Abstract
Prolonged B-cell depletion due to anti-CD20 monoclonal antibody (mAbs) therapy impairs the adaptive immune response, causing severe manifestations during COronaVIrus Disease-2019 (COVID-19). The cases of two patients under anti-CD20 therapy who experienced prolonged and severe COVID-19 successfully treated with mAbs against Severe Acute Respiratory Syndrome-CoV-2 spike proteins are reported.
Keywords: Anti-CD20 therapy, SARS-CoV-2 infection, B-cell depletion, Prolonged infection
Introduction
Patients with pre-existing comorbidities and immunosuppression, including anti-CD20 monoclonal antibody, widely used to treat hematological malignancies or autoimmune disease, are at greater risk for persistent Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-CoV-2) infection (He et al., 2020). Prolonged B-cell depletion impairs the adaptive immune response and the ability to produce neutralizing antibodies, causing severe manifestations and a prolonged course of COVID-19 (Mehta et al., 2020, Hueso et al., 2020). Here, we report the cases of two patients, admitted to the Lazzaro Spallanzani National Institute for Infectious Diseases (INMI), under anti-CD20 therapy who experienced prolonged and severe COVID-19, who were successfully treated with casirivimab and imdevimab mAbs against Severe Acute Respiratory Syndrome-CoV-2 (SARS-CoV-2) spike proteins.
Materials and methods
Cell-mediated immunity and inflammation
The frequency of B (CD19+), T (CD3+), CD4+ and CD8+ T cells, as well as the expression of CD38 on CD4+ and CD8+ T cells, was evaluated in the peripheral blood and in BAL by flow cytometry (OneFlow LST tube, BD Biosciences). The frequency of Spike-specific T-cells was quantified by ELISpot assay (Mabtech). Briefly, peripheral blood mononuclear cells (PBMC) were isolated and stimulated for 20 h with peptides overlapping the S protein (0.1 μg/ml, Miltenyi Biotech) and with PHA as a positive control. At the end of incubation, the assay was developed according to the manufacturer’s instruction. Results are shown as spot-forming cells (CFS) per million PBMC. Finally, inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α) were quantified by automatic ELISA (ELLA, Biotechne).
Microneutralization assay
For the microneutralization test, patients’ sera were heat-inactivated, diluted 1:10 in serum-free medium, and titrated in duplicate in two-fold dilutions. Equal volumes of 100 TCID50/well SARS-CoV-2 (2019-nCoV/Italy-INMI1; GISAID accession ID: EPI_ISL_412974) and serum dilutions were mixed and incubated at 37 °C for 30 min. Virus-serum mixtures were added to sub-confluent Vero E6 cells and incubated at 37 °C and 5% CO2 for two days. Then, a Crystal Violet 2% Formaldehyde solution was added to each well and removed after 30 min. The cytopathic effect was measured by photometer at 595 nm (Synergy HTX Biotek). To standardize inter-assay procedures, positive control samples showing high (1:160) and low (1:40) neutralizing activity were included. The highest serum dilution inhibiting at least 90% of the CPE was indicated as the neutralization titer and expressed as the reciprocal of serum dilution (MNA90). Serum from the National Institute for Biological Standards and Control, UK (NIBSC) with known neutralization titer was used as a reference in MNA (Research reagent for anti-SARS-CoV-2 Ab NIBSC code 20/130).
Case presentation
A 54-year-old female patient (pt1) with a history of multiple sclerosis treated with ocrelizumab since December 2018 (last administration on July 2020) and a 54-year-old male patient (pt2) with a diagnosis of stage 4 follicular lymphoma, treated with a single chemotherapy cycle followed by rituximab on October 2020, are described.
