Summary
Chronic stress has adverse consequences on many organ systems and physiological processes. However, existing protocols show large variability in response and are not suitable for female mice. Here, we provide a step-by-step protocol for establishing a reliable chronic stress model in mice that can be used in a variety of physiological settings. This protocol has been tested to be effective to produce a consistent response to stress in several mouse strains (C57BL/6J, 129X1/SvJ, B6.V-Lepob/J) and both sexes.
For complete details on the use and execution of this protocol, please refer to Ip et al. (2019).
Subject areas: Metabolism, Model Organisms, Neuroscience
Graphical abstract

Highlights
-
•
Protocol to establish a chronic stress mouse model for studying energy homeostasis
-
•
Detailed description to pair the stress paradigm with various post-analysis methods
-
•
Tips for avoiding pre-selection bias for long-term physiological study
Chronic stress has adverse consequences on many organ systems and physiological processes. However, existing protocols show large variability in response and are not suitable for female mice. Here, we provide a step-by-step protocol for establishing a reliable chronic stress model in mice that can be used in a variety of physiological settings. This protocol has been tested to be effective to produce a consistent response to stress in several mouse strains (C57BL/6J, 129X1/SvJ, B6.V-Lepob/J) and both sexes.
Before you begin
Experimental considerations
-
1.
Ensure all the research and animal care procedures are approved by your institute-based animal welfare and ethics Committee for the use in animal research.
-
2.
The development of a stress-induced metabolic phenotype may vary in each individual animal, in order to minimize such variability, it is critical to control the age of the animal as well as to perform pre-treatment monitoring of basal body composition, corticosterone level, glucose tolerance, and energy metabolic state (energy expenditure, food intake, respiratory exchange ratio) and then utilize such information to help to group them accordingly to make sure that no basal difference on any of these parameters pre-exists.
-
3.
For medium- to long-term studies it is advised to use the same batch of diets throughout the study to minimize batch effects.
-
4.
With our experience we have seen that there are physiological differences such as corticosterone level, body weight, and food intake depending on the time of the day, therefore we suggest maintaining the standard monitoring and experimental time throughout your experiment. For example, we monitor our mice and perform body composition scans at 14:00 h.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-c-Fos polyclonal | Santa Cruz Biotechnology | Cat# SC-52-G; RRID: AB_2629503 |
| Alexa 594 secondary anti-rabbit | Molecular Probes | Cat# A-11012; RRID: AB_ AB_2534079 |
| Chemicals, peptides, and recombinant proteins | ||
| Fluoroshield with DAPI | Sigma-Aldrich | Cat# F6057 |
| 50 mL Glucose 50% (w/v%) | Phebra | Cat# inj 128 |
| Phosphate buffered saline | Sigma-Aldrich | Cat# P3813 |
| Paraformaldehyde | Sigma-Aldrich | Cat# 441244 |
| Critical commercial assays | ||
| Sensitive Rat Insulin RIA | Merck Millipore | Cat# SRI-13K |
| Corticosterone DA 125I | MP Biomedicals | Cat# 07120102 |
| Experimental models: organisms/strains | ||
| C57BL/6J (both genders with age between 8 to 10 weeks) | The Jackson Laboratory | Cat# 000664 |
| 129X1/SvJ (both genders with age between 8 to 10 weeks) | The Jackson Laboratory | Cat# 000691 |
| B6.V-Lepob/J (both genders with age between 8 to 10 weeks) | The Jackson Laboratory | Cat# 000632 |
| Software and algorithms | ||
| Expedata-P Data Analysis Software | Sable Systems International | N/A |
| Meta screen Analysis Software | Sable Systems International | N/A |
| Other | ||
| Promethion metabolic cage system | Sable Systems International | N/A |
| Accu-Chek Go glucometer | Roche | N/A |
| Lunar PIXImus DEXA | Piximus | N/A |
| 60 mm TC-treated Cell Culture Dish | Falcon | Cat# 353001 |
Step-by-step method details
Establishing a chronic stress mouse model
Acclimatisation and pre-monitoring period (2 weeks)
-
1.
