Abstract
Postural stability is a multi-factorial skill maintained implicitly. Components of quiet standing can decline with Alcohol Use Disorder (AUD), cause instability, and disrupt activities of daily living (ADL). To examine how stability factors contribute to ADL and balance, 638 force platform testing sessions measured sway paths acquired during quiet standing in 151 AUD and 96 control men and women, age 25–75. Structural equation (seq) path analysis estimated contributions from age, diagnosis, and sensory perception to sway and measures of ADL and roadside ataxia testing. Whether eyes were open or closed, older AUD and control participants had longer sway paths than younger ones; older men had longer sway paths than older women. Although each sensory ability tested declined with aging, different factor constellations influenced ADL, ataxia scores, or sway path. Seq-path analysis indicated that ADL was strongly dependent on sensory (but not cognitive) systems with sway-path length accounting for upwards of 25% of variance. Within the AUD group, an index of historically-experienced withdrawal symptoms was a common predictor of stability regardless of vision condition. The greatest variance measured by the seq-path model was for predicting platform sway and simple ataxia testing of one-leg standing even though these measures were affected by different predictor variables: strong predictors of one-leg standing were diagnosis and age (R2=39.6%−43.2%), whereas strong predictors of sway-path length were sensory factors and withdrawal index (R2=22.0%−22.9%). These findings present evidence for appreciating selective factors that contribute to declining postural stability and to liability for compromised quality of life in AUD.
Keywords: alcohol, balance, sensory, quality of life, neuropathy
INTRODUCTION
The ability to stand with stability becomes an implicitly maintained, multicomponent skill typically acquired in the first year of life. Components that contribute to standing include touch and pressure cues on the soles of the feet that inform distal and proximal muscular-skeletal coordination with input from vestibular, visual, and cerebellar interactions (e.g.,1,2–6). With normal aging and neuropsychiatric conditions, components of this multifactorial skill can degrade and disrupt postural stability7–11, presenting an acquired liability for falling, a leading cause of morbidity and mortality especially in older adults, and risks are further heightened in those with a history of Alcohol Use Disorder (AUD) [https://www.who.int/news-room/fact-sheets/detail/falls].
Quantitative studies in AUD report postural instability enduring (e.g.,12,13–15) well beyond acute intoxication (cf.,16). Identified mechanisms of dysfunction include volume deficits in anterior superior cerebellum15,17–20, abnormal ankle-hip interaction implicating disturbed distal-core skeletomuscular interaction1,7,9,21,22 (review,23), and compromised corticospinal white matter tracts as contributing to excessive truncal tremor in sober individuals with AUD24. That individuals with AUD who maintain sobriety can show significant improvement in upright stability13,25–28 supports excessive alcohol consumption as a causative factor of compromised performance. Taken together, these studies provide critical evidence to construct elements of postural stability that can independently be disrupted by AUD. Yet to be considered in these mechanistic studies are the real-life ramifications of instability of static upright posture, which is central to a wide scope of life’s usual activities, considered activity of daily living (ADL), such as one-leg balance needed for dressing (e.g., putting on pants) or getting out of a car. To the extent that the maintenance of postural stability is a relatively implicit skill, we questioned whether diminished cognitive status, short of dementia levels, could play a role in disturbing balance or whether balance, measured as sway path length, would be unrelated to cognitive status.
Postural stability is readily measured using a force plate platform that records the participant’s center of gravity while standing quietly. The more unstable the static balance, the more the center of gravity is displaced over time and the greater is a “path” connecting the change in force over time. Further, the amount of displacement is also influenced by external factors such as visual cues with greater postural stability evident with eyes open than with eyes closed (e.g.,15,29,30).
Herein, we applied a novel physiological assessment of the consequences of AUD on the postural stability evidenced as disrupted standing balance with real-world consequences. Accordingly, this study had three major aims: 1) to test aging, sex, and AUD effects on postural stability with sway path analysis using combined cross-sectional and longitudinal data across a five-decade age range; 2) to identify physiological, cognitive, and motor correlates of sway path that have the potential of explaining group differences; and 3) to use structural equation path analysis to test the strength of the contributions from age, diagnosis, sensory perception variables, and general cognitive status tested with the Dementia Rating Scale31 to the physiological measure of sway and the behavioral outcome measures of ataxia and ADL. Knowledge about influential factors on instability may reveal specific areas for mitigating balance problems and potential falls (cf.,10).
METHODS
Participants
From 12 April 2006 to 29 July 2019, 638 force platform test sessions were acquired in 247 participants meeting study criteria based on clinical interview and age at study entry (25 to 75 years). Longitudinal data were acquired in 40 of the 96 controls and in 39 of the 151 AUD participants (Table 1). Exclusive of the 96 controls was one man with a history of spinal stenosis surgery; in addition, 2 control men and 1 control woman whose sway paths exceeded 3 SD for their age from the remaining control cohort were considered outliers and excluded. The final groups were matched on age, sex, and body mass index (BMI), but the AUD group had fewer years of education and lower socioeconomic status than the control group. Demographic statistics are presented in Table 2.
Table 1.
Number of participants with single and multiple visits
| Control | AUD | ||||||
|---|---|---|---|---|---|---|---|
| Sway Path Sessions | Male | Female | Total | Male | Female | Total | |
| 1 | 35 | 21 | 56 | 83 | 29 | 112 | |
| 2 | 12 | 8 | 20 | 17 | 3 | 20 | |
| 3 | 4 | 6 | 10 | 6 | 7 | 13 | |
| 4 | 1 | 2 | 3 | 3 | 2 | 5 | |
| 5 | 4 | 2 | 6 | 0 | 0 | 0 | |
| 6 | 1 | 0 | 1 | 0 | 1 | 1 | |
| Total individuals= | 57 | 39 | 96 | 109 | 42 | 151 | |
| Total visits= | 101 | 73 | 174 | 147 | 70 | 217 |
Table 2.
Study entry demographics of the study groups: mean (SD) or frequency count
| Control | AUD | Group Differences by lm: t, p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | Diagnosis | Sex | Age | ||
| Age (yrs) | 51.20 | 48.89 | 50.26 | 50.05 | 50.23 | 50.10 | t=−0.180, p=.857 | t=0.556, p=.579 | — | |
| (13.37) | (14.55) | (13.83) | (10.83) | (8.96) | (10.32) | C=A | M=F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| Education (yrs) | 15.68 | 16.18 | 15.89 | 13.12 | 13.76 | 13.30 | t=−8.321, p=.6.33e-15 | t=−1.920, p=.056 | t=1.980, p=.049 | |
| (2.41) | (2.45) | (2.43) | (2.29) | (2.03) | (2.24) | C>A | M<F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| Socioeconomic status | 27.5 | 25.1 | 26.5 | 43.13 | 38.38 | 41.81 | t=8.277, p=.8.41e-15 | t=2.031, p=0.043 | t=−1.096, p=0.274 | |
| (lower score=higher status) | (12.74) | (10.59) | (11.91) | (14.98) | (13.39) | (14.67) | C<A | M>F | ||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| Height (inches) | 70.09 | 64.85 | 67.96 | 70.44 | 65.34 | 69.02 | t=1.018, p=.310 | t=12.909, p<2e-16 | t=−1.251, p=.212 | |
| (2.66) | (2.50) | (3.66) | (3.08) | (3.28) | (3.88) | C=A | M>F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| Weight (lbs) | 186.86 | 153.81 | 173.44 | 191.67 | 167.31 | 184.90 | t=1.866, p=.063 | t=6.356, p=1.02e-09 | t=0.949, p=.344 | |
| (28.88) | (31.02) | (33.80) | (33.92) | (32.70) | (35.22) | C=A | M>F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| Body Mass Index (BMI) | 26.73 | 25.77 | 26.34 | 27.09 | 27.60 | 27.23 | t=1.510, p=.132 | t=0.150, p=.881 | t=1.664, p=.097 | |
| (3.80) | (5.29) | (4.46) | (4.15) | (5.27) | (4.48) | C=A | M=F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| pNART IQ | 112.53 | 114.12 | 113.18 | 106.66 | 107.93 | 107.02 | t=−5.251, p=3.51e-07 | t=−1.219, p=.224 | t=2.057, p=.041 | |
| (8.11) | (6.95) | (7.66) | (8.73) | (9.48) | (8.93) | C>A | M=F | |||
| n= | 52 | 36 | 88 | 100 | 40 | 140 | ||||
| pWTAR FSIQ | 104.45 | 106.66 | 105.35 | 96.93 | 98.68 | 97.43 | t=−5.091, p=7.54e-07 | t=−1.270, p=.205 | t=2.032, p=.043 | |
| (10.44) | (9.08) | (9.91) | (11.61) | (12.83) | (11.95) | C>A | M=F | |||
| n= | 52 | 36 | 88 | 100 | 40 | 140 | ||||
| DRS total score (max=144) | 139.96 | 139.83 | 139.91 | 136.06 | 137.09 | 136.33 | t=−5.777, p=2.7e-08 | t=−0.747, p=.456 | t=−0.753, p=.452 | |
| (2.81) | (2.86) | (2.82) | (5.19) | (4.60) | (5.04) | C>A | M=F | |||
| n= | 51 | 36 | 87 | 95 | 33 | 128 | ||||
| Total Lifetime Alcohol Drunk (kg) | 38.73 | 22.79 | 32.26 | 1350.41 | 886.44 | 1221.36 | t=12.191, p<2e-16 | t=−2.595, p=.010 | t=3.889, p=.00013 | |
| (65.44) | (31.83) | (54.70) | (1040.79) | (595.43) | (959.35) | C<A | M>F | |||
| n= | 57 | 39 | 96 | 109 | 42 | 151 | ||||
| AUD onset age | — | — | — | 24.50 | 26.77 | 25.13 | — | — | — | |
| — | — | — | (9.10) | (9.98) | (9.38) | — | — | — | ||
| n= | — | — | — | 109 | 42 | 151 | — | — | — | |
