Abstract
Background
Previous fMRI studies of posttraumatic stress disorder (PTSD) have investigated region-specific alterations in intrinsic connectivity but connectome-wide changes in connectivity are yet to be characterized. Understanding the neurobiology of this is important to develop novel treatment interventions for PTSD. This study aims to identify connectome-wide disruptions in PTSD to provide a more comprehensive analysis of nseural networks in this disorder.
Methods
A functional MRI scan was completed by 138 individuals (67 PTSD and 71 non-trauma-exposed healthy controls [HC]). For every individual, inter-regional intrinsic functional connectivity was estimated between 436 brain regions, comprising intra and inter-network connectivity of eight large-scale brain networks. Group-wise differences between PTSD and HC were investigated using network-based statistics at a family-wise error rate of p < 0.05. Significant network differences were then further investigated in 27 individuals with trauma exposure but no PTSD [TC]).
Results
Compared to HC, PTSD displayed lower intrinsic functional connectivity in a network of 203 connections between 420 regions within and between mid-posterior default mode, central executive, limbic, visual and somatomotor regions. Additionally, PTSD displayed higher connectivity across a network of 50 connections from thalamic and limbic to sensory and default-mode regions. Connectivity in TC in both these networks was intermediate and significantly different to PTSD and HC.
Conclusion
A large-scale imbalance between hypoconnectivity of higher-order cortical networks and hyperconnectivity of emotional and arousal response systems seems to occur on a sliding scale from trauma exposure to clinical manifestation as PTSD. Novel interventions that target this systemic functional imbalance could provide potential mitigation of PTSD.
Keywords: Connectome, Posttraumatic stress disorder, fMRI, Functional connectivity, Network, Neuropathology
Highlights
-
•
This study investigates intrinsic whole-brain functional connectivity (FC) in PTSD and trauma-exposed controls.
-
•
We identify an imbalance between dysconnectivity of the cortex and hyperconnectivity of subcortical emotional and arousal response systems.
-
•
This disruption in intrinsic FC was more extensive than previously identified, impacting all major functional brain networks.
-
•
Degree of dysconnectivity in PTSD was further associated with dissociative symptoms.
-
•
Alterations in functional connectivity are also present in trauma-exposed controls but are less severe.
1. Introduction
The past few decades have seen many neuroimaging investigations of posttraumatic stress disorder (PTSD), and these have focused on dysfunctional systems of executive function, contextual processing, threat detection, fear learning and emotional regulation (Shalev et al., 2017; Kunimatsu et al., 2019). These studies have typically investigated these systems in isolation, focusing on a specific region (Kennis et al., 2015; Olson et al., 2019) or network (Sripada et al., 2012; DiGangi et al., 2016). Functional MRI studies over the past decade have revealed that the brain is intrinsically organized into distinct, functionally coherent networks that underlie cognitive and emotional processes (Power et al., 2011; Yeo et al., 2011). Investigating the functional architecture of the brain by examining task-free intrinsic connectivity provides insight into fundamental neural functions such as the maintenance of arousal states, consolidation of memories, preparation for the future and introspection (Buckner and Vincent 2007). This is particularly pertinent to the study of PTSD, which is characterized by alterations in many of these functions (Shalev et al., 2017).
Abnormal patterns of connectivity between specific brain networks during the resting state have been previously identified in PTSD using specific seeds or regions of interest (ROIs) to focus on specific circuitry. These studies have identified alterations within and between regions of the default mode and salience network (DiGangi et al., 2016; Akiki et al., 2017) as well as within regions of the executive control network (Fan et al., 2016), with some inconsistency in the direction of findings depending on the ROI, age of trauma occurrence and types of trauma (Bluhm et al., 2009; Sripada et al., 2012). While these findings are valuable in terms of identifying single systems that may be important in PTSD, they are potentially subject to bias depending on the choice of seed and are therefore limited to present a narrow view of changes in regions that are directly connected to the seed. However when taken together, as in a recent review paper of seed-based resting-state connectivity studies in PTSD, lower functional connectivity across seed-based studies has been observed in 97 distinct targets and while higher functional connectivity has been observed in 43 distinct targets suggesting much more global effects are at play (Ross and Cisler 2020).
Other fMRI analysis approaches used to tackle the issue of seed-based bias include independent component analysis (ICA) and graph-based network analyses. Independent component analysis (ICA) is data-driven approach that identifies functionally related neural networks (these commonly conform to the canonical neural networks identified by large-scale parcellation schemes) and correlate them to measures of interest (Beckmann et al., 2005). This approach allows studies to investigate connectivity within an identified network as single measure independent of a particular region in the network. Studies using this approach in PTSD, have found both higher DMN connectivity at rest (Patriat et al., 2016) and lower DMN functional connectivity during rest (Shang et al., 2014; Zhang et al., 2015; Reuveni et al., 2016). Studies have also observed decreased functional connectivity between the DMN and the SN, and decreased connectivity of specific CEN regions (Shang et al., 2014) in PTSD, with one study identifying increased excitatory connections between the CEN and the posterior DMN in subjects with PTSD (Ke et al., 2018). These studies largely support seed-based findings of a tri-network model of dysfunction in PTSD. However, while they are able to explore networks without seed bias these ICA studies have still focused on specific networks of interest rather than the whole brain.
