Abstract
Heat stress (HS) results in health problems in animals. This study was conducted to investigate the effect and the underlying mechanism of HS on the proliferation and differentiation process of 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were treated at 37 °C or 41.5 °C. HS up-regulated the mRNA and protein expression level of heat shock protein 70 (HSP70). Furthermore, the proliferation of 3T3-L1 preadipocytes were significantly inhibited after HS treatment for 2 days. A large number of accumulated lipid droplets were observed under the microscope after HS treatment for 8 days. Notably, the result of oil red O staining showed that the number of lipid droplets increased significantly and the differentiation ability of the cells was enhanced after HS. Moreover, after 2 and 8 d of differentiation, HS increased the transcription levels of fat synthesis genes including peroxisome proliferators activated receptor γ (PPARγ), fatty acid binding protein 2 (AP2), fatty acid synthase (FAS) and CCAAT enhancer binding protein α (CEBPα) genes, while decreasing the transcription levels of lipid decomposition genes including ATGL and HSL genes. In addition, HS reduced the expression of AMPK and PGC-1α, as well as the dephosphorylation of AMPK. 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) can eliminate HS induced lipogenesis by activating AMPK. These results indicated that HS inhibited the proliferation of 3T3-L1 preadipocytes and promoted lipid accumulation by inhibiting the AMPK-PGC-1α signaling pathway in 3T3-L1 preadipocytes. This work lays a theoretical foundation for improving the effect of HS on meat quality of livestock and provides a new direction for the prevention of obesity caused by HS.
Keywords: Heat stress, 3T3-L1 preadipocyte, Proliferation, Differentiation, AMPK, PGC-1α
Introduction
The sustainable development of livestock production system is affected by climate change. In high temperatures, when the body temperature exceeds the specific range of heat balance (the total heat load exceeds the heat dissipation capacity), the animal is in heat stress (HS) (Feder and Hofmann 1999). HS reaction results in health problems and metabolic disorders in animals, which has become one of the main threats affecting livestock production causing serious economic losses in husbandry all over the world (Li et al. 2019; Purohit et al. 2015). The activation of heat shock proteins (HSP) that is known to be involved in restoring cell function and in enhancing cell viability under heat and other pressures was considered to be an important adaptive response in heat adaptation (Horowitz et al. 1997). The measure to deal with HS is to improve the body’s tolerance to stress through the high expression of heat shock protein (HSP) (Horowitz et al. 1997). Among them, HSP70 is the most sensitive to temperature fluctuation and can be used as an important molecular biomarker of animal HS (Min et al. 2015).
Lipid regulation plays an important role in meat quality of livestock and poultry (Liu et al. 2018; Wood et al. 2008). HS response also involves changes in lipid metabolism. The lipid content in the subcutaneous adipose tissue of pigs increased under HS conditions (Qu et al. 2015b; Qu et al. 2016b). The mRNA expression level of fat synthesis genes in HS broilers increased significantly, resulting in lipid accumulation in the liver (Liu et al. 2018). When young rats were exposed to high temperature, the amount of lipids and cholesterol increased in the liver, and the fat composition was significantly increased (Katsumata et al. 1990). In addition, HS promoted the expression of CCAAT enhancer binding protein α (CEBPα), fatty acid binding protein 2 (AP2), and fatty acid synthase (FAS) genes in porcine subcutaneous adipocytes (Qu et al. 2015). At the same time, HS promoted the synergistic effect between CEBPα and peroxisome proliferators activated receptor γ (PPARγ) (Qu et al. 2015). Thermal stimulation after adipogenesis induced by mouse preadipocytes also proved that there was no inhibitory effect on lipid accumulation (Ezure and Amano 2009). There is also evidence that HS increased lipid accumulation by reducing the expression of adipolysis related genes including adipose triglyceride lipase (ATGL) and hormone sensitive triglayceride lipase (HSL) in pigs. However, the underlying molecular mechanism of HS promoting fat deposition has not been well understood.
Adenosine-activated protein kinase (AMP-activated protein kinase, AMPK) plays an important role as an intracellular energy sensor in the regulation of lipid metabolism (Kodiha et al. 2007; Zhang et al. 2016). Under physiological or pathological stimulation, AMPK is allosterically activated by the increase of intracellular AMP/ATP ratio or the phosphorylation of threonine 172 in α subunit (Min et al. 2015). 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is a mimic of AMP, which can activate AMPK without changing the level of adenine nucleotides in cells (Thomson 2018). When AMPK was activated, it can regulate the expression of downstream key factor peroxisome proliferators activated receptor gamma co-activator 1 α (PGC-1α) to regulate fatty acid and glucose homeostasis and plays the role of cell energy sensor (Coughlan et al. 2013). After HS, the expression of AMPK and PGC-1α were downregulated, thus reducing their antioxidant capacity in chicken myoblasts (Xue et al. 2017). Mild HS induced mitochondrial biogenesis by activating the AMPK-PGC-1α pathway in C2C12 myotubes (Liu and Brooks 2012). However, it is not clear whether HS can regulate lipid metabolism through the AMPK-PGC-1α pathway. In this study, 3T3-L1 preadipocytes were selected as experimental materials, and an in vitro adipocyte differentiation model was used to observe the response of adipocyte differentiation under HS. The aim of this study is to investigate the proliferation of preadipocytes and the changes of lipid metabolism during differentiation in a HS environment.
Materials and methods
Cell culture and differentiation
3T3-L1 preadipocytes were provided by the Genetics and Breeding Laboratory of Guangxi University. 3T3-L1 preadipocytes were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) with 10% fetal bovine serum (FBS, Gibco, USA), incubated at 37 °C with 5% CO2, changing the medium every 2 d (Day2, D2). After 2 d of complete fusion, the cells were treated at 37 °C (Control group) or 41.5 °C (HS group) under 5% CO2 with induced differentiation medium including 10% FBS, 90% DMEM, 1% penicillin/streptomycin (Sigma-Aldrich, USA), 1 μM dexamethasone (Sigma-Aldrich, USA), 10 μg/ml insulin (Gibco, USA), and 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, USA). Cultured for 2 d, then replaced the differentiation medium with 10% FBS, 90% DMEM and 10 μg/ml insulin continued to develop to the 8 d (Day8, D8), cell differentiation mature.
