Abstract
The NHLBI and the Cardiovascular Medical Research and Education Fund held a workshop on the application of pulmonary vascular disease omics data to the understanding, prevention, and treatment of pulmonary vascular disease. Experts in pulmonary vascular disease, omics, and data analytics met to identify knowledge gaps and formulate ideas for future research priorities in pulmonary vascular disease in line with NHLBI Strategic Vision goals. The group identified opportunities to develop analytical approaches to multi-omic datasets, to identify molecular pathways in pulmonary vascular disease pathobiology, and to link novel phenotypes to meaningful clinical outcomes. The committee suggested support for interdisciplinary research teams to develop and validate analytical methods, a national effort to coordinate biosamples and data, a consortium of preclinical investigators to expedite target evaluation and drug development, longitudinal assessment of molecular biomarkers in clinical trials, and a task force to develop a master clinical trials protocol for pulmonary vascular disease.
Keywords: Pulmonary hypertension, integrative omics, systems biology, drug repurposing, precision medicine, master clinical trial protocol
Condensed Abstract:
Pulmonary hypertension is diagnosed on the basis of a hemodynamic definition that encompasses a heterogenous group of diseases with disparate etiologies, pathobiologies, and management strategies. A classification strategy based on shared disease mechanisms defined by clinically meaningful biomarkers are urgently needed to improve diagnosis, prognosis, and treatment responses. Several international efforts have begun deep clinical and molecular phenotyping of these patients. Here, we report key findings from a joint NHLBI and CMREF working group on how best to leverage these vast omics datasets to improve patient outcomes.
Tweet:
Key findings on the integration of clinical, molecular, and radiologic datasets to redefine pulmonary vascular disease phenotypes from a joint @nih_nhlbi and CMREF working group.
Introduction
Pulmonary hypertension, defined as a resting mean pulmonary artery pressure (mPAP) greater than 20 mmHg (1), contributes to the increased morbidity and mortality of millions of affected patients (2,3). Importantly, this hemodynamic definition encompasses a heterogeneous group of diseases with disparate etiologies, pathobiologies, and management strategies. The World Symposium on Pulmonary Hypertension (WSPH) classification scheme groups these patients into five categories on the basis of similar clinical characteristics and cardiopulmonary hemodynamics with group assignment informing treatment recommendations (1,4-6). While clinically useful, each of the WSPH groups also comprises a diversity of phenotypes of which only two subgroups, heritable pulmonary arterial hypertension (PAH) and pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis, can be linked to molecular pathogenic mechanisms. Moreover, many patients fit poorly into a single WSPH group, such as a patient with COPD (WSPH Group 3) found to have an “out-of-proportion” increase in mPAP suggestive of a primary pulmonary arteriopathy (WSPH Group 1). With the exception of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension (CTEPH, WSPH Group 4), treatment responses are modest regardless of classification, and true disease-modifying therapies are currently unavailable. These observations highlight the limitations of a disease taxonomy lacking clear connections to pathobiology. Furthermore, they emphasize the need for a revised classification system based on biomarkers of shared disease mechanisms.
To help address this need, the National Heart, Lung, and Blood Institute (NHLBI) launched an initiative, Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics (PVDOMICS), with a goal of reconstructing the classification system for pulmonary hypertension on the basis of deep clinical and molecular phenotyping (7). This effort grew from strategic planning workshops hosted by the NHLBI over the past decade (8,9) where experts emphasized the importance of improved phenotyping as the foundation for future mechanistic studies of disease pathobiology, for focusing efforts at drug development, for identifying new biomarkers of treatment response or high-risk clinical phenotypes, and for targeting enrollment in clinical trials of novel therapeutics.
As PVDOMICS, the consortium created from the NHLBI initiative, transitions from subject recruitment to data analysis, the NHLBI, with support from the Cardiovascular Medical Research and Education Fund (CMREF), convened a workshop to discuss how best to leverage the wealth of clinical and molecular data generated by PVDOMICS and other similar multifaceted phenotyping efforts to improve patient outcomes. This workshop took place at the NHBLI on November 14-15, 2019. Workshop participants included experts in the study and treatment of pulmonary vascular disease as well as authorities on the use of various omics technologies and novel analytical approaches. Participants were selected from diverse disciplines and backgrounds including basic scientists, biomedical engineers, clinical trialists, epidemiologists, and systems biologists. Seven sessions were followed by a group discussion. Session topics included: genomics; proteomics; transcriptomics; metabolomics; coagulomics and lipidomics; new technologies and resources in omics studies including data storage, public access, and data interpretation; and the use PVDOMICS data as clinical biomarkers to inform trial design, diagnosis, treatment responses, and prognosis. Challenges were identified and areas for future development were identified. These areas were formulated through in-person discussions and the drafting of this manuscript over the past year. This report summarizes the working group’s major findings (Table 1, Central Illustration).
Table 1:
Summary of Workshop Outcomes
| KEY PRINCIPLE | COMPONENTS | GOALS |
|---|---|---|
| 1. Develop novel analytical approaches to large datasets |
|
|
| 2. Incentivize dataset integration & facilitate data accessibility |
|
|
| 3. Organize preclinical studies of new targets and new experimental disease models |
|
|
| 4. Support acquisition of longitudinal data |
|
|
| 5. Lay the foundation for a master protocol for pulmonary vascular disease trials |
|
|
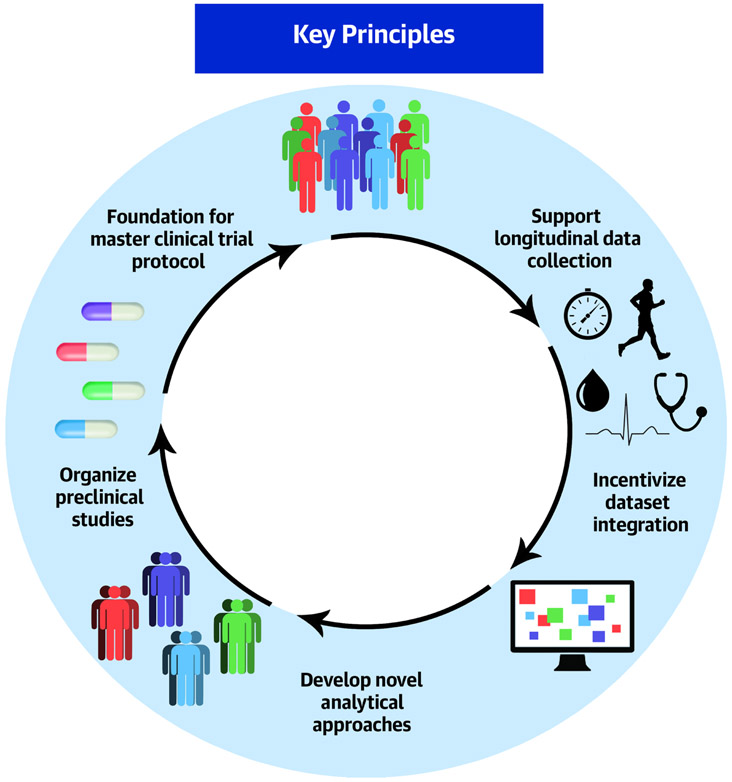
Central Illustration. Key principles identified for using deep phenotyping to improve patient care in patients with pulmonary vascular disease.
