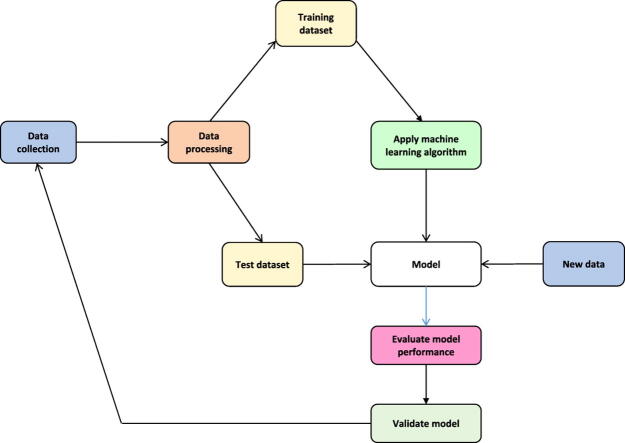
Graphical abstract
Keywords: Heart failure, Risk score, Predictive modelling, Machine learning, Sub-Saharan Africa, Mortality, Hospitalization
Abstract
Objective
The partnership between humans and machines can enhance clinical decisions accuracy, leading to improved patient outcomes. Despite this, the application of machine learning techniques in the healthcare sector, particularly in guiding heart failure patient management, remains unpopular. This systematic review aims to identify factors restricting the integration of machine learning derived risk scores into clinical practice when treating adults with acute and chronic heart failure.
Methods
Four academic research databases and Google Scholar were searched to identify original research studies where heart failure patient data was used to build models predicting all-cause mortality, cardiac death, all-cause and heart failure-related hospitalization.
Results
Thirty studies met the inclusion criteria. The selected studies' sample size ranged between 71 and 716 790 patients, and the median age was 72.1 (interquartile range: 61.1–76.8) years. The minimum and maximum area under the receiver operating characteristic curve (AUC) for models predicting mortality were 0.48 and 0.92, respectively. Models predicting hospitalization had an AUC of 0.47 to 0.84. Nineteen studies (63%) used logistic regression, 53% random forests, and 37% of studies used decision trees to build predictive models. None of the models were built or externally validated using data originating from Africa or the Middle-East.
Conclusions
The variation in the aetiologies of heart failure, limited access to structured health data, distrust in machine learning techniques among clinicians and the modest accuracy of existing predictive models are some of the factors precluding the widespread use of machine learning derived risk calculators.
1. Introduction
Predictive analytics is applied across many industries, typically for insurance underwriting, credit risk scoring and fraud detection [1], [2], [3]. Both statistical methods and machine learning algorithms are used to create predictive models [4]. In heart failure, machine learning algorithms create risk scores estimating the likelihood of a heart failure diagnosis and the probability of outcomes such as all-cause mortality, cardiac death and hospitalization [5], [6], [7], [8], [9], [10], [11], [12], [13].
Clinicians treating heart failure patients may underestimate or overestimate the risk of complications and may battle with dose titration, failing to reach target dosages when prescribing oral medication such as beta-blockers [14], [15]. Despite these challenges, risk calculators are still not widely used to guide the management of heart failure patients. Most clinicians find risk calculation time consuming and are not convinced of the value of the information derived from predictive models [15], [16]. Moreover, the lack of integration of risk scores predicting heart failure outcomes into management guidelines may diminish clinicians’ confidence when using risk calculators. Also, clinicians may question the integrity of unsupervised machine learning and deep learning methods since algorithms single-handedly select features (predictors) without human input.
Machine learning and its subtype, deep learning, have shown an impressive performance in medical image analysis and interpretation [17]. Convolutional neural networks (CNN) were trained to classify chest radiographs as pulmonary tuberculosis (TB) or normal using chest radiographs from 685 patients. The ensemble of CNN’s performed well with an area under the receiver operating characteristic curve (AUC) of 0.99 [17]. These impressive results have resulted in the commercialization of chest x-ray interpretation software [18]. The availability of such software can play a critical role in remote areas with limited or no access to radiologists, as CNN can potentially identify subtle manifestations of TB on chest radiographs, leading to prompt initiation therapy, curbing further transmission of TB. Amid these capabilities, the uptake of machine learning techniques in the healthcare sector remains limited. This systematic review aims to identify models predicting mortality and hospitalization in heart failure patients and discuss factors that restrict the widespread clinical use of risk scores created with machine learning algorithms.
2. Methods
2.1. Search strategy for identification of relevant studies
A systematic literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Literature searches were conducted in MEDLINE, Google Scholar, Springer Link, Scopus, and Web of Science. The search string contained the following terminology: (Mortality OR Death OR Readmission OR Hospitalization) AND (Machine Learning OR Deep Learning) AND (Heart Failure OR Heart Failure, Diastolic OR Heart Failure, Systolic).
2.2. Review methods and selection criteria
Studies reported in languages other than English were not included. A single reviewer screened titles, abstracts and full-text articles and made decisions regarding potential eligibility. Studies were eligible if they reported models predicting all-cause or cardiac mortality or all-cause or heart failure-related hospitalization in heart failure patients. Models included in the study were created using machine learning algorithms and/or deep learning. We did not include studies using solely logistic regression for a classification task. Logistic regression analysis is a machine learning algorithm borrowed from traditional statistics. When logistic regression is used as a machine learning algorithm, the algorithm is initially trained to identify clinical data patterns using a dataset with labelled classes, a process known as supervised learning. After that, the logistic regression algorithm attempts to classify new data into two or more categories based on “posteriori knowledge.”
2.3. Data extraction
The following items were extracted: study region, data collection period, sample size, age, gender, cause of heart failure (ischaemic vs non-ischaemic), predictor variables, handling of missing data, internal and external validation, all-cause mortality and cardiovascular death rate, all-cause hospitalization rate and performance metrics (sensitivity, accuracy, AUC or c-statistics and F-score). Summary statistics were generated with STATA MP version 13.0 (StataCorp, Texas).
