria Inês Pavão, Maria Machín-Sánchez, Maria Vale, Mariano Rego, Marisa Toste, Marlene Terra, Marta Coca, Miguel Frada, Mikel Mendizabal, Miguel Furtado, Miguel Matias, Miriam Gutierrez, Mutue Toyota Fujii, Natália Jardim, Nikola Zic, Nil Alvarez Segura, Nuno Vaz Álvaro, Núria Vila, O. Laclaustra, Olaia Morán, Olalla Torrontegi, Olivie Laroche, Patrícia Madeira, Patrícia Pereira, Paula Avelar, Paulo Azevedo, Paulo Custódio, Paulo Torres, Pedro Cavazin, Pedro Cerqueira, Pedro Raposeiro, Pedro Rodrigues, Rafael Fraga, Raquel Torres, Renato Calado, Ricardo Lacerda, Rita F. Patarra, Rita Grilo, Rita Norberto, Robert Fletcher, Rocio Sanchez, Roger Fuste, Ruben Couto, Rui Costa, Rui Jesus, Rui Moreira, Rui Patrício, Rui Sousa, Sabrina Garcia, Sandra Monteiro, Sara Peres, Sérgio Ávila, Silvia Escarduça, Sofia Carreiro, Susan Clayden, Tarso Costa, Valeria Cassano and William Farnham.
Preliminary in situ identifications were undertaken by: Ana I Neto, Andrea Z. Botelho, Andreia Levi, Daniela Gabriel, David Milla-Figueras, Edgar F. Rosas-Alquicira, Enric Ballesteros, Eva Cacabelos, Francisco Wallenstein, Heather Baldwin, Ian Tittley, Ignacio Moreu, Karla León-Cisneros, Manuela I. Parente, Maria Machín-Sanchez, Marlene Terra, Mutue Toyota Fujii, Nuno Vaz Álvaro, Raquel Torres, Robert Fletcher, Ruben Couto, Valeria Cassano and William Farnham.
Final species identification were undertaken by Ana I. Neto, Daniela Gabriel, Edgar F. Rosas-Alquicira, Enric Ballesteros, Eva Cacabelos, Ian Tittley, Ignacio Moreu, Karla León-Cisneros, Manuela I. Parente, Maria Machín-Sanchez, Marlene Terra, Mutue Toyota Fujii, Robert Fletcher, Valeria Cassano and William Farnham.
Voucher specimen management was mainly undertaken by Afonso C.L. Prestes, Ana I. Neto, Eunice Nogueira, Manuela I. Parente, Natália Cabral, Rita Patarra and Roberto Resendes.
Study area description
The Azores Archipelago (38°43′49″N, 27°19′10″W, Fig. 1), isolated in the mid-Atlantic Ocean, comprises nine volcanic Islands and several islets spread over 500 km in a WNW–ESE direction, emerging from the Azores Plateau and located above an active triple junction between three of the world's largest tectonic plates (the North American Plate, the Eurasian Plate and the African Plate, Hildenbrand et al. 2014).
Figure 1.
The Azores, its location in the Atlantic and São Miguel Island highlighted in black (by Nuno V. Álvaro).
The Archipelago comprises nine volcanic Islands and several small Islets in three separate groups (eastern, central and western).
São Miguel (in black in Fig. 1), approximately 750 km² in size, is the largest and most volcanically-active Island (Gaspar et al. 2015). Located in the eastern group of the Archipelago (37°54'58''N, -25°51'52''W, Fig. 2), its formation followed a series of volcanic events, with different parts of the Island having different ages. The oldest portion (4 M years old) is the eastern side, Nordeste, where Pico da Vara (the highest mountain of the Island with 1103 m a.s.l.) is located. The Island was then progressively formed to the west: Povoação (2 M years); Furnas (750,000 years); Serra de Água de Pau (250,000 years). The Sete Cidades complex appeared 500,000 years ago and only later (50,000 years ago) was connected to Serra de Agua de Pau through the Serra Gorda and its succeeding line of peaks (Zbyszewski et al. 1958, Zbyszewski and Ferreira 1959).
Figure 2.
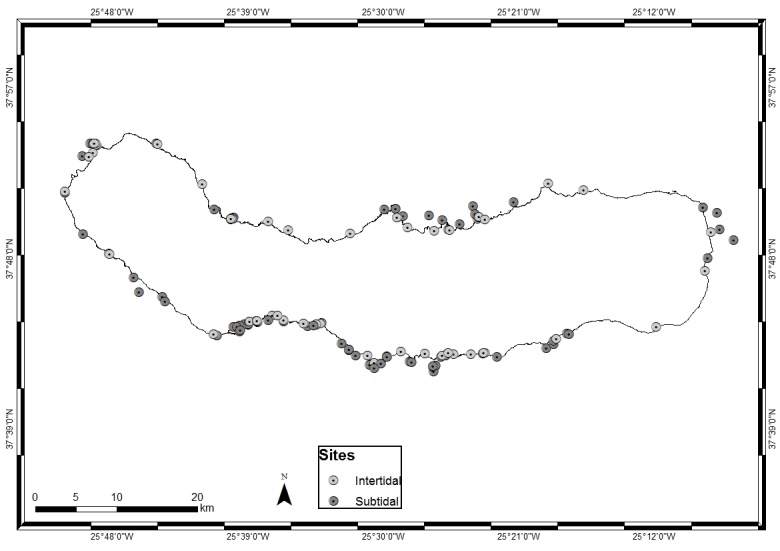
São Miguel Island with indication of the sampling locations (by Nuno V. Álvaro).
As in the other Azorean Islands, the climate is considerably influenced by the surrounding ocean and is characterised by regular rainfall, medium levels of relative humidity and persistent winds, mainly during the winter and autumn seasons (Morton et al. 1998). The tidal range is small (< 2 m) and the coastal extension is restricted, with deep waters occurring within a few kilometres offshore (Hidrográfico 1981). Most sea-shores are subject to swell and surge most of the year and few are sheltered, except for some bays and harbours. Extremely heavy seas occur during winter (Neto et al. 2005).
São Miguel has the longest coastline in the archipelago, about 155 km, corresponding to 25.3% of the whole Azorean coastline. The coastal topology, resulting from the effect of the maritime agitation, responsible for the predominance of erosive morphologies, is mainly composed of high, steep cliffs with a variety of stack, arch and gully formations and is mostly difficult to access by land. Most of the cliffs and coastal slopes are less than 50 m a.s.l. (Borges 2003) and fall directly into the sea. The coastline is mainly composed of irregular compact, bedrock platforms, alternating with boulder and cobble locations. On some shores, boulders entrap coarse sand and gravel and there are a few sandy beaches (Wallenstein et al. 2009b).
Intertidal communities of São Miguel Island, as on the other islands of the Archipelago, are primarily dominated by macroalgae, which mainly exhibit a mosaic and/or zoned distribution pattern and have a predominance of algal turfs that cover the rocks as a carpet (Wallenstein et al. 2009), best seen when rocks are uncovered at low tide. There is a very distinct horizontal pattern of species distribution, with three major zones commonly found on bedrock and boulder shores (Neto 2000, Neto et al. 2005, Wallenstein et al. 2009b). The uppermost intertidal level is dominated by littorinids (Fig. 3), while the mid-level zone is usually characterised by a fringe of chthamalid barnacles (Fig. 4), in which sometimes algae and limpets can occur (Fig. 5), followed by a lower area, in which either algal turf (generally monospecific and usually composed of Caulacanthus ustulatus (Turner) Kützing) dominates (Fig. 6) or patches of the brown alga Fucus spiralis Linnaeus and the red agarophyte Gelidium microdon Kützing (Fig. 7) grow interspaced with barnacles and algal turf. The lowest intertidal zone, representing the transition to the sublittoral envrironment, is either dominated by algal turf (generally multispecific and commonly dominated by coralline algae, Fig. 8) or by various species of frondose algae growing in bands (e.g. the brown alga Gongolaria abies-marina (S.G.Gmelin) Kuntze, Fig. 9) or forming patches amongst and over turf species (e.g. the agarophyte Pterocladiella capillacea (S.G.Gmelin) Santelices & Hommersand and the calcareous Ellisolandia elongata (J.Ellis & Solander) K.R.Hind & G.W.Saunders, Fig. 10). The brown alga Colpomenia sinuosa (Mertens ex Roth) Derbès & Solier is very common at this level, growing epiphytically on several other algae. Seasonally, the red algae Porphyra/ Neopyropia and/or Nemalion elminthoides (Velley) Batters can be seen growing in patches at the mid-intertidal level. In some locations, the brown crust Nemoderma tingitanum Schousboe ex Bornet can be common at this shore level (Neto et al. 2005, Wallenstein et al. 2009b).
Figure 3.
Littorinids, a characteristic gastropod species of the Azorean high intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 4.
Chthamalid barnacles on São Miguel mid-intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 5.
Chthamalid barnacles, algal turf and limpet on São Miguel mid-intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 6.

Algal turf, dominated by the red alga Caulacanthus ustulatus, on São Miguel mid-intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 7.
The brown alga Fucus spiralis and the red agarophyte Gelidium microdon on São Miguel mid-intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 8.
Multispecific algal turf and the coralline red alga Ellisolandia elongata on São Miguel low intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 9.
The brown alga Gongolaria abies-marina growing in bands at the low shore level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 10.
Patches of the agarophyte Pterocladiella capillacea and the calcareous Ellisolandia elongata at the low intertidal level (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
In spring and summer, considerable amounts of the introduced red alga Asparagopsis armata Harvey can be seen at the lower intertidal level, normally as an epiphyte on other algae (Neto, personal observation).
At cobble locations, the zonation pattern of macroalgae species is not clear (Costa 1994). The many microhabitats and substrate instability tend to mask and attenuate the limits of the biological zones. Nevertheless, in locations where cobbles are large and their size enlarges towards the sea (e.g. Fenais da Luz, north shore), the profile is steeper and usually the mid-intertidal level is dominated by the green macroalgae Ulva linza Linnaeus, U. clathrata (Roth) C.Agardh and U. rigida C.Agardh; the lower level is characterised by the presence of algal turf, mainly composed of Jania crassa J. V. Lamouroux and Corallina officinalis Linnaeus, with epiphytic Rhodophyta, such as Asparagopsis armata Harvey, phase Falkenbergia rufolanosa (Harvey) F.Schmitz, Centroceras clavulatum (C.Agardh) Montagne, Ceramium ciliatum (J.Ellis) Ducluzeau, C. deslongchampsii Chauvin ex Duby and Polysiphonia atlantica Kapraun & J.N.Norris. In locations with small cobbles (e.g. Caloura, south coast) or where there is a mixture of large and small cobbles (e.g. Povoação, south coast), the mid-intertidal level is usually characterised by fast growing algae, such as Cyanobacteria and the green algae Ulva spp., whereas the lowest level is also dominated by algal turf, but here mainly composed of C. officinalis and C. clavulatum (Caloura) or by C. clavulatum, Chondracanthus acicularis (Roth) Fredericq, Jania sp. and Lophosiphonia sp. (Povoação).
Important habitats at the shore level in bedrock locations are rock pools (Fig. 11). Differing in shape and size, they recreate a shallow subtidal habitat which may contain a rich diversity of marine algae and other marine organisms (Neto et al. 2005, Wallenstein et al. 2010).
Figure 11.

Low shore pool (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
The macroalgae diversity varies according to the pool location on the shore. Pools in the upper shore region are dominated by green algae, whilst those lower on the shore are dominated by red and brown algae. Similarly, faunal diversity in rock pools is greater at lower intertidal levels.
The adjacent submerged zone is also dominated by algal vegetation, with the rocky bottoms covered by more frondose macrophytes (Neto 2001, Wallenstein et al. 2009b), such as the red algae Asparagopsis taxiformis (Delile) Trevisan, Ellisolandia elongata, Jania spp., Plocamium cartilagineum (Linnaeus) P.S.Dixon, Pterocladiella capillacea and Sphaerococcus coronopifolius Stackhouse and the brown algae Dictyopteris polypodioides (A.P.De Candolle) J.V.Lamouroux, Dictyota spp., Gongolaria abies marina (S.G.Gmelin) Kuntze, Halopteris scoparia (Linnaeus) Sauvageau and Zonaria tournefortii (J.V.Lamouroux) Montagne (Fig. 12). The introduced red alga, Symphyocladia marchantioides (Harvey) Falkenberg, can be locally abundant below 15 m depth, usually as an ephiphyte on calcareous crusts; Hypnea musciformis (Wulfen) J.V.Lamouroux and Dasya spp. are other red algal species that can be locally abundant. The green species Codium elisabethiae (Fig. 13) and the brown species Padina pavonica (Linnaeus) Thivy can also be locally common, mainly in locations with sand influence (Neto 2001).
Figure 12.

The frondose brown algae Zonaria tournefortii and Dictyota spp. at the deepest level sampled (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 13.
The Macaronesian endemic Codium elisabethiae on the shallow bottoms of São Miguel Island (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Design description
The sampling referred to in this study was performed across littoral and sublittoral levels down to approximately 40 m. Each sampling location was visited several times and, on each occasion, a careful and extensive survey was undertaken to provide a good coverage of the area. Both presence recording and physical collections were made by walking over the intertidal shores during low tides or by SCUBA diving in the subtidal. The specimens collected were taken to the laboratory for identification and preservation and the resulting vouchers were deposited at the AZB Herbarium Ruy Telles Palhinha and the LSM - Molecular Systematics Laboratory at the Faculty of Sciences and Technology of the University of the Azores.
