Abstract
The poultry industry plays a significant role in boosting the economy of several countries, particularly developing countries, and acts as a good, cheap, and affordable source of animal protein. A stress-free environment is the main target in poultry production. There are several stressors, such as cold stress, heat stress, high stocking density, and diseases that can affect birds and cause several deleterious changes. Stress reduces feed intake and growth, as well as impairs immune response and function, resulting in high disease susceptibility. These effects are correlated with higher corticosteroid levels that modulate several immune pathways such as cytokine-cytokine receptor interaction and Toll-like receptor signaling along with induction of excessive production of reactive oxygen species (ROS) and thus oxidative stress. Several approaches have been considered to boost bird immunity to overcome stress-associated effects. Of these, dietary supplementation of certain nutrients and management modifications, such as light management, are commonly considered. Dietary supplementations improve bird immunity by improving the development of lymphoid tissues and triggering beneficial immune modulators and responses. Since nano-minerals have higher bioavailability compared to inorganic or organic forms, they are highly recommended to be included in the bird’s diet during stress. Additionally, light management is considered a cheap and safe approach to control stress. Changing light from continuous to intermittent and using monochromatic light instead of the normal light improve bird performance and health. Such changes in light management are associated with a reduction of ROS production and increased antioxidant production. In this review, we discuss the impact of stress on the immune system of birds and the transcriptome of oxidative stress and immune-related genes, in addition, how nano-minerals supplementations and light system modulate or mitigate stress-associated effects.
Keywords: Immune stimulant, Immunity, Light management, Nanoparticles, Poultry, Stress
Development of immune response in poultry
Birds’ defense mechanism starts to develop during embryonic life; however, immunocompetence only appears few days post-hatch (Yasuda et al. 2003). During incubation of the egg and shortly after hatching (approximately 1 month), embryos and chicks are transiently protected against microbial infection by maternal antibodies found in the yolk (Hamal et al. 2006). Thus, vaccination at 1-day-old chick is not effective because it does not trigger antibody production. However, the complete development of the secondary lymphoid tissues takes place at 1 week post-hatch that could allow an effective immune response with specific antibody production when chicks are subjected to vaccination (Yasuda et al. 2003). Therefore, the post-hatching period is critical, approximately 1 week, because of the absence of the continuous supply of maternal immunity in contrast to mammalian colostrum.
During the growth, reproductive stages, dietary changes, as well as stress, there are many changes in immunocompetence. This could be attributed to certain receptors on the immune cells that variably respond to sex and metabolic hormones and other endocrine-signaling molecules (O’Neal and Ketterson 2012). These hormones may induce immunosuppressive or immunostimulatory effects to rebalance the immune system (i.e., immunomodulation) that include increases or decreases of some effector mechanisms. Such rebalancing of immunity functions to integrate the immune system into the environmental changes that birds face and life history stages (Lee et al. 2008; Martin et al. 2008).
Stress types and their impact on the immune system of birds
Stress has many different definitions; however, it can be defined as an adaptive response to hazards that threaten a bird’s homeostasis (Dohms and Metz 1991). Stressors can be external or internal stimuli; and the bird’s response differs according to the severity and duration of the stressor and bird’s physiological status. Stressors include light (e.g., ultraviolet light), temperature, air quality (e.g., ammonia, ozone), environmental contaminants (e.g., mycotoxins, pesticides), general sanitation including infectious agents, and food composition changes (Dietert et al. 1994).
Temperature stressors
Birds are homoeothermic animals. They can maintain a relatively constant temperature of their internal organs only when raised in a thermoneutral zone (approximately at 21–28 °C; comfortable zone) (Soliman and Safwat 2020). When the environmental temperature exceeds the lower or upper limits of the thermoneutral zone, it results in heat or cold stress in animals. Heat stress negatively impacts poultry production, quality of their products (meat or eggs), reproduction, and disease resistance. This is because high temperature and relative humidity prevent birds to express their genotypes of high productive traits (Soliman and Safwat 2020). Heat stress reduces feed intake, increases feed conversion ratio, and decreases body weight (El-Naggar et al. 2019); besides, it decreases meat quality after slaughtering (Liu et al. 2019). Birds are more sensitive to heat stress than other domestic animals (Wang et al. 2018) because of the absence of sweat glands, as well as their feather covering. Moreover, bird’s resistance to heat stress varies among different chicken strains, where birds of high growth rate are markedly less resistant (Rimoldi et al. 2015). In this regard, the rapid growth and higher metabolic rate of the commercial broilers make them more sensitive to heat stress (El-Naggar et al. 2019). Therefore, it is crucial to get attention to the thermoregulation ability when selecting a broiler strain to be used in hot and warm climate conditions (Yalcin et al. 2001).
Extremely high temperature influences a bird’s immune response as it generally inhibits lymphocyte-mediated responses (Dohms and Metz 1991), particularly lymphocyte proliferation, resulting in variable changes in humoral immune responses (Regnier and Kelley 1981). Heat stress also lowers the relative weights of immune organs and decreases the levels of circulating antibodies such as IgM and IgG and lymphocytes (Hofmann et al. 2020). In addition, heat stress caused inflammation of the jejunal epithelium and triggered more pathological lesion of Salmonella enteritidis, possibly as a result from altered macrophage’s function (Quinteiro-Filho et al. 2012a; Quinteiro-Filho et al. 2012b).
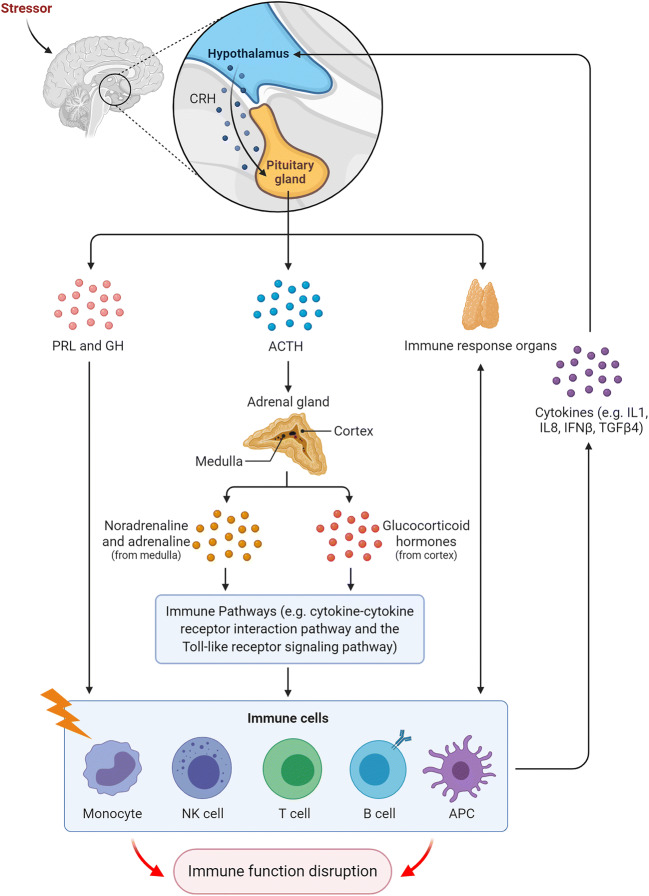
Heat stress stimulates the hypothalamic-pituitary-adrenal (HPA) axis (Fig. 1), which results in increases in corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) that lead to an increase of corticosteroid level, which in turn increases the fatty acid synthesis, fat accumulation, and protein catabolism and impairs the GIT functions, such as decreased jejunum villi height and increased permeability to microorganisms (Hangalapura 2006). Additionally, heat stress modulates a bird’s immune response through the induction of heat shock protein (HSP) expression in immune cells such as heterophils, macrophages, and lymphocytes (Dietert et al. 1994).
Fig. 1.
Stress-associated modulation of the hypothalamic-pituitary-adrenal axis and immune response by the central nervous system. Stress triggers a series of hormonal releases. For example, the stress-associated increased level of corticosteroids induces several immune pathways (e.g., cytokine-cytokine receptor interaction and the Toll-like receptor signaling pathways). Together, these reactions lead to impaired immune function and high levels of immune mediators such as interleukins. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; GH, growth hormone; IFN, interferon; IL, interleukin; PRL, prolactin; TGFβ4, transforming growth factor beta 4. (Figure created using BioRender.com)
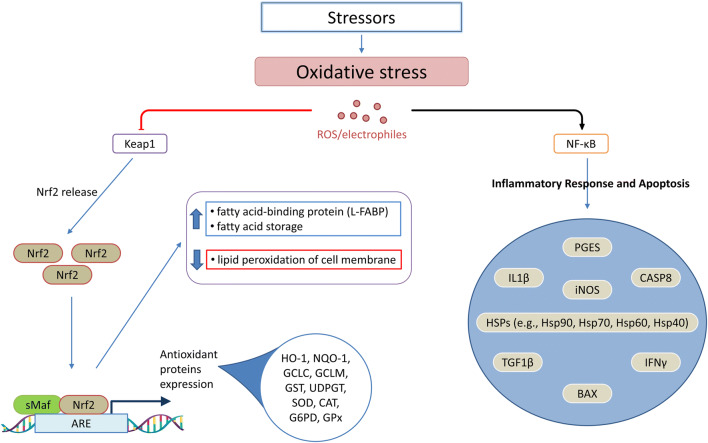
Exposure to a lower temperature (cold stress) is one of the potential environmental stressors that can modulate the immune response and influence the severity of infectious diseases (Hangalapura 2006). Cold stress influences a bird’s performance: it reduces body mass and/or egg production. It also activates the hypothalamus-pituitary-adrenal axis, leading to the release of stress hormones, which controls the restoration of homeostasis (Freeman 1987). Prolonged exposure to low temperature and thereby elevated levels of corticosteroids cause metabolic imbalances or diseases and structural alterations and functional dysfunction of the lymphoid tissue (Siegel 1985), resulting in increased bird susceptibility to diseases (Tsiouris et al. 2015a). For example, it increased pulmonary hypertension syndrome (Sato et al. 2002) and necrotic enteritis in broilers (Tsiouris et al. 2015a) and colibacillosis in turkey poults (Huff et al. 2007). The effect of cold stress is mediated through the suppression of plasma corticosterone levels and the increases of thyroid hormone, triiodothyronine (T3), levels (Hangalapura et al. 2004) to generate additional metabolic heat to help maintain body temperature in colder environments (Gupta 2011). On the immunity level, cold stress exposure in chickens triggers oxidative stress that is characterized by overexpression of iNOS (inducible nitric oxide synthase) mRNA and increased abundance of free Nrf2 (nuclear factor (erythroid-derived 2)-like 2; NFE2L2) protein within the cytosol (Chen et al. 2015). Nrf2 is a transcriptional mediator of antioxidants and triggers antioxidative stress response mediated by the Nrf2/ARE (antioxidant response element) signaling pathway (Fig. 2), which upregulates the expression of the fatty acid–binding protein (L-FABP) that inactivates lipid peroxidation of the cell membrane and promotes fatty acid storage (Chen et al. 2015). In addition, cold stress is reported to suppress humoral immunity and cell-mediated immunity of chickens (Zhao et al. 2014b). Interleukin (IL) 10 (Sesti-Costa et al. 2012) and IL2 (Janský et al. 1996) were upregulated following exposure to cold stress, while phagocytic activity and tumor necrosis factor (TNF) α and IL6 production were diminished (Sesti-Costa et al. 2012).
