Abstract
Background
Potts shunt has been suggested as an effective palliative therapy for patients with pulmonary artery hypertension (PAH) not associated with congenital heart disease.
Materials and methods
This is a prospective single-center study performed to assess outcomes of Potts shunt in patients with PAH who are in functional class III or IV.
Results
52 patients in functional class III/IV with pulmonary arterial hypertension without significant intra or extracardiac shunt on maximal medical therapy were evaluated and counseled for undergoing Potts shunt/patent ductus arteriosus (PDA) stenting. 16/52 patients (13 females) consented for the procedure; 14 patients underwent surgical creation of Potts, and 2 underwent transcatheter stenting of PDA, which physiologically acted like a Potts shunt. Standard medical therapy was continued in patients who did not consent for the procedure. 12/16 patients survived the procedure. Patients who did not survive the procedure were older, with severe right ventricular systolic dysfunction, and functional class IV. Patients who survived the procedure were followed up in the pulmonary hypertension clinic. The Median follow-up was 17 months (1–40 months). 11/13 patients discharged after the operation showed sustained clinical, echocardiographic, and biochemical improvement, which reduced need for pulmonary vasodilator therapy in 10/13 patients. There was one death in the follow-up period 16 months post-surgery due to lower respiratory tract infection.
Conclusion
Potts shunt is feasible in patients with PAH without significant intra or extracardiac shunts. It can be done safely with an acceptable success rate. Patient selection, preoperative stabilization, and meticulous postoperative management are essential. It should be performed at the earliest sign of clinical, echocardiographic, or laboratory deterioation for optimal outcomes.
Long-term follow-up is required to see a sustained improvement in functional class and the need for a lung transplant in the future.
Keywords: Reverse Potts shunt, Right ventricular dysfunction, Bridge to transplant
1. Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease that can present at any age, from infancy to adulthood. Despite advances in medical therapy, prognosis remains guarded, with 5-year survival ranging from 57 to 75%.1 Moreover, prostacyclin analogues, which significantly reduce mortality are still unavailable or cannot be afforded in some developing countries. Until recently, lung or heart-lung transplant was thought to be the only surgical option available, which is also cost-prohibitive with limited availability and guarded long term outcomes.2 Creating Potts shunt is considered as an alternative option in both adult and pediatric patients with PAH who show symptomatic deterioration on maximal medical therapy.3,4 This innovative therapy postulates that by creating an unrestrictive communication between the descending aorta and left pulmonary artery, there would be a reduction in the right ventricular afterload with an improvement of the right ventricular function and right ventricle to pulmonary artery coupling. This would result in the conversion of an idiopathic PAH physiology to Eisenmenger physiology with better functional capacity and survival.5,6 Potts shunt can be effectively created surgically, or as an alternative, one can stent the patent ductus arteriosus (PDA) in patients with probe patent ductus.7,8 The creation of Potts shunt has shown morbidity and mortality benefits with a reduction in the requirement of pulmonary vasodilators.3,9 We report clinical outcomes of the first 16 patients with PAH who underwent Potts shunt at our institute from April 2015–September 2019.
2. Material and methods
This is a prospective observational study from a single tertiary care center from India. Institutional ethics committee clearance was obtained.
Patient selection: Secondary causes of PAH were ruled out as per the protocol.10 Patients with the following criteria were considered for performing Potts shunt.
2.1. Inclusion criteria
-
•
Patients with group I PAH without intra or extracardiac shunt or PAH out of proportion to the shunt
-
•
They were receiving maximal doses of phosphodiesterase 5 inhibitors and endothelin receptor antagonists for at least six months prior to the procedure.
-
•
Patients who could afford prostacyclin analogues were on maximal doses of the same.
-
•
Functional class IV or deterioration in functional class on maximal medical therapy and
-
•
Consented for undergoing Potts shunt.
2.2. Exclusion criteria
-
•
Patients/legal guardians not consenting for undergoing Potts shunt
-
•
Significant intra or extracardiac shunt
Clinical symptoms of syncope, functional class, and right heart failure were asked for in all the patients. Clinical evaluation included upper and lower limb oxygen saturations, signs of right failure like the elevation of the jugular venous pulse, presence of hepatomegaly, and edema feet were noted. X-ray chest and electrocardiography was performed in all the patients. A detailed echocardiogram was performed prior to the procedure, at the time of discharge, and during follow-up in all the patients.
The following parameters were studied during echocardiography.
-
1.
Structural heart defects were ruled out in the initial evaluation
-
2.
Accurate estimation of pulmonary artery pressures whenever possible
-
3.
Right ventricular size and function
-
4.
Pulmonary artery acceleration time (PAAT) and RV Ejection time (ET) were measured using 2 d echocardiogram and Doppler study.
-
5.
Assessment of right atrial pressure, pericardial effusion
N terminal pro-brain natriuretic peptide (NT-proBNP) was measured before, immediately after the procedure and reassessed on follow up.
Computerized tomography (CT) with a pulmonary angiogram was performed in all patients prior to the procedure. CT angiogram was used to ascertain the size of the interposition graft or PDA stent during the procedure (80% of the size of the descending aorta at the level of the diaphragm).9
Cardiac catheterization was performed on 10/16. Six patients did not undergo right heart catheterization due to their vulnerable clinical scenario and unstable hemodynamic condition. Hemodynamic data were obtained in these six patients after induction of general anesthesia prior to surgery. Swan Ganz catheter of appropriate size was inserted through the right internal jugular vein for the same.
