Abstract
The circadian rhythmicity of endogenous metabolic and hormonal processes is controlled by a complex system of central and peripheral pacemakers, influenced by exogenous factors like light/dark-cycles, nutrition and exercise timing. There is evidence that alterations in this system may be involved in the pathogenesis of metabolic diseases. It has been shown that disruptions to normal diurnal rhythms lead to drastic changes in circadian processes, as often seen in modern society due to excessive exposure to unnatural light sources. Out of that, research has focused on time-restricted feeding and exercise, as both seem to be able to reset disruptions in circadian pacemakers. Based on these results and personal physical goals, optimal time periods for food intake and exercise have been identified. This review shows that appropriate nutrition and exercise timing are powerful tools to support, rather than not disturb, the circadian rhythm and potentially contribute to the prevention of metabolic diseases. Nevertheless, both lifestyle interventions are unable to address the real issue: the misalignment of our biological with our social time.
Keywords: intermittent fasting, circadian rhythm, exercise, metabolism, stress hormones
1. Introduction
Humans are responsive to a multitude of metabolic and hormonal “background” processes dictating daily life [1]. From the days of the Stone Age until the 21st century, humans have submitted to the fundamental daily light/dark cycle on Earth, which is the foundation of our wake/sleep cycle called the circadian rhythm [2]. This highly complex cycle has a major influence on our metabolic and hormonal health [3,4]. Throughout human evolution, wake/sleep cycles have influenced our lives as much as our dietary intake. The human genome evolved during the hunter-gatherer period, which led to a selection of genes and traits that define the humane gene pool today [5,6]. A major contributing factor in defining our genome is the interplay of food scarcity and food abundance that accompanied humans throughout their evolution, enabling human metabolism to store surplus energy [7,8].
In earlier days, extended periods of fasting led to metabolic stress, which increased the demand for food or promoted pathways of energy preservation by extending sleep periods. Across all species on earth, sleeping behavior has shown a remarkable flexibility to counteract metabolic stress, whereas the circadian rhythm remains the fundament, yet duration, sleep architecture and timing of sleep may adapt autonomously.
With industrialization and the ubiquitous availability of food, the ability of the human body to be as efficient as possible at storing surplus energy gave rise to several common diseases and the “global burden of diabetes” [9]. However, this burden to public health has not only emerged due to a disturbed metabolic milieu leading to an excessive gain in body weight but also due to our modern lifestyle, which is characterized by physical inactivity and overconsumption of food [10,11]. Our metabolism demands physical activity since similar to our metabolic response to the absence/presence of food, our genomes support a “physical activity cycle” as discussed by Chakravarthy et al., which, if ignored can be an origin of disease [6]. The sheer complexity in maintaining a balanced calorie intake, sufficient physical activity and adequate sleep has previously been the subject of investigation [12,13]. However, the hormonal background, potential interactions and confounding variables remain a matter of investigation.
In recent years, intermittent fasting in particular time restricted feeding, described as the limited consumption of foods and calorie-containing beverages in a set window of a few hours per day and constantly abstaining from calorie-dense products for the remaining hours, has shown health benefits [14,15]. The effects of fasting and the interaction with the circadian rhythm and its hormonal response in “re-setting the clock” have previously been discussed in several reviews [16,17,18]. However, it is unclear how physical exercise can be incorporated into this complex metabolic and hormonal interaction. Even though advocated as the “poly-pill” for the treatment of certain diseases, prescribing exercise in type, intensity, duration and mode may have a major impact on circadian rhythm and intermittent fasting strategies [19,20]. Therefore, this present review aimed to summarize novel information on fasting strategies, intermittent fasting, circadian rhythm and exercise with respect to metabolic and hormonal responses to define recommendations of how to incorporate exercise for a healthy lifestyle.
2. Materials and Methods
For this narrative review, a literature search on PubMed was conducted in December 2020 for research studies involving intermittent fasting (16/8), circadian rhythm, metabolic pathways and their interaction with physical exercise. Key articles from these broad areas of research were included.
3. Results
3.1. Circadian Rhythm
The obligate need for a chocolate brownie after lunch and craving for sweet beverages during the day when actually being overfed, or being overexcited when you are tired, sometimes seems like an arbitrary reaction of the body. Nevertheless, metabolic processes in the human sleep/wake and feeding/fasting cycle are influenced by a complex network of circadian pacemakers. They are synchronized by the suprachiasmatic nucleus (SCN) [21,22], which is located at the base of the hypothalamus, functioning as a central clock, and several peripheral tissue-specific clocks. The rhythmicity, which is sculpted by a central transcription-translation autoregulatory feedback loop [23], roughly reflects the 24 h daily rhythm [24]. Anticipatory adjustments of rhythmicity of both, the SCN and peripheral clocks, are induced by changes in the exogenous physical environment. This leads to the formation of individual circadian rhythms. These rhythms interact with several physiological processes, like sleep homeostasis [25,26,27], metabolic function [28,29,30] and the immune system [31,32]. Additionally, numerous peripheral modulators are responsible for the control of tissue-specific processes. They are partly located in other brain regions like the pineal or pituitary gland [33], but are also localized outside the brain like cardiomyocytes, liver and muscle cells [34,35,36,37].
3.1.1. Hormonal Pathways and Its Effects on the Circadian Rhythm
The circadian rhythmicity in mammals is driven by the primary transcriptional-translational autoregulatory feedback loop in the SCN including the key transcriptional activators, circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt-like 1 (BMAL1) [38]. The CLOCK:BMAL1 heterodimer subsequently formed initiates the expression of Period (Per) and Cryptochrome (Cry) genes. Specific messenger ribonucleic acid (mRNA) is then translated into the mPER and mCRY proteins in the cytoplasm. The PER:CRY heterodimers translocate to the nucleus and inhibit their own transcription by interacting with CLOCK:BMAL1 [24,39,40,41]. mPER and mCRY proteins form a part of the negative limb of the feedback loop. A second regulatory loop is initiated by CLOCK:BMAL1-activated transcription of retinoic acid-related orphan receptors Rev-erbα,β and Rorα,β,γ. The translated proteins, REV-ERBα,β and RORα,β,γ, bind to retinoic acid-related orphan receptor response elements (ROREs). ROREs, which are present in the BMAL1 promoter, modulate transcription of Bmal1. REV-ERBα,β and RORα,β,γ influence expression in different ways. RORα,β,γ enhances Bmal1 mRNA levels, while REV-ERBα,β represses them [42,43]. Both proteins are crucial in modulating the positive site of the autoregulatory feedback loop. Besides the central clock gene (CG) expression, many other peripheral cells show a rhythmical oscillation of CGs, as seen in liver, gut, heart, testis or adipose tissue [44,45,46,47]. In addition to CGs, peripheral tissues show transcription of thousands of clock-controlled genes (CCGs) regulated by clock output genes [48]. They influence, for example, the circadian rhythm of endocrine processes, including the formation of adrenocorticotropic hormone (ACTH) or other glucocorticoids [49,50] or rhythmicity of metabolism [51,52].
