Abstract
Although oxaliplatin is an effective chemotherapeutic drug commonly used for colorectal cancer (CRC) treatment, drug resistance usually occurs during the long-term use of it. It is urgent to create strategies to reduce the resistance of CRC cells to oxaliplatin. Oxaliplatin-resistant CRC cells (OR-SW480 and OR-HT29) were acquired through long-term exposure of CRC cells to oxaliplatin. It was found that OR-SW480 and OR-HT29 cells exhibited obvious lower sensitivity and a higher metabolism rate of glucose compared to their parental SW480 and HT29 cells, respectively. However, combination with scutellarin significantly resensitized the OR-SW480 and OR-HT29 cells to oxaliplatin-induced cytotoxicity. Mechanically, overexpression of pyruvate kinase isoenzyme M2 (PKM2) was responsible for the resistance to oxaliplatin in OR-SW480 and OR-HT29. Combination with scutellarin was able to inhibit the PKM2 activity and thus reduced the production of adenosine triphosphate (ATP) to sensitize the oxaliplatin-induced mitochondrial apoptosis pathway in both OR-SW480 and OR-HT29 cells. It was indicated that scutellarin resensitizes oxaliplatin-resistant CRC cells to oxaliplatin treatment through inhibition of PKM2.
Keywords: oxaliplatin, colorectal cancer, pyruvate kinase isoenzyme M2, adenosine triphosphate, resistance, glucose, scutellarin, metabolism, overexpression
Graphical abstract

Oxaliplatin, an effective chemotherapeutic drug, is commonly used for colorectal cancer (CRC) treatment, but drug resistance usually occurs during its long-term use. We constructed oxaliplatin-resistant CRC cells using SW480 and HT29 cells and found that combination with scutellarin and oxaliplatin resensitized the oxaliplatin-resistant CRC cells through inhibition of PKM2.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide. Unfortunately, the incidence of CRC continues to increase, and its mortality rate is high. Despite the advancement of CRC treatment in recent decades, the 5-year survival rate of CRC patients remains poor due to the high incidence of liver metastasis.1,2 Although surgical resection is the most effective approach for CRC treatment, chemotherapy is still indispensable for postoperative adjuvant treatment and metastatic cancer treatment.3,4 In addition, many patients’ tumors showed obvious chemoresistance.5,6 Therefore, it is urgent to explore novel strategies to enhance the chemosensitivity in CRC.
Oxaliplatin, a third generation of platinum compound, is known to induce formation of intra-strand guanine-guanine and guanine-adenine DNA links in cancer cells. As the result of DNA damage, oxaliplatin induces apoptotic cell death of cancers. Nowadays, oxaliplatin-based chemotherapy is considered to be the first-line treatment for CRC patients.7, 8, 9 Oxaliplatin is used for patients with both colon cancer and rectal cancer, but, for example, chemotherapy including oxaliplatin is regularly used neoadjuvantly for the treatment of resectable rectal cancer.10 However, all of the CRC cells eventually become resistant to oxaliplatin because of the long-term use of it.11,12 Novel approaches are required to overcome the oxaliplatin resistance in CRC. Scutellarin, a flavone glycoside, is a major active component of the traditional Chinese herb Erigeron breviscapus. As a natural drug, scutellarin exhibits a low toxicity in humans.13 Besides the clinical use in treatment with cardiovascular diseases, sleep disorders, and depression, scutellarin is also found to show some anti-tumor activities in some cancers such as human tongue squamous carcinoma and breast cancer.14,15 In this study, we hypothesized that scutellarin may exert its special functions in oxaliplatin-resistant CRC.
Pyruvate kinases (PK) are the key enzymes of glycometabolism. Among them, the PK isoenzyme M1 (PKM1) is expressed in normal tissues while the PKM2 is strongly overexpressed in cancers.16, 17, 18, 19 As one function of PK, it converts phosphoenolpyruvate and adenosine diphosphate (ADP) to pyruvate and adenosine triphosphate (ATP), suggesting that cellular level of PK determines the glycometabolism rate and production of ATP.16,17 To meet the need of high proliferation and high glycometabolism rate, cancer cells usually show a high glycolytic rate with production through changes of the glycolytic isoenzyme.18,19 Therefore, PKM2 is special in the glycometabolism pathway in cancer cells. Here, we explored the effects and the mechanisms of scutellarin and oxaliplatin in oxaliplatin-resistant CRC.