2.3. Preoperative stabilization
All patients were admitted 3 ± 1-day prior to the pediatric cardiac intensive care unit. All of them received inotropes (Dopamine), inodilators (milrinone), diuretics, intravenous sildenafil, and oral endothelin receptor blocker (Ambrisentan). Inhaled iloprost was continued in 3 patients. Inhaled nitric oxide was administered pre-operatively in 4/13 patients. Serial NT –Pro BNP was monitored, and the patients were taken up for the procedure after demonstrating a serial drop in NT-pro BNP over 3–5 days and improvement in right heart function on echocardiogram. At least a 30% fall in NT pro-BNP and a similar improvement in the RV functional assessment on echocardiogram was considered sufficient for taking up the patient for Potts shunt.
Procedure: Patients with a probe patent PDA identified on CT angiogram or on cardiac catheterization underwent PDA stent. The remaining patients underwent surgical creation of Potts shunt using an interposition graft.
2.4. Surgical details of Potts shunt
After initial stabilization, 14 out of 16 patients underwent surgical Potts shunt using an interposing tube graft through a left lateral thoracotomy approach without cardiopulmonary bypass (CPB) through the 4th intercostal space. We ensured that we could put the patient on CPB if required via aortic and pulmonary artery cannulation. Polytetrafluoroethylene (PTFE) graft was used for the shunt, and the size of the graft used was 80% of the size of the descending aorta.9(Table 1).
Table 1.
Preoperative demographic profile of the patients.
| Serial number | Age (Years) | Height (Cm) | Weight (Kg) | BSA (m2) | WHO FC | Procedure | Cause of PAH | Size of conduit/stent (mm) | Last follow up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 98 | 14 | 0.6 | IV | PDA stenting | Idiopathic | 6 | 20 |
| 2 | 0.8 | 70 | 6.5 | 0.4 | IV | PDA stenting | Idiopathic | 6 | 28 |
| 3 | 35 | 140 | 51.4 | 1.4 | III | Surgical | SLE | 12 | Expired |
| 4 | 23 | 172 | 49.9 | 1.6 | III | Surgical | Idiopathic | 12 | Expired |
| 5 | 6 | 113 | 15.4 | 0.7 | IV | Surgical | Idiopathic | 7 | 26 |
| 6 | 10 | 122 | 21.4 | 0.9 | IV | Surgical | Idiopathic | 8 | 31 |
| 7 | 5 | 95 | 9.5 | 0.5 | IV | Surgical | Idiopathic | 7 | 24 |
| 8 | 11 | 123 | 28 | 0.6 | III | Surgical | Idiopathic | 8 | 15 |
| 9 | 32 | 154 | 57 | 1.54 | IV | Surgical | Idiopathic | 12 | 17 |
| 10 | 17 | 156 | 42.9 | 1.38 | IV | Surgical | Post VSD closure | 10 | Expired |
| 11 | 1.5 | 85 | 10.6 | 0.5 | IV | Surgical | S/pASO | 7 | 20a |
| 12 | 14 | 165 | 54.5 | 1.6 | IV | Surgical | Idiopathic | 10 | 40 |
| 13 | 8 | 124 | 19.7 | 0.82 | III | Surgical | Idiopathic | 8 | 16 |
| 14 | 0.9 | 70 | 7 | 0.36 | IV | Surgical | Idiopathic | 6 | Expired |
| 15 | 16 | 156 | 62 | 1.4 | IV | Surgical | Idiopathic | 10 | 7 |
| 16 | 18 | 146 | 46 | 1.2 | IV | Surgical | Idiopathic | 10 | 5 |
BSA= Body surface area. WHOFC: World health organization functional class, PDA = patent ductus arteriosus.S/p ASO: S/p arterial switch operation for TGA with intact inter ventricular septum done at 5 days of life.
Expired 20 months post procedure secondary to lower respiratory tract infection.
2.5. Stenting of the duct
Potts shunt can also be created by stenting the PDA if present.11,12 Two patients underwent PDA stenting. The procedure was performed under general anesthesia. The femoral artery and venous accesses were obtained. An aortic angiogram was done in the lateral view to demonstrate the PDA. The PDA was stented using the antegrade approach in one and retrograde approach in the second patient. 6 mm bare-metal stents were used in both the patients.
2.6. Post-operative management
All the patients were managed postoperatively in the pediatric cardiac ICU. They were shifted to the PCICU on inhaled nitric oxide (20–30 ppm), IV sildenafil 1.6 mg/kg/day, IV milrinone (0.5–0.7 mcg/kg/min) and adrenaline infusion (0.04–0.08 mcg/kg/min). Postoperative monitoring included arterial pressure, upper limb and lower limb saturations, and Pao2. The inotropes and pulmonary vasodilators were finely tuned to achieve a difference in upper and lower limb Spo2 of 15–20% while maintaining normal cardiac output.
2.7. Discharge
Pulmonary vasodilators were continued at the time of discharge; anti-failure medications were continued as clinically indicated; additionally, all patients received antiplatelet agents.
2.8. Follow up
All patients who survived the procedure were followed up in the institutional PAH clinic. Pulmonary vasodilators were adjusted based on the functional class, echocardiographic findings, and difference in the upper and lower limb saturations. Improvement in functional class, shunting of the blood across Potts shunt, improvement in right ventricular function, and decrease in NT-proBNP levels were used as criteria to modify the pulmonary vasodilators on follow up.
Survival analyses: Survival analysis was performed for the entire cohort as well as comparing patients who underwent Potts shunt/PDA stenting by using Kaplan Meier graphs. Patients who underwent Potts shunt/PDA stenting were divided into two groups depending on the age (<16 years/≥ 16 years) right ventricular function (TAPSE ≤ 12 mm/TAPSE >12 mm), right atrial pressure (RAP < 8 mm Hg/≤ 8 mm Hg) and cardiac index (CI > 2.5 l/min/m2/CI ≤ 2.5 l/min/m2) and the survival was compared using Kaplan Meier graphs.
