Abstract
Pheochromocytomas and paragangliomas (PCPGs) are catecholamine-producing neuroendocrine tumors. Accumulating evidences indicate that the blockade of antioxidative pathways might be a novel therapeutic approach to the treatment of PCPG. NIX has been confirmed to play a key role in maintaining redox homeostasis in tumors, while the function of NIX in PCPG remains unclear. In this study, the analyses of the disease-free survival (DFS) showed that high NIX protein level is related to poor prognosis in patients of PCPG. Consistent with this, high level of NIX protein upregulates the level of p-NF-κB and promotes the migration of PC12 cells. In NIX-over-expressing PC12 cells, the level of reactive oxygen species (ROS) is decreased while trolox-equivalent antioxidant capacity (TEAC) increased. But in NIX-silencing cells, ROS level is increased, while TEAC reversely reduced, consequently antioxidase and phase II enzymes of NRF2 signaling were activated, and elevated endoplasmic reticulum (ER) stress was observed. Additionally, the apoptosis induced by luminespib/NVP-AUY922, an inhibitor of heat shock protein 90 (HSP90, a cellular stress response factor), was enhanced in NIX-silencing cells but reduced in the NIX-over-expressing cells. All of these results indicated that high NIX protein level enhances antioxidant capacity of PC12 cells and reduces the apoptosis caused by cell stress, such as induced by luminespib/NVP-AUY922. Therefore, luminespib/NVP-AUY922 might be effective only for PCPG with low NIX level, while targeting NIX could be a further supplement to the therapeutic treatment strategy for PCPG patients with high NIX protein level.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-021-01193-6.
Keywords: NIX, PCPG, PC12 cells, ROS, Luminespib/NVP-AUY922, HSP90 inhibitor
Introduction
Pheochromocytomas and paragangliomas (PCPGs) are catecholamine-producing neuroendocrine tumors, which arise from adrenal medulla in pheochromocytomas (PCCs), or from extra-adrenal sympathetic/parasympathetic ganglia in paragangliomas (PGLs), respectively (Koopman et al. 2019). The incidence of diagnosed PCC/PGL (PCPG) is about 0.8 per 100,000 persons per year, and around 10% of PCPG patients are metastatic (Nölting et al. 2019b). Multi-therapeutic strategies, including surgery, radiotherapy, and chemotherapy, are used for non-metastatic PCPG. However, the clinical outcome and the prognosis of metastatic tumors remain extremely poor (Nölting et al. 2019a). Targeted therapies, which include using inhibitors respectively targeting PI3K, mTORC1, HIF-2α, PARP, and HDAC, SSTR2 analogs and DNA demethylating agents, are currently under investigation and are regarded as the future personalized therapy approaches. Nonetheless, the intrinsic or acquired resistance and dose-limiting toxicities have limited their medicinal application. Thus, there is an urgent need to optimize the therapeutic regimens and to improve the selectivity and efficacy of treatment (Pang et al. 2019).
Several investigations have revealed the elevated generation of reactive oxygen species (ROS) and the activated antioxidative pathways in IDH-mutate or SDH-deficient cancers, suggesting the blockade of antioxidative pathway might be a novel therapeutic strategy for PCPG (Liu et al. 2019; Chouchani et al. 2014). Mitochondria are major organelles that cause the generation of ROS during tumorigenesis, and eliminating mitochondria through mitophagy has been confirmed to prevent the generation of ROS (Panigrahi et al. 2019). BNIP3L/NIX, a BH3-only member of the Bcl-2 family and proved significant receptor of mitophagy (Drake et al. 2017), has been identified as a pro-apoptotic protein (Panigrahi et al. 2019; Zhang and Ney 2009). Recently, it was found that upregulation of NIX in tumors promoted the mitochondrial ROS clearance, the activity of the NF-κB pathway, and the cell proliferation and invasiveness (Lu et al. 2012; Melser et al. 2013; Humpton et al. 2019; Jung et al. 2019). While all these studies indicate that NIX might play important roles during tumorigenesis, the role of NIX in PCPG remains obscure. Here, we investigated the correlation between NIX and the disease-free survival (DFS) of PCPG and evaluated the function of NIX in cell migration, antioxidant capacity, nuclear factor-erythroid 2-related factor 2 (NRF2) signaling, and endoplasmic reticulum (ER) stress in PC12 cells. Furthermore, the potential functions of NIX in PC12 cells while treated by H2O2 or a heat shock protein 90 (HSP90) inhibitor luminespib/NVP-AUY922 were also examined.
