Abstract
Heat shock protein 70 (HSP70) is a key member of the HSP family that contributes to a pre-cancerous environment; however, its role in lung cancer remains poorly understood. The present study used geranylgeranylacetone (GGA) to induce HSP70 expression, and transforming growth factor-β (TGF-β) was used to construct an epithelial-mesenchymal transition (EMT) model by stimulating A549 cells in vitro. Western Blot was performed to detect protein levels of NADPH oxidase 4 (NOX4) and the EMT-associated proteins E-cadherin and vimentin both before and after HSP70 expression. Cell morphological changes were observed, and the effect of HSP70 on cell migration ability was detected via the wound healing. The results demonstrated that GGA at 50 and 200 μmol/L could significantly induce HSP70 expression in A549 cells (P < 0.05). Furthermore, HSP70 induced by 200 μmol/L GGA significantly inhibited the changes of E-cadherin, vimentin, and cell morphology induced by TGF-β (P < 0.05), while HSP70 induced by 50 μmol/L GGA did not. The results of the wound healing assay indicated that 200 μmol/L GGA significantly inhibited A549 cell migration induced by TGF-β. Taken together, the results of the present study demonstrated that overexpression of HSP70 inhibited the TGF-β induced EMT process and changed the cell morphology and migratory ability induced by TGF-β in A549 cells.
Keywords: Lung cancer, A549 cells, Heat shock protein 70, Transforming growth factor-β, Epithelial-mesenchymal transition, Migration
Introduction
Lung cancer is one of the most dangerous malignancies to human health and life, whereby the morbidity and mortality rates are rapidly increasing (Ferlay et al. 2015; Siegel et al. 2019). According to the American Cancer Society, 154,050 mortalities among 234,030 new cancer cases were reported in 2018 (Hechtner et al. 2019). Lung cancer is predominantly divided into two subtypes, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), of which NSCLC accounts for 85% of lung cancer cases (Ali et al. 2013).
Epithelial-mesenchymal transition (EMT) is crucial to the progression of lung cancer (Pastushenko and Blanpain 2019). EMT is an key biological process by which epithelial cells acquire a mesenchymal-like phenotype, resulting in the loss of cell polarity, detachment from the basement membrane, and acquisition of migration, invasion, anti-apoptosis, and degradation of extracellular matrix characteristics (Lu and Kang 2019; Stemmler et al. 2019). Despite several studies focusing on the EMT mechanism, a complete EMT-targeted tumor therapy drug has not yet been developed.
HSP70 is a key member of the HSP family, and it has been extensively studied since its discovery (Tissiéres et al. 1974). HSP70 contributes to a pre-cancerous environment (Gabai et al. 2016; Teng et al. 2012); however, it has also been reported to exert a protective role against different types of cancer. Overexpression of HSP70 failed to exhibit increased carcinogenesis in mice, which had a longer survival time compared with wild-type mice (Naka et al. 2014). Furthermore, cancer cells transition to a mesenchymal state, and the migratory ability of cancer cells is enhanced in the absence of HSP70 (Kasioumi et al. 2019).
The present study hypothesized that HSP70 may be a potential therapeutic molecule targeted against lung cancer (Kasioumi et al. 2019; Yang et al. 2015). Thus, this study aimed to elucidate the role of overexpression of HSP70 in the EMT process, and cell migration induced by transforming growth factor-β (TGF-β).
Materials and methods
Cell lines and cell culture
The human lung adenocarcinoma A549 cell line was purchased from the Cell Bank of the Institute of Biochemistry and Cell Biology (Shanghai, China). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, without antibiotics at 37 °C in a humidified atmosphere of 5% CO2. Cells were collected at logarithmic growth phase.
Antibodies and reagents
Geranylgeranylacetone (GGA) was purchased from MCE Biological Company of America. TGF-β was purchased from PeproTech, Inc. The horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from Wuhan Sanying Biotechnology. Antibody against NOX4 (ab133303) was purchased from Abcam China. Antibodies against E-cadherin (14472S), vimentin (5741S), HSP70 (4873S), and GAPDH (5174S) were purchased from Cell Signaling Technology, Inc.
Observation of cell morphology
In order to observe and photograph cell morphology, the computer was connected with the living cell workstation. After cells were washed three times with phosphate-buffered saline (PBS), the cell culture bottle was placed on the platform of the living cell workstation, and the morphological changes of each group of cells were observed and photographed on an inverted microscope (Nikon Eclipse E100, Tokyo, Japan) (magnification, ×100).