The goal is to develop a new classification system for pulmonary vascular disease based on shared clinical and molecular phenotypes to improve outcomes for patients with pulmonary vascular disease. By applying novel analytical approaches to these disease phenotypes, new molecular targets will be identified, and therapeutics developed. Several key principles were identified in this joint NIH/NHBLI and Cardiovascular Medical Research and Education Fund workshop that should focus future efforts in this area.
Deep Phenotyping in Pulmonary Vascular Disease
Much of the research in pulmonary vascular disease has focused on the pathobiology of pulmonary arterial hypertension (PAH), a particularly severe form of pulmonary hypertension characterized by a primary pulmonary arterial vasculopathy causing obliteration of the vessel lumens and leading to right ventricular failure and death. PAH is a rare disease, affecting between 15-50 patients per million population (10), which has catalyzed the formation of national and international networks to assemble the patient numbers necessary to draw robust conclusions from molecular phenotyping efforts (Table 2) (11). Such networks include the PH Breakthrough Initiative and the PAH Biobank in the U.S.; the National Cohort Study of PAH in the U.K.; the French Network on PAH; and the International Consortium for Genetic Studies in PAH (PAH ICON). Notably, each of these networks has focused primarily on the genetic pathobiology of the subset of pulmonary hypertension patients diagnosed with PAH, although their exploration of other molecular features of the disease is expanding rapidly (12).
Table 2:
Pulmonary Vascular Disease Research Networks
| NETWORK | LOCATION | WEB |
|---|---|---|
| Pulmonary Hypertension Breakthrough Initiative | U.S. | www.ipahresearch.org/services.html |
| National Biological Sample and Data Repository for PAH (PAH Biobank) | U.S. | www.pahbiobank.org/ |
| Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics (PVDOMICS) | U.S. | www.phassociation.org/pvdomics/ |
| United States Pulmonary Hypertension Scientific Registry (USPHSR) | U.S. | |
| National Cohort Study of Idiopathic and Heritable PAH | U.K. | www.ipahcohort.com |
| French Network on PAH | France | |
| International Consortium for Genetic Studies in PAH (PAH ICON) | U.S., U.K., France, Italy, Austria, Germany, Canada, Belgium, Netherlands, Spain | www.pahicon.com |
By contrast, PVDOMICS was designed to expand clinical and molecular phenotyping efforts across all WSPH groups of pulmonary hypertension (Figure 1) (7). Toward this end, the study enrolled ~1,200 adult participants with pulmonary hypertension, with risk factors for pulmonary hypertension, or healthy controls. The majority of patients were prevalent cases of disease. As it began enrollment prior to the recent WSPH, which lowered the mPAP for the diagnosis of pulmonary hypertension, PVDOMICS used a mPAP ≥ 25 mmHg to define pulmonary hypertension. Importantly, the investigators also enrolled a carefully defined group of at-risk subjects matched to WSPH group. These participants included subjects with a mPAP between 20-25 mmHg; exercise-induced pulmonary hypertension; post-capillary pulmonary hypertension; and left heart disease, pulmonary disease, or chronic pulmonary embolism without pulmonary hypertension.
Figure 1. Deep phenotyping performed as part of the PVDOMICS study.
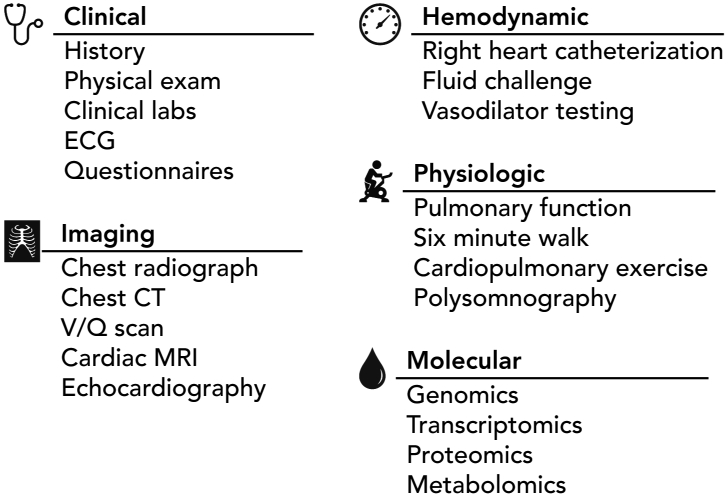
PVDOMICS collected data across a broad range of clinical, imaging, and molecular parameters. A standard clinical evaluation was supplemented with quality-of-life questionnaires. Hemodynamic assessment by pulmonary artery catheterization incorporated provocative testing with fluid challenge and pulmonary vasodilators. Molecular profiling was performed on blood samples using leukocyte DNA for genomics, whole-blood RNA for transcriptomics, and plasma for aptamer-based proteomics and untargeted metabolomics.
