Highlights
-
•
Local ablative radiotherapy is associated with high rates of local control.
-
•
Prognosis in oligometastatic disease may be improved by metastases-directed therapy.
-
•
OLIGOMA assesses the role of radiotherapy in oligometastatic breast cancer patients.
Keywords: Stereotactic body radiotherapy, Stereotactic ablative radiotherapy, Radiosurgery, Metastatic breast cancer, Quality of life, Progression-free survival
Abstract
Background
Several recent randomized therapeutic exploratory trials demonstrated improvement of progression-free survival and in some even overall survival using stereotactic body radiotherapy in patients with oligometastatic disease. However, only very few patients enrolled in these trials had breast cancer, and results from confirmatory trials are lacking.
Methods/design
The OLIGOMA-trial is a randomized controlled multi-national multi-center therapeutic confirmatory trial studying the role of local ablative radiotherapy as an additive treatment in patients with oligometastatic breast cancer receiving standard systemic therapy. Patients will be randomized 1:1 to standard systemic therapy according to national guidelines with or without radiotherapy to all metastatic sites. Randomization will be stratified according to type and line of systemic therapy, which has to be determined by a multidisciplinary tumor board before enrollment. Patients with up to five metastatic lesions are eligible, including patients with up to three brain metastases (only in case of extracranial disease) and with locoregional recurrence (only in case of additional metastatic lesions). In the standard arm, palliative radiotherapy to symptomatic metastases is permitted if at least one lesion remains untreated. The co-primary endpoints are progression-free survival and quality of life. The primary hypothesis is that progression-free survival in the experimental arm will be superior to the standard arm while simultaneously demonstrating non-inferiority of quality of life at 12 weeks after randomization. Secondary endpoints are feasibility, overall survival, toxicity, quality of life and patient satisfaction. A translational sub-study with collection of ctDNA will be conducted.
Discussion
The OLIGOMA-trial will provide high level evidence on the use of and benefit from local ablative radiotherapy for patients with oligometastatic breast cancer.
Trial registration
The OLIGOMA-trial is registered at clinicialtrials.gov under the identification NCT04495309. The related information was first posted on July 31st 2020.
Introduction/Rationale
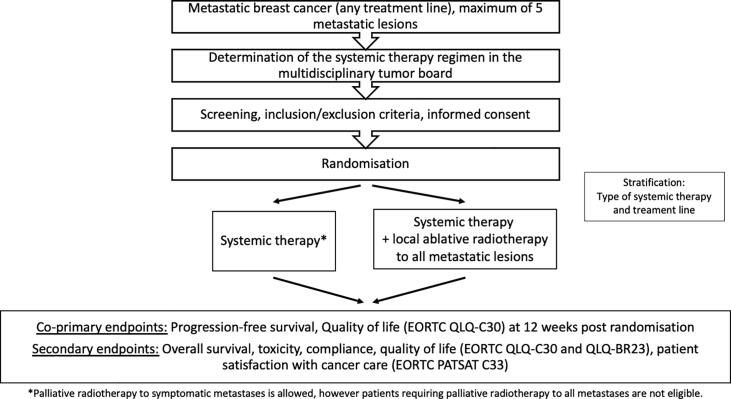
The concept of oligometastatic disease as a transitional state between locally confined disease and widespread metastatic disease was first defined by Hellman and Weichselbaum in the 1990 s [1], [2]. This was guided by the observation that there are patients with limited metastatic disease who achieve long lasting-remissions or even cure after local therapy of metastases (i.e. surgery, radiotherapy, radiofrequency ablation). Up to this point, there were two main competing hypotheses of breast cancer. In the late 19th century, William Halsted proposed that breast cancer spreads in an orderly fashion with serial involvement from the primary tumor to lymph nodes and distant organs. This led to the establishment of radical breast cancer surgery as a means of achieving long term cure. Based on experiments of tumor biology and metastatic spread, Fisher at al. established the alternative hypothesis (or systemic hypothesis) in the 1960 s. This hypothesis states that there is no orderly metastatic spread and that breast cancer is a systemic disease even in patients with early stage breast cancer (for review see [3]). The hypothesis by Hellman and Weichselbaum which defined the term “oligometastases” is also referred to as the spectrum hypothesis as it refers to breast cancer representing a spectrum of diseases ranging from diseases that remain local during the course of the disease to those that are characterized by early systemic spread [1], [2], [3].Fig. 1.
Fig. 1.
Workflow for the OLIGOMA trial.
There is no uniform definition of oligometastases, but most authors have defined this disease state as a maximum of 3–5 metastatic lesions (for review [4]). Recently, a consensus statement by the European Society of Radiotherapy and Oncology (ESTRO) and the American Society of Radiation Oncology (ASTRO) was published [4]. Here, the authors argue against the use of a threshold but instead recommend to determine the maximum number of metastases individually based on the possibility to safely deliver ablative radiotherapy to all lesions.