Results
Oxaliplatin-resistant SW480 and HT29 cells exhibit a higher rate of glycometabolism
First, the oxaliplatin-resistant CRC model was established using SW480 and HT29 cell lines. Our data showed that the OR-SW480 and OR-HT29 cells exhibited obvious resistance to oxaliplatin treatment compared to their parental SW480 and HT29 cells, respectively (Figure 1A). Specifically, inhibitory concentration (IC50) of oxaliplatin to OR-SW480 and OR-HT29 was 11.2- and 6.5-fold higher than that to SW480 and HT29 cells, respectively (p < 0.05; Figure 1B). Next, we compared the glucose metabolism between oxaliplatin-resistant CRC and routine CRC cells and found that uptake of glucose in OR-SW480 and OR-HT29 was significantly higher than that in SW480 and HT29, respectively (p < 0.05; Figure 1C). Furthermore, OR-SW480 and OR-HT29 produced more lactate (Figure 1D) and ATP (Figure 1E) than the SW480 and HT29 cells (p < 0.05). Taken together, we demonstrated that oxaliplatin-treated CRC cells exhibited obvious oxaliplatin resistance and a high rate of glycometabolism compared to the routine CRC cells.
Figure 1.
Oxaliplatin resistance and glycometabolism rate in OR-SW480 and OR-HT29
(A) Cell viability of SW480, OR-SW480, HT29, and OR-HT29 cells after treatment with different concentrations of oxaliplatin. (B) IC50 of SW480, OR-SW480, HT29, and OR-HT29 to oxaliplatin. (C) Relative glucose uptake in SW480, OR-SW480, HT29, and OR-HT29 cells. (D) Relative lactate generation in SW480, OR-SW480, HT29, and OR-HT29 cells. (E) Relative ATP production in SW480, OR-SW480, HT29, and OR-HT29 cells. ∗p < 0.05.
Expression of PKM2 determines glycometabolism rate and oxaliplatin sensitivity in CRC cells
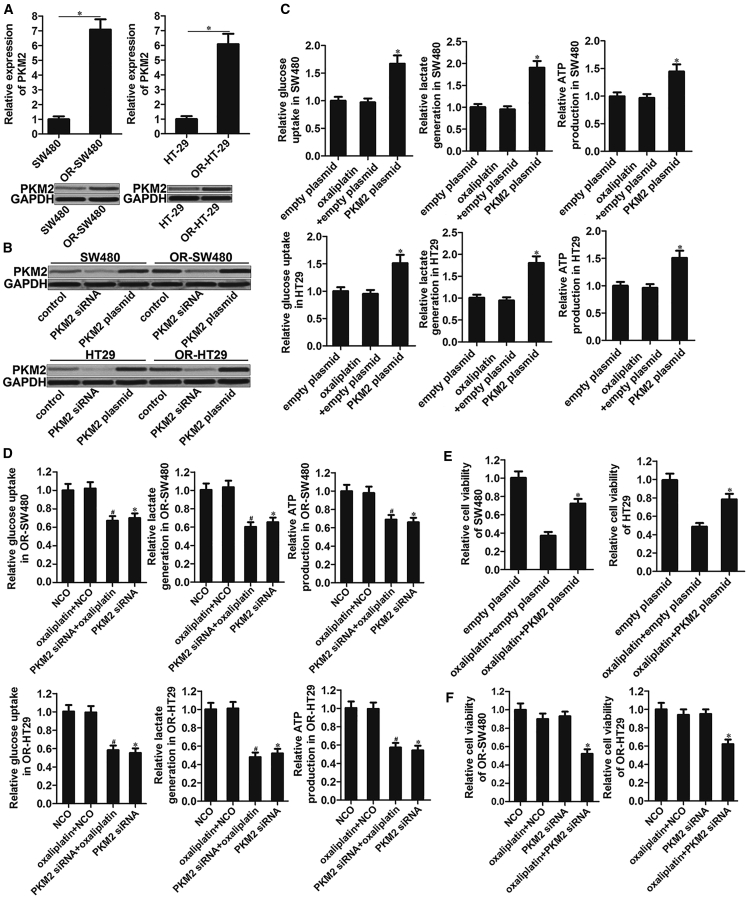
Results of quantitative real-time polymerase chain reaction (PCR) and western blot analysis showed that mRNA and protein expression of PKM2 was increased significantly in OR-SW480 and OR-HT29 cells compared to the SW480 and HT29 cells, respectively (p < 0.05; Figure 2A). Next, we investigated the association between PKM2 expression and chemoresistance in oxaliplatin-resistant CRC and routine CRC cells. To perform the gain- and loss-of-function of PKM2 in SW480, OR-SW480, HT29, and OR-HT29 cells, these CRC cells were transfected with PKM2 plasmid or its specific small interfering RNA (siRNA). PKM2 expression was increased in PKM2 plasmid transfected cell lines while it was decreased significantly in PKM2 siRNA transfected cell lines (p < 0.05; Figure 2B), indicating good transfection efficiency of PKM2 plasmid or its specific siRNA. Overexpression of PKM2 increased glucose up-intake, lactate, and ATP in both SW480 and HT29 cells (p < 0.05; Figure 2C), suggesting that PKM2 determined the glycometabolism rate in CRC. By contrast, knockdown of PKM2 directly in OR-SW480 and OR-HT29 reduced the glucose up-intake, lactate, and ATP obviously (p < 0.05; Figure 2D). Furthermore, we found that overexpression of PKM2 in SW480 and HT29 cells significantly decreased their sensitivity to oxaliplatin treatment (p < 0.05; Figure 2E). Most importantly, knockdown of PKM2 sensitized the OR-SW480 and OR-HT29 cells to oxaliplatin treatment (p < 0.05; Figure 2F). Taken together, we indicated that PKM2 determines the glycometabolism rate and sensitivity to oxaliplatin in CRC, and it may be a potent target for reversing the oxaliplatin resistance in OR-SW480 and OR-HT29.
