Summary
Conditioned place avoidance assays are broadly used in mammals to study different cognitive aspects of operant learning. Here, we introduce a series of experimental designs for training juvenile zebrafish in short-term and long-term conditioned place avoidance assays. Our goal is to promote standardization of animal handling procedures and setup conditions to improve animal welfare and reproducibility while studying operant learning behaviors in juvenile zebrafish.
For complete details on the use and execution of this protocol, please refer to Palumbo et al. (2020).
Subject areas: Model Organisms, Neuroscience, Behavior
Graphical abstract
Highlights
-
•
We describe juvenile zebrafish handling procedures for operant learning behavior
-
•
We detail experimental designs for short- and long-term learning
-
•
We introduce potential pitfalls for chemogenetic ablation of neurons
-
•
We highlight the importance of animal welfare for successful learning performance
Conditioned place avoidance assays are broadly used in mammals to study different cognitive aspects of operant learning. Here, we introduce a series of experimental designs for training juvenile zebrafish in short-term and long-term conditioned place avoidance assays. Our goal is to promote standardization of animal handling procedures and setup conditions to improve animal welfare and reproducibility while studying operant learning behaviors in juvenile zebrafish.
Before you begin
Before describing all the steps of our behavioral experiments in detail we want to emphasize the importance of proper and reproducible zebrafish husbandry to ensure reproducibility of most behavioral assays, including our conditioned place avoidance learning assay (Palumbo et al., 2020) described in the following section.
Fish maintenance and husbandry
Note: The animal facilities and maintenance of the zebrafish, Danio rerio, were approved by the Norwegian Food Safety Authority.
-
1.Keep fish in 3.5-liter tanks in a Techniplast Zebtech Multilinking system at constant conditions:
-
a.Temperature=28°C
-
b.pH=7
-
c.Water conductivity=600 μSiemens
-
d.Dark/light cycle=14:10 h light/dark cycle
-
e.Maximum animals per tank= 35; 10 animals per liter of water at adult stage
-
f.Diet: dry food two times a day (SDS 100–400, dependent of age) and, after the 5th day of development, also Artemia nauplii once a day (ZM Brine Shrimp Cysts Premium 250 Grade, ZM Systems). We notice an increase in animal health and growth since we started feeding them Artemia nauplii as soon as 5 days post fertilization.
-
a.
Note: Variations from the above-mentioned parameters in fish husbandry will affect animal welfare and stress level, and it might affect the outcome of the experiment.
Note: We recommend standardizing the breeding procedures to minimize experimental variability. We have optimized the following procedure.
-
2.
Place animals for breeding before the evening feeding in subgroups of 5 to 10 fish.
-
3.
On the following day, collect the eggs in a petri dish containing egg water, a solution of 0.1% methylene blue in artificial fish water (AFW, 0.2 g/L instant ocean salt in reverse osmosis water (RO)), and incubate at 28.5°C.
-
4.
Clean the Petri dish and exchange the egg water on a daily basis.
-
5.
At the 5th day post fertilization screen the animals for positive expression of the transgenic fluorescent protein of interest.
-
6.
Transfer the larvae zebrafish to the zebrafish facility into 0.7 L nursery nets installed in 3,5 L tanks (see key resources table) until 3–4 weeks post fertilization. Pay attention to keep the number of animal larvae around 100 per tank.
-
7.
Keep the water flow outside of the nursery net for the first 7–10 days (from day 5 until day 15 post fertilization). This will limit mechanical stress on the young animals, while ensuring a high rate of water turnover surrounding the nursery nets.
Note: Putting the animal into nursery nets will provide them with a high exchange of fresh water while keeping them in a confined space and protected by the mechanical stress of the water flow.
Fish handling
Timing: 20 min
Stress and anxiety can severely impact animal behavior to a point of disrupting its learning capability. Therefore, caution in handling the animals is essential both before and during behavioral testing. We therefore optimized a series of good practices to ensure we minimize animal stress caused by experimenter handling. We recommend observing animal behavior during and after any procedure is performed.
-
8.
Collect the zebrafish, once the desired age is reached, from the zebrafish facility in the same morning of the planned experiments, after the morning feeding. We observed that the morning feeding is particularly important for better welfare and successful learning performance. This will prevent the animal from being food deprived over a long period of time since the last feeding time would have been the previous evening.
-
9.
Use a 7 mL plastic pipette and cut the first 5–8 mm of the pipette’s tip to increase its cross-section. This reduces the mechanical stress on the animals while transferring them. Pay attention to always pipette the animal from its front or its back and never from the side. The animal’s length is greater than the pipette cross-section therefore, if pipetted from the side, animal body can bend unnaturally.
-
10.
Place the animals immediately in a 50 mL falcon tube containing AFW at around 28C°. Cover the falcon tube (any opaque material is fine) during transportation and avoid any sudden movement of the falcon tube to reduce animals’ stress. We recommend avoiding taking the stairs if possible.
-
11.
Once in the experimental area, transfer the fish immediately to a petri dish in AFW, at ± 28°C, and place it in an incubator (± 28°C). We recommend to always store a bottle of AFW in the incubator to have available pre-warmed AFW and avoid any thermal shock for the animal.
Note: Further fish handling and monitoring during chemogenetic ablation and the behavior experiment are described later in the step-by-step methods details.
CRITICAL: It is crucial that the animals are never exposed to water colder than 25°C to ensure good health (see problem 1).
CRITICAL: Always wash the behavioral chamber before and after an experiment. Use RO water first and AFW later, to remove any olfactory trace.
CRITICAL: 3–4 weeks old zebrafish are transitioning from larval to adult stage. During this developmental stage fish size is a highly variable parameter. Hence, we recommend selecting animals of a similar size within the same age group (see problem 3). In our study the size of 4 weeks old zebrafish is in the range of 7±2 mm2 (Palumbo et al., 2020).
CRITICAL: As animals develop expression patterns of transgenic markers may change. Larval zebrafish usually display a broader unspecific expression of the Gal4:UAS construct, which becomes more specific once the animal reach the juvenile/adult stage. Therefore, it is a good practice to confirm the transgenic expression patterns for chemogenetic ablation once at larval stage and later at juvenile stage.
Note: Keep in mind that different genetic backgrounds/strains might lead to substantial differences in animal behavior. It is important to design a control experiment in which you test for the effect of the different transgenics strains. In our article we show that pigmentless nacre mutants expressing Gcamp6s (Vladimirov et al., 2014) show no differences in CPA learning performance, when compared to pigmented AB wildtype zebrafish (https://www.ezrc.kit.edu/, Cat# ZDB-GENO-960809-7).
