Abstract
Objective: We aimed to explore the prognostic value of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) genes in gastric cancer (GC). Methods: The RNA-sequencing (RNA-seq) expression data for 351 GC patients and other relevant clinical data were acquired from The Cancer Genome Atlas (TCGA). Survival analysis and a genome-wide gene set enrichment analysis (GSEA) were performed to define the underlying molecular value of the ADAMTS genes in GC development. Besides, qRT-PCR and immunohistochemistry were all employed to validate the relationship between the expression of these genes and GC patient prognosis. Results: The Log rank test with both Cox regression and Kaplan–Meier survival analyses showed that ADAMTS6 expression profile correlated with the GC patients clinical outcome. Patients with a high expression of ADAMTS6 were associated with poor overall survival (OS). Comprehensive survival analysis of the ADAMTS genes suggests that ADAMTS6 might be an independent predictive factor for the OS in patients with GC. Besides, GSEA demonstrated that ADAMTS6 might be involved in multiple biological processes and pathways, such as the vascular endothelial growth factor A (VEGFA), kirsten rat sarcoma viral oncogene (KRAS), tumor protein P53, c-Jun N-terminal kinase (JNK), cadherin (CDH1) or tumor necrosis factor (TNF) pathways. It was also confirmed by immunohistochemistry and qRT-PCR that ADAMTS6 is highly expressed in GC, which may be related to the prognosis of GC patients. Conclusion: In summary, our study demonstrated that ADAMTS6 gene could be used as a potential molecular marker for GC prognosis.
Keywords: ADAMTS, gastric cancer, mRNA, prognosis
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second cause of mortality worldwide [1]. The median survival time of patients with GC recurrence and metastasis is less than 1 year [2,3]. According to the 2015 data at the National Cancer Center, there were ∼679000 new stomach cancer cases and 498000 deaths [4]. China accounts for 430000 new GC cases and 300000 deaths every year [5]. The GC development may be a process of long-term synergistic action of multiple factors. GC might be triggered by pathogenic infections, such as Helicobacter pylori (HP) [6–9] or gastric ulcers [10], chronic atrophic gastritis [11], carcinogens such as nitrite in food [12], smoking [13,14], and long-term drinking [15]. Despite the immense progress made in the clinical management of GC, the prognosis and survival rates of patients remain poor. Low early diagnosis rate and high local recurrence cases coupled with distant metastasis rates for advanced GC results in the poor GC prognosis in China. It is, therefore, vital to further interrogate the carcinogenesis and development of GC in order to develop new prognostic molecular markers and targeted therapy.
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) genes are zinc-dependent metalloproteases. Previous research has demonstrated a close association between ADAMTS and tumor invasion and metastasis [16]. ADAMTS participate in multiple biological pathways, including histomorphogenesis, pathophysiological reconstruction, inflammation, angiogenesis, or tumorigenesis [17,18]. However, the diagnostic, prognostic, or therapeutic value of the ADAMTS genes in the development of GC is yet to be defined. Here, using The Cancer Genome Atlas (TCGA) and the Kaplan–Meier plotter (KM plotter) tools, we explored the diagnostic and underlying prognostic value of the ADAMTS family of genes in stomach cancer.
Methods
Public database source
The RNA-sequencing (RNA-seq) expression data for 383 patients and relevant clinical data were acquired from the TCGA database (https://portal.gdc.cancer.gov/; accessed 15 May 2019). The mined data comprised 351 GC tumors and 32 normal gastric samples. The raw data were normalized via the DESeq (https://www.bioconductor.org/packages/release/bioc/html/DESeq.html) [19].
Bioinformatics analysis
We used GraphPad Prism 8 to draw the scatter diagram and receiver operating characteristic (ROC) curves of the expression distribution for both the GC and the normal samples. The unpaired t test was used to compare the differences shown in the scatter diagram and the area under ROC curve. Using the Database for Annotation, Visualization and Integrated Discovery (DAVID, (https://david.ncifcrf.gov/home.jsp; accessed 1 December 2019; v.6.8), we then investigated the Gene Ontology (GO) enrichment of ADAMTS genes [20]. We used the gene multiple association network integration algorithm (Gene MANIA; http://www.genemania.org/; accessed 1 December 2019) to construct the gene–gene networks [21,22] and the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING v.10.0; https://string-db.org/; accessed 1 December 2019) to define the protein–protein interaction (PPI) networks [23,24].
Comprehensive survival analysis of ADAMTS genes
Using the median values for the gene expression profile, GC patients were classified into two categories based on survival analysis. Both the ADAMTS expression and clinical data in GC tissues or adjacent tissues were analyzed using the log-rank test, while the clinical characteristics related to overall survival (OS) were selected and adjusted by the multivariate Cox regression survival analysis. Besides, the clinical pathological features were further analyzed in the subgroups. We then conducted full survival analysis using the prognosis-related ADAMTS genes, and assessed the survival ROC curves using the R package platform, as well as the total and subgroup survival analysis. Finally, the prognostic relationship between ADAMTS family of genes and GC was verified by the KM plotter database (www.kmplot.com).
Gene set enrichment analysis
We used gene set enrichment analysis (GSEA) (http://software.broadinstitute.org/gsea/index.jsp; accessed 15 December 2020) [25,26] to study the biological differences and pathways affected by the differential expression of the ADAMTS gene pool and their relationship with GC clinical outcome. The GSEA software applies the Molecular Signatures Database (MSigDB) c2 (c2.cp.kegg.v7.0.symbols.gmt) and c5 (c5.all.v7.0.symbols.gmt) [27] in gene set analysis. Then a probability value of <0.05 and a false discovery rate (FDR) < 0.25 for GSEA were considered statistically significant.
qRT-PCR
We collected 24 pairs of GC and adjacent normal tissue samples surgically from December 2020 to February 2021 at Guangxi Medical University Affiliated Tumor Hospital for RNA extraction. A cDNA reverse transcription kit (RR036A; TaKaRa, U.S.A.) and TB Green kit (RR820A; TaKaRa, U.S.A.) were used for reverse transcription into cDNA and qRT-PCR. Primers used were: ADAMTS6-F: 5′-CCTCCCAAGCGTGACTTTCT-3′; ADAMTS6-R: 5′-AGACACCAGAGCTCTCTACACACTT-3′. GAPDH-F: 5′-CAGGAGGCATTGCTGATGAT-3′, and GAPDH-R: 5′-GAAGGCTGGGGCTCATTT-3′.
Immunohistochemistry
We collected 18 pairs of GC tissues and paired paracancerous tissue samples in the Guangxi Medical University Affiliated Tumor Hospital from January 2018 to December 2018. The experiments were approved by the Ethical Committee of the Guangxi Medical University Affiliated Tumor Hospital and written informed consent was signed by each participant. The sections were placed in an oven at 65°C for 2 h, dewaxed with xylene, hydrated with a graded series of ethanol, and repaired with antigen repair buffer using the EDTA method. Endogenous antigens were blocked with 3% hydrogen peroxide. The sections were incubated with Rabbit Anti-ADAMTS6 antibody (bs-8009R) overnight at 4°C. The sections were heated for 30 min to bring them to room temperature and incubated with secondary antibody for 20 min. Staining was visualized using DAB color developing solution. The sections were stained with Hematoxylin, differentiated in 1% hydrochloric acid in alcohol, and dehydrated. Thereafter, the sections were dried naturally in a fume hood, transparentized with xylene, and sealed with neutral resin. The negative control contained PBS instead of primary antibody, and a proven positive section served as the positive control. The staining index was scored according to staining intensity (0, no staining; 1, weak, light yellow; 2, moderate, yellow brown; 3, strong, brown) and the proportion of positive cells (0, 0%; 1, <10%; 2, <50%; 3, <75%; 4, >76%). An ‘immunoreactive score’ was determined to be the product of the intensity and percentage of positive cells, which ranged from 0 to 12. Cases with scores of 0–7 were defined as the negative group and those with scores of 8–12 were the positive group [28].
Statistical analysis
We employed the Benjamini–Hochberg program to adjust FDRs in GSEA for multiple tests [29–31]. The statistical analysis was conducted via SPSS version 23.0 (IBM Corporation, Armonk, NY, U.S.A.). A probability value of P<0.05 was considered statistically significant.
Results
In determining the potential diagnostic value of ADAMTS family in distinguishing between GC tumor tissue and adjacent normal tissue, the results of unpaired t test showed that ADAMTS1 and ADAMTS8 mRNA expression was down-regulated in GC tumor tissues as compared with the adjacent normal tissues (P<0.05). On the other side, ADAMTS2, ADAMTS6, and ADAMTS7 genes mRNA expression were up-regulated in GC tissues (P<0.05), as shown in Figure 1. The ROC curves for the GC and adjacent normal samples showed that the tumor tissues could be effectively identified based on the risk score (Figure 2).
Figure 1. The scatter plot of ADAMTS mRNA expression in GC and adjacent tissues based on TCGA database.
****P<0.0001.
Figure 2. The ROC curve of ADAMTS mRNA expression in GC and adjacent tissues based on TCGA database.