Wound healing
For the migration assay, cells were plated into 6-well plates; once the cells reached 80–90% confluence, the monolayers of cells were scratched using a 10-μl pipette tip to generate a cell-free gap, 0.1 mm in width. Cells were washed three times with phosphate-buffered saline (PBS) and photographed to record the wound width at 0 h. Subsequently, cells were treated for 24 h in serum-free medium according to the different experimental groups. Finally, cells were observed on a microscope (Nikon Eclipse E100, Tokyo, Japan), and the MiE software was used to capture the images to record the wound width at 24 h.
Western blotting
Total proteins were extracted using RIPA lysis buffer (50 mM Tris/HCL pH 8.0, 150 mM NaCL, 1% Nonidet-P40, 1% sodium deoxycholate, 0.1% SDS) and quantified via Bradford method, using NanoVue spectrophotometer (General Electric Company, USA). The protein samples were boiled in SDS sample buffer for 5 min. Equal amounts of lysate protein was separated via SDS-PAGE, transferred onto polyvinylidene membranes (CoWin Biosciences), and subsequently blocked with 5% skim milk for 2 h. Membranes were washed three times with Tris Buffer Solution Tween (TBST) for 30 minutes, prior to incubation with primary antibodies overnight at 4 °C. Membranes were re-washed three times with Tris Buffer Solution Tween prior to incubation with HRP-conjugated secondary antibody for 1 h. Finally, antibody-antigen complexes were visualized by ECL reagent (Absin Bioscience Inc.).
Statistical analysis
Statistical analysis was performed using SPSS software (version 21.0; IBM Corp.). Each experiment repeated 3 times. The data was presented as the mean ± standard deviation from three independent experiments. One-way analysis of variance followed by the Bonferroni post hoc test was used to compare differences between multiple groups. P < 0.05 was considered to indicate a statistically significant difference.
Results
A549 cells were stimulated by TGF-β to construct the EMT model
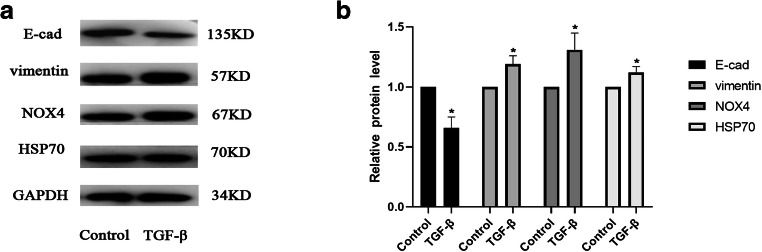
Based on the preliminary experiments, 5 μg/L of TGF-β was used to irritate A549 cells in order to construct the EMT model. Western blot analysis was performed to detect the protein expression levels of HSP70, NOX4, and the EMT-associated proteins E-cadherin and vimentin (Fig. 1a and b). The results demonstrated that compared with the blank group, the epithelial cell marker protein was downregulated, while interstitial cell marker protein was elevated, and HSP70 and NOX4 were also elevated when TGF-β was used to stimulate A549 cells (P < 0.05; Fig. 1a and b).
Fig. 1.
A549 cells were stimulated by TGF-β to construct the EMT model. A549 cells were exposed to 5 μg/L of TGF-β, and the expression of E-cadherin, vimentin, HSP70, and NOX4 in the TGF-β group and control group were detected by Western blot (A and B). The result was expressed as mean ± SD. *P < 0.05 versus the control group
Screen for the optimum concentration and time of GGA
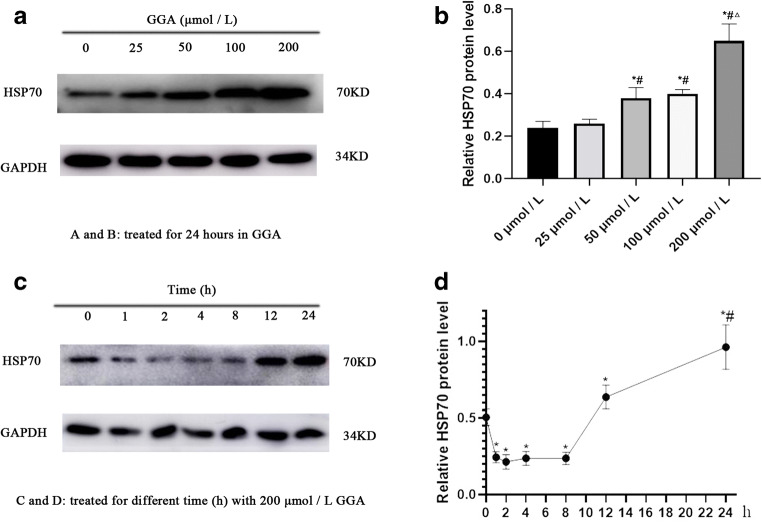
A549 cells were treated with different concentrations of GGA for 24 h to induce HSP70 expression. Proteins from each group were collected, and HSP70 expression was detected via Western blotting, in order to determine the optimum concentration of GGA (Fig. 2a). A549 cells were subsequently stimulated with the optimum concentration of GGA (200 μmol/L) at various time points (0, 1, 2, 4, 8, 12, and 24 h) to induce HSP70 expression. Similarly, proteins at the various time points were collected, and HSP70 expression was detected via Western blotting, in order to determine the optimal time of GGA (Fig. 2c). The results indicated that HSP70 expression increased over time. Notably, HSP70 protein expression significantly increased at 12 h compared with 0 h, while HSP70 protein levels were higher at 24 h compared with 12 h (Fig. 2d). Thus, the optimal time of GGA to stimulate A549 cells, in order to induce HSP70, was selected at 24 h. The results demonstrated differences when 50 and 200 μmol/l GGA were used (Fig. 2b). Thus, the effects of HSP70 on cell morphology, EMT, and cell migration was investigated, following treatment of cells 50 and 200 μmol/l GGA.