3. Results
3.1. The review process
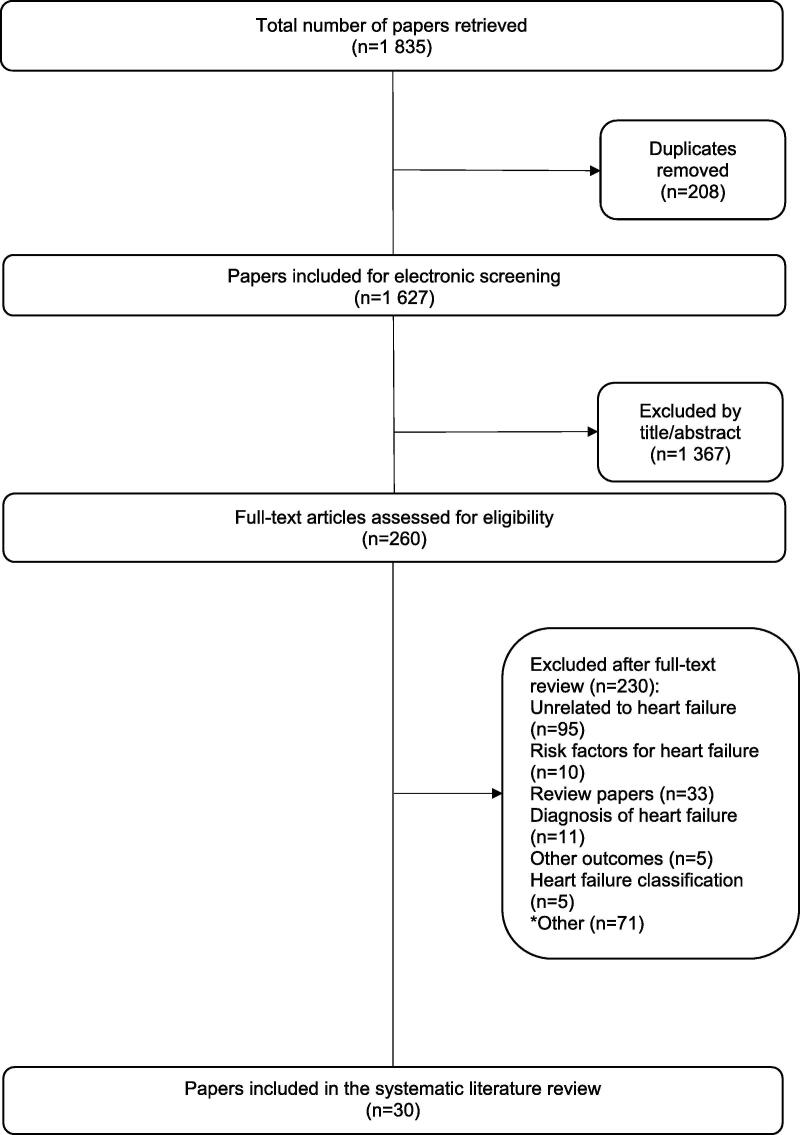
The initial search yielded 1 835 research papers. After screening titles and abstracts, 1 367 did not meet the inclusion criteria. Excluded papers were predominantly theoretical reviews and conference papers in the field of computer science. Two hundred and sixty full-text articles were assessed for eligibility. A further 230 studies were excluded, leaving thirty papers legible for analysis (Fig. 1). Reasons for excluding 230 studies are provided as supplementary data.
Fig. 1.
Flow chart of the systematic literature search.
3.2. Characteristics of the included studies
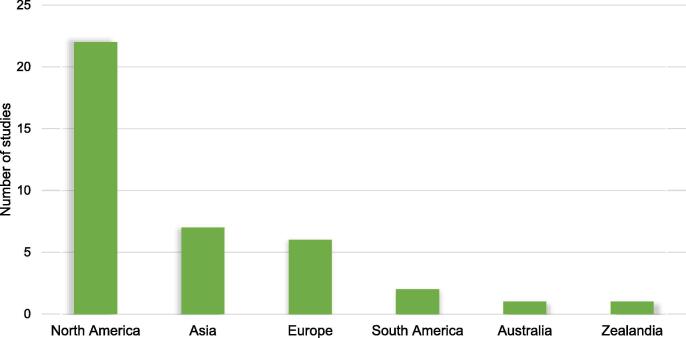
The source of data in the majority of the studies were electronic health records (EHR) (n = 16), followed by claims data (n = 5), trial data (n = 3), registry (n = 3) and data obtained from research cohorts (n = 3). Data was collected from hospitalized patients in twelve studies. The sample size in the predictive models ranged between 71 and 716 790, with the smallest sample size used to predict survival in patients with advanced heart failure managed with second-generation ventricular assist devices [19]. Within the 30 studies, twelve studies created models predicting mortality. Another 13 studies predicted hospitalization, and five studies predicted both mortality and hospitalization. The data used to create predictive models was collected between 1993 and 2017 (Table 1). Of the 30 included studies, 22 included data originating from North America, seven from Asia and six from Europe. There were no studies conducted in Africa or Middle-East (Fig. 2).
Table 1.
Characteristics of the included studies.