Methods
Cell culture and treatment
The rat adrenal pheochromocytoma PC12 cell line was obtained from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). The PC12 cells were cultured in DMEM medium containing 10% (v/v) fetal bovine serum (BI), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco) in a 5% CO2 atmosphere at 37°C. To establish in vitro cell models of over-expressing or silencing of NIX, PC12 cells were infected with NIX lentivirus (Gene ID:NM_080888; Hanheng, Shanghai, China), NIX short hairpin RNA (shRNA) lentivirus (shRNA sequences: sense 5′-GGA AGA GTG GAG CCA TGA AGA TTC AAG AGA TCT TCA TGG CTC CAC TCT TCC-3′ and antisense 5′-GGA AGA GTG GAG CCA TGA AGA TCT CTT GAA TCT TCA TGG CTC CAC TCT TCC-3′; Hanheng) or corresponding control lentivirus respectively for 24 h and then treated with 10 μg/mL puromycin (Alomone, Jerusalem, Israel) for 14 days, according to the manufacturer’s protocol.
Survival analysis
Based on the data from GEPIA 2 (http://gepia2.cancer-pku.cn/#index) and Kaplan-Meier Plotter (http://kmplot.com/analysis/) (Nagy et al. 2018; Tang et al. 2017), the effects of NIX protein on the disease-free survival (DFS) of patients with PCPG were analyzed. In brief, 64 patients of subtype “pseudohypoxia” of PCPG were analyzed by using median cutoff in GEPIA 2, and 159 patients of PCPG were analyzed by using auto select best cutoff in Kaplan-Meier Plotter.
RNA isolation and qRT-PCR
Total RNA from the PC12 cells was isolated, using a Cell Total RNA Isolation Kit (FOREGENE, Chengdu, China). cDNA was prepared using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, MA, USA). The qRT-PCR analysis was performed in a CFX ConnectTM Real-Time PCR System (Bio-Rad, CA, USA), using PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific). The primers used in this study were synthesized by TSINGKE (TSINGKE, Chengdu, China) and are listed in Table 1. The relative quantification (RQ) of mRNA expression was calculated by using the 2–ΔΔCt method.
Table 1.
Primers used for qRT-PCR
| Genes | Primer sequences |
|---|---|
| NQO1 | Forward: 5′-GCG TCT GGA GAC TGT CTG GG-3′ |
| Reverse: 5′-CGG CTG GAA TGG ACT TGC-3′ | |
| GSTM2 | Forward: 5′-CAC AAG ATC ACC CAG AGC AA-3′ |
| Reverse: 5′-CCA TAG CCT GGT TCT CCA AA-3′ | |
| GCLC | Forward: 5′-GTC CTC AGG TGA CAT TCC AAG C-3′ |
| Reverse: 5′-TGT TCT TCA GGG GCT CCA GTC-3′ | |
| GPx | Forward: 5′-CGG TTT CCC GTG CAA TCA GT-3′ |
| Reverse: 5´-ACA CCG GGG ACC AAA TGA TG-3′ | |
| NRF2 | Forward: 5′-ATA TAC GCA GGA GAG GGA AG-3′ |
| Reverse: 5′-TCC CAT CCT CAT CAC GTA AC-3′ | |
| GAPDH | Forward: 5′-CCG TGT TCC TAC CCC CAA TG-3′ |
| Reverse: 5′-TCC TCA GTG TAG CCC AGG AT-3′ |
GSTM2 glutathione S-transferase mu 2, NQO1 NAD(P) H quinone dehydrogenase 1, GPx glutathione peroxidase, GCLC glutamate-cysteine ligase catalytic subunit, GAPDH glyceraldehyde-3-phosphate dehydrogenase
Western blot analysis
The total proteins of the PC12 cells were prepared using a RIPA Lysis Buffer (Beyotime, Shanghai, China). Protein samples were separated by 10% SDS-PAGE, and then transferred to 0.22-μm PVDF membranes (Millipore, MA, USA). After blocking with 5% (w/v) nonfat milk in TBS-T buffer (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) at 37°C for 1 h, the membranes were incubated overnight at 4°C with primary antibodies to β-actin (1:5000; ZEN BIO, Chengdu, China), NIX (1:1000; Cell Signaling Technology, MA, USA), NQO1 (1:1000; ABclonal, Wuhan, China), CHOP (1:1000; Bimake, TX, USA), Keap1 (1:1000; Proteintech, Wuhan, China), NRF2 (1:1000; ABclonal), NF-κB/p65 (1:1000; ABclonal), p-NF-κB/p65-S276 (1:1000; ABclonal), GCLC (1:1000; ABclonal), GSTM2 (1:1000; ABclonal), and GRP78/BIP (1:1000; Absin, Shanghai, China), respectively. The next day, after washing away the first antibodies, the membranes were incubated with secondary antibodies (1:2000; Beyotime) at 37°C for 1 h. Protein bands were detected by an ECL chemiluminescent detection kit (Millipore).