| Days since last drink | — | — | — | 210.98 | 389.95 | 261.94 | — | — | — | |
| — | — | — | (409.81) | (876.56) | (584.20) | — | — | — | ||
| range= | — | — | — | 0 – 2296 | 1 – 4598 | 0 – 4598 | — | — | — | |
| n= | 103 | 41 | 144 | |||||||
| History of detox/Tx Y/N | — | — | — | 98/11 | 32/10 | 130/21 | — | — | — | |
| History of drank to stop Y/N | — | — | — | 87/19 | 31/11 | 118/30 | — | — | — | |
| Reported seizures Y/N | — | — | — | 15/94 | 2/40 | 17/134 | — | — | — | |
| Smoker Y/N | 6/47 | 0/38 | 6/85 | 71/35 | 28/12 | 99/47 | χ2=85.132, p=.00001 | |||
| Other drugs Y/N | 0 | 0 | 0 | 78/31 | 22/20 | 100/51 | ||||
| Marijuana Y/N | 0 | 0 | 0 | 49/60 | 9/33 | 58/93 | ||||
| Cocaine Y/N | 0 | 0 | 0 | 54/55 | 16/26 | 70/81 | ||||
| Amphetamines Y/N | 0 | 0 | 0 | 27/82 | 9/33 | 36/115 | ||||
| Opiates Y/N | 0 | 0 | 0 | 19/90 | 4/38 | 23/128 | ||||
| Self-Defined Ethnicity | χ2=31.324, p=.00001 | |||||||||
| Asian | — | — | 21 | — | — | 4 | ||||
| African American | — | — | 17 | — | — | 58 | ||||
| Caucasian | — | — | 53 | — | — | 75 | ||||
| Other/unknown | — | — | 5 | — | — | 14 | ||||
Regression to adjust NART IQ and WTAR FSIQ to be comparable
Bonferroni correction for 10 comparisons p≤.005
Participants underwent interviews with the Structured Clinical Interview for DSM-IV revised32 to determine diagnosis for alcohol dependence and in later years DSM-5 diagnosis for AUD33 and to determine study eligibility. All participants in the AUD group met criteria for alcohol dependence and for AUD. Interviews were conducted by research clinicians in our laboratory and included structured health questionnaires, a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption34,35, and the Clinical Institute Withdrawal Assessment for Alcohol-revised (CIWA-Ar)36. Comorbid drug use was determined with SCID diagnosis and was not exclusionary. Allowable drug use could only be secondary to alcohol use and was typically more remote than alcohol use in a participant’s history. Subjects were excluded for significant history of medical (including but not limited to HIV infection, epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 minutes), psychiatric (i.e., schizophrenia, bipolar I disorder, or PTSD), or neurological disorders (e.g., neurodegenerative disease).
AUD participants were recruited during times of abstinence from post-detoxification, area sober living environments. Follow-up periods varied by person as did their interim drinking; for some, the recovery period did involve relapse. As a naturalistic study, some participants at re-study had relapsed; thus, participants were re-tested irrespective of their relapse status. Abstinence was confirmed on the days of testing with an alcohol breathalyzer, which required a reading of 0.0 breath alcohol level (BrAL) to carry on with testing on a day. Anyone whose BrAL exceeded 0.0 was asked to return for testing when abstinent at a minimum of overnight.
At study entry, general cognitive ability was assessed with the National Adult Reading Test (NART)37 or Wechsler Test of Adult Reading (WTAR)38 as estimates of premorbid intelligence quotient (IQ). The scores of the AUD group were lower than those of the control group (Table 2). The AUD group also scored lower than the control group on the Dementia Rating Scale, second edition (DRS-2)31, which assessed current cognitive status. No control scored in the impaired range on the DRS-2, but 4 men and 2 women in the AUD group had DRS-2 scores between 119 and 124, which is in the impaired range.
Quiet Standing Quantified with Force Platform Analysis
Test Conditions.
Subjects wore rubber-soled socks and stood still on a force plate with feet together with arms relaxed at one’s side. The test comprised three, 45 sec. trials in each of two conditions, both with feet together: standing with eyes open and then eyes closed.
Sway Analysis.
Balance was assessed with a microcomputer-controlled force plate (model 9284; Kistler, Amherst, NY) with multiple transducers and analog-digital converters. Data were sampled at 1000Hz; raw data were three 45 sec. continuous trials of center-of-pressure displacements (x-y pairs). Data were subjected to a 10Hz low-pass filter (99 terms, −50db Gibbs); sway path length (P) was expressed as the line integral (cm):
Walk-a-Line Ataxia Test (“Ataxia”)
Participants completed a gait and balance battery, which is similar to road-side sobriety testing. Each task, done first with eyes open and then with eyes closed, included the following: stand heel-to-toe on a line with arms folded across the chest for a maximum of 60 sec.; walk a line heel-to-toe for 10 steps; stand on the left foot for 30 sec.; stand on the right foot for 30 sec. Each condition was conducted twice unless the subject achieved a perfect score on the first trial, in which case the second trial was also given a perfect score. The scores for each condition equaled the sum of the number of seconds or steps completed14,39.
Sensory Testing
Most participants underwent sensory testing of the lower extremities to assess the contribution of cutaneous and other peripheral sensory impairment on metrics of quiet standing40. High scores were in the impaired direction.
Perception of great toe vibration (noted as “Vibration” in the causal path figures).
The subject lay supine on the examination table. The examiner then struck a tuning fork and placed it on distal interphalangeal joint of the great toe on the dominant side and asked when the vibration stopped. The time from touch to response was timed with a stopwatch. The procedure was repeated on the other great toe. Each toe was scored as follows and the final score was the mean of the left and right toes: 0= >10 sec. (normal); 1= 6–10 sec. (mild loss); 2= ≤5 sec. (moderate loss); 3= no feeling of vibration (severe loss). The timed vibratory test is considered a sensitive noninvasive method of detecting mild to moderate impairments in vibratory sensation and is used to detect sensory neuropathy41.
2-point discrimination (“2-Point”).
Using a 3-point aesthesiometer, the examiner touched the sole of one foot with 1 or 2 points, to which the subject (with eyes closed) responded “one” or “two.” Testing avoided calloused skin and proceeded with descending limits (starting at 50mm distance between points); the threshold was the shortest distance on which fewer than 3 errors were made42. The procedure was repeated with the other foot. As a control comparison measure, 2-point discrimination was tested on the palms of the hands.
Sensory symptoms of peripheral neuropathy (“Sensory”).
Subjects were asked to rate discomfort in feet or legs that might be symptomatic of peripheral neuropathy43. Rating was done usually on the day of balance testing for each of three categories: 1) pain, aching, burning; 2) pins and needles; 3) numbness; scores were 0=none, 1=mild, 2=moderate, 3=severe. Herein, this subjective assessment of peripheral neuropathy is referred to as sensory symptoms.
Withdrawal Index (“Withdrawal”)
A withdrawal index was based on self-report from the SCID interview to determine 1) history of withdrawal seizures, 2) medical detoxifications, and 3) times when one drank to stop alcohol withdrawal symptoms. The index was calculated as the sum of presence (1) or absences (0) of any of these three signs, yielding possible scores of 0, 1, 2 or 3.
Activities of Daily Living (“ADL”)
This questionnaire had two parts: Instrumental ADL with 7 questions (e.g., using the telephone, managing money) and Physical ADL with 9 questions (e.g., pushing or pulling large objects, reaching above or below shoulder level)44,45. For each activity, participants categorized their ability as needs no help (score=2 for each item), need some help (score=1), or unable to do at all (score=0). To reduce the number of variables used in the path analysis, the ADLs were pooled, rendering the maximum score possible as 32; thus, high scores indicated high level of well-being.
Statistical analysis
Statistical analysis was performed using R 3.5.1 [htpp://www.r-project.org/]. The primary metrics were sway path length derived from the force platform. Additional measures were demographics and scores on sensory, ataxia, withdrawal index, and ADL testing. Analysis was conducted in three major parts: 1) Longitudinal sway data were analyzed with a mixed-effects general linear model (lmer) separately predicting each balance score as a function of diagnosis (control vs. AUD) + age + sex. The lmer computes a slope across observations for each individual; thus, the number of observations typically increases the accuracy of each slope and gives more weight to individuals with more observations. Interactions were also examined with the model including 2-way and 3-way interactions. The model outputs produced t and p significance values for group differences. Additional posthoc testing used lm analysis to examine diagnosis + sex + age effects for the cross-sectional, baseline scores only. 2) Group differences on performance of measures entered into the sway analyses to identify significant predictors of the primary dependent variables and of ADL were examined with lm predicting each score as a function of diagnosis + sex +age. Correlations examined relations of age, alcohol consumption variables, metrics of test performance with sway metrics with family-wise Bonferroni correction applied. 3) Structural equation modeling path analyses were generated using R packages (e.g., semPaths) to estimate the strength of selective independent (i.e., exogenous) variables (diagnosis, age, pedal sensory perception metrics, DRS score, and withdrawal index) in predicting sway path length and behavioral outcome measures reflecting life condition, measured as ADL and ataxia scores.
