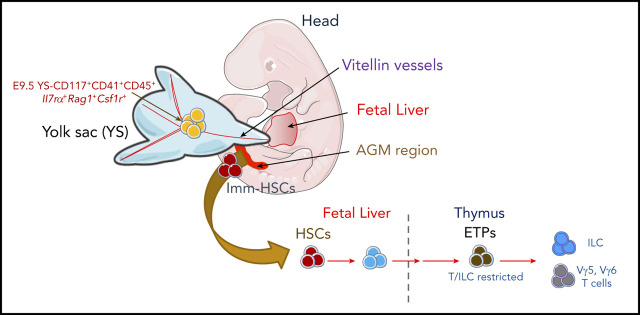
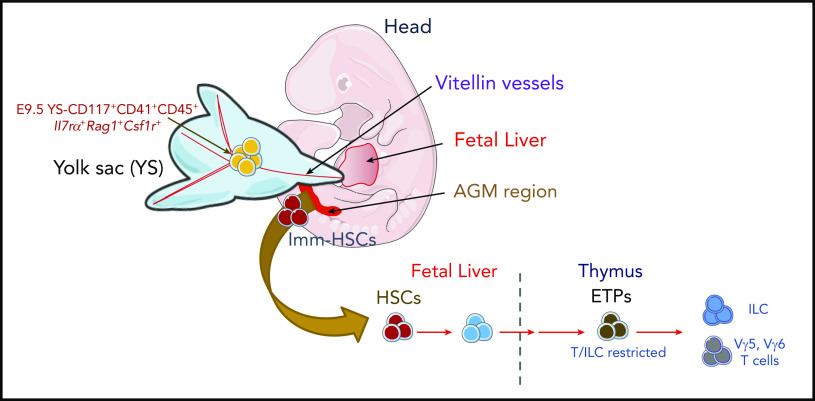
There is a Blood Commentary on this article in this issue.
Key Points
Embryonic-restricted bipotent T/innate lymphoid cell progenitors shape the thymic architecture by controlling mTEC maturation.
These cells do not originate from YS but rather from intraembryonic-derived progenitors.
Abstract
During embryonic development, multiple waves of hematopoietic progenitors with distinct lineage potential are differentially regulated in time and space. Two different waves of thymic progenitors colonize the fetal thymus where they contribute to thymic organogenesis and homeostasis. The origin, the lineage differentiation potential of the first wave, and their relative contribution in shaping the thymus architecture, remained, however, unclear. Here, we show that the first wave of thymic progenitors comprises a unique population of bipotent T and innatel lymphoid cells (T/ILC), generating a lymphoid tissue inducer cells (LTi's), in addition to invariant Vγ5+ T cells. Transcriptional analysis revealed that innate lymphoid gene signatures and, more precisely, the LTi-associated transcripts were expressed in the first, but not in the second, wave of thymic progenitors. Depletion of early thymic progenitors in a temporally controlled manner showed that the progeny of the first wave is indispensable for the differentiation of autoimmune regulator–expressing medullary thymic epithelial cells (mTECs). We further show that these progenitors are of strict hematopoietic stem cell origin, despite the overlap between lymphopoiesis initiation and the transient expression of lymphoid-associated transcripts in yolk sac (YS) erythromyeloid-restricted precursors. Our work highlights the relevance of the developmental timing on the emergence of different lymphoid subsets, required for the establishment of a functionally diverse immune system.
Visual Abstract
Introduction
The embryonic thymus is colonized by 2 waves of distinct hematopoietic progenitors, named early thymic progenitors (ETPs).1 The first wave of ETP follows a unique developmental program as they share phenotype, transcriptional signature, and restricted T-cell differentiation potential with HSAlo α4β7− fetal liver (FL) common lymphoid progenitors (CLPs).2,3 After embryonic day 15.5 (E15.5), the thymus is colonized by a second wave of ETPs that share phenotype, transcriptional signature, and differentiation potential with lymphomyeloid-primed progenitors (LMPPs).2-5
Three lymphoid subsets are exclusively developed during embryonic life: the skin-resident Vγ5+ T cells, the lungs and the genital urinary tract resident Vγ6+, and a subset of group 3 innate lymphoid cells (ILCs; ILC3) known as lymphoid tissue inducers (LTi’s).1 Both Vγ5+ T cells and LTi’s are required for Aire+ medullary thymic epithelial cell (mTEC) development before birth.6-9 Aire expression is concomitant with that of CD80, a marker of mTEC maturation, and neonatal Aire expression is necessary for inducing lifelong T-cell tolerance throughout life.8,10,11 LTi’s are also required for secondary lymphoid tissue organization12,13 whereas Vγ6 T cells contribute to tissue remodeling.14
The majority of embryonic thymic ILCs are LTi’s gradually lost after birth and only a small population of ILC2 is maintained throughout life.15 The first ETPs generate Vγ5+ T cells,3,16 however, it is important to determine their origin, whether they generate other embryonic lymphoid cells such as thymic LTi’s, and what is their contribution to shaping the thymic architecture in a time-dependent manner.8
Most hematopoietic cells are constantly differentiating from hematopoietic stem cells (HSCs) although few lineages are HSC independent and exclusively produced during embryonic development.1,17 For example, yolk sac (YS) erythromyeloid progenitors (EMPs) contribute to tissue-resident macrophages that persist throughout life.18 This layered organization of the hematopoietic system raises the possibility that, similar to tissue macrophages, the first innate lymphocytes could also be YS derived. Accordingly, cells identified as lymphomyeloid-restricted progenitors (LMPs) were identified in E9.5 YS before HSC activity is detected.18 They were shown to express lymphoid-associated genes (Il7r, Rag2, Rag1) and were proposed as the origin of the first wave of ETP.19 Independent reports have also converged to support the notion that Vγ5+ and the Vγ6+ T cells might originate from YS progenitors, independent of HSCs.20,21 In contrast, Vγ5+ and B1a B cells were shown to be preferentially derived from a particular FL-transient HSC-like subset marked by a history of Flk2 expression.22
In this report, we studied the developmental origin, differentiation potential, and the temporal requirement of the first ETPs for shaping the thymus microenvironment. We showed, in single-cell differentiation assays, that the first ETPs comprise bipotent T/LTi progenitors that generate the thymic LTi’s. Colonization of Rag2−/−γc−/− thymic lobes with the first, but not the second, wave ETPs reconstituted the LTi compartment. Depletion of the first wave of lymphoid progenitors resulted in lower numbers of Vγ5+ T cells, LTi’s, and severely reduced numbers of CD80+ mTECs after birth. Therefore, the major role of the first ETPs appear to be providing cells that drive thymic organogenesis and tissue homeostasis.1,10,23
We further demonstrated, using an inducible lineage tracer model, that the first ETPs originate from HSCs. YS-derived progenitors, although showing a history of expression of lymphoid-associated genes such as Il7r, Rag2 and Rag1, failed to generate a lymphoid progeny in vivo or in vitro. This transient expression, also found in other FL myeloid progenitors, is restricted to embryonic hematopoiesis and uncoupled from differentiation potential. Altogether, our data highlight the impact of embryonic developmental timing on lymphocyte production, gene expression, organogenesis, and heterogeneity of the immune system.
Methods
Detailed methods are available in supplemental Methods (available on the Blood Web site).
Animals
Mice were bred in house under specific pathogen-free conditions. Il7rCre,24 Rag2−/−γc−/− CD45.1,25 ROSA26Tomato,26 and ROSA26YFP mice were on C57BL/6 background, Csf1rMeriCreMer mice27 were on FVB background. All animal manipulations were performed according to the French Agriculture ministry and to the European Parliament Directive 2010/63/EU.
Fate mapping of Il7r+ hematopoietic progenitors
Il7rCre female mice were crossed to homozygous ROSA26YFP. Indicated embryonic tissues and adult tissues were analyzed by flow cytometry.
Lineage tracing of Csf1r+ progenitors
Csf1rMeriCreMer were crossed with either ROSA26YFP or ROSA26Tomato reporter mice. A single dose of 75 µg/g (body weight) of 4-Hydroxytamoxifen (4OHT; Sigma) injected either at E8.5 or E9.5 induced reporter recombination and was supplemented with 37.5 µg/g (body weight) progesterone (Sigma-Aldrich) (to counteract the mixed estrogen agonist effects of 4OHT).28 To induce recombination at E10.5, female mice received a single dose of 1.2 mg of 4OHT and 0.6 mg of progesterone.
