Abstract
Objective
To compare the outcome of suture mediated vascular closure device Perclose Proglide (PP) with manual compression (MC) following transfemoral access for coronary interventions (CI).
Methods
It is a retrospective, observational, single centre study from January 2018 to September 2019. Consecutive patients undergoing interventions through transfemoral access were divided into PP and MC groups. Those with less than 3 months follow up were excluded. Two groups were compared for baseline characteristics and various complications at 24 h and at 30 days.
Results
Out of 1743 patients studied, PP group included 1343 and MC group, 400 patients. Both groups were comparable in baseline characteristics, sheath size and use of antiplatelets and anticoagulation. PP group had significantly less minor bleeding (P = .01, CI 0.34–4.03) and hematoma (P = .0007, CI 0.95–5.10) at 24 h. At 30 days, minor bleeding (P < .0001, CI 0.97–4.25), hematoma (P = .0002, CI 1.05–4.93) and pseudo-aneurysm (P = .0095, CI 0.03–1.18) were also significantly less in PP group. Obesity (OR 3.5, CI 1.29–9.49) and hypertension (OR 2.41, CI 1.12–5.19) were associated with increased minor bleeding at 24 h. Device failure rate was 2.38%.
Conclusions
PP device is safe, effective and is associated with fewer complications than MC in CI. Device failure rate is low. Obesity and hypertension are associated with increased minor bleeding in both groups.
Keywords: Coronary intervention, Femoral access site complication, Manual compression, Percutaneous coronary intervention, Suture mediated vascular closure device
1. Introduction
Percutaneous coronary interventions (PCI) are on the rise worldwide with more complex coronary lesions being taken up for PCI as an alternative to surgery.1,2 However, despite miniaturization of the catheterization equipment and advances in compression devices, the rate of access site complications (ASC) remains high.3 This has led to increase in the usage of radial artery for coronary interventions as it is associated with considerable reduction in ASC4,5,.6 In recent years, radial approach has gained popularity and has become the standard of care for routine PCI.7,8 However, the choice of hardware is limited by the smaller size of radial artery in coronary interventions.7,8 Large size of the femoral artery permits a wider range of hardware which might be required for the treatment of complex coronary lesions. Traditionally femoral access site hemostasis is achieved by manual compression (MC) which is associated with various drawbacks such as patient discomfort during compression, vasovagal reaction, prolonged immobilization and bleeding.9,10
Femoral access site complications (FASC) remain the Achilles heel of interventional cardiology. Hence adopting measures and acquiring skills to reduce FASC is imperative. Various vascular closure devices (VCD) have evolved over a period of time and these devices have improved patient comfort and reduced local complications.11 Perclose Proglide (Abbot Vascular, Santa Clara, CA, USA) is one such commonly used suture mediated vascular closure device (SMVCD). Perclose Proglide (PP) device works by placing a suture at the puncture site, and this, unlike the other VCD, allows for even immediate re-puncturing of the artery at the site of its deployment. There are only two small observational studies without the control arm on PP device published from India.9,12 The present study was designed with the aim to understand the performance and clinical outcome of femoral artery closure with PP in patients undergoing PCI for coronary lesions compared to the conventional MC.
