Highlights
-
•
Acute coronary occlusions without ST elevation criteria suffer double mortality without reperfusion.
-
•
ECG findings other than ST elevation criteria can identify occlusion myocardial infarction sooner and more accurately.
-
•
Our results justify further research to evaluate the external validity of advanced ECG interpretation.
Keywords: Acute coronary syndromes, ST elevation myocardial infarction, Occlusion myocardial infarction, Electrocardiography
Abbreviations: ACS, Acute coronary syndrome; AMI, acute myocardial infarction; ECG, Electrocardiogram; ED, Emergency department; NOMI, Non-occlusion myocardial infarction; NSTEMI, Non-ST-segment elevation myocardial infarction; OMI, Occlusion myocardial infarction; STD, ST-segment depression; STE, ST-segment elevation; STEMI, ST-segment elevation myocardial infarction; MIRO, Myocardial Infarction Ruled Out; LBBB, Left Bundle Branch Block; VPR, Ventricular Paced Rhythm; MSC, Modified Sgarbossa Criteria
Abstract
Objective
In the STEMI paradigm of Acute Myocardial Infarction (AMI), many NSTEMI patients have unrecognized acute coronary occlusion MI (OMI), may not receive emergent reperfusion, and have higher mortality than NSTEMI patients without occlusion. We have proposed a new OMI vs. Non-Occlusion MI (NOMI) paradigm shift. We sought to compare the diagnostic accuracy of OMI ECG findings vs. formal STEMI criteria for the diagnosis of OMI. We hypothesized that blinded interpretation for predefined OMI ECG findings would be more accurate than STEMI criteria for the diagnosis of OMI.
Methods
We performed a retrospective case-control study of patients with suspected acute coronary syndrome. The primary definition of OMI was either 1) acute TIMI 0–2 flow culprit or 2) TIMI 3 flow culprit with peak troponin T 1.0 ng/mL or I 10.0 ng/mL.
Results
808 patients were included, of whom 49% had AMI (33% OMI; 16% NOMI). Sensitivity, specificity, and accuracy of STEMI criteria vs Interpreter 1 using OMI ECG findings among 808 patients were 41% vs 86%, 94% vs 91%, and 77% vs 89%, and for Interpreter 2 among 250 patients were 36% vs 80%, 91% vs 92%, and 76% vs 89%. STEMI(−) OMI patients had similar infarct size and mortality as STEMI(+) OMI patients, but greater delays to angiography.
Conclusions
Blinded interpretation using predefined OMI ECG findings was superior to STEMI criteria for the ECG diagnosis of Occlusion MI. These data support further investigation into the OMI vs. NOMI paradigm and suggest that STEMI(−) OMI patients could be identified rapidly and noninvasively for emergent reperfusion using more accurate ECG interpretation.
1. Introduction
1.1. Background and importance
Reperfusion of an acute coronary occlusion (ACO) results in decreased mortality and morbidity in patients with acute myocardial infarction (AMI) [1]. The only placebo-controlled trials of reperfusion therapy were conducted in the thrombolytic era, and ECGs were classified simply as ST Elevation (STE), ST Depression (STD), or neither. The large meta-analysis of these trials showed that STE correlated with decreased mortality from thrombolytics [1]. Although these studies contained no angiographic information and STE was poorly characterized, STE became the surrogate term for ACO, or near-ACO, causing AMI requiring emergent reperfusion. There have been no further interventional trials examining the relationship between STE (or any other ECG findings) and reperfusion for ACO. Nevertheless, the medical community and international guidelines use the term “STEMI” as the surrogate for acute coronary occlusion myocardial infarction (“Occlusion MI”, or “OMI”). Under the current STEMI vs. NSTEMI paradigm, 25–30% of NSTEMI have unrecognized acute total occlusion (OMI) discovered on delayed angiogram (in these studies, an average of 24 h after presentation) and have approximately double short and long-term mortality compared to NSTEMI patients without OMI (Non-Occlusion MI, or NOMI) [2]. Conversely, 15–35% of cath lab activations due to perceived STEMI criteria are found to be false positives without even a culprit lesion [3], [4], [5].
Investigators find many patients with OMI have STE that does not meet STEMI criteria, and have found many other ECG indicators of OMI, including hyperacute T-waves, terminal QRS distortion, low QRS amplitude, and more [6]. Aslanger et al. recently reclassified 28% of NSTEMIs as OMI with structured interpretation using predefined OMI ECG findings, identifying a group with similar lesions and outcomes as STEMI [7]. Furthermore, some OMI have no ECG manifestations and must be diagnosed by a combination of clinical suspicion, ongoing symptoms, biomarker elevation, echocardiography, or even coronary computed tomography angiography [8], [9], [10]. Even so, the diagnosis may not be evident until angiography.
Objections to this new OMI/NOMI classification center around studies that purport to show that early angiography for undifferentiated NSTEMI patients does not result in better outcomes. These objections fail to take into account that these studies excluded patients with persistent symptoms, or did not actually use very early intervention [11], [12], [13], [14], [15], [16], [17], [18], [19]. In the largest such study, patients with persistent symptoms were excluded and “early” angiography was at a mean of 16 h; even so, patients with a GRACE (Global Registry of Acute Coronary Events) score of >140 did indeed benefit from earlier reperfusion [11], [12]. In studies that did not exclude patients with persistent symptoms, and patients underwent truly early intervention, outcomes were better in the group randomized to early intervention.
Thus, international guidelines for NSTEMI, explicitly recognizing the limitations of the aforementioned randomized trials, recommend emergent angiography for patients with symptoms highly suspicious for ACS and with instability or persistent symptoms, even in the absence of ECG or biomarker evidence of AMI [20], [21]. By recommending multiple other diagnostic adjuncts in addition to the ECG, these guidelines reinforce the underlying assumption that acute coronary occlusion is the underlying pathology that warrants emergent reperfusion, rather than the ECG millimeter criteria which may or may not accompany it. Despite the fact that NSTEMIs with missed occlusion have double the mortality of NSTEMIs without occlusion, no randomized trial has ever assessed this, and probably could not be performed for ethical reasons.
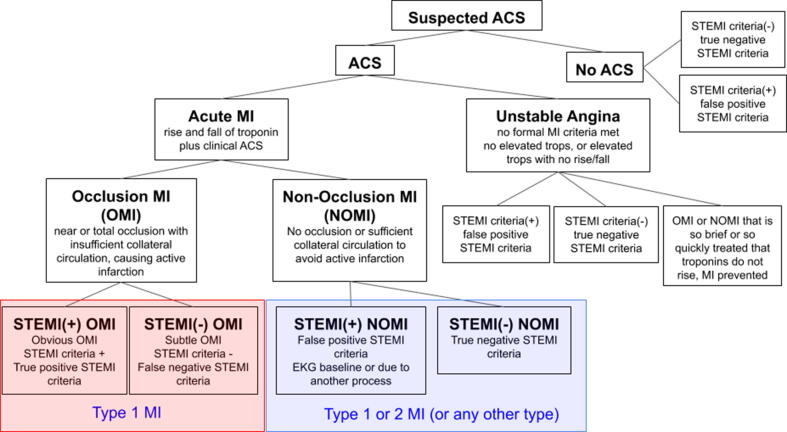
We have proposed a replacement paradigm known as Occlusion MI (OMI) [22], [23]. OMI is defined conceptually as acute coronary occlusion or near occlusion with insufficient collateral circulation, such that downstream myocardium will undergo imminent infarction without timely reperfusion. The OMI paradigm emphasizes the underlying pathology rather than insufficient surrogate test results (STE). Table 1 defines terms relevant to both paradigms. Fig. 1 shows the ACS paradigm with incorporation of the OMI vs. NOMI concept.