In vivo anti-IL7Rα (A7R34) antibody injections
Interleukin 7 receptor α (IL-7Rα) blockade was performed by 3 successive IV injections of 1 mg of anti-IL7Rα antibody (A7R34)29 (a gift from Shin Nishikawa, Riken, Wako, Saitama, Japan) in pregnant female mice at E10.5, E12.5, and E14.5 of gestation.
Results
E13 ETP retain T/LTi lineage potential in vitro and generate LTi cells in a fetal thymic microenvironment
The analysis of the transcriptional profiles of the first (E13) and the second (E18) ETP waves identified the overexpression, in the former, of LTi-associated transcripts (Rorc, Cd4, Cxcr5, Il1r1, and Ltb)3,30 (supplemental Figure 1a). Genes involved in γδ T-cell development (Bhlhe40, Cited4, B4galnt2, Tgm2, and Txk) were also differentially expressed between E13 and E18 ETPs (supplemental Figure 1a). Of note, Sell and Ly6a were upregulated in E18 ETPs, in line with their LMPP affiliation.2,3 The E14 double negative 3 (DN3) thymocytes were devoid of terminal deoxynucleotidyl transferase (TdT)activity (supplemental Figure 1b) generating T cells with a restricted T-cell repertoire, suggesting that they do not contribute significantly to the adaptive T cell.
We performed a 2-step culture2,5 (Figure 1A) to detect the potential of a single ETP (sorted as in Ramond et al,3 Lin−CD117+CD44+CD24low) to generate all major lymphoid lineages (T, B, ILC, natural killer [NK]) and myeloid cells (granulocyte/macrophage [GM]) (Figure 1B). We found that E13 ETPs only generated T and ILC whereas E18 ETPs retained B and myeloid potential, consistent with our previous observations.3 Most ETPs gave rise to T and ILCs, although a few (6% to 15%) only generated T cells and none gave ILCs only. Approximately 59% of the clones derived from E18 ETPs generate all subsets of ILC and NK cells in contrast to 24% of E13 ETPs (Figure 1B-C), suggesting that E13 ETPs have a more restricted differentiation potential.
Figure 1.
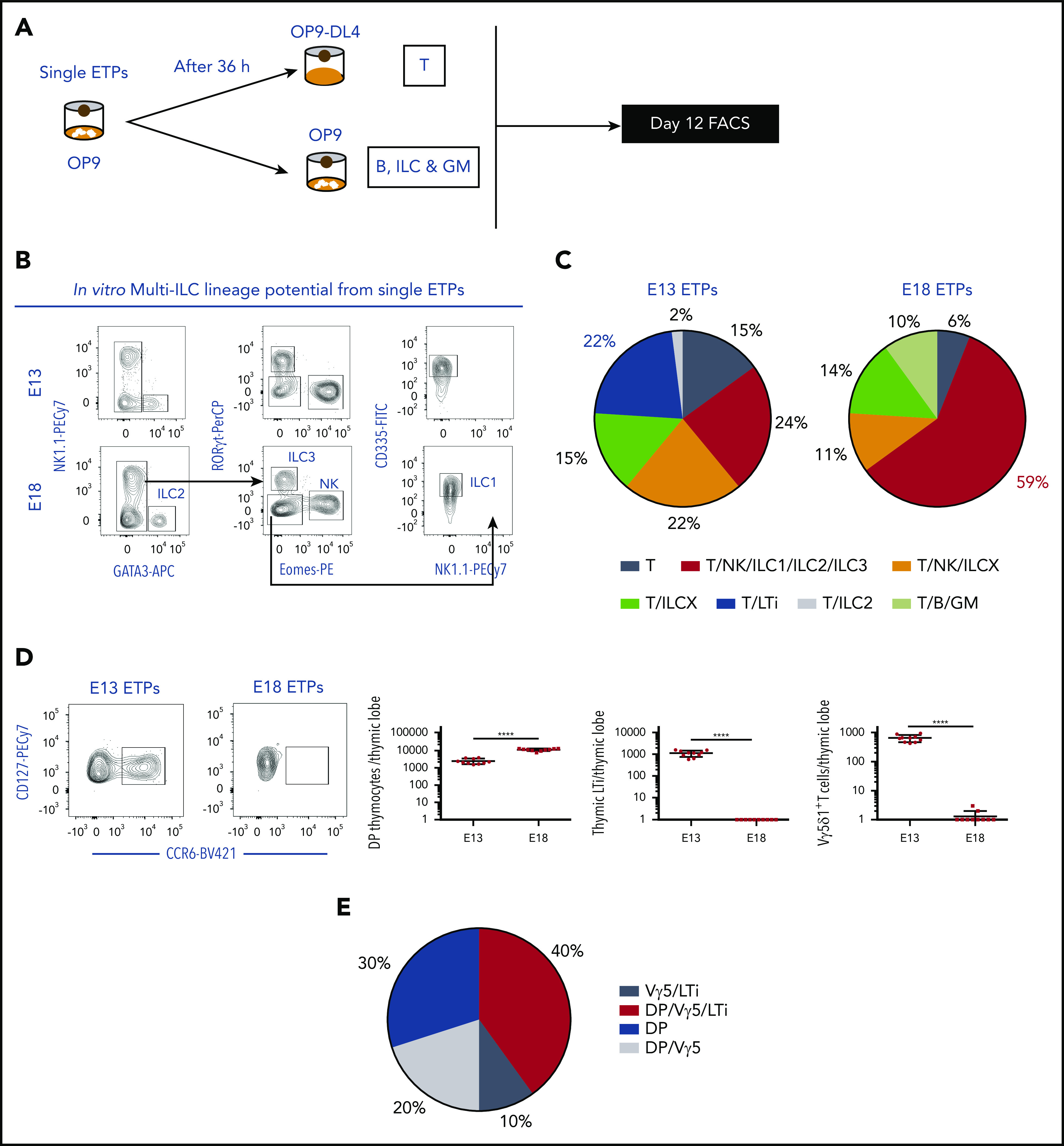
E13 ETPs retain T/LTi lineage potential in vitro and generate LTi cells in a fetal thymic microenvironment. (A) Experimental strategy for combined T, B, and ILC lineage potential analysis of single ETPs. Single ETPs from either E13 or E18 were sorted onto OP9 stromal cells supplemented with IL-7, Flt3L, KitL, and IL-2. After 36 hours, clones were subdivided in conditions that sustain differentiation of T cells (OP9-DL4 supplemented with IL-7, Flt3L, KitL, and IL-2) or B, myeloid, and ILC/LTi (OP9 supplemented with IL-7, Flt3L, KitL, IL-2, and GM-CSF). (B) Representative flow cytometry plots outlining the gating strategy used to identify ILC1, ILC2, ILC3, and NK cells of E13 and E18 ETP single-cell–derived multi-ILC lineage clone. (C) Pie chart indicating all phenotypic combinations in individual clones detected after in vitro differentiation of E13 (81 clones analyzed of 240 sorted cells; cloning efficiency [CE], 34%) or E18 ETPs (86 clones analyzed of 240 sorted cells; CE, 36%) in 2 independent experiments. Frequency of clones generating only T cells (steel blue); T, NK, ILC1, ILC2, and ILC3 (red) (different in E13 and E18 clones; P < .0001); T and LTi (Rorc+CCR6+) (blue) (different in E13 and E18 clones; P < .0001); T, NK, LCX, 2 other ILC subsets (orange); T and ILC2 (gray); T and 2 other ILC subsets (light green); T, B, and myeloid cells (lime green) (different in E13 and E18 clones; P = .0036). All other combinations were not significantly different in E13 and E18 clones. (D) Flow cytometry plots of Rag2−/−γc−/− E14.5 thymic lobes reconstituted with 500 E13 ETPs or 500 E18 ETPs per lobe and gated CD3− and stained for the expression of CD127 and CCR6 (left plots). Numbers of double-positive (DP) thymocytes per lobe (right plot), thymic LTi per lobe (middle plot) Vγ5Vδ1 γδT cells (left plot). FTOCs were analyzed at day 12 after reconstitution. ****P < .0001. (E) Single E13 ETP were processed as in panel A. After 36 to 48 hours in culture, clones were harvested and colonized single Rag2−/−γc−/− E14.5 thymic lobes. After 24 to 48 hours in hanging drop lobes were transferred into filters and analyzed at day 12 (n = 10 lobes).
The striking difference between the 2 subsets was the high frequency of bipotent T/LTi precursors (22%) only observed in E13 ETPs (Figure 1C). Accordingly, a subset of E13-derived ILC3 expressed the LTi marker CCR6 (Figure 1D).