For prognostic purposes, additional clinical factors need to be considered, among them the disease-free interval since the last oncologic treatment, previous lines of treatment and response to these treatments, timing of metastatic disease (synchronous vs. metachronous), pattern of organ involvement, and histology. The ESTRO and the European Organisation for Research and Treatment of Cancer (EORTC) have recently developed the first systematic classification of oligometastatic disease [5].
In the past decade, there has been a rapidly increasing interest in oligometastatic disease due to advances in imaging and ablative treatment modalities. Prospective and retrospective studies have shown that approximately 50% of patients with metastatic breast cancer present with ≤ 2 metastatic sites [6]. Thus, there is considerable potential in terms of eligibility for local ablative treatment strategies in patients with metastatic breast cancer.
Numerous local ablative treatment modalities are available, among them surgery, radiofrequency ablation, irreversible electroporation, microwave ablation, and radiotherapy.
Stereotactic radiosurgery (SRS) has been used for intracranial tumors since the 1950 s and represents the standard of care for patients with limited brain metastases. While the available randomized controlled trials comparing SRS alone to SRS plus whole-brain irradiation for brain metastases have included patients with up to 4 brain metastases [7], [8], [9], [10], prospective data on patients treated with SRS for up to 10 brain metastases have been published [11].
Stereotactic body radiotherapy (SBRT) was first introduced in the clinic in the 1990 s and has been extensively studied in patients with pulmonary, osseous and hepatic metastases. Several prospective and retrospective studies have analyzed the outcome of patients with oligometastatic disease treated with SBRT (for review see [12], [13]) and have shown promising local control and low rates of grade ≥ 2 adverse events.
The SABR-COMET trial was the first randomized controlled trial of SBRT in patients with oligometastatic disease [14]. Patients were eligible if they had up to 5 metastatic lesions with a good performance status (ECOG 0-1) and a life expectancy > 6 months. 99 patients were randomized 2:1 to SBRT or palliative standard of care. The overwhelming majority (92%) of patients had 1–3 metastatic lesions and 18% suffered from metastatic breast cancer. Progression-free survival (PFS) was 12 months in patients treated with SBRT and 6 months in patients treated with palliative care alone (p = 0.001). There was an improvement in median overall survival (OS) in patients treated with SBRT (41 months vs. 28 months; p = 0.09) that was statistically significant at the pre-defined level of 0.2. Grade ≥ 2 adverse events occurred significantly more often in the SBRT-arm (30% vs. 9%; p = 0.022). There were 3 grade 5-events in the SBRT-arm. Long term results were recently published and confirmed the OS-benefit without additional safety concerns [15].
Furthermore, two randomized controlled trials have shown a significant improvement in PFS [16], [17] and one also in OS [18] with local ablative therapy to all metastatic sites in patients with de novo oligometastatic non-small cell lung cancer who had stable disease or partial response after first-line systemic therapy.
For prostate cancer patients, two randomized controlled phase II-trials demonstrated improvements in PFS or androgen-deprivation therapy-free survival with metastasis-directed treatment, however, both trials were small and did not use an active comparator [19], [20].
There are only few studies specifically addressing the outcome of patients with oligometastatic breast cancer (for review see [6], [21], [22]).
Several retrospective and prospective reports of SBRT in patients with oligometastatic disease have suggested that patients with breast cancer have a favorable prognosis compared to other tumor types and achieve higher rates of local and distant control [23], [24], [25], [26], [27].
There are several small, single arm prospective trials of SBRT in patients with oligometastatic breast cancer (Table 1).
Table 1.
Prospective trials of stereotactic body radiotherapy for patients with oligometastatic breast cancer.