Recruit a group of animals with minimal age gap, e.g., for our studies we generally use mice with an age of 8 weeks, however, mice at younger and older ages respond similar to this protocol.
-
2.
Pair-house two mice in each cage (32 cm × 15 cm × 23 cm dimension), and acclimatize them for at least one week before the experiment starts. Maintain the animal under conditions of controlled temperature (22°C–24°C for standard laboratory temperature) and duration of light-dark cycle (12 h light cycle, lights on at 07:00 h) throughout all the studies.
CRITICAL: Pair-housing is important to maintain the wellbeing of the mouse as solitary housing can trigger other forms of social distress that can lead to changes in behaviour as well as in thermogenesis. It is possible to increase the number of mice per cage, however, it is important to make sure that all treatment groups are the same, as that may alter baseline food intake and energy metabolism (Nagy et al., 2002; Bastias-Pérez et al., 2020). In addition, one needs to consider that with increasing numbers of mice the formation of social hierarchy will occur that can have influences on the basal stress levels of the individual mice. If larger numbers of mice per cage are used it is advisable to choose animals with similar bodyweight to minimize dominance and fighting, especially with male mice. We advise that when deciding on the number of mice to be placed into one cage the user should take into the consideration on pre-testing the animal’s social dominancy, especially when compared the phenotype between male and female (van Den Berg et al., 2015; Williamson et al., 2019; Karamihalev et al., 2020)
-
3.At the pre-monitoring week (Figure 1), measure body composition, glucose tolerance, and basal metabolic profiles following the subsequent steps.Note: Sophisticated monitoring systems might not be accessible for all laboratories. It is possible to limit the parameters to determine the randomised pre-grouping to body weight, glucose tolerance, and food intake.Monitoring of the establishment of social hierarchies during this phase is also advisable.
-
a.Body composition monitoring on Monday
 Timing: [2 h / 8 mice]
Timing: [2 h / 8 mice]
-
i.Perform body composition scan on Monday using either the dual-energy X-ray absorptiometry (DXA; Lunar PIXImus2) or MRI (EchoMRI) at a designated time e.g., 14:00 – 16:00 h.
-
ii.Obtain baseline data on lean mass (g), whole body fat mass (g), bone mineral content and bone mineral density (g/cm2).Note: We found that stress exposure of less than 4 weeks seldom lead to a change in bone metabolism, therefore fat mass and lean mass are the most critical data for the pre-grouping stage. However, longer stress exposure may also affect the bone phenotype, hence it is advice to obtain this data if the duration of the study is longer than 6 weeks (Baldock et al., 2014).
-
i.
-
b.Glucose tolerance test (GTT) on Tuesday
 Timing: [2 h / 8 mice]
Timing: [2 h / 8 mice]
-
i.Fast all mice at 9:00 h by removing foods from the hopper as well as change to new bedding to avoid the mice from feeding on spillages.
-
ii.At 14:00 h, use fresh injectable glucose at 50% strength (0.5 mg / 1 μL; Phebra), and then dilute it into 10% strength with saline ( 0.1 mg / 1 μL). Administer the 10% glucose aliquot at 10 μL per gram of body weight via intraperitoneal injection. For example, animal with a body weight of 25 g will be injected with 250 μL of the 10% glucose aliquot.
-
iii.Then measure tail-blood glucose level at 0, 15, 30, 60 and 90 min time interval by using the Accu-chek Go glucometer (Roche, Dee Why, Australia).
-
iv.In between each interval, we suggest collecting additional 80 μL of blood at room temperature (RT) in order to examine the efficiency of insulin secretion in response to glucose challenge and the baseline by using the commercial assays for insulin (Merck Millipore)
 CRITICAL: To avoid proteins in the serum from degrading it is important to spin down blood during each time interval immediately at 2000 ×g RT for 5 min. 50 μL of serum can be extracted from about 80 μL of blood. Collecting higher volumes of blood is not recommended as this may exhaust the animal which will affect its metabolism and body weight.