2.9. Statistical analyses
Statistical analyses were performed using SPSS 20 software. Parametric data are expressed as mean ± standard deviation, and non-parametric data are expressed as median with ranges. Student t-test and Mann Whitney U tests were performed to compare the parametric and non-parametric data, respectively. Kaplan Mein survival graph was plotted, and the Log-rank test was used to compare survival between groups.
3. Results
Fifty-two patients with pulmonary arterial hypertension without significant intra or extracardiac shunt in functional class III/IV on maximal medical therapy were evaluated and counseled for undergoing Potts shunt/PDA stenting.
16/52 (32%) patients (13 females) consented for the procedure and underwent Potts shunt (14 surgical and 2 PDA stent) for PAH in our center. The median age was eight years. Demographic and hemodynamic data are presented in Table 1, Table 2, respectively. Echocardiographic data is presented in Table 3. 10/14 patients in the surgical group and 2/2 patients of the stenting group survived the procedure.
Table 2.
Hemodynamic data parameters.
| Serial number | SPAP | DPAP | MPAP | RAP | SAoP | DAoP | MAoP | LAP | CI | PVRI | SVRI | Rp/Rs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | 44 | 62 | 6 | 92 | 46 | 61 | 6 | 3.3 | 17.0 | 16.7 | 1.02 |
| 2 | 66 | 32 | 44 | 8 | 100 | 68 | 79 | 8 | 3.1 | 11.6 | 22.9 | 0.51 |
| 3a | 116 | 76 | 89 | 16 | 108 | 68 | 81 | 4 | 2.1 | 34.8 | 36.7 | 0.95 |
| 4a | 126 | 82 | 97 | 14 | 110 | 68 | 82 | 7 | 2.2 | 37.7 | 34.1 | 1.11 |
| 5 | 116 | 82 | 100 | 16 | 106 | 60 | 82 | 13 | 2.8 | 30.0 | 24.6 | 1.22 |
| 6a | 98 | 46 | 63 | 10 | 92 | 46 | 61 | 11 | 2.4 | 22.1 | 20.8 | 1.06 |
| 7a | 88 | 44 | 59 | 12 | 84 | 46 | 59 | 10 | 2.1 | 22.4 | 23.3 | 0.96 |
| 8 | 96 | 64 | 76 | 8 | 88 | 60 | 72 | 8 | 3.1 | 21.9 | 20.6 | 1.06 |
| 9a | 116 | 76 | 89 | 16 | 106 | 72 | 83 | 6 | 2.8 | 26.1 | 27.5 | 0.95 |
| 10a | 136 | 78 | 97 | 18 | 114 | 72 | 86 | 4 | 2.2 | 35.9 | 37.3 | 0.96 |
| 11 | 98 | 62 | 74 | 12 | 88 | 58 | 68 | 6 | 2.1 | 29.5 | 29.5 | 1.00 |
| 12 | 126 | 58 | 81 | 10 | 119 | 52 | 74 | 8 | 3.2 | 22.2 | 20.6 | 1.08 |
| 13 | 110 | 62 | 78 | 8 | 100 | 58 | 72 | 10 | 3.4 | 20.6 | 18.2 | 1.13 |
| 14 | 88 | 58 | 68 | 16 | 76 | 50 | 59 | 8 | 2.4 | 21.7 | 21.3 | 1.02 |
| 15 | 120 | 64 | 83 | 8 | 110 | 64 | 79 | 6 | 3.1 | 24.2 | 23.5 | 1.03 |
| 16 | 116 | 68 | 84 | 6 | 100 | 60 | 73 | 4 | 3.5 | 22.3 | 19.7 | 1.13 |
In all other patients the data is obtained in the cath lab under local anesthesia, SPAP = systolic pulmonary artery pressures, DPAP = diastolic mean PA pressure, MPAP = Mean PA pressure, RAP = mean right atrial pressure, SAoP = Systolic aortic pressure, DAoP = Diastolic aortic pressure, MAoP = Mean aortic pressure, MAoP = Mean aortic pressure, LAP: mean left atrial pressure, CI = cardiac index, PVRI = pulmonary vascular resitance index, SVRI = systemic vascular resistance index, Rp/RS = ratio of pulmonary and systemic vascular resistance index. Pressures recorded as mm Hg, cardiac output = l/min/m2, Vascular resistance = Woods units.m.2.
Hemodynamic data obtained in the operating room after induction of general anesthesia prior to surgery.
Table 3.
Echocardiographic parameters, pre-operative and at last follow up.
| Serial number | TAPSE Z score |
RVFAC |
PAAT |
|||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| 1 | −4.5 | 1.2 | 15 | 28 | 0.3 | 0.7 |
| 2 | −3 | 0 | 12 | 32 | 0.4 | 0.9 |
| 3 | −5 | Expired | 10 | Expired | 0.2 | Expired |
| 4 | −5.3 | Expired | 8 | Expired | 0.2 | Expired |
| 5 | −3.8 | 1.1 | 17 | 36 | 0.4 | 0.8 |
| 6 | −4 | −1.5 | 13 | 26 | 0.5 | 1 |
| 7 | −4.4 | −2.3 | 15 | 32 | 0.4 | 0.8 |
| 8 | −4 | −4.2 | 10 | 10 | 0.3 | 0.8 |
| 9 | −3 | −1 | 17 | 32 | 0.5 | 0.9 |
| 10 | −5.8 | Expired | 13 | Expired | 0.3 | Expired |
| 11a | −2 | −1.8 | 16 | 15 | 0.5 | 0.4 |
| 12 | −5.7 | −3.1 | 8 | 18 | 0.4 | 0.6 |
| 13 | −2.3 | −1.1 | 20 | 28 | 0.5 | 0.6 |
| 14 | −4.8 | Expired | 10 | Expired | 0.2 | Expired |
| 15 | −2.2 | −1.6 | 22 | 30 | 0.6 | 0.6 |
| 16 | −2.6 | −1.0 | 18 | 32 | 0.4 | 0.5 |
| Cohort median | −4 | −1.3 | 14 | 29 | 59 | 98 |
| % change between median | 67.5 | 107 | 66 | |||
| P value | 0.03 | 0.004 | 0.01 | |||
TAPSE: tricuspid annular peak systolic excursion, RVFAC = RV fractional area change, PAAT: Pulmonary artery acceleration time.