Detection of ROS
Intracellular ROS was detected with a Reactive Oxygen Species Assay Kit (Beyotime). In brief, PC12 cells were seeded into 6-well plates at a density of 3×105 per well for 24 h. Then, the cells were treated with 10 μM DCFH-DA, a cell-permeable non-fluorescent probe useful for sensitive and rapid quantitation of oxygen-reactive species in response to oxidative metabolism, for 20 min. The green fluorescence signal was detected by flow cytometric analysis (BD Accuri™ C6 Plus, NJ, USA).
Total antioxidant capacity assay
Total antioxidants were measured by using a Total Antioxidant Capacity Assay Kit with the ABTS method (Beyotime). Briefly, the supernatant aliquots of the PC12 cells were added to a 96-well plate, and absorbance was obtained at 734 nm. A series of concentrations (0.15, 0.3, 0.6, 0.9, 1.2, 1.5 mM) of Trolox, a cell-permeable, water-soluble derivative of vitamin E working as antioxidant standard substance, were used to establish the standard curve. The trolox-equivalent antioxidant capacity (TEAC) of PC12 cells was calculated by the equation of the standard curve, and then averaged by the concentrations of proteins.
Detection of GSH and GSSG
The intracellular amount of GSH and GSSG of PC12 cells were detected with a GSH/GSSG Assay Kit (Beyotime). Briefly, the supernatant aliquots of the PC12 cells were added to a 96-well plate with total glutathione detection solution. After incubation at 25°C for 5 min, the NADPH was added. The absorbance at 412 nm was read at an interval of 5 min for 25 min continuously. A series of concentrations of 0.5, 1, 2, 5, 10, 15 μM GSSG were set to establish the standard curve. The levels of GSH and GSH + GSSG in the PC12 cells were averaged by the concentrations of proteins.
Detection of NADP+ and NADPH
Intracellular NADP+ and NADPH were detected with a NADP/NADPH Quantification Colorimetric Kit (BioVision, CA, USA). Briefly, the supernatant aliquots of the PC12 cells were filtered through 10 kDa molecular weight cut off filters to remove the cell enzymes. The total NADP/NADPH (NADPt) and NADPH were detected separately, and a series of dilutions of 0, 20, 40, 60, 80, 100 pmol/well NADPH were used to establish the standard curve. The NADPH/NADP+ ratio was calculated as NADPH/(NADPt–NADPH).
Assay of GPx
The glutathione peroxidase (GPx) assay was conducted using a Total GPx Assay Kit (Beyotime). In brief, the supernatant aliquots of PC12 cells, GPx detection solution, and detection buffer were added to a 96-well plate. The absorbance was read at wavelength of 340nm by an interval of 4 min for 20 min continuously. GPx activities in the PC12 cells were averaged by the concentrations of proteins.
Assay of GR
The glutathione reductase (GR) assay was conducted using a GR Assay Kit (Beyotime). Briefly, the supernatant aliquots of the PC12 cells were added to a 96-well plate with GR detection solution and detection buffer. The absorbance at 412 nm was read at an interval of 2 min for 10 min continuously. GR activities in the PC12 cells were calculated as [A412/min (sample)−A412/min (blank)]/0.01415 and averaged by the concentrations of proteins.
Apoptosis assay
The apoptosis of PC12 cells was assayed as described in previous study (Tusi and Khodagholi 2014; Lian et al. 2017). In brief, PC12 cells were seeded in 24-well plates at a density of 5×104 per well for 24 h, then incubated with 500 μM H2O2 for 12 h to induce oxidative injury or incubated with 200, 400, 800 nM luminespib/NVP-AUY922 (Selleck Chemicals, TX, USA) for 48 h. After staining with Hoechst 33342 (Beyotime) for 10 min, the cells were detected with an Olympus BX-53 fluorescence microscope (Olympus, Tokyo, Japan), and the apoptosis ratios of the PC12 cells were analyzed. Apoptosis was also identified by using Annexin V-FITC Apoptosis Detection Kit (Beyotime). Briefly, PC12 cells were seeded in 6-well plates at a density of 3×105 per well for 24 h, then incubated with 200, 400, 800 nM luminespib. After 48 h, the cells were incubated as described by the manufacturers. The apoptosis ratios of the PC12 cells were measured by flow cytometric analysis (BD Accuri™ C6 Plus), and at least 10,000 events were analyzed for each sample.
Migration assay
The motilities of PC12 cells were tested with a scratch wound assay. In brief, PC12 cells were seeded in 6-well plates at a density of 6×105 per well for 24 h. Then, the monolayer cells were scratched using a P200 pipette tip. The scratch gaps of PC12 cells were photographed at 0h and 24h respectively, and distances between the edges of the scratches were measured by Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA) to quantitatively evaluate cell migration.
