Abstract
Background
Parkinson's Disease (PD) is among one of the common comorbidities in older patients. People with PD may be more vulnerable to severe pneumonia, due to the impairment of pulmonary function. Currently, the association between PD and COVID-19 is not yet established. This study aims to analyze the relationship between PD and in-hospital outcomes of COVID-19.
Materials and methods
We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until December 25th, 2020. All articles published on COVID-19 and Parkinson's Disease were retrieved. The quality of the study was assessed using the Newcastle Ottawa Scale (NOS) tool for observational studies and Joanna Briggs Institute (JBI) Critical Appraisal Tools for cross-sectional studies. Statistical analysis was done using Review Manager 5.4 software.
Results
A total of 12 studies with 103,874 COVID-19 patients were included in this meta-analysis. This meta-analysis showed that Parkinson's Disease was associated with poor in-hospital outcomes [[OR 2.64 (95% CI 1.75–3.99), p < 0.00001, I2 = 81%] and its subgroup which comprised of severe COVID-19 [OR 2.61 (95% CI 1.98–3.43), p < 0.00001, I2 = 0%] and mortality from COVID-19 [RR 2.63 (95% CI 1.50–4.60), p = 0.0007, I2 = 91%]. Meta-regression showed that the association was influenced by age (p = 0.05), but not by gender (p = 0.46) and dementia (p = 0.23).
Conclusions
Extra care and close monitoring should be provided to Parkinson's Disease patients to minimize the risk of infections, preventing the development of severe and mortality outcomes.
Keywords: Coronavirus disease 2019, COVID-19, Parkinson's disease, Movement disorder, Neurologic disease
1. Introduction
A novel coronavirus disease (COVID-19) was initially reported in the region of Wuhan around December 2019. This disease, which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), have made a national outbreak of severe pneumonia in China and spread worldwide just in a few months. On March 11th, 2020, the World Health Organization (WHO) have declared COVID-19 as a global pandemic. As of December 21, 2020, there have been 75,479,471 confirmed cases of COVID-19, including 1,686,267 deaths, globally [1].
Although most patients are thought to have a favorable prognosis, some individuals are more prone to develop severe manifestations, including fatal pneumonia that eventually results in death [2,3]. People with pre-existing medical conditions (e.g. hypertension, diabetes, coronary heart disease), older age, and male sex appear linked to more severe manifestations and mortality of COVID-19 [[4], [5], [6], [7], [8], [9], [10]]. Parkinson's Disease (PD) is the second most common age related neurodegenerative disease after Alzheimer's disease, affecting over 1% of the population over the age of 60 [11]. People with PD may be more vulnerable to severe pneumonia, due to its motor and non-motor symptoms. Moreover, the emotional impact of this pandemic, the social isolation, and prolonged immobility imposed by lockdown, may indirectly exacerbate both motor and psychic symptoms of PD, leading to worsening symptoms of the disease [12]. However, there is currently insufficient evidence regarding the association between Parkinson's Disease (PD) and the in-hospital outcomes from COVID-19. The aims of this systematic review and meta-analysis study is to explore the potential association between PD and in-hospital outcomes of COVID-19 infection.
2. Materials and Methods
2.1. Eligibility criteria
Studies were included in this review if met the following inclusion criteria: have all the features from the PICO (Population, Intervention, Control, and Outcome) criteria (P: hospitalized patients with positive/confirmed cases of COVID-19; I: a group of patients with Parkinson's Disease as their comorbidity; C: a group of patients without Parkinson's disease; O: in-hospital outcomes which comprise of severe COVID-19 OR mortality), type of study was a cohort or case-cohort design, and if the full-text article was available. The following types of articles were excluded: randomized control trial, clinical trial, cross-over design, articles other than original research (e.g., review articles, letters, or commentaries); case reports; articles not in the English language; articles on research in pediatric populations (17 years of age or younger); and articles on research in pregnant women.
2.2. Search strategy and study selection
A systematic search of the literature was conducted on PubMed and Europe PMC using the keywords “Parkinson's Disease” OR “parkinsonism” OR “movement disorders” AND “coronavirus disease 2019″ OR “COVID-19″, between 2019 and present time (December 25th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of Parkinson's Disease in COVID-19 patients with a clinically validated definition of “severe disease” and “mortality” were included in this meta-analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles. The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13].
