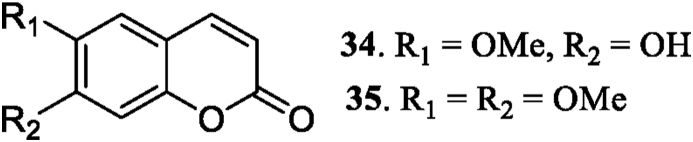Abstract
Plants are the key source for the production of novel therapeutic products for new medicines. The biological properties of the plant species used world wide are mainly accountable for their secondary metabolites obtained from plants. The goal of this analysis is to summarize the chemical composition and biological effects of the genus Sida (Malvaceae) to identify potential research opportunities. This analysis draws on the literature review of scientific journals, and books from libraries, and electronic sources like ScienceDirect, Springer, PubMed, ResearchGate, Google Scholar, and the Website. Some groups of secondary metabolite compounds isolated from the genus Sida include alkaloids, flavonoids, coumarin, and others. Pharmacological experiments found that there are a wide variety of biological activities in extracts and compounds isolated from the genus Sida comprising antimalarial, antiplasmodial, antimicrobial, analgesic, antibacterial, antioxidant, vasorelaxant, wound healing, antifungal activities, the inhibition of quinone reductase, and mouse mammary organ culture.
Keywords: Sida, Alkaloids, Flavonoids, Coumarin, Anticancer, Antidiabetic
Sida, Alkaloids, Flavonoids, Coumarin, Anticancer, Antidiabetic
1. Introduction
Metabolites are the transitional products of metabolism and restricted to small molecules. Plant produces a lot of chemicals that can be categorized into primary metabolites and secondary metabolites. Primary metabolites are necessary for cell function and they are omnipresent. Secondary metabolites are useful for human because of their diverse applications (Dufour and Rao, 2011; Olivoto et al., 2017; Yang et al., 2018).
The genus sida has relatively 200 species scattered in every part of tropical and subtropical regions in the world (Mabberley, 1997). The genus sida is originated in Brazil (Bovini, 2015). This plant can be used as traditional medicine, but no reviews have been made on the phytochemical, toxicological and pharmacological properties of the genus Sida. The main focus of this review is phytochemistry, pharmacological properties, botanical aspects of the herbs, their experimental application and translational investigation of genus sida (see Table 1).
Table 1.
Isolated compounds from Genus Sida and their biological activities.
| Compound name | Species | Biological activities | References |
|---|---|---|---|
| Ψ-(peudo)-ephedrine (1) | S. cordifolia | Ghosh and Dutta (1930) | |
| β- phenethylamine (2) | S. cordifolia | Ghosal et al. (1975) | |
| ephedrine (3) | S. cordifolia | Ghosal et al. (1975) | |
| Ψ-ephedrine (4), | S. cordifolia | Ghosal et al. (1975) | |
| S-(+) Nb-methyltrypto-phanmethyl ester (5) | S. cordifolia | Ghosal et al. (1975) | |
| hypaphorine (6) | S. cordifolia | Ghosal et al. (1975) | |
| vasicinone (7) | S. cordifolia | Ghosal et al. (1975) | |
| vasicine(8) | S. cordifolia | Ghosal et al. (1975) | |
| vasicinol (9) | S. cordifolia | Ghosal et al. (1975) | |
| quindolinone (10) | S. acuta | Quinone reductase | Jang et al. (2003) |
| cryptolepinone (11) | S. acuta, S. rhombifolia | Vasorelaxant, Mouse mammary organ culture, Quinone reductase | Jang et al. (2003), Chaves et al. (2013) |
| 11-methoxyquindoline (12) | S. acuta | Quinone reductase | Jang et al. (2003) |
| N-trans-feruloyltyramine (13) | S. acuta | Mouse mammary organ culture | Jang et al. (2003) |
| 5′-hydroxymethyl-1′-(1,2,3,9-tetrahydro-pyrrolo[2,1-b] quinazolin-1-yl)-heptan-1-one (14) | S. cordifolia | Sutradhar et al. (2006) | |
| 1,2,3,9-tetrahydro-pyrrolo [2,1-b] quinazolin-3-ylamine (15) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| 2-(1′-amino-butyl) indol-3-one (16) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| 2′-(3H-indol-3-ylmethyl)-butan-1′-ol (17) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| a salt of cryptolepine (18) | S. cordifolia | Chaves et al. (2013) | |
| 11-methoxy-quindoline (19) | S. cordifolia | Chaves et al. (2013) | |
| quindoline (20) | S. cordifolia | Chaves et al. (2013) | |
| 1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-amine (21) | Sida glutinosa | Das et al. (2011) | |
| 3,4,5,6-tetrahydro-3-methyl-β-carboline-5carboxylic acid (22) | Sida szechuensis | Yao and Xu (2000) | |
| cryptolepine (23) | S. acuta | Antiplasmodial | Banzouzi et al. (2004) |
| 3'-(3″,7″-dimethyl-2″,6″-octadiene)-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucoside (24) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| 3'-(3″,7″-dimethyl-2″,6″-octadiene)-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucosyl [1→4]-β-D-glucoside (25) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| 6-(3″-methyl2″-butene)-3′-methoxyl-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucosyl [1→4]-β-D-glucoside 3, 3′-(3″, 7″-Dimethyl 2″,6″ otadiene)-8-C β-D-glucosylkeampferol 3-O-β-D-glucoside (26) | S. cordifolia | Sutradhar et al., 2007a, Sutradhar et al., 2007b | |
| 5,7-dihydroxy-3-isoprenylflavone (27) | S. cordifolia | Sutradhar et at., (2008) | |
| 5-hydroxy-3-isoprenyl Flavone (28) | S. cordifolia | Sutradhar et at., (2008) | |
| 5,7-dihydroxy-4′-methoxyflavone (29) | S. rhombifolia | Chaves et al. (2013) | |