Statistical analysis
Data were expressed as the means ± SD. Statistical analysis was performed by using SPSS 19.0. The comparisons between two groups were performed with t test, while comparisons among three or more groups were performed using ANOVA with post hoc Tukey’s test to correct multiple comparisons. The statistical significance was defined as a P value < 0.05.
Results
High NIX protein level correlated with poor prognosis in patients of PCPG and promoted migration of PC12 cells
In order to validate the role of NIX protein during tumorigenesis, the correlation between NIX protein expression level and patient prognosis of PCPG was evaluated. As the data demonstrated in Fig. 1a, b, 64 patients or 159 patients were classified into NIX high-level group (n=32, 41) or low-level group (n=32, 148) by using median cutoff in GEPIA 2 or auto select best cutoff in Kaplan-Meier Plotter, respectively. Both DFS curves consistently showed better prognosis for NIX low-level groups (P=0.04 or P=0.022).
Fig. 1.
High NIX protein level correlated with poor prognosis in patients of PCPG and promoted migration of PC12 cells. a The effect of NIX (bnip3l) protein level on DFS of PCPG patients was analyzed by using GEPIA 2 (http://gepia2.cancer-pku.cn/#index). b The effect of NIX (BNIP3L) protein on DFS of PCPG patients was analyzed by using Kaplan-Meier plotter (http://kmplot.com/analysis/). c PC12 cells were infected with LV-Puro, LV-NIX, LV-Puro-shRNA, or LV-NIX-shRNA lentivirus respectively, and the protein expression levels of NIX, NF-κB and p-NF-κB were detected by western blot. d The scratch gaps of PC12 cells were photographed at 0 h and 24 h respectively, and the migrations of PC12 cells were analyzed. Scale bar = 500 μm. Data were shown as mean ± SD from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, n.s. no significant difference
To further explore the function of NIX in PCPG, the PC12 cells were infected with LV-NIX or LV-NIX-shRNA lentivirus respectively, and the protein expression levels of NIX (Fig. 1c, Supplementary Fig. 1), NF-κB, and p-NF-κB were detected by western blot (Fig. 1c). The results showed a higher level of p-NF-κB in the NIX-over-expressing cells, and lower levels of NF-κB and p-NF-κB in the NIX-silencing cells (Fig. 1c). The motilities of the PC12 cells were also tested by using scratch wound assay, which showed that over-expressing of NIX promoted migration of PC12 cells, but silencing of NIX obviously attenuated it (Fig. 1d).
NIX protein-upregulated antioxidant capacity of PC12 cells
To investigate whether NIX plays a role in maintaining redox homeostasis in PCPG, NIX were over-expressed or silenced in PC12 cells, followed by ROS and trolox-equivalent antioxidant capacity (TEAC) examination. As shown in Fig. 2a and b, decreased ROS level and increased TEAC were observed in NIX-over-expressing cells while the reversed results were obtained in NIX-silencing cells. Furthermore, the levels of GSH (Fig. 2c), NADPH (Fig. 2d) and GPx mRNA (Fig. 2g) as well as the activities of GPx (Fig. 2f) and GR (Fig. 2e) in these cells were also examined. While compared with the corresponding controls, although total GSH (GSH + GSSG) was not influenced in NIX-over-expressing PC12 cells, the ratios of GSH/GSSG, the level of GPx mRNA, and the activities of GPx and GR were all increased, and the ratio of NADPH/NADP+ was decreased; but in NIX-silencing PC12 cells, total GSH (GSH + GSSG) and the ratios of GSH/GSSG increased, while GPx activity decreased, and there was no significance difference for GPx mRNA level and GR activity. These data demonstrated that the antioxidant capacity was enhanced in NIX-over-expressing cells but reduced in NIX-silencing cells.
Fig. 2.
NIX protein-upregulated antioxidant capacity of PC12 cells. a Relative amount of ROS (DCFH-DA fluorescence) of PC12 cells were analyzed by flow cytometry. b The total antioxidant capacity (TEAC) of PC12 cells was evaluated by ABTS method. c, d The amount of GSH, GSSG, NADP+, and NADPH of PC12 cells was detected, and total GSH (GSH + GSSG); the ratios of GSH/GSSG and NADPH/NADP+ were calculated respectively. e, f The activities of GPx and GR of PC12 cells were calculated and averaged by the concentrations of proteins. g The mRNA expression level of GPx detected by qRT-PCR was reported as relative quantification =2−∆∆Ct. h PC12 cells treated with 500 μM H2O2 for 12 h were stained by Hoechst 33342, and the apoptosis ratios were calculated by analyzing about 4000 cells for each sample. Scale bar = 50 μm. Data were shown as mean ± SD from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, n.s. no significant difference
Moreover, H2O2 was also employed to evaluate the effects of NIX on the antioxidant capacity of PC12 cells under oxidative stress. As shown in Fig. 2h, exposure to single H2O2 treatment for 12 h led to apoptosis in all PC12 cells (NIX either over-expressed or silenced), displaying typical apoptosis morphology changes after staining with Hoechst 33342. Surprisingly, this cytotoxic effect was obviously relieved not only in NIX-over-expressing cells but also in NIX-silencing cells, with lower apoptosis ratio of 11.14±2.35% and 12.28±1.59%, compared with 23.57±3.79% and 21.47±1.59% in corresponding controls, respectively. The protein expression levels of NIX treated by H2O2 were further tested, as shown in Supplementary Fig. 2; treatment of H2O2 upregulated the protein expression level of NIX in PC12 cells, and there was a dose effect between the protein level of NIX and concentration of H2O2. Furthermore, treatment of antioxidant agent N-acetyl-L-cysteine (NAC) downregulated the protein expression level of NIX in PC12 cells (Supplementary Fig. 3). These results showed that the protein expression level of NIX could be reversely regulated by ROS level.