CRITICAL: To avoid proteins in the serum from degrading it is important to spin down blood during each time interval immediately at 2000 ×g RT for 5 min. 50 μL of serum can be extracted from about 80 μL of blood. Collecting higher volumes of blood is not recommended as this may exhaust the animal which will affect its metabolism and body weight. -
v.Snap freeze serum samples by placing them into liquid nitrogen and store them at −80°C until use.
-
i.
-
c.Metabolic profiling on Thursday
 Timing: [2 h / 8 mice]
Timing: [2 h / 8 mice]
-
i.Acclimatize individual mice in each metabolic chamber (Sable systems international) for 24 h and then start the recording over 3 nights e.g., starting at 17:00 h on Friday until 10:00 h on Monday.
-
ii.To record the most reliable physiological and behavioral results only use data (energy expenditure, food intake, respiratory exchange ratio and physical activity) recorded from 09:00 h Saturday to 9:00 h Monday to allow the mice to be familiarized themselves with the new environment and all parameters have reach a stable baseline.
-
i.
-
a.
Figure 1.
Schematic summary of the chronic stress paradigm
A full 7 weeks protocol to establish a reliable chronic stress mouse model. ACC, acclimatization; Wk-BW, weekly body weight monitoring; DXA, dual-energy X-ray absorptiometry scan; GTT, glucose tolerance test; P. analysis, post-analysis; FI, food intake monitoring.
Caution: After the single-house monitoring of their metabolic profiles it is possible that when they are being placed back to their home cage they may start to fight with each other. We suggest to provide them an extra dome to give additional space to each individual mouse as well as the user should ensure the animal holding room has no other large groups of female mouse being held in the room.
Chronic stress inducing period (4 weeks)
-
4.
Once the mice are pre-grouped, start any feeding interventions under the stress condition depending on your interest of the diets, e.g., Chow diet, high sugar diet or high fat diet. We perform the following stress paradigm 3 times each week on Monday, Wednesday, and Friday at 14:00 h – 15:00 h.
CRITICAL: To induce stress, use an empty cage with the same specifications as the home cage. Make sure that the cage of choice contains no side platform or edges the mice can rest on to avoid exposure to the stressor. It is also important to use a container or cage with a height above 18 cm to avoid that the mice can escape or getting injured from jumping onto the edges.
CRITICAL: It is important to close the lid immediately to avoid the mice from escaping, as stress exposure may induce acute hyper-activity.
-
5.
Pre-fill empty cage with 1-cm depth ice-supplemented water (200 g of ice in 100 mL of water). Does not need to be top up with ice throughout the stress exposure (Figure 2).
-
6.
Pre-record body weight before the stress paradigm and then place each animal from the same cage gently into a separate stressor cage and monitor their activity for 1 h for the first exposure to stress to observe any extreme discomfort to the mice. Full hour monitoring will not be necessary at subsequent occasions if the mice did not show any heightened response at the first round.
CRITICAL: During the first stress exposure it is important to closely monitor the animal every 5 min, as the first time may trigger extreme hyperventilation and hyperactivity in some animals. If such situation happens, remove the animal immediately from the water and restart on the next round of stress. The tolerance of the mice in response to the cold water will gradually improve. Generally, 90% of animals react without any acute adverse effects to this method.