Table 1.
Definitions and terminology among paradigms. AMI, Acute myocardial infarction, ECG, Electrocardiogram, STEMI, ST-segment elevation myocardial infarction.
| Definitions and Terminology of Paradigms | |
|---|---|
| STEMI | Refers to AMI with ECG findings meeting the definition of STEMI criteria in the fourth universal definition of MI[21] |
| False positive STEMI | Refers to a patient with ECG features meeting formal STEMI criteria, but the STE is not a result of ischemia, as evidenced by absence of OMI on angiogram and absence of any evolution on subsequent ECGs. |
| STEMI(+) OMI (“True positive STEMI”) | Refers to a patient with ECG features meeting formal STEMI criteria, who is found to have OMI as the cause of the STE and the AMI. |
| Occlusion MI (OMI) | Refers to type 1 ACS involving acute occlusion or near occlusion of a major epicardial coronary vessel with insufficient collateral circulation, resulting in imminent necrosis of downstream myocardium without emergent reperfusion. OMI is the anatomic and pathophysiologic substrate of STEMI, but not all OMI manifests as STEMI. |
| Non-Occlusion MI (NOMI) | Refers to AMI without angiographic, laboratory, or clinical evidence of OMI (NSTEMI without occlusion). Many but not all NOMI have culprit lesions and are type 1 MI. |
| STEMI(−) OMI | Refers to OMI without the ECG meeting STEMI criteria. (NSTEMI with occlusion) |
| MIRO, MI Ruled Out | Refers to a patient in whom AMI has been ruled out. MIRO cases may still have mildly elevated troponins but are adjudicated as non-AMI acute myocardial injury. |
Fig. 1.
The ACS spectrum using the Occlusion MI (OMI) vs. Non-OMI (NOMI) paradigm primarily. The proposed paradigm of MI divides AMI into OMI and NOMI. OMI are those for whom thrombolytics and percutaneous coronary intervention were conceptually designed and indicated, but many OMI do not manifest STEMI criteria. ACS, acute coronary syndrome, MI, myocardial infarction, STEMI, ST-segment elevation MI, OMI, Occlusion MI, NOMI, Non-Occlusion MI.
Although STE is insufficient to detect many cases of OMI, there are many other ECG findings [6] of OMI, which may improve both the sensitivity and specificity of the ECG compared to STEMI criteria, as Aslanger showed among cardiologist interpreters [7]. However, this has not yet been studied among emergency physicians.
We aimed to compare the accuracy of ECG interpretation using predefined OMI ECG findings versus STEMI criteria for identification of OMI. Secondarily, we planned to precisely explore ECG findings of OMI cases without STEMI criteria, and to quantify the delay between OMI ECG findings and progression to STEMI criteria, if and when such progression occurred. We evaluated interobserver reliability between two interpreters for both 1) presence of STEMI criteria and 2) presence of OMI ECG findings. Finally, we evaluated all available patient and disease-oriented outcomes (cardiac troponin [cTn] I or T, angiographic findings, clinical outcomes) among various groups according to STEMI and OMI ECG findings. Infarct size was estimated by peak cTn [24], [25], [26], [27]. We hypothesized that OMI ECG findings would be significantly more sensitive than STEMI criteria, while maintaining specificity, for the detection of OMI, and that STEMI(−) OMI patients would have adverse outcome markers similar to STEMI(+) OMI patients when both groups are compared against patients with NOMI.
2. Material and methods
2.1. Study design and setting
This retrospective case-control study was the primary analysis of the Diagnosis of Occlusion MI And Reperfusion by Interpretation of the electrocardioGram in Acute Thrombotic Occlusion (DOMI ARIGATO) database (clinical trials.gov number NCT03863327), which is a 2-site collaboration designed to investigate OMI. Stony Brook University Hospital (SBUH) is a suburban, academic hospital serving as regional cardiac catheterization referral center. Hennepin County Medical Center (HCMC) ED is urban and academic. Both have more than 100,000 ED visits per year. IRB approval was obtained at both. There was no extramural funding.
2.2. Selection of participants
We retrospectively collected patients who presented to the ED with symptoms suggestive of possible ACS. Due to the rarity of OMI among all ED patients with potential ACS, we had insufficient resources to perform a prospective, consecutive cohort study, and instead we performed a retrospective cohort study to maximize both the number of OMI patients and the number of Non-AMI patients with abnormal ECGs. First, each site searched the cardiac catheterization laboratory activation database (all urgent and emergent left heart catheterizations over a 1 year period), which provided both cases (OMI) and controls (without OMI). At SBUH, we added a previously collected prospective population of ED patients who were admitted to the cardiology service with suspected ACS during a six month time period (again contributing both cases and controls). To ensure that the final cohort also contains a substantial number of control patients with abnormal ECGs, we added additional controls from HCMC identified by searching the UTROPIA database for patients without OMI but with STE, STD, or T-wave inversion, approximately 1/3 of whom were diagnosis with NOMI [28]. Patients were excluded if there were no ECGs in the electronic medical record or if there was insufficient retrospective information available to determine the primary outcome (the presence or absence of our OMI definition).
2.3. Measurements
Chart review was performed by four EM physicians after training with a standardized data coding manual. Primary and senior authors (HPM and SWS) were available for on-demand questions, feedback, and re-training. Demographics, clinical and laboratory results, serial ECGs, and angiographic findings were collected using the web-based Research and Electronic Data Capture (REDCap) site hosted by SBUH [29]. We collected all available transfer, prehospital, and study site ECGs.