Alternatively, graph-based network analyses capture brain-wide functional interaction by focusing on the topological structure of brain regions and networks (van den Heuvel and Sporns 2011; Fornito et al., 2015). These measures primarily use the number and distribution of connections to describe how segregated or integrated regions or networks are (Fornito et al., 2015). These studies, despite inconsistencies, have described wide-spread alterations in the functional organization of the connectome in PTSD (Lei et al., 2015; Jung et al., 2016). However, while graph-based studies reflect alterations in non-trivial organizational and topological properties, they do not capture the strength of association between individual links within the connectome and how these may differ in PTSD. In contrast, the network-based statistic (NBS) captures both the strength of association between individual links in the brain and the scope of alterations across the brain. This allows whole-brain seed-free comparisons of connectivity while controlling for multiple comparisons (Zalesky et al., 2010a). This is conducive for identification of connected networks across the whole brain that may be associated with trauma exposure/PTSD. This approach is important as region-specific changes are unlikely to exist given the interconnectedness of the brain (Sporns 2011; Fornito et al., 2015). Instead alterations in any given connection are likely to influence adjacent connections, leading to a ripple effect on brain function (Zalesky et al., 2010a).
Here, we implemented a thorough brain-wide approach to investigate large-scale intrinsic functional brain network changes that characterize PTSD. We use the NBS to investigate differences in functional connectivity/organization between 67 PTSD and 71 healthy controls (HC) across 436 cortical and subcortical brain regions (Fan et al., 2016; Schaefer et al., 2018). Observed differences were further investigated in an additional 29 individuals who experienced trauma but did not develop PTSD to evaluate whether these brain connectivity alterations are specific to the experience of trauma or development of the disorder. Based on previous studies, we hypothesized that there would be wide-spread differences (likely decreases) in connectivity involving the salience, default mode and executive function networks in the PTSD group.
2. Methods and materials
2.1. Participant information
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by Western Sydney Area Health Service Human Ethics Committee. Written informed consent was obtained from all participants. Eighty-one PTSD participants were recruited at the Westmead Institute for Medical Research (Australia) to complete a clinical interview and a structural and functional MRI battery. PTSD diagnosis (as defined by DSM-IV) was obtained using a structured interview and the Clinical Administered PTSD Scale (CAPS10) (Blake et al., 1995). Participants were also assessed for comorbid Axis I disorders using the Mini International Neuropsychiatric Interview (MINI version 5.5) (Sheehan et al., 1998). Participants also completed the Beck Depression Inventory-2 to assess severity of depressive symptoms (BDI) (Beck et al., 1996), a 21-item self-report inventory measuring depressive symptoms in the past two week, and the Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al., 1998); a 27-item scale with 19 subject-rated items and 8 clinician-rated items, to measure dissociative symptoms. Participants with a history of neurological disorder, psychosis, or current substance dependence were excluded. Participants on a psychotropic medication were eligible to be included if they were on a stable dosage for at least two months prior to testing.
Of the 81 participants recruited, two failed to complete the MRI and 12 were excluded due to movement during the MRI scan (details below and in supplementary methods). This resulted in data from 67 PTSD participants being available for analysis. PTSD participants were matched for age and sex with 71 HC, who underwent the same study protocol. An additional 32 trauma-exposed controls (TC), were recruited and tested as above, with three excluded due to in-scanner motion, leading to a total of 29 included in the analysis. TC participants were defined as being exposed to a traumatic event but not meeting criteria for more than one cluster of PTSD symptoms. The demographics for each group are shown in Table 1.
Table 1.
Group demographics.
| PTSD (n = 67) | HC (n = 71) | TC (n = 29) | F/t/χ2 | p | ||
|---|---|---|---|---|---|---|
| Age, y, m±SD | 39.5 ± 11.4 | 36.3 ± 12.0 (19–65) | 36.8 ± 12.23 | F = 1.356 | 0.261 | |
| (range) | (19–63) | (19–63) | ||||
| Sex, female (%) | 46(68.66) | 53(74.65) | 10 (34.48) | χ2= 15.22 | P < 0.001 | PTSD > TC |
| HC > TC | ||||||
| CAPS, m±SD (range) | 65.99 ± 20.7 (21–115) | NA | 8.66 ± 10.50 (0–42) | t = 17.95 | P<0.001 | PTSD > TC |
| CADSS, m±SD (range) | 20.6 ± 13.17 (0–62) | NA | 6.60 ± 11.33 (0–47) | t = 5.04 | P<0.001 | PTSD > TC |
| BDI, m±SD (range) | 31.72 ± 12.36 (4–58) | NA | 7.81 ± 10.10 (0–40) | t = 9.66 | P<0.001 | PTSD > TC |
| Time since trauma, y | 20.51 ± 15.35 (0.75–51) | NA | 10.89 ± 8.81 (0.42–29) | t = -4.98 | P<0.001 | PTSD > TC |
| (range) | ||||||
| Trauma type, n (%) | ||||||
| Child abuse | 26(38.81) | NA | 0 | |||
| Road accident | 5(7.46) | NA | 11(37.9) | |||
| Assault | 13(19.4) | NA | 6 (20.7) | |||
| Death of loved one | 4(5.97) | NA | 4 (13.8) | |||
| Witnessed violence/police | 14(20.9) | NA | 4 (13.8) | |||
| Domestic violence | 5(7.46) | NA | 2 (6.89) | |||
| Current Medication, n (%) | ||||||
| SNRI | 11(16.42) | NA | NA | |||
| SSRI | 15(22.39) | NA | 1 (3.45) | |||
| Antidepressant | 13(19.4) | NA | NA | |||
| Antipsychotic | 10(14.93) | NA | NA | |||
| Benzodiazepam | 12(17.91) | NA | NA | |||
| Stimulant | 1(1.49) | NA | 1 (3.45) | |||
| Comorbid diagnoses, n (%) | ||||||
| Major Depression | 45(67.16) | NA | 2 (6.89) | |||
| Panic Disorder | 1(1.49) | NA | NA | |||
| Agoraphobia | 22(32.84) | NA | NA | |||
| Social Phobia | 30(44.78) | NA | 1 (3.45) | |||
| OCD | 11(16.42) | NA | NA | |||
| GAD | 28(41.79) | NA | 2(3) |
PTSD – Post-traumatic Stress Disorder, HC – Healthy Controls, TC – Trauma Controls, CAPS – Clinician Administered PTSD Scale, CADDS – Clinician Administered Dissociative States Scale, BDI – Becks Depression Inventory, SNRI – Serotonin Norepinephrine Reuptake Inhibitors, SSRI – Selective Serotonin Reuptake Inhibitors, OCD – Obsessive Compulsive Disorder.