| kaempferol (30) | S. rhombifolia | Chaves et al. (2017) | |
| kaempferol-3-O-β-D-glycosyl-6″-α-D-rhamnose (31) | S. rhombifolia | Chaves et al. (2017) | |
| Glutinoside (32) | S. glutinosa | Das et al. (2012) | |
| Chrysin (33) | S. glutinosa | Das et al. (2012) | |
| scopoletin (34) | S. acuta, S. rhombifolia | Jang et al. (2003) | |
| scoporone (35) | S. rhombifolia | Jang et al. (2003) | |
| 20-hydroxy,24-hydroxymethyl ecdysone (36) | S. spinosa | Darwish and Reinecke (2003); Jadhav et al. (2007) | |
| 20-hydroxyecdysone (37) | S. spinosa | Darwish and Reinecke (2003); Jadhav et al. (2007) | |
| turkesterone (38) | S. spinosa | Darwish and Reinecke (2003) | |
| makisterone C (39) | S. spinosa | Darwish and Reinecke (2003) | |
| 20-Hydroxyecdysone-20,22-monoacetonide (40) | S. spinosa | Darwish and Reinecke (2003) | |
| 24(28)-Dehydromakisterone A (41) | S. glutinosa | Das et al. (2012) | |
| 2β,3β,14α,20,21,22R,24-heptahydroxycholest-7-en-6-one (42) | S. szechuensis | Yao and Xu (2000) | |
| polypodine B (43) | S. szechuensis | Yao and Xu (2000) | |
| α-ecdysone (44) | S. szechuensis | Yao and Xu (2000) | |
| 25-acetoxy-20-hydroxyecdysone-3-O-β-D-glucopyranoside (45) | S. rhombifolia | Jadhav et al. (2007) | |
| Pterosterone-3-O-β-D-glucopyranoside ecdysteroid (46) | S. rhombifolia | Jadhav et al. (2007) | |
| ecdysone-3-O-β-D-glucopyranoside (47) | S. rhombifolia | Jadhav et al. (2007) | |
| 2-deoxy-20-hydroxyecdysone-3-O-β-D-glucopyranoside (48) | S. rhombifolia | Jadhav et al. (2007) | |
| 20-hydroxyecdysone-3-O-β-D-glucopyranoside (49) | S. rhombifolia | Jadhav et al. (2007) | |
| taraxast-1,20(30)-dien-3-one (50) | S. acuta | Antioxidant | Chen et al. (2007) |
| taraxasterone (51) | S. acuta | Antioxidant | Chen et al. (2007) |
| 7a-methoxy-α-tocopherol (52) | S. acuta | Antioxidant | Chen et al. (2007) |
| β-tocopherol (53) | S. acuta | Chen et al. (2007) | |
| α-tocopherol (54) | S. acuta | Chen et al. (2007) | |
| α-tocospiro B (55) | S. acuta | Chen et al. (2007) | |
| vomifoliol (56) | S. acuta | Jang et al. (2003) | |
| Ioliolide (57) | S. acuta | Jang et al. (2003) | |
| 4-ketopinoresinol (58) | S. acuta | Jang et al. (2003) | |
| evofolin-A (59) | S. acuta | Jang et al. (2003) | |
| evofolin-B (60) | S. acuta | Jang et al. (2003) | |
| glyceryl-1-eicosanoate (61) | S. spinosa | Darwish and Reinecke (2003) | |
| p-hydroxyphenethyl trans-ferulate (62) | S. spinosa | Darwish and Reinecke (2003) | |
| triacontane (63) | S. spinosa | Darwish and Reinecke (2003) | |
| 1-eicosene (64) | S. spinosa | Darwish and Reinecke (2003) | |
| 9-hydroxy-cis-11-octadecenoic acid (65) | S. spinosa | Darwish and Reinecke (2003) | |
| 1-O-β-D-Glucopyranosyl-(2S,3S,4R,8Z)-2-[(2′R)-2′-hydroxypalmito-ylamino]-8-octadecene-1,3,4′-triol (66) | S. spinosa | Darwish and Reinecke (2003) | |
| phaeophytin A (67) | S. rhombifolia | Chaves et al. (2013) | |
| 173-ethoxypheophorbide A (68) | S. rhombifolia | Chaves et al. (2013) | |
| 132-hydroxy phaeophytin B (69) | S. rhombifolia | Chaves et al. (2013) | |
| 173-ethoxypheophorbide B (70) | S. rhombifolia | Chaves et al. (2013) |
2. Botanical description
2.1. Sida cordifolia
Sida cordifolia is an erect perennial growing up to 50–200 cm high. Leaves are oblong, hair-coated and 3.5 cm long. The trunks are green-yellow, thick, long and short. The cycle of flora and fruit is from October to December (Shetu et al., 2019). Sida cordifolia plants according to Khurana et al. (2016) have the following categorization:
| Kingdom | Plantae |
| Subkingdom | Tracheobionta |
| Super division | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Dilleniidae |
| Superorder | Malvanae |
| Order | Malvales |
| Family | Malvaceae |
| Subfamily | Malvoideae |
| Tribe | Malveae |
| Genus | Sida |
| Species | S. cordifolia |
| Scientific name | S. cordifolia L. |
2.2. Sida acuta
There was no literature for plant description of Sida acuta. Sida acuta plants according to Mohideen et al. (2002) have the following categorization:
| Kingdom | Plantae |
| Class | Dicotyledoneae |
| Order | Malvales |
| Family | Malvaceae |
| Genus | Sida |
| Specie | S. acuta |
| Scientific name | Sida acuta Burman f. |
2.3. Sida rhombifolia
Sida rhombifolia is a menber of the genus Sida which belongs to the Malvaceae family. Local names are called guri, sidaguri, saliguri (Sumatra), sadagori, sidaguri, otok-otok, taghuri, sidagori (Java), kahindu, mistaken (Nusa Tenggara) and hutugamo, bitumu, digo, sosapu (Maluku) (Dalimartha, 2003). Sidaguri plants (Sida rhombifolia L) according to Sivarajan and Pradeep (1994), have the following classification:
| Divisio | Spermatophyta |
| Sub Divisio | Angiospermae |
| Classis | Dicotyledoneae |
| Sub classis | Dialypetalae |
| Ordo | Malvales/Columniferae |
| Familia | Malvaceae |
| Genus | Sida |
| Species | S. rhombifolia |
| Scientific name | Sida rhombifolia L. |
2.4. Sida spinosa