AICAR-treated cells
The cells were divided into four groups: 37 °C group, 37 °C + AICAR group, 41.5 °C group, and 41.5 °C + AICAR group. The 37 °C group and 41.5 °C group were added with differentiation medium, the 37 °C + AICAR group and 41.5 °C + AICAR group were added with differentiation medium containing 0.2 mM (Rao et al. 2015) AICAR (Selleck, China). After 2 days of culture, the 37 °C group and 41.5 °C group were changed to differentiation medium, the 37 °C + AICAR group and 41.5 °C + AICAR group were changed to differentiation medium containing 0.2 mM AICAR.
Cell viability assay
3T3-L1 preadipocytes were seeded in 96 well plates with a density of 3 × 103 cells/well. After 24 h of culture, HS treatment was performed. After 2, 4, 6, 8, and 10 d of treatment, CCK-8 kit (Solarbio, Beijing, China) was used to measure cell viability according to the kit instruction. Cell activity was quantified by measuring the absorbance of each hole at 450 nm.
Flow cytometry analysis
The effect of HS on cell apoptosis and cell cycle distribution was analyzed by flow cytometry. The cells with a density of 1×105 were inoculated into the six-pore plate. After HS treatment for 2 d, PE annexin V and 7-AAD were stained with the apoptosis detection kit (BD Pharmingen, USA) according to the manufacturer's instructions. It was detected by flow cytometry and analyzed by Flowjo (BD, USA) software.
After HS treatment for 2 d, 1×106 cells were collected. The cells were washed once with PBS and 1 ml DNA standing solution, and 10 ul permeability solution were added, mixed well with vortex and shaken, and incubated at room temperature and in the dark for 30 min; cell cycle was detected on a flow cytometer and analyzed with Modfit LT (Verity software house, USA) software.
Oil red O staining
After 8 d of induced differentiation culture, the cells were washed with PBS and fixed with 10% formalin solution for 10 min. The formalin was discarded, the cells were rinsed with PBS and then washed with 60% isopropanol. Diluted Oil Red O (Solarbio, Beijing, China) solution was added for 20 min. Finally, the excess dye solution was cleaned with PBS and photographed with an inverted microscope.
RNA extraction and cDNA synthesis
RNAios (TaKaRa, Dalian, China) was used to extract the total RNA from 3T3-L1 cells. After the concentration and purity of the total RNA were detected, it was reverse transcribed into cDNA by Reverse transcription Kit (TaKaRa, Dalian, China). Real-time PCR was performed with cDNA as template. The PCR reaction mixture contained 5 μL of TB GreenTM Premix Ex Taq ™ II (TaKaRa, Dalian, China), 0.25 μL of forward and reverse primers respectively, 1 μg of cDNA and 2 μL of RNase Free H2O in a 10 μL reaction system. The reaction procedure is as follows: pre-denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, extension at 60 °C for 30 s, 45 cycles, extension at 65 °C for 5 s, extension at 95 °C for another 5 min. The primers used in this study were shown in Table 1. The mRNA abundance of each gene was calculated by 2−ΔΔCt.
Table 1.
Real-time PCR primers sequence
| Genes | Forward | Reverse |
|---|---|---|
| HSP70 | ATCACCATCACCAACGAC | ACTTGTCCAGCACCTTCTT |
| PPARγ | GATGGAAGACCACTCGCATT | AACCATTGGGTCAGCTCTTG |
| CEBPα | TGGACAAGAACAGCAACGAG | TCACTGGTCAACTCCAGCAC |
| FAS | CCCTTGATGAAGAGGGATCA | ACTCCACAGGTGGGAACAAG |
| AP2 | TCAGCGTAAATGGGGATTTGG | GTCTGCGGTGATTTCATCGGA |
| ATGL | ATGGTGCCCTACACGCTG | GCCTGTCTGCTCCTTTATCC |
| HSL | CTTTGCGGGTATTCGGGAACA | ATGCTGCGGCGGTTGGA |
| AMPK | CACCCTGAAAGAGTACCGT | CATTTTGCCTTCCGTACACCT |
| PGC-1α | TATGGAGTGACATAGAGTGTGCT | GTCGCTACACCACTTCAATCC |
| 18S rRNA | CCCACGGAATCGAGAAAGAG | TTGACGGAAGGGCACCA |
Western blot
Extraction of proteins by radioimmunoprecipitation assay (RIPA, Solarbio, Beijing, China). First, the cells were cleaned twice with cold PBS and then suspended in RIPA buffer containing a certain proportion of commercial protease and phosphatase inhibitor (Sigma-Aldrich, USA), at 4 °C 15,000 rpm for 15 min. The protein content of the supernatant was determined using a protein concentration determination kit (Beyotime, China), and reagents were prepared according to the instructions. 1× loading buffer was added and boiled in a water bath for 10 min. The loading volume of each well was 50 μg of proteins to the 10% SDS polyacrylamide gel on 100 V electrophoresis 1.5 h. Proteins were transferred to a nitrated cellulose membranes (Bio-Rad, USA.) at 100 V and sealed overnight with 5% non-fat milk at 4 °C (Difco TM Skim milk, BD, USA). After that, membranes were imprinted with the corresponding primary antibodies such as anti-GAPDH, anti-HSP70, anti-P-AMPK, anti-PGC-1α (Abclonal, China) and anti-AMPK (OmnimAbs, Japan); diluted with primary antibodies dilution of 1:2000–500, incubated at 37 °C for 2 h, and then rinsed with TBST 3 times. The membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Abclonal, China) for 1h at room temperature. Cleaned the membranes three times with TBST. In order to be able to see the exposed target protein, the membranes were with eECL Western Blot Kit (CWbio, China) to detect protein bands. The imaging system was used for exposure imaging (Bio-Rad, USA). Image J software was used to analyze the gray level of protein bands, SPSS software was used for statistical analysis, and GraphPad software was used for graphic analysis of the data.
Statistical analysis
One-way ANOVA data from IBM SPSS statistics 20 software was used for statistical analysis. The results were expressed as mean ± SEM. P < 0.05 means significant difference, and P < 0.01 or P < 0.001 indicates extremely significant difference. All quantitative experiments were repeated three times.
Results
Heat stress promotes expression of HSP70 in 3T3-L1 preadipocytes
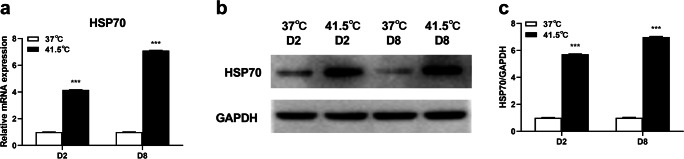
Previous research has shown that HS can cause cell heat shock response (Chen et al. 2018). One highly conserved gene HSP70 in the heat shock protein family was detected in this study. After HS treatment for 2 d, the mRNA and protein expression level of HSP70 were upregulated (P = 0.0001, 0.0003), and after HS treatment for 8 d, the mRNA and protein expression level of HSP70 were also up-regulated (P = 0.0006, 0.0003) (FigS. 1a–c). The results showed that we had established the heat stress model in vitro successfully.