3.1.2. External Stressors of the Circadian Rhythm
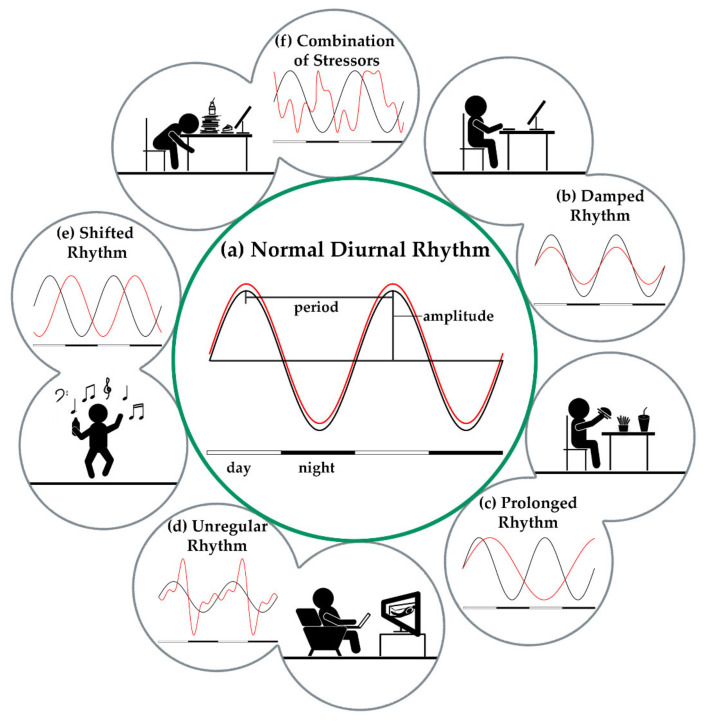
Whenever the natural circadian rhythm is altered by external factors, this has physiological (metabolic stress) as well as psychological (cognitive function and mental health) consequences [53]. The level of information about potential relationships between shift work and various diseases of civilization such as various types of cancer, metabolic or cardiovascular disease, or in combination, can be considered as inconsistent and rather inconclusive [54]. However, a factor for an increased risk of developing disease is a shifted rhythm of the sleep hormone melatonin, which has been shown previously [55,56]. Melatonin is responsible for controlling thermoregulatory processes. The lowering of core body temperature when melatonin is released, results in the onset of sleepiness in the evening and a drop in melatonin concentration in the morning leads to awakening [57]. Exposure to light at night leads to an inhibition of melatonin release and, in shift workers, results in a shortened over-all sleep duration on workdays [58] and an increased prevalence of sleep disorders due to a shifted sleep-wake cycle [59,60]. In the morning, decreasing melatonin levels go hand in hand with formation of cortisol, which is regulated by the hypothalamic-pituitary-adrenal (HPA) axis. Elevated total cortisol levels right after waking up [61] as well as a rise in absolute cortisol concentrations [62] were observed in shift workers previously. This indicates a disruption of the pituitary-adrenal response to light stimuli, which can already be observed after only five days of nighttime work [63]. The influence of increased cortisol and thus decreased melatonin levels on glucose metabolism was reviewed by Cipolla-Neto et al. They discussed studies on pinealectomized animals with a loss in endogenous pineal melatonin production, showing an increased peripheral and central insulin resistance, as well as decreased glucose tolerance and a reduction in glucose transporter type 4 (GLUT4) mRNA in adipose and muscle tissue [64,65,66]. Therefore, an appropriate melatonin replacement therapy for populations with a lack in diurnal melatonin production, like in shift workers and in the elderly, for either preventing, eliminating, or both, metabolic diseases is recommended [67]. This demonstrates the distinct influence of shift work on glucose homeostasis. Shift work also affects other endocrine functions such as the secretion of ghrelin and leptin. Leptin, a satiety hormone produced by the adipose tissue, is important in regulating appetite and energy metabolism [68]; its synthesis is regulated by nutritional status. Ghrelin can be understood as an antagonist to leptin and is produced in the pancreas and the gastric mucosa. Higher ghrelin levels promote a decreased fat oxidation that leads to an increase of adipose tissue and food intake [69], whereas higher serum leptin levels represent an adequate nutritional status [70]. Leptin is reduced and higher ghrelin levels are observed in subjects with shorter sleep durations [71], as regularly seen in shift workers [58,60,72], leading to a state of increased metabolic stress, which serves as a driver for development of metabolic diseases [73]. In addition, stress, irregular and unhealthy eating patterns and sedentary lifestyle may also lead to disturbances in circadian processes (Figure 1).
Figure 1.
Different Stressors modify the normal diurnal rhythmicity. The circadian rhythm is controlled by the light/dark-cycle that influences the rhythmicity of central pacemaker (black) and thus all peripheral processes (red) are synchronized with each other (a). Different external stressors can lead to disturbances in the highly complex interaction of the central and peripheral oscillators. Damped responses of peripheral pacemakers (b) are observed especially in insulin resistance or night eating. High fat meals lead to prolonged cycles (c) and thus a shift of the periods against each other. Irregular rhythms (d) may be caused by irregular meals or generally an irregular lifestyle. A decoupling of the sleep/wake cycle from the light/dark cycle, as seen in shift work or due to modern lifestyle prolonged waking times, can additionally shift the periods against each other (e). A combination of several factors can result in a partial or complete decoupling of the peripheral from the central pacemaker (f).
3.2. Intermittent Fasting as a Lifestyle
Intermittent fasting (IF) has become the center of public attention and, therefore, of more research in recent years [74,75,76]. IF means food deprivation in a daily or hourly time window. Methods carried out on a daily basis are usually linked to the aims of caloric restriction and weight loss. Probably the foremost known forms of day-long fasts are the so-called Leangains and Warrior Diet, in which an 8 h or 4 h eating window is implemented from lunch to dinner or at the end of the day. These fasting forms, also known as time restricted feeding (TRF), are intended to be suitable as a long-term form of nutrition and will therefore be discussed in more detail in the following section.
3.2.1. Intermittent Fasting and Its Influence on Central Clock Genes/Hormones
Recent studies in humans reported that intermittent fasting (IF), especially when feeding time is early or in the middle of the day, results in decreased body weight and fat mass as well as improved blood pressure and insulin sensitivity [77,78,79,80,81]. Due to the time restricted food intake, the body uses less glucose and more lipids and ketones as energy resources [82], resulting in improved glucose and lipid homeostasis [83], preventing mitochondrial ageing processes [84] and deoxyribonucleic acid (DNA) repair by enhancing ketone body levels, indicators for a healthier metabolic phenotype [85]. In addition, a stimulated thermogenic activity in brown adipose tissue leads to a reduction in body fat [86]. Many effects of TRF on either hypothalamic, pituitary, or both, hormones have already been described in literature [18,87,88,89] so its influence on metabolic processes, whether in health or in disease, cannot be neglected.