Funding
This study was mainly financially supported by the following projects/scientific expeditions:
- Projects:
- ABLA/MAC – “Associações Biológicas do Litoral Açoreano/Moluscos, Algas e Crustáceos”, funded by the Portuguese Science and Technology Foundation (1987-1991);
- Azorean Algal Flora – “Studies on algal communities of São Miguel, Azores”, partially funded by CIRN/DB/UAc (1992-1996);
- BIA - “Biodiversity of Azores Archipelago”, funded by the Portuguese Science and Technology Foundation. PRAXIS/2/2.1/BIA/169/94 (1996-1999);
- BIOTOPE – “ Classification, mapping and modelling of Azorean littoral biotopes”, funded by the Portuguese Science and Technology Foundation, POCTI MGS/45319/2002 (2003-2006);
- CAMAG/ORI – “Characterization of coastal water bodies on the islands of Santa Maria and São Miguel”, funded by the Regional Government of the Azores, Regional Secretariat for the Environment and the Sea, Regional Directorate for Planning and Water Resources (2008-2012);
- GESMAR – “ Sustainable management of marine Resources”, funded by the EU Funding Programme III B 2000-2006, Açores-Madeira-Canárias, GESMAR/MAC/2/C068 (2009-2012);
- PATELGENE – “ Genetic Structure of Azorean Limpets: Implications for Conservation and Marine Protected Areas”, funded by the Portuguese Science and Technology Foundation, PTDC/BIA-BIC/115837/2009 (2011-2014);
- MACROBIOMOL – “Macroalgae biodiversity under a molecular view - for a better understanding of North Atlantic Biogeography”, funded by PTDC/MAR/114613/2009 (2011-2015);
- ASMAS – “Açores: Stop-over for Marine Alien Species?”, funded by the Government of the Azores - Regional Secretariat for the Sea, Science and Technology, M2.1.2/I/032/2011 (2012-2016);
- BUS – “ Urban Structures: a driver of biodiversity change in coastal ecosystems?”, funded by the Portuguese Science and Technology Foundation, PTDC/MAR-EST/2160/2012 (2013-2015);
- ECOSUBVEG – “ Changes in submersed vegetation: assessing loss in ecosystems services from frondose to depauperate systems dominated by opportunistic vegetation”, funded by the Voluntary Scheme for Biodiversity and Ecosystem Services in Territories of the EU Outermost Regions and Oversees Countries and Territories, BEST 07.032700/2012/635752/SUB/B2 (2013-2016);
- LAUMACAT - “ Diversity and phylogenetic relationships on the benthic marine algae with pharmacological potential: the Laurencia complex (Rhodophyta) in Macaronesian archipelagos, tropical and subtropical Atlantic”, funded by the Ministerio de Ciencia e Innovación, Dirección General de Investigación y Gestión del Plan Nacional de R+D+i, Subdirección General de Proyectos de Investigación, Gobierno de España (2010-2013) and by the São Paulo State Research Support Foundation (FAPESP), Brazil, Proc. 2014 / 00012-1 (2013 a 2016);
- BALA – “Elaboration of the implementation program of the marine strategy framework directive - biodiversity of the coastal environments of the Azores” (2 /DRAM /2015), funded by the Government of the Azores - Regional Secretariat for the Sea, Science and Technology, Regional Directorate for Sea Affairs, GRA /SRMCT-DRAM, (2015);
- PIMA – “Elaboration of the implementation program of the Marine Strategy Framework Directive - Marine Invasion Program in the Azores” (3/DRAM /2015), funded by the Government of the Azores - Regional Secretariat for the Sea, Science and Technology, Regional Directorate for Sea Affairs, GRA /SRMCT-DRAM, (2015);
- ASPAZOR – “Ecosystem impacts and socioeconomic benefits of Asparagopsis armata in the Azores”, funded by the Regional Direction for Science, Technology. ACORES-01 -0145-FEDER-000060 (2016-2020);
- PORBIOTA - “ACORES-01-0145-FEDER-000072 - AZORES BIOPORTAL”, funded by the Operational Programme Azores 2020 (85% ERDF and 15% regional funds) (2019-2021);
- Scientific Expeditions and campaigns:
- “Campaign Macaronesia 2000”, under the project Macaronesia 2000 (2000-2001);
- “Waitt Foundation”, under the projects BALA and PIMA (2016);
- “BALA/PIMA”, under the projects BALA and PIMA (2018);
- “PORBIOTA/2019” under the project ACORES-01-0145-FEDER-000072 - AZORES BIOPORTAL – PORBIOTA (2019);
- Other funds:
- Portuguese National Funds, through FCT – the Portuguese Science and Technology Foundation, within the projects UID/BIA/00329/2013, 2015-2019, UID/BIA/00329/2020-2023 and UID/BIA/50027/2019, UID/BIA/50027/2013-2020, UID/Multi/04423/2013, PEst-C/MAR/LA0015/2013 and POCI-01-0145-FEDER-006821;
- European Regional Development (ERD) funds through the Operational Programme for Competitiveness Factors (COMPETE);
- Portuguese Regional Funds, through DRCT - Regional Directorate for Science and Technology, within several projects, 2019 and 2020 and SRMCT /DRAM - Regional Secretariat for the Sea, Science and Technology, Regional Directorate for Sea Affairs;
- CIRN/DB/UAc (Research Centre for Natural Resources, Universidade dos Açores, Departamento de Biologia);
- CIIMAR (Interdisciplinary Centre of Marine and Environmental Research, Porto, Portugal).
Sampling methods
Study extent
The present publication includes sampling performed over a relatively large area, covering littoral and sublittoral levels down to approximately 40 m around the Island (Table 1, Fig. 2).
Table 1.
Information and location of the sampling sites on São Miguel Island
| Location N0 | Location ID | Municipality | Locality | Latitude / Longitude | Littoral zone |
| 1 | SMG_L_APs | Lagoa | Água de Pau | Subtidal | 37°43'08''N, 25°27'45''W | Subtidal |
| 2 | SMG_L_APsE | Lagoa | Água de Pau | Subtidal E | 37°43'10''N, 25°27'44''W | Subtidal |
| 3 | SMG_L_APsW | Lagoa | Água de Pau | Subtidal W | 37°43'27''N, 25°27'16''W | Subtidal |
| 4 | SMG_L_Avb | Lagoa | Atalhada | Viteleiro | baía | 37°44'38''N, 25°23'23''W | Subtidal |
| 5 | SMG_L_Avem | Lagoa | Atalhada | Viteleiro | Entre-marés | 37°44'43''N, 25°23'24''W | Intertidal |
| 6 | SMG_L_Cbab | Lagoa | Caloura | Baixa da Areia | baía | 37°42'50''N, 25°28'11''W | Subtidal |
| 7 | SMG_L_Ccb | Lagoa | Caloura | Cerco | baía | 37°42'24''N, 25°29'30''W | Subtidal |
| 8 | SMG_L_CcbW | Lagoa | Caloura | Cerco | baía W | 37°42'21''N, 25°29'07''W | Subtidal |
| 9 | SMG_L_Ccem | Lagoa | Caloura | Cerco | Entre-marés | 37°42'27''N, 25°29'27''W | Intertidal |
| 10 | SMG_L_Cepgb | Lagoa | Caloura | Entre Porto e Galera | baía | 37°42'24''N, 25°29'52''W | Subtidal |
| 11 | SMG_L_Cgb | Lagoa | Caloura | Galera | baía | 37°42'10''N, 25°29'27''W | Subtidal |
| 12 | SMG_L_Chem | Lagoa | Caloura | Hotel | Entre-marés | 37°42'50''N, 25°28'59''W | Intertidal |
| 13 | SMG_L_Cpb | Lagoa | Caloura | porto | baía | 37°42'45''N, 25°30'15''W | Subtidal |
| 14 | SMG_L_Cpem | Lagoa | Caloura | porto | Entre-marés | 37°42'47''N, 25°30'15''W | Intertidal |
| 15 | SMA_L_Crcb | Lagoa | Caloura | Ribeira Chã | baía | 37°42'45''N, 25°30'16''W | Subtidal |
| 16 | SMG_L_Lbp | Lagoa | Lagoa | Bairro dos Pescadores | 37°44'23''N, 25°25'00''W | Subtidal |
| 17 | SMG_L_Lcrb | Lagoa | Lagoa | Cruzeiro | baía | 37°44'34''N, 25°25'56''W | Subtidal |
| 18 | SMG_L_Lcrem | Lagoa | Lagoa | Cruzeiro | Entre-marés | 37°44'32''N, 25°25'49''W | Intertidal |
| 19 | SMG_L_Lovem | Lagoa | Lagoa | Observatório vulcanológico | Entre-marés | 37°44'31''N, 25°24'44''W | Intertidal |
| 20 | SMG_L_Lpib | Lagoa | Lagoa | Piscina | baía | 37°44'26''N, 25°25'33''W | Subtidal |
| 21 | SMG_L_Lpiem | Lagoa | Lagoa | Piscina | Entre-marés | 37°44'29''N, 25°25'34''W | Intertidal |
| 22 | SMG_L_Lpb | Lagoa | Lagoa | porto | baía | 37°44'26''N, 25°25'25''W | Subtidal |
| 23 | SMG_L_Pepem | Lagoa | Pisão | Entre praias | Entre-marés | 37°43'02''N, 25°31'12''W | Intertidal |
| 24 | SMG_N_AN | Nordeste | Achada do Nordeste | 37°51'34''N, 25°43'23''W | Intertidal |
| 25 | SMG_N_LGpsem | Nordeste | Lombo Gordo | Ponta do Sossego | Entre-marés | 37°47'18''N, 25°51'27''W | Intertidal |
| 26 | SMG_N_Nb6 | Nordeste | Nordeste | baía 6 | 37°50'21''N, 25°52'15''W | Subtidal |
| 27 | SMG_N_Nb7 | Nordeste | Nordeste | baía 7 | 37°48'55''N, 25°53'22''W | Subtidal |
| 28 | SMG_N_Npinb | Nordeste | Nordeste | Piscinas Naturais | baía | 37°50'39''N, 25°51'17''W | Subtidal |
| 29 | SMG_N_Npmb | Nordeste | Nordeste | Ponta da Madrugada | baía | 37°49'30''N, 25°52'25''W | Subtidal |
| 30 | SMG_N_Npmqb | Nordeste | Nordeste | Ponta da Marquesa | baía | 37°47'59''N, 25°51'35''W | Subtidal |
| 31 | SMG_N_Npaem | Nordeste | Nordeste | Ponta do Arnel | Entre-marés | 37°49'21''N, 25°51'49''W | Intertidal |
| 32 | SMG_PD_Bab | Ponta Delgada | Bretanha | Ajuda | baía | 37°54'02''N, 25°14'56''W | Subtidal |
| 33 | SMG_PD_Baem | Ponta Delgada | Bretanha | Ajuda | Entre-marés | 37°54'00''N, 25°15'00''W | Intertidal |
| 34 | SMG_PD_Cb | Ponta Delgada | Candelária | baía | 37°49'15''N, 25°10'04''W | Subtidal |
| 35 | SMG_PD_CPpb | Ponta Delgada | Capelas | porto | baía | 37°50'32''N, 25°18'46''W | Subtidal |
| 36 | SMG_PD_FLem | Ponta Delgada | Fenais da Luz | Entre-marés | 37°49'54''N, 25°22'23''W | Intertidal |
| 37 | SMG_PD_Fb | Ponta Delgada | Ferraria | baía | 37°51'26''N, 25°08'51''W | Subtidal |
| 38 | SMG_PD_Fem | Ponta Delgada | Ferraria | Entre-marés | 37°51'30''N, 25°08'50''W | Intertidal |
| 39 | SMG_PD_Ftb | Ponta Delgada | Feteiras | baía | 37°46'57''N, 25°13'24''W | Subtidal |
| 40 | SMG_PD_Ftem | Ponta Delgada | Feteiras | Entre-marés | 37°48'13''N, 25°11'47''W | Intertidal |
| 41 | SMG_PD_Ftpfgs | Ponta Delgada | Feteiras | Ponta da Fonte Grande | Subtidal | 37°46'12''N, 25°13'48''W | Subtidal |
| 42 | SMG_PD_Ftpem | Ponta Delgada | Feteiras | Porto | Entre-marés | 37°48'12''N, 25°11'49''W | Intertidal |
| 43 | SMG_PD_Mib | Ponta Delgada | Mosteiros | Ilhéus | baía | 37°53'20''N, 25°10'00''W | Subtidal |
| 44 | SMG_PD_Mpinb | Ponta Delgada | Mosteiros | piscinas naturais | baía | 37°54'02''N, 25°10'31''W | Subtidal |
| 45 | SMG_PD_Mpinemb | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Blocos | 37°53'56''N, 25°10'58''W | Intertidal |
| 46 | SMG_PD_Mpinemc1 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Calhau 1 | 37°53'58''N, 25°10'45''W | Intertidal |
| 47 | SMG_PD_Mpinemc2 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Calhau 2 | 37°53'57''N, 25°10'56''W | Intertidal |
| 48 | SMG_PD_Mpineme1 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Escoada 1 | 37°54'01''N, 25°10'50''W | Intertidal |
| 49 | SMG_PD_Mpineme2 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Escoada 2 | 37°53'59''N, 25°10'44''W | Intertidal |
| 50 | SMG_PD_Mpineme3 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Escoada 3 | 37°53'59''N, 25°10'46''W | Intertidal |
| 51 | SMG_PD_Mpineme4 | Ponta Delgada | Mosteiros | piscinas naturais | Entre-marés | Escoada 4 | 37°54'02''N, 25°10'54''W | Intertidal |
| 52 | SMG_PD_Mpinpo | Ponta Delgada | Mosteiros | piscinas naturais | Poças | 37°54'01''N, 25°10'48''W | Intertidal |
| 53 | SMG_PD_Mpem | Ponta Delgada | Mosteiros | porto | Entre-marés | 37°53'33''N, 25°10'42''W | Intertidal |
| 54 | SMG_PD_Mpremw | Ponta Delgada | Mosteiros | Praia | Entre-marés (W) | 37°53'20''N, 25°10'27''W | Intertidal |
| 55 | SMG_PD_Pdcacb | Ponta Delgada | Ponta Delgada | Calheta | atrás da cadeia | baía | 37°44'30''N, 25°20'46''W | Subtidal |
| 56 | SMG_PD_PDeb | Ponta Delgada | Ponta Delgada | Etar | baía | 37°44'27''N, 25°21'02''W | Subtidal |
| 57 | SMG_PD_PDeem | Ponta Delgada | Ponta Delgada | Etar | Entre-marés | 37°44'29''N, 25°21'02''W | Intertidal |
| 58 | SMG_PD_PDeem | Ponta Delgada | Ponta Delgada | Etar | Entre-marés | 37°44'29''N, 25°21'02''W | Subtidal |
| 59 | SMG_PD_PDmle | Ponta Delgada | Ponta Delgada | marina | lado externo | 37°44'22''N, 25°20'35''W | Subtidal |
| 60 | SMG_PD_Pdmleem | Ponta Delgada | Ponta Delgada | marina | lado externo | Entre-marés | 37°44'22''N, 25°20'29''W | Intertidal |
| 61 | SMG_PD_PDmli | Ponta Delgada | Ponta Delgada | marina | lado interno | 37°44'26''N, 25°20'29''W | Subtidal |
| 62 | SMG_PD_PDmn | Ponta Delgada | Ponta Delgada | marina nova | 37°44'20''N, 25°20'05''W | Intertidal |
| 63 | SMG_PD_PDmn | Ponta Delgada | Ponta Delgada | marina nova | 37°44'20''N, 25°20'05''W | Subtidal |
| 64 | SMG_PD_PDpe | Ponta Delgada | Ponta Delgada | pesqueiro | 37°44'21''N, 25°20'18''W | Subtidal |
| 65 | SMG_PD_PDple | Ponta Delgada | Ponta Delgada | porto | lado externo | 37°44'06''N, 25°20'28''W | Subtidal |
| 66 | SMG_PD_PDpli | Ponta Delgada | Ponta Delgada | porto | lado interno | 37°44'11''N, 25°20'32''W | Subtidal |
| 67 | SMG_PD_PDscb | Ponta Delgada | Ponta Delgada | Santa Clara | baía | 37°43'53''N, 25°18'59''W | Subtidal |