ECG interpretation was performed, blinded to all patient information except age and sex, by SWS and HPM using a standardized data form including various ECG findings, objective measurements, predefined OMI ECG findings, and subjective interpretations. One interpreter (HPM) was just out of residency; the other has been an Emergency Medicine attending for 30 years. HPM had been trained by SWS in identification of OMI on the ECG. SWS interpreted all ECGs in the database, while HPM interpreted all ECGs from HCMC only (because HPM was a data collector and adjudicator for SBUH cases, thus unable to be blinded). All ECG interpretations were made blinded to all clinical data other than age and sex, which are required to determine the presence of STEMI criteria. Serial ECGs were interpreted sequentially, with the interpreter unable to change prior interpretations, and blinded to the baseline ECG until after interpreting the first ECG of the series, then re-interpreted that first ECG with access to the baseline ECG. STEMI criteria were defined according to the fourth universal definition of MI, and thus measured in millimeters using the QRS onset (PQ junction) and the J-point [30]. If any ECG prior to angiogram met STEMI criteria, the patient was considered to be STEMI(+); if not, then STEMI(−). Interobserver variation to the nearest 0.5 mm has been previously established [31], [32], [33], [34]. We assessed interobserver reliability between HPM and SWS for all cases interpreted by both. Furthermore, all 108 consecutive OMI cases from the prospective cohort were reviewed for STEMI criteria by a cardiologist blinded to the outcome and the study goals. In addition to obvious STE (suspected true positive STEMI criteria), our proposed OMI ECG findings included the following eight findings which indicate high likelihood of OMI: subtle STE not meeting criteria, hyperacute T waves (including de Winter pattern), reciprocal ST depression and/or negative hyperacute T waves, STD maximal in V1-V4 indicative of posterior OMI, suspected acute pathologic Q waves (meaning Q waves associated with subtle STE which cannot be attributed to old MI), terminal QRS distortion (absence of S-wave preceding any subtle STE, where an S-wave would be expected) [35], any STE in inferior leads with any STD or T wave inversion in lead aVL, and positive modified Sgarbossa criteria (MSC) for a patient with left bundle branch block (LBBB) [34], [40] or ventricular paced rhythm (VPR) [55]. Like the STEMI criteria [3], [36], [37], these OMI ECG findings are inherently subjective and interpreter-dependent.
2.4. Outcomes
OMI vs. NOMI was adjudicated by structured chart review. Diagnosis of any AMI in absence of angiography was based on final diagnosis in the patient’s record. The retrospective diagnosis of OMI cannot be based solely on the culprit TIMI flow because, due to dynamic thrombus, the state of the artery may differ between the ECG time and the angiogram time. Proven STEMI has TIMI-3 flow in 16–19% of cases [38], [39]. For these reasons, the retrospective definition of OMI was reproduced from prior studies [34], [40], [41], comprised of either (1) “confirmed OMI” on cardiac catheterization (defined as an acute culprit lesion with TIMI 0–2 flow), or (2) “presumed OMI with significant cardiac outcome”, defined as any of the following: (a) acute but non-occlusive culprit lesion with large infarct size as demonstrated by highly elevated cTn (contemporary cTnT ≥ 1.0 ng/mL [Roche Diagnostics Elecsys, reference range ≤ 0.01 ng/mL] or contemporary cTnI ≥ 10.0 ng/mL [Abbott Architect 4th generation, reference range ≤ 0.030 ng/mL]).; (b) if no angiography, then highly elevated cTn and a new or presumed new regional echocardiographic wall motion abnormality; or (c) STEMI(+) ECG with death before angiogram. Formal adjudication was made using all available data, including ECGs, cTns, and angiograms. If TIMI flow was not reported, the cineangiogram was reviewed by an experienced cardiologist. The definition of “highly elevated” cTn was chosen previously as the most accurate cutoff for differentiating STEMI from NSTEMIs using various cTn assays [25], [42], [43], [44], [45], [46], and has subsequently been internally and externally validated [34], [40], [47]. We performed separate exploratory analyses using variations of this OMI definition including altering the TIMI score cutoff to 0–1 and lowering the cTn cutoff to 50%, 30%, 20%, and 10% of the primary definition.
2.5. Analysis
We calculated summary statistics for cases and controls, and diagnostic utility for each interpreter. Interobserver agreement was calculated using κ values for categorical variables. Subject characteristics and outcomes were compared between groups using Mann-Whitney U or Kruskal-Wallis tests for continuous measurements and Pearson’s chi-squared or Fisher’s exact test for categorical measures. All tests were two-sided, and statistical significance was accepted at the 0.05 level, with Bonferroni corrections applied when applicable. Descriptive statistics were performed in REDCap, while other statistical tests and graphs were performed with Microsoft Excel (Version 1905; Redmond, WA, USA). The primary analysis was the comparison of diagnostic accuracy characteristics (sensitivity, specificity, and accuracy) between OMI ECG findings and STEMI criteria for the diagnosis of OMI using 2-tailed McNemar’s test.
3. Results
3.1. Characteristics of study subjects
The case and control searches at HCMC yielded 72 OMI cases and 181 controls. The case search at SBUH yielded 94 OMI cases, while the prospective consecutive ACS cohort yielded another 467 patients (108 OMIs and 359 controls). The combined total was 814 patients, of which 6 were excluded due to insufficient data to categorize as either OMI or No Occlusion, leaving 808 patients (265 OMI and 543 controls) for final analysis.
Table 2 (online appendix) shows the clinical characteristics and outcomes. There were 808 patients with a combined total of 3421 ECGs. There were 396 AMI (49%), 265 (33%) of them OMI; 108 of 265 OMI (41%) met STEMI criteria. There were 108 true positive STEMIs (STEMI[+] OMI) and 288 NSTEMIs; there were 265 OMIs and 131 NOMIs. There were an additional 34 patients without OMI who had false positive STEMI criteria on ECG. The cardiac catheterization lab was emergently activated in 218 (27%) cases, whether true OMI or not, and angiography was performed during the index visit in 635 (79%) cases. Twenty-four (3%) patients died during the index visit, 5 (0.7%) were discharged to hospice, and 6 more died within 3 months of discharge.
4. Main results
4.1. Accuracy of STEMI criteria vs. OMI ECG findings
The sensitivity, specificity, and overall accuracy of STEMI criteria as measured by Interpreter 1 for detection of OMI in all 808 patients were 41%, 94%, and 77%. The same statistics in the HCMC-only cohort (250 patients, evaluated by both interpreters) were 30%, 92%, and 74% by Interpreter 1 and 36%, 91%, and 76% by Interpreter 2. When Interpreter 1 selected positive STEMI criteria and also interpreted the ECG as OMI, the specificity was 97.2%.
The sensitivity of Interpreter 1 for OMI was significantly greater when using OMI ECG findings than the STEMI criteria (86% vs. 41% p < 0.0001), while the specificity was slightly less (91% vs. 94%, p = 0.008), with overall greater accuracy (89% vs. 77%, p < 0.0001). Table 3 shows the sensitivity, specificity, and accuracy across various alterations of the OMI definition. As the definition threshold of OMI was lowered, the accuracy of Interpreter 1 using OMI ECG findings did not change, but that of STEMI criteria lowered significantly, such that the accuracy difference widened further (88% vs. 72%, p < 0.0001 as the cTn threshold was lowered to 10%).
Table 3.