All subjects underwent an extensive clinical evaluation. Each had a thorough medical interview and physical examination including documentation of New York Heart Association functional class and body composition by bioimpedance measurements. They completed quality of life questionnaires. Routine laboratories were obtained including serum electrolytes, cell count and differential, coagulation tests, and serologies. Lung structure and function were assessed by pulmonary function testing, non-contrast computed tomography of the chest, ventilation-perfusion scintigraphy, and home polysomnography. Cardiac structure and function were assessed by electrocardiography, two-dimensional echocardiography, and contrast-enhanced cardiac magnetic resonance imaging. Cardiopulmonary hemodynamic measurements were obtained by right heart catheterization with provocative testing including 100% oxygen, inhaled nitric oxide, and fluid challenge when invasive testing was not performed. Cardiopulmonary exercise testing was performed either non-invasively or invasively with pulmonary and radial arterial catheters in place.
Blood samples were obtained from peripheral vein, pulmonary artery, and distal to pulmonary artery occlusion for molecular phenotyping using multiple omics approaches. Sample analysis is currently ongoing. The planned studies include next generation sequencing of leukocyte DNA and whole-blood RNA for genomics and transcriptomics; plasma proteomics (Olink); and untargeted metabolomics (Metabolon). Urine samples were collected and stored for future study.
After the initial evaluation, all enrollees will be followed annually by phone to assess the incidence of death or transplantation. A subset of approximately 400 patients is participating in additional follow-up testing to include a detailed history and physical examination, anthropometric measurements, quality-of-life surveys, echocardiography, six-minute walk testing, and blood collection for repeat omics.
Altogether, PVDOMICS will generate a tremendous amount of data from patients with pulmonary hypertension, at-risk subjects, and healthy controls. Indeed, approximately 2,000 clinical variables, 1,000 metabolites, 1,000 proteins, and 30,000 transcripts will be measured plus whole genome sequences generated for ~1,200 participants. This study will provide the most comprehensive clinical and molecular description of pulmonary hypertension available. Its successful completion will mark a turning point in our struggle against this devastating disease. Moving forward, what are the most promising avenues of future investigation with the highest likelihood of improving patient outcomes?
New Horizons in Pulmonary Hypertension Research
The data provided by PVDOMICS and other research consortia provide a solid foundation for future investigations in a variety of areas, from data analytics to clinical trial design, that promise to have far-reaching impact on all areas of biomedical science. At least as important is the function of PVDOMICS as a “use case” for how investigators can interact with large mixed data sets that include complex molecular and clinical data types, and how findings can be prioritized for more in-depth mechanistic investigations leading as quickly as possible to clinical trials.
Transforming data into knowledge
Compared to similar large, multi-omic initiatives, such as the Cancer Genome Atlas (13), PVDOMICS stands apart owing to the diversity of clinical and molecular data that were obtained. Indeed, the breadth of clinical, imaging, functional, and molecular data available from these participants is truly unique. Moreover, the inclusion of healthy and at-risk subjects further enhances the utility and the complexity of the dataset. How best to transform these data into insights that improve patient care remains the most important unanswered question raised by this and similar studies.
Perhaps the simplest analyses of a complex omics dataset like PVDOMICS would include identifying the disease signature contained within a given collection of omics data (i.e., what is the transcriptomic signature of pulmonary hypertension?). On this basis, PVDOMICS has the opportunity to validate similar studies that have been completed previously (14-19) as well as advance radiomic (20) or other phenomic studies (21) of patients with pulmonary vascular disease of all types.
Moving beyond single omic analyses toward multi-omic integration offers the promise of a more holistic understanding of disease pathobiology. Just as there has been an explosion in the acquisition of omics data, there have been rapid advances in analytical approaches for multi-omic analyses (22-26). A variety of methods have been developed and may be broadly classified into clustering/dimensionality reduction, predictive modeling, pairwise integration, network methods, or some combination of these approaches (22), with new approaches, such as developments in deep learning applications, under active development (27,28).
While initial efforts have focused on integrating molecular omics data that share a related biological framework, recent studies have also begun to explore methods to link molecular and clinical datasets (e.g., radiogenomics, (29)). Overall, the analytical field of integrative omics is developing rapidly, and best practices remain to be developed. Moreover, bridging the gap between molecular and clinical omics data remains a challenge. Indeed, the unique breadth of phenomic, imaging, and physiologic data contained within the PVDOMICS dataset poses a novel challenge in this regard.
Thus, the results of PVDOMICS provide an outstanding opportunity for analytical method development related to integrative omics. The methods developed here will apply broadly across the biomedical research enterprise. Specific questions will likely employ different analytical techniques, and some of these are discussed further in the sections below.
Identifying novel pathophenotypes
One overarching goal of PVDOMICS is to identify subphenotypes of pulmonary hypertension on the basis of shared molecular features. The hypothesis is that such a classification scheme would be more clinically and biologically meaningful than the current method of grouping patients based on hemodynamic assessment and medical comorbidities. The hope for this approach is that it will facilitate experimental dissection of pathobiologic mechanisms and the development of more effective and targeted therapies. α1-antitrypsin deficiency illustrates the value of molecular phenotyping. These patients present with dyspnea, airflow obstruction, and emphysema that is often clinically indistinguishable from smoking-related chronic obstructive pulmonary disease; however, they benefit from targeted enzyme replacement therapy. In a similar way, the goal of molecular phenotyping of patients with pulmonary hypertension will identify subpopulations of patients that share a common prognosis or therapeutic response.
In addition, molecular insights obtained from the identification of novel pathophenotypes in patient subsets may have a broad impact on the treatment of all patients with pulmonary hypertension. For example, gain-of-function mutations in the PCSK9 gene were identified through genetic studies of families affected by autosomal dominant hypercholesterolemia (30,31). Loss-of-function mutations in PCSK9 were subsequently found to be associated with low LDL cholesterol (32). Within a decade, anti-PCSK9 therapy was shown to markedly reduce LDL cholesterol levels and cardiovascular events (33,34).
The endeavor to identify meaningful molecular phenotypes in pulmonary hypertension will rely heavily on statistical learning approaches that can be broadly divided into supervised and unsupervised methods. In supervised methods, algorithms use parameters (e.g., plasma metabolite concentrations) to optimize assignment to pre-determined groups (e.g., good v. poor prognosis). These approaches have been successfully applied in studies of PAH to identify plasma biomarkers that distinguish patients with PAH from controls and to prognosticate outcomes in those with the disease (12,15,16,18). Currently the clinical adoption of these biomarkers hinges on their validation in prospective clinical trials.