Bioinformatics analysis
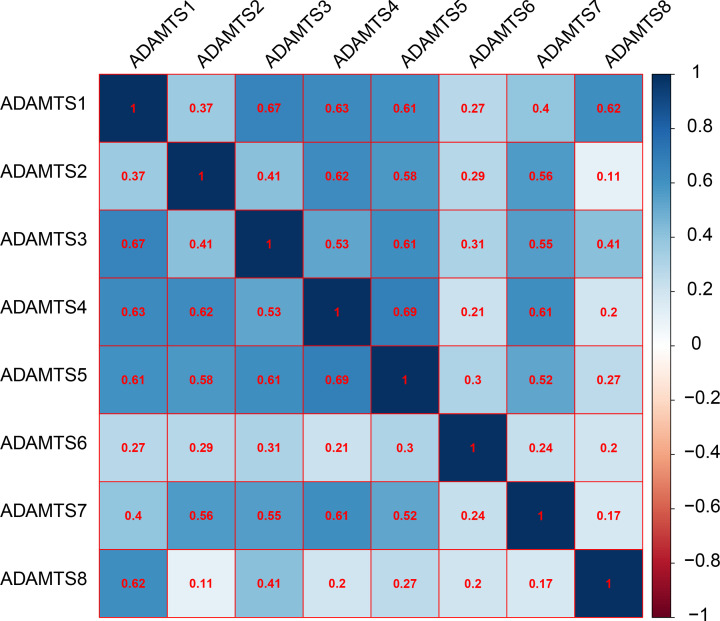
The GO analysis indicated that the ADAMTS genes were linked with extracellular matrix/structure organization, extracellular matrix disassembly, cell adhesion molecule binding, or collagen catabolic/metabolic processes, as shown in Figure 3. Our interaction network analysis showed that ADAMTS genes and other related genes formed an intricate network together. The gene–gene interaction network showed that the ADAMTS genes are strongly co-expressed (Figure 4A), while the PPI network analysis showed that the ADAMTS proteins directly interact with each other (Figure 4B). Furthermore, there was co-expression among the ADAMTS1–8 genes in the GC tumor tissues (Figure 5), demonstrating that the genes are positively correlated with GC development (Pearson correlation coefficient range: 0.11–0.69; P<0.05). Besides, our analysis of the ADAMTS genes expression in different tumor stages and histologic grades showed that the ADAMTS6 expression was significantly different in G1/G2 and G3 tumor tissues (Figure 6A). However, there was no significant difference in the expression of ADAMTS genes in stages I/II and III/IV tumor samples (Figure 6B). Together, these data show that ADAMTS6 overexpression is associated with G3 tumor tissues samples in GC patients, P=0.01 (Figure 6A).
Figure 3. GO enrichment analysis of ADAMTS genes.
Figure 4. Gene and protein interaction networks of ADAMTS genes.
(A) Gene multiple association network integration algorithm. (B) PPI networks.
Figure 5. Co-expression matrix of ADAMTS genes in GC tumor tissues, and demonstrated that ADAMTS1–8 were positively correlated and co-expressed with each other in GC tumor tissues.
Figure 6. Gene expression distribution of ADAMTS genes in different GC histologic grades and tumor stages.
(A) Gene expression distribution of ADAMTS genes in different GC histologic grades. (B) Gene expression distribution of ADAMTS genes in different GC tumor stages. *P<0.05.
ADAMTS1–8 genes and OS in GC patients
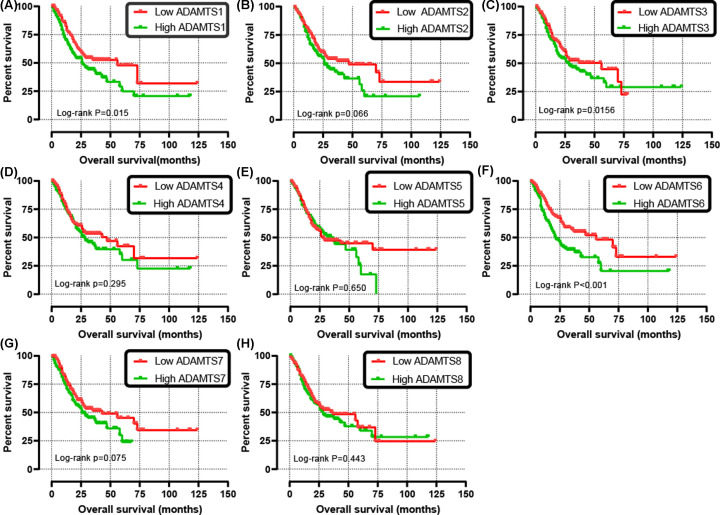
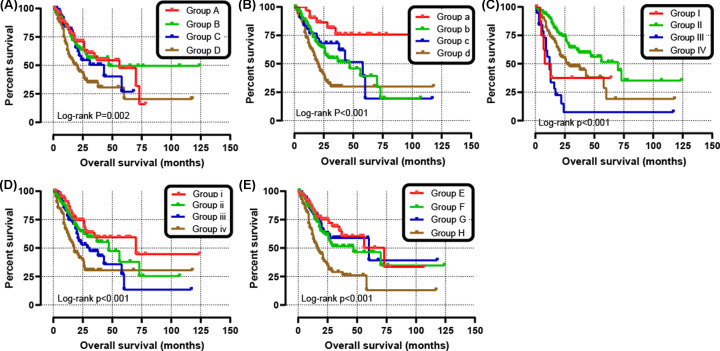
The clinical characteristics of the 351 GC patients is shown in Table 1. The results from the univariate analysis found that the high ADAMTS1 and ADAMTS6 mRNA expression were associated with poor survival rates of GC patients (hazard ratio (HR) = 1.52, 95% confidence interval (CI) = 1.09–2.12, P=0.013 and HR = 1.80, 95% CI = 1.29–2.51, P=0.001, respectively) as shown in Table 2. Unlike the other ADAMTS genes, the survival analysis indicated that the up-regulation of ADAMTS1, ADAMTS3, or ADAMTS6 increased risk of death in GC patients (P<0.05) (Figure 7A–H). Clinical parameters such as age, TNM stage, cancer status, primary therapy outcome, residual tumor, and therapy were remarkably correlated with OS in GC. What needs to be adjusted in multivariate Cox regression and indicated that ADAMTS6 overexpression was remarkably raised death rate in GC (adjusted HR = 1.89, 95% CI = 1.19–3.01, adjusted P=0.007, Table 2, Figure 7F) and accelerated a worse OS (high ADAMTS6 vs low ADAMTS6; 21 vs 56 months, Table 2, Figures 7F and 8A). There was, however, no correlation between other ADAMTS genes and the GC OS. In addition, as shown in Table 3, subgroup analysis indicated that overexpression of the ADAMTS6 genes increased the death rate in GC patients who are ≥60 years old; female patients; patients with gastric antrum cancer; intestinal type adenocarcinoma; G2, G3 histologic grade; microsatellite instability-altitude (MSI-H), MMS; lymph node metastasis; T3, T4 stage; tumor free status; pathologic stage III; R0 resection; those untreated with radiation therapy or targeted therapy, without distant metastasis and HP infection. Time-dependent ROC analysis proved that ADAMTS6 expression profile could reliably predict OS in GC patients. The area under the 1-, 2-, or 3-year curve (AUC) was 0.613, 0.607, or 0.759, respectively (Figure 8B). Besides, we demonstrate that ADAMTS6, together with the OS-related clinical characteristics give superior performance in the prediction OS in GC patients (Figure 9A–E and Table 4).
Table 1. Correlation between OS and clinicopathologic features of GC patients in TCGA cohort.
| Variables | Events/total | MST (months) | HR (95% CI) | P-value |
|---|---|---|---|---|
| Age (years) | 144/348 | 29 | 0.022 | |
| <60 | 36/108 | 60 | 1 | |
| ≥60 | 108/240 | 26 | 1.55 (1.06–2.27) | |
| Missing | 3 | |||
| Gender | 144/351 | 29 | 0.178 | |
| Male | 100/226 | 29 | 1 | |
| Female | 44/125 | 35 | 0.78 (0.55–1.12) | |
| Missing | 0 | |||
| Anatomic neoplasm | 138/337 | 29 | 0.919 | |
| Gastroesophageal junction | 36/84 | 26 | 1 | |
| Fundus gastric body | 50/123 | 28 | 0.92 (0.60–1.41) | |
| Gastric antrum | 52/130 | 35 | 0.94 (0.61–1.43) | |
| Missing | 14 | |||
| HP infection | 66/161 | 43 | 0.304 | |
| Positive | 6/18 | 58 | 1 | |
| Negative | 60/143 | 43 | 1.55 (0.67–3.61) | |
| Missing | 190 | |||
| Histologic type | 144/350 | 29 | 0.057 | |
| Intestinal | 64/160 | 38 | 1 | |
| Diffuse type | 24/61 | 60 | 1.00 (0.63–1.60) | |
| Signet ring type | 8/11 | 13 | 2.52 (1.20–5.25) | |
| Other types | 48/118 | 26 | 1.29 (0.88–1.88) | |
| Missing | 1 | |||
| Histologic grade | 140/342 | 29 | 0.169 | |
| G1 | 2/9 | NA | 1 | |
| G2 | 48/127 | 43 | 1.67 (0.41–6.86) | |
| G3 | 90/206 | 26 | 2.22 (0.55–9.01) | |
| Missing | 9 | |||
| MSS1 | 144/350 | 29 | 0.225 | |
| MSI-H | 99/240 | 28 | 1 | |
| MSI-L | 22/51 | 29 | 1.26 (0.79–2.01) | |
| MMS | 23/59 | 35 | 0.76 (0.48–1.19) | |
| Missing | 1 | |||
| Pathological M | 138/336 | 29 | 0.012 | |
| M1 | 13/23 | 12 | 1 | |
| M0 | 125/313 | 35 | 0.49 (0.28–0.86) | |
| Missing | 15 | |||
| Pathological N | 139/341 | 29 | 0.004 | |
| N0 | 28/103 | 60 | 1 | |
| N+ | 111/238 | 25 | 1.83 (1.21–2.76) | |
| Missing | 10 | |||
| Pathological T | 140/347 | 31 | 0.009 | |
| T1/T2 | 28/91 | 70 | 1 | |
| T3/T4 | 112/256 | 26 | 1.73 (1.14–2.63) | |
| Missing | 4 | |||
| TNM stage | 136/338 | 29 | <0.001 | |
| Stage I | 11/47 | 73 | 1 | |
| Stage II | 34/109 | 56 | 1.61 (0.81–3.18) | |
| Stage III | 69/147 | 26 | 2.44 (1.29–4.61) | |
| Stage IV | 22/35 | 16 | 3.79 (1.84–7.82) | |
| Missing | 13 | |||
| Cancer status | 121/324 | 37 | <0.001 | |
| Tumor free | 35/206 | NA | 1 | |
| With tumor | 86/118 | 17 | 5.53 (3.70–8.26) | |
| Missing | 27 | |||
| Primary therapy outcome | 114/303 | 38 | <0.001 | |
| CR | 55/209 | 73 | 1 | |
| PR | 4/5 | 17 | 4.23 (1.52–11.78) | |
| SD | 7/25 | 31 | 1.89 (0.86–4.18) | |
| PD | 48/64 | 13 | 4.33 (2.91–6.45) | |
| Missing | 47 | |||
| Radiation therapy | 135/328 | 31 | 0.001 | |
| Yes | 19/62 | NA | 1 | |
| No | 116/266 | 26 | 2.32 (1.42–3.80) | |
| Missing | 23 | |||
| Residual tumor | 121/316 | 37 | <0.001 | |
| R1 | 9/14 | 13 | 1 | |
| R2 | 12/14 | 9 | 1.88 (0.95–3.73) | |
| R0 | 100/288 | 47 | 7.19 (3.88–13.34) | |
| Missing | 35 | |||
| Targeted therapy | 134/326 | 31 | 0.022 | |
| Yes | 56/151 | 43 | 1 | |
| No | 78/175 | 26 | 1.49 (1.06–2.10) | |
| Missing | 25 |
Abbreviations: CR, complete response; G1, highly differentiated; G2, moderately differentiated; G3, poorly differentiated; MSI-L, microsatellite instability-altitude; MST, median survival time; PD, progressive disease; PR, partial response; R0, no residual tumor; R1, microscopic residual tumor; R2, macroscopic residual tumor; SD, stable disease.