Fig. 2.
Induction of oxidative stress by, e.g., cold or heat stressors. Oxidative stress triggers Nrf2/ARE signaling and NF-κB and eventually induces antioxidant protein expression and inflammatory response and apoptosis. ARE, antioxidant response element; BAX, BCL2 associated X; CASP8, caspase-8; CAT, catalase; G6PD, glucose 6 phosphate dehydrogenase; GCLC and GCLM, glutamate cysteine ligase catalytic and regulatory subunits; GPx, glutathione peroxidase-1; GST, glutathione S-transferase; HO-1, heme oxygenase-1; HSP, heat shock proteins; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; Keap1, Kelch-like ECH-associated protein 1; NF-κB, nuclear factor kappa light-chain enhancer of activated B cells; NQO-1, NAD(P)H quinone oxidoreductase-1; Nrf2, Nuclear factor erythroid 2 (NF-E2)-related factor 2; PGES, prostaglandin E synthase; ROS, reactive oxygen species; sMaf, small musculoaponeurotic fibrosarcoma; SOD, superoxide dismutase; TGF1β, transforming growth factor beta 1; UDPGT, uridine diphosphate glucuronosyltransferase
Antioxidant production is also influenced by exposure to a lower temperature (Mujahid 2010). Cold stress decreased superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities, while it increased malondialdehyde (MDA) levels (Li et al. 2011). This was associated with an induction of oxidative stress characterized by a high expression of HSP such as Hsp27, Hsp40, Hsp60, Hsp70, and Hsp90 as a protecting response against cold-induced oxidative stress (Zhao et al. 2014b). Such modulations depend on the stress time and intensity (Hangalapura et al. 2003).
Other environmental stressors
Changing rearing systems, stocking density, farm location, and other physical environments can induce a stress response and thus modulate the immune response (Kamal et al. 2018). The slatted floors and the high breeding density modulated the cutaneous basophil hypersensitivity responses, increased the heterophil-to-lymphocyte (H/L) ratio and duration of tonic immobility, and reduced the antibody titers (El-Lethey et al. 2003; Tella et al. 2001). Moreover, when the laying hens are allowed to access perches as natural behavior, this decreased the H/L ratios (Campo et al. 2005). Farm location modulates the immune responses that vary according to species. For example, raising birds, such as sparrows (Passer domesticus), in a temperate environment elevated the baseline corticosterone levels compared with those reared in tropical environments due to changes in the photoperiod and increased parasite loads (Martin II et al. 2005; Martin et al. 2008).
Overcrowding negatively modulates the immune response of poultry by suppressing the growth of immune organs (Heckert et al. 2002). It is considered as a predisposing factor that induces enteritis (Tsiouris et al. 2015b), decreases nutrient absorption, and thus reduces growth performance (Gomes et al. 2014). In addition, high stocking density decreased the plasma IgG levels and the macrophage phagocytic activity (Gomes et al. 2014). This was associated with an increase in the corticosterone level via the interaction with the hypothalamic-pituitary-adrenal axis. In general, the unfavorable environments suppress the normal antibody- and cell-mediated responses. These effects are not only directed through the central pathway (the network of the nervous system) but also through an extra pituitary and extra hypothalamic “ACTH-like” and “β-endorphin-like” peptides that are produced by cells of the immune system, which may act as initiators of immune response and interact with the central pathway (Siegel 1987). In addition, exposure to contaminations or a pathogenic challenge during development may significantly increase the impact of other stressors (Fair and Ricklefs 2002; Tuerkyilmaz 2008).
Role of stress in regulating the immune response of birds
When an organism, including poultry, is exposed to a stressor, an acute stress response takes place that is driven by catecholamines such as dopamine, epinephrine, and norepinephrine (Kaspers and Schat 2012). Concurrently, vasopressin and hypothalamic corticotrophin-releasing factor (CRF) stimulate the ACTH release that results in the secretion of glucocorticoid from the adrenal cortex (reviewed in Dohms and Metz 1991; Kaiser et al. 2009). This in turn initiates a series of immunological, physiological, and metabolic processes that allow animals to adapt and survive and to maintain normal homeostasis (Koutsos and Klasing 2014). Stress hormones can directly stimulate the immune cells, such as lymphocytes that have high-affinity receptors for glucocorticoids and ACTH (reviewed in Mumma et al. 2006). The stimulation of bursal and thymic cells induces more production and release of corticosteroids (Lechner et al. 2001), which leukocytes can synthesize corticosterone, ACTH, and other neuroendocrine mediators (Mashaly et al. 1998).
The effect of stress hormones on the immune system mainly depends on the duration of exposure to stressors (reviewed in Koutsos and Klasing 2014; Martin II et al. 2005; Martin et al. 2008; Mashaly et al. 1998; Merrill et al. 2012; Shini et al. 2010a). Short exposure to an environmental stressor induces corticosterone production and consequently increases heterophil mobilization from the bone marrow, resulting in an increased H/L ratio (Shini et al. 2008a). Heterophils and lymphocytes showed an induced expression of some pro-inflammatory cytokines such as IL1β, IL6, IL18, and transforming growth factor beta 4 (TGFβ4) and the chemokines such as CCLi2, CCL5, CCL16, and CXCLi1 (Shini and Kaiser 2009; Shini et al. 2008a; Shini et al. 2008b; Shini et al. 2010b). Moreover, dexamethasone induces thymic and bursal lymphocyte apoptosis (Compton et al. 1990a; Compton et al. 1990b). Relatively long-term exposure to corticosterone, dexamethasone, and ACTH (i.e., 7 days) decreases the size of immune organs and disrupts their normal function (Dohms and Metz 1991; Shini et al. 2010a), resulting in shifting of the immune system from high activity during acute stress to an immunosuppressed and anti-inflammatory state during chronic exposure. This is accompanied by decreases in the antibody response (Stier et al. 2009), phytohemagglutinin (PHA) response (Martin II et al. 2005; Mumma et al. 2006), concanavalin A–induced lymphocyte proliferation, IL2 and gamma interferon (INFγ) production, and monocyte phagocytic activity (Fowles et al. 1993; Isobe and Lillehoj 1992).
The bird’s immune response varies according to the exposure time of stress. Additionally, in ovo, exposure to stress hormones affects future immune responses (Kaspers and Schat 2012). Glucocorticoid levels of the hen influence the embryo’s future physiology, and hens with elevated glucocorticoids tend to have higher levels of glucocorticoids in their egg yolk (Merrill et al. 2012). Furthermore, chicks hatched from these eggs exhibited decreased cutaneous basophil hypersensitivity responses, increased adrenocortical responses to acute stress, and slow growth rates (reviewed in Hayward and Wingfield 2004).
Transcriptomic profile changes during stress
Transcriptomic profile changes of immune-related genes during stress
Stress reduces the growth of the bursa of Fabricius, thymus, and spleen and impairs their function (Liu et al. 2014). This could be reflected on the immune cell population, for example, reduce the counts of blood leukocyte and lymphocyte and serum immunoglobulins (IgM, IgG, IgA) (Roushdy et al. 2020). Thus, stress impairs immune responses and disease resistance of poultry (Monson et al. 2019). In broilers, thymic transcriptome responses to lipopolysaccharide were modulated by acute heat stress and thereby contribute to the negative effects on thymocyte survival and trafficking. In fact, expression changes were more pronounced in response to exposure to lipopolysaccharide than birds exposed to lipopolysaccharide and heat stress (Monson et al. 2019).
Stress is known to disrupt various physiological functions, thereby increasing disease susceptibility and suppressing immune responses (Abo-Al-Ela and Faggio 2021; Burgos-Aceves et al. 2021; Dawood et al. 2020; Zhang et al. 2018). Corticosterone is a glucocorticoid hormone that significantly increases in the circulation during stresses in chickens (Scanes 2016). In an established stress model using corticosterone in chicken (Gallus gallus), stress exhibited 199 upregulated and 1235 downregulated genes in the bursa of Fabricius. The key influenced genes included myeloid differentiation primary response protein 88 (MYD88), vascular endothelial growth factor (VEGFA), Toll-like receptor (TLR) 4, and IL15, which seemed to play crucial roles in response to stress. Such genes mainly contribute to the Toll-like receptor signaling pathway (mainly MYD88 and TLR4) and cytokine-cytokine receptor interaction pathway (mainly VEGFA and ILs) (Zhang et al. 2018). These were accompanied by elevated serum levels of TNFα, IL1β, and IL6 and a decreased CD3+ and CD4+, resulting in an inflammatory response. This inflammatory response was associated with marked upregulation of cathelicidin-B1-like (CATHB1, also known as CATHL1) (Zhang et al. 2018). CATHB1 has an anti-inflammatory action, presumably, through the induction of IL10 expression. CATH-B1 downregulated the expression of the pro-inflammatory cytokines (IL1β, IL6, IL8, and IFNβ) induced by influenza A virus and avian pathogenic Escherichia coli in vitro (Peng et al. 2020a; Peng et al. 2020b). A possible explanation might be that CATHB1 can bind lipopolysaccharide, which suggests that CATHB1 reduces TLR4-dependent activation by avian pathogenic E. coli (Peng et al. 2020b). Similarly, an aggregation between the CATHB1 and the influenza A virus was seen after their incubation, and thereby it affected the infectivity of the virus in vitro (Peng et al. 2020a). Mostly, this scenario may be different during the stress in the absence of infection.
During Newcastle disease virus infection, chicken exposed to heat stress showed a downregulation in prominent immune pathways, such as IL8 signaling, NF-κB signaling, and B cell receptor signaling in the lung tissue (Saelao et al. 2018). Heat- and lipopolysaccharide-induced changes in the bursal immune transcriptome were more pronounced than heat-induced changes alone. Additionally, the combined treatments decreased the expression in WNT signaling pathways (Monson et al. 2018). WNT signaling regulates the proliferation of blood and immune progenitor cells and cell fate specification of stem cells. WNT proteins regulate dendritic cell maturation and effector T-cell development and activation (Staal et al. 2008). In a similar study, the chicken monocyte/macrophage cell line responded with increases in the expression of the pro-inflammatory cytokines (IL1β) and chemokines (IL8, CCL4, and CCL5) and iNOS when exposed to heat stress and lipopolysaccharide stimuli. This action seemed to be synergistically accompanied by induction of HSP expression (HSPA2 that encodes HSP70, HSP25, and HSPH1) (Slawinska et al. 2016). This could reflect how stress modulates and represses immune responses in different ways.
HSP are a group of proteins and have protective actions with anti-apoptosis properties, immune and cellular functions, and anti-inflammatory effects (Beere et al. 2000; Jiang et al. 2020; Yenari et al. 2005). HSPA8, HSPA2, and IL8L1 seemed to play important regulatory roles in the immune function during corticosterone-induced stress effects (Guo et al. 2020). Environmental temperature and stocking density disrupt the levels of various related molecules; for example, they cause significant increases in HSP70 expression and circulating corticosterone in broiler chickens (Najafi et al. 2015). The role of the HSP proteins is likely linked to oxidative stress, which will be discussed in more detail in the next section. There is compelling evidence for close interaction between oxidative stress and immune response (Abo-Al-Ela 2018; Abo-Al-Ela and Burgos-Aceves 2021; Chen et al. 2019; Slawinska et al. 2016). For example, the oxidative stress induced by H2S exposure is mediated by FOS/IL8 signaling (upregulation) (Chen et al. 2019). In addition, stress is mostly associated with high levels of corticosteroid and reactive oxygen species (ROS) that suppress the function and effectiveness of the immune system (El-Lethey et al. 2003; Hangalapura 2006; Lauridsen 2019).