Fig. 2.
Screen out of the optimum concentration and time of GGA. The expression of HSP70 induced by various concentrations (0, 25, 50, 100, 200 μmol/L) of GGA for 24 hours was detected by Western blot (a and b). *P < 0.05 versus 0 μmol/L group, #P < 0.05 versus 25 μmol/L group, ΔP < 0.05 versus 100 μmol/L group. The expression of HSP70 induced by 200 μmol/L GGA at various times (0, 1, 2, 4, 8, 12, and 24 h) was detected by Western blot (c and d). *P < 0.05 versus 0 h, #P < 0.05 versus 12 h
Overexpression of HSP70 inhibits EMT induced by TGF-β in A549 cells
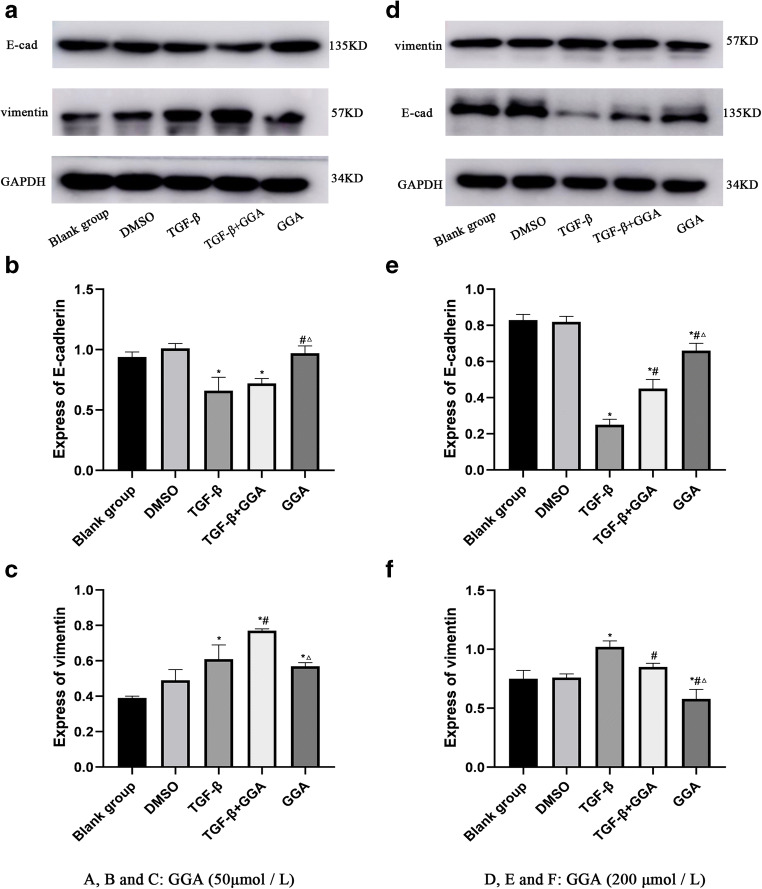
In order to investigate the effects of HSP70 on EMT, HSP70 expression was induced with 50 and 200 μmol/l GGA in A549 cells, and the EMT-associated proteins were detected via Western blot analysis. The results demonstrated that the epithelial marker protein, E-cadherin, did not change following treatment with 50 μmol/l GGA, compared with TGF-β group (P > 0.05), while the mesenchymal cell marker protein, vimentin, increased in the TGF-β+GGA group (P < 0.05). Furthermore, E-cadherin expression increased (P < 0.05), while vimentin expression decreased (P < 0.05) in the GGA group compared with the TGF-β+GGA group (Fig. 3a,b,c). Similarly, the EMT-associated proteins, E-cadherin and vimentin, were detected via Western blotting following treatment of cells with 200 μmol/l GGA (Fig. 3d). E-cadherin expression increased, while vimentin expression decreased in both the TGF-β+GGA and GGA groups, compared with the TGF-β group (Fig. 3e and f). Furthermore, E-cadherin expression increased, and vimentin expression decreased in the GGA group compared with the TGF-β+GGA group (P < 0.05; Fig. 3e and f). Taken together, these results suggested that HSP70 induced by 200 μmol/l GGA increased E-cadherin and decreased vimentin expression. However, these effects were less prominent when a lower concentration of GGA was used. Collectively, these results indicated that overexpression of HSP70 can inhibit the changes of E-cadherin and vimentin in TGF-β-induced EMT, suppressing the EMT process.