Table 2. Prognostic values of ADAMTS genes expression in GC OS of TCGA cohort.
| Gene expression | Events/total (n=351) | MST (months) | Crude HR (95% CI) | Crude P-value | Adjusted HR (95% CI) | Adjusted P-value1 |
|---|---|---|---|---|---|---|
| ADAMTS1 | ||||||
| Low | 61/176 | 56 | 1 | 1 | ||
| High | 83/175 | 26 | 1.52 (1.09–2.12) | 0.013 | 1.39 (0.91–2.11) | 0.125 |
| ADAMTS2 | ||||||
| Low | 64/176 | 47 | 1 | 1 | ||
| High | 80/175 | 26 | 1.38 (0.99–1.92) | 0.055 | 1.30 (0.85–1.98) | 0.226 |
| ADAMTS3 | ||||||
| Low | 63/176 | 37 | 1 | 1 | ||
| High | 81/175 | 27 | 1.25 (0.90–1.73) | 0.19 | 1.36 (0.88–2.09) | 0.168 |
| ADAMTS4 | ||||||
| Low | 63/176 | 43 | 1 | 1 | ||
| High | 81/175 | 28 | 1.21 (0.87–1.68) | 0.266 | 1.34 (0.87–2.07) | 0.189 |
| ADAMTS5 | ||||||
| Low | 70/176 | 27 | 1 | 1 | ||
| High | 74/175 | 35 | 1.09 (0.79–1.52) | 0.599 | 0.84 (0.54–1.31) | 0.441 |
| ADAMTS6 | ||||||
| Low | 59/176 | 56 | 1 | 1 | ||
| High | 85/175 | 21 | 1.80 (1.29–2.51) | 0.001 | 1.89 (1.19–3.01) | 0.007 |
| ADAMTS7 | ||||||
| Low | 64/176 | 43 | 1 | 1 | ||
| High | 80/175 | 26 | 1.33 (0.95–1.85) | 0.095 | 1.41 (0.91–2.18) | 0.120 |
| ADAMTS8 | ||||||
| Low | 67/176 | 37 | 1 | 1 | ||
| High | 77/175 | 28 | 1.15 (0.83–1.60) | 0.401 | 1.52 (0.97–2.39) | 0.067 |
Abbreviation: MST, median survival time.
Adjusted for age, TNM stage, cancer status, primary therapy outcome, residual tumor, targeted molecular therapy, and radiation therapy.
Figure 7. Kaplan–Meier survival curves for ADAMTS genes in GC of TCGA cohort.
OS stratified by ADAMTS1 (A), ADAMTS2 (B), ADAMTS3 (C), ADAMTS4 (D), ADAMTS5 (E), ADAMTS6 (F), ADAMTS7 (G), ADAMTS8 (H).
Figure 8. Prognostic value evaluation of ADAMTS6 in patients with GC.
(A) From top to bottom: are the expression values of ADAMTS6, patients’ survival status distribution, and the expression heat map of ADAMTS6 in the low- and high-expression groups. (B) ROC curve for predicting OS in GC patients by the ADAMTS6.
Table 3. Stratified analysis of ADAMTS6 gene expression in clinicopathologic features of GC cases.
| Variables | Cases | HR (95% CI) | Log-rank P-value |
|---|---|---|---|
| Age (years) | 348 | ||
| <60 | 108 | 1.01 (0.46–2.21) | 0.102 |
| ≥60 | 240 | 1.94 (1.25–3.01) | 0.048 |
| Gender | 351 | ||
| Male | 226 | 1.66 (1.05–2.62) | 0.021 |
| Female | 125 | 2.26 (1.14–4.50) | 0.293 |
| Anatomic neoplasm | |||
| Gastroesophageal | 337 | ||
| Junction | 84 | 0.79 (0.29–2.15) | 0.501 |
| Fundus gastric body | 123 | 0.70 (0.39–1.24) | 0.001 |
| Gastric antrum | 130 | 1.97 (1.01–3.85) | 0.001 |
| HP infection | 161 | ||
| Positive | 18 | 3.61 (0.22–59.81) | 0.180 |
| Negative | 143 | 1.20 (0.65–2.23) | 0.005 |
| Histologic type | 350 | ||
| Intestinal | 160 | 2.53 (1.43–4.46) | P<0.001 |
| Diffuse type | 61 | 0.67 (0.28–1.59) | 0.158 |
| Signet ring type | 11 | 0.64 (0.02–26.22) | 0.312 |
| Other types | 118 | 1.96 (1.01–3.82) | 0.005 |
| Histologic grade | 342 | ||
| G1 | 9 | NA | NA |
| G2 | 127 | 1.15 (0.59–2.26) | 0.006 |
| G3 | 206 | 2.38 (1.49–3.81) | P<0.001 |
| MSS1 | 350 | ||
| MSI-H | 240 | 1.67 (1.08–2.59) | P<0.001 |
| MSI-L | 51 | 2.15 (0.81–5.73) | 0.099 |
| MMS | 59 | 2.62 (0.88–7.81) | 0.005 |
| Pathological M | 336 | ||
| M1 | 23 | 1.19 (0.07–19.35) | 0.870 |
| M0 | 313 | 1.63 (1.12–2.38) | P<0.001 |
| Pathological N | 139/341 | ||
| N0 | 103 | 0.90 (0.35–2.30) | 0.005 |
| N+ | 111/238 | 2.20 (1.41–3.42) | P<0.001 |
| Pathological T | 347 | ||
| T1/T2 | 91 | 0.60 (0.24–1.51) | 0.118 |
| T3/T4 | 256 | 2.24 (1.44–3.50) | P<0.001 |
| Pathologic stage | 136/338 | ||
| Stage I | 47 | 1.60 (0.48–5.29) | 0.402 |
| Stage II | 109 | 1.55 (0.79–3.07) | 0.478 |
| Stage III | 147 | 2.00 (1.16–3.44) | 0.039 |
| Stage IV | 35 | 3.11 (0.70–13.86) | 0.132 |
| Cancer status | 121/324 | ||
| Tumor free | 206 | 2.26 (1.07–4.80) | P<0.001 |
| With tumor | 118 | 1.71 (1.06–2.76) | 0.108 |
| Radiation therapy | 135/328 | ||
| Yes | 62 | 2.26 (0.80–6.39) | 0.287 |
| No | 266 | 1.75 (1.78–2.59) | P<0.001 |
| Residual tumor | 121/316 | ||
| R1 | 14 | NA | NA |
| R2 | 14 | 0.78 (0.02–41.15) | 0.531 |
| R0 | 288 | 1.71 (1.13–2.58) | 0.090 |
| Targeted therapy | 134/326 | ||
| Yes | 151 | 1.36 (0.77–2.39) | 0.388 |
| No | 175 | 1.92 (1.18–3.12) | P<0.001 |
Abbreviations: CR, complete response; G1, highly differentiated; G2, moderately differentiated; G3, poorly differentiated; MSI-L, microsatellite instability-low; MST, median survival time; M, metastasis; N, node; NA, not applicable; PD, progressive disease; PR, partial response; R0, no residual tumor; R1, microscopic residual tumor; R2, macroscopic residual tumor; SD, stable disease; T, tumor.
Figure 9. Joint effects analysis of OS stratified by ADAMTS6 and GC clinical parameters.
Joint effects analysis stratified by ADAMTS6 and following clinical parameters: histologic grade (A), radiation therapy (B), radical resection (C), targeted molecular therapy (D), stage (E).
Table 4. Joint effects survival analysis of clinical factors and the ADAMTS6 expression with OS.
| Group | ADAMTS6 | Variables | Events/total | MST (months) | Crude HR (95% CI) | Crude P-value | Adjusted HR (95% CI) | Adjusted P-value1 |
|---|---|---|---|---|---|---|---|---|
| Histologic grade | ||||||||
| A | Low expression | G1 + G2 | 27/74 | 56 | 1 | 1 | ||
| B | Low expression | G3 + G4 | 32/98 | 47 | 1.005 (0.602–1.680) | 0.984 | 0.734 (0.394–1.371) | 0.332 |
| C | High expression | G1 + G2 | 23/62 | 31 | I.226 (0.701–2.142) | 0.475 | 1.171 (0.600–2.286) | 0.644 |
| D | High expression | G3 + G4 | 58/108 | 18 | 2.045 (1.291–3.237) | 0.002 | 1.815 (1.035–3.184) | 0.038 |
| Radiation therapy | ||||||||
| a | Low expression | Yes | 6/31 | 47 | 1 | 1 | ||
| b | Low expression | No | 47/131 | 58 | 3.104 (1.321–7.2940 | 0.009 | 3.265 (1.276–8.353) | 0.014 |
| c | High expression | Yes | 13/31 | 20 | 2.821 (1.071–7.432) | 0.036 | 2.644 (0.954–7.324) | 0.062 |
| d | High expression | No | 69/135 | 31 | 5.665 (2.434–13.188) | <0.001 | 5.917 (2.349–14.900) | <0.001 |
| Radical resection | ||||||||
| I | Low expression | R0 | 44/155 | 70 | 1 | 1 | ||
| II | Low expression | R1+R2 | 5/9 | 8 | 3.046 (1.204–7.707) | 0.019 | 3.308 (0.931–11.751) | 0.064 |
| IV | High expression | R0 | 56/133 | 27 | 1.829 (1.230–2.720) | 0.003 | 2.055 (1.349–3.129) | 0.001 |
| III | High expression | R1+R2 | 16/19 | 12 | 5.009 (2.807–8.937) | <0.001 | 3.485 (1.719–7.067) | 0.001 |
| Targeted molecular therapy | ||||||||
| i | Low expression | Yes | 22/74 | 70 | 1 | 1 | ||
| ii | Low expression | No | 31/89 | 47 | 1.400 (0.810–2.420) | 0.228 | 0.928 (0.478–1.801) | 0.825 |
| iii | High expression | Yes | 34/77 | 29 | 1.829 (1.068–3.133) | 0.028 | 1.626 (0.891–2.966) | 0.113 |
| iv | High expression | No | 47/86 | 18 | 3.002 (1.802–5.001) | <0.001 | 2.030 (1.087–3.793) | 0.026 |
| Stage | ||||||||
| E | Low expression | I + II | 24/89 | 56 | 1 | 1 | ||
| F | Low expression | III+IV | 34/84 | 47 | 1.398 (0.829–2.358) | 0.209 | 1.839 (0.975–3.469) | 0.06 |
| G | High expression | I + II | 21/67 | 60 | 1.214 (0.675–2.181) | 0.517 | 1.579 (0.794–3.142) | 0.193 |
| H | High expression | III+IV | 57/98 | 17 | 2.916 (1.807–4.704) | <0.001 | 3.897 (2.117–7.176) | <0.001 |
Abbreviation: MST, median survival time.