Transcriptomic profile changes during oxidative stress
Exposure to several stressors in the poultry environment, such as heat, toxin, heavy metals, transportation, handling, and pathogenic infection, induces oxidative damage through excess production of ROS (Surai and Fisinin 2016a; Surai and Fisinin 2016b; Surai et al. 2019). ROS is among the most detrimental consequences faced by cells (Pamplona and Costantini 2011). ROS are formed as a result of normal metabolic activity in the cells that can be tolerated by the antioxidant defense mechanisms (Pamplona and Costantini 2011). However, excess production of such molecules can exceed the antioxidant system capacity that results in oxidative stress damage and ultimately apoptotic or necrotic cell death (Genestra 2007; Trachootham et al. 2008).
Oxidative stress enhances transcriptional regulatory cascades that include Nrf2, NF-κB (nuclear factor kappa light-chain enhancer of activated B cells), and heat shock factor (HSFs) (Fig. 2) (Ma and He 2012; Paul et al. 2018). Nrf2 is considered as a master regulator of the cell stress response (Itoh et al. 2010). Upon Nrf2 activation by a high level of ROS, it translocates to the nucleus, where it modulates the expression of several stress response genes (Bhakkiyalakshmi et al. 2015; Keum and Choi 2014). Nrf2 signaling pathway induces the expression of several protective molecules and detoxifying genes such as SOD, GPx, catalase (CAT), glutathione S-transferase (GST), heme oxygenase-1 (HO-1), NAD(P)H:quinone acceptor oxidoreductases 1 (NQO1), and glucose-6-phosphate dehydrogenase (G6PD) through the cis-acting ARE located in the promoter region (Itoh et al. 1999; Nguyen et al. 2009). Furthermore, Nrf2 helps in the restoration of cellular protein quality after oxidative stress, in which it upregulates the expression of the protective protein and directs proteasome subunits to repair the unfolded and damaged protein (Bellezza et al. 2018). Nrf2 was found to modulate detrimental changes of oxidative stress via enhancing ROS-scavenging elements (Bellezza et al. 2018; Ishii et al. 2000). However, excessive stress downregulates the Nrf2 expression and thus inhibits its protective signaling pathway (Surai et al. 2019). On the other side, NF-κB is the principal modulator involved in pro-inflammatory responses (Lushchak 2011). A high concentration of NF-κB impairs the cellular function, which induces inflammatory tissue damage (Liu and Malik 2006).
HSPs have a vital role in monitoring the immune response during stress conditions through modifying the pro-inflammatory and anti-inflammatory cytokine expression. HSPs can prevent or arrest inflammatory damage, in which they enhance the production of anti-inflammatory cytokines (Borges et al. 2012). The anti-inflammatory effect of HSP may be due to their inhibitory action of signaling pathways NF-κB and NF-κB/nuclear factor kappa B (IκB), possibly through the prevention of IκB kinase activation that stabilizes IkB (Janus et al. 2011; Lyu et al. 2020). Induced Hsp70 inhibited the production of pro-inflammatory cytokines, such as TNFα, IL1β, and IL6 in vitro (Wang et al. 2017).
Dysfunction of the antioxidant defense could modulate the cellular macromolecules, such as lipids, nucleic acids, and proteins, leading to the aggregation of non-native proteins and ultimately cellular death. Thus, the cells increase the production of chaperone proteins (HSPs and HSFs) as protective molecules to alleviate the aggregation of non-native proteins (Morimoto et al. 1996). Gu et al. (2012) showed that expression of HSP is positively correlated with the antioxidant response. The suppression of the intestinal HSP70 expression by quercetin (a plant pigment) exaggerated the severity of acute heat stress in broiler; there was a significant increase of the serum corticosterone and a reduction in the antioxidant enzyme activity. HSP and HSF expressions are regulated by different factors: duration, severity, and type of stressor (Pirkkala et al. 2001).
Ross and Cobb broilers of 21 days old were heat-stressed (33°C/5h a day for 4 days), and they exhibited degenerative and oxidative changes in the hepatic tissue. They showed a downregulation of the SOD and CAT gene expression and enzyme activity and an upregulation of the HSP70, HSP90, HSF1, and HSF3 (Abdo et al. 2017). Heat stress induced oxidative stress in male broiler chicks (Ross 308) reared at a temperature of 34 °C for 8 h/day for 42 days. The oxidative stress was evidenced by a decreased serum SOD and GPx enzyme activity and increased MDA. Furthermore, heat stress significantly increased the muscle Keap1 expression and decreased the muscle Nrf2 expression (Sahin et al. 2016). A 15-day-old broiler chicken was heat-stressed at 35±1°C for 15 days and showed a significantly reduced body growth performance and a downregulated GPx expression (El-Deep et al. 2016).
Cyclic chronic heat stress upregulated the expression of hepatic NF-κB, cyclooxygenase-2, and HSPs and downregulated the Nrf2 expression in Japanese quails exposed to 34 °C for 8 h per day for 12 weeks that result in decreased tissue damage and apoptosis (Orhan et al. 2013; Sahin et al. 2010). Commercial broilers exposed to a cyclic chronic heat stress (33°C for 5 h per day for 2 successive weeks) exhibited an induced liver oxidative damage and showed a downregulation of the SOD, CAT, and GPx1 gene expression levels and a differential modulation of HSP70, HSP90, and HSF3 mRNA levels (El-Kassas et al. 2018). Moreover, an inflammatory response was induced, in which NF-κB was significantly upregulated in the spleen. This was accompanied by an upregulation of the pro-apoptotic genes prostaglandin E synthase (PGES), IL1β, TGF1β, IFNγ, BAX (BCL2 associated X, apoptosis regulator), and CASP8 (encodes caspase-8 protein) (El-Kassas et al. 2018).
Heavy metals induce oxidative stress damage in poultry by interfering with the Nrf2 expression. Mercury reduced the Nrf2 protein level and suppressed the Nrf2/Keap1 pathway in the ovary, liver, and kidney tissue of laying hens, and the expression of Keap1 protein was increased (Ma et al. 2018a; Ma et al. 2018b). Mycotoxins in the poultry diet such as aflatoxin B1 is another stress factor. They cause hepatic oxidative damage, impaired mitochondrial function, increased ROS generation, and downregulated Nrf2, NQO1, and SOD (Li et al. 2019). Aflatoxin B1 also induced inflammation and upregulated the expression of apoptosis gene caspase-9 and caspase-3 and Bax and significantly downregulated Nrf2 and hemeoxygenase-1 (HO-1) expression and protein levels in broiler chick liver (Muhammad et al. 2018).
Mineral nanoparticle supplementation alleviates the detrimental effect of stress
Nutrition is a key influencer of the immune system function that is reflected on the generated immune response. Such an effect results from both nutrient and non-nutrient ingredients, which can affect the development and maintenance of the immune system response (Kaspers and Schat 2012). Proper nutrition mitigates the stress-associated immune suppression in birds. It has been said “Diets nourish immune cells, modulate them and facilitate the establishment of commensal microflora, but diets shouldn’t normally stimulate the immune system. Let pathogens do that” (Klasing 2007). Essential trace minerals, such as zinc (Zn), copper (Cu), and selenium (Se), play essential roles in normal immune function and antioxidative enzymes (Lauridsen 2019).
Nutrition affects the bird’s immune function through modulating the development of lymphoid tissues, synthesis of immune modulators, mucus production, cell proliferation and activation, and phagocytosis, ultimately controlling the immune response. It has been found that nutrient deficiencies hinder the bird’s ability to produce an efficient immune response (Kidd 2004).
Nanotechnology is a promising field and deals with nanoparticles that are applied in various sectors such as biology, physics, and chemistry (Buzea et al. 2007). Nanotechnology has been successfully applied in poultry and livestock production to improve animal welfare and production that could supply human with high-quality, safe poultry and animal products (El Sabry et al. 2018). The application of nanotechnology in animal production included various possibilities of feed additives and medications, diagnosis and treatment of diseases, animal records (e.g., follow up its products (meat, milk, eggs)), and hormonal immunosensors that can monitor reproductive performance (Scott 2005).
The mode of action of nanoparticles was elucidated by Chen et al. (2006), in which they suggested that nanoscale allows more surface area of interaction in different biological process, increases the shelf-life of compounds in the gastrointestinal tract, decreases intestinal clearance mechanisms, achieves more penetration effect in tissues via fine capillaries as well as epithelial lining fenestration, is easily uptaken by cells, and can be efficiently delivered to target sites.
Nano-minerals are widely studied and used in poultry because of their high bioavailability and low antagonistic effect in the gut (Gopi et al. 2017). Besides, nano-minerals exhibited lower required levels compared to normal conditions and thus low environmental dissemination and contamination (environmentally friendly) (Hassan et al. 2020b). The high bioavailability of mineral nanoparticles is attributed to novel characteristics, such as great surface area, high catalytic efficiency and surface activity, and great absorption efficiencies, transport, and uptake that allow more delivery efficiency and effect (Zhang et al. 2001).
Zinc nanoparticles
Zn is an essential trace element of various biological processes such as growth, metabolism, immune function, fertility, and wound healing (Liu et al. 2011). It plays a crucial role in the antioxidant defense mechanism as a structural component of SOD (Suttle 2010). Zn nanoparticle levels were concentrated in the liver of broilers while decreased in breast and thigh muscle tissues. This possibly could explain the increased MDA content in thigh muscle of broilers at 7 days post-Zn supplementation (Ramiah et al. 2019) and being the liver as the main organ of Zn metabolism (Grüngreiff et al. 2016). Many studies investigated the impact of Zn nanoparticle supplementation on poultry in terms of intestinal morphology, growth performance, and immune response. The majority of studies used zinc oxide nanoparticles, possibly because of their excellent biocompatibility and low toxicity and cost (Jiang et al. 2018).
Zhao et al. (2014a) reported that a low concentration of nano-Zn supplementation (20 ppm) significantly improved feed efficiency, growth performance, and antioxidant function (total antioxidant capability, SOD, and catalase activity) in broilers. Also, the same conclusion was obtained when 40 and 60 mg/kg concentrations were used (Ramiah et al. 2019). In laying hens, Zn nanoparticle supplementation at 60 or 30 mg/kg improved the laying performance—enhanced egg production and reduced cracked shell percentage—and improved liver and kidney functions, as well as enhanced the immune response and oxidative defenses (El-katcha et al. 2018).