| 68 | SMG_PD_PDscfem | Ponta Delgada | Ponta Delgada | Santa Clara | Farol | Entre-marés | 37°43'58''N, 25°18'46''W | Intertidal |
| 69 | SMG_PD_PDscpoem | Ponta Delgada | Ponta Delgada | Santa Clara | pontão | Entre-marés | 37°43'58''N, 25°18'45''W | Intertidal |
| 70 | SMG_PD_Popmem | Ponta Delgada | Pópulo | Praia das milícias | Entre-marés | 37°44'58''N, 25°22'38''W | Intertidal |
| 71 | SMG_PD_Poppem | Ponta Delgada | Pópulo | Praia pequena | Entre-marés | 37°44'56''N, 25°22'59''W | Intertidal |
| 72 | SMG_PD_Rb | Ponta Delgada | Relva | baía | 37°45'57''N, 25°15'21''W | Subtidal |
| 73 | SMG_R_bes | Ponta Delgada | Relva | Baixa do Espelho | Subtidal | 37°45'41''N, 25°15'29''W | Subtidal |
| 74 | SMG_PD_SAACem | Ponta Delgada | Santo António Além Capelas | Entre-marés | 37°51'52''N, 25°18'00''W | Intertidal |
| 75 | SMG_PD_SRd | Ponta Delgada | São Roque | Dori | 37°44'42''N, 25°22'22''W | Subtidal |
| 76 | SMG_PD_SRfcem | Ponta Delgada | São Roque | Forno da Cal | Entre-marés | 37°44'39''N, 25°21'34''W | Intertidal |
| 77 | SMG_PD_SRpb | Ponta Delgada | São Roque | Pranchinha | baía | 37°44'37''N, 25°21'08''W | Subtidal |
| 78 | SMG_PD_SRpem | Ponta Delgada | São Roque | Pranchinha | Entre-marés | 37°44'38''N, 25°21'07''W | Intertidal |
| 79 | SMG_PD_SRrcb | Ponta Delgada | São Roque | Rosto do Cão | baía | 37°44'36''N, 25°21'39''W | Subtidal |
| 80 | SMG_PD_SRrcem1 | Ponta Delgada | São Roque | Rosto do Cão | Entre-marés 1 | 37°44'40''N, 25°21'37''W | Intertidal |
| 81 | SMG_PD_SRrcem2 | Ponta Delgada | São Roque | Rosto do Cão | Entre-marés 2 | 37°44'38''N, 25°21'40''W | Intertidal |
| 82 | SMG_PD_SRrcem3 | Ponta Delgada | São Roque | Rosto do Cão | Entre-marés 3 | 37°44'40''N, 25°21'39''W | Intertidal |
| 83 | SMG_PD_SVpobe | Ponta Delgada | São Vicente | Poços | baía (E) | 37°50'06''N, 25°20'04''W | Subtidal |
| 84 | SMG_PD_SVpobw | Ponta Delgada | São Vicente | Poços | baía (W) | 37°50'04''N, 25°19'55''W | Subtidal |
| 85 | SMG_PD_SVpoeme | Ponta Delgada | São Vicente | Poços | Entre-marés (E) | 37°50'02''N, 25°19'53''W | Intertidal |
| 86 | SMG_PD_SVpoemw | Ponta Delgada | São Vicente | Poços | Entre-marés (W) | 37°50'03''N, 25°19'53''W | Intertidal |
| 87 | SMG_PD_SVpoi | Ponta Delgada | São Vicente | Poços | ilhéu | 37°50'03''N, 25°20'02''W | Subtidal |
| 88 | SMG_P_bls | Povoação | Baixa da Lobeira | Subtidal | 37°43'14''N, 25°40'52''W | Subtidal |
| 89 | SMG_P_brqs | Povoação | Baixa da Ribeira Quente | Subtidal | 37°43'26''N, 25°41'22''W | subtidal |
| 90 | SMG_P_FTem | Povoação | Faial da Terra | Entre-marés | 37°44'20''N, 25°48'12''W | Intertidal |
| 91 | SMG_P_RQbf | Povoação | Ribeira Quente | baía | Fumarolas | 37°43'36''N, 25°41'22''W | Subtidal |
| 92 | SMG_P_RQbr | Povoação | Ribeira Quente | Baixa da Ribeira | 37°43'59''N, 25°42'16''W | Subtidal |
| 93 | SMG_P_RQborb | Povoação | Ribeira Quente | Boca da Ribeira | baía | 37°43'59''N, 25°42'23''W | Subtidal |
| 94 | SMG_P_RQrcem | Povoação | Ribeira Quente | Rua do Castelo | Entre-marés | 37°43'43''N, 25°41'32''W | Intertidal |
| 95 | SMG_RG_apgrpcpm21s | Ribeira Grande | Área Protegida de Gestão de Recursos da Ponta do Cintrão – Ponta da Maia (SMG21) | Subtidal | 37°50'34''N, 25°30'51''W | Subtidal |
| 96 | SMG_RG_FAem | Ribeira Grande | Fenais da Ajuda | Entre-marés | 37°51'56''N, 25°41'01''W | Intertidal |
| 97 | SMG_RG_Mamem | Ribeira Grande | Maia | Alameda do Mar | Entre-marés | 37°50'03''N, 25°36'17''W | Intertidal |
| 98 | SMG_RG_M_cms | Ribeira Grande | Maia | Cabecinhos da Maia | Subtidal | 37°50'44''N, 25°36'00''W | Subtidal |
| 99 | SMG_RG_Mfmb | Ribeira Grande | Maia | Frade da Maia | baía | 37°50'17''N, 25°36'07''W | Subtidal |
| 100 | SMG_RG_Mlmb | Ribeira Grande | Maia | Lomba da Maia | baía | 37°50'56''N, 25°38'43''W | Subtidal |
| 101 | SMG_RG_Mpinpb | Ribeira Grande | Maia | Piscinas naturais | pontas | baía | 37°50'08''N, 25°36'20''W | Subtidal |
| 102 | SMG_RG_Mpinpem | Ribeira Grande | Maia | Piscinas naturais | pontas | Entre-marés | 37°50'10''N, 25°36'23''W | Intertidal |
| 103 | SMG_RG_Mpem | Ribeira Grande | Maia | porto | Entre-marés | 37°50'02''N, 25°36'47''W | Intertidal |
| 104 | SMG_RG_PFbE | Ribeira Grande | Porto Formoso | baía E | 37°50'00''N, 25°33'58''W | Subtidal |
| 105 | SMG_RG_PFpb | Ribeira Grande | Porto Formoso | porto | baía | 37°49'29''N, 25°34'22''W | Subtidal |
| 106 | SMG_RG_PFpem | Ribeira Grande | Porto Formoso | porto | Entre-marés | 37°49'27''N, 25°34'27''W | Intertidal |
| 107 | SMG_RG_Pfprem | Ribeira Grande | Porto Formoso | praia | Entre-marés | 37°49'25''N, 25°33'25''W | Intertidal |
| 108 | SMG_RG_PFpmb | Ribeira Grande | Porto Formoso | Praia dos moinhos | baía | 37°50'13''N, 25°33'05''W | Subtidal |
| 109 | SMG_RG_PFsbb | Ribeira Grande | Porto Formoso | São Brás | baía | 37°49'46''N, 25°35'08''W | Subtidal |
| 110 | SMG_RG_Pfztem | Ribeira Grande | Porto Formoso | Zona termal | Entre-marés | 37°49'36''N, 25°31'38''W | Intertidal |
| 111 | SMG_RG_RPcem | Ribeira Grande | Rabo de Peixe | Calhetas | Entre-marés | 37°49'28''N, 25°23'42''W | Intertidal |
| 112 | SMG_RG_RGbem | Ribeira Grande | Ribeira Grande | Bandejo | Entre-marés | 37°49'17''N, 25°27'51''W | Intertidal |
| 113 | SMG_RG_Rfpb | Ribeira Grande | Ribeirinha | Furna da Pataca | baía | 37°50'35''N, 25°30'52''W | Subtidal |
| 114 | SMG_RG_Rpcb | Ribeira Grande | Ribeirinha | Ponta do Cintrão | baía | 37°50'11''N, 25°31'20''W | Subtidal |
| 115 | SMG_RG_Rpsib | Ribeira Grande | Ribeirinha | Porto de Santa Iria | baía | 37°50'08''N, 25°30'59''W | Subtidal |
| 116 | SMG_RG_RpsibW | Ribeira Grande | Ribeirinha | Porto de Santa Iria | baía W | 37°50'34''N, 25°30'06''W | Subtidal |
| 117 | SMG_RG_Rpsiem | Ribeira Grande | Ribeirinha | Porto de Santa Iria | Entre-marés | 37°50'08''N, 25°30'56''W | Intertidal |
| 118 | SMG_PD_SVpoemw | Ribeira Grande | São Vicente | Poços | Entre-marés (W) | 37°50'03''N, 25°19'53''W | Intertidal |
| 119 | SMG_VF_AA_bgs | Vila Franca do Campo | Água de Alto | Baixa da Garoupa | Subtidal | 37°42'31''N, 25°31'47''W | Subtidal |
| 120 | SMG_VF_AAbr | Vila Franca do Campo | Água de Alto | Boca da Ribeira | 37°42'56''N, 25°32'48''W | Intertidal |
| 121 | SMA_VF_AAtm | Vila Franca do Campo | Água de Alto | Três Marias | 37°42'29''N, 25°31'56''W | Subtidal |
| 122 | SMG_VF_PGb | Vila Franca do Campo | Ponta Garça | baía | 37°42'56''N, 25°36'41''W | Intertidal |
| 123 | SMG_VF_PGb | Vila Franca do Campo | Ponta Garça | baía | 37°42'56''N, 25°36'41''W | Subtidal |
| 124 | SMG_VF_PGbE | Vila Franca do Campo | Ponta Garça | baía E | 37°42'46''N, 25°37'36''W | Subtidal |
| 125 | SMG_VF_PGem1 | Vila Franca do Campo | Ponta Garça | Entre-marés 1 | 37°42'58''N, 25°36'48''W | Intertidal |
| 126 | SMG_VF_PGem2 | Vila Franca do Campo | Ponta Garça | Entre-marés 2 | 37°42'59''N, 25°36'41''W | Intertidal |
| 127 | SMG_VF_RPem | Vila Franca do Campo | Ribeira da Praia | Entre-marés | 37°42'54''N, 25°34'43''W | Intertidal |
| 128 | SMG_VF_RTem | Vila Franca do Campo | Ribeira das Tainhas | Entre-marés | 37°42'55''N, 25°35'52''W | Intertidal |
| 129 | SMG_VF_VFile | Vila Franca do Campo | Vila Franca do Campo | Ilhéu | lado externo | 37°42'14''N, 25°33'27''W | Subtidal |
| 130 | SMG_VF_VFilep | Vila Franca do Campo | Vila Franca do Campo | Ilhéu | lado externo | picos | 37°42'00''N, 25°33'22''W | Subtidal |
| 131 | SMG_VF_VFileE | Vila Franca do Campo | Vila Franca do Campo | Ilhéu | lado externo E | 37°42'18''N, 25°33'32''W | Subtidal |
| 132 | SMG_VF_VFileS | Vila Franca do Campo | Vila Franca do Campo | Ilhéu | lado externo S | 37°42'14''N, 25°33'20''W | Subtidal |
| 133 | SMG_VF_VFileSW | Vila Franca do Campo | Vila Franca do Campo | Ilhéu | lado externo SW | 37°42'15''N, 25°33'19''W | Subtidal |
| 134 | SMG_VF_VFmem | Vila Franca do Campo | Vila Franca do Campo | marina | Entre-marés | 37°42'54''N, 25°34'16''W | Intertidal |
| 135 | SMG_VF_VFmli | Vila Franca do Campo | Vila Franca do Campo | marina | lado interno | 37°42'50''N, 25°34'12''W | Subtidal |
| 136 | SMG_VF_VFpbw | Vila Franca do Campo | Vila Franca do Campo | porto | baía (W) | 37°42'45''N, 25°33'53''W | Subtidal |
| 137 | SMG_VF_VFpemw | Vila Franca do Campo | Vila Franca do Campo | porto | Entre-marés (W) | 37°42'50''N, 25°33'58''W | Intertidal |
| 138 | SMG_VF_VFpem | Vila Franca do Campo | Vila Franca do Campo | praia | Entre-marés | 37°42'59''N, 25°34'22''W | Intertidal |
Sampling description
Sampling involved species presence recording and/or specimen collecting at each sampling location. Species recording data were gathered by registering all species present in the sampled locations (Fig. 14). Destructive samples were obtained by scraping and/or manually collecting one or two specimens of every species found (Fig. 15). Intertidal collections were made during low tide by walking over the shores. Subtidal collections were made by SCUBA diving.
Figure 14.
Quantitative recording of the presence and coverage of macroalgal species at the intertidal rocky habitat (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Figure 15.
Collecting macroalgae in the subtidal of São Miguel Island (by the Island Aquatic Ecology Subgroup of cE3c-ABG).
Quality control
Each specimen collected was identified by trained taxonomists and involved morphological and anatomical observations of whole specimens by eye and/or of histological preparations under the microscope to determine the main diagnostic features of each species as described in literature.
Step description
At the laboratory, specimen sorting and macroalgae identification followed standard procedures. A combination of morphological and anatomical characters and reproductive structures was used for species identification. For small and simple thalli, this required the observation of the entire thallus with the naked eye and/or using dissecting and compound microscopes. For larger and more complex algae, investigation of the thallus anatomy required histological preparations (longitudinal and transverse sections) or squashed preparations of mucilaginous thalli, sometimes after staining, to observe vegetative and reproductive structures and other diagnostic features.
The Azorean algal flora has components from several geographical regions, which implies difficulties in species identification. Floras and keys for the North Atlantic, Tropical Atlantic and Western Mediterranean were, therefore, used (e.g. Schmidt 1931, Taylor 1967, Taylor 1978, Levring 1974, Dixon and Irvine 1977, Lawson and John 1982, Irvine 1983, Gayral and Cosson 1986, Fletcher 1987, Afonso-Carrillo and Sansón 1989, Burrows 1991, Boudouresque et al. 1992, Cabioc'h et al. 1992, Maggs and Hommersand 1993, Irvine and Chamberlain 1994, Brodie et al. 2007, Lloréns et al. 2012, Rodríguez-Prieto et al. 2013). For more critical and taxonomically-difficult taxa, specimens were taken to the Natural History Museum (London) for comparison with collections there.
A reference collection was made for all collected specimens by assigning them a herbarium code number and depositing them at the AZB Herbarium Ruy Telles Palhinha and the LSM - Molecular Systematics Laboratory, University of Azores. Depending on the species and on planned further research, different methods of preservation were used, namely (i) wet collections using 5% buffered formaldehyde seawater and then replacing it by the fixing agent Kew (Bridsen and Forman 1999); (ii) dried collections, either by pressing the algae (most species) as described by Gayral and Cosson (1986) or by letting them air dry (calcareous species); and (iii) silica gel collections for molecular studies.
Nomenclatural and taxonomic status used here follow Algaebase (Guiry and Guiry 2021). The database was organised on FileMaker Pro.
Geographic coverage
Description
São Miguel Island Description: Azores, Portugal (approximately 37°54'58''N, 25°51'52''W).
Coordinates
37°42'45''N and 37°54'57''N Latitude; 25°52'10''W and 25°08'06''W Longitude.
Taxonomic coverage
Description
All macroalgae were identified to genus or species level. In total, 431 taxa were identified belonging to 36 orders and 83 families, distributed amongst the phyla Rhodophyta (20 orders and 50 families), Chlorophyta (5 orders and 14 families) and Ochrophyta (11 orders and 19 families).
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| phylum | Rhodophyta | Red algae |
| phylum | Chlorophyta | Green algae |
| phylum | Ochrophyta | Brown algae |
Temporal coverage
Notes
The sampling was performed on several occasions in the period between 1989 and 2019.