2.3. Data extraction and quality assessment
Data extraction was performed independently by two authors, we used standardized forms that include author, year, study design, number of participants, age, number of patients with Parkinson's Disease, and proportion of patients with each outcome of COVID-19.
The outcome of interest was in-hospital outcomes that comprised of severe COVID-19 and mortality. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) or admission into ICU. Mortality outcome from COVID-19 was defined as the number of patients who were dead because of COVID-19.
Two investigators independently evaluated the quality of the included cohort and case-control studies using the Newcastle–Ottawa Scale (NOS) [14]. The selection, comparability, and outcome of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality. Meanwhile, the quality of the included cross-sectional studies was assessed by using the Joanna Briggs Institute (JBI) Critical Appraisal Tools For Analytical Cross Sectional Studies [15].
2.4. Statistical analysis
A meta-analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. We used the Generic Inverse Variance formula with random-effects models to calculate each outcome's risk in the Parkinson's Disease group compared with non-Parkinson's Disease group. The heterogeneity was assessed by using the I2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. If there was significant heterogeneity amongst the results, a further sensitivity analysis was conducted to determine the source of heterogeneity. After significant clinical heterogeneity was excluded, the randomized effects model was used for meta-analysis. The effect estimate was reported as odds ratio (OR) along with its 95% confidence intervals (CIs) for dichotomous outcomes, respectively. P-value was two-tailed, and the statistical significance was set at ≤0.05. Random effects meta-regression was performed using a maximum likelihood for pre-specified variables including age, gender, hypertension, diabetes, and dementia. We performed Begg's funnel-plot analysis to qualitatively assess the risk of publication bias.
3. Results
3.1. Study selection and characteristics
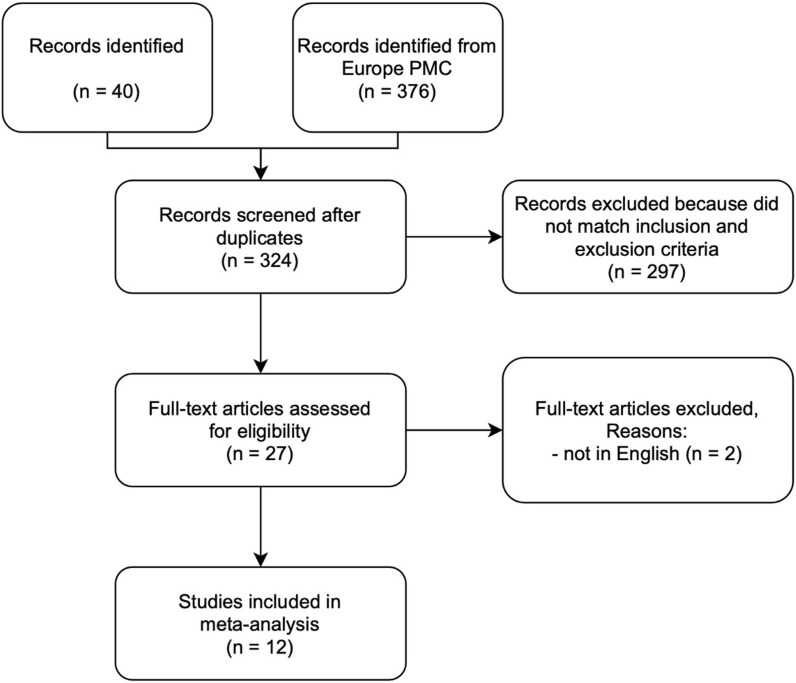
A total of 416 records were obtained through systematic electronic searches. After the removal of duplicates, 324 records remained. A total of 297 records were excluded after screening the titles/abstracts because they did not match our inclusion and exclusion criteria. After evaluating 27 full-texts for eligibility, 8 full-text articles were excluded because they do not have the control/comparison group, 5 full-text articles were excluded because they do not have the outcome of interest (severe COVID-19 OR mortality), 2 full-text articles were excluded because the articles were not in English, and finally, 12 studies [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]] with a total of 103,874 COVID-19 patients were included in the meta-analysis (Fig. 1 ). Of a total of 12 included studies, 9 studies were retrospective cohort, 1 study was prospective cohort, 1 study was case-control study, while the remaining 1 study was a cross-sectional study. The essential characteristics of the included studies are summarized in Table 1 .
Fig. 1.
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1.