We colonized Rag2−/−γc−/− non-irradiated fetal thymic organ culture (FTOC) with limited numbers of E13 or E18 ETPs and found that only the former could generate thymic LTi’s (Figure 1D). In addition, only E13 ETPs generated Vγ5+ γδ T cells and both waves generated double-positive (DP) thymocytes (Figure 1D), in line with previous report from our laboratory.3 To ensure E13 ETP bipotency in a thymic environment, we repeated the experiment in Figure 1A, and used the expanded progeny of single E13 ETPs in Rag2−/−γc−/− FTOCs. Results indicate that 50% of ETPs generated Vg5 expressing γδ T cells and LTi’s in fetal thymic (Figure 1E).
Altogether, E12.5-15.5 ETPs comprise unique bipotent progenitors, LTi biased, that generate thymic LTi and Vγ5+ γδ T cells3 within a thymic microenvironment.
First-wave ETPs have an LTi-primed transcriptional profile
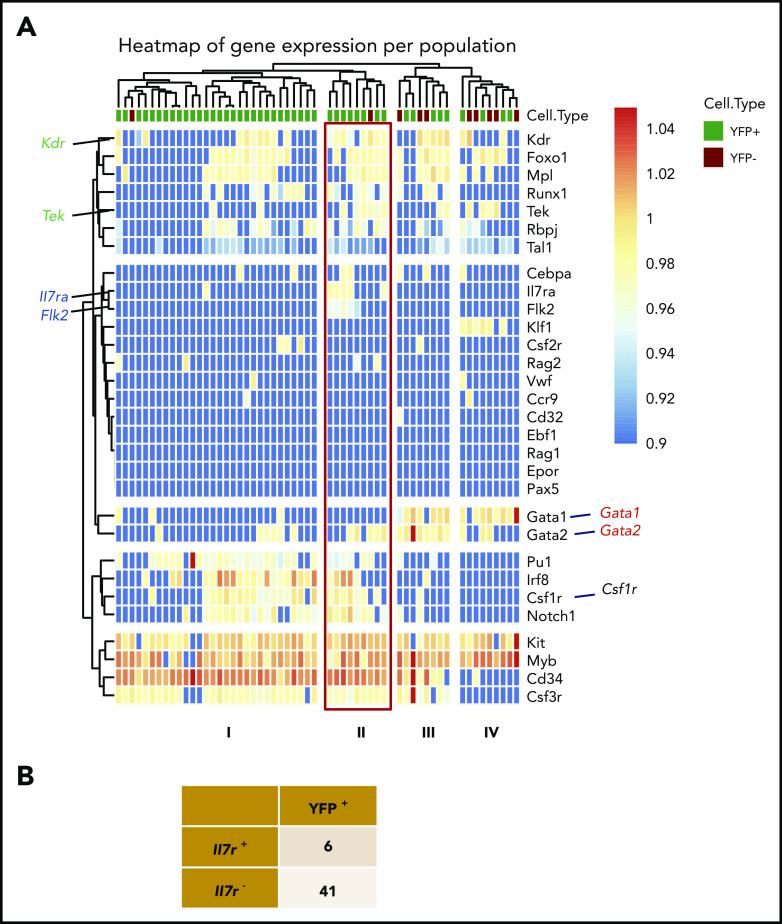
To understand the molecular basis of the E13 ETP LTi lineage potential, we performed single-cell transcriptional analysis by multiplex quantitative reverse transcription polymerase chain reaction for the expression of 41 lymphoid-associated genes. Unsupervised hierarchical clustering identified 4 distinct clusters (Figure 2A). Cluster I, comprising a majority (76%) of E18 ETPs, showed no ILC transcript expression, indicating that they are not primed toward a specific ILC lineage, consistent with their multipotent ILC differentiation capacity. E13 ETPs, found in clusters II and IV, expressed the ILC-associated transcripts Tox, Tcf7, and Gata3. Expression of LTi transcripts Cxcr6, Cd4, Rorc, Cxcr5, Il1r1, and Ltb was found in cells within cluster IV, consistent with LTi-differentiation potential.
Figure 2.
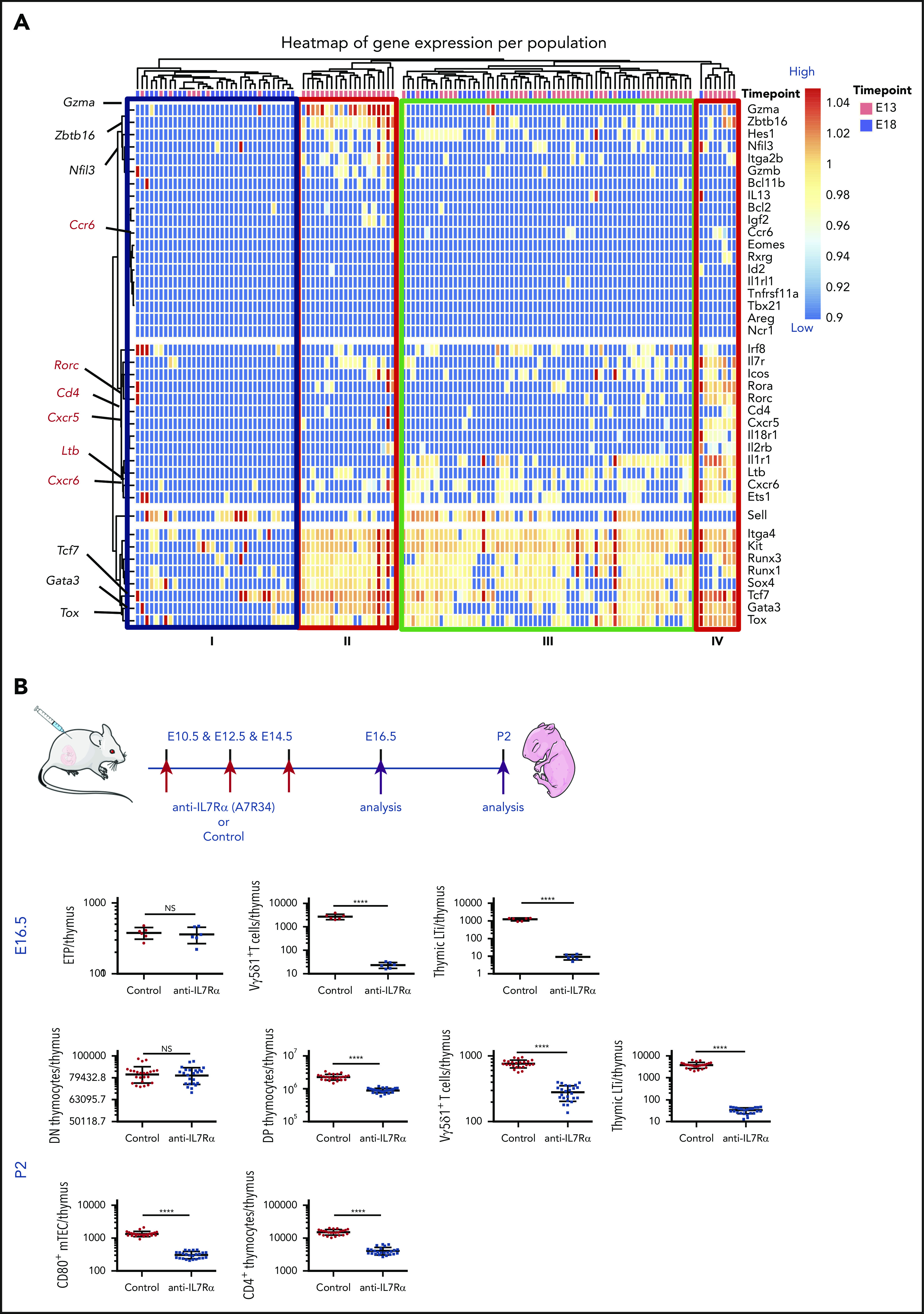
E13 ETPs have a transcriptional LTi signature and are required for the maturation (CD80+) of mTECs. (A) Single-cell multiplex qPCR analyzed by hierarchical clustering of single E13 and E18 ETPs (80 cells each) for the expression of ILC lineage-specific transcripts (right margin). Each column represents a cell (E13 ETP in salmon, E18 ETP in blue) and each row represents 1 gene (of 41 genes). Highlighted are ILC-associated transcripts (black) and LTi-associated transcripts (in red). Analysis was done by normalizing expression to 2 independent housekeeping genes (Gapdh and Actinb) in R package Phenograph as in Perchet et al63 and represented in a code color where red represents high expression and blue low expression. Data are pooled from 2 independent experiments. (B) Pregnant female mice were injected at E10.5, E12.5, and E14.5 with 1 mg of anti-IL-7Ra antibody A7R34 (red arrows indicate time points of anti–IL-7Ra injections and purple arrows indicate time points of analysis). Thymic lobes were analyzed at E16.5 for the presence of ETP (Lin−CD117+CD44+CD24low), for Vγ5Vδ1 γδT cells, and for LTi (CD127+CCR6+TcR−CD3− cells) (n = 6), or at P2 for the presence of DN (CD4−CD8−γδ−), DP(CD4+CD8+), CD4+ thymocytes, and of Vγ5Vδ1 γδT cells, for LTi (CD127+CCR6+TcR−CD3− cells), and for CD80+ mTECs (EpCAM+Ly51−UEA.1+CD45−) (n = 24). Plots show numbers of the respective populations per thymus. Data from 2 independent experiments. ****P < .0001.