Fig. 3.
Exploring the effect of HSP70 on EMT induced by TGF-β in A549 cells. The study included the following groups: (1) blank group, (2) DMSO group, (3) TGF-β group, (4) TGF-β+GGA group, and (5) GGA group. Western blot analysis was used to detect the impact of HSP70 induced by 50 μmol/L GGA on the expression of EMT-related proteins E-cadherin and vimentin (a, b, and c). The same method was used to detect the impact of HSP70 induced by 200 μmol/L GGA on the EMT (d, e, and f). *P < 0.05 versus the control group, #P < 0.05 versus TGF-β group, ΔP < 0.05 versus TGF-β+GGA group
Overexpression of HSP70 inhibits the changes in cell morphology induced by TGF-β in A549 cells
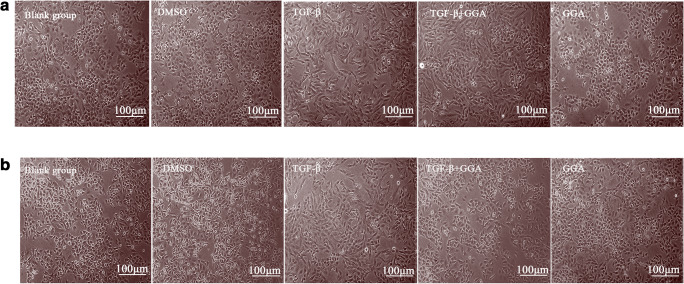
The present study assessed the changes in cell morphology following treatment with 50 and 200 μmol/l GGA. The changes in cell morphology were less prominent following treatment with 50 μmol/l GGA in the TGF-β+GGA group compared with the TGF-β group. Cells treated with 50 μmol/l GGA only exhibited a more round appearance compared with the TGF-β and TGF-β+GGA groups (Fig. 4a). Compared with the TGF-β group, cells in the TGF-β+GGA group became shorter and more round following treatment with 200 μmol/l GGA (Fig. 4b). HSP70 induced by 50 μmol/l GGA had a less prominent effect on the TGF-β-induced cell morphology changes, whereas HSP70 induced by 200 μmol/l GGA had a prominent effect on the TGF-β-induced cell morphology, and the cells exhibited a more round morphology in the TGF-β+GGA group. Collectively, these results indicated that overexpression of HSP70 can inhibit the changes in A549 cell morphology induced by TGF-β.
Fig. 4.
Exploring the effect of HSP70 on cell morphology induced by TGF-β in A549 cells. Cells were photographed at × 100 magnification using an inverted microscope when cells were treated by 50 μmol/L (a) and 200 μmol/L GGA (b). The scale bars = 100 μm
Overexpression of HSP70 inhibits the changes of migration ability induced by TGF-β in A549 cells
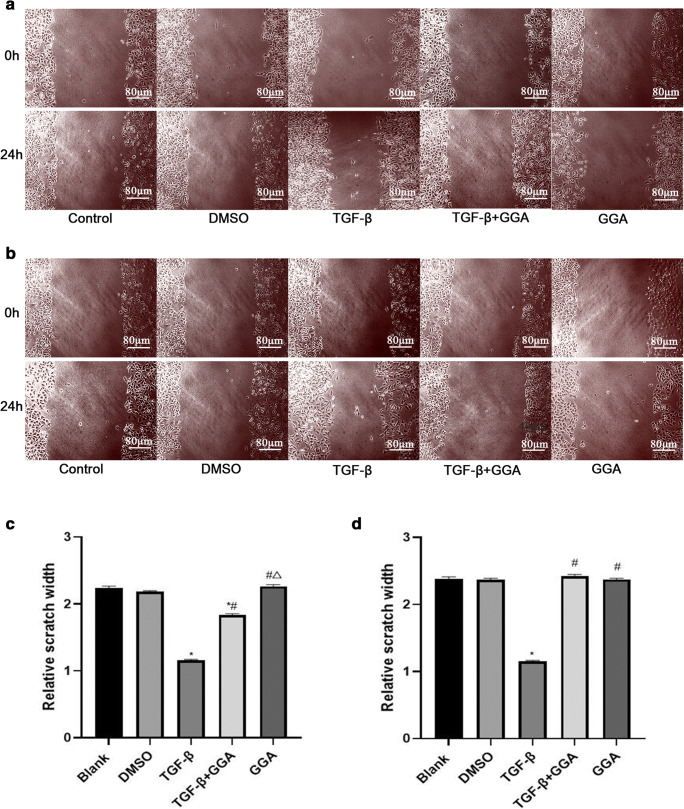
Increasing evidences suggest that EMT is a crucial mechanism regulating the migration of tumor cells (Aiello and Kang 2019; Aiello et al. 2018; Mittal 2018). Thus, the present study investigated the effects of HSP70 on the migratory ability of cells treated with 50 and 200 μmol/l GGA. The results demonstrated that the wound widths of the TGF-β group narrowed following treatment with 50 μmol/l GGA. Furthermore, the wound widths of the TGF-β+GGA group and the GGA group were widened compared with the TGF-β group (P < 0.05; Fig. 5a and c).