Sida spinosais recognized as Kantakinibala (prickly fanpetals) is an erect perennial shrub. This plant is primarily found in the warmer parts of India at the height of 4400 ft. The plant is stellate, with filiform leaves 30 cm–1 m high, stipules 2–5 mm long, petiole length 2–20 mm, 1–3 spiny tubercles present on the stem at the base of petiole, lanceolate to ovate, oblong or rather orbicular, round at the base, acute or obtuse at the apex, and serrate at the apex. Moreover, flowers in terminal branches, axillary, solitary or 2–5 in fascicles, 2–5 mm pedicel, 0.2 cm long fruits, attached close to the center or tip. Calyx is 4–5 mm long and the fruits are compressed spherical with pubescent above. Furthermore, the length of the reddish brown glabrous seeds are 1.5 mm (Lin et al., 2010). Sida spinosa plants according to Singh and Navneet (2018) have the following classification:
| Kingdom | Plantae |
| Division | Tracheophyta |
| Class | Mangnoliopsida |
| Order | Malvales |
| Genus | Sida |
| Species | S. spinosa |
| Scientific name | Sida spinosa |
2.5. Sida tuberculata
In an area of South America, Sida tuberculata is mostly grown and is a well-known drink such as infusion or tea. Sida tuberculata is an herbaceous or subshrub plant that grows between 40 and 80 cm in length and expands by kernels. leaf blades elliptic or subrhombic to narrowly linear, upper surface with stellate trichomes; flowers and fruits crowded in subsessile axillary glomerules or, if solitary, the pedicels up to 15 mm long; calyx lobes with stellate trichomes and often with sparse simple trichomes in addition; and mericarps 6–8, muticous to submuticous, indehiscent (Rosa et al., 2015). Sida tuberculata plants according to https://www.earth.com/earthpedia/plant/no/sida-tuberculata/have the following classification:
| Kingdom | Plantae |
| Class | Mangnoliopsida |
| Order | Malvales |
| Family | Malvaceae |
| Genus | Sida |
| Species | S. tuberculata |
| Scientific name | Sida tuberculata |
2.6. Sida cordata
Sida cordata (Burm.f.), a medicinal plant, has been used for several illnesses. This plant belongs to the family Malvaceae which commonly grow in India. The plant is 30–80 cm tall. Soft hairy grows in its stems, petioles and pedicels pubescent. The heart-shaped leaves are 1–5.5 cm long and one at every node. The yellow flowers are produced on the axils of the leaf. Furthermore, flowers are axillary, solitary, 8–10 mm in diameter, Calyx 5 × 6 mm across, campanulate, 5-fid, simple and some stellate-hairy outside, glabrous within and around the margin, while the length of petioles is 1.5–30 mm. Moreover, the width of Corolla orange-yellow is 10 mm, the size of petals is 6 × 5 mm, obovate, base ciliate. There are 4 × 3 mm schizocarps, globose, enclosed in persistent calyx; mericarps 5, awnless. The colour of the seeds is brownish black. The plant is blooming and fruiting throughout the year, but it is mainly produced from September through November. The plant is also best to live in unused lands, damp and cool which needs up to one thousand and five hundred meter of area (Ugborogho, 1980). Sida cordata plants according to https://www.gbif.org/species/5406739 have the following classification:
| Kingdom | Plantae |
| Phylum | Tracheophyta |
| Class | Mangnoliopsida |
| Order | Malvales |
| Family | Malvaceae |
| Genus | Sida |
| Species | S. cordata |
| Scientific name | Sida cordata (Burm. f) |
2.7. Sida glutinosa
Sida glutinosa is different from other species. It has a viscid dense stem with glandular and cutaneous hair, sometimes with star hair; leaves 2–4 × 1–3 cm, symmetrical, concolor, heart-shaped oval; solitary interest in axils or in diffuse terminal panicles; calyx 4.5–5.0 mm, unaccented, densely covered by viscid glandular hair and mixed with simple hair and stellate, obtrullate sepals; 1–2 cm in diameter; symmetrical petals, retuse, yellow with or without a red center, stamens 12–20, free filaments leading to the apex; and merger 5, apical 2-spined, dense thorn covered with antrorse hair (Baracho and Agra, 2016; Abat et al., 2017). This species can be found in tropical America, from northwestern Mexico and the Caribbean to Argentina and Brazil (Krapovickas, 2006). Sida glutinosa plants according to http://plantamor.com/species/info/sida/glutinosa have the following classification:
| Kingdom | Plantae |
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Dilleniidae |
| Order | Malvales |
| Family | Malvaceae |
| Genus | Sida |
| Species | S. glutinosa |
| Scientific name | Sida glutinosa Comm. ex Cav. |
2.8. Sida szechuensis
Sida szechuensis is a Vietnamese plant. Its main uses are as an herb, the petiole and pedicle have slightly thick hair, penninerved leaves, axillary, solitary or in panicles; calyx campanulate, with 5 triangular lobes; pale yellow or orange crown, with pointed petals, mostly more or less obliquely; staminal column ca. 5 mm long with long hair; ovaries with hair like a star; style more than 7; the outside of the protruding proticulately veined, short-tent; reniform seeds, dark brown or black, with white hair (Xuyen, 2006). Sida szechuensis plants according to https://www.gbif.org/species/119199119 have the following classification:
| Kingdom | Plantae |
| Family | Malvsceae |
| Genus | Sida |
| Species | S. szechuensis |
| Scientific name | S. szechuensis Matsuda |
2.9. Methods
The documentation for this review was compiled from various data sources likes ScienceDirect, Springer, PubMed, ResearchGate, Google Scholar, and the Website.