Key resource table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Metronidazole (MTZ) | Sigma-Aldrich | Cat#M1547 |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | Cat#276855 |
| Phosphate-buffered saline (PBS) | Thermo Fisher Scientific | Cat#BR0014G |
| Formaldehyde | Sigma-Aldrich | Cat#F8775 |
| Bovine Serum Albumin Fraction V | AppliChem/Panreac | Cat#A1391.0100 |
| LMP Agarose | Fisher Scientific | Cat# 16520100 |
| MS222 (Tricaine methanesulfonate) | Sigma-Aldrich | Cat# E10521 |
| Gibco Trypsin 2.5% | Thermo Fisher Scientific - Gibco | 11538876 |
| DAPI | Thermo Fisher Scientific | Cat#P36931 |
| Goat serum | Sigma-Aldrich | Cat#G9023 |
| Triton X-100 | Merck | Cat#108643 |
| Glycerol | Sigma-Aldrich | Cat# 1.04092 |
| Critical commercial assays | ||
| In Situ Cell Death Detection Kit, Fluorescein | Sigma - Roche | 11684795910 |
| Experimental models: organisms/strains | ||
| Tg(elavl3 :GCaMP6s) | Vladimirov et al., 2014 | ZFIN Cat# ZDB-ALT-141023-1, RRID:ZFIN_ZDB-ALT-141023-1 |
| Tg(narp:GAL4VP16) | Agetsuma et al., 2010 | ZFIN ID: ZDB-ALT-110215-5 |
| Tg(UAS-E1b:NTR-mCherry) | Agetsuma et al., 2010 | ZFIN ID: ZDB-ALT-070316-1 |
| Wildtype zebrafish | EZRC (https://www.ezrc.kit.edu/) | ZFIN Cat# ZDB-GENO-960809-7 |
| Software and algorithms | ||
| Fiji/ImageJ | Schindelin et al., 2012 | https://imagej.net/Fiji.html#Downloads |
| MATLAB 2019b | https://se.mathworks.com/ | N/A |
| Open CV3.0 | https://opencv.org/opencv-3-0/ | N/A |
| QtCreator5.2 | https://www.qt.io/ | https://doc.qt.io/qt-5/gettingstarted.html#installing-qt |
| Deposited data | ||
| Custom scripts | https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish | N/A |
| Other | ||
| Confocal microscope (20× plan NA 0.8 objective) | Zeiss | Examiner Z1 |
| Confocal microscope (20× plan NA 1.0, Plan-Apochromat) | Zeiss | Examiner Z1 |
| Fish dry food | Sparos | ZEBRAFEED |
| Artemia nauplii – ZM Brine Shrimp Cysts Premium 250 Grade | ZM Systems | N/A |
| Instant Ocean Salt | https://www.webzoo.net/ | AS 1010006 |
| Methylene blue | Sigma-Aldrich | M9140-25G |
| Plastic pipets | VWR | 612-1681 |
| ZebTEC nursery nets (bottom pore size = 300 μm) | Scanbur | 80-ZB300BTI |
| Falcon tubes 50 mL | VWR | 525-0158 or 525-0610 |
| Petri dishes | VWR | 391-0440 |
| Zebrafish tank 3.5 L | Scanbur – Tecniplast ZebTech | 80ZB30TK |
| Gosselin™ Square Petri Dish, 120×120 mm, Corning® | VWR | 10799-777 |
| GigE Vision camera | Allied Vision | Manta G-235B |
| Excalibur low-noise high-speed precision operational amplifiers | Texas Instrument | TLE2142 |
| Arduino Due | https://www.arduino.cc/ | https://store.arduino.cc/arduino-due |
| Tungsten wire | Sigma-Aldrich | 267554-9.5G |
| Vortex | https://www.ika.com/ | 0003617000 |
| Shaker (PMR-30 Mini Rocker-Shaker) | VWR | 444-0341 |
Step-by-step method details
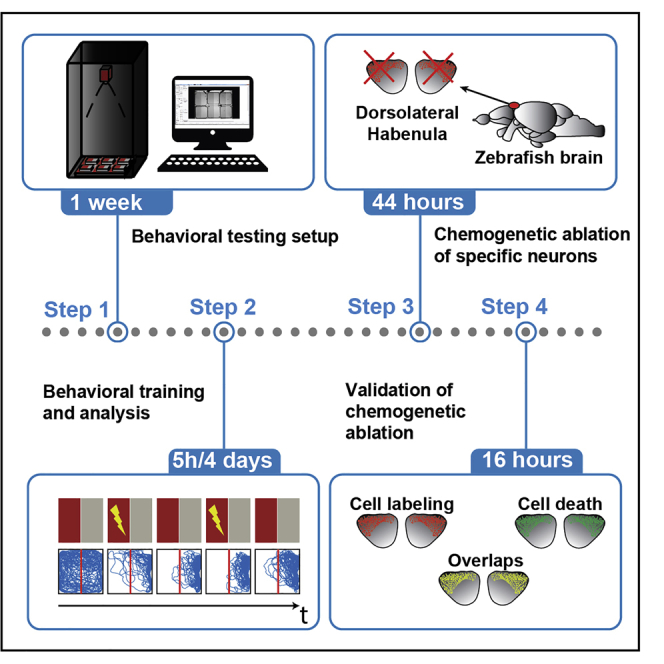
Behavioral testing setup
Timing: varies, up to 1 week
-
1.
Software. To implement the custom-made real-time tracking software (Kermen et al., 2020, Palumbo et al., 2020) we use the OpenCV 3.0 library and the QtCreator5.2 developing platform. With this STAR protocol, we publish the source code to implement the Object class “Tracker”, which would allow you to create instances of “trackers” and allocate each of them in a different thread, if you go for a multi-threaded approach. The only step you will have to implement is the image collection from a camera of your choice and send them to the object for processing. Once processed, the object “tracker” will return the detected position of the animals together with its size. For further details see documentations for source code, header and instruction on how to implement it in your code: https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish.
-
2.Implementation. We use a class of algorithm called “Adaptive Background Gaussian Mixture Model for foreground segmentation” (KaewTraKulPong and Bowden, 2002, Lech et al., 2014, Zivkovic, 2004, Power and Schoonees, 2002).
-
a.The principle behind this algorithm is the following: the intensity value of each pixel in the image is modeled by an adaptive parametric mixture model of N, typically three or five, Gaussian distributions (KaewTraKulPong and Bowden, 2002, Power and Schoonees, 2002). Once the image has been modeled, the posterior probability is estimated, to define the current state of the pixel: background or foreground (Power and Schoonees, 2002).
-
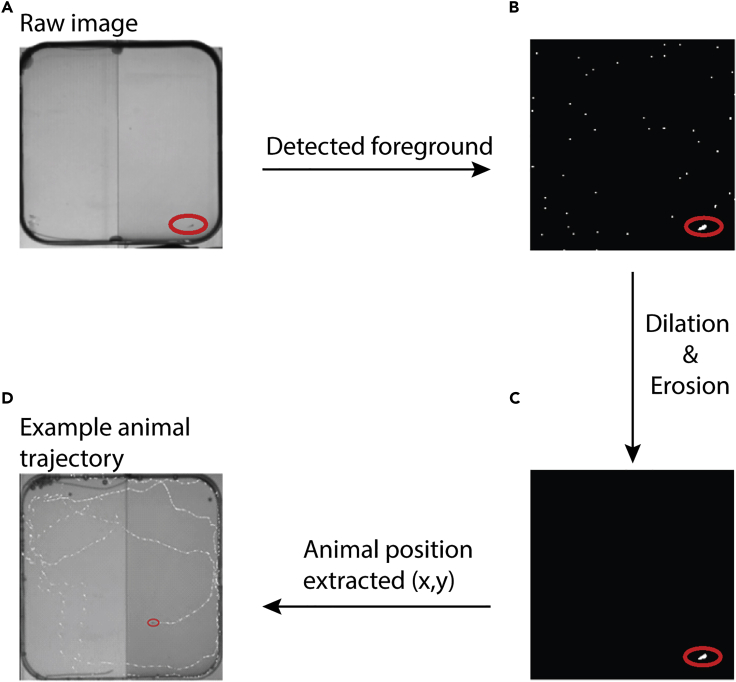
b.The implementation of this algorithm is described more in detail in Figure 1 and in attached documentation,https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish. Once the pixels corresponding to the juvenile zebrafish are identified their center of mass defines the animal’s coordinates in the arena (Figures 1A–1C). As a quality control of the detected fish, two criteria must be satisfied by the area detected:
-
i.The size of the detected zebrafish must be within a physiological range for the developmental stage of the analyzed fish (1–2 week=2–4 mm2, 3–4 weeks= 4–10 mm2).
