Abstract
Mild cognitive impairment (MCI) and dementia are clinically prevalent in the elderly. There is a high risk of cognitive decline in patients diagnosed with MCI or dementia. This review describes the effectiveness of Ginkgo biloba leaf special extract EGb 761® for the treatment of dementia syndromes and EGb 761® combination therapy with other medications for symptomatic dementia. This drug has shown convincing results, improving cognitive function, neuropsychiatric symptoms and consequent reduction of caregiver stress and maintenance of autonomy in patients with age-related cognitive decline, MCI and mild to moderate dementia. Currently, there is little evidence to support the combination therapy with anti-dementia drugs and, therefore, more evidence is needed to evaluate the role of EGb 761® in mixed therapy.
Keywords: mild cognitive impairment (MCI), mild dementia, Alzheimer’s disease, Ginkgo biloba (Egb761®), Tebonin, anti-dementia drugs, randomized controlled trials
1. Introduction
Mild cognitive impairment (MCI) is a clinically relevant health problem in the elderly and it is considered an intermediate state between normal aging and dementia [1]. This state can progress to dementia, and Alzheimer’s disease (AD) is the most common form of neurodegenerative disorder [2]. The prevalence of MCI in the population over 60 is approximately 5.9%, it tends to increase with age, and is more common in men. The MCI development varies according to numerous risk factors that, beyond age, also include genetics, comorbidities, chronic diseases as vascular risk factors, pulmonary diseases, depression, metabolic risk such as diabetes mellitus, hypertension and also tobacco utilization [3].
Currently, despite pharmacological new findings, there is still no specific drug approved by the Food and Drug Administration (FDA) for the treatment of MCI. The only drugs used, approved by the FDA for the treatment of mild and moderate AD, are Acetylcholinesterase (AChE) inhibitors: AChEIs or memantines, although their effects are not very effective and have numerous side effects such as nausea, bradycardia, fatigue [4]. Moreover, non-pharmacological treatments such as behavioral interventions, psychosocial support, physical activity including rehabilitation programs, diet and cognitive stimulation shown a benefit to patients. [4,5].
Additionally, an important factor involved in the etiology of cognitive decline is inflammation and the subsequent development of oxidative stress.
Consequently, several studies suggested an important role of diet in the prevention of neurodegenerative diseases, showing a protective role against the damaging effects of neuroinflammation and oxidative stress [4,5].
Indeed, the Mediterranean diet has been shown to reduce the incidence of mild cognitive impairment (MCI) and, possibly, the conversion of MCI to dementia [6]. Vitamins, minerals, polyphenols have been associated to the prevention of cognitive impairment due to their antioxidant effects, such as the reduction of free radical species and therefore the oxidative stress development [7,8,9,10].
Considering polyphenols deriving from plants, Ginkgo biloba is the oldest living tree species in the world and is one of the most studied herbals for cognitive disorders and AD [11,12].
In traditional medicine Ginkgo leaves were used mainly for the treatment of respiratory and cardiovascular disorders while in Chinese medicine, Ginkgo biloba seeds were used to treat pulmonary symptoms, alcohol abuse and bladder infections [13]. The modern use of Ginkgo biloba extract is all about leaf-based preparations and numerous health benefits have been attributed to its utilization. Recent studies showed an important role of Ginkgo biloba in cognitive improvement [14].
In particular, it has been observed that Ginkgo biloba extract EGb 761® could play protective effect roles and it is an effective treatment in both Alzheimer’s diseases and vascular dementia [15]. Its pharmacological effect is based on antioxidant, anti-inflammatory and anti-apoptotic action and the defense against mitochondrial dysfunction [16]. These mechanisms are considered to contribute to cognitive improvement, impeding the evolvement of neurodegenerative diseases.
Indeed, vegetal origin drug based on EGb 761® active principle, registered as “well established use” is authorized in many European States and it is considered the only drug treatment recommended in the guidelines for the treatment of MCI.
This review describes the main clinical and preclinical human studies highlighting the efficacy of the Ginkgo biloba EGb 761® for the treatment of MCI and dementia syndrome, and EGb 761® combination therapy with other symptomatic drugs for dementia.
1.1. Mild Cognitive Impairment (MCI) and Mild Dementia: Recent Classification Criteria, Pathogenesis, Therapeutic Aspects
Neurodegenerative diseases involve different groups of pathologies of the central nervous system, characterized by a progressive loss of synaptic transmission in some specific neuronal circuits. This information flow deficit, without any clear structural damage, is then followed by progressive neuronal death with loss of volume and obvious accumulation of toxic substances in both extra- (e.g., amyloid beta plaques) and intra-neuronal spaces (e.g., neurofibrillary tangles) [17,18,19]. Moreover, these disorders are classified considering clinical features (Parkinson or dementia), anatomic distribution of neurodegeneration (frontotemporal degenerations, spinocerebellar degenerations), or as a result of molecular abnormalities [20]. Depending on the type of disease, neuronal deterioration can result in cognitive deficits, dementia, motor alterations, behavioral and psychological disorders [21].
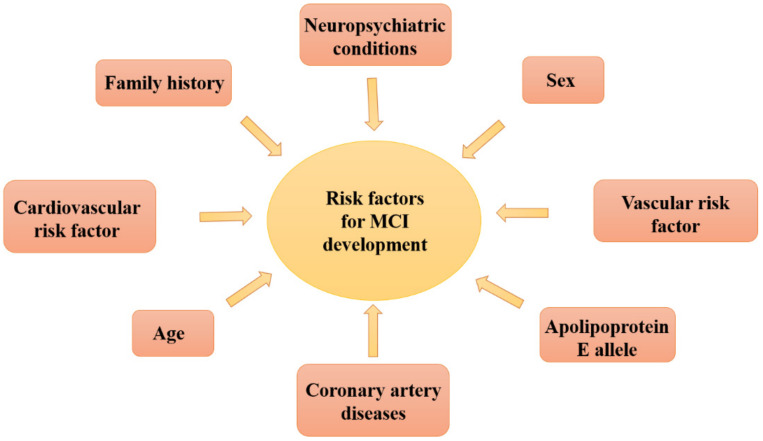
Specifically, mild cognitive impairment (MCI) and dementia are symptoms of various neurodegenerative diseases characterized by cognitive decline, the most common are Alzheimer’s disease (AD), vascular dementia and Lewy Body’s disease [22]. Unlike MCI, in mild dementia the interference with daily life is evident [2]. In recent years, MCI prevalence in the over 60 population is estimated to be around 6–22% [23], depending on the method of evaluation, the population examined and other factors. MCI is clinically heterogeneous and different factors can increase the risk of MCI development (Figure 1).
Figure 1.
Risk factors for MCI development.
Diagnostic criteria employed to identify MCI include a decrease in the performance of several cognitive functions, in one or more domains, related to memory, orientation or verbal skills [17,18]. This cognitive decline, which is still common in the elderly population, is not necessarily indicative of incipient dementia.
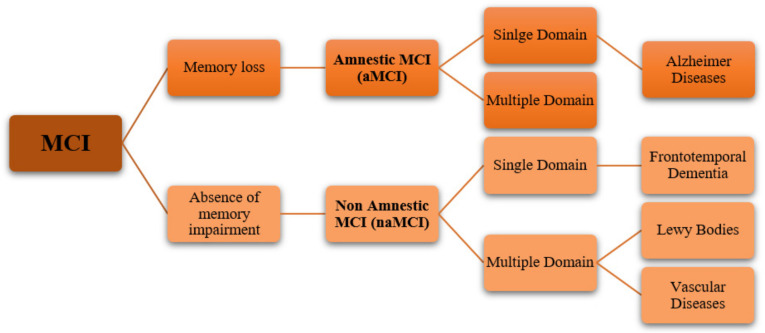
A MCI classification has been proposed to distinguish between amnestic MCI (aMCI, where memory is significantly impaired) and non-amnestic MCI (naMCI, where memory remains intact). Furthermore, MCI may involve impairment in a single cognitive domain or in multiple cognitive domains (Figure 2). The combination of the several clinical subtype with degenerative, psychiatric or vascular etiology could be an important predicting factor of the type of dementia that the MCI patient would develop (AD, vascular dementia, frontotemporal dementia, Lewy bodies) [24]. The progression of different MCI subtypes into a particular type of dementia has not yet been well understood [25].
Figure 2.
Schematic representation of MCI subtypes.
Therefore, patient’s history is critical to recognize and detect various clinical signs and making a diagnosis.
Cognitive decline in the elderly is a common problem and healthcare providers are often the first point of contact for patients and their families. Generally only elderly with moderate to severe dementia are subject to medical treatment, while MCI patients are often not even diagnosed by primary care providers [26].
MCI has attracted a lot of interest within the scientific community because it represents a stage between normal age-related cognitive function and a clinically probable diagnosis of several dementias [27]. It has become the “target” of many recent clinical trials with potentially disease-modifying drugs.
One of the scientific and public health problems is the fact that neuropsychological tests do not distinguish MCI patients who will never develop dementia from those who are in a prodromal-to-dementia condition [22].
