Highlights
-
•
We tested gender differences in brain volumes of alcohol dependent vs control groups.
-
•
Group differences in brain volumes emerged as gross and widespread.
-
•
Group-by-gender effects emerged in selected brain regions (cerebellum, amygdala)
-
•
In dependent users, greater alcohol use predicted smaller amygdala and larger cerebellum GM volume.
-
•
Our results highlight the need to account for gender differences in MRI studies of alcohol dependence.
Keywords: Alcohol, Sex, Alcohol dependence, Gender differences, Neuroimaging, MRI
Abstract
Gender-related differences in the susceptibility, progression and clinical outcomes of alcohol dependence are well-known. However, the neurobiological substrates underlying such differences remain unclear. Therefore, this study aimed to investigate gender differences in the neuroanatomy (i.e. regional brain volumes) of alcohol dependence. We examined the volume of a priori regions of interest (i.e., orbitofrontal cortex, hippocampus, amygdala, nucleus accumbens, caudate, putamen, pallidum, thalamus, corpus callosum, cerebellum) and global brain measures (i.e., total grey matter (GM), total white matter (WM) and cerebrospinal fluid). Volumes were compared between 660 people with alcohol dependence (228 women) and 326 controls (99 women) recruited from the ENIGMA Addiction Working Group, accounting for intracranial volume, age and education years. Compared to controls, individuals with alcohol dependence on average had (3–9%) smaller volumes of the hippocampus (bilateral), putamen (left), pallidum (left), thalamus (right), corpus callosum, total GM and WM, and cerebellar GM (bilateral), the latter more prominently in women (right). Alcohol-dependent men showed smaller amygdala volume than control men, but this effect was unclear among women. In people with alcohol dependence, more monthly standard drinks predicted smaller amygdala and larger cerebellum GM volumes. The neuroanatomical differences associated with alcohol dependence emerged as gross and widespread, while those associated with a specific gender may be confined to selected brain regions. These findings warrant future neuroscience research to account for gender differences in alcohol dependence to further understand the neurobiological effects of alcohol dependence.
1. Introduction
Alcohol use disorders can be chronic relapsing disorders that, together with harmful alcohol use, account for 5% of the total global burden of disease and cause three million deaths per year (World Health Organization, 2018). Gender differences are expressed in patterns of alcohol consumption and related behaviours, including alcohol use disorders (Erol and Karpyak, 2015). For instance, historically, women consume less alcohol, start drinking later and have lower rates of alcohol dependence than men (Brennan et al., 2011, Chung et al., 2012, World Health Organization, 2018). However, women transition from first alcohol use to alcohol dependence more rapidly than men, and amongst heavy drinkers, women have a higher risk of somatic and psychiatric comorbidities (Flensborg-Madsen et al., 2011, Mann et al., 2005, Mann et al., 2004). Yet, women with alcohol dependence show less severe withdrawal symptoms and those who enter treatment programs achieve better long-term outcomes (e.g., lower alcohol consumption) (Bravo et al., 2013, Deshmukh et al., 2003). Such gender differences in patterns of alcohol use and related outcomes may derive (in part) from sex/gender-specific neurobiological mechanisms (Erol and Karpyak, 2015, Nixon et al., 2014, Logrip et al., 2018), but this remains to be elucidated.
Alcohol dependence has been associated with neuroanatomical differences including smaller volumes of the medial temporal and orbitofrontal cortices, the cerebellum, the striatum, total grey matter (GM) and white matter (WM) and larger volume of the cerebrospinal fluid (Mackey et al., 2019, Zahr, 2014). The contribution of gender to these findings has been examined by a minority of studies to date (Lind et al., 2017), which led to mixed findings (Nixon et al., 2014, Ruiz et al., 2013, Sawyer et al., 2016, Verplaetse et al., 2021). More pronounced volume differences have been shown in alcohol-dependent women than men (compared to their control counterparts), including smaller hippocampus (Agartz et al., 1999, Agartz et al., 2003), corpus callosum (Hommer et al., 1996), total GM and total WM, and larger CSF (Hommer et al., 2001). In contrast, greater volume changes were also noted in alcohol-dependent men than women in overlapping regions (i.e. corpus callosum, total WM, CSF) (Pfefferbaum et al., 2001, Ruiz et al., 2013) and in total reward network (including dorsolateral prefrontal, orbitofrontal (OFC) and cingulate cortices, and temporal pole, insula, amygdala, hippocampus, nucleus accumbens (NAcc) and ventral diencephalon) (Sawyer et al., 2016). Yet, other MRI studies failed to find gender differences between men and women with alcohol dependence in either overlapping (i.e., corpus callosum, total GM and WM) or other (i.e., frontal cortices, hippocampus, insula, cerebellum, pons, thalamus) brain areas (Demirakca et al., 2011, Mechtcheriakov et al., 2007, Sawyer et al., 2016).
While the literature to date suggests that neuroanatomical gender differences in alcohol dependence may exist, the inconsistent findings cannot be readily integrated due to methodological limitations (Nixon et al., 2014, Sawyer et al., 2016). First, a systematic assessment of gender differences in the literature thus far has been hindered by the fact that most studies include male-only or largely male samples, the lack of testing or reporting group-by-gender interactions and the use of small samples (Lind et al., 2017). Second, the role of confounders in the findings to date is poorly understood as these were inconsistently accounted for in statistical analyses (e.g., age, education, tobacco use) (Durazzo et al., 2014, Gilbertson et al., 2008).
The level of alcohol exposure may also drive neuroanatomical differences in a gender-dependent fashion. Specifically, alcohol use measures (i.e., number of daily drinks, heavy drinking years or lifetime monthly standard drinks) have been associated with the volume of distinct brain regions in alcohol-dependent men (e.g., corpus callosum, cerebellum, parietal lobe) and women (e.g., frontal, temporal, ventricles) (Ruiz et al., 2013, Sawyer et al., 2016) or with the volume of both men and women but with opposite direction (e.g., temporal pole, cingulate cortex) (Sawyer et al., 2017). Thus, alcohol exposure may drive distinct neurobiological differences in alcohol dependent men and women.
Here we report an analysis of previously published datasets collected at multiple sites, intended to address the limitations of the literature to date and investigate gender differences in the neuroanatomical (brain volumetric) correlates of alcohol dependence in a large and well-characterized sample of 986 adults recruited from 10 distinct research sites that participate in the ENIGMA Addiction Working Group (www.enigmaaddictionconsortium.com) (SI Appendix, Tables S1 and S2). These comprised 326 people without alcohol dependence (99 women; henceforth labelled as ‘controls’) and 660 people with alcohol dependence (228 women).
We focused on 26 a-priori brain regions of interest (ROIs) in which volumetric differences have been most consistently shown in people with alcohol dependence compared to controls: the OFC, medial temporal (i.e., hippocampus, amygdala) and striatal areas (i.e., NAcc, caudate, putamen, pallidum, thalamus), the corpus callosum, the cerebellum and global brain estimates (i.e., total GM, total WM and CSF) (Mackey et al., 2019, Nixon et al., 2014).
Based on previous structural MRI studies, we hypothesized that people with alcohol dependence relative to controls would show smaller volume of a priori ROIs, total GM and total WM and larger CSF volume. Moreover, we explored whether group-by-gender interactions would emerge in the hippocampus, corpus callosum, global estimates (Nixon et al., 2014) or in other a priori ROIs where these effects have not been examined (or found) so far. Lastly, in people with alcohol dependence, we explored whether gender, monthly standard drinks (and their interaction) or monthly standard drinks separately in men and women would predict brain volumes of those ROIs that demonstrated significant group-by-gender effects.
2. Materials and methods
The study protocol was pre-registered in the Open Science Framework (https://osf.io/yku8j) after screening the sample as per inclusion and exclusion criteria, and before running the statistical analysis. This is detailed in the SI Appendix. Participants’ data including structural MRI, gender, age, education, monthly standard drinks and monthly cigarettes was gathered from 10 research sites in the ENIGMA Addiction Working Group. All sites obtained local ethics approval and participants’ written informed consent.
The original sample (N = 1348) was screened against the following exclusion criteria (i) lifetime and/or current primary psychiatric comorbidities and/or current substance dependence other than alcohol (n = 191); (ii) abstinence > 30 days (n = 15); (iii) IQ < 80 (n = 15); (iv) MRI artifacts (n = 1); (iv) missing data for key variables including gender (n = 68) and education (n = 72). The final sample included 986 people, consisting of 326 controls (227 men, 99 women) with a mean age of 30.2 ± 10.4 years and 660 alcohol dependent participants (432 men, 228 women) with a mean age of 33.9 ± 10.5 years. Table S1 overviews each study’s assessment site, inclusion/inclusion criteria, MRI acquisition parameters and instruments used to measure sample characteristics of each study while Table S2 describes participants’ demographic and substance use characteristics, by imaging site (see SI Appendix).
2.1. Structural MRI data acquisition and processing
Structural T1-weighted MRI scans were prepared locally using FreeSurfer 5.3 (http://sufrer.nmr.mgh.harvard.edu/), a fully automated MRI processing pipeline (Dale et al., 1999, Desikan et al., 2006). Quality control procedures (i.e., detection of outliers and data visual inspection) were run via standardized ENIGMA protocols (http://enigma.ini.usc.edu/protocols/imaging-protocols/). We extracted the volume of 11 bilateral ROIs (medial OFC, lateral OFC, hippocampus, amygdala, NAcc, caudate, putamen, globus pallidus, thalamus (all GM), cerebellum GM and cerebellum WM); five corpus callosum ROIs (anterior, middle-anterior, central, middle-posterior, posterior) and three global brain estimates (total GM, total WM and CSF). Left and right hemispheres were considered separately for each bilateral ROI while the five corpus callosum ROIs were summed in a single ROI before the statistical analysis. Therefore, a total of 26 ROIs were included in the analysis.
2.2. Statistical analyses
Chi-square tests were run to test differences between groups (alcohol-dependent vs. controls) in gender distribution.
A series of mixed-effect models were run to examine group, gender and group-by-gender effects for age, education, monthly standard drinks, monthly cigarettes and brain volumes.
This technique statistically accommodates dependency between observations in a nested design (i.e., participants within sites) (Aarts et al., 2014). Site was treated as a random intercept to account for the systematic site-level variation in the dependent variables expected to occur from differences in scanners, protocols and assessments. The extent of variation explained by site-level differences was estimated as an intra-class correlation (ICC).