Collection data
Collection name
AZB | Marine macroalgae collection of São Miguel Island (Azores)-Campaign Macaronesia 2000; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Occasional sampling; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project ABLA/MAC; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project ASPAZOR; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project Azorean Algal Flora; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project BIA (Biodiversity of Azores Archipelago); AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project BIOTOPE; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project BUS; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project ECOSUBVEG; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project GESMAR; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project LAUMACAT; AZB | Marine macroalgae collection of São Miguel Island (Azores)-Project PATELGENE; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Nordeste Expedition; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Occasional sampling; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Postdoc Manuela I Parente; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Project LusoMarBol; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Sabrina Expedition; LSM | Marine macroalgae collection of São Miguel Islands (Azores)-Master Project Artur Oliveira; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Occasional sampling; LSM | Marine macroalgae collection of São Miguel Island (Azores)-Project MACROBIOMOL; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project BIA (Biodiversity of Azores Archipelago); AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project BIOTOPE; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project BUS; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project GESMAR; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Occasional sampling; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project Azorean Algal Flora; AZB | Marine macroalgae occurrence of São Miguel Island (Azores)-Project CAMAG-ORI-SMG; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-Occasional sampling; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-Campaign Waitt Foundation/2016; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-Campaign Waitt Foundation-BALA/PIMA/2016; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-PhD Andrea Z Botelho; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-PIMA/2016; LSM | Marine macroalgae occurrence of São Miguel Island (Azores)-PIMA/BALA/2019
Collection identifier
3cee8546-66d5-49c1-b63f-8efd4227ccc9; 5f198d55-a6ad-42f8-9342-3d96513fe808; 15c76196-2b68-40cf-b49a-9392237f8d4d; c063f27f-5530-4e08-b50a-03843d61fb77; 996ba65a-d07a-4f4f-a17f-511a40983710; 445ca45c-3ba5-4a95-9289-39b944d6894e; 90fb970e-9be1-4caf-89ea-d3823235908a; 21215ae4-0e1f-44f0-95a8-dbed8022cfb0; 3dee61eb-0b56-4794-9d4e-75f2dce26918; 2840e5e1-4353-40f1-81c7-9c29ade05d2c; 7e12ab6e-a568-48aa-8ec3-0697ca734dac; ee3acc7e-abcf-4dec-9c08-961e15d4c029; c4d4cb43-19d4-4633-b252-0e73e6600800; 1fbe2045-3ccb-4e38-9058-d7927e76db62; 22941d45-0678-49fb-bdfe-8b0052ceb298; ef2b1875-8520-4520-b6e0-48a1039f9b1f; 64a1cad8-6242-4075-b762-64b84350864c; 4a97a5db-4970-4f54-b598-d1125a6b8c63; 494c9846-f867-4203-b476-42eb0789ca39; 54b8c165-ab17-4489-9382-e19f7a6af090; 3353c5f1-a12d-4c21-a0a2-c6c080d5d9fc; 2d7e1c20-23be-4e81-b349-4dba060ef8de; 5b048916-da07-4366-97f6-31741d804e51; 7a3f16c8-fb0e-4118-bf69-54dd20024146; 15799ce3-106e-48ff-933d-3d6a0a5b079d; bd4dddc7-c708-4b0d-b912-e9a0a14a87f8; f9a307a6-1137-4265-baae-85924ef72ae7; b252cbd3-385a-4808-bd39-05add8d8eca0; 5efb9cd5-d89c-4ee9-b29d-9c4ce401e186; fd958ad3-143a-4be7-bc89-0b58879c36ef; 52e0ab57-3fe9-436e-90d8-5ea2e1f4899e; 57fc6968-c1f3-4802-9c73-0609a61b8b10; f5b2f2cc-58c8-4bdf-9ab3-1fe3e5feea65
Parent collection identifier
AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; LSM - Molecular Systematics Laboratory, Faculty of Sciences and Technology of the University of the Azores; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable; Not applicable
Specimen preservation method
Air dry, Dried and pressed; Wet (Formalin; fixing agent Kew), Silica gel
Curatorial unit
AZB Herbarium Ruy Telles Palhinha, Faculty of Sciences and Technology of the University of the Azores
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
Data resources
Data package title
Marine algal flora of São Miguel Island, Azores
Resource link
http://ipt.gbif.pt/ipt/resource?r=sao_miguel_macroalgal_flora
Alternative identifiers
https://www.gbif.org/dataset/322b5629-997c-4986-ada9-7d9d078d8648; https://doi.org/10.15468/xtuzd3
Number of data sets
1
Data set 1.
Data set name
Marine algal flora of São Miguel Island, Azores
Data format
Darwin Core Archive
Number of columns
50
Download URL
http://ipt.gbif.pt/ipt/resource?r=sao_miguel_macroalgal_flora&v=1.0
Data format version
1.3
Description
This data paper presents physical and occurrence data from macroalgal surveys undertaken on São Miguel Island between 1989 and 2019 (Neto et al. 2021b). The dataset submitted to GBIF is structured as a sample event dataset, with two tables: event (as core) and occurrences. The data in this sampling event resource have been published as a Darwin Core Archive (DwCA), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data table contains 506 records (eventID). The extension data table has 12,781 occurrences. An extension record supplies extra information about a core record. The number of records in each extension data table is illustrated in the IPT link. This IPT archives the data and thus serves as the data repository. The data and resource metadata are available for downloading in the downloads section.
Data set 1.
| Column label | Column description |
|---|---|
| eventID | Identifier of the event, unique for the dataset |
| country | Country of the sampling site |
| countryCode | Code of the country where the event occurred |
| stateProvince | Name of the region |
| island | Name of the island |
| municipality | Name of the municipality |
| locality | Name of the locality |
| locationID | Identifier of the location |
| decimalLatitude | The geographic latitude of the sampling site |
| decimalLongitude | The geographic longitude of the sampling site |
| geodeticDatum | The spatial reference system upon which the geographic coordinates are based |
| coordinateUncertaintyInMetres | The horizontal distance (in metres) from the given decimalLatitude and decimalLongitude describing the smallest circle containing the whole of the Location |
| eventDate | Time interval when the event occurred |
| year | The year of the event |
| samplingProtocol | Sampling method used during an event |
| locationRemarks | Zonation level |
| minimumDepthInMetres | The minimum depth in metres where the specimen was found |
| maximumDepthInMetres | The maximum depth in metres where the specimen was found |
| eventRemarks | Notes about the event |
| occurrenceID | Identifier of the record, coded as a global unique identifier |
| institutionID | The identifier for the institution having custody of the object or information referred to in the record |
| institutionCode | The acronym of the institution having custody of the object or information referred to in the record |
| collectionID | An identifier of the collection to which the record belongs |
| collectionCode | The name of the collection from which the record was derived |
| datasetName | The name identifying the dataset from which the record was derived |
| kingdom | Kingdom name |
| phylum | Phylum name |
| class | Class name |
| order | Order name |
| family | Family name |
| genus | Genus name |
| specificEpithet | The name of the first or species epithet of the scientificName |
| infraspecificEpithet | The name of the lowest or terminal infraspecific epithet of the scientificName, excluding any rank designation |
| acceptedNameUsage | The specimen accepted name, with authorship |
| previousIdentifications | Previous name of the specimen, with authorship |
| scientificName | The name without authorship applied on the first identification of the specimen |
| scientificNameAuthorship | The authorship information for the scientificName formatted according to the conventions of the applicable nomenclaturalCode |
| taxonRank | The taxonomic rank of the most specific name in the scientificName |
| basisOfRecord | The specific nature of the data record |
| habitat | Description of the habitat where the specimen was found |
| organismQuantityType | The type of quantification system used to quantify the organisms |
| organismQuantity | Percentage of the organism coverage |
| recordedBy | Person(s) responsible for sampling |
| catalogNumber | Identifying code for a unique sample lot in a biological collection |
| identifiedBy | Person(s) responsible for taxa identification |
| type | The nature of the resource |
| preparations | The preservation method used for the specimen |
| establishmentMeans | The establishment status of the organism in the study region |
| occurrenceRemarks | New record status assignment |
| licence | Reference to the licence under which the record is published |
Additional information
This paper accommodates the 12,781 specimens of macroalgae recorded from São Miguel Island in 431 taxa comprising 323 confirmed species (Tables 2, 3) and 108 taxa identified only to genus level. The confirmed species (Table 3) include 212 Rhodophyta, 48 Chlorophyta and 63 Ochrophyta (Phaeophyceae). Of these, 61 species are newly recorded to the Island (42 Rhodophyta, 9 Chlorophyta and 10 Ochrophyta). Most species are native, Predaea feldmannii subsp. azorica is an Azorean endemic, whereas the rhodophyta Botryocladia macaronesica, Laurencia viridis, Millerella tinerfensis, Phyllophora gelidioides and the Chlorophyta Codium elisabethiae are Macaronesian endemics. Nineteen species represent introductions to the algal flora (the Rhodophyta Antithamnion diminuatum Wollaston, Antithamnion hubbsii E.Y.Dawson, Antithamnionella spirographidis (Schiffner) E.M.Wollaston, Antithamnionella ternifolia (J.D.Hooker & Harvey) Lyle, Asparagopsis armata, Grallatoria reptans M.Howe, Gymnophycus hapsiphorus Huisman & Kraft, Laurencia brongniartii J.Agardh, Laurencia dendroidea J.Agardh, Neoizziella divaricata (C.K.Tseng) S.-M.Lin, S.-Y.Yang & Huisman, Scageliopsis patens E.M.Wollaston, Symphyocladia marchantioides, Xiphosiphonia pennata (C.Agardh) Savoie & G.W.Saunders and Xiphosiphonia pinnulata (Kützing) Savoie & G.W.Saunders; the Chlorophyta Caulerpa prolifera (Forsskål) J.V.Lamouroux and Codium fragile subsp. fragile (Suringar) Hariot; and the Ochrophyta Papenfussiella kuromo (Yendo) Inagaki and Petalonia binghamiae (J.Agardh) K.L.Vinogradova. Thirty-two species have an uncertain status (21 Rhodophyta, 5 Chlorophyta and 6 Ochrophyta).
Table 2.
Macroalgal species recorded from São Miguel Island, with information on relative abundance, origin and status.
| Phylum | Species (Accepted Name) | Number of records | Establishment means | Occurrence remarks |
| Rhodophyta | Acrosorium ciliolatum (Harvey) Kylin | 826 | Native | |
| Rhodophyta | Agardhinula browneae (J.Agardh) De Toni | 1 | Native | |
| Rhodophyta | Aglaothamnion bipinnatum (P.Crouan & H.Crouan) Feldmann & G. Feldmann | 24 | Native | New record |
| Rhodophyta | Aglaothamnion pseudobyssoides (P.Crouan & H.Crouan) Halos | 1 | Native | |
| Rhodophyta | Aglaothamnion tenuissimum (Bonnemaison) Feldmann-Mazoyer | 14 | Uncertain | |
| Rhodophyta | Ahnfeltiopsis devoniensis (Greville) P.C.Silva & DeCew | 5 | Native | |
| Rhodophyta | Amphiroa beauvoisii J.V.Lamouroux | 12 | Native | |
| Rhodophyta | Amphiroa cryptarthrodia Zanardini | 4 | Native | |
| Rhodophyta | Amphiroa rigida J.V.Lamouroux | 3 | Native | |
| Rhodophyta | Anotrichium barbatum (C.Agardh) Nägeli | 8 | Native | |
| Rhodophyta | Anotrichium furcellatum (J.Agardh) Baldock | 14 | Uncertain | |
| Rhodophyta | Anotrichium tenue (C.Agardh) Nägeli | 1 | Native | |
| Rhodophyta | Antithamnion cruciatum (C.Agardh) Nägeli | 11 | Native | New record |
| Rhodophyta | Antithamnion decipiens (J.Agardh) Athanasiadis | 1 | Native | |
| Rhodophyta | Antithamnion diminuatum Wollaston | 6 | Introduced | |
| Rhodophyta | Antithamnion hubbsii E.Y.Dawson | 7 | Introduced | New record |
| Rhodophyta | Antithamnionella boergesenii (Cormaci & G.Furnari) Athanasiadis | 1 | Uncertain | |