| Study ID | Data collection period | No. of patients | Setting | Data source | No. of features | Primary outcome assessed |
|---|---|---|---|---|---|---|
| Adler, E.D (2019) [10] | 2006–2017 | 5 822 | Inpatient and outpatient | EHR and Trial | 8 | All-cause mortality |
| Ahmad, T (2018) [30] | 2000–2012 | 44 886 | Inpatient and outpatient | Registry | 8 | 1-year all-cause mortality |
| Allam, A (2019) [31] | 2013 | 272 778 | Inpatient | Claims dataset | 50 | 30-day all-cause readmission |
| Angraal, S (2020) [13] | 2006–2013 | 1 767 | Inpatient | Trial | 26 | All-cause mortality and HF hospitalization |
| Ashfaq, A (2019) [32] | 2012–2016 | 7 655 | Inpatient and outpatient | EHR | 30-day all-cause readmission | |
| Awan, SE (2019) [33] | 2003–2008 | 10 757 | Inpatient and outpatient | EHR | 47 | 30-day HF-related readmission and mortality |
| Chen, R (2019) [34] | 2014–2017 | 98 | Inpatient | Prospective Clinical and MRI | 32 | Cardiac death, heart transplantation and HF-related hospitalization |
| Chicco, D (2020) [11] | 2015 | 299 | Inpatient | Medical records | 13 | One year survival |
| Chirinos, J (2020) [35] | 2006–2012 | 379 | Inpatient | Trial | 48 | Risk of all-cause death or heart failure-related hospital admission |
| Desai, R.J (2020) [6] | 2007–2014 | 9 502 | Inpatient and outpatient | Claims data and EHR | 62 | All-cause mortality and HF hospitalization, total costs for hospitalization, outpatient visits, and medication |
| Frizzell, J.D (2017) [36] | 2005–2011 | 56 477 | Inpatient | Registry and claims data | All-cause readmission 30-days after discharge | |
| Gleeson, S (2017) [37] | 2010–2015 | 295 | Inpatient | Echo database & EHR | 291 | All-cause mortality and heart failure admissions |
| Golas, S.B (2018) [12] | 2011–2015 | 11 510 | Inpatient and outpatient | EHR | 3 512 | All-cause 30-day readmission, healthcare utilization cost |
| Hearn, J (2018) [38] | 2001–2017 | 1 156 | EHR and Cardiopulmonary stress test data | All-cause mortality | ||
| Hsich, E (2011) [9] | 1997–2007 | 2 231 | Cardiopulmonary stress test data | 39 | All-cause mortality | |
| Jiang, W (2019) [39] | 2013–2015 | 534 | Inpatient | EHR | 57 | 30-day readmission |
| Kourou, K (2016) [19] | 71 | Pre and post-operative data | 48 | 1-year all-cause mortality | ||
| Krumholz, H (2019) [40] | 2013–2015 | 716 790 | Inpatient | Claims dataset | All-cause death within 30-days of admission | |
| Kwon, J (2019) [5] | 2016–2017 | 2 165 (training dataset) | Inpatient | Registry | 12 and 36-month in-hospital mortality | |
| Liu, W (2020) [41] | 303 233 (heart failure) | Inpatient | Readmission database | Admission 3H myocardial infarction, congestive heart failure and pneumonia 30-day readmission | ||
| Lorenzoni, G (2019) [7] | 2011–2015 | 380 | Inpatient | Research data | Hospitalization among patients with heart failure | |
| Maharaj, S.M (2018) [42] | 2015 | 1 778 | Inpatient | EHR | 56 | 30-day readmission |
| McKinley, D (2019) [20] | 2012–2015 | 132 | Inpatient | EHR | 29 | All-cause readmission within 30-days |
| Miao, F (2017) [43] | 2001–2007 | 8 059 | Public database | 32 | 1-year in-hospital mortality | |
| Nakajima, K (2020) [24] | 2005–2016 | 526 | Multicentre database | 13 | 2-year life-threatening arrhythmic events and heart failure death | |
| Shameer, K (2016) [44] | 1 068 | Inpatient | EHR | 4 205 | 30-day readmission | |
| Shams, I (2015) [45] | 2011–2012 | 1 674 | Inpatient | EHR | 30-day readmission | |
| Stampehl, M (2020) [46] | 2010–2014 | 206 644 | Inpatient | EHR | 30-day and one-year post-discharge all-cause mortality | |
| Taslimitehrani, V (2016) [47] | 1993–2013 | 5 044 | Inpatient | EHR | 43 | 1,2 and 5-year survival after HF diagnosis |
| Turgeman, L (2016) [27] | 2006–2014 | 4 840 | Inpatient | EHR | Readmission |
CVD = cardiovascular disease; EHR = electronic health record; HF = heart failure; MRI = magnetic resonance imaging.
Fig. 2.
Study population region.
3.3. Clinical characteristics of patients with heart failure
The majority of studies reported the patients’ age (93%) and gender (87%). The median age was 72.1 (61.1–76.85) years. Between 14.0 and 83.9% of the extracted studies' participants had ischaemic heart disease (Table 2). In total, 30% of studies mentioned Black patients. Between 0.95% and 100% of the individuals were Black, with one study enrolling only African American males with heart failure [20].
Table 2.
Characteristics of heart failure patients included in the 30 models predicting mortality and hospitalization.