| Patient number | Inclusion criteria | Dose and fractionation | Follow up | Local control | PFS | OS | |
|---|---|---|---|---|---|---|---|
| Milano 2008 | 40 pat./85 met. |
|
10x5 Gy @ 80%-isodose | median 50 months (survivors) | 4 y 89% | 2 y 44% / 4 y 38% | 2 y 76% / 4 y 59% |
| Scorsetti 2016 | 33 pat./47 met. |
|
3x19-25 Gy @ 95%-isodose, 4x12 Gy @ 95%-isodose | median 24 months | 2 y 90% | 2 y 27% | 2 y 66% |
| Trovo 2018 | 54 pat./92 met. |
|
3x10-15 Gy, 25x2.4 (IMRT) | median 30 months | 2 y 97% | 2 y 53% | 2 y 95% |
| David 2019 | 15 pat. / 19 met. |
|
1x20 Gy @ 80%-isodose | minimum 24 months | 2 y 100% | 2 y 65% | 2 y 100% |
PFS = progression-free survival; OS = overall survival; KI = Karnofsky index, Gy = Gray; ECOG-PS = Eastern Cooperative Oncology Group-Performance Score; NaF = Natriumfluoride; y = year
Milano et al. conducted a prospective trial of SBRT in patients with up to 5 metastatic sites, including 40 patients with 85 lesions from breast cancer treated with curative intent [28]. The most common fractionation regimen was 10x5 Gy to the 80%-isodose. >70% of breast cancer patients had 1–2 metastatic lesions, with involvement of the liver, lung or bone. Overall, the 4-year local control, PFS and OS were 80%, 38% and 59%, respectively. Milano et al. recently published an updated analysis of breast cancer patients from different prospective trials [29]. They demonstrated that patients with bone-only oligometastases had a significantly better overall survival and freedom from widespread metastatic disease on multivariate analysis. Patients with bone-only oligometastases had a 10-year OS of 75%.
From 2010 to 2014, Scorsetti et al. enrolled 33 patients with 47 lung or liver metastases who were treated with 48–75 Gy in 3–4 fractions [30]. At 2 years, local control, PFS and OS were 90%, 27% and 66%, respectively. No acute or chronic toxicity ≥ grade 2 were observed.
Trovo et al. conducted a single-arm trial of patients with up to 5 extracranial metastases on FDG-PET/CT and a controlled primary tumor [31]. 54 patients with 92 lesions were treated with SBRT consisting of 30–45 Gy in 3 fractions or intensity-modulated radiotherapy consisting of 60 Gy in 25 fractions. 50% of patients had only one metastatic lesion, 74% had synchronous metastatic disease and 60 and 23 lesions were bone and lymph node metastases, respectively. 89% of patients received concomitant systemic therapy. After a median follow-up of 30 months, 2-year PFS and local control were 53% and 95%, respectively. Neither number of metastatic lesions, nor pattern or timing of metastatic disease were significantly associated with PFS. There was no grade ≥ 3-toxicity.
A prospective trial enrolling patients with bone-only oligometastatic disease was recently published by David [32]. All patients received a sodium fluoride positron emission tomography and were treated with a single fraction of 20 Gy to the 80%-isodose. 15 patients with 19 metastases were enrolled. 73% of patients had luminal breast cancer. At two years, no local progression or death from any cause had been observed. PFS was 65% at two years. There was no grade ≥ 3-toxicity.
In summary, local control rates of ≥ 90% can be achieved with SBRT in patients with oligometastatic breast cancer with PFS at 2 years ranging between 27% and 65%.
Notwithstanding these excellent results, the value of local therapy in oligometastatic disease has been questioned due to the efficacy of systemic therapy for most breast cancer subtypes.
Key differences between the OLIGOMA trial and three other ongoing randomized controlled trials are listed in Table 2.
Table 2.
Randomized controlled trials of local treatment in patients with oligometastatic breast cancer.
| OLIGOMA (NCT04495309) | NRG-BR002 (NCT02364557) | Fudan University (NCT04413409) | STEREO-SEIN (NCT02089100) | Chinese Academy of Sciences (NCT04646564) | |
|---|---|---|---|---|---|
| Primary tumor | Locoregional recurrence allowed as target lesion* | Controlled | Local recurrence not allowed | Treated with curative intent | Controlled |
| Brain metastases | Allowed** | Not allowed | Not allowed | Not allowed | Not allowed |
| Maximum number of metastatic lesions | 5 (any number of involved organs) | 2 | 3 (only lung or liver metastases, < 5 cm) | 5 (≤10 cm / ≤ 50 ml) | 5 (≤5 cm) |
| Setting | Any line, any tumor biology | First line palliative therapy, ≤ 365 days after diagnosis of metastatic breast cancer, any tumor biology | First line metastatic setting, any tumor biology | First line metastatic setting, hormone-receptor positive | Metachronous recurrence > 3 months after surgery, any tumor biology |
| Type of local therapy | Radiotherapy | Radiotherapy or surgery | Surgery | Radiotherapy | Radiotherapy |
| Primary endpoint | PFS | PFS/OS | OS | PFS | PFS |
| Primary hypothesis | Median PFS 12 months → 16 months (HR 0.75) | Phase IIR: Median PFS 10.5 months → 19 months (HR 0.55) | n.s. | n.s. | n.s. |
| Phase III: 5-year OS 28% →42.5% (HR 0.67) | |||||
| Sample size | 564 patients | Phase IIR: 146 patients | 172 patients | 280 patients | 170 patients |
| Phase III: 256 patients |
PFS = progression-free survival, OS = overall survival, HR = hazard ratio, n.s. = not stated.