3. Chemical constituents
3.1. Alkaloid
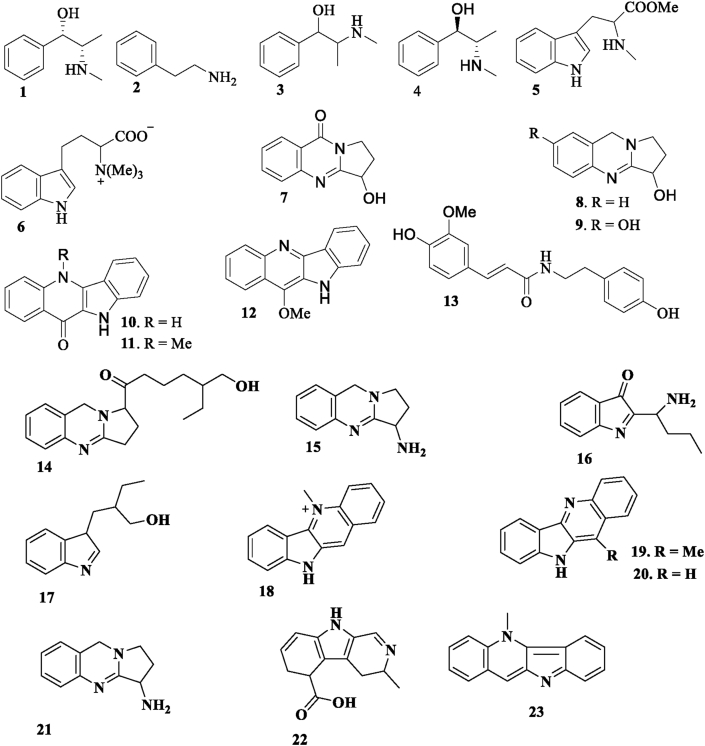
Ghosh and Dutt (1930) isolated Ψ-(peudo)-ephedrine (1) from the S. cordifolia. From the roots of S. cordifolia, Ghosal et al. (1975), reported β-phenethylamine (2), ephedrine (3), Ψ-ephedrine (4), S-(+) Nb-methyltryptophanmethyl ester (5), hypaphorine (6), vasicinone (7), vasicine (8), and vasicinol (9) Jang et al. (2003), isolated quindolinone (10), cryptolepinone (11), 11-methoxyquindoline (12), N-trans-feruloyltyramine (13) from the whole plants of S. acuta. From the aerial parts of S. cordifolia, Sutradhar et al. (2006), isolated a new alkaloid, 5′-hydroxymethyl-1′-(1,2,3,9-tetrahydro-pyrrolo [2,1-b] quinazolin-1-yl)-heptan-1-one (14). Sutradhar et al., 2007a, Sutradhar et al., 2007b, studied the aerial parts of S. cordifolia revealed the presence of four new alkaloids., 1,2,3,9-tetrahydro-pyrrolo [2,1-b] quinazolin-3-ylamine (15), 5′-hydroxymethyl-1′-(1,2,3,9-tetrahydropyrrolo [2, 1-b] quinazolin-1-yl)-heptan-1-one (14), 2-(1′-amino-butyl) indol-3-one (16), and 2′-(3H-indol-3-ylmethyl)-butan-1′-ol (17). Chaves et al. (2013), isolated cryptolepinone (11) and a salt of cryptolepine (18). From the aerial parts of S. rhombifolia. Chaves et al. (2017), reported quindolinone (10), the cryptolepine salt (18), 11-methoxy-quindoline (19), and quindoline (20) from the aerial parts of S. rhombifolia. Das et al. (2011) isolated 1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-amine (21) from the aerial parts of Sida glutinosa. Yao and Xu (2000) reported 3,4,5,6-tetrahydro-3-methyl-β-carboline-5carboxylic acid was isolated from Sida szechuensis. Banzouzi et al. (2004), isolated cryptolepine (23) from the ethanolic extract of S. acuta. Alkaloid group compounds in the genus Sida are presented in Figure 1.
Figure 1.
Alkaloids 1–23 isolated from genus Sida.
3.2. Flavonoid
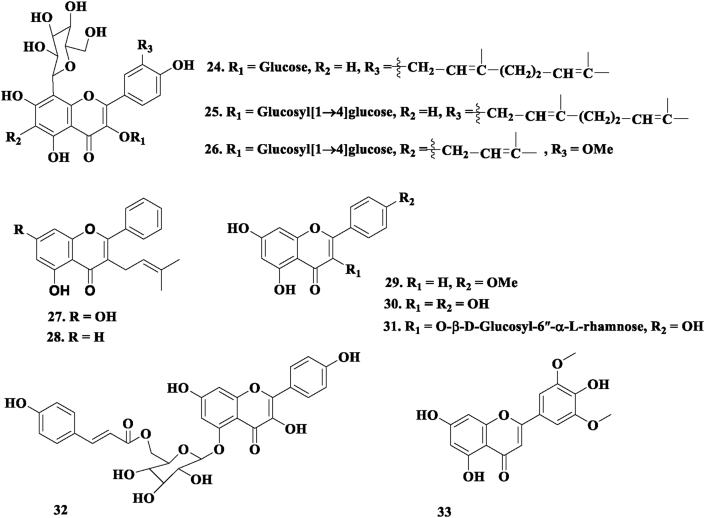
Sutradhar et al., 2007a, Sutradhar et al., 2007b, isolated three new flavonol C-glycosides: 3'-(3″,7″-dimethyl-2″,6″-octadiene)-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucoside (24), 3'-(3″,7″-dimethyl-2″,6″-octadiene)-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucosyl [1→4]-β-D-glucoside (25), and 6-(3″-methyl2″-butene)-3′-methoxyl-8-C-β-D-glucosyl-kaempferol 3-O-β-D-glucosyl [1→4]-β-D-glucoside 3, 3′-(3″, 7″-Dimethyl 2″,6″ otadiene)-8-C β-D-glucosylkeampferol 3-O-β-D-glucoside (26) from the aerial parts of S. cordifolia. Sutradhar et at., (2008), isolated two new flavones, 5,7-dihydroxy-3-isoprenylflavone (27) and 5-hydroxy-3-isoprenyl Flavone (28) from the chloroform extract of S. cordifolia. In 2013, Chaves et al. isolated 5,7-dihydroxy-4′-methoxyflavone (29) from the aerial parts of S. rhombifolia. Chaves et al. (2017), also isolated kaempferol (30) and kaempferol-3-O-β-D-glycosyl-6″-α-D-rhamnose (31) from the aerial parts of S. rhombifolia. Das et al. (2012) isolated Glutinoside (32) and Chrysin (33) from the aerial parts of Sida glutinosa. Figure 2 presents the reported flavonoid compounds isolated from the genus Sida.
Figure 2.
Flavonoids 24–33 isolated from genus Sida.
3.3. Coumarins
Jang et al. (2003), isolated scopoletin (34) from the whole plants of S. acuta. Chaves et al. (2017), reported scopoletin (34) and scoporone (35) from the aerial parts of S. rhombifolia. The two structures of coumarin compound are shown in Figure 3.
Figure 3.
Coumarins 34–35 isolated from genus Sida.