Currently, medical history and mental health examination are the most commonly used tools to diagnose MCI or mild dementia [28]. Specifically, through medical history, the clinician determines whether or not there is a decline in the patient’s daily functions while, through the mental health examination, the clinician establishes a patient’s noticeable cognitive impairment [28]. Another important tool that could be performed for MCI and dementia diagnosis is the general neurological examination whose role in the diagnostic process is to understand the etiology of cognitive disorder and to exclude/confirm the presence of modifiable factors (i.e., subdural hematoma, frontal lobe neoplasm, dysmetabolic syndromes, etc.) [26]. Consequently, over the last twenty years, several attempts have been made to draw up general investigations for this disorder [29,30].
Therefore, after a comparison with normal subjects of the same age, a greater degree of memory impairment has been noted in MCI patients with little or absent involvement of common activities of daily living [31]. As the disease progresses, a sub-sequential loss of cognitive function, a loss of functional independence, and the development of behavioral problems occur. Therefore, early diagnosis and treatment may delay the progression of symptoms.
For this reason, the concept of “biomarkers” is now emerging, as instrumental examinations that, in association with neuropsychological tests, could be used to identify individuals with MCI who are already in a prodromal-to-dementia stage [22]. Due to the general interest in this area, the Ministry of Health and Italian Drug Agency (AIFA) has designed the INTERCEPTOR project that aims to compare 6 different biomarkers (Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET), neuropsychological tests, liquor for beta and tau, electroencephalogram (EEG) for connectivity through graphs, magnetic resonance imaging (MRI) for hippocampal volumetry, genetics for Apolipoprotein E (ApoE)) and an organizational model to validate a sustainable, non-invasive and widely widespread method across the country for the diagnosis of prodromal MCI. This will be important to identify high-risk individuals, to find the resources for early pharmacological and non-pharmacological-treatments, as well as for the delivery of any disease-modifying drugs, which should be effective among several trials.
1.2. Therapeutic Approaches in Mild Cognitive Impairment and Mild Dementia
Currently, only pharmacological treatments with modest value are approved for mild dementia due to AD, while none have been approved for MCI in Italy. In fact, despite numerous randomized clinical trials (RCTs) being conducted in MCI patients, none have been able to demonstrate the effectiveness at delaying disease progression [30,32]. In addition, it is important to note that (when symptoms are disabling) the delaying or slowing down the onset or the worsening of dementia-related symptoms can have a major impact on the social and health costs. An extension of the total/partial autonomy time can reduce the total costs of the disease by about 50% [33].
To date, the only drugs used to treat symptoms are cholinesterase inhibitors and memantines.
Indeed, several studies identified a cholinergic deficit in subjects with dementia [34]. By Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI), a reduction in AChE activity and an atrophy of the nucleus basalis of Meynert (the main source of AChE, the origin of cholinergic neurotransmission and projections to the cortical brain areas associated with learning and memory) was shown. Therefore, actual therapeutic hypothesis in AD is to restore physiological levels of acetylcholine through the inhibition of acetylcholinesterase enzyme activity [35]. Reversible AChEIs drugs are available; they are lipophilic enough to overcome the blood–brain barrier to act preferably on the central nervous system [36].
Among these drugs, the most studied are donepezil, physostigmine (no longer in use), rivastigmine and galantamine, approved by FDA for the treatment of mild dementia due to AD, but no treatment has yet been approved by the US FDA for MCI.
Another important therapeutic approach involves the use of drugs that act directly on the glutamatergic system, such as memantine [35]. In particular, memantine is a noncompetitive N-methyl-D-aspartate receptor (NMDA) receptors-antagonist and provides symptomatic treatment of dementia inhibiting the pathological activation of NMDA receptors [37]. Its neuroprotective effects have been demonstrated in several neurological disorders. Indeed, studies conducted in ischemic models showed that an increase in NMDA receptor antagonist drugs, in the blood, led to a decrease in glucose metabolism, thus supporting the memantine neuroprotective effect.
Memantine is used for cognitive disorders in patients with moderate to severe AD. No drugs have been approved for the prevention of these disorders [34].
For this reason, the focus has shifted towards non-pharmacological treatments that involve behavioral interventions, psychosocial support and cognitive training. Such measures are usually supplemented with drug treatment, and their positive effectiveness in the overall clinical patient’s management have been demonstrated [22].
Cognitive training (of different types, and with different functional goals: Reality-Orientation Therapy, Validation Therapy, Reminiscence Therapy, various cognitive stimulation therapy programs—Cognitive Stimulation Therapy, etc.) showed results both in stimulating and reinforcing neuro-cognitive abilities, as well as in improving the execution of daily life tasks [22].
The effect of moderate physical and motor activity, especially in the intermediate stages of the disease, seems to be positive for the tone of mood, the physical wellness- and the regularization of behavioral disorders, sleep and nutrition [22].
Furthermore, inflammation and therefore oxidative stress are important factors involved in cognitive decline [14]. Particularly, oxidative stress appears to be involved in the early phase of AD and MCI and, therefore, could be considered as a prodromal phase of dementia. For this reason, various antioxidant therapies have been shown to influence the onset and progression of AD [38].
Natural polyphenolic compounds confer an antioxidant effect thus reducing the development of free radicals, restoring the endogenous antioxidant defense and carrying out important neuroprotective effects for the body [14].
1.3. Ginkgo Biloba: From Traditional Chinese Medicine to Anti-Dementia Drug Based on Scientific Evidence
Ginkgo biloba is one of the oldest tree species in the planet used especially for its health properties [13].
Ginkgo and derived pharmaceutical formulations have a tradition in the Chinese medicine; at the beginning seeds and then, in the modern phytotherapy, leaves extracts were used [13,39].
In the 1960s, Dr Willmar Schwabe pharmaceuticals introduced a drug based on leaf extract that contained terpenoids and flavonoid glycosides and organic acids. Several studies, conducted in cell and animal models, showed the neuroprotective effects of this drug.
Later, this product has been modified to improve the good characteristics and decrease side effects; in the 1980s, it wasproposed with the name of EGb 761® with an enrichment in flavonoids, ginkgolides and bilobalide, and a reduction of ginkgolic acids [39].
Herbal medicinal products with Ginkgo biloba leaf extracts such as EGb 761® active principle, belonging to the cognitive drug category (Ginkgo biloba, ATC cod: NO6DX02) authorized in many European States.
Ginkgo biloba L. (Family: Ginkgoaceae) dry leaves are used to satisfy the European pharmacopeia and specific pharmaceutical companies requisites. Tebonin indication is approved by EMA report and monograph, as a vegetal drug for the improvement of cognitive deterioration (linked to age) and life quality in the mild dementia.
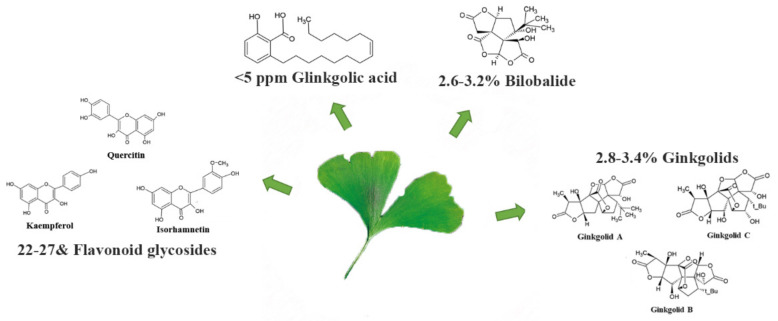
Particularly, EGb 761® is a light yellow–brown/yellow–orange bitter powder that has to satisfy all the requisites of European pharmacopeia actual monograph “Ginkgo dry extract, refined and quantified”. The flavonoid fraction is responsible for the antioxidant properties of EGb 761®. Particularly, EGb 761® contains between the 22 and 27% flavonoid glycosides (i.e., kaempferol, quercetin and isorhamnetin) that inhibit oxidation of tert-butylhydroperoxide [40], 2.8–3.4% ginkgolids A, B and C, 2.6–3.2% bilobalide and contains less than 5 ppm ginkgolic acids (Figure 3). The Ginkgo flavonoids and the terpene lactones (ginkgolide A, B, and C diterpenes and the sesquiterpene bilobalide, all provided with three lactone rings) are EGb 761® ingredients with therapeutic characteristics (Figure 3). In particular, ginkgolide A has been demonstrated to lack the ability to scavenge the superoxide, while the superoxide scavenging activity of bilobalide and the ginkgolides B, C is still not sure [41].
Figure 3.
EGb 761® active components.
From the beginning, EGb 761® has been used for peripheral and central vascular disorder diseases [42]. Currently, EGb 761® utilization has been abandoned in the most peripheral vascular diseases, as well as Raynaud syndrome, the intermitted claudication and peripheral arteriopathy, despite the beneficial evidences obtained in placebo-controlled trials [42,43]. Positive effects have been observed also for cerebrovascular diseases, above all for vascular cognitive impairment.
Later, it was also used for cognitive impairment associated with aging, demonstrating beneficial in different clinical trials, in line with the international diagnostic criteria modification but with several limitation about the most updated guidelines for Alzheimer’s disease drugs development [6,13,44,45,46].