Cells in cluster II expressed Zbtb16, Gzmb, Nfil3, Itga2b, and Gzma associated with innate-like T cells.31 Cluster III comprised two-thirds of E13 and one-third of E18 ETPs and was characterized by the lower expression of Tox, Gata3, Tcf7, Sox4, Runx1, and Runx3 indicative of common ILC priming and consistent with a broad ILC-differentiation potential (Figure 2A). Altogether, these results indicated that the first ETPs are unique in expressing LTi and invariant T cells transcripts, indicative of transcriptional priming.
First-wave ETPs contribute to the maturation of the thymic mTECs
To assess the role of the first ETPs and their progeny in mTEC maturation, we used in vivo injection of A7R34 anti-IL7Rα32-34 antibody in pregnant female mice. We injected A7R34 antibody at E10.5, E12.5, and E14.5, and analyzed the embryos at E16.6 and 2 days after birth (P2) newborn mice. At E16.5, we observed severely reduced numbers of Vγ5+ T and LTi cells in the thymus although the second-wave ETPs3 were not altered (Figure 2B).
P2-treated mice had normal numbers of DN thymocytes consistent with an unaffected second wave of thymic colonization. P2 had significantly lower numbers of Vγ5+ T, and drastically lower numbers of LTi, compared with controls, indicating efficient depletion of the first ETPs and their progeny (Figure 2B). Interestingly, CD80+ mTECs (mature Aire-expressing cells)8 were reduced approximately threefold, indicating that the first ETPs are required for mTEC maturation, around birth. CD4+CD8+ DP cells were also reduced by less than threefold. Our results indicate that the progeny of the first-wave ETPs contribute to mTEC maturation, required for conventional T-cell negative selection.
Neonatal thymectomy does not impact numbers of tissue-resident Vγ5+ and Vγ6+ γδ T cells
Vγ5+ and Vγ6+ γδ T cells in the skin and in the lymph nodes (LNs), respectively, are of embryonic origin,16,35 although they develop asynchronously. To assess the dependency of these γδ subsets on thymic output, indicating their origin in the first or second wave of ETPs, we performed neonatal thymectomy. Six weeks after neonatal thymectomy or sham operation as control (Figure 3A), complete thymectomy was ascertained by the absence of DP CD4/CD8 cells in the tissue around the thymus location (Figure 3B). Thymectomized mice (n = 6) were severely lymphopenic as shown by the numbers of CD4 and CD8 αβ T cells in the inguinal (i) LN (Figure 3D) and in the spleen. Splenic CD44− αβ T cells were severely reduced (Figure 3E), a sign of activation of the T-cell compartment consequent to the lymphopenia and consistent with the absence of a functional thymus in these mice. The numbers of Vγ5 + γδ T cells in the skin and of iLN Vγ4− IL17-producing γδ T cells (considered as Vγ6+) were similar in sham and in thymectomized animals (Figure 3C-D). By contrast, iLN Vγ4+ IL-17–producing γδ T cells were severely reduced (Figure 3D). These experiments suggest that these 2 embryonic γδ T-cell subsets are likely derived from the first wave ETPs. All other αβ or γδ T depend on postnatal thymic output and thus are issued from the second wave of ETPs.
Figure 3.
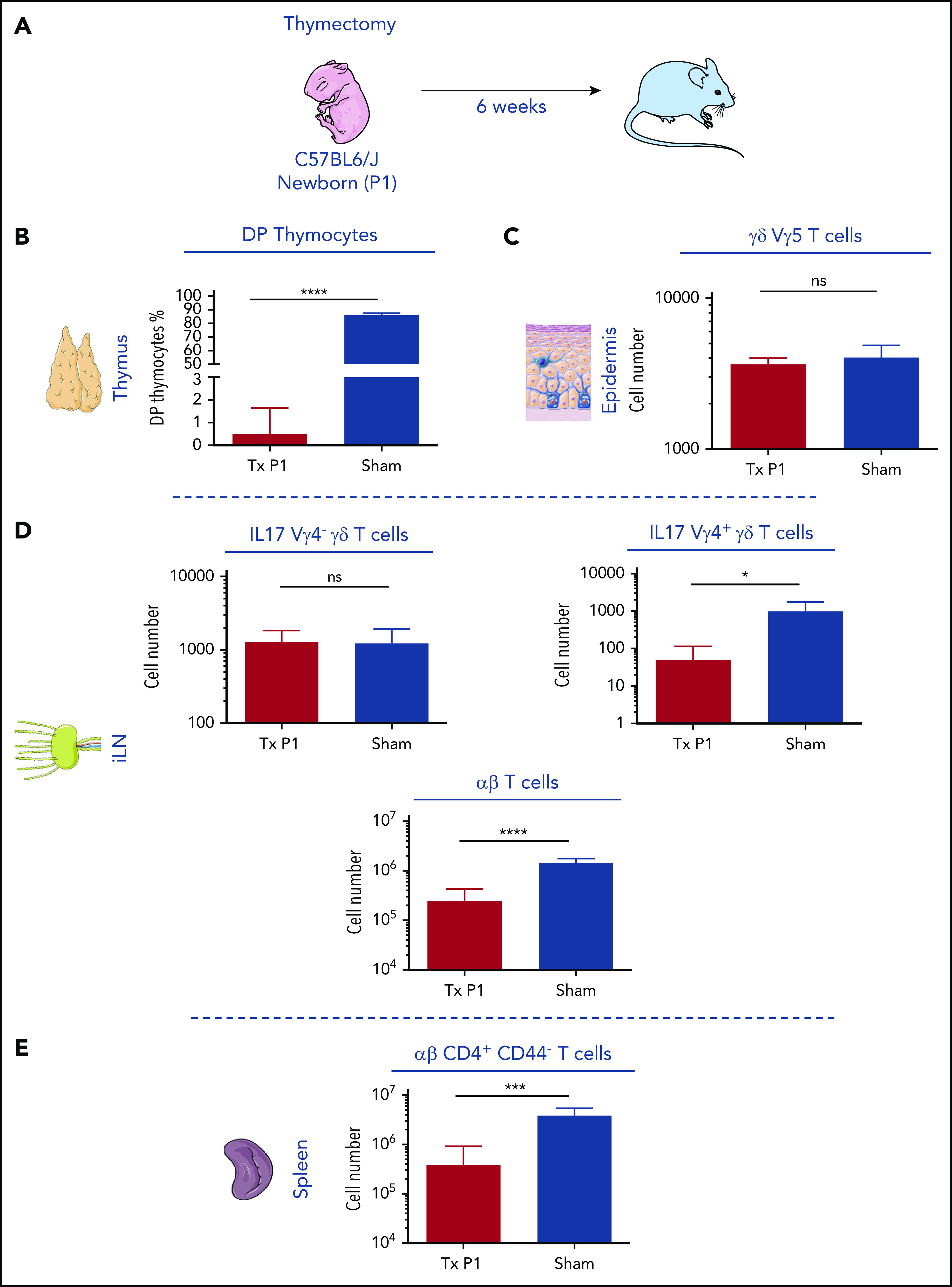
Neonatal thymectomy has no effect on embryonic-derived Vγ5 and Vγ6 γδ T cells. (A) Schematic diagram of neonatal thymectomy experiment. P1 newborn mice were thymectomized and analyzed 6 weeks later. (B) Frequencies of double-positive CD4/CD8 T cells found in the thymus and perithymic tissue isolated from sham and thymectomized mice, respectively. (C) Number of Vγ5+ γδ T cells in the epidermis isolated from 1 ear of sham and thymectomized mice. (D) Number of Vγ6 IL17 γδ T cells, Vγ4 IL17 γδ T cells, and αβ T cells in 1 inguinal lymph node, isolated from sham and thymectomized mice. (E) Number of αβ CD4+CD44− T cells in the spleen. *P < .05, ***P < .001, and ****P < .0001 (Student t test). Data are representative of at least 4 mice in each group from 2 independent experiments. Data are depicted as mean plus or minus standard error of the mean (SEM).