In unsupervised methods, algorithms derive clusters on the basis of underlying patterns in high-dimensional datasets. These approaches are inherently unbiased and are likely better able to capture biologically meaningful differences among patients with heterogeneous diseases than clinical classification schemes. Recently, consensus clustering, an unsupervised statistical learning method, identified four distinct immune phenotypes from plasma cytokine profiles in patients with PAH (35). Interestingly, these immune phenotypes demonstrated differences in clinical risk and were unrelated to commonly recognized PAH subtypes. Moreover, this analysis highlighted key cytokine signaling networks associated with each cluster, thereby providing a foundation for future mechanistic studies. Similar approaches have been successfully applied to SARS-CoV-2 infection (36), exercise intolerance (21), heart failure (37), and cardiometabolic disease (38).
Developing new drug targets
The detailed molecular pathophenotypes of pulmonary hypertension that emerge from PVDOMICS will open new avenues of research investigation by identifying novel molecular pathways implicated in disease pathobiology and associated with distinct clinical phenotypes. These pathways not only provide insight into disease mechanisms, but also suggest new targets for drug development.
Several omics integration methods have been used for mechanistic discovery (22,39,40). For example, genetic variation can be linked to transcript expression by identifying expression quantitative trait loci (eQTL). In a similar way, loci that account for the variance in protein levels (pQTL) or metabolite concentrations (metQTL) can be determined (41). These QTL have utility in determining causality in Mendelian randomization analyses such as that recently performed in PAH finding no causal association of iron deficiency (42). Correlation analyses may be applied to “downstream” omics data, such as between transcripts and metabolites. While these methods do not incorporate any additional biological information, other methods do build upon previously identified relationships among various molecular species. For example, combined over-representation analyses can identify pathways enriched for differentially regulated proteins or metabolites (43). Similarly, network-based methodologies offer a powerful and flexible method to interrogate molecular biomarkers against a broader tableau of previously determined molecular relationships.
Biological networks illustrate how various nodes representing distinct biological entities (e.g., individual proteins) are related to one another. These relationships, or edges, can represent a variety of connections including physical interactions, correlations, or regulatory relationships. Many approaches may be used to interrogate these networks in order to identify novel relationships. For example, mapping miRNA targets onto a network of pulmonary hypertension genes extracted from the consolidated human interactome (44) identified several key miRNA families involved in the pathobiology of pulmonary vascular disease (45,46). Similar network-based filtering approaches identified NEDD9 as a critical mediator of pulmonary vascular fibrosis (47). Since network nodes can represent different entities, these graphs offer a particularly straightforward means of integrating disparate datasets. Indeed, integrating disease associations and drug targets into the consolidated human interactome to generate a disease-gene-drug network suggested new targets for drug repurposing (48).
One important limitation of the “blood biopsies” performed as part of human phenotyping studies is the indirect relationship between the pathobiology of affected tissues (e.g., pulmonary vessel walls or cardiac myocytes) and the composition of molecular biomarkers in the blood. The extent to which disease pathways manifest in blood omics studies overlap with tissue disease pathways is unclear. Moreover, this question is particularly challenging to address in human subjects with pulmonary vascular disease where tissue samples are available only at the time of death or transplant. These observations suggest a potential role for tissue-plasma multi-omics investigations using animal models to develop methodological approaches to link the findings from human plasma biomarkers to molecular disease mechanisms within the pulmonary vasculature.
Clearly, multiple avenues lead to molecular drivers of disease and novel therapeutic targets. As new targets are identified, the talented basic-translational pulmonary vascular disease research community will need to provide the biological validation necessary to link human omics insights to disease pathobiology. Given the potential for identifying a considerable number of new targets, there may be opportunities to achieve economies of scale through a pre-clinical consortium of investigators who can prioritize promising targets and develop core facilities for large and small animal studies, omics measurements, tissue culture, human sample procurement and analyses, medicinal chemistry, etc. Many successful laboratories throughout the country are equipped to perform many facets of pre-clinical research in pulmonary vascular disease; however, data quality and experimental throughput may increase while costs decrease through laboratory specialization and collaboration. Moreover, a diversity of expertise will be advantageous in prioritizing new molecular pathways for therapeutic development. Many metrics may be valuable in weighing the most promising avenues for investigation including analytical tools such as network-based drug target proximity (49-53), as well as pragmatic judgements about biological plausibility, therapeutic efficacy, and patient tolerability.
In addition to maximizing the yield of current model systems, developing new and more relevant models of human pulmonary vascular disease should be a priority for the research community. Novel molecular insights from PVDOMICS and other similar pulmonary vascular disease programs may lead to new genetic models of pulmonary hypertension. Although most laboratories study rodent models of pulmonary hypertension, large animals models, such as the simian immunodeficiency virus-infected macaque (54) or CTEPH pig (55), may offer unique opportunities to study disease pathogenesis that more closely recapitulates human pulmonary vascular disease. Beyond animal models, microfluidic-based organ-on-a-chip technologies to study cell-cell interactions under conditions of flow have been underutilized and may be useful in the study of pulmonary vascular disease (56,57).
PVDOMICS will be a fount of new hypotheses for preclinical studies in pulmonary vascular disease. To maximize its return on investment and to validate new analytical pipelines, the research community must aggressively pursue the validation of novel targets in preclinical models. Rather than relying on the specific interests of individual laboratories, the community could consider a translational PVDOMICS-like initiative to identify a more expeditious route through which to bring new drugs to clinical testing.
Clinical applications of novel pathophenotypes
PVDOMICS will identify new pathophenotypes of pulmonary vascular disease and will, thereby, provide insights into the molecular underpinnings of clinical phenotypes. As a result of its primarily cross-sectional design and the limited availability of longitudinal data at present, the clinical utility of these phenotypes for informing patient prognosis or response to specific therapies may be limited until such follow-up data is collected and analyzed. Certainly, continued monitoring of this patient population would provide important clinical information for biological validation of novel phenotypic clusters. A similar approach has been successful in identifying protein and metabolite biomarkers of prognosis in a U.K. patient cohort (15,16). Moreover, these clinical data can be used to guide supervised statistical learning approaches to identify biomarkers associated with patient outcomes or drug responses.