| Rhodophyta | Antithamnionella floccosa (O.F.Müller) Whittick | 1 | Native | New record |
| Rhodophyta | Antithamnionella spirographidis (Schiffner) E.M.Wollaston | 1 | Introduced | New record |
| Rhodophyta | Antithamnionella ternifolia (J.D.Hooker & Harvey) Lyle | 2 | Introduced | |
| Rhodophyta | Aphanocladia stichidiosa (Funk) Ardré | 2 | Native | |
| Rhodophyta | Apoglossum ruscifolium (Turner) J.Agardh | 2 | Native | New record |
| Rhodophyta | Asparagopsis armata Harvey | 188 | Introduced | |
| Rhodophyta | Asparagopsis armata Harvey, phase Falkenbergia rufolanosa (Harvey) F.Schmitz | 34 | Introduced | |
| Rhodophyta | Asparagopsis taxiformis (Delile) Trevisan | 154 | Native | |
| Rhodophyta | Asteromenia peltata (W.R.Taylor) Huisman & A.J.K.Millar | 6 | Native | |
| Rhodophyta | Balliella cladoderma (Zanardini) Athanasiadis | 3 | Native | New record |
| Rhodophyta | Bangia atropurpurea (Mertens ex Roth) C.Agardh | 7 | Native | |
| Rhodophyta | Bonnemaisonia asparagoides (Woodward) C.Agardh | 8 | Native | |
| Rhodophyta | Bonnemaisonia hamifera Hariot | 1 | Introduced | |
| Rhodophyta | Bornetia secundiflora (J.Agardh) Thuret | 6 | Native | |
| Rhodophyta | Bostrychia scorpioides (Hudson) Montagne | 1 | Native | New record |
| Rhodophyta | Botryocladia botryoides (Wulfen) Feldmann | 4 | Native | |
| Rhodophyta | Botryocladia macaronesica Afonso-Carillo, Sobrino, Tittley & Neto | 52 | Macaronesian endemism | |
| Rhodophyta | Callithamnion corymbosum (Smith) Lyngbye | 7 | Native | |
| Rhodophyta | Callithamnion granulatum (Ducluzeau) C. Agardh | 38 | Native | |
| Rhodophyta | Callithamnion tetragonum (Withering) S.F.Gray | 15 | Native | New record |
| Rhodophyta | Callithamnion tetricum (Dillwyn) S.F.Gray | 1 | Native | |
| Rhodophyta | Carradoriella denudata (Dillwyn) Savoie & G.W.Saunders | 26 | Uncertain | |
| Rhodophyta | Carradoriella elongata (Hudson) A.M.Savoie & G.W.Saunders | 1 | Native | |
| Rhodophyta | Catenella caespitosa (Withering) L.M.Irvine | 5 | Native | |
| Rhodophyta | Caulacanthus ustulatus (Turner) Kützing | 121 | Uncertain | |
| Rhodophyta | Centroceras clavulatum (C.Agardh) Montagne | 82 | Native | |
| Rhodophyta | Ceramium botryocarpum A.W.Griffiths ex Harvey | 12 | Native | |
| Rhodophyta | Ceramium ciliatum (J.Ellis) Ducluzeau | 25 | Native | |
| Rhodophyta | Ceramium cimbricum H.E.Petersen | 8 | Native | New record |
| Rhodophyta | Ceramium circinatum (Kützing) J.Agardh | 3 | Native | |
| Rhodophyta | Ceramium deslongchampsii Chauvin ex Duby | 6 | Native | |
| Rhodophyta | Ceramium diaphanum (Lightfoot) Roth | 9 | Native | |
| Rhodophyta | Ceramium echionotum J.Agardh | 10 | Native | |
| Rhodophyta | Ceramium pallidum (Kützing) Maggs & Hommersand | 24 | Native | New record |
| Rhodophyta | Ceramium secundatum Lyngbye | 19 | Native | New record |
| Rhodophyta | Ceramium tenuicorne (Kützing) Waern | 11 | Native | New record |
| Rhodophyta | Ceramium virgatum Roth | 23 | Native | |
| Rhodophyta | Ceratodictyon intricatum (C.Agardh) R.E.Norris | 2 | Native | |
| Rhodophyta | Champia parvula (C.Agardh) Harvey | 10 | Native | |
| Rhodophyta | Chondracanthus acicularis (Roth) Fredericq | 203 | Native | |
| Rhodophyta | Chondracanthus teedei (Mertens ex Roth) Kützing | 57 | Native | |
| Rhodophyta | Chondria capillaris (Hudson) M.J.Wynne | 2 | Native | |
| Rhodophyta | Chondria coerulescens (J.Agardh) Sauvageau | 29 | Uncertain | |
| Rhodophyta | Chondria dasyphylla (Woodward) C.Agardh | 22 | Uncertain | |
| Rhodophyta | Coelothrix irregularis (Harvey) Børgesen | 2 | Native | |
| Rhodophyta | Compsothamnion decompositum (J.Agardh) Maggs & L'Hardy-Halos | 2 | Native | |
| Rhodophyta | Corallina ferreyrae E.Y.Dawson, Acleto & Foldvik | 30 | Native | New record |
| Rhodophyta | Corallina officinalis Linnaeus | 140 | Native | |
| Rhodophyta | Cottoniella filamentosa (M.Howe) Børgesen | 15 | Native | New record |
| Rhodophyta | Crouania attenuata (C.Agardh) J.Agardh | 5 | Native | |
| Rhodophyta | Cruoria pellita (Lyngbye) Fries | 2 | Native | New record |
| Rhodophyta | Cryptonemia crenulata (J.Agardh) J.Agardh | 1 | Uncertain | New record |
| Rhodophyta | Cryptonemia seminervis (C.Agardh) J.Agardh | 2 | Native | |
| Rhodophyta | Cryptopleura ramosa (Hudson) L.Newton | 9 | Native | |
| Rhodophyta | Dasya baillouviana (S.G.Gmelin) Montagne | 6 | Uncertain | |
| Rhodophyta | Dasya caraibica Børgesen | 1 | Native | New record |
| Rhodophyta | Dasya corymbifera J.Agardh | 3 | Native | |
| Rhodophyta | Dasya crouaniana J.Agardh | 1 | Native | New record |
| Rhodophyta | Dasya hutchinsiae Harvey | 6 | Native | |
| Rhodophyta | Dasya ocellata (Grateloup) Harvey | 3 | Native | |
| Rhodophyta | Dermocorynus dichotomus (J.Agardh) Gargiulo, M.Morabito & Manghisi | 67 | Native | |
| Rhodophyta | Diplothamnion jolyi C.Hoek | 8 | Native | |
| Rhodophyta | Drachiella heterocarpa (Chauvin ex Duby) Maggs & Hommersand | 5 | Native | New record |
| Rhodophyta | Dudresnaya crassa M.Howe | 7 | Native | |
| Rhodophyta | Dudresnaya verticillata (Withering) Le Jolis | 2 | Native | |
| Rhodophyta | Ellisolandia elongata (J.Ellis & Solander) K.R.Hind & G.W.Saunders | 67 | Native | |
| Rhodophyta | Erythrocystis montagnei (Derbès & Solier) P.C.Silva | 4 | Native | |
| Rhodophyta | Erythrodermis traillii (Holmes ex Batters) Guiry & Garbary | 10 | Uncertain | |
| Rhodophyta | Erythroglossum laciniatum (Lightfoot) Maggs & Hommersand | 1 | Native | New record |
| Rhodophyta | Erythrotrichia carnea (Dillwyn) J.Agardh | 1 | Uncertain | |
| Rhodophyta | Eupogodon planus (C.Agardh) Kützing | 2 | Native | |
| Rhodophyta | Gaillona gallica (Nägeli) Athanasiadis | 19 | Native | |
| Rhodophyta | Gaillona hookeri (Dillwyn) Athanasiadis | 9 | Native | |
| Rhodophyta | Gastroclonium clavatum (Roth) Ardissone | 1 | Native | |
| Rhodophyta | Gastroclonium ovatum (Hudson) Papenfuss | 17 | Native | |
| Rhodophyta | Gastroclonium reflexum (Chauvin) Kützing | 23 | Native | |
| Rhodophyta | Gayliella flaccida (Harvey ex Kützing) T.O.Cho & L.J.McIvor | 8 | Native | |
| Rhodophyta | Gelidium corneum (Hudson) J.V.Lamouroux | 2 | Native | |
| Rhodophyta | Gelidium microdon Kützing | 228 | Native | |
| Rhodophyta | Gelidium pusillum (Stackhouse) Le Jolis | 52 | Native | |
| Rhodophyta | Gelidium spinosum (S.G.Gmelin) P.C.Silva | 150 | Native | |
| Rhodophyta | Gigartina pistillata (S.G.Gmelin) Stackhouse | 20 | Native | |
| Rhodophyta | Gracilaria gracilis (Stackhouse) Steentoft, L.M.Irvine & Farnham | 3 | Native | New record |
| Rhodophyta | Gracilaria multipartita (Clemente) Harvey | 1 | Native | |
| Rhodophyta | Gracilariopsis longissima (S.G.Gmelin) Steentoft, L.M.Irvine & Farnham | 12 | Native | |
| Rhodophyta | Grallatoria reptans M.Howe | 2 | Introduced | |
| Rhodophyta | Grateloupia filicina (J.V.Lamouroux) C.Agardh | 21 | Native | |
| Rhodophyta | Griffithsia corallinoides (Linnaeus) Trevisan | 2 | Native | |
| Rhodophyta | Griffithsia phyllamphora J.Agardh | 2 | Uncertain | |
| Rhodophyta | Gymnogongrus crenulatus (Turner) J.Agardh | 107 | Native | |
| Rhodophyta | Gymnogongrus griffithsiae (Turner) C.Martius | 115 | Native | |
| Rhodophyta | Gymnophycus hapsiphorus Huisman & Kraft | 1 | Introduced | |
| Rhodophyta | Gymnothamnion elegans (Schousboe ex C.Agardh) J.Agardh | 4 | Native | |
| Rhodophyta | Halarachnion ligulatum (Woodward) Kützing | 26 | Native | |
| Rhodophyta | Halurus flosculosus (J.Ellis) Maggs & Hommersand | 4 | Native | |
| Rhodophyta | Haraldia lenormandii (Derbès & Solier) Feldmann | 1 | Native | New record |
| Rhodophyta | Haraldiophyllum bonnemaisonii (Kylin) A.D.Zinova | 17 | Native | New record |
| Rhodophyta | Herposiphonia secunda (C.Agardh) Ambronn | 19 | Native | |
| Rhodophyta | Heterosiphonia crispella (C.Agardh) M.J.Wynne | 5 | Native | |
| Rhodophyta | Hildenbrandia rubra (Sommerfelt) Meneghini | 8 | Native | |
| Rhodophyta | Hypnea arbuscula P.J.L.Dangeard | 1 | Native | |
| Rhodophyta | Hypnea cervicornis J.Agardh | 2 | Native | New record |
| Rhodophyta | Hypnea musciformis (Wulfen) J.V.Lamouroux | 148 | Uncertain | |
| Rhodophyta | Hypoglossum hypoglossoides (Stackhouse) Collins & Hervey | 16 | Native | |
| Rhodophyta | Itonoa marginifera (J.Agardh) Masuda & Guiry | 5 | Native | |
| Rhodophyta | Jania crassa J.V.Lamouroux | 3 | Native | |
| Rhodophyta | Jania longifurca Zanardini | 11 | Uncertain | |
| Rhodophyta | Jania pedunculata var. adhaerens (J.V.Lamouroux) A.S.Harvey, Woelkerling & Reviers | 4 | Native | |
| Rhodophyta | Jania rubens (Linnaeus) J.V.Lamouroux | 14 | Native | |
| Rhodophyta | Jania squamata (Linnaeus) J.H.Kim, Guiry & H.-G.Choi | 2 | Native | |
| Rhodophyta | Jania verrucosa J.V.Lamouroux | 9 | Native | |
| Rhodophyta | Jania virgata (Zanardini) Montagne | 9 | Uncertain | |
| Rhodophyta | Kallymenia reniformis (Turner) J.Agardh | 37 | Native | |
| Rhodophyta | Laurencia brongniartii J.Agardh | 2 | Introduced | New record |
| Rhodophyta | Laurencia dendroidea J.Agardh | 1 | Introduced | |
| Rhodophyta | Laurencia intricata J.V.Lamouroux | 2 | Native | New record |
| Rhodophyta | Laurencia microcladia Kützing | 1 | Native | New record |
| Rhodophyta | Laurencia obtusa (Hudson) J.V.Lamouroux | 23 | Native | |
| Rhodophyta | Laurencia pyramidalis Bory ex Kützing | 8 | Native | |
| Rhodophyta | Laurencia tenera C.K.Tseng | 1 | Native | |
| Rhodophyta | Laurencia viridis Gil-Rodríguez & Haroun | 21 | Macaronesian endemism | |
| Rhodophyta | Laurenciella marilzae (Gil-Rodríguez, Sentíes, Díaz-Larrea, Cassano & M.T.Fujii) Gil-Rodríguez, Sentíes, Díaz-Larrea, Cassano & M.T.Fujii | 6 | Native | New record |
| Rhodophyta | Leptosiphonia brodiei (Dillwyn) Savoie & G.W.Saunders | 14 | Uncertain | |
| Rhodophyta | Liagora distenta (Mertens ex Roth) J.V.Lamouroux | 21 | Native | New record |
| Rhodophyta | Liagora viscida (Forsskål) C.Agardh | 11 | Native | |
| Rhodophyta | Lomentaria articulata (Hudson) Lyngbye | 128 | Native | |
| Rhodophyta | Meredithia microphylla (J.Agardh) J.Agardh | 55 | Native | New record |
| Rhodophyta | Millerella tinerfensis (Seoane-Camba) S.M.Boo & J.M.Rico | 4 | Macaronesian endemism | New record |
| Rhodophyta | Monosporus pedicellatus (Smith) Solier | 1 | Native | |
| Rhodophyta | Myriogramme minuta Kylin | 12 | Native | |
| Rhodophyta | Nemalion elminthoides (Velley) Batters | 41 | Native | |
| Rhodophyta | Neoizziella divaricata (C.K.Tseng) S.-M.Lin, S.-Y.Yang & Huisman | 14 | Introduced | |
| Rhodophyta | Neopyropia leucosticta (Thuret) L.-E.Yang & J.Brodie | 1 | Native | New record |
| Rhodophyta | Nitophyllum punctatum (Stackhouse) Greville | 19 | Native | |
| Rhodophyta | Osmundea hybrida (A.P.de Candolle) K.W.Nam | 12 | Native | |
| Rhodophyta | Osmundea oederi (Gunnerus) G.Furnari | 1 | Native | |
| Rhodophyta | Osmundea pinnatifida (Hudson) Stackhouse | 151 | Native | |
| Rhodophyta | Osmundea truncata (Kützing) K.W.Nam & Maggs | 7 | Native | |
| Rhodophyta | Palisada corallopsis (Montagne) Sentíes, Fujii & Díaz-Larrea | 2 | Native | New record |
| Rhodophyta | Peyssonnelia squamaria (S.G.Gmelin) Decaisne ex J.Agardh | 71 | Native | |
| Rhodophyta | Phyllophora crispa (Hudson) P.S.Dixon | 37 | Native | |
| Rhodophyta | Phyllophora gelidioides P.Crouan & H.Crouan ex Karsakoff | 2 | Macaronesian endemism | |
| Rhodophyta | Phyllophora sicula (Kützing) Guiry & L.M.Irvine | 5 | Native | |
| Rhodophyta | Platoma cyclocolpum (Montagne) F.Schmitz | 139 | Native | |
| Rhodophyta | Platysiphonia delicata (Clemente) Cremades | 3 | Native | New record |
| Rhodophyta | Pleonosporium borreri (Smith) Nägeli | 7 | Native | |
| Rhodophyta | Plocamium cartilagineum (Linnaeus) P.S.Dixon | 173 | Native | |
| Rhodophyta | Pneophyllum confervicola (Kützing) Y.M.Chamberlain | 1 | Native | New record |
| Rhodophyta | Polysiphonia atlantica Kapraun & J.N.Norris | 6 | Native | |
| Rhodophyta | Polysiphonia havanensis Montagne | 1 | Native | |
| Rhodophyta | Polysiphonia opaca (C.Agardh) Moris & De Notaris | 1 | Native | |
| Rhodophyta | Polysiphonia stricta (Mertens ex Dillwyn) Greville | 3 | Native | |
| Rhodophyta | Porphyra umbilicalis Kützing | 14 | Native | |
| Rhodophyta | Porphyrostromium ciliare (Carmichael) M.J.Wynne | 3 | Native | |
| Rhodophyta | Predaea feldmannii Børgesen | 3 | Native | New record |
| Rhodophyta | Predaea feldmannii subsp. azorica Gabriel | 4 | Azorean endemism | |
| Rhodophyta | Pterocladiella capillacea (S.G.Gmelin) Santelices & Hommersand | 377 | Native | |
| Rhodophyta | Pterothamnion crispum (Ducluzeau) Nägeli | 54 | Native | |