Additionally, El-Katcha et al. (2017) figured out that 30 mg of Zn nanoparticles/kg diet is equivalent to the inorganic Zn requirement, in which it was sufficient to improve bird performance, intestinal health, and immune response. Zn nanoparticles (40 and 60 mg/kg diet) ameliorated the negative impact of heat stress through lowering the serum corticosterone levels and lipid peroxidation and the enhancement of the antioxidant defense system (Ramiah et al. 2019). Zn nanoparticles enhanced the antioxidant capacity by enhancing the activities of anti-oxidases and decreasing the levels of free radicals in broilers (Lina et al. 2009). Hafez et al. (2020) reported that Zn nanoparticles increased the activity of SOD and CAT enzyme and reduced the oxidative stress, indicated by lowered levels of MDA and increased production of IgY, and enhanced cellular immunity in broiler chickens injected with the immunogenic protein antigen dinitrophenyl hapten-keyhole limpet hemocyanin.
Selenium nanoparticles
Se is a fundamental part of selenoproteins (e.g., GPxs). Such enzymes play essential roles in fighting oxidative damage through free radical scavengers; protection of biomolecules such as lipid, protein, and DNA; and maintenance of membrane cellular integrity (Suttle 2010). Supplementation of Se elicits the immune response, increases disease resistance, and improves growth and reproductive performance. However, Se deficiency increases the risk of exudative diathesis—a disorder arising from vitamin E deficiency in chicks—immunodeficiency, pancreatic dystrophy, nutritional muscular dystrophy, myopathy, and reduced performance in poultry (Suttle 2010).
It has been reported that Se nanoparticle supplementation improved growth performance (daily weight gain and feed conversion ratio) and survival rate and increased activity of GPx enzyme in broilers (Ahmadi et al. 2018; Wang 2009; Zhou and Wang 2011) and productive performance in laying hens (El-Deep et al. 2017). Nano-Se-supplemented birds showed significantly improved meat quality and lowered abdominal fat (Ahmadi et al. 2018; Zhou and Wang 2011). Cai et al. (2012) found no significant differences in meat color and immune organ index (spleen, bursa, and thymus) in broilers fed different doses of nano-Se. Additionally, the optimal concentration of nano-Se ranges between 0.3 and 0.5 mg/kg and is limited to a 1-mg/kg diet to avoid negative effects on the immune and antioxidative systems (Cai et al. 2012).
As an important compound of the GPx enzyme, nano-Se supplementation increased the activity and expression of GPx and cytokines (IL2 and IL6) in the liver under both normal and heat stress conditions (El-Deep et al. 2016; Safdari-Rostamabad et al. 2017). Nano-Se can alleviate heat and oxidative stresses by reducing MDA levels in the liver, breast muscle, and serum of broilers and layers (El-Deep et al. 2016; Meng et al. 2019). In addition, nano-Se improved the total antioxidant capacity and the levels of MDA, SOD, IgG, and IgM during heat stress in Ross and Arbor broilers (Hassan et al. 2020a); increased phagocytic activity of laying hens subjected to heat stress (El-Deep et al. 2017); and alleviated the heat stress and reduced production and performance in Sinai hens (Rizk et al. 2017). Possibly, Se restored the high levels of FAS, FASL, and TNFα (apoptosis-associated proteins) induced by heat stress in the follicular cells of laying hens (Fang et al. 2018; Li et al. 2020).
Boostani et al. (2015) stated that Se supplementation (organic, inorganic, or nano) increased the antioxidant enzyme activities, decreased MDA levels, and increased IgM and IgG levels, and these effects were the highest in nano-Se supplementation in broilers under oxidative stress. Se nanoparticles were supplemented to birds challenged with E. coli, which showed an enhanced performance, immunological response, antioxidant activity, and protection from the tissue pathological effect and a lowered bacterial load in the intestine of broilers (Ali et al. 2020).
Copper nanoparticles
Cu is an essential trace element that is involved in different physiological functions and biochemical processes in the body, such as bone and connective tissue growth, fetal and early stages of development, proper nerve health and functions (required for the formation of myelin sheath), and as a cofactor of enzymatic processes (McDowell 2003; Suttle 2010). Cu is a part of the structure of several metalloenzymes such as cytochrome oxidase and SOD (Sharma et al. 2005), as well as Cu neutralizes free radicals (Failla 2003). Cu is also involved in enhancing the immune response of animals to combat infection and repair damaged tissue (Failla 2003) and required for the formation of antibodies and white blood cells (Sharma et al. 2005). Cu nanoparticles showed no adverse effects on the growth performance of turkeys (Jankowski et al. 2020).
The in ovo injection of Cu nanoparticles significantly improved the energy and nitrogen utilization and thus feed conversion ratio, average daily gain, and final body weight compared to that of copper sulfate and the control (Scott et al. 2018). Cu nanoparticles increased the relative weight and growth of the bursa of Fabricius (Scott et al. 2018). El-Kazaz and Hafez (2020) found that Cu nanoparticles improved blood profile and lymphoid organs’ index weight as well as positively affected the behavioral patterns and reduced H/L ratio, erythrocyte sedimentation rate, MDA, and corticosterone hormone.
Ognik et al. (2018) pointed out that supplementing chicken diets with nano-Cu up to 12 mg/bird for 6 weeks increased their antioxidant and immune defense capacities. Dietary nano-Cu improved the redox status indicators, as well as increased the bursa glutathione levels and SOD activity in the small intestine (Jankowski et al. 2020). El-Katcha et al. (2020) reported that 7.5-ppm Cu nanoparticles (approximately close to average requirement of copper, 6–8 ppm) improved phagocytic capacity and lysosomal and bactericidal activities, increased serum CAT and SOD activity, and mitigated the toxic effect of oxidized oil on the broiler liver.
When broiler chickens were subjected to heat stress, Cu nanoparticles at 50% of average requirement enhanced the serum lysozyme and phagocytic activities, downregulated the expressions of immune response mediators, and mitigated the degenerative changes of heat stress in the spleen (El-Kassas et al. 2018). Additionally, Cu nanoparticle supplementation during heat stress improved the performance of broilers; decreased liver MDA concentration; upregulated the expression and activities of the SOD, catalase, and GPx; reduced liver degenerative changes; and modulated the HSPs (El-Kassas et al. 2020). Cu nanoparticles also alleviated the stress-induced degenerative changes and enhanced the antioxidant gene expression levels (hepatic HSP70, HSP90, and HSF3) and enzyme activities in the hepatic tissue. Moreover, it reduced heat stress–induced inflammatory responses via downregulating the inflammatory gene expression levels and suppression of the degenerative changes in the spleen (El-Kassas et al. 2020). Nano-Cu reduced the inflammatory effects of lipopolysaccharide through lowering the serum levels of IFNγ and TNFα and the expression of TNFα and IL1β in the spleen and thymus of yellow feathered birds (Fan et al. 2020).
Chromium nanoparticles
Chromium (Cr) is an essential micro-element involved in different nutrient metabolisms and has immunomodulatory effects (Pechova and Pavlata 2007). Zha et al. (2009) found that broiler chicks exposed to heat stress and supplemented with Cr nanoparticles or Cr picolinate (0.5 mg/kg) showed improved performance and decreased abdominal fat. Cr nanoparticles were effective in regulating the gene expression of IFNγ and reducing the serum cortisol and the negative effects of heat stress, as well as had an immunomodulatory effect (Hamidi et al. 2021; Hamidi et al. 2016). Moreover, Berenjian et al. (2018) reported that nano-Cr ameliorated the physiological stress–associated impacts resulted from high corticosteroid (dexamethasone, 0.6-mg/kg body weight) in quails through improving nutrient utilization efficiency, performance, and immune responses. Moreover, Hajializadeh et al. (2017) found that supplementation of nano-Cr at a level of 1000 ppb increased the antibody titers against infectious bronchitis and avian influenza in chickens reared in heat stress conditions.
Light management ameliorates the negative effects of stress
Light is one of the most important environmental factors influencing animal performance (Pan et al. 2014; Yang et al. 2016; Yang et al. 2015). Its effect varies according to intensity, duration, and wavelength (color) (Guo et al. 2018). Light intensity significantly influences bird’s health (Blatchford et al. 2009). In this regard, low light intensity negatively affects broiler’s early uniformity, carcass traits, and meat tenderness (Blatchford et al. 2012; Deep et al. 2012; Fidan et al. 2015; Rault et al. 2017; Senaratna et al. 2016), while high light intensity improves bird’s activity through enhancing bone health (Blatchford et al. 2012; Blatchford et al. 2009; Rault et al. 2017), increasing growth and breast muscle percentage (Deep et al. 2013) and inducing a comfort behavior for broilers (Alvino et al. 2009). Despite these positive impacts, too strong light stimulates aggressive behavior of broilers leading to compromised broiler’s health and welfare (Kjaer and Vestergaard 1999). Thus, duration of exposure and light level modulate the bird’s health and growth performance under either normal or stress conditions.
In young chickens, the change from continuous light to intermittent light causes an initial decrease of growth; however, the final body weight is similar to or higher than that of birds reared in continuous light exposure (Rahimi et al. 2005). The intermittent light program is effective during heat stress; it improves the body weight reduction by increasing plasma T3 level, which modulates the metabolism of carbohydrate, fat, and protein substrates and feed consumption and decreases the levels of the pro-inflammatory cytokines as a cause of growth inhibition (Rahimi et al. 2005).
The light color is considered a powerful factor that can alter many physiological, immunological, and behavioral pathways in birds under normal housing and stressed conditions (Abdo et al. 2017; Mohamed et al. 2014; Xie et al. 2008). Birds sense light through retinal photoreceptors in their eyes and extra-retinal photoreceptors of photosensitive cells in the brain. Bird’s eyes contain four types of retinal cones, unlike three only in the human eye (Osorio et al. 1999), that make them sensitive to short wavelength, long wavelength, and mid-wavelength as well as ultraviolet wavelength (Archer 2018). Thus, birds have maximum visual sensitivity at 415, 455, 508, and 571 nm (Prescott et al. 2003). Additionally, their retinal cones have oil droplets, which filter incident light before it reaches the pigments and eventually reduce the spectral overlap between pigments, increasing the amount of color a bird can perceive (Gongruttananun 2011).
At the reproductive level, red light enhances reproductive performance by stimulating the development of gonads, whereas the blue and green lights have little or no effect on the activity of the reproductive organs (Gongruttananun 2011). Blue and green colors increase body weight by stimulating muscle growth more than red light (Cao et al. 2008). Blue light significantly improved meat quality, decreased lipid peroxidation, and improved antioxidative status compared with red light and white light. Blue light significantly increased SOD, GPx, and total antioxidant capability activities while reduced MDA level in the breast and thigh muscles (Ke et al. 2011). The effects of blue and green lights start early at the embryo stage where the green light stimuli during embryogenesis boost the post-hatch body weight of male broilers, increase breast muscle growth, and improve the feed conversion ratio (Zhang et al. 2012).
Using different monochromatic light (red, green, and blue) influences the immune response. Green and blue monochromatic lights improve the cellular immune response of broiler by promoting T-lymphocyte proliferation. The enhancing effect of green light occurred at an earlier stage of growth (21 days of age), while that of blue light is at 49 days of age (Xie et al. 2008). Both blue and green lights maintain and promote effective antibody production and humoral immune function in broilers (Xie et al. 2008). In addition, blue and green lights alleviate stress-induced negative effects by reducing the IL1β production (Mohamed et al. 2014; Xie et al. 2011; Xie et al. 2008). Monochromatic blue light reduces the heat stress–induced oxidative stress by lowering MDA level and enhancing the antioxidant SOD and CAT expression and activities and regulating the HSP70, HSP90, HSF1, and HSF3 gene expression (Abdo et al. 2017).