Embryonic ETPs have multi-ILC lineage potential in vivo
To assess E13 and E18 ETP ILC lineage potential in vivo, CD45.2+ E13 or E18 ETPs were IV transferred into 1-day old CD45.1+ Rag2−/−γc−/− mice and E13 FL HSCs (Lin−CD117+Sca-1+CD150+CD48−) were used as a positive control (supplemental Figure 2a). Five weeks after transfer,36 mice engrafted with HSCs and E18 ETPs developed B cells, ILCs, and myeloid cells (supplemental Figure 2c and data not shown). By contrast, mice engrafted with E13 ETPs generated only ILC (supplemental Figure 2b), consistent with the E13 ETP-restricted lymphoid potential. Analysis of PB 3 and 6 weeks or splenic cells 5 weeks after transplantation show a total absence of B and myeloid progeny from E13 ETPs (supplemental Figure 2b,e). Few T cells were generated in these chimeras because Rag2−/−γc−/− mice have an atrophic thymus and ETPs downregulate CCR7 and CCR9 once in the thymus, consequently losing their ability to home back to the thymus.37
Analysis of intestinal lamina propria, where all ILC lineages coexist, showed that both waves of ETPs generated NK cells (EOMES+), ILC1 (EOMES−), ILC3 (RORɣt+), and ILC2 (GATA3+). In the lung, we found ILC2; in the spleen, NK cells; and in the liver, NK cells and ILC1 (supplemental Figure 2c-d). These results indicated that E13 and E18 ETPs have the capacity to generate all ILC subsets in vivo recapitulating the expected tissue distribution.
Emergence of Il7r-expressing YS progenitors in E9.5 embryos
We used a lineage tracer mouse line, Il7rαCreRosa26YFP,24 to identify the origin of the first Il7r+ ETPs. YFP-expressing cells were undetectable in E8.5 embryos, in YS and embryo proper, in either the Kit+ or Kit− fraction (data not shown). Consistent with previous studies, YFP+ progenitors were first detected in Lin−Kit+CD41+ E9.5 YS (Figure 4A-C) and undetected in the aorta-gonad-mesonephros (AGM) or in the placenta (Figure 4D). Five percent to 8% of the c-kit+ YS cells were YFP+ (Figure 4B-C), expressed CD31, CD16/32, and lacked surface expression of CD115, CD127, CD135, the phenotype of YS EMPs (Lin−Kit+CD41+YFP−; Figure 4E).38,39
Figure 4.
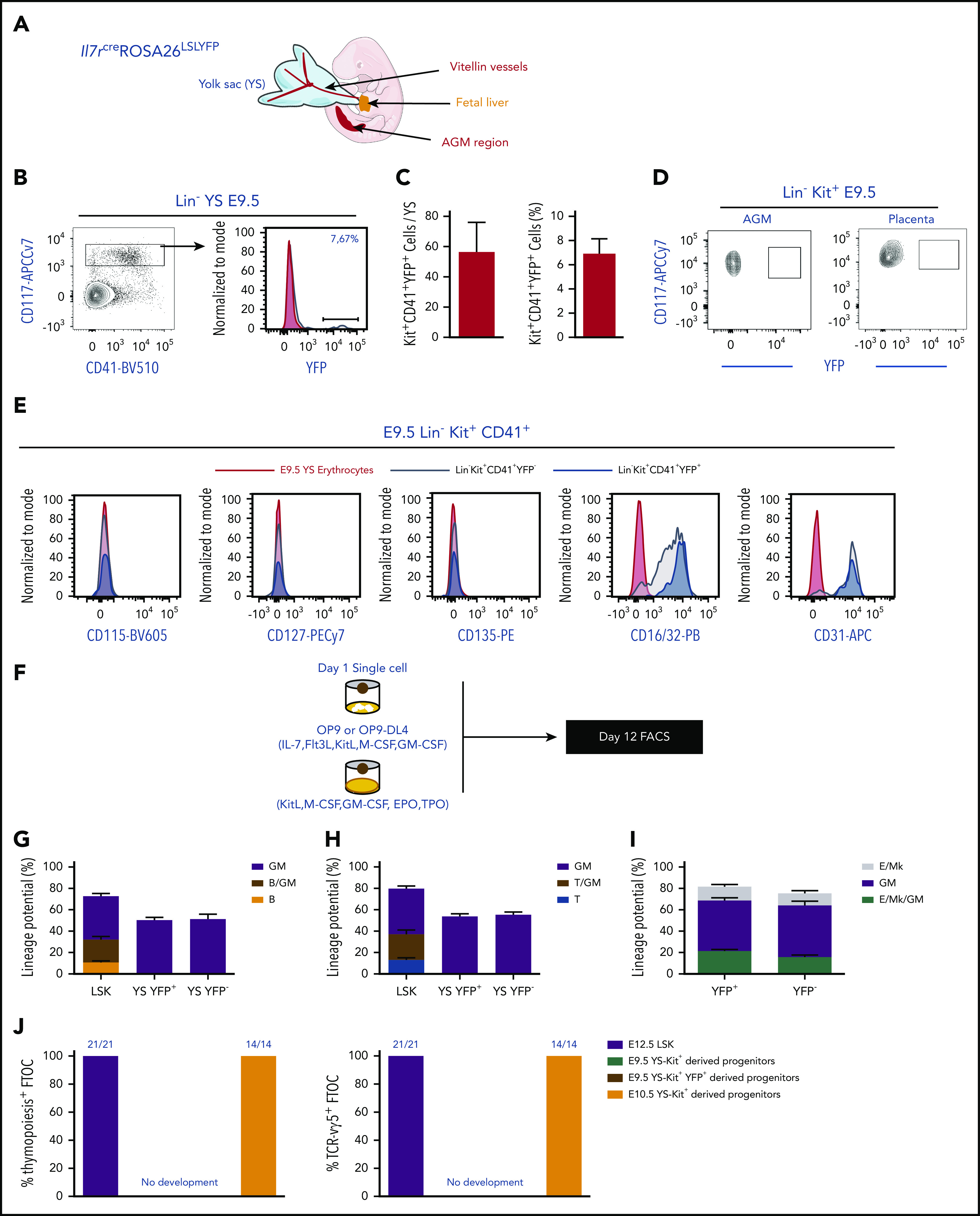
Emergence of Il7R⍺+expressing YS progenitors in E9.5 embryos. (A) Schematic representation of the anatomical sites analyzed in mouse embryos: YS, head, FL and the AGM region. (B) Representative flow cytometry plot showing YFP expression on live Lin−CD117+CD41+ E9.5 YS cells from Il7rcreROSA26LSLYFP. (C) Number and percentage of YFP+ per YS (mean plus or minus SEM). Data representative of 14 embryos from 4 independent experiments. (D) Representative flow cytometry plot showing YFP expression on live Lin−CD117+ E9.5 placenta and AGM region cells. (E) Expression of CD115, CD127, CD135, CD16/32, and CD31 within the E9.5 YS Lin−CD117+CD41+ YFP+ and YFP− populations. (F) Experimental strategy for single-cell lineage potential assay of E9.5 YS Lin−CD117+CD41+ YFP+ and YFP− populations. Frequency of wells containing (G) B cells or (H) T cells in cultures of single-sorted E9.5 YS Lin−CD117+CD41+ YFP+ and YFP− cells (180 cells from each population analyzed in 3 independent experiments). (I) Frequency of erythroid and megakaryocytic (E/Mk), myeloid (GM), or mixed E/Mk/GM colonies of single-sorted E9.5 YS Lin−CD117+CD41+ YFP+ and YFP− cells. (J) Frequency of thymic lobes irradiated and reconstituted with CD117+ E9.5 YS YFP+ or YFP−, or with E12.5 LSK or E10.5 YS as controls that contained CD4/CD8 DP cells (left plot) or TCR-Vγ5+ T cells (right plot). FTOCs were analyzed at day 12 after reconstitution.
To investigate the lineage potential of YS YFP+ cells, single cells were cultured into monolayers of OP9-DL4 (T/GM) or OP9 (B/GM) stroma (Figure 4F). Although YFP+ YS cells showed myeloid potential, they lacked detectable T- or B-cell potential (Figure 4G-H). Liquid-culture colony assays showed that both YFP+ and YFP− Lin−Kit+ YS cells exhibited comparable GM and MkE potential (Figure 4I). These results indicated that YFP expression in the E9.5 YS embryos does not equate with lymphoid potential, and both YFP+ and YFP− E9.5 YS Kit+ cells showed a lineage potential similar to EMP.28,38,39
E9.5 YS-derived cells did not generate Vγ5+ T cells or initiate thymopoiesis, whereas E10.5 YS-Kit+ progenitors and E12 FL LSK readily generated T cells including Vγ5+ T cells in FTOCs of thymic lobes (Figure 4J). E10.5 YS-Kit+ progenitor-derived T cells originate from AGM pre-HSCs, known to enter circulation between E9.5 and E11.540,41 and were used to probe the sensitivity of the T-cell assay.