Characteristics of included studies.
| Study | Sample size | Design | Outcome | Age (years) | Male n (%) | Hypertension n (%) | Diabetes n (%) | Dementia n (%) | Patients with PD n (%) | Patients without PD n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Brown EG et al. [16] 2020 | 77 | Cross-sectional | Severitya | 64.6 ± 16.3 | 26 (33.7%) | 21 (27.2%) | N/A | N/A | 51 (66.2%) | 26 (33.8%) |
| Clift AK et al. [17] 2020 | 10776 | Retrospective cohort | Mortality | 69.6 ± 17.9 | 5962 (55.3%) | N/A | 3153 (29.2%) | 1235 (11.4%) | 218 (2%) | 10558 (98%) |
| Hong KS et al. [18] 2020 | 98 | Retrospective cohort | Severitya | 55.4 ± 17.1 | 38 (38.8%) | 30 (30.6%) | 9 (9.2%) | 3 (3.1%) | 1 (1%) | 97 (99%) |
| Hwang J et al. [19] 2020 | 103 | Retrospective cohort | Mortality | 67.6 ± 15.3 | 52 (50%) | 57 (55%) | 35 (34%) | 11 (11%) | 2 (2%) | 101 (98%) |
| Jang JG et al. [20] 2020 | 110 | Retrospective cohort | Severityb | 56.9 ± 17 | 48 (43.6%) | 37 (33.6%) | 29 (26.4%) | 4 (3.6%) | 1 (0.9%) | 109 (99.1%) |
| Ji W et al. [21] 2020 | 7341 | Case-control | Severityc | 47 ± 19 | 2970 (40.5%) | 1628 (22.2%) | 1043 (14.2%) | 368 (5%) | 265 (3.6%) | 7076 (96.4%) |
| Mendes A et al. [22] 2020 | 235 | Retrospective cohort | Mortality | 86.3 ± 6.5 | 102 (43.4%) | 168 (71.5%) | 54 (23%) | 119 (50.6%) | 8 (3.5%) | 227 (96.5%) |
| Poblador-Plou B et al. [23] 2020 | 4412 | Retrospective cohort | Mortality | 67.7 ± 20.7 | 1819 (41.2%) | 1520 (34.4%) | 528 (11.9%) | 468 (10.6%) | 53 (1.2%) | 4359 (98.8%) |
| Rutten JJS et al. [24] 2020 | 1538 | Prospective cohort | Mortality | 84 ± 8.7 | 554 (36%) | N/A | 52 (3.3%) | 122 (7.9%) | 92 (6%) | 1446 (94%) |
| Yin R et al. [25] 2020 | 106 | Retrospective cohort | Severityd | 72.7 ± 11.8 | 64 (60.4%) | 72 (67.9%) | 37 (34.9%) | 20 (18.9%) | 2 (1.9%) | 104 (98.1%) |
| Zhang Q et al. [26] 2020 | 79049 | Retrospective cohort | Mortality | 58.4 ± 18.3 | N/A | N/A | N/A | N/A | 694 (0.8%) | 78355 (99.2%) |
| Zhao J et al. [27] 2020 | 29 | Retrospective cohort | Severityd | 51.1 ± 25.5 | 14 (48.3%) | N/A | 7 (24.1%) | N/A | 1 (3.4%) | 28 (96.6%) |
admission into intensive care unit (ICU).
respiratory distress (≥30 breaths per min) or admission into ICU.
oxygen saturation at rest ≤93%.
any of the followings: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; (4) critical complications.
3.2. Quality of study assessment
Studies with various study designs including cohort and case-control were included in this review and assessed accordingly with the appropriate scale or tool. The Newcastle Ottawa Scale (NOS) was used to assess the cohort and case-control studies (Table 2 ), while the Joanna Briggs Institute Critical Appraisal checklist was used for case series studies (Table 3 ). All included studies were rated ‘good’ based on the criteria used in the Newcastle Ottawa Scale (NOS) and the Joanna Briggs Institute Critical Appraisal checklist. In conclusion, all studies were deemed fit to be included in the meta-analysis.
Table 2.