| First Author (year) | Study Region | No. of patients | % Black | Age | % male | % Hypertension | % IHD |
|---|---|---|---|---|---|---|---|
| Adler, E.D (2019) [10] | USA and Europe | 5 822 | 60.3 | ||||
| Ahmad, T (2018) [30] | Europe | 44 886 | 73.2 | 63 | |||
| Allam, A (2019)[31] | USA and Europe | 272 778 | 73 ± 14 | 51 | |||
| Angraal, S (2020)[13] | USA, Canada, Brazil, Argentina, Russia, Georgia | 1 767 | 72 (64–79) | 50 | |||
| Ashfaq, A (2019) [32] | Europe | 7 655 | 78.8 | 57 | |||
| Awan, SE (2019) [33] | Australia | 10 757 | 82 ± 7.6 | 49 | 67 | 55 | |
| Chen, R (2019) [34] | China | 98 | 47 ± 14 | 79 | 23 | ||
| Chicco, D (2020) [34] | Pakistan | 299 | 40–95* | 65 | |||
| Chirinos, J (2020) [35] | USA, Canada, Russia | 379 | 7.4 | 70 (62–77) | 53.5 | 94.5 | 30.6 |
| Desai, R.J (2020) [6] | USA | 9 502 | 5.1 | 78 ± 8 | 45 | 87.1 | 22 |
| Frizzell, J.D (2017) [36] | USA | 56 477 | 10 | 80 (74–86) | 45.5 | 75.7 | 58 |
| Gleeson, S (2017) [37] | New Zealand | 295 | 62 | 74 | 43 | ||
| Golas, S.B (2018) [12] | USA | 11 510 | 7.9 | 75.7 (64–85) | 52.8 | ||
| Hearn, J (2018) [38] | Canada | 1 156 | 54 | 74.6 | |||
| Hsich, E (2011) [9] | USA | 2 231 | 54 ± 11 | 73 | 41 | ||
| Jiang, W (2019) [39] | USA | 534 | 28 | 74.8 | 46 | ||
| Kourou, K (2016) [19] | Belgium | 71 | 48.07 ± 14.82 | 80.3 | |||
| Krumholz, H (2019) [40] | USA | 716 790 | 11.3 | 81.1 ± 8.4 | 45.6 | ||
| Kwon, J (2019) [5] | Asia | 2 165 | 69.8 | 59.7 | |||
| Liu, W (2019) [41] | USA | 303 233 | 72.5 | 50.9 | |||
| Lorenzoni, G (2019) [7] | Italy | 380 | 78 (72–83) | 42.9 | 18.9 | ||
| Maharaj, S.M (2018) [42] | USA | 1 778 | 0.95 | 72.3 ± 12.1 | 97.6 | 14 | |
| McKinley, D (2019) [20] | USA | 132 | 100 | 59.25 | 100 | 91 | |
| Miao, F (2017) [43] | USA | 8 059 | 73.7 | 54 | 25 | 23.2 | |
| Nakajima, K (2020) [24] | Japan | 526 | 66 ± 14 | 72 | 53 | 37 | |
| Shameer, K (2016) [44] | USA | 1 068 | |||||
| Shams, I (2015) [45] | USA | 1 674 | 70.4 | 69.9 | 96 | ||
| Stampehl, M (2020) [46] | USA | 206 644 | 12.6 | 80.5 ± 11.2 | 38.3 | 96.5 | 0.4 |
| Taslimitehrani, V (2016) [47] | USA | 5 044 | 78 ± 10 | 52 | 81 | 70.2 | |
| Turgeman, L (2016) [27] | USA | 4 840 | 69.3 ± 11.02 | 96.5 | 84.9 |
Age showed as mean ± standard deviation, median (25th-75th percentile interquartile range) or minimum and maximum value.* IHD: ischaemic heart disease; USA: United States of America.
3.4. Machine learning algorithms
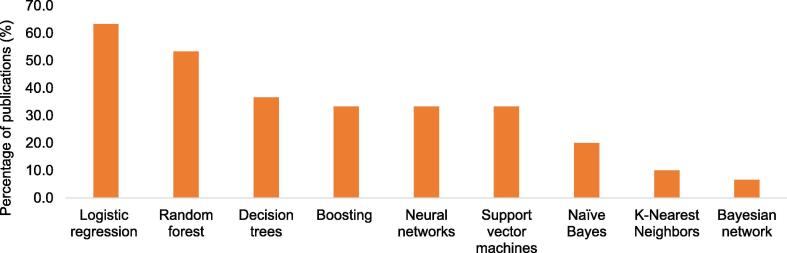
Only eight (27%) studies used a single algorithm to build a predictive model. Nineteen studies (63%) used logistic regression, 53% random forests, and 36% of studies used decision trees to create predictive models. The rest of the algorithms are depicted in Fig. 3.
Fig. 3.
Number of studies using machine learning algorithms.
3.5. Predictors
Twelve (36.4%) studies did not report on the number of predictors or features used. The number of predictors in the identified studies were between 8 and 4 205. Some authors only mentioned the number of predictors and did not list them. Age, gender, diastolic blood pressure, left ventricular ejection fraction (LVEF), estimated glomerular filtration rate, haemoglobin, serum sodium, and blood urea nitrogen were some of the predictors of mortality identified in the extracted studies [10], [11], [13]. Predictors of hospitalization included ischaemic cardiomyopathy, age, LVEF, hypotension, haemoglobin, creatinine, and potassium serum levels [7].
3.6. Model development, internal and external validation
When creating a predictive model using machine learning, data is generally partitioned into three or four datasets. In the studies extracted, between 60 and 80% of the data was used for training models, while the rest was used for testing and/or internally validating the models. Although the data on model validation was scanty, external validation was explicitly mentioned in two studies. None of the models were externally validated using data originating from Africa or the Middle-East.
3.7. Model performance and evaluation metrics
Parameters used to evaluate model performance were the confusion matrix, reporting sensitivity, specificity, positive and negative predictive value, accuracy, and precision. Most studies also reported the f-score, AUC, concordance statistic (C-statistic), and recall. The minimum and maximum AUC for models predicting mortality were 0.477 and 0.917, and models predicting hospitalization had an AUC between 0.469 and 0.836 (Table 3).
Table 3.
Performance metrics of algorithms predicting mortality and hospitalization in heart failure.