Case 1
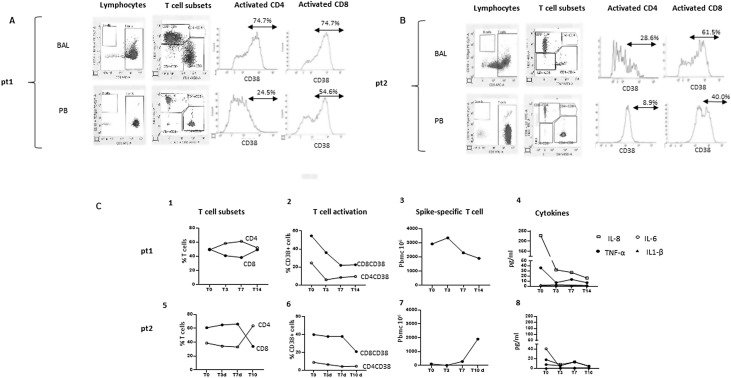
Pt1 was diagnosed with a paucisymptomatic SARS-COV-2 infection with nasopharyngeal swabs on October 24, 2020. For the persistence of fever on November 13, she was admitted at INMI, and a first chest CT scan showed the presence of widespread bilateral ground-glass opacities. Multiple nasopharyngeal swabs for SARS-CoV-2 detection were negative. On November 24, she underwent bronchoalveolar lavage (BAL) because a new chest CT scan showed two additional lesions in the upper right and lower left lobes. Due to fever persistence, a third chest CT scan showed the regression of the previous lesions and a new area in the lower right lobe. Other infections were excluded. On December 15, she performed a second BAL with SARS-CoV-2 positive real-time- reverse-transcriptase-polymerase-chain-reaction (rRT-PCR) assay results (gene S: cycle threshold (Ct) 24.7; gene ORF1ab: Ct 24.9. LIAISON MDX - Simplexa COVID-19 Direct, DiaSorin Molecular LLC, CA). Viral isolation from the BAL was negative. Subsequently, rRT-PCR SARS-CoV-2 positive detection was confirmed even in the first BAL sample. B-lymphocytes were undetectable in both BAL and peripheral blood, and the CD38+ marker of T lymphocytes immune activation was highly expressed on CD8+ and CD4+ cells (Figure 1 A). On December 18, she started antiviral (iv remdesivir, 200 mg on the first day followed by 100 mg on days two to five) and systemic steroid therapy (iv methylprednisone, 40 mg twice daily) followed, on December 24 by mAbs against SARS-CoV-2 spike protein (SARS-CoV-2-mAbs, casirivimab and imdevimab, single iv infusion of 1200 mg of both drugs). Treatment did not significantly modulate T-cell subsets (Figure 1/C1). After treatment, a significant reduction of CD4+ and CD8+ T-cell activation (Figure 1/C2) and inflammatory plasmatic cytokines (Figure 1/C4) was observed. Moreover, a strong T-cell response directed against Spike protein was not modified by mAbs treatment (Figure 1/C3). About the sero-virological findings, before treatments, the patient showed very low levels of SARS-CoV-2 specific antibodies IgM (Index: 1.3) in the absence of positivity for IgA and IgG (ENZY-WELL SARS-CoV-2 IgA, IgM and IgG, DIESSE, IT) with a neutralizing titer 1:20 (Supplementary data). After mAbs treatment, neutralizing antibodies reached a titer ≥1:640 and remained stable until the last follow-up. The patient, fully recovered, was discharged with both sputum and nasopharyngeal swabs negative for SARS-CoV-2 on day 45.
Figure 1.
A and B: Immune parameters in BAL and in peripheral blood before treatment. The frequency of B cells (CD19+), T-cell subsets (CD3+, CD4+, CD8+), and T-cell activation (CD38+ expression) were evaluated in bronchoalveolar lavage and in the peripheral blood by Flow cytometry before monoclonal treatment. Representative dot and histogram plots related to pt1 (A) and pt2 (B) are shown. C: Impact of monoclonal treatment on immune homeostasis and specific T-cell response. The kinetics of T-cell subsets (1,5), T-cell activation (2,6), Spike-specific T-cells (3,7), and inflammatory cytokines (4,8) were evaluated in pt1 and pt2 before (T0) and after three, seven, ten, or 14 days of monoclonal treatment (T3, T7, T10, T14). Specifically, T-cell subsets and T-cell activation were evaluated by flow cytometry and Spike–specific T-cells response by ELISpot assay after overnight stimulation of peripheral blood mononuclear cells with peptides overlapping the Spike protein. The inflammatory cytokines (IL1-β, IL-6, IL-8, TNF-α) were quantified by automatic ELISA.