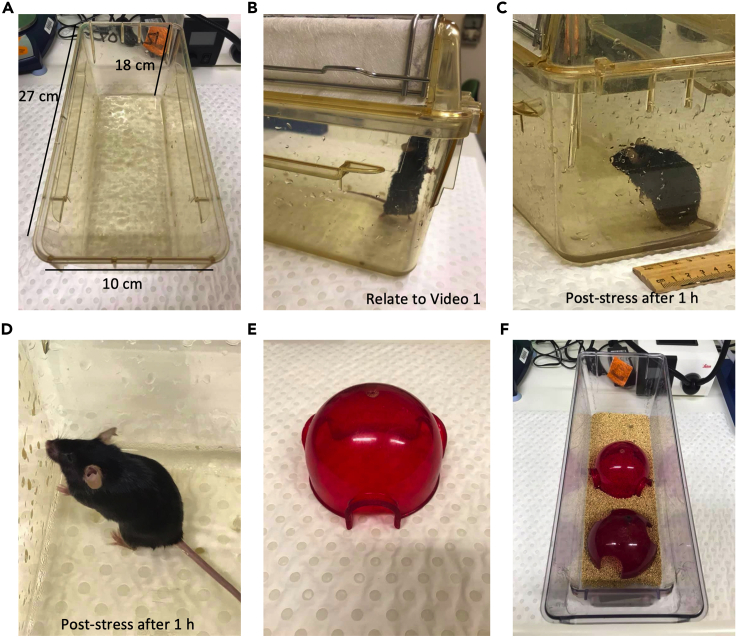
CRITICAL: Place each mouse gently to avoid the cold water from splashing onto their fur, as this may cause significant decrease in their body temperature. We prefer to only place one mouse into the stressor cage under dimension 27 cm × 10 cm × 18 cm to avoid the cage being overly crowded (Figure 2 and Methods video S1). Also observe that at the end of the stress paradigm the fur of the mouse is dry (Figure 2 and Methods video S2).
Figure 2.
Stress exposure set up example
(A) Pre-fill cage (27 cm × 10 cm × 18 cm) with ice supplemented water.
(B) Mouse avoiding ice water by standing up.
(C) Mouse behavior at 1 h after stress.
(D) Dry fur at 1 h after stress.
(E) Mouse dome used for home cage.
(F) 2 domes used for 2 mice inside the same cage.
Note: We confirmed that the stress phenotype induced by this protocol is not due to the coldness of the water, as monitoring of the body temperature throughout the stress protocol compared to control conditions employing an infrared thermo-camera showed no significant difference between the groups (Ip et al., 2019)
-
7.
While the stress paradigm is under way, change new bedding in all cages as well as pre-weigh food in each cage to allow the monitoring of food intake after stress exposure. Weigh food again before the next stress including the spillages on the bedding.
-
8.
Perform the same stress method 3 times a week with 1 day rest apart between each stress exposure for four weeks. At the end of the second week, perform progress monitoring for body composition and metabolic profiling again. (steps described in pre-monitoring and acclimatisation period).
-
9.
At the end of the fourth weeks of stress exposure, perform again body composition, metabolic as well as GTT monitoring (Figure 1).
-
10.
At the end of the experiment, there are two options to either examine activity changes in the brain or process brain and organs from the periphery for protein and/or transcriptome analysis.
Option 1: Post-stress analysis of neural activity (3 days)
-
11.
To examine activity changes in the brain such increases in cFos after the chronic stress exposure, perfuse the animal by transcardial perfusion with freshly prepared 4% paraformaldehyde (PFA), extract and incubate the brain overnight with 4% PFA at 4°C followed by another day of incubation with 30% glucose at 4°C until the brain sinks to the bottom in the liquid. Remove all the glucose before freezing at −80°C. (To examine for transcriptional changes please refer to option 2 below. )
Note: if perfusion is done in a separate experimental room, acclimatize the animal in the same room for at least 3 h to minimise non-specific activation of neuronal activity. Alternatively, the animal can be anesthetised in the same room and carry to the experimental room.
Pause point: perfused brain can be kept at −80°C for up to 1 year. Longer than 1 year may lead to potential tissue damage due to dehydration.
-
12.
Section brain coronally at 30 μm and then perform immunofluorescence analysis by using a rabbit raised anti-cFos antibody (1:1200; Santa Cruz Biotechnology). Sections can also be stored in cryoprotectant at −20°C for later use.