These experiments showed that E9.5 YS progenitors are devoid of lymphoid potential under culture conditions sufficiently sensitive to detect few circulating T-cell progenitors.
Expression of lymphoid-associated transcripts in YS progenitors is transient
To further characterize YFP+ YS progenitors, we analyzed the expression of transcripts associated with multiple hematopoietic lineages in single E9.5 YS YFP+ and YFP− Lin−CD117+CD41+ cells. Unsupervised hierarchical clustering did not segregate YFP+ and YFP− cells, indicating that they do not differ significantly in the expression of the analyzed transcripts (Figure 5A). Cluster I–associated Pu-1, Irf8 and Csf1r expressions with low or no Kdr-, Tek-, Runx1-, Mp-, Foxo1-, IL7ra-, and Flk2-expressing cells, a pattern of expression corresponding to EMPs42 (Figure 5A). Cluster II singled out EMP-expressing Pu-1, Irf8, and Csf1r but also Kdr, Tek, Runx1, Mpl, and Foxo1, suggesting recent specification from hemogenic endothelium.42-44 These cells also exhibited Il7ra and Flk2. Clusters III and IV comprised YFP+ and YFP− cells with high levels of Gata1 indicating their erythroid lineage engagement. Cells in cluster IV also expressed Klf1, indicating that they are more advanced into the erythroid lineage differentiation. As expected, no lymphoid-associated transcripts were detected in cells from cluster III and IV. Ebf1, Pax5, or Rag1 were not detected in any of the analyzed YS cells. Within the 47 YFP+ cells analyzed only 6 coexpressed Il7ra transcripts reinforcing the notion that Il7ra expression is transient in E9.5 YS progenitors (Figure 5B).
Figure 5.
Gene-expression analysis of single E9.5 YS Lin−CD117+ CD41+ YFP+ and YFP−cells. (A) Single-cell multiplex qPCR analyzed by hierarchical clustering of single YFP+ (47 cells) and YFP− (10 cells) E9.5 Lin−CD117+CD41+ cells analyzed for the expression of lymphoid-associated (Il7r, Rag1, Rag2, Flt3), myeloid-associated (Cebpa, Cd32, Csf1r, Csf2r, Csf3r, Irf8, Pu1), B-cell–associated (Pax5, Ebf1), E/Mk-associated (EpoR, Gata1, Mpl, Tal1, Vwf, Klf1), and hematopoiesis-associated (Gata2, Myb, Runx1, Kit, Cd34) genes. (B) Number of Il7r+ and Il7r− cells within E9.5 YS YFP-expressing cells.
Interestingly, analyzed at the population level, YFP+ cells expressed significantly higher levels of Rag1, Rag2, and Il7r than YFP− cells albeit 10-fold lower than CLPs (supplemental Figure 3).
These experiments indicated that expression of lymphoid-associated genes in emerging YS-derived EMPs is transient and dissociated from differentiation potential.
Multiple FL hematopoietic progenitors express lymphoid-associated genes
We further assessed whether YFP expression also occurs in other embryonic hematopoietic cells. We found that unlike in adult bone marrow (BM),24 YFP expression in the FL was found in multipotent and myeloid progenitors (supplemental Figure 4a-b). FL and fetal blood (FB), short-term HSCs (ST-HSCs), multipotent progenitors (MPPs), GM progenitors (GMPs), common myeloid progenitors (CMPs) and megakaryocytes-erythroid progenitors (MEPs) were labeled at frequencies ranging from 10% to 40% (supplemental Figure 4a-b; supplemental Figure 5 for gating strategy). The frequency of YFP+ ST-HSCs and myeloid progenitors progressively decreased after birth and was undetectable in adult mice24 (supplemental Figure 4b). FL YFP+ GMPs and CMPs also lacked any detectable B-cell potential in vitro (supplemental Figure 4c).
We found that 40% of E18 brain microglia, the FL Kupffer cells, and the skin mast and Langerhans cells also expressed YFP, in line with a recent report45 (supplemental Figure 6a-b). These observations indicated that this model does not faithfully trace the origin of embryonic lymphoid cells.
Thymopoiesis-initiating cells develop exclusively from HSC-derived progenitors
To define the origin of the first ETPs, we used a fate-mapping mouse model expressing the tamoxifen-inducible Cre (MerCreMer) under the control of the Csf1r promoter27 (Figure 6A). Injection of hydroxy-tamoxifen (4-OH-TAM) in either Csf1rMeriCreMerRosa26YFP or Rosa26Tomato (depending on the availability of fluorochrome-labeled antibodies for the analysis) induced recombination and permanently labeled Csf1r-expressing cells and their progeny. Csf1r is first expressed in E8.5 YS progenitors,28 later it is expressed in E9.5 YS Il7raCreRosa26YFP (Figure 5A; supplemental Figure 3)18 and at E10.5 Csf1r is also expressed in pre-HSC43 (supplemental Figure 7d).
Figure 6.
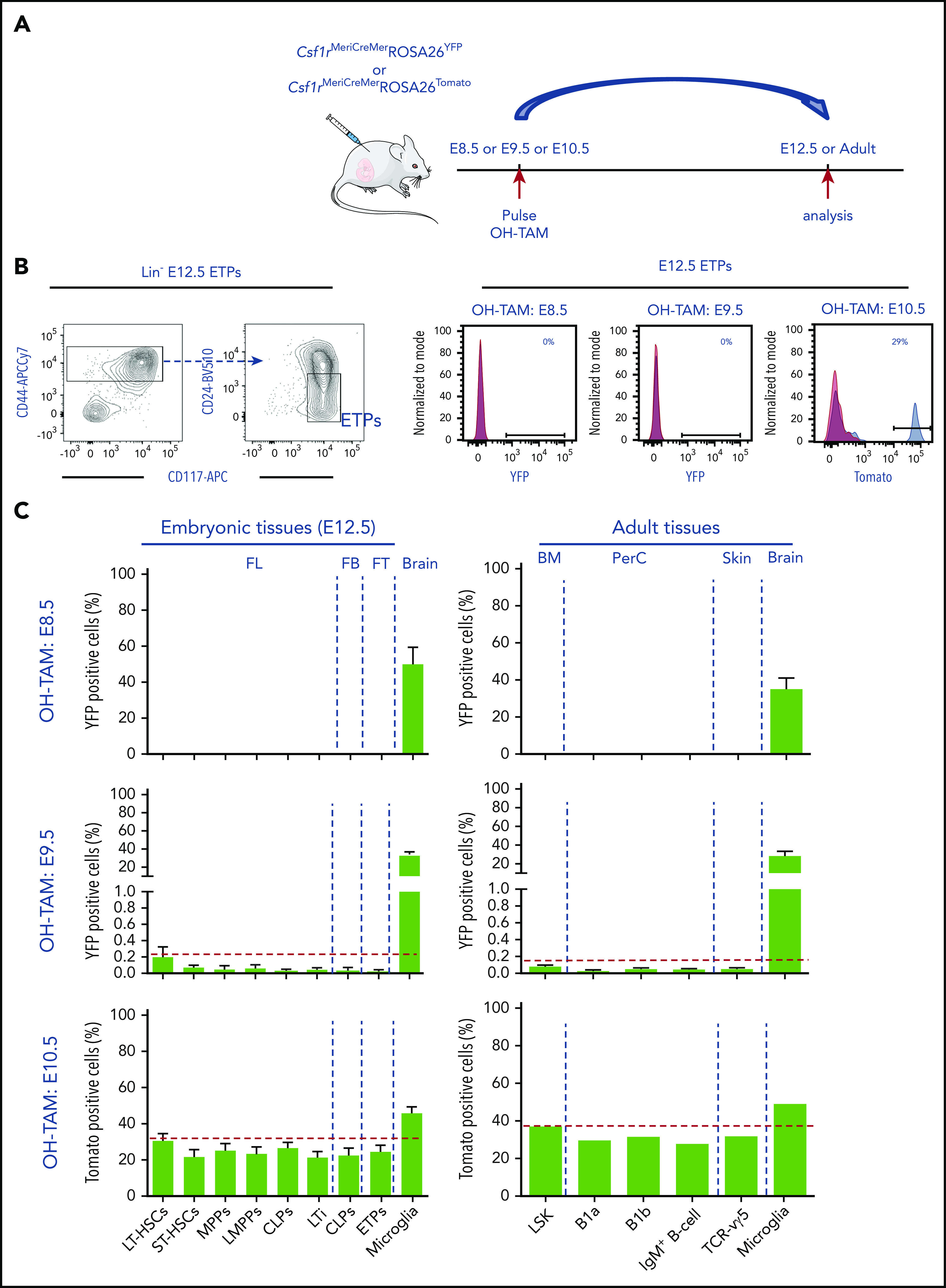
Thymopoiesis-initiating cells develop exclusively from HSC-derived progenitors. (A) Experimental design of lineage-tracing analysis of Csf1r-expressing cells after OH tamoxifen (OH-TAM) administration at E8.5, E9.5, or E10.5. Arrows indicate pulse and analysis time points. (B) Flow cytometry analysis of E12.5 early thymic progenitors (ETPs). E12.5 thymic lobes of embryos pulsed at indicated time points were analyzed. Left plots show the gating strategy to identify ETP. Right representative histograms show the frequency of Tomato+ or YFP+ cells in ETP. For each experiment, 1 representative analysis is shown. (C) Frequencies of YFP- or Tomato-labeled hematopoietic progenitors and LTi’s in E12.5 FL, fetal blood (FB), and thymi (left plots) and of LSK, B1 B cells, B cells, and Vγ5+ T cells in adult tissues (right plots) of animals pulsed at E8.5, E9.5, or E10.5. Microglia served as controls for labeling efficiency. All data are pooled from minimally 2 independent experiments for embryos analyzed at E12.5 (pulsed at E8.5, n = 8; E9.5, n = 10; E10.5, n = 10); for adult tissues, animals were analyzed between 10 and 12 weeks of age (pulsed at E8.5, n = 6; E9.5, n = 6; E10.5, n = 2). Data are depicted as mean plus or minus SEM except for adult animals pulsed at E10.5 (mean). BM, bone marrow; FT, fetal thymus; PerC, peritoneal cavity.