Fig. 1.
HS promotes expression of heat shock protein 70 in 3T3-L1 adipocytes. The cells were treated with control group (37 °C) or HS (41.5 °C) for 2 and 8 d. The mRNA expression levels of HSP70 a was detected by real-time PCR. The expression of HSP70 protein abundance was detected by Western blot b, c. The data represent the mean ± SEM (n = 3). *** The mean difference were extremely significant ( P < 0.001), compared with 37 °C
Effects of heat stress on 3T3-L1 preadipocytes
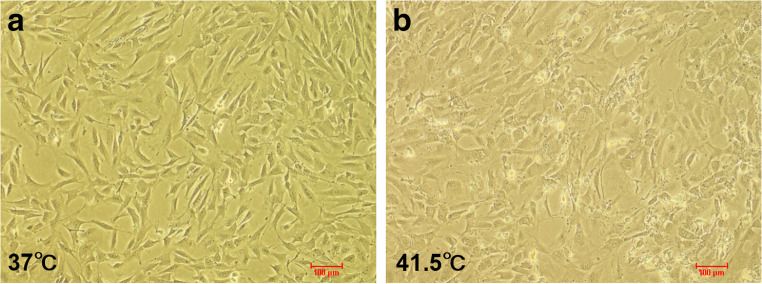
Firstly, we studied the growth and morphology of 3T3-L1 preadipocytes at 41.5 °C (Fig. 2). After 2 d of HS treatment, the cells tended to be irregular under the microscope, and the dead cells could be seen floating in the medium without changing the medium. The results showed that HS could induce 3T3-L1 preadipocyte apoptosis.
Fig. 2.
The effect of heat stress on 3T3-L1 preadipocytes. a, b Control group (37 °C) and HS (41.5 °C) were treated for 2 d (n = 3)
Heat stress inhibits 3T3-L1 preadipocytes proliferation
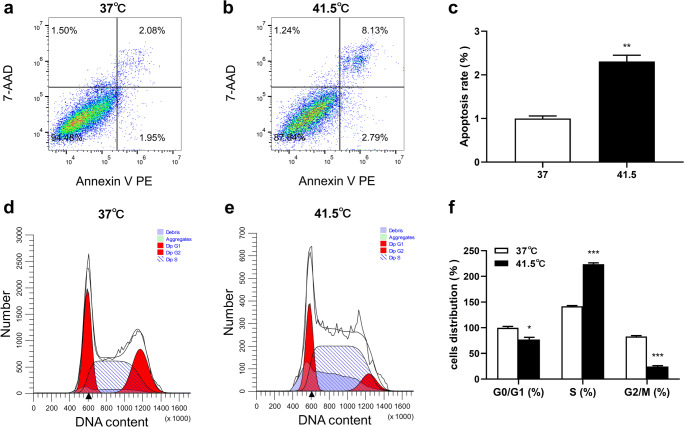
To investigate the effects of HS on the proliferation of 3T3-L1 preadipocytes, cells were treated at high temperature. After HS 2 d, cell viability was decreased in a time-dependent manner, but the decline trend slowed down (Table 2). In order to determine whether the decline of cell viability was caused by proliferation inhibition, the proliferation of 3T3-L1 preadipocytes were determined by flow cytometry. As shown in Fig. 3, after HS treatment for 2 d, cell proliferation was analyzed by cell apoptosis and cell cycle kit combined with flow cytometry. The apoptosis rate was significantly increased (P = 0.0016), and the cell allocation in S phase was significantly upregulated (P = 0.0001). Cells were moving from the normal stage to the apoptotic stage and apparently arresting in the S phase. It was suggested that HS reduced cell viability and induced apoptosis, which may be caused by cell arrested in S phase.
Table 2.
Effect of heat stress on the viability of 3T3-L1 preadipocytes
| Cell viability (%) | ||||||
|---|---|---|---|---|---|---|
| Condition | D0 | D2 | D4 | D6 | D8 | D10 |
| 37 °C | 100.00 ± 0.02a | 110.40 ± 0.21a | 131.65 ± 0.23a | 155.32 ± 0.05a | 167.40 ± 0.09a | 168.18 ± 0.41a |
| 41.5 °C | 99.91 ± 0.03a | 108.66 ± 0.32b | 119.67 ± 0.15b | 120.54 ± 0.15b | 126.44 ± 0.18b | 127.27 ± 0.10b |
| P value | 0.0134 | 0.0432 | 0.0023 | 0.0064 | 0.0238 | 0.0463 |
Cell viability assay was repeated three times with 5 wells in each group. D: Day; a, b: Different superscripts within the same column, the average values of different letters were significantly different, (P < 0.05)
Fig. 3.
The effect of HS on proliferation of 3T3-L1 preadipocytes was investigated by detecting cell apoptosis and cell cycle. a-c HS induced apoptosis of 3T3-L1 preadipocytes. The cells were exposed to 37 °C or 41.5 °C for 2 d, analyzed by flow cytometry. In the two-dimensional FCM analysis, the cells were divided into 4 subgroups: the lower left quadrant was the living cell group, the lower right quadrant was the early apoptotic cells, the upper right quadrant was the late apoptotic cells, and the upper left quadrant was the necrotic cells. d-f HS induced S phase arrest of 3T3-L1 preadipocytes. The cells were exposed to 37 °C or 41.5 °C for 2 d, analyzed by flow cytometry. The data represent the mean ± SEM (n = 3). * The mean difference was significant (P < 0.05) and ** or *** mean difference were extremely significant (P < 0.01, P < 0.001), compared with 37 °C
Heat stress promotes adipogenic differentiation of 3T3-L1 preadipocytes
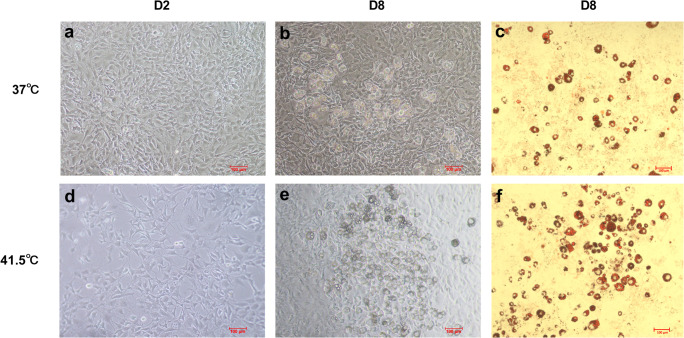
In order to detect whether HS directly affects the adipogenic differentiation of 3T3-L1 preadipocytes, the lipid droplet formation of 3T3-L1 preadipocytes induced by HS was observed under inverted microscope. The result showed that there was a little cell death after HS 2 d and the differentiation degree of the HS treatment group was higher than that of the control group at 8 d induced differentiation. The result of oil red O staining showed that the number of lipid droplets were increased significantly and the differentiation ability of the cells was enhanced after HS (Fig. 4).