Pause point: Brain sections can be kept in cryoprotectant at −20°C for up to 6 months. We haven’t tested the time course beyond this point on section quality.
-
13.
To perform the immunofluorescence step against neuronal markers such as cFos, the following “free-floating” protocol has been optimized by our laboratory.
-
14.
On the day of the experiment, pre-wash the stored sections 3 times at room temperature (RT) for 5 min in triton X-100 supplemented phosphate-buffered saline (PBST; 0.2% v/v) with gentle orbital shaking. This PBST buffer concentration will be used throughout the washing steps in this protocol.
-
15.
Next, prepare the citrate acid-based antigen retrieval reagent (mix 1.125 mL of 0.1 M citric acid, 5.125 mL 0.1 M tri-sodium citrate and 62.5 mL water together). Antigen retrieval step is performed in a 2 mL Eppendorf tube. 2 mL of the citrate buffer will be used for each brain containing approximately 20 sections of the 30 μm cut-brain slides.
-
16.
Heat the brain sections in a 60°C water bath for 5 min.
Note: It is also better to pre-heat your citrate buffer before adding it to the brain sections to ensure the antigen retrieval step is performed at the most optimal condition.
-
17.
After the antigen retrieval step, wash the brain sections 3 times at RT with PBST buffer by removing the supernatant in the 2 mL Eppendorf tube with gentle orbital shaking.
-
18.
Then block sections in 2 mL of PBST supplemented with normal goat serum (5% v/v %) for 2 h at RT with gentle orbital shaking.
-
19.
Remove blocking buffer and then add 2 mL of the cFos antibody containing PBST buffer supplemented with bovine serum albumin (1:1000; Stock concentration at 1 mg/mL) into the section and incubate at 4°C overnight with gentle orbital shaking.
-
20.
Wash the brain sections 3 times at RT with 2 mL of PBST and then add Alexa 594 secondaries anti-rabbit antibody (Molecular probes) into the sections and incubate at RT for 1 h. Wash the brain sections again 3 times at RT with 2 mL of PBST. Finally, mount the sections with fluoroshield with DAPI (Sigma) onto the slides and visualize under fluorescence microscope (Leica).
Option 2: Fresh tissue collection to examine body fat depots and organs (3 h for 8 mice)
In option 1 the procedure presents a limitation due to PFA fixation, which prevents the use of various peripheral tissues for other purposes. In order to examine precisely the mass of various organs, or to perform protein and transcriptome analysis on these organs as well as in the brain one can collect tissues freshly by following the subsequent steps.
-
21.
After stress exposure, cull mice at 14:00 h by cervical dislocation followed by decapitation and immediate serum collection using the method as described. Serum collected after cull can be used for the corticosterone assay to confirm the stress phenotype.
-
22.
Collect the brain immediately and rinse them quickly on phosphate-buffered saline (PBS). It is also possible to dissect out different parts of the brain in chilled dissection buffer (1× HBSS, 2.5 mM HEPES-KOH [pH 7.4], 35 mM Glucose, and 4 mM NaHCO3), in a 6 cm petric dish on ice, to allow for the study of gene expression changes in specific nucleus (Figure 4).
-
23.
To dissect out various peripheral tissues freshly we collect and weight them at the following sequence: brown fat, inguinal fat, muscles, perigonadal fat, reproductive organs, perirenal fat, kidney, spleen, pancreas, mesenteric fat, liver, and the heart.
-
24.
To preserve tissues for transcriptome and protein analysis, rinse each organ with phosphate-buffered saline (PBS) to remove blood at RT and snap freeze them progressively by placing them on dry ice.
Note: Due to the weighing time it is impractical to collect all the tissues without potential degradation, hence we recommend two options: 1. To collect all the tissues without weighing 2. To focus only on a couple of tissues such as the brain and the fat depots. (The choice of interest varies depending on your project.)
-
25.