First, we examined the impact of factors including group (control, alcohol-dependent), gender (man, woman) and group-by-gender on the volume of a-priori ROIs as dependent variables, accounting for age, education years and intracranial volume (ICV). Separate models were run for each ROI. Significant group-by-gender effects were interrogated using pairwise post-hoc comparisons. In the text, we expressed the significant difference between two mean volumes as a percentage (%) difference (formula: [(predicted mean 1 - predicted mean 2 / (predicted mean 1 + predicted mean 2) / 2) *100]).
Second, in people with alcohol dependence, we explored whether gender or monthly standard drinks (model A), gender-by-standard drinks (model B) or monthly standard drinks separately in men and women (models C and D) predicted the volume of those ROIs that demonstrated significant group-by-gender effects, controlling for age, education years and ICV.
Both analyses were replicated on a sensitivity subsample where men and women with alcohol dependence were matched by monthly standard drinks (SI Appendix, Table S3).
Additional analyses including tobacco use (i.e., monthly cigarettes) as a covariate, were run in a subsample where this data was also available (465 alcohol dependent participants and 140 controls). Tobacco use (i.e., presence versus absence) did not significantly affect the results and was not included as a covariate in final models (SI Appendix, Table S4).
Alcohol use was positively skewed (skewness = 2.40) and was square-root transformed (skewness = 1.03) prior to statistical analyses (SI Appendix, Figure S1). Volumetric results were corrected for multiple comparisons using Benjamini and Yekutieli’s modified False Discovery Rate (FDR) method (Benjamini and Yekutieli, 2001, Newson, 2010) that was applied independently to each beta coefficient (for example the P-values for group comparisons were corrected separately from the P-values for sex comparisons, etc.). Cohen’s d was used to estimate effect sizes of the differences between groups, based on the marginal means predicted by the model. All statistical analyses were performed using STATA 14 (StataCorp; 2015).
3. Results
Table 1 shows demographic and substance use characteristics for the pooled sample. Women were fewer than men in both the alcohol-dependent and the control group. Specifically, the female-to-male ratio was 35/65 and 30/70, respectively. Compared to controls, people with alcohol dependence were (on average) three years older, had a one-year lower education and consumed six times more standard drinks and four times more cigarettes. Alcohol-dependent men used (on average) almost twice as many monthly standard drinks as alcohol-dependent women.
Table 1.
Demographic and substance use characteristics of the sample
| Control | Alcohol-dependent | Group (Alcohol-dependent vs Control) | Gender (Men vs Women) | Group-by-Gender | Site§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | Var | |
| Gender, N (%) | 227 (69.6) | 99 (30.4) | 432 (65.4) | 228 (34.6) | – | – | – | – | – | – | – |
| Age | 30.44 (10.57); 18–68 | 29.76 (10.15); 18–58 | 34.43 (10.42); 21–66 | 32.78 (10.55); 18–62 | 5.52 (2.94, 8.10) | <0.001** | 0.74 (-1.37, 2.85) | 0.490 | 0.61 (-1.90, 3.12) | 0.635 | 0.39 |
| Education, years | 15.14 (2.95) | 15.63 (2.86) | 14.00 (2.36) | 14.35 (2.55) | -1.80 (-2.55, -1.05) | <0.001** | -0.26 (-0.89, 0.36) | 0.402 | -0.09 (-0.83, 0.66) | 0.818 | 0.11 |
| Alcohol use, StDr/mo | 28.66 (30.15) | 17.51 (22.05) | 189.29 (181.42) | 107.08 (107.31) | 205.24 (160.39, 250.09) | <0.001** | 10.28 (-24.32, 44.90) | 0.560 | 40.75 (-0.53, 80.97) | 0.047*a | 0.37 |
| Tobacco use, Cig/mo | 25.07 (91.27) | 42.85 (101.39) | 178.50 (235.98) | 141.96 (232.88) | 253.76 (157.84, 349.66) | <0.001** | -23.56 (-100.63, 53.52) | 0.549 | 45.39 (-39.86, 130.64) | 0.297 | 0.19 |
Note: Cig = cigarettes, mo = monthly, StDr = standard drink, Var = variance. Differences in sex distribution measured with chi2 test (χ2 = 1.72, p = 0.190). Values for age are mean (SD) and range. Values for education, alcohol use and tobacco use are mean (SD). § Site-level variation estimated as an intraclass correlation (ICC). a Alcohol dependent men > Alcohol dependent women (β = 51.04, p < .001). * p < .05, ** p < .001.
Volumetric measures by group and gender are shown in Table 2. People with alcohol dependence compared to controls had 6% smaller volume of the bilateral hippocampus, bilateral cerebellum GM and total WM. Furthermore, volume reductions in the alcohol dependent group amounted to 9% for the corpus callosum, 7% for the left putamen, 5% for the right thalamus, 4% and for the left globus pallidus and 3% for total GM.
Table 2.
Brain volumes of a-priori regions of interest in people with alcohol dependence versus controls
| Control | Alcohol-dependent | Group (Alcohol-dependent vs Control) |
Gender (Men vs Women) |
Group-by-Gender | Site§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 227) |
Women (n = 99) |
Men (n = 432) |
Women (n = 228) |
β (95% CI) | p | d | β (95% CI) | p | d | β (95% CI) | p | Var | ||
| Brain region | ||||||||||||||
| OFC medial |
L | 5246.85 (751.08) | 4701.97 (680.31) | 5110.90 (653.54) | 4604.22 (658.99) | 22.19 (-147.48, 191.86) | 0.798 | 0.02 | 147.21 (3.76, 290.65) | 0.044* | 0.03 | -123.71 (-286.34, 38.91) | 0.136 | 0.23 |
| R | 5322.67 (743.46) | 5006.09 (644.98) | 5068.84 (645.96) | 4680.69 (624.30) | -149.38 (-317.89, 19.13) | 0.082 | 0.07 | 25.14 (-17.70, 167.98) | 0.730 | 0.01 | -1.83 (-160.21, 163.88) | 0.982 | 0.19 | |
| lateral | L | 7982.47 (992.69) | 7303.38 (862.22) | 7488.18 (883.58) | 7035.66 (853.90) | -119.43 (-330.60, 91.73) | 0.268 | 0.09 | 166.98 (-11.28, 345.23) | 0.066 | 0.01 | -277.37 (-479.41, -75.33) | 0.007* | 0.26 |
| R | 7640.83 (1074.53) | 6998.46 (989.36) | 6972.16 (857.41) | 6510.53 (819.16) | -153.02 (-378.43, 72.38) | 0.183 | 0.08 | 89.15 (-101.09, 279.38) | 0.358 | 0.01 | -180.13 (-395.74, 35.48) | 0.102 | 0.27 | |
| Hippocampus | L | 4349.54 (517.54) | 4053.65 (450.20) | 4160.07 (511.82) | 3981.50 (447.46) | -199.39(-326.64,-72.14) | 0.002** | 0.14 | 71.66 (-36.33, 179.65) | 0.193 | 0.01 | -89.92 (-212.31, 32.47) | 0.150 | 0.23 |
| R | 4453.53 (544.65) | 4162.94 (446.34) | 4278.94 (499.62) | 4042.30 (427.36) | -212.91(-340.10,-85.72) | 0.001** | 0.12 | 55.69 (-52.41, 163.78) | 0.313 | 0.02 | -44.69 (-167.25, 77.86) | 0.475 | 0.21 | |
| Amygdala | L | 1737.01 (272.17) | 1547.13 (261.50) | 1595.45 (220.39) | 1490.99 (188.20) | -14.83 (-77.10, 47.43) | 0.641 | 0.06 | 116.40(63.80,169.00) | <0.001** | 0.05 | -86.45 (146.00, -26.89) | 0.004* | 0.37 |
| R | 1833.44 (296.25) | 1600.01 (230.75) | 1609.28 (216.01) | 1492.73 (210.90) | 7.59 (-55.74, 70.92) | 0.814 | 0.05 | 129.71(76.24,183.17) | <0.001** | 0.04 | -113.98(-174.51,-53.45) | <0.001** | 0.40 | |
| Nucleus Accumbens | L | 690.10 (200.32) | 647.63 (169.96) | 532.40 (127.78) | 480.09 (125.29) | -15.84 (-49.02, 17.35) | 0.350 | 0.04 | 40.03 (13.00, 67.97) | 0.005 | 0.02 | -31.84 (-63.45, -0.24) | 0.048* | 0.63 |
| R | 684.93 (161.57) | 625.70 (147.35) | 609.55 (127.06) | 567.78 (118.14) | -23.36 (-54.25, 7.53) | 0.138 | 0.02 | 42.82(16.81, 68.83) | 0.001** | 0.02 | -34.81 (-64.24, -5.37) | 0.020* | 0.56 | |
| Caudate | L | 3929.38 (478.33) | 3631.15 (443.44) | 3817.82 (504.10) | 3505.09 (471.41) | -14.69 (-139.84, 110.47) | 0.818 | 0.02 | 17.72 (-88.44, 123.87) | 0.744 | 0.00 | -26.54 (-146.84, 93.76) | 0.665 | 0.24 |
| R | 4094.54 (542.91) | 3735.19 (501.98) | 3887.83 (519.70) | 3543.45 (516.76) | -19.93 (-153.74, 113.88) | 0.770 | 0.02 | 57.13 (-55.80, 170.05) | 0.321 | 0.01 | -50.39 (-178.23, 77.45) | 0.440 | 0.41 | |