In Germany, the E commission has approved the monograph which defines the use of Ginkgo biloba undefined leaves preparation, for cerebral and arterial blood circulation, for vertigo and the reinforcement of the vascular system, (i.e., veins), for the stimulation of blood circulation (i.e., after psychotropic and neurotropic therapy). The specific monograph of the purified and titrated product, extracted from Ginkgo biloba leaf (DER 35-67:1), described its use in the symptomatic treatment of cerebral insufficiency, as a part of a general therapeutic strategy for dementia with the following principal symptoms: deficit of memory, concentration and mood disturbance, vertigo, tinnitus and headache [47,48].
The main target group includes primary degenerative dementia and/or vascular dementia patients. Moreover, it has been used to improve the free from pain motor skills in patients with second stage of peripheral occluding arteriopathy, according to Fontaine classification.
The 2003 European Scientific Cooperative On Phytotherapy (ESCOP) monograph [49] describes the Ginkgo biloba leaf utilization for the symptomatic treatment of moderate and mild dementia, involving degenerative primary dementia, vascular dementia, mixed forms and cerebral impairment; for neurosensorial impediments as well as dizziness/vertigo and tinnitus; for cognitive performance improvement; for the symptomatic treatment of occlusive peripheral arteries disease.
The 2006 British Herbal Compendium [50] lists the following indications for Ginkgo biloba leaves: symptomatic treatment of moderate and mild dementia, including primary degenerative dementia, such as Alzheimer’s, multi-infarct dementia and mixed forms; treatment of symptoms of cerebral vascular impairment and concentration/memory problems, confusion, deficit of energy and initiative, anxiety and depression; improvement of cognitive performances; neuro-sensorial disturbance as vertigo and visual dysfunction.
1.3.1. EGb 761® in Basic Research: The Mechanism of Action
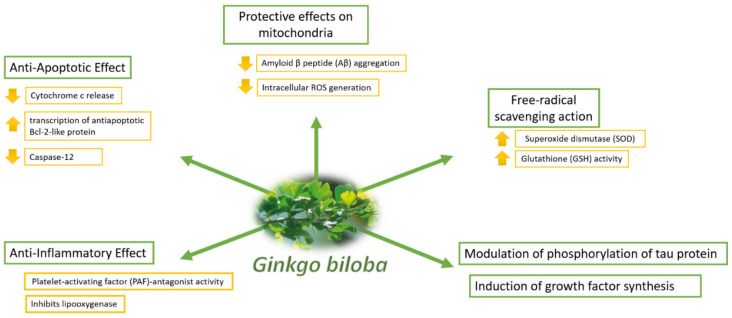
EGb 761® pharmacological effect is based on four main neurobiological mechanisms: 1. increase in neurogenesis and synaptogenesis, 2. mitochondrial DNA oxidation prevention, followed by stabilization of mitochondrial membranes which slows down aging, 3. neuro-transmission improvement and 4. improved microcirculation.
Figure 4 shows the possible mechanisms of action.
Figure 4.
Mechanisms of action of Ginkgo biloba [41].
The different extract active components act simultaneously, in the cerebral context, with different mechanisms to enhance beneficial effects on cognitive functions and improve neuro-protection. Several preclinical experimental studies showed that EGb 761® has antioxidant proprieties due to its flavonoids [51,52,53]. These components protect against several neurotoxic agents and in a specific manner against the neurotoxicity from amyloid beta oligomers [47]. The antioxidant action can be explicated by a direct reactive oxygen species (ROS) scavenging or through the modulation of specific mechanisms of signaling and transcription factors, able to stimulate the cellular repair system and to amplify the endogenous antioxidant defenses [48,54,55].
A peculiar EGb 761® activity is to improve mitochondrial dysfunction during the aging and in cerebral cognitive impairment [53,56,57,58,59,60]. This mechanism underlines EGb 761® neuroprotection. Thus, several studies demonstrated that functional mitochondrial homeostasis plays a critical role in neuro degeneration and the cognitive deterioration associated with dementia [48,61].
EGb 761® reduces mitochondrial ROS production and protects mitochondria and mitochondrial complexes of the respiratory chain from ROS, it increases the energetic metabolism and ATP availability [53,57,62,63,64,65].
Preclinical studies demonstrated that EGb 761® also has a positive role in neuroinflammation [66,67], including a specific inhibitory action of inflammatory molecules transcription factors at neuronal level and in the microglia [68]. Moreover, EGb 761® can inhibit the aggregation and production of amyloid beta [66,69,70,71,72,73,74,75,76,77]. Recently, it has been observed that EGb 761®, and in particular its ginkgolid A (but not ginkgolid B and C), and flavonoid components, enhanced autophagic activity and degradation of phosphorylated TAU protein in lysosomes and neuron [78]. Moreover, EGb 761® stimulated the dopaminergic and noradrenergic neurotransmission [79,80], thus improving cognitive functions in the elderly [81].
Cellular and animal studies demonstrated that EGb 761® helps also synaptic functions and the neuronal plasticity, including the neuritogenesis, the spinal cord density, the long-term potentiation, and neurogenesis. These effects are stronger in experimental models of hypoglycemia, hypoxia, amyloid beta exposition, oxidative stress [58,82,83].
1.3.2. EGb 761® in Preclinical and Clinical Dementia: Evidence of Efficacy
Although it has been used in the treatment of neuro-cognitive disorders for several years, the expert evaluation of EGb 761® has become unanimously positive over the last years [15,44]; however, in the last few years, scientific reviews and meta-analysis studies results have been pivotal in the achievement of EGb 761® as a drug for dementia, as underlined in the guidelines of cognitive disorders treatments. Table 1 describes the different types of dementia and the types of pharmacological treatments currently in use [84,85,86,87,88,89].
Table 1.
Clinical studies on EGb761EGb 761® in patients with MCI, vMCI, neurocognitive deficit and dementia.
| Dementia | Diagnosis | References |
|---|---|---|
| Mild cognitive impairment (MCI) | Neuropsychological syndrome characterized by emerging cognitive impairment | [84] |
| Alzheimer’s disease (AD) | Neurodegenerative disorder characterized by loss of neurons and synapses in the cerebral cortex and certain subcortical regions. | [85] |
| Vascular dementia (VaD) | Cognitive dysfunctions resulting from brain tissue death due to ischemia caused by vascular disease. | [86] |
| Frontotemporal lobar degeneration (FTLD) |
Neurodegenerative disorders characterized by progressive changes in behavior, personality. | [87] |
| Dementia with Lewy bodies (DLB) | Several cognitive, behavioral and neurological symptoms characterized by memory loss, hallucinations, rapid eye movement | [88] |
| Behavioral and psychological and symptoms of dementia” (BPSD) | Neuropsychiatric symptoms and behavioral manifestations (apathy, depression, aggression agitation) associated with dementia. | [89] |
1.3.3. EGb 761® in vMCI and Dementia Prevention
Prospective observational studies, such as the clinical study conducted by Amieva et al., 2013 [90] which involved 3612 patients, aged over 65, from the South of France, highlighted a slower progression of cognitive impairment in the EGb 761® group of patients than in Piracetam ones. This difference has been confirmed also through a multiple-choice visual memory test (Benton Visual Retention Test (BVRT)) and language skills test (Isaacs Set Test (IST)). Moreover, EGb 761® patients showed a significant reduction of psychotropic drug consumption, considering that, in dementia experimental clinical trials, ADAS-Cog is the more used formulation (Table 2).
Table 2.
Clinical studies on EGb 761® in patients with MCI, vMCI, and dementia.
| Inclusion Criteria | Treatment Groups | Results | References |
|---|---|---|---|
| EGb 761® in vMCI, MCI and dementia prevention | |||
| Non-demented patients | EGb 761® or Piracetam and placebo, data collected on cognitive function over a period of twenty years | Patients treated with EGb 761® highlighted a slower cognitive impairment than in Piracetam group. Moreover, EGb 761® patients showed a significant reduction of psychotropic drugs assumption. | [90] |
| Patients with very mild cognitive impairment and low functioning | EGb 761® (240 mg/die) or placebo, for 12 weeks | Patients treated with EGb 761® showed an improvement of memory performances, measured through Wechsler Memory Scale III (human face recognition in pictures) and a significant attention improvement, by using the Vienna Test System Work Performance Series (a computerized math test to keep concentration). | [91] |
| EGb 761® MCI, neurocognitive deficit and dementia | |||
| Patients with amnesic MCI | EGb 761® (240 mg/die) or placebo, for 24 weeks | Patients treated with EGb 761® showed improvement in all the neuropsychiatric symptoms, measured through Neuropsychiatrics Inventory (NPI) sympoms. | [94] |
| Patients with normal cognitive function or MCI | EGb 761® (240 mg/die) or placebo, patients were evaluated every 6 months | This study didn’t demonstrate any significant benefit to prevent dementia development with EGb 761® treatment versus placebo. | [95] |
| Outpatients with mild to moderate dementia (AD or VaD) | EGb 761® (240 mg/die) or placebo, for 24 weeks | Patients treated with EGb 761® demonstrated neuro psychiatric improvement for Neuropsychiatric Inventory (NPI) symptoms. | [96] |
| Outpatients 24-week with mild to moderate dementia (Alzheimer’s disease or vascular dementia) associated with neuropsychiatric symptoms | EGb 761® (240 mg/die) or placebo, for 24 weeks | Treatment with EGb 761® led to a significant and clinically relevant improvement in patients’ cognition, psychopathology, functional measures and quality of life. | [97] |
A clinical study, conducted by Grass-Kapanke et al., in 2011 [91], observed EGb 761® positive effects with very mild cognitive impairment (vMCI). In this study, 300 vMCI patients, aged 45 to 65 years, received EGb 761® (240 mg/die) or placebo, for 12 weeks. These patients showed an improvement of memory performances, measured through Wechsler Memory Scale III (human face recognition in pictures) and a significant attention improvement, by using the Vienna Test System Work Performance Series (a computerized math test to keep concentration) (Table 2).