Figure 2.
Expression of PKM2 determines glycometabolism rate and sensitivity to oxaliplatin in SW480, OR-SW480, HT29, and OR-HT29 cells
(A) Expression of PKM2 in SW480, OR-SW480, HT29, and OR-HT29 was evaluated by quantitative real-time PCR and western blot analysis. ∗p < 0.05. (B) Transfection efficiency of PKM2 siRNA (50 pmol/mL) and PKM2 plasmid (2 μg/mL) in SW480, OR-SW480, HT29, and OR-HT29 cells. (C) Glucose, lactate, and ATP assays were performed to evaluate the glycometabolism of SW480 and HT29 cells after treatment with oxaliplatin (1 μM) and PKM2 plasmid (2 μg/mL). ∗p < 0.05 versus empty plasmid group. (D) Glucose, lactate, and ATP assays were performed to evaluate the glycometabolism of OR-SW480 and OR-HT29 cells after treatment with oxaliplatin (10 μM) and PKM2 siRNA (50 pmol/mL). ∗p < 0.05 versus NCO group. #p < 0.05 versus Oxaliplatin + NCO group. (E) Effect of PKM2 plasmid (2 μg/mL) on inducing the oxaliplatin resistance in SW480 and HT29. ∗p < 0.05 versus Oxaliplatin + empty plasmid group. (F) Effect of PKM2 siRNA (50 pmol/mL) on reversing the oxaliplatin resistance in OR-SW480 and OR-HT29. ∗p < 0.05 versus oxaliplatin + NCO group.
Scutellarin resensitizes OR-SW480 and OR-HT29 cells to oxaliplatin treatment
To study the effect of scutellarin on oxaliplatin resistance in CRC, we co-treated the OR-SW480 and OR-HT29 cells with 2 μM scutellarin and oxaliplatin at concentrations of 0, 5, 10, 15, 20, and 30 μM. Cytotoxicity was shown in Figure 3A, after SW480, OR-SW480, HT29, and OR-HT29 cells were treated with scutellarin at concentrations of 0, 2, 5, 10, 20, and 40 μM. The data proved that cytotoxicity of 2 μM scutellarin to SW480, OR-SW480, HT29, and OR-HT29 was slight, so this concentration of scutellarin (2 μM) was used for co-treatment with oxaliplatin. Results of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays showed that scutellarin significantly increased the cytotoxicity of various concentrations of oxaliplatin to OR-SW480 and OR-HT29 (p < 0.05). Specifically, scutellarin decreased the IC50 of oxaliplatin to OR-SW480 and OR-HT29 by 79.5% and 75.2%, respectively (p < 0.01; Figure 3B). In addition, scutellarin decreased the IC50 of oxaliplatin to SW480 and HT29 by 32.5% and 53.3%, respectively (p < 0.05; Figure 3C). It was suggested that OR-SW480 and OR-HT29 cells were more sensitive to the synergistic effect of scutellarin compared to the SW480 and HT29 cells, indicating that scutellarin was able to resensitize the OR-SW480 and OR-HT29 cells to oxaliplatin treatment.
Figure 3.
Scutellarin resensitizes OR-SW480 and OR-HT29 cells to oxaliplatin treatment
(A) After single treatment with different concentrations of scutellarin (0–40 μM) in OR-SW480, OR-HT29, SW480, and HT29 cells for 24 h or 48 h, cytotoxicity of scutellarin was evaluated by MTT assays. (B) Effect of scutellarin (2 μM) on reducing the IC50 of oxaliplatin to OR-SW480 and OR-HT29. ∗p < 0.05 versus control group. (C) Effect of scutellarin (2 μM) on decreasing the IC50 of oxaliplatin to SW480 and HT29. ∗p < 0.05 versus control group.