3.2.2. Intermittent Fasting and Hormonal Pathways
The hypothalamus, part of the endocrine system and nervous system, forms the interface between the nervous and hormonal regulation of metabolism [90,91]. The SCN influences metabolism in peripheral organs by transmitting the time information via circadian CCGs to target organs, but peripheral CGs also show autonomous regulation of metabolic processes and influences CCGs, underlining the bidirectional character of circadian control. The connection between both is described in the review of Mazzoccoli et al., showing that misalignment of the central circadian clock in SCN with unusual light/dark cycles, or alterations in peripheral metabolic processes can both lead to disturbances in circadian rhythmicity [51]. The hormones produced in the hypothalamus can be divided into two different classes—releasing hormones (RH) and inhibiting hormones (IH)—according to their function. RH promotes the release of other hormones like corticotropin releasing hormone (CRH) for secretion of ACTH, whereas IH mitigates their release, like CRH-induced secretion of somatostatin, resulting in suppression of the release of growth hormone (GH) [92,93,94]. After the secretion of RH and IH, they reach the anterior pituitary gland (adenohypophysis) via the portal system, transmitting the hormones to trope cells and stimulating or inhibiting the secretion of somatotropic and glandotropic hormones [95]. They control the hormone release in peripheral glands, like glucocorticoids, influencing cortisol, insulin and glucagon secretion [96]. The two hormones, glucagon and insulin, secreted by the α-(glucagon) and β-(insulin) cells of the pancreas, mainly regulate blood glucose levels [97]. Plasma insulin secretion is increased when blood glucose levels are elevated [98] for stimulating glucose transport to muscle and adipocytes, while gluconeogenesis in the liver is inhibited [99]. In contrast, during hypoglycemia, glucagon secretion is promoted, which increases blood glucose levels by hepatic glucose production [100]. The peak of the circadian insulin secretion is reached in the early morning. Besides this internal rhythm, food intake has a great effect on insulin secretion, resulting in a postprandial increase of insulin and glucose levels [93]. In contrary, TRF reduces fasting insulin levels when implementing in the morning, indicating an enhanced insulin sensitivity [78,101]. Whole-body insulin sensitivity decreases throughout the day [102,103], which is why it seems that food intake at the beginning of the day is preferable, making early TRF the chosen method for improving and preventing metabolic diseases. Insulin and glucagon as the main hormones influencing glucose homoeostasis are regulated by SCN during fasting times but their secretion is sufficiently driven by tissue-specific CCGs during feeding cycles [104]. A change of the eating time from active to inactive phase, can thereby lead to a reset of peripheral clock machinery, leading to shifted phases of peripheral and circadian clocks [105,106]. Organs involved in this complex process are the liver, pancreas, adipose and muscle tissue. The liver has a central role in maintaining glucose homeostasis and is governed by SCN control [107]. However, implementation of TRF has shown that alteration in the fasting/feeding time window results in complete decoupling of the liver clock from the hypothalamic clock. Many of the circadian transcripts found in the liver influence CG Per2 independently of the hepatocyte clock, highlighting the influence of additional external, hormonal or behavioral clocks [108]. Here, administration of insulin has been shown to induce a phase-advance of PER2. Conversely, a liver-specific Bmal1 knockout affects glucose homeostasis and Cry knockout alters glucagon-induced gluconeogenesis. Both lead to an improvement in glucose tolerance. [109,110]. In muscle, Rev-Erbα is the central CG. A deficiency in Rev-Erbα results in a deterioration of mitochondrial function [111], implying a deficiency in glucose metabolism, while a knockout of Bmal1 is associated with impaired muscle glucose uptake and metabolism [112]. Adipocyte-specific Bmal1 knockout leads to obesity, displaying the increase in food consumption when lacking Bmal1 [113]. However, in contrast to liver-specific knockout of Bmal1 [109], no increase in insulin sensitivity could be detected. This finding is supported by results of Basse et al., which demonstrated a different diurnal rhythm of glucose uptake in skeletal muscle and adipose tissue compared to diurnal whole-body insulin tolerance in mice [114]. Metabolic processes in adipose tissue are also driven by other endogenous clocks like adipokines, with adiponectin regulating insulin sensitivity with a peak in the active phase [115]. Adipokines show a circadian rhythmicity but can also be altered with changes in sleep/wake and feeding/fasting cycles, which could explain the increased prevalence of metabolic disorders in shift workers [116]. Furthermore, in pancreatic cells, further autonomous circadian rhythms have been found. Glucose-induced insulin secretion seems to be dependent on the two pancreatic-CGs, Bmal1 and Clock. This was demonstrated in Clock and Bmal1 knockout mice, showing a disturbed glucose tolerance and decrease in insulin secretion [30]. Clock and Bmal1 are also involved in expression of some islet genes and therefore a normal pancreas-specific CG expression is required for sufficient insulin release for glucose homoeostasis [117,118].
During fasting, glucagon stimulates hepatic glucose production. Several studies suggest that glucagon levels also influence the expression of central CGs [119,120]. The effect of restricted feeding on glucagon levels and its influence on CGs was demonstrated by Mukherji et al. They measured a starvation-induced increase in glucagon and free fatty acids (FFA), leading to activation of transcriptional factor cAMP response element-binding protein (CREB) through glucagon and peroxisome proliferator-activated receptor alpha (PPARα) activation through enhanced FFA levels. This results in activated Per1 and Per2 expression (glucagon) and an increase in Rev-Erbα expression activated by FFA-liganded PPARα [121]. The interplay between peripheral pacemakers and CG expression was also investigated by Jakubowicz et al., who compared the influence of glucagon-like peptide 1, a peptide hormone regulating glucagon and insulin levels [122,123], on CG expression after a single omission of breakfast in healthy individuals compared to individuals with type 2 diabetes. They detected large differences between both groups after breakfast and on fasting days, resulting in the expression of different CGs in healthy compared to type 2 diabetes. In addition, a difference in gene expression was detected between breakfast and fasting in both groups. This shows that metabolism in type 2 diabetes is associated with specific changes in gene expression and breakfast skipping shows metabolic effects in health and disease [124]. The highly complex relationship between feeding/fasting cycles and CGs and CCGs and its positive effect for resetting the circadian clock and preventing chronic metabolic diseases was discussed in more detail in the literature [89,101,125,126,127].
Another metabolism influencing hormone is adiponectin, an adipokine expressed in adipose tissue like leptin, sensitizing the tissue to insulin [128]. Adiponectin level is lower at higher body weights and leads to insulin resistance, a higher fat mass and central fat distribution [129,130,131]. TRF appears to be a valuable method for enhancing adiponectin levels, which was proven by Moro et al. [81]. They randomized 34 resistance-trained males to a regular diet group and to TRF (16 h fasting, 8 h eating). After 8 weeks of intervention they detected a highly significant increase in adiponectin levels whereas leptin levels were lowered, which is in accordance with other literature [132]. In non-obese mice with type 1 diabetes, it was further shown that substitution of insulin with leptin or supplementation of leptin instead of insulin therapy can mimic the effect of insulin monotherapy [133]. Glycated hemoglobin (HbA1c), indicator of long-term glycemic control, normalization was achieved with significantly lower glucose fluctuations. Furthermore, a reduction in plasma and tissue lipids was demonstrated, showing the comprehensive effect of leptin on metabolism.