* Enrollment is only possible in case of additional metastatic sites.
** Only patients with 1–3 asymptomatic brain metastases and extracranial disease activity are eligible.
The OLIGOMA-trial is the only trial enrolling patients with locoregional recurrence and patients with brain metastases. However, enrolment in both situations will only be possible in the case of concurrent extracranial metastatic disease. While most other trials exclusively treat patients with de novo oligometastatic disease, OLIGOMA will enroll patients from all treatment lines. Since radiotherapy is currently the only local treatment modality with consistent improvement in PFS, and in some cases OS, in clinical trials of patients with oligometastatic disease of different primaries [14], [15], [16], [17], [18], [19], [20], it was chosen as the only local treatment modality for the OLIGOMA-trial. Surgery of large brain metastases or spine metastases with postoperative radiotherapy is allowed in selected cases.
The response to prior systemic therapy is not an inclusion criterion. Thus, patients with oligoprogression or patients with oligopersistence may be enrolled. Patients with de novo oligometastatic breast cancer are eligible, however, it is recommended to complete treatment of the primary tumor before enrollment. While one might argue that including patients with various courses of disease is problematic [42] and these different presentations certainly have a prognostic impact [43], [44], we believe that it is important to gather clinical data across all situations. Disease state according to the ESTRO/EORTC-classification of oligometastatic disease [5] will be collected, and randomization is stratified for line and type of systemic treatment. Systemic therapy has to be specified by a multidisciplinary tumor board prior to enrollment, thus reducing the risk of bias related to different intensity of systemic therapy between the trial arms. While normofractionated and moderately hypofractionated regimens are allowed in the case of large metastases or proximity to critical organs at risk, there is a clear preference for using hypofractionated regimens as SRS/SBRT with single doses > 5 Gy.
Since cross-over is allowed after progression, we believe that an improvement in OS is not realistic and PFS was hence chosen as the primary endpoint. OS will be reported as a secondary endpoint. However, we chose to include quality of life at 12 weeks after randomization as a co-primary endpoint. The goal is to show that local ablative radiotherapy improves PFS without a detrimental effect on quality of life.
Design
The OLIGOMA trial (NCT04495309) is a randomized controlled multi-national multi-center therapeutic confirmatory-trial studying the role of local ablative radiotherapy as an additive treatment in patients with oligometastatic breast cancer. Patients will be recruited at 50 sites in Germany and Austria. Inclusion and exclusion criteria are shown in Table 3. Patients will be randomized 1:1 to systemic therapy either with or without ablative radiotherapy. Systemic therapy is administered according to national guidelines [33], [34] and will be determined before enrollment by a multidisciplinary tumor board.
Table 3.
Inclusion and exclusion criteria.
| Inclusion criteria: |
|---|
|
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
Central randomization (permuted blocks of variable length) will be stratified according to systemic therapy and line of treatment (first-line endocrine therapy vs. ≥ second-line endocrine therapy vs. first-line chemotherapy +/- HER2-targeted therapy vs. ≥ second line chemotherapy +/- HER2-targeted therapy). Endocrine-based therapy with CDK4/6-inhibitors or mTOR-inhibitors will be considered as endocrine therapy for this purpose.
Assessment with a CT of chest and abdomen (contrast-enhanced MRI or ultrasonography of the abdomen is also allowed), mammography (within the last 6 months) and a bone scintigraphy are required for enrollment. Staging with FDG-PET/CT is optional. MRI of the brain is only recommended in patients with clinical suspicion of brain metastases.
Treatment description
Ablative radiotherapy should preferentially be administered as SRS or SBRT, if technically feasible. Typical dose and fractionation regimens are listed in Table 4. For brain metastases, SRS is recommended, although fractionated stereotactic radiotherapy may be used for larger lesions or for lesions with close proximity to organs at risk. SBRT in 3–5 fractions is the recommended regimen for bone, lung and liver metastases. If SBRT is not feasible due to the size of the lesion or proximity to organs at risk, hypo- or normofractionated intensity-modulated or 3D-conformal radiotherapy is allowed. The minimal total dose should be 45 Gy administered in 25 fractions over 5 weeks. Surgery of large brain metastases or spine metastases with postoperative radiotherapy is allowed in selected cases.
Table 4.