Scutellarin targets PKM2 to reduce the oxaliplatin resistance and glycometabolism in OR-SW480 and OR-HT29 cells
To explore the mechanism by which scutellarin reversed the oxaliplatin resistance in OR-SW480 and OR-HT29, we detected the expression of PKM2 in OR-SW480 and OR-HT29 cells after treatment with scutellarin and oxaliplatin. We found that scutellarin but not oxaliplatin obviously reduced the protein expression of PKM2 in both OR-SW480 and OR-HT29 cells (Figure 4A), suggesting that PKM2 may be the target of scutellarin in OR-SW480 and OR-HT29 cells. Thus, we “rescued” the OR-SW480 and OR-HT29 cells with PKM2 plasmid after combination treatment with oxaliplatin and scutellarin. Results of cell viability assays showed that scutellarin significantly increased the cytotoxicity of oxaliplatin against OR-SW480 and OR-HT29 (p < 0.05; Figure 4B). However, overexpression of PKM2 in OR-SW480 and OR-HT29 cells was found to protect them from the cytotoxicity of co-treatment of oxaliplatin and scutellarin (Figure 4B). Scutellarin treatment significantly weakened the glycometabolism of OR-SW480 and OR-HT29 cells, expressed as the decreased glucose uptake, lactate, and ATP (p < 0.05; Figure 4C). Moreover, enforced expression of PKM2 almost completely abolished the effect of scutellarin on changes of the glycometabolism in OR-SW480 and OR-HT29 cells (Figure 4C). The above findings demonstrated that scutellarin reduced the oxaliplatin resistance and glycometabolism in OR-SW480 and OR-HT29 cells through inhibition of PKM2.
Figure 4.
Scutellarin decreases the oxaliplatin resistance through inhibition of PKM2 in OR-SW480 and OR-HT29 cells
(A) Effect of scutellarin (2 μM), oxaliplatin (10 μM), and PKM2 plasmid (2 μg/mL) on changing the expression of PKM2 in OR-SW480 and OR-HT29 cells. (B) Effect of PKM2 plasmid (2 μg/mL) on protecting the OR-SW480 and OR-HT29 cells from the cytotoxicity of co-treatment with oxaliplatin (10 μM) and scutellarin (2 μM). ∗p < 0.05 versus oxaliplatin + empty plasmid group. #p < 0.05 versus oxaliplatin + scutellarin + empty plasmid group. (C) PKM2 plasmid (2 μg/mL) increased the glycometabolism rate in scutellarin-treated (2 μM) OR-SW480 and OR-HT29 cells. ∗p < 0.05 versus empty plasmid group. #p < 0.05 versus oxaliplatin + empty plasmid group. &p < 0.05 versus oxaliplatin + scutellarin + empty plasmid group.
Scutellarin promotes oxaliplatin-induced mitochondrial apoptosis in OR-SW480 and OR-HT29 cells
To investigate the damage of mitochondria in oxaliplatin and scutellarin co-treated OR-SW480 and OR-HT29 cells, we evaluated mitochondrial membrane potential (Δϕ) by flow cytometry. The results showed that scutellarin obviously enhanced the effect of oxaliplatin on reducing the Δϕ of OR-SW480 and OR-HT29 cells (Figure 5A), suggesting that scutellarin promoted oxaliplatin-dependent mitochondria damage in OR-SW480 and OR-HT29 cells. Next, we found that scutellarin obviously promoted the release of cytochrome c and apoptosis-inducing factor (AIF) from mitochondria into cytosol in oxaliplatin-treated OR-SW480 and OR-HT29 cells (Figure 5B). As shown in the results, cleaved caspase-9 and cleaved caspase-3 protein expression in OR-SW480 and OR-HT29 were increased by oxaliplatin and scutellarin (Figure 5C). And finally, severe apoptosis occurred in OR-SW480 and OR-HT29 cells, which were co-treated with oxaliplatin and scutellarin (p < 0.05; Figure 5D). We therefore demonstrated that adjuvant treatment with scutellarin can promote oxaliplatin-induced mitochondrial apoptosis in OR-SW480 and OR-HT29 cells through suppression of PKM2.
Figure 5.
Promotion of scutellarin on oxaliplatin-induced mitochondrial apoptosis pathway
(A) Mitochondrial membrane potential (Δϕ) of OR-SW480 and OR-HT29 cells treated with oxaliplatin (10 μM), scutellarin (2 μM), and PKM2 plasmid (2 μg/mL) was measured by flow cytometry. (B) Release of cytochrome c and AIF was evaluated by western blot analysis after removal of mitochondria from cytosol of OR-SW480 and OR-HT29 cells treated with oxaliplatin (10 μM), scutellarin (2 μM), and PKM2 plasmid (2 μg/mL). (C) Western blot analysis was performed to detect the cleavage of caspase-9 and caspase-3 in OR-SW480 and OR-HT29 cells treated with oxaliplatin (10 μM), scutellarin (2 μM), and PKM2 plasmid (2 μg/mL). (D) Flow cytometry analysis was performed to detect the apoptotic rate of OR-SW480 and OR-HT29 cells treated with oxaliplatin (10 μM), scutellarin (2 μM), and PKM2 plasmid (2 μg/mL). ∗p < 0.05 versus empty plasmid group. #p < 0.05 versus oxaliplatin + empty plasmid group. &p < 0.05 versus oxaliplatin + scutellarin + empty plasmid group.