3.3. Exercise as a Lifestyle
Within the last decades, physical exercise shifted towards the center of attention due to its multifactorial properties [20,134]. It can be applied in nearly any population of any age-group to improve physiological and psychological parameters and general quality of life [135,136,137,138]. Due to these properties, medicine has started to apply exercise with a personalized approach and moved forward towards an adjuvant therapy in different cohorts [139,140,141]. Besides its features in treating disease, it has also become part of daily life in healthy populations [142,143]. The World Health Organization (WHO) recommends at least 150 min of moderate-intensity, or 75 min of vigorous intensity per week [144]. From this perspective, living a physically active lifestyle is desirable and the urge of physiological improvement to remain healthy and live with a high quality of life has become present through all age groups by an increased application of physical activity trackers, food apps or sleep trackers [145,146,147].
Users of these applications receive plenty of information from their devices; however, it is not entirely clear how nutrition, exercise and sleep interact. Of course, it is well known that a certain number of calories with a specific composition should be consumed daily to perform well in any kind of physical activity. However, dependent on the goal of the individual, a calorie surplus or a reduction of calories is important. Furthermore, composition and meal timing play a major role in physiological performance. Since the management of exercise timing, intensity, duration and frequency and consumption of food in a specific manner, calorie amount and composition of food has not been complex enough, sleep and recovery should also be incorporated. Lack of sleep is the cause of severe psychosocial diseases and also the origin of metabolic diseases since the hormonal cycle is reliant on a regular sleep and wake cycle. Once this cycle is disturbed, our physiological performance decreases rapidly.
As a consequence, it is of interest to shed some light onto a topic that might appear trivial, yet the fragility and complexity of the simple and popular mantra “eat, train, sleep—repeat” might lead us further away from what it is actually supposed to be—a retreat.
3.3.1. Macronutrient Timing
Carbohydrates, mainly glucose, are used in particular by the brain and muscles during physical activity [148,149]. A higher glucose tolerance in the morning [150] seems to make carbohydrate intake more reasonable in the early hours. This has been underpinned by a cross-over study in 10 healthy men that showed an increase in core body temperature (CBT) of up to 8 h and suppression of nocturnal melatonin production following a single carbohydrate-rich evening meal on 3 consecutive days [151], and thus, a shift in the circadian clock. However, eating patterns must be considered in a differentiated way for athletes when a performance limiting factor is the availability of carbohydrates [152]. Moreover, for short recovery times between training sessions (<8 h), recommendations move towards immediate carbohydrate intake after the end of training whereas longer recovery times (>24 h) allow a more flexible intake [153].
Along with carbohydrates, fats represent the main energy reserve in the body. The influence of a nightly high-fat meal was studied in 25 healthy adults [154]. The postprandial response was compared during the day (1:30 p.m.) and at night (1:30 a.m.), with significantly higher circulating triacylglycerol (TAG) levels detected at night. In another trial, there was shown that TAG levels are lower after lunch as compared to breakfast [155], which could be explained by an increased uptake of TAGs into skeletal muscle and brown adipose tissue during the active phase [156]. Burdge et al. concluded that metabolic disease could possibly be prevented if high-fat meals were consumed sooner at lunch.
After their breakdown into amino acids and peptides, proteins are taken up by peptide transporters, mainly peptide transporter 1 (PEPT1), in the intestine [157]. Animal studies suggest that PEPT1 levels exhibit a diurnal rhythmicity, with increased levels at the beginning of the active phase [158]. This implies that protein intake, as well as carbohydrate intake, should occur at the beginning of the day.
3.3.2. Exercise Timing
When it comes to the question of whether exercise should rather take place in the morning or evening, opinions differ tremendously. While some consider their training sessions to be a good way to end the day, others cannot get any rest after a late sports unit. Looking at the normal circadian rhythm, this seems surprising at first. For both strength and endurance training, maximum performance is observed in the afternoon and evening hours [159,160,161]. This is caused by the diurnal rhythm of the core body temperature (CBT). Rhythmicity is controlled by changes in blood flow, and thus, skin temperature of the distal limbs, which reaches its maximum in the late evening and its minimum in the morning [162]. The same processes controlling CBT are responsible for thermoregulation in exercise. This was demonstrated by comparing the initial response of temperature to moderate activity at different times of the day (5:00 a.m., 11:00 a.m., 5:00 p.m. and 11:00 p.m.). Thus, the increase of about 0.75 °C in CBT in the morning is significantly larger than 0.45 °C at the other experimental time points, whereas the increase in skin blood flow measured at the forearm was smaller [163], which is consistent with other literature [164,165]. This indicates that at times of most efficient thermoregulation, the greatest aerobic performance can be observed. For example, in a ramp test performed in 11 cyclists, up to 95% of the maximum power (Pmax), the time to exhaustion (TTE) was determined [166]. Thus, TTE in the evening (6:00 p.m.) was on average 40 s (270 ± 105 s vs. 234 ± 85 s) longer than in the morning (6:00 a.m.). In contrast to 15% increase in power output (±16%), no difference was measured in VO2mean and VO2peak. Similar results were obtained in a TTE (increase of 3.66% VO2max/s to a maximum load of 95% of VO2max) in eight women. This is in agreement with other studies [167,168,169]. However, recent results highlight that large variations in performance can be observed between morning and evening types [170,171,172]. Thomas et al. concluded that exercise in the evening could lead to misalignments in early chronotypes [173].
The results of anaerobic performance and muscle strength tests do not always show consistent results. However, the trend also shows that peak performance tends to be elevated in the afternoon hours [174]. When comparing a force-velocity and a multi-jump test at three different times of the day (9:00 a.m., 2:00 p.m. and 6:00 p.m.; 23 subjects), a 3% increase in performance was detected in the wheel test and 5–7% in the jump test [175]. A further investigation on 19 subjects with extended test times (2:00 a.m., 6:00 a.m., 10:00 a.m., 2:00 p.m., 6:00 p.m., 10:00 p.m.) demonstrated that for Pmax, an increase of 7% at the peak clock time at 17:10 ± 00:52 h was observed. Furthermore, Ppeak and Pmean, determined by Wingate test, resulted in an increase of 7.6% and 11.3% and a peak clock time at 5:24 p.m. ± 00:36 h and 6:00 p.m. ± 01:01 h, respectively [176]. This is consistent with other research [177,178,179]. The peak performance in the afternoon hours can also be observed for muscle strength tests [180]. Thus, the research is clear for most types of exercise and indicate that performance is critically influenced by intrinsic factors. This demonstrates that knowledge of chronotype and the general circadian rhythmicity of thermoregulation is an important tool in exercise planning, especially if training sessions are to be performed at maximal load with the lowest possible risk of injury [181].