RESULTS
Group Differences in Sway Path
The means and standard deviations (SD) for sway paths and the lmer statistical results of diagnosis, age, and sex effects are presented in Table 3 and described below. Examples of sway paths appear in Figure 1.
Table 3.
Group differences on sway path and velocity: mean (SD), p-value
| Control | AUD | Effect Size† | lmer or lm Effects: t (df), p | Interactions with Group: t (df), p | Age-by-Sex Interaction: t (df), p | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | All | Male | Female | All | Male | Female | All | Group | Age | Sex | Group-by-Age | Group-by-Age-by-Sex | Control only | AUD only | ||||||
| Sway Path: longitudinal (lmer): N= | 102 | 75 | 177 | 148 | 69 | 217 | |||||||||||||||
| Eyes closed (mm) | 67.12 | 56.82 | 62.75 | 76.00 | 66.73 | 73.05 | 0.41 | 0.60 | 0.51 | 3.204 (219.0), 0.00156 | 5.215 (296.6), 3.45e-07 | 2.283 (241.1), 0.0233 | 1.141 (293.3), 0.2546 | 0.350 (313.5), 0.7267 | 2.629 (126.0), 0.0096 | 1.742 (196.9), 0.0831 | |||||
| (21.64) | (16.47) | (20.22) | (33.26) | (25.40) | (31.22) | ||||||||||||||||
| Eyes open (mm) | 48.86 | 41.82 | 45.88 | 54.78 | 48.56 | 52.80 | 0.33 | 0.62 | 0.44 | 3.182 (209.6), 0.00168 | 8.198 (283.9), 8.48e-15 | 2.351 (228.2), 0.01957 | 0.850 (280.5), 0.3963 | 0.505 (296.5), 0.6140 | 1.927 (119.6), 0.0564 | 1.714 (193.2), 0.0882 | |||||
| (17.87) | (10.78) | (15.63) | (22.08) | (19.67) | (21.49) | ||||||||||||||||
| Sway Path: cross-sectional (lm): N= | 57 | 39 | 96 | 109 | 42 | 151 | |||||||||||||||
| Eyes closed (mm) | 65.84 | 56.17 | 61.91 | 75.60 | 68.05 | 73.50 | 0.47 | 0.68 | 0.58 | 2.861, 0.004595 | 3.723, 0.000244 | 2.053, 0.041101 | — | — | — | — | |||||
| (20.83) | (17.45) | (20.01) | (35.36) | (29.16) | (33.83) | ||||||||||||||||
| Eyes open (mm) | 46.37 | 40.31 | 43.91 | 53.49 | 48.62 | 52.14 | 0.45 | 0.70 | 0.56 | 3.111, 0.00209 | 5.886, 1.31e-08 | 1.882, 0.0611 | — | — | — | — | |||||
| (15.99) | (11.93) | (14.72) | (23.21) | (22.28) | (22.99) | ||||||||||||||||
Glass’s delta
Figure 1.
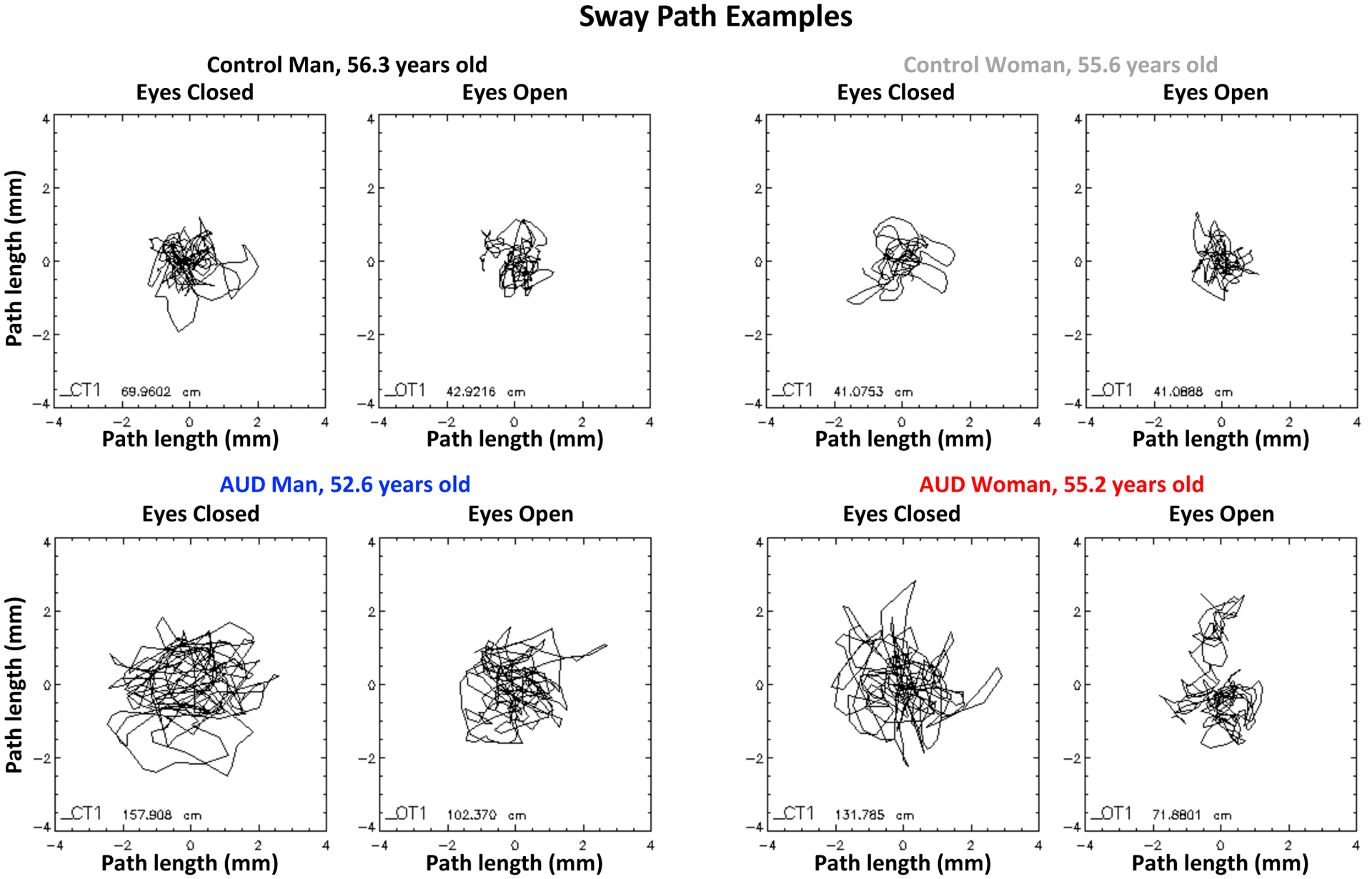
Examples of sway paths detected by the force plate as each person attempted to stand still with feet together and eyes closed (left panel of a pair) or eyes open (right panel of a pair). The top two pairs are of a control man (left) and control woman (right); the bottom pairs are of an AUD man and AUD woman. Note the longer excursion of the paths with eyes closed than open and in the AUD participants than the controls despite similar ages.
The lmer for the eyes closed condition yielded significant group, age, and sex effects. Specifically, age was significant for both groups, indicating longer sway paths with advancing age (Figure 2). A significant age-by-sex interaction for the controls indicated that the sway paths of older men were disproportionately longer than for the older women; this interaction was a trend for the AUD group. This pattern of results was the same when using cross-sectional linear modeling (lm) to test effects limited to the first assessment but was confirmed with longitudinal data. Entering socioeconomic status (SES) and years of education into the model did not alter the identified effects.
Figure 2.