Fig. 4.
HS promotes adipogenic differentiation of 3T3-L1 preadipocytes. Control group (37 °C) and HS (41.5 °C) were treated for 2 d a, d and 8 d b, e and oil red O staining for 8 d c, f were observed (n = 3)
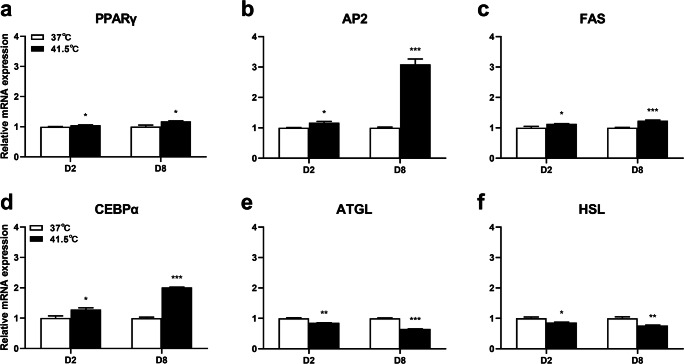
Heat stress affects the mRNA expression of genes related to fat synthesis and decomposition
In order to further verify the effect of HS on differentiation of 3T3-L1 preadipocytes, the mRNA expression levels of genes related to fat synthesis were detected. After HS for 2 d, the mRNA expression of PPARγ, AP2, FAS, and CEBPα were significantly upregulated (P = 0.0271, 0.0233, 0.0417, 0.0395), and after HS for 8 d, the mRNA expression of PPARγ, AP2, FAS, and CEBPα were significantly upregulated (P = 0.0312, 0.0005, 0.0005, 0.0002) (Figs. 5a–d). Next, we studied the effect of HS on cell lipolysis genes. After HS for 2 d, the results of real-time PCR showed that the mRNA expression of ATGL and HSL genes were decreased (P = 0.0021, 0.0351), and after HS for 8 d, the results of real-time PCR showed that the mRNA expression of ATGL and HSL genes were decreased (P = 0.0009, 0.0095) (Fig. 5e, f). Therefore, HS increased the transcription levels of fat synthesis genes and decreased the transcription levels of lipid decomposition genes.
Fig. 5.
Effect of HS on mRNA expression of fat synthesis and decomposition genes in 3T3-L1 adipocytes. The cells were treated with control group (37 °C) or HS (41.5 °C) for 2 and 8 d. The mRNA expression levels of PPARγ a, AP2 b, FAS c, CEBPα d, ATGL e and HSL f were detected by real-time PCR. The data represent the mean ± SEM (n = 3). * The mean difference was significant (P < 0.05) and ** or *** mean difference were extremely significant (P < 0.01, P < 0.001), compared with 37 °C
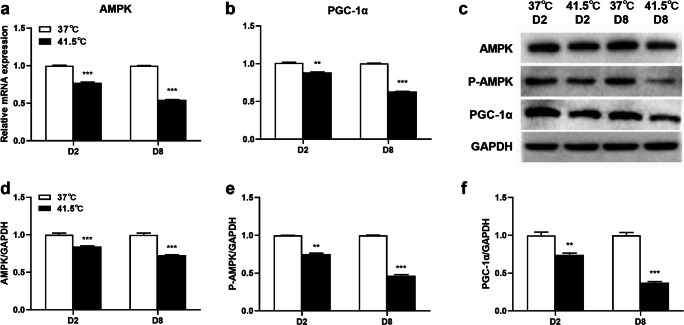
Heat stress inhibits AMPK-PGC-1α signaling pathway
AMPK-PGC-1α signaling pathway plays a key regulatory role in preadipocyte differentiation. In order to determine whether HS can affect AMPK-PGC-1α signaling pathway during 3T3-L1 preadipocytes differentiation, the effects of HS on AMPK and PGC-1α expressions were detected after 2 and 8 d of differentiation. After HS for 2 d, HS reduced the mRNA expression levels of AMPK and PGC-1α, compared with the control group (P = 0.0003, 0.0064), and after HS for 8 d, HS reduced the mRNA expression levels of AMPK and PGC-1α, compared with the control group (P = 0.0008, 0.0005). After HS for 2 d, western blot analysis showed that cells were induced rapid dephosphorylation of AMPK thr172 and reduced the protein abundance of AMPK and downstream key gene PGC-1α at 41.5 °C (P = 0.0031, 0.0006, 0.0062). After HS for 8 d, western blot analysis showed that the abundance of AMPK, PGC-1α and ampk-thr172 phosphorylated protein decreased at 41.5 °C (P = 0.0007, 0.0005, 0.0005) (Fig. 6). Therefore, the results suggested that HS can inhibit AMPK-PGC-1α signaling pathway, which may be related to the promotion of HS on adipogenesis.
Fig. 6.