Expired 20 months post procedure secondary to lower respiratory tract infection.
After completion of the procedure, the patient’s saturations were 10–20% lower in the lower extremity compared to the upper extremity.
The average ventilation duration was 16 ± 4 hours. Inotropes were continued until the patients were hemodynamically stable. The postoperative duration of ICU stay was 4 ± 2 days, and the hospital stay was 10 ± 2 days.
3.1. Immediate postoperative mortality
There were four deaths in the immediate postoperative period. 2 patients did not tolerate the intra-operative clamping of the pulmonary artery, and in 2 patients, there was pulmonary hemorrhage with respiratory failure requiring extracorporal membrane oxygenator in 1.
All the surviving patients were discharged home on PDE5 inhibitors (Tadalafil/Sildenafil), endothelin receptor antagonist (Ambresentan/Bosentan), antiplatelet, and anti-failure medications. Inhaled iloprost was continued in 2/3 patients with pre-operative prostacyclin requirement.
3.2. Follow up
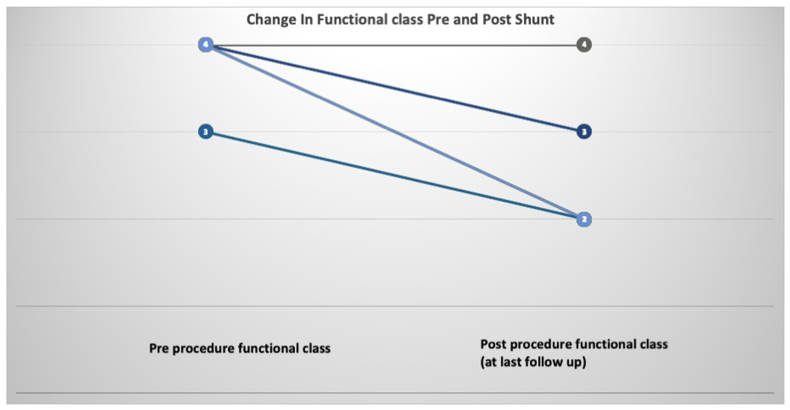
All the survivors were followed up in the PAH clinic. The median duration of follow-up was 17 months (1month–40 months). 10/12 patients who survived the procedure had improvement in a functional class by at least one grade (Fig. 1). One of the two patients who did not improve post-procedure expired 20 months later due to lower respiratory tract infection. And the second is currently listed for transplant.
Fig. 1.
Change in World Heat organization functional class in patients pre Potts shunt and at last follow up. All except two patients demonstrated improvement in functional class by at least 1 grade after the shunt.
3.3. Echocardiographic parameters
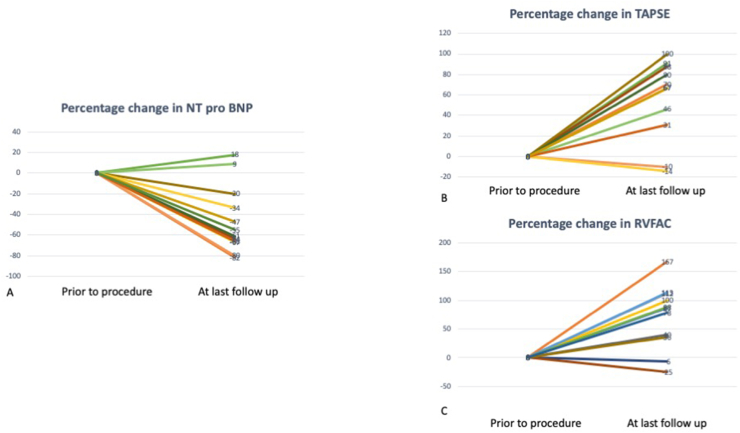
TAPSE z score improved from – 3.9 ± 1.2 to -1.3 ± 1.5 (p = 0.03) (Fig. 2) and RV fractional area change improved from 14 ± 4% to 26 ± 7% (p = 0.004). PAAT increased from 59.2 ± 11.4 to 84 ± 26 msec(p = 0.01)(Fig. 2).
Fig. 2.
Improvement in echocardiographic and laboratory parameters pre and post shunt. A. N terminal pro Brain natriuretic peptide (NT pro BNP) decreased by medina of 63% (+18,-82%). B. Longitudinal right ventricular function measured as tricuspid annular peak systolic excursion increased by median of 70% (−14, 100%). B. Right ventricular function measured as right ventricular fractional area change (RVFAC) increased by median of 88% (−25,167%).
3.4. Laboratory parameters
NT-Pro BNP decreased from median of 4947 pg/ml (1143–13204) to 1106 (389–14327) p = 0.001 at last follow up (Fig. 2).
3.5. Pulmonary vasodilators
10/12 surviving the patients received dual pulmonary vasodilators, and 2 received inhaled iloprost in addition to the oral medications till three months after surgery/PDA stent. At three months follow-up, patients with improvement in functional class and right ventricular function allowed discontinuation of iloprost in both the patients and endothelin receptor antagonists in 5/8 patients. Overall, the 10/16 (63%) of the patients benefited from the creation of the Potts shunt.