| Putamen | L | 6315.64 (933.10) | 5851.62 (864.67) | 5729.71 (784.46) | 5250.93 (685.07) | -363.18(-567.16,-159.19) | <0.001** | 0.08 | 173.54 (1.40, 345.67) | 0.048 | 0.04 | -18.95 (213.81, 175.91) | 0.849 | 0.41 |
| R | 6037.24 (874.82) | 5527.51 (779.47) | 5483.38 (723.77) | 5010.99 (681.49) | -191.89 (-377.17, -6.61) | 0.042* | 0.06 | 226.13 (69.83, 382.43) | 0.005 | 0.05 | -70.92 (-247.84, 106.01) | 0.432 | 0.43 | |
| Globus Pallidus | L | 1683.30 (290.45) | 1537.25 (304.76) | 1704.88 (296.07) | 1500.54 (268.04) | -105.59(177.30, -33.88) | 0.004** | 0.04 | 36.39 (-24.15, 96.93) | 0.239 | 0.06 | 72.38 (3.84, 140.93) | 0.038* | 0.39 |
| R | 1682.90 (228.64) | 1557.33 (210.71) | 1543.72 (219.07) | 1379.71 (193.51) | -58.54 (-116.95, -0.12) | 0.050* | 0.06 | 59.53 (10.16, 108.90) | 0.018* | 0.05 | -15.17 (-71.08, 40.74) | 0.595 | 0.35 | |
| Thalamus | L | 8324.35 (1059.85) | 7688.46 (950.51) | 8297.12 (885.16) | 7715.98 (870.11) | -138.63 (-352.99, 75.72) | 0.205 | 0.06 | 190.77 (9.90, 371.64) | 0.039* | 0.01 | -219.93 (-424.68, -15.18) | 0.035* | 0.42 |
| R | 7704.08 (749.21) | 7085.12 (623.99) | 7690.73 (831.02) | 7038.23 (775.77) | -349.85(-535.57-164.14) | <0.001** | 0.11 | 161.19 (4.06, 318.32) | 0.044 | 0.05 | -13.75 (-191.72, 164.22) | 0.880 | 0.31 | |
| Corpus Callosum | 3377.26 (598.73) | 3214.01 (478.03) | 3254.95 (554.98) | 3172.03 (534.12) | -259.58(-411.98,-107.18) | 0.001** | 0.14 | -75.07 (-204.23, 54.08) | 0.255 | 0.06 | -47.64 (-194.14, 98.87) | 0.524 | 0.19 | |
| Cerebellum | ||||||||||||||
| GM | L | 49748.97 (12223.38) | 45350.75 (10674.25) | 49700.22 (10184.27) | 47046.38 (5347.57) | -4860.64 (-6581.92,-3139.35) | <0.001** | 0.07 | -3029.93 (-4474.17,-1585.69) | <0.001** | 0.03 | 2584.59 (949.00, 4219.50) | 0.002* | 0.53 |
| R | 50681.09 (12255.99) | 46880.50 (10597.60) | 51297.01 (10320.89) | 48469.18 (5571.48) | -4881.83 (-6633.11,-3130.55) | <0.001** | 0.06 | -3314.29 (-4783.61,-1844.96) | <0.001** | 0.03 | 2968.63(1305.35, 4631.92) | <0.001** | 0.53 | |
| WM | L | 14762.27 (3327.16) | 14037.49 (3261.05) | 15479.50 (3233.58) | 15526.19 (3301.02) | -542.28 (-1320.94, 236.38) | 0.172 | 0.03 | -1326.91 (-1981.97,-671.85) | <0.001** | 0.08 | 235.24 (-506.70, 977.18) | 0.534 | 0.37 |
| R | 14917.15 (3477.19) | 14031.98 (3071.54) | 15777.43 (3419.94) | 15845.76 (2880.35) | -675.26 (-1431.84, 81.32) | 0.080 | 0.04 | -1178.86 (-1814.96,-542.76) | <0.001** | 0.07 | 178.05 (-542.33, 898.42) | 0.628 | 0.40 | |
| Total GM | 645538.63 (69823.91) | 587412.59 (63758.74) | 627384.45 (65861.02) | 580684.82 (58709.93) | -14172.81 (-23770.19,-4575.44) | 0.004** | 0.11 | 6160.45 (-1907.42, 14228.32) | 0.135 | 0.01 | -10852.99 (-19989.47, -1716.50) | 0.020* | 0.40 | |
| Total WM | 509147.64 (53259.68) | 445501.06 (44096.16) | 489192.50 (57031.89) | 436520.55 (48983.21) | -22379.95 (-33688.79,-1071.10) | <0.001** | 0.09 | 8726.59 (-755.27, 18208.44) | 0.071 | 0.00 | -11894.12 (-22626.19, -1162.07) | 0.030* | 0.59 | |
| CSF | 984.87 (278.31) | 947.42 (243.35) |
1131.61 (240.76) | 991.96 (210.11) | 62.21 (-3.46, 127.88) | 0.063 | 0.10 | -13.38 (-69.17, 42.41) | 0.638 | 0.01 | 28.32 (-35.00, 91.65) | 0.381 | 0.17 | |
| ICV (10^6) | 1.44 (0.25) | 1.24 (0.23) | 1.62 (0.21) | 1.42 (0.17) | -0.03 (-0.075, 0.013) | 0.169 | 0.00 | 0.19 (0.15, 0.22) | <0.001** | 0.17 | 0.043 (0.00, 0.09) | 0.049* | 0.58 | |
Note: β = beta, CI = confidence interval, CSF = Cerebrospinal fluid, GM = grey matter, L = left, OFC = orbitofrontal cortex, R = right, Var = variance, WM = white matter. Values for brain volumes are mean (SD). Bolded values are β (95% CI), p and d of the significant effects (FDR-corrected). § Site-level variation estimated as an intraclass correlation (ICC). * p(uncorrected) < 0.05, ** p(FDR) < 0.05.
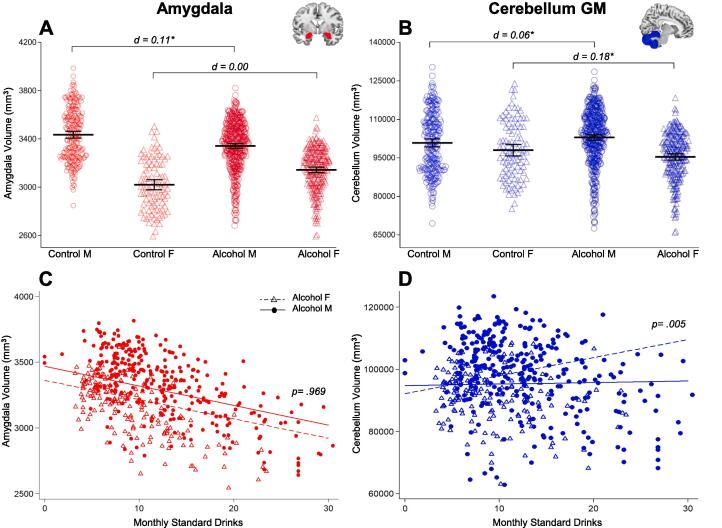
There were group-by-gender interactions in the cerebellum GM and amygdala volumes (Fig. 1, panels A and B). As detailed in Table 3 pairwise comparisons showed that alcohol-dependent men had 6% smaller right amygdala volume than control men, while this effect was unclear among women. They also showed that right cerebellum GM volume was smaller in both alcohol-dependent men and women (4% and 9%, respectively) compared to their control counterparts, but the effect was more marked among women. Comparable interactions emerged in the left hemisphere of these ROIs, but at lower significance level.
Fig. 1.
Overview of significant group-by-gender effects. Plots of the A Amygdala and B Cerebellum GM volumes comprising individual data stratified by group (Control, Alcohol) and gender (M = males; F = females). The group average of the estimated marginal means predicted by the models is indicated by the solid horizontal black line, with a vertical bar representing 95% confidence interval. The bottom panel shows regression plots for the volume of the C Amygdala and D Cerebellum GM by monthly standard drinks (square root-transformed) in alcohol dependent women (Alcohol F) and men (Alcohol M), adjusted for intracranial volume, age and education years. Only the gender-by-standard drinks interaction in the Cerebellum GM was significant. Volumes have been averaged across hemispheres.
Table 3.
Results of the pairwise comparisons of significant group-by-gender interactions in people with alcohol dependence versus controls
| Brain regions | β (95% CI) | p | d | β (95% CI) | p | d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | R | HC men | vs | HC women | 129.71 (76.24, 183.17 | <0.001*** | 0.16 | L | 116.40 (63.79, 169.00) | <0.001*** | 0.17 |
| ALC women | vs | HC women | 7.59 (-55.74, 70.92) | 0.814 | 0.01 | -14.83 (-77.10, 47.43) | 0.641 | 0.02 | |||
| ALC men | vs | HC women | 23.32 (-39.22, 85.86) | 0.465 | 0.02 | 15.11 (-46.38, 76.61) | 0.630 | 0.02 | |||
| ALC women | vs | HC men | -122.12 (-180.16,-64.07) | <0.001*** | 0.15 | -131.23 (-188.27, -74.19) | <0.001*** | 0.17 | |||
| ALC men | vs | HC men | -106.39 (-156.77,-56.00) | <0.001*** | 0.13 | -101.28 (-150.80, -51.77) | <0.001*** | 0.10 | |||
| ALC men | vs | ALC women | 15.73 (-23.45, 54.91) | 0.431 | 0.01 | 29.95 (-8.5915, 68.49) | 0.128 | 0.03 | |||
| Cerebellum GM | R | HC men | vs | HC women | -3314.29 (-4783.61, -1844.96) | <0.001*** | 0.13 | L | -3029.93 (-4474.17, -1585.69) | <0.001*** | 0.12 |
| ALC women | vs | HC women | -4881.83 (-6633.11, -3130.55) | <0.001*** | 0.18 | -4860.64 (-6581.92, -3139.35) | <0.001*** | 0.18 | |||
| ALC men | vs | HC women | -5227.49 (-6951.98, -3503) | <0.001*** | 0.14 | -5305.98 (-7000.94, -3611.02) | <0.001*** | 0.15 | |||
| ALC women | vs | HC men | -1567.55 (-3180.63, 45.54) | 0.57 | 0.05 | -1830.71 (-3416.08, -245.33) | 0.024* | 0.06 | |||
| ALC men | vs | HC men | -1913.20 (-3309.66, -516.75) | 0.007** | 0.05 | -2276.05 (-3648.52, -903.58) | 0.001** | 0.06 | |||
| ALC men | vs | ALC women | -345.65 (-1425.67, 734.36) | 0.530 | 0.01 | -445.34 (-1506.90, 616.21) | 0.411 | 0.01 |
Note: ALC = alcohol dependent, β = beta, CI = confidence interval, GM = grey matter, HC = controls, L = left, R = right. Only the right amygdala and the right cerebellum GM demonstrated significant group-by-gender effects after FDR correction however, we present both the right and the left hemispheres of these brain regions as the coefficients of their interaction effect are very similar (see Table 2). * p < 0.05; ** p < 0.01; *** p < 0.001.
Group-by-gender effects within the amygdala and the cerebellum GM were confirmed in the sensitivity subsample where alcohol-dependent men and women consumed the same amount of standard drinks (Table 4, Table 5). Interestingly, in this subsample the effect in the left cerebellum GM remained significant after FDR correction.
Table 4.