Fig. 5.
To explore the effect of HSP70 on cell migration ability induced by TGF-β in A549 cells. The impact of HSP70 induced by GGA (50 μmol/L) on cell migratory ability was detected by wound healing (a and c). The monolayers were scratched using a 10-μl pipette tip once the cells reached 80–90% confluence. Cells were washed three times with PBS and subsequently treated according to the different experimental groups. The same method was used to detect the impact of HSP70 induced by 200 μmol/L GGA on the migratory ability (b and d). The result was expressed as mean ± SD. *P < 0.05 versus the control group, #P < 0.05 versus TGF-β group, ΔP < 0.05 versus TGF-β+GGA group. The scale bars = 80 μm
The wound width of the TGF-β group narrowed, while the widths of the TGF-β+GGA and GGA groups widened following treatment with 200 μmol/l GGA compared with the blank group. Furthermore, the wound widths of both the TGF-β+GGA and GGA groups widened compared with the TGF-β group (P < 0.05; Fig. 5b and d). Taken together, these results suggested that HSP70 induced by both 50 and 200 μmol/l GGA inhibited the migratory ability of cells induced by TGF-β; however, it is evident that migration induced by TGF-β cannot be completely inhibited by treatment with 50 μmol/l GGA. Thus, we speculate that overexpression of HSP70 inhibits the changes of migratory ability induced by TGF-β in A549 cells (Fig. 6).
Fig. 6.
Schematic representation of the cell signaling status of HSP70, GGA, TGF-β, and EMT that shows overexpression of HSP70 inhibited the TGF-β induced EMT process and changed the cell morphology and migratory ability induced by TGF-β in A549 cells
Discussion
The results of the present study demonstrated that overexpression of HSP70 inhibited the changes in EMT-associated proteins, cell morphology, and the migratory ability induced by TGF-β in A549 cells.
TGF-β is a member of the TGF family that is considered crucial to proliferation, differentiation, and apoptosis of epithelial cells (Caja et al. 2018). TGF-β induces EMT in different types of tissues and cells, such as the kidney (Loeffler 2019), colorectal (Vu and Datta 2017), and breast (Nami and Wang 2017). TGF-β promotes EMT initiation by regulating the expression levels of growth factors, cytokines, chemokines, and developmental signaling pathways (Sisto et al. 2020). Thus, TGF-β was used in the present study to establish an EMT model in vitro. During this process, E-cadherin, a marker protein of epithelial cells, and vimentin, a marker protein of mesenchymal cells, were selected as indicators for observation.
E-cadherin is a typical marker of epithelial cells, and epithelial polarity and cellular connectivity are lost during EMT (Tae-Young Na et al. n.d.). E-cadherin maintains tight cellular connections and prevents cell invasion and migration (Chen et al. 2012). Vimentin is a typical marker of mesenchymal cells that is involved in several aspects of tumorigenesis and progression, including tumor initiation, EMT, and metastasis of tumor cells (Wu et al. 2019). Furthermore, vimentin affects cell-cell and cell-extracellular matrix (ECM) adhesion and thus is considered a key regulator of cell movement (Bhuyan et al. 2019). The results of the present study demonstrated that E-cadherin expression decreased and vimentin expression increased in the TGF-β group, suggesting that the EMT model induced by TGF-β in A549 cells was successful in vitro.