A randomized, double blind, placebo controlled, multicentric study (GuidAge), including French people with subjective memory deficit and enrolled for primary clinical care for Alzheimer’s evaluation, prospective followed for more than 5 years [92], showed that EGb 761® treatment could not prevent dementia incidence.
A post hoc analysis of a subgroup of people on going EGb 761® for at least 4 years, showed a significant reduction of dementia development (50% more than placebo group). This long-term effect made scientists doubt and confirm the failure of the statistical models used [93].
In conclusion, these studies affirm a positive effect of Ginkgo biloba in delaying cognitive impairment and improving memory performance in people with vMCI. Currently, the question is still open whether EGb 761® could potentially prevent dementia yet.
1.4. EGb 761® in MCI
Gavrilova and colleagues, in 2014 [94], evaluated EGb 761® (240 mg/die) versus placebo for 24 weeks in 160 patients with amnesic MCI (age > 55 years old). All the neuropsychiatric symptoms, measured through Neuropsychiatric Inventory (NPI), improved in patients treated with EGb 761®. They reached a better diagnosis also for anxiety, depression and visual-motor and cognitive aspect (Table 2).
1.4.1. EGb 761® Efficacy in Mild and Moderate Dementia with or without Neuropsychiatric Disorders
The older controlled clinical trials using EGb 761® versus placebo, have demonstrated a moderate improvement of cognitive functions and activities of daily living (ADL).
In the last 10 years, randomized and controlled trials and sub sequential meta-analysis, considering people affected by AD and MCI associated with neuro-psychiatric symptoms (NPS), have renewed interest on EGb 761® benefits at the dosage of 240 mg/die.
In particular, neuro-psychiatric symptoms, known as “behavioral and psychological dementia symptoms (BPSD)” represent various not-cognitive symptoms groups and behavioral attitude of dementia patients, which dramatically increase during the development of pathology, compromising also “caregivers” life quality. In the last years, EGb 761® has been largely studied in BPSD; in addition to neuro psychiatric symptoms improvement, also the recovery of behavioral ability enhancing has been detected. Indeed, three EGb 761® studies [95,96,97] have demonstrated neuro psychiatric improvement for Neuropsychiatric Inventory (NPI) symptoms not only for Alzheimer’s but also on vascular dementia and mixed forms. AD and Vascular Dementia (VaD) have been included in these studies, where the Caregiver Distress Score was improved in patients on going EGb 761®, by showing a stress reduction for patient’s relatives (Table 2) [90,91,92,93,94,95,96,97].
EGb 761® in people affected by MCI associated with NPS was also studied, observing an improvement in NPS and in cognitive performance in EGb 761® patients [94].
1.4.2. EGb 761® Efficacy and Combined (AChEIs and EGb 761® Association) or Compared (AChEIs versus EGb 761®) Therapy in Mild or Moderate Dementia
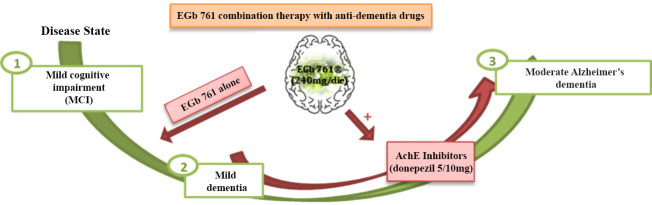
EGb 761® in association with anti-dementia drugs have been studied. The first result derived from GINDON study [98], which analyzed the potential benefit of a combined therapy with EGb 761® and donepezil, after 22 weeks in 96 Alzheimer’s patients with neuro psychiatric disorders (Figure 5).
Figure 5.
EGb 761® in association with anti-dementia drugs.
This study has showed a moderate but not significant benefit at the EGb 761® 240 mg/die + donepezil 5/10 mg/die dosage, in monotherapy, versus the single treatment with EGb 761® 240 mg/die or the single treatment with donepezil 5/10 mg/die, considering cognitive, physic and functional outcomes and neuro psychiatric disorders (Figure 5).
Further evidence regarding EGb 761® and AChEIs combination cognitive performances benefits derive from a prospective ICTUS study, involving 828 patients with mild and moderate Alzheimer’s, for 1 year [99]. These patients were treated with donepezil (55%), rivastigmin (27%) or galantamin (18%) with or without co-administration of EGb 761® (120 mg/die). After 12 months, patients ongoing also EGb 761® showed better results of Mini Mental State Examination (MMSE) than patients using only AChEIs (+1.9 points on MMSE, p = 0.005).
However, recent studies observed a similar effect among patients with AD who received EGb 761® or AChEI.
In particular, Rapp and colleagues, in 2018 [100], evaluated the efficacy of EGb 761® (240 mg/die) or donepezil (5/10 mg) in 189 patients with Alzheimer’s disease (≥age 80 years old). Similar effects on cognitive symptoms, measured by MMSE over 12 months, resulting from the use of EGb 761® and donepezil in patients with AD were observed in this study.
Similarly, Mazza et al., in 2006, conducted a study on 76 patients (aged 50 to 80 years) with mild to moderate dementia, who received Ginkgo biloba (160 mg/die), donepezil (5 mg/die) or placebo for 24-week [101].
Results of this study also showed no differences in the efficacy of EGb 761® and donepezil in the treatment of mild to moderate Alzheimer’s dementia.
1.4.3. EGb 761® Efficacy Verified through RCTs Meta-Analysis in MCI, Mild and Moderate Dementia with or without Behavior Deficits
In the last 10 years, meta-analysis and RCTs results on patients affected by dementia, associated with psycho-behavioral deficits, have led to a new interest for EGb 761®. Tan and colleagues [44] estimated EGb 761® effect on 2561 patients with cognitive deficit and dementia (9 RCTs) after 22–26 weeks (Table 3) [15,44].
Table 3.
Meta-analysis studies on the efficacy of EGb 761® in patients with MCI, mild and moderate dementia with or without behavioral deficits.
| Meta-Analysis Studies (Inclusion Criteria) |
Treatment Groups | Results | References |
|---|---|---|---|
| Patients affected by dementia, associated with psycho-behavioral deficits | EGb 761® (240 mg/die) or placebo |
Patients treated with EGb 761® showed benefits on cognitive decline stabilization or slowing down, on ADL and neuro psychiatric deficit for MCI, Alzheimer’s and dementia (with/without neuropsychiatric problems) patients. | [44] |
| Patients with a diagnosis of AD, VaD, or mixed dementia | EGb 761® (120 mg/die) or EGb 761® (240 mg/die) or placebo |
EGb 761® (240 mg/die; best dosage) has been demonstrated efficacy on cognitive functions, including significant neuropsychiatric deficits in patients with dementia. | [15] |
| Patients with AD or AD + VaD patients | EGb 761® (120 mg/die) or EG b761® (240 mg/die) or placebo |
Patients treated with EGb 761® (240 mg/die) showed benefits versus placebo for cognitive deficits, through Syndrom–Kurztest [SKT] analysis. | [104] |
| Patients with the diagnosis of AD, VaD or mixed dementia with behavioral and psychological symptoms (BPSD) | EGb 761® (240 mg/die) or placebo |
EGb 761® ginkgo biloba extract (240 mg/die) improved the patients’ cognitive performance, BPSD, functional abilities and general condition. | [105] |
| Patients with MCI and dementia (AD, VaD or AD + VaD, mixed dementia) | EGb 761® (240 mg/die) or placebo |
EGb 761® showed benefit versus placebo for cognition, behavior and activities of daily living in patients with MCI and dementia. | [106] |
| Patients with dementia (probable AD, VaD or AD +CVD) | EGb 761® (240 mg/die) or placebo |
EGb 761® improved neuro-psychiatric symptoms, in AD, VaD or AD + CVD patients, except for delirium, hallucination and euphoria. | [107] |
The results of this work demonstrated EGb 761® benefits on cognitive decline stabilization or slowing down, on ADL and neuro psychiatric deficit for MCI, Alzheimer’s and dementia (with/without neuro psychiatric problems) patients. Other analysis in the same work showed differences in the efficacy of EGb 761® different dosages, underling the best treatment with 240 mg/die. Tan and colleagues, 2015, [44], as in the case of Cochrane meta-analysis [102,103], considered only randomized studies of patients with cognitive function compromised or dementia, with the same EGb 761® dosage and similar follow up timing, with the only difference in the selection of the best trials (better dementia diagnosis). Moreover, Tan and coworkers identified and included three RCTs published in that period [94,96,97] and showing an EGb 761® benefit at the dosage of 240 mg/die, for dementia, AD and MCI associated with NPS. After that, a Gauthier and Schlaefke, 2014, [15] meta-analysis confirmed EGb 761® efficacy and tolerability in patients with dementia (Table 3).