In embryos pulsed at E8.5, YFP+ cells were detected in the E9.5 YS, expressed Csf1r, Il7r, and were devoid of lymphoid potential (supplemental Figure 7a-c). In embryos pulsed at either E8.5 or E9.5 and analyzed at E12.5, YFP+ HSCs, HSC-derived lymphoid progenitors, and ETPs were either not detectable or represented <1% in FL and thymus, respectively (Figure 6B-C). In contrast, 30% to 50% of microglia cells were labeled, indicating the labeling efficiency. Embryos pulsed at E10.5 and analyzed at E12.5 showed significant labeling in populations of FL HSCs, multipotent, lymphoid, and myeloid progenitors (Figure 6C). Interestingly, the frequency of Tomato+ cells in E12.5 ETPs (25%) and in FL LTi (22%) (supplemental Figure 7e for gating strategy) paralleled that of HSCs (31%).
These fate-mapping experiments demonstrated that under physiological conditions, no detectable ETPs or lymphoid progenitors originate from YS cells, rather their origin is coincident in time with that of pre-HSCs.
Innate-like Vγ5+ T cells or B1-a cells that differentiate during a limited time window of embryonic development have been proposed to derive from HSC-independent precursors.
Adult Csf1rMeriCreMerRosa26YFP or Csf1rMeriCreMerRosa26Tomato mice pulsed at E8.5 showed no YFP-labeled lymphocytes and those pulsed at E9.5 showed <1% of Vγ5+ T or B-1a–labeled cells. In both cases, 30% to 40% of microglia cells were YFP labeled (Figure 6C). Embryos pulsed at E10.5 showed a frequency of Vγ5+ T- or B-1a–labeled cells similar to that found in HSCs (Figure 6C) (supplemental Figure 7f for gating strategy). These results are consistent with a strict HSC origin of all lymphoid cells including the first wave of ETPs and innate-like lymphocytes of embryonic origin.
Discussion
Here, we show that the first ETPs comprise a unique and novel population of bipotent cells that generate the invariant T and LTi lineages, and together induce the maturation of medullary thymic epithelial cells.8,9 This observation was further supported by a transcriptional analysis showing a cluster of E13.5 ETPs that are primed toward the LTi lineage with high expression of Rorc, Cd4, Cxcr5, Il1r1, and Ltb.30 The first ETPs were devoid of TdT activity giving rise to T cells without N sequences and thus with a restricted T-cell repertoire. Low TdT expression, together with the poor proliferative capacity,3 suggested that the contribution of the first ETPs to the conventional T-cell compartment is limited.1 Together, these observations indicate that the first wave of ETPs is dedicated to induce early maturation of the thymic epithelia and to generate invariant T cells that ensure tissue homeostasis. Neonatal Aire expression is necessary for inducing lifelong T-cell tolerance11 and it was shown that both Vγ5+ γδ cells and LTi’s play an important role in the maturation of CD80+mTECs.8 Our results highlight the necessity of a normal development of the first ETPs and their progeny to ensure Aire expression at birth and therefore induction of neonatal thymic tolerance and avoiding autoimmunity. A recent report analyzing human embryonic thymopoiesis suggests that similar sequence of events might be shared between mouse and human.46
Using Rag1-GFP mice, it was argued that E11.5 ETPs retained myeloid potential and resembled PIRA/B+ LMPPs in phenotype, lineage potential, and molecular signature.19 Our data show that the first ETPs, isolated using generally accepted surface markers3,47 and not based on reporter mouse models, are T/ILC restricted with poor B and myeloid potential.
The analysis of YFP+ cells under the control of the Il7r-regulatory sequences throughout embryonic life indicated that, unlike adult hematopoietic progenitors, multipotent- and myeloid-restricted progenitors express lymphoid-associated genes, albeit in a transient manner, without any consequent restriction in their differentiation potential.45,48,49 These data argue against the notion that the embryonic thymus is initially seeded at E11.5 by Rag1-expressing lymphomyeloid-restricted progenitors.19 These observations established that in hematopoiesis, gene expression does not necessarily equate with lineage potential and care should be taken before extrapolating to embryonic development findings obtained by the analysis of adult hematopoiesis.
By analogy with tissue-resident macrophages,17,28 it was proposed that also early developing innate-like T cells, and therefore the first ETPs, were HSC independent.18-21,50-52 Inducible lineage tracing of Cdh5- and Runx1-expressing cells20 suggested that Vγ5+ T cells were generated from YS-derived hematopoietic progenitors. Cdh5, Runx1, and Tie2 are expressed in endothelial cells of both the YS, that produces EMPs, and the AGM that is the source of HSCs.53 Therefore, endothelial cells and their hematopoietic progeny will be labeled in both generation sites with different frequencies at different induction time points.20,28,54-56 To unambiguously assign the contribution of each anatomical site to blood cell formation, we used the Csf1rMeriCreMer lineage-tracing model that marks the progeny of cells generated in each of these hematopoietic sites in a nonoverlapping spatiotemporal manner. Recombination induced at E8.5-E9.5 marks the YS-derived progenitors (including lymphoid-associated gene-expressing progenitors), in which virtually no Vγ5+ T cells or early ETPs were labeled. Recombination induced at E10.5 traced both YS-derived Csf1r+ myeloid cells, HSCs, and also E12.5 ETPs and CLPs, and adult Vγ5+ γδ T cells and B1a B cells. These experiments are the first in vivo evidence that ETPs, innate-like lymphocytes, and lymphoid progenitors originate in the embryo from Csf1r+ HSC-derived progeny. However compelling, these results are also compatible with a late expression of Csf1r in subsets of YS cells. We think that this is unlikely because induction at E9.5 leads to virtually no contribution of Csf1r+ cells to the lymphoid compartment, which would be expected from an ongoing process and because it was previously reported that between E9 and E11 the frequency of Csf1r+ YS cells continuously decrease.54 It is, however, possible that AGM-derived MPPs bypass the HSC stage and directly differentiate and contribute to the first-wave ETPs, as recently reported in zebrafish57 and in mice.50 Altogether, these data indicate that lymphopoiesis is a hallmark of HSCs and HSC-derived progenitors.
The ability of adult ETPs to generate ILCs in vitro has been previously documented,31,58,59 however, the capacity of E13 ETPs to generate thymic LTi’s within a thymic microenvironment is the first evidence that these cells can originate also from hematopoietic progenitors other than the classical innate lymphoid cells progenitors (ILCP),60,61 and in organs other than the FL where hematopoiesis occurs62 (Figure 7).
Figure 7.
Model depicting the origin and the lineage potential of thymopoiesis-initiating progenitors.