Scutellarin reverses oxaliplatin resistance of CRC in vivo
To investigate the effect of scutellarin on reversing the oxaliplatin resistance of CRC in vivo, we established the in vivo CRC model using OR-SW480 cells in mice. Although oxaliplatin single treatment failed to inhibit the tumor growth of OR-SW480 efficiently, we observed that combination treatment with scutellarin could enhance the anti-tumor effect of oxaliplatin dramatically on the oxaliplatin-resistant CRC mice model (Figures 6A and 6B). After analyzing the cleavage of caspase-9 and caspase-3 through western blot assay, we found that scutellarin decreased cleaved caspase-9 and caspase-3 protein expression induced by oxaliplatin, indicating that scutellarin sensitized the oxaliplatin-induced apoptosis in the OR-SW480 mice model (Figure 6C). Next, we found that scutellarin significantly decreased the protein expression of PKM2 (Figure 6D) and production of ATP (Figure 6E) in the resected tumors. These results indicated that scutellarin negatively regulated the glycometabolism of oxaliplatin-resistant CRC in vivo. We thus demonstrated that scutellarin can reverse the oxaliplatin resistance of CRC by targeting the PKM2/glycometabolism/ATP pathway.
Figure 6.
Scutellarin sensitizes oxaliplatin-resistant CRC cells to oxaliplatin treatment in vivo
(A) Tumor growth of mice bearing OR-SW480 cells after treatment with oxaliplatin (10 mg/kg) and scutellarin (10 mg/kg) twice a week. (B) Resected tumors from mice in each group. (C) Western blot assay was performed to detect the cleavage of caspase-9 and caspase-3 in the resected tumors. (D) Western blot assay was performed to analyze the expression of PKM2 in the resected tumors. (E) Production of ATP in the purified tumor cells in each group. ∗p < 0.05 versus control group. #p < 0.05 versus oxaliplatin group.
Discussion
Drug combinations are often used to overcome drug resistance, and numerous studies have identified novel drug combinations to improve therapeutic efficiency.20,21 Recent studies have demonstrated that combination drug treatments are able to reduce the chemoresistance and thus improve the efficacy of chemotherapy on CRC cells.22, 23, 24 Oxaliplatin is usually used with 5-fluorouracil and considered as the first-line treatment for CRC.25 In the present study, we proved that our established oxaliplatin-resistant CRC cells show significant resistance to oxaliplatin treatment. However, we found that combination treatment with scutellarin is able to resensitize the oxaliplatin-resistant CRC cells to oxaliplatin-induced cytotoxicity both in vitro and in vivo, demonstrating the effect of scutellarin on reversing the oxaliplatin resistance in CRC.
PKM2 is overexpressed in cancer cells and associated with cancer development.26 Previous studies have reported that overexpression of PKM2 is responsible for induction of drug resistance and has been found to promote the cancer process and indicate poor prognosis of cancer patients.27 PKM2 plays an important role in maintaining the metabolic process of cancer cells and is considered as a potential target for cancer therapy.28 Chemoresistance is associated with dysregulation of multiple factors, including mutation of K-Ras and p53, overexpression of anti-apoptotic proteins, and change of metabolism.29, 30, 31 We focused on the role of PKM2 in changing the oxaliplatin sensitivity in CRC, because one important target of scutellarin is PKM2.32 In this study, we observed significant upregulation of PKM2 protein expression in OR-SW480 and OR-HT29 cells compared to their parental SW480 and HT29 cells, respectively. Furthermore, we found that knockdown of PKM2 directly in OR-SW480 cells and OR-HT29 sensitizes these cells to oxaliplatin-induced cytotoxicity, whereas enforced expression of PKM2 in routine SW480 and HT29 induced obvious resistance of these CRC cells to oxaliplatin treatment. We proved the role of PKM2 in determining the sensitivity of CRC cells to oxaliplatin.
A previous study has demonstrated that glucose metabolism determines cell survival and chemosensitivity in cancers.24 Previous studies also have demonstrated that glycometabolism rates and intracellular ATP levels are pivotal determinants of chemoresistance in tumor cells. It is reported that depletion of glycometabolism and intracellular ATP induces the depression of drug efflux system and sensitizes cells to mitochondrial dysfunction-induced apoptosis pathway.33, 34, 35, 36 Thus, a high level of glycometabolism and intracellular ATP facilitates the occurrence of chemoresistance of cancer cells. In this study, we explored the effect of scutellarin on reversing the oxaliplatin resistance in CRC. Mechanically, we found that scutellarin inhibits the PKM2 expression and thus reduces the glycometabolism rate and the production of ATP. The lower level of ATP then facilitated the oxaliplatin-induced mitochondrial dysfunction, as determined by a decrease of Δϕ. As the results, cytochrome c and AIF, the mitochondria-derived pro-apoptotic inducers,37,38 were released from the mitochondria into the cytosol. Subsequently, these apoptotic inducers activate the effector caspases and cause the final occurrence of apoptosis (Figure 7).
Figure 7.