This is a well-designed meta-analysis, involving good quality placebo-controlled studies with at least 200 randomized patients. EGb 761® efficacy has been demonstrated on cognitive functions (p = 0.03), ADL (p < 0.001) and on the clinical impression scales (p = 0.01).
The best result in term of EGb 761® best dosage on cognitive functions has been obtained with 240 mg/die, in studies including significant neuro psychiatric deficits patients. In the seven selected studies of this meta-analysis, four of them enrolled only patients with significative neuro psychiatric symptoms, two of them patients with neuro psychiatric symptoms, and one study excluded patients with significant neuro psychiatric deficits.
A 2015 meta-analysis [104], demonstrated EGb 761® benefits versus placebo for cognitive deficits, through Syndrom–Kurztest [SKT] analysis, in AD and AD + VaD patients (Table 3) [15,44,104,105,106,107].
Another meta-analysis on patient with dementia and neuropsychiatric deficits [105] showed that 240 mg/die of EGb 761® significantly improved the cognitive functions, NPS, caregiver stress associated with NPS, ADL and clinical impression, versus placebo in AD, VaD and AD+ Cardio Vascular Disease (CVD) (p < 0.001 for all the analysis) out patients.
A 2016 meta-analysis [106] showed an important activity of Ginkgo extract in improving cognition, behavior and activities of daily living in MCI and dementia. One of the most important outcomes was that the efficacy was dose-dependent and only convincing with a daily dose of 240 mg. Its utilization was safe and the adverse events (AEs) were at placebo level. In the sub-group of Alzheimer’s patients, less AEs arose compared to placebo. Cases of dizziness, Angina pectoris and headache were also less frequent in the active substance group.
Zhang 2016’s publication [106] is the result of an analysis of ten systematic reviews and meta-analyses, in which the efficacy of Ginkgo special extract for cognitive disorders was assessed (Table 3) [15,44,104,105,106,107].
At the end, in a recent meta-analysis of Savaskan and colleagues, in 2018 [107], it has been demonstrated that EGb 761® versus placebo is more efficient for neuro psychiatric symptoms, measured through NPI in AD, VaD or AD + CVD patients, except for delirium, hallucination and euphoria (Table 3) [15,44,104,105,106,107].
Moreover, EGb 761® reduced also the risk of new neuro psychiatric symptoms onset, as the caregiver stress.
2. Discussion and Conclusions
MCI and mild dementia are important diseases affecting our society and, currently, no drug treatment for MCI has been approved in Italy [108]. Specifically, MCI represents an intriguing clinical entity and, until now, the only clinically diagnosed pre-dementia stage.
This review describes the efficacy of the Ginkgo biloba leaves extract in the treatment of MCI and dementia syndrome [30].
The published evidence investigating the association between EGb 761® 240 mg/die and placebo for cognitive deficits were reviewed and a meta-analysis was performed.
Papers meting the inclusion criteria were analyzed. Our meta-analysis included a total of eight studies [96,97,109,110,111,112,113,114], encompassing 1120 cases and 1128 healthy controls (Figure 6). As indicated in Figure 6, EGb 761® was significantly effectiveness to placebo as indicated by the dementia scores SKT (SMD = −0.52, 95% CI = −0.99–0.05, p = 0.031).
Figure 6.
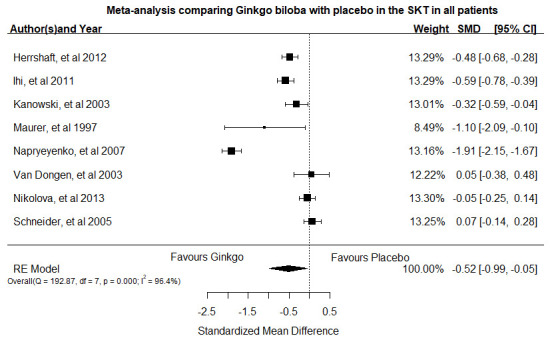
Forest plot of meta-analysis for EGb 761® 240 mg/die and placebo using random-effect model. Meta-analysis of the data obtained from the systematic literature review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis [116]. We searched the PubMed, MEDLINE, EMBASE, PsycINFO, CINAHL, Cochrane Database of Systematic Reviews with the terms Ginkgo* or Gingko* or EGB761 or “EGB 761” or EGB-761. For continuous data, standardized mean differences (SMD) was used. Given the expected heterogeneity, we a priori used a random-effects model. Overall, SMD with 95% CI was estimated with DerSimonian–Laird random-effects models. Publication bias was evaluated by a funnel plot and Egger’s linear regression analysis and p < 0.05 was set as the level of significant. This meta-analysis was performed using R 3.6.2.
Meta-analysis resulted in a statistically significant difference in favor of EGb 761® therapy respect to placebo (SMD = −0.52, 95% CI = −0.99–0.05, p = 0.031).
There was significant heterogeneity I2 = 96.4% (p < 0.0001) [115].
Our funnel plot and statistical test showed no evidence of publication bias (Egger’s test z = −0.5069, p = 0.612). These analysis supports the notion that EGb 761® is effective in these patients.
EGb 761® treatment has also been studied in patients with psychological and behavioral symptoms of dementia” (BPSD), highlighting an improvement in neuro psychiatric symptoms as well as a clear recovery in behavioral abilities. There is also further evidence of the benefit of the combination of EGb 761® in association with AChEIs in cognitive performance [98,99]. The exact mechanism of action is still unclear. Human pharmacological data show increased potency of EEG in older subjects, a reduction in blood viscosity and improved brain perfusion in specific areas in healthy male subjects (60–70 years) [117,118,119].
In addition, more evidences are needed to assess the role of EGb 761® in combination therapy with AChEIs or memantine or triple, EGb 761®, AChEIs and memantine together.
This compound combination, in the future, may have a role in the therapy and in the drug management of dementia, also improving patient rehabilitation. Finally, future investigation will need neuroimaging criteria to better understand the mechanism of action of EGb 761®.
Author Contributions
All authors contributed equally to the drafting of the manuscript. Conceptualization, C.T. and P.M.R.; methodology, S.I.; formal analysis, S.P.; resources, G.Z.; data curation, S.I. and P.R.; writing—original draft preparation, S.I., F.M. and V.M.; writing—review and editing, C.T., P.R., C.M. and V.S.; supervision, P.M.R.; project administration, G.S.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study is also supported by the Italian Ministry of Health [ricerca corrente].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
For all data used in the review there is a reference.