Comparison of proposed OMI criteria vs. STEMI criteria accuracy for the diagnosis of OMI. Presence of STEMI criteria above does not imply that the interpreter believes the ECG to represent true positive STEMI criteria, or STEMI(+) OMI, only that the interpreter identified STE that meets the definition of STEMI criteria. The definitions of OMI are listed from left to right in order of decreasing thresholds, with the primary outcome definition bolded. For example, “0–1 cTn-10” included a culprit lesion with TIMI 0–1 flow, or any culprit lesion with a peak cTn of > 10.0 ng/mL for cTnI and 1.0 ng/mL for cTnT. “0–2 Trop-2” would mean a culprit lesion with TIMI 0–2 flow, peak cTn threshold 1.0 ng/mL for cTnI and 0.10 ng/mL for cTnT.
|
Expert 1 vs. STEMI Criteria. Diagnostic Accuracy for all 808 Patients | |||||||
|---|---|---|---|---|---|---|---|
| OMI Definition (TIMI range and cTn threshold) | 0–1 cTn-10 | 0–2 cTn-10 (Primary Outcome) | 0–2 cTn-5 | 0–2 cTn-3 | 0–2 cTn-2 | 0–2 cTn-1 | |
| Sensitivity | Expert 1 | 90% | 86% | 84% | 81% | 81% | 79% |
| Criteria | 44% | 41% | 39% | 38% | 37% | 36% | |
| Specificity | Expert 1 | 88% | 91% | 92% | 92% | 93% | 93% |
| Criteria | 94% | 94% | 95% | 95% | 95% | 95% | |
| Accuracy | Expert 1 | 89% | 89% | 89% | 88% | 89% | 88% |
| Criteria | 79% | 77% | 75% | 74% | 73% | 72% | |
| Expert 2 vs. STEMI Criteria Diagnostic Accuracy for 250 HCMC Patients | |||||||
| OMI Definition (TIMI range cTn threshold) | 0–1 cTn-10 | 0–2 cTn-10 (Primary Outcome) | 0–2 cTn-5 | 0–2 cTn-3 | 0–2 cTn-2 | 0–2 cTn-1 | |
| Sensitivity | Expert 2 | 86% | 80% | 80% | 79% | 79% | 78% |
| Criteria | 42% | 36% | 35% | 34% | 34% | 32% | |
| Specificity | Expert 2 | 89% | 92% | 94% | 95% | 95% | 96% |
| Criteria | 91% | 91% | 91% | 91% | 91% | 91% | |
| Accuracy | Expert 2 | 88% | 89% | 90% | 90% | 90% | 90% |
| Criteria | 80% | 76% | 75% | 74% | 74% | 72% | |
Interpreter 2 interpreted all 250 HCMC patients and showed significantly higher sensitivity for OMI using the proposed OMI ECG findings compared to STEMI criteria (80% vs. 36%, p < 0.0001), statistically similar specificity (92% vs. 91%, p = 0.85), and higher overall accuracy (89% vs. 76%, p < 0.0001, see Table 3).
4.2. Interrater reliability
Interpreters 1 and 2 interpreted the same group of 250 HCMC patients, resulting in 97.2% agreement for the determination of STEMI criteria ( = 0.893), and 94.0% agreement for the diagnosis of OMI ( = 0.849). Interpreter 2 and a third blinded reviewer (a cardiologist blinded to all study objectives and hypothesis) both reviewed 108 consecutive OMI patients, resulting in 87% agreement for the presence of STEMI criteria ( = 0.735, 95% CI 0.607–0.863). Importantly, the third reviewer classified 59 of the OMI patients as STEMI(+), while Interpreter 2 classified 67 as STEMI(+), suggesting that the study interpreters did not undercall the number of STEMI (+) OMI and, in fact, may have overcalled that number.
4.3. Time between OMI ECG findings and STEMI criteria diagnosis of OMI
Each ECG was time stamped such that we could evaluate the relationship between the time elapsed since presentation and the presence or progression of ECG findings over serial ECGs both before and after angiogram. For each OMI patient we calculated the time difference between the time of OMI diagnosis by OMI ECG findings and the time of OMI diagnosis by STEMI criteria. For either set of ECG criteria, if the patient never met criteria before cardiac catheterization, then the time of OMI diagnosis was considered the time of cardiac catheterization (because the angiogram revealed OMI that was missed by the surrogate criteria). For an example of this methodology, see the supplementary appendix A.
Of the 265 OMI patients in the database, 146 (55%) were diagnosed earlier by Interpreter 1 using OMI ECG findings than by STEMI paradigm (by STEMI paradigm, we mean by either STEMI criteria or by angiogram, whichever diagnosed OMI first). 120 of 146 never met STEMI criteria on any serial ECG recorded before the angiogram, and the remaining 26 were initially diagnosed only by OMI ECG findings which later, before the angiogram, evolved to meet STEMI criteria on serial ECGs. Twenty of these 146 patients were then excluded due to >24 h earlier diagnosis by OMI ECG findings compared to STEMI criteria (these OMIs were missed by STEMI criteria, identified by OMI ECG findings, and had extremely delayed catheterization greater than 24–48 h). The final analysis included 126 OMI patients who were diagnosed by Interpreter 1 an average of 3.00 hours earlier (95% CI 2.69–3.31 hours), and a median of 1.3 hours earlier (IQR 0.58–2.76) by OMI ECG findings compared to either STEMI criteria or later angiogram. Conversely, 119 (45%) of all 265 OMI patients were not diagnosed earlier by OMI ECG findings: 82 patients had both STEMI criteria and OMI ECG findings simultaneously on the first available ECG, and 37 OMIs were missed by both STEMI criteria and OMI ECG findings.
4.4. ECG findings in OMI cases detected earlier by OMI ECG findings
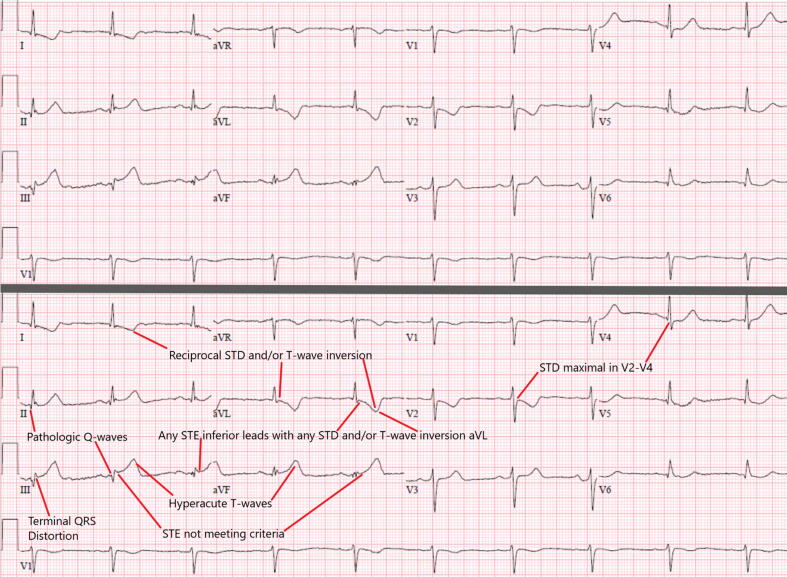
Interpreter 1 identified 146 OMI cases (55%) earlier using OMI ECG findings compared to STEMI criteria, with the two most common OMI ECG findings being “subtle STE not meeting STEMI criteria” (83%) and “reciprocal ST depression and/or reciprocal T-wave inversion” (82%). LBBB and VPR with positive MSC were very rare, and the prevalence of the other seven OMI ECG findings are listed in Table 6 (online appendix). Only 6 cases (4%) had none of these 7 findings, while 134 (92%) had 2 or more. Fig. 2 provides an example of a STEMI criteria (−) but OMI Criteria(+) OMI which displayed all 7 findings.