Table 4 demonstrates the differences between the patients who benefited from Potts shunt vs. those who did not benefit. The patients who did not benefit from the procedure were older, had higher functional class, and worse RV function on echocardiogram, higher right atrial pressure, lower cardiac index, and higher NT –pro-BNP levels (Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Table 4.
Comparison between patients who benefited from Potts shunt (Group1) vs patients who did not benefit from Potts shunt (group 2).
| Parameters | Group1 (Benefited from Potts shunt) | Group 2 (Did not benefit from Potts shunt) | P value | |
|---|---|---|---|---|
| Demographic | Number | 10 | 6 (4 deaths) | |
| Age | 9.5 (1–32) | 12.1 (1–35) | 0.3 | |
| Height (cm) | 122.5 (70–156) | 148 (70–172) | 0.29 | |
| Weight (Kg) | 20.5 (6–62) | 46.5 (7–54) | 0.4 | |
| BSA | 0.78 | 1.2 | 0.3 | |
| WHO Functional class | IV (2 in functional class III, remaining all in class IV) | IV (all were in functional class IV) | 0.6 | |
| Echocardiogram | TAPSE Z score | −3.14 (−4, −2) | −5.2 (−6, −4) | <0.0001 |
| RVFAC (%) | 15.7 (10,22) | 10 (8,17) | 0.02 | |
| LV eccentricity index | 2.5 (1.7,3.3) | 5 (2.0,5.5) | 0.04 | |
| Laboratory | NT Pro-BNP (pg/ml) | 2444 (1143–6543) | 9866 (6648–13204) | <0.001 |
| Cardiac catheterization | Mean PAP (mmHg) | 75 (44,89) | 91.7 (68,100) | 0,2 |
| Mean AoP (mmHg) | 72 (59,83) | 81.3 (59,86) | 0.17 | |
| Mean RAP (mmHg) | 8.8 (6,16) | 15.5 (10,18) | 0.003 | |
| Mean LAP (mmHg) | 7.3 (4,11) | 7.3 (4,13) | 0.9 | |
| Cardiac index (L/min/m2) | 3.1 (2,4) | 2.2 (2,3) | 0.009 | |
| PVRi (Woods units.m2) | 22.2 (11.6,29.5) | 21.4 (22.4,31.7) | 0.01 | |
| SVRi (Woods units.m2) | 21.8 (16.7,29.5) | 29.3 (20.6,37.3) | 0.4 | |
| Rp/Rs | 1.02 (0.5,1.13) | 1.04 (0.95,1.2) | 0.4 | |
| Type of procedure | Stent 2, surgery 8 | Surgery 6 | ||
BSA: body surface area, TAPSE: tricuspid annular systolic excursion velocity, RVFAC: Right ventricular fractional area change, NT pro BNP: N terminal pro brain natriuretic peptide (pg/ml). PAP = pulmonary artery pressure, AoP = aortic pressure, RAP = Right atrial pressure, LAP = left atrial pressure, PVRi = pulmonary vascular resistance index. SVRi = systemic vascular resistance index, Rp/Rs = ratio of pulmonary and systemic vascular resistance.
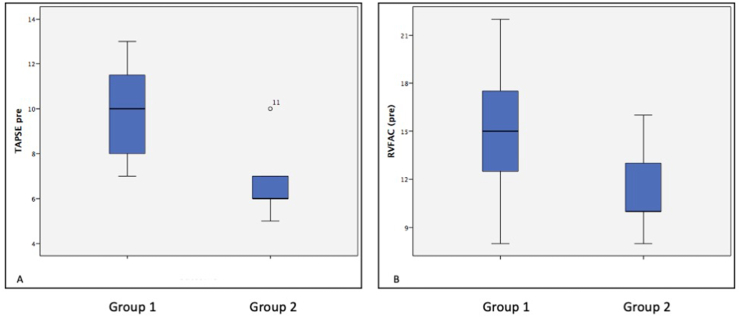
Fig. 3.
Box and Whisker plot comparing the echocardiographic features of group 1 (benefited from Potts shunt) and group 2 (Did not benefit from the Potts shunt). Patients with group 1 had significantly better tricuspid annular peak systolic excursion (TAPSE) (Figure A), p value < 0.001 and B shows the difference in Right ventricular fractional area change (RVFAC), (figure B) p value = 0.02.
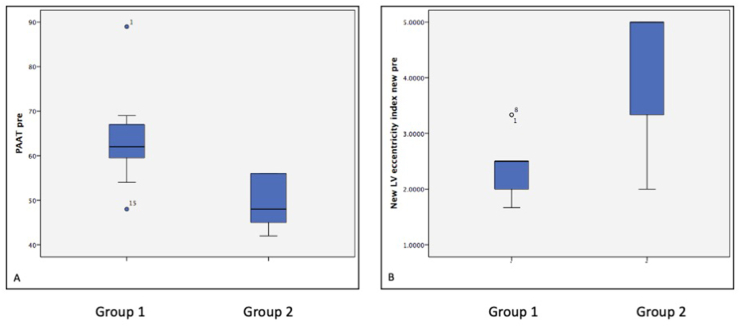
Fig. 4.
Box and Whisker plot comparing the echocardiographic features of group 1 (benefited from Potts shunt) and group 2 (Did not benefit from the Potts shunt). Patients with group 1 had significantly higher pulmonary artery acceleration time (PAAT) (Figure A), p value = 0.02 and B shows the difference in left ventricular eccentricity index (figure B) p value = 0.04.
Fig. 5.