Adjusted for histologic grade, radiation therapy, radical resection, targeted molecular therapy, and stage.
K–M plotter survival analysis
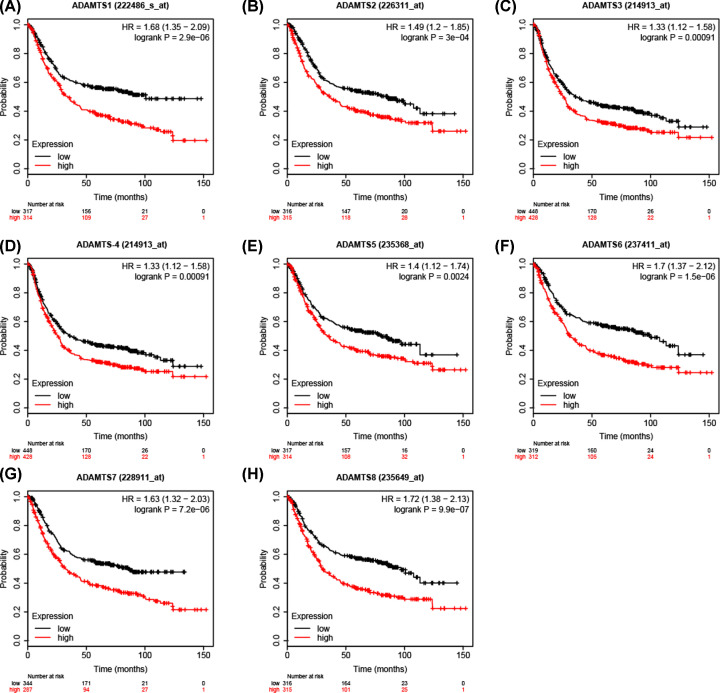
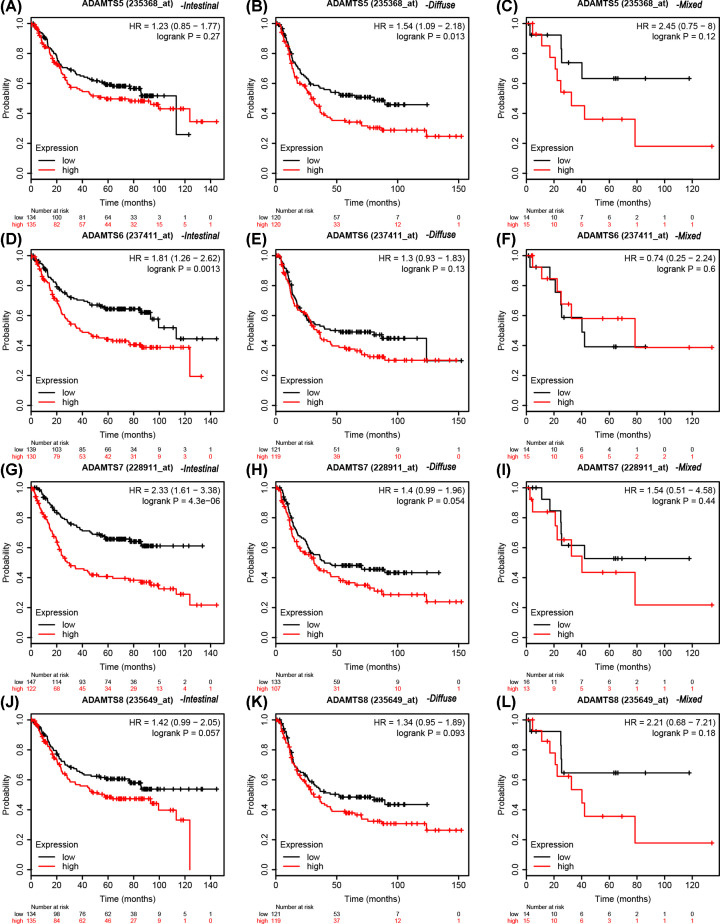
The expression profile for the ADAMTS genes in the K–M plotter database demonstrated that patients with high expression had poor clinical outcomes (Figure 10 and Table 5). The data from the Lauren classification of the ADAMTS mRNA expression of the GC patients showed that the patients with high expression of diffuse ADAMTS5 (Affymetrix ID: 235368_at) gene, intestinal ADAMTS1 (Affymetrix ID: 222486_s_at), ADAMTS2 (Affymetrix ID: 226311_at), ADAMTS3 (Affymetrix ID: 214913_at), ADAMTS4 (Affymetrix ID: 214913_at), ADAMTS6 (Affymetrix ID: 237411_at) or ADAMTS7 (Affymetrix ID: 228911_at) had shorter survival time compared with those with low expression (Figures 11 and 12 and Table 6). Tables 7-10 show the stratified results of the ADAMTS expression in TNM stage, tumor differentiation degree, different treatment strategies, and HER2 status of the GC cases. The high expression of ADAMTS1 in tumor stage IV (adjusted P=0.036, adjusted HR = 1.52, 95% CI = 1.02−2.27), ADAMTS2 in stage III (adjusted P=0.02, adjusted HR = 1.56, 95% CI = 1.07−2.28), ADAMTS5 in stages III and IV (adjusted P=0.002, adjusted HR = 1.81, 95% CI = 1.24−2.64 and adjusted P=0.04, adjusted HR = 1.51, 95% CI = 1.02−2.25), ADAMTS7 in stages III and IV (adjusted P=0.0026, adjusted HR = 1.77, 95% CI = 1.21−2.57 and adjusted P=0.0047, adjusted HR = 1.76, 95% CI = 1.18−2.63) increased the mortality rate, while a high expression of ADAMTS2 in tumor stage I (adjusted P=0.03, adjusted HR = 0.26, 95% CI = 0.07−0.96) increased the survival rate in GC patients. Similarly, the high expression in G2 degree of tumor differentiation of ADAMTS7 (adjusted P=0.0074, adjusted HR = 2.39, 95% CI = 1.24−4.59) increased the death rate in GC patients. In addition, the mortality rate in patients with high expression of ADAMTS genes after surgery was higher than those with low expression. The HER2 state subgroup analysis showed that the high expression of ADAMTS family of genes increased mortality in patients with HER2 positive and HER2 negative status, but not in ADAMTS5, ADAMTS7 HER2 positive patients.
Figure 10. Survival analysis of ADAMTS mRNA expression in GC based on K–M plotter database.
Survival analysis of ADAMTS1 (A), ADAMTS2 (B), ADAMTS3 (C), ADAMTS4 (D), ADAMTS5 (E), ADAMTS6 (F), ADAMTS7 (G), ADAMTS8 (H).
Table 5. Survival analysis of ADAMTS gene mRNA expression in GC cases in KM plotter database.
| Gene/Affymetrix ID | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|
| ADAMTS1/222486_s_at | 317/314 | 1.68 (1.35–2.09) | 2.9e-06 |
| ADAMTS2/226311_at | 316/315 | 1.49 (1.2–1.85) | 3e-04 |
| ADAMTS3/214913_at | 448/428 | 1.33 (1.12–1.58) | 0.00091 |
| ADAMTS4/214913_at | 448/428 | 1.33 (1.12–1.58) | 0.00091 |
| ADAMTS5/235368_at | 317/314 | 1.4 (1.12–1.74) | 0.0024 |
| ADAMTS6/237411_at | 319/312 | 1.7 (1.37–2.12) | 1.5e-06 |
| ADAMTS7/228911_at | 344/287 | 1.63 (1.32–2.03) | 7.2e-06 |
| ADAMTS8/235649_at | 316/315 | 1.72 (1.38–2.13) | 9.9e-07 |
Figure 11. Stratified analysis of ADAMTS1–4 gene mRNA in Lauren typing of GC cases in K–M plotter database.
OS curves for (A) intestinal-type ADAMTS1, (B) diffuse-type ADAMTS1, (C) mixed-type ADAMTS1, (D) intestinal-type ADAMTS2, (E) diffuse-type ADAMTS2, (F) mixed-type ADAMTS2, (G) intestinal-type ADAMTS3, (H) diffuse-type ADAMTS3, (I) mixed-type ADAMTS3, (J) intestinal-type ADAMTS4, (K) diffuse-type ADAMTS4, (L) mixed-type ADAMTS4.
Figure 12. Stratified analysis of ADAMTS5-8 gene mRNA in Lauren typing of GC cases in K–M plotter database.
OS curves for (A) intestinal-type ADAMTS5, (B) diffuse-type ADAMTS5, (C) mixed-type ADAMTS5, (D) intestinal-type ADAMTS6, (E) diffuse-type ADAMTS6, (F) mixed-type ADAMTS6, (G) intestinal-type ADAMTS7, (H) diffuse-type ADAMTS7, (I) mixed-type ADAMTS7, (J) intestinal-type ADAMTS8, (K) diffuse-type ADAMTS8, (L) mixed-type ADAMTS8.