Schema of the predicted mechanisms implicated in OR-SW480 cells response to oxaliplatin
Scutellarin inhibits PKM2 expression and thus reduces the glycometabolism rate and the production of ATP. The lower level of ATP facilitates the oxaliplatin-induced mitochondrial dysfunction, as determined by a decrease in Δϕ. As a result, cytochrome c and AIF are released from the mitochondria into the cytosol. Subsequently, these apoptotic inducers activate the effector caspases and cause the final occurrence of apoptosis.
In conclusion, we indicate the effect of scutellarin on resensitizing oxaliplatin-resistant CRC cells to oxaliplatin treatment through inhibition of PKM2/glycometabolism/ATP pathway. Combination with scutellarin may represent a novel strategy for efficient application of oxaliplatin in CRC treatment.
Materials and methods
Establishment of an oxaliplatin-resistant CRC cell model
Human CRC cell line SW480 derived from colon cancers was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, CA, USA) with 10% fetal bovine serum (FBS, GIBCO, CA, USA) at 37°C in a humidified incubator with 5% CO2. To establish the oxaliplatin-resistant CRC cell model, we exposed the SW480 cells with gradually increasing concentrations of oxaliplatin. Briefly, SW480 cells were initially treated with 0.5 μM oxaliplatin for 2 months. Subsequently, the oxaliplatin concentration was increased every 1 week by 0.1 μM up to a final concentration of 2 μM. The established oxaliplatin-resistant SW480 cells were named as OR-SW480 cells. Human CRC cell line HT29 derived from colon cancers and oxaliplatin-resistant HT29 cell model were kindly provided by Professor Bai from Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute.
Gain- and loss-of-function of PKM2
For enforced expression of PKM2, PKM2 expression vector was conducted by cloning the open reading frame of the PKM2 gene into the pcDNA3.1 plasmid (Invitrogen, Carlsbad, CA, USA). For direct knockdown of PKM2, PKM2 siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). To perform the gain- and loss-of-function experiments of PKM2, we transient transfected cells with 2 μg/mL PKM2 plasmid or 50 pmol/mL PKM2 siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Negative control oligonucleotides (NCOs), used as the general negative control RNA, were purchased from Genechem (Shanghai, China), and empty pcDNA3.1 plasmids were used as the internal control for transfection with PKM2 siRNA and PKM2 plasmids, respectively.
Quantitative real-time PCR
Relative expression of PKM2 was detected by quantitative real-time PCR on an Applied Biosystems ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA, USA). Briefly, total RNAs were isolated from CRC cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was reverse transcribed by Moloney’s mouse leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA) followed by real-time PCR amplification with SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan). The program included: 1 cycle at 94°C for 10 min and 38 cycles at 94°C for 45 s, 58°C for 30 s, and 72°C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as normalization control to determine the relative expression of PKM2.
Cell viability detection
MTT assay was performed to detect the viability of CRC cells. Briefly, cells were seeded into 96-well plates at a density of 5 × 103/well and cultured at 37°C. After treatment with oxaliplatin (Sigma-Aldrich, Steinheim, Germany) and scutellarin (Sigma-Aldrich Steinheim, Germany), 20 μL of MTT reagent (Sigma-Aldrich, Steinheim, Germany; 5 mg/mL) was added into the culture medium followed by 4 h incubation at 37°C. Cells were then suspended in 150 μL of dimethyl sulfoxide before detection of the absorbance at 490 nm by an ELISA microplate reader (Sunrise Microplate Reader, TECAN, Switzerland). Half maximal IC50 of oxaliplatin was calculated according to the cell viability curve.
Western blot analysis
Total proteins were extracted by using radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA, USA) before separation with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were then blocked with 5% non-fat milk in Tris-buffered saline (TBS) with 0.1% Tween 20 at 37°C for 1 h, and probed with specific antibodies of PKM2, cytochrome c, AIF, caspase-9, caspase-3, and GAPDH (Cell Signaling, Danvers, MA, USA) overnight at 4°C. The membranes were washed with TBST for 15 min, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 h. Membranes were then visualized by an enhanced chemiluminescence detection kit (Pierce, Rockford, IL, USA). In addition, to evaluate the release of cytochrome c and AIF from mitochondria into cytosol, we separated cellular mitochondria using mitochondria/cytosol fraction kits (BioVision, Milpitas, CA, USA).
Glucose, lactate, and ATP assays
Cells were collected and washed with phosphate-buffered saline (PBS) twice. Relative glucose uptake, lactate production, and ATP production were detected by Amplex Red Glucose/Glucose Oxidase Assay kits (Molecular Probes, Eugene, OR, USA), Lactate Assay kits (BioVision, Milpitas, CA, USA) and ATP Colorimetric/Fluorometric Assay kits (BioVision Milpitas, CA, USA) according to the manufacturer’s instructions, respectively.