Brain volumes of people with alcohol dependence versus controls from the sensitivity subsample where alcohol-dependent men and women were matched by standard drinks
| Control | Alcohol-dependent | Group (Alcohol-dependent vs Control) |
Gender (Men vs Women) |
Group-by-Gender | Site§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men n = 227) |
Women (n = 99) |
Men (n = 298) |
Women (n = 228) |
β (95% CI) | p | d | β (95% CI) | p | d | β (95% CI) | p | Var | ||
| Brain region | ||||||||||||||
| OFC medial |
L | 5246.85 (751.08) | 4701.97 (680.31) | 5149.45 (658.21) | 4604.22 (658.99) | 75.14 (-108.08, 258.39) | 0.422 | 0.00 | 171.14 (25.16, 316.96) | 0.022* | 0.04 | -129.14 (-296.20, 37.91) | 0.130 | 0.243 |
| R | 5322.67 (743.46) | 5006.09 (644.98) | 5130.29 (662.65) | 4680.69 (624.30) | -88.73 (-270.60, 93.14) | 0.339 | 0.04 | 49.21 (-96.17, 194.59) | 0.507 | 0.03 | 9.89 (-156.69, 176.46) | 0.907 | 0.20 | |
| lateral | L | 7982.47 (992.69) | 7303.38 (862.22) | 7578.05 (872.0047) | 7035.66 (853.90) | -140.17 (-365.49, 85.15) | 0.223 | 0.11 | 186.48 (7.04, 365.91) | 0.042* | 0.01 | -273.62 (-479.08, -68.16 | 0.009* | 0.24 |
| R | 7640.83 (1074.53) | 6998.46 (989.36) | 7022.96 (885.40) | 6510.53 (819.16) | -209.29 (-451.53, 32.98) | 0.090 | 0.10 | 93.61 (-99.28, 286.52) | 0.342 | 0.01 | -189.36 (-410.23, 31.51) | 0.093 | 0.24 | |
| Hippocampus | L | 4349.54 (517.54) | 4053.65 (450.20) | 4208.25 (479.66) | 3981.50 (447.46) | -164.97 (-296.66, -33.29) | 0.014* | 0.00 | 53.67 (-51.61, 158.95) | 0.318 | 0.00 | -85.21 (-205.68, 35.22) | 0.165 | 0.27 |
| R | 4453.53 (544.65) | 4162.94 (446.34) | 4331.36 (486.84) | 4042.30 (427.36) | -169.44 (-304.58, -34.30) | 0.014* | 0.11 | 45.33 (-63.03, 153.69) |
0.412 | 0.02 | -27.84 (-151.88, 96.19) |
0.660 | 0.24 | |
| Amygdala | L | 1737.01 (272.17) | 1547.13 (261.50) | 1608.40 (212.72) | 1490.99 (188.20) | -9.67 (-76.17, 56.83) | 0.776 | 0.07 |
120.40 (67.49,173.31) |
<0.001** | 0.07 | -83.65 (-144.14, -23.16) | 0.007* | 0.36 |
| R | 1833.44 (296.25) | 1600.01 (230.75) | 1625.36 (215.71) | 1492.73 (210.90) | 6.67 (-62.22, 75.55) | 0.850 | 0.00 |
127.47 (72.72,182.21) |
<0.001** | 0.05 | -104.11(-166.68,-41.53) | 0.001** | 0.41 | |
| Nucleus accumbens | L | 690.10 (200.32) | 647.63 (169.96) | 527.41 (119.16) | 480.09 (125.29) | -4.70 (-40.31, 30.92) | 0.796 | 0.03 |
41.28 (13.12,69.44) |
0.004** | 0.02 | -32.36 (-64.51, -0.20) | 0.049* | 0.64 |
| R | 684.93 (161.57) | 625.70 (147.35) | 613.37 (121.91) | 567.78 (118.14) | -18.45 (-50.68, 13.77) | 0.262 | 0.05 | 44.98(19.48,70.48) | 0.001** | 0.03 | -34.37 (-63.49, -5.25) | 0.021* | 0.56 | |
| Caudate | L | 3929.38 (478.33) | 3631.15 (443.44) | 3849.49 (493.42) | 3505.09 (471.41) | -21.95 (-153.71, 109.81) | 0.744 | 0.02 | 22.56 (-83.10, 128.21) | 0.676 | 0.01 | -16.20 (-137.15, 104.74) | 0.793 | 0.23 |
| R | 4094.54 (542.91) | 3735.19 (501.98) | 3901.28 (515.46) | 3543.45 (516.76) | -18.55 (-162.43, 125.34) | 0.801 | 0.02 | 64.75 (-49.59, 179.10) | 0.267 | 0.01 | -51.56 (-182.26, 79.12) | 0.439 | 0.40 | |
| Putamen | L | 6315.64 (933.10) | 5851.62 (864.67) | 5757.26 (739.00) | 5250.93 (685.07) | -319.53 (-531.52, -107.55) | <0.003* | 0.10 | 162.92 (-5.31, 331.14) | 0.058 | 0.04 | -32.32 (-224.54, 159.91) | 0.742 | 0.44 |
| R | 6037.24 (874.82) | 5527.51 (779.47) | 5497.12 (692.21) | 5010.99 (681.49) | -179.80 (-374.44, -14.83) | 0.070 | 0.06 | 222.43 (-265.78, 87.14) | 0.005* | 0.05 | -89.32 (-265.78, 87.14) | 0.321 | 0.45 | |
| Globus Pallidus | L | 1683.30 (290.45) | 1537.25 (304.76) | 1740.77 (286.21) | 1500.54 (268.04) | -93.16 (-168.69, 17.63) | 0.016* | 0.03 | 38.36 (-21.65, 98.38) |
0.210 | 0.07 | 84.16 (15.57, 152.75) | 0.016* | 0.40 |
| R | 1682.90 (228.64) | 1557.33 (210.71) | 1554.24 (206.50) | 1379.71 (193.51) | -48.54 (-110.00, -12.90) | 0.122 | 0.05 | 62.48 (13.55, 111.40 | 0.012* | 0.06 | -1.96 (-57.90, 53.97) | 0.945 | 0.35 | |
| Thalamus | L | 8324.35 (1059.85) | 7688.46 (950.51) | 8368.97 (840.83) | 7715.98 (870.11) | -63.67 (-291.62, 164.27) | 0.584 | 0.05 | 214.41 (33.45, 395.36) | 0.020* | 0.00 | -226.66 (-433.44, -19.86 | 0.032* | 0.43 |
| R | 7704.08 (749.21) | 7085.12 (623.99) | 7776.97 (777.07) | 7038.23 (775.77) | -290.04 (-481.24, -98.84) | <0.003* | 0.10 | 176.81 (24.42, 329.20) | 0.023* | 0.06 | -11.23 (-185.49, 163.03) | 0.899 | 0.33 | |
| Corpus Callosum | 3377.26 (598.73) | 3214.01 (478.03) | 3321.33 (543.45) | 3172.03 (534.12) | -230.75 (-392.35, -69.14) | 0.005* | 0.12 | -75.05 (-204.42, 54.32) | 0.256 | 0.05 | -12.13 (-160.39, 136.812) | 0.873 | 0.19 | |
| Cerebellum | ||||||||||||||
| GM | L | 49748.97 (12223.38) | 45350.75 (10674.25) | 51033.07 (9298.11) | 47046.38 (5347.57) | -4143.84(-5989.08,-2298.61) | <0.001** | 0.07 | -2811.73(-4263.04,-1360.42) | <0.001** | 0.04 | 2719.10(1060.74,4377.46) | 0.001** | 0.53 |
| R | 50681.09 (12255.99) | 46880.50 (10597.60) | 52635.32 (9567.45) | 48469.18 (5571.48) |
-4247.65 (-6138.42,-2356.88) |
<0.001** | 0.08 | -3133.30(4620.48, -1646.12) | <0.001** | 3085.40(1386.05,4784.76) | 0.001** | 0.53 | ||
| WM | L | 14762.27 (3327.16) | 14037.49 (3261.05) | 15761.19 (3147.76) | 15526.19 (3301.02) | -488.42 (-1340.27, 363.43) | 0.261 | 0.02 | -1245.57(-1919.25,-571.89) | <0.001** | 0.08 | 304.99 (-465.49, 1075.47) | 0.438 | 0.35 |
| R | 14917.15 (3477.19) | 14031.98 (3071.54) | 16035.98 (3326.17) | 15845.76 (2880.35) | -684.87 (-1497.42, 127.68) | 0.099 | 0.00 | -1157.45(-1799.12,-515.78) | <0.001** | 0.08 | 183.46 (-550.23, 917.15) | 0.624 | 0.39 | |
| Total GM | 645538.63 (69823.91) | 587412.59 (63758.74) | 635692.40 (64282.99) | 580684.82 (58709.93) | -9923.09 (-20151.40, 16304.97) | 0.057 | 0.09 | 8210.167 (137.94, 16282.41) | 0.046* | 0.00 | -10866.91 (-20095.91, -1637.93) | 0.021* | 0.41 | |
| Total WM | 509147.64 (53259.68) | 445501.06 (44096.16) | 491191.73 (56801.08) | 436520.55 (48983.21) | -19547.76 (-31944.64, -7150.88) | 0.002* | 0.09 | 9775.49 (33.20, 19517.78) | 0.049* | 0.01 | -10222.08 (-21352.75, 908.59) | 0.072 | 0.57 | |
| CSF | 984.87 (278.31) | 947.42 (243.35) | 1114.05 (232.64) | 991.96 (210.11) | 30.12 (-39.11, 99.34) | 0.394 | 0.07 | -18.28 (-74.21, 37.65) | 0.522 | 0.00 | 30.36 (-33.84, 94.56) | 0.354 | 0.14 | |
| ICV (10^6) | 1.44 (0.25) | 1.24 (0.23) | 1.63 (0.20) | 1.42 (0.17) | -0.00 (-0.04, 0.05) | 0.904 | 0.02 | 0.19 (0.16,0.23) | <0.001** | 0.18 | 0.04 (0.00, 0.08) | 0.082 | 0.58 | |
Note: β = beta; CI = confidence interval; CSF = Cerebrospinal fluid; GM = grey matter; L = left; OFC = orbitofrontal cortex; R = right; Var = variance; WM = white matter. Values for brain volumes are mean (SD). Bolded values are β (95% CI), p and d of the significant effects (FDR-corrected). § Site-level variation estimated as an intraclass correlation (ICC). * p(uncorrected) < 0.05, ** p(FDR) < 0.05.