-
ii.The distance between the detected animal position in two consecutive frames must be smaller than a threshold defined by the physiological maximum velocity for the zebrafish. We use 3 cm displacement between two frames, corresponding to 66 ms, to avoid noise detection from the distant borders of the arena.
-
i.
-
c.The result of our tracking algorithm has proven to be very stable and reliable even in conditions of weak contrast between the fish and the surrounding environment (Figures 1A–1C), compared to a background subtraction algorithm in our assay. In our CPA training assay, image acquisition frequency of 15 Hz was sufficient to accurately track the fish without computationally overload the software. An example two minutes’ pathway for one juvenile is shown in Figure 1D.
-
a.
Alternative: The approach we follow to implement our behavioral software is only one of the possible solutions. It is worth to mention the recent sotfware package, Stytra (https://github.com/portugueslab/stytra), published by Portugues lab (Štih et al., 2019). This software package provides a platform which will allow the experimenter to perform many different behavioral protocols, requiring little programming knowledge by the user thanks to a user-friendly interface and documentation.
Figure 1.
Image processing flow chart to detect juvenile zebrafish in low contrast images
(A) Pixels corresponding to the zebrafish are detected by the tracking algorithm.
(B) Dilations and erosion operations are performed to reduce the noise in the background pixels.
(C) Coordinates of the biggest area detected, which match the animal size range defined by the user, are extracted and processed by the software.
(D) Example of two min raw trace of fish position superimposed on the raw image detected by the camera. Dashed white lines shows 2 min of detected fish position, recorded at 15 frames per second.
-
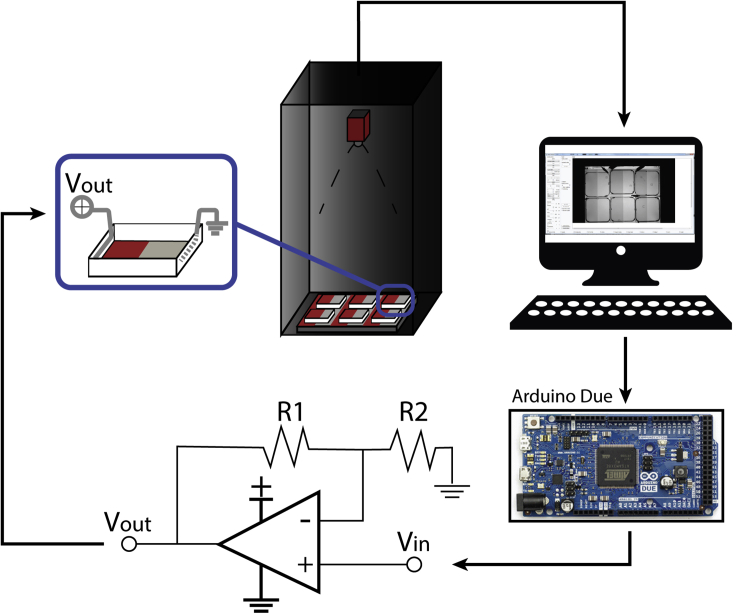
3.Hardware. To implement the behavioral setup there are specific design requirements that are important to maximize animal welfare and minimize external influence during the experiment. We implemented a setup design, shown in Figure 2, which fulfills the following requirements:
-
a.Prevent light\sound contamination from the surrounding environment as good as possible.
-
b.Prevent visual contact between the animals in different chambers during the behavioral testing.
-
c.The water temperature should be maintained constant and never below 25°C/26°C. We used a heater in the room to buffer temperature fluctuations.
-
d.Prevent any intervention by the experimenter during the entire protocol.
-
a.
-
4.
Behavioral arena. To construct the behavioral arena using standard components, we recommend Gosselin square shaped Petri dishes (120 mm × 120 mm × 15.8 mm). The walls of each arena were covered with standard white opaque electric tape to avoid any interaction between the fish in neighboring chambers. This is particularly important, since the juvenile zebrafish exhibit social interactions (Dreosti et al., 2015, Tunbak et al., 2020, Groneberg et al., 2020, Hinz and de Polavieja, 2017). Using these large petri dishes allows the animal to explore the arena freely without any restrictions and increase the richness of the behavioral repertoire. These small details play a crucial role on the resulting behavior since we are not constraining animal movement and enhance flexibility. Finally, using square shaped petri dishes allows easier implementation of the electrodes for establishing a uniform electric field.
-
5.
Electrodes. We recommend the use of inert tungsten wire electrodes, 12 cm of length and 0.25 mm of diameter, for delivering mild aversive electric stimulation to the fish. We observed that tungsten electrodes were better than silver electrodes which showed toxic effects in water. However, tungsten wires exhibit fast decay in conductivity due to the accumulation of ions over one of the electrodes, generating a coating layer within few seconds under constant voltage. To avoid coating and establish constant current, we use 10 milliseconds long 1.2 mA current pulses at 1.33 Hz repetition rate and alternate the polarity on the electrodes between conditioning sessions. All these additional measures prevent oxidation of tungsten wires throughout 2–3 h long behavioral training sessions. This allows us to reuse the same electrodes across experiments extending their lifetime indefinitely (we have used the same electrodes for 4 years now and they still work well). (See problem 2 for troubleshooting)
-
6.
Electric stimulation circuit. To deliver mildly aversive electric stimuli separately to individual fish, we used an Arduino Due based circuit. Arduino board received 3,3 V digital input from the tracking software, when the zebrafish is detected in the condition zone, during conditioning period, and delivered 18 V (10 millisecond, 1.2 mA current at 1.33 Hz) to each fish separately. For each fish, a separate integrated circuit was built and used, connecting an Operational-Amplifier (Opamp, TLE2142 EXCALIBUR low-noise high-speed precision, Texas Instrument) in a non-inverted amplified configuration. The Voltage at the output is given by the relation (see Figure 2):
-
7.Visual stimuli presentation. Visual stimuli are presented to the fish form the bottom using a horizontally positioned LCD monitor. The LCD monitor gives the flexibility to change the visual stimuli, to control its timing, and to synchronize with other electronic systems.
-
a.We use a simple visual pattern dividing the arena half in red and half in gray colors. We match the luminosity of colored illumination, when choosing the red and gray patterns marking different compartment of the arena. This is important since zebrafish respond differently to different illumination and ambient light levels (Spence and Smith, 2008, Avdesh et al., 2012, Oliveira et al., 2015, Guggiana-Nilo and Engert, 2016, Cheng et al., 2017, Cheng et al., 2016).Note: It is very important to keep the water in the behavioral chambers warm, which is crucial especially for extended behavioral testing.To ensure that the water in each behavioral arena never drops below 26°C:
-
b.Measure the water temperature in each arena before and after performing a behavioral experiment.
-
c.Perform the behavioral experiments in a heated room. We installed a passive heater element in our behavioral room which helps to keep the room temperature around 26°C over time.
-
a.
-
8.Experimental design. When designing behavioral experiments, the first rule you need to have in mind is “minimize animals’ discomfort, while maximizing animal welfare and scientific rigor of the experimental output.” To do so it is very important that you tune all the parameters of your behavioral protocol with this goal in mind.