Besides the influence of normal circadian variations of CBT on exercise performance, the peripheral muscle clock might have a crucial impact on diurnal energy metabolism. Initial results on metabolomic and transcriptomic investigations demonstrated that exercise in the early active phase influences other metabolic processes than exercise in the early rest phase [182]. Adenosine triphosphate (ATP) production and utilization are dependent of hypoxia-inducible factor 1α (HIF1α) production in exercising skeletal muscle under hypoxic conditions. Mason et al. demonstrated that a shift from glycolytic to oxidative metabolic processes can be observed in HIF1α knockout mice, resulting in increased exercise times in concentric and decreased times in eccentric exercise [183]. In this regard, a high HIF1α muscle content seems to be beneficial during exercise with a high content of glycolytic metabolic processes, whereas low levels seem to be advantageous during aerobic exercise. Sato et al. found higher levels of HIF1α only during exercise in the early active phase and a reduction in the levels of transcripts involved in mitochondrial processes, whereas glycolysis-related transcripts were elevated. In addition, they were able to detect increased fat and protein utilization in the early active phase. On the other hand, an increase in energy expenditure was only detected when exercising in the early rest phase. They concluded that energy depletion in skeletal muscle cells is highest in early active hours and thus leads to changes in metabolic processes between the different times of exercise. For this reason, it seems advisable that concentric exercise with high mitochondrial capacity is better performed in the late active phase [182].
3.3.3. Exercise and Other Hormonal Pathways
The effect of exercise on hormonal pathways has often been discussed in literature [184,185,186,187]. A central point of scientific research is the investigation of insulin-independent GLUT4 skeletal muscle expression while exercising [188,189]. GLUT4 is a glucose transporter that regulates glucose uptake in skeletal, cardiac muscles and fat cells, increasing insulin sensitivity and improving glucose metabolism, making exercise an early treatment in insulin resistance and diabetes.
Cortisol is a frequently studied hormone in connection with exercise. Its secretion in adrenal cortex is initiated through HPA axis after release of ACTH [92]. Besides its function as the primary stress hormone [190], cortisol levels also represent the response to metabolic processes by releasing glucocorticoids which increases macronutrient utilization [191]. External factors influencing cortisol levels are meals, hydration and exercise. During exercise, hydration influences the cortisol concentrations, as shown by Maresh et al. Thus, in a dehydrated state (hypohydrated by 5% of body mass), cortisol concentrations were higher than in euhydrated state. Furthermore, an increase in exercise intensity from 70% to 80% VO2max resulted in higher cortisol concentrations before and 20 min after exercise [192]. Cortisol secretion is particularly important during prolonged exercise, as it prevents the esterification of FFAs and stimulates glucose production in the liver [193,194]. Cortisol, which is elevated during chronic stress can lead to the development of disease, from chronic pain to cardiac diseases [190,195]. However, after exercise it falls rapidly and can lead to a decrease in basal cortisol levels during sleep. This was shown in 17 athletes in whom cortisol response to (1) a moderate training, (2) two high-intensity training sessions or (3) no training was measured at night [196]. This phenomenon, termed the exercise-glucocorticoid paradox by Chen et al. 2016 [197], shows that acute exercise induces a stress response, but the long-term effect leads to a reduction in stress hormone production. The cortisol response to exercise at different times of the day (7:00 a.m., 7:00 p.m., 12:00 a.m.) was also studied and it resulted in different cortisol levels (highest cortisol levels at 7:00 a.m., suppression of cortisol release at 12:00 a.m.) [198]. The greatest influence on the increase in cortisol between rest days and exercise days was observed in the measurement at 12:00 a.m., since a lower basal cortisol concentration was determined here, due to the circadian rhythmicity of cortisol secretion (integrated cortisol concentrations 07:00 a.m.: 127.64 ± 6.46 vs. 135.91 ± 12.23; 12:00 a.m.: 74.77 ± 14.29 vs. 103.94 ± 13.36 min·nmol/L on control vs. exercise days). Subsequently, in people with primary chronic insomnia, their condition was found to be associated with increased cortisol secretion and increased HPA axis activity [199]; implementation of high-intensity exercise late in the evening could have a short-term negative effect on sleep quality. This issue has also been investigated by Buman et al. which used surveys to collect data on exercise behavior and sleep quality from 1000 individuals [200]. They distinguished between physical activity >8 h, 4–8 h and <4 h before bedtime and their influence on sleep quality. In their data, no significant difference between the groups was found, which could be associated with a worsening in sleep quality when exercising in the evening. However, the low significance due to the subjectivity of the answers and the lack of physiological measurement data must be viewed critically. Another point that should be considered when discussing exercise and associated cortisol levels, is the adrenal clock of cortisol production. Cortisol concentrations peak in the early morning. This is mediated by adrenal CGs, triggered by light stimuli [93]. Cortisol, besides other glucocorticoids, was found to generate a catabolic status of muscle tissue [201,202]. Therefore, it seems, that especially anaerobic exercise and strength training should be performed in the afternoon, when cortisol levels decrease. This is supported by Bird and Tarpenning, as they showed that the cortisol response of 13 weight-trained men to heavy strength training was significantly lower, when performing at 6:00 p.m. compared to exercise at 6:00 a.m. [203].
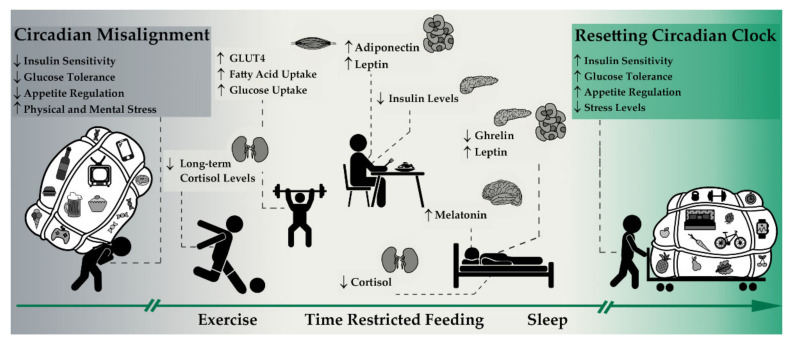
As demonstrated in the previous sections, implementing normal day/night rhythm, exercise and time restricted feeding significantly influence metabolic processes and can thus help to rebalance circadian processes as also shown in Figure 2.
Figure 2.
Resetting the circadian clock. Sleep, time restricted feeding (TRF) and exercise affect circadian rhythmicity helping to resynchronize the central with peripheral pacemakers by influencing different metabolic pathways. Exercise particularly affects peripheral processes in muscle and adipose tissues and influences glucose homeostasis. In addition, regular exercise can reduce stress levels by influencing cortisol levels. Besides, glucose homeostasis and hunger/satiety hormones are controlled by TRF. Despite these peripheral influences, sleep also affects central rhythmicity through a direct impact on melatonin and cortisol secretion.