NADPH oxidase (NOXs) is a class of enzymes capable of producing superoxide and hydrogen peroxide (Konate et al. 2020). NOX4 is one subtype of NOXs that is frequently expressed in several tumor cell lines (Cheng et al. 2001). NOX4 expression was increased in the present EMT model. The result of the present study was consistent with the previous finding that NOX4 expression increased in the EMT model of rat lens epithelial cell constructed by TGF-β; however, ROS expression decreased following addition of the NOX4 inhibitor, attenuating the EMT process (Pastushenko and Blanpain 2019). Furthermore, in breast cancer, TGF-β can enhance the activity of the NOX4 promoter that triggers EMT process by relying on the SMAD3 signaling pathway. Conversely, silencing of NOX4 can attenuate the EMT process, suggesting that NOX4 plays a positive role in the process of TGF-β induced EMT model (Bronsert et al. 2014).
GGA is clinically used to treat gastric ulcers and is a non-toxic inducer of HSP70 (Hirakawa et al. 1996). GGA can attenuate hepatic fibrosis by upregulating HSP70 expression (He et al. 2015). GGA can directly activate the HSP70 gene to stimulate transcription in cultured gastric mucosal cells (Hirakawa et al. 1996). GGA may enhance heat shock response through the activation of heat shock factor 1 (HSF1) in rat liver cells (Ikeyama et al. 2001). Furthermore, HSP70 induced by GGA depended on HSF1 (Tanaka et al. 2007).
Both 50 and 200 μmol/L GGA were used to induce HSP70 in the present study. HSP70 induced by 200 μmol/l GGA inhibited the changes of E-cadherin, vimentin, and cell morphology induced by TGF-β, while HSP70 induced by 50 μmol/l GGA did not completely. Following treatment with 200 μmol/l GGA, E-cadherin expression increased, vimentin expression decreased, and the cells became shorter and more round in the TGF-β+GGA group compared with the TGF-β group that was more inclined to the cell morphology of the blank group. Consistent with the results of the present study, previous findings demonstrated that HSP70 gene silencing caused cells to become more elongated in morphology and to lose contact with neighboring cells in MCF7, A549, and HeLa cell lines (Kasioumi et al. 2019). Similarly, HSP70 attenuated EMT in rat lens epithelial cells; after the lens epithelial cells treated with TGF-β were heated at 45 °C for 90 min, the EMT process of lens epithelial cells was attenuated (Banh et al. 2007). Besides, the absence of ΗSP70 decreased the accumulation of E-cadherin and caused the cells to acquire a mesenchymal-like phenotype (Kasioumi et al. 2019). HSP70 inhibited advanced glycation end-products-induced EMT by inhibiting the TGF-β/SMAD and MAPK/ERK signaling pathways in peritoneal mesothelial cells (Yang et al. 2015). Furthermore, HSP70 is involved in inhibiting TGF-β signaling (Yun et al. 2010) and blocking TGF-β-induced EMT by decreasing the receptor-dependent phosphorylation of Smad2 (Li et al. 2011).
GGA at 50 and 200 μmol/L were used to investigate the effect of HSP70 on the cell migratory ability of A549 cells. The wound healing assay results demonstrated that overexpression of HSP70 inhibited cell migration induced by TGF-β. There was evidence that HSP70 was involved in the process of cell migration. In glioma, overexpression of HSP70 can promote cell migration (Sun et al. 2019). Conversely, in HeLa cells, silencing HSP70 gene expression promotes cell migration (Kasioumi et al. 2019). This may be due to different cell types. The inhibitor of HSP90 can upregulate HSP70 expression that can effectively inhibit the production of ROS from NOX in human pulmonary artery endothelial cells, thereby inhibiting the cell migration (Chen et al. 2012). Inhibiting the expression of E-cadherin or blocking its function can alter the morphology of cells that is favorable for cell migration and invasion (Pastushenko and Blanpain 2019). In breast cancer, the loss of partial or total function of E-cadherin is associated with invasion, migration, and poor prognosis of tumor cells (Xu et al. 2020). The expression of vimentin may play a key role in cell migratory ability, since migratory ability can be inhibited when vimentin expression is suppressed (Wawruszak et al. 2019). The expression level of vimentin is positively associated with the invasive and migratory ability of tumor cells, such as ovarian cancer, cervical cancer, endometrial cancer (Zhou et al. 2014), breast cancer (Xu et al. 2020), tongue squamous cell carcinoma (Hussein et al. 2018), lung cancer (Kielbus et al. 2019), and prostate cancer (Kaufhold and Bonavida 2014). In the present study, overexpression of HSP70 can downregulate the expression of vimentin and abate the process of EMT; however, the specific molecular mechanism and signaling pathways involved remain unclear; thus, further studies are required. Based on the results of the present study, we conclude that HSP70 may be a therapeutic target for cell migration; however, further investigations are required to confirm our findings.
A major limitation of the present study is the lack of investigation on how HSP70 affects EMT and migration. Thus, prospective studies will focus on investigating the underlying molecular mechanisms of HSP70 in this process
Conclusion
Taken together, the results of the present study demonstrated that overexpression of HSP70 can inhibit the TGF-β induced EMT process and change the cell morphology and migratory ability induced by TGF-β in A549 cells. Thus, HSP70 may be a crucial target in the EMT process.