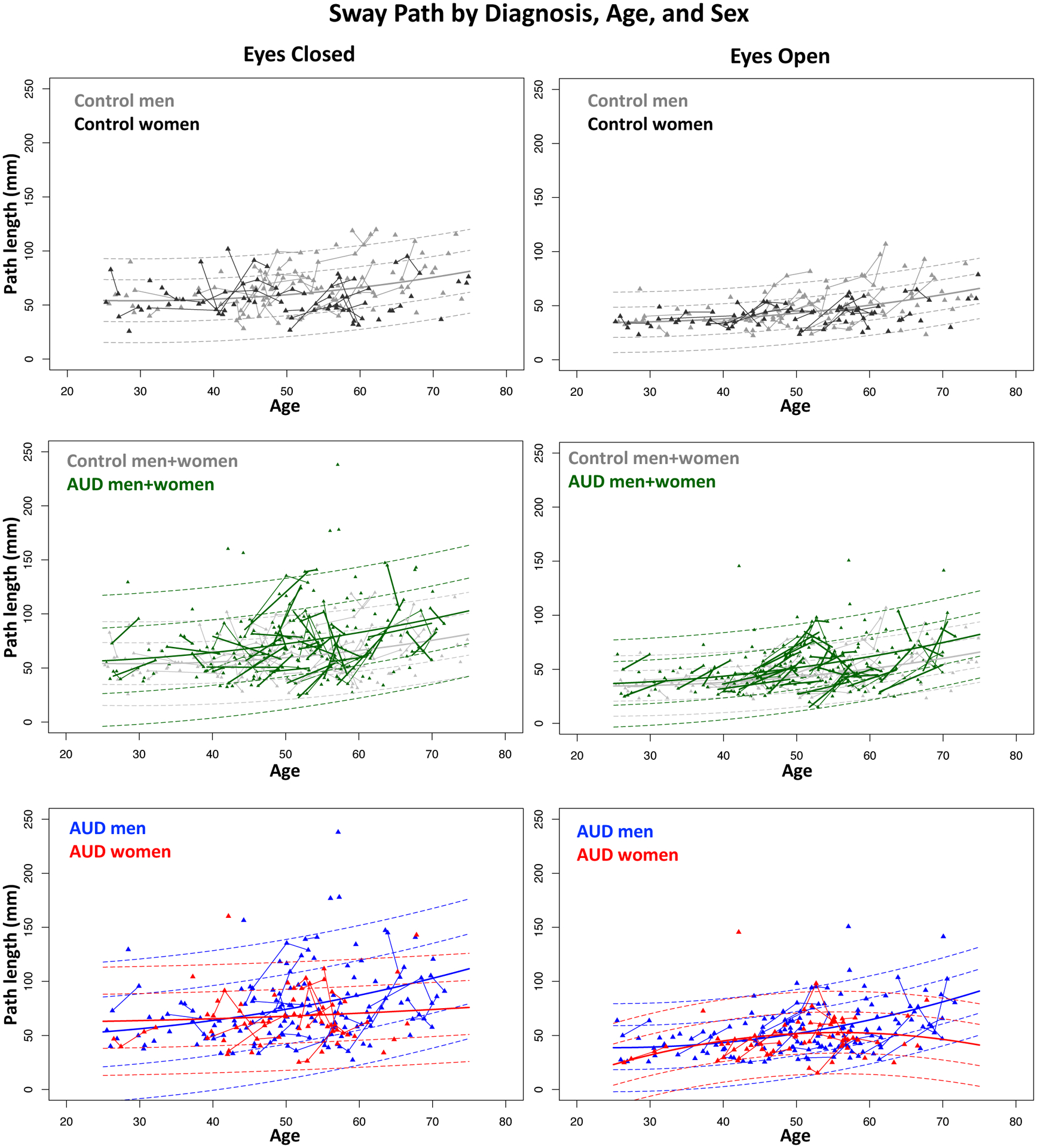
Scatterplots of the mean sway path length of each participant (triangles) at each test session; longitudinal session triangles are connected with lines. Individual AUD trajectories are drawn in thick lines in the middle two panels. The mean regression over age is the solid line; ±1 and ±2 standard deviations are plotted as dashed lines. All values are color coded by diagnosis and sex. The general trend is for longer sway paths to be associated with older ages.
As observed for eyes closed, the lmer for the eyes open condition yielded significant group, age, and sex effects with age-by-sex interactions at a trend level only. The results were similar for the initial assessment only, although the sex effect favoring balance by the women was reduced to a trend (Table 3). Entering SES and years of education into the model did not alter the effects.
Group Differences in Ataxia, Sensory, and ADL Scores and Correlations with Sway Path
The AUD group achieved poorer scores than the control group on all measures, and the differences were significant for 2-point discrimination of the feet but not the hands, five of the six ataxia conditions (the exception was taking steps with eyes closed), subjective peripheral neuropathy rating, and ADL (Table 4). Although the sex effect was not significant for any measure, the age effect was significant for all measures for the combined groups, such that poorer scores were associated with older age.
Table 4.
Group differences on sensory, ataxia, and ADL tests: †mean (SD): longitudinal observations (lmer)
| lmer Effects: t (df), p | |||||
|---|---|---|---|---|---|
| Control | AUD | Group | Age | Sex | |
| Sensory Testing: longitudinal (lmer) | |||||
| 2-point discrimination: L+R hand (mm) | 15.65 | 18.35 | 2.514 (180.3), 0.0128 | 4.796 (268.2), 2.69e-06 | −1.842 (208.2), 0.0669 |
| (5.09) | (8.14) | ||||
| n= | 151 | 200 | |||
| 2-point discrimination: L+R foot (mm) | 25.69 | 29.57 | 2.794 (159.8), 0.00585 | 9.431 (224.2), 2e-16 | −0.833 (169.6), 0.406 |
| (11.56) | (13.51) | ||||
| n= | 151 | 200 | |||
| Perceived vibration: L+R great toe (sec.) | 0.28 | 0.36 | 1.665 (185.5), 0.0976 | 7.291 (253.6), 3.91e-12 | 2.470 (203.7), 0.0143 |
| (0.56) | (0.59) | ||||
| n= | 151 | 202 | |||
| Subjective peripheral neuropathy | 0.07 | 0.25 | 2.766 (161.2), 0.00634 | 3.297 (225.1), 0.00114 | −0.150 (173.8), 0.88075 |
| (0.33) | (0.67) | ||||
| n= | 153 | 200 | |||
| Ataxia Testing: eyes closed | |||||
| Stand heel-to-toe (max=120 sec.) | 51.86 | 32.82 | −3.189 (161.4), 0.00171 | −5.666 (211.4), 4.75e-08 | 0.451 (174.7), 0.653 |
| (44.28) | (39.34) | ||||
| n= | 130 | 161 | |||
| Stand on one foot: L+R (max=60 sec.) | 17.96 | 9.92 | −4.675 (175.7), 5.84e-06 | −9.670 (233.5), < 2e-16 | −0.939 (196.0), 0.349 |
| (16.17) | (9.69) | ||||
| n= | 130 | 158 | |||
| Take steps (max=20 steps) | 4.90 | 4.11 | −1.503 (122.8), 0.1354 | −3.645 (17.8), 0.000351 | −1.787 (133.8), 0.0762 |
| (3.46) | (3.07) | ||||
| n= | 129 | 157 | |||
| Ataxia Testing: eyes open | |||||
| Stand heel-to-toe (max=120 sec.) | 109.80 | 87.48 | −4.075 (168.4), 7.08e-05 | −5.073 (215.8), 8.43e-07 | −1.021 (180.4), 0.308 |
| 27.34 | (43.06) | ||||
| n= | 130 | 161 | |||
| Stand on one foot: L+R (max=60 sec.) | 49.96 | 33.12 | −6.369 (168.6), 1.74e-09 | −9.028 (230.2), < 2e-16 | −0.450 (188.1), 0.653 |
| 15.66 | (21.50) | ||||
| n= | 132 | 160 | |||
| Step heel-to-toe (max=20 steps) | 17.29 | 14.31 | −3.864 (156.2), 0.000163 | −5.989 (207.1), 9.2e-09 | −0.003 (166.6), 0.9978 |
| 4.96 | (6.39) | ||||
| n= | 132 | 161 | |||
| ADL (max=32) | 30.70 | 28.54 | −5.220 (196.7), 4.53e-07 | −3.427 (250.7), 0.000714 | 1.800 (210.2), 0.0733 |
| (2.20) | (3.79) | ||||
| n= | 152 | 204 | |||
Bold font: alpha=.05, 1-tailed, family-wise Bonferroni for 11 comparisons, p≤0.009
Lower mean values for sensory testing and higher mean values for ataxia and ADL testing reflect better performance.
Simple correlations confirmed the age effect in both groups, where sway paths were longer with older age (Table 5, Figure 2, and Supplemental Figure 1). The only other significant correlation that was common to both groups occurred between impaired perceived vibration and longer sway paths for eyes open and closed. Additionally, in the AUD group, longer sway paths correlated with poorer scores on ADL and several ataxia measures and showed a selective correlation with poorer two-point discrimination of the feet but not the hands.
Table 5.