4. Discussion
Since the circadian rhythm is already a highly complex system of central and peripherally controlled hormonal and metabolic processes, this system becomes even more complex once TRF and exercise are incorporated [161,204,205]. TRF has been shown to be a tool in regulating glucose and lipid metabolism and increasing insulin sensitivity by directly regulating the blood glucose levels by means of hormones, insulin and glucagon, among others [78,101,119]. This is enhanced by the expression of adiponectin, sensitizing the adipose tissue to insulin and leptin, mimicking the effect of insulin monotherapy [133]. The blood glucose-lowering effect is also observed with the implementation of exercise, here the blood glucose-regulation runs through the peripheral insulin-independent expression of GLUT4 in muscle cells [188,189]. Considering the normal circadian rhythm of macronutrient uptake, with an increased glucose tolerance and a peak activity of PEPT1 for most efficient protein uptake in the morning and with an increased uptake of TAGs in the middle of the day, it seems that TRF with an early feeding phase is the most natural way of feeding, as metabolism is most efficient at this time and the ingested food can thus be best utilized. Some studies also showed more positive effects when feeding time fell in the morning hours compared to late feeding cycles. The late fasting cycle is therefore able to prevent the harmful effects of a nightly high fat diet and the associated increase in TAGs as well as an increased postprandial CBT after an excessive carbohydrate intake and thus a suppression of melatonin expression in the evening. Even though thermoregulatory processes are more efficient in the afternoon and therefore maximal performance is usually maintained at this time, for recreational sports with mostly short durations or moderate intensities no or hardly any negative effect in performance should be noticed by switching exercise time from evening to morning hours. This allows a high flexibility in planning fasting/feeding cycles with exercise, especially for healthy individuals. The situation is different if TRF is used for health purposes like support in the treatment of type 2 diabetes. In this example, exercise should be performed before feeding time, as it results in a significantly higher improvement in blood glucose control [206] and lipid metabolism [207] in contrast to post-meal exercise. However, for other diseases or exercise types, the individual condition of the person must be taken into account. A differentiated metabolic response to morning (fasting) and afternoon resistance exercise was observed in type 1 diabetics, in whom a greater incidence of hyperglycemia events occurred after morning than after afternoon exercise [208]. Summarizing these results, an early feeding time with exercise before feeding seems to result in the greatest improvements in metabolic processes and is preferable for the treatment of diseases, but must always be adjusted to the individual. For athletes, a proper exercise timing as well as an adequate carbohydrate supply during either high-intensity, long duration, or both, exercises seemed to be essential as it could be a limiting factor in maintaining exercise performance [209,210]. Furthermore, replenishing used glycogen immediately after training sessions for better regeneration is important [211]. A slower regeneration is not a problem in recreational exercise with a recommendation of 150 min of moderate-intensity, or 75 min of vigorous intensity per week, if a sufficient amount of carbohydrates is supplied within 24 h following exercise as suggested by Burke et al. However, this should be considered for ambitious recreational athletes as well as competitive athletes with several intensive training sessions per week or day. In that case it should be carefully weighed up whether the eating window should be placed in the midday and evening hours, or—preferably to be applied in the case of an early chronotype—the intensive units should be postponed to the morning hours. Depending on the goal—whether maximum physical performance or health purposes—attention should also be given to a sufficient timeframe for the feeding period. Tinsley et al. showed that a limitation to 4 h of eating per day led to a reduced energy intake. This could be beneficial for health aspects, such as a reduction of body fat in overweight people, but could have a negative effect on athletes’ performances. TRF and exercise have been shown to mainly influence peripheral hormonal processes in specific tissues, such as adipose tissue, pancreas and muscle cells, with less influence on the central control mechanisms [15,18,78,81,89]. In contrast, the normal day/night rhythm may have an enormous influence on the central pacemaker—the SCN—and thus on the melatonin production controlled by the light/dark cycle [212]. Shifts in the circadian melatonin rhythmicity lead to shifts of the central and peripheral pacemaker against each other, which subsequently induces a suppression of the amplitude of the phase, or free running periods of hormone and gene expression [63,213,214]. These disturbances can be considered as the cause of metabolic diseases. Disturbances in the complex circadian system can be observed especially in shift workers since sleep deprivation increases disease risk factors. Furthermore, social jet-lag, in other words, the shift of the social clock against the normal circadian rhythm of the metabolism, as well as the excessive use of light-intensive devices until late in the evening, lead to an increased prevalence of modern diseases [215,216].
5. Conclusions
As shown in Figure 2, exercise and TRF are simple options implementable in daily life situations to potentially eliminate symptoms of sleep deprivation and other unhealthy lifestyle behaviors (Figure 1), leading to a disturbed circadian rhythm. However, the potential cause of metabolic disease arising from sleep deprivation may not solely be eliminated by the aforementioned implementation. Therefore, not only symptoms must be treated, but a holistic approach is necessary, which first identifies the disturbing factors on the sleep/wake cycles and successively eliminates them. Adding TRF and exercise is reasonable, and since for most people a significantly higher meal frequency than exercise frequency is achieved and more comprehensive processes can be regulated by TRF compared to exercise, we should probably go from: eat, train, sleep—retreat to sleep, eat, train—retreat.