In addition to longitudinal data on patient outcomes, longitudinal measurements of clinical and molecular biomarkers would provide much-needed data on the natural molecular history of pulmonary vascular disease. When coupled to clinical information, such as hemodynamic measurements, imaging assessments, or medication changes, molecular signatures of disease progression or treatment response could be defined. By coupling clinical and molecular assessments, a patient’s care could be more responsive to their disease trajectory and outcomes may be improved.
A major strength of PVDOMICS is the enrollment of patients with cardiac or pulmonary disease without pulmonary hypertension (mPAP < 20 mmHg) and those with mild pulmonary hypertension (mPAP 20-24 mmHg) as important disease comparators. These patients have comorbidities that place them at risk for incident disease, and longitudinal clinical and molecular follow-up of these individuals could provide valuable insights into disease pathogenesis and longitudinal course of pulmonary vascular disease. A careful analysis of this cohort may suggest new approaches for screening at-risk populations that lead to earlier diagnosis and, hopefully, improved outcomes (58).
While performing deep phenotyping of every patient with pulmonary hypertension is presently unrealistic, the PVDOMICS initiative will begin to identify the clinical and molecular variables that segregate meaningful phenotypes most effectively. This pulmonary hypertension panel could be used in clinical trials, both at enrollment and over the duration of the trial, to inform clinical response. Similarly, the PVDOMICS data will help answer the question of which “ome” is the most valuable in this regard, as the cost of measuring multiple individual markers rapidly approaches and may even exceed the cost of a full omic analysis. In other words, if one could choose only one “ome” to assess in a given patient, which “ome” should that be? Or is some limited multi-omic or oligo-omic signature more informative? In sum, a critical analysis of the information value contained within each of the assessments performed during PVDOMICS will enable cost effective evaluations of patient phenotypes in future studies.
Rethinking clinical trials in pulmonary vascular disease
Despite showing preclinical promise, a multitude of pulmonary hypertension therapies have failed in clinical trials. Every NHLBI workshop on pulmonary vascular disease over the last decade has identified a need for improved clinical trial design for affected patients (8,9,59). Biomarker-driven trial design strategies have been proposed as a solution. PVDOMICS offers the opportunity to develop, incorporate, and evaluate novel biomarkers in clinical trial design. Biomarkers may be used to enrich study cohorts based, guide the development of stratified study enrollment or adaptive trial design, define responses within clinical trials, and identify patient phenotypes unlikely to benefit from current therapies. PVDOMICS also provides the opportunity to broaden pharmacogenomic assessments of treatment outcomes in clinical trials to include and account for changes in the placebo arm that might be derived from the natural history of the disease or physiologically induced placebo effects (60). Future clinical trial designs should seek to incorporate molecular phenotyping to address these issues.
In addition, the biological insights generated from PVDOMICS and other similar studies will almost certainly generate “a need to answer more [clinical] questions more efficiently and in less time (61).” One approach to fulfilling this need is to develop clinical trials designed to assess multiple treatments in multiple patient phenotypes within the same overall trial structure. These so-called “master protocols” can incorporate biomarker-driven randomization (both traditional and response-adaptive randomization), maximize subject screening efficiency, provide economies of scale, and reduce time for new drug evaluation. PVDOMICS has contributed not only the molecular signatures that can be used for targeted therapies, but PVDOMICS investigators have also developed detailed protocols for the clinical and molecular assessments of patients with pulmonary vascular disease that can provide a starting point for future clinical trial design. During this next phase of target identification and drug repurposing, efforts to prepare for the rapid clinical testing of novel therapies should be undertaken.
Key Principles and Opportunities Identified
Develop novel analytical approaches to large datasets
As described above, the breadth and depth of information collected by efforts like PVDOMICS offers a unique testbed for the development of novel analytical approaches focused on the integration of clinical and molecular omics data. Moreover, this and similar datasets provide an opportunity to address key analytical challenges in high-dimensional data analysis. Perhaps among the least interesting but most frustrating are confounding associated with non-biological variation from differences in sample collection, storage, processing or batch effects from analytical platforms. As a multi-center, multi-omic, multi-year endeavor, PVDOMICS is well-positioned to set the standard for identifying and mitigating the effects of non-biological variation in omics studies. One characteristic of these datasets is that the number of subjects is vastly outnumbered by the number of parameters used for modeling, a problem compounded somewhat by the study of a rare disease like pulmonary hypertension. Optimizing feature selection algorithms and dimensionality reduction approaches will be important for model fitting as well as comparing data sets with varying numbers of variables (e.g., hemodynamic measurements v. differentially expressed transcripts). Furthermore, the construction of interpretable models should be emphasized, particularly as the connections between parameters and phenotypes will provide the foundation for further mechanistic studies into disease pathobiology.
The advances in bioinformatic methods will be as valuable as the biological insights they reveal. Encouraging their development and validation should be a goal of future funding opportunities. Importantly, this research requires a diversity of expertise including “wet lab” biologists, computational biologists, epidemiologists, statisticians, and omics experts in addition to clinical investigators and experts in pulmonary vascular disease. Moreover, these research endeavors are inherently founded on “discovery science” driven by big data analytics that is coupled to the use of more familiar reductionist approaches to validate their findings. Evaluating these research proposals will require a similar breadth of expertise and an acknowledgement of the importance of hypothesis generating experimentation. Opportunities to collaborate with foundation (e.g., CMREF) or industry partners to advance the findings of PVDOMICS should be pursued, perhaps in the form of co-sponsored workshops or “hackathons” addressing targeted analytical challenges as have been sponsored by the National Center for Biotechnology Information.