Effects of HS on AMPK-PGC-1α signaling pathway in 3T3-L1 cells. The cells were treated with control group (37 °C) or HS (41.5 °C) for 2 and 8 d. The mRNA expression levels of AMPK a and PGC-1α b were detected by real-time PCR. The expression of AMPK, P-AMPK and PGC-1α protein abundance were detected by Western blot c-f. The data represent the mean ± SEM (n = 3). ** or *** mean difference were extremely significant (P < 0.01, P < 0.001), compared with 37 °C
Activation of AMPK inhibits 3T3-L1 preadipocyte differentiation
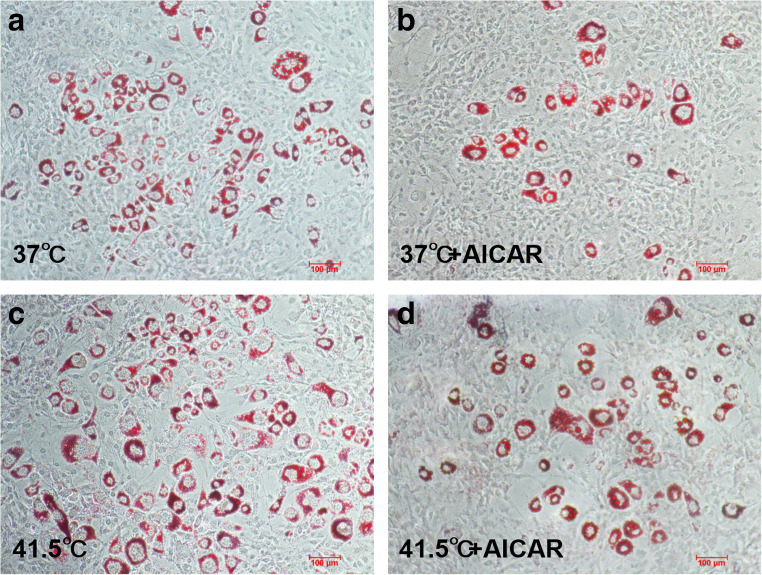
Next, we investigated whether activation of AMPK inhibits HS-induced enhanced adipogenesis. The cells were treated with AMPK activator (AICAR) and HS (Fig. 7). AICAR is an adenosine analogue, which is absorbed by adenosine transporter on cell membrane and then phosphorylated to form 5-amino-4-imidazolecarboxamide Ribotide (ZMP). Therefore, ZMP mimics AMP and stimulates the phosphorylation of AMPK in cells (Merrill et al. 1997). AICAR alone could reduce fat accumulation, while HS alone could significantly increase fat accumulation. When AICAR and HS were combined, the accumulation of fat was significantly reduced.
Fig. 7.
AICAR can inhibit fat accumulation induced by HS. a-d Cells treated with HS or AICAR (0.2 mM) for 8 d were stained with oil red O (n = 3)
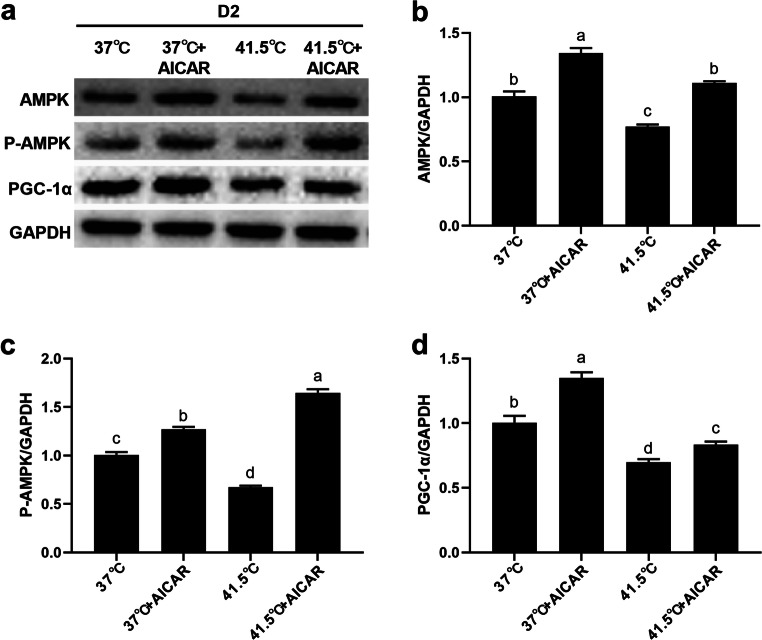
Activation of AMPK upregulates the protein expression of AMPK-PGC-1α signaling pathway key genes
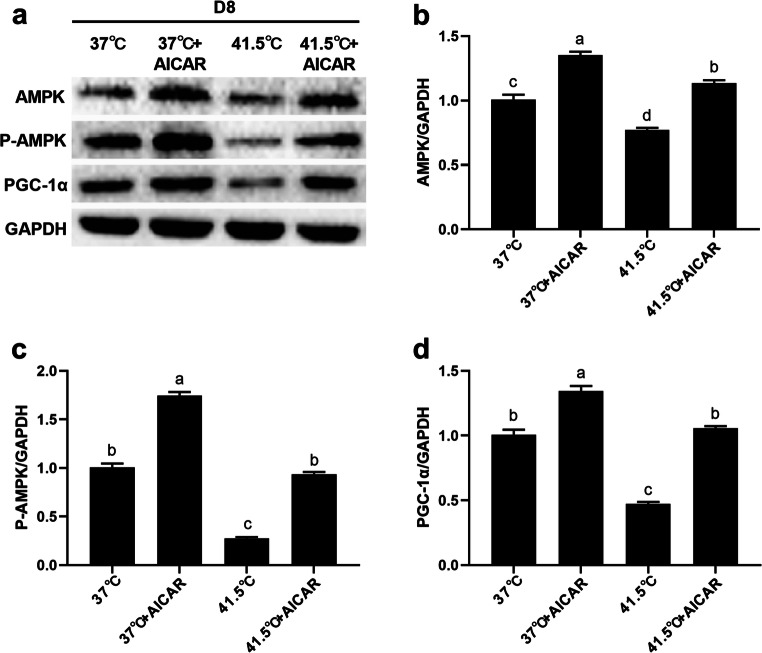
Figures 8 and 9 show the protein expression levels of AMPK-PGC-1α signaling pathway key genes at 2 and 8 d after AICAR and HS treatment. After HS for 2 d, AICAR alone significantly increased the protein expression of AMPK、 P-AMPK, and PGC-1α (P = 0.0064, 0.0078, 0.0013), and after HS for 8 d, AICAR alone significantly increased the protein expression of AMPK、P-AMPK, and PGC-1α (P = 0.0031, 0.0045, 0.0026) compared with the 37 °C group. After HS for 2 d, the protein expressions of AMPK, P-AMPK, and PGC-1α were significantly decreased in HS-treated group (P = 0.0092, 0.0036, 0.0077), and after HS for 8 d, the protein expressions of AMPK, P-AMPK, and PGC-1α were significantly decreased in HS-treated group (P = 0.0076, 0.0084, 0.0061) compared with the 37 °C group. Compared with cells treated with HS alone, after HS for 2 d, the protein expression of AMPK, P-AMPK, and PGC-1α in cells treated with HS and AICAR was significantly increased (P = 0.0055, 0.0081, 0.0029), and after HS for 8 d, the protein expression of AMPK, P-AMPK, and PGC-1α were significantly increased (P = 0.0072, 0.0012, 0.0031). These results suggest that the activation of AMPK can eliminate HS-induced adipogenesis, which reversely verifies that HS promotes adipogenesis through AMPK-PGC-1α signaling pathway.
Fig. 8.