Table 5.
Results of the pairwise comparisons of significant group-by-gender interactions in people with alcohol dependence versus controls from the sensitivity subsample where alcohol-dependent men and women were matched by standard drinks
| Brain regions | β (95% CI) | p | d | β (95% CI) | p | d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | R | HC men | vs | HC women | 127.47 (72.72, 182.21) | <0.001** | 0.17 | L | 120.40 (67.49, 173.31) | <0.001** | 0.14 |
| ALC women | vs | HC women | 6.67 (-62.22, 75.55) | 0.850 | 0.01 | -9.67 (-76.170, 56.83) | 0.776 | 0.01 | |||
| ALC men | vs | HC women | 30.02 (-40.26, 100.31) | 0.402 | 0.03 | 27.08 (-40.78, 94.95) | 0.434 | 0.04 | |||
| ALC women | vs | HC men | -120.80 (-184.49, -57.11) | <0.001** | 0.14 | -130.07 (-191.51, -68.63) | <0.001** | 0.17 | |||
| ALC men | vs | HC men | -97.44 (-155.55, -39.34) | 0.001* | 0.11 | -93.31 (-149.39, -37.25) | 0.001** | 0.11 | |||
| ALC men | vs | ALC women | 23.36 (-18.99, 65.70) | 0.280 | 0.03 | 36.75 (-4.16, 77.67) | 0.078 | 0.04 | |||
| Cerebellum GM | R | HC men | vs | HC women | -3133.30 (-4620.476, -1646.119) | <0.001** | 0.12 | L | -2811.73 (-4263.04, -1360.42) | <0.001** | 0.11 |
| ALC women | vs | HC women | -4247.65 (-6138.42, -2356.88) | <0.001** | 0.16 | -4143.84 (-5989.08, -2298.61) | <0.001** | 0.16 | |||
| ALC men | vs | HC women | -4295.55 (-6217.30, -2373.79) | <0.001** | 0.14 | -4236.47 (-6111.93, -2361.01) | <0.001** | 0.14 | |||
| ALC women | vs | HC men | -1114.35 (-2873.91, 645.20) | 0.215 | 0.04 | -1332.12 (-3049.35, 385.11) | 0.128 | 0.04 | |||
| ALC men | vs | HC men | -1162.25 (-2762.20, 437.70) | 0.155 | 0.04 | -1424.74 (-2986.19, 136.71) | 0.074 | 0.05 | |||
| ALC men | vs | ALC women | -47.894 (-1201.34, 1105.55) | 0.935 | 0.00 | -92.62 (-1218.26, 1033.01) | 0.872 | 0.00 |
Note: ALC = alcohol dependent, β = beta, CI = confidence interval, GM = grey matter, HC = controls, L = left, R = right. Only the right amygdala and the left and right cerebellum GM demonstrated significant group-by-gender differences after FDR correction however, both the right and the left amygdala are presented as the coefficients of the interaction effect were very similar across the two hemispheres (see Table 4). * p < 0.01; ** p < 0.001.
The effect sizes of all group and group-by-gender differences were small and ranged from d = 0.03 to d = 0.18.
Results presented in Table 2, Table 3, Table 4, Table 5 show that, regardless of significance level, the coefficient of group-by-gender interactions for the left and right amygdala were consistent, as were those for the left and right cerebellum GM. As such, we took into consideration both the left and the right volume of these two ROIs when exploring the association between gender, monthly standard drinks and brain volume within the alcohol dependent group (Table 6).
Table 6.
Associations between gender, monthly standard drinks and brain volumes that demonstrated significant group-by-gender interactions in people with alcohol dependence
| Mixed-effect model adjusted for age, education and ICV | Alcohol-dependent (n = 484, 36% women) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R Amygdala | L Amygdala | R Cerebellum GM | L cerebellum GM | ||||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Model A | Gendera | 47.78 (0.28, 95.28) | 0.049* | 58.19 (9.92, 106.45) | 0.018* | -1255.93 (-2455.24, -56.62) | 0.040 | -1453.91 (-2648.76, -259.06) | 0.017* |
| StDr/mo | -7.66(-11.79,-3.52) | <0.001** | -7.139(-11.33,-2.95) | 0.001** | 89.84 (-14.52, 194.20) | 0.092 | 82.34 (-21.63, 186.32) | 0.121 | |
| Model B | Gender-by-StDr/mo | 0.84 (-6.78, 8.47) | 0.829 | -1.06 (-8.88, 6.75) | 0.790 | -267.73(-458.04,-77.43) | 0.006** | -262.53(-452.18,-72.89) | 0.007** |
| Model C (men only) |
StDr/mo | -8.92(-13.67,-4.17) | <0.001** | -8.66(-13.61,-3.71) | 0.001** | 70.80 (-53.69, 195.29) | 0.265 | 69.62 (-55.88, 195.12) | 0.277 |
| Model D (women only) |
StDr/mo | -7.28 (-15.40, 0.85) | 0.079 | -8.01 (-15.63, -0.39) | 0.039 | 148.6 (-36.38, 333.58) | 0.115 | 20.05 (-142.87, 182.97) | 0.809 |
Note: three separate models were run to explore the association between ROIs volume and gender, standard drinks (model A), gender-by-standard drinks (model B) or standard drinks separately in men and women (model C, D). Only the right amygdala and the right cerebellum GM demonstrated significant group-by-gender interactions after FDR correction however, both the right and the left hemisphere of these regions are presented as the coefficient of their interaction effects was very similar (see table 2). β = beta, CI = confidence interval, GM = grey matter, L = left, R = right, StDr/mo = monthly standard drinks. a Me(FDR-corrected).
Bolded values are β (95% CI), p and d of the significant effects (FDR-corrected). * p(uncorrected) < 0.05; ** p(FDR) < 0.05.
A greater amount of standard drinks predicted smaller amygdala volume (Fig. 1, panel C). However, this association emerged in men only when alcohol-dependent men and women were considered separately. Also, a sex-by-standards drinks interaction emerged in the cerebellum GM. Specifically, more monthly standard drinks were associated with larger cerebellum GM volume, and this association was more marked in women compared to men (Fig. 1, panel D).
The negative association between standard drinks and amygdala volume was replicated in the subsample of alcohol-dependent men and women that consumed the same amount of standard drinks. For the cerebellum, a positive association of the standard drinks and cerebellum GM was observed, but the significance of the sex-by-standard drinks interaction was not replicated in this sensitivity subsample (Table 7).
Table 7.
Associations between gender, monthly standard drinks and brain volumes that demonstrated significant group-by-gender interactions in alcohol-dependent men and women matched by standard drinks (sensitivity subsample)
| Mixed-effect model adjusted for age, education and ICV | Alcohol-dependent (n = 350, 50% women) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R Amygdala | L Amygdala | R Cerebellum GM | L cerebellum GM | ||||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Model A | Gender | 47.19 (-6.46, 100.83) | 0.085 | 60.37 (7.92, 112.81) | 0.024* | -844.52 (-2087.74, 398.71) | 0.183 | -916.08 (-2133.65, 301.49) | 0.140 |
| StDr/mo | -10.37(-16.07,-4.67) | <0.001** | -9.21(-14.84,-3.58) | 0.001** | 194.65(63.46,325.84) | 0.004** | 198.81(70.35,327.28) | 0.002** | |
| Model B | Gender -by-StDr/mo | -5.61 (-15.31, 4.07) | 0.256 | -6.11 (-15.76, 3.55) | 0.215 | -216.76 (-438.94, 5.42) | 0.056 | -170.76 (-388.71, 47.18) | 0.125 |
| Model C (men only) |
StDr/mo | -14.30(-22.25,-6.35) | <0.001** | -12.81(-20.94,-4.67) | 0.002** | 31.99 (-134.11, 198.79) | 0.707 | 179.08 (-5.62, 363.78) | 0.057 |
| Model D (women only) |
StDr/mo | -7.28 (-15.40, 0.85) | 0.079 | -8.01 (-15.63, -0.39) | 0.039* | 148.61 (-36.38, 333.58) | 0.115 | 20.05 (-142.87, 182.97) | 0.809 |
Note: Three separate models were run to explore the association between ROIs volume and gender or standard drinks (model A); gender-by-standard drinks (model B) or standard drinks separately in men and women (model C, D). Only the right amygdala and the right and left cerebellum GM demonstrated significant group-by-gender effects after FDR correction however, both the right and the left amygdala are presented as the coefficient of their interaction effects was very similar (see Table 2). β = beta, CI = confidence interval, GM = grey matter, L = left, R = right, StDr/mo = monthly standard drinks. a Men = 1, Women = 0. Bolded values are β (95% CI), p and d of the significant effects (FDR-corrected). * p(uncorrected) < 0.05, ** p(FDR) < 0.05.
4. Discussion
We examined the impact of alcohol dependence, alcohol dosage and gender on a set of a priori ROIs in a composed sample of 660 alcohol-dependent individuals from ENIGMA addiction workgroup studies. First, we confirmed prior evidence of widespread smaller volumes in people with alcohol dependence versus controls in the hippocampus (Agartz et al., 1999, Beresford et al., 2006), striatum (Boutte et al., 2012, Chanraud et al., 2007, Sullivan et al., 2005), corpus callosum (Pfefferbaum et al., 1996, Ruiz et al., 2013), cerebellum GM (Boutte et al., 2012, Chanraud et al., 2007, Sullivan et al., 2005) and global brain estimates (total GM and WM) (Hommer et al., 2001, Pfefferbaum et al., 2001). Second, we found group-by-gender interactions in selected brain regions i.e., the cerebellum GM and the amygdala. Third, we showed that a higher number of monthly standard drinks predicted smaller amygdala volume (Mackey et al., 2019) and larger cerebellum GM volume of people with alcohol dependence.
Our findings suggest that alcohol dependence is associated with gross alteration of the mesolimbic pathway that encompasses the midbrain, striatum and basal ganglia and mediates learning, stress and reward processing (Koob and Volkow, 2010). However, to date, it remains to be clarified whether such differences are the results of neuroadaptations associated with the development of addiction (Koob, 2014) or due to neurotoxicity related to chronic exposure to ethanol (Volkow et al., 2017).
Yet, shrinkage of global estimates (i.e. total GM and WM volumes) resemble those observed in ageing, suggesting that alcohol may accelerate brain ageing (Pfefferbaum et al., 2018, Sullivan et al., 2018). Future longitudinal studies are warranted to test the ageing hypothesis.