Fig. 2.
This patient was found to have OMI of the mid-RCA (pre-intervention TIMI 1 flow, 99% stenosis with thrombus), correctly diagnosed on the first ECG by the OMI criteria but missed by STEMI criteria despite 5 ECGs prior to angiogram, with a delay of 21.4 h (cath performed next day due to “NSTEMI”). Although it is not subtle, the ECG does not meet STEMI criteria because only one lead (III) has 1 mm STE, without 1 mm in adjacent leads (II and aVF). The documentation states: “…substernal chest pain and pressure which radiated to the jaw area and found to have ruled in for a NSTEMI via positive cTns. Referred for cardiac catheterization.” This presentation ECG shows all 7 of the above findings (top panel without annotation, bottom panel with). This patient had a very high peak cTnT of 3.74 ng/mL, a new inferoposterior wall motion abnormality, and a newly depressed EF of 40%, but survived to discharge.
4.5. Analysis of OMI ECG findings “False Positive” cases
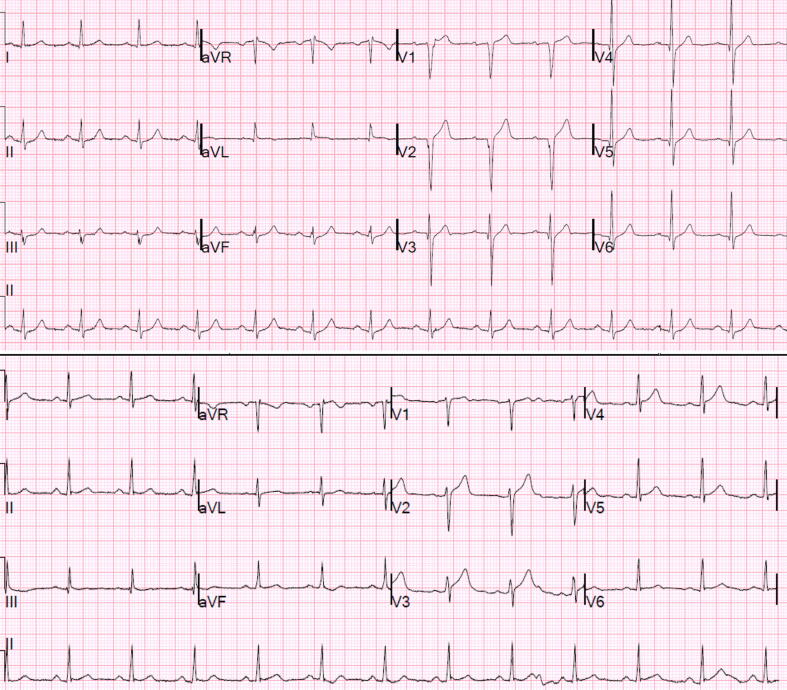
There were 51 cases with positive OMI ECG findings which did not meet the primary outcome definition of OMI. However, 20 of these “false positives” had acute culprit lesions (meaning they had an acute culprit lesion with TIMI 3 flow but peak troponin lower than our cutoff), one patient required urgent CABG, and four suffered cardiac arrest. Fig. 3 illustrates two examples of meaningful cases which were counted as “false positives.”
Fig. 3.
Top panel: This 37 year-old male was found to have an acute thrombotic 90% lesion in the proximal LAD with TIMI 3 flow at the time of cath. cTnI rose from undetectable to 5.80 ng/mL within three hours with no further serial cTns measured. Bottom Panel: This 46 year-old male was found to have an acute thrombotic 90% lesion in the mid-LAD with TIMI 3 flow at the time of cath; cTnI peaked at 4.44 ng/mL. Both interpreters diagnosed both patients as OMI of the LAD using only the ECG and age (no other history provided). Both cases were counted as “false positives” because the TIMI flow of the lesions and the peak recorded cTns were insufficient according to our primary outcome definition. Neither case had any ECG (out of 7 total) meeting STEMI criteria (bottom panel measurements at J-point, relative to QRS onset per 4th Universal Definition of MI: V1 0 mm, V2 1.2 mm, V3 1.6 mm, V4 1.1 mm, V5 0.8 mm V6 0.7 mm). In both cases, serial ECGs evolved in confirmation of abnormal subtle STE.
4.6. Comparison of STEMI(+) OMI, STEMI(−) OMI, and NOMI groups
For the 727 patients who had sufficient cTn data available, Table 7 shows the cTn, angiographic, and clinical outcome characteristics. Mean and median peak cTnT at SBUH were not different for STEMI(−) OMI vs. STEMI(+) OMI at SBUH, but were significantly higher than for NOMI. At HCMC, STEMI(+) OMI did have significantly higher peak cTnI than STEMI(−) OMI, and both were significantly higher than NOMI. A new or presumed new wall motion abnormality was found in 42% of NOMI, 89.0% of STEMI(+) OMI, and 83.2% of STEMI(−) OMI (p < 0.0001 for both STEMI[+] and STEMI[−] OMI compared to NOMI, p = 0.234 between STEMI[+] and STEMI[−] OMI).
Table 7.
Clinical outcome measures among the 727 of 808 who underwent angiography, by group: STEMI(+) OMI, STEMI(−) OMI, NOMI, and MIRO groups. SBUH, Stony Brook University Hospital. HCMC, Hennepin County Medical Center.