Author Contributions
Conceptualization, S.H., M.L.E., A.W. and O.M.; writing—original draft preparation, S.H., M.L.E. and A.W.; writing—review and editing, S.H., M.L.E., A.W., R.T.Z., N.B.W. and O.M.; visualization, S.H. and R.T.Z.; supervision, O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schott M., Bornstein S.R., Stratakis C.A. Hormone and Metabolic Research: 50 Years of Research. Horm. Metab. Res. 2019;51:8–10. doi: 10.1055/a-0818-8930. [DOI] [PubMed] [Google Scholar]
- 2.Eckel-Mahan K., Sassone-Corsi P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher G., Sassone-Corsi P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Challet E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019;15:393–405. doi: 10.1038/s41574-019-0210-x. [DOI] [PubMed] [Google Scholar]
- 5.Booth F.W., Chakravarthy M.V., Gordon S.E., Spangenburg E.E. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarthy M.V., Booth F.W. Eating, exercise, and “thrifty” genotypes: Connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 7.Danguir J., Nicolaidis S. Dependence of sleep on nutrients’ availability. Physiol. Behav. 1979;22:735–740. doi: 10.1016/0031-9384(79)90240-3. [DOI] [PubMed] [Google Scholar]
- 8.Halberg N., Henriksen M., Söderhamn N., Stallknecht B., Ploug T., Schjerling P., Dela F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005;99:2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- 9.Dobbs R., Sawers C., Thompson F., Manyika J., Woetzel J., Child P., McKenna S., Spatharou A. Overcoming Obesity: An Initial Economic Analysis. The McKinsey Global Institute; New York, NY, USA: 2014. [Google Scholar]
- 10.Bergouignan A., Rudwill F., Simon C., Blanc S. Physical inactivity as the culprit of metabolic inflexibility: Evidence from bed-rest studies. J. Appl. Physiol. 2011;111:1201–1210. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 11.Kohl H.W., Craig C.L., Lambert E.V., Inoue S., Alkandari J.R., Leetongin G., Kahlmeier S. The pandemic of physical inactivity: Global action for public health. Lancet. 2012;380:294–305. doi: 10.1016/S0140-6736(12)60898-8. [DOI] [PubMed] [Google Scholar]
- 12.Markwald R.R., Melanson E.L., Smith M.R., Higgins J., Perreault L., Eckel R.H., Wright J.K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chennaoui M., Arnal P.J., Sauvet F., Léger D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015;20:59–72. doi: 10.1016/j.smrv.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Rynders C., Weltman A., Delgiorno C., Balagopal P., Damaso L., Killen K., Mauras N. Lifestyle Intervention Improves Fitness Independent of Metformin in Obese Adolescents. Med. Sci. Sports Exerc. 2012;44:786–792. doi: 10.1249/MSS.0b013e31823cef5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Templeman I., Gonzalez J.T., Thompson D., Betts J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020;79:76–87. doi: 10.1017/S0029665119000636. [DOI] [PubMed] [Google Scholar]
- 16.Northeast R.C., Vyazovskiy V.V., Bechtold D.A. Eat, sleep, repeat: The role of the circadian system in balancing sleep–wake control with metabolic need. Curr. Opin. Physiol. 2020;15:183–191. doi: 10.1016/j.cophys.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parr E.B., Heilbronn L.K., Hawley J.A. A Time to Eat and a Time to Exercise. Exerc. Sport Sci. Rev. 2020;48:4–10. doi: 10.1249/JES.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo V.D., Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen B.K., Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports. 2006;16:3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 20.Fiuza-Luces C., Garatachea N., Berger N.A., Lucia A. Exercise is the Real Polypill. Physiology. 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 21.Schibler U., Sassone-Corsi P. A Web of Circadian Pacemakers. Cell. 2002;111:919–922. doi: 10.1016/S0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 22.Patton A.P., Hastings M.H. The suprachiasmatic nucleus. Curr. Biol. 2018;28:R816–R822. doi: 10.1016/j.cub.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Bass J., Takahashi J.S. Circadian Integration of Metabolism and Energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreani T.S., Itoh T.Q., Yildirim E., Hwangbo D.-S., Allada R. Genetics of Circadian Rhythms. Sleep Med. Clin. 2015;10:413–421. doi: 10.1016/j.jsmc.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patke A., Murphy P.J., Onat O.E., Krieger A.C., Özçelik T., Campbell S.S., Young M.W. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell. 2017;169:203–215.e13. doi: 10.1016/j.cell.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toh K.L., Chen B., Eddaoudi M., Hyde S.T., O’Keeffe M., Yaghi O.M. An hPer2 Phosphorylation Site Mutation in Familial Advanced Sleep Phase Syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 27.Hirano A., Shi G., Jones C.R., Lipzen A., Pennacchio L.A., Xu Y., Hallows W.C., McMahon T., Yamazaki M., Ptáček L.J., et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. eLife. 2016;5:e16695. doi: 10.7554/eLife.16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudic R.D., McNamara P., Curtis A.-M., Boston R.C., Panda S., HogenEsch J.B., Fitzgerald G.A. BMAL1 and CLOCK, Two Essential Components of the Circadian Clock, Are Involved in Glucose Homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimba S., Ishii N., Ohta Y., Ohno T., Watabe Y., Hayashi M., Wada T., Aoyagi T., Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H., Ivanova G., Omura C., Mo S., Vitaterna M.H., et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nat. Cell Biol. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheiermann C., Gibbs J., Ince L., Loudon A. Clocking in to immunity. Nat. Rev. Immunol. 2018;18:423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 32.Curtis A.M., Bellet M.M., Sassone-Corsi P., O’Neill L.A. Circadian Clock Proteins and Immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Abe M., Herzog E.D., Yamazaki S., Straume M., Tei H., Sakaki Y., Menaker M., Block G.D. Circadian Rhythms in Isolated Brain Regions. J. Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durgan D.J., Trexler N.A., Egbejimi O., McElfresh T.A., Suk H.Y., Petterson L.E., Shaw C.A., Hardin P.E., Bray M.S., Chandler M.P., et al. The Circadian Clock within the Cardiomyocyte Is Essential for Responsiveness of the Heart to Fatty Acids. J. Biol. Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 35.Stokkan K.-A., Yamazaki S., Tei H., Sakaki Y., Menaker M. Entrainment of the Circadian Clock in the Liver by Feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 36.Storch K.-F., Paz C., Signorovitch J., Raviola E., Pawlyk B., Li T., Weitz C.J. Intrinsic Circadian Clock of the Mammalian Retina: Importance for Retinal Processing of Visual Information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyar K.A., Ciciliot S., Wright L.E., Biensø R.S., Tagliazucchi G.M., Patel V.R., Forcato M., Paz M.I., Gudiksen A., Solagna F., et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko C.H., Takahashi J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 39.Lee C., Etchegaray J.-P., Cagampang F.R.A., Loudon A.S.I., Reppert S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/S0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 40.Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z.S., Eichele G., Bradley A., et al. Nonredundant Roles of the mPer1 and mPer2 Genes in the Mammalian Circadian Clock. Cell. 2001;105:683–694. doi: 10.1016/S0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 41.Kume K., Zylka M.J., Sriram S., Shearman L.P., Weaver D.R., Jin X., Maywood E.S., Hastings M.H., Reppert S.M. mCRY1 and mCRY2 Are Essential Components of the Negative Limb of the Circadian Clock Feedback Loop. Cell. 1999;98:193–205. doi: 10.1016/S0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 42.Preitner N., Damiola F., Molina L.-L., Zakany J., Duboule D., Albrecht U., Schibler U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell. 2002;110:251–260. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 43.Guillaumond F., Dardente H., Giguère V., Cermakian N. Differential Control of Bmal1 Circadian Transcription by REV-ERB and ROR Nuclear Receptors. J. Biol. Rhythm. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 44.Leibetseder V., Humpeler S., Svoboda M., Schmid D., Thalhammer T., Zuckermann A., Marktl W., Ekmekcioglu C. Clock Genes Display Rhythmic Expression in Human Hearts. Chronobiol. Int. 2009;26:621–636. doi: 10.1080/07420520902924939. [DOI] [PubMed] [Google Scholar]