Table 6. Stratified analysis of ADAMTS gene mRNA in Lauren typing in K–M plotter.
| Gene | Lauren typing | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|---|
| ADAMTS1 | Intestinal | 136/133 | 1.79 (1.23–2.59) | 0.0019 |
| Diffuse | 120/120 | 1.3 (0.92–1.83) | 0.13 | |
| Mixed | 14/15 | 1.96 (0.6–6.37) | 0.26 | |
| ADAMTS2 | Intestinal | 136/133 | 1.55 (1.08–2.23) | 0.017 |
| Diffuse | 120/120 | 1.37 (0.97–1.94) | 0.069 | |
| Mixed | 14/15 | 0.9 (0.3–2.68) | 0.85 | |
| ADAMTS3 | Intestinal | 162/158 | 1.55 (1.13–2.13) | 0.0063 |
| Diffuse | 120/121 | 1.35 (0.96–1.9) | 0.088 | |
| Mixed | 16/16 | 2.72 (0.92–8.06) | 0.061 | |
| ADAMTS4 | Intestinal | 162/158 | 1.55 (1.13–2.13) | 0.0063 |
| Diffuse | 120/121 | 1.35 (0.96–1.9) | 0.088 | |
| Mixed | 16/16 | 2.72 (0.92–8.06) | 0.061 | |
| ADAMTS5 | Intestinal | 134/135 | 1.23 (0.85–1.77) | 0.27 |
| Diffuse | 120/120 | 1.54 (1.09–2.18) | 0.013 | |
| Mixed | 14/15 | 0.9 (0.3–2.68) | 0.85 | |
| ADAMTS6 | Intestinal | 139/130 | 1.81 (1.26–2.62) | 0.0013 |
| Diffuse | 121/119 | 1.3 (0.93–1.83) | 0.13 | |
| Mixed | 14/15 | 0.74 (0.25–2.24) | 0.6 | |
| ADAMTS7 | Intestinal | 147/122 | 2.33 (1.61–3.38) | 4.3e-06 |
| Diffuse | 133/107 | 1.4 (0.99–1.96) | 0.054 | |
| Mixed | 16/13 | 1.54 (0.51–4.58) | 0.44 | |
| ADAMTS8 | Intestinal | 134/135 | 1.42 (0.99–2.05) | 0.057 |
| Diffuse | 121/119 | 1.34 (0.95–1.89) | 0.093 | |
| Mixed | 14/15 | 2.21 (0.68–7.21) | 0.18 |
Table 7. Stratified analysis of ADAMTS gene mRNA in stage in K–M plotter database.
| Gene | Stage | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|---|
| ADAMTS1 | I | 31/31 | 1.95 (0.6–6.4) | 0.26 |
| II | 69/66 | 1.57 (0.83–2.97) | 0.16 | |
| III | 98/99 | 1.11 (0.77–1.62) | 0.57 | |
| IV | 70/70 | 1.52 (1.02–2.27) | 0.036 | |
| ADAMTS2 | I | 31/31 | 0.26 (0.07–0.96) | 0.03 |
| II | 68/67 | 0.62 (0.33–1.19) | 0.15 | |
| III | 98/99 | 1.56 (1.07–2.28) | 0.02 | |
| IV | 70/70 | 1.29 (0.87–1.91) | 0.2 | |
| ADAMTS3 | I | 35/32 | 1.18 (0.43–3.23) | 0.75 |
| II | 70/70 | 1.52 (0.83–2.76) | 0.17 | |
| III | 152/153 | 1.13 (0.85–1.5) | 0.4 | |
| IV | 74/74 | 1.46 (1–2.15) | 0.051 | |
| ADAMTS4 | I | 35/32 | 1.18 (0.43–3.23) | 0.75 |
| II | 70/70 | 1.52 (0.83–2.76) | 0.17 | |
| III | 152/153 | 1.13 (0.85–1.5) | 0.4 | |
| IV | 74/74 | 1.46 (1–2.15) | 0.051 | |
| ADAMTS5 | I | 31/31 | 0.48 (0.16–1.49) | 0.19 |
| II | 68/67 | 1.66 (0.86–3.18) | 0.13 | |
| III | 98/99 | 1.81 (1.24–2.64) | 0.002 | |
| IV | 71/69 | 1.51 (1.02–2.25) | 0.04 | |
| ADAMTS6 | I | 32/30 | 0.41 (0.12–1.33) | 0.12 |
| II | 68/67 | 1.4 (0.74–2.63) | 0.29 | |
| III | 98/99 | 1.36 (0.93–1.98) | 0.11 | |
| IV | 70/70 | 1.31 (0.88–1.95) | 0.18 | |
| ADAMTS7 | I | 34/28 | 2.56 (0.77–8.56) | 0.11 |
| II | 82/53 | 1.35 (0.71–2.57) | 0.35 | |
| III | 110/87 | 1.77 (1.21–2.57) | 0.0026 | |
| IV | 74/66 | 1.76 (1.18–2.63) | 0.0047 | |
| ADAMTS8 | I | 31/31 | 1.87 (0.56–6.23) | 0.3 |
| II | 68/67 | 1.52 (0.8–2.88) | 0.19 | |
| III | 98/99 | 1.2 (0.82–1.74) | 0.34 | |
| IV | 70/70 | 1.4 (0.94–2.09) | 0.096 |
Table 8. Stratified analysis of ADAMTS gene mRNA in degree of tumor differentiation in K–M plotter.
| Gene | Differentiation | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|---|
| ADAMTS1 | G1 | 2/3 | 1142066032.99 (0–Inf) | 0.41 |
| G2 | 34/33 | 0.89 (0.46–1.7) | 0.72 | |
| G3 | 60/61 | 0.87 (0.53–1.4) | 0.55 | |
| ADAMTS2 | G1 | 2/3 | 1142066039.57 (0–Inf) | 0.41 |
| G2 | 34/33 | 1.31 (0.69–2.52) | 0.41 | |
| G3 | 60/61 | 1.34 (0.82–2.18) | 0.24 | |
| ADAMTS3 | G1 | 16/16 | 1.52 (0.64–3.61) | 0.34 |
| G2 | 34/33 | 1.35 (0.71–2.59) | 0.36 | |
| G3 | 82/83 | 1.23 (0.82–1.83) | 0.32 | |
| ADAMTS4 | G1 | 16/16 | 1.52 (0.64–3.61) | 0.34 |
| G2 | 34/33 | 1.35 (0.71–2.59) | 0.36 | |
| G3 | 82/83 | 1.23 (0.82–1.83) | 0.32 | |
| ADAMTS5 | G1 | 2/3 | 1142066032.99 (0–Inf) | 0.41 |
| G2 | 34/33 | 0.96 (0.5–1.84) | 0.91 | |
| G3 | 60/61 | 1.16 (0.72–1.88) | 0.54 | |
| ADAMTS6 | G1 | 2/3 | 1142066039.57 (0–Inf) | 0.41 |
| G2 | 36/31 | 1.49 (0.78–2.84) | 0.23 | |
| G3 | 62/59 | 1.07 (0.66–1.73) | 0.78 | |
| ADAMTS7 | G1 | 2/3 | 1142066042.6 (0–Inf) | 0.41 |
| G2 | 40/27 | 2.39 (1.24–4.59) | 0.0074 | |
| G3 | 60/61 | 0.77 (0.48–1.26) | 0.3 | |
| ADAMTS8 | G1 | 2/3 | 1142066039.57 (0–Inf) | 0.41 |
| G2 | 34/33 | 1.43 (0.75–2.74) | 0.28 | |
| G3 | 60/61 | 1.1 (0.68–1.79) | 0.69 |
Table 9. Stratified analysis of ADAMTS genes mRNA in treatment method in K–M plotter.
| Gene | Treatment method | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|---|
| ADAMTS1 | Surgery | 189/191 | 1.66 (1.24–2.22) | 0.00065 |
| 5-FU | 17/17 | 0.99 (0.4–2.47) | 0.98 | |
| ADAMTS2 | Surgery | 192/188 | 1.32 (0.99–1.76) | 0.061 |
| 5-FU | 17/17 | 2.26 (0.89–5.76) | 0.08 | |
| ADAMTS3 | Surgery | 194/186 | 1.54 (1.15–2.06) | 0.0032 |
| 5-FU | 77/76 | 0.88 (0.63–1.24) | 0.47 | |
| ADAMTS4 | Surgery | 194/186 | 1.54 (1.15–2.06) | 0.0032 |
| 5-FU | 77/76 | 0.88 (0.63–1.24) | 0.47 | |
| ADAMTS5 | Surgery | 190/190 | 1.28 (0.96–1.71) | 0.096 |
| 5-FU | 17/17 | 0.68 (0.27–1.71) | 0.42 | |
| ADAMTS6 | Surgery | 190/190 | 1.75 (1.31–2.34) | 0.00014 |
| 5-FU | 17/17 | 2.04 (0.81–5.16) | 0.12 | |
| ADAMTS7 | Surgery | 209/171 | 1.57 (1.17–2.09) | 0.0021 |
| 5-FU | 17/17 | 1 (0.4–2.47) | 1 | |
| ADAMTS8 | Surgery | 190/190 | 1.48 (1.11–1.98) | 0.0075 |
| 5-FU | 17/17 | 2.25 (0.85–5.95) | 0.094 |
Table 10. Stratified analysis of ADAMTS gene mRNA in HER2 state in K–M plotter database.