Conflicts of Interest
IRCCS San Raffaele Roma received an “Unrestricted educational grant” from Schwabe Italia srl. The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jongsiriyanyong S., Limpawattana P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimers Dis. Dement. 2018;33:500–507. doi: 10.1177/1533317518791401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondi M.W., Edmonds E.C., Salmon D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017;23:818–831. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma L. Depression, Anxiety, and Apathy in Mild Cognitive Impairment: Current Perspectives. Front. Aging Neurosci. 2020;12:9. doi: 10.3389/fnagi.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlachos G.S., Scarmeas N. Dietary interventions in mild cognitive impairment and dementia. Dialog. Clin. Neurosci. 2019;21:69–82. doi: 10.31887/DCNS.2019.21.1/nscarmeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrattan A.M., McEvoy C.T., McGuinness B., McKinley M.C., Woodside J.V. Effect of dietary interventions in mild cognitive impairment: A systematic review. Br. J. Nutr. 2018;120:1388–1405. doi: 10.1017/S0007114518002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivera-Pueyo J., Pelegrin-Valero C. Dietary supplements for cognitive impairment. Actas Esp. Psiquiatr. 2017;45:37–47. [PubMed] [Google Scholar]
- 7.Ilari S., Giancotti L.A., Lauro F., Dagostino C., Gliozzi M., Malafoglia V., Sansone L., Palma E., Tafani M., Russo M.A., et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020;157:104851. doi: 10.1016/j.phrs.2020.104851. [DOI] [PubMed] [Google Scholar]
- 8.Lauro F., Giancotti L.A., Ilari S., Dagostino C., Gliozzi M., Morabito C., Malafoglia V., Raffaeli W., Muraca M., Goffredo B.M., et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE. 2016;11:e0156039. doi: 10.1371/journal.pone.0156039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauro F., Ilari S., Giancotti L.A., Ventura C.A., Morabito C., Gliozzi M., Malafoglia V., Palma E., Paolino D., Mollace V., et al. Pharmacological effect of a new idebenone formulation in a model of carrageenan-induced inflammatory pain. Pharmacol. Res. 2016;111:767–773. doi: 10.1016/j.phrs.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Ilari S., Giancotti L.A., Lauro F., Gliozzi M., Malafoglia V., Palma E., Tafani M., Russo M.A., Tomino C., Fini M., et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants. 2020;9:1103. doi: 10.3390/antiox9111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Ye M., Guo H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2019;10:1688. doi: 10.3389/fphar.2019.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramassamy C., Longpre F., Christen Y. Ginkgo biloba extract (EGb 761) in Alzheimer’s disease: Is there any evidence? Curr. Alzheimer Res. 2007;4:253–262. doi: 10.2174/156720507781077304. [DOI] [PubMed] [Google Scholar]
- 13.Bonassi S., Prinzi G., Lamonaca P., Russo P., Paximadas I., Rasoni G., Rossi R., Ruggi M., Malandrino S., Sanchez-Flores M., et al. Clinical and genomic safety of treatment with Ginkgo biloba L. leaf extract (IDN 5933/Ginkgoselect(R)Plus) in elderly: A randomised placebo-controlled clinical trial [GiBiEx] BMC Complement. Altern. Med. 2018;18:22. doi: 10.1186/s12906-018-2080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S.K., Srivastav S., Castellani R.J., Plascencia-Villa G., Perry G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics. 2019;16:666–674. doi: 10.1007/s13311-019-00767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier S., Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: A systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Interv. Aging. 2014;9:2065–2077. doi: 10.2147/CIA.S72728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng M., Ai D., Sun L., Xu X., Cao X. EGb 761 inhibits Abeta1-42-induced neuroinflammatory response by suppressing P38 MAPK signaling pathway in BV-2 microglial cells. Neuroreport. 2019;30:434–440. doi: 10.1097/WNR.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 17.D’Amelio M., Rossini P.M. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: From animal models to human findings. Prog. Neurobiol. 2012;99:42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Perez E.J., Gallegos S., Armijo-Weingart L., Araya A., Riffo-Lepe N.O., Cayuman F., Aguayo L.G. Changes in neuronal excitability and synaptic transmission in nucleus accumbens in a transgenic Alzheimer’s disease mouse model. Sci. Rep. 2020;10:19606. doi: 10.1038/s41598-020-76456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campora M., Francesconi V., Schenone S., Tasso B., Tonelli M. Journey on Naphthoquinone and Anthraquinone Derivatives: New Insights in Alzheimer’s Disease. Pharmaceuticals. 2021;14:33. doi: 10.3390/ph14010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugger B.N., Dickson D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matej R., Tesar A., Rusina R. Alzheimer’s disease and other neurodegenerative dementias in comorbidity: A clinical and neuropathological overview. Clin. Biochem. 2019;73:26–31. doi: 10.1016/j.clinbiochem.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Rossini P.M., Di Iorio R., Granata G., Miraglia F., Vecchio F. From Mild Cognitive Impairment to Alzheimer’s Disease: A New Perspective in the “Land” of Human Brain Reactivity and Connectivity. J. Alzheimers Dis. 2016;53:1389–1393. doi: 10.3233/JAD-160482. [DOI] [PubMed] [Google Scholar]
- 23.Costa A., Bak T., Caffarra P., Caltagirone C., Ceccaldi M., Collette F., Crutch S., Della Sala S., Demonet J.F., Dubois B., et al. The need for harmonisation and innovation of neuropsychological assessment in neurodegenerative dementias in Europe: Consensus document of the Joint Program for Neurodegenerative Diseases Working Group. Alzheimers Res. Ther. 2017;9:27. doi: 10.1186/s13195-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam C.L.M., Yiend J., Lee T.M.C. Imaging and neuropsychological correlates of white matter lesions in different subtypes of Mild Cognitive Impairment: A systematic review. NeuroRehabilitation. 2017;41:189–204. doi: 10.3233/NRE-171471. [DOI] [PubMed] [Google Scholar]
- 25.Guan H., Liu T., Jiang J., Tao D., Zhang J., Niu H., Zhu W., Wang Y., Cheng J., Kochan N.A., et al. Classifying MCI Subtypes in Community-Dwelling Elderly Using Cross-Sectional and Longitudinal MRI-Based Biomarkers. Front. Aging Neurosci. 2017;9:309. doi: 10.3389/fnagi.2017.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoi Y., Takano H., Sakata M., Maruo K., Nakagome K., Matsuda H. Discrete effect of each mild behavioural impairment category on dementia conversion or cognitive decline in patients with mild cognitive impairment. Psychogeriatrics. 2019;19:591–600. doi: 10.1111/psyg.12447. [DOI] [PubMed] [Google Scholar]
- 27.Wahlman C., Doyle T.M., Little J.W., Luongo L., Janes K., Chen Z., Esposito E., Tosh D.K., Cuzzocrea S., Jacobson K.A., et al. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. Pain. 2018;159:1025–1034. doi: 10.1097/j.pain.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langa K.M., Levine D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 30.Petersen R.C. Mild Cognitive Impairment. Continuum. 2016;22:404–418. doi: 10.1212/CON.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris J.C., Price J.L. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J. Mol. Neurosci. 2001;17:101–118. doi: 10.1385/JMN:17:2:101. [DOI] [PubMed] [Google Scholar]
- 32.Sanford A.M. Mild Cognitive Impairment. Clin. Geriatr. Med. 2017;33:325–337. doi: 10.1016/j.cger.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Khachaturian Z. The five-five, ten-ten plan for Alzheimer’s disease. Neurobiol. Aging. 1992;13:197–198. doi: 10.1016/0197-4580(92)90030-2. [DOI] [PubMed] [Google Scholar]
- 34.Karakaya T., Fusser F., Schroder J., Pantel J. Pharmacological Treatment of Mild Cognitive Impairment as a Prodromal Syndrome of Alzheimer s Disease. Curr. Neuropharmacol. 2013;11:102–108. doi: 10.2174/1570159X11311010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanacore N., Bianchi C., Da Cas R., Rossi M. Use of antiparkinsonian drugs in the Umbria Region. Neurol. Sci. 2003;24:221–222. doi: 10.1007/s10072-003-0140-0. [DOI] [PubMed] [Google Scholar]
- 36.Ibach B., Haen E. Acetylcholinesterase inhibition in Alzheimer’s Disease. Curr. Pharm. Des. 2004;10:231–251. doi: 10.2174/1381612043386509. [DOI] [PubMed] [Google Scholar]
- 37.Algin D.I., Atalay S.D., Ozkan S., Adapinar D.O., Sivrioz I.A. Memantine improves semantic memory in patients with amnestic mild cognitive impairment: A single-photon emission computed tomography study. J. Int. Med. Res. 2017;45:2053–2064. doi: 10.1177/0300060517715166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez L.J., Barbagallo M. Nutritional prevention of cognitive decline and dementia. Acta Biomed. 2018;89:276–290. doi: 10.23750/abm.v89i2.7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caesar W. Of Ginkgo EGb on GBL—A long path to rational phytopharmacy. Pharm. Unserer Zeit. 2009;38:400–405. doi: 10.1002/pauz.200900327. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X.W., Chen J.Y., Ouyang D., Lu J.H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020;21:493. doi: 10.3390/ijms21020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi C., Liu J., Wu F., Yew D.T. Ginkgo biloba extract in Alzheimer’s disease: From action mechanisms to medical practice. Int. J. Mol. Sci. 2010;11:107–123. doi: 10.3390/ijms11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeFeudis F.V. Ginkgo Biloba Extract (EGb 761): From Chemistry to the Clinic. Ullstein Medical; Berlin, Germany: 1998. [Google Scholar]