| Author | Algorithms | Sensitivity | Accuracy | AUC (mortality) | AUC (Hospitalization) | F-score |
|---|---|---|---|---|---|---|
| Adler, E.D (2019) [10] | Boosted decision trees | 0.88 (0.85–0.90) | ||||
| Ahmad, T (2018) [30] | Random forest | 0.83 | ||||
| Allam, A (2019) [31] | Recurrent neural network | 0.64 (0.640–0.645) | ||||
| Logistic regression l2-norm regularization (LASSO) | 0.643 (0.640–0.646) | |||||
| Angraal, S (2020) [13] | Logistic regression | 0.66 (0.62–0.69) | 0.73 (0.66–0.80) | |||
| Logistic regression with LASSO regularization | 0.65 (0.61–0.70) | 0.73 (0.67–0.79) | ||||
| Gradient descent boosting | 0.68 (0.66–0.71) | 0.73 (0.69–0.77) | ||||
| Support vector machines (linear kernel) | 0.66 (0.60–0.72) | 0.72 (0.63–0.81) | ||||
| Random forest | 0.72 (0.69–0.75) | 0.76 (0.71–0.81) | ||||
| Ashfaq, A (2019) [32] | Long Short-Term Memory (LSTM) neural network | 0.77 | 0.51 | |||
| Awan, SE (2019) [33] | Multi-layer perceptron (MLP) | 48.4 | 0.62 | |||
| Chen, R (2019) [34] | Naïve Bayes | 0.827 | 0.855 0.887 0.890 0.877 0.852 0.847 0.705 0.797 |
|||
| Naïve Bayes + IG | 0.857 | |||||
| Random forest | 0.817 | |||||
| Random forest + IG | 0.827 | |||||
| Decision trees (bagged) | 0.827 | |||||
| Decision trees (bagged) + IG | 0.816 | |||||
| Decision trees (boosted) | 0.735 | |||||
| Decision trees (boosted) + IG | 0.806 | |||||
| Chicco, D (2020) [11] | Random forest | 0.740 | 0.800 | 0.547 | ||
| Decision tree | 0.737 | 0.681 | 0.554 | |||
| Gradient boosting | 0.738 | 0.754 | 0.527 | |||
| Linear regression | 0.730 | 0.643 | 0.475 | |||
| One rule | 0.729 | 0.637 | 0.465 | |||
| Artificial neural network | 0.680 | 0.559 | 0.483 | |||
| Naïve Bayes | 0.696 | 0.589 | 0.364 | |||
| SVM (radial) | 0.690 | 0.749 | 0.182 | |||
| SVM (linear) | 0.684 | 0.754 | 0.115 | |||
| K-nearest neighbors | 0.624 | 0.493 | 0.148 | |||
| Chirinos, J (2020) [35] | Tree-based pipeline optimizer | 0.717 (0.643–0.791) | ||||
| Desai, R.J (2020) [6] | Logistic regression (traditional) | 0.749 (0.729–0.768) | 0.738 (0.711–0.766) | |||
| LASSO | 0.750 (0.731–0.769) | 0.764 (0.738–0.789) | ||||
| CART | 0.700 (0.680–0.721) | 0.738 (0.710–0.765) | ||||
| Random forest | 0.757 (0.739–0.776) | 0.764 (0.738–0.790) | ||||
| GBM | 0.767 (0.749–0.786) | 0.778 (0.753–0.802) | ||||
| Frizzell, J.D (2017) [36] | Random forest | 0.607 | ||||
| GBM | 0.614 | |||||
| TAN | 0.618 | |||||
| LASSO | 0.618 | |||||
| Logistic regression | 0.624 | |||||
| Gleeson, S (2017) [37] | Decision trees | 0.7505 | ||||
| Golas, S.B (2018) [12] | Logistic regression | 0.626 | 0.664 | 0.435 | ||
| Gradient boosting | 0.612 | 0.650 | 0.425 | |||
| Maxout networks | 0.645 | 0.695 | 0.454 | |||
| Deep unified networks | 0.646 | 0.705 | 0.464 | |||
| Hearn, J (2018) [38] | Staged LASSO | 0.827 (0.785–0.867) | ||||
| Staged neural network | 0.835 (0.795–0.880) | |||||
| LASSO (breath-by-breath) | 0.816 (0.767–0.866) | |||||
| Neural network (breath-by-breath) | 0.842 (0.794–0.882) | |||||
| Hsich, E (2011) [9] | Random survival forest | 0.705 | ||||
| Cox proportional hazard | 0.698 | |||||
| Jiang, W (2019) [39] | Logistic and beta regression (ML) | 0.73 | ||||
| Kourou, K (2016) [19] | Naïve Bayes | 85 | 0.86 | |||
| Bayesian network | 85.9 | 0.596 | ||||
| Adaptive boosting | 78 | 0.74 | ||||
| Support vector machines | 90 | 0.74 | ||||
| Neural networks | 87 | 0.845 | ||||
| Random forest | 75 | 0.65 | ||||
| Krumholz, H (2019) [40] | Logistic regression (ML) | 0.776 | ||||
| Kwon, J (2019) [5] | Deep learning | 0.813 (0.810–0.816) | ||||
| Random forest | 0.696 (0.692–0.700) | |||||
| Logistic regression | 0.699 (0.695–0.702) | |||||
| Support vector machine | 0.636 (0.632–0.640) | |||||
| Bayesian network | 0.725 (0.721–0.728) | |||||
| Liu, W (2019) [41] | Logistic regression | 0.580 (0.578–0.583) | ||||
| Gradient boosting | 0.602 (0.599–0.605) | |||||
| Artificial neural networks | 0.604 (0.602–0.606) | |||||
| Lorenzoni, G (2019) [7] | GLMN | 77.8 | 0.812 | 0.86 | ||
| Logistic regression | 54.7 | 0.589 | 0.646 | |||
| CART | 44.3 | 0.635 | 0.586 | |||
| Random forest | 54.9 | 0.726 | 0.691 | |||
| Adaptive Boosting | 57.3 | 0.671 | 0.644 | |||
| Logitboost | 66.7 | 0.625 | 0.654 | |||
| Support vector machines | 57.3 | 0.699 | 0.695 | |||
| Artificial neural networks | 61.6 | 0.682 | 0.677 | |||
| Maharaj, S.M (2018) [42] | Boosted tree | 0.719 | ||||
| Spike and slab regression | 0.621 | |||||
| McKinley, D (2019) [20] | K-nearest neighbor | 0.773 | 0.768 | |||
| K-nearest neighbor (randomized) | 0.477 | 0.469 | ||||
| Support vector machines | 0.545 | 0.496 | ||||
| Random forest | 0.682 | 0.616 | ||||
| Gradient boosting machine | 0.614 | 0.589 | ||||
| LASSO | 0.614 | 0.576 | ||||
| Miao, F (2017) [43] | Random survival forest | 0.804 | ||||
| Random survival forest (improved) | 0.821 | |||||
| Nakajima, K (2020) [24] | Logistic regression | 0.898 | ||||
| Random forest | 0.917 | |||||
| GBT | 0.907 | |||||
| Support vector machine | 0.910 | |||||
| Naïve Bayes | 0.875 | |||||
| k-nearest neighbors | 0.854 | |||||
| Shameer, K (2016) [44] | Naïve Bayes | 0.832 | 0.78 | |||
| Shams, I (2015) [45] | Phase type Random forest | 91.95 | 0.836 | 0.892 | ||
| Random forest | 88.43 | 0.802 | 0.865 | |||
| Support vector machine | 86.16 | 0.775 | 0.857 | |||
| Logistic regression | 83.40 | 0.721 | 0.833 | |||
| Artificial neural network | 82.39 | 0.704 | 0.823 | |||
| Stampehl, M (2020) [46] | CART | |||||
| Logistic regression | ||||||
| Logistic regression (stepwise) | 0.74 | |||||
| Taslimitehrani, V (2016) [47] | CPXR(Log) | 0.914 | ||||
| Support vector machine | 0.75 | |||||
| Logistic regression | 0.89 | |||||
| Turgeman, L (2016) [27] | Naïve Bayes | 48.9 | 0.676 | |||
| Logistic regression | 28.1 | 0.699 | ||||
| Neural network | 8.9 | 0.639 | ||||
| Support vector machine | 23.0 | 0.643 | ||||
| C5 (ensemble model) | 43.5 | 0.693 | ||||
| CART (boosted) | 22.6 | 0.556 | ||||
| CART (bagged) | 9.0 | 0.579 | ||||
| CHAID Decision trees (boosted) | 30.3 | 0.691 | ||||
| CHAID Decision trees (bagged) | 10.5 | 0.707 | ||||
| Quest decision tree (boosted) | 20.3 | 0.487 | ||||