This highly orchestrated sequence of events highlights the requirement of a layered immune cell production whereby the first wave of ETPs is essential for thymic organogenesis and T-cell tolerance.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank S. Novault, S. Megharba, and S. Schmutz from the flow cytometry core facility of the Institut Pasteur for technical support, and the staff of the animal facility of the Institut Pasteur for mouse care. The authors thank H.-R. Rodewald for providing Il7raCreRosa26YFP mice and S. E. W. Jacobsen for fruitful discussions.
This work was supported by the Institut Pasteur, INSERM, the Pasteur-Weizmann Foundation, and Agence Nationale de la Recherche (ANR; grant Twothyme and Epi-Dev) through grants (A.C.); by REVIVE (Investissement d’Avenir, ANR-10-LABX-73) (A.C. and R.E.); by recurrent funding from the Institut Pasteur, the Centre National de la Recherche Scientifique (CNRS), Revive (Investissement d’Avenir, ANR-10-LABX-73), and by European Research Council (ERC) investigator award 2016-StG-715320; E.G.P.).
Footnotes
For original data, please contact the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.E. designed and performed most experiments, analyzed data, and wrote the manuscript; S.M. analyzed chimeric mice and contributed to discussions; F.S.-d.-S. and T.P. performed Biomark analysis; O.B.-D. performed neonatal thymectomy; E.G.P., L.F., and L.I. performed fate-mapping experiments; A.B. performed adoptive transfer in neonatal mice; R.G., P.V., A.B., and P.P. contributed to the discussions; A.C. directed the research, designed experiments, analyzed data, and wrote the manuscript; and all authors contributed to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ana Cumano, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: ana.cumano@pasteur.fr; and Ramy Elsaid, Institut Pasteur, 25 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: ramy.elsaid@pasteur.fr; ramyelsaid@rocketmail.com.
REFERENCES
- 1.Cumano A, Berthault C, Ramond C, et al. New molecular insights into immune cell development. Annu Rev Immunol. 2019;37:497-519. [DOI] [PubMed] [Google Scholar]
- 2.Berthault C, Ramond C, Burlen-Defranoux O, et al. Asynchronous lineage priming determines commitment to T cell and B cell lineages in fetal liver. Nat Immunol. 2017;18(10):1139-1149. [DOI] [PubMed] [Google Scholar]
- 3.Ramond C, Berthault C, Burlen-Defranoux O, et al. Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat Immunol. 2014;15(1):27-35. [DOI] [PubMed] [Google Scholar]
- 4.Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26(6):678-689. [DOI] [PubMed] [Google Scholar]
- 5.Luc S, Luis TC, Boukarabila H, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat Immunol. 2012;13(4):412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves NL, Takahama Y, Ohigashi I, et al. Serial progression of cortical and medullary thymic epithelial microenvironments. Eur J Immunol. 2014;44(1):16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyden LM, Lewis JM, Barbee SD, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40(5):656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts NA, White AJ, Jenkinson WE, et al. Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36(3):427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi SW, Kim M-Y, Leibbrandt A, et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204(6):1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson J, Anderson G. Thymic epithelial cells. Annu Rev Immunol. 2017;35:85-118. [DOI] [PubMed] [Google Scholar]
- 11.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206(6):1245-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cupedo T, Mebius RE. Role of chemokines in the development of secondary and tertiary lymphoid tissues. Semin Immunol. 2003;15(5):243-248. [DOI] [PubMed] [Google Scholar]
- 13.Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3- cells in lymphoid organ development. Immunol Rev. 2002;189:41-50. [DOI] [PubMed] [Google Scholar]
- 14.Papotto PH, Ribot JC, Silva-Santos B. IL-17+ γδ T cells as kick-starters of inflammation. Nat Immunol. 2017;18(6):604-611. [DOI] [PubMed] [Google Scholar]
- 15.Jones R, Cosway EJ, Willis C, et al. Dynamic changes in intrathymic ILC populations during murine neonatal development. Eur J Immunol. 2018;48(9):1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikuta K, Kina T, MacNeil I, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62(5):863-874. [DOI] [PubMed] [Google Scholar]
- 17.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böiers C, Carrelha J, Lutteropp M, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13(5):535-548. [DOI] [PubMed] [Google Scholar]
- 19.Luis TC, Luc S, Mizukami T, et al. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nat Immunol. 2016;17(12):1424-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentek R, Ghigo C, Hoeffel G, et al. Epidermal γδ T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J Exp Med. 2018;215(12):2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spidale NA, Sylvia K, Narayan K, et al. Interleukin-17-producing γδ T cells originate from SOX13+ progenitors that are independent of γδTCR signaling. Immunity. 2018;49(5):857-872.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaudin AE, Boyer SW, Perez-Cunningham J, et al. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19(6):768-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White AJ, Lucas B, Jenkinson WE, Anderson G. Invariant NKT cells and control of the thymus medulla. J Immunol. 2018;200(10):3333-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlenner SM, Madan V, Busch K, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32(3):426-436. [DOI] [PubMed] [Google Scholar]
- 25.Colucci F, Soudais C, Rosmaraki E, et al. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J Immunol. 1999;162(5):2761-2765. [PubMed] [Google Scholar]
- 26.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian B-Z, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudo T, Nishikawa S, Ohno N, et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90(19):9125-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishizuka IE, Chea S, Gudjonson H, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17(3):269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Obaldia ME, Bhandoola A. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu Rev Immunol. 2015;33:607-642. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida H, Kawamoto H, Santee SM, et al. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167(5):2511-2521. [DOI] [PubMed] [Google Scholar]
- 33.Hashizume T, Togawa A, Nochi T, et al. Peyer’s patches are required for intestinal immunoglobulin A responses to Salmonella spp. Infect Immun. 2008;76(3):927-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider C, Lee J, Koga S, et al. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity. 2019;50(6):1425-1438.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas JD, Ravens S, Düber S, et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37(1):48-59. [DOI] [PubMed] [Google Scholar]
- 36.Xu W, Cherrier DE, Chea S, et al. An Id2RFP-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity. 2019;50(4):1054-1068.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115(10):1897-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106(9):3004-3011. [DOI] [PubMed] [Google Scholar]
- 39.McGrath KE, Frame JM, Fegan KH, et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11(12):1892-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4(1):97-106. [DOI] [PubMed] [Google Scholar]
- 41.McGrath KE, Koniski AD, Malik J, Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101(5):1669-1676. [DOI] [PubMed] [Google Scholar]
- 42.Mass E, Ballesteros I, Farlik M, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353(6304):aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron CS, Kester L, Klaus A, et al. Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat Commun. 2018;9(1):2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasaai B, Caolo V, Peacock HM, et al. Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Sci Rep. 2017;7:43817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung GA, Cool T, Valencia CH, et al. The lymphoid-associated interleukin 7 receptor (IL7R) regulates tissue-resident macrophage development. Development. 2019;146(14):dev176180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tieppo P, Papadopoulou M, Gatti D, et al. The human fetal thymus generates invariant effector γδ T cells. J Exp Med. 2020;217(3):e20190580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20(6):735-745. [DOI] [PubMed] [Google Scholar]
- 48.Gautier EL, Shay T, Miller J, et al. ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heng TSP, Painter MW; Immunological Genome Project Consortium . The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9(10):1091-1094. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M, Shelley WC, Seo W, et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc Natl Acad Sci USA. 2014;111(33):12151-12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108(4):1468-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimoto M, Porayette P, Glosson NL, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elsaid R, Yang J, Cumano A. The influence of space and time on the establishment of B cell identity. Biomed J. 2019;42(4):209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42(4):665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gentek R, Ghigo C, Hoeffel G, et al. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 2018;48(6):1160-1171.e5. [DOI] [PubMed] [Google Scholar]
- 56.Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542-546. [DOI] [PubMed] [Google Scholar]
- 57.Tian Y, Xu J, Feng S, et al. The first wave of T lymphopoiesis in zebrafish arises from aorta endothelium independent of hematopoietic stem cells. J Exp Med. 2017;214(11):3347-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13(3):229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q, Li F, Harly C, et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. 2015;16(10):1044-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diefenbach A, Gnafakis S, Shomrat O. Innate lymphoid cell-epithelial cell modules sustain intestinal homeostasis. Immunity. 2020;52(3):452-463. [DOI] [PubMed] [Google Scholar]
- 61.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41(3):354-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745-785. [DOI] [PubMed] [Google Scholar]
- 63.Perchet Thibaut, Petit M, Banchi E-G, et al. The Notch signaling pathway is balancing type 1 innate lymphoid cell immune functions. Front Immunol. 2018;9: doi:10.3389/fimmu.2018.01252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.