After collection the frozen tissues can be kept at −80°C for at least 2 years, however, we don’t have data to support the maximal time the tissues can be stored without degradation. For the exact RT-qPCR protocol for post-analysis, please refer to Ip et al., 2019.
Figure 4.
Arcuate nucleus dissection for transcriptome analysis
(A) Isolate the whole brain and place it into a 6 cm petri dish containing dissection buffer.
(B) Position the brain upside down and cut coronally at the hypothalamic region indicated by the two dotted lines.
(C) Cut a triangle at the Arc region.
(D) Place Arc in a 1.5 Eppendorf tube and snap freeze it immediately by place the tube into liquid nitrogen for transcriptome analysis.
Expected outcomes
The result generated by this stress paradigm has been proven to be highly effective when compared to other forms of stressors in inducing physiological and metabolic changes in both the brain and in the periphery (Kuo et al., 2007; Ip et al., 2019). This protocol is highly versatile which can be combined with other diets or drug interventions, as well as to study a particular gene function in response to stress by using genetic knockout models, and this protocol when combined with other interventions can provide valuable data for understanding a wider dimension of impact of chronic stress to different organ system.
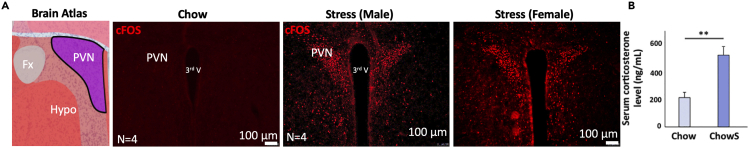
To confirm for the successfulness of the mice in perceiving stress we examined the cFos activity in the paraventricular nucleus of the hypothalamus (PVN), a critical area in triggering stress responses through activation of the hypothalamic pituitary axis (Smith and Vale, 2006). As expected after 4 weeks, the number of cFos positive cells was significantly higher in the stressed mice (in both male and female mice) when compared to the control mice in the PVN (Figure 3). Consistent with this result the level of serum corticosterone was also significantly higher in the stress cohort, confirming that this is an effective method to induce stress in mice.
Figure 3.
Chronic stress induced response in the PVN and in the blood
(A) cFos (Red) visualization in the PVN of the 4 weeks stress exposed mice compared to the non-stress control.
(B) Serum corticosterone measurement at the mid-stage monitoring (Result adapted from Ip et al., 2019).
∗p < 0.05; ∗∗p < 0.01. Hypo, hypothalamus; Fx, columns of the fornix.
Limitations
In order to gain an in-depth understanding of the influence of chronic stress on metabolism and physiology this protocol carries a high number of active monitoring parameters at different stages (pre-,mid-,end-stage) which can potentially limit the use of any other additional parameters. For example, we perform GTT to monitor glucose metabolism only at the pre- and end-treatment stage as we found that additional GTT monitoring in the mid-stage along with other parameters may exhaust the animal significantly, leading to an extreme exhaustion phenotype which may introduce unreliable results such as reduced food intake and body weight. Therefore we suggest not to overwhelm mice with additional parameters. Moreover, the chronic stress may also trigger other behavioral responses in mice and additional cohort may be required to run behavioral test such as open-field test, marble burying test and sucrose preference test at the pre-treatment and end-stage.
During the exposure to repeated stress this may enhance the possibility of fighting between the mice in the cage. However, we do not recommend to overcome this problem by single house them as it will introduce another form of social distress due to solitary isolation. For this some other suggestions are provided under Troubleshooting.
Troubleshooting
Problem 1
Mice increased fighting after stress exposure.
Potential solution
While the protocol can establish a stress induced phenotype effectively it is also possible that you may observe increased fighting between the two mice from the same cage, depending on the strains of the mice used - C57BL/6 was used in our protocol. To avoid territory invasion between the two mice it is possible to increase the dimension of your cage used, or place additional shielding device (a red colored dome) to allow the mouse to hide from the dominant one (Figure 2).
Also, it is advised that the animal holding room in which the user host the animal should not be co-housed with female mice as the presence of female mouse scent can trigger aggressiveness.