3.4. Ecdysteroids
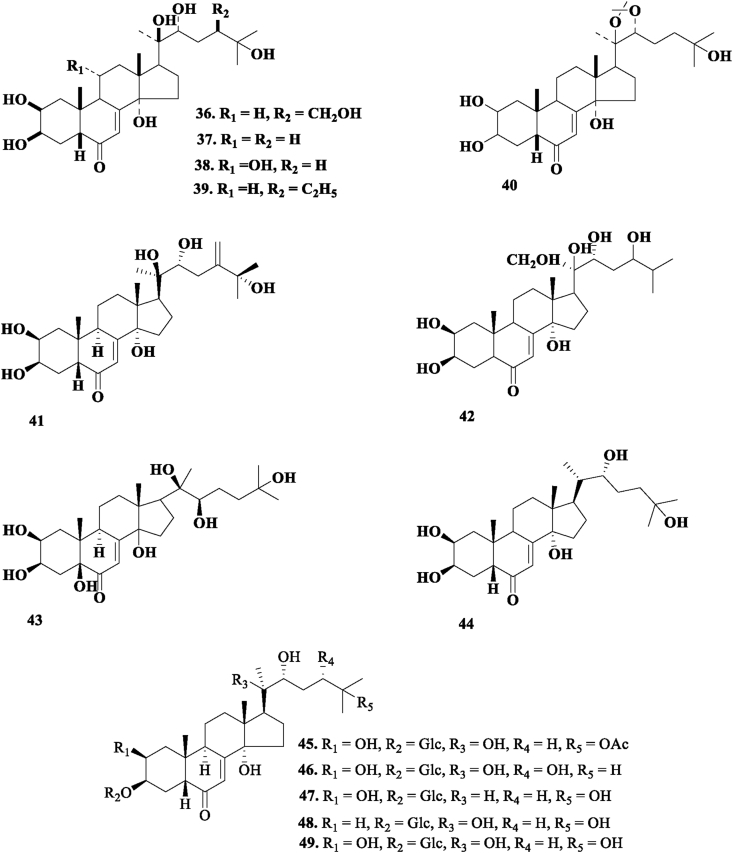
From the aerial parts of S. spinosa, Darwish and Reinecke (2003), reported five ecdysteroids, 20-hydroxy,24-hydroxymethyl ecdysone (36), 20-hydroxyecdysone (37), turkesterone (38), makisterone C (39), and 20-Hydroxyecdysone-20,22-monoacetonide (40). Das et al. (2012) reported 24(28)-Dehydromakisterone A (41) found in dried aerial parts of Sida glutinosa. Yao and Xu (2000) isolated 2β,3β,14α,20,21,22R,24-heptahydroxycholest-7-en-6-one (42), polypodine B (43), and α-ecdysone (44) from Sida szechuensis. Jadhav et al. (2007) isolated ecdysone (36), 20-hydroxyecdysone (37), 25-acetoxy-20-hydroxyecdysone-3-O-β-D-glucopyranoside (45), Pterosterone-3-O-β-D-glucopyranoside ecdysteroid (46), ecdysone-3-O-β-D-glucopyranoside (47), 2-deoxy-20-hydroxyecdysone-3-O-β-D-glucopyranoside (48), and 20-hydroxyecdysone-3-O-β-D-glucopyranoside from the whole plant of S. rhombifolia. Figure 4 shows the molecular structure of the ecdysteroid group of compound of the genus Sida.
Figure 4.
Ecdysteroids 36–49 isolated from genus Sida.
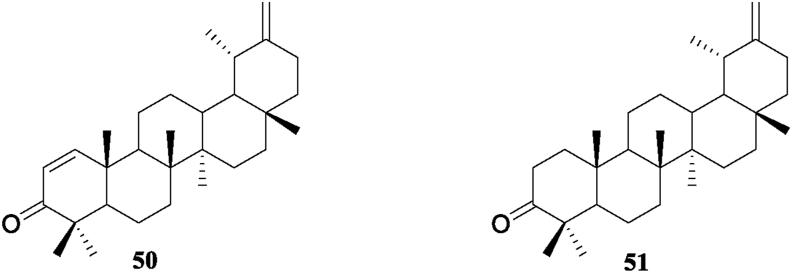
3.5. Triterpenes
Chen et al. (2007), reported two triterpenes, including a new taraxast-1,20(30)-dien-3-one (50) and taraxasterone (51) from the whole plant of S. acuta. The two triterpenes structure can be seen in Figure 5.
Figure 5.
Triterpenes 50–51 isolated from genus Sida.
3.6. Tocopherols
Chen et al. (2007), reported a new tocopherol derivative, 7a-methoxy-α-tocopherol (52), along with β-tocopherol (53), α-tocopherol (54), and α-tocospiro B (55) from the whole plant of S. acuta. The structure of the tocopherol's derivatives is shown in Figure 6.
Figure 6.
Tocopherols 52–55 isolated from genus Sida.
3.7. Other compounds
Jang et al. (2003), isolated vomifoliol (56), Ioliolide (57), 4-ketopinoresinol (58), evofolin-A (59), and evofolin-B (60) from the whole plants of S. acuta. Darwish and Reinecke (2003) reported two new compounds, namely glyceryl-1-eicosanoate (61) and p-hydroxyphenethyl trans-ferulate (62), together with four known compounds, triacontane (63), 1-eicosene (64), 9-hydroxy-cis-11-octadecenoic acid (65), and 1-O-β-D-Glucopyranosyl-(2S,3S,4R,8Z)-2-[(2′R)-2′-hydroxypalmito-ylamino]-8-octadecene-1,3,4′-triol (66), from the aerial parts of S. spinosa,. Chaves et al. (2013), isolated phaeophytin A (67), 173-ethoxypheophorbide A (68), 132-hydroxy phaeophytin B (69), 173-ethoxypheophorbide B (70), from the aerial parts of S. rhombifolia. The structure of compounds's 56–70 is presented in Figure 7.
Figure 7.
Other compounds 56–70 isolated from genus Sida
4. Biological activities
4.1. Antimalarial activity
Karou et al. (2003), reported that various fractions of S. acuta showed the antimalarial activity with the IC50 values in the range of 0.05–57.04 μg/mL.