| Rhodophyta | Pterothamnion plumula (J.Ellis) Nägeli | 6 | Native | |
| Rhodophyta | Ptilothamnion pluma (Dillwyn) Thuret | 4 | Uncertain | |
| Rhodophyta | Radicilingua thysanorhizans (Holmes) Papenfuss | 8 | Native | New record |
| Rhodophyta | Rhodophyllis divaricata (Stackhouse) Papenfuss | 7 | Native | |
| Rhodophyta | Rhodymenia holmesii Ardissone | 92 | Native | |
| Rhodophyta | Rhodymenia pseudopalmata (J.V.Lamouroux) P.C.Silva | 18 | Native | |
| Rhodophyta | Scagelia pylaisaei (Montagne) M.J.Wynne | 4 | Native | |
| Rhodophyta | Scageliopsis patens E.M.Wollaston | 1 | Introduced | |
| Rhodophyta | Schimmelmannia schousboei (J.Agardh) J.Agardh | 29 | Native | New record |
| Rhodophyta | Schizymenia apoda (J.Agardh) J.Agardh | 89 | Native | |
| Rhodophyta | Schottera nicaeensis (J.V.Lamouroux ex Duby) Guiry & Hollenberg | 28 | Uncertain | |
| Rhodophyta | Scinaia furcellata (Turner) J.Agardh | 33 | Native | |
| Rhodophyta | Scinaia interrupta (A.P.de Candolle) M.J.Wynne | 34 | Native | |
| Rhodophyta | Sebdenia dichotoma Berthold | 19 | Native | |
| Rhodophyta | Sebdenia rodrigueziana (Feldmann) Codomier ex Athanasiadis | 41 | Native | |
| Rhodophyta | Spermothamnion repens (Dillwyn) Magnus | 5 | Native | |
| Rhodophyta | Sphaerococcus coronopifolius Stackhouse | 68 | Native | |
| Rhodophyta | Spyridia filamentosa (Wulfen) Harvey | 1 | Native | |
| Rhodophyta | Stichothamnion cymatophilum Børgesen | 1 | Native | |
| Rhodophyta | Stylonema alsidii (Zanardini) K.M.Drew | 2 | Native | |
| Rhodophyta | Stylonema cornu-cervi Reinsch | 3 | Native | |
| Rhodophyta | Symphyocladia marchantioides (Harvey) Falkenberg | 85 | Introduced | |
| Rhodophyta | Taenioma nanum (Kützing) Papenfuss | 1 | Native | New record |
| Rhodophyta | Tenarea tortuosa (Esper) Me.Lemoine | 3 | Native | |
| Rhodophyta | Vertebrata foetidissima (Cocks ex Bornet) Díaz-Tapia & Maggs | 1 | Native | |
| Rhodophyta | Vertebrata fruticulosa (Wulfen) Kuntze | 5 | Native | |
| Rhodophyta | Vertebrata fucoides (Hudson) Kuntze | 7 | Uncertain | |
| Rhodophyta | Vertebrata furcellata (C.Agardh) Kuntze | 6 | Native | |
| Rhodophyta | Vertebrata hypnoides (Welwitsch) Kuntze | 4 | Uncertain | |
| Rhodophyta | Vertebrata nigra (Hudson) Díaz-Tapia & Maggs | 1 | Native | New record |
| Rhodophyta | Vertebrata reptabunda (Suhr) Díaz-Tapia & Maggs | 4 | Uncertain | |
| Rhodophyta | Vertebrata tripinnata (Harvey) Kuntze | 3 | Native | |
| Rhodophyta | Wrangelia penicillata (C.Agardh) C.Agardh | 6 | Native | New record |
| Rhodophyta | Wurdemannia miniata (Sprengel) Feldmann & Hamel | 1 | Native | |
| Rhodophyta | Xiphosiphonia ardreana (Maggs & Hommersand) Savoie & G.W.Saunders | 4 | Native | |
| Rhodophyta | Xiphosiphonia pennata (C.Agardh) Savoie & G.W.Saunders | 2 | Introduced | |
| Rhodophyta | Xiphosiphonia pinnulata (Kützing) Savoie & G.W.Saunders | 2 | Introduced | |
| Chlorophyta | Anadyomene stellata (Wulfen) C.Agardh | 4 | Uncertain | |
| Chlorophyta | Blidingia marginata (J.Agardh) P.J.L.Dangeard ex Bliding | 1 | Native | New record |
| Chlorophyta | Blidingia minima (Nägeli ex Kützing) Kylin | 2 | Native | |
| Chlorophyta | Bryopsis cupressina J.V.Lamouroux | 27 | Native | |
| Chlorophyta | Bryopsis duplex De Notaris | 1 | Native | |
| Chlorophyta | Bryopsis hypnoides J.V.Lamouroux | 28 | Native | |
| Chlorophyta | Bryopsis pennata J.V.Lamouroux | 6 | Native | New record |
| Chlorophyta | Bryopsis plumosa (Hudson) C.Agardh | 71 | Native | |
| Chlorophyta | Caulerpa prolifera (Forsskål) J.V.Lamouroux | 10 | Introduced | |
| Chlorophyta | Chaetomorpha aerea (Dillwyn) Kützing | 10 | Native | |
| Chlorophyta | Chaetomorpha linum (O.F.Müller) Kützing | 26 | Native | |
| Chlorophyta | Chaetomorpha pachynema (Montagne) Kützing | 2 | Native | |
| Chlorophyta | Cladophora albida (Nees) Kutzing | 5 | Native | |
| Chlorophyta | Cladophora coelothrix Kützing | 15 | Native | |
| Chlorophyta | Cladophora conferta P.Crouan & H.Crouan | 5 | Native | |
| Chlorophyta | Cladophora dalmatica Kützing | 1 | Uncertain | |
| Chlorophyta | Cladophora hutchinsiae (Dillwyn) Kützing | 1 | Native | |
| Chlorophyta | Cladophora laetevirens (Dillwyn) Kützing | 7 | Uncertain | |
| Chlorophyta | Cladophora lehmanniana (Lindenberg) Kützing | 5 | Native | |
| Chlorophyta | Cladophora liebetruthii Grunow | 1 | Native | New record |
| Chlorophyta | Cladophora prolifera (Roth) Kützing | 82 | Native | |
| Chlorophyta | Cladophoropsis membranacea (Hofman Bang ex C.Agardh) Børgesen | 1 | Uncertain | |
| Chlorophyta | Codium adhaerens C. Agradh | 115 | Native | |
| Chlorophyta | Codium decorticatum (Woodward) M.Howe | 10 | Native | |
| Chlorophyta | Codium elisabethiae O.C.Schmidt | 84 | Macaronesian endemism | |
| Chlorophyta | Codium fragile (Suringar) Hariot | 9 | Native | |
| Chlorophyta | Codium fragile subsp. atlanticum (A.D.Cotton) P.C.Silva | 3 | Native | New record |
| Chlorophyta | Codium fragile subsp. fragile (Suringar) Hariot | 15 | Introduced | |
| Chlorophyta | Codium tomentosum Stackhouse | 2 | Native | New record |
| Chlorophyta | Codium vermilara (Olivi) Delle Chiaje | 2 | Native | New record |
| Chlorophyta | Gayralia oxysperma (Kützing) K.L.Vinogradova ex Scagel & al. | 5 | Native | |
| Chlorophyta | Lychaete pellucida (Hudson) M.J.Wynne | 3 | Native | |
| Chlorophyta | Microdictyon boergesenii Setchell | 3 | Native | New record |
| Chlorophyta | Microdictyon umbilicatum (Velley) Zanardini | 3 | Native | New record |
| Chlorophyta | Pseudochlorodesmis furcellata (Zanardini) Børgesen | 1 | Native | New record |
| Chlorophyta | Pseudorhizoclonium africanum (Kützing) Boedeker | 5 | Native | |
| Chlorophyta | Ulothrix flacca (Dillwyn) Thuret | 2 | Native | |
| Chlorophyta | Ulva clathrata (Roth) C.Agardh | 12 | Native | |
| Chlorophyta | Ulva compressa Linnaeus | 12 | Native | |
| Chlorophyta | Ulva intestinalis Linnaeus | 37 | Native | |
| Chlorophyta | Ulva lactuca Linnaeus | 2 | Uncertain | |
| Chlorophyta | Ulva linza Linnaeus | 6 | Native | |
| Chlorophyta | Ulva polyclada Kraft | 1 | Native | |
| Chlorophyta | Ulva prolifera O.F.Müller | 6 | Native | |
| Chlorophyta | Ulva ralfsii (Harvey) Le Jolis | 1 | Native | |
| Chlorophyta | Ulva rigida C.Agardh | 198 | Native | |
| Chlorophyta | Valonia macrophysa Kützing | 3 | Native | |
| Chlorophyta | Valonia utricularis (Roth) C. Agardh | 7 | Native | |
| Ochrophyta | Ascophyllum nodosum (Linnaeus) Le Jolis | 8 | Native | |
| Ochrophyta | Bachelotia antillarum (Grunow) Gerloff | 2 | Native | |
| Ochrophyta | Canistrocarpus cervicornis (Kützing) De Paula & De Clerck | 2 | Native | New record |
| Ochrophyta | Carpomitra costata (Stackhouse) Batters | 16 | Native | |
| Ochrophyta | Cladostephus spongiosus (Hudson) C.Agardh | 33 | Native | |
| Ochrophyta | Colpomenia sinuosa (Mertens ex Roth) Derbès & Solier | 472 | Native | |
| Ochrophyta | Compsonema saxicola (Kuckuck) Kuckuck | 28 | Native | |
| Ochrophyta | Cutleria multifida (Turner) Greville | 27 | Uncertain | |
| Ochrophyta | Cutleria multifida (Turner) Greville phase Aglaozonia parvula (Greville) Zanardini | 4 | Uncertain | |
| Ochrophyta | Cystoseira compressa (Esper) Gerloff & Nizamuddin | 81 | Native | |
| Ochrophyta | Cystoseira foeniculacea (Linnaeus) Greville | 46 | Native | |
| Ochrophyta | Cystoseira humilis Schousboe ex Kützing | 21 | Native | |
| Ochrophyta | Dictyopteris polypodioides (A.P.De Candolle) J.V.Lamouroux | 21 | Native | |
| Ochrophyta | Dictyota bartayresiana J.V.Lamouroux | 9 | Native | |
| Ochrophyta | Dictyota ciliolata Sonder ex Kützing | 7 | Native | New record |
| Ochrophyta | Dictyota cyanoloma Tronholm, De Clerck, A.Gómez-Garreta & Rull Lluch | 7 | Native | |
| Ochrophyta | Dictyota dichotoma (Hudson) J.V.Lamouroux | 189 | Native | |
| Ochrophyta | Dictyota dichotoma var. intricata (C.Agardh) Greville | 10 | Native | New record |
| Ochrophyta | Dictyota fasciola (Roth) J.V.Lamouroux | 5 | Native | New record |
| Ochrophyta | Dictyota implexa (Desfontaines) J.V.Lamouroux | 9 | Native | New record |
| Ochrophyta | Ectocarpus fasciculatus Harvey | 7 | Native | |
| Ochrophyta | Ectocarpus siliculosus (Dillwyn) Lyngbye | 6 | Uncertain | |
| Ochrophyta | Elachista flaccida (Dillwyn) Fries | 1 | Native | New record |
| Ochrophyta | Feldmannia irregularis (Kützing) Hamel | 1 | Native | |
| Ochrophyta | Feldmannia mitchelliae (Harvey) H.-S.Kim | 10 | Native | |
| Ochrophyta | Feldmannia paradoxa (Montagne) Hamel | 3 | Native | |
| Ochrophyta | Fucus spiralis Linnaeus | 89 | Uncertain | |
| Ochrophyta | Gongolaria abies-marina (S.G.Gmelin) Kuntze | 265 | Native | |
| Ochrophyta | Halopteris filicina (Grateloup) Kützing | 217 | Native | |
| Ochrophyta | Halopteris scoparia (Linnaeus) Sauvageau | 207 | Native | |
| Ochrophyta | Hapalospongidion macrocarpum (Feldmann) León-Álvarez & González-González | 16 | Native | New record |
| Ochrophyta | Hecatonema terminale (Kützing) Kylin | 20 | Native | |
| Ochrophyta | Hincksia ovata (Kjellman) P.C.Silva | 4 | Native | |
| Ochrophyta | Hydroclathrus clathratus (C.Agardh) M.Howe | 111 | Native | |
| Ochrophyta | Leathesia marina (Lyngbye) Decaisne | 6 | Uncertain | |
| Ochrophyta | Lobophora variegata (J.V.Lamouroux) Womersley ex E.C.Oliveira | 88 | Native | |
| Ochrophyta | Mesogloia vermiculata (Smith) S.F.Gray | 9 | Native | New record |
| Ochrophyta | Myriactula rivulariae (Suhr ex Areschoug) Feldmann | 20 | Native | |
| Ochrophyta | Myrionema strangulans Greville | 13 | Native | New record |
| Ochrophyta | Nemoderma tingitanum Schousboe ex Bornet | 50 | Native | |
| Ochrophyta | Padina pavonica (Linnaeus) Thivy | 231 | Native | |
| Ochrophyta | Papenfussiella kuromo (Yendo) Inagaki | 11 | Introduced | |
| Ochrophyta | Petalonia binghamiae (J.Agardh) K.L.Vinogradova | 177 | Introduced | |
| Ochrophyta | Petalonia fascia (O.F.Müller) Kuntze | 3 | Native | |
| Ochrophyta | Petrospongium berkeleyi (Greville) Nägeli ex Kützing | 12 | Native | |
| Ochrophyta | Pseudolithoderma adriaticum (Hauck) Verlaque | 1 | Native | |
| Ochrophyta | Pseudolithoderma roscoffense Loiseaux | 48 | Native | |
| Ochrophyta | Punctaria tenuissima (C.Agardh) Greville | 4 | Native | |
| Ochrophyta | Ralfsia verrucosa (Areschoug) Areschoug | 92 | Native | |
| Ochrophyta | Sargassum cymosum C.Agardh | 20 | Native | |
| Ochrophyta | Sargassum furcatum Kützing | 37 | Native | |
| Ochrophyta | Sargassum vulgare C.Agardh | 15 | Native | |
| Ochrophyta | Scytosiphon lomentaria (Lyngbye) Link | 127 | Native | |
| Ochrophyta | Scytosiphon lomentaria (Lyngbye) Link, phase Microspongium gelatinosum Reinke | 43 | Native | |
| Ochrophyta | Sphacelaria cirrosa (Roth) C.Agardh | 7 | Native | |
| Ochrophyta | Sphacelaria plumula Zanardini | 2 | Native | |
| Ochrophyta | Sphacelaria rigidula Kützing | 1 | Native | |
| Ochrophyta | Sphacelaria tribuloides Meneghini | 5 | Uncertain | |
| Ochrophyta | Sphaerotrichia divaricata (C.Agardh) Kylin | 41 | Uncertain | |
| Ochrophyta | Sporochnus pedunculatus (Hudson) C.Agardh | 2 | Native | |
| Ochrophyta | Stragularia clavata (Harvey) Hamel | 1 | Native | New record |
| Ochrophyta | Stypopodium zonale (J.V.Lamouroux) Papenfuss | 8 | Native | |
| Ochrophyta | Taonia atomaria (Woodward) J.Agardh | 41 | Native | |
| Ochrophyta | Zanardinia typus (Nardo) P.C.Silva | 7 | Native | |
| Ochrophyta | Zonaria tournefortii (J.V.Lamouroux) Montagne | 248 | Native |
Table 3.
Summary of the macroalgal flora of the Island of São Miguel (N spec- number of specimens; N taxa- taxa; N spp- number of species) with information on the species origin and status (Introd- introduced; Uncrt- uncertain origin; Azo end- Azores endemism; Mac end- Macaronesia endemism; New rec- new record).
| Phyllum | Order | Family | N spec | N taxa | N spp | Native | Introd | Uncrt | Azo end | Mac end | New rec |
| Rhodophyta | 20 | 50 | 7510 | 284 | 212 | 171 | 15 | 21 | 1 | 4 | 42 |
| Chlorophyta | 5 | 14 | 1103 | 59 | 48 | 40 | 2 | 5 | 1 | 9 | |
| Ochrophyta | 11 | 19 | 4168 | 88 | 63 | 55 | 2 | 6 | 10 | ||
| Total | 36 | 83 | 12781 | 431 | 323 | 266 | 19 | 32 | 1 | 5 | 61 |
Many species were only sporadically recorded, but 10 were widely recorded around the Island and occurred quite abundantly in some locations, namely: the Rhodophyta Acrosorium ciliolatum (Harvey) Kylin, Chondracanthus acicularis, Gelidium microdon and Pterocladiella capillacea; and the Ochrophyta Colpomenia sinuosa, Gongolaria abies-marina, Halopteris filicina (Grateloup) Kützing, Halopteris scoparia, Padina pavonica and Zonaria tournefortii.