-
a.The stimulus design is a critical step when building a conditioned place avoidance task. We chose the minimum stimulus intensity that elicits a brief escape response. We therefore recommend minimizing the intensity and the duration of the aversive electric stimuli to maximize animals’ welfare and consequently their performance.
-
b.The duration of each training and test session of the CPA protocol is another important parameter to optimize.
-
c.Additionally, we recommend verifying the CPA learning results by flipping the color assignment of the conditioned zone from red to gray, in separate CPA experiments.
-
d.We recommend our CPA protocols below.
-
a.
Figure 2.
Schematic representation of the behavioral setup that is used for conditioned place avoidance
Six square shaped petri dishes are placed on an LCD screen that display specific visual patterns from the bottom. A high-resolution camera, Manta 235B (Allied Vision) with a wide-angle objective, is used to acquire images of the animals. A custom-made software is used to acquired and process the images in real time. Depending on the protocol that is performed, the software communicates with an Arduino Due that controls 6 separate non-inverting amplifier Opamp circuit for delivering the mildly aversive electric stimulation only when animals are detected in the conditioned zone. Each arena is equipped with two tungsten wires, covering the entire length of it two opposing sides. Figure adapted with permission from (Palumbo et al., 2020)
Behavioral training
Timing: varies, 5h for steps 9–10, 4 days for step 11
-
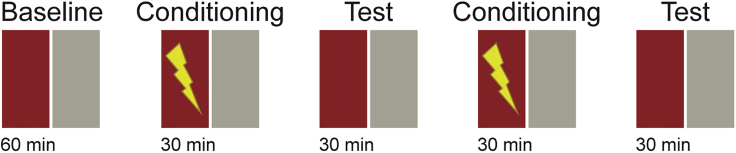
9.Single day CPA training. For single day CPA training, we recommend the experimental design in Figure 3. Also keep in mind:
-
a.Establish a long enough baseline session to ensure that the animals are relaxed and habituated to the environment, evenly exploring the arena without bias. We choose to have a baseline session of 1 h. This will give you the opportunity to evaluate possible innate preference of the animal for any of the arena parameters (location, pattern displayed, etc.). If a naïve animal displays a clear preference for specific compartment of the arena after a long baseline session, it might be necessary to adjust the visual patterns to reduce such bias. We suggest to always maintain a homogeneous luminosity all over the arena (Methods video S1).Methods video S1. Example video of baseline exploration of the arena by a 3-week-old juvenile zebrafish, related to steps 9–11Example video recording of animal behavior for 5 min (speed-up 5×) of the baseline session of a three weeks old juvenile zebrafish. The explored path is marked by white dots. Please note that the animal does not exhibits neither freezing behavior neither erratic swimming. Arena dimensions are 12 cm × 12 cm. Example video from (Palumbo et al., 2020).Download video file (12.1MB, flv)
-
b.Minimize the duration of the conditioning sessions, as much as possible. This will reduce the discomfort of the animal, substantially. We recommend performing multiple short conditioning sessions, separated by test sessions.
-
c.Administrate the negative stimulus to the animal (1.2 mA for 10 ms at 1.33 Hz) every time the animal enters the conditioned zone.
-
d.Terminate stimulus administration as soon as the animal exits the conditioned zone.
-
e.We recommend two 30 min conditioning sessions and two 30 min test sessions. Together with a 1-h long baseline session, the experiment will last around 3 h, which has no negative impact on animals’ health and welfare, as we show in our previous publication (Palumbo et al., 2020).
-
f.We do not recommend protocols longer than 5 h (the longest duration we have tested). This might have a negative impact on animals’ health since they will not be fed for a long time and the water is not changed during the training.
-
a.
Note: In our study (Palumbo et al., 2020), we do not observe freezing behavior or anxiety-like phenotype in animal trained in 1 day protocols up to 5 h long. We therefore recommend to keeping any behavioral protocol shorter than 5 h.
Note: We do recommend collecting the animal from the facility only when the behavioral protocol is ready to begin. This will allow the experimenter to transport the animal to the experimental area and to initiate the behavioral training immediately. This will minimize the total time the animal is handled outside the facility.
-
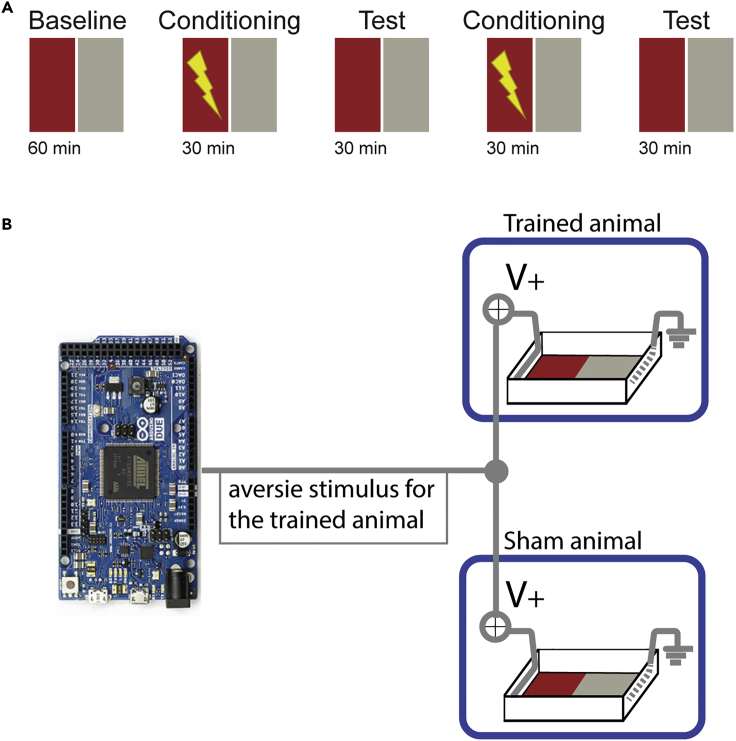
10.Sham-training experiments. In our recent study focusing on CPA learning in juvenile zebrafish (Palumbo et al., 2020), we proposed a sham-training protocol to distinguish the effects of animals’ aversive experience due to mildly aversive electric stimulation (US), from the CPA learning related adaptations of animal behavior, as described in Figure 4A. This sham training protocol decouples animals’ spatial position in the arena from animals’ subjective experiences in response to the delivery of electric stimuli (US). This allows us to compare the behaviors of CPA trained animals with sham-trained animals with same subjective experience of the US. To do so:
-
a.Assign, for each animal which undergoes a training session, a sibling animal as a sham-control. Animals will undergo the training session in couples, one trained and one sham-trained.
-
b.The two arenas need to be identical. We also recommend alternating the arena used for training with the arena used for sham-training, in different sessions. This will control for any asymmetry in the arena of which the experimenter is not aware of.
-
c.Connect both arenas to the same stimulation cable, the one controlled by the trained fish (Figure 4B). Doing so, both animals will be exposed to the same stimulation, controlled exclusively by the trained fish.
-
d.Perform a new series of sham-training experiment any time a new training protocol is implemented.
-
a.