| Gene | HER2 state | Low/high expression cases | HR (95% CI) | P-value |
|---|---|---|---|---|
| ADAMTS1 | Negative | 214/215 | 1.6 (1.23–2.1) | 0.00049 |
| Positive | 101/101 | 1.94 (1.32–2.84) | 0.00063 | |
| ADAMTS2 | Negative | 214/215 | 1.46 (1.12–1.91) | 0.0049 |
| Positive | 101/101 | 1.59 (1.09–2.32) | 0.015 | |
| ADAMTS3 | Negative | 266/266 | 1.49 (1.19–1.87) | 0.00053 |
| Positive | 172/172 | 1.26 (0.97–1.63) | 0.082 | |
| ADAMTS4 | Negative | 266/266 | 1.49 (1.19–1.87) | 0.00053 |
| Positive | 172/172 | 1.26 (0.97–1.63) | 0.082 | |
| ADAMTS5 | Negative | 216/213 | 1.44 (1.1–1.87) | 0.0077 |
| Positive | 102/100 | 1.37 (0.94–1.99) | 0.1 | |
| ADAMTS6 | Negative | 215/214 | 1.75 (1.33–2.29) | 4.2e-05 |
| Positive | 101/101 | 1.59 (1.09–2.31) | 0.015 | |
| ADAMTS7 | Negative | 225/204 | 1.85 (1.42–2.43) | 4.6e-06 |
| Positive | 104/98 | 1.33 (0.92–1.93) | 0.13 | |
| ADAMTS8 | Negative | 214/215 | 1.75 (1.34–2.29) | 3.7e-05 |
| Positive | 101/101 | 1.64 (1.12–2.38) | 0.0096 |
GSEA
The GSEA data indicated that, in the c2 category, the high expression of ADAMTS6 may participate in extracellular matrix organization, development of advanced GC, metastasis, ECM receptor interaction, vascular endothelial growth factor A (VEGFA), kirsten rat sarcoma viral oncogene (KRAS), c-Jun N-terminal kinase (JNK), and cadherin (CDH1) signaling pathways (Figure 13A–I). In the c5 category, down-regulation of the ADAMTS6 expression might be linked with DNA damage, cell cycle, apoptosis, glycolysis, fatty acid metabolism, mRNA catabolism, tumor protein p53, and tumor necrosis factor (TNF) signaling pathways (Figure 14A–I).
Figure 13. GSEA of C2 gene sets for high ADAMTS6 expression groups.
(A) ‘Extracellular matrix organization’, (B) ‘Gastric cancer advanced vs early up’, (C) ‘ECM receptor interaction’, (D) ‘Tavazoie metastasis’, (E) ’Alonso metastasis EMT up’, (F) ‘KRAS targets up’ (G) ‘VEGFA targets 12HR’, (H) ‘JNK signaling up’ and (I) ‘CDH1 signaling pathway’ were enriched in the ADAMTS6 high-expression groups.
Figure 14. GSEA of C5 gene sets for low ADAMTS6 expression groups.
The GO terms (A) ‘Intrinsic apoptotic signaling pathway’, (B) ‘Regulation of apoptotic signaling pathway’, (C) ‘Intrinsic apoptotic signaling pathway by P53 class mediator’, (D) ‘G1 DNA damage checkpoint’, (E) ‘Glycolysis process’, (F) ‘Fatty acid metabolic process’, (G) ‘Regulation of mRNA catabolic process’, (H) ‘Signal transduction by P53 class mediator’ and (I) ‘Tumor necrosis factor-mediated signaling pathways’ were enriched in the ADAMTS6 low-expression groups.
qRT-PCR and immunohistochemistry
Although the application of bioinformatics has given us directions, we still needed to combine sample verification to provide guidance for clinical research. We found that ADAMTS6 is mainly expressed in the cytoplasm of GC tissues. The positive expression of ADAMTS6 was observed in 10 (55.56%) and 4 (22.22%) cases of GC and adjacent normal tissues, respectively (Table 11, P<0.05), which proved that ADAMTS6 was up-regulated in GC compared with adjacent tissues (Figure 15A,B), consistent with TCGA findings (Figure 1, P<0.05).
Table 11. ADAMTS6 expression in GC and paracarcioma tissues.
| ADAMTS6 expression | P-value | ||
|---|---|---|---|
| Positive, n (%) | Negative, n (%) | ||
| GC tissue (n=18) | 10 (55.56) | 8 (44.44) | 0.04 |
| Paracarcioma tissues (n=18) | 4 (22.22) | 14 (77.78) | |
Figure 15. ADAMTS6 expression in GC patients.
(A) Representative images of immunostaining for ADAMTS6 in GC and adjacent normal tissues. (B) ADAMTS6 levels were detected in 24 pairs of GC tissues by qRT-PCR, revealing higher ADAMTS6 expression in GC tissues relative to paracancerous tissues. *P<0.05.
Discussions
Invasive growth and distant metastasis are the two key features that characterize malignancy, which are also the primary reason for high mortality. Rapid proliferation and metastasis of tumors is facilitated by emergence of new blood vessels in the stroma [32]. Thus, inhibiting angiogenesis may be an effective strategy to inhibit cancer growth. The most significant feature of the ADAMTS family of enzymes are their diversity in thrombospondin type 1 (TSP1) motifs at the C-terminus. The TSP1 motifs are highly conserved and are an endogenous inhibitor of angiogenesis. It inhibits endothelial cell proliferation, induce endothelial cell apoptosis and anti-angiogenesis through its interaction with CD36 receptor [33].
In our study, GO enrichment analysis revealed that ADAMTS gene family is related to extracellular matrix/structure organization, extracellular matrix disassembly, and binding of cell adhesion molecules. Cell adhesion molecules mediate the interaction between cells or between the cells and matrix [34]. These adhesion molecules are synthesized and secreted by a variety of cells, and participate in the occurrence and metastasis of tumors [35]. In preventing tumor metastasis, extracellular matrix organization plays an important regulatory roles and its dissolution can promote tumor growth and metastasis. Previous data have suggested that the ADAMTS family of genes plays a vital role in the degradation of extracellular matrix [36]. Thus, the ADAMTS genes might be key anti-invasive molecules. The co-expression of the ADAMTS1–8 genes shows positive correlation with GC tumors. Nevertheless, data on the molecular expression and the role of ADAMTS set of genes in GC are still scant.
Several studies have revealed that ADAMTS1 is down-regulated in a variety of tumors [37–39], as well as in GC tumor tissue [40]. In our study, there was low expression of ADAMTS1 mRNA in GC tissues compared with those in adjacent tissues, P<0.001. Besides, the survival analysis also demonstrated that up-regulation of the ADAMTS1 is associated with poor survival time in GC patients (P=0.013), but not in multifactor analysis (P>0.05). Whereas the ADAMTS1 gene expression level correlates with OS in stomach cancer patients, it is not an independent prognostic biomarker for GC, thus more validation studies are required.
Compared with the normal tissues, ADAMTS2 expression in GC tumor cells and fibroblasts was significantly increased, and the up-regulation of the ADAMTS2 was associated with poor prognosis of GC patients [41]. Our data demonstrated that the expression of ADAMTS2 increases in GC, P<0.001, but the high expression did not affect mortality in GC (P>0.05).
It has also been observed that the increased expression of ADAMTS3 gene takes a major part in the development of myocardial infarction, osteoarthritis, and breast cancer [42]. Our study found the expression level of ADAMTS3 gene is not associated with the GC OS. Whereas ADAMTS4 expression was significantly up-regulated in invasive breast cancer tissues [43] and human glioma [44], our study showed that the expression of ADAMTS4 gene does not affect the OS of GC patients. Similarly, unlike previous studies [45–48], our findings shows that the expression profile of ADAMTS5 gene does not correlate with survival of GC patients. In addition, ADAMTS7 is involved in the migration and proliferation of smooth muscle and the development of atherosclerosis and restenosis [49]. Our current findings indicates the expression level of the ADAMTS7 gene is not related to OS in GC patients. Previous studies have shown that the ADAMTS8 expression is down-regulated in breast cancer, brain cancer, and non-small cell lung cancer [50–52]. Whereas our data showed down-regulation of the ADAMTS8 mRNA expression in GC, P<0.001, the low expression was not correlated with the risk of GC-specific mortality (P>0.05).
The up-regulation of ADAMTS6 is a molecular marker for poor prognosis in esophageal squamous cell carcinoma [53]. Besides, some researches have demonstrated that ADAMTS6 is dysregulated in breast cancer [54], prolactin tumors [55], and colorectal cancer [56]. In this study, we revealed that ADAMTS6 mRNA is overexpressed in GC tissues, P<0.001. ADAMTS6 expression is largely correlated with tumor stage, targeted molecular therapy, radical resection, radiation therapy, and histological grade of the GC. It was confirmed by immunohistochemistry and qRT-PCR that ADAMTS6 is up-regulated in GC, consistent with TCGA findings, which may be related to the prognosis of GC patients. Stratified analysis of the clinic pathological parameters such as age, gender, and TNM stage also showed that patients with the high expression of ADAMTS6 have reduced survival than those with low expression. Similarly, the multivariate survival and stratified analysis showed that the ADAMTS6 mRNA was up-regulated in GC patients and led to poor survival time. In addition, the ADAMTS6 gene was shown to promote the occurrence and development of stomach cancer. Our study revealed that ADAMTS6 is a tumor promoter, whose overexpression mediates occurrence, proliferation, invasion, or metastasis of stomach tumor, thus leading to a high mortality. Therefore, the ADAMTS6 gene may be a potential therapeutic target for GC.
The GSEA indicated that ADAMTS6 enriched cancer-related pathways, such as apoptosis, VEGF, KRAS, P53, JNK, CDH1, or TNF pathways, which may affect GC prognosis. Apoptosis plays a significant role in maintaining the stability of the internal environment. The balance between cell proliferation and apoptosis is pivotal to the stability of human internal environment, otherwise, any perturbation of this state might lead to tumorigenesis [57]. VEGF mediates angiogenesis, which has been considered as the strongest cytokine promoting tumor angiogenesis [58]. It has been shown that inhibition of the VEGF signaling pathway inhibits neovascularization, thus blocking the occurrence and metastasis of tumors [59]. KRAS is the most common mutation type in the RAS family of genes that affects the development of tumors [60,61]. Once KRAS mutates, it will lose the activity of GTP hydrolase, and thus continue to activate, promoting the uncontrolled cell proliferation and carcinogenesis. Besides, mutations in the p53 gene renders it ineffective in regulating cell growth, apoptosis, DNA repair and so on, causing cell transformation and cancer [62,63]. Deveci et al. demonstrated that the P53 gene is associated with the occurrence and development of GC [64]. JNK signaling pathway plays a significant part in regulation of cell cycle, reproduction, apoptosis, and cell stress. Moreover, Yan et al., emphasized that inhibition of JNK signaling pathway may lead to apoptosis and metastasis of GC [65,66]. CDH1 gene mutation is a marker for poor GC prognosis [67–70]. TNF is involved in the inflammation and cellular immune response as well as tumor regulatory mechanisms [71–73]. The findings infer that ADAMTS6 may be involved in cancer-related pathways, including VEGF, KRAS, P53, JNK, CDH1, or TNF pathways, which play a crucial role in GC prognosis.