Acknowledgements
In addition, we are thankful to Professor Fang Zhou for her advice and support in this study.
Abbreviations
- HSP70
Heat shock protein 70
- EMT
Epithelial-mesenchymal transition
- TGF-β
Transforming growth factor-β
- NOX4
NADPH oxidase 4
Authors’ contributions
Fang Zhou and Yanan Ren conceived and designed the experiments. Yanan Ren, Fengxian Shi, and Mingze Ma participated in the in experiments. Fengxian Shi wrote and edited the manuscript. Ruonan Zhai reviewed the manuscript. All the authors contributed greatly to the manuscript and approved it.
Funding
The study was funded by the National Natural Science Foundation of China (Grant No.8183206) and the Ministry of Education of Science and Technology research foundation of Henan province (Grant No.172102310267).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fang Zhou, Email: zhoufang23@sina.com.
Wu Yao, Email: yaowu@zzu.edu.cn.
References
- Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216:1016–1026. doi: 10.1084/jem.20181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello NM, et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev Cell. 2018;45:681–695. doi: 10.1016/j.devcel.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20:300–306. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh A, Deschamps PA, Vijayan MM, Sivak JG, West-Mays JA. The role of Hsp70 and Hsp90 in TGF-beta-induced epithelial-to-mesenchymal transition in rat lens epithelial explants. Mol Vis. 2007;13:2248–2262. [PubMed] [Google Scholar]
- Bhuyan G, Arora R, Ahluwalia C, Sharma P. Epithelial-mesenchymal transition in serous and mucinous epithelial tumors of the ovary. J Cancer Res Ther. 2019;15:1309–1315. doi: 10.4103/jcrt.JCRT_35_18. [DOI] [PubMed] [Google Scholar]
- Bronsert P, et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol. 2014;234:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I (2018) TGF-beta and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci 19. 10.3390/ijms19051294 [DOI] [PMC free article] [PubMed]
- Chen F, et al. Opposing actions of heat shock protein 90 and 70 regulate nicotinamide adenine dinucleotide phosphate oxidase stability and reactive oxygen species production. Arterioscler Thromb Vasc Biol. 2012;32:2989–2999. doi: 10.1161/ATVBAHA.112.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gabai VL, et al. Anticancer effects of targeting Hsp70 in tumor stromal cells. Cancer Res. 2016;76:5926–5932. doi: 10.1158/0008-5472.CAN-16-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, et al. Geranylgeranylacetone attenuates hepatic fibrosis by increasing the expression of heat shock protein 70. Mol Med Rep. 2015;12:4895–4900. doi: 10.3892/mmr.2015.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtner M, Eichler M, Wehler B, Buhl R, Sebastian M, Stratmann J, Schmidberger H, Gohrbandt B, Peuser J, Kortsik C, Nestle U, Wiesemann S, Wirtz H, Wehler T, Bals R, Blettner M, Singer S. Quality of life in NSCLC survivors - a multicenter cross-sectional study. J Thorac Oncol. 2019;14:420–435. doi: 10.1016/j.jtho.2018.11.019. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111:345–357. doi: 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]
- Hussein AA, Forouzanfar T, Bloemena E, de Visscher J, Brakenhoff RH, Leemans CR, Helder MN. A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br J Cancer. 2018;119:724–736. doi: 10.1038/s41416-018-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S, Kusumoto K, Miyake H, Rokutan K, Tashiro S. A non-toxic heat shock protein 70 inducer, geranylgeranylacetone, suppresses apoptosis of cultured rat hepatocytes caused by hydrogen peroxide and ethanol. J Hepatol. 2001;35:53–61. doi: 10.1016/s0168-8278(01)00053-8. [DOI] [PubMed] [Google Scholar]
- Kasioumi P, Vrazeli P, Vezyraki P, Zerikiotis S, Katsouras C, Damalas A, Angelidis C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int J Oncol. 2019;54:821–832. doi: 10.3892/ijo.2018.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res. 2014;33:62. doi: 10.1186/s13046-014-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielbus M, Czapinski J, Kalafut J, Wos J, Stepulak A, Rivero-Muller A (2019) Genetically engineered lung cancer cells for analyzing epithelial-mesenchymal transition. Cells 8. 10.3390/cells8121644 [DOI] [PMC free article] [PubMed]