Case 2
Pt2, on November 7, 2020, showed a positive nasopharyngeal swab for SARS-CoV-2 requested for routine screening. He was paucisymptomatic for several weeks and, on December 7, SARS-CoV-2 nasopharyngeal swabs were negative. Nevertheless, on December 16, he was admitted to INMI for acute onset of fever and dyspnoea. Chest-CT scan showed multiple bilateral ground-glass opacities, and BAL was positive for SARS-CoV-2 rRT-PCR (gene S: Ct 21.2; gene ORF1ab: Ct 20.7), and SARS-CoV-2 was isolated on Vero-E6 cell line from BAL samples (Colavita et al., 2020). B lymphocytes were undetectable in both BAL and peripheral blood, and CD38+ marker of T lymphocytes immune activation was strongly expressed in CD8+ and CD4+ cells (Figure 1B). SARS-CoV-2 IgA, IgM, and IgG detection were negative. Other infections were excluded. The patient experienced moderate acute respiratory distress syndrome (ARDS). He received oxygen support through a Venturi Mask (FiO2 40%), steroid therapy (iv methylprednisolone, 40 mg twice per day), anticoagulants (subcutaneous enoxaparin, 4000 IU twice per day), and empiric antimicrobial therapy. On December 31, non-invasive ventilation, antiviral (iv remdesivir), and SARS-CoV-2-mAbs (casirivimab and imdevimab, single iv infusion of 1200 mg of both drugs) were prescribed. After seven days, non-invasive ventilation was discontinued, and steroid therapy progressively tapered with marked improvement of clinical conditions until discharge. After mAbs treatment, T-cell subsets came back to a normal CD4/CD8 T-cell ratio (Figure 1C/5), and a significantly decreased CD4 and CD8 T-cell activation (Figure 1C/6) and plasmatic IL-6 (Figure 1C/8) were observed. Moreover, a weak spike-specific T-cells response was observed before and shortly after mAbs treatment, significantly increasing after ten days from the treatment initiation (Figure 1C/7). The low T-cell response before treatment, together with the lack of B-cells with consequent absence of Ab response, may be at least partially responsible for a high SARS-CoV-2 titer observed in this patient. Immediately after treatment, neutralizing antibodies reached a titer ≥1:640 and remained stable until the last follow-up (Supplementary data). The patient, fully recovered, was discharged with negative SARS-CoV-2 respiratory samples on day 60.
Discussion
Risk factors associated with severe COVID-19 include older age, male sex, and comorbidities such as obesity, diabetes, hypertension, coronary heart disease, chronic pulmonary or kidney disease (Williamson et al., 2020). An immunocompromised patient, such as with cancer or hematological malignancies or patients receiving immunosuppressive drugs, appears to be at higher risk of severe COVID-19 disease and mortality. In the context of multiple sclerosis (MS), it is currently debated whether MS patients are at an increased risk of SARS-CoV-2 infection or severe course of COVID-19 (Hughes et al., 2020, Sormani et al., 2021). Anti-CD20 mAbs are widely used to treat hematological malignancies or autoimmune diseases (Hawker et al., 2009). These long-lasting therapies are associated with an increased risk of infections such as tuberculosis, hepatitis B virus, and herpes virus reactivation and, for a few months, SARS-CoV-2 infection (Cantini et al., 2019, Iannetta et al., 2020). Anti-CD20 treatment targets specific Fab domains of CD20+ or CD19+ B lymphocytes, causing the selective depletion of circulating B-cells through natural killer cell-mediated antibody-dependent-cellular cytotoxicity, complement-dependent cytotoxicity, and antibody-triggered apoptosis (Crawford et al., 2006). B-cell depletion and consequently decreased immunoglobulin G level in both patients were associated with a persistent SARS-CoV-2 infection. Although anti-CD20 treatment does not appear to impact cellular-mediated responses (Chisari et al., 2021), our patients showed high levels of immune activated T-cells and pro-inflammatory cytokines, likely due to the prolonged SARS-CoV-2 infection. Interestingly, despite several negative SARS-CoV-2 swabs, pt2 experienced a severe disease with a protracted viral positive culture from BAL and low systemic inflammation probably caused by the recent cycle of chemotherapy for the B follicular lymphoma. Considering the clinical, virological, and immunological parameters, a tailored approach based on off-label remdesivir, systemic steroid, and mAbs against SARS-CoV-2 spike protein was adopted. Remdesivir treatment is to date the only COVID-19 antiviral treatment with a reported efficacy (Jiang et al., 2021); recently, multiple studies have described the development and characterization of potent neutralizing mAbs targeting the SARS-CoV-2 spike glycoprotein. Casirivimab and imdevimab, providing neutralizing SARS-CoV-2 Abs, lead to an effective and well-balanced immune response (Weinreich et al., 2020, Moderbacher et al., 2020). Overall, the combined therapy (steroid, mAbs, and remdesivir) brought to a reduced viral load, T-cell activation, and inflammatory cytokines without affecting the functionality of the peripheral blood spike-specific T-cell response. In the scenario of the COVID-19 pandemic, immunosuppression should be considered a red flag for unconventional clinical presentations and the occurrence of unexpected different viral kinetics. During the COVID-19 pandemic, anti-CD20 treatment is a matter of debate whether it should be delayed, avoided, or continued. While the use of anti-CD20 poses a risk of prolonged and/or severe SARS-COV-2 infection, the progression of hematologic or autoimmune disease must be avoided. To date, given the limited data available, clinicians must share the therapeutic path, taking into account the individual risk-benefit. Further studies are needed to understand the best clinical and therapeutical management of anti-CD20 recipients, such as the use of antiviral agents, systemic steroid, passive and active immunotherapy, and their impact on anti-SARS-CoV-2-specific antibody production.