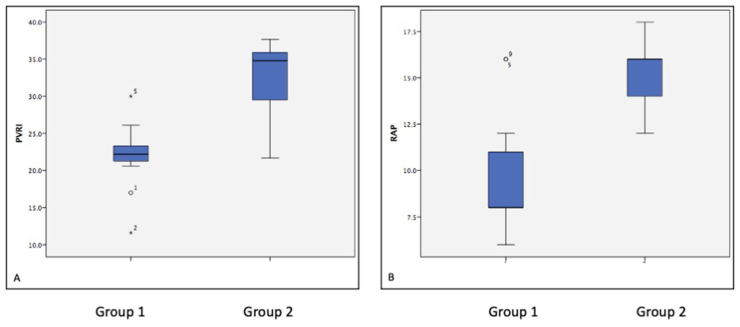
Box and Whisker plot comparing the cardiac catheterization features of group 1 (benefited from Potts shunt) and group 2 (Did not benefit from the Potts shunt). Patients with group 1 had significantly higher pulmonary vascular resistance indexed to body surface area (PVRI) (Figure A), p value = 0.01 and higher right atrial pressure (RAP) (figure B) p value = 0.003.
Fig. 6.
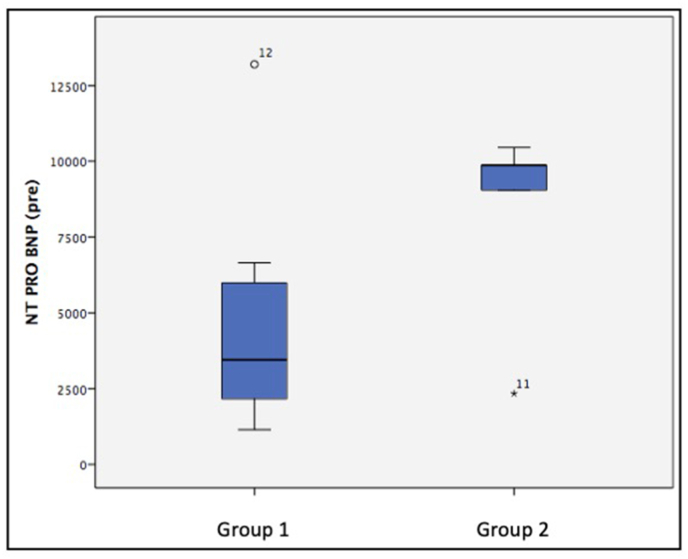
Box and Whisker plot comparing the levels of N terminal Pro brain natriuretic peptide (NT pro BNP) of group 1 (benefited from Potts shunt) and group 2 (Did not benefit from the Potts shunt). Patients in group 2 had significantly higher NT pro BNP levels as compared to those in group 1 (p value < 0.001).
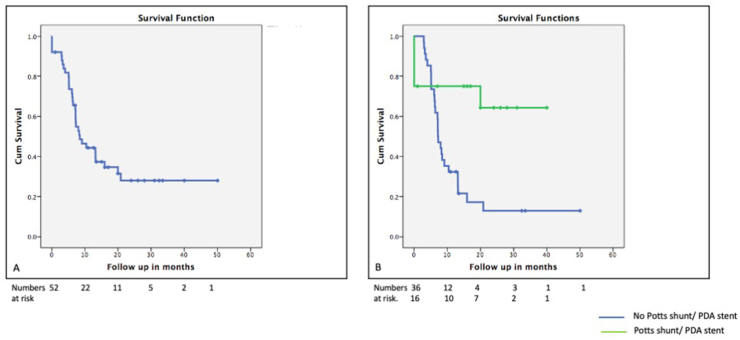
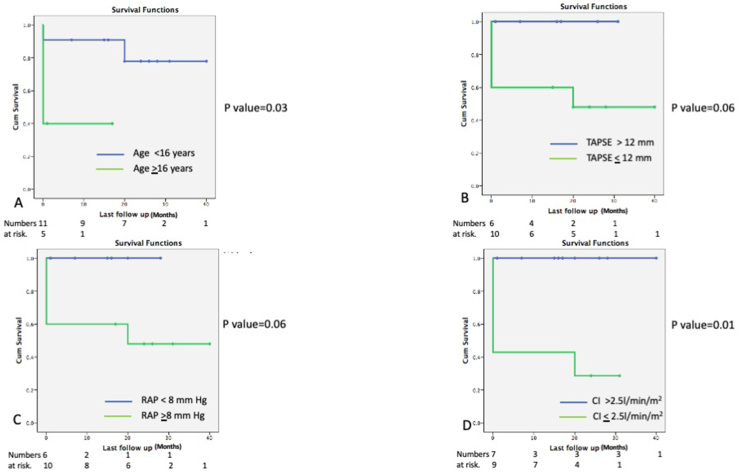
Survival analyses: Kaplan Meier survival graphs were plotted; survival of the entire cohort was 44%, 34%, and 28% at six months, one year, and two years, respectively. The mean survival for patients undergoing Potts shunt was 28 ± 4 months vs. 13.6 ± 2.7 months (p = 0.007), which was significantly better as compated to the patients who did not consent for the procedure (Fig. 7). Age less than 16 years, TAPSE more than 13 mm, right atrial pressure less than 8 mm Hg, and cardiac index more than 2.5 l/m/m2 were associated with better survival (Fig. 8).
Fig. 7.
Kaplan Meir survival graph demonstrating survival of 70% at 40 months after the Potts shunt surgery.
Fig. 8.
Kaplan Meir survival graph comparing survival in patients who were older (Age > 16 years) (Figure A), severe right ventricular systolic dysfunction as measures by tricuspid annular peak systolic excursion (TAPSE) less than 13 mm (figure B), right atrial pressure (RAP) > 8 mm Hg (Figure C) and cardiac index lesser than 2.5 l/min/m2 (Figure D). Comparison between the groups was done using Log rank test.