Our study has several limitations. Our study is dependent on data from a public database, thus the ADAMTS and survival analysis findings require further validation. Therefore, further experiment verification of the expression and function of ADAMTS is very necessary to improve the credibility of our current study. Besides, the underlying molecular mechanisms by which ADAMTS6 affects the occurrence and prognosis of GC needs further interrogation. In addition, because the information on TCGA database is incomplete, we were unable to clearly evaluate the relationship between the mRNA expression of the ADAMTS family of genes and protein expression. Subsequent studies need to reveal the biological mechanism of ADAMTS6 in the development and metastasis of GC from various aspects.
Despite these limitations, the present study is the first to investigate the correlation between ADAMTS mRNA expression and survive time in patients with stomach cancer. Log-rank test with Cox regression survival analysis and K–M survival analysis method were performed and found that ADAMTS6 expression level was largely correlated to GC patient clinical prognosis outcome. Thus, the prognostic relationship between ADAMTS family and GC was verified in the KM plotter database. Finally, GSEA found the differences in biological processes and related tumor pathways. Once these consequences are confirmed, we anticipate that ADAMTS6-targeted therapy drugs will be used in GC patients.
Conclusions
Our research reveals that the up-regulation of ADAMTS6 is significantly associated with poor prognosis, and might be used as an independent predictive factor for GC. The potential mechanism of ADAMTS6 in GC prognosis was involved in cancer-related biologic processes and pathways, including apoptosis, VEGF, KRAS, P53, JNK, CDH1, or TNF pathways. Nevertheless, the potential mechanism of ADAMTS6 still need further verification and investigation.
Acknowledgements
The authors thank the contributors of TCGA (https://cancergenome.nih.gov/) and UCSC Xena (http://xena.ucsc.edu/) for sharing the GC data on open access. The manuscript has been presented as Pre-print in researchsquare according to the following link: https://www.researchsquare.com/article/rs-41038/v1.
Abbreviations
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- CDH1
cadherin
- CI
confidence interval
- FDR
false discovery rate
- GC
gasctric cancer
- GO
Gene Ontology
- GSEA
Gene set enrichment analysis
- HP
Helicobacter pylori
- HR
hazard ratio
- JNK
c-Jun N-terminal kinase
- KM plotter
Kaplan–Meier plotter
- KRAS
kirsten rat sarcoma viral oncogene
- MMS
microsatellite stability
- OS
overall survival
- PPI
protein–protein interaction
- ROC
receiver operating characteristic
- TCGA
The Cancer Genome Atlas
- TNF
tumor necrosis factor
- TNM
T, tumour; N, node; M, metastasis
- TSP1
thrombospondin type 1
Data Availability
The data used to support the findings of the presewnt study are available from the corresponding author.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported in part by the Self-Raised Scientific Research Fund of the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region [grant number Z2014253]; and the Scientific Research Fund of the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region [grant number S2017096].
Author Contribution
Ya-zhen Zhu wrote this manuscript and performed the relevant experiments. Yi Liu and Xi-wen Liao designed this manuscript, collected and analyzed the data. Shan-shan Luo guided the writing.
References
- 1.Jemal A., Center M.M., DeSantis C. and Ward E.M. (2010) Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Prev. 19, 1893–1907 10.1158/1055-9965.EPI-10-0437 [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A. and Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Kim Y., Byeon S.J., Hur J., Lee K., Kim D., Ahn J.H.et al. (2019) High delta-like ligand 4 expression correlates with a poor clinical outcome in gastric cancer. J. Cancer 10, 3172–3178 10.7150/jca.30257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson P.T., Ho S., Williams R. and Johnson P.J. (2001) HLA class II alleles in Chinese patients with hepatocellular carcinoma. Liver 21, 143–148 10.1034/j.1600-0676.2001.021002143.x [DOI] [PubMed] [Google Scholar]
- 5.Chen W.Q., Li H., Sun K.X., Zheng R.S., Zhang S.W., Zeng H.M.et al. (2018) Report of Cancer Incidence and Mortality in China, 2014. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol.] 40, 5–13 [DOI] [PubMed] [Google Scholar]
- 6.Akgollu E., Bilgin R., Akkiz H., Ulger Y., Kaya B.Y., Karaogullarindan U.et al. (2017) Association between chronic hepatitis B virus infection and HLA-DP gene polymorphisms in the Turkish population. Virus Res. 232, 6–12 10.1016/j.virusres.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez C.A., Megraud F., Buissonniere A., Lujan Barroso L., Agudo A., Duell E.J.et al. (2012) Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann. Oncol. 23, 1320–1324 10.1093/annonc/mdr384 [DOI] [PubMed] [Google Scholar]
- 8.Lochhead P. and El-Omar E.M. (2007) Helicobacter pylori infection and gastric cancer. Best Pract. Res. Clin. Gastroenterol. 21, 281–297 10.1016/j.bpg.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Plummer M., Franceschi S., Vignat J., Forman D. and de Martel C. (2015) Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 136, 487–490 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- 10.Sogaard K.K., Farkas D.K., Pedersen L., Lund J.L., Thomsen R.W. and Sorensen H.T. (2016) Long-term risk of gastrointestinal cancers in persons with gastric or duodenal ulcers. Cancer Med. 5, 1341–1351 10.1002/cam4.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L., Cheng Y.J., Cheng M.L., Yao Y.M., Zhang Q., Zhao X.K.et al. (2015) Quantitative assessment of common genetic variations in HLA-DP with hepatitis B virus infection, clearance and hepatocellular carcinoma development. Sci. Rep. 5, 14933 10.1038/srep14933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricker A.R. and Preussmann R. (1991) Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 259, 277–289 10.1016/0165-1218(91)90123-4 [DOI] [PubMed] [Google Scholar]
- 13.Freedman N.D., Abnet C.C., Leitzmann M.F., Mouw T., Subar A.F., Hollenbeck A.R.et al. (2007) A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am. J. Epidemiol. 165, 1424–1433 10.1093/aje/kwm051 [DOI] [PubMed] [Google Scholar]
- 14.Steevens J., Schouten L.J., Goldbohm R.A. and van den Brandt P.A. (2010) Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut 59, 39–48 10.1136/gut.2009.191080 [DOI] [PubMed] [Google Scholar]
- 15.Dong J. and Thrift A.P. (2017) Alcohol, smoking and risk of oesophago-gastric cancer. Best Pract. Res. Clin. Gastroenterol. 31, 509–517 10.1016/j.bpg.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Rocks N., Paulissen G., El Hour M., Quesada F., Crahay C., Gueders M.et al. (2008) Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 90, 369–379 10.1016/j.biochi.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 17.Porter S., Clark I.M., Kevorkian L. and Edwards D.R. (2005) The ADAMTS metalloproteinases. Biochem. J. 386, 15–27 10.1042/BJ20040424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelwick R., Desanlis I., Wheeler G.N. and Edwards D.R. (2015) The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 16, 113 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anders S. and Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis G. Jr, Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C.et al. (2003) DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4, P3 10.1186/gb-2003-4-5-p3 [DOI] [PubMed] [Google Scholar]
- 21.Mostafavi S., Ray D., Warde-Farley D., Grouios C. and Morris Q. (2008) GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 9, S4 10.1186/gb-2008-9-s1-s4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warde-Farley D., Donaldson S.L., Comes O., Zuberi K., Badrawi R., Chao P.et al. (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38, W214–W220 10.1093/nar/gkq537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P. and Snel B. (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 31, 258–261 10.1093/nar/gkg034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Mering C., Jensen L.J., Snel B., Hooper S.D., Krupp M., Foglierini M.et al. (2005) STRING: known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 33, D433–D437 10.1093/nar/gki005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mootha V.K., Lindgren C.M., Eriksson K.-F., Aravind Subramanian1 S.S., Lehar J., Puigserver P.et al. (2003) PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A.et al. (2005) Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov Jill P. and Tamayo P. (2015) The molecular signatures database hallmark gene set collection. Cell Syst. 1, 417–425 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y., Gou Q., Xie K., Wang Z., Wang Y. and Zheng H. (2016) ADAMTS6 suppresses tumor progression via the ERK signaling pathway and serves as a prognostic marker in human breast cancer. Oncotarget 7, 61273–61283 10.18632/oncotarget.11341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y., Drai D., Elmer G., Kafkafi N. and Golani I. (2001) Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 10.1016/S0166-4328(01)00297-2 [DOI] [PubMed] [Google Scholar]
- 30.Reiner A., Yekutieli D. and Benjamini Y. (2003) Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19, 368–375 10.1093/bioinformatics/btf877 [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y. and Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. 57, 289–300 [Google Scholar]
- 32.Hanahan D. and Folkman J. (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 10.1016/S0092-8674(00)80108-7 [DOI] [PubMed] [Google Scholar]
- 33.Gantus M.A., Nasciutti L.E., Cruz C.M., Persechini P.M. and Martinez A.M. (2006) Modulation of extracellular matrix components by metalloproteinases and their tissue inhibitors during degeneration and regeneration of rat sural nerve. Brain Res. 1122, 36–46 10.1016/j.brainres.2006.09.016 [DOI] [PubMed] [Google Scholar]