Newcastle-Ottawa quality assessment of observational studies.
| First author, year | Study design | Selectiona | Comparabilityb | Outcomec | Total score | Result |
|---|---|---|---|---|---|---|
| Clift AK et al. [17] 2020 | Cohort | **** | ** | *** | 9 | Good |
| Hong KS et al. [18] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Hwang J et al. [19] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Jang JG et al. [20] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Ji W et al. [21] 2020 | Case-control | *** | ** | *** | 8 | Good |
| Mendes A et al. [22] 2020 | Cohort | *** | ** | **** | 9 | Good |
| Poblador-Plou B et al. [23] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Rutten JJS et al. [24] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Yin R et al. [25] 2020 | Cohort | *** | ** | *** | 8 | Good |
| Zhang Q et al. [26] 2020 | Cohort | ** | ** | *** | 7 | Good |
| Zhao J et al. [27] 2020 | Cohort | *** | ** | *** | 8 | Good |
(1) representativeness of the exposed cohort; (2) selection of the non-exposed cohort; (3) ascertainment of exposure; (4) demonstration that outcome of interest was not present at start of study.
(1) comparability of cohorts on the basis of design or analysis, (maximum two stars).
(1) assessment of outcome; (2) was follow-up long enough for outcomes to occur; (3) adequacy of follow up of cohorts.
Table 3.
Joanna Briggs Institute Critical Appraisal tool for cross-sectional study.
| Brown EG et al. [16] 2020 | |
|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | Yes |
| 2. Were the study subjects and the setting described in detail? | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes |
| 4. Were objective, standard criteria used for measurement of the condition? | Yes |
| 5. Were confounding factors identified? | Yes |
| 6. Were strategies to deal with confounding factors stated? | Yes |
| 7. Were the outcomes measured in a valid and reliable way? | Yes |
| 8. Was appropriate statistical analysis used? | Yes |
| Quality | Include study |
3.3. Parkinson's disease and outcomes
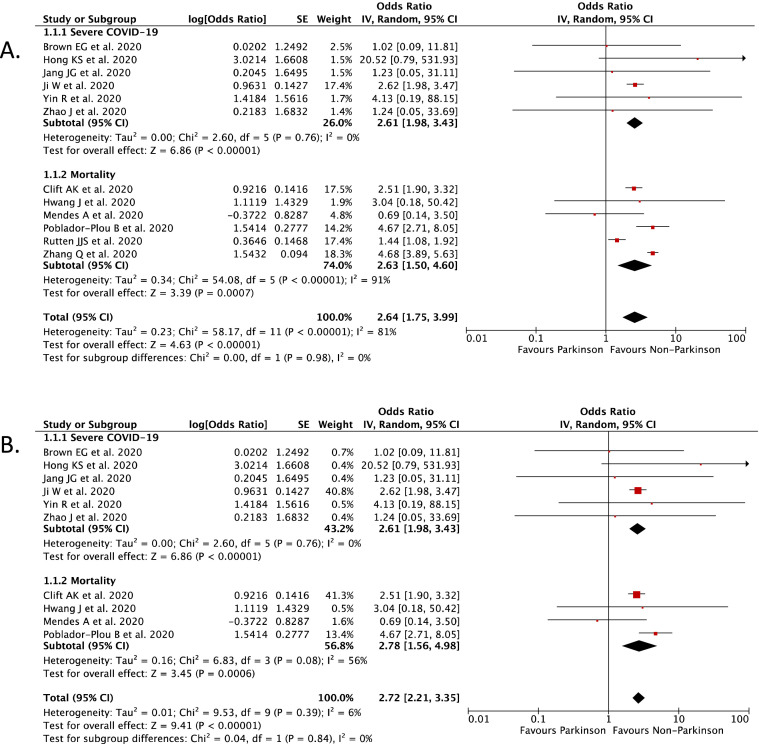
Our pooled analysis showed that Parkinson's Disease (PD) was associated with poor in-hospital outcomes of COVID-19 [OR 2.64 (95% CI 1.75–3.99), p < 0.00001, I 2 = 81%] (Fig. 2 A). Subgroup analysis showed that Parkinson's Disease (PD) was associated with severe COVID-19 [OR 2.61 (95% CI 1.98–3.43), p < 0.00001, I 2 = 0%], and mortality from COVID-19 [RR 2.63 (95% CI 1.50–4.60), p = 0.0007, I 2 = 91%].
Fig. 2.
(A) Forest plot that demonstrates the association of Parkinson's Disease with in-hospital outcomes and its subgroup which comprises of severe COVID-19 and mortality.