Correlations between the mean of each variable and the mean of sway paths over available visits
| Correlations with Sway Paths | Control | AUD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eyes Closed | N | r | p | Rho | p | N | r | p | Rho | p | |
| Lifetime alcohol drunk (Kg) | 96 | 0.351 | 0.0005 | 0.222 | 0.0294 | 151 | −0.125 | 0.1256 | −0.051 | 0.5359 | |
| Days since last drink | 89 | −0.060 | 0.5752 | −0.221 | 0.0376 | 144 | −0.040 | 0.6342 | −0.057 | 0.5008 | |
| 2-point discrimination: L+R feet | 73 | 0.106 | 0.3716 | 0.083 | 0.4828 | 134 | 0.223 | 0.0096 | 0.258 | 0.0026 | |
| 2-point discrimination: L+R hands | 73 | 0.162 | 0.1703 | 0.180 | 0.1274 | 134 | 0.021 | 0.8094 | 0.051 | 0.5557 | |
| Perceived vibration (L+R) | 73 | 0.364 | 0.0016 | 0.248 | 0.0341 | 136 | 0.330 | 0.0001 | 0.245 | 0.0041 | |
| Subjective neuropathy | 78 | 0.018 | 0.8767 | 0.017 | 0.8855 | 139 | 0.177 | 0.0374 | 0.148 | 0.0822 | |
| Activities of daily living (ADL) | 72 | −0.075 | 0.5286 | 0.098 | 0.4142 | 139 | −0.260 | 0.0020 | −0.196 | 0.0211 | |
| Stand heel-to-toe | 63 | −0.130 | 0.3106 | −0.111 | 0.3873 | 102 | −0.146 | 0.1440 | −0.227 | 0.0220 | |
| Step heel-to-toe | 63 | 0.017 | 0.8944 | −0.042 | 0.7412 | 102 | −0.283 | 0.0039 | −0.318 | 0.0011 | |
| Stand on one leg (L+R) | 63 | −0.284 | 0.0241 | −0.254 | 0.0448 | 102 | −0.265 | 0.0070 | −0.335 | 0.0006 | |
| BMI | 96 | −0.072 | 0.4876 | −0.114 | 0.2705 | 151 | 0.012 | 0.8859 | 0.010 | 0.9056 | |
| Age | 96 | 0.294 | 0.0037 | 0.238 | 0.0199 | 151 | 0.251 | 0.0019 | 0.259 | 0.0013 | |
| Eyes Open | N | r | p | Rho | p | N | r | p | Rho | p | |
| Lifetime alcohol drunk (Kg) | 96 | 0.289 | 0.0043 | 0.183 | 0.0744 | 151 | −0.055 | 0.5026 | 0.011 | 0.8911 | |
| Days since last drink | 89 | −0.047 | 0.6596 | −0.135 | 0.2060 | 144 | −0.064 | 0.4448 | −0.045 | 0.5961 | |
| 2-point discrimination: L+R feet | 73 | 0.132 | 0.2673 | 0.124 | 0.2970 | 134 | 0.268 | 0.0017 | 0.420 | 0.0000 | |
| 2-point discrimination: L+R hands | 73 | 0.234 | 0.0461 | 0.229 | 0.0508 | 134 | 0.093 | 0.2860 | 0.174 | 0.0441 | |
| Perceived vibration (L+R) | 73 | 0.415 | 0.0003 | 0.298 | 0.0104 | 136 | 0.309 | 0.0040 | 0.301 | 0.0004 | |
| Subjective neuropathy | 78 | 0.060 | 0.6008 | 0.088 | 0.4447 | 139 | 0.240 | 0.0045 | 0.200 | 0.0184 | |
| Activities of daily living (ADL) | 72 | −0.141 | 0.2390 | 0.079 | 0.5097 | 139 | −0.323 | 0.0001 | −0.292 | 0.0005 | |
| Stand heel-to-toe | 63 | −0.322 | 0.0101 | −0.259 | 0.0407 | 102 | −0.187 | 0.0597 | −0.201 | 0.0425 | |
| Step heel-to-toe | 63 | −0.191 | 0.1344 | −0.170 | 0.1826 | 102 | −0.201 | 0.0430 | −0.167 | 0.0940 | |
| Stand on one leg (L+R) | 63 | −0.283 | 0.0246 | −0.243 | 0.0545 | 102 | −0.476 | 0.0000 | −0.470 | 0.0000 | |
| BMI | 96 | −0.079 | 0.4429 | −0.109 | 0.2888 | 151 | 0.019 | 0.8137 | 0.032 | 0.6994 | |
| Age | 96 | 0.434 | 1.0E-05 | 0.406 | 0.0000 | 151 | 0.327 | 0.0000 | 0.336 | 0.0000 | |
Bonferroni correction for 12 comparisons (1-tailed): p≤.008
r=Pearson parametric correlation
Rho=Spearman nonparametric correlation
Given the high prevalence of non-alcohol drug use in the AUD group, we conducted lm that examined age, sex, cannabis, and non-cannabis drug use as predictors of sway path. For both vision conditions, only age was significant (eyes closed t=3.066, p=.0026; eyes open t=4.021, p=.00009). Neither sex (eyes closed t=1.465, p=.145; eyes open t=1.433, p=.154) nor drug use variables (cannabis eyes closed t=−0.0290, p=.772; eyes open t=−0.648, p=.518; non-cannabis eyes closed t=0.570, p=.570; eyes open t=1.352, p=.178) were significant. Similarly, history of tobacco smoking was not a significant factor in sway path differences.
Structural Equation Path Analyses: Independent Predictors of Behavioral Measures
Guided by results of the simple correlations, structural equation modeling path analysis predictor variables were those that were significant with sway path length. Accordingly, the sensory measures of 2-point discrimination of the feet, perceived vibration, and sensory symptoms (i.e., subjective peripheral neuropathy) ratings were chosen as independent predictors of sway path, which was tested as a dependent or moderator variable of each of three behavioral outcome measures in separate path analyses: ADL and ataxia scores for stepping heel-to-toe and for standing on one foot. For each path analysis, one set of models included diagnosis as a predictor, and a second set tested predictors for the AUD group only. The withdrawal index was also included as a predictor in the AUD-only analyses. The resulting statistical path analyses with path coefficients appear in Figures 4–6 and summary statistics are presented in Supplemental Tables 1–3 and Supplemental Figure 2.
Figure 4.
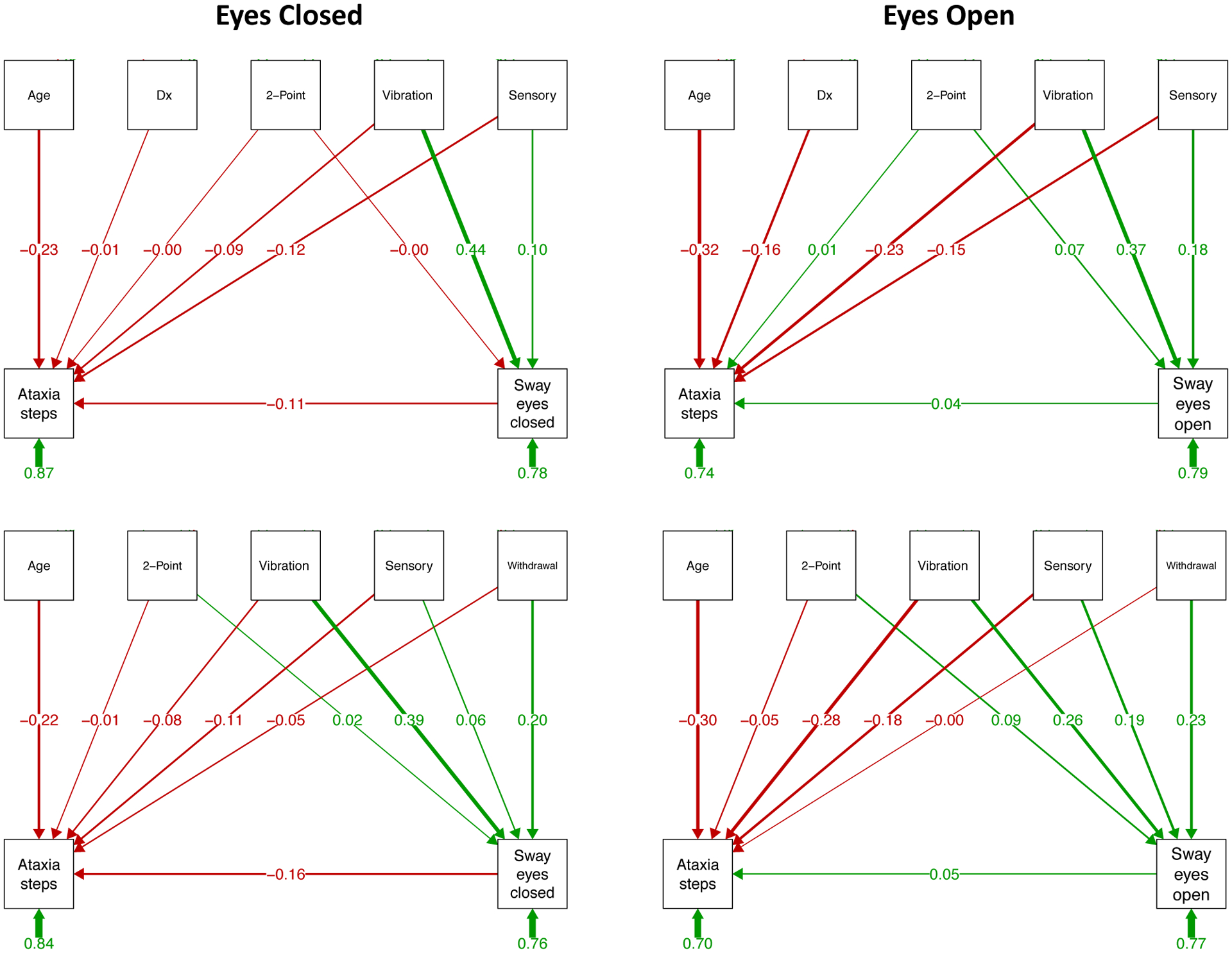
Prediction of ataxia steps and sway paths: As described in the caption for Figure 3, structural equation path analyses based on testing with eyes closed (left panels) and eyes open (right panels). The top two sets of paths include diagnosis (Dx) as a predictor, and the bottom two sets examine relations within the AUD group only and include the alcohol withdrawal index, which is meaningful for the AUD group, as a predictor. In this model, vibration sense is a consistent predictor of ataxia steps and sway path length in all path models. See Supplemental Table 3 for statistical test output.
Predicting ADL (Figure 3 and Supplemental Table 1).
Figure 3.