- 34.Freitas V.M., do Amaral J.B., Silva T.A., Santos E.S., Mangone F.R., Pinheiro Jde J.et al. (2013) Decreased expression of ADAMTS-1 in human breast tumors stimulates migration and invasion. Mol. Cancer 12, 2 10.1186/1476-4598-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagstaff L., Kelwick R., Decock J. and Edwards D.R. (2011) The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front. Biosci. 16, 1861–1872 10.2741/3827 [DOI] [PubMed] [Google Scholar]
- 36.van Goor H., Melenhorst W.B., Turner A.J. and Holgate S.T. (2009) Adamalysins in biology and disease. J. Pathol. 219, 277–286 10.1002/path.2594 [DOI] [PubMed] [Google Scholar]
- 37.Choi J.E., Kim D.S., Kim E.J., Chae M.H., Cha S.I., Kim C.H.et al. (2008) Aberrant methylation of ADAMTS1 in non-small cell lung cancer. Cancer Genet. Cytogenet. 187, 80–84 10.1016/j.cancergencyto.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Gustavsson H., Wang W., Jennbacken K., Welén K. and Damber J.-E. (2009) ADAMTS1, a putative anti-angiogenic factor, is decreased in human prostate cancer. BJU Int. 104, 1786–1790 10.1111/j.1464-410X.2009.08676.x [DOI] [PubMed] [Google Scholar]
- 39.Masui T., Tuji S., Ida J., Nakajima S., Kawaguch M. and Koizumi M. (2001) Expression of METH-1 and METH-2 in pancreatic cancer. Clin. Cancer Res. 7, 3437–3443 [PubMed] [Google Scholar]
- 40.Lind G.E., Kleivi K., Meling G.I., Teixeira M.R., Thiis-Evensen E., Rognum T.O.et al. (2006) ADAMTS1, CRABP1, a n d NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell. Oncol. 28, 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang C., Zhou Y., Huang Y., Wang Y., Wang W. and Kuai X. (2019) Overexpression of ADAMTS-2 in tumor cells and stroma is predictive of poor clinical prognosis in gastric cancer. Hum. Pathol. 84, 44–51 10.1016/j.humpath.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 42.Alper M., Aydemir A.T. and Kockar F. (2015) Induction of human ADAMTS-2 gene expression by IL-1alpha is mediated by a multiple crosstalk of MEK/JNK and PI3K pathways in osteoblast like cells. Gene 573, 321–327 10.1016/j.gene.2015.07.064 [DOI] [PubMed] [Google Scholar]
- 43.Kelwick R., Desanlis I., Wheeler G.N. and Edwards D.R. (2015) The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 16, 113 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Held-Feindt J., Paredes E.B., Blömer U., Seidenbecher C., Stark A.M., Mehdorn H.M.et al. (2006) Matrix-degrading proteases ADAMTS4 and ADAMTS5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomas. Int. J. Cancer 118, 55–61 10.1002/ijc.21258 [DOI] [PubMed] [Google Scholar]
- 45.Gu J., Chen J., Feng J., Liu Y., Xue Q., Mao G.et al. (2016) Overexpression of ADAMTS5 can regulate the migration and invasion of non-small cell lung cancer. Tumour Biol. 37, 8681–8689 10.1007/s13277-015-4573-x [DOI] [PubMed] [Google Scholar]
- 46.Li C., Xiong Y., Yang X., Wang L., Zhang S., Dai N.et al. (2015) Lost expression of ADAMTS5 protein associates with progression and poor prognosis of hepatocellular carcinoma. Drug Des. Dev. Ther. 9, 1773–1783 10.2147/DDDT.S77069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J., Liao Y., Huang J., Sun Y., Chen H., Chen C.et al. (2018) Epigenetic silencing of ADAMTS5 is associated with increased invasiveness and poor survival in patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 144, 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakada M., Miyamori H., Kita D., Takahashi T., Yamashita J., Sato H.et al. (2005) Human glioblastomas overexpress ADAMTS-5 that degrades brevican. Acta Neuropathol. (Berl.) 110, 239–246 10.1007/s00401-005-1032-6 [DOI] [PubMed] [Google Scholar]
- 49.Pu X., Xiao Q., Kiechl S., Chan K., Ng F.L., Gor S.et al. (2013) ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am. J. Hum. Genet. 92, 366–374 10.1016/j.ajhg.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi G.C., Li J., Wang Y., Li L., Zhong L., Ma B.et al. (2014) The metalloprotease ADAMTS8 displays antitumor properties through antagonizing EGFR-MEK-ERK signaling and is silenced in carcinomas by CpG methylation. Mol. Cancer Res. 12, 228–238 10.1158/1541-7786.MCR-13-0195 [DOI] [PubMed] [Google Scholar]
- 51.Dunn J.R., Panutsopulos D., Shaw M.W., Heighway J., Dormer R., Salmo E.N.et al. (2004) METH-2 silencing and promoter hypermethylation in NSCLC. Br. J. Cancer 91, 1149–1154 10.1038/sj.bjc.6602107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koo B.H., Coe D.M., Dixon L.J., Somerville R.P., Nelson C.M., Wang L.W.et al. (2010) ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am. J. Pathol. 176, 1494–1504 10.2353/ajpath.2010.090655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L., Yang Z., Ni W. and Xuan Y. (2018) ADAMTS-6 is a predictor of poor prognosis in patients with esophageal squamous cell carcinoma. Exp. Mol. Pathol. 104, 134–139 10.1016/j.yexmp.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 54.Porter S., Scott S.D., Sassoon E.M., Williams M.R., Jones J.L., Girling A.C.et al. (2004) Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 10, 2429–2440 10.1158/1078-0432.CCR-0398-3 [DOI] [PubMed] [Google Scholar]
- 55.Wierinckx A., Auger C., Devauchelle P., Reynaud A., Chevallier P., Jan M.et al. (2007) A diagnostic marker set for invasion, proliferation, and aggressiveness of prolactin pituitary tumors. Endocr. Relat. Cancer 14, 887–900 10.1677/ERC-07-0062 [DOI] [PubMed] [Google Scholar]
- 56.Xiao W.H., Qu X.L., Li X.M., Sun Y.L., Zhao H.X., Wang S.et al. (2015) Identification of commonly dysregulated genes in colorectal cancer by integrating analysis of RNA-Seq data and qRT-PCR validation. Cancer Gene Ther. 22, 278–284 10.1038/cgt.2015.20 [DOI] [PubMed] [Google Scholar]
- 57.Espinosa M., Cantu D., Herrera N., Lopez C.M., De la Garza J.G., Maldonado V.et al. (2006) Inhibitors of apoptosis proteins in human cervical cancer. BMC Cancer 6, 45 10.1186/1471-2407-6-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan X., Krieg S., Kuo C.J., Wiegand S.J., Rabinovitch M., Druzin M.L.et al. (2008) VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 22, 3571–3580 10.1096/fj.08-111401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui-Zhuo X., Shuang-Zhen L., Si-Qi X. and Xiao-Bo X. (2011) HIF-1α siRNA reduces retinal neovascularization in a mouse model of retinopathy of prematurity. Chin. J. Contemp. Pediatr. 13, 680–683 [PubMed] [Google Scholar]
- 60.Albertini A.F., Raoux D., Neumann F., Rossat S., Tabet F., Pedeutour F.et al. (2017) Detection of RAS genes mutation using the Cobas((R)) method in a private laboratory of pathology: Medical and economical study in comparison to a public platform of molecular biology of cancer. Bull. Cancer 104, 662–674 10.1016/j.bulcan.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 61.Liu Z.M., Liu L.N., Li M., Zhang Q.P., Cheng S.H. and Lu S. (2009) Mutation detection of KRAS by high-resolution melting analysis in Chinese with gastric cancer. Oncol. Rep. 22, 515–520 10.3892/or_00000465 [DOI] [PubMed] [Google Scholar]
- 62.Chari N.S., Pinaire N.L., Thorpe L., Medeiros L.J., Routbort M.J. and McDonnell T.J. (2009) The p53 tumor suppressor network in cancer and the therapeutic modulation of cell death. Apoptosis 14, 336–347 10.1007/s10495-009-0327-9 [DOI] [PubMed] [Google Scholar]
- 63.Machado-Silva A., Perrier S. and Bourdon J.C. (2010) p53 family members in cancer diagnosis and treatment. Semin. Cancer Biol. 20, 57–62 10.1016/j.semcancer.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 64.Deveci M.S. and Deveci G. (2007) Prognostic value of p53 protein and MK-1 (a tumor-associated antigen) expression in gastric carcinoma. Gastric Cancer 10, 112–116 10.1007/s10120-007-0418-7 [DOI] [PubMed] [Google Scholar]
- 65.Choi Y., Ko Y.S., Park J., Choi Y., Kim Y., Pyo J.S.et al. (2016) HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J. Gastroenterol. 22, 9141–9153 10.3748/wjg.v22.i41.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan H., Xiao F., Zou J., Qiu C., Sun W., Gu M.et al. (2018) NR4A1-induced increase in the sensitivity of a human gastric cancer line to TNFalpha-mediated apoptosis is associated with the inhibition of JNK/Parkin-dependent mitophagy. Int. J. Oncol. 52, 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Cho J., Ahn S., Son D.S., Kim N.K., Lee K.W., Kim S.et al. (2019) Bridging genomics and phenomics of gastric carcinoma. Int. J. Cancer 145, 2407–2417 10.1002/ijc.32228 [DOI] [PubMed] [Google Scholar]
- 68.Cho S.Y., Park J.W., Liu Y., Park Y.S., Kim J.H., Yang H.et al. (2017) Sporadic early-onset diffuse gastric cancers have high frequency of somatic cdh1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology 153, 536.e26–549.e26 10.1053/j.gastro.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guilford P., Humar B. and Blair V. (2010) Hereditary diffuse gastric cancer: translation of CDH1 germline mutations into clinical practice. Gastric Cancer 13, 1–10 10.1007/s10120-009-0531-x [DOI] [PubMed] [Google Scholar]
- 70.Li X., Wu W.K., Xing R., Wong S.H., Liu Y., Fang X.et al. (2016) Distinct subtypes of gastric cancer defined by molecular characterization include novel mutational signatures with prognostic capability. Cancer Res. 76, 1724–1732 10.1158/0008-5472.CAN-15-2443 [DOI] [PubMed] [Google Scholar]
- 71.Hoesel B. and Schmid J.A. (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12, 86–100 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lebrec H., Ponce R., Preston B.D., Iles J., Born T.L. and Hooper M. (2015) Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr. Med. Res. Opin. 31, 557–574 10.1185/03007995.2015.1011778 [DOI] [PubMed] [Google Scholar]
- 73.Wang L., Zhao Y., Liu Y., Akiyama K., Chen C., Qu C.et al. (2013) IFN-gamma and TNF-alpha synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFkappaB signaling. Stem Cells 31, 1383–1395 10.1002/stem.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the presewnt study are available from the corresponding author.