2. Material and methods
In a retrospectively designed study, all consecutive patients who underwent coronary interventions through femoral access between January 2018 and September 2019 at a tertiary care centre were studied after taking approval from the institutional ethics committee. Coronary interventions (CI) included in the study were interventions in calcified coronary lesions, bifurcation stenosis anywhere in the coronary tree including distal left main, saphenous vein graft stenosis and interventions requiring rotablation or distal protection devices. Femoral artery puncture was done under fluoroscopic guidance in all patients as per institutional practice. Vascular doppler or angiography from contralateral side were not used. Femoral sheath angiogram was done in all patients before PP device deployment. Dual antiplatelets, glycoprotein (Gp) IIb/IIIa inhibitors and unfractionated heparin were used as per current recommendations.13 Activated Clotting Time (ACT) was monitored periodically during the procedures and maintained between 250 and 300 s. Heparin reversal was not done in any patient. It was the operator’s discretion to opt for either PP or MC. Depending on the method used for hemostasis femoral access site, the study population was divided into two groups. PP group included all CI patients who received PercloseProglide device in the study period. In the same period, those who received manual compression were screened and only age and sex matched patients from a larger pool were included in the MC group. All the PP devices were deployed by operators who had the experience of having deployed at least 50 such devices earlier, as per standard recommended techniques.14 For arteriotomies of size 5French (F) to 8 F, one PP has to be used and for arteriotomy of size > 8 F two or more PPs to be used.14 In our study, single PP was used in all except in those who had device failure. In the absence of any post procedure complications, patients in PP and MC groups were ambulated at 4–6 h and 8–12 h respectively. Data collection was done from the digital record of all the patients. Demographic details, atherosclerotic risk factors, medication administered along with details of the cardiovascular intervention were noted. Patients whose follow up records were not available were excluded from the study.
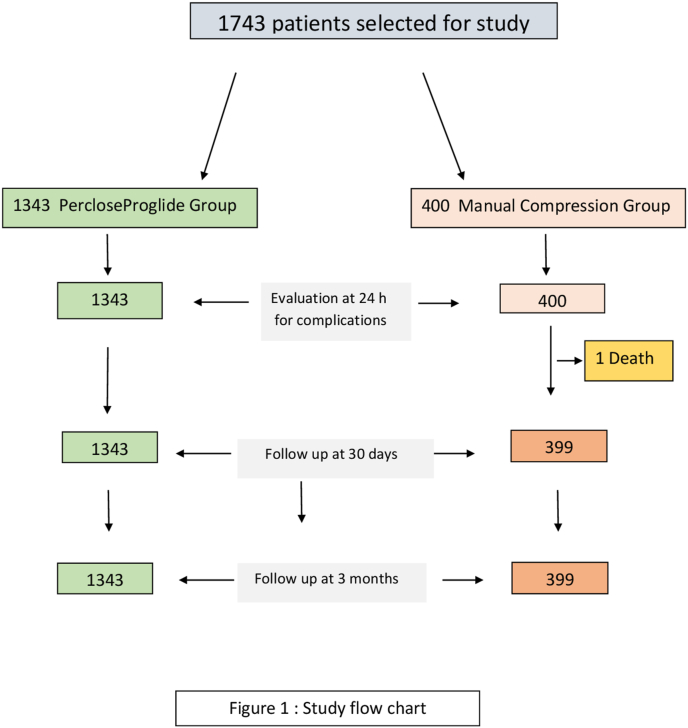
Study population was followed up for a minimum period of 3 months with first review in the cardiology outpatient department at 4–6 weeks post PCI; thereafter patients were reviewed at 3 months or earlier if clinically indicated. A total of 1743 patients were included in the study after scrutinizing the records. There were 1343 patients in PP group and 400 patients in MC group as shown in Fig. 1.
Fig. 1.
Study flow chart.
2.1. Definitions for the study
Device failure –Inability of the PP device to achieve hemostasis due to any of the under mentioned reasons.
-
(a)
Breakage of suture during plunger withdrawal
-
(b)
Failure to form the knot/place the suture despite using correct technique
-
(c)
Failure to achieve complete hemostasis and requirement of manual compression for more than 5 min
Major complication: Following constituted major complications.
-
(a)Major bleeding: Any of the under mentioned condition could qualify as major bleeding
-
•Bleeding causing hemodynamic compromise
-
•Bleeding leading to fall in Hb > 3 g/dl or decrease in hematocrit > 15%
-
•Bleeding requiring blood transfusion of at least 2 units of packed red blood cells
-
•Retroperitoneal bleeding
-
•
-
(b)
Ischemia of limb leading to percutaneous or surgical intervention
-
(c)
Death
-
(d)
Pseudoaneurysm
-
(e)
Major hematoma: Hematoma was defined as localized collection of blood in the tissues outside the blood vessel. Major Hematoma was defined as measuring >5 cm and causing hemodynamic compromise and/or requiring blood transfusion.15
Minor complication: Following constituted minor complications.