-
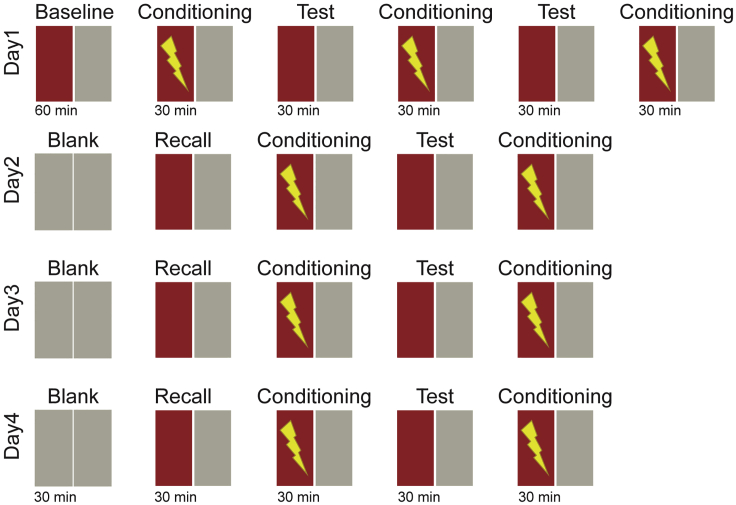
11.Multi-day CPA training. In our recent study, we showed that long term training of juvenile zebrafish leads to better CPA learning performance and memory recall (Palumbo et al., 2020). Such long-term training is best achieved by multiple training sessions spread over consecutive days. This allows the animal to rest, feed and recover between training days. Training juvenile zebrafish over multiple days requires the experimenter to plan animal husbandry and handling accordingly. For multi-day CPA training, we recommend the experimental design in Figure 5. Also, keep in mind:
-
a.Minimize the amount of time for transferring animals between behavioral chambers and temporary holding between training days. After the first training day, we keep individual juvenile zebrafish in petri-dishes (90 mm radius) in a 28°C incubator from the end of the behavioral training (circa 5:00 pm) until the next day training session (circa 10:00 am) We feed the fish before placing them in the incubator. The next morning, we refresh the water and we feed the animals 30 min before the start of the new training session.
-
b.We recommend, before performing a 30 min recall session, to begin each additional day of training with a 30 min habituation session without any visual pattern presented to the animal. This will allow the animal to habituate to the behavioral arena without eliciting any memory recall related to the training visual stimuli, since they are not displayed. The arena is illuminated with light gray, of the same luminosity of the pattern used on day1, see Figure 5 for a graphical representation.
-
c.We recommend concluding each training day with a conditioning session (but not a test session), as it might help promote memory consolidation.
-
d.Wash each behavioral chamber thoroughly with RO water, before and after each training day.
-
a.
Figure 3.
Schematic description of the protocol recommended for one day training in juvenile zebrafish
Baseline is 60 min long; conditioning and test sessions are 30 min long with no delay in between sessions. During the conditioning session, mildly aversive electric stimuli are delivered at 1.33hz, every time the animal enters the conditioned zone (red). We recommend verifying the CPA learning results by filliping the color assignment of the conditioned zone from red to gray, in separate CPA experiments. Figure adapted with permission from (Palumbo et al., 2020).
Figure 4.
Schematic description of the Sham experiment in juvenile zebrafish
(A) Schematic description of the protocol recommended for one day training in juvenile zebrafish. Baseline is 60 min long; conditioning and test sessions are 30 min long with no delay in between sessions.
(B) Hardware configuration of the behavioral setup to perform sham training. Each trained animal is coupled, via direct hardwiring, to another animal which will be sham trained. During the conditioning session, mildly aversive electric stimuli are delivered at 1.33hz to both animals simultaneously, every time the trained animal enters the conditioned zone (red). We recommend verifying the CPA learning results by filliping the color assignment of the conditioned zone from red to gray, in separate CPA experiments. Figure adapted with permission from (Palumbo et al., 2020).
Figure 5.
Schematic description of the protocol recommended for multi-day training in juvenile zebrafish
Baseline and blank sessions are 60 min long, recall, conditioning and test sessions are 30 min long with no delay in between sessions. During the conditioning session, mildly aversive electric stimuli are delivered at 1.33hz, every time the animal enters the conditioned zone (red). Each day is concluded with a conditioning session to avoid memory extinction overnight. From day two, each protocol starts with a blank session during which the animals are habituated to the arena without any pattern presented. Figure adapted with permission from (Palumbo et al., 2020).
Behavioral analysis
Timing: varies, up to 15–30 min to adapt and run the codes we provide
For designing the analysis pipeline, we recommend using multiple complementary CPA learning measures, some of which are also used in previous studies (Palumbo et al., 2020, Valente et al., 2012, Millot et al., 2014, Yashina et al., 2019, Kermen et al., 2020). This approach allows quantification of animal behavior from different perspectives with different strengths and weaknesses.
-
12.We suggest quantifying the following parameters to assess CPA learning:
-
a.Average density of zebrafish position in the CPA arena.
-
b.Relative time spent in the conditioned zone.
-
c.Relative swimming distance in the conditioned zone.
-
d.Animals’ distance from the boundary between safe and conditioned zones.
-
e.Conditioned Place Avoidance learning index (Palumbo et al., 2020).
-
f.Swim direction near the boundary between safe and conditioned zone.
-
g.Avoidance of entry to conditioned zone, near the boundary between safe and conditioned zones.
-
a.
-
13.We suggest quantifying following parameters to assess animals state and health:
-
a.Swim velocity during the experiment.
-
b.Percentage of time that the zebrafish exhibit no swimming (freezing), which we defined as less than 2 mm swimming for 2 s (adapted from (Agetsuma et al., 2010)).
-
c.Swim velocity, one second before (b) and after (a) the delivery of aversive unconditioned stimuli (US).
-
a.
Note: To see the implementation of these measures, please refer to the documentation in https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish and in our earlier study (Palumbo et al., 2020).
Note: In order to have a complete and complementary overview of animal health and performance, we highlight the importance of using multiple metrics while evaluating animal behavior.
Chemogenetic ablation of specific neural populations in zebrafish brain
Timing: 44 h
This protocol describes the use of a transgenic zebrafish line Tg(narp:GAL4VP16; UAS-E1b:NTR-mCherry) to ablate dorsolateral habenula (dlHb) neurons. This transgenic line expresses Nitroreductase (NTR) fusion protein tagged by a mCherry specifically in dlHb (Agetsuma et al., 2010) (Methods video S2). As shown before in adult zebrafish (Agetsuma et al., 2010), bath application of Metronidazole (MTZ) chemogenetically ablate NTR positive dlHb neurons in juvenile zebrafish (Palumbo et al., 2020).
-
14.Prepare MTZ solution
-
a.Needed: 50 mL tubes, petri dishes (50 mL), aluminum foil, microbalance, vortex, and shaker (see key resources table), incubator (± 28°C).
-
b.Prepare 50 mL [10 mM] MTZ solution in a 50 mL tube. Fill the 50 mL tube with AFW and add DMSO, subsequently add the MTZ powder. Proportion is described in Table 1).
-
c.Given the photodegradable nature of the resulting MTZ solution, wrap the entire 50 mL tube, once securely locked, into aluminum foil and make sure that no light comes into contact with the solution. Pay particular attention to this step.
 CRITICAL: MTZ is photodegradable. Prepare a fresh batch of MTZ solution for each ablation protocol. 2 × 50 mL is needed for treating control and experimental groups simultaneously.
CRITICAL: MTZ is photodegradable. Prepare a fresh batch of MTZ solution for each ablation protocol. 2 × 50 mL is needed for treating control and experimental groups simultaneously. CRITICAL: We recommend leaving the animal for at least 12h (overnight) in fish water after removing them from the MTZ bath to allow the MTZ to wash out. During this time, frequently refresh the water in the petri dish and feed the animal.