(B). Sensitivity analysis performed by removing study by Rutten JJS et al. and Zhang Q et al. which cause significant heterogeneity still showed significant association between Parkinson's Disease with in-hospital outcomes of COVID-19.
We then performed a sensitivity analysis to found the studies which are responsible for the significant heterogeneity. After removing the study by Rutten JJS et al. [24] and Zhang Q et al. [26], the heterogeneity was dropped from 81% into 6% and the pooled analysis still showed that Parkinson's Disease (PD) was associated with poor in-hospital outcomes of COVID-19, but with low heterogeneity [OR 2.72 (95% CI 2.21–3.35), p < 0.00001, I 2 = 6%] (Fig. 2B).
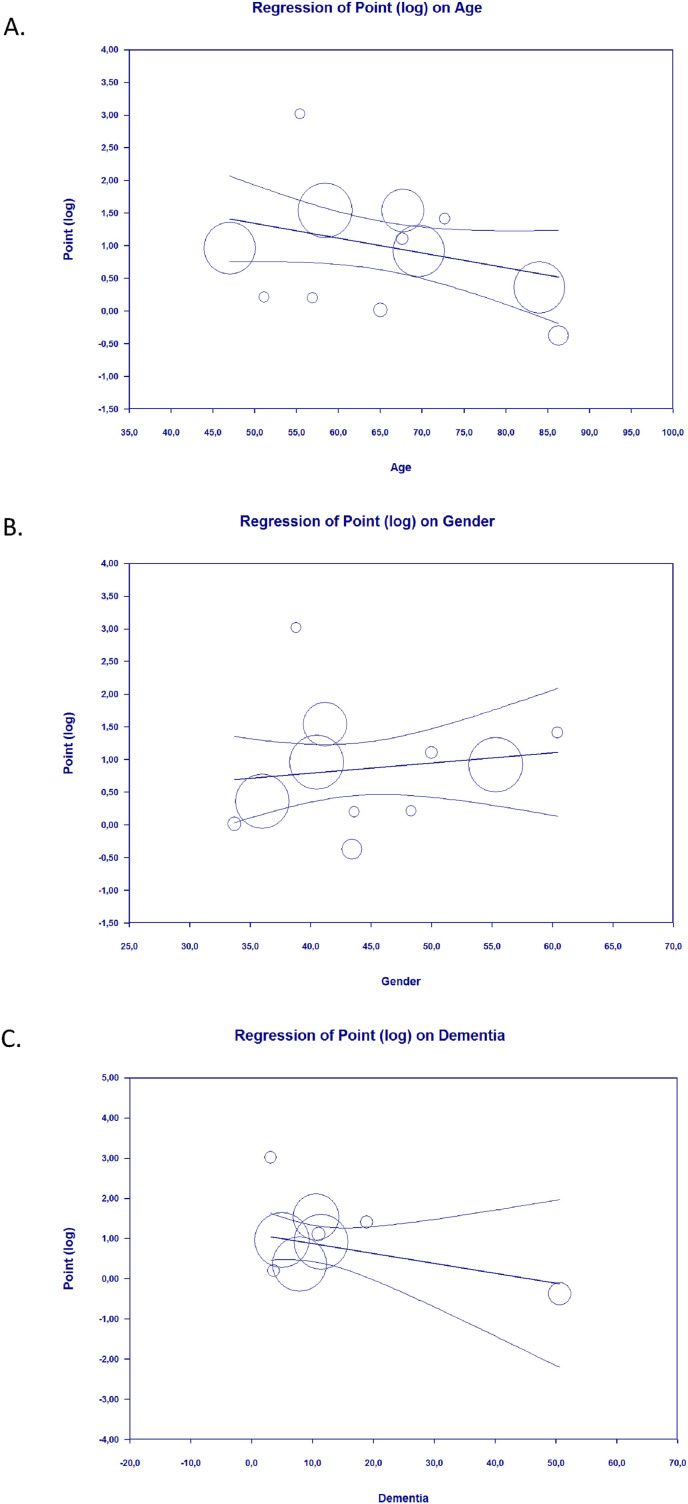
3.4. Meta-regression
Meta-regression showed that the association between Parkinson's Disease and in-hospital outcomes of COVID-19 was affected by age (p = 0.05) (Fig. 3 A), but not affected by gender (p = 0.46) (Fig. 3B), hypertension (p = 0.44), diabetes (p = 0.58), and dementia (p = 0.23) (Fig. 3C).