- 43.Gardner C.D., Taylor-Piliae R.E., Kiazand A., Nicholus J., Rigby A.J., Farquhar J.W. Effect of Ginkgo biloba (EGb 761) on treadmill walking time among adults with peripheral artery disease: A randomized clinical trial. J. Cardiopulm. Rehabil. Prev. 2008;28:258–265. doi: 10.1097/01.HCR.0000327184.51992.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan M.S., Yu J.T., Tan C.C., Wang H.F., Meng X.F., Wang C., Jiang T., Zhu X.C., Tan L. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2015;43:589–603. doi: 10.3233/JAD-140837. [DOI] [PubMed] [Google Scholar]
- 45.Cave A.E., Chang D.H., Munch G.W., Steiner G.Z. Efficacy of Cognition Support Formula(R) on cognitive function in older adults with subjective cognitive impairment: A protocol for a 26-week, randomised, double-blind, placebo-controlled trial. Trials. 2019;20:345. doi: 10.1186/s13063-019-3431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandiah N., Ong P.A., Yuda T., Ng L.L., Mamun K., Merchant R.A., Chen C., Dominguez J., Marasigan S., Ampil E., et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761((R)) CNS Neurosci. Ther. 2019;25:288–298. doi: 10.1111/cns.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L., Zhang C., Kalionis B., Wan W., Murthi P., Chen C., Li Y., Xia S. EGb761 protects against Abeta1-42 oligomer-induced cell damage via endoplasmic reticulum stress activation and Hsp70 protein expression increase in SH-SY5Y cells. Exp. Gerontol. 2016;75:56–63. doi: 10.1016/j.exger.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Smith J.V., Luo Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J. Alzheimers Dis. 2003;5:287–300. doi: 10.3233/JAD-2003-5404. [DOI] [PubMed] [Google Scholar]
- 49.ESCOP. Phytotherapy (ESCOP) ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. European Scientific Cooperative on Phytotherapy; Yasit, UK: 2003. [Google Scholar]
- 50.Bone K., Mills S. Principles and Practice of Phytotherapy—E-Book: Modern Herbal Medicine. Elsevier Health Sciences; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 51.Haramaki N., Aggarwal S., Kawabata T., Droy-Lefaix M.T., Packer L. Effects of natural antioxidant ginkgo biloba extract (EGB 761) on myocardial ischemia-reperfusion injury. Free Radic. Biol. Med. 1994;16:789–794. doi: 10.1016/0891-5849(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 52.Bridi R., Crossetti F.P., Steffen V.M., Henriques A.T. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother. Res. 2001;15:449–451. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 53.Eckert A., Keil U., Kressmann S., Schindowski K., Leutner S., Leutz S., Muller W.E. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36 (Suppl. 1)(Suppl. 1):S15–S23. doi: 10.1055/s-2003-40449. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X., Nie B., Fu S., Hu J., Yin L., Lin L., Wang X., Lu P., Xu X.M. EGb761 protects hydrogen peroxide-induced death of spinal cord neurons through inhibition of intracellular ROS production and modulation of apoptotic regulating genes. J. Mol. Neurosci. 2009;38:103–113. doi: 10.1007/s12031-008-9140-0. [DOI] [PubMed] [Google Scholar]
- 55.Martin R., Mozet C., Martin H., Welt K., Engel C., Fitzl G. The effect of Ginkgo biloba extract (EGb 761) on parameters of oxidative stress in different regions of aging rat brains after acute hypoxia. Aging Clin. Exp. Res. 2011;23:255–263. doi: 10.1007/BF03337752. [DOI] [PubMed] [Google Scholar]
- 56.Sastre J., Millan A., De La Asuncion J.G., Pla R., Juan G., Pallardo F.V., O’Connor E., Martin J.A., Droy-Lefaix M.-T., Viña J. A Ginkgo biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free Radic. Biol. Med. 1998;24:298–304. doi: 10.1016/S0891-5849(97)00228-1. [DOI] [PubMed] [Google Scholar]
- 57.Abdel-Kader R., Hauptmann S., Keil U., Scherping I., Leuner K., Eckert A., Muller W.E. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761) Pharmacol. Res. 2007;56:493–502. doi: 10.1016/j.phrs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Muller W.E., Heiser J., Leuner K. Effects of the standardized Ginkgo biloba extract EGb 761(R) on neuroplasticity. Int. Psychogeriatr. 2012;24 (Suppl. S1)(Suppl. S1):S21–S24. doi: 10.1017/S1041610212000592. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A., Singh A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015;6:206. doi: 10.3389/fphar.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leuner K., Hauptmann S., Abdel-Kader R., Scherping I., Keil U., Strosznajder J.B., Eckert A., Muller W.E. Mitochondrial dysfunction: The first domino in brain aging and Alzheimer’s disease? Antioxid. Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 61.Muller W.E., Eckert A., Eckert G.P., Fink H., Friedland K., Gauthier S., Hoerr R., Ihl R., Kasper S., Moller H.J. Therapeutic efficacy of the Ginkgo special extract EGb761((R)) within the framework of the mitochondrial cascade hypothesis of Alzheimer’s disease. World J. Biol. Psychiatry. 2019;20:173–189. doi: 10.1080/15622975.2017.1308552. [DOI] [PubMed] [Google Scholar]
- 62.Janssens D., Michiels C., Delaive E., Eliaers F., Drieu K., Remacle J. Protection of hypoxia-induced ATP decrease in endothelial cells by ginkgo biloba extract and bilobalide. Biochem. Pharmacol. 1995;50:991–999. doi: 10.1016/0006-2952(95)00227-Q. [DOI] [PubMed] [Google Scholar]
- 63.Eckert A., Keil U., Scherping I., Hauptmann S., Muller W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005;1056:474–485. doi: 10.1196/annals.1352.023. [DOI] [PubMed] [Google Scholar]
- 64.Rhein V., Giese M., Baysang G., Meier F., Rao S., Schulz K.L., Hamburger M., Eckert A. Ginkgo biloba extract ameliorates oxidative phosphorylation performance and rescues abeta-induced failure. PLoS ONE. 2010;5:e12359. doi: 10.1371/journal.pone.0012359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baliutyte G., Trumbeckaite S., Baniene R., Borutaite V., Toleikis A. Effects of standardized extract of Ginkgo biloba leaves EGb761 on mitochondrial functions: Mechanism(s) of action and dependence on the source of mitochondria and respiratory substrate. J. Bioenerg. Biomembr. 2014;46:493–501. doi: 10.1007/s10863-014-9590-8. [DOI] [PubMed] [Google Scholar]
- 66.Liu X., Hao W., Qin Y., Decker Y., Wang X., Burkart M., Schotz K., Menger M.D., Fassbender K., Liu Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 2015;46:121–131. doi: 10.1016/j.bbi.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Wan W., Zhang C., Danielsen M., Li Q., Chen W., Chan Y., Li Y. EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp. Gerontol. 2016;81:92–100. doi: 10.1016/j.exger.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Gargouri B., Carstensen J., Bhatia H.S., Huell M., Dietz G.P.H., Fiebich B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine. 2018;44:45–55. doi: 10.1016/j.phymed.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Ahlemeyer B., Mowes A., Krieglstein J. Inhibition of serum deprivation- and staurosporine-induced neuronal apoptosis by Ginkgo biloba extract and some of its constituents. Eur. J. Pharmacol. 1999;367:423–430. doi: 10.1016/S0014-2999(98)00903-0. [DOI] [PubMed] [Google Scholar]
- 70.Schindowski K., Leutner S., Kressmann S., Eckert A., Muller W.E. Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761) J. Neural Transm. 2001;108:969–978. doi: 10.1007/s007020170016. [DOI] [PubMed] [Google Scholar]
- 71.Luo Y., Smith J.V., Paramasivam V., Burdick A., Curry K.J., Buford J.P., Khan I., Netzer W.J., Xu H., Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colciaghi F., Borroni B., Zimmermann M., Bellone C., Longhi A., Padovani A., Cattabeni F., Christen Y., Di Luca M. Amyloid precursor protein metabolism is regulated toward alpha-secretase pathway by Ginkgo biloba extracts. Neurobiol. Dis. 2004;16:454–460. doi: 10.1016/j.nbd.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 73.Blasko I., Kemmler G., Krampla W., Jungwirth S., Wichart I., Jellinger K., Tragl K.H., Fischer P. Plasma amyloid beta protein 42 in non-demented persons aged 75 years: Effects of concomitant medication and medial temporal lobe atrophy. Neurobiol. Aging. 2005;26:1135–1143. doi: 10.1016/j.neurobiolaging.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Longpre F., Garneau P., Christen Y., Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 75.Shi C., Zhao L., Zhu B., Li Q., Yew D.T., Yao Z., Xu J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Biol. Interact. 2009;181:115–123. doi: 10.1016/j.cbi.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Wan W.B., Cao L., Liu L.M., Kalionis B., Chen C., Tai X.T., Li Y.M., Xia S.J. EGb761 provides a protective effect against Abeta1-42 oligomer-induced cell damage and blood-brain barrier disruption in an in vitro bEnd.3 endothelial model. PLoS ONE. 2014;9:e113126. doi: 10.1371/journal.pone.0113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie H., Wang J.R., Yau L.F., Liu Y., Liu L., Han Q.B., Zhao Z., Jiang Z.H. Quantitative analysis of the flavonoid glycosides and terpene trilactones in the extract of Ginkgo biloba and evaluation of their inhibitory activity towards fibril formation of beta-amyloid peptide. Molecules. 2014;19:4466–4478. doi: 10.3390/molecules19044466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin Y., Zhang Y., Tomic I., Hao W., Menger M.D., Liu C., Fassbender K., Liu Y. Ginkgo biloba Extract EGb 761 and Its Specific Components Elicit Protective Protein Clearance Through the Autophagy-Lysosomal Pathway in Tau-Transgenic Mice and Cultured Neurons. J. Alzheimers Dis. 2018;65:243–263. doi: 10.3233/JAD-180426. [DOI] [PubMed] [Google Scholar]