2.2. fMRI acquisition
fMRI data was acquired using an 8-channel phased-array head coil on a 3 T GE Signa Twinspeed HDxT MR Scanner (GE Healthcare, Milwaukee, WI). Participants performed five functional MRI tasks during the MRI scan and a T1-weighted structural image was obtained. The details of the acquisition and pre-processing has been described previously (Goldstein-Piekarski et al., 2018) and is detailed in the supplementary methods. Intrinsic functional connectivity data was derived from the concatenated residuals time series of the functional fMRI tasks using a previously validated method (Fair et al., 2007; Korgaonkar et al., 2013; Korgaonkar et al., 2014). For each of the five fMRI tasks, the blood oxygen level dependent (BOLD) responses for each experimental condition were modelled in the general linear model framework. The mean signal time course derived from the cerebral spinal fluid (CSF) and white matter masks, as well as the temporal masks for movement outliers described below, were also included as covariates in the model to remove physiological noise. The variance in BOLD signal associated with each of the stimuli in the task was then modelled as a covariate and the remaining residual images represented the task-derived resting state signal. After this, a band-pass filter (0.009 Hz < f < 0.08 Hz) was applied. This process results in a movement and task-effect-corrected time series of 600 vol (120 vol x 5) from which intrinsic-resting state connectivity (correlation of change in BOLD signal between voxels/nodes across time) can be measured. While this “intrinsic connectivity” is different from pure resting-state connectivity previous studies have shown that it exhibits a very similar functional connectivity pattern reflecting the inherent connectivity of the large-scale networks in the brain (Korgaonkar et al., 2014; Ball et al., 2017). Movement outliers were identified as volumes where movement of the head from one volume to the next was 0.3 mm or greater or had a difference in scaled signal intensity greater than 10, as well as the two volumes before and one after (Achaibou et al., 2016; Goldstein-Piekarski et al., 2018; Power et al., 2012; Power et al., 2014; Siegel et al., 2014). The Volterra expansion of twenty-four realignment parameters was also modelled for each task (Friston et al., 1996). Participants with outliers covering more than 30 percent of total scan time or with a mean FD of greater than 0.2 were excluded. The number of motion outliers was matched between groups, however there were differences between group in mean FD and max translation. A summary of these and a replication of the analysis with motion parameters controlled for is included in the supplementary material to illustrate that motion is appropriately controlled for and not influencing the findings.
2.3. Generation of whole-brain functional connectomes
For each participant the average time series was extracted from 400 cortical regions derived from a recent functional parcellation of the cerebral cortex by Schaefer et al., 2018 (Schaefer et al., 2018). Schaefer et al., 2018 used a gradient-weighted Markov Random Field model applied to resting-state data from 1489 participants to obtain parcells at various resolutions. These parcels were further clustered into 7 large scale intrinsic connectivity networks based on binarized correlations of voxel time-series averaged across each parcel, similar to what was previously done by Yeo et al. (2011) (Yeo et al., 2011; Schaefer et al., 2018). We additionally included 36 regions from the subcortex derived from the Brainnetome Atlas (Fan et al., 2016). The BOLD time-series for each participants intrinsic-resting state scan was then extracted for each parcel and correlated pair-wise with the time-series of every other parcel and Fisher-Z transformed to create a 436 × 436 interregional functional correlation matrix for each participant. As negative connectivity is susceptible to artificial enhancement by fMRI preprocessing methods aiming to reduce signal noise we are unable to ascertain exactly what information is contained in negative connections (Qian et al., 2018), thus connections within these matrices that were on-average negative (based on mean connectivity values for all 138 PTSD and HC participants) were removed. This process was repeated with an additional structural (AAL) (Tzourio-Mazoyer et al., 2002); and functional parcellation scheme (Gordon et al., 2014; Tian et al., 2020) to investigate the impact of parcellation scheme on the network analysis (reported in Supplementary Results, Table s3-s5) (Wang et al., 2009; Fornito et al., 2010; Zalesky et al., 2010b).
2.4. Statistical analysis
The NBS (Zalesky et al., 2010a), was used to analyze whole-brain resting-state connectivity differences between PTSD and HC at a corrected p < 0.001 level (t-stat > 3.5). The NBS is a validated non-parametric statistical approach that addresses the multiple comparison problem by testing the null hypothesis based on interconnected subnetworks rather than individual connections (described in detail in Supplementary Methods). A supplementary NBS analysis between PTSD and TC was also performed (see Supplementary Results) to investigate if any connections unique to differences between these groups were present.
Functional connectivity values for each connection identified in significant subnetworks using NBS were extracted for PTSD, HC and the TC subgroup. Total network connectivity for the subnetworks was calculated as a mean of all significant connections and, to compare groups, a one-sample ANOVA between PTSD, TC and HC was performed in R (v3.6.2). Multivariate regression was used to investigate association of mean connectivity with BDI, CADSS and CAPS scores evaluated for PTSD (Bonferroni multiple-comparison correction was applied), controlling for age and sex. Supplementary analyses were also done to examine the potential impact of years of education, medication, comorbidity, time since trauma, child abuse and trauma type on functional connectivity of the subnetwork across the groups.