This paper increases the total of macroalgae species previously listed for São Miguel Island by 63 (44 Rhodophyta, 9 Chlorophyta and 10 Ochrophyta). When compared with the other Azorean Islands (Table 4), São Miguel has the highest number of species in all phylla, which reflects the greater amount of research undertaken on this Island on a more regular basis, involving both temporal and long-term studies.
Table 4.
Number of macroalgae species on the Azorean Islands: Santa Maria (Neto et al. 2020d); São Miguel (the present paper); Terceira (Neto et al. 2020a); Graciosa (Neto et al. 2020c); São Jorge and Faial (authors' unpublished data); Pico (Neto et al. 2020b); Flores and Corvo (Neto et al. 2021a).
| Phyllum | Santa Maria | São Miguel | Terceira | Graciosa | São Jorge | Pico | Faial | Flores | Corvo |
| Rhodophyta | 103 | 212 | 73 | 126 | 35 | 142 | 59 | 80 | 22 |
| Chlorophyta | 29 | 48 | 24 | 31 | 17 | 41 | 16 | 22 | 8 |
| Ochrophyta | 44 | 63 | 16 | 38 | 10 | 42 | 8 | 26 | 13 |
| Total | 176 | 323 | 113 | 195 | 62 | 225 | 83 | 128 | 43 |
In general and in keeping with other warm-water areas of the North Atlantic Ocean, the Azorean macroalgae flora has a larger proportion of red seaweeds.
A relatively high number of non-native species has been recorded on São Miguel Island (see Tables 2, 3), similarly to what has been reported for Santa Maria (Neto et al. 2020d), Terceira (Neto et al. 2020a), Graciosa (Neto et al. 2020c), Pico (Neto et al. 2020b) and Flores and Corvo (Neto et al. 2021a). Research over the past 15 years (Cardigos et al. 2006, Micael et al. 2014, Vaz-Pinto et al. 2014, Chainho et al. 2015, Cacabelos et al. 2019, Cacabelos et al. 2020, Martins et al. 2019, Parente et al. 2019) has indicated that the arrival of non-native species is increasing in the Azores, which is very likely to be related to the geostrategic position of the Archipelago as a “crossroad” in the distribution of marine algae in the North Atlantic, with documented exchanges with European, Mediterranean and American coasts. This may favour the arrival of new species via maritime traffic, both commercial and recreational (hull fouling, ballast waters), which may be a high risk to local marine ecosystems, as non-indigenous species can become invasive, resulting in impacts on ecosystem services and biodiversity (e.g. Katsanevakis et al. 2014).
The discovery of the new macroalgae records on São Miguel Island (present study) and on the other Azorean Islands (Neto et al. 2020a, Neto et al. 2020b, Neto et al. 2020c, Neto et al. 2020d, Neto et al. 2021a), demonstrates the need for continuing taxonomic and floristic studies in this region of the Atlantic Ocean. The biogeographically-variable nature of the new records found confirms the overall mixed nature of the marine algal flora of the Azores with elements shared with Macaronesia, the Mediterranean Sea, Atlantic Europe and the subtropical and tropical Atlantic America.
Taxonomic mismatch
A mismatch regarding the GBIF backbone taxonomy of some of the macroalgae species names was identified as detailed in Suppl. material 1.
Supplementary Material
DP-SMG-id_15785_normalized.csv
Ana I Neto
Data type
Macroalgae taxonomic mismatching
Brief description
GBIF does not have the more actualised nomenclature for some of the macroalgae species names. Therefore, the matching tools of its platform were applied to the species list, as required by Pensoft's data auditor, to identify the problematic taxonomic situations. The resulting file (DP-SMG-id_15785_normalized.csv) is included here, since the names will not be immediately updated in the GBIF Taxonomic Backbone. A request was already sent to GBIF helpdesk to solve this situation.
File: oo_505943.csv
Acknowledgements
This research was supported by several projects, expeditions and campaigns (see Funding above) and lately by the project “ACORES-01-0145-FEDER-000072” funded the Operational Programme Azores 2020 (85% ERDF and 15% regional funds). Thanks are due to the campaign teams for their critical involvement in this project (Abel Sentíes, Aina del Alcázar, Ana Alfaya, Ana Belén Villalba Lapeña, Ana Santos, Ana Sofia Carreiro, André Amaral, Andrea Tracana, Ane Laborda, Anna Lloveras Armengol, António Brigos Plafon, Berta Solé Nadal, Camille Fontaine, Carlos Rius, Carles Mir, Caroline Terral, Catarina Santos, Cláudia Hipólito, Daniela Gabriel, Edward Hehre, Emanuel Xavier, Eduardo García, Enrique Almira, Esteban Belles, Eunice Nogueira, Fátima Vaz Pinto, Francisco Wallenstein, Gustavo M Martins, Heather Baldwin, Isadora Moniz, Jana Verdura, Joana Pombo, João Brum, João Faria Santos, João Ferreira, Laura Busquier, Marco Enoch, Maria Ana Dionísio, Maria Machín-Sánchez, Maria Vale, Marlene Terra, Mónica Martínez, Mutue Toyota Fujii, Patrícia Madeira, Pedro Raposeiro, Richard Fralick, Richard Thompson, Rocío Sánchez, Ruben Couto, Rubén Mosquera, Rui Sousa, Sara Peres, Tarso Costa, Tito Silva, Valeria Cassano, Virginie Leyendecker). Edgar Rosas Alquicira and Karla León Cisneros were supported by the Programme AlBan, the European Union Programme of High Level Scholarships for Latin America (through scholarships E05D060221MX and E05D060520MX), “Consejo Nacional de Ciencia y Tecnología” (doctoral scholarships 176162 and 157904) and the UNAMUNO Programme of PhD Scholarships for Europe. Eva Cacabelos was supported by a postdoctoral grant (Project M1420-09-5369-FSE-000002) from ARDITI (Regional Agency for Development of Research, Technology and Innovation of Madeira). Andrea Z. Botelho was supported by a PhD grant (M3.1.a/F/083/2015), awarded by Fundo Regional da Ciência e Tecnologia (FRCT). Afonso C.L. Prestes was supported by a PhD grant (M3.1.a/F/083/2015), awarded by Fundo Regional da Ciência e Tecnologia (FRCT). Rita F. Patarra was supported by a Science and Technology Management Fellowship grant (SFRH/BGCT/135478/2018), awarded by Fundação para a Ciência e a Tecnologia (FCT I.P.). Manuela I. Parente was supported by a Postdoc grant (SFRH/BPD/34246/2006), awarded by Fundação para a Ciência e a Tecnologia (FCT).
Funding Statement
This work is financed by the ERDF (85%) and by regional funds (15%), through the Operational Programme Azores 2020, within the scope of the project “ACORES-01-0145-FEDER-000072”.
Author contributions
AIN: Conceptualisation; Methodology; Research (field and laboratory work); Resources; Data Curation; Formal analysis and interpretation; Paper writing
IM: Research (field and laboratory work); Data Curation; Formal analysis and interpretation; Paper writing
EFRA: Research (field and laboratory work); Data Curation; Paper writing
KLC: Research (field and laboratory work); Data Curation; Paper writing
EC: Research (field and laboratory work); Data Curation; Paper writing
AZB: Research (field and laboratory work); Data Curation
JM: Research (field and laboratory work); Data Curation
ACC: Research (field and laboratory work); Resources; Data Curation
RMAN: Data Curation; Formal analysis and interpretation
JMNA: Research (field work and laboratory work); Formal analysis and interpretation
SM: Research (field and laboratory work); Data Curation
RR: Resources; Data Curation
PA: Resources
ACLP: Research (field and laboratory work); Data Curation
RFP: Research (field and laboratory work); Data Curation; Paper writing
NVA: Research (field work); Maps elaboration
DM-F: Research (field and laboratory work); Data Curation
EB: Research (fieldwork and laboratory work); Resources; Data Curation; Paper writing
RF: Methodology; Research (fieldwork and laboratory work); Data Curation; Paper writing
WF: Methodology; Research (fieldwork and laboratory work); Data Curation; Paper writing
IT: Methodology; Research (fieldwork and laboratory work); Resources; Data Curation; Paper writing
MIP: Research (field and laboratory work); Resources; Data Curation; Formal analysis and interpretation; Paper writing
References
- Abecasis R. C., Afonso P., Colao A., Longnecker N., Clifton J., Schmidt L., Santos R. S. Marine conservation in the Azores: Evaluating marine protected area development in a remote island context. Frontiers in Marine Science. 2015;2:1–16. doi: 10.3389/fmars.2015.00104. [DOI] [Google Scholar]
- Afonso-Carrillo J., Sansón M. Clave ilustrada para la determinación de los macrófitos marinos bentónicos de las Islas Canarias. Departamento de Biología Vegetal (Botánica), Universidad de La Laguna; La Laguna: 1989. 55 [Google Scholar]
- Amorim P., Atchoi E., Berecibar E., Tempera F. Infralittoral mapping around an oceanic archipelago using MERIS FR satellite imagery and deep kelp observations: A new tool for assessing MPA coverage targets. Journal of Sea Research. 2015;100:141–151. doi: 10.1016/j.seares.2014.10.002. [DOI] [Google Scholar]
- Borges P. J.S.A. Ambientes litorais nos grupos central e oriental do arquipélago dos Açores. Tese de doutoramento. Universidade dos Açores; 2003. xxvii+413 [Google Scholar]
- Boudouresque C. - F., Meinesz A., Verlaque M. Mediterranée. In: Boudouresque C. - F., et al., editors. Guide des Algues des Mers d'Europe. Delachaux et Niestlé; Paris: 1992. 138-231 [Google Scholar]
- Bridsen D., Forman L., editors. The Herbarium Handbook. 3. The Board of Trustees of the Royal Botanic Gardens; key: 1999. xii + 334. [Google Scholar]
- Brodie J., Maggs C., John D. M., editors. The green seaweeds of Britain and Ireland. British Phycological Society; Dunmurry: 2007. xii + 242 [Google Scholar]
- Bruno de Sousa C., Cox C. J., Brito L., Pavo M. M., Pereira H., Ferreira A., Ginja C., Campino L., Bermejo R., Parente M., Varela J. Improved phylogeny of brown algae Cystoseira (Fucales) from the Atlantic-Mediterranean region based on mitochondrial sequences. PLOS One. 2019;14(1):e0210143. doi: 10.1371/journal.pone.0210143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows E. M. Seaweeds of the British lsles. 2. Chlorophyta. Natural History Museum; London: 1991. xii + 238 [Google Scholar]
- Cabioc'h J., Floc'h J. Y., Le Toquin A. Manche et Atlantique. In: Boudouresque C. - F., et al., editors. Guide des Algues des Mers d'Europe. Delachaux et Niestlé; Paris: 1992. 30-136 [Google Scholar]
- Cacabelos E., Faria J., Martins G. M., Mir C., Parente M. I., Gabriel D., Snchez R., Altamirano M., Costa A. C., Reine W. F., Neto A. I. First record of Caulerpa prolifera (Forsskl) J.V.Lamouroux in the Azores. Botanica Marina. 2019;62(2):155–160. doi: 10.1515/bot-2018-0075. [DOI] [Google Scholar]
- Cacabelos E., Martins G. M., Faria J., Prestes A. C.L., Costa T., Moreu I., Neto A. I. Limited effects of marine protected areas on the distribution of invasive species, despite positive effects on diversity in shallow-water marine communities. Biological Invasions. 2020;22(3):1169–1179. doi: 10.1007/s10530-019-02171-x. [DOI] [Google Scholar]
- Cardigos F., Tempera F., Ávila S., Gonçalves J., Colaço A., Santos R. S. Nonindigenous marine species of the Azores. Helgoland Marine Research. 2006;60:160–169. doi: 10.1007/s10152-006-0034-7. [DOI] [Google Scholar]
- Cardoso P., Erwin T., Borges P. V., New T. The seven impediments in invertebrate conservation and how to overcome them. Biological Conservation. 2011;144(11):2647–2655. doi: 10.1016/j.biocon.2011.07.024. [DOI] [Google Scholar]
- Chainho P., Fernandes A., Amorim A., Ávila S. P., Canning-Clode J., Castro J. J., Costa A. C., Costa J. L., Cruz T., Gollasch S., Grazziotin-Soares C., Melo R., Micael J., Parente M. I., Semedo J., Silva T., Sobral D., Sousa M., Torres P., Veloso V., Costa M. J. Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuarine, Coastal and Shelf Science. 2015;167:199–211. doi: 10.1016/j.ecss.2015.06.019. [DOI] [Google Scholar]
- Costa A. Brachyura intertidais: estudo das populações de três praias de calhau rolado da Ilha de S. Miguel, Açores. Trabalho de síntese submetido no âmbito das provas de APCC. Universidade dos Açores; 1994. 9 [Google Scholar]
- Dixon S. P., Irvine L. M. Seaweeds of the British Isles. Vol. I Rhodophyta. Part 1 Introduction, Nemaliales, Gigartinales. British Museum (Natural History); London: 1977. xi + 252 [Google Scholar]
- Faria J., Navas D., Prestes A., Cacabelos E., Moreu I., Martins G. M., Pereira L., Neto A. I. A guide for harvesting practices of macroalgae in Azores (NE Atlantic): The project ASPAZOR and the case study of Asparagopsis spp. Azorean Biodiversity Group- Centre for Ecology, Evolution and Environmental Changes (ABG-cE3c); 2020. 47. [DOI] [Google Scholar]
- Faria J., Navas D., Prestes A., Cacabelos E., Moreu I., Martins G. M., Pereira L., Neto A. I. Guia de boas práticas para a apanha de algas nos Açores - O projeto ASPAZOR e o caso-estudo de Asparagopsis spp. Azorean Biodiversity Group- Centre for Ecology, Evolution and Environmental Changes (ABG-cE3c); 2020. 15. [DOI] [Google Scholar]
- Fletcher R. L. Seaweeds of the British Isles. Vol. III. Fucophyceae (Phaeophyceae). Part 1. British Museum (Natural History); London: 1987. x + 359 [Google Scholar]
- Freitas R., Romeiras M., Silva L., Cordeiro R., Madeira P., González J. A., Wirtz P., Falcón J. M., Brito A., Floeter S. R., Afonso P., Porteiro F., Viera-Rodríguez M. A., Neto A. I., Haroun R., Farminho J. N.M., Rebelo A. C., Baptista L., Melo C. S., Martínez A., Núñez J., Berning B., Johnson M. E., Ávila S. P. Restructuring of the Macaronesia biogeographic unit: A marine multi-taxon biogeographical approach. Scientific Reports. 2019;9:15792. doi: 10.1038/s41598-019-51786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar J. L., Guest J. E., Duncan A. M., Chester D. K., Barriga F. J.A.S. Volcanic geology of São Miguel Island (Azores Archipelago): Introduction. In: Gaspar J. L., Guest J. E., Duncan A. M., Barriga F. J.A.S., Chester D. K., editors. Volcanic Geology of São Miguel Island. Vol. 44. Geological Society, London, Memoirs; London: 2015. 1-3. [DOI] [Google Scholar]
- Gayral P., Cosson J. Connaitre et reconnaitre les algues marines. Ouest France; 1986. 220 [Google Scholar]
- Guiry M. D., Guiry G. M. AlgaeBase. World-wide electronic publication. https://www.algaebase.org. [2021-01-29T00:00:00+02:00];
- Haroun R., Machín-Snchez M., Neto A. I., Viera-Rodríguez M. A. A review of current uses and new biotechnological roads for the seaweed resources of the Macaronesian region (Central-east Atlantic Ocean) Journal of Applied Phycology. 2019;31(6):3777–3790. doi: 10.1007/s10811-019-01889-4. [DOI] [Google Scholar]
- Hidrográfico I, editor. Roteiro do Arquipélago dos Açores. PUB. (N) -lli-128-SN, Lisboa 1981.