Downregulation of NIX-activated NRF2 signaling in PC12 cells
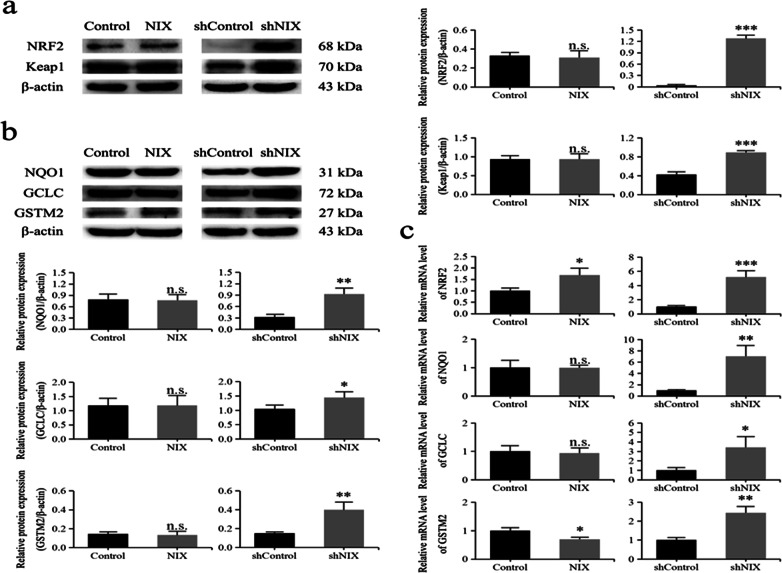
The NRF2 signaling was originally identified to play a key role in maintaining redox homeostasis (Kahroba et al. 2019; Ryoo et al. 2016). To further clarify the function of NIX in regulating the activity of NRF2 signaling, western blot was performed to assess the levels of NRF2 and its cytoplasmic inhibitor Keap1 in PC12 cells. As shown in Fig. 3a, the protein expression levels of NRF2 and Keap1 were surprisingly both significantly increased in NIX-silencing cells but were not influenced in NIX-over-expressing cells. Consistently, qRT-PCR analysis showed that the mRNA expression level of NRF2 was also obviously increased by approximately 5.2-fold in NIX-silencing cells, but just mildly increased by approximately 1.7-fold in NIX-over-expressing cells (Fig. 3c)
Fig. 3.
Downregulation of NIX-activated NRF2 signaling in PC12 cells. a, b The protein expression levels of NRF2, Keap1, NQO1, GCLC, and GSTM2 were detected by western blot. c The mRNA expression levels of NRF2, NQO1, GCLC, and GSTM2 detected by qRT-PCR were reported as relative quantification =2−∆∆Ct. Data were shown as mean ± SD from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, n.s. no significant difference
Furthermore, the protein/mRNA levels of NRF2-targeted genes, including antioxidase GCLC as well as phase II enzymes NQO1 and GSTM2, were measured by western blot and qRT-PCR, respectively (Fig. 3b, c). Consistent with the change of NRF2, the protein expression levels of NQO1, GCLC, and GSTM2 were significantly increased in NIX-silencing cells but were not influenced in NIX-over-expressing cells. The qRT-PCR analysis showed that their mRNA expression levels were also obviously increased by approximately 7.0-, 3.4-, and 2.4-fold in NIX-silencing cells, but NQO1 and GCLC were not influenced, and GSTM2 was decreased in NIX-over-expressing cells. These findings indicated that the antioxidase and phase II enzymes of NRF2 signaling might be effectively activated in NIX-silencing cells.