The alleviating effect of blue light is likely because of its calming effect that reduces stress and increases immune response, which in turn reduces the risk and the cost of infections and increases survivability (Lewis and Morris 2000). In this context, broilers reared under monochromatic blue light manifest a low fear and stress response during the transportation and pre-slaughter handling that are expressed by decreased H/L ratio and IL1β level (Mohamed et al. 2014). Furthermore, blue and green monochromatic lights improved gut health through improving mechanical and immunological barriers of the intestinal mucous membrane as shown by the increased villus height of the small intestine and numbers of goblet cells, intestinal intraepithelial lymphocytes, and IgA+ cells (Xie et al. 2011).
Conclusion and future directions
It clearly appears that stressors mainly induce oxidative stress, which is characterized by overproduction of ROS. ROS stimulates a pro-inflammatory response that leads to the upregulation of several pro-inflammatory cytokines such as interleukins and interferon through the activation of Toll-like receptor signaling and cytokine to cytokine pathways. Simultaneously, an anti-inflammatory response is recruited. Nano-nutrient dietary supplementation and effective light management such as monochromatic lights improve the immune response of birds by stimulating antioxidative stress response. Future studies are recommended to further elucidate how nanoparticles and light management enhance and mitigate stress-associated effects. Previous studies showed that the combination of photoperiod changes and supplementation with certain nutrients, such as melatonin and tryptophan (Blair et al. 1993; Kliger et al. 2000) or light-emitting diodes (LEDs) with L-tryptophan (Sharideh and Zaghari 2021), can enhance the immune system and decrease the mortality of poultry. However, it is recommended to develop a rearing system that includes both dietary nano-nutrients and effective light management using monochromatic light.
Data Availability
Not applicable
Declarations
Ethics approval
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haitham G. Abo-Al-Ela, Email: haitham.aboalela@frc.suezuni.edu.eg, Email: haithamgamal2@gmail.com
Seham El-Kassas, Email: seham.elkassas@vet.kfs.edu.eg.
References
- Abdo SE, El-Kassas S, El-Nahas AF, Mahmoud S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxidative Med Cell Longev. 2017;2017:1351945–1351913. doi: 10.1155/2017/1351945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Al-Ela HG. An introduction to selected innate immune-relevant genes in fish. Appl Ecol Env Res. 2018;16:955–976. doi: 10.15666/aeer/1602_955976. [DOI] [Google Scholar]
- Abo-Al-Ela HG, Burgos-Aceves MA. Exploring the role of microRNAs in axolotl regeneration. J Cell Physiol. 2021;236:839–850. doi: 10.1002/jcp.29920. [DOI] [PubMed] [Google Scholar]
- Abo-Al-Ela HG, Faggio C. MicroRNA-mediated stress response in bivalve species. Ecotoxicol Environ Saf. 2021;208:111442. doi: 10.1016/j.ecoenv.2020.111442. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Ahmadian A, Seidavi A. Effect of different levels of nano-selenium on performance, blood parameters, immunity and carcass characteristics of broiler chickens. Poult Sci J. 2018;6:99–108. doi: 10.22069/psj.2018.13815.1276. [DOI] [Google Scholar]
- Ali A, Soliman E, Hamad R, El-Borad O, Hassan R, Helal M. Preventive, behavioral, productive, and tissue modification using green synthesized selenium nanoparticles in the drinking water of two broiler breeds under microbial stress. Braz J Poult Sci. 2020;22:eRBCA-2019-1129. doi: 10.1590/1806-9061-2019-1129. [DOI] [Google Scholar]
- Alvino GM, Archer GS, Mench JA. Behavioural time budgets of broiler chickens reared in varying light intensities. Appl Anim Behav Sci. 2009;118:54–61. doi: 10.1016/j.applanim.2009.02.003. [DOI] [Google Scholar]
- Archer G. Color temperature of light-emitting diode lighting matters for optimum growth and welfare of broiler chickens. Animal. 2018;12:1015–1021. doi: 10.1017/S1751731117002361. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Berenjian A, Sharifi SD, Mohammadi-Sangcheshmeh A, Ghazanfari S. Effect of chromium nanoparticles on physiological stress induced by exogenous dexamethasone in Japanese quails. Biol Trace Elem Res. 2018;184:474–481. doi: 10.1007/s12011-017-1192-y. [DOI] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–114. doi: 10.1016/j.phrs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Blair R, Newberry RC, Gardiner EE. Effects of lighting pattern and dietary tryptophan supplementation on growth and mortality in broilers. Poult Sci. 1993;72:495–502. doi: 10.3382/ps.0720495. [DOI] [PubMed] [Google Scholar]
- Blatchford R, Klasing K, Shivaprasad H, Wakenell P, Archer G, Mench J. The effect of light intensity on the behavior, eye and leg health, and immune function of broiler chickens. Poult Sci. 2009;88:20–28. doi: 10.3382/ps.2008-00177. [DOI] [PubMed] [Google Scholar]
- Blatchford R, Archer G, Mench J. Contrast in light intensity, rather than day length, influences the behavior and health of broiler chickens. Poult Sci. 2012;91:1768–1774. doi: 10.3382/ps.2011-02051. [DOI] [PubMed] [Google Scholar]
- Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci. 2015;178:330–336. doi: 10.1016/j.livsci.2015.05.004. [DOI] [Google Scholar]
- Borges T, Wieten L, van Herwijnen M, Broere F, Van Der Zee R, Bonorino C, Van Eden W (2012) The anti-inflammatory mechanisms of Hsp70. Front Immunol 3. 10.3389/fimmu.2012.00095 [DOI] [PMC free article] [PubMed]
- Burgos-Aceves MA, Abo-Al-Ela HG, Faggio C. Physiological and metabolic approach of plastic additive effects: immune cells responses. J Hazard Mater. 2021;404:124114. doi: 10.1016/j.jhazmat.2020.124114. [DOI] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci J. 2012;91:2532–2539. doi: 10.3382/ps.2012-02160. [DOI] [PubMed] [Google Scholar]
- Campo J, Gil M, Davila S, Munoz I. Influence of perches and footpad dermatitis on tonic immobility and heterophil to lymphocyte ratio of chickens. Poult Sci. 2005;84:1004–1009. doi: 10.1093/ps/84.7.1004. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu W, Wang Z, Xie D, Jia L, Chen Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J Appl Poult Res. 2008;17:211–218. doi: 10.3382/japr.2007-00043. [DOI] [Google Scholar]
- Chen H, Weiss J, Shahidi F. Nanotechnology in nutraceuticals and functional foods. Food Technol. 2006;3:30–36. [Google Scholar]
- Chen X, Li R, Geng Z. Cold stress initiates the Nrf2/UGT1A1/L-FABP signaling pathway in chickens. Poult Sci. 2015;94:2597–2603. doi: 10.3382/ps/pev253. [DOI] [PubMed] [Google Scholar]
- Chen M, Li X, Shi Q, Zhang Z, Xu S. Hydrogen sulfide exposure triggers chicken trachea inflammatory injury through oxidative stress-mediated FOS/IL8 signaling. J Hazard Mater. 2019;368:243–254. doi: 10.1016/j.jhazmat.2019.01.054. [DOI] [PubMed] [Google Scholar]
- Compton MM, Gibbs PS, Johnson LR. Glucocorticoid activation of deoxyribonucleic acid degradation in bursal lymphocytes. Poult Sci. 1990;69:1292–1298. doi: 10.3382/ps.0691292. [DOI] [PubMed] [Google Scholar]
- Compton MM, Gibbs PS, Swicegood LR. Glucocorticoid-mediated activation of DNA degradation in avian lymphocytes. Gen Comp Endocrinol. 1990;80:68–79. doi: 10.1016/0016-6480(90)90149-g. [DOI] [PubMed] [Google Scholar]
- Dawood MAO, Abo-Al-Ela HG, Hasan MT. Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020;97:268–282. doi: 10.1016/j.fsi.2019.12.054. [DOI] [PubMed] [Google Scholar]
- Deep A, Schwean-Lardner K, Crowe TG, Fancher BI, Classen HL. Effect of light intensity on broiler behaviour and diurnal rhythms. Appl Anim Behav Sci. 2012;136:50–56. doi: 10.1016/j.applanim.2011.11.002. [DOI] [Google Scholar]
- Deep A, Raginski C, Schwean-Lardner K, Fancher B, Classen H. Minimum light intensity threshold to prevent negative effects on broiler production and welfare. Br Poult Sci. 2013;54:686–694. doi: 10.1080/00071668.2013.847526. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Golemboski KA, Austic RE. Environment-immune interactions. Poult Sci. 1994;73:1062–1076. doi: 10.3382/ps.0731062. [DOI] [PubMed] [Google Scholar]
- Dohms JE, Metz A. Stress—mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991;30:89–109. doi: 10.1016/0165-2427(91)90011-z. [DOI] [PubMed] [Google Scholar]
- El Sabry MI, McMillin KW, Sabliov CM. Nano-technology considerations for poultry and livestock production systems – a review. Ann Anim Sci. 2018;18:319–334. doi: 10.1515/aoas-2017-0047. [DOI] [Google Scholar]
- El-Deep MH, Ijiri D, Ebeid TA, Ohtsuka A. Effects of dietary nano-selenium supplementation on growth performance, antioxidative status, and immunity in broiler chickens under thermoneutral and high ambient temperature conditions. J Poult Sci. 2016;53:274–283. doi: 10.2141/jpsa.0150133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deep MH, Shabaan M, Assar MH, Attia KM, Sayed MAM. Comparative effects of different dietary selenium sources on productive performance, antioxidative properties and immunity in local laying hens exposed to high ambient temperature. J Animal and Poultry Prod. 2017;9:335–343. doi: 10.21608/JAPPMU.2017.45998. [DOI] [Google Scholar]
- El-Kassas S, Abdo SE, El-Naggar K, Abdo W, Kirrella AAK, Nashar TO Ameliorative effect of dietary supplementation of copper oxide nanoparticles on inflammatory and immune responses in commercial broiler under normal and heat-stress housing conditions. J Therm Biol. 2018;78:235–246. doi: 10.1016/j.jtherbio.2018.10.009. [DOI] [PubMed] [Google Scholar]
- El-Kassas S, El-Naggar K, Abdo SE, Abdo W, Kirrella AAK, El-Mehaseeb I, Abu El-Magd M. Dietary supplementation with copper oxide nanoparticles ameliorates chronic heat stress in broiler chickens. Anim Prod Sci. 2020;60:254–268. doi: 10.1071/AN18270. [DOI] [Google Scholar]
- El-Katcha M, Soltan MA, El-badry M. Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alex J Vet Sci. 2017;55:129–145. doi: 10.5455/ajvs.266925. [DOI] [Google Scholar]
- El-katcha MI, Soltan MA, Arafa MM, El-Naggar K, Kawarei E-SR. Impact of dietary replacement of inorganic zinc by organic or nano sources on productive performance, immune response and some blood biochemical constituents of laying hens. Alex J Vet Sci. 2018;59:48–59. doi: 10.5455/ajvs.301885. [DOI] [Google Scholar]
- El-Katcha MI, Soltan MA, Khalifa E, Fadl SE, Hassan A, El-Shimey OK, El-Naggar K. Growth and immune response of broiler chicks fed on oxidized oil containing diets and supplemented with different copper sources and levels. Alex J Vet Sci. 2020;66:15–26. doi: 10.5455/ajvs.98495. [DOI] [Google Scholar]