4. Discussion
Pulmonary arterial hypertension is a chronic and progressive disease with very high morbidity and mortality.13,14 PAH associated mortality has decreased in the last few years, mostly secondary to early diagnosis, better risk stratification, and upfront dual and triple therapy in high-risk individuals.15 However, prostacyclin analogues, which form the cornerstone of this management strategy, are not marketed in India and are beyond the reach of many. Similarly lung transplant is available in very few centers in India with limited medium term survival.2
Patients with repeated syncopal episodes or evidence of right ventricular failure are traditionally referred for balloon atrial septostomy. However, it is limited by a very high incidence of decreasing in size and spontaneous closure over time.16 Recently, use of atrial septal flow regulator has mitigated the risk of spontaneous decrease in size over time. However, the echocardiographic parameters, as well as the BNP, did not show significant improvement.17 Also the need for pulmonary vasodilators remains unchanged after creation of an interatrial communication.
4.1. Advantages of Potts shunt over creation of interatrial communication
Creation of a non-restrictive communication between the left pulmonary artery and the descending aorta (Potts shunt) has shown significant mortality and morbidity benefits in patients with PAH.3,12 Unlike atrial septostomy, Potts shunt does not create arterial desaturation in the upper part of the body including cerebral and coronary circulation and the shunt remains open throughout the cardiac cycle.18 Improvement in functional class, reduction in need for PAH specific medications and improvement in right ventricular function has been demonstrated after creation of the Potts shunt.19, 20, 21
4.2. Appropriate selection of cases and preoperative stabilization
Timing of the Potts shunt, as well as preoperative stabilization, is critical for successful outcome. Creation of a Potts shunt is a high-risk procedure, the risk increases incrementally with worsening of functional class and deterioration in the right heart function. All the four immediate postoperative deaths and the two patients who did not show improvement after the surgery in our series were older were in functional class IV, had worse echocardiographic and hemodynamic parameters. Age more than 16 years, right atrial pressure more than 8 mm Hg, TAPSE <13 mm, and cardiac index less than 2.5 l/min/m2 were associated with poor short and intermediate outcomes. Hence, it might be prudent to perform this high-risk procedure at the first sign of clinical/echocardiographic deterioration and not to wait until the patient is in functional class IV or severe right ventricular dysfunction ensues.
Two of our patients had sub-systemic PA pressures at the time of performing Potts shunt. Both of them had a history of syncope on exertion, which disappeared after the procedure. PA pressure and PVRI are dynamic in nature and are known to increase on effort. Decompression of the RV by the Potts shunt during such episodic pulmonary hypertensive crisis offered symptomatic relief to these patients.
4.3. Pre-operative stabilization and creation of the Potts shunt
Pre-operative stabilization with milrinone, IV sildenafil, and nitric oxide is essential for successful post-operative outcomes. This is especially true in the Indian scenario where prostacyclin analogues are not readily available.
Potts shunt can be done surgically through left lateral thoracotomy or in the cardiac catheterization laboratory by stenting the patent ductus arteriosus.7,22,23 In our series, two patients with probe patent ductus underwent PDA stenting. Although transcatheter creation of Potts shunt in the absence of a probe patent ductus has been reported, procedural risks and long term follow up needs to be looked at carefully.24
Surgically Potts shunt can be done by using an interposition graft or direct side to side anastomosis between the left pulmonary artery and the descending aorta. The creation of the shunt using a unidirectional valved conduit has also been described.25 In our series, an interposition graft was used in all the patients who had surgical Potts shunt. The advantage of the interposition graft is that the flow can be controlled by putting a band across it if required. We could successfully discharge 12/16 patients who underwent the procedure at our center. Overall survival in patients undergoing Potts shunt/PDA stent was significantly higher than those who did not undergo the said procedure.
4.4. Clinical, echocardiographic and laboratory improvements on follow up
On follow up, all but two surviving patients demonstrated at least 1-grade improvement in functional class, reduced RV afterload and improvement in the right ventricular function in echocardiographic as well as laboratory parameters.26 Most of the above factors have been shown to predict clinical outcomes in adult as well as pediatric patients with PAH.15 Hence improvement in these factors could translate into better clinical outcomes.
We could successfully wean iloprost and endothelial receptor antagonists in patients demonstrating improvement of the above parameters. This might be an important indication to perform Potts shunt in a developing country like India, where either PAH-specific medications are not readily available or are beyond the reach of the majority of the population.
The experience of our center is similar to the results described in other countries.3,9,22 In addition, we have demonstrated that early referral for Potts shunt before severe RV dysfunction ensues is essential for optimal results. The immediate goal in creating Potts shunt in patients with PAH is to create physiology like Eisenmenger syndrome, thereby ensuring that the right ventricle never faces more than systemic pressures.
5. Conclusion
Potts shunt/PDA stenting is feasible in patients with PAH; it can be done safely with an acceptable success rate. Patient selection, preoperative stabilization, and meticulous intra and postoperative management are essential. For optimal outcomes, it should be performed at the earliest sign of clinical, echocardiographic, or laboratory deterioration before severe right ventricular dysfunction sets in. Long term follow-up is required to ascertain sustainable improvement in functional class and the need for a lung transplant.
6. Limitations of the study
Single-center experience with limited duration of follow up.
Funding
None.
Declaration of competing interest
None.
Acknowledgement
None.