- Hildenbrand A., Weis D., Madureira P., Marques F. O. Recent plate re-organization at the Azores Triple Junction: Evidence from combined geochemical and geochronological data on Faial, S Jorge and Terceira volcanic Islands. Lithos. 2014;210-211:27–39. doi: 10.1016/j.lithos.2014.09.009. [DOI] [Google Scholar]
- Hortal J., Bello F., Diniz-Filho J. A.F., Lewinsohn T. M., Lobo J. M., Ladle R. J. Seven shortfalls that beset large-scale knowledge of biodiversity. Annual Review of Ecology, Evolution, and Systematics. 2015;46:523–549. doi: 10.1146/annurev-ecolsys-112414-054400. [DOI] [Google Scholar]
- Irvine L. M. Seaweeds of the British Isles. Vol. I Rhodophyta. Part 2 A Cryptonemiales (sensu stricto), Palmariales, Rhodymeniales. British Museum (Natural History); London: 1983. xii + 115 [Google Scholar]
- Irvine L. M., Chamberlain Y. M. Seaweeds of the British Isles. Vol. 1. Rhodophyta. Part 2B. Corallinales, Hildenbrandiales. Natural History Museum; London: 1994. vii + 276 [Google Scholar]
- Katsanevakis S., Wallentinus I., Zenetos A., Leppakoski E., Çinar M. E., Ozturk B., Grabowski M., Golani D., Cardoso A. C. Impacts of marine invasive alien species on ecosystem services and biodiversity: a pan-European review. Aquatic Invasions. 2014;9:391–423. doi: 10.3391/ai.2014.9.4.01. [DOI] [Google Scholar]
- Kellaris A., Gil A., Faria J., Amaral R., Badia I. M., Neto A. I., Yesson C. Using low-cost drones to monitor heterogeneous submerged seaweed habitats: a case study in the Azores. Aquatic Conservation: Marine and Freshwater Ecosystems. 2019;29:1909–1922. doi: 10.1002/aqc.3189. [DOI] [Google Scholar]
- Lawson G. W., John D. M. The marine algae and coastal environment of Tropical West Africa. Beihefte zur Nova Hedwigia, J. CRAMER; Vaduz: 1982. 455 [Google Scholar]
- Leon-Cisneros K., Tittley I., Terra M. R., Nogueira E. M., Neto A. I. The marine algal (seaweed) flora of the Azores: 4, further additions. Arquipelago - Life and Marine Sciences. 2012;29:25–32. [Google Scholar]
- Levring T. The marine algae of the archipelago of Madeira. http://publications.cm-funchal.ptljspui/handle/1 00/1231 Boletim do Museu Municipal do Funchal. 1974;28(125):5–111. [Google Scholar]
- Lloréns J. L. P., Cabrero I. H., Lacida R. B., González G. P., Murillo F. G. B., Oñate J. J. V. Flora marina del litoral gaditano. Biología, ecología, usos y guía de identificación. mCN Monografias de Ciencias de la Naturaleza. Servicio de Publicaciones de la Universidad de Cádiz; Cádiz: 2012. 368 [Google Scholar]
- Maggs C. A., Hommersand M. H. Seaweeds of the British Isles. Vol 1. Rhodophyta. Part 3A. Ceramiales. Natural History Museum; London: 1993. xv + 444 [Google Scholar]
- Martins G. M., Cacabelos E., Faria J., Álvaro N. M., Prestes A. C.L., Neto A. I. Patterns of distribution of the invasive alga Asparagopsis armata Harvey: a multi-scaled approach. Aquatic Invasions. 2019;14(4):582–593. doi: 10.3391/ai.2019.14.4.02. [DOI] [Google Scholar]
- Micael J., Parente M. I., Costa A. C. Tracking macroalgae introductions in North Atlantic oceanic islands. Helgoland Marine Research. 2014;68(2):209–219. doi: 10.1007/s10152-014-0382-7. [DOI] [Google Scholar]
- Morton B., Britton J. C., Martins A. M.F. Coastal ecology of the Azores. Sociedade Afonso Chaves, Ponta Delgada; 1998. 249 [Google Scholar]
- Neto A. I. Contribution to the taxonomy and ecology of the Azorean benthic marine algae. Biological Journal of the Linnean Society. 1992;46:163–176. doi: 10.1111/j.1095-8312.1992.tb00858.x. [DOI] [Google Scholar]
- Neto A. I. Checklist of the benthic marine algae of the Azores. http://hdl.handle.net/10400.3/1171. Arquiplago. Life and Marine Sciences. 1994;12A:15–34. [Google Scholar]
- Neto A. I. Studies on algal communities of São Miguel, Azores. Tese de Doutoramento em Biologia, especialidade de Biologia Marinha. Universidade dos Açores; Ponta Delgada: 1997. x+309 [Google Scholar]
- Neto A. I. Ecology and dynamics of two intertidal algal communities on the littoral of the Island of São Miguel (Azores) Hydrobiologia. 2000;432:135–147. doi: 10.1023/A:1004042808901. [DOI] [Google Scholar]
- Neto A. I. Macroalgal species diversity and biomass of subtidal communities of São Miguel (Azores) Helgoland Marine Research. 2001;55:101–111. doi: 10.1007/s101520100074. [DOI] [Google Scholar]
- Neto A. I., Tittley I., Raposeiro P. Flora Marinha do Litoral dos Açores. Secretaria Regional do Ambiente e do Mar, Açores; 2005. 156. [Google Scholar]
- Neto A. I., Viera M. A., Haroun R. A synthetic overview of marine phycological studies in the Macaronesian Archipelagos. Silva Lusitana. 2014;22:217–244. [Google Scholar]
- Neto A. I., Prestes A. C.L., Álvaro N. V., Resendes R., Neto R. M.A., Moreu I. Marine algal (seaweed) flora of Terceira Island, Azores. Biodiversity Data Journal. 2020;8:e57462. doi: 10.3897/BDJ.8.e57462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A. I., Prestes A. C.L., Álvaro N. V., Resendes R., Neto R. M.A., Tittley I., Moreu I. Marine algal flora of Pico Island, Azores. Biodiversity Data Journal. 2020;8:e57461. doi: 10.3897/BDJ.8.e57461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A. I., Parente M. I., Botelho A. Z., Prestes A. C.L., Resendes R., Afonso C. L.P., Álvaro N. V., Milla-Figueras D., Neto R. M.A., Tittley I., Moreu I. Marine algal flora of Graciosa Island, Azores. Biodiversity Data Journal. 2020;8:e57201. doi: 10.3897/BDJ.8.e57201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A. I., Parente M. I., Cacabelos E., Costa A. C., Botelho A. Z., Ballesteros E., Monteiro S., Resendes R., Afonso P., Afonso C. L.P., Patarra R. F., Álvaro N. V., Milla-Figueras D., Neto R. M. A., Azevedo J. M. N., Moreu I. Marine algal flora of Santa Maria Island, Azores. v1.1. Dataset/Samplingevent. Universidade dos Açores; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A. I., Parente M. I., Tittley I., Fletcher R. L., Farnham W. F., Costa A. C., Botelho A. Z., Monteiro S., Resendes R., Afonso P., Prestes A. C. L., Álvaro N. V., Milla-Figueras D., Neto R. M. A., Azevedo J. M. N., Moreu I. Marine algal flora of Flores and Corvo Islands, Azores. Biodiversity Data Journal. 2021;9:e60929. doi: 10.3897/BDJ.9.e60929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A. I., Moreu I., Rosas-Alquicira E. F., León-Cisneros K., Cacabelos E., Botelho A. Z., Micael J., Costa A. C., Neto R. M. A., Azevedo J. M. N., Monteiro S., Resendes R., Afonso P., Prestes A. C. L., Patarra R. F., Álvaro N. V., Milla-Figueras D., Ballesteros E., Fletcher R. L., Farnham W. F., Tittley I., Parente M. I. Marine algal flora of São Miguel Island, Azores. v1.2. Dataset/Samplingevent. Universidade dos Açores via GBIF.; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente MI. List of marine macroalgae (Rhodophyta, Chlorophyta and Phaeophyceae) In: Borges P. A.V., Costa A. C., Cunha R., et al., editors. Description of the Terrestrial and marine biodiversity of the Azores. A list of the terrestrial and marine biota from the Azores. Princípia Editora; Cascais, Lisboa: 2010. 275-286. [Google Scholar]
- Parente M. I., Gabriel D., Micael J., Botelho A. Z., Ballesteros E., Milla D., Santos R., Costa A. C. First report of the invasive macroalga Acrothamnion preissii (Rhodophyta, Ceramiales) in the Atlantic Ocean. Botanica Marina. 2019;61(1):85–90. doi: 10.1515/bot-2017-0060. [DOI] [Google Scholar]
- Parente M. I., Fletcher R. L., Costa F. O., Saunders G. W. Taxonomic investigation of Ralfsia-like (Ralfsiales, Phaeophyceae) taxa in the North Atlantic Ocean based on molecular and morphological data, with descriptions of Pseudoralfsiaceae fam. nov., Pseudoralfsia azorica gen. et sp. nov. and Nuchella vesicularis gen. et sp. nov. European Journal of Phycology. 2020 doi: 10.1080/09670262.2020.1753245.. [DOI]
- Patarra R. F., Carreiro A. S., Lloveras A. A., Abreu M. H., Buschmann A. H., Neto A. I. Effects of light, temperature and stocking density on Halopteris scoparia growth. Journal of Applied Phycology. 2017;29:405–411. doi: 10.1007/s10811-016-0933-1. [DOI] [Google Scholar]
- Patarra R. F., Lloveras A. A., Carreiro A. S., Abreu M. H., Buschmann A. H., Neto A. I. Short term effects of irradiance on the growth of Pterocladiella capillacea (Gelidiales, Rhodophyta) Arquipelago. Life and Marine Sciences. 2019;36:85–94. [Google Scholar]
- Patarra R. F., Iha C., Pereira L., Neto A. I. Concise review of biological data on Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand. Journal of Applied Phycology. 2020;32:787–808. doi: 10.1007/s10811-019-02009-y. [DOI] [Google Scholar]
- Rodríguez-Prieto C., Ballesteros E., Boisset F., Afonso-Carrillo J. Guía de las macroalgas y fanerógamas marinas del Mediterráneo Occidental. Omega, S.A.; Barcelona: 2013. 656 [Google Scholar]
- Rosas-Alquicira E. F., Riosmena-Rodríguez R., Afonso-Carrillo J., Neto A. I. Taxonomic biodiversity of geniculate coralline red algae (Corallinales; Rhodophyta) from the Macaronesia region: summary and analysis. Helgoland Marine Research. 2011;65(2):133–153. doi: 10.1007/s10152-010-0209-0. [DOI] [Google Scholar]
- Schmidt O. C. Die marine vegetation der Azoren in ihren Grundzügen dargestellt. Bibliotheca Botanica, 24 (102); 1931. ix+116 [Google Scholar]
- Sousa C. B.De, Cox C. J., Brito L., Pavao M. M., Pereira H., Ferreira A., Ginja C., Campino L., Bermejo R., Parente M. I. Improved phylogeny of brown algae Cystoseira (Fucales) from the Atlantic- Mediterranean region based on mitochondrial sequences. PLoS One. 2019;14: e0210143 doi: 10.1371/journal.pone.0210143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. Marine algae of the northeastern coasts of North America. The University of Michigan Press; 1967. viii+509 [Google Scholar]
- Taylor W. R. Marine algae of the eastern tropical and subtropical coasts of the Americas. The University of Michigan Press; 1978. xxi+870 [Google Scholar]
- Tittley I., Neto A. I. The marine algal flora of the Azores and its biogeographical affinities. http://hdl.handle.net/10400.3/1771 Boletim do Museu Municipal do Funchal. 1995;4:747–766. [Google Scholar]
- Tittley I. Seaweed diversity in the North Atlantic Ocean. Arquiplago Life and Marine Sciences. 2003;19A:13–25. [Google Scholar]
- Tittley I., Neto A. I. The marine algal (seaweed) flora of the Azores: additions and amendments. Botanica Marina. 2005;48:248–255. doi: 10.1515/BOT.2005.030. [DOI] [Google Scholar]
- Tittley I., Neto A. I. The marine algal flora of the Azores: Island isolation or Atlantic stepping stones? Occasional papers of the Irish Biogeographical Society. 2006;9:40–54. [Google Scholar]
- Vaz-Pinto F., Torrontegi O., Prestes A. C.L., Álvaro N. V., Neto A. I., Martins G. M. Invasion success and development of benthic assemblages: effect of timing, duration of submersion and substrate type. Marine Environmental Research. 2014;94:72–79. doi: 10.1016/j.marenvres.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Vieira C., Henriques F., D'hondt S., Neto A. I., Almada C. H., Kauffman M., Sansón M., Sangil C., De Clerck O. Lobophora (Dictyotales) species richness, ecology and biogeography across the North-eastern Atlantic archipelagos and description of two new species. Journal of Phycology. 2020;56(2):346–357. doi: 10.1111/JPY.12956. [DOI] [PubMed] [Google Scholar]
- Wallenstein F. M., Terra M. R., Pombo J., Neto A. I. Macroalgal turfs in the Azores. Marine Ecology-An Evolutionary Perspective. 2009;30(Suppl. 1):113–117. doi: 10.1111/j.1439-0485.2009.00311.x. [DOI] [Google Scholar]
- Wallenstein F. M., Neto A. I., Álvaro N. V., Tittley I., Azevedo J. M. N. Guia para definição de biótopos costeiros em ilhas oceânicas. Secretaria Regional do Ambiente e do Mar; 2009. [Google Scholar]
- Wallenstein F. M., Peres S. D., Xavier E. D., Neto A. I. Phytobenthic communities of intertidal rock pools in the eastern islands of Azores and their relation to position on shore and pool morphology. http://hdl.handle.net/10400.3/666 Arquiplago. Life and Marine Sciences. 2010;27:9–20. [Google Scholar]
- Zbyszewski G., Almeida F. M. O., Ferreira O. V. Carta geológica de Portugal. Notícia Explicativa da Folha "B'' da Ilha de S. Miguel (Açores) Serviços Geológicos de Portugal; Lisboa: 1958. 35 [Google Scholar]
- Zbyszewski G., Ferreira O. V. Carta geológica de Portugal. Notícia Explicativa da Folha "A" da Ilha de S. Miguel (Açores). Serviços Geológicos de Portugal; Lisboa: 1959. 22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DP-SMG-id_15785_normalized.csv
Ana I Neto
Data type
Macroalgae taxonomic mismatching
Brief description
GBIF does not have the more actualised nomenclature for some of the macroalgae species names. Therefore, the matching tools of its platform were applied to the species list, as required by Pensoft's data auditor, to identify the problematic taxonomic situations. The resulting file (DP-SMG-id_15785_normalized.csv) is included here, since the names will not be immediately updated in the GBIF Taxonomic Backbone. A request was already sent to GBIF helpdesk to solve this situation.
File: oo_505943.csv