Downregulation of NIX elevated the level of ER stress and upregulation of NIX reduced the apoptosis induced by luminespib/NVP-AUY922 in PC12 cells
To study the effect of NIX on the endoplasmic reticulum (ER) stress, the two associated proteins, CCAAT/enhancer-binding protein homologous protein (CHOP) and glucose-regulated protein 78/binding immunoglobulin protein (GRP78/BIP), were analyzed by western blot in PC12 cells, NIX either over-expressed or silenced. As shown in Fig. 4a, the protein expression levels of CHOP and BIP were significantly increased in NIX-silencing cells but were not influenced in NIX-over-expressing cells, suggesting a rise of the ER stress in NIX-silencing cells.
Fig. 4.
Downregulation of NIX elevated the level of ER stress and upregulation of NIX reduced the apoptosis induced by luminespib/NVP-AUY922 in PC12 cells. a The protein expression levels of CHOP and BIP, the endoplasmic reticulum (ER) stress proteins, were detected by western blot. b PC12 cells treated with 200, 400 and 800 nM luminespib for 48 h were detected by flow cytometry, and the apoptosis ratios were calculated as the sum of early apoptosis (lower right) and late apoptosis (upper right) for each sample. c PC12 cells treated with 200, 400, and 800 nM luminespib were stained by Hoechst 33342, and the apoptosis ratios were analyzed by about 4000 cells for each sample. Data were shown as mean ± SD from at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, n.s. no significant difference
Moreover, the apoptosis of PC12 cells treated by luminespib/NVP-AUY922, an inhibitor of HSP90 which is a cell stress response factor, was tested using flow cytometry (Annexin V-PI) and Hoechst 33342 staining method. As shown in Fig. 4b, c, exposure to luminespib led to a significant apoptosis in luminespib groups compared with DMEM control group, and there was a dose effect between the apoptosis ratio and concentration of luminespib both in the NIX-over-expressing cells and control cells. In 400 and 800 nM luminespib groups, the results of flow cytometry showed the apoptosis percentages were reduced in the NIX-over-expressing cells compared with control cells. Hoechst 33342 staining also showed unanimous conclusion; in the luminespib groups, the rate of apoptosis was also reduced in NIX-over-expressing cells compared with control cells. However, the results of flow cytometry and Hoechst 33342 staining both showed that, in the luminespib group, more apoptotic cells were detected in NIX-silencing cells compared with control cells (Supplementary Fig. 4). These results demonstrated that NIX might confer resistance to luminespib/NVP-AUY922 in PC12 cells, but silencing of NIX would obviously attenuate it.
Discussion
Several studies have reported that NIX may be a pro-apoptotic factor which induced hypoxia-dependent suppression of tumors (Panigrahi et al. 2019; Zhang and Ney 2009). However, evidences indicated that high expression level of NIX may also serve as a progression marker for cancers (Lu et al. 2012; Melser et al. 2013; Humpton et al. 2019; Jung et al. 2020). In this manuscript, the analyses of the DFS showed that NIX protein correlated with poor prognosis in patients of PCPG, and further in vitro assay indicated that over-expressing of NIX promoted the migration of PC12 cells, which suggested that there might be a side of oncogene function for NIX in PCPG. Moreover, as revealed in glioblastoma, NIX might positively regulate tumorigenesis through activating the NF-κB pathway (Lu et al. 2012). Our study also showed a higher expression of p-NF-κB in the NIX-over-expressing cells and proposed a similar mechanism that might be underlying the promotion effect of NIX on various types of tumors. However, there was no relevance between the protein level of NIX and that of p-NF-κB in PC12 cells treated by H2O2 (Supplementary Fig. 2a), which suggested that the ROS clearance function of NIX might not rely on the NF-κB pathway.
It was revealed that the tumor promotion role of NIX might benefit from the redox-homeostasis via mitophagy (Humpton et al. 2019). We found the level of ROS decreased in NIX-over-expressing cells while increased in NIX-silencing cells. Therefore, high expression of NIX reduced the level of ROS might be due to its role in eliminating mitochondria (Jung et al. 2020). In addition, our results showed the antioxidant capacity was enhanced in NIX-over-expressing cells but reduced in NIX-silencing cells, which suggested high expression of NIX might also enhance the cellular antioxidant capacity. To confirm that, we further investigated in these cells the level of GSH, which was considered as a central molecule in antioxidant system (Hansen et al. 2019). In NIX-over-expressing cells, the level of total GSH (GSH + GSSG) was not influenced, but the ratios of GSH/GSSG and the activities of GPx and GR were all increased, indicating the higher activation of glutathione antioxidant system in NIX-over-expressing cells. However, in NIX-silencing cells, the level of total GSH (GSH + GSSG) and the activity of GPx were both decreased, revealing lower activation of the glutathione antioxidant system. These results showed that the overexpressed NIX enhanced antioxidant capacity of PC12 cells, which might be due to the NIX-mediated mitophagy, or other mechanisms remain unclear. Moreover, we also discovered that the treatment of H2O2 upregulated the protein expression level of NIX in PC12 cells, while the treatment of NAC downregulated it. These results also confirmed the reverse regulation of the protein expression level of NIX by the ROS level (Jung et al. 2020).