| Quest decision tree (bagged) | 7.2 | 0.579 | ||||
| Naïve network + Logistic regression | 38.2 | 0.653 | ||||
| Naïve network + Neural network | 26.3 | 0.635 | ||||
| Naïve network + SVM | 35.8 | 0.649 | ||||
| Logistic regression + Neural network | 16.8 | 0.59 | ||||
| Logistic regression + SVM | 26.2 | 0.607 | ||||
| Neural network + SVM | 16.5 | 0.577 | ||||
AUC: area under the receiver operating characteristic curve; CART: classification and regression tree; CPXR: contrast pattern aided logistic regression; GBM: gradient-boosted model; HR: hazard ratio; IG: information gain; LASSO: least absolute shrinkage and selection operator; ML: machine learning; SVM: support vector machine; TAN: tree augmented Bayesian network. The AUC is displayed under both the mortality and hospitalization column if the authors did not specify the outcome predicted.
4. Discussion
This systematic review highlights several factors that restrict the use of risk scores created with machine learning algorithms in the clinical setting. The existence of clinical information with prognostic significance such as the New York Heart Association functional class in the free-text format in EHR systems may result in models with low predictive abilities if such critical data is omitted when building predictive models. Fortunately, newer emerging techniques such as bidirectional long short-term memory with a conditional random fields layer have been introduced to remedy the problem of free-text in EHR [21], [22].
Risk scores derived from heart failure patients residing in North America or Europe may not be suitable for application in low and middle-income countries (LMIC). In high income countries (HIC), the predominant cause of heart failure is ischaemic heart disease (IHD), whereas, in sub-Saharan Africa, hypertension is still the leading cause of heart failure [23]. Also, healthcare services' availability and efficiency differ significantly between countries, suggesting that algorithms trained using data from HIC should be retrained using local data before adopting risk calculators.
Despite the endemicity of heart failure in LMIC, risk scores derived from patients residing in LMIC are scanty or non-existent. The lack of EHR systems, registries, and pooled data from multicentre studies is responsible for the absence of risk scores derived from patients in LMIC. If digital structured health data were available in LMIC, models predicting outcomes could be created instead of extrapolating from studies conducted in HIC. The absence of structured health data in LMIC resulted in the underrepresentation of this population in the training and test datasets included in this systematic review.
The AUC was one of the most commonly reported performance metric in the extracted studies. The highest AUC for models predicting mortality was 0.92, achieved by the random forest algorithm in a study by Nakajima et al., where both clinical and physiological imaging data were used to train algorithms [24]. A model with an AUC equal to or below 0.50 is unable to discriminate between classes. One might as well toss a coin when making predictions. Some of the reasons for the modest performance metrics demonstrated by machine learning algorithms include a training dataset with excessive missing data or few predictors, absence of ongoing partnership between clinicians and data scientists and class imbalance. In most instances, when handling healthcare data, the negative class tends to outnumber positive classes. The learning environment is rendered unfavourable since there are fewer positive observations or patterns for an algorithm to learn from. For example, when predicting mortality, the class with patients that demised is frequently smaller than the class with alive patients.
Models with perfect precision and recall have an F-measure, also known as the F-Score or F1 Score, equal to one [25]. Sensitivity, also known as recall, measures a proportion of positive classes accurately classified as positive [26]. Machine learning algorithms in the extracted studies had a sensitivity rate between 7.2 and 91.9%. The low sensitivity, reported by Turgeman and May, improved to 43.5% when they used an ensemble method to combine multiple predictive models to produce a single model [27].
Although the random forest algorithm appeared to have the highest predictive abilities in most studies, one cannot conclude that it should be the algorithm of choice whenever one attempts to create a predictive model. The random forest algorithm's main advantage is that it is an ensemble-based classifier that takes random samples of data, exposing them to multiple decision tree algorithms. Decision trees are intuitive and interpretable and can immediately suggest why a patient is stratified into a high-risk category, hence guiding subsequent risk reduction interventions. The interpretability of decision trees is a significant advantage in contrast to deep learning methodologies such as artificial neural networks with a “black box” nature. Once random samples of data have been exposed to multiple decision tree algorithms, the decision trees' ensemble identifies the class with the highest number of votes when making predictions. Random forests also perform well in large datasets with missing data, a common finding when handling healthcare data, and can rank features (predictors) in the order of importance, based on predictive powers [28].
Predictors of mortality identified by machine learning algorithms in the extracted studies were explainable and included features such as the LVEF, hypotension, age and blood urea nitrogen levels. Whether these predictors should be considered significant risk factors for all heart failure, irrespective of genetic makeup, is debatable. The youngest patient in the studies reviewed was 40 years old, but most of the patients included in the predictive models were significantly older, with a median age of 72 years. Risk scores derived from older patients may reduce the applicability of the existing risk calculators in the sub-Saharan African (SSA) context, considering that patients with heart failure in SSA are generally a decade younger [29].