4.2. Antiplasmodial activity
The EtOH roots extract of S. acuta displayed the antiplasmodial activity with the IC50 values in the range of 3.9–5.4 μg/mL. In addition, compound 23 from S. acuta also showed potent antiplasmodial activity (Banzouzi et al., 2004).
4.3. Antimicrobial activity
Momin et al. (2014), evaluated the antimicrobial activity of S. cordifolia EtOH extract using five pathogenic bacteria with standard antibiotic kanamycin. The extract of S. cordifolia has no antimicrobial activity against five selected bacteria.
4.4. Analgesic activity
Momin et al. (2014), evaluated the analgesic activity S. cordifolia EtOH extract using acetic acid-induced writhing reflex model in mice with standard drug diclofenac. The extract displayed a significant (P > 0.001) decrease in the writhing reflex of mice induce acetic acid at a dose of 500 mg/kg. Moreover, the aqueous acetone extract of S. acuta and S. cordifolia was reported to inhibit dose-dependent analgesic activity (Konaté et al., 2012).
4.5. Anti-inflammatory activity
Moreover, aqueous leaves extract of S. cordifolia was found to inhibit the carrageenin-induced rat paw edema at a dose of 400 mg/kg administrated orally (Franzotti et al., 2000).
4.6. Antibacterial activity
Islam et al. (2003) studied the antibacterial activity of various extracts of S. rhombilolia with Gram-positive and Gram-negative test organisms. The results revealed that all extracts exhibited weak activity. Karou et al. (2005), conducted the antibacterial activity of S. acuta alkaloids fraction using agar-well diffusion assay and broth microdilution assay. The highest zone inhibition diameters were identified by Gram-positive bacteria in accordance with agar-well diffusion assay. From the broth microdilution assay, the MIC and MBC values observed in the range of 16–400 μg/mL and 80–400 μg/mL. In 2007, Oboh et al. evaluated the antibacterial activity of the 90% EtOH extract of S. acuta aerial parts with agar-dilution method. The results of the MIC were found values in the range of 5–10 mg/mL. In other studies, Assam et al. (2010) evaluated antibacterial activity of the aqueous-methanol extract of S. rhombifolia (1:4, v/v) using agar disc diffusion and agar dilution methods. The results found that aqueous-methanol extract of S. rhombifolia displayed moderate activity. Moreover, Halilu et al. (2016) evaluated antibacterial activity of S. cordifolia ethanolic leaves extract using cup plate method against Gram-positive and Gram-negative microorganisms. The results showed that the extract has potent activity on all tested organisms.
4.7. Antioxidant activity
In 2003, Auddy et al. (2003) assessed the antioxidant activity of EtOH extract and water infusions of S. cordifolia with ABTS radical cation decolorization assay and lipid peroxidation. According to the ABTS assay, the EtOH extract displayed potent activity (IC50 16.07 μg/mL). Chen et al. (2007), studied the antioxidant activity of compounds 52–55 with DPPH assay, and the result showed that compound 52, 53, and 54 displayed significant activities with the EC50 values of 86.9, 68.2, and 70.9 μM. Momin et al. (2014), evaluated the antioxidant activity of EtOH extract of S. cordifolia using DPPH assay with standard ascorbic acid. According to the results, the IC50 values of the extract and standard ascorbic acid was 50 and 1.16 μg/mL. In addition, Kumar et al. (2019), assessed the antioxidant activity of various extracts of S. cordifolia using DPPH assay with standard ascorbic acid. The results found that various extracts and standard ascorbic acid displayed concentration-dependent percent inhibition of DPPH radical.
4.8. Vasorelaxant activity
In another study, the vasorelaxant activity was detected in the rodent isolated mesenteric arteries by the compound 11 (Chaves et al., 2013).
4.9. Wound healing activity
Pawar et al. (2013), informed that the EtOH extract of S. cordifolia displayed wound healing activity in rats. Moreover, Francis et al. (2018), also informed that EtOH leaves extract of S. rhombifolia has a prospective advantage in enhancing wound healing. In another study, Kumar et al. (2019), studied wound healing activity of various extract of S. cordifolia against dexamethasone-induced retardation in rats. According to the results, aqueous extract of S. cordifolia displayed significant activity.
4.10. Antifungal activity
Rosa et al. (2015), evaluated the antifungal activity of aqueous infusion from S. tuberculata leaves and roots, using the broth micro-dilution method against 37 clinical isolates of opportunistic yeasts. The MIC (minimal inhibitory concentration) and MFC (minimal fungicidal concentration) were identified toward Candida krusei isolates.
4.11. Antidiabetic activity
Ahmad et al. (2014) documented that the alcoholic extract of S. cordifolia displayed antihyperglycemic action when administered to streptozotocin-induced diabetic rats.
4.12. Toxicity
Islam et al. (2003) evaluated the toxicity of various extracts of S. rhombilolia using brine shrimp bioassay with standard reference gallic acid. According to the results, EtOAc extract of S. rhombilolia exhibited significant cytotoxic activity with LC50 value of 5.41 ppm. In addition, the aqueous extract of S. cordifolia has low acute toxicity in mice (Franzotti et al., 2000).
4.13. Antiarthritic activity
Gupta et al. (2009) assessed the antiarthritic activity of various extracts of S. rhombifolia. According to the results, EtOH and aqueous extracts displayed significant activity.
4.14. Quinone reductase induction assay
Jang et al. (2003), evaluated quinine reductase induction activity of compounds (10–14, 34, and 56–60) with culture mouse Hepa 1c1c7 cell. The results showed that compounds 10–11 have the most potent activity.
4.15. Mouse mammary organ culture assay
Jang et al. (2003), studied the prospective of compounds (10–14, 34, and 56–60) to inhibit the 7,12-dimethylbenzene (DMBA)- induced preneoplastic lesion in a mouse mammary organ culture. According to the results, compounds 11 (83.3%) and 13 (75.0%) displayed the activity to inhibit 7,12-dimethylbenzene (DMBA)- induced preneoplastic lesion at a dose of 10 μg/mL.