CRITICAL: We recommend leaving the animal for at least 12h (overnight) in fish water after removing them from the MTZ bath to allow the MTZ to wash out. During this time, frequently refresh the water in the petri dish and feed the animal. CRITICAL: Perform the behavioral experiments as soon as the MTZ washout is completed, before any neurogenesis that might occur over the course of the next few days.
CRITICAL: Perform the behavioral experiments as soon as the MTZ washout is completed, before any neurogenesis that might occur over the course of the next few days. CRITICAL: As control animals, we recommend using sibling zebrafish that do not express the UAS-E1b:NTR-mCherry transgene.
CRITICAL: As control animals, we recommend using sibling zebrafish that do not express the UAS-E1b:NTR-mCherry transgene. -
d.Shake by hand, vortex thoroughly, and leave the tube on a shaker for at least 30 min, allowing the metronidazole to dissolve properly.
-
a.
-
15.Chemogenetic ablation
-
a.Before you start treating the animals make sure you have:
-
i.2× 50 mL [10 mM] MTZ solution
-
ii.Aluminum foil
-
iii.Fish already in the experimental area and fed prior to the treatment.
-
iv.Incubator at ± 28°C.
-
v.1 petri dish must contain at most 3 juvenile zebrafish.
-
vi.Prepare the fish ready for the experiment. Make sure that the fish had access to food before the start of the ablation protocol. Limit the number of fish (max 3) per dish, to avoid mortality due to hypoxia or excessive ammonia. Make sure that the animals to be treated are size matched.
-
i.
-
b.Fill two petri dishes with 50 mL [10 mM] each, and make sure the temperature of the solution is not lower than 26°C
-
c.Transfer the zebrafish, one at a time in the petri dishes with the MTZ solution, using a 7 mL pipette. Make sure to cut the tip of the pipette, as described earlier. To prevent reduction of MTZ concentration in the petri dish, transfer the fish in a small bubble of water.
 CRITICAL: We observed that the lower MTZ concentrations (1 and 5 mM) did not yield desired effects in juvenile zebrafish. Moreover 10 mM MTZ solution did not elicit any toxicity or alteration of animal behavior in control animals of nacre or Ab wild-type genetic background.
CRITICAL: We observed that the lower MTZ concentrations (1 and 5 mM) did not yield desired effects in juvenile zebrafish. Moreover 10 mM MTZ solution did not elicit any toxicity or alteration of animal behavior in control animals of nacre or Ab wild-type genetic background. -
d.Label the petri dish with necessary information (fish line, date, experimental group, experimenters initial, etc.)
-
e.Metronidazole is light-sensitive, therefore wrap the dish in aluminum foil. Exposure to light can decrease effectiveness of the ablation and will turn the fish water brown/yellow.
-
f.Label the aluminum foil again with the same info as in point 3.
-
g.Place the petri dishes in the incubator (± 28°C).Note: Be extra careful when moving the dish around since even small movements can be very stressful for the animals.
-
h.Incubate for 24 h in MTZ.Note: For these 24 h, the animal will be kept in darkness.
-
i.After 24 h, transfer the fish to a new petri dish containing clean fish water. Make sure to refresh the water twice in each petri dish in the first few hours after the MTZ treatment.
-
j.Incubate again (± 28°C) for 12 h in AFW. Make sure to feed the animal before incubating.
-
k.The next morning make sure to refresh the fish water in each petri dish and feed the animal at least 30 min before the start of the behavioral protocol.
-
l.The fish are now ready for the behavior protocol. Ideally start between 9 and 12 am, to keep consistency for the timing of the experiments, and animals’ circadian rhythm.
-
a.
-
16.
Validation of chemogenetic ablation (Timing: 16 h)
To verify successful chemogenetic ablation of neurons we recommend TUNEL assay (Agetsuma et al., 2010). The protocol was adapted from the manufacturer’s protocol (In Situ Cell Death Detection Kit, Fluorescein, 11684795910 Roche) and optimized for whole mount labeling and confocal imaging (Methods video S3).-
a.Euthanize fish in ice cold water and fix it in cooled 4% PFA in 0.25% PBTx (0.25% Triton X-100 in 1× PBS) for 12 h at 4°C.
-
b.Permeabilize samples in 0.050% Trypsin-EDTA on ice for 40 min and wash 3 times with 0.25% PBTx.
-
c.Incubate the samples in 50μL TUNEL reaction mix for 1 h at 37°C humidified atmosphere, in a water bath covered by aluminum foil the dark. Subsequently wash it in 0.25% PBTx.
-
d.Place the samples on microscopy slides after washing steps in increasing glycerol concentration of 25%, 50%, and 75%. Then, use 75% Glycerol as a medium for mounting the samples on glass slides for confocal imaging (see problems 4 and 5).
-
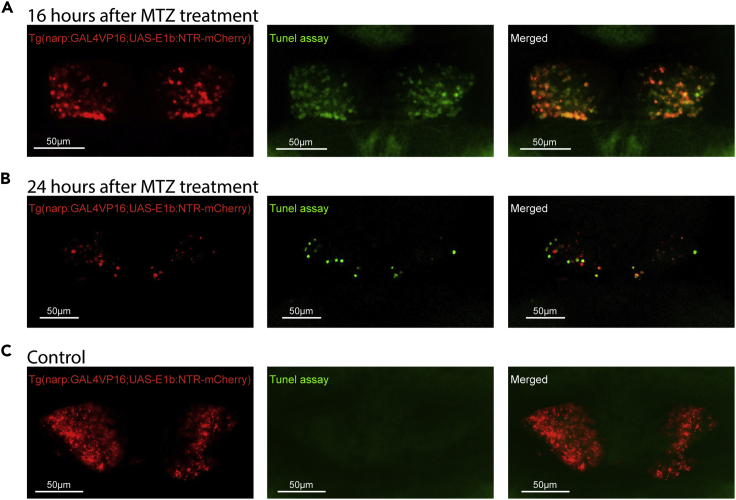
e.The TUNEL assay is based on detection of apoptotic cells. Apoptosis is a form of programmed cell death and can be affected by numerous factors (e.g., uptake of the drug, passage through the blood brain barrier, concentration, etc.). To visualize the ablated cells at different apoptotic stages, we performed TUNEL assay at 16 h (Figure 6A and 6C) and 24 h (Figure 6B) after the start of the MTZ treatment. While apoptotic cells are clearly visible in 16 h after chemogenetic ablation, 24 h after the ablation apoptotic fragments are less detectible with TUNEL assay (Figure 6B).
Methods video S3. TUNEL assay in a 3-week-old narp:Gal4;UAS-E1b:NTR-mCherry zebrafish following MTZ treatment, related to Figure 6A and step 16Confocal microscopy of the two habenulas in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. TUNEL signal, detecting apoptosis, is marked with green fluorescence, while red fluorescence is related to debriefs of NTR-mCherry protein. Fish orientation: anterior to the top.Download video file (2.9MB, flv) -
a.
Note: Zebrafish brain have exceptional regenerative capacity (Celikkaya et al., 2019; Kizil, 2018; Cosacak et al., 2019; Lange et al., 2020; Kesavan et al., 2020; Schwarzer et al., 2020). Therefore, we recommend that the behavioral testing is done no longer than 2 days after the chemogenetic ablation, otherwise neuroregeneration may affect subsequent experiments. We observed addition of new habenular neurons in juvenile zebrafish, already 2 days after chemogenetic ablation (Figure 7; Methods video S4).
Table 1.