Geographically unique heart failure aetiologies and diverse clinical presentations call for predictive models that incorporate genomic, clinical and imaging data. We recommend that clinicians treating heart failure patients focus on establishing structured EHR systems and comparing outcomes such as mortality and hospitalization in patients managed with and without risk scores. Clinicians without access to EHR systems should carefully study the cohort used to create risk scores before implementing risk scores in their clinical practice.
5. Limitations
This systematic literature review has several limitations. The systematic literature search was conducted by a single reviewer, predisposing the review to selection bias. We only included original research studies published after 2009. The rationale for including studies published in the past 11 years was to avoid including studies where rule-based expert systems were used instead of newer machine learning techniques. Although the data used to create predictive models was grossly heterogeneous, a meta-analytic component as part of the review would have provided a broader perspective on machine learning algorithms' performance metrics when predicting heart failure patient outcomes.
6. Conclusion
The variation in the aetiologies of heart failure, limited access to structured health data, distrust in machine learning techniques among clinicians and the modest accuracy of predictive models are some of the factors precluding the widespread use of machine learning derived risk calculators.
7. Grant support
The study did not receive financial support. The primary author Dr Dineo Mpanya is a full-time PhD Clinical Research fellow in the Division of Cardiology, Department of Internal Medicine at the University of the Witwatersrand. Her PhD is funded by the Professor Bongani Mayosi Netcare Clinical Scholarship, the Discovery Academic Fellowship (Grant No. 039023), the Carnegie Corporation of New York (Grant No. b8749) and the South African Heart Association.
Declaration of Competing Interest
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100773.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Boodhun N., Jayabalan M. Risk prediction in life insurance industry using supervised learning algorithms. Compl. Intell. Syst. 2018;4:145–154. [Google Scholar]
- 2.Carcillo F., Le Borgne Y.-A., Caelen O., Bontempi G. Streaming active learning strategies for real-life credit card fraud detection: assessment and visualization. Int. J. Data Sci. Analyt. 2018;5:285–300. [Google Scholar]
- 3.Moradi S., Mokhatab Rafiei F. A dynamic credit risk assessment model with data mining techniques: evidence from Iranian banks. Financ. Innovat. 2019;5:15. [Google Scholar]
- 4.Mpanya D., Celik T., Klug E., Ntsinjana H. Machine learning and statistical methods for predicting mortality in heart failure. Heart Fail Rev. 2020 doi: 10.1007/s10741-020-10052-y. [DOI] [PubMed] [Google Scholar]
- 5.Kwon J.M., Kim K.H., Jeon K.H., Lee S.E., Lee H.Y., Cho H.J. Artificial intelligence algorithm for predicting mortality of patients with acute heart failure. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai R.J., Wang S.V., Vaduganathan M., Evers T., Schneeweiss S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.18962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.G. Lorenzoni, S. Santo Sabato, C. Lanera, D. Bottigliengo, C. Minto, H. Ocagli, et al., Comparison of machine learning techniques for prediction of hospitalization in heart failure patients, J. Clin. Med. 2019;8. [DOI] [PMC free article] [PubMed]
- 8.Blecker S., Sontag D., Horwitz L.I., Kuperman G., Park H., Reyentovich A. Early identification of patients with acute decompensated heart failure. J. Card Fail. 2018;24:357–362. doi: 10.1016/j.cardfail.2017.08.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsich E., Gorodeski E.Z., Blackstone E.H., Ishwaran H., Lauer M.S. Identifying important risk factors for survival in patient with systolic heart failure using random survival forests. Circulat.: Cardiovas. Qual. Outcomes. 2011;4:39–45. doi: 10.1161/CIRCOUTCOMES.110.939371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler E.D., Voors A.A., Klein L., Macheret F., Braun O.O., Urey M.A. Improving risk prediction in heart failure using machine learning. Eur. J. Heart Fail. 2020;22:139–147. doi: 10.1002/ejhf.1628. [DOI] [PubMed] [Google Scholar]
- 11.Chicco D., Jurman G. Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. BMC Med. Inf. Decis. Making. 2020;20 doi: 10.1186/s12911-020-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golas S.B., Shibahara T., Agboola S., Otaki H., Sato J., Nakae T. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med. Inform. Decis. Mak. 2018;18:44. doi: 10.1186/s12911-018-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angraal S., Mortazavi B.J., Gupta A., Khera R., Ahmad T., Desai N.R. Machine learning prediction of mortality and hospitalization in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:12–21. doi: 10.1016/j.jchf.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiade M., Albert N.M., Curtis A.B., Thomas Heywood J., McBride M.L., Inge P.J. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012;18:9–17. doi: 10.1111/j.1751-7133.2011.00250.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanratty B., Hibbert D., Mair F., May C., Ward C., Capewell S. Doctors' perceptions of palliative care for heart failure: focus group study. BMJ. 2002;325:581–585. doi: 10.1136/bmj.325.7364.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichler K., Zoller M., Tschudi P., Steurer J. Barriers to apply cardiovascular prediction rules in primary care: a postal survey. BMC Family Pract. 2007;8:1. doi: 10.1186/1471-2296-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakhani P., Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284:574–582. doi: 10.1148/radiol.2017162326. [DOI] [PubMed] [Google Scholar]
- 18.Khan F.A., Majidulla A., Tavaziva G., Nazish A., Abidi S.K., Benedetti A. Chest x-ray analysis with deep learning-based software as a triage test for pulmonary tuberculosis: a prospective study of diagnostic accuracy for culture-confirmed disease. Lancet Digit Health. 2020;2:e573–e581. doi: 10.1016/S2589-7500(20)30221-1. [DOI] [PubMed] [Google Scholar]