Funding
This work was supported by Line1 Ricerca Corrente “Infezioni Emergenti e Riemergenti” and by Progetto Covid-2020-12371675 both funded by Italian Ministry of Health.
Ethical approval
Casirivimab and imdevimab were provided by Regeneron Pharmaceuticals, Inc. under a compassionate use protocol approved by the INMI Ethical Board for compassionate use and by the Italian Drug Agency (AIFA) for import authorization. Off-label remdesivir use was approved by AIFA. Patients’ written informed consent for publication was collected.
Conflict of interest
All authors have no conflict of interest to declare.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
Spallanzani COVID-19 Case Investigation Team: Angela Corpolongo, Laura Scorzolini, Luciana Lepore, Claudia Palazzolo, Nazario Bevilacqua, Maria Letizia Giancola, Tommaso Ascoli Bartoli, Giuseppe Ippolito, Silvia Rosati, Francesca Colavita, Michele Bibas, Saeid Najafi Fard, Stefania Notari, Eleonora Cimini, Silvia Meschi.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.04.068.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Cantini F., Niccoli L., Capone A., Petrone L., Goletti D. Risk of tuberculosis reactivation associated with traditional disease-modifying anti-rheumatic drugs and non-anti-tumor necrosis factor biologics in patients with rheumatic disorders and suggestion for clinical practice. Expert Opin Drug Saf. 2019;18(5):415–425. doi: 10.1080/14740338.2019.1612872. [DOI] [PubMed] [Google Scholar]
- Chisari C.G., Sgarlata E., Arena S., Toscano S., Luca M., Patti F. Rituximab for the treatment of multiple sclerosis: a review. J Neurol. 2021;8:1–25. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita F., Lapa D., Carletti F., Lalle E., Messina F., Rueca M., et al. Virological characterization of the first two COVID-19 patients diagnosed in Italy: phylogenetic analysis, virus shedding profile from different body sites and antibody response kinetics. Open Forum Infect Dis. 2020;10(7) doi: 10.1093/ofid/ofaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A., Macleod M., Schumacher T., Corlett L., Graye D. Primary T-cell expansion and differentiation in vivo requires antigen presentation by B-cells. J Immunol. 2006;176(6):3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- Hawker K., O’Connor P., Freedman M.S., Calabresi P.A., Antel J., Simon J., et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- He W., Chen L., Chen L., Yuan G., Fang Y., Chen W., et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueso T., Pouderoux C., Péré H., Beaumont A., Raillon L., Ader F., et al. Convalescent plasma therapy for B-cell depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R., Whitley L., Fitovski K., Schneble H.M., Muros E., Sauter A., et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2020;30(49) doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M., Cesta N., Stingone C., Malagnino V., Teti E., Vitale P., et al. Mild clinical manifestations of SARS-CoV-2 related pneumonia in two patients with multiple sclerosis under treatment with ocrelizumab. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Chen D., Cai D., Yi Y., Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: a network meta-analysis. J Med Virol. 2021;93(2):1171–1174. doi: 10.1002/jmv.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Porter J.C., Chambers R.C., Isenberg D.A., Reddy V. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol. 2020;2(10):E589–E590. doi: 10.1016/S2665-9913(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderbacher C.R., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.038. 996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2020;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.