To our knowledge, we are the first to report group-by-gender effects in the cerebellum GM of people with alcohol dependence. Specifically, smaller volumes were found in both men and women with alcohol dependence (compared to their control counterparts) but these were more marked in women. Our findings are in line with those from previous studies where gender differences were not examined (Boutte et al., 2012, Chanraud et al., 2007) and corroborate neuroscientific theories of addiction that have recently reconsidered the cerebellum as a key modulator between motor and reward, motivation and cognitive control (Miquel et al., 2016, Moulton et al., 2014).
More pronounced cerebellar GM reductions in alcohol-dependent women than men is consistent with previous evidence of women being more vulnerable to the neurotoxic effect of alcohol (Agartz et al., 1999, Hommer et al., 1996, Hommer et al., 2001, Mann et al., 2005). The results of the sensitivity analysis corroborate this hypothesis. Specifically, when comparing alcohol dependent men and women that consumed the same amount of standard drinks with their same-gender control counterparts, the effect in alcohol-dependent females was replicated whereas the effect in alcohol-dependent men become unclear.
However, our data does not support that cerebellar GM reductions were driven by alcohol dosage. Indeed, smaller cerebellum GM volume was predicted by lower (not higher) numbers of monthly standard drinks. Smaller volumes in our sample might be driven by chronic alcohol exposure, such as years of heavy drinking and age at first drinking, as demonstrated in previous work (Chanraud et al., 2007, Sawyer et al., 2016), which we were not able to consistently obtain in this multi-site study.
Preclinical and clinical studies show that γ-aminobutyric acid (GABA) dependent neurotransmission is a potential mechanism for ethanol-induced cerebellar toxicity (Ravindran et al., 2007, Rossi and Richardson, 2018). Interestingly, gender differences emerged in the GABAergic signalling of the cerebellum following prolonged alcohol exposure (Devaud et al., 1998, Lingford-Hughes et al., 2000). However, results are controversial suggesting greater ethanol-related sensitivity either in men or women.
Within the amygdala, we found that men with alcohol dependence had smaller volumes than control men, an effect that was not clear among women. Our results are in line with those of a previous study showing smaller total reward network volume (including amygdala) in alcohol-dependent men versus control men (Sawyer et al., 2017). However, our findings contrast with other works showing group (but not group-by-gender) differences in amygdala volume between people with alcohol dependence and controls (Mackey et al., 2019, Makris et al., 2008, Wrase et al., 2008). Possibly, this is due to the fact that previous work had only male samples (Makris et al., 2008) and those including both men and women had small sample sizes (i.e., n ≤ 43 participants with alcohol dependence) (Fein et al., 2006) or used gender as a covariate rather than testing group-by-gender interactions in their analysis (Mackey et al., 2019, Wrase et al., 2008). Smaller amygdala volumes have been associated with behaviours that characterize alcohol dependence, such as increased alcohol seeking (Chaudhri et al., 2013), greater alcohol craving and relapse risk (Wrase et al., 2008). As our study design was cross-sectional, one could speculate that either greater alcohol consumption implicates excessive drinking as contributing to the smaller size of the amygdala, or that gender differences in the volume of the amygdala could be a vulnerability factor for men drinking about a double amount alcohol dosage than women (World Health Organization, 2018).
We also showed that amygdala volumes were significantly smaller in alcohol-dependent people who reported the highest numbers of monthly standard drinks. Thus, ethanol exposure may drive amygdala volume reduction (Mackey et al., 2019), but this needs to be elucidated by future longitudinal studies examining the neural correlates of the gender-specific effect of alcohol consumption over time. The robustness of this finding was demonstrated by the fact that it was replicated in both the original sample and in the alcohol-dependent subsample where men and women consumed an equal amount of standard drinks. Of note, when alcohol-dependent men and women were considered separately, the association between higher numbers of standard drinks and smaller amygdala volume emerged only in men (both in the original sample and the sensitivity subsample). This suggests that men may be more sensitive than women to amygdala neurotoxicity induced by prolonged alcohol exposure, as previously shown in other brain regions (e.g., corpus callosum, cerebellum, parietal lobe) (Fein et al., 2009, Ruiz et al., 2013, Sawyer et al., 2016). Moreover, this is also in line with preclinical studies suggesting a greater alcohol-related amygdala neuroadaptation for males than females (see Logrip et al., 2018 for a recent review).
Overall, we could not determine whether the group-by-gender effects in selected brain regions (i.e., cerebellum GM and amygdala) reflect either a (partly) gender-dependent neurobiology of alcohol dependence or phenotype differences between men and women of our sample. Indeed, variables such as distinct drinking behaviours (Erol and Karpyak, 2015) or sex hormones (Erol et al., 2019) may have driven the observed group-by-gender interactions. Of note, we found that alcohol-dependent men consumed more monthly standard drinks than their female counterparts. To mitigate for gender differences in alcohol consumption we ran a sensitivity analysis using a subsample of men and women with alcohol dependence matched for alcohol standard drinks, and we replicated the findings. Yet, we cannot exclude that other drinking patterns that are systematically different between men and women (e.g., age of onset of alcohol use, lifetime alcohol dosage, years of heavy drinking, severity of alcohol dependence) (Erol and Karpyak, 2015) could have driven the observed effects.
The influence of sex hormones may also contribute to the differences in the brains of men and women with alcohol dependence (Erol et al., 2019), especially within the reward circuitry (Witt, 2007). For instance, preclinical studies show that estrogens (i.e., estradiol) might modulate ethanol-induced cerebellar toxicity (Hedges et al., 2012) and protect against the alteration of endocannabinoid signalling induced by alcohol withdrawal within the amygdala (Henricks et al., 2017). Future clinical studies are warranted to study the interaction between alcohol and sex hormones and alcohol dependence on brain outcomes.
Alternatively, group and group-by-gender effects observed in our study may reflect a vulnerability predating alcohol dependence (Hill et al., 2011, Squeglia et al., 2017) or driven by other factors influencing the development of alcohol dependence (e.g. personality characteristics, family history, psychiatric symptoms, high stress levels, early-life adverse events) (Benegal et al., 2007, Gilbertson et al., 2008, Gondré-Lewis et al., 2016, Ramchandani et al., 2018, Schulte et al., 2009).
Lastly, the results of the pairwise comparisons show that group-by-gender interactions observed in this study could have been driven by gender differences within the control group. Gender differences in brain volumes of normative samples have been widely shown in large-scale MRI studies, above and beyond the effect of differences in brain size (Liu et al., 2020, Lotze et al., 2019). However, there is also evidence showing that gender differences diminish (Ritchie et al., 2018) or disappear (Jäncke et al 2015) when adjusting for overall brain size. This discrepancy raise question about the adequacy of adjusting for ICV to control for the effect of overall brain size in gender-related analysis (Sanchis-Segura et al, 2020). Future studies applying different ICV correction methods may help disentangle this issue.
In contrast with our hypothesis, we failed to replicate group differences for the volumes of selected ROIs (i.e. OFC, amygdala, NAcc, cerebellar WM and CSF) (Boutte et al., 2012, Cardenas et al., 2007, Fein et al., 2006). However, we do realize that p values threshold is arbitrary and non-significant effect should not be over-interpreted as they could indicate very different things (e.g., a true null result, an underpowered genuine effect or an ambiguous effect) (Makin and de Xivry, 2019). For instance, this discrepancy may be due to methodological differences between the present and previous studies. Previous works had small sample size, as such, their findings may have been false positive (Button et al., 2013, Ioannidis, 2008). Yet, we used a more stringent statistical approach. This issue may have limited the power to detect group differences with small effect sizes in our but not previous work (i.e., range from d = 0.04 to d = 0.14). Unlike previous work, we (i) controlled for multiple comparisons and thereby used a more stringent statistical threshold, (ii) accounted for gender differences and controlled for multiple confounders (i.e., age, education years and ICV), thus reducing the power to detect existing effects; (iii) we screened for major confounders and excluded 191 participants for major psychiatric and substance use disorders to mitigate the impact of confounders that may affect brain volumes independent or in interaction with alcohol dependence.
Of note, methodological differences (e.g., distinct sample sizes and ROIs, brain indices and confounders considered) may also account for discrepancies between our results and those of recent work from the ENIGMA addiction workgroup where people with alcohol dependence showed smaller volumes of the hippocampus, globus pallidus, putamen and thalamus but also of the amygdala and NAcc, and the OFC thickness (Mackey et al., 2019).
This study has a number of limitations. First, the cross-sectional study design precluded the understanding of whether neuroanatomical differences predated or followed the onset of alcohol dependence and exacerbated with its course. Second, we had limited measures of alcohol exposure (i.e., monthly standard drinks). The role of drinking patterns on volumetric reductions in alcohol dependence remains unclear and is to be examined by future work e.g., age of onset of alcohol use and dependence, cumulative lifetime exposure to alcohol, number of binge episodes, number of detoxifications, length of abstinence prior to MRI and severity of alcohol dependence (Ruiz et al., 2013, Sawyer et al., 2016). Third, we lack data on additional variables that also may influence the neuroanatomy of people with alcohol dependence (e.g., personality characteristics, mental health symptoms, sex hormones, other substances used, nutrition status). The limited data available was due to heterogeneous testing protocols between sites and warrants the development of a minimum set of standardized tools to assess alcohol dependence, related psychosocial outcomes and comorbid drug use, so that future (including multi-site) studies can measure the specificity and the functional significance of the findings. Also, our findings may have been confounded by noise due to inter-site heterogeneity in MRI methodology (e.g., MR strength, manufacturer, acquisition parameters that lead to distinct MRI image quality), behavioural testing protocols, samples’ demographic (e.g., age) and mental health characteristics. These were mitigated using standardized MR quality check protocols (Thompson et al., 2014, van Erp et al., 2016) and a multilevel statistical approach that accounts for dependency between observations in nested designs (Aarts et al., 2014). Also, we controlled for differences in demographics (i.e. age, education) in all analyses. As such, we are confident that we accounted for the impact of these variables in estimating the results. Of note, large-scale longitudinal brain imaging studies report that adults’ brain volume is relatively stable until 35–40 years and decreases thereafter, with mean percentage changes per year between 0.2% and 0.8% (Hedman et al., 2012, Ritchie et al., 2015). If we consider that alcohol-dependent individuals and controls in our sample were (on average) under their 35s, it is unlikely that a 2-years age differences may have driven the observed effects. Lastly, previous evidence shows that cerebellum's close proximity to the base of the skull causes image contrast nonuniformity, resulting in inferior GM/WM discrimination (Carass et al., 2018, Price et al., 2014). As such, we cannot exclude that differences in head/brain size between men and women in our sample may have influenced the segmentation of the cerebellum GM and WM. Yet, this was the largest structural neuroimaging study to date to examine gender differences in a sample of participants with current alcohol dependence confirmed with standardized clinical tools i.e., DSM-IV, screened for comorbid psychopathologies and substance dependence.