5. Conclusion
Eight species of the genus Sida have been explained and discussed in this review article. The eight species produce a variety of secondary metabolite compounds, namely alkaloids, flavonoids, coumarines, ecdysteroids, triterpenes, tocopherols, and other compounds. The compounds depicted a variety of interesting biological activities. So they can be used as a reference for the researchers to develop further research or exploration.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The work is supported by Universitas Airlangga (UNAIR), IPB, UGM, and ITB through “RISET KOLABORASI INDONESIA-WCU (WORLD CLASS UNIVERSITY)” (252/UN3.14/PT/2020).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abat J.K., Kumar S., Mohanty A. Ethnomedicinal, phytochemical and ethnopharmacological aspects of four medicinal plants of malvaceae used in Indian traditional medicines: a review. Medicines. 2017;4(4):75. doi: 10.3390/medicines4040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Prawez S., Sultana M., Raina R., Pankaj N.K., Verma P.K., Rahman S. Anti-hyperglycemic, anti-hyperlipidemic and antioxidant potential of alcoholic-extract of Sida cordifolia (areal part) in streptozotocin-induced-diabetes in wistar-rats. Proc. Natl. Acad. Sci. India B Biol. Sci. 2014;84:397–405. [Google Scholar]
- Assam A.J., Dzoyem J.P., Pieme C.A., Penlap V.B. In vitro antibacterial activity and acute toxicity studies of aqueous-methanol extract of Sidarhombifolia Linn. (Malvaceae) BMC Compl. Alternative Med. 2010;10:40. doi: 10.1186/1472-6882-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auddy B., Ferreira M., Blasina F., Lafon L., Arredondo F., Dajas F., Tripathi P.C., Seal T., Mukherjee B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003;84(2-3):131–138. doi: 10.1016/s0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Banzouzi J.-T., Prado R., Menan H., Valentin A., Roumestan C., Mallié M., Pelissier Y., Blache Y. Studies on medicinal plants of Ivory Coast: investigation of Sida acuta for in vitro antiplasmodial activities and identification of an active constituent. Phytomedicine. 2004;11(4):338–341. doi: 10.1078/0944711041495245. [DOI] [PubMed] [Google Scholar]
- Baracho G.S., Agra M.D.F. A new record of Sida glutinosa (Malvaceae), a rare species of the Caatinga in northeastern Brazil, with lectotypifications and taxonomic notes on the allied Sida glabra. Phytotaxa. 2016;282(1):37–45. [Google Scholar]
- Bovini M.G. Jardim Botânico do Rio de Janeiro; Rio de Janeiro, Brazil: 2015. Sida in Lista de Espécies da Flora do Brasil. [Google Scholar]
- Chaves O.S., Gomes R.A., Tomaz A.C.A., Fernandes M.G., Mendes Junior L.D.G., Agra M.F., Braga V.A., Souza M.F.V. Secondary metabolites from Sida rhombifolia L. (Malvaceae) and the vasorelaxant activity of cryptolepinone. Molecules. 2013;18:2769–2777. doi: 10.3390/molecules18032769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves O.S., Teles Y.C.F., Monteiro M.M.D.O., Junior L.D.G.M., Agra M.D.F., Braga V.D.A., Silva T.M.S., Souza M.D.F.V.D.S. Alkaloids and phenolic compounds from Sida rhombifolia L. (Malvaceae) and vasorelaxant activity of two indoquinoline alkaloids. Molecules. 2017;22(1):94. doi: 10.3390/molecules22010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.R., Chao L.H., Pan M.H., Liao Y.W., Chang C.I. Tocopherols and triterpenoids from Sida acuta. J. Chin. Chem. Soc. 2007;54:41–45. [Google Scholar]
- Dalimartha S. EdisiKetiga; PuspaSwara, Jakarta: 2003. Atlas Tumbuhan Obat Indonesia. [Google Scholar]
- Darwish F.M., Reinecke M.G. Ecdysteroids and other constituents from Sida spinosa L. Phytochemistry. 2003;62(8):1179–1184. doi: 10.1016/s0031-9422(03)00021-9. [DOI] [PubMed] [Google Scholar]
- Das N., Achari B., Harigaya Y., Dinda B. A new flavonol glucoside from the aerial parts of Sida glutinosa. J. Asian Nat. Prod. Res. 2011;13:965–971. doi: 10.1080/10286020.2011.602343. [DOI] [PubMed] [Google Scholar]
- Das N., Nath J., Dinda B. Antioxidant phytochemicals from Sida glutinosa. J. Pharm. Res. 2012;5:4845–4848. [Google Scholar]
- Dufour N., Rao R.P. Secondary metabolites and other small molecules as intercellular pathogenic signals. FEMS Microbiol. Lett. 2011;314(1):10–17. doi: 10.1111/j.1574-6968.2010.02154.x. [DOI] [PubMed] [Google Scholar]
- Francis P., Masimba P., Awakigonja A.R. Evaluation of the wound healing activity of formulated ointments and water preparation from Sida rhombifolia leaf extract. Tanzan. J. Health Res. 2018;20(4):1–8. [Google Scholar]
- Franzotti E.M., Santos C.V., Rodrigues H.M., Mourao R.H., Andrade M.R., Antoniolli A.R. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca) J. Ethnopharmacol. 2000;72:273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- Ghosal S., Ballav R., Chauhan P.S., Mehta R. Alkaloids from Sida cordifolia. Phytochemistry. 1975;14:830–832. [Google Scholar]
- Ghosh S., Dutt A. Chemical examination of Sida cordifolia Linn. J. Indian Chem. Soc. 1930;7:825–829. [Google Scholar]
- Gupta S.R., Nirmal S.R., Patil R.Y., Asane G.S. Anti-arthritic activity of various extracts of Sida rhombifolia aerial parts. Nat. Prod. Res. 2009;23:689–695. doi: 10.1080/14786410802242778. [DOI] [PubMed] [Google Scholar]
- Halilu M.E., Muhammad I., Dangoggo S.M., Farouq A.A., Ahmed A., Shamsuddeen A.A. Phytochemical and antibacterial screening of petroleum ether and ethanol extracts of Sida cordifolia leaves. J. Chem. Soc. Niger. 2016;41:137–142. [Google Scholar]