Incentivize dataset integration and facilitate data accessibility
Biodata collected from future clinical studies of pulmonary hypertension should be aggregated in a web-based repository that can be shared and correlated with any and all other research projects. Such a resource would provide a valuable historical control dataset that could be utilized in the design of prospective trials (62). The scope of data collected from PVDOMICS would make this a good template through which to harmonize future datasets. In addition, supporting the incorporation of datasets obtained through other NHLBI-supported projects, such as the Pulmonary Hypertension Breakthrough Initiative (PHBI), the PAH Biobank, and other repositories like the Pulmonary Hypertension Association Registry and the United States Pulmonary Hypertension Scientific Registry (63,64), would increase investigational power for the study of both rare and common forms of pulmonary vascular disease. Moreover, it is imperative to consider the development of international partnerships for data sharing to increase the power for subgroup analyses in this rare disease and provide for experimental cross-validation of molecular phenotypes identified through PVDOMICS.
These efforts will likely be more comprehensive, successful, and fruitful with financial and infrastructure support, such as the NHLBI-supported biomedical data platform BioData Catalyst (65), the NCBI Database of Genotypes and Phenotypes (dbGap) (66), or the NHLBI TOPMed database (67) to which PVDOMICS data will be deposited. Implementation of the FAIR Data Principles to make research data Findable, Accessible, Interoperable, and Reusable (68) will optimize reuse of these extensive biological data. Furthermore, these principles should extend to ensuring methodological vigilance, code availability, and full transparency with methodological details related to the application of these non-traditional analytical approaches. These resources will likely be as important to investigators outside the pulmonary vascular disease field as it is to investigators studying pulmonary hypertension.
Organize preclinical studies of new targets and new experimental disease models
Analysis of the PVDOMICS data will undoubtedly provide numerous new molecular targets for further investigation. The translational promise of these findings may be best realized through the concerted efforts of a consortium of preclinical investigators that could prioritize promising targets and develop core facilities for animal modeling and hemodynamic measurements, omics acquisition and analyses, molecular biology, and bio-sample collection. Rather than relying on the distributed efforts of individual laboratories, the consortium approach offers the advantages of enhancing experimental throughput, improving economies-of-scale, and increasing data quality and data standardization/harmonization.
Beyond target validation, new animal models based on these novel targets or representing new disease phenotypes are needed, including large animal models of various types of pulmonary vascular disease. In addition, the utility of alternative models, including microfluidic devices and in silico modeling, remains relatively unexplored.
Support acquisition of longitudinal data
While cross-sectional data is invaluable for phenotypic clustering of patients with pulmonary hypertension, they are less helpful for addressing the key clinical questions for which longitudinal assessments are essential. To utilize these data to address questions of diagnosis, prognosis, or pharmaceutical response will require the assessment of clinical outcomes data associated with repeated clinical and molecular phenotyping information. Early insights from these observations may then be incorporated in future clinical trials to validate their utility as biomarkers.
Lay the foundation for a master protocol for pulmonary vascular disease trials
Finally, now is the time to begin planning a master clinical protocol for pulmonary vascular disease trials to expedite the third phase of complex multi-featured data initiatives such as PVDOMICS, where novel molecular insights, and particularly “druggable” targets, are rapidly translated to clinical practice after validation in this second phase of preclinical investigation. Perhaps as important as the forthcoming data, the PVDOMICS Steering Committee established rigorous protocols for clinical testing and sample collection (7,69) that should serve as a model for future studies of this and similar populations of patients with cardiovascular and pulmonary diseases. Several important issues remain to be addressed in this area, including the development and evaluation of more meaningful endpoints such as patient-centered outcomes; the incorporation of newly identified biomarkers in trial randomization, longitudinal assessments, and as surrogate endpoints; the role of adaptive trial design; and the role of drug withdrawal trials. All of these approaches will reshape the field into one more focused on precision medicine strategies and transformative therapies. Innovative clinical trials will also need to satisfy the concerns of regulatory authorities, industry, patients, and clinicians. Several clinical trial networks provide examples of how to resolve these concerns and implement a master protocol successfully (70-72). Importantly, the pulmonary vascular disease research community can begin the important process of planning and consensus building immediately. Indeed, a commitment to adopting a common approach to clinical trials at individual centers or in smaller studies would likely strengthen the justification for the creation of a larger network. Hopefully, the insights generated from PVDOMICS and similar initiatives will catalyze the formation of such a network to address a critical need to improve outcomes for patients with pulmonary vascular disease.
Conclusions
The conclusion of the first phase of PVDOMICS is another milestone on the road to improved diagnosis and treatment of pulmonary vascular disease. This large dataset will fuel innovations in analytical approaches and discovery of novel molecular targets for therapeutic development. Leveraging these insights to improve patient care will require additional investments in preclinical studies of disease mechanisms, longitudinal collection of additional clinical and biomarker data, and the clinical framework to conduct clinical trials efficiently building on these findings.
Highlights.
Pulmonary hypertension can arise from a heterogeneous array of pathological processes.
Clinical, genetic, and molecular phenotyping provide data that inform new approaches to classification of pulmonary vascular diseases.
A biomarker-based classification system can organize research and improve precision in patient evaluation and management.
Clinical advances will require integration of novel analytical approaches, development of translational research pathways, and incorporation of biomarkers into clinical trial designs.
Acknowledgments
Funding: Several authors report funding support from the NIH/NHLBI (K08HL128802 to W.M.O.; R35HL140019 to M.A.A.; R01HL146588 to E.L.B.; R01HL135142, R01HL137927, R01HL089856, and R01HL147148 to M.H.C.; R01HL136603 to A.A.D.; K01HL146980 to R.S.K.; to J.A.L.; to B.M.). W.M.O. received a research grant from the Cardiovascular Medical Research and Education Fund (CMREF). J.A.L. was supported by grants from the American Heart Association. M.R.W. was supported by the British Heart Foundation (RE/18/4/34215, SP/18/10/33975).
Abbreviations
- PVDOMICS
Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics (NCT02980887)
- WSPH
World Symposium on Pulmonary Hypertension
- CTEPH
chronic thromboembolic pulmonary hypertension
- mPAP
mean pulmonary artery pressure
- PAH
pulmonary arterial hypertension
- NHLBI
National Heart, Lung, and Blood Institute
- QTL
quantitative trait loci
- CMREF
Cardiovascular Medical Research and Education Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the view of the National Institutes of Health or United States Department of Health and Human Services. The authors report no disclosures relevant to the submitted manuscript. They do report the following unrelated industry relationships. A.H.R. has served as a consultant for Actelion, Bayer, Complexa, and United Therapeutics and owns shares in PHPrecisionMed.