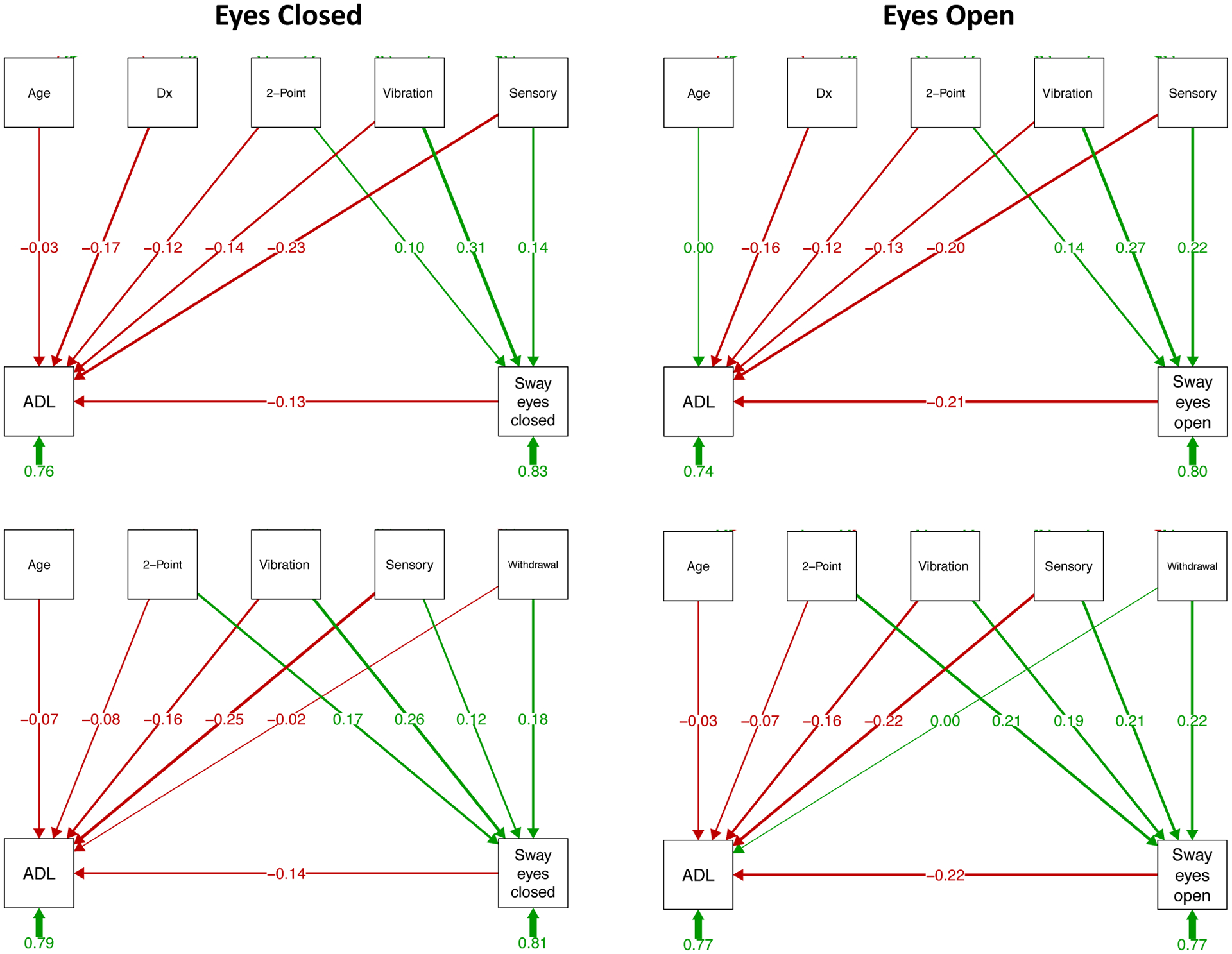
Prediction of ADL and sway paths: Structural equation path analyses based on testing with eyes closed (left panels) and eyes open (right panels). The top two sets of paths include diagnosis (Dx) as a predictor, and the bottom two sets examine relations within the AUD group only and include the alcohol withdrawal index, which is meaningful for the AUD group, as a predictor. The variables in the top boxes of each path analysis are predictors of the dependent measures in the bottom boxes; the number under each short green arrow under the bottom boxes refers to the “completely standardized solution,” which includes variance associated with latent and observed variables. The long green lines with arrows and numbers represent positive correlations or path predictions, and red ones represent negative correlations or path predictions; the thicker the line connecting a predictor and outcome measure, the stronger the relation. For example, moderate to strong predictors of ADL are subjective ratings of sensory symptoms of peripheral neuropathy (“Sensory”) regardless of vision and sway path with eyes open; vibration sense and 2-point discrimination on the soles of the feet are contributors of sway path length. See Supplemental Table 1 for statistical test output.
The seq path analysis that included diagnosis for conditions done with eyes closed (Figure 3, top left) indicated that ADL was dependent on diagnosis (z=−2.523, p=.012) and sensory symptoms (z=−3.304, p=.001) and that sway path length was dependent on perceived vibration (z=4.171, p=.000) and sensory symptoms (z=2.063, p=.039). The remaining dependencies were weak. The overall variance accounted in predicting ADL was 24.0% and was 16.9% for sway.
Within the AUD group alone (Figure 3, bottom left), only sensory symptoms were a significant predictor of ADL (z=−2.937, p=.003), whereas the strongest dependencies with eyes closed sway path were with perceived vibration (z=2.890, p=.004) and the withdrawal index (z=2.105, p=.035). The overall variance accounted in predicting ADL was 21.1% and was 18.9% for sway.
The seq path analysis including diagnosis for conditions done with eyes open indicated that ADL was dependent on diagnosis (z=−2.3382, p=.017), sensory symptoms (z=−2.943, p=.003), and sway path length (z=−2.961, p=.003) and that sway path length was dependent on 2-point discrimination (feet) (z=1.958, p=.05), perceived vibration (z=3.792, p=.000), and sensory symptoms (z=3.217, p=.001). The variance accounted in predicting ADL was 25.9% and was 20.2% for sway path.
Within the AUD group alone, ADL was dependent on sway path (z=−2.431, p=.015) and sensory symptoms (z=−2.605, p=.009). Sway path was dependent on all four predictive factors: 2-point discrimination (feet) (z=2.503, p=.012), perceived vibration (z=2.232, p=.026), sensory symptoms (z=2.585, p=.010), and withdrawal index (z=2.739, p=.006). The variance accounted in predicting ADL was 23.0% and was 23.3% for sway path.
To test whether general cognitive status influenced the path predictions of ADL, the total DRS-2 score was included as a predictor in the group and the within-AUD group path analyses. In neither case did DRS-2 score contribute significantly to the predictions nor did they significantly modulate the contributions from the other predicting variables (Supplemental Figure 2 and Supplemental Table 2).
Predicting stepping (Figure 4 and Supplemental Table 3).
The contribution of diagnosis to the eyes closed conditions was negligible; thus, the results of the path analysis were essentially the same with or without diagnosis in the model. In both cases, stepping performance was dependent on age (with diagnosis z=−2.680, p=.007; within AUD z=−2.027, p=.043), whereas sway was dependent on perceived vibration (with diagnosis z=5.780, p=.000; within AUD z=4.039, p=.000) and withdrawal index in the AUD group (z=2.160, p.031). The total variance accounted in predicting stepping was 13.5% and 22.0% for sway with diagnosis and was 16.4% for stepping and 24.3% for sway within AUD.
For the eyes open conditions, diagnosis was a relevant contributor to stepping (z=−2.197, p=.028) as were age (z=−3.930, p=.000), perceived vibration (z=−2.762, p=.006), and sensory symptoms (z=−1.991, p=.047). The latter two were also strong predictors of sway (vibration z=5.132, p=.000; sensory symptoms z=2.437, p=.015).
The same pattern of predictors was identified when diagnosis was omitted from the model: age (z=−3.024, p=002), perceived vibration (z=−2.801, p=.005), and sensory symptoms (z=−1.972, p=.049) predicted stepping performance, and perceived vibration (z=2.674, p=.007), sensory symptoms (z=2.077, p=.038), and withdrawal index (z=2.443, p=.015) predicted sway. The variance accounted in predicting stepping when including diagnosis in the model was 26.4% and was 21.1% for sway; within the AUD group the variance accounted for was 30.2% for stepping and was 22.9% for sway.
Predicting standing on one leg (Figure 5 and Supplemental Table 4).
Figure 5.