-
(a)
Minor bleeding: Persistent oozing/bleeding after initial manual compression of 20 min in MC group. Any other bleeding not meeting criteria for major bleeding.
-
(b)
Persistent pain/inflammation at access site (c) Access site infection
-
(d)
Minor hematoma: Hematoma <5 cm in size and requiring no therapy or requiring only local compression
Procedural success: Achievement of complete haemostasis at access site after PP deployment without requirement of any manual compression or with requirement of minimal compression less than 5 min.
2.2. Statistical methods
Continuous variables were summarized by descriptive statistics such as mean and standard deviation. Categorical variables were reported by frequency and percentage. Continuous variables in the two groups were compared using Student’s t test and categorical variables with Chi-square test or Fisher’s exact test. Risk factors for various complications were analysed using multivariate logistic regression model. Odds ratio (OR) and 95% confidence interval were reported by a table embedded Forest plot. P-value less than 0.05 was considered as the statistical significance. The statistical software R version 3.6.2 (R Core Team, 2019) was used for data analysis.
3. Results
A total of 1743 patients were studied (PP group - 1343 & MC group - 400 patients). PP group patients received a total of 1402 PP devices. The demographic characteristics of the patients and types of interventions performed are shown in Table 1. There was no difference in the baseline characteristics of both the groups. There was no difference in use of antiplatelets, heparin or Gp IIb/IIIa inhibitors in both the groups (Table- 2). Majority of the coronary interventions were done using 7 F sheath (Table 2).
Table 1.
Baseline Characteristics of the study population.
| PP Group (n = 1343) | MC Group (n = 400) | p Value | |
|---|---|---|---|
| Mean Age ± SD (Years) | 51.85 ± 11.06 | 52.78 ± 11.86 | 0.15 |
| Male | 853 (63.5%) | 244 (61%) | 0.36 |
| Diabetes | 606 (45.12%) | 174 (43.5%) | 0.57 |
| Hypertension | 517 (38.5%) | 165 (41.25%) | 0.32 |
| Dyslipidemia | 501 (37.3%) | 155 (38.8%) | 0.59 |
| Obesity | 761 (56.7%) | 234 (58.5%) | 0.52 |
| Smoking | 515 (38.4%) | 142 (35.5%) | 0.29 |
| Chronic Kidney Disease | 29 (2.2%) | 11 (2.8%) | 0.48 |
| PTA for Peripheral Arterial Disease | 40 (2.97%) | 12 (3%) | 0.98 |
| PTCA | 1303 (97.03%) | 388 (97%) | 0.98 |
PP Group:Perclose Proglide group, MC Group: Manual compression group; PTCA: Percutaneous transluminal coronary angioplasty; PTA:Percutaneous transluminal angioplasty.
Table 2.
Details of antiplatelets, anticoagulation and access in two groups.
| PP Group (n = 1343) | MC Group (n = 400) | p value | |
|---|---|---|---|
| Antiplatelets | |||
| Aspirin + Clopidogrel | 1209 (90.02%) | 364 (91%) | 0.56 |
| Aspirin + Ticagrelor | 108 (8.04%) | 32 (8%) | 0.98 |
| Aspirin + Prasugrel | 26 (1.93%) | 4 (1%) | 0.21 |
| Gp IIb/IIIa inhibitors | 68 (5.06%) | 18 (4.5%) | 0.65 |
| Heparin | |||
| 7000–8000 U | 1088 (81.01%) | 318 (79.5%) | 0.50 |
| 10000 U | 255 (18.99%) | 82 (20.5%) | 0.50 |
| Sheath size | |||
| 6 French | 348 (25.91%) | 107 (26.75%) | 0.74 |
| 7or 8 French | 995 (74.09%) | 293 (73.25%) | 0.74 |
The complications and outcome in the two groups are shown in Table 3. At 24 h, major bleeding was noted to be less in PP group but this was not statistically significant (p = .07). All cases of major bleeding were managed with blood transfusion as dictated by the clinical profile. There was one death of a 63-year-old hypertensive and diabetic lady due to major bleeding in MC group. She had triple vessel disease. Following PCI to left anterior descending artery (LAD), she developed a large hematoma along with hypotension. Bed side ultrasound examination showed large groin hematoma with no retroperitoneal extension. She was started on blood transfusion along with continuous groin compression; however, she developed sudden cardiac arrest and could not be revived. Minor bleeding and hematoma were significantly higher in MC group at 24 h. Minor bleeding was managed using either manual compression or Femostop -femoral compression system (Abbott Cardiovascular, CA, USA).