5. Conclusions
Our findings validate and advance existing knowledge on the role of alcohol dependence, gender differences, and alcohol dosage on neuroanatomy using a large-scale multi-site structural neuroimaging study, in a carefully selected sample screened for major psychiatric comorbidities and using a robust statistical design. Alcohol dependence was associated with widespread smaller volumetric brain regions encompassing medial temporal (hippocampus), striatal (putamen, pallidum, thalamus), corpus callosum, and global brain volumes (total GM and WM). Gender differences in people with alcohol dependence emerged in the volumes of specific brain regions. These include the cerebellar GM, which was reduced more prominently in women, and the amygdala, which showed a significant reduction only in dependent men. The effects size of our findings was small. As such, communicating the nature of this study's findings will inform the work of future researchers’ on the neurotology of gender differences in alcohol dependence, with estimates for power for sample size calculations. This is particularly true for studies that address novel research questions, such as that the study of group-by-gender differences in substance dependence hereby conducted.
The findings of this study have implications to advance neuroscientific theories that implicate the amygdala and cerebellum in addiction but do not account for gender differences. Our results highlight the need to account for gender differences in neuroscientific studies of alcohol dependence using samples carefully matched on gender and other important demographic characteristics (e.g., age), multimodal imaging techniques that map distinct properties of neural integrity (e.g., brain anatomy, function and neurotransmitters), longitudinal designs and comprehensive standardized assessments of alcohol dependence and relevant psychosocial outcomes. This work will be necessary to shed some light on the mechanisms underlying gender differences in trajectories in and out of alcohol dependence related psychosocial/treatment outcomes and to match the treatment demands posed by increasing rates of substance use disorders in women (Greenfield et al., 2010, Heidari et al., 2016).
Data availability
The code of the statistical analysis is provided in the supplementary material. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
V.L. and P.P. designed the research; M.G.R. performed research;
M.G.R. and P.P. analyzed data and described the procedures; M.G.R. wrote the paper with the help of V.L; N.B.A., A.B., R.L.C.; Y.Y.C., J.C., A.E.G., R.H., K.H., R.M., R.M., R.S., L.S., Z.S., N.S., C.S., R.J.V.H., D.V., M.Y., made substantial contributions to the data collection;
V.L., S.M., H.G., P.C., made substantial contributions to the interpretation.
All authors contributed to revising the manuscript critically for important intellectual content and final approval.
Funding sources
Data collection: Drs. Sjoerds and Veltman received funding from Netherlands Organization for Health Research and Development (ZonMW) grant 31160004 from NWO. Drs. Goudriaan and van Holst received funding from ZonMW grant 91676084 from NWO. Drs. Cousijn and Goudriaan received funding for the Cannabis Prospective study from ZonMW grant 31180002 from NWO. Dr. Momenan was supported by the Intramural Clinical and Biological Research Program of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Dr. Sinha received funds from NIDA (PL30-1DA024859-01), the NIH National Center for Research Resources (UL1-RR24925-01), and NIAAA (R01-AA013892). Dr. Solowij received funding from the Clive and Vera Ramaciotti Foundation for Biomedical Research National and Health and Medical Research Council Project grant 459111 and was supported by Australian Research Council Future Fellowship FT110100752. Prof. Yücel was supported by National Health and Medical Research Council Fellowship 1117188 and the David Winston Turner Endowment Fund. Dr Lorenzetti was supported by The Australian Catholic University through a competitive scheme.
Declaration of interest
P.T. received partial grant support from Biogen, Inc. (Boston, USA) for research unrelated to this manuscript. M.Y. has received funding from several law firms in relation to expert witness reports.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102636.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aarts E., Verhage M., Veenvliet J.V., Dolan C.V., van der Sluis S. A solution to dependency: using multilevel analysis to accommodate nested data. Nat. Neurosci. 2014;17(4):491–496. doi: 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- Agartz I., Momenan R., Rawlings R.R., Kerich M.J., Hommer D.W. Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry. 1999;56(4):356. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Agartz I., Shoaf S., Rawlings R.R., Momenan R., Hommer D.W. CSF monoamine metabolites and MRI brain volumes in alcohol dependence. Psychiatry Res. Neuroim. 2003;122(1):21–35. doi: 10.1016/s0925-4927(02)00084-7. [DOI] [PubMed] [Google Scholar]
- Benegal V., Antony G., Venkatasubramanian G., Jayakumar P.N. Imaging study: gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict. Biol. 2007;12(1):122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001:1165–1188. [Google Scholar]
- Beresford T.P., Arciniegas D.B., Alfers J., Clapp L., Martin B., Du Y., Liu D., Shen D., Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcohol. Clin. Exp. Res. 2006;30(11):1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Boutte D., Calhoun V.D., Chen J., Sabbineni A., Hutchison K., Liu J. Association of genetic copy number variations at 11 q14. 2 with brain regional volume differences in an alcohol use disorder population. Alcohol. 2012;46(6):519–527. doi: 10.1016/j.alcohol.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo F., Gual A., Lligoña A., Colom J. Gender differences in the long-term outcome of alcohol dependence treatments: An analysis of twenty-year prospective follow up. Drug Alcohol Rev. 2013;32(4):381–388. doi: 10.1111/dar.12023. [DOI] [PubMed] [Google Scholar]
- Brennan P.L., Schutte K.K., Moos B.S., Moos R.H. Twenty-year alcohol-consumption and drinking-problem trajectories of older men and women. J. Stud. Alcohol Drugs. 2011;72(2):308–321. doi: 10.15288/jsad.2011.72.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Carass A., Cuzzocreo J.L., Han S., Hernandez-Castillo C.R., Rasser P.E., Ganz M., Beliveau V., Dolz J., Ben Ayed I., Desrosiers C., Thyreau B., Romero J.E., Coupé P., Manjón J.V., Fonov V.S., Collins D.L., Ying S.H., Onyike C.U., Crocetti D., Landman B.A., Mostofsky S.H., Thompson P.M., Prince J.L. Comparing fully automated state-of-the-art cerebellum parcellation from magnetic resonance images. Neuroimage. 2018;183:150–172. doi: 10.1016/j.neuroimage.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas V.A., Studholme C., Gazdzinski S., Durazzo T.C., Meyerhoff D.J. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S., Martelli C., Delain F., Kostogianni N., Douaud G., Aubin H.-J., Reynaud M., Martinot J.-L. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chaudhri N., Woods C.A., Sahuque L.L., Gill T.M., Janak P.H. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur. J. Neurosci. 2013;38(5):2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W., Lim S., Lee S. Why is high-risk drinking more prevalent among men than women? Evidence from South Korea. BMC Public Health. 2012;12:101. doi: 10.1186/1471-2458-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Demirakca T., Ende G., Kämmerer N., Welzel-Marquez H., Hermann D., Heinz A., Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol. Clin. Exp. Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh A., Rosenbloom M.J., Sassoon S., O'Reilly A., Pfefferbaum A., Sullivan E.V. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. J. Stud. Alcohol. 2003;64(3):375–379. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devaud L.L., Fritschy J.-M., Morrow A.L. Influence of gender on chronic ethanol-induced alterations in GABAA receptors in rats. Brain Res. 1998;796(1-2):222–230. doi: 10.1016/s0006-8993(98)00357-6. [DOI] [PubMed] [Google Scholar]
- Durazzo T.C., Mon A., Pennington D., Abé C., Gazdzinski S., Meyerhoff D.J. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict. Biol. 2014;19(1):132–143. doi: 10.1111/j.1369-1600.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A., Ho A.-C., Winham S.J., Karpyak V.M. Sex hormones in alcohol consumption: a systematic review of evidence. Addict. Biol. 2019;24(2):157–169. doi: 10.1111/adb.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A., Karpyak V.M. Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Fein G., Landman B., Tran H., McGillivray S., Finn P., Barakos J., Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32(3):1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., Shimotsu R., Chu R., Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin. Exp. Res. 2009;33(10):1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flensborg-Madsen T., Becker U., Grønbæk M., Knop J., Sher L., Mortensen E.L. Alcohol consumption and later risk of hospitalization with psychiatric disorders: prospective cohort study. Psychiatry Res. 2011;187(1-2):214–219. doi: 10.1016/j.psychres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Gilbertson R., Prather R., Nixon S.J. The role of selected factors in the development and consequences of alcohol dependence. Alcohol Res. Health J. Nat. Inst. Alcohol Abuse Alcohol. 2008;31:389–399. [PMC free article] [PubMed] [Google Scholar]
- Gondré-Lewis M.C., Warnock K.T., Wang H., June H.L., Bell K.A., Rabe H., Tiruveedhula V.V.N.P.B., Cook J., Lüddens H., Aurelian L., June H.L. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress (Amsterdam, Netherlands) 2016;19(2):235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S.F., Back S.E., Lawson K., Brady K.T. Substance abuse in women. Psychiatric Clin. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges V.L., Ebner T.J., Meisel R.L., Mermelstein P.G. The cerebellum as a target for estrogen action. Front. Neuroendocrinol. 2012;33(4):403–411. doi: 10.1016/j.yfrne.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman A.M., van Haren N.E.M., Schnack H.G., Kahn R.S., Hulshoff Pol H.E. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari S., Babor T.F., De Castro P., Tort S., Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res. Integrity Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks A.M., Berger A.L., Lugo J.M., Baxter-Potter L.N., Bieniasz K.V., Petrie G., Sticht M.A., Hill M.N., McLaughlin R.J. Sex-and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharmacology. 2017;124:121–133. doi: 10.1016/j.neuropharm.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Hill S.Y., Wang S., Carter H., Tessner K., Holmes B., McDermott M., Zezza N., Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. Neuroim. 2011;194(3):304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D., Momenan R., Rawlings R., Ragan P., Williams W., Rio D., Eckardt M. Decreased corpus callosum size among alcoholic women. Arch. Neurol. 1996;53(4):359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Hommer D.W., Momenan R., Kaiser E., Rawlings R.R. Evidence for a gender-related effect of alcoholism on brain volumes. Am. J. Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P. Why Most Discovered True Associations Are Inflated. Epidemiology. 2008;19(5):640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- Jäncke L., Mérillat S., Liem F., Hänggi J. Brain size, sex, and the aging brain. Hum. Brain Mapp. 2015;36(1):150–169. doi: 10.1002/hbm.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Neurocircuitry of alcohol addiction: synthesis from animal models. Handbook Clinical Neurology Elsevier. 2014:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K.E., Gutierrez E.J., Yamamoto D.J., Regner M.F., McKee S.A., Tanabe J. Sex disparities in substance abuse research: evaluating 23 years of structural neuroimaging studies. Drug Alcohol Depend. 2017;173:92–98. doi: 10.1016/j.drugalcdep.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A., Acton P., Gacinovic S., Boddington S., Costa D., Pilowsky L., Ell P., Marshall E., Kerwin R. Levels of γ-Aminobutyric acid-benzodiazepine receptors in abstinent, alcohol-dependent women: preliminary findings from an 123I-Iomazenil single photon emission tomography study. Alcohol. Clin. Exp. Res. 2000;24:1449–1455. [PubMed] [Google Scholar]