- Konate MM, Antony S, Doroshow JH. Inhibiting the activity of NADPH oxidase in cancer. Antioxid Redox Signal. 2020;33:435–454. doi: 10.1089/ars.2020.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang X, Wang Q. HSP70 decreases receptor-dependent phosphorylation of Smad2 and blocks TGF-beta-induced epithelial-mesenchymal transition. J Genet Genomics. 2011;38:111–116. doi: 10.1016/j.jgg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Loeffler I. MKP2 suppresses TGF-beta1-induced epithelial-to-mesenchymal transition through JNK inhibition. Clin Sci (Lond) 2019;133:545–550. doi: 10.1042/CS20180881. [DOI] [PubMed] [Google Scholar]
- Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361–374. doi: 10.1016/j.devcel.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- Naka KK, Vezyraki P, Kalaitzakis A, Zerikiotis S, Michalis L, Angelidis C. Hsp70 regulates the doxorubicin-mediated heart failure in Hsp70-transgenic mice. Cell Stress Chaperones. 2014;19:853–864. doi: 10.1007/s12192-014-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami B, Wang Z (2017) HER2 in breast cancer stemness: a negative feedback loop towards trastuzumab resistance. Cancers (Basel) 9. 10.3390/cancers9050040 [DOI] [PMC free article] [PubMed]
- Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis trends. Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Sisto M, Lorusso L, Ingravallo G, Ribatti D, Lisi S. TGFbeta1-Smad canonical and -Erk noncanonical pathways participate in interleukin-17-induced epithelial-mesenchymal transition in Sjogren's syndrome. Lab Investig. 2020;100:824–836. doi: 10.1038/s41374-020-0373-z. [DOI] [PubMed] [Google Scholar]
- Stemmler MP, Eccles RL, Brabletz S, Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21:102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- Sun G, Cao Y, Xu Y, Huai D, chen P, Guo J, Li M, Dai Y. Overexpression of Hsc70 promotes proliferation, migration, and invasion of human glioma cells. J Cell Biochem. 2019;120:10707–10714. doi: 10.1002/jcb.28362. [DOI] [PubMed] [Google Scholar]
- Tae-Young Na LS, Mendonsa AM, Gumbiner BM (n.d.) The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Cell Biol 117:5931–5937. 10.1073/pnas.1918167117/-/DCSupplemental [DOI] [PMC free article] [PubMed]
- Tanaka K, et al. Genetic evidence for a protective role of heat shock factor 1 against irritant-induced gastric lesions. Mol Pharmacol. 2007;71:985–993. doi: 10.1124/mol.106.033282. [DOI] [PubMed] [Google Scholar]
- Teng Y, Ngoka L, Mei Y, Lesoon L, Cowell JK. HSP90 and HSP70 proteins are essential for stabilization and activation of WASF3 metastasis-promoting protein. J Biol Chem. 2012;287:10051–10059. doi: 10.1074/jbc.M111.335000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissiéres A, Mitchell H, Tracy UM. Protein synthesis in salivary glands of drosophila melanogaster : relation to chromosome PufFs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Vu T, Datta PK (2017) Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 9. 10.3390/cancers9120171 [DOI] [PMC free article] [PubMed]
- Wawruszak A, Kalafut J, Okon E, Czapinski J, Halasa M, Przybyszewska A, Miziak P, Okla K, Rivero-Muller A, Stepulak A (2019) Histone deacetylase inhibitors and phenotypical transformation of cancer cells. Cancers (Basel) 11. 10.3390/cancers11020148 [DOI] [PMC free article] [PubMed]
- Wu Q, Wang J, Liu Y, Gong X. Epithelial cell adhesion molecule and epithelial-mesenchymal transition are associated with vasculogenic mimicry, poor prognosis, and metastasis of triple negative breast cancer. Int J Clin Exp Pathol. 2019;12:1678–1689. [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shi J, Tang W, Jiang P, Guo M, Zhang B, Ma G. ROR2 promotes the epithelial-mesenchymal transition by regulating MAPK/p38 signaling pathway in breast cancer. J Cell Biochem. 2020;121:4142–4153. doi: 10.1002/jcb.29666. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhu T, Liu X, Zhang L, Yang Y, Zhang J, Guomicron M. Heat shock protein 70 protects rat peritoneal mesothelial cells from advanced glycation end-products-induced epithelial-to-mesenchymal transition through mitogenactivated protein kinases/extracellular signal-regulated kinases and transforming growth factor-beta/Smad pathways. Mol Med Rep. 2015;11:4473–4481. doi: 10.3892/mmr.2015.3271. [DOI] [PubMed] [Google Scholar]
- Yun CH, et al. Geldanamycin inhibits TGF-beta signaling through induction of Hsp70. Arch Biochem Biophys. 2010;495:8–13. doi: 10.1016/j.abb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumour Biol. 2014;35:9523–9530. doi: 10.1007/s13277-014-2537-1. [DOI] [PubMed] [Google Scholar]