Problem 2
The level of corticosterone was not induced in some of the mice.
Potential solution
It is very important to control the time of blood collection and the environment where the serum is collected for corticosterone measurement. If you observed a large variation of serum corticosterone level in the blood you can modify the protocol by removing the food hoppers from 9:00 h and then collect at 14:00 h in the same experimental room. Also, do not single house them for blood collection.
It is also important to maintain the quality of the serum by extracting them from blood not long after the collection, preferably within 15 min after collection, and then snap freeze them in liquid nitrogen.
Problem 3
Mice had significant drop of body weight and food intake after the first week of stress exposure.
Potential solution
From our data we know that our stress protocol does not affect overall body weight significantly under chow diet due to the reduced food intake and energy expenditure phenotype (Ip et al., 2019). Therefore, a significant reduction in body weight after the start of the stress protocol is not likely due to this stressor alone. Depending on the mouse strain used some may be more vulnerable to stress, and when combined with many active monitoring procedures this may further enhance the stress phenotype. Therefore, if you observe a large number of mice had a significant drop on body weight -more than 10 % each week and food intake you may want to consider to reduce the number of monitoring used e.g., perform GTT test only on a separate cohort of animal.
Problem 4
Can you incorporate behavioral monitoring together with metabolic monitoring for the study?
Potential solution
Phenotyping the behaviors may be complicated due to the diverse nature of animal activities, we recommend running a separate cohort to characterize for any behavioral changes in mice after the stress protocol, without any GTT monitoring.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, k.ip@garvan.org.au.
Materials availability
This study did not generate new materials.
Data and code availability
This study did not generate new code or data.
Acknowledgments
This research was supported by the National Health and Medical Research Council (NHMRC) with project grant 1066809.
Author contributions
C.K.I. optimized the method, designed and conducted the experiments, and wrote the article.
Declaration of interests
The author declares no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100448.
References
- Baldock P.A., Lin S., Zhang L., Karl T., Shi Y., Driessler F., Zengin A., Hormer B., Lee N.J., Wong I.P. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J. Bone Miner. Res. 2014;29:2238–2249. doi: 10.1002/jbmr.2205. [DOI] [PubMed] [Google Scholar]
- Bastias-Perez M., Zagmutt S., Soler-Vazquez M.C., Serra D., Mera P., Herrero L. Impact of adaptive thermogenesis in mice on the treatment of obesity. Cells. 2020;9:316. doi: 10.3390/cells9020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C.K., Zhang L., Farzi A., Qi Y., Clarke I., Reed F., Shi Y.C., Enriquez R., Dayas C., Graham B., Begg D., Bruning J.C., Lee N.J., Hernandez-Sanchez D., Gopalasingam G., Koller J., Tasan R., Sperk G., Herzog H. Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab. 2019;30:111–128 e6. doi: 10.1016/j.cmet.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Karamihalev S., Brivio E., Flachskamm C., Stoffel R., Schmidt M.V., Chen A. Social dominance mediates behavioral adaptation to chronic stress in a sex-specific manner. Elife. 2020;9:e58723. doi: 10.7554/eLife.58723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L.E., Kitlinska J.B., Tilan J.U., Li L., Baker S.B., Johnson M.D., Lee E.W., Burnett M.S., Fricke S.T., Kvetnansky R., Herzog H., Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Nagy T.R., Krzywanski D., Li J., Meleth S., Desmond R. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes. Res. 2002;10:412–415. doi: 10.1038/oby.2002.57. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Berg W.E., Lamballais S., Kushner S.A. Sex-specific mechanism of social hierarchy in mice. Neuropsychopharmacology. 2015;40:1364–1372. doi: 10.1038/npp.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson C.M., Lee W., Decasien A.R., Lanham A., Romeo R.D., Curley J.P. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Sci. Rep. 2019;9:7324. doi: 10.1038/s41598-019-43747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate new code or data.