- El-Kazaz SE, Hafez MH. Evaluation of copper nanoparticles and copper sulfate effect on immune status, behavior, and productive performance of broilers. J Adv Vet Anim Res. 2020;7:16–25. doi: 10.5455/javar.2020.g388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Lethey H, Huber-Eicher B, Jungi TW. Exploration of stress-induced immunosuppression in chickens reveals both stress-resistant and stress-susceptible antigen responses. Vet Immunol Immunopathol. 2003;95:91–101. doi: 10.1016/s0165-2427(02)00308-2. [DOI] [PubMed] [Google Scholar]
- El-Naggar K, El-Kassas S, Abdo SE, Kirrella AAK, Al Wakeel RA. Role of gamma-aminobutyric acid in regulating feed intake in commercial broilers reared under normal and heat stress conditions. J Therm Biol. 2019;84:164–175. doi: 10.1016/j.jtherbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr. 2003;133:1443s–1447s. doi: 10.1093/jn/133.5.1443S. [DOI] [PubMed] [Google Scholar]
- Fair J, Ricklefs R. Physiological, growth, and immune responses of Japanese quail chicks to the multiple stressors of immunological challenge and lead shot. Arch Environ Contam Toxicol. 2002;42:77–87. doi: 10.1007/s002440010294. [DOI] [PubMed] [Google Scholar]
- Fan Q, et al. Influence of mushroom polysaccharide, nano-copper, copper loaded chitosan, and lysozyme on intestinal barrier and immunity of LPS-mediated yellow-feathered chickens. Animals. 2020;10:594. doi: 10.3390/ani10040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Zheng Z, Yang Z, Peng X, Zuo Z, Cui H, Ouyang P, Shu G, Chen Z, Huang C. Ameliorative effects of selenium on the excess apoptosis of the jejunum caused by AFB1 through death receptor and endoplasmic reticulum pathways. Toxicol Res. 2018;7:1108–1119. doi: 10.1039/c8tx00068a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan ED, Turkyilmaz MK, Nazligul A. Effects of noise and light intensities on stress and fear reactions in broilers. Indian J Anim Sci. 2015;85:1375–1378. [Google Scholar]
- Fowles JR, Fairbrother A, Fix M, Schiller S, Kerkvliet NI. Glucocorticoid effects on natural and humoral immunity in mallards. Dev Comp Immunol. 1993;17:165–177. doi: 10.1016/0145-305X(93)90026-M. [DOI] [PubMed] [Google Scholar]
- Freeman B. The stress syndrome. Worlds Poult Sci J. 1987;43:15–19. doi: 10.1079/WPS19870002. [DOI] [Google Scholar]
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Gomes A, et al. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathol. 2014;43:82–90. doi: 10.1080/03079457.2013.874006. [DOI] [PubMed] [Google Scholar]
- Gongruttananun N. Influence of red light on reproductive performance, eggshell ultrastructure, and eye morphology in Thai-native hens. Poult Sci. 2011;90:2855–2863. doi: 10.3382/ps.2011-01652. [DOI] [PubMed] [Google Scholar]
- Gopi M, Pearlin B, Kumar RD, Shanmathy M, Prabakar G. Role of nanoparticles in animal and poultry nutrition: Modes of action and applications in formulating feed additives and food processing. Int J Pharmacol. 2017;13:724–731. doi: 10.3923/ijp.2017.724.731. [DOI] [Google Scholar]
- Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15:7–16. doi: 10.5604/16652681.1184191. [DOI] [PubMed] [Google Scholar]
- Gu XH, Hao Y, Wang XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress1. Poult Sci. 2012;91:790–799. doi: 10.3382/ps.2011-01628. [DOI] [PubMed] [Google Scholar]
- Guo Y, Ma S, Du J, Chen J. Effects of light intensity on growth, anti-stress ability and immune function in yellow feathered broilers. Braz J Poult Sci. 2018;20:79–84. doi: 10.1590/1806-9061-2017-0542. [DOI] [Google Scholar]
- Guo Y, Jiang R, Su A, Tian H, Zhang Y, Li W, Tian Y, Li K, Sun G, Han R, Yan F, Kang X. Identification of genes related to effects of stress on immune function in the spleen in a chicken stress model using transcriptome analysis. Mol Immunol. 2020;124:180–189. doi: 10.1016/j.molimm.2020.06.004. [DOI] [PubMed] [Google Scholar]
- Gupta A. Ascites syndrome in poultry: a review. Worlds Poult Sci J. 2011;67:457–468. doi: 10.1017/S004393391100050X. [DOI] [Google Scholar]
- Hafez A, Nassef E, Fahmy M, Elsabagh M. Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ Sci Pollut Res. 2020;27:19108–19114. doi: 10.1007/s11356-019-04344-6. [DOI] [PubMed] [Google Scholar]
- Hajializadeh F, Ghahri H, Talebi A. Effects of supplemental chromium picolinate and chromium nanoparticles on performance and antibody titers of infectious bronchitis and avian influenza of broiler chickens under heat stress condition. Vet Res Forum. 2017;8:259–264. [PMC free article] [PubMed] [Google Scholar]
- Hamal K, Burgess SC, Pevzner I, Erf G. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- Hamidi O, Mohammad C, Hasan G, Ali A, Hassan M. Effects of chromium (III) picolinate and chromium (III) picolinate nanoparticles supplementation on growth performance, organs weight and immune function in cyclic heat stressed broiler chickens. Kafkas Univ Vet Fak Derg. 2016;1:10–16. doi: 10.9775/kvfd.2015.14736. [DOI] [Google Scholar]
- Hamidi O, Chamani M, Ghahri H, Sadeghi AA, Malekinejad H, Palangi V (2021) Effects of supplemental chromium nanoparticles on IFN-γ expression of heat stress broilers. Biol Trace Elem Res. 10.1007/s12011-021-02634-0 [DOI] [PubMed]
- Hangalapura BN (2006) Cold stress and immunity: do chickens adapt to cold by trading-off immunity for thermoregulation? PhD Thesis. Wageningen Institute of Animal Sciences, Wageningen University and Research Centre.
- Hangalapura BN, Nieuwland MG, de Vries RG, Heetkamp MJ, Van den Brand H, Kemp B, Parmentier HK. Effects of cold stress on immune responses and body weight of chicken lines divergently selected for antibody responses to sheep red blood cells. Poult Sci. 2003;82:1692–1700. doi: 10.1093/ps/82.11.1692. [DOI] [PubMed] [Google Scholar]
- Hangalapura BN, Nieuwland MGB, Buyse J, Kemp B, Parmentier HK. Effect of duration of cold stress on plasma adrenal and thyroid hormone levels and immune responses in chicken lines divergently selected for antibody responses. Poult Sci. 2004;83:1644–1649. doi: 10.1093/ps/83.10.1644. [DOI] [PubMed] [Google Scholar]
- Hassan RA, Soliman ES, Hamad RT, El-Borady OM, Ali AA, Helal MS. Selenium and nano-selenium ameliorations in two breeds of broiler chickens exposed to heat stress. South Afr J Anim Sci. 2020;50:215–232. doi: 10.4314/sajas.v50i2.5. [DOI] [Google Scholar]
- Hassan S, Hassan F-U, Rehman MS-U. Nano-particles of trace minerals in poultry nutrition: potential applications and future prospects. Biol Trace Elem Res. 2020;195:591–612. doi: 10.1007/s12011-019-01862-9. [DOI] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Heckert RA, Estevez I, Russek-Cohen E, Pettit-Riley R. Effects of density and perch availability on the immune status of broilers. Poult Sci. 2002;81:451–457. doi: 10.1093/ps/81.4.451. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schmucker SS, Bessei W, Grashorn M, Stefanski V. Impact of housing environment on the immune system in chickens: a review. Animals. 2020;10:1138. doi: 10.3390/ani10071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff GR, Huff WE, Rath NC, de Los Santos FS, Farnell MB, Donoghue AM. Influence of hen age on the response of turkey poults to cold stress, Escherichia coli challenge, and treatment with a yeast extract antibiotic alternative. Poult Sci. 2007;86:636–642. doi: 10.1093/ps/86.4.636. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Isobe T, Lillehoj HS. Effects of corticosteroids on lymphocyte subpopulations and lymphokine secretion in chickens. Avian Dis. 1992;36:590–596. doi: 10.2307/1591753. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Otowski K, Kozłowski K, Pietrzak P, Ferenc K, Ognik K, Juśkiewicz J, Sawosz E, Zduńczyk Z. Effect of different levels of copper nanoparticles and copper sulfate on morphometric indices, antioxidant status and mineral digestibility in the small intestine of turkeys. Ann Anim Sci. 2020;20:975–990. doi: 10.2478/aoas-2020-0013. [DOI] [Google Scholar]
- Janský L, Pospíšilová D, Honzova S, Uličný B, Šrámek P, Zeman V, Kaminkova J. Immune system of cold-exposed and cold-adapted humans. Eur J Appl Physiol Occup Physiol. 1996;72:445–450. doi: 10.1007/BF00242274. [DOI] [PubMed] [Google Scholar]
- Janus P, Pakuła-Cis M, Kalinowska-Herok M, Kashchak N, Szołtysek K, Pigłowski W, Widlak W, Kimmel M, Widlak P. NF-κB signaling pathway is inhibited by heat shock independently of active transcription factor HSF1 and increased levels of inducible heat shock proteins. Genes to Cells. 2011;16:1168–1175. doi: 10.1111/j.1365-2443.2011.01560.x. [DOI] [PubMed] [Google Scholar]
- Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1062562–1062518. doi: 10.1155/2018/1062562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Mohammed AA, Jacobs JA, Cramer TA, Cheng HW. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult Sci. 2020;99:142–150. doi: 10.3382/ps/pez571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P, Wu Z, Rothwell L, Fife M, Gibson M, Poh TY, Shini A, Bryden W, Shini S. Prospects for understanding immune-endocrine interactions in the chicken. Gen Comp Endocrinol. 2009;163:83–91. doi: 10.1016/j.ygcen.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Kamal R, Dey A, Mondal KG, Chandran PC. Impact of environmental stressors on the performance of backyard poultry. Proc Natl Acad Sci, India, Sect B Biol Sci. 2018;88:79–84. doi: 10.1007/s40011-016-0741-z. [DOI] [Google Scholar]
- Kaspers B, Schat KA. Avian immunology (Second Edition) Boston: Academic Press; 2012. [Google Scholar]
- Ke YY, Liu WJ, Wang ZX, Chen JL. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poult Sci. 2011;90:2632–2637. doi: 10.3382/ps.2011-01523. [DOI] [PubMed] [Google Scholar]
- Keum Y-S, Choi BY. Molecular and chemical regulation of the Keap1-Nrf2 signaling pathway. Molecules. 2014;19:10074–10089. doi: 10.3390/molecules190710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd MT. Nutritional modulation of immune function in broilers. Poult Sci. 2004;83:650–657. doi: 10.1093/ps/83.4.650. [DOI] [PubMed] [Google Scholar]
- Kjaer JB, Vestergaard K. Development of feather pecking in relation to light intensity. Appl Anim Behav Sci. 1999;62:243–254. doi: 10.1016/S0168-1591(98)00217-2. [DOI] [Google Scholar]
- Klasing KC. Nutrition and the immune system. Br Poult Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Kliger CA, Gehad AE, Hulet RM, Roush WB, Lillehoj HS, Mashaly MM. Effects of photoperiod and melatonin on lymphocyte activities in male broiler chickens. Poult Sci. 2000;79:18–25. doi: 10.1093/ps/79.1.18. [DOI] [PubMed] [Google Scholar]