The effects of H2O2-induced cytotoxicity were both obviously relieved in NIX-over-expressing cells and NIX-silencing cells. It was contradicted with our results that NIX-over-expressing and silencing led to opposing effects in ROS level and antioxidant capacity in the cells. Therefore, we further investigated in these cells the activation level of NRF2 signaling, which played key roles in maintaining cell redox homeostasis. However, the protein/mRNA levels of NRF2 were all significantly increased in NIX-silencing cells, but did not change in NIX-over-expressing cells, implying effective activation of NRF2 signaling might be a consequence of increased level of ROS in NIX-silencing cells. Consistently, the protein/mRNA levels of antioxidase GCLC, phase II enzymes NQO1 and GSTM2, the downstream targets of NRF2, were also significantly increased in NIX-silencing cells. Therefore, these results highly hinted that the observed cytoprotection in NIX-silencing cells treated by H2O2 might partially be due to the high activities of antioxidase or the xenobiotic detoxification via phase II enzymes of NTF2 signaling.
In addition to oxidative stress caused by the increased level of ROS, endoplasmic reticulum (ER) stress was detected in NIX-silencing cells, as shown by the enhanced protein expression levels of CHOP and BIP detected in NIX-silencing cells. It was found that HSP90, which played important roles in many crucial cellular processes (Tukaj and Węgrzyn 2016), was upregulated under conditions of cellular stress (Kudze et al. 2018). And the HSP90 inhibitor, luminespib/NVP-AUY922, has been proven to inhibit proliferation of malignant PCC in vitro and in vivo (Lian et al. 2017). We further investigated the potential functions of NIX in PC12 cells while treated by luminespib/NVP-AUY922. Our results showed the apoptosis percentages induced by luminespib were reduced in NIX-over-expressing cells but significantly increased in NIX-silencing cells. These results demonstrated that NIX might enhance resistance to luminespib/NVP-AUY922 in PC12 cells. And the higher apoptosis induced by luminespib in NIX-silencing cells might be due to disturbance of cell homeostasis, such as upregulated unfolded protein response (UPS) under the high levels of oxidative stress (Graner et al. 2017). Therefore, our results showed luminespib/NVP-AUY922, being proposed as a promising novel molecule for the treatment of malignant PCC, might be more effective in patients with low expression level of NIX.
In conclusion, the high expression of NIX enhances antioxidant capacity of and reduced the apoptosis induced by HSP90 inhibitor luminespib/NVP-AUY922 in PC12 cells; and possibly due to the disturbance of cell homeostasis for elevated levels of ROS, the low expression of NIX upregulated the apoptosis induced by luminespib/NVP-AUY922. Our results may provide a new insight into the treatment of PCPG; only patients with low NIX level can benefit from luminespib/NVP-AUY922, while for those with high NIX level, targeting NIX might be a further supplement to the present therapeutic strategy.
Supplementary information
(DOCX 1.69 mb).
Funding
This work was supported by the Application Foundation Research Project of Sichuan Provincial Department of Science and Technology (No. 2017JY0182), Scientific Research Foundation of the Education Department of Sichuan Province, China (No. 17ZA0100), Collaborative Innovation Center of Sichuan for Elderly Care and Health, Chengdu Medical College (No. YLZBZ1811), Innovation and entrepreneurship training program for College Students, Chengdu Medical College (No. 201713705062).
Footnotes
The original version of this article was revised: the online supplementary information (ESM 1) contained an error in supplementary Fig. 4.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong Zhang, Fanghui Ge, Xindong Shui and Yuling Xiang contributed equally to this work.