AICAR could up-regulate the protein expression of key genes in the AMPK-PGC-1α signaling pathway after HS 2 d. The cells were treated with HS (41.5 °C) or AICAR (0.2 mM) for 2 d. a-d The expression of AMPK, P-AMPK and PGC-1α protein abundance were detected by Western blot. Numbers represent mean ± SEM (n = 3). Means with different letters were significantly different at P < 0.01
Fig. 9.
AICAR could up-regulate the protein expression of key genes in the AMPK-PGC-1α signaling pathway after HS 8 d. The cells were treated with HS (41.5 °C) or AICAR (0.2 mM) for 8 d. a-d The expression of AMPK, P-AMPK and PGC-1α protein abundance were detected by Western blot. Numbers represent mean ± SEM (n = 3). Means with different letters were significantly different at P < 0.01
Discussion
In this study, we found that HS treatment promoted HSP70 expression, which indicated the HS model in the introduction was established successfully. HS reduced cell viability and induced apoptosis. Furthermore, HS inhibited 3T3-L1 preadipocytes proliferation. Lipid accumulation was stimulated during the differentiation of 3T3-L1 preadipocytes. In addition, we showed that HS increased the transcription level of fat synthesis genes and decreased the transcription levels of lipid decomposition genes. Activation of AMPK eliminated HS-induced lipogenesis. Mechanistically, we proved that HS promoted lipid accumulation during 3T3-L1 preadipocytes differentiation by inhibiting the AMPK-PGC-1α pathway.
HSP is an evolutionarily highly conserved protein family with molecular chaperones, heat resistance, anti-peroxidation damage, and other characteristics (Beere et al. 2000; Ohashi et al. 2000). They are most significantly synthesized after cells are stressed (Feder and Hofmann 1999; Park et al. 2005). Heat shock proteins are divided into HSP27, HSP60, HSP70, HSP90, and other protein families according to their molecular weight (Xie et al. 2014; Yan et al. 2009). HSP70 has been found to play a key role in the folding and activity of hundreds of thermodynamically unstable “client” proteins among them and is considered an important regulatory factor in the process of adaptation to HS in animals (Bharati et al. 2017; Zeni et al. 2017). Therefore, the expression level of HSP70 can be used as the standard for the establishment of an HS model. In this study, the mRNA and protein expression levels of HSP70 gene were increased, consistent with the research result of Bernabucci et al. (Bernabucci et al. 2009), which successfully established an HS model.
Cell survival rates decrease within the temperature range of 40–47 °C (Raaphorst et al. 1979). Due to long-term heat resistant cells, below 42.5 °C, survival rates exponentially decline, declining more slowly after 4–5 h (Sapareto et al. 1978; Urano 1988). In this study, we found that a few dead cells could be observed under the microscope after HS treatment, and cell activity decreased in a time-dependent manner. However, with the extension of HS time, the rate of decline slowed down. In cells continuously heated at 41.5 °C, the significant effect was S phase arrest, and the cells could not carry out a normal repair process, leading to cell death (Dewey et al. 1971; Mackey et al. 1996). After HS treatment for 2 d, the apoptotic rate increased and the cells were arrested in S phase. The reduction of cell viability and apoptosis induced by HS may be caused by cell arrest in S phase, which was consistent with previous studies.
Next, we investigated the effect of HS in the process of differentiation of 3T3-L1 preadipocytes. In a previous study, HS stimulated the formation of lipid droplets in porcine subcutaneous adipocytes and increased the content of serum triglyceride (TAG) (Qu et al. 2015). In this study, we observed that HS significantly improved the degree of cell differentiation. In addition, oil red O staining showed that HS treatment increased the number of lipid droplets. This is direct evidence that HS affects the differentiation ability of 3T3-L1 preadipocytes.
We observed an apparent increase in the number of lipid droplets in 3T3-L1 preadipocytes stimulated by HS. Next, we detected the mRNA expression of several key genes involved in fat synthesis. PPARγ plays an important role in fat production, fatty acid oxidation, storage and transportation (Mansour 2014; Takano and Komuro 2009). Previous research with 3T3-L1 cells showed that the mRNA expression of PPARγ increased significantly in a temperature-dependent manner (Bernabucci et al. 2009). HS promoted the expression of CEBPα homologue, FAS and AP2 in adipose tissue (Qu et al. 2015). After CEBPα and PPARγ were activated, they regulated each other to promote fatty acid uptake and TAG synthesis, thus promoting adipocyte differentiation (Jang 2016; Miller et al. 1996). In this study, the mRNA expression levels of PPARγ, AP2, FAS, and CEBPα in 3T3-L1 preadipocytes differentiation were significantly up-regulated by HS treatment. Under the action of enzymes, fat cells or adipose tissue decompose step by step, and finally produce free fatty acids and glycerol, which is called lipolysis. ATGL and HSL are the key enzymes in lipolysis. ATGL is located on the surface of lipid droplets and participates in the first step of lipolysis and is the rate limiting enzyme of fat hydrolysis. ATGL hydrolyzes TAG to diacylglycerols and non-esterified fatty acids (NEFAs) (Bertile and Raclot 2011; Nie et al. 2010). HSL can hydrolyze a variety of acyl esters, including TAG, diacylglycerol, and monoacylglycerol (Gaidhu et al. 2010; Small et al. 1989). During HS, the mRNA expression levels of ATGL and HSL were significantly decreased in the subcutaneous adipose tissue of growing pigs (Kellner et al. 2016; Victoria et al. 2015). Our results showed that the mRNA expression of ATGL and HSL genes were decreased after HS in 3T3-L1 preadipocytes differentiation. Therefore, taken together, these results showed that HS increased the mRNA expression of fat synthesis genes and decreased the mRNA expression of fat decomposition genes in 3T3-L1 preadipocytes, suggesting that the lipid accumulation effect of HS could be mediated by an improved adipogenesis capacity and a suppressed fatty acid synthesis capacity.