Specifics of the 10 mM MTZ solution
| Component | Volume/Weight | Final concentration |
|---|---|---|
| Metronidazole (MTZ) | 0.086 g | 0.01 M [10 mM] |
| Dimethyl Sulfoxide (DMSO) | 0.250 mL | 0.5% |
| Artificial fish water (AFW, 0.2 g/L instant ocean salt in reverse osmosis water (RO)) | 49.750 mL | N/A |
Specification about the chemicals can be found in the key resources table.
Figure 6.
TUNEL assay for detecting apoptosis after chemogenetic ablation with metrodiniazole
(A–C)Confocal microscopy images of three weeks old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish 16 h (A) and 24 h (B) after the start of Metronidazole (MTZ) treatement. Green fluorescent labels TUNEL signal which is a direct measure of cell death (apoptosis). Note that green TUNEL signal is not visible in control animals (C). Scale bar is 50 μm.
Figure 7.
Regeneration of new neurons 48 h after chemogenetic ablation with metrodiniazole
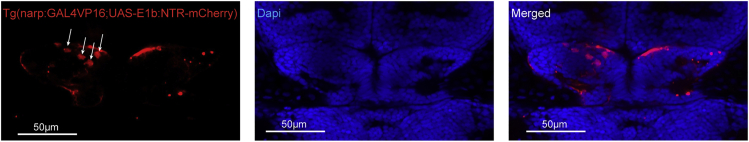
Confocal microscopy image of three weeks old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, 48h after Metrodiniazole treatement. The new-born neurons that are marked with white arrows are already integrating into developing habenula. Scale bar is 50 μm.
Confocal microscopy image of the entire brain in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. Please note that the narp:Gal4 expression is exclusively in the dorsolateral habenula and its projections to midbrain. Fish orientation: anterior to the left. Video re-used with permission from (Palumbo et al., 2020)
Note: Health monitoring. In the rare cases, if we observe that a fish has abnormal swim or no movement, we euthanized these animals and did not perform any behavioral study
Confocal microscopy image of the two habenulas in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. Please note that the few NTR-mCherry (red fluorescence) positive cells are now located dorso-medially in the habenula. In blue a DAPI staining has been performed for anatomical reference. Fish orientation: anterior to the top.
Expected outcomes
Once the conditioned protocol has been performed, following all the instruction described above, the behavioral phenotype of the animal will replicate the one we describe in our previous publication (Palumbo et al., 2020).
Most importantly, the animal will not show any 1) Freezing behavior, 2) Erratic swimming, or 3) Thigmotaxis.
The animal will spend most of his time in the safe zone of the arena once the training session is completed.
For more details, in our publication (Palumbo et al., 2020) we provide several analysis of animal behavior over the course of the behavioral training.
Limitations
We only detect the animal’s “center of mass” to obtain its position. We did not detect/analyzed animal posture or animal swim pattern.
In our setup we did not implement a conditioned place learning protocol based on reward. This challenging task will require development of repeated and robust reward delivery systems that require further design.
Implementing more computational power and higher spatiotemporal resolution of video monitoring would lead to better image quality for posture estimation and allow the processing of larger number of zebrafish in parallel.
Troubleshooting
Problem 1
During the baseline session, the animal shows reduced swimming behavior, with a high freezing time (step 9–11).
Potential solution
Control the water temperature in the behavioral arena, it is likely too cold for the animal. Additionally, make sure that the animal is fed before it is transported (always using warm water) to the experimental area. Heating up the room in which you perform the behavioral training can also contribute to maintain the right water temperature.
See Methods video S1 for an example of animal baseline behavior.
Problem 2
The animal does not respond to the electric stimulation during the conditioned sessions (step 5–11).
Potential solution
As a first approach always make sure that all the hardware is properly connected and secured (no loose wires around). Moreover, be sure of alternating cathode and anode across conditioning sessions to avoid electrode passivation.
If none of the above solves the problem, then it is likely that the stimulus amplitude is the problem: too low amplitude will not elicit any response from the animal, while a too strong stimulus will elicit at first a strong animal reaction, but it will quickly lead to a helplessness behavior. Therefore, pay close attention to see in which of these two conditions you find yourself and adjust the stimulus amplitude accordingly.
Problem 3
The animal used for experiments at 3-week post fertilization is small in size compared to previous experiments (step 9–11).
Potential solution
This is probably due to crowded nursery tank in the fish facility together with a non-appropriate feeding routine. We do recommend keeping no more than 25 larvae per liter of water and feeding them twice a day with dry food (morning and evening) and once with Artemia naplii (around 13:00) (see key resources table). Keeping the waterflow outside the nursery net also improve animal growth and health.
Problem 4
The fluorescent signal of the whole mount TUNEL staining decreases with the imaging depth (step 16).
Potential solution
When dissecting the brain remove the skin and dura on top of the brain. This allows a better penetration of the antibodies into the brain. Additionally, when imaging, make sure that the cover slip is right on top of the brain, as close as possible. Pay attention however not to squeeze the brain.
Problem 5
The sample is not stable during the imaging process (step 16).
Potential solution
We recommend using a 4% gelatin - 65% glycerol solution as mounting medium. It improves the stability of the sample, since it has better mechanical properties at room temperature (20°C ± 2°C) than 75% glycerol, which remains viscous.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Emre Yaksi (emre.yaksi@ntnu.no)
Materials availability
This study did not generate any new unique reagent
Data and code availability
All custom scripts described in this article are available at:
https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish
Acknowledgments
We thank Hitoshi Okamoto, Misha Ahrens (for transgenic lines), Siv Eggen, Vy Nguyen, Andreas Nygard (for technical assistance), and all Yaksi lab members (for stimulating discussions). This work was funded by ERC grant 335561 (F.P. and E.Y.), RCN FRIPRO grant 314212 (E.Y.), Kavli Foundation, and NTNU.
Author contributions
Conceptualization, F.P. and E.Y.; methodology and data, F.P. and B.S.; codes repository, F.P.; data analysis, F.P.; investigation, all authors; writing, F.P. and E.Y.; review & editing, all authors; funding acquisition and supervision, E.Y.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100465.
Contributor Information
Fabrizio Palumbo, Email: fabrizio.palumbo@ntnu.no.
Emre Yaksi, Email: emre.yaksi@ntnu.no.