References
- 1.Ivy D.D., Abman S.H., Barst R.J. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D117–D126. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein B.S., Sweet S.C., Mao J., Huddleston C.B., Grady R.M. Lung transplantation in children with idiopathic pulmonary arterial hypertension: an 18-year experience. J Heart Lung Transplant. 2011;30(10):1148–1152. doi: 10.1016/j.healun.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Baruteau A.E., Belli E., Boudjemline Y. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur J Cardio Thorac Surg. 2015;47(3):e105–e110. doi: 10.1093/ejcts/ezu445. [DOI] [PubMed] [Google Scholar]
- 4.Keogh A.M., Nicholls M., Shaw M., Dhital K., Weintraub R., Winlaw D.S. Modified Potts shunt in an adult with pulmonary arterial hypertension and recurrent syncope - three-year follow-up. Int J Cardiol. 2015;182:36–37. doi: 10.1016/j.ijcard.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins W.E., Waggoner A.D. Severe pulmonary hypertension without right ventricular failure: the unique hearts of patients with Eisenmenger syndrome. Am J Cardiol. 2002;89(1):34–38. doi: 10.1016/s0002-9149(01)02159-2. [DOI] [PubMed] [Google Scholar]
- 6.Diller G.P., Dimopoulos K., Broberg C.S. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J. 2006;27(14):1737–1742. doi: 10.1093/eurheartj/ehl116. [DOI] [PubMed] [Google Scholar]
- 7.Schranz D., Kerst G., Menges T. Transcatheter creation of a reverse Potts shunt in a patient with severe pulmonary arterial hypertension associated with Moyamoya syndrome. EuroIntervention. 2015;11(1):121. doi: 10.4244/EIJV11I1A21. [DOI] [PubMed] [Google Scholar]
- 8.Kula S., Atasayan V. Surgical and transcatheter management alternatives in refractory pulmonary hypertension: Potts shunt. Anatol J Cardiol. 2015;15(10):843–847. doi: 10.5152/AnatolJCardiol.2015.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady R.M., Eghtesady P. Potts shunt and pediatric pulmonary hypertension: what we have learned. Ann Thorac Surg. 2016;101(4):1539–1543. doi: 10.1016/j.athoracsur.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 10.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 11.D’Alto M., Santoro G., Palladino M.T., Parisi F., Russo M.G. Patent ductus arteriosus stenting for palliation of severe pulmonary arterial hypertension in childhood. Cardiol Young. 2015;25(2):350–354. doi: 10.1017/S1047951114001152. [DOI] [PubMed] [Google Scholar]
- 12.Boudjemline Y., Patel M., Malekzadeh-Milani S., Szezepanski I., Levy M., Bonnet D. Patent ductus arteriosus stenting (transcatheter Potts shunt) for palliation of suprasystemic pulmonary arterial hypertension: a case series. Circ Cardiovasc Interv. 2013;6(2) doi: 10.1161/CIRCINTERVENTIONS.112.000091. e18–20. [DOI] [PubMed] [Google Scholar]
- 13.Vonk Noordegraaf A., Chin K.M., Haddad F. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert M., Guignabert C., Bonnet S. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galie N., Channick R.N., Frantz R.P. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval J., Gaspar J., Pena H. Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J. 2011;38(6):1343–1348. doi: 10.1183/09031936.00072210. [DOI] [PubMed] [Google Scholar]
- 17.Rajeshkumar R., Pavithran S., Sivakumar K., Vettukattil J.J. Atrial septostomy with a predefined diameter using a novel occlutech atrial flow regulator improves symptoms and cardiac index in patients with severe pulmonary arterial hypertension. Cathet Cardiovasc Interv. 2017 Dec 1;90(7):1145–1153. doi: 10.1002/ccd.27233. [DOI] [PubMed] [Google Scholar]
- 18.Delhaas T., Koeken Y., Latus H., Apitz C., Schranz D. Potts shunt to Be preferred above atrial septostomy in pediatric pulmonary arterial hypertension patients: a modeling study. Front Physiol. 2018;9:1252. doi: 10.3389/fphys.2018.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausknecht M.J., Sims R.E., Nihill M.R., Cashion W.R. Successful palliation of primary pulmonary hypertension by atrial septostomy. Am J Cardiol. 1990;65(15):1045–1046. doi: 10.1016/0002-9149(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 20.Collins T.J., Moore J.W., Kirby W.C. Atrial septostomy for pulmonary hypertension. Am Heart J. 1988;116(3):873–874. doi: 10.1016/0002-8703(88)90353-5. [DOI] [PubMed] [Google Scholar]
- 21.Rich S., Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol. 1983;51(9):1560–1561. doi: 10.1016/0002-9149(83)90678-1. [DOI] [PubMed] [Google Scholar]
- 22.Gorbachevsky S.V., Shmalts A.A., Barishnikova I.Y., Zaets S.B. Potts shunt in children with pulmonary arterial hypertension: institutional experience. Interact Cardiovasc Thorac Surg. 2017;25(4):595–599. doi: 10.1093/icvts/ivx209. [DOI] [PubMed] [Google Scholar]
- 23.Latus H., Apitz C., Moysich A. Creation of a functional Potts shunt by stenting the persistent arterial duct in newborns and infants with suprasystemic pulmonary hypertension of various etiologies. J Heart Lung Transplant. 2014;33(5):542–546. doi: 10.1016/j.healun.2014.01.860. [DOI] [PubMed] [Google Scholar]
- 24.Esch J.J., Shah P.B., Cockrill B.A. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32(4):381–387. doi: 10.1016/j.healun.2013.01.1049. [DOI] [PubMed] [Google Scholar]
- 25.Salna M., van Boxtel B., Rosenzweig E.B., Bacchetta M. Modified Potts shunt in an adult with idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc. 2017;14(4):607–609. doi: 10.1513/AnnalsATS.201701-057LE. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal M., Grady R.M., Choudhry S., Anwar S., Eghtesady P., Singh G.K. Potts shunt improves right ventricular function and coupling with pulmonary circulation in children with suprasystemic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2018;11(12) doi: 10.1161/CIRCIMAGING.118.007964. [DOI] [PubMed] [Google Scholar]