Flow cytometry analysis
Cell apoptosis and Δϕ were detected by flow cytometry analysis. For measurement of apoptotic rate, Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kits (Sigma-Aldrich, Steinheim, Germany) were used to detect and calculate the Annexin V-positive cells. Briefly, OR-SW480 and OR-HT29 cells were grown in 6-well plates for 24 h and then transfected with empty plasmid, scutellarin + empty plasmid, oxaliplatin + empty plasmid, oxaliplatin + scutellarin + empty plasmid, and oxaliplatin + scutellarin + PKM2 + empty plasmid for 48 h. Next, cells were digested with trypsin and washed with PBS, followed by resuspending in 1× binding buffer, and stained with propidium iodide (PI) and FITC-Annexin V for 15 min at 25°C in the dark. Cells were finally detected by a flow cytometer (Beckman Coulter, Fullerton, CA, USA). For detection of Δϕ, cells were stained with 5,5′,6,6’-tetrachloro-1,1’,3,3′-tetraethylbenzimidazoly-lcarbocyanine iodide (JC-1, Molecular Probes, Eugene, OR, USA) to detect the red fluorescence according to the manufacturer’s instructions.
Xenograft on nude mice
The animal care and experimental protocols were approved by the Animal Care Committee of Shengjing Hospital of China Medical University. 4-week-old female immunodeficient nude BALB/c mice were purchased from Shanghai Super-B&K Laboratory Animal (Shanghai, China). A total of 5 × 106 OR-SW480 cells were subcutaneously injected into the right armpit. Oxaliplatin (10 mg/kg) and scutellarin (10 mg/kg) were administrated by intraperitoneal injection twice a week. Tumor size was measured every 5 days. Tumor volume was calculated according to the following formula: volume (V) = 1/2 × length × width2.
Statistical analysis
Experiments were independently repeated at least three times to obtain the data. Non-paired t test was used to estimate the statistical differences between two groups. One-way analysis of variance (ANOVA) was used to determine the differences between three or more groups. Data were analyzed by Statistical Package for the Social Science (SPSS) 15.0 software (SPSS, Chicago, IL, USA). A p value < 0.05 was considered to indicate a statistically significant difference.
Acknowledgments
None.
Author contributions
W.S. is responsible for the integrity of the entire study, study design, definition of intellectual content, data analysis, statistical analysis, and manuscript preparation and editing; Y.G. and J.C. are responsible for the literature research, clinical studies, data acquisition, and manuscript editing; Y.Y. is responsible for the literature research, experimental studies, data acquisition, and manuscript editing; B.L. is responsible for the integrity of the entire study, study concepts and design, and manuscript review. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., Bouvier A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciombor K.K., Wu C., Goldberg R.M. Recent therapeutic advances in the treatment of colorectal cancer. Annu. Rev. Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., van de Velde C.J., Watanabe T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 6.Marin J.J., Sanchez de Medina F., Castaño B., Bujanda L., Romero M.R., Martinez-Augustin O., Moral-Avila R.D., Briz O. Chemoprevention, chemotherapy, and chemoresistance in colorectal cancer. Drug Metab. Rev. 2012;44:148–172. doi: 10.3109/03602532.2011.638303. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt J.A., Mayer R.J. Systemic therapy for colorectal cancer. N. Engl. J. Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y., Wan G., Spizzo R., Ivan C., Mathur R., Hu X., Ye X., Lu J., Fan F., Xia L. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol. Oncol. 2014;8:83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gramont A., Buyse M., Abrahantes J.C., Burzykowski T., Quinaux E., Cervantes A., Figer A., Lledo G., Flesch M., Mineur L. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J. Clin. Oncol. 2007;25:3224–3229. doi: 10.1200/JCO.2006.10.4380. [DOI] [PubMed] [Google Scholar]
- 10.Díaz Beveridge R., Aparicio J., Tormo A., Estevan R., Artes J., Giménez A., Segura Á., Roldán S., Palasí R., Ramos D. Long-term results with oral fluoropyrimidines and oxaliplatin-based preoperative chemoradiotherapy in patients with resectable rectal cancer. A single-institution experience. Clin. Transl. Oncol. 2012;14:471–480. doi: 10.1007/s12094-012-0826-y. [DOI] [PubMed] [Google Scholar]
- 11.Temraz S., Mukherji D., Alameddine R., Shamseddine A. Methods of overcoming treatment resistance in colorectal cancer. Crit. Rev. Oncol. Hematol. 2014;89:217–230. doi: 10.1016/j.critrevonc.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.J., Li A.J., Han Y., Yin L., Lin M.B. Inhibition of Girdin enhances chemosensitivity of colorectal cancer cells to oxaliplatin. World J. Gastroenterol. 2014;20:8229–8236. doi: 10.3748/wjg.v20.i25.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Cui L., Duan X., Ma B., Zhong D. Pharmacokinetics and metabolism of the flavonoid scutellarin in humans after a single oral administration. Drug Metab. Dispos. 2006;34:1345–1352. doi: 10.1124/dmd.106.009779. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Huang D., Gao Z., Chen Y., Zhang L., Zheng J. Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int. J. Oncol. 2013;42:1674–1681. doi: 10.3892/ijo.2013.1873. [DOI] [PubMed] [Google Scholar]