Table 3.
Complications and outcome.
| PP Group (n = 1343) number (%) | MC Group (n = 400) number (%) | p Value | |
|---|---|---|---|
| Complications at 24 h | |||
| Major Bleeding | 1 (0.074%) | 2 (0.5%) | 0.07 |
| Death related to bleeding | Nil | 01 (0.25%) | 0.06 |
| Minor bleeding | 16 (1.19%) | 12 (3%) | 0.01 |
| Hematoma> 5 cm | 18 (1.34%) | 16 (4%) | 0.0007 |
|
Complications at 30 days | |||
| Minor bleeding | 04 (0.298%) | 10 (2.5%) | <0.0001 |
| Hematoma | 12 (0.89%) | 14 (3.5%) | 0.0002 |
| Pseudoaneurysm | 0 | 02 (0.5%) | 0.0095 |
| Access site infection | 02 (0.15%) | 02 (0.5%) | 0.20 |
| Pain and Induration at access site | 16 (1.19%) | 4 (1%) | 0.75 |
Follow up at 30 days revealed that hematoma and minor bleeding were significantly higher in MC group. There were two pseudoaneurysms noted in MC group at 30 days. These were managed with ultrasound guided compression. One of them required one attempt and the other three attempts. Both resolved at 12 weeks of follow up. There was no pseudoaneurysm in PP group. Access site infection was similar in the two groups and responded well to oral antibiotics. Pain and induration at access site were similar in both the groups which resolved without any active intervention. None of the patients at 3 months follow up were noted to have any access site complication.
There were no arterial occlusions or thrombosis, arteriovenous fistula, distal embolization or nerve injury in either group. There were 32 (2.38%) device failures in PP group. Twenty-two device failures were due to breakage of suture while the plunger was being withdrawn. All these cases were bailed out by using a second device. Ten device failures were due to failure to form or place the knot over the femoral artery despite using correct technique. All of these were managed with manual compression.
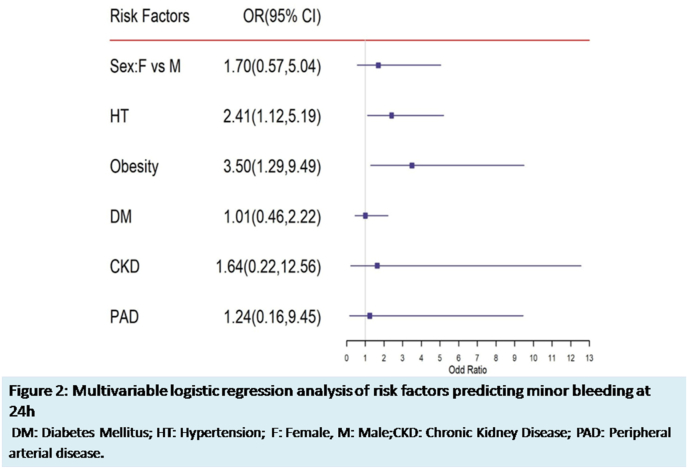
Multivariable logistic regression analysis model was applied to identify the risk factors associated with various complications at 24 h and 30 days. Minor bleeding at 24 h was significantly associated with obesity (OR 4.53, 95% CI 1.63–12.58) and hypertension (OR 2.43, 95% CI 1.12–5.24) as shown in Fig. 2. None of the risk factors predicted the other complications noted at 24 h and 30 days.