S.Y.C. has served as a consultant for Zogenix, Aerpio, and United Therapeutics; S.Y.C. is a director, officer, and shareholder in Synhale Therapeutics; S.Y.C. has held research grants from Actelion and Pfizer; S.Y.C. has filed patent applications regarding the targeting of BMP signalling and metabolic pathways in PH. M.H.C. has received grant support from Bayer and GSK and served as a consultant for Genentech, AstraZeneca, and Illumina. A.A.D. has served as a consultant for Novartis. B.M. has received honoraria from AstraZeneca, Genentech, GSK, Regeneron, Sanofi, and Teva for consulting or lectures in the past, and has current research funding from Genentech and GSK. C.J.R. has received personal advisory board fees from Janssen and United Therapeutics. M.R.W. has consulted with Actelion, Novartis, GSK, and MorphogenIX in the development of biomarkers for pulmonary hypertension.
References
- 1.Simonneau G, Montani D, Celermajer DS et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huston JH, Maron BA, French J et al. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maron BA, Hess E, Maddox TM et al. Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016;133:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vachiery JL, Tedford RJ, Rosenkranz S et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53. [DOI] [PubMed] [Google Scholar]
- 5.Nathan SD, Barbera JA, Gaine SP et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NH, Delcroix M, Jais X et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemnes AR, Beck GJ, Newman JH et al. PVDOMICS: A Multi-Center Study to Improve Understanding of Pulmonary Vascular Disease Through Phenomics. Circ Res 2017;121:1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins IM, Moore TM, Blaisdell CJ, Abman SH. National Heart, Lung, and Blood Institute Workshop: improving outcomes for pulmonary vascular disease. Circulation 2012;125:2165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erzurum S, Rounds SI, Stevens T et al. Strategic plan for lung vascular research: An NHLBI-ORDR Workshop Report. Am J Respir Crit Care Med 2010;182:1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007;30:104–9. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes CJ, Batai K, Bleda M et al. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med 2019;7:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbaum L, Rhodes CJ, Otero-Nunez P, Wharton J, Wilkins MR. The application of 'omics' to pulmonary arterial hypertension. Br J Pharmacol 2020. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research N, Weinstein JN, Collisson EA et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013;45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraidenburg DR, Machado RF. A Review of Transcriptome Analysis in Pulmonary Vascular Diseases. Methods Mol Biol 2018;1783:259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes CJ, Wharton J, Ghataorhe P et al. Plasma proteome analysis in patients with pulmonary arterial hypertension: an observational cohort study. Lancet Respir Med 2017;5:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes CJ, Ghataorhe P, Wharton J et al. Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension. Circulation 2017;135:460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis GD, Ngo D, Hemnes AR et al. Metabolic Profiling of Right Ventricular-Pulmonary Vascular Function Reveals Circulating Biomarkers of Pulmonary Hypertension. J Am Coll Cardiol 2016;67:174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes CJ, Otero-Nunez P, Wharton J et al. Whole-Blood RNA Profiles Associated with Pulmonary Arterial Hypertension and Clinical Outcome. Am J Respir Crit Care Med 2020;202:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elinoff JM, Mazer AJ, Cai R et al. Meta-analysis of blood genome-wide expression profiling studies in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2020;318:L98–L111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med 2017;38:122–139. [DOI] [PubMed] [Google Scholar]
- 21.Oldham WM, Oliveira RKF, Wang RS et al. Network Analysis to Risk Stratify Patients With Exercise Intolerance. Circ Res 2018;122:864–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arneson D, Shu L, Tsai B, Barrere-Cain R, Sun C, Yang X. Multidimensional Integrative Genomics Approaches to Dissecting Cardiovascular Disease. Front Cardiovasc Med 2017;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Chaudhary K, Garmire LX. More Is Better: Recent Progress in Multi-Omics Data Integration Methods. Front Genet 2017;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon-Mimila P, Wang J, Huertas-Vazquez A. Relevance of Multi-Omics Studies in Cardiovascular Diseases. Front Cardiovasc Med 2019;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S, Ince-Dunn G, Suomalainen A, Elo LL. Integrative omics approaches provide biological and clinical insights: examples from mitochondrial diseases. J Clin Invest 2020;130:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin E, Han J, Luo W et al. On fusion methods for knowledge discovery from multi-omics datasets. Comput Struct Biotechnol J 2020;18:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng J, Zhang H, Li F. Investigating the relevance of major signaling pathways in cancer survival using a biologically meaningful deep learning model. BMC Bioinformatics 2021;22:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eicher T, Kinnebrew G, Patt A et al. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanfardino M, Franzese M, Pane K et al. Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases. J Transl Med 2019;17:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abifadel M, Varret M, Rabes JP et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–6. [DOI] [PubMed] [Google Scholar]
- 31.Cameron J, Holla OL, Ranheim T, Kulseth MA, Berge KE, Leren TP. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum Mol Genet 2006;15:1551–8. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161–5. [DOI] [PubMed] [Google Scholar]
- 33.Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 34.Sabatine MS, Giugliano RP, Wiviott SD et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9. [DOI] [PubMed] [Google Scholar]
- 35.Sweatt AJ, Hedlin HK, Balasubramanian V et al. Discovery of Distinct Immune Phenotypes Using Machine Learning in Pulmonary Arterial Hypertension. Circ Res 2019;124:904–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overmyer KA, Shishkova E, Miller IJ et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst 2021;12:23–40 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaptein YE, Karagodin I, Zuo H et al. Identifying Phenogroups in patients with subclinical diastolic dysfunction using unsupervised statistical learning. BMC Cardiovasc Disord 2020;20:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shomorony I, Cirulli ET, Huang L et al. An unsupervised learning approach to identify novel signatures of health and disease from multimodal data. Genome Med 2020;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee LY, Loscalzo J. Network Medicine in Pathobiology. Am J Pathol 2019; 189:1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allum F, Hedman AK, Shao X et al. Dissecting features of epigenetic variants underlying cardiometabolic risk using full-resolution epigenome profiling in regulatory elements. Nat Commun 2019;10:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun BB, Maranville JC, Peters JE et al. Genomic atlas of the human plasma proteome. Nature 2018;558:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich A, Wharton J, Thayer TE et al. Mendelian randomisation analysis of red cell distribution width in pulmonary arterial hypertension. Eur Respir J 2020;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinu FR, Beale DJ, Paten AM et al. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menche J, Sharma A, Kitsak M et al. Disease networks. Uncovering disease-disease relationships through the incomplete interactome. Science 2015;347:1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh VN, Jin RC, Rabello S et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 2012;125:1520–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertero T, Lu Y, Annis S et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest 2014;124:3514–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samokhin AO, Stephens T, Wertheim BM et al. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng F, Kovacs IA, Barabasi AL. Network-based prediction of drug combinations. Nat Commun 2019;10:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wishart DS, Feunang YD, Guo AC et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 2018;46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guney E, Menche J, Vidal M, Barabasi AL. Network-based in silico drug efficacy screening. Nat Commun 2016;7:10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng F, Desai RJ, Handy DE et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun 2018;9:2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng F, Lu W, Liu C et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat Commun 2019;10:3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song JS, Wang RS, Leopold JA, Loscalzo J. Network determinants of cardiovascular calcification and repositioned drug treatments. FASEB J 2020;34:11087–11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarantelli RA, Schweitzer F, Simon MA et al. Longitudinal Evaluation of Pulmonary Arterial Hypertension in a Rhesus Macaque (Macaca mulatta) Model of HIV Infection. Comp Med 2018;68:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stam K, van Duin RWB, Uitterdijk A, Cai Z, Duncker DJ, Merkus D. Exercise facilitates early recognition of cardiac and vascular remodeling in chronic thromboembolic pulmonary hypertension in swine. Am J Physiol Heart Circ Physiol 2018;314:H627–H642. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Nakajima M, Tokuda S, Ogawa A. Fluidic Culture and Analysis of Pulmonary Artery Smooth Muscle Cells for the Study of Pulmonary Hypertension. Anal Sci 2016;32:1217–1221. [DOI] [PubMed] [Google Scholar]
- 57.Ribas J, Sadeghi H, Manbachi A et al. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl In Vitro Toxicol 2016;2:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev 2012;21:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newman JH, Rich S, Abman SH et al. Enhancing Insights into Pulmonary Vascular Disease through a Precision Medicine Approach. A Joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop Report. Am J Respir Crit Care Med 2017;195:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall KT, Loscalzo J, Kaptchuk T. Pharmacogenomics and the Placebo Response. ACS Chem Neurosci 2018;9:633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med 2017;377:62–70. [DOI] [PubMed] [Google Scholar]
- 62.Hall KT, Vase L, Tobias DK et al. Historical Controls in Randomized Clinical Trials: Opportunities and Challenges. Clin Pharmacol Ther 2020. [DOI] [PubMed] [Google Scholar]
- 63.Elliott CG, Austin ED, Badesch D et al. United States Pulmonary Hypertension Scientific Registry (USPHSR): rationale, design, and clinical implications. Pulm Circ 2019;9:2045894019851696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badlam JB, Badesch DB, Austin ED et al. United States Pulmonary Hypertension Scientific Registry (USPHSR): Baseline Characteristics. Chest 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Heart L, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services The NHLBI BioData Catalyst. 2020. [Google Scholar]
- 66.Mailman MD, Feolo M, Jin Y et al. The NCBI dbGaP database of genotypes and phenotypes. Nat Genet 2007;39:1181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taliun D, Harris DN, Kessler MD et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021;590:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkinson MD, Dumontier M, Aalbersberg IJ et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang WHW, Wilcox JD, Jacob MS et al. Comprehensive Diagnostic Evaluation of Cardiovascular Physiology in Patients With Pulmonary Vascular Disease: Insights From the PVDOMICS Program. Circ Heart Fail 2020;13:e006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ivanova A, Israel E, LaVange LM et al. The precision interventions for severe and/or exacerbation-prone asthma (PrecISE) adaptive platform trial: statistical considerations. J Biopharm Stat 2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The IPFnet Strategy: Creating a comprehensive approach in the treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:527–8. [DOI] [PubMed] [Google Scholar]
- 72.Huang DT, Angus DC, Moss M et al. Design and Rationale of the Reevaluation of Systemic Early Neuromuscular Blockade Trial for Acute Respiratory Distress Syndrome. Ann Am Thorac Soc 2017;14:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Biodata collected from future clinical studies of pulmonary hypertension should be aggregated in a web-based repository that can be shared and correlated with any and all other research projects. Such a resource would provide a valuable historical control dataset that could be utilized in the design of prospective trials (62). The scope of data collected from PVDOMICS would make this a good template through which to harmonize future datasets. In addition, supporting the incorporation of datasets obtained through other NHLBI-supported projects, such as the Pulmonary Hypertension Breakthrough Initiative (PHBI), the PAH Biobank, and other repositories like the Pulmonary Hypertension Association Registry and the United States Pulmonary Hypertension Scientific Registry (63,64), would increase investigational power for the study of both rare and common forms of pulmonary vascular disease. Moreover, it is imperative to consider the development of international partnerships for data sharing to increase the power for subgroup analyses in this rare disease and provide for experimental cross-validation of molecular phenotypes identified through PVDOMICS.
These efforts will likely be more comprehensive, successful, and fruitful with financial and infrastructure support, such as the NHLBI-supported biomedical data platform BioData Catalyst (65), the NCBI Database of Genotypes and Phenotypes (dbGap) (66), or the NHLBI TOPMed database (67) to which PVDOMICS data will be deposited. Implementation of the FAIR Data Principles to make research data Findable, Accessible, Interoperable, and Reusable (68) will optimize reuse of these extensive biological data. Furthermore, these principles should extend to ensuring methodological vigilance, code availability, and full transparency with methodological details related to the application of these non-traditional analytical approaches. These resources will likely be as important to investigators outside the pulmonary vascular disease field as it is to investigators studying pulmonary hypertension.