Fig. 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between Parkinson's Disease and in-hospital outcomes was affected by age [A], but not by gender [B] and dementia [C].
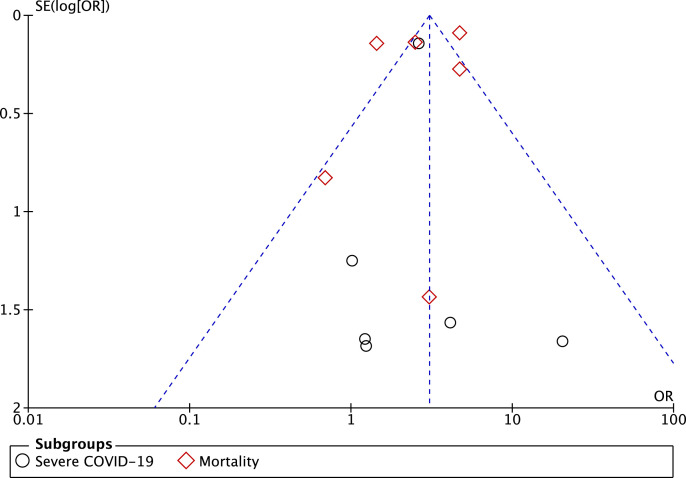
3.5. Publication bias
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between Parkinson's Disease and in-hospital outcomes of COVID-19 (Fig. 4 ), showing no indication of publication bias.
Fig. 4.
Funnel plot analysis for the association of Parkinson's Disease with in-hospital outcomes and its subgroup which comprises of severe COVID-19 and mortality.
4. Discussion
This is the first systematic review and meta-analysis which analyze the potential association between Parkinson's Disease (PD) and in-hospital outcomes of COVID-19. Based on our pooled analysis of available data, PD seems to be associated with an enhanced risk of severity and mortality from COVID-19. This association was influenced by age, but not by gender, hypertension, diabetes, and dementia. Several reasons can be proposed to explain this result. First, most of the patients with Parkinson's Disease (PD) were old and have other co-existing comorbid conditions that could increase the severity of infections [28,29]. Parkinson's Disease was most often diagnosed in people over age of 60 and only 4% of all cases are diagnosed before age 50 [29,30]. Patients of older age tend to present with atypical symptoms of COVID-19. They may present afebrile with non-respiratory symptoms, such as delirium or isolated functional decline without any obvious physical symptoms [31]. These atypical presentations of COVID-19 may impede the early recognition of the disease, increase COVID-19 spread, and mortality. Moreover, in a meta-analysis of 611,583 COVID-19 patients, age over 60 years was found to be a risk factor for disease progression [32]. This fact could be influenced by both the physiological aging process and, especially, the greater prevalence in older adult patients and patients with Parkinson's Disease of frailty and comorbidities that contribute to a decrease in functional reserve that reduces intrinsic capacity and resilience and hinders the fight against infections [32,33]. Older people will have a condition called “immune senescence” which consist of (1) decreased neutrophil phagocytosis and oxidative burst; (2) decreased macrophages chemotaxis and phagocytosis; and (3) decreased number of peripheral naïve T and B cells which will lead to a reduced ability to respond to new antigen [34]. Elderly patients with Parkinson's Disease may also experience respiratory muscle rigidity and impaired cough reflex alongside pre-existing dyspnea, which could lead to increased COVID-19 severity [35]. Second, patients with Parkinson's Disease may experience worsening of both motor and non-motor symptoms during SARS-CoV-2 infection. A recently published studies have shown that 31.9% of patients with pre-existing neurological disorders, including Parkinson's Disease experienced a worsening of neurological symptoms during COVID-19 infection, independent of age and disease duration [36,37]. Worsening of Parkinson's Disease symptoms is either due to the effects of the acute systemic inflammatory response, altered dopaminergic signaling or the pharmacokinetics changes during infection [38]. This condition can caused a significant stress, depression or even anxiety to Parkinson's Disease patients, where all of these can lead to reduction of quality of life (QoL) and finally higher mortality from the disease [39]. Third, patients with Parkinson's Disease (PD) depend on the medications to control their symptoms. Meanwhile, COVID-19 pandemic has caused interruption in the PD-related care, including medical care, exercise, and social activities. Some hospitals even closed the Parkinson's clinics because of this pandemic [40]. The disruption in the medical care can caused irregular visit to the hospitals and more difficulties in obtaining PD medications because PD medications needs prescription, which may force the Parkinson's Disease patients to decrease or even stop their medications [40]. This can also lead to worsening of Parkinson's Disease symptoms which increase their mortality rate, as described previously.