Fig. 2.
Multivariable logistic regression analysis of risk factors predicting minor bleeding at 24 h. DM: Disbetes Mellitus; HT: Hypertension; F: Female; M: Male; CKD: Chronic Kidney Disease; PAD: Peripheral arterial disease.
4. Discussion
The safety of the PP device post diagnostic coronary catheterization has been established.9,10 Use of multiple PPs in ‘preclose manner’ has been studied in femoral arteriotomies for TEVAR/EVAR/TAVR up to 24 F.16 However, the safety of use of single PP device without heparin reversal has not been tested in CI which require relatively larger sheaths along with higher dose of anticoagulant and antiplatelet agents. In addition, it is not yet proven whether safety of SMVCD is a class effect or not. In this single-center retrospective study, we describe our experience of PP versus MC in patients undergoing CI with majority of patients having relatively large arterial punctures (7 or 8 F). This is the first comparative study from the Indian subcontinent which has not only demonstrated the safety of the PP or SMVCD but also a significant reduction in ASC with its use in CI with relatively larger access sheaths (7 or 8 F).
The risk factor profile of these patients is in conformity with other published Indian studies which have shown wide heterogeneity in risk factor prevalence.17,18 Our patients were relatively younger compared to western population as coronary artery disease in Indians occurs a decade earlier with higher prevalence of triple vessel disease, complex calcified lesions and higher mortality.17,18
A high procedural success rate of PP deployment (97.6%) was noted in the present study with device failure rate of only 2.4 percent. Earlier studies have reported device failure rate of 3–7 percent.9,10,12,19, 20, 21, 22 An Indian study has reported a failure rate of 4% in 323 patients studied.9 Device failure rates have reduced over time with evolving operator experience. But it has not reached the desired perfection. Breakage of suture while withdrawing the plunger was the mechanism of failure in majority (22 out of 32 failures) of cases in our study. However, this could be managed by reintroducing the 0.035” guide wire and deploying a second device in all these cases. Failure of hemostasis post removal of device from the vessel mandated groin compression either manually or by using a Femo-stop. It is strongly recommended to maintain the wire access to femoral artery while deploying the device in high stake situations to overcome the bleeding due to device failure or due to any other reason.
Majority of the complications were reduced in PP group which is consistent with most of the recent studies.9,10,12,19,20 Major bleeding was less in PP group but this did not reach statistical significance (p = .07). In literature, rate of major bleeding has ranged from 0 to 1.9%.9,10,12,19, 20, 21, 22 A few studies have reported no major bleeding at all with PP10; but this has not been reproduced in other studies. In the present study, groin hematoma and minor bleeding were significantly less with PP at both 24 h and at 30 days, and similar findings have been reported by others.9,10,12,19, 20, 21, 22 There was no pseudoaneurysm formation in PP group despite a large number of device deployments compared to two pseudoaneurysms in MC group. This is consistent with other studies which have reported no or very few pseudoaneurysms with PP devices.9,10,12,21,23 The rate of pseudoaneurysm formation even in MC group was less (0.5%) compared to other studies where it was noted to be 2.9–8% following PCI.24,25 This is probably due to the standard practice at our centre of fluoroscopic guided femoral punctures which has been demonstrated to reduce the complications associated with femoral artery puncture.21,23 SMVCD facilitates early patient ambulation and results in greater patient comfort and satisfaction.9,10 We observed that the patients in PP group could be ambulated early (4–6 h) as compared to a delayed ambulation in MC group (8–12 h). Reduced time to ambulation and greater patient comfort has been well established with use of SMVCD10,21 which comes with an upfront additional cost of the device.