- Islam M.E., Haque M., Mosaddik M.A. Cytotoxicity and antibacterial activity of Sida rhombifolia (Malvaceae) grown in Bangladesh. Phytother Res. 2003;17:1182–1186. doi: 10.1002/ptr.1294. [DOI] [PubMed] [Google Scholar]
- Jadhav A.N., Pawar R.S., Avula B., Khan I.A. Ecdysteroid glycosides from Sida rhombifoliaL. Chem. Biodivers. 2007;4:2225–2230. doi: 10.1002/cbdv.200790180. [DOI] [PubMed] [Google Scholar]
- Jang D.S., Park E.J., Kang Y.P., Su B.N., Hawthorne M.E., Vigo J.S., Graham J.G., Cabieses F., Fong H.H.S., Mehta R.G., Pezzuto J.M., Douglas Kinghorn A. Compounds obtained from Sida acuta with the potential to induce quinone-reductase and to inhibit 7, 12-dimethylbenz [a]-anthracene-induced prenoplastic lesions in a mouse mammary organ culture model. Arch. Pharm. Res. 2003;26:585–590. doi: 10.1007/BF02976704. [DOI] [PubMed] [Google Scholar]
- Karou D., Dicko M.H., Sanon S., Simpore J., Traore A.S. Antimalarial activity of Sida acuta burm. F. (malvaceae) and pterocarpus erinaceus poir. (Fabaceae) J. Ethnopharmacol. 2003;89(2-3):291–294. doi: 10.1016/j.jep.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Karou D., Savadogo A., Canini A., Yameogo S., Montesano C., Simpore J., Colizzl V., Traore A.S. Antibacterial activity of alkaloids from Sidaacuta. Afr. J. Biotechnol. 2005;4(12):1452–1457. [Google Scholar]
- Khurana N., Sharma N., Patil S., Asmita G. Phytopharmacological properties of Sida cordifolia: a review of folklore use and pharmacological activities. Asian J. Pharmaceut. Clin. Res. 2016;2:52–58. [Google Scholar]
- Konaté K., Bassolé I.H.N., Hilou A., Aworet-Samseny R.R.R., Souza A., Barro N., Dicko M.H., Datte J.Y., Batchi B.M. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f . and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Compl. Alternative Med. 2012;12:120. doi: 10.1186/1472-6882-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapovickas A. Las especies argentinas Y de países vecinos de Sida Secc. Nelavaga (malvaceae, Malveae) Bonplandia. 2006;15(1/2):5–45. [Google Scholar]
- Kumar S., Lakshmi P.K., Sahi C., Pawar R.S. Sida cordifolia accelerates wound healing process delayed by dexamethasone in rats: effect on ROS and probable mechanism of action. J. Ethnopharmacol. 2019;10(235):279–292. doi: 10.1016/j.jep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Lin H.W., Wang C.M., Tseng Y.H. Sida spinosa L. (Malvaceae), a newly naturalized plant in Taiwan. J. Natl. Taiwan Mus. 2010;32:1–6. [Google Scholar]
- Mabberley D.J. second ed. cambridge University Press; cambridge: 1997. The Plant Book: A Portable Dictionary of the Vascular Plants. [Google Scholar]
- Mohideen S., Sasikala E., Gopal V. Pharmacognostic studies on Sida acuta. Burm.f. Anc. Sci. Life. 2002;22(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- Momin M.A., Bellah S.F., Rahman S.M., Rahman A.A., Murshid G.M., Emran T.B. Phytopharmacological evaluation of ethanol extract of Sida cordifolia L. roots. Asian Pac. J. Trop. Biomed. 2014;4(1):18–24. doi: 10.1016/S2221-1691(14)60202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivoto T., Nardino, Carvalho I.R., Follmann D.N., Szareski J., Ferrari M., de Pelegrin A.J., de Souza V.Q. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: a review. Afr. J. Agric. Res. 2017;12(2):71–84. [Google Scholar]
- Pawar R.S., Chaurasiya P.K., Rajak H., Singour P.K., Toppo F.A., Jain A. Wound healing activity of Sida cordifolia Linn. in rats. Indian J. Pharmacol. 2013;45(5):474–478. doi: 10.4103/0253-7613.117759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa H.S., Camargo V.B., Camargo G., Garcia C.V., Fuentefria A.M., Mendez A.S. Ecdysteroids in Sidatuberculata RE Fries (Malvaceae): chemical composition by LC-ESI-MS and selective anti-Candida krusei activity. Food Chem. 2015;182:193–199. doi: 10.1016/j.foodchem.2015.02.144. [DOI] [PubMed] [Google Scholar]
- Shetu H.J., Durdana N., Farjana A., Nuzhat Z., Pritesh R.D. 2019. Pharmacological and Phytochemicalimportance of Sidacordifolia: a Review; pp. 60–73. [Google Scholar]
- Singh A., Navneet Ethnomedicinal, pharmacological properties and phytochemistry of Sida spinosa Linn. A mini review. J. Phytopharm. 2018;7(1):88–91. [Google Scholar]
- Sivarajan V.V., Pradeep A.K. Taxonomy of the Sida rhombifolia (Malvaceae) complex in India. Contrib. Bot. 1994;16(1):63–78. [Google Scholar]
- Sutradhar R.K., Rahman A.K., Ahmad M.U., Bachar S.C., Saha A., Guha S.K. Bioactive alkaloid from Sidacordifolia Linn. With analgesic and antiinflammatory activities. Iran. J. Pharmacol. Ther. 2006;5(2):175–178. [Google Scholar]
- Sutradhar R.K., Rahman A.K.M.M., Ahmad M.U., Saha K. Alkaloids of Sida cordifolia L. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2007;46(11):1896–1900. [Google Scholar]
- Sutradhar R.K., Rahman A.K.M.M., Ahmad M.U. Three new flavonol C-glycosides from Sida cordifolia Linn. J. Iran. Chem. Soc. 2007;4:175–181. [Google Scholar]
- Sutradhar R.K., Rahman A.K., Ahmad M.U., Bachar S.C. Bioactive flavones of Sida cordifolia. Phytochem. Lett. 2008;1(4):179–182. [Google Scholar]
- Ugborogho R.E. The taxonomy of Sida L. (Malvaceae) in Nigeria. Boletin da Sociedade Broteriana Ser. 1980;54:99–119. [Google Scholar]
- Xuyen D.T. Sida azechuensis Matuda (Malvaceae), one new species for the flora of Vietnam. Vienam Acad. J. Biol. 2006;28(3):40–42. [Google Scholar]
- Yang L., Wen K.S., Ruan X., Zhao Y.X., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23(4):762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Xu Y. Phytoecdysone from Sidaszechuensis. Yunan Zhiwu Yanjiu. 2000;22:503–506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.