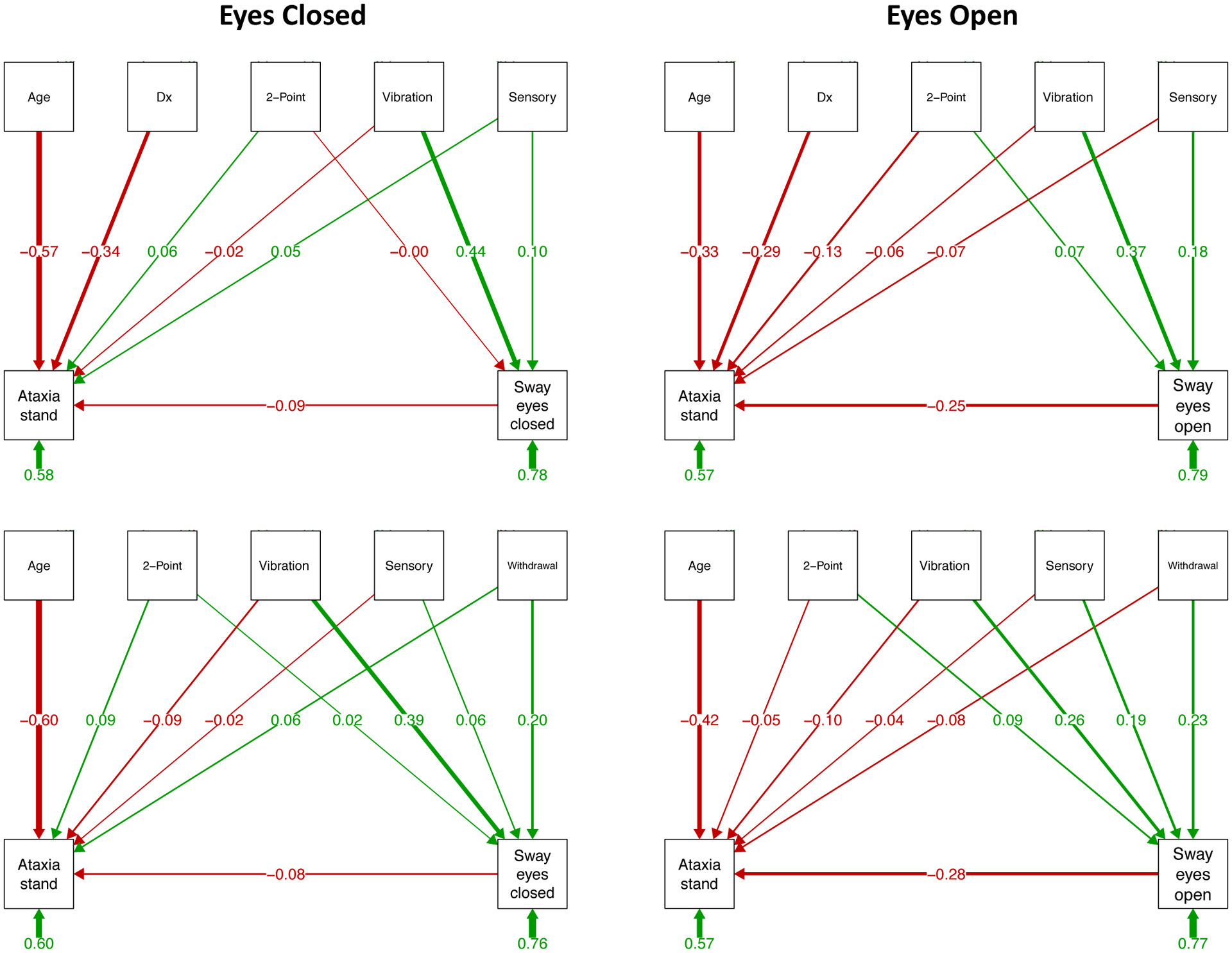
Prediction of ataxia (on-foot balance) and sway paths: As described in the caption for Figure 3, structural equation path analyses based on testing with eyes closed (left panels) and eyes open (right panels). The top two sets of paths include diagnosis (Dx) as a predictor, and the bottom two sets examine relations within the AUD group only and include the alcohol withdrawal index, which is meaningful for the AUD group, as a predictor. Here, age is a moderate to strong predictor of standing on one leg (“Ataxia stand”); in all path models, vibration sense is a consistent predictor of sway path length, which itself is a predictor of “Ataxia stand” with eyes open, where the longer the sway path, the less time one could stand on one leg. See Supplemental Table 4 for statistical test output.
The predictor pattern was different for eyes closed and open. For eyes closed, diagnosis (z=−5.088, p=.000) and age (z=−7.950, p=.000) were strong predictors of standing on one leg but sway was not, whereas perceived vibration predicted sway (z=5.780, p=.000). The variance accounted in predicting one-leg standing was 41.8% and was 22.0% for sway. When diagnosis was removed from the model, only age was a significant predictor of one-leg standing (z=−6.496, p=.000), whereas perceived vibration (z=4.039, p=.000) and withdrawal index (z=2.160, p=.031) were significant predictors of sway. The total variance account for was 39.6% for one-leg standing variance and 24.3% for sway.
For eyes open, diagnosis (z=−4.401, p=.000), age (z=−4.606, p=.000), and sway (z=−3.559, p=.000) were strong predictors of standing on one leg, whereas perceived vibration (z=4.756, p=.000) and sensory symptoms (z=2.437, p=.015) predicted sway. The variance accounted in predicting standing was 42.6% and was 21.1% for sway path. Without diagnosis in the model, age (z=−4,685, p=.000) and sway (z=−3.228, p=.001) were predictors of one-leg standing, whereas perceived vibration (z=2.674, p=.007) and withdrawal index (z=2.443, p=.015) were predictors of sway, together accounting for 43.2% of the one-leg standing variance and was 22.9% of the sway variance.
DISCUSSION
Significantly diminished postural stability manifest as longer sway paths in older control and AUD participants that were even longer in men than women, but significant age-by-AUD interactions were not forthcoming. Further analysis indicated that although all of the sensory skills tested declined with aging in both groups, different constellations of these factors appeared to influence ADL, ataxia scores, or sway path and were differentially related to the presence or absence of visual information. Consideration of these findings is presented next.
Influence of Age, Sex, and AUD on Balance
The detrimental effects on postural stability of age in the normal population and in AUD even with sustained sobriety are well established15,27,28,46 and were replicated herein. We were unable, however, to detect a reliable relation between historical alcohol consumption variables, such as amount of alcohol drunk over a lifetime or age of onset and balance measures, as reported previously13,15,47. Another series of studies found recovery on the roadside-like ataxia test used herein after a short sobriety interval48 but no further recovery with a year of abstinence12. Other measures of drinking might be more successful in heralding change. To wit, a recent study revealed the sensitivity of using a consumption metric that categorically rated the degree of reduction of drinking as predictive of the degree of frontal cortical volume recovery49. Nonetheless, in addition to the broad alcohol history differences related to diagnosis, we did find that history of withdrawal episodes, which indexes severity of alcoholism’s enduring effect, was a strong predictor of sway path length. Similarly, the Fein and Greenstein48 study reported a modest relation between standing heel-to-toe with eyes open and severity of withdrawal symptoms reported by their nontreatment seeking individuals with alcohol dependence12.
Regarding sex, both AUD and control men were more unstable than AUD or control women, and the age-sex interaction significant in the control group indicated a greater untoward effect of aging in the men than women (cf.,15). Although the AUD group exhibited a tendency for accelerated aging in sway path length compared with controls, the interaction was not significant. Despite the high prevalence of drug and nicotine dependence in the AUD group, such comorbidity did not contribute significantly to the observed group differences in sway path and so did not replicate previously reported exacerbated effects of comorbid drug48 or tobacco use13 with AUD. In fact, the nonsmoking abstinent AUD group showed recovery early into sobriety that improved to normal levels sustained with longer, continued sobriety, whereas smoking alcohol abstainers showed delayed and attenuated improvement in balance stability compared with their nonsmoking counterparts13. As relevant as age and sex were to sway path length in both groups, their contributions were diminished when considered in the context of physiological sensory factors, considered next.
Physiological Substrates of Static Balance and Quality of Life
We used two classes of measures of static balance: a traditional road-side sobriety test of ataxia and the force platform measure of sway path. Both approaches are quantitative and identified significant instability in quiet standing in the AUD group, and both were sensitive to age-related worsening of stability especially without the aid of visual cues. Critically, however, the statistical path analyses revealed different physiological mechanisms that contributed to each measurement class and that differed depending on conditions with or without vision. In particular, whereas age and diagnosis were notable mediators of ability to stand on one leg, sensory factors were strong mediators of sway path length. In turn, sway path was a strong predictor of one-leg stance with eyes open but not closed, thus suggesting vision as a common, stabilizing force regardless of class of measure. Conversely, the sway path/ataxia stance relation broke down without vision, providing further support for the dissociable processes assessed by each metric class. Another factor contributing uniquely to sway path but little to one-leg standing was the alcohol withdrawal index, thereby providing evidence for a unique influence of alcoholism-related physiological sequelae on postural instability.
The structural equation modeling path analysis structure that predicted ataxia steps, that is, the number of steps one could take walking tandem on a line, was largely unlinked to the force platform sway path length. In addition to the effects of age and diagnosis on steps taken, vibration sense exerted a notable influence on sway and tandem stepping especially with vision. These trade-offs of variance sources provide evidence that different constellations of factors are more or less likely to support or disrupt active stepping versus static standing, even though quiet standing requires an exerted, coordinated effort to maintain upright posture and balance. To the extent that both balance activities are implicit and likely depend on unconscious motor learning, it may be of little surprise that postural instability was largely not related to current cognitive status measured with the DRS.
To determine a practical interpretation of the identified factor relations, we used ADL as an outcome measure moderated by age, diagnosis, and physiological sources and ultimately mediated by sway path. The most prominent contributors to ADL were diagnosis, withdrawal index, and sensory symptoms together with sway path, especially with eyes open, but not the DRS. That result supported the interpretation that greater postural instability measured with sway path was a strong predictor of ADL beyond influences from cognitive status or age. Indeed, only diagnosis and sensory symptoms were predictors of ADL after sway path, thereby indicating a central role for lower limb and pedal discomfort in affecting quality of life.