- 15.Hou L., Chen L., Fang L. Scutellarin Inhibits Proliferation, Invasion, and Tumorigenicity in Human Breast Cancer Cells by Regulating HIPPO-YAP Signaling Pathway. Med. Sci. Monit. 2017;23:5130–5138. doi: 10.12659/MSM.904492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazurek S., Boschek C.B., Hugo F., Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Israelsen W.J., Dayton T.L., Davidson S.M., Fiske B.P., Hosios A.M., Bellinger G., Li J., Yu Y., Sasaki M., Horner J.W. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y.H., Li X.F., Liu J.T., Wang H., Fan L.L., Li J., Sun G.P. PKM2, a potential target for regulating cancer. Gene. 2018;668:48–53. doi: 10.1016/j.gene.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Tyers M., Wright G.D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019;17:141–155. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- 21.Lehár J., Krueger A.S., Avery W., Heilbut A.M., Johansen L.M., Price E.R., Rickles R.J., Short G.F., 3rd, Staunton J.E., Jin X. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009;27:659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Gramont A., Figer A., Seymour M., Homerin M., Hmissi A., Cassidy J., Boni C., Cortes-Funes H., Cervantes A., Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 23.Healey E., Stillfried G.E., Eckermann S., Dawber J.P., Clingan P.R., Ranson M. Comparative effectiveness of 5-fluorouracil with and without oxaliplatin in the treatment of colorectal cancer in clinical practice. Anticancer Res. 2013;33:1053–1060. [PubMed] [Google Scholar]
- 24.Martinez-Outschoorn U.E., Lin Z., Ko Y.H., Goldberg A.F., Flomenberg N., Wang C., Pavlides S., Pestell R.G., Howell A., Sotgia F., Lisanti M.P. Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10:2521–2528. doi: 10.4161/cc.10.15.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassidy J., Clarke S., Díaz-Rubio E., Scheithauer W., Figer A., Wong R., Koski S., Lichinitser M., Yang T.S., Rivera F. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z., Deng X., Liu Y., Liu Y., Sun L., Chen F. PKM2, function and expression and regulation. Cell Biosci. 2019;9:52. doi: 10.1186/s13578-019-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y., Li X., Yang W., Hawke D.H., Zheng Y., Xia Y., Aldape K., Wei C., Guo F., Chen Y., Lu Z. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol. Cell. 2014;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W.R., Tian M.X., Yang L.X., Lin Y.L., Jin L., Ding Z.B., Shen Y.H., Peng Y.F., Gao D.M., Zhou J. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget. 2015;6:846–861. doi: 10.18632/oncotarget.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deschoemaeker S., Di Conza G., Lilla S., Martín-Pérez R., Mennerich D., Boon L., Hendrikx S., Maddocks O.D., Marx C., Radhakrishnan P. PHD1 regulates p53-mediated colorectal cancer chemoresistance. EMBO Mol. Med. 2015;7:1350–1365. doi: 10.15252/emmm.201505492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze-Bergkamen H., Ehrenberg R., Hickmann L., Vick B., Urbanik T., Schimanski C.C., Berger M.R., Schad A., Weber A., Heeger S. Bcl-x(L) and Myeloid cell leukaemia-1 contribute to apoptosis resistance of colorectal cancer cells. World J. Gastroenterol. 2008;14:3829–3840. doi: 10.3748/wjg.14.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He J., Xie G., Tong J., Peng Y., Huang H., Li J., Wang N., Liang H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem. Biophys. 2014;70:1343–1350. doi: 10.1007/s12013-014-0062-x. [DOI] [PubMed] [Google Scholar]
- 32.You L., Zhu H., Wang C., Wang F., Li Y., Li Y., Wang Y., He B. Scutellarin inhibits Hela cell growth and glycolysis by inhibiting the activity of pyruvate kinase M2. Bioorg. Med. Chem. Lett. 2017;27:5404–5408. doi: 10.1016/j.bmcl.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Kabanov A.V., Batrakova E.V., Alakhov V.Y. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J. Control. Release. 2003;91:75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Tozzi F., Chen J., Fan F., Xia L., Wang J., Gao G., Zhang A., Xia X., Brasher H. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen S., Zhu D., Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 37.Liang J., Yu Y., Wang B., Lu B., Zhang J., Zhang H., Ge P. Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release. Molecules. 2013;18:12777–12792. doi: 10.3390/molecules181012777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoshan-Barmatz V., Keinan N., Abu-Hamad S., Tyomkin D., Aram L. Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: release of cytochrome c, AIF and Smac/Diablo. Biochim. Biophys. Acta. 2010;1797:1281–1291. doi: 10.1016/j.bbabio.2010.03.003. [DOI] [PubMed] [Google Scholar]