- 79.Fehske C.J., Leuner K., Muller W.E. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol. Res. 2009;60:68–73. doi: 10.1016/j.phrs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Yoshitake T., Yoshitake S., Kehr J. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010;159:659–668. doi: 10.1111/j.1476-5381.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beck S.M., Ruge H., Schindler C., Burkart M., Miller R., Kirschbaum C., Goschke T. Effects of Ginkgo biloba extract EGb 761(R) on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment—A randomized double-blind placebo-controlled trial. Hum. Psychopharmacol. 2016;31:227–242. doi: 10.1002/hup.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tchantchou F., Xu Y., Wu Y., Christen Y., Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 83.Tchantchou F., Lacor P.N., Cao Z., Lao L., Hou Y., Cui C., Klein W.L., Luo Y. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J. Alzheimers Dis. 2009;18:787–798. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- 84.Kasper S., Bancher C., Eckert A., Forstl H., Frolich L., Hort J., Korczyn A.D., Kressig R.W., Levin O., Palomo M.S.M. Management of mild cognitive impairment (MCI): The need for national and international guidelines. World J. Biol. Psychiatry. 2020;21:579–594. doi: 10.1080/15622975.2019.1696473. [DOI] [PubMed] [Google Scholar]
- 85.Massoud F., Leger G.C. Pharmacological treatment of Alzheimer disease. Can. J. Psychiatry. 2011;56:579–588. doi: 10.1177/070674371105601003. [DOI] [PubMed] [Google Scholar]
- 86.Baskys A., Hou A.C. Vascular dementia: Pharmacological treatment approaches and perspectives. Clin. Interv. Aging. 2007;2:327–335. doi: 10.1016/j.exger.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boxer A.L., Gold M., Feldman H., Boeve B.F., Dickinson S.L., Fillit H., Ho C., Paul R., Pearlman R., Sutherland M., et al. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimers Dement. 2020;16:131–143. doi: 10.1016/j.jalz.2019.06.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hershey L.A., Coleman-Jackson R. Pharmacological Management of Dementia with Lewy Bodies. Drugs Aging. 2019;36:309–319. doi: 10.1007/s40266-018-00636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young J.J., Balachandran S., Garg G., Balasubramaniam M., Gupta A., Tampi D.J., Tampi R.R. Personality and the risk factors for developing behavioral and psychological symptoms of dementia: A narrative review. Neurodegener. Dis. Manag. 2019;9:107–118. doi: 10.2217/nmt-2018-0044. [DOI] [PubMed] [Google Scholar]
- 90.Amieva H., Meillon C., Helmer C., Barberger-Gateau P., Dartigues J.F. Ginkgo biloba extract and long-term cognitive decline: A 20-year follow-up population-based study. PLoS ONE. 2013;8:e52755. doi: 10.1371/journal.pone.0052755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grass-Kapanke B., Busmane A., Lasmanis A., Hoerr R., Kaschel R. Effects of Ginkgo biloba special extract EGb761(R) in very mild cognitive impairment (vMCI) Neurosci. Med. 2011;2:48–56. doi: 10.4236/nm.2011.21007. [DOI] [Google Scholar]
- 92.Vellas B., Coley N., Ousset P.J., Berrut G., Dartigues J.F., Dubois B., Grandjean H., Pasquier F., Piette F., Robert P., et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–859. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 93.Scherrer B., Andrieu S., Ousset P.J., Berrut G., Dartigues J.F., Dubois B., Pasquier F., Piette F., Robert P., Touchon J., et al. Analysing Time to Event Data in Dementia Prevention Trials: The Example of the GuidAge Study of EGb761. J. Nutr. Health Aging. 2015;19:1009–1011. doi: 10.1007/s12603-015-0661-2. [DOI] [PubMed] [Google Scholar]
- 94.Gavrilova S.I., Preuss U.W., Wong J.W., Hoerr R., Kaschel R., Bachinskaya N., Group G.I.S. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: A randomized, placebo-controlled, double-blind, multi-center trial. Int. J. Geriatr. Psychiatry. 2014;29:1087–1095. doi: 10.1002/gps.4103. [DOI] [PubMed] [Google Scholar]
- 95.DeKosky S.T., Williamson J.D., Fitzpatrick A.L., Kronmal R.A., Ives D.G., Saxton J.A., Lopez O.L., Burke G., Carlson M.C., Fried L.P., et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ihl R., Bachinskaya N., Korczyn A.D., Vakhapova V., Tribanek M., Hoerr R., Napryeyenko O., Group G.S. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomized controlled trial. Int. J. Geriatr. Psychiatry. 2011;26:1186–1194. doi: 10.1002/gps.2662. [DOI] [PubMed] [Google Scholar]
- 97.Herrschaft H., Nacu A., Likhachev S., Sholomov I., Hoerr R., Schlaefke S. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012;46:716–723. doi: 10.1016/j.jpsychires.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Yancheva S., Ihl R., Nikolova G., Panayotov P., Schlaefke S., Hoerr R., Group G.S. Ginkgo biloba extract EGb 761(R), donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: A randomised, double-blind, exploratory trial. Aging Ment. Health. 2009;13:183–190. doi: 10.1080/13607860902749057. [DOI] [PubMed] [Google Scholar]
- 99.Canevelli M., Adali N., Kelaiditi E., Cantet C., Ousset P.J., Cesari M., Group I.D. Effects of Gingko biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: Data from the ICTUS study. Phytomedicine. 2014;21:888–892. doi: 10.1016/j.phymed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Rapp M., Burkart M., Kohlmann T., Bohlken J. Similar treatment outcomes with Ginkgo biloba extract EGb 761 and donepezil in Alzheimer’s dementia in very old age: A retrospective observational study. Int. J. Clin. Pharmacol. Ther. 2018;56:130–133. doi: 10.5414/CP203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazza M., Capuano A., Bria P., Mazza S. Ginkgo biloba and donepezil: A comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur. J. Neurol. 2006;13:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 102.Birks J., Grimley E.V., Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2002;4:1–19. doi: 10.1002/14651858.CD003120. [DOI] [PubMed] [Google Scholar]
- 103.Birks J., Evans J.G. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2009;1:CD003120. doi: 10.1002/14651858.CD003120.pub3. [DOI] [PubMed] [Google Scholar]
- 104.Hashiguchi M., Ohta Y., Shimizu M., Maruyama J., Mochizuki M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015;1:14. doi: 10.1186/s40780-015-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Von Gunten A., Schlaefke S., Uberla K. Efficacy of Ginkgo biloba extract EGb 761((R)) in dementia with behavioural and psychological symptoms: A systematic review. World J. Biol. Psychiatry. 2016;17:622–633. doi: 10.3109/15622975.2015.1066513. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H.F., Huang L.B., Zhong Y.B., Zhou Q.H., Wang H.L., Zheng G.Q., Lin Y. An Overview of Systematic Reviews of Ginkgo biloba Extracts for Mild Cognitive Impairment and Dementia. Front. Aging Neurosci. 2016;8:276. doi: 10.3389/fnagi.2016.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Savaskan E., Mueller H., Hoerr R., von Gunten A., Gauthier S. Treatment effects of Ginkgo biloba extract EGb 761(R) on the spectrum of behavioral and psychological symptoms of dementia: Meta-analysis of randomized controlled trials. Int. Psychogeriatr. 2018;30:285–293. doi: 10.1017/S1041610217001892. [DOI] [PubMed] [Google Scholar]
- 108.Knopman D.S., Petersen R.C. Mild cognitive impairment and mild dementia: A clinical perspective. Mayo Clin. Proc. 2014;89:1452–1459. doi: 10.1016/j.mayocp.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanowski S., Hoerr R. Ginkgo biloba extract EGb 761 in dementia: Intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry. 2003;36:297–303. doi: 10.1055/s-2003-45117. [DOI] [PubMed] [Google Scholar]
- 110.Maurer K., Ihl R., Dierks T., Frolich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. J. Psychiatr. Res. 1997;31:645–655. doi: 10.1016/S0022-3956(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 111.Napryeyenko O., Borzenko I., Group G.-N.S. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11. doi: 10.1055/s-0031-1296579. [DOI] [PubMed] [Google Scholar]
- 112.Van Dongen M., van Rossum E., Kessels A., Sielhorst H., Knipschild P. Ginkgo for elderly people with dementia and age-associated memory impairment: A randomized clinical trial. J. Clin. Epidemiol. 2003;56:367–376. doi: 10.1016/S0895-4356(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 113.Nikolova G., Yancheva S., Raychev I., Hoerr R., PLAGIN Study Group Ginkgo biloba extract in dementia: A 22-week randomised, placebo-controlled, double-blind trial. Bulg. Neurol. 2013;14:139–143. [Google Scholar]
- 114.Schneider L.S., DeKosky S.T., Farlow M.R., Tariot P.N., Hoerr R., Kieser M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr. Alzheimer Res. 2005;2:541–551. doi: 10.2174/156720505774932287. [DOI] [PubMed] [Google Scholar]
- 115.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 117.Halil M., Cankurtaran M., Yavuz B.B., Ozkayar N., Ulger Z., Dede D.S., Shorbagi A., Buyukasik Y., Haznedaroglu I.C., Arogul S. No alteration in the PFA-100 in vitro bleeding time induced by the Ginkgo biloba special extract, EGb 761, in elderly patients with mild cognitive impairment. Blood Coagul. Fibrinolysis. 2005;16:349–353. doi: 10.1097/01.mbc.0000172695.62363.57. [DOI] [PubMed] [Google Scholar]
- 118.Sollier C.B.D., Caplain H., Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clin. Lab. Haematol. 2003;25:251–253. doi: 10.1046/j.1365-2257.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 119.Wolf H.R. Does Ginkgo biloba special extract EGb 761 provide additional effects on coagulation and bleeding when added to acetylsalicylic acid 500 mg daily? Drugs R D. 2006;7:163–172. doi: 10.2165/00126839-200607030-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For all data used in the review there is a reference.