Change history
7/1/2021
A Correction to this paper has been published: 10.1007/s12192-021-01218-0
References
- Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake LE, Springer MZ, Poole LP, Kim CJ, Macleod KF. Expanding perspectives on the significance of mitophagy in cancer. Semin Cancer Biol. 2017;47:110–124. doi: 10.1016/j.semcancer.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner AN, Hellwinkel JE, Lencioni AM, Madsen HJ, Harland TA, Marchando P, Nguyen GJ, Wang M, Russell LM, Bemis LT, Anchordoquy TJ, Graner MW. HSP90 inhibitors in the context of heat shock and the unfolded protein response: effects on a primary canine pulmonary adenocarcinoma cell line. Int J Hyperth. 2017;33:303–317. doi: 10.1080/02656736.2016.1256503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Porosk R, Mahlapuu R, Kairane C, Kilk K, Soomets U. GSH synthetic analogue O-methyl-L-tyrosinylglutathione regulates Nrf2-mediated expression of GCLc and GCLm. J Chem. 2019;2019:1–9. doi: 10.1155/2019/3841219. [DOI] [Google Scholar]
- Humpton TJ, Alagesan B, Denicola GM, Lu D, Yordanov GN, Leonhardt CS, Yao MA, Alagesan P, Zaatari MN, Park Y, Skepper JN, Macleod KF, Perez-Mancera PA, Murphy MP, Evan GI, Vousden KH, Tuveson DA. Oncogenic KRAS induces NIX-mediated mitophagy to promote pancreatic cancer. Cancer Discov. 2019;9:1268–1287. doi: 10.1158/2159-8290.CD-18-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zhang Y, Celiku O, Zhang W, Song H, Williams BJ, Giles AJ, Rich JN, Abounader R, Gilbert MR, Park DM (2019) Mitochondrial NIX promotes tumor survival in the hypoxic niche of glioblastoma. Cancer Res 79(20):5218–5232. 10.1158/0008-5472.CAN-19-0198.Mitochondrial [DOI] [PMC free article] [PubMed]
- Kahroba H, Shirmohamadi M, Hejazi MS, Samadi N. The role of Nrf2 signaling in cancer stem cells: from stemness and self-renewal to tumorigenesis and chemoresistance. Life Sci. 2019;239:116986. doi: 10.1016/j.lfs.2019.116986. [DOI] [PubMed] [Google Scholar]
- Koopman K, Gaal J, de Krijger RR. Pheochromocytomas and paragangliomas: new developments with regard to classification, genetics, and cell of origin. Cancers. 2019;11:1–14. doi: 10.3390/cancers11081070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudze T, Mendez-Dorantes C, Jalloh CS, McClellan AJ (2018) Evidence for interaction between Hsp90 and the ER membrane complex. Cell Stress and Chaperones 23(5):1101–1115. 10.1007/s12192-018-0908-z [DOI] [PMC free article] [PubMed]
- Lian J, Lin D, Xie X, Xu Y, Xu L, Meng L, Zhu Y. NVP-AUY922, a novel HSP90 inhibitor, inhibits the progression of malignant pheochromocytoma in vitro and in vivo. OncoTargets Ther. 2017;10:2219–2226. doi: 10.2147/OTT.S130236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu Y, Celiku O, Li A, Wu Q, Zhou Y, Yang C. Targeting IDH1-mutated malignancies with NRF2 blockade. J Natl Cancer Inst. 2019;111:1033–1041. doi: 10.1093/jnci/djy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang L, He M, Huang W, Li H, Wang Y, Kong J, Qi S, Ouyang J, Qiu X. Nix protein positively regulates NF-κB activation in gliomas. PLoS One. 2012;7:3–9. doi: 10.1371/journal.pone.0044559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melser S, Chatelain EH, Lavie J, Mahfouf W, Jose C, Obre E, Goorden S, Priault M, Elgersma Y, Rezvani HR, Rossignol R, Bénard G. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 2013;17:719–730. doi: 10.1016/j.cmet.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Nagy Á, Lánczky A, Menyhárt O, Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. doi: 10.1038/s41598-018-27521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nölting S, Grossman A, Pacak K. Metastatic phaeochromocytoma: spinning towards more promising treatment options. Exp Clin Endocrinol Diabetes. 2019;127:117–128. doi: 10.1055/a-0715-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nölting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A, Pacak K (2019b) Current management of pheochromocytoma/paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers 11. 10.3390/cancers11101505 [DOI] [PMC free article] [PubMed]
- Pang Y, Liu Y, Pacak K, Yang C. Pheochromocytomas and paragangliomas: from genetic diversity to targeted therapies. Cancers. 2019;11:1–16. doi: 10.3390/cancers11040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Patra S, Behera BP, Mishra SR, Bhutia SK (2019) The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin Cancer Biol 0-1. 10.1016/j.semcancer.2019.07.015 [DOI] [PubMed]
- Ryoo IG, Lee SH, Kwak MK (2016) Redox modulating NRF2: a potential mediator of cancer stem cell resistance. Oxidative Med Cell Longev 2016:1–14. 10.1155/2016/2428153 [DOI] [PMC free article] [PubMed]
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Węgrzyn G (2016) Anti-Hsp90 therapy in autoimmune and inflammatory diseases: a review of preclinical studies. Cell Stress and Chaperones 21(2):213–218. 10.1007/s12192-016-0670-z [DOI] [PMC free article] [PubMed]
- Tusi SK, Khodagholi F. Salvia macilenta exhibits antiglycating activity and protects PC12 cells against H2O2-induced apoptosis. Cytotechnology. 2014;66:169–179. doi: 10.1007/s10616-013-9550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1.69 mb).