Recommended dose and fractionation regimens.
| Number of fractions | Preferred dose | Accepted dose | Protocol violation |
|---|---|---|---|
| 1 | 20–27 Gy | 16–19 Gy / 28–30 Gy | <16 Gy or > 30 Gy |
| 3 | 30–42 Gy | 24–29 Gy / 43–45 Gy | <24 Gy or > 45 Gy |
| 5 | 35–50 Gy | 30–34 Gy / 51–55 Gy | <30 Gy or > 55 Gy |
| 10 | 45–60 Gy | 35–44 Gy / 61–65 Gy | <35 Gy or > 65 Gy |
| 15 | 45 Gy | 37.5–75 Gy | <37.5 Gy or > 75 Gy |
| 25 | 50 Gy | 45–75 Gy | <45 or > 75 Gy |
Regarding SRS/SBRT, dose will be prescribed to the 60–80%-isodose encompassing the planning target volume (PTV), with at least 98% of the PTV receiving the prescription dose (PTV D98%). For intensity-modulated radiotherapy (IMRT) and 3D-conformal radiotherapy (3D-CRT), dose will be prescribed to the PTV D50%. The use of a simultaneous integrated boost is allowed.
The margin from gross tumor volume (GTV) to the clinical target volume (CTV) should be kept to a clinically acceptable minimum. Larger CTV-margins of up to 5 mm may be used if clinically necessary. The CTV-to-PTV margin should be chosen according to the technique, immobilization as well as image guidance and motion management strategies. The CTV-to-PTV margin should be no>1–2 mm for intracranial targets. For target volume delineation of brain, liver and bone metastases, an additional MRI should be performed.
4D-planning CT should be performed for pulmonary and hepatic metastases and the use of motion compensation techniques such as gating or tracking is highly recommended for targets in the lower lung or liver.
Radiotherapy should be started as early as possible after enrollment, usually within 2–4 weeks after initiation of endocrine therapy or after the first or second chemotherapy cycle. Usually, no>3 metastatic lesions should be treated simultaneously. SRS or SBRT with a fraction dose ≥ 5 Gy should not be administered on the same day as chemotherapy. Radiotherapy has to be started within 4–6 weeks after randomization.
The indication for systemic therapy as well as the specific regimen have to be determined by a multidisciplinary tumor board according to national guidelines [33], [34] before enrollment. Systemic therapy may include endocrine therapy, chemotherapy, targeted therapy or immunotherapy without preference for any specific regimen. Delay of systemic therapy due to radiotherapy should be avoided.
Follow-up assessments will be conducted according to national guidelines [33], [34]. They will take place every 12 weeks and include a brief medical history with physical examination, imaging with CT or MRI (same diagnostic tool as at enrollment), assessment of toxicity using Common Terminology Criteria For Adverse Effects, version 5.0 [35], ECOG performance status and quality of life with the EORTC QLQ-C30 [36] and BR23 [37] questionnaires. At 12 weeks and starting from week 60, Radiation Therapy and Oncology Group-classification [38] is used to assess late toxicity. At 12 and 36 weeks after randomization, patient satisfaction with cancer care will be evaluated using the EORTC PATSAT C33 questionnaire [39].
When patients have disease progression, the use of ablative radiotherapy to new or progressive metastases is allowed in both arms. The use of radiotherapy and further systemic therapy after progression will be documented.
Endpoints
There are two co-primary endpoints, PFS during 1–4 years and quality of life at 12 weeks after randomization. The hypothesis is that the experimental arm is superior in terms of PFS and non-inferior in terms of quality of life 12 weeks after randomization.
Progression will be determined according to RECIST 1.1 [40]. The primary assessment will be performed by the local investigator at the treating site. In case of suspected progression, the trial leadership should be contacted. Regular virtual study meetings will be conducted to discuss exemplary cases. Central radiology assessment of all events of progression with final assessment of the primary endpoint is planned. Observations will be considered censored at the last visit with sufficient examinations and imaging to assess progression, if two or more visits in a row are missed, regardless of negative findings later that will be used for secondary analyses. If later examinations show a progression, its date is set at six weeks into the hiatus of follow-ups and at the day of imaging otherwise.
Quality of life at 12 weeks after randomization (at least two weeks after the end of radiotherapy) will be assessed using the sum score of the EORTC QLQ-C30 [41].
The two co-primary hypotheses will be tested at multiple significance level 5% in a Bonferroni-Holm procedure while adjusting for the stratification used in randomization.
Secondary endpoints are feasibility (proportion of patients treated per protocol), toxicity (CTCAE/RTOG), quality of life using the EORTC QLQ-C30 and QLQ-BR23 throughout the course of the trial and patient satisfaction with cancer care using the EORTC PATSAT C33.
A translational sub-study will evaluate the prognostic and predictive value of circulating tumor DNA, which will be collected at randomization and 12 weeks as well as 36 weeks after randomization.
Statistics
Estimated PFS in the control arm is 12 months based on published literature for metastatic breast cancer (a list of publications used for PFS estimation can be found in Supplementary Material 1). The trial is designed to show an improvement in PFS from 12 to 16 months with a hazard ratio of 0.75 at a two-sided significance level of 0.05 with power of 0.8. This requires a total number of 380 PFS-events.