Obesity and hypertension were significantly associated with increased minor bleeding at 24 h in our study. Higher rate of groin complications has been noted with increasing age, female sex, hypertension and obesity in other studies.26, 27, 28 Female sex was associated with higher bleeding in our study but did not reach statistical significance. Age was not found to be a risk factor for groin complications possibly due to relatively younger subjects in our study compared to western population. Thorough literature search did not reveal any study assessing predictors of complications at access site in Indian population.
The strength of the present study lies in the fact that it is one of the largest studies from Indian subcontinent comparing PP with MC in CI. Our study reported not only in-hospital outcomes but also included follow up for 3 months thereby demonstrating the long-term safety of the PP device. The limitation of present study is that it is a single centre study with no randomization. Thereby the possibility of bias in patient selection for PP device cannot be ruled out.
5. Conclusions
PercloseProglide suture mediated closure device is safe and effective at femoral access site compared to manual compression in patients undergoing coronary interventions. Device failure rate is low. Obesity and hypertension are associated with increased minor bleeding.
| ‘What is Already Known?’ SMVCDs are safe and effective in diagnostic catheterization What this Study Adds?’ SMVCD has lesser complications compared to MC in coronary interventions. Device failure rates with PP are low. Obesity and hypertension are associated with increased minor bleeding. |
Source(s) of support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Nil.
Acknowledgements
We sincerely thank Mr SS Rawat, Mr A Patra and Mr S K Tripathi for the help rendered in data collection and Dr S Ghosh for stasitical analysis.
Contributor Information
Ajay Kumar Dabas, Email: ajay13dabas@gmail.com.
Davinder Singh Chadha, Email: agiamu@gmail.com.
Ajay Jagannath Swamy, Email: ajayswamy@rediffmail.com.
References
- 1.Young M.N., Kolte D., Cadigan M.E. Multidisciplinary heart Team Approach for complex coronary artery disease: single center clinical presentation. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu D., Qu J., Zhang H. Revascularization for coronary artery disease: principle and challenges. Adv Exp Med Biol. 2020;1177:75–100. doi: 10.1007/978-981-15-2517-9_3. [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Schüpke S., Helde S., Gewalt S. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. J Am Med Assoc. 2014;312:1981–1987. doi: 10.1001/jama.2014.15305. [DOI] [PubMed] [Google Scholar]
- 4.Alkatiri A.A., Firman D., Haryono N. Comparison between radial versus femoral percutaneous coronary intervention access in Indonesian hospitals, 2017-2018: a prospective observational study of a national registry. Int J Cardiol Heart Vasc. 2020;27:100488. doi: 10.1016/j.ijcha.2020.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante G., Rao S.V., Jüni P. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv. 2016;9:1419–1434. doi: 10.1016/j.jcin.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Kolkailah A.A., Alreshq R.S., Muhammed A.M. Transradial versus transfemoral approach for diagnostic coronary angiography and percutaneous coronary intervention in people with coronary artery disease. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD012318.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel P.K., Menon A., Mullasari A.S. Transradial access for coronary diagnostic and interventional procedures: consensus statement and recommendations for India: advancing Complex CoronariES Sciences through TransRADIAL intervention in India - ACCESS RADIAL™: clinical consensus recommendations in collaboration with Cardiological Society of India (CSI) Indian Heart J. 2018;70:922–933. doi: 10.1016/j.ihj.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason P.J., Shah B., Tamis-Holland J.E. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/HCV.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 9.Vinayakumar D., Kayakkal S., RajasekharanS 24 h and 30 day outcome of PercloseProglide suture mediated vascular closure device: an Indian experience. Indian Heart J. 2017;69:37–42. doi: 10.1016/j.ihj.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekhar A., Sutton B.S., Raheja P. Femoral arterial closure using ProGlide® is more efficacious and cost-effective when ambulating early following cardiac catheterization. Int J Cardiol Heart Vasc. 2016;13:6–13. doi: 10.1016/j.ijcha.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Applegate R.J. Vascular closure device failure: we are getting better but not there yet. JACC Cardiovasc Interv. 2012;5:845–847. doi: 10.1016/j.jcin.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Vijayvergiya R., Rana N., Khanal S. To study the safety and efficacy of vascular closure devices (VCD) after transfemoral PCI. Indian Heart J. 2015;67(S1):S60–S61. [Google Scholar]
- 13.Windecker S., Kolh P., Alfonso F. 2014 ESC/EACTS guidelines on myocardial revascularization:the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS)developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 14.PercloseProGlide 6F Suture-Mediated Closure (SMC) System Instructions for Use: IFU (Full Version). https://eifu.abbottvascular.com/content/dam/av/eifu/EL2122075 Artwork.pdf.