- Koutsos EA, Klasing KC. Chapter 17 - factors modulating the avian immune system. In: Schat KA, Kaspers B, Kaiser P, editors. Avian Immunology. 2. Boston: Academic Press; 2014. pp. 299–313. [Google Scholar]
- Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poult Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- Lechner O, Dietrich H, Wiegers GJ, Vacchio M, Wick G. Glucocorticoid production in the chicken bursa and thymus. Int Immunol. 2001;13:769–776. doi: 10.1093/intimm/13.6.769. [DOI] [PubMed] [Google Scholar]
- Lee KA, Wikelski M, Robinson WD, Robinson TR, Klasing KC. Constitutive immune defences correlate with life-history variables in tropical birds. J Anim Ecol. 2008;77:356–363. doi: 10.1111/j.1365-2656.2007.01347.x. [DOI] [PubMed] [Google Scholar]
- Lewis PD, Morris TR. Poultry and coloured light. Worlds Poult Sci J. 2000;56:189–207. doi: 10.1079/Wps20000015. [DOI] [Google Scholar]
- Li Q, Zhang M, Chen Y-J, Wang Y-J, Huang F, Liu J. Oxidative damage and HSP70 expression in masseter muscle induced by psychological stress in rats. Physiol Behav. 2011;104:365–372. doi: 10.1016/j.physbeh.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Li S, Muhammad I, Yu H, Sun X, Zhang X. Detection of aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol Environ Saf. 2019;176:137–145. doi: 10.1016/j.ecoenv.2019.03.089. [DOI] [PubMed] [Google Scholar]
- Li G-M, Liu LP, Yin B, Liu YY, Dong WW, Gong S, Zhang J, Tan JH. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems. Poult Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lina T, Fenghua Z, Huiying R, Jianyang J, Wenli L. Effects of nano-zinc oxide on antioxidant function in broilers. Chin J Anim Nutr. 2009;21:534–539. [Google Scholar]
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Lu L, Li SF, Zhang LY, Xi L, Zhang KY, Luo XG. Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult Sci. 2011;90:1782–1790. doi: 10.3382/ps.2010-01215. [DOI] [PubMed] [Google Scholar]
- Liu LL, He JH, Xie HB, Yang YS, Li JC, Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu W, Yuan Y, Sun C, Balasubramanian B, Zhao Z, An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol. 2011;153:175–190. doi: 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Lyu Q, Wawrzyniuk M, Rutten VPMG, van Eden W, Sijts AJAM, Broere F. Hsp70 and NF-kB mediated control of innate inflammatory responses in a canine macrophage cell line. Int J Mol Sci. 2020;21:6464. doi: 10.3390/ijms21186464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev. 2012;64:1055–1081. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zheng YX, Dong XY, Zou XT. Effect of mercury chloride on oxidative stress and nuclear factor erythroid 2-related factor 2 signalling molecule in liver and kidney of laying hens. J Anim Physiol Anim Nutr. 2018;102:1199–1209. doi: 10.1111/jpn.12920. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhu M, Miao L, Zhang X, Dong X, Zou X. Mercuric chloride induced ovarian oxidative stress by suppressing Nrf2-Keap1 signal pathway and its downstream genes in laying hens. Biol Trace Elem Res. 2018;185:185–196. doi: 10.1007/s12011-018-1244-y. [DOI] [PubMed] [Google Scholar]
- Martin LB, II, Gilliam J, Han P, Lee K, Wikelski M. Corticosterone suppresses cutaneous immune function in temperate but not tropical house sparrows, Passer domesticus. Gen Comp Endocrinol. 2005;140:126–135. doi: 10.1016/j.ygcen.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos Trans R Soc Lond Ser B-Biol Sci. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaly MM, Trout JM, Hendricks G, III, Al-Dokhi LM, Gehad A. The role of neuroendocrine immune interactions in the initiation of humoral immunity in chickens. Domest Anim Endocrinol. 1998;15:409–422. doi: 10.1016/s0739-7240(98)00023-x. [DOI] [PubMed] [Google Scholar]
- McDowell LR (2003) Minerals in animal and human nutrition (Second Edition). Elsevier, Amsterdam. 10.1016/B978-0-444-51367-0.X5001-6
- Meng T, Liu YL, Xie CY, Zhang B, Huang YQ, Zhang YW, Yao Y, Huang R, Wu X. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol Trace Elem Res. 2019;189:548–555. doi: 10.1007/s12011-018-1490-z. [DOI] [PubMed] [Google Scholar]
- Merrill L, Angelier F, O’Loghlen AL, Rothstein SI, Wingfield JC. Sex-specific variation in brown-headed cowbird immunity following acute stress: a mechanistic approach. Oecologia. 2012;170:25–38. doi: 10.1007/s00442-012-2281-4. [DOI] [PubMed] [Google Scholar]
- Mohamed RA, Eltholth MM, El-Saidy NR. Rearing broiler chickens under monochromatic blue light improve performance and reduce fear and stress during pre-slaughter handling and transportation. Biotechnol Anim Husb. 2014;30:457–471. doi: 10.2298/BAH1403457M. [DOI] [Google Scholar]
- Monson MS, Van Goor AG, Ashwell CM, Persia ME, Rothschild MF, Schmidt CJ, Lamont SJ. Immunomodulatory effects of heat stress and lipopolysaccharide on the bursal transcriptome in two distinct chicken lines. BMC Genomics. 2018;19:643. doi: 10.1186/s12864-018-5033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson MS, Van Goor AG, Persia ME, Rothschild MF, Schmidt CJ, Lamont SJ. Genetic lines respond uniquely within the chicken thymic transcriptome to acute heat stress and low dose lipopolysaccharide. Sci Rep. 2019;9:13649. doi: 10.1038/s41598-019-50051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. In: Feige U, Yahara I, Morimoto RI, Polla BS, editors. Stress-Inducible Cellular Responses. Basel: Birkhäuser Basel; 1996. pp. 139–163. [DOI] [PubMed] [Google Scholar]
- Muhammad I, Wang X, Li S, Li R, Zhang X. Curcumin confers hepatoprotection against AFB1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol Biol Rep. 2018;45:1775–1785. doi: 10.1007/s11033-018-4323-4. [DOI] [PubMed] [Google Scholar]
- Mujahid A. Acute cold-induced thermogenesis in neonatal chicks (Gallus gallus) Comp Biochem Physiol A-Mol Integr Physiol. 2010;156:34–41. doi: 10.1016/j.cbpa.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Mumma JO, Thaxton JP, Vizzier-Thaxton Y, Dodson WL. Physiological stress in laying hens. Poult Sci. 2006;85:761–769. doi: 10.1093/ps/85.4.761. [DOI] [PubMed] [Google Scholar]
- Najafi P, Zulkifli I, Amat Jajuli N, Farjam AS, Ramiah SK, Amir AA, O’Reily E, Eckersall D. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int J Biometeorol. 2015;59:1577–1583. doi: 10.1007/s00484-015-0964-3. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal DM, Ketterson ED. Life-history evolution, hormones, and avian immune function. In: Demas GE, Nelson RJ, editors. Ecoimmunology. New York, NY, USA: Oxford University Press, Inc.; 2012. [Google Scholar]
- Ognik K, Sembratowicz I, Cholewińska E, Jankowski J, Kozłowski K, Juśkiewicz J, Zduńczyk Z. The effect of administration of copper nanoparticles to chickens in their drinking water on the immune and antioxidant status of the blood. Anim Sci J. 2018;89:579–588. doi: 10.1111/asj.12956. [DOI] [PubMed] [Google Scholar]
- Orhan C, Tuzcu M, Gencoglu H, Sahin N, Hayirli A, Sahin K. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry. Br Poult Sci. 2013;54:447–453. doi: 10.1080/00071668.2013.806787. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M, Jones C. Colour vision of domestic chicks. J Exp Biol. 1999;202:2951–2959. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol. 2011;301:R843–R863. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- Pan JM, Yang YF, Yang B, Yu YH. Artificial polychromatic light affects growth and physiology in chicks. Plos One. 2014;9:e113595. doi: 10.1371/journal.pone.0113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Ghosh S, Mandal S, Sau S, Pal M. NRF2 transcriptionally activates the heat shock factor 1 promoter under oxidative stress and affects survival and migration potential of MCF7 cells. J Biol Chem. 2018;293:19303–19316. doi: 10.1074/jbc.RA118.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechova A, Pavlata L. Chromium as an essential nutrient: a review. Vet Med. 2007;52:1–18. doi: 10.17221/2010-VETMED. [DOI] [Google Scholar]
- Peng L, Du W, Balhuizen MD, Haagsman HP, de Haan CAM, Veldhuizen EJA (2020a) Antiviral activity of chicken cathelicidin b1 against influenza A virus. Front Microbiol 11. 10.3389/fmicb.2020.00426 [DOI] [PMC free article] [PubMed]
- Peng L, Scheenstra MR, van Harten RM, Haagsman HP, Veldhuizen EJA. The immunomodulatory effect of cathelicidin-B1 on chicken macrophages. Vet Res. 2020;51:122. doi: 10.1186/s13567-020-00849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Prescott N, Wathes CM, Jarvis J. Light, vision and the welfare of poultry. Anim Welf. 2003;12:269–288. [Google Scholar]
- Quinteiro-Filho WM, Gomes AVS, Pinheiro ML, Ribeiro A, Ferraz-de-Paula V, Astolfi-Ferreira CS, Ferreira AJP, Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho WM, Rodrigues MV, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sá LRM, Ferreira AJP, Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J Anim Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Rahimi G, Rezaei M, Hafezian H, Saiyahzadeh H. The effect of intermittent lighting schedule on broiler performance. Int J Poult Sci. 2005;4:396–398. doi: 10.3923/ijps.2005.396.398. [DOI] [Google Scholar]
- Ramiah SK, Awad EA, Mookiah S, Idrus Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult Sci. 2019;98:3828–3838. doi: 10.3382/ps/pez093. [DOI] [PubMed] [Google Scholar]
- Rault J-L, Clark K, Groves PJ, Cronin GM. Light intensity of 5 or 20 lux on broiler behavior, welfare and productivity. Poult Sci. 2017;96:779–787. doi: 10.3382/ps/pew423. [DOI] [PubMed] [Google Scholar]
- Regnier JA, Kelley KW. Heat-and cold-stress suppresses in vivo and in vitro cellular immune responses of chickens. Am J Vet Res. 1981;42:294–299. [PubMed] [Google Scholar]
- Rimoldi S, Lasagna E, Sarti FM, Marelli SP, Cozzi MC, Bernardini G, Terova G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. 2015;6:17–25. doi: 10.1016/j.mgene.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk YS, Ibrahim AF, Mansour MK, Mohamed HS, El-Slamony AE, Soliman AAM. Effect of dietary source of selenium on productive and reproductive performance of Sinai laying hens under heat stress conditions. Egypt Poult Sci. 2017;37:461–489. [Google Scholar]
- Roushdy EM, Zaglool AW, Hassan FAM. Thermal stress consequences on growth performance, immunological response, antioxidant status, and profitability of finishing broilers: transcriptomic profile change of stress-related genes. Trop Anim Health Prod. 2020;52:3685–3696. doi: 10.1007/s11250-020-02405-4. [DOI] [PubMed] [Google Scholar]