We assumed that the difference in the quality of life sum score between treatment arms is 5 points and that the standard deviation of the sum score of all patients is 20 points. The non-inferiority margin (the minimal clinically relevant difference) is defined as 10 points in the sum score of the EORTC QLQ-C30. 508 evaluations are needed to show non-inferiority with a one-sided level of 0.025 with power of 0.8.
Assuming a dropout rate of 5%, a censoring rate of 5% per year for PFS and a dropout rate of 10% for quality of life, 564 patients need to be randomized.
All statistical analyses will be described in detail in the statistical analysis plan which will be finalized before the randomization of the last patient. Reporting and visualization comply with the CONSORT guidelines.
The primary analysis will be performed on the full analysis set based on the intention to treat principle. The per protocol population will consist of patients treated according to treatment protocol. For safety purposes, patients will be analyzed as treated and being irradiated at least once. No interim analysis is planned.
Explorative subgroup analyses are planned for number of metastatic lesions (1 vs. 2–3 vs. 4–5), number and type of involved organs (lung, liver, bone, brain, others; 1 vs. 2 vs. 3 or more), systemic therapy and Eastern Cooperative Oncology Group (ECOG) performance status (0 vs. 1–2).
Planned timeline
The estimated duration of recruitment is 36 months. Minimum follow-up will be 12 months. The estimated end of study will be 2024. A preliminary discontinuation of the trial is possible in case of unforeseen toxicity, insufficient recruitment or if new scientific data show that the study hypothesis is invalid.
Ethical and legal considerations
The study protocol was approved by the DEGRO-expert commission and the leading institutional review board at the University of Kiel (ID D500/20). Approval by the respective institutional review board relevant to each site will be collected before opening new sites. Written informed consent will be obtained by each participant. The study is monitored by ZKS Lübeck. The trial is supported by the Arbeitsgemeinschaft Radiologische Onkologie.
Funding
The trial is funded by Stiftung Deutsche Krebshilfe (ID 70112962).
Author contributions
David Krug: Conceptualization, Writing - Initial draft, Writing - review & editing. Reinhard Vonthein: Conceptualization, Methodology, Writing - review & editing. Alicia Illen: Conceptualization, Project administration, Writing - review & editing. Denise Olbrich: Conceptualization, Project administration, Writing - review & editing. Jörg Barkhausen: Writing - review & editing. Julia Richter: Writing - review & editing. Wolfram Klapper: Writing - review & editing. Claudia Schmalz: Writing - review & editing. Achim Rody: Writing - review & editing. Nicolai Maass: Writing - review & editing. Dirk Bauerschlag: Conceptualization, Writing - review & editing. Nicole Heßler: Methodology, Writing - review & editing. Inke R.König: Conceptualization, Methodology, Writing - review & editing. Kathrin Dellas: Conceptualization, Funding acquisition, Writing - review & editing. Jürgen Dunst: Conceptualization, Funding acquisition, Writing - Initial draft, Writing - review & editing.
Declaration of Competing Interest
DK has received honoraria from Merck Sharp & Dome outside the submitted work. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.03.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. JCO. 1994;12(10):2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S., Weichselbaum R.R. Oligometastases. JCO. 1995;13(1):8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B., Anderson S.J. The Breast Cancer Alternative Hypothesis: Is There Evidence to Justify Replacing It? JCO. 2010;28(3):366–374. doi: 10.1200/JCO.2009.26.8292. [DOI] [PubMed] [Google Scholar]
- 4.Lievens Y., Guckenberger M., Gomez D., Hoyer M., Iyengar P., Kindts I. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiotherapy and Oncology. The Authors. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Guckenberger M., Lievens Y., Bouma A.B., Collette L., Dekker A., deSouza N.M., Dingemans A.-M., Fournier B., Hurkmans C., Lecouvet F.E., Meattini I., Méndez Romero A., Ricardi U., Russell N.S., Schanne D.H., Scorsetti M., Tombal B., Verellen D., Verfaillie C., Ost P. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 6.Salama J.K., Chmura S.J. The Role of Surgery and Ablative Radiotherapy in Oligometastatic Breast Cancer. Semin Oncol Elsevier. 2014;41:790–797. doi: 10.1053/j.seminoncol.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama H., Shirato H., Tago M., Nakagawa K., Toyoda T., Hatano K., Kenjyo M., Oya N., Hirota S., Shioura H., Kunieda E., Inomata T., Hayakawa K., Katoh N., Kobashi G. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases: A Randomized Controlled Trial. JAMA. 2006;295(21):2483. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 8.Brown P.D., Jaeckle K., Ballman K.V., Farace E., Cerhan J.H., Anderson S.K., Carrero X.W., Barker F.G., II, Deming R., Burri S.H., Ménard C., Chung C., Stieber V.W., Pollock B.E., Galanis E., Buckner J.C., Asher A.L. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316(4):401. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang E.L., Wefel J.S., Hess K.R., Allen P.K., Lang F.F., Kornguth D.G. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol Elsevier Ltd. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 10.Kocher M., Soffietti R., Abacioglu U., Villa S., Fauchon F., Baumert B.G. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M., Serizawa T., Shuto T., Akabane A., Higuchi Y., Kawagishi J., Yamanaka K., Sato Y., Jokura H., Yomo S., Nagano O., Kenai H., Moriki A., Suzuki S., Kida Y., Iwai Y., Hayashi M., Onishi H., Gondo M., Sato M., Akimitsu T., Kubo K., Kikuchi Y., Shibasaki T., Goto T., Takanashi M., Mori Y., Takakura K., Saeki N., Kunieda E., Aoyama H., Momoshima S., Tsuchiya K. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 12.Tree A.C., Khoo V.S., Eeles R.A., Ahmed M., Dearnaley D.P., Hawkins M.A., Huddart R.A., Nutting C.M., Ostler P.J., van As N.J. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14(1):e28–e37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 13.Salama J.K., Milano M.T. Radical irradiation of extracranial oligometastases. J Clin Oncol. 2014;32:2902–2912. doi: 10.1200/JCO.2014.55.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., Mulroy L., Lock M., Rodrigues G.B., Yaremko B.P., Schellenberg D., Ahmad B., Griffioen G., Senthi S., Swaminath A., Kopek N., Liu M., Moore K., Currie S., Bauman G.S., Warner A., Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. The Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 15.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. Journal of Clinical Oncology. 2020;:JCO.20.00818–10. [DOI] [PMC free article] [PubMed]
- 16.Gomez D.R., Blumenschein G.R., Jr, Lee J.J., Hernandez M., Ye R., Camidge D.R., Doebele R.C., Skoulidis F., Gaspar L.E., Gibbons D.L., Karam J.A., Kavanagh B.D., Tang C., Komaki R., Louie A.V., Palma D.A., Tsao A.S., Sepesi B., William W.N., Zhang J., Shi Q., Wang X.S., Swisher S.G., Heymach J.V. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar P., Wardak Z., Gerber D.E., Tumati V., Ahn C., Hughes R.S., Dowell J.E., Cheedella N., Nedzi L., Westover K.D., Pulipparacharuvil S., Choy H., Timmerman R.D. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMAOncol. 2018;4(1):e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez D.R., Tang C., Zhang J., Blumenschein G.R., Jr, Hernandez M., Lee J.J. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non–Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019 doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ost P., Reynders D., Decaestecker K., Fonteyne V., Lumen N., De Bruycker A. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 20.Phillips R., Shi W.Y., Deek M., Radwan N., Lim S.J., Antonarakis E.S. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer. JAMA Oncol. 2020:1–10. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent C.L., McDuff S.G.R., Salama J.K. Oligometastatic breast cancer: where are we now and where are we headed?—a narrative review. Ann Palliat Med. 2020;9:62–72. doi: 10.21037/apm-20-1128. [DOI] [PubMed] [Google Scholar]
- 22.Possanzini M., Greco C. Stereotactic radiotherapy in metastatic breast cancer. The Breast Elsevier Ltd. 2018;41:57–66. doi: 10.1016/j.breast.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Andratschke N., Alheid H., Allgäuer M., Becker G., Blanck O., Boda-Heggemann J. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer. 2018;18:1–11. doi: 10.1186/s12885-018-4191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard V., Bishop A.J., Allen P.K., Amini B., Wang X.A., Li J. Heterogeneity in Treatment Response of Spine Metastases to Spine Stereotactic Radiosurgery Within “Radiosensitive” Subtypes. Int J Radiat Oncol Biol Phys. 2017;99:1207–1215. doi: 10.1016/j.ijrobp.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Habermehl D., Herfarth K.K., Bermejo J.L., Hof H., Rieken S., Kuhn S., Welzel T., Debus J., Combs S.E. Single-dose radiosurgical treatment for hepatic metastases - therapeutic outcome of 138 treated lesions from a single institution. Radiat Oncol. 2013;8(1) doi: 10.1186/1748-717X-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milano M.T., Katz A.W., Zhang H., Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Ricco A., Davis J., Rate W., Yang J., Perry D., Pablo J. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry’s experience. Radiat Oncol. 2017;12:1–8. doi: 10.1186/s13014-017-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milano M.T., Zhang H., Metcalfe S.K., Muhs A.G., Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115(3):601–608. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 29.Milano M.T., Katz A.W., Zhang H., Huggins C.F., Aujla K.S., Okunieff P. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: Some patients survive longer than a decade. Radiother Oncol. 2019;131:45–51. doi: 10.1016/j.radonc.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Scorsetti M., Franceschini D., De Rose F., Comito T., Villa E., Iftode C., Navarria P., D'Agostino G.R., Masci G., Torrisi R., Testori A., Tinterri C., Santoro A. Stereotactic body radiation therapy: A promising chance for oligometastatic breast cancer. The Breast. 2016;26:11–17. doi: 10.1016/j.breast.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Trovo M., Furlan C., Polesel J., Fiorica F., Arcangeli S., Giaj-Levra N., Alongi F., Del Conte A., Militello L., Muraro E., Martorelli D., Spazzapan S., Berretta M. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177–180. doi: 10.1016/j.radonc.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 32.David S., Tan J., Savas P., Bressel M., Kelly D., Foroudi F., Loi S., Siva S. Stereotactic ablative body radiotherapy (SABR) for bone only oligometastatic breast cancer: A prospective clinical trial. The Breast. 2020;49:55–62. doi: 10.1016/j.breast.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wöckel A, Festl J, Stüber T, Brust K, Krockenberger M, Heuschmann PU, et al. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of Breast Cancer. Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017) - Part 2 with Recommendations for the Therapy of Primary, Recurrent and Advanced Breast Cancer. Geburtshilfe Frauenheilkd. Georg Thieme Verlag KG; 2018;78:1056–88. [DOI] [PMC free article] [PubMed]
- 34.Thill M., Jackisch C., Janni W., Müller V., Albert U.-S., Bauerfeind I., Blohmer J., Budach W., Dall P., Diel I., Fasching P.A., Fehm T., Friedrich M., Gerber B., Hanf V., Harbeck N., Huober J., Kolberg-Liedtke C., Kreipe H.-H., Krug D., Kühn T., Kümmel S., Loibl S., Lüftner D., Lux M.P., Maass N., Möbus V., Müller-Schimpfle M., Mundhenke C., Nitz U., Rhiem K., Rody A., Schmidt M., Schneeweiss A., Schütz F., Sinn H.-P., Solbach C., Solomayer E.-F., Stickeler E., Thomssen C., Untch M., Wenz F., Witzel I., Wöckel A., Ditsch N. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2019. Breast Care. 2019;14(4):247–255. doi: 10.1159/000500999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U S DEPARTMENT OF HEALTH AND HUMAN SERVICES. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 [Internet]. 2017 [cited 2020 Aug 9]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 36.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., Haes J.C.J.M.d., Kaasa S., Klee M., Osoba D., Razavi D., Rofe P.B., Schraub S., Sneeuw K., Sullivan M., Takeda F. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J Natl Cancer Institute. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 37.Sprangers M.A., Groenvold M., Arraras J.I., Franklin J., te Velde A., Muller M., Franzini L., Williams A., de Haes H.C., Hopwood P., Cull A., Aaronson N.K. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. JCO. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 38.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 39.Brédart A., Anota A., Young T., Tomaszewski K.A., Arraras J.I., Moura De Albuquerque Melo H., Schmidt H., Friend E., Bergenmar M., Costantini A., Vassiliou V., Hureaux J., Marchal F., Tomaszewska I.M., Chie W.-C., Ramage J., Beaudeau A., Conroy T., Bleiker E., Kulis D., Bonnetain F., Aaronson N.K. Phase III study of the European Organisation for Research and Treatment of Cancer satisfaction with cancer care core questionnaire (EORTC PATSAT-C33) and specific complementary outpatient module (EORTC OUT-PATSAT7) Eur J Cancer Care. 2018;27(1):e12786. doi: 10.1111/ecc.12786. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Giesinger J.M., Kieffer J.M., Fayers P.M., Groenvold M., Petersen M.A., Scott N.W., Sprangers M.A.G., Velikova G., Aaronson N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88. doi: 10.1016/j.jclinepi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Chang D.T., Pollom E.L., Keane F.K., Wo J.Y. Treating Oligometastatic Disease With SABR: More Than Just a Numbers Game? Int J Radiat Oncol Biol Phys. 2020;107:257–260. doi: 10.1016/j.ijrobp.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Pembroke C.A., Fortin B., Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol. 2018;127(3):493–500. doi: 10.1016/j.radonc.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Patel P.H., Palma D., McDonald F., Tree A.C. The Dandelion Dilemma Revisited for Oligoprogression: Treat the Whole Lawn or Weed Selectively? Clin Oncol Elsevier Ltd. 2019;31:824–833. doi: 10.1016/j.clon.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.