- Logrip M.L., Milivojevic V., Bertholomey M.L., Torregrossa M.M. Sexual dimorphism in the neural impact of stress and alcohol. Alcohol. 2018;72:49–59. doi: 10.1016/j.alcohol.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M., Domin M., Gerlach F.H., Gaser C., Lueders E., Schmidt C.O., Neumann N. Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Sci. Rep. 2019;9(1):1–7. doi: 10.1038/s41598-018-38239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Seidlitz J., Blumenthal J.D., Clasen L.S., Raznahan A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl. Acad. Sci. 2020;117(31):18788–18798. doi: 10.1073/pnas.1919091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S., Allgaier N., Chaarani B., Spechler P., Orr C., Bunn J., Allen N.B., Alia-Klein N., Batalla A., Blaine S., Brooks S., Caparelli E., Chye Y.Y., Cousijn J., Dagher A., Desrivieres S., Feldstein-Ewing S., Foxe J.J., Goldstein R.Z., Goudriaan A.E., Heitzeg M.M., Hester R., Hutchison K., Korucuoglu O., Li C.-S., London E., Lorenzetti V., Luijten M., Martin-Santos R., May A., Momenan R., Morales A., Paulus M.P., Pearlson G., Rousseau M.-E., Salmeron B.J., Schluter R., Schmaal L., Schumann G., Sjoerds Z., Stein D.J., Stein E.A., Sinha R., Solowij N., Tapert S., Uhlmann A., Veltman D., van Holst R., Whittle S., Wiers R., Wright M.J., Yücel M., Zhang S., Yurgelun-Todd D., Hibar D.P., Jahanshad N., Evans A., Thompson P.M., Glahn D.C., Conrod P., Garavan H. Mega-analysis of gray matter volume in substance dependence: general and substance-specific regional effects. Am. J. Psychiatry. 2019;176(2):119–128. doi: 10.1176/appi.ajp.2018.17040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., de Xivry J.J.O. Science Forum: Ten common statistical mistakes to watch out for when writing or reviewing a manuscript. Elife. 2019;8 doi: 10.7554/eLife.48175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Oscar-Berman M., Jaffin S.K., Hodge S.M., Kennedy D.N., Caviness V.S., Marinkovic K., Breiter H.C., Gasic G.P., Harris G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K., Ackermann K., Croissant B., Mundle G., Nakovics H., Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol. Clin. Exp. Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Mann K., Hintz T., Jung M. Does psychiatric comorbidity in alcohol-dependent patients affect treatment outcome? Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:172–181. doi: 10.1007/s00406-004-0465-6. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S., Brenneis C., Egger K., Koppelstaetter F., Schocke M., Marksteiner J. A wide-spread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J. Neurol. Neurosurg. Psychiatry. 2007;78(6):610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M., Vazquez-Sanroman D., Carbo-Gas M., Gil-Miravet I., Sanchis-Segura C., Carulli D., Manzo J., Coria-Avila G.A. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci. Biobehav. Rev. 2016;60:1–11. doi: 10.1016/j.neubiorev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Moulton E.A., Elman I., Becerra L.R., Goldstein R.Z., Borsook D. The cerebellum and addiction: insights gained from neuroimaging research. Addict. Biol. 2014;19(3):317–331. doi: 10.1111/adb.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson R.B. Frequentist q-values for multiple-test procedures. Stata journal. 2010;10(4):568–584. [Google Scholar]
- Nixon S.J., Prather R., Lewis B. Sex differences in alcohol-related neurobehavioral consequences. Handbook Clinical Neurology. Elsevier. 2014:253–272. doi: 10.1016/B978-0-444-62619-6.00016-1. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Lim K.O., Desmond J.E., Sullivan E.V. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol. Clin. Exp. Res. 1996;20(4):752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Deshmukh A., Sullivan E.V. Sex differences in the effects of alcohol on brain structure. Am. J. Psychiatry. 2001;158(2):188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Zahr N.M., Sassoon S.A., Kwon D., Pohl K.M., Sullivan E.V. Accelerated and premature aging characterizing regional cortical volume loss in human immunodeficiency virus infection: contributions from alcohol, substance use, and hepatitis C coinfection. Biol. Psychiatry Cogn. Neurosci. Neuroim. 2018;3(10):844–859. doi: 10.1016/j.bpsc.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M., Cardenas V.A., Fein G. Automated MRI cerebellar size measurements using active appearance modeling. Neuroimage. 2014;103:511–521. doi: 10.1016/j.neuroimage.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani V.A., Stangl B.L., Blaine S.K., Plawecki M.H., Schwandt M.L., Kwako L.E., Sinha R., Cyders M.A., O'Connor S., Zakhari S. Stress vulnerability and alcohol use and consequences: From human laboratory studies to clinical outcomes. Alcohol (Fayetteville. 2018;72:75–88. doi: 10.1016/j.alcohol.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran C.R., Mehta A.K., Ticku M.K. Effect of chronic administration of ethanol on the regulation of the δ-subunit of GABAA receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S.J., Dickie D.A., Cox S.R., Valdes Hernandez M.D.C., Corley J., Royle N.A., Pattie A., Aribisala B.S., Redmond P., Muñoz Maniega S., Taylor A.M., Sibbett R., Gow A.J., Starr J.M., Bastin M.E., Wardlaw J.M., Deary I.J. Brain volumetric changes and cognitive ageing during the eighth decade of life. Hum. Brain Mapp. 2015;36(12):4910–4925. doi: 10.1002/hbm.22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S.J., Cox S.R., Shen X., Lombardo M.V., Reus L.M., Alloza C., Harris M.A., Alderson H.L., Hunter S., Neilson E., Liewald D.C.M., Auyeung B., Whalley H.C., Lawrie S.M., Gale C.R., Bastin M.E., McIntosh A.M., Deary I.J. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex. 2018;28:2959–2975. doi: 10.1093/cercor/bhy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D.J., Richardson B.D. The Cerebellar GABA A R System as a Potential Target for Treating Alcohol Use Disorder. The Neuropharmacology of Alcohol. Springer. 2018:113–156. doi: 10.1007/164_2018_109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S.M., Oscar-Berman M., Sawyer K.S., Valmas M.M., Urban T., Harris G.J. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol. Clin. Exp. Res. 2013;37(1):110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C., Ibañez-Gual M.V., Aguirre N., Cruz-Gómez Á.J., Forn C. Effects of different intracranial volume correction methods on univariate sex differences in grey matter volume and multivariate sex prediction. Sci. Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-69361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K.S., Oscar-Berman M., Mosher Ruiz S., Gálvez D.A., Makris N., Harris G.J., Valera E.M. Associations between cerebellar subregional morphometry and alcoholism history in men and women. Alcohol. Clin. Exp. Res. 2016;40(6):1262–1272. doi: 10.1111/acer.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K.S., Oscar-Berman M., Barthelemy O.J., Papadimitriou G.M., Harris G.J., Makris N. Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Res. Neuroim. 2017;263:15–25. doi: 10.1016/j.pscychresns.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte M.T., Ramo D., Brown S.A. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin. Psychol. Rev. 2009;29(6):535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L.M., Ball T.M., Jacobus J., Brumback T.y., McKenna B.S., Nguyen-Louie T.T., Sorg S.F., Paulus M.P., Tapert S.F. Neural predictors of initiating alcohol use during adolescence. Am. J. Psychiatry. 2017;174(2):172–185. doi: 10.1176/appi.ajp.2016.15121587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Deshmukh A., De Rosa E., Rosenbloom M.J., Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol. Psychiatry. 2005;57(7):768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Zahr N.M., Sassoon S.A., Thompson W.K., Kwon D., Pohl K.M., Pfefferbaum A. The role of aging, drug dependence, and hepatitis C comorbidity in alcoholism cortical compromise. Jama Psychiatry. 2018;75(5):474–483. doi: 10.1001/jamapsychiatry.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Stein J.L., Medland S.E., Hibar D.P., Vasquez A.A., Renteria M.E., Toro R., Jahanshad N., Schumann G., Franke B. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Im. Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp T.G., Hibar D.P., Rasmussen J.M., Glahn D.C., Pearlson G.D., Andreassen O.A., Agartz I., Westlye L.T., Haukvik U.K., Dale A.M. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. 2016;21:547. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse T.L., Cosgrove K.P., Tanabe J., McKee S.A. Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. J. Neurosci. Res. 2021;99(1):309–323. doi: 10.1002/jnr.24625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wiers C.E., Shokri-Kojori E., Tomasi D., Wang G.-J., Baler R. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: studies with positron emission tomography. Neuropharmacology. 2017;122:175–188. doi: 10.1016/j.neuropharm.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Witt E.D. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol. Teratol. 2007;29(1):81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Global status report on alcohol and health 2018. World Health Organization; 2018. [Google Scholar]
- Wrase J., Makris N., Braus D.F., Mann K., Smolka M.N., Kennedy D.N., Caviness V.S., Hodge S.M., Tang L., Albaugh M., Ziegler D.A., Davis O.C., Kissling C., Schumann G., Breiter H.C., Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry. 2008;165(9):1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Zahr N.M. Structural and microstructral imaging of the brain in alcohol use disorders. Handbook of Clinical Neurology. Elsevier. 2014:275–290. doi: 10.1016/B978-0-444-62619-6.00017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code of the statistical analysis is provided in the supplementary material. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.