- 19.Kourou K., Rigas G., Exarchos K.P., Goletsis Y., Exarchos T.P., Jacobs S. Prediction of time dependent survival in HF patients after VAD implantation using pre- and post-operative data. Comput. Biol. Med. 2016;70:99–105. doi: 10.1016/j.compbiomed.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 20.McKinley D., Moye-Dickerson P., Davis S., Akil A. Impact of a pharmacist-led intervention on 30-Day readmission and assessment of factors predictive of readmission in African American men with heart failure. Am. J. Men's Health. 2019;13 doi: 10.1177/1557988318814295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagannatha A.N., Yu H. Bidirectional RNN for medical event detection in electronic health records. Proc. Conf. 2016;2016:473–482. doi: 10.18653/v1/n16-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagannatha A.N., Yu H. Structured prediction models for RNN based sequence labeling in clinical text. Proc. Conf. Empir. Methods Nat. Lang. Process. 2016;2016:856–865. doi: 10.18653/v1/d16-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agbor V.N., Essouma M., Ntusi N.A.B., Nyaga U.F., Bigna J.J., Noubiap J.J. Heart failure in sub-Saharan Africa: a contemporaneous systematic review and meta-analysis. Int. J. Cardiol. 2018;257:207–215. doi: 10.1016/j.ijcard.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima K., Nakata T., Doi T., Tada H., Maruyama K. Machine learning-based risk model using 123I-metaiodobenzylguanidine to differentially predict modes of cardiac death in heart failure. J. Nucl. Cardiol.: Off. Publ. Am. Soc. Nucl. Cardiol. May. 2020 doi: 10.1007/s12350-020-02173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hripcsak G., Rothschild A.S. Agreement, the f-measure, and reliability in information retrieval. J. Am. Med. Informat. Assoc.: JAMIA. 2005;12:296–298. doi: 10.1197/jamia.M1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T., Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turgeman L., May J.H. A mixed-ensemble model for hospital readmission. Artif. Intell. Med. 2016;72:72–82. doi: 10.1016/j.artmed.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Uddin S., Khan A., Hossain M.E., Moni M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inf. Decis. Making. 2019;19 doi: 10.1186/s12911-019-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloomfield G.S., Barasa F.A., Doll J.A., Velazquez E.J. Heart failure in sub-Saharan Africa. Curr. Cardiol. Rev. 2013;9:157–173. doi: 10.2174/1573403X11309020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad T., Lund L.H., Rao P., Ghosh R., Warier P., Vaccaro B. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allam A., Nagy M., Thoma G., Krauthammer M. Neural networks versus Logistic regression for 30 days all-cause readmission prediction. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-45685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashfaq A., Sant'Anna A., Lingman M., Nowaczyk S. Readmission prediction using deep learning on electronic health records. J. Biomed. Inform. 2019;97 doi: 10.1016/j.jbi.2019.103256. [DOI] [PubMed] [Google Scholar]
- 33.Awan S.E., Bennamoun M., Sohel F., Sanfilippo F.M., Chow B.J., Dwivedi G. Feature selection and transformation by machine learning reduce variable numbers and improve prediction for heart failure readmission or death. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R., Lu A., Wang J., Ma X., Zhao L., Wu W. Using machine learning to predict one-year cardiovascular events in patients with severe dilated cardiomyopathy. Eur. J. Radiol. 2019;117:178–183. doi: 10.1016/j.ejrad.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Chirinos J.A., Orlenko A., Zhao L., Basso M.D., Cvijic M.E., Li Z. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2020;75:1281–1295. doi: 10.1016/j.jacc.2019.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frizzell J.D., Liang L., Schulte P.J., Yancy C.W., Heidenreich P.A., Hernandez A.F. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMACardiol. 2017;2:204–209. doi: 10.1001/jamacardio.2016.3956. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson S., Liao Y.W., Dugo C., Cave A., Zhou L., Ayar Z. ECG-derived spatial QRS-T angle is associated with ICD implantation, mortality and heart failure admissions in patients with LV systolic dysfunction. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hearn J., Ross H.J., Mueller B., Fan C.P., Crowdy E., Duhamel J. Neural networks for prognostication of patients with heart failure. Circulation Heart Failure. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005193. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W., Siddiqui S., Barnes S., Barouch L.A., Korley F., Martinez D.A. Readmission risk trajectories for patients with heart failure using a dynamic prediction approach: Retrospective study. J. Med. Int. Res. 2019;21 doi: 10.2196/14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krumholz H.M., Coppi A.C., Warner F., Triche E.W., Li S.X., Mahajan S. Comparative effectiveness of new approaches to improve mortality risk models from medicare claims data. JAMA Network Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W., Stansbury C., Singh K., Ryan A.M., Sukul D., Mahmoudi E. Predicting 30-day hospital readmissions using artificial neural networks with medical code embedding. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahajan S.M., Mahajan A.S., King R., Negahban S. Predicting risk of 30-day readmissions using two emerging machine learning methods. Stud. Health Technol. Inform. 2018;250:250–255. [PubMed] [Google Scholar]
- 43.Miao F., Cai Y.P., Zhang Y.X., Fan X.M., Li Y. Predictive modeling of hospital mortality for patients with heart failure by using an improved random survival forest. IEEE Access. 2018;6:7244–7253. [Google Scholar]
- 44.Shameer K., Johnson K.W., Yahi A., Miotto R., Li L.I., Ricks D. Predictive modeling of hospital readmission rates using electronic medical record-wide machine learning: a case-study using mount sinai heart failure cohort. Pac. Symp. Biocomput. 2017;22:276–287. doi: 10.1142/9789813207813_0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shams I., Ajorlou S., Yang K. A predictive analytics approach to reducing 30-day avoidable readmissions among patients with heart failure, acute myocardial infarction, pneumonia, or COPD. Health Care Manage. Sci. 2015;18:19–34. doi: 10.1007/s10729-014-9278-y. [DOI] [PubMed] [Google Scholar]
- 46.Stampehl M., Friedman H.S., Navaratnam P., Russo P., Park S., Obi E.N. Risk assessment of post-discharge mortality among recently hospitalized Medicare heart failure patients with reduced or preserved ejection fraction. Curr. Med. Res. Opin. 2020;36:179–188. doi: 10.1080/03007995.2019.1662654. [DOI] [PubMed] [Google Scholar]
- 47.Taslimitehrani V., Dong G., Pereira N.L., Panahiazar M., Pathak J. Developing EHR-driven heart failure risk prediction models using CPXR(Log) with the probabilistic loss function. J. Biomed. Inform. 2016;60:260–269. doi: 10.1016/j.jbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.