While only the subnetwork as a whole can be considered significant at a corrected level when using NBS, to aide in interpretability, connections found in the significant subnetwork that joined parcels belonging to intrinsic connectivity networks (ICNs), assigned and validated by the Schaefer parcellation (Schaefer et al., 2018), were grouped together and average connectivity for these intra- and inter-network pair combinations was estimated. A further exploratory analysis of between-group differences of ICNs in the subnetwork were evaluated post-hoc to examine differences relative to trauma controls, Bonferroni corrected for multiple comparisons of the 32 (8 × 8/2) possible within or between network connectivity values (p < 0.0015).
3. Results
3.1. Demographics and clinical characteristics
Participant characteristics are presented in Table 1. There was no significant difference between PTSD and controls on age [t = −1.60, p = 0.112] or sex [χ2 = 0.351, p = 0.554]. Both PTSD and HC differed from TC on sex [χ2 = 15.22, p < 0.001] but not on age [F = 1.356, p = 0.261]. PTSD patients had significantly higher CAPS [t = 17.95, p < 0.001], CADDS [t = 5.0356, p < 0.001] and BDI [t = 9.6568, p < 0.001] scores compared to TC and greater average time since trauma [t = −4.9761, p < 0.001].
3.2. Whole-brain connectivity differences between PTSD and HC
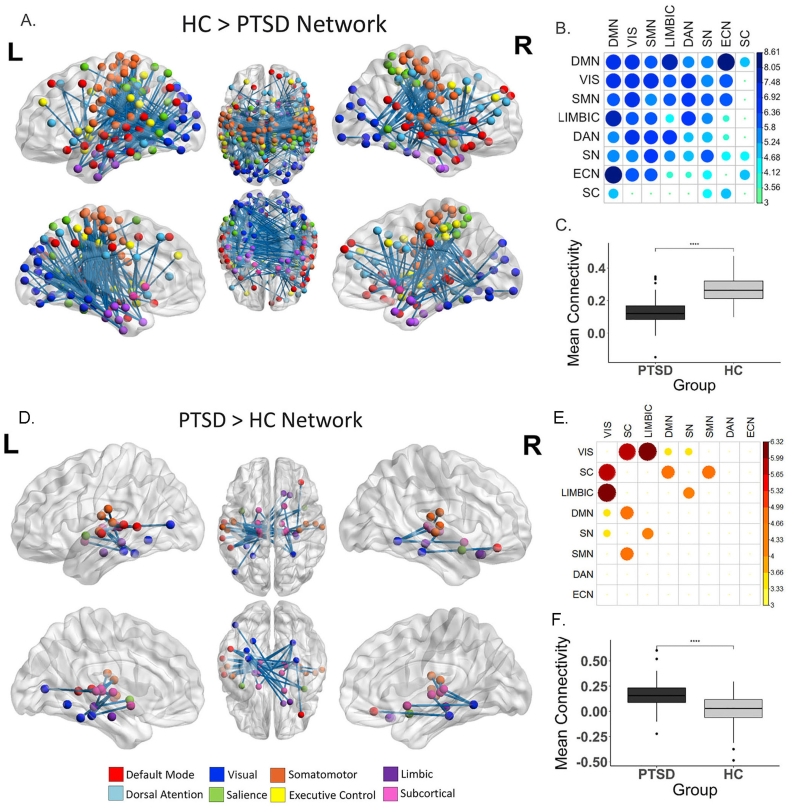
The NBS analysis identified a subnetwork (HC > PTSD) comprised of 420 connections across 203 nodes where the PTSD group had significantly lower functional connectivity than HC (p < 0.001, FWE-corrected α < 0.05), shown in Fig. 1a. Connections within this subnetwork were spread extensively across the cortex, involving internetwork connections between all of primary brain networks and intra-network connections within six of the eight primary brain networks (executive control-network and subcortical intra-network connectivity not impacted, see central diagonal row in Fig. 1b.). Decreases in connectivity within and to and from the DMN explained the most difference in connectivity between the group, followed by visual and somatomotor connectivity, then limbic, dorsal attention, salience and executive network connections (Fig. 1b). Overall, connections between the default mode & executive control network demonstrated the greatest effect-size between the groups (Table s1).
Fig. 1.
Differences in connectivity in PTSD compared to HC identified with network-based statistics. For HC > PTSD, a network comprised of 203 regions and 420 edges (connections) was identified (p < 0.001, FWE-corrected) (A–C). For PTSD > HC a network comprised of 34 regions and 50 edges (connections) was identified (D–F). A & D) all significant nodes and edges of HC > PTSD subnetwork visualized on the surface of the brain (created using BrainNet viewer) B & E) a heatmap of the mean t-statistic of significantly different connections within and between the eight primary functional networks. Larger circle size and darker colour represent greater mean t-statistic of connections (larger difference in HC and PTSD connectivity). Networks are ordered based on their overall contribution to the difference in connectivity between PTSD and HC. C & F) Shows the difference in mean connectivity across all significant regions between groups. PTSD – Posttraumatic Stress Disorder, HC – Healthy Controls.
The NBS analysis identified a second subnetwork (PTSD > HC) comprised of 34 nodes and 50 connections where the PTSD group had significantly greater functional connectivity than HC (p < 0.038, FWE-corrected α < 0.05), shown in Fig. 1d and f. The strongest differences in connectivity were between the visual nodes and the subcortical and limbic nodes. Further differences were characterized by connections between subcortical with the DMN and somatomotor network nodes and between the limbic and salience network nodes (Fig. 1e & Table s2).
Post-hoc tests using mean connectivity of the HC > PTSD network found connectivity associations with age (corr = −4.23, p < 0.001, PTSD only: corr = -3.31, p = 0.002, HC only: corr = −2.53, p = 0.014) but not sex (Figure s3) but remained significantly different between groups controlling for these measure. Connectivity for the PTSD > HC network was not associated with either age or sex.
3.3. Association of connectivity in significant networks to clinical measures
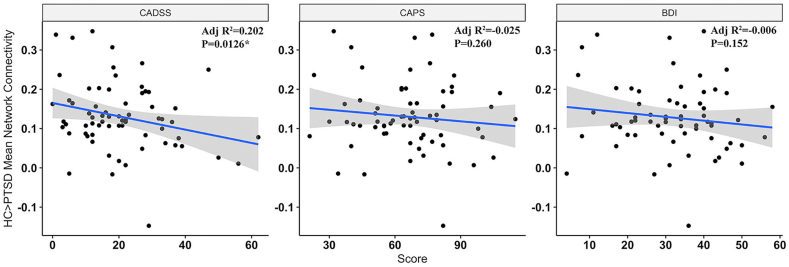
Multiple regression analyses assessed relationship of mean connectivity of the HC > PTSD network to BDI, CADSS and CAPS scores, measuring depression, dissociation and PTSD severity respectively, controlling for age and sex. A trend-level correlation between greater CADDS score and lower connectivity was identified and is shown in Fig. 2 (p = 0.012, ß = −0.283) but this did not survive multiple comparisons. Mean connectivity of the PTSD > HC network had no significant association with BDI, CADSS or CAPS scores. Connectivity was not associated with medication load, comorbidity, trauma-type (Figure s5 and s6), time since trauma (Figure s4) or a history of childhood abuse (Figure s7) in either network. We corrected for multiple comparison at Bonferroni α = 0.05/8 = 0.006.
Fig. 2.
Correlation between PTSD network connectivity and BDI, CADSS and CAPS, controlling for age and sex. Lower dissociation score is associated with greater connectivity in HC > PTSD network only. PTSD – Posttraumatic Stress Disorder, BDI – Beck's Depression Inventory, CADSS – Clinician Administered Dissociative States Scale, CAPS – Clinician Administered PTSD Scale.
3.4. Connectome signature in trauma controls
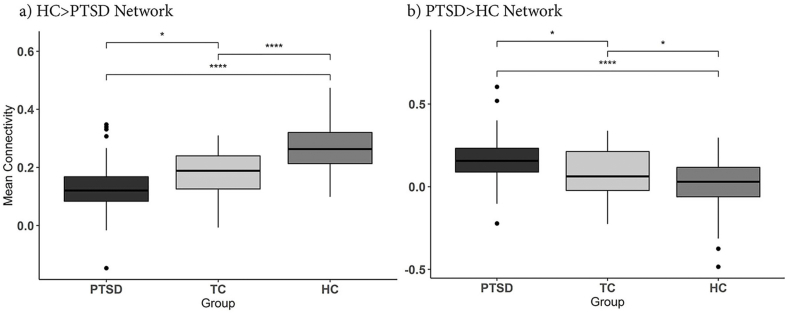
One-way ANOVA between groups showed significant differences between groups for both the HC > PTSD Network (F2,164 = 49.38, p < 0.001) and the PTSD < HC Network (2,164 F = 19.90, p < 0.001). Post-hoc tests (Tukey) showed TC were distinct relative to both PTSD (p = 0.0366) and HC (p < 0.001) for the HC > PTSD Network (Fig. 3a) and were also distinct relative to both PTSD (p = 0.0384) and HC (p = 0.0455) for the PTSD > HC network (Fig. 3b). Exploratory analysis of these differences broken up into primary functional networks is shown in Figure s9 & s10. Mean network connectivity was also correlated with BDI, CADSS and CAPS scores across both TC and PTSD but did not survive Bonferroni correction (see Figure s8). Supplemental NBS analysis between TC and PTSD found small, significant networks involving visual and hippocampal regions to be more connected in PTSD compared to TC (Figure s11), and involving visual, fusiform and precuneus region to be greater in TC compared to HC (Figure s12).
Fig. 3.
Comparison between TC, HC and PTSD connectivity in significant networks. Connectivity for significant networks was extracted from TC subgroup and compared post-hoc with HC and PTSD. One-way ANOVA showed all three groups to have significantly difference connectivity for both networks (p < 0.001). Post-hoc tests found that TC participants mean connectivity values were in between and significantly difference from HC and PTSD groups for both networks. For the HC > PTSD Networks in a) PTSD < TC: p = 0.0366 & HC > TC: p < 0.001. For the PTSD > HC Network in b) PTSD > TC: p = 0.038 & HC < TC: p = 0.046). PTSD – Posttraumatic Stress Disorder, TC – Trauma-exposed Control, HC – Healthy Control. Significance levels are denoted as **** for p < 0.001 and * for p < 0.05.
4. Discussion
This study provides a thorough and updated investigation of the functional connectome in PTSD that builds on previous literature and demonstrates that intrinsic functional connectivity was reduced in PTSD throughout an extensive network of cortical regions, primarily within the mid-caudal part of the brain. We also found a second smaller network involving connections between subcortical regions and a small subset of visual and temporal regions where PTSD individuals had greater connectivity relative to HC. Additionally, we also evaluated the level of connectivity of these two significant networks in a smaller group of trauma-exposed controls in order to assess if these connectivity changes were due to the experience of trauma alone or to the etiology and/or progression of the disorder. We found the level of functional connectivity in TC to be distinct from both PTSD and HC and was at an intermediate level between PTSD and HC. This suggests that a degree of alteration in connectivity is associated with the general experience of trauma but this appears to worsen in PTSD, and specifically with the degree of dissociative symptoms. This may indicate a load effect of chronic stress on connectivity imbalances in the brain following trauma. Identifying such widespread neural dysfunction in PTSD uniquely highlights the contribution of altered whole-brain connectivity, rather than individual areas or networks, to disorder pathology and progression.
The extent of connectivity loss observed in PTSD in this study here finds connections in every major functional network were impacted. This supports global synaptic loss and dysfunction in neurotransmission identified in previous in neurobiological models of trauma-induced chronic stress (McEwen 2017; Abdallah et al., 2019). However, there are specific intra and inter-network connections that dominate the overall pattern of dysconnectivity. In particular, loss of connectivity was centred around the DMN, visual network, SMN and limbic regions in the temporal pole and amygdala and specifically between the DMN and ECN. Although previous studies have been methodologically limited to identify such broad changes, they have reported lower connectivity related to the DMN and ECN (Jung et al., 2016), but also alterations (in both directions) in between-network connectivity of DMN to SN and higher connectivity of SN in PTSD (Lei et al., 2015; Akiki et al., 2017; Sripada et al., 2012). The combination of disordered internal mentation, reduced top-down regulation and increased arousal as a result of this dysfunctional tri-network system has been proposed to explain PTSD pathophysiology (Akiki et al., 2017). Supporting these findings, we see here a strong weakening of DMN inter and intra-network connectivity, particularly between the DMN and ECN. In contrast to previous seed-based studies, we also see a reduction (though to a lesser extent than other networks) in SN inter and intra-network connectivity and no reduction in connectivity within the ECN. Two other studies which used a similar whole-brain connectivity approach, but using a lower-resolution parcellation scheme and graph theoretical analyses to select regions for the NBS analysis, also found decreased functional connectivity in both posterior DMN and SN regions (Lei et al., 2015), which supports our findings, as well as alterations between thalamic, visual and ECN nodes, however in the opposite direction to our findings, but this may be specific to the paediatric sample used (Suo et al., 2015). In general, these inconsistencies suggest the direction of connectivity findings may be susceptible to study-specific factors, in particular seed-choice, and emphasises the importance of seed-independent approaches, such as is implemented here, to identify more global patterns of pathophysiology. Further these findings were found to also be independent of parcellation choice, replicating when a second high-resolution parcellation was used and partially replicating in a low resolution parcellation (Supplementary Figure s1 and s2). This suggests a connectome-based approach may have the capacity to provide a replicable whole-brain signature of PTSD.
Functional connectivity in our identified network was also found to have a trend level negative correlation with dissociative symptoms. This pattern accords with evidence of distinct neural connectivity in PTSD individuals with more dissociative symptoms (Nicholson et al., 2015). The maintenance of dissociative states has been proposed to be a coping mechanism to deal with high-levels of arousal in PTSD (Lanius et al., 2012), this is further supported by the trending relationship between dissociative symptoms and the PTSD > HC network involving regions moderating arousal. Dissociative symptoms have been previously found to be correlated with DMN hypoconnectivity and DMN-ECN dysconnectivity (Tursich et al., 2015), which characterize the PTSD connectome identified here. A lack of connectivity within the DMN in combination with reduced inputs from sensory circuits and impaired executive control may be associated with a disordered sense of self and reality contributing to dissociation.
In addition to the loss of connectivity to these key cortical networks, we also observed lower connectivity in PTSD centred more focally around visual, subcortical and limbic regions. Specifically, we saw lower connectivity in PTSD between visual regions and regions of the nucleus accumbens, thalamus and hippocampus, as well as between thalamic regions and mid-temporal regions of the DMN and SMN. We also observed lower limbic, specifically hippocampal, connectivity to the SN. This finding concurs with proposals that one key dysfunctional network in PTSD relates to disturbed contextual processing and arousal, involving the hippocampus and thalamus (Shalev et al., 2017). The nucleus accumbens has also been tied to fear learning in particular (McCutcheon et al., 2012). Higher connectivity from these regions to sensory systems, in particularly visual processing, and to the DMN and SN, could be mediating hypervigilance to threat, impaired fear learning and disordered remembering (Dunkley et al., 2014; Liberzon and Abelson 2016; Zhang et al., 2016). In combination with the lower connectivity observed within and between cortical networks this could reflect an imbalance between the higher-order regulatory functions of the cortex and subcortical systems involved in emotion, memory and arousal following trauma that extends far beyond dysfunction of the DMN, SN and ECN alone.
Finally, we were able to relate this connectome-wide alteration in connectivity not just to PTSD but to general trauma exposure. We found that the level of connectivity through these two networks that differentiated PTSD from HC, was also altered in TC but not to the same extent as in those with a PTSD diagnosis. This pattern was identified both in post-hoc analyses and when directly comparing TC to HC in NBS (Figure s13). While very little work has examined functional connectivity across PTSD, trauma-exposure without PTSD, and HC, one other study found disruptions in topological features of the connectome were intermediate in trauma-exposed controls (Jung et al., 2016). These findings support the idea that there may be a continuum of connectome-wide connectivity change related to trauma ranging from healthy trauma-exposed individuals through to patients who develop PTSD. Examining patterns of connectivity across intrinsic networks present in NBS subnetworks (Figure s8 and s9) which is backed up by direct comparison of TC and PTSD and HC in NBS (Figure s11 and s12) provides additional evidence for specific functional connections that may be compensating or providing resilience to PTSD symptoms. In particular connections between the ECN, attentional networks and visual networks are increased in TC relative to PTSD. This could reflect better regulation of emotion and identification of emotionally salient sensory information in TC. This is supported by findings in task-based fMRI studies that show increased cognitive control network activity during emotion regulation tasks is associated with resilience to PTSD symptoms in trauma-exposed individuals (Blair et al., 2013; White et al., 2018). Additionally, the NBS analysis suggests hyperconnectivity within limbic, the temporal pole and hippocampus, and visual regions in PTSD compared to TC, which may suggest key differences in consolidation of memories in these two groups. Overall these findings suggest that a range of connectome-wide changes may occur response to the experience of trauma itself, but that protective mechanisms and/or compensatory alterations in specific networks could provide varying degrees of resilience to some, while in others the extent of alterations in global connectivity continue to contribute to the development of PTSD.
The study had the following limitations. Both a strength and limitation of our study is the use of healthy, non-trauma-exposed controls as our primary comparative group, followed up by investigating network differences in a much smaller group of trauma-exposed controls. Previous seed-based fMRI studies have shown that TC differ from both HC and PTSD and results can differ dramatically depending on which group is used as controls (Kennis et al., 2015; Wang et al., 2016). We attempted to explore this by investigating TC connectivity within the framework of differences observed between PTSD and HC as well as with a supplementary analysis of TC and PTSD using NBS which can better identify specific differences between these groups. The TC and PTSD patients also differed significantly in time since trauma and experience of childhood trauma, and while we found no statistical evidence, these factors may be contributing to connectivity differences. Additionally, we did not have enough detailed data regarding severity, onset, age of and type of trauma to fully understand how these factors may be related to degree of connectivity change. Finally, it is important to emphasise that the intrinsic connectivity measure derived here differs from pure resting state functional connectivity. In particular, the connectivity of these networks, derived from task-based fMRI, may reflect a primed state of connectivity, rather than spontaneous ongoing neural activity observed in pure resting state which may better reflect the trait-based neural architecture of the brain (Seeley et al., 2007).
In conclusion, this research identified extensive connectome-wide differences in PTSD. This is important as previous studies which isolate areas of interest may bias the field and development of treatments to specific areas when in fact global connectivity changes are occurring. This study provides a novel neurobiological understanding of PTSD, presenting a characteristic connectome of hypo-connected cortical networks relative to hyper-connected autonomic and limbic networks. The imbalance across these two systems could help explain the symptoms of PTSD. Additionally, alterations of connectivity for both these networks in trauma-controls suggested there may be a ‘sliding-scale’ of neural changes following trauma and in the development of PTSD. Further study of how measures of global connectivity characterize PTSD could contribute to the identification of biomarkers for risk, diagnosis and optimal treatment of PTSD.
Funding and conflicts of interest
The study was funded by the National Health and Medical Research Council, Australia (1073041). The authors have no completing financial interests to disclose in relation to the work described.
Data availability
Data is not publicly available due to ethical restrictions, however we are happy to make the data available upon request.
CRediT authorship contribution statement
Isabella A. Breukelaar: Investigation, Methodology, Formal analysis, Writing – original draft, preparation. Richard A. Bryant: Conceptualization, Methodology, Writing – review & editing, Supervision, Resources, Funding acquisition, Project administration. Mayuresh S. Korgaonkar: Conceptualization, Methodology, Writing – review & editing, Supervision, Resources, Project administration.
Declaration of competing interest
All authors report no conflict of interest.
Acknowledgements
We acknowledge the support of the staff at Westmead Radiology for assistance collecting the MRI data and the clinicians of the UNSW Posttraumatic Stress Clinic for performing clinical interviews and assessing patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100321.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abdallah C.G., Averill L.A., Akiki T.J., Raza M., Averill C.L., Gomaa H., Adikey A., Krystal J.H. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu. Rev. Pharmacol. Toxicol. 2019;59:171–189. doi: 10.1146/annurev-pharmtox-010818-021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achaibou A., Loth E., Bishop S.J. Distinct frontal and amygdala correlates of change detection for facial identity and expression. Soc. Cognit. Affect Neurosci. 2016;11(2):225–233. doi: 10.1093/scan/nsv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatr. Rep. 2017;19(11):81. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.M., Goldstein-Piekarski A.N., Gatt J.M., Williams L.M. Quantifying person-level brain network functioning to facilitate clinical translation. Transl. Psychiatry. 2017;7:e1248. doi: 10.1038/tp.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Vythilingam M., Crowe S.L., McCaffrey D.E., Ng P., Wu C.C., Scaramozza M., Mondillo K., Pine D.S., Charney D.S., Blair R.J.R. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol. Med. 2013;43(1):85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatr. Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Krystal J.H., Putnam F.W., Southwick S.M., Marmar C., Charney D.S., Mazure C.M. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS) J. Trauma Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Vincent J.L. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37(4):1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097-1099. [DOI] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Rauch S.A.M., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley B.T., Doesburg S.M., Sedge P.A., Grodecki R.J., Shek P.N., Pang E.W., Taylor M.J. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage: Clinical. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L., Miezin F.M., Dosenbach N.U., Wenger K.K., Fox M.D., Snyder A.Z., Raichle M.E., Petersen S.E. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L., Yang Z., Chu C., Xie S., Laird A.R., Fox P.T., Eickhoff S.B., Yu C., Jiang T. The human brainnetome Atlas: a new brain Atlas based on connectional architecture. Cerebr. Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16(3):159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E. Network scaling effects in graph analytic studies of human resting-state fMRI data. Front. Syst. Neurosci. 2010;4:22. doi: 10.3389/fnsys.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Goldstein-Piekarski A.N., Staveland B.R., Ball T.M., Yesavage J., Korgaonkar M.S., Williams L.M. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry. 2018;8(1):57. doi: 10.1038/s41398-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebr. Cortex. 2014;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W.H., Chang K.J., Kim N.H. Disrupted topological organization in the whole-brain functional network of trauma-exposed firefighters: a preliminary study. Psychiatr. Res. Neuroimaging. 2016;250:15–23. doi: 10.1016/j.pscychresns.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Xu Q., Zhong Y., Liu T., Li J., Lu G., Chen F. Typhoon-related post-traumatic stress disorder and trauma might lead to functional integration abnormalities in intra- and inter-resting state networks: a resting-state fmri independent component analysis. Cell. Physiol. Biochem. 2018;48(1):99–110. doi: 10.1159/000491666. [DOI] [PubMed] [Google Scholar]
- Kennis M., Rademaker A.R., van Rooij S.J.H., Kahn R.S., Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum. Brain Mapp. 2015;36(1):99–109. doi: 10.1002/hbm.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Grieve S.M., Etkin A., Koslow S.H., Williams L.M. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38(5):863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Ram K., Williams L.M., Gatt J.M., Grieve S.M. Establishing the resting state default mode network derived from functional magnetic resonance imaging tasks as an endophenotype: a twins study. Hum. Brain Mapp. 2014;35(8):3893–3902. doi: 10.1002/hbm.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu A., Yasaka K., Akai H., Kunimatsu N., Abe O. MRI findings in posttraumatic stress disorder. J. Mag. Resonance Imag. 2019 doi: 10.1002/jmri.26929. in Press. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Brand B., Vermetten E., Frewen P.A., Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress. Anxiety. 2012;29(8):701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- Lei D., Li K., Li L., Chen F., Huang X., Lui S., Li J., Bi F., Gong Q. Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology. 2015;276(3):818–827. doi: 10.1148/radiol.15141700. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Abelson James L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92(1):14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J., Ebner S., Loriaux A., Roitman M. Encoding of aversion by dopamine and the nucleus accumbens. Front. Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. vol. 1. 2017. Neurobiological and systemic effects of chronic stress. (Chronic Stress). Thousand Oaks, Calif. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W., McKinnon M.C., Lanius R.A. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology : Off. Pub. Am. College Neuropsychopharmacol. 2015;40(10):2317–2326. doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E.A., Kaiser R.H., Pizzagalli D.A., Rauch S.L., Rosso I.M. Regional prefrontal resting-state functional connectivity in posttraumatic stress disorder. Biol. Psychiatr.: Cognitive Neuroscience and Neuroimaging. 2019;4(4):390–398. doi: 10.1016/j.bpsc.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(4):319–327. doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Diez I., Ortiz-Terán L., Bonadio C., Liddell T., Goñi J., Sepulcre J. Positive connectivity predicts the dynamic intrinsic topology of the human brain network. Front. Syst. Neurosci. 2018;12:38. doi: 10.3389/fnsys.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni I., Bonne O., Giesser R., Shragai T., Lazarovits G., Isserles M., Schreiber S., Bick A.S., Levin N. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp. 2016;37(2):589–599. doi: 10.1002/hbm.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M.C., Cisler J.M. Altered large-scale functional brain organization in posttraumatic stress disorder: a comprehensive review of univariate and network-level neurocircuitry models of PTSD. Neuroimage: Clinical. 2020;27:102319. doi: 10.1016/j.nicl.2020.102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.N., Holmes A.J., Eickhoff S.B., Yeo B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebr. Cortex. 2018;28(9):3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A., Liberzon I., Marmar C. Post-traumatic stress disorder. N. Engl. J. Med. 2017;376(25):2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Liao W., Zhang W. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 1998;59(Suppl. 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Siegel J.S., Power J.D., Dubis J.W., Vogel A.C., Church J.A., Schlaggar B.L., Petersen S.E. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp. 2014;35(5):1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann. N. Y. Acad. Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo X., Lei D., Li K., Chen F., Li F., Li L., Huang X., Lui S., Li L., Kemp G.J., Gong Q. Disrupted brain network topology in pediatric posttraumatic stress disorder: a resting-state fMRI study. Hum. Brain Mapp. 2015;36(9):3677–3686. doi: 10.1002/hbm.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Margulies D.S., Breakspear M., Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 2020;23(11):1421–1432. doi: 10.1038/s41593-020-00711-6. [DOI] [PubMed] [Google Scholar]
- Tursich M., Ros T., Frewen P.A., Kluetsch R.C., Calhoun V.D., Lanius R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 2015;132(1):29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31(44):15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang L., Zang Y., Yang H., Tang H., Gong Q., Chen Z., Zhu C., He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum. Brain Mapp. 2009;30(5):1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Huang H., Zhu H., Kemp G.J., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep. 2016;6 doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.F., Costanzo M.E., Thornton L.C., Mobley A.M., Blair J.R., Roy M.J. Increased cognitive control and reduced emotional interference is associated with reduced PTSD symptom severity in a trauma-exposed sample: a preliminary longitudinal study. Psychiatr. Res. Neuroimaging. 2018;278:7–12. doi: 10.1016/j.pscychresns.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Harding I.H., Cocchi L., Yucel M., Pantelis C., Bullmore E.T. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50(3):970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen H., Long Z., Cui Q., Chen H. Altered effective connectivity network of the thalamus in post-traumatic stress disorder: a resting-state FMRI study with granger causality method. Appl. Inf. 2016;3(1):8. [Google Scholar]
- Zhang Y., Liu F., Chen H., Li M., Duan X., Xie B., Chen H. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is not publicly available due to ethical restrictions, however we are happy to make the data available upon request.