Limitations
Like most studies, this one has limitations that can only be remedied by further investigations that include the following considerations. Despite a longitudinal component, most participants were examined only once. Although this restriction limits conclusions about aging, the longitudinal results were essential in supporting and verifying cross-sectional suggestions of age-related declines in stability measures. Further, we were unable to detect relations between sway path length and either total amount of alcohol drunk in a lifetime or duration of sobriety before testing (cf.,15) (Table 5). Such relations may be forthcoming with more longitudinal assessment and tracking of alcohol consumption variables within individuals. Metrics should recognize the importance of drinking reduction rather than total abstinence in producing harm reduction and associated improvement in performance measures49,50 that might result in diminution of sway path length and truncal tremor derived from frequency analysis of sway51 (but see24). Moreover, information about engagement in physical activities, regular exercise, and balance training may ultimately provide insight into vulnerability and resilience to postural instability in AUD (e.g.,23,52,53). Additionally, the moderate to strong influence of self-reported sensory discomfort suggestive of peripheral neuropathy to the balance measures indicates the need for further study using quantitative measures of sensory neuropathy.
Implications
The overarching aim of this analysis was to identify sensory physiological, motor, and cognitive factors that have the potential of moderating instability while standing still and to test whether postural instability, in turn, contributes to problems with activities of everyday life in normal health and in AUD (cf.,54). This endeavor is relevant to any condition that disrupts postural stability, such as normal aging and chronic alcoholism as studied herein, and to myriad neurological disorders affecting stability especially those related to aging. Our statistical path analyses identified a constellation of physiological factors that did mediate instability, including lower extremity vibration sense and self-reported sensory symptoms of peripheral neuropathy. Within the alcohol group, history of withdrawal symptoms was a major contributor to postural instability with or without vision. While age exerted defining effects on simple ataxia scores, especially standing on one leg, ADLs were most influenced by sway path length and sensory symptoms of lower limb discomfort with little contribution from age, diagnosis, or cognitive status. Recognizing the heightened vulnerability to falling as we age, these findings present ample evidence for appreciating unchecked declining postural stability, even with visual cues, as a liability for compromised quality of life and poor balance especially during one-leg standing, which we do unwittingly throughout the day, for example, while getting dressed, towel drying after bathing, or getting out of a car.
Supplementary Material
Acknowledgment
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA010723, AA005965).
Footnotes
Conflict of Interest
All authors contributed significantly to this research project, and none has any conflict with this work. This work is not being considered for publication elsewhere.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Amiridis IG, Hatzitaki V, Arabatzi F. Age-induced modifications of static postural control in humans. Neurosci Lett. 2003;350:137–140. [DOI] [PubMed] [Google Scholar]
- 2.Balmer TS, Trussell LO. Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poortvliet P, Hsieh B, Cresswell A, Au J, Meinzer M. Cerebellar transcranial direct current stimulation improves adaptive postural control. Clin Neurophysiol. 2018;129:33–41. [DOI] [PubMed] [Google Scholar]
- 4.Qiu F, Cole MH, Davids KW, et al. Enhanced somatosensory information decreases postural sway in older people. Gait Posture. 2012;35:630–635. [DOI] [PubMed] [Google Scholar]
- 5.Schmuckler MA, Tang A. Multisensory factors in postural control: Varieties of visual and haptic effects. Gait Posture. 2019;71:87–91. [DOI] [PubMed] [Google Scholar]
- 6.Surgent OJ, Dadalko OI, Pickett KA, Travers BG. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture. 2019;71:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughton CA, Slavin M, Katdare K, et al. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18:101–108. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Nussbaum MA, Madigan ML. Direct parameterization of postural stability during quiet upright stance: effects of age and altered sensory conditions. J Biomech. 2008;41:406–411. [DOI] [PubMed] [Google Scholar]
- 9.Patel M, Magnusson M, Kristinsdottir E, Fransson PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol. 2009;105:167–173. [DOI] [PubMed] [Google Scholar]
- 10.van den Hoorn W, Kerr GK, van Dieen JH, Hodges PW. Center of Pressure Motion After Calf Vibration Is More Random in Fallers Than Non-fallers: Prospective Study of Older Individuals. Front Physiol. 2018;9:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TY, Lin SI. Sensitivity of plantar cutaneous sensation and postural stability. Clin Biomech (Bristol, Avon). 2008;23:493–499. [DOI] [PubMed] [Google Scholar]
- 12.Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt TP, Pennington DL, Durazzo TC, Meyerhoff DJ. Postural stability in cigarette smokers and during abstinence from alcohol. Alcohol Clin Exp Res. 2014;381753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006;16:1077–1086. [DOI] [PubMed] [Google Scholar]
- 16.Goebel JA, Dunham DN, Rohrbaugh JW, Fischel D, Stewart PA. Dose-related effects of alcohol on dynamic posturography and oculomotor measures. Acta Otolaryngol Suppl. 1995;520 Pt 1:212–215. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- 18.Diener HC, Dichgans J, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984;57:134–142. [DOI] [PubMed] [Google Scholar]
- 19.Cardenas VA, Hough CM, Durazzo TC, Meyerhoff DJ. Cerebellar Morphometry and Cognition in the Context of Chronic Alcohol Consumption and Cigarette Smoking. Alcohol Clin Exp Res. 2020;44:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melgaard B, Ahlgren P. Ataxia and cerebellar atrophy in chronic alcoholics. J Neurol. 1986;23313–15. [DOI] [PubMed] [Google Scholar]
- 21.Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci. 2004;59:166–171. [DOI] [PubMed] [Google Scholar]
- 22.Masani K, Sayenko DG, Vette AH. What triggers the continuous muscle activity during upright standing? Gait Posture. 2013;37:72–77. [DOI] [PubMed] [Google Scholar]
- 23.Vassar RL, Rose J. Motor systems and postural instability. Handb Clin Neurol. 2014;125:237–251. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Cognitive demands during quiet standing elicit truncal tremor in two frequency bands: differential relations to tissue integrity of corticospinal tracts and cortical targets. Frontiers in human neuroscience. 2015;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000;14:178–188. [PubMed] [Google Scholar]
- 26.Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with change in brain structure. Psychiatry Res. 2007;155:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcohol Clin Exp Res. 2011;35:2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan EV, Rose J, Pfefferbaum A. Mechanisms of postural control in alcoholic men and women: biomechanical analysis of musculoskeletal coordination during quiet standing. Alcohol Clin Exp Res. 2010;34:528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choy NL, Brauer S, Nitz J. Changes in postural stability in women aged 20 to 80 years. J Gerontol A Biol Sci Med Sci. 2003;58:525–530. [DOI] [PubMed] [Google Scholar]
- 31.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2: Professional Manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 32.First M. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (DSM-V). Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 34.Skinner HA. Statistical approaches to the classification of alcohol and drug addiction. Br J Addict. 1982;77:259–273. [DOI] [PubMed] [Google Scholar]
- 35.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–1357. [DOI] [PubMed] [Google Scholar]
- 37.Nelson HE. National Adult Reading Test (NART): Test Manual. Windsor, Berks: NFER-NELSON; 1982. [Google Scholar]
- 38.Wechsler D Wechsler Test of Adult Reading (WTAR). San Antonio, TX: Pearson Education, Inc; 2001. [Google Scholar]
- 39.Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerospace Medicine. 1972;43:395–399. [PubMed] [Google Scholar]
- 40.Bigley GK. Sensation. In: rd Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: 1990. [PubMed] [Google Scholar]
- 41.Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. [DOI] [PubMed] [Google Scholar]
- 42.Corkin S, Milner B, Rasmussen T. Somatosensory thresholds--contrasting effects of postcentral-gyrus and posterior parietal-lobe excisions. Arch Neurol. 1970;23:41–58. [DOI] [PubMed] [Google Scholar]
- 43.Zahr NM, Pohl KM, Pfefferbaum A, Sullivan EV. Central Nervous System Correlates of “Objective” Neuropathy in Alcohol Use Disorder. Alcohol Clin Exp Res. 2019;43:2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 45.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 46.Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57:768–776. [DOI] [PubMed] [Google Scholar]
- 47.Diener HC, Dichgans J, Bacher M, Guschlbauer B. Improvement of ataxia in alcoholic cerebellar atrophy through alcohol abstinence. J Neurol. 1984;231:258–262. [DOI] [PubMed] [Google Scholar]
- 48.Fein G, Smith S, Greenstein D. Gait and balance in treatment-naive active alcoholics with and without a lifetime drug codependence. Alcohol Clin Exp Res. 2012;36:1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerhoff DJ, Durazzo TC. Not all is lost for relapsers: Relapers with low WHO risk drinking levels and complete abstainers have comparable regional gray matter volumes. Alcoholism Clinical and Experimental Research. 2020:in press. [Google Scholar]
- 50.Witkiewitz K, Heather N, Falk DE, et al. World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom. Addiction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad S, Rohrbaugh JW, Anokhin AP, Sirevaag EJ, Goebel JA. Effects of lifetime ethanol consumption on postural control: a computerized dynamic posturography study. J Vestib Res. 2002;12:53–64. [PubMed] [Google Scholar]
- 52.Ilg W, Synofzik M, Brotz D, Burkard S, Giese MA, Schols L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73:1823–1830. [DOI] [PubMed] [Google Scholar]
- 53.Clemson L, Fiatarone Singh MA, Bundy A, et al. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ. 2012;345:e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witkiewitz K, Kranzler HR, Hallgren KA, et al. Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder. Alcohol Clin Exp Res. 2018;42:2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