| STEMI(+) OMI (STEMI on any ECG) | STEMI(−) OMI | NOMI (Non-Occlusion MI) | MIRO (No Occlusion and MI Ruled Out) | |
|---|---|---|---|---|
| N, 727 | 92 | 118 | 205 | 312 |
| Time to cath Avg (SD) [mins] | 265 (1227) | 1181 (3022) | 3235 (3303) | 2328 (2526) |
| Time to cath Median (IQR) [mins] | 55 (30–106) | 175 (57–1028) | 2139 (1191–4359) | 1471 (629–3095) |
| Cath < 90 mins from presentation | 65 (71%) | 45 (38%) | 11 (6%) | 13 (7%) |
| Average (SD) peak cTnT ng/mL (SBUH) | 6.06 (7.77) | 5.29 (11.29) | 0.30 (0.53) | 0.00 (0.00) |
| Median (IQR) peak cTnT ng/mL (SBUH) | 3.87 (2.25–7.84) | 2.94 (1.28–4.78) | 0.12 (0.03–0.33) | 0.00 (0.00–0.00) |
| Average (SD) peak cTnI ng/mL (HCMC) | 141.94 (168.25) | 32.79 (29.60) | 1.09 (2.30) | 0.01 (0.01) |
| Median (IQR) peak cTnI ng/mL (HCMC) | 79.02 (26.31–177.33) | 21.34 (8.34–59.31) | 0.09 (0.05–0.70 | 0.01 (0.00–0.02) |
| % WMA | 81, 89.0% | 94, 83.2% | 66, 41.8% | 25, 21.2% |
| Pre-cath cardiac arrest | 10, 10.8% | 12, 10.2% | 9, 4.4% | 0, 0% |
| Death during index visit | 3, 3.3% | 9, 7.6% | 5, 2.4% | 0, 0% |
| Arrest/Death/Hospice/Death 3mo | 17, 18.5% | 25, 21.2% | 18 (8.7%) | 2 (0.6%) |
Median times from presentation to angiogram for STEMI(+) OMI, STEMI(−) OMI, and NOMI were 55 (30–106), 175 (57–1028), and 2139 min (1191–4359) (p < 0.0001 between each pair of the three groups). The STEMI(+) OMI group was significantly more likely to receive emergent (<90 min) angiogram than the STEMI(−) OMI group (71 vs. 38%, p < 0.00001).
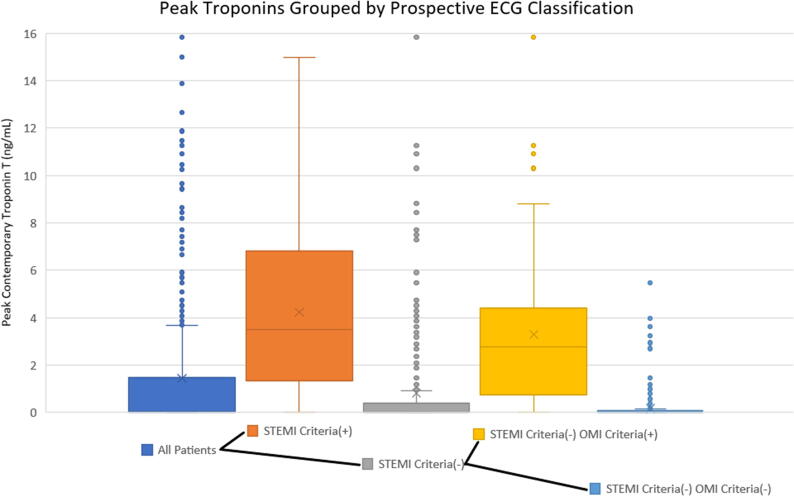
Clinical outcomes for each group, including pre-cath cardiac arrest and death during index visit, are listed in Table 7. From left to right, Fig. 4 shows the peak cTn levels first among all patients, then divided into the current paradigm’s STEMI and NSTEMI categories, and finally the NSTEMI group is further subdivided into NSTEMIs with and without the OMI ECG findings.
Fig. 4.
Graph showing peak cTnT by MI category for SBUH patients. From left to right, the initial group of all patients is divided into STEMI(+) and STEMI(−) based on ECG criteria, as per the current paradigm. Next, the STEMI(−) group is further divided based on the presence of OMI criteria, showing the result of the OMI paradigm. The STEMI(−) OMI criteria(+) category shows the subset of patients with large infarcts due to OMI which are missed by the STEMI paradigm but diagnosed by the proposed OMI criteria.
4.7. Limitations
Each of our proposed ECG findings are inherently subjective and require dedicated training and experience to accurately identify them, which represents an important limitation to external validity. However, the current STEMI criteria are also highly subjective, with notoriously poor interrater reliability, [3], [36], [37], [56] and were never required to meet any external validity standards before becoming the universal approach to ECG ischemia interpretation. Until we have a more reliable machine learning solution, ECG interpretation remains subjective and requires years of deliberate practice. The high interrater reliability between our two interpreters (94% absolute agreement) shows that these skills can be taught and learned. Although this exact set of ECG criteria have not been externally validated, Aslanger et al. also showed that cardiologists were able to successfully reclassify a significant portion of NSTEMI patients as acute coronary occlusion using expert interpretation.
We did not have the resources to study OMI in consecutive ED patients who present with undifferentiated symptoms of ACS because the incidence of STEMI (1–3%) and OMI (2–5%) is very low in such a cohort [48], [49], [50], [51]. By studying a cohort with more OMI than would be represented in such a consecutive series, the population is much higher risk than the general Emergency Department chest pain population, and this inherently biases the interpretation of the reviewers and limits the external validity.
Additionally, ECG adjudication by study interpreters as STEMI(−) vs. STEMI(+) OMI may have been biased in borderline cases in favor of STEMI(−) OMI. To evaluate for this limitation we had a cardiologist blinded to the study goals and hypothesis review all 108 cases of OMI from the consecutive prospective cohort. The cardiologist classified even fewer cases as STEMI(+) than both of our study interpreters (59 vs. 67, or 55% vs. 62%), suggesting that our interpreters may have been biased towards calling more STEMI.
Because we did not have access to long-term follow up data, we used peak cTn as a surrogate marker of infarct size, as it correlates with mortality, incidence of adverse events, and decreased quality of life in survivors [25], [26], [42], [52], [53], [54]. Table 5 (online appendix) shows the correlation between peak cTn and TIMI flow of culprit lesion.
Finally, comparison of outcomes of STEMI(+) OMI vs. STEMI(−) OMI is confounded by the shorter door to balloon time in the STEMI(+) OMI. It is possible that had STEMI(−) OMI been intervened upon as quickly as STEMI(+) OMI, that markers of infarct size would have been more different. Only a randomized trial of immediate vs. delayed angiography for ECG-diagnosed STEMI(−) OMI on the ECG, or a randomized trial of immediate vs. delayed intervention for STEMI(−) OMI proven by immediate angiogram, could definitively answer this question. Such trials may be justified, but are unlikely to be performed.
5. Discussion
This study represents the largest existing database containing both detailed angiographic outcome data as well as detailed ECG interpretation for patients with acute coronary occlusion. The poor sensitivity of STEMI criteria for OMI in this study (41% by primary analysis) is supported by a recent, large, prospective study in which STEMI criteria had 30% sensitivity for OMI using all available serial ECGs; blinded cardiologists reading the same ECGs had 49% sensitivity for OMI [49]. In contrast, our blinded interpreters were able to more than double the sensitivity of the STEMI criteria for OMI, with similar specificity. Our ability to reclassify NSTEMIs as STEMI(−) OMI externally confirms the results of Aslanger et al. who also recently demonstrated the potential of the OMI paradigm [7]. Our interpreters exhibited very high interrater reliability among many ECGs, which is likely attributable to the fact that Interpreter 2 has been trained extensively by Interpreter 1. As in all operator-dependent skills in medicine, this confirms that the skills used in ECG interpretation can be taught and reproduced with appropriate training.