References
- Agetsuma M., Aizawa H., Aoki T., Nakayama R., Takahoko M., Goto M., Sassa T., Amo R., Shiraki T., Kawakami K. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- Avdesh A., Martin-Iverson M.T., Mondal A., Chen M., Askraba S., Morgan N., Lardelli M., Groth D.M., Verdile G., Martins R.N. Evaluation of color preference in zebrafish for learning and memory. J. Alzheimer's Dis. 2012;28:459–469. doi: 10.3233/JAD-2011-110704. [DOI] [PubMed] [Google Scholar]
- Celikkaya H., Cosacak M.I., Papadimitriou C., Popova S., Bhattarai P., Biswas S.N., Siddiqui T., Wistorf S., Nevado-Alcalde I., Naumann L. GATA3 promotes the neural progenitor state but not neurogenesis in 3D traumatic injury model of primary human cortical astrocytes. Front. Cell. Neurosci. 2019;13:23. doi: 10.3389/fncel.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.-K., Krishnan S., Lin Q., Kibat C., Jesuthasan S. Characterization of a thalamic nucleus mediating habenula responses to changes in ambient illumination. BMC Biol. 2017;15:104. doi: 10.1186/s12915-017-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.K., Krishnan S., Jesuthasan S. Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci. Rep. 2016;6:20788. doi: 10.1038/srep20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosacak M.I., Bhattarai P., Reinhardt S., Petzold A., Dahl A., Zhang Y., Kizil C. Single-cell transcriptomics analyses of neural stem cell heterogeneity and contextual plasticity in a zebrafish brain model of amyloid toxicity. Cell Rep. 2019;27:1307–1318.e3. doi: 10.1016/j.celrep.2019.03.090. [DOI] [PubMed] [Google Scholar]
- Dreosti E., Lopes G., Kampff A.R., Wilson S.W. Development of social behavior in young zebrafish. Front. Neural Circuits. 2015;9:39. doi: 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg A.H., Marques J.C., Martins A.L., Diez Del Corral R., De Polavieja G.G., Orger M.B. Early-life social experience shapes social avoidance reactions in larval zebrafish. Curr. Biol. 2020;30:4009–4021.e4. doi: 10.1016/j.cub.2020.07.088. [DOI] [PubMed] [Google Scholar]
- Guggiana-Nilo D.A., Engert F. Properties of the visible light phototaxis and UV avoidance behaviors in the larval zebrafish. Front. Behav. Neurosci. 2016;10:160. doi: 10.3389/fnbeh.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz R.C., De Polavieja G.G. Ontogeny of collective behavior reveals a simple attraction rule. Proc. Natl. Acad. Sci. U S A. 2017;114:2295–2300. doi: 10.1073/pnas.1616926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewtrakulpong P., Bowden R. An improved adaptive background mixture model for real-time tracking with shadow detection. In: Remagnino P., Jones G.A., Paragios N., RegazzonI C.S., editors. Video-Based Surveillance Systems: Computer Vision and Distributed Processing. Springer US; 2002. [Google Scholar]
- Kermen F., Darnet L., Wiest C., Palumbo F., Bechert J., Uslu O., Yaksi E. Stimulus-specific behavioral responses of zebrafish to a large range of odors exhibit individual variability. BMC Biol. 2020;18:66. doi: 10.1186/s12915-020-00801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan G., Machate A., Hans S., Brand M. Cell-fate plasticity, adhesion and cell sorting complementarily establish a sharp midbrain-hindbrain boundary. Development. 2020;147:dev186882. doi: 10.1242/dev.186882. [DOI] [PubMed] [Google Scholar]
- Kizil C. Mechanisms of pathology-induced neural stem cell plasticity and neural regeneration in adult zebrafish brain. Curr. Pathobiol. Rep. 2018;6:71–77. doi: 10.1007/s40139-018-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Rost F., Machate A., Reinhardt S., Lesche M., Weber A., Kuscha V., Dahl A., Rulands S., Brand M. Single cell sequencing of radial glia progeny reveals the diversity of newborn neurons in the adult zebrafish brain. Development. 2020;147:dev185595. doi: 10.1242/dev.185595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech M., Dalka P., Szwoch G., Czyżewski A. Examining quality of hand segmentation based on gaussian mixture models. In: Dziech A., Czyżewski A., editors. Multimedia Communications, Services and Security: 7th International Conference, MCSS 2014, Krakow, Poland, June 11-12, 2014. Proceedings. Springer International Publishing; 2014. [Google Scholar]
- Millot S., Cerqueira M., Castanheira M.F., Øverli Ø., Martins C.I.M., Oliveira R.F. Use of conditioned place preference/avoidance tests to assess affective states in fish. Appl. Anim. Behav. Sci. 2014;154:104–111. [Google Scholar]
- Oliveira J., Silveira M., Chacon D., Luchiari A. The zebrafish world of colors and shapes: preference and discrimination. Zebrafish. 2015;12:166–173. doi: 10.1089/zeb.2014.1019. [DOI] [PubMed] [Google Scholar]
- Palumbo F., Serneels B., Pelgrims R., Yaksi E. The zebrafish dorsolateral habenula is required for updating learned behaviors. Cell Rep. 2020;32:108054. doi: 10.1016/j.celrep.2020.108054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power P.W., Schoonees J.A. Proceedings of Image and Vision Computing New Zealand. IVCNZ; 2002. Understanding background mixture models for foreground segmentation; pp. 266–271. [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer S., Asokan N., Bludau O., Chae J., Kuscha V., Kaslin J., Hans S. Neurogenesis in the inner ear: the zebrafish statoacoustic ganglion provides new neurons from a Neurod/Nestin-positive progenitor pool well into adulthood. Development. 2020;147:dev176750. doi: 10.1242/dev.176750. [DOI] [PubMed] [Google Scholar]
- Spence R., Smith C. Innate and learned colour preference in the Zebrafish, Danio rerio. Ethology. 2008;114:582–588. [Google Scholar]
- Štih V., Petrucco L., Kist A.M., Portugues R. Stytra: An open-source, integrated system for stimulation, tracking and closed-loop behavioral experiments. PLoS Comput. Biol. 2019;15:e1006699. doi: 10.1371/journal.pcbi.1006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbak H., Vazquez-Prada M., Ryan T.M., Kampff A.R., Dreosti E. Whole-brain mapping of socially isolated zebrafish reveals that lonely fish are not loners. Elife. 2020;9:e55863. doi: 10.7554/eLife.55863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A., Huang K.H., Portugues R., Engert F. Ontogeny of classical and operant learning behaviors in zebrafish. Learn. Mem. 2012;19:170–177. doi: 10.1101/lm.025668.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov N., Mu Y., Kawashima T., Bennett D.V., Yang C.T., Looger L.L., Keller P.J., Freeman J., Ahrens M.B. Light-sheet functional imaging in fictively behaving zebrafish. Nat. Methods. 2014;11:883–884. doi: 10.1038/nmeth.3040. [DOI] [PubMed] [Google Scholar]
- Yashina K., Tejero-Cantero Á., Herz A., Baier H. Zebrafish exploit visual cues and geometric relationships to form a spatial memory. iScience. 2019;19:119–134. doi: 10.1016/j.isci.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic Z. Proceedings of the Pattern Recognition, 17th International Conference on (ICPR'04) Volume 2 - Volume 02. IEEE Computer Society; 2004. Improved adaptive gaussian mixture model for background subtraction. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example video recording of animal behavior for 5 min (speed-up 5×) of the baseline session of a three weeks old juvenile zebrafish. The explored path is marked by white dots. Please note that the animal does not exhibits neither freezing behavior neither erratic swimming. Arena dimensions are 12 cm × 12 cm. Example video from (Palumbo et al., 2020).
Confocal microscopy of the two habenulas in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. TUNEL signal, detecting apoptosis, is marked with green fluorescence, while red fluorescence is related to debriefs of NTR-mCherry protein. Fish orientation: anterior to the top.
Confocal microscopy image of the entire brain in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. Please note that the narp:Gal4 expression is exclusively in the dorsolateral habenula and its projections to midbrain. Fish orientation: anterior to the left. Video re-used with permission from (Palumbo et al., 2020)
Confocal microscopy image of the two habenulas in three-week-old Tg(narp:Gal4;UAS-E1b:NTR-mCherry) zebrafish, dorsal view. Please note that the few NTR-mCherry (red fluorescence) positive cells are now located dorso-medially in the habenula. In blue a DAPI staining has been performed for anatomical reference. Fish orientation: anterior to the top.
Data Availability Statement
All custom scripts described in this article are available at:
https://github.com/fabrizio-palumbo/Conditioned-behaviour-in-juvenile-zebrafish