- Saelao P, et al. Integrated proteomic and transcriptomic analysis of differential expression of chicken lung tissue in response to NDV infection during heat stress. Genes. 2018;9:579. doi: 10.3390/genes9120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdari-Rostamabad M, Hosseini-Vashan SJ, Perai AH, Sarir H. Nanoselenium supplementation of heat-stressed broilers: effects on performance, carcass characteristics, blood metabolites, immune response, antioxidant status, and jejunal morphology. Biol Trace Elem Res. 2017;178:105–116. doi: 10.1007/s12011-016-0899-5. [DOI] [PubMed] [Google Scholar]
- Sahin K, Orhan C, Tuzcu M, Ali S, Sahin N, Hayirli A. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult Sci. 2010;89:2251–2258. doi: 10.3382/ps.2010-00749. [DOI] [PubMed] [Google Scholar]
- Sahin K, Orhan C, Tuzcu M, Sahin N, Hayirli A, Bilgili S, Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult Sci. 2016;95:1088–1095. doi: 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- Sato T, Tezuka K, Shibuya H, Watanabe T, Kamata H, Shirai W. Cold-induced ascites in broiler chickens and its improvement by temperature-controlled rearing. Avian Dis. 2002;46:989–996. doi: 10.1637/0005-2086(2002)046[0989:CIAIBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Scanes CG. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult Sci. 2016;95:2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- Scott NR. Nanotechnology and animal health. Rev Sci Tech. 2005;24:425–432. doi: 10.20506/rst.24.1.1579. [DOI] [PubMed] [Google Scholar]
- Scott A, Vadalasetty KP, Łukasiewicz M, Jaworski S, Wierzbicki M, Chwalibog A. Effect of different levels of copper nanoparticles and copper sulphate on performance, metabolism and blood biochemical profiles in broiler chicken. J Anim Physiol Anim Nutr. 2018;102:e364–e373. doi: 10.1111/jpn.12754. [DOI] [PubMed] [Google Scholar]
- Senaratna D, Samarakone TS, Gunawardena WWDA. Red color light at different intensities affects the performance, behavioral activities and welfare of broilers. Asian-Australas J Anim Sci. 2016;29:1052–1059. doi: 10.5713/ajas.15.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti-Costa R, Ignacchiti M, Chedraoui-Silva S, Marchi L, Mantovani B. Chronic cold stress in mice induces a regulatory phenotype in macrophages: correlation with increased 11β-hydroxysteroid dehydrogenase expression. Brain Behav Immun. 2012;26:50–60. doi: 10.1016/j.bbi.2011.07.234. [DOI] [PubMed] [Google Scholar]
- Sharideh H, Zaghari M. Effect of dietary L-tryptophan supplementation and light-emitting diodes on growth and immune response of broilers. Vet Res Forum. 2021;12:63–67. doi: 10.30466/vrf.2019.96558.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MC, Joshi C, Pathak NN, Kaur H. Copper status and enzyme, hormone, vitamin and immune function in heifers. Res Vet Sci. 2005;79:113–123. doi: 10.1016/j.rvsc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Shini S, Kaiser P. Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress. 2009;12:388–399. doi: 10.1080/10253890802526894. [DOI] [PubMed] [Google Scholar]
- Shini S, Kaiser P, Shini A, Bryden WL. Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comp Biochem Physiol B-Biochem Mol Biol. 2008;149:324–333. doi: 10.1016/j.cbpb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Shini S, Kaiser P, Shini A, Bryden WL. Differential alterations in ultrastructural morphology of chicken heterophils and lymphocytes induced by corticosterone and lipopolysaccharide. Vet Immunol Immunopathol. 2008;122:83–93. doi: 10.1016/j.vetimm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Shini S, Huff GR, Shini A, Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult Sci. 2010;89:841–851. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- Shini S, Shini A, Kaiser P. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress. 2010;13:185–194. doi: 10.3109/10253890903144639. [DOI] [PubMed] [Google Scholar]
- Siegel HS. Immunological responses as indicators of stress. Worlds Poult Sci J. 1985;41:36–44. doi: 10.1079/WPS19850003. [DOI] [Google Scholar]
- Siegel HS. Effects of behavioural and physical stressors on immune responses. In: Wiepkema PR, Van Adrichem PWM, editors. Biology of Stress in Farm Animals: An Integrative Approach. Dordrecht, The Netherlands: Springer Netherlands; 1987. pp. 39–54. [Google Scholar]
- Slawinska A, Hsieh JC, Schmidt CJ, Lamont SJ. Heat stress and lipopolysaccharide stimulation of chicken macrophage-like cell line activates expression of distinct sets of genes. Plos One. 2016;11:e0164575. doi: 10.1371/journal.pone.0164575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, Safwat AM. Climate change impact on immune status and productivity of poultry as well as the quality of meat and egg products. In: Ewis Omran E-S, Negm AM, editors. Climate Change Impacts on Agriculture and Food Security in Egypt: Land and Water Resources—Smart Farming—Livestock, Fishery, and Aquaculture. Cham: Springer International Publishing; 2020. pp. 481–498. [Google Scholar]
- Staal FJT, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Stier KS, Almasi B, Gasparini J, Piault R, Roulin A, Jenni L. Effects of corticosterone on innate and humoral immune functions and oxidative stress in barn owl nestlings. J Exp Biol. 2009;212:2085–2091. doi: 10.1242/jeb.024406. [DOI] [PubMed] [Google Scholar]
- Surai PF, Fisinin VI. Vitagenes in poultry production: part 1. Technological and environmental stresses. Worlds Poult Sci J. 2016;72:721–734. doi: 10.1017/S0043933916000714. [DOI] [Google Scholar]
- Surai PF, Fisinin VI. Vitagenes in poultry production: part 2. Nutritional and internal stresses. Worlds Poult Sci J. 2016;72:761–772. doi: 10.1017/S0043933916000726. [DOI] [Google Scholar]
- Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle NF. The mineral nutrition of livestock (4th Edition) CABI Publishing. UK: Oxfordshire; 2010. [Google Scholar]
- Tella JL, Forero MG, Bertellotti M, Donázar JA, Blanco G, Ceballos O. Offspring body condition and immunocompetence are negatively affected by high breeding densities in a colonial seabird: a multiscale approach. Proc R Soc Lond Ser B-Biol Sci. 2001;268:1455–1461. doi: 10.1098/rspb.2001.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Valle NR-D, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V, Georgopoulou I, Batzios C, Pappaioannou N, Ducatelle R, Fortomaris P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:430–435. doi: 10.1080/03079457.2015.1083094. [DOI] [PubMed] [Google Scholar]
- Tsiouris V, Georgopoulou I, Batzios C, Pappaioannou N, Ducatelle R, Fortomaris P. High stocking density as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:59–66. doi: 10.1080/03079457.2014.1000820. [DOI] [PubMed] [Google Scholar]
- Tuerkyilmaz MK. The effect of stocking density on stress reaction in broiler chickens during summer. Turk J Vet Anim Sci. 2008;32:31–36. [Google Scholar]
- Wang Y. Differential effects of sodium selenite and nano-Se on growth performance, tissue Se distribution, and glutathione peroxidase activity of avian broiler. Biol Trace Elem Res. 2009;128:184–190. doi: 10.1007/s12011-008-8264-y. [DOI] [PubMed] [Google Scholar]
- Wang L-C, Liao LX, Lv HN, Liu D, Dong W, Zhu J, Chen JF, Shi ML, Fu G, Song XM, Jiang Y, Zeng KW, Tu PF. Highly selective activation of heat shock protein 70 by allosteric regulation provides an insight into efficient neuroinflammation inhibition. EBioMedicine. 2017;23:160–172. doi: 10.1016/j.ebiom.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Saelao P, Chanthavixay K, Gallardo R, Bunn D, Lamont SJ, Dekkers JM, Kelly T, Zhou H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult Sci. 2018;97:770–780. doi: 10.3382/ps/pex363. [DOI] [PubMed] [Google Scholar]
- Xie D, Wang ZX, Dong YL, Cao J, Wang JF, Chen JL, Chen YX. Effects of monochromatic light on immune response of broilers. Poult Sci. 2008;87:1535–1539. doi: 10.3382/ps.2007-00317. [DOI] [PubMed] [Google Scholar]
- Xie D, Li J, Wang ZX, Cao J, Li TT, Chen JL, Chen YX. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult Sci. 2011;90:2697–2704. doi: 10.3382/ps.2011-01416. [DOI] [PubMed] [Google Scholar]
- Yalcin S, Özkan S, Türkmut L, Siegel PB. Responses to heat stress in commercial and local broiler stocks. 1. Performance traits. Br Poult Sci. 2001;42:149–152. doi: 10.1080/00071660120048375. [DOI] [PubMed] [Google Scholar]
- Yang YF, Jin SF, Zhong ZT, Yu YH, Yang B, Yuan HB, Pan JM. Growth responses of broiler chickens to different periods of artificial light. J Anim Sci. 2015;93:767–775. doi: 10.2527/jas2014-8096. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yu Y, Pan J, Ying Y, Zhou H. A new method to manipulate broiler chicken growth and metabolism: response to mixed LED light system. Sci Rep. 2016;6:25972. doi: 10.1038/srep25972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Kajiwara E, Ekino S, Taura Y, Hirota Y, Horiuchi H, Matsuda H, Furusawa S. Immunobiology of chicken germinal center: I. Changes in surface Ig class expression in the chicken splenic germinal center after antigenic stimulation. Dev Comp Immunol. 2003;27:159–166. doi: 10.1016/s0145-305x(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Zha L, Zeng J, Chu X, Mao L, Luo H. Efficacy of trivalent chromium on growth performance, carcass characteristics and tissue chromium in heat-stressed broiler chicks. J Sci Food Agric. 2009;89:1782–1786. doi: 10.1002/jsfa.3656. [DOI] [Google Scholar]
- Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. BioFactors. 2001;15:27–38. doi: 10.1002/biof.5520150103. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang HJ, Qiao X, Yue HY, Wu SG, Yao JH, Qi GH. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Sun G, Li K, Li Z, Su A, Liu X, Li G, Jiang R, Han R, Tian Y, Kang X, Yan F. Transcriptome profile in bursa of Fabricius reveals potential mode for stress-influenced immune function in chicken stress model. BMC Genomics. 2018;19:918. doi: 10.1186/s12864-018-5333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C-Y, Tan S-X, Xiao X-Y, Qiu X-S, Pan J-Q, Tang Z-X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res. 2014;160:361–367. doi: 10.1007/s12011-014-0052-2. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Zhang ZW, Qu JP, Yao HD, Li M, Li S, Xu SW. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19:635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang Y. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult Sci. 2011;90:680–686. doi: 10.3382/ps.2010-00977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable