Abstract
Background
In patients with ACS, risk assessment at hospital discharge has not received much consideration in prior risk scoring systems. Hence, there is a need for a reliable and simple tool to identify patients with high mortality risk at discharge form the hospital.
Methods
In a 1-year observational, prospective study, 1012 patients admitted with ACS were followed up for 6 months after discharge. From 26 potential variables, a new risk score to predict 6-month mortality was developed.
Results
A multi-variant Cox regression analysis with forward stepwise variable selection was performed and 10 highly significant independent predictors of 6-month mortality were identified. These include previous history of ACS, higher Killip class at admission, NYHA class at discharge, recurrent ischemia during hospital stay, heart failure, requiring ionotropic supports, requiring hemodialysis, presence of arrhythmia, left ventricular dysfunction detected on echocardiography and elevated admission blood glucose levels. Points were given to each variable and a total score was calculated. A risk score of 0–4 (low risk) predicted a mortality of 3.7%,a risk score of 5–15 (Intermediate risk) predicted a mortality of 16.4% and a risk score of 11–15 predicted a mortality of 32.0% over a 6-month period. The new risk score was noninferior to GRACE risk score in its predictive accuracy of 6-month mortality in the same cohort of patients (p < 0.05).
Conclusion
The risk score developed in our study can be easily calculated at the bedside and is aimed at identifying high risk patients who require more intense follow up after discharge.
Keywords: Acute coronary syndrome, Hospital discharge, Risk score, Mortality
1. Introduction
The prognosis of a patient admitted with acute coronary syndrome (ACS) surviving up to hospital discharge varies. In patients admitted with ACS, post-discharge morbidity and mortality remains a main concern that needs to be addressed.1 A number of risk scores have been developed to assess mortality risk. However, in-hospital mortality has been included in the analysis of most of these risk prediction tools. Thus, the earlier risk score of ACS has been calculated from the time of admission in most of the studies.2,3
Earlier studies have looked at the risk scores in ACS at the time of admission.4 However; the risk assessment at hospital discharge has not been given much consideration. There are only a few risk scores developed previously regarding six-month mortality post discharge after ACS. One such score is the large multinational observational Global Registry of Acute Coronary Events (GRACE) risk score. GRACE risk score has been used for deriving regression models to predict death during hospital stay5 and death after discharge in patients across entire spectrum of ACS.6
The data collected from 1999 to 2003 was used to develop and validate GRACE risk score compiling nine predictors.6 Management of ACS has changed in the past decade with more widespread use of newer medications and more patients undergoing increasing number of interventional procedures like PCI. The present study is done to identify the strongest parameters independently associated with mortality in individual patients, up to six months after discharge following hospitalization with an ACS and to develop a risk score based on the factors which are significant in multi-variant analysis.
2. Methods
The present study was conducted at a tertiary care hospital as a prospective observational study for a period of one year from 1st March, 2016 to 28th February, 2017. All patients more than 18 years of age who were admitted with the diagnosis of ACS including ST Elevation Myocardial Infarction (STEMI) and non-ST-elevation acute coronary syndrome (NSTE-ACS)7,8 to the intensive cardiac care unit (ICCU) of the hospital and who survived to hospital discharge were included in the study. The patients excluded from the study included those who were admitted for chest pain but who were not diagnosed to have ACS at discharge. Patients who did not give consent for the study, who died during first hospital stay and those who could not be contacted during follow up were also excluded from the study.
The 26 potential predictor variables studied included age, gender, previous history of ACS and heart failure, initial heart rate, blood pressure, Killip class, cardiac arrest on admission, left ventricular ejection fraction (LVEF), elevated initial cardiac markers, ST segment deviation, requiring coronary angiography, in-hospital percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), New York Heart Association (NYHA) class at discharge, serum creatinine concentration at discharge, requiring hemodialysis, admission glucose and hemoglobin. Cardiac complications included were recurrent ischemia during hospital stay, cardiogenic shock, requiring inotropic supports, heart failure, use of intra-aortic balloon pumps, presence of any arrhythmia (supraventricular arrhythmias, atrial fibrillation, ventricular arrhythmias, brady-arrhythmias), requiring anti-arrhythmic agents, requiring direct current (DC) cardioversion and requiring temporary pacemaker (TPM) insertion.
The patients were followed up for 6 months after discharge. The period of observation ended at 6 months’ post-discharge or the death of the patient whichever was earlier.
3. Statistical analysis
From the 26 potential predictor variables (variables identified from patient’s history, at the time of hospital admission, during hospital stay and at the time of discharge), a new risk score for 6-month mortality was developed using Cox proportional hazard model. Univariate relation between all the parameters and 6-month mortality was assessed by Cox regression analysis. All the variables found significant on univariant analysis were tested by multivariate stepwise Cox regression analysis (forward elimination). A new risk score was developed using highly significant parameters identified on multivariate analysis to predict the 6-month mortality. GRACE risk score calculation was done in all the patients in the present study since GRACE score considered patients with entire spectrum of ACS at the time of discharge as in the case of our study. Age, history of heart failure, history of myocardial infarction, increased heart rate at rest, low systolic blood pressure on admission, increased initial serum creatinine; increased cardiac biomarkers, ST-segment depression and not having in-hospital PCI were used for GRACE scoring. C-statistic or area under the receiver operator characteristic curve was used to assess the predicted accuracy of the risk score. The newly developed risk score was compared with GRACE risk score by differences between 2 areas under the curve in the 95% confidence interval (CI).
The area under curve for the new risk score was calculated using Receiver Operating Characteristics Curve. To validate the new risk score, it was compared with Grace Score by using the formula and p-value = 2∗(1-NORMSDIST (z)). Where Area1, Area2, S.EArea1 and S.EArea2 were calculated using ROC. A p value of <0.05 was considered statistically significant. All statistical analysis was performed using SPSS (Statistical Packages for Social Sciences, version 21.0. Armonk, NY: IBM corp.).
4. Results
Out of 1082 patients who presented with an acute coronary syndrome from 1st March, 2016 to 28th February, 2017 and discharged alive, 1012 (93.5%) had complete 6-month follow-up and represented the development cohort for a model to assess the patient risk score. Seventy patients (6.5%) were lost to follow-up. Seventy-four patients (7.3%) died within six months of discharge.
Two hundred and thirty (230) patients had STEMI (22.7%) and 782 patients had NSTE-ACS (77.3%). The baseline characteristics are presented in Table 1, Table 2. The cardiac complications suffered by the patients with ACS were studied (Table 3). Recurrent ischemia during hospital stay was present in 8.55% of patients. Cardiogenic shock was present in 6.9% of patients and 35.6% of patients developed heart failure. Arrhythmias were noted in 18.6% of patients of which 9.8% patients had supraventricular arrhythmias, 4.3% had ventricular arrhythmias and 5.3% had brady-arrhythmias. Anti-arrhythmic agents were used in 14.1% of patients, 1.6% required DC Cardioversion, and a temporary pacemaker was inserted in 3.4% of patients.
Table 1.
Demographic and Clinical profile of patients.
| Characteristics | Total (n = 1012) | STEMI (n = 230) | NSTE-ACS (n = 782) |
|---|---|---|---|
| Age (years) | 62 ± 12.39 | 59 ± 10.77 | 63 ± 12.64 |
| Gender n (%) | |||
| Male | 656 (64.8) | 168 (73.0) | 488 (62.4) |
| Female | 356 (35.2) | 62 (27.0) | 294 (37.6) |
| Heart Rate | 89 ± 25.20 | 82 ± 19.91 | 91 ± 26.23 |
| Systolic Blood Pressure | 129 ± 29.25 | 121 ± 27.96 | 131 ± 29.28 |
| Diastolic Blood Pressure | 80 ± 15.79 | 76 ± 16.91 | 80 ± 15.33 |
| Cardiac enzymes | 518 (51.2) | 230 (100.0) | 288 (36.8) |
| LVEF | 46 ± 10.41 | 44 ± 8.27 | 47 ± 10.91 |
| Creatinine | 1 ± 0.78 | 1 ± 0.84 | 1 ± 0.77 |
| Admitting Glucose | 171 ± 83.46 | 173 ± 92.21 | 170 ± 80.76 |
| Hemoglobin | 12 ± 2.13 | 13 ± 2.16 | 12 ± 2.09 |
Table 2.
Clinical characteristics.
| Characteristics | Total (n = 1012) |
STEMI (n = 230) |
NSTE-ACS (n = 782) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Previous history of ACS | 264 (26.1) | 44 (19.1) | 220 (28.1) |
| Killip class | |||
| 1 | 620 (61.3) | 154 (67.0) | 466 (59.6) |
| 2 | 268 (26.5) | 38 (16.5) | 230 (29.4) |
| 3 | 56 (5.5) | 10 (4.3) | 46 (5.9) |
| 4 | 68 (6.7) | 28 (12.2) | 40 (5.1) |
| Cardiac arrest on admission | 6 (0.6) | 2 (0.9) | 4 (0.5) |
| ST-segment Deviation | 726 (71.7) | 230 (100.0) | 496 (63.4) |
| Requiring CAG | 870 (86.0) | 230 (100.0) | 640 (81.8) |
| In hospital PCI | 155 (15.3) | 60 (26.1) | 95 (12.1) |
| NYHA at discharge | |||
| 1 | 906 (89.5) | 212 (92.2) | 694 (88.7) |
| 2 | 102 (10.1) | 18 (7.8) | 84 (10.7) |
| 3 | 4 (0.4) | 0(0) | 4 (0.5) |
| 4 | 0(0) | 0 (0) | 0(0) |
| Requiring Hemodialysis | 6 (0.6) | 2 (0.9) | 4 (0.5) |
Table 3.
Cardiac complications.
| Characteristics | Total (n = 1012) |
STEMI (n = 230) |
NSTE-ACS (n = 782) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Recurrent ischemia during hospital stay | 86 (8.5) | 28 (12.2) | 58 (7.4) |
| Cardiogenic Shock | 70 (6.9) | 30 (13.0) | 40 (5.1) |
| Requiring Inotropes | 68 (6.7) | 24 (10.4) | 44 (5.6) |
| Heart failure | 360 (35.6) | 54 (23.5) | 306 (39.1) |
| Use of Intra-Aortic Balloon Pump | 2 (0.2) | 0 (0) | 2 (0.3) |
| Any Arrhythmia | 188 (18.6) | 46 (20.0) | 142 (18.2) |
| a) Supraventricular Arrhythmia | 99 (9.8) | 9 (3.9) | 90 (11.5) |
| b) Ventricular Arrhythmia | 44 (4.3) | 16 (7.0) | 28 (3.6) |
| c) Brady Arrhythmia | 54 (5.3) | 26 (11.3) | 28 (3.6) |
| Requiring Anti-Arrhythmic Agents | 143 (14.1) | 25 (10.9) | 118 (15.1) |
| Requiring DC Cardioversion | 16 (1.6) | 8 (3.5) | 8 (1.0) |
| Requiring Temporary Pacemaker (TPM) Insertion | 34 (3.4) | 18 (7.8) | 16 (2.0) |
On univariant analysis, 20 variables were found to be statistically significant as potential predictors of 6-month mortality. They include age, previous history of ACS and heart failure, initial heart rate, blood pressure, Killip class, left ventricular ejection fraction, ST segment deviation, requiring coronary angiography, in-hospital PCI/CABG, NYHA class at discharge, serum creatinine concentration at discharge, requiring hemodialysis, admission glucose and hemoglobin, recurrent ischemia during hospital stay, cardiogenic shock, requiring inotropic supports, heart failure, presence of any arrhythmia, requiring anti-arrhythmic agents.
From all of the available 20 candidate variables, a Cox proportional hazard model was used with forward stepwise variable selection and 10 highly significant independent predictors of six-month mortality were identified. By multi-variant Cox regression analysis, the 10 independent factors that increased the risk of 6-month mortality include previous history of ACS, higher Killip class at admission, NYHA at discharge, recurrent ischemia during hospital stay, heart failure, requiring inotropic supports, requiring hemodialysis, presence of arrhythmia, left ventricular dysfunction and hyperglycemia at admission (Table 4).
Table 4.
Multivariate analysis for predictors of 6 month mortality.
| Coefficient | p-value | Hazard Ratio | 95% C.I |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Previous history of ACS | 1.721 | <0.001 | 5.590 | 3.027 | 8.730 |
| Requiring Hemodialysis | 1.289 | 0.05 | 3.629 | 0.980 | 11.438 |
| Recurrent ischemia | 1.327 | <0.001 | 3.768 | 2.032 | 6.987 |
| Requiring Inotropes | 0.768 | 0.027 | 2.156 | 1.092 | 4.257 |
| Heart failure | 1.264 | 0.001 | 3.540 | 1.642 | 7.111 |
| Any Arrhythmia (Supraventricular and Ventricular Tachy-arrhythmias, and Brady-arrhythmias) | 1.325 | <0.001 | 3.762 | 2.185 | 6.480 |
| Killip class ≥ 3 | 0.654 | 0.039 | 1.923 | 1.035 | 3.573 |
| NYHA at discharge ≥ 2 | 1.424 | <0.001 | 4.156 | 2.235 | 7.726 |
| Glucose > 160 | 1.276 | 0.030 | 3.582 | 1.637 | 6.373 |
| LVEF < 30% | 1.726 | <0.001 | 5.617 | 2.794 | 11.291 |
| LVEF 31–50% | 1.283 | <0.001 | 3.607 | 1.825 | 6.355 |
A new risk score was developed using the 10 independent variables identified on multi-variant Cox regression analysis associated with 6-month mortality. The lowest hazard ratio was 1.923. 1 point was given for a hazard ratio <3.7 and 2 points were given for hazard ratio from 3.7 to 7. The calculated new risk score was a simple sum of variables identified on the multivariate-adjusted risk relation. After analysis, the parameters for which 1 point was given included Killip class 3 or 4 at admission, NYHA class of breathlessness at discharge, heart failure, requiring inotropic supports, requiring hemodialysis, left ventricular dysfunction with LVEF between 31% and 50%.
The significant individual parameters for which 2 points were given included - previous history of ACS, recurrent ischemia during hospital stay, presence of arrhythmia, left ventricular dysfunction with LVEF less than or equal to 30% and sugar levels more than 160 mg/dl at admission (Table 5). The maximum calculated score was 15.
Table 5.
Risk score points for 6 Month mortality.
| PARAMETERS | RISK SCORE POINTS | |
|---|---|---|
| Killip class at admission | ≥3 | 1 |
| NYHA at discharge | ≥2 | 1 |
| Requiring hemodialysis | Yes | 1 |
| Heart failure during hospital stay | Yes | 1 |
| Requiring inotropic supports | Yes | 1 |
| LVEF (%) | 31–50 | 1 |
| LVEF (%) | <30 | 2 |
| Glucose levels (mg/dl) | >160 | 2 |
| Previous history of ACS | Yes | 2 |
| Recurrent ischemia during hospital stay | Yes | 2 |
| Any arrhythmia during hospital stay (Supraventricular and Ventricular Tachy-arrhythmias, and Brady-arrhythmias) | Yes | 2 |
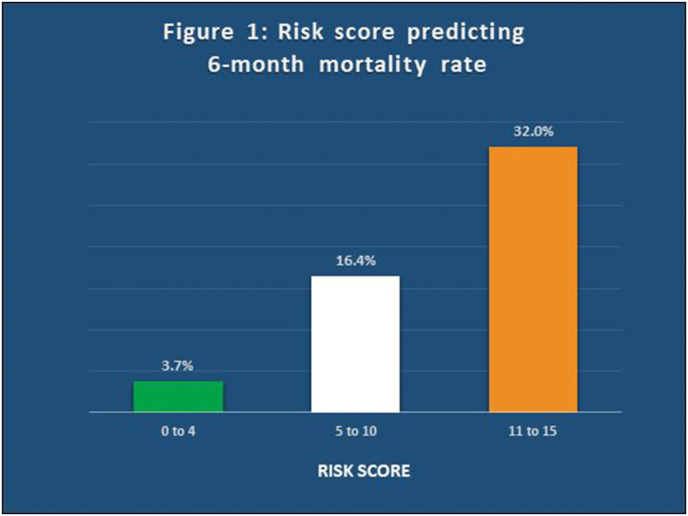
The newly developed risk score was calculated in all the 1012 patients of our study. The mortality rate of each score was also studied. After analysis, patients were grouped into 3 categories and mortality rates were compared. Those patients with risk score between 0 and 4 were considered as low risk with a mortality rate of 3.7%. Those with risk score between 5 and 10 were considered as having intermediate risk with mortality rate of 16.4%. The patients with risk score between 11 and 15 were considered as high risk with mortality rate of 32%. Higher risk scores were associated with higher mortality (Fig. 1).
Fig. 1.
New Risk Score predicting 6-month mortality rate after acute coronary syndrome. Patients were grouped into three categories-low risk (risk score between 0 and 4), intermediate risk (risk score between 5 and 10) and high risk (risk score between 11 and 15).
5. Discussion
The risk score developed in our study can estimate a patient’s post discharge risk of 6-month mortality in all forms of ACS, regardless of their initial electrocardiogram or biomarker results. It provides simplicity and accuracy for long term prognosis by adding independent highly significant parameters.
In patients with ACS, the initial stratification of patients always aims to identify those suitable for reperfusion therapy based on clinical symptoms and ST segment changes on electrocardiogram. Most of the risk scores, like TIMI (Thrombolysis in Myocardial Infarction) and PURSUIT (Platelet Glycoprotein IIb/IIIa In Unstable Angina: Receptor Suppression Using Integrilin) risk scores developed 20 years back, targets this aspect so as to guide the clinicians to decide on an early invasive strategy.2,3 These scores developed for patients with NSTE-ACS considered short term follow up - events at 30 days for PURSUIT Risk score2 and at 14 days for TIMI Risk Score.3 In-hospital mortality was included in their statistics which can give confabulated long-term mortality results. Therefore, there is a need to conduct more studies to assess long term morbidity and mortality after excluding patients who have died during hospital stay.
Killip classification is used to group patients on the basis of simple examination findings into various risk groups. It is a reflection of heart failure and also assesses hypotension. Khot UN et al studied this and concluded that higher Killip class is associated with higher mortality.9 Moreover, Killip class was one of the most powerful predictors of mortality in the GUSTO I trial.10 Higher Killip class of the patient came as a highly significant predictor of all-cause mortality in our study also. The SHOCK (Should we emergently revascularize occluded coronaries for cardiogenic shock) Trial, which included patients with cardiogenic shock and acute myocardial infarction, showed that one of the main causes of cardiogenic shock was ventricular septum rupture in addition to left ventricular dysfunction.11,12 In our study, it was noted that patients requiring inotropic supports for cardiogenic shock was an independent poor prognostic factor on multivariate analysis. A strong association exists between NYHA class of breathlessness and outcomes in patients with left ventricular dysfunction. A higher NYHA class was independently associated with poor outcomes and increased mortality.13
Armstrong et al14 studied patients enrolled in the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO)-II b trial and showed that the recurrent ischemia was associated with a twofold increase in mortality among patients with STEMI and a threefold increase in mortality in patients with non- ST-elevation ACS. A higher risk of mortality in patients with ACS is associated with left ventricular systolic dysfunction.15 Our study also showed a higher all-cause mortality rate at 6 months in patients with left ventricular dysfunction and recurrent ischemia. It was noted that patients requiring hemodialysis were having a higher mortality rate on follow up and was included in the parameters being used to develop a risk score in the present study.
Arrhythmias like atrial fibrillation may exacerbate ischemia and predispose the patient to develop heart failure. Sustained ventricular tachycardia or ventricular fibrillation may cause cardiac arrest, especially if electrical cardioversion is unavailable. Sinus bradycardia and atrioventricular block may occur in patients with inferior wall infarction. But when advanced atrioventricular block complicates anterior infarction, it mostly denotes extensive myocardial injury and ventricular pacing is nearly always required.16 We studied the occurrence of various type of arrhythmias (supraventricular arrhythmias, atrial fibrillation, ventricular arrhythmias, brady-arrhythmias) during hospital stay. When we analyzed the occurrence of these arrhythmias individually and as a group, we found them to be statistically significant on univariant analysis. Therefore, presence of any arrhythmia was included in our risk score.
A study by Lenzen et al17 showed that in patients with CAD with 1-year follow-up, diabetes mellitus is an independent risk factor for mortality and myocardial infarction. But this study also showed that impaired glucose regulation was not an independent predictor of major adverse outcomes in their patients. Our study confirms that patients with blood glucose levels more than 160 mg/dl have a higher risk of mortality at 6 months and has been included in the risk score developed for 6-month mortality.
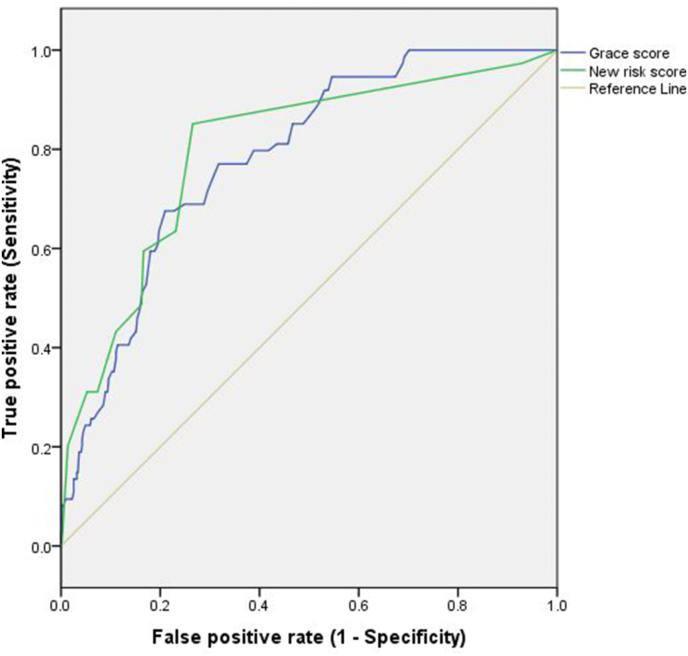
It can be seen that higher risk scores were associated with higher mortality. Hence, risk stratification of all patients is possible at the time of discharge using the score developed in our study. Many patients are apprehensive about their long-term prognosis and how frequently they need to follow up with the clinician after discharge. Aggressive treatment strategies with more frequent follow visits up are needed in those patients who are at high risk of developing a major adverse event. Accuracy for 6-month mortality by the GRACE score and the newly developed risk score in the present study were 0.78 (area under the receiver operator characteristic curve, CI 0.73 to 0.83) and 0.80 (CI 0.75 to 0.85) respectively with p value < 0.05 (Fig. 2). This showed that the newly developed risk score is as good as GRACE Risk Score in predicting mortality.
Fig. 2.
Comparison of new risk score and Grace Score for 6-month mortality. Receiver operator characteristic curves for new risk score (green line), and Global Registry of Acute Coronary Events score (blue line) and no discrimination (Grey line) for 6-month mortality in patients with acute coronary syndrome.
6. Conclusion
Though it may be argued that a simple method may not always be the best, it is more likely that a clinician may calculate a simple risk score rather than a complex one. A simple risk scoring system is less time consuming and it does not require a web-based calculator. Therefore, the simpler risk score developed in our study may find its application, especially in a developing country like India.
The risk score developed in our study can be easily calculated at bedside and is aimed at identifying high risk patients who require a closer follow up. Intense risk factor modification and lifestyle changes are warranted after hospital discharge in all patients following ACS, especially those with higher risk scores.
7. Limitations of our study
1] The population selected is not from a multicenter, and multinational sample, but from a regional Indian population. So, race and ethnic variation was not taken into consideration in our study. 2] Although all parameters in our risk score were known strong predictors of mortality in various multinational studies, further validation on a worldwide registry may be needed for widespread use of our risk model. 3] Patients with atrial fibrillation and those with LV dysfunction have a high chance of thromboembolism and stroke which can lead to increased morbidity and hence affect the outcome. This was not evaluated in our study. 4] The predictive value of this score stops at 6 months after discharge. In order to strengthen the value of the score, longer follow-up is needed.
Contributor Information
Anish John Padiyara, Email: anishjp3@rediffmail.com.
Rajneesh Kumar Calton, Email: caltonrajneesh@yahoo.in.
References
- 1.Fox K.A.A., Carruthers K.F., Dunbar D.R. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study) Eur Heart J. 2010;31:2755–2764. doi: 10.1093/eurheartj/ehq326. [DOI] [PubMed] [Google Scholar]
- 2.Boersma E., Pieper K.S., Steyerberg E.W. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–2567. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- 3.Antman E.M., Cohen M., Bernink P.J. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. J Am Med Assoc. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 4.Bueno H., Fernández-Avilés F. Use of risk scores in acute coronary syndromes. Heart Br Card Soc. 2012;98:162–168. doi: 10.1136/heartjnl-2011-300129. [DOI] [PubMed] [Google Scholar]
- 5.Granger C.B., Goldberg R.J., Dabbous O. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 6.Eagle K.A., Lim M.J., Dabbous O.H. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. J Am Med Assoc. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 7.Roffi M., Patrono C., Collet J.-P. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Kardiol Pol. 2015;73:1207–1294. doi: 10.5603/KP.2015.0243. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 9.Khot U.N., Jia G., Moliterno D.J. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: the enduring value of Killip classification. J Am Med Assoc. 2003;290:2174–2181. doi: 10.1001/jama.290.16.2174. [DOI] [PubMed] [Google Scholar]
- 10.Lee K.L., Woodlief L.H., Topol E.J. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 11.Hochman J.S., Sleeper L.A., Webb J.G. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 12.Valente S., Lazzeri C., Chiostri M., Zucchini M., Giglioli C., Gensini G.F. Intra-aortic balloon pump in intensive cardiac care: a registry in Florence. Int J Cardiol. 2011;146:238–239. doi: 10.1016/j.ijcard.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Muntwyler J., Abetel G., Gruner C., Follath F. One-year mortality among unselected outpatients with heart failure. Eur Heart J. 2002;23:1861–1866. doi: 10.1053/euhj.2002.3282. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong P.W., Fu Y., Chang W.C. Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998;98:1860–1868. doi: 10.1161/01.cir.98.18.1860. [DOI] [PubMed] [Google Scholar]
- 15.Volpi A., De Vita C., Franzosi M.G. Determinants of 6-month mortality in survivors of myocardial infarction after thrombolysis. Results of the GISSI-2 data base. The Ad hoc Working Group of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-2 Data Base. Circulation. 1993;88:416–429. doi: 10.1161/01.cir.88.2.416. [DOI] [PubMed] [Google Scholar]
- 16.Timmis A. Acute coronary syndromes. BMJ. 2015;351:h5153. doi: 10.1136/bmj.h5153. [DOI] [PubMed] [Google Scholar]
- 17.Lenzen M., Ryden L., Ohrvik J. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J. 2006;27:2969–2974. doi: 10.1093/eurheartj/ehl363. [DOI] [PubMed] [Google Scholar]