Fifty-five percent (146) of OMIs were correctly diagnosed a median of 1.5 h earlier by our OMI ECG findings than by either STEMI ECG criteria or by angiogram if it never met STEMI criteria. Although studies showing the benefit of earlier intervention involved STEMI(+) patients, until proven otherwise, any OMI must be assumed to benefit from earlier reperfusion regardless of the ECG findings manifested.
At first glance, our study appears to suggest that the proposed OMI ECG findings were not able to significantly improve upon the already high specificity of the STEMI criteria. Upon further review, however, the “false positive” group identified by OMI ECG findings actually had a high rate of acute coronary lesions, elevated troponins, and need for interventions, suggesting that these patients may also be important to identify earlier than the general ACS population.
Our data overall support the growing notion that the NSTEMI (STEMI[−]) population is actually comprised of two importantly different subgroups: STEMI(−) OMI patients (who have acute large vessel coronary occlusion and similar outcome severity to STEMI[+] OMI patients) and NOMI (Non-Occlusion MI) patients (identified by subsequent troponin elevation but not by ECG OMI findings) who usually need an intervention but not emergently. Despite the fact that STEMI(−) OMI patients have similar angiographic findings, highly elevated cTns, and a high risk of pre-cath cardiac arrest or index visit mortality similar to the STEMI(+) OMI group, the STEMI(−) OMI patients were much less likely to receive emergent cardiac catheterization than the STEMI(+) OMI group (38% vs 71%).
6. Conclusions
We found that OMI ECG findings were superior to STEMI criteria for the blinded ECG diagnosis of Occlusion MI in the hands of highly trained electrocardiographers. STEMI(−) OMI patients had significant delays to cardiac catheterization but similarly severe clinical, angiographic, and laboratory features as the STEMI(+) OMI group when compared to the No Occlusion group. STEMI(−) OMI patients are an under-identified population with the potential to benefit from emergent intervention, and our results suggest that they may be rapidly and noninvasively identified using OMI ECG findings. Our findings support further investigation into the OMI vs. NOMI paradigm shift, including methods to disseminate ECG expertise and achieve external validity, as well as interventional trials to evaluate the potential benefit of earlier reperfusion therapy for STEMI(−) OMI.
Funding
There was no funding for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100767.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group, Lancet 343 (1994) 311–322. [PubMed]
- 2.Khan A.R. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur. Heart J. 2017;38:3082–3089. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 3.McCabe J.M. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch. Intern. Med. 2012;172:864–871. doi: 10.1001/archinternmed.2012.945. [DOI] [PubMed] [Google Scholar]
- 4.Larson D.M. ‘False-positive’ cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 5.Kontos M.C. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST-segment elevation myocardial infarction. Ann. Emerg. Med. 2010;55:423–430. doi: 10.1016/j.annemergmed.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Miranda D.F., Lobo A.S., Walsh B., Sandoval Y., Smith S.W. New insights into the use of the 12-lead electrocardiogram for diagnosing acute myocardial infarction in the emergency department. Can. J. Cardiol. 2018;34:132–145. doi: 10.1016/j.cjca.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Aslanger E.K. DIagnostic accuracy oF electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction (DIFOCCULT Study) IJC Heart Vascul. 2020;30 doi: 10.1016/j.ijcha.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowland-Fisher A., Smith S., Laudenbach A., Reardon R. Diagnosis of acute coronary occlusion in patients with non-STEMI by point-of-care echocardiography with speckle tracking. Am. J. Emerg. Med. 2016;34(1914):e3–e6. doi: 10.1016/j.ajem.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Eek C. Strain echocardiography predicts acute coronary occlusion in patients with non-ST-segment elevation acute coronary syndrome. Eur. J. Echocardiogr. 2010;11:501–508. doi: 10.1093/ejechocard/jeq008. [DOI] [PubMed] [Google Scholar]
- 10.Linde J.J. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J. Am. Coll. Cardiol. 2020;75:453–463. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Mehta S.R., Granger C.B., Boden W.E. Early versus delayed invasive intervention in acute coronary syndromes. N. Engl. J. Med. 2009;360:2165–2175. doi: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 12.S.R. Mehta, Personal communication regarding methods for TIMACS trial: were patients with refractory ischemia excluded? (2014).
- 13.Montalescot G., Cayla G., Collet J.-P. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA. 2009;302:947–954. doi: 10.1001/jama.2009.1267. [DOI] [PubMed] [Google Scholar]
- 14.Milosevic A., Vasiljevic-Pokrajcic Z., Milasinovic D. Immediate versus delayed invasive intervention for non-STEMI patients: the RIDDLE-NSTEMI study. JACC Cardiovasc Interv. 2016;9:541–549. doi: 10.1016/j.jcin.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Neumann F.-J., Kastrati A., Pogatsa-Murray G. Evaluation of prolonged antithrombotic pretreatment (‘‘cooling-off’’ strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA. 2003;290:1593–1599. doi: 10.1001/jama.290.12.1593. [DOI] [PubMed] [Google Scholar]
- 16.Reuter P.-G., Rouchy C., Cattan S. Early invasive strategy in high-risk acute coronary syndrome without ST-segment elevation. The Sisca randomized trial. Int J Cardiol. 2015;182:414–418. doi: 10.1016/j.ijcard.2014.12.089. [DOI] [PubMed] [Google Scholar]
- 17.Hoedemaker N.P.G., Damman P., Woudstra P. Early Invasive versus selective strategy for non-ST-segment elevation acute coronary syndrome: the ICTUS trial. J Am Coll Cardiol. 2017;69:1883–1893. doi: 10.1016/j.jacc.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 18.van’t Hof A.W.J., de Vries S.T., Dambrink J.-H.E. A comparison of two invasive strategies in patients with non-ST elevation acute coronary syndromes: results of the Early or Late Intervention in unStable Angina (ELISA) pilot study. 2b/3a upstream therapy and acute coronary syndromes. Eur. Heart J. 2003;24:1401–1405. doi: 10.1016/s0195-668x(03)00259-8. [DOI] [PubMed] [Google Scholar]
- 19.Thiele H., Rach J., Klein N. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction: the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial) Eur Heart J. 2012;33:2035–2043. doi: 10.1093/eurheartj/ehr418. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam E.A. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez B. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 22.H.P. Meyers, S.D. Weingart, S.W. Smith, The OMI Manifesto. Dr. Smith’s ECG Blog http://hqmeded-ecg.blogspot.com/2018/04/the-omi-manifesto.html (2018).