AMPK is a key regulator of lipid catabolism and anabolism (Han et al. 2018). AMPK has been shown to play a negative regulatory role in adipocyte differentiation (Bu et al. 2019; Liu et al. 2018). Activation of AMPK can directly regulate the downstream key gene PGC-1α located in the downstream of AMPK signaling, which plays an important role in promoting fatty acid oxidation, regulating glucose and lipid metabolism (Chau et al. 2010; Schnyder and Handschin 2015). Therefore, the AMPK-PGC-1α signaling pathway is a potential target for regulating lipid metabolism. Under HS, AMPK was expressed differently in different species. Min et al. (Min et al. 2015) discovered that HS caused activation of AMPK signaling molecules in blood of dairy cows. The mRNA expression of AMPK and PGC-1α was increased in the soleus tissue of HS rats (Sanders et al. 2009). Also of note, after HS, the expression and phosphorylation levels of AMPK in rodent C2C12 cell lines were inhibited (Wang et al. 2010). The expression of AMPK and PGC-1α decreased in broilers and myoblasts after HS (Xue et al. 2017). Consistent with this view, our results indicated that HS resulted in the downregulation of AMPK and PGC-1α mRNA levels and protein abundance, as well as the dephosphorylation of AMPK. AICAR is known to be a purine nucleoside with a wide range of metabolic effects, including activation of AMPK (Awwad et al. 2018). AICAR can activate AMPK through reversible phosphorylation of upstream kinase at thr172 site in its α subunit (Stein et al. 2000). Activation of AMPK by AICAR significantly inhibited lipid-mediated endoplasmic reticulum stress in hepatocytes (Wang et al. 2011). Studies have shown that AICAR reverses HS-induced dephosphorylation of AMPK α in HepG2 cells (Wang et al. 2010). Our results show that AICAR can downregulate the increase of lipid droplets induced by HS, activate AMPK, promote the phosphorylation of AMPK and the expression of PGC-1 α, and eliminate HS-induced lipogenesis. Therefore, these results suggest that the lipid accumulation during the differentiation of 3T3-L1 preadipocytes under HS may be caused by the inhibition of the AMPK-PGC-1α signaling pathway.
Conclusion
In conclusion, HS stimulates lipid accumulation during 3T3-L1 preadipocytes differentiation by inhibiting the AMPK-PGC-1α signaling pathway. These results enhance our understanding of HS regulating fat metabolism and provide useful evidence for the metabolic reaction of adipose tissue in the process of HS. This study provides a new direction for improving the meat quality of livestock and treating obesity diseases caused by HS.
Acknowledgments
This work was supported by the grants from National Natural Science Foundation of China (31760672 and 31460606).
Ethics statement
All experimental procedures were approved by the Institutional Animal Care and Welfare Committee of Guangxi University (Nanning, China).
Declarations
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanna Huang and Hongyue Xie contributed equally to this work.
References
- Awwad O, Coperchini F, Pignatti P, Denegri M, Massara S, Croce L, di Buduo CA, Abbonante V, Balduini A, Chiovato L, Rotondi M. The AMPK-activator AICAR in thyroid cancer: effects on CXCL8 secretion and on CXCL8-induced neoplastic cell migration. J Endocrinol Investig. 2018;41:1275–1282. doi: 10.1007/s40618-018-0862-8. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bernabucci U, Basiricò L, Morera P, Lacetera N, Ronchi B, Nardone A. Heat shock modulates adipokines expression in 3 T3-L1 adipocytes. J Mol Endocrinol. 2009;42:139–147. doi: 10.1677/JME-08-0068. [DOI] [PubMed] [Google Scholar]
- Bertile F, Raclot T. ATGL and HSL are not coordinately regulated in response to fuel partitioning in fasted rats. J Nutr Biochem. 2011;22:372–379. doi: 10.1016/j.jnutbio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bharati J, Dangi SS, Chouhan VS, Mishra SR, Bharti MK, Verma V, Shankar O, Yadav VP, Das K, Paul A, Bag S, Maurya VP, Singh G, Kumar P, Sarkar M. Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle. Int J Biometeorol. 2017;61:1017–1027. doi: 10.1007/s00484-016-1281-1. [DOI] [PubMed] [Google Scholar]
- Bu S, Yuan CY, Xue Q, Chen Y, Cao FL. Bilobalide Suppresses Adipogenesis in 3 T3-L1 Adipocytes via the AMPK Signaling Pathway. Molecules. 2019;24:3503. doi: 10.3390/molecules24193503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1- PGC-1α pathway. Proc Natl Acad Sci U S A. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Feder ME, Kang L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol Ecol. 2018;27:3040–3054. doi: 10.1111/mec.14769. [DOI] [PubMed] [Google Scholar]
- Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2013) Nutrient excess in AMPK downregulation and insulin resistance. J Endocrinol Diabetes Obes 1:1008 [PMC free article] [PubMed]
- Dewey WC, Westra A, Miller HH, Nagasawa H. Heat-induced lethality and chromosomal damage in synchronized chinese hamster cells treated with 5-bromodeoxyuridine. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20:505–520. doi: 10.1080/09553007114551421. [DOI] [PubMed] [Google Scholar]
- Ezure T, Amano S (2009) Heat stimulation reduces early adipogenesis in 3T3-L1 preadipocytes. Endocrine 35:402–408. 10.1007/s12020-009-9164-4 [DOI] [PubMed]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am J Phys. 2010;298:C961–C971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- Han MH, Kim HJ, Jeong JW, Park C, Choi YH. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of vitis coignetiae pulliat is associated with the activation of ampk signaling pathway. Toxicol Res. 2018;34:13–21. doi: 10.5487/TR.2018.34.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Maloyan A, Shlaier J (1997) HSP70 kDa dynamics in animals undergoing heat stress superimposed on heat acclimation. Ann NY Acad Sci 813:617–619. 10.1111/j.1749-6632.1997.tb51755.x [DOI] [PubMed]
- Jang BC. Artesunate inhibits adipogeneis in 3 T3-L1 preadipocytes by reducing the expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, perilipin A, and STAT-3. Biochem Biophys Res Commun. 2016;474:220–225. doi: 10.1016/j.bbrc.2016.04.109. [DOI] [PubMed] [Google Scholar]
- Katsumata M, Yano H, Ishida N, Miyazaki A (1990) Influence of a high ambient temperature and administration of clenbuterol on body composition in rats. J Nutr Sci Vitaminol 36:569–578. 10.3177/jnsv.36.569 [DOI] [PubMed]