The limitation of this study is that the data/information regarding the other confounding factors such as patients' nutritional status, duration of Parkinson's Disease, severity of motor symptoms, and daily medication that can affect the relationship between Parkinson's Disease and clinical outcomes of COVID-19 were lacking in the included studies, therefore cannot be analyzed and should still be considered. However, with this study, we hope that Parkinson's Disease can further be considered as an important factor and comorbidity in COVID-19 patients.
5. Conclusions
Our study suggests that patients with Parkinson's Disease were likely to develop poor in-hospital outcomes of COVID-19. Hence, patients with Parkinson's Disease should be given extra care and monitoring to minimize exposure to the virus. Physicians should be aware of the risk of atypical COVID-19 manifestations and exacerbations of Parkinson's Disease symptoms. Telemedicine-based practice with neurologists can also be used to maintain patient's treatment and provide remote evaluations in PD patients to minimize their visit to the hospitals. Physicians should also be engaged in close monitoring of Parkinson's Disease patients with suspected COVID-19, for early diagnosis and treatment to avoid severe infections. Finally, Parkinson's Disease should be regarded as an important factor in future risk stratification models for COVID-19.
Funding
None.
Declaration of competing interest
None.
Acknowledgment
None.
References
- 1.World Health Organization Coronavirus disease (COVID-19): weekly epidemiological update – 22 December 2020. 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020
- 2.Hariyanto T.I., Rizki N.A., Kurniawan A. Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: a meta-analysis. Int. Arch. Otorhinolaryngol. 2020 doi: 10.1055/s-0040-1719120. 10.1055/s-0040-1719120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariyanto T.I., Kristine E., Jillian Hardi C., Kurniawan A. Efficacy of lopinavir/ritonavir compared with standard care for treatment of coronavirus disease 2019 (COVID-19): a systematic review. Infect. Disord. - Drug Targets. 2020 doi: 10.2174/1871526520666201029125725. [DOI] [PubMed] [Google Scholar]
- 4.Gold M.S., Sehayek D., Gabrielli S., Zhang X., McCusker C., Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad. Med. 2020;132(8):749–755. doi: 10.1080/00325481.2020.1786964. [DOI] [PubMed] [Google Scholar]
- 5.Hariyanto T.I., Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus. Apher. Sci. 2020:102926. doi: 10.1016/j.transci.2020.102926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(6):1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariyanto T.I., Putri C., Arisa J., Situmeang R.F.V., Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020;93:104299. doi: 10.1016/j.archger.2020.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur. Arch. Psychiatr. Clin. Neurosci. 2020:1–3. doi: 10.1007/s00406-020-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is associated with severe coronavirus disease 2019 (COVID-19) infection. Am. J. Med. Sci. 2020 doi: 10.1016/j.amjms.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14(100):19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmich R.C., Bloem B.R. The impact of the COVID-19 pandemic on Parkinson's disease: hidden sorrows and emerging opportunities. J. Parkinsons Dis. 2020;10(2):351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulis A.V., Pladevall M., Riera-Guardia N., Varas-Lorenzo C., Hazell L., Berkman N.D., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: Joanna Briggs Institute Reviewer's Manual. Aromataris E., Munn Z., editors. The Joanna Briggs Institute; 2017. Chapter 7: systematic reviews of etiology and risk.https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 16.Brown E.G., Chahine L.M., Goldman S.M., Korell M., Mann E., Kinel D.R., et al. The effect of the COVID-19 pandemic on people with Parkinson's disease. J. Parkinsons Dis. 2020;10(4):1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong K.S., Lee K.H., Chung J.H., Shin K.C., Choi E.Y., Jin H.J., et al. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in daegu, South Korea: a brief descriptive study. Yonsei Med. J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang J.M., Kim J.H., Park J.S., Chang M.C., Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol. Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in daegu, korea. J. Kor. Med. Sci. 2020;35(23):e209. doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji W., Huh K., Kang M., Hong J., Bae G.H., Lee R., et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in korea: a nationwide case-control study. J. Kor. Med. Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendes A., Serratrice C., Herrmann F.R., Genton L., Périvier S., Scheffler M., et al. Predictors of in-hospital mortality in older patients with COVID-19: the COVIDAge study. J. Am. Med. Dir. Assoc. 2020;21(11):1546–1554. doi: 10.1016/j.jamda.2020.09.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int. J. Environ. Res. Publ. Health. 2020;17(14):5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutten J.J.S., van Loon A.M., van Kooten J., van Buul L.W., Joling K.J., Smalbrugge M., et al. Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. J. Am. Med. Dir. Assoc. 2020;21(12):1791–1797. doi: 10.1016/j.jamda.2020.10.034. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin R., Yang Z.Q., Wei Y.X., Li Y.M., Chen H., Liu Z., et al. Clinical characteristics of 106 patients with neurological diseases and co-morbid coronavirus disease 2019: a retrospective study. medRxiv. 2020 doi: 10.1101/2020.04.29.20085415. [DOI] [Google Scholar]
- 26.Zhang Q., Schultz J.L., Aldridge G.M., Simmering J.E., Narayanan N.S. Coronavirus disease 2019 case fatality and Parkinson's disease. Mov. Disord. 2020;35(11):1914–1915. doi: 10.1002/mds.28325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J., Gao H.Y., Feng Z.Y., Wu Q.J. A retrospective analysis of the clinical and epidemiological characteristics of COVID-19 patients in henan provincial people's hospital, zhengzhou, China. Front. Med. 2020 Jun 5;7:286. doi: 10.3389/fmed.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Zeng F., Jin W.S., Zhu C., Wang Q.H., Bu X.L., et al. Comorbidity burden of patients with Parkinson's disease and Parkinsonism between 2003 and 2012: a multicentre, nationwide, retrospective study in China. Sci. Rep. 2017;7(1):1671. doi: 10.1038/s41598-017-01795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeve A., Simcox E., Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14(100):19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tysnes O.B., Storstein A. Epidemiology of Parkinson's disease. J. Neural. Transm. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 31.D'Adamo H., Yoshikawa T., Ouslander J. Coronavirus disease 2019 in geriatrics and long‐term care: the ABCDs of COVID ‐19. J. Am. Geriatr. Soc. 2020;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 32.Bonanad C., García-Blas S., Tarazona-Santabalbina F., Sanchis J., Bertomeu-González V., Fácila L., et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peball M., Mahlknecht P., Werkmann M., Marini K., Murr F., Herzmann H., et al. Prevalence and associated factors of sarcopenia and frailty in Parkinson's disease: a cross-sectional study. Gerontology. 2019;65(3):216–228. doi: 10.1159/000492572. [DOI] [PubMed] [Google Scholar]
- 34.Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M., et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Wamelen D.J., Leta V., Johnson J., Ocampo C.L., Podlewska A.M., Rukavina K., et al. Drooling in Parkinson's disease: prevalence and progression from the non-motor international longitudinal study. Dysphagia. 2020;35(6):955–961. doi: 10.1007/s00455-020-10102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubota T., Kuroda N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin. Neurol. Neurosurg. 2020:106349. doi: 10.1016/j.clineuro.2020.106349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cilia R., Bonvegna S., Straccia G., Andreasi N.G., Elia A.E., Romito L.M., et al. Effects of COVID-19 on Parkinson's disease clinical features: a community-based case-control study. Mov. Disord. 2020;35(8):1287–1292. doi: 10.1002/mds.28170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brugger F., Erro R., Balint B., Kägi G., Barone P., Bhatia K.P. Why is there motor deterioration in Parkinson's disease during systemic infections-a hypothetical view. NPJ Parkinsons Dis. 2015;1:15014. doi: 10.1038/npjparkd.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalash A., Roushdy T., Essam M., Fathy M., Dawood N.L., Abushady E.M., et al. Mental Health, physical activity, and quality of life in Parkinson's disease during COVID-19 pandemic. Mov. Disord. 2020;35(7):1097–1099. doi: 10.1002/mds.28134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo D., Han B., Lu Y., Lv C., Fang X., Zhang Z., et al. Influence of the COVID-19 pandemic on quality of life of patients with Parkinson's disease. Parkinson's Dis. 2020;2020:1–6. doi: 10.1155/2020/1216568. [DOI] [PMC free article] [PubMed] [Google Scholar]