- 23.Meyers H.P., Smith S.W. Prospective, real-world evidence showing the gap between ST elevation myocardial infarction (STEMI) and occlusion MI (OMI) Int. J. Cardiol. 2019;293:48–49. doi: 10.1016/j.ijcard.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Licka M. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524. doi: 10.1136/heart.87.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannitsis E. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J. Am. Coll. Cardiol. 2008;51:307–314. doi: 10.1016/j.jacc.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Steen H. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J. Am. Coll. Cardiol. 2006;48:2192–2194. doi: 10.1016/j.jacc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Remppis A. Cardiac troponin T levels at 96 hours reflect myocardial infarct size: a pathoanatomical study. Cardiology. 2000;93:249–253. doi: 10.1159/000007034. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval Y. Single high-sensitivity cardiac troponin I to rule out acute myocardial infarction. Am. J. Med. 2017;130:1076–1083.e1. doi: 10.1016/j.amjmed.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Harris P.A. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thygesen K. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 31.Smith S.W. Electrocardiographic differentiation of early repolarization from subtle anterior ST-segment elevation myocardial infarction. Ann. Emerg. Med. 2012;60:45–56. doi: 10.1016/j.annemergmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Smith S.W. T/QRS ratio best distinguishes ventricular aneurysm from anterior myocardial infarction. Am. J. Emerg. Med. 2005;23:279–287. doi: 10.1016/j.ajem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Smith S.W. ST elevation in anterior acute myocardial infarction differs with different methods of measurement. Acad. Emerg. Med. 2006;13:406–412. doi: 10.1197/j.aem.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Meyers H.P. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: a retrospective case-control study. Am. Heart J. 2015;170:1255–1264. doi: 10.1016/j.ahj.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Lee D.H., Walsh B., Smith S.W. Terminal QRS distortion is present in anterior myocardial infarction but absent in early repolarization. Am. J. Emerg. Med. 2016;34:2182–2185. doi: 10.1016/j.ajem.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 36.Carley S.D., Gamon R., Driscoll P.A., Brown G., Wallman P. What’s the point of ST elevation? Emerg. Med. J. 2002;19:126–128. doi: 10.1136/emj.19.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandberg D., Kastendieck K.D., Meskin S. Observer variation in measured ST-segment elevation. Ann. Emerg. Med. 1999;34:448–452. doi: 10.1016/s0196-0644(99)80045-6. [DOI] [PubMed] [Google Scholar]
- 38.Cox D.A. Comparative early and late outcomes after primary percutaneous coronary intervention in ST-segment elevation and non-ST-segment elevation acute myocardial infarction (from the CADILLAC trial) Am. J. Cardiol. 2006;98:331–337. doi: 10.1016/j.amjcard.2006.01.102. [DOI] [PubMed] [Google Scholar]
- 39.Stone G.W. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104:636–641. doi: 10.1161/hc3101.093701. [DOI] [PubMed] [Google Scholar]
- 40.Smith S.W., Dodd K.W., Henry T.D., Dvorak D.M., Pearce L.A. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann. Emerg. Med. 2012;60:766–776. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 41.Aslanger E. A simplified formula discriminating subtle anterior wall myocardial infarction from normal variant ST-segment elevation. Am. J. Cardiol. 2018;122:1303–1309. doi: 10.1016/j.amjcard.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 42.Antman E.M. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N. Engl. J. Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 43.Hallén J. Relation of cardiac troponin I measurements at 24 and 48 hours to magnetic resonance-determined infarct size in patients with ST-elevation myocardial infarction. Am. J. Cardiol. 2009;104:1472–1477. doi: 10.1016/j.amjcard.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Chia S. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2008;1:415–423. doi: 10.1016/j.jcin.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Dumas F. Can early cardiac troponin I measurement help to predict recent coronary occlusion in out-of-hospital cardiac arrest survivors? Crit. Care Med. 2012;40:1777–1784. doi: 10.1097/CCM.0b013e3182474d5e. [DOI] [PubMed] [Google Scholar]
- 46.D’Souza M. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am. J. Med. 2015;128:852–860. doi: 10.1016/j.amjmed.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 47.Baro R., Haseeb S., Ordoñez S., Costabel J.P. High-sensitivity cardiac troponin T as a predictor of acute total occlusion in patients with non-ST-segment elevation acute coronary syndrome. Clin. Cardiol. 2018 doi: 10.1002/clc.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wereski R. High-Sensitivity cardiac troponin concentrations at presentation in patients with ST-segment elevation myocardial infarction. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillinger P. Prospective validation of current quantitative electrocardiographic criteria for ST-elevation myocardial infarction. Int. J. Cardiol. 2019 doi: 10.1016/j.ijcard.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 50.Sandoval Y. Diagnosis of type 1 and type 2 myocardial infarction using a high-sensitivity cardiac troponin I assay with sex-specific 99th percentiles based on the third universal definition of myocardial infarction classification system. Clin. Chem. 2015;61:657–663. doi: 10.1373/clinchem.2014.236638. [DOI] [PubMed] [Google Scholar]
- 51.Miller C.D. Is the initial diagnostic impression of ‘noncardiac chest pain’ adequate to exclude cardiac disease? Ann. Emerg. Med. 2004;44:565–574. doi: 10.1016/j.annemergmed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 52.Bøhmer E., Hoffmann P., Abdelnoor M., Seljeflot I., Halvorsen S. Troponin T concentration 3 days after acute ST-elevation myocardial infarction predicts infarct size and cardiac function at 3 months. Cardiology. 2009;113:207–212. doi: 10.1159/000201991. [DOI] [PubMed] [Google Scholar]
- 53.van Domburg R.T., Cobbaert C., Kimman G.J., Zerback R., Simoons M.L. Long-term prognostic value of serial troponin T bedside tests in patients with acute coronary syndromes. Am. J. Cardiol. 2000;86:623–627. doi: 10.1016/s0002-9149(00)01040-7. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs S. Prognostic value of cardiac troponin I re-elevation following percutaneous coronary intervention in high-risk patients with acute coronary syndromes. Am. J. Cardiol. 2001;88:129–133. doi: 10.1016/s0002-9149(01)01606-x. [DOI] [PubMed] [Google Scholar]
- 55.Tran V., Huang H.D., Diez J.G. Differentiating ST-elevation myocardial infarction from nonischemic ST-elevation in patients with chest pain. Am J Cardiol. 2011;108(8) doi: 10.1016/j.amjcard.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Dodd KW, Zvosec DL, Hart M, Glass G, Bannister LE, Body R. Boggust BA, Brady WJ, Chang AM, Cullen LC, Gomez-Vicente R, Huis in’t Veld M, Karim RM, Meyers HP, Miranda DF, Mitchell GJ, Rice C, Salverda BJ, Stellpflug SJ, Tolia VM, Walsh BM, White JL, Smith SW. Electrocardiographic Diagnosis of Acute Coronary Occlusion Myocardial Infarction in Ventricular Paced Rhythm Using the Modified Sgarbossa Criteri. In Press. Annals of Emergency Medicine. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.