- 15.Ortiz D., Jahangir Singh. Access site complications after peripheral vascular interventions: incidence, predictors, and outcomes. Circulation: Cardiovascular Interventions. 2014;7:821–828. doi: 10.1161/CIRCINTERVENTIONS.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vierhout B.P., Pol R.A., ElMoumni M. Editor’s choice - arteriotomy closure devices in EVAR, TEVAR, and TAVR: a systematic review and meta-analysis of randomised clinical trials and cohort studies. Eur J Vasc Endovasc Surg. 2017;54:104–115. doi: 10.1016/j.ejvs.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakaran D., Jeemon P., Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605–1620. doi: 10.1161/CIRCULATIONAHA.114.008729. [DOI] [PubMed] [Google Scholar]
- 18.KauL U., Natrajan S., Dalal J. Prevalence and control of cardiovascular risk factors in stable coronary artery outpatients in India compared with the rest of the world: an analysis from international CLARIFY registry. Indian Heart J. 2017;69:447–452. doi: 10.1016/j.ihj.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin J.L., Pratsos A., Magargee E. A randomized trial comparing compression, PercloseProglide and Angio-Seal VIP for arterial closure following percutaneous coronary intervention: the CAP trial. Cathet Cardiovasc Interv. 2008;71:1–5. doi: 10.1002/ccd.21333. [DOI] [PubMed] [Google Scholar]
- 20.Bilge M., Alemdar R., Ali S. Efficacy and safety of percutaneous suture-mediated closure devices in interventional cardiology: outcomes of the largest series of percutaneous vascular closure in Turkey. J Am Coll Cardiol. 2013;62(suppl 2) [Google Scholar]
- 21.Bangalore S., Arora N., Resnic F.S. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv. 2009;2:549–556. doi: 10.1161/CIRCINTERVENTIONS.109.877407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidi V.D., Matheny M.E., Govindarajulu U.S. Vascular closure device failure in contemporary practice. JACC Cardiovasc Interv. 2012;5:837–844. doi: 10.1016/j.jcin.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun E.J. Ultrasonographic evaluation of complications related to transfemoral arterial procedures. Ultrasonography. 2018;37:164–173. doi: 10.14366/usg.17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone P.A., Campbell J.E., AbuRahma A.F. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg. 2014;60:1359–1366. doi: 10.1016/j.jvs.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Schneider C., Malisius R., Küchler R. A prospective study on ultrasound-guided percutaneous thrombin injection for treatment of iatrogenic post-catheterisation femoral pseudoaneurysms. Int J Cardiol. 2009;131:356–361. doi: 10.1016/j.ijcard.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Sherev D.A., Shaw R.E., Brent B.N. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Cathet Cardiovasc Interv. 2005;65:196–202. doi: 10.1002/ccd.20354. [DOI] [PubMed] [Google Scholar]
- 27.Al-momani M.S., Aburuz M.E. Incidence and predictors of groin complications early after coronary artery intervention: a prospective observational study. BMC Nurs. 2019;18:24. doi: 10.1186/s12912-019-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz D., Jahangir A., Singh M., Allaqaband S. Access site complications after peripheral vascular interventions:incidence, predictors, and outcomes. Circ Cardiovasc Interv. 2014:821–828. doi: 10.1161/CIRCINTERVENTIONS.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]