- Kellner TA, Baumgard LH, Prusa KJ, Gabler NK, Patience JF. Does heat stress alter the pig's response to dietary fat? J Anim Sci. 2016;94:4688–4703. doi: 10.2527/jas.2016-0756. [DOI] [PubMed] [Google Scholar]
- Kodiha M, Rassi JG, Brown CM, Stochaj U (2007) Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK→ ERK1/2 pathway. Am J Physiol 293:C1427–C1436. 10.1152/ajpcell.00176.2007 [DOI] [PubMed]
- Li M, Li D, Xiong T, Nan X, Wang G (2019) Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: a review. Int J Biometeorol 63:1283–1302. 10.1007/s00484-019-01744-8 [DOI] [PubMed]
- Liu CT, Brooks GA (2012) Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol 112:354–361. 10.1152/japplphysiol.00989.2011 [DOI] [PMC free article] [PubMed]
- Liu H, Wang J, Liu M, Zhao H, Yaqoob S, Zheng M, Cai D, Liu J. Antiobesity Effects of Ginsenoside Rg1 on 3 T3-L1 Preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. 2018;10:830. doi: 10.3390/nu10070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey MA, Zhang XF, Hunt CR, Sullivan SJ, Blum J, Laszlo A, Roti Roti JL. Uncoupling of M-phase kinase activation from the completion of S-phase by heat shock. Cancer Res. 1996;56:1770–1774. [PubMed] [Google Scholar]
- Mansour M. The roles of peroxisome proliferator-activated receptors in the metabolic syndrome. Prog Mol Biol Transl Sci. 2014;121:217–266. doi: 10.1016/B978-0-12-800101-1.00007-7. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273:E1107–E1112. 10.1152/ajpendo.1997.273.6.E1107 [DOI] [PubMed]
- Miller SG, de Vos P, Guerre-Millo M, Wong K, Hermann T, Staels B, Briggs MR, Auwerx J. The adipocyte specific transcription factor C/EBPalpha modulates human ob gene expression. Proc Natl Acad Sci U S A. 1996;93:5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Cheng JB, Shi BL, Yang HJ, Zheng N, Wang JQ (2015) Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J Zhejiang Univ Sci B:541-548. 10.1631/jzus.B1400341 [DOI] [PMC free article] [PubMed]
- Nie QH, Fang MX, Xie L, Shen X, Liu J, Luo ZP, Shi JJ, Zhang XQ. Associations of ATGL gene polymorphisms with chicken growth and fat traits. J Appl Genet. 2010;51:185–191. doi: 10.1007/BF03195726. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit GK, Mahanty A, Suar M, Sharma AP, Mohanty S (2015) Investigating hsp gene expression in liver of channa striatus under heat stress for understanding the upper thermal acclimation. Biomed Res Int 2014:1–10. 10.1155/2014/381719 [DOI] [PMC free article] [PubMed]
- Qu H, Donkin SS, Ajuwon KM. Heat stress enhances adipogenic differentiation of subcutaneous fat depot-derived porcine stromovascular cells. J Anim Sci. 2015;93:3832–3842. doi: 10.2527/jas.2015-9074. [DOI] [PubMed] [Google Scholar]
- Qu H, Yan H, Lu H, Donkin SS, Ajuwon KM. Heat stress in pigs is accompanied by adipose tissue-specific responses that favor increased triglyceride storage. J Anim Sci. 2016;94:1884–1896. doi: 10.2527/jas.2015-0084. [DOI] [PubMed] [Google Scholar]
- Raaphorst GP, Dewey ML, Freemanw C. Radiosensitivity and recovery from radiation damage in cultured cho cells exposed to hyperthermia at 42.5 or 45.5 °C. RADIATION RES. 1979;79:390–402. doi: 10.2307/3575104. [DOI] [PubMed] [Google Scholar]
- Rao Y, Liu H, Gao L, Yu H, Tan JH, Ou TM, Huang SL, Gu LQ, Ye JM, Huang ZS. Discovery of natural alkaloid bouchardatine as a novel inhibitor of adipogenesis/lipogenesis in 3 T3-L1 adipocytes. Biorg Med Chem. 2015;23:4719–4727. doi: 10.1016/j.bmc.2015.05.057. [DOI] [PubMed] [Google Scholar]
- Sanders SR, Cole LC, Flann KL, Baumgard LH, Rhoads RP (2009) Effects of acute heat stress on skeletal muscle gene expression associated with energy metabolism in rats. FASEB J 23. doi:10.1096/fasebj.23.1_supplement.598.7
- Sapareto SA, Hopwood LE, Dewey WC, Raju MR, Gray JW. Effects of hyperthermia on survival and progression of Chinese hamster ovary cells. Cancer Res. 1978;38:393–400. [PubMed] [Google Scholar]
- Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone. 2015;80:115–125. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CA, Garton AJ, Yeaman SJ. The presence and role of hormone-sensitive lipase in heart muscle. Biochem J. 1989;258:67–72. doi: 10.1042/bj2580067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–443. doi: 10.1042/bj3450437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H, Komuro I. Peroxisome proliferator-activated receptor γ and cardiovascular diseases. Circ J. 2009;73:214–220. doi: 10.1253/circj.cj-08-1071. [DOI] [PubMed] [Google Scholar]
- Thomson DM (2018) The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration. Int J Mol Sci 19. 10.3390/ijms19103125 [DOI] [PMC free article] [PubMed]
- Urano D (1988) Thermal effects on cells and tissues. VSP,
- Victoria SFM, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Phys Rep. 2015;3:e12315. doi: 10.14814/phy2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Yu QJ, Chen JA, Deng B, Qian LH, Le YY. PP2A Mediated AMPK Inhibition Promotes HSP70 Expression in Heat Shock Response. PLoS One. 2010;5:e13096. doi: 10.1371/journal.pone.0013096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Z, Li D, Wang D, Wang X, Feng X, Xia M. Involvement of oxygen-regulated protein 150 in AMP-activated protein kinase-mediated alleviation of lipid-induced endoplasmic reticulum stress. J Biol Chem. 2011;286:11119–11131. doi: 10.1074/jbc.M110.203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD et al (2008) Fat deposition, fatty acid composition and meat quality: A review. Meat Sci 78:343–358. 10.1016/j.meatsci.2007.07.019 [DOI] [PubMed]
- Xie J, Tang L, Lu L, Zhang L, Xi L, Liu HC, Odle J, Luo X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus) PLoS One. 2014;9:e102204. doi: 10.1371/journal.pone.0102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Song J, Liu L, Luo J, Tian G, Yang Y. Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat-stressed broilers. Arch Anim Nutr. 2017;71:362–372. doi: 10.1080/1745039X.2017.1355129. [DOI] [PubMed] [Google Scholar]
- Yan J, Bao E, Yu J. Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature. Res Vet Sci. 2009;86:533–538. doi: 10.1016/j.rvsc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Zeni O, Simkó M, Scarfi M, Mattsson M. Cellular response to elf-mf and heat: evidence for a common involvement of heat shock proteins? Front Public Health. 2017;5:280. doi: 10.3389/fpubh.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang Y, Liu F, Zhang F, Ding W (2016) Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J Inorg Biochem 162:1–8. 10.1016/j.jinorgbio.2016.06.013 [DOI] [PubMed]