- 45.Polidarová L., Soták M., Sládek M., Pácha J., Sumová A. Temporal Gradient in the Clock Gene and Cell-Cycle Checkpoint KinaseWee1Expression along the Gut. Chronobiol. Int. 2009;26:607–620. doi: 10.1080/07420520902924889. [DOI] [PubMed] [Google Scholar]
- 46.Liu S., Cai Y., Sothern R.B., Guan Y., Chan P. Chronobiological Analysis of Circadian Patterns in Transcription of Seven Key Clock Genes in Six Peripheral Tissues in Mice. Chronobiol. Int. 2007;24:793–820. doi: 10.1080/07420520701672556. [DOI] [PubMed] [Google Scholar]
- 47.Zvonic S., Ptitsyn A.A., Conrad S.A., Scott L.K., Floyd Z.E., Kilroy G., Wu X., Goh B.C., Mynatt R.L., Gimble J.M. Characterization of Peripheral Circadian Clocks in Adipose Tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 48.Bozek K., Relógio A., Kielbasa S.M., Heine M., Dame C., Kramer A., Herzel H. Regulation of Clock-Controlled Genes in Mammals. PLoS ONE. 2009;4:e4882. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oster H., Damerow S., Kiessling S., Jakubcakova V., Abraham D., Tian J., Hoffmann M.W., Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Son G.H., Cha H.K., Chung S., Kim K. Multimodal Regulation of Circadian Glucocorticoid Rhythm by Central and Adrenal Clocks. J. Endocr. Soc. 2018;2:444–459. doi: 10.1210/js.2018-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzoccoli G., Pazienza V., Vinciguerra M. Clock Genes and Clock-Controlled Genes in the Regulation of Metabolic Rhythms. Chronobiol. Int. 2012;29:227–251. doi: 10.3109/07420528.2012.658127. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh M.-C., Yang S.-C., Tseng H.-L., Hwang L.-L., Chen C.-T., Shieh K.-R. Abnormal expressions of circadian-clock and circadian clock-controlled genes in the livers and kidneys of long-term, high-fat-diet-treated mice. Int. J. Obes. 2009;34:227–239. doi: 10.1038/ijo.2009.228. [DOI] [PubMed] [Google Scholar]
- 53.Vogel M., Braungardt T., Meyer W., Schneider W. The effects of shift work on physical and mental health. J. Neural Transm. 2012;119:1121–1132. doi: 10.1007/s00702-012-0800-4. [DOI] [PubMed] [Google Scholar]
- 54.Wang X.-S., Armstrong M.E.G., Cairns B.J., Key T.J., Travis R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sack R.L., Blood M.L., Lewy A.J. Melatonin Rhythms in Night Shift Workers. Sleep. 1992;15:434–441. doi: 10.1093/sleep/15.5.434. [DOI] [PubMed] [Google Scholar]
- 56.Roden M., Koller M., Pirich K., Vierhapper H., Waldhauser F. The circadian melatonin and cortisol secretion pattern in permanent night shift workers. Am. J. Physiol. Integr. Comp. Physiol. 1993;265:R261–R267. doi: 10.1152/ajpregu.1993.265.1.R261. [DOI] [PubMed] [Google Scholar]
- 57.Kräuchi K., Cajochen C., Pache M., Flammer J., Wirz-Justice A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol. Int. 2006;23:475–484. doi: 10.1080/07420520500545854. [DOI] [PubMed] [Google Scholar]
- 58.Brum M.C.B., Filho F.F.D., Schnorr C.C., Bertoletti O.A., Bottega G.B., Rodrigues T.D.C. Night shift work, short sleep and obesity. Diabetol. Metab. Syndr. 2020;12:1–9. doi: 10.1186/s13098-020-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drake C.L., Roehrs T., Richardson G., Walsh J.K., Roth T. Shift Work Sleep Disorder: Prevalence and Consequences Beyond that of Symptomatic Day Workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 60.Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occup. Med. 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]
- 61.Li J., Bidlingmaier M., Petru R., Gil F.P., Loerbroks A., Angerer P. Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. J. Occup. Med. Toxicol. 2018;13:1–9. doi: 10.1186/s12995-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manenschijn L., Van Kruysbergen R.G.P.M., De Jong F.H., Koper J.W., Van Rossum E.F.C. Shift Work at Young Age Is Associated with Elevated Long-Term Cortisol Levels and Body Mass Index. J. Clin. Endocrinol. Metab. 2011;96:E1862–E1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 63.Leese G., Chattington P., Fraser W., Vora J., Edwards R., Williams G. Short-term night-shift working mimics the pituitary-adrenocortical dysfunction in chronic fatigue syndrome. J. Clin. Endocrinol. Metab. 1996;81:1867–1870. doi: 10.1210/jcem.81.5.8626849. [DOI] [PubMed] [Google Scholar]
- 64.Zanquetta M.M., Seraphim P.M., Sumida D.H., Cipolla-Neto J., Machado U.F. Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J. Pineal Res. 2003;35:141–148. doi: 10.1034/j.1600-079X.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 65.Diaz B., Blázquez E. Effect of Pinealectomy on Plasma Glucose, Insulin and Glucagon Levels in the Rat. Horm. Metab. Res. 1986;18:225–229. doi: 10.1055/s-2007-1012279. [DOI] [PubMed] [Google Scholar]
- 66.Mellado C., Rodríguez V., Diego J.G., Alvarez E., Blázquez E. Effect of Pinealectomy and of Diabetes on Liver Insulin and Glucagon Receptor Concentrations in the Rat. J. Pineal Res. 1989;6:295–306. doi: 10.1111/j.1600-079X.1989.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 67.Cipolla-Neto J., Amaral F.G., Afeche S.C., Tan D.X., Reiter R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014;56:371–381. doi: 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- 68.Harris R.B.S. Leptin—Muchmorethan asatietysignal. Annu. Rev. Nutr. 2000;20:45–75. doi: 10.1146/annurev.nutr.20.1.45. [DOI] [PubMed] [Google Scholar]
- 69.Meier U., Gressner A.M. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin. Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 70.Rohner-Jeanrenaud F., Jeanrenaud B. The discovery of leptin and its impact in the understanding of obesity. Eur. J. Endocrinol. 1996;135:649–650. doi: 10.1530/eje.0.1350649. [DOI] [PubMed] [Google Scholar]
- 71.Taheri S., Lin L., Austin D., Young T., Mignot E. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohayon M.M., Lemoine P., Arnaud-Briant V., Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. J. Psychosom. Res. 2002;53:577–583. doi: 10.1016/S0022-3999(02)00438-5. [DOI] [PubMed] [Google Scholar]
- 73.Ulhôa M.A., Marqueze E.C., Burgos L.G.A., Moreno C.R.C. Shift Work and Endocrine Disorders. Int. J. Endocrinol. 2015;2015:1–11. doi: 10.1155/2015/826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng H., Zhu L., Kord-Varkaneh H., Santos H.O., Tinsley G.M., Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: A systematic review and meta-analysis. Nutrition. 2020;77:110801. doi: 10.1016/j.nut.2020.110801. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z., Dai X., Zhang H., Shi R., Hui Y., Jin X., Zhang W., Wang L., Wang Q., Wang D., et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K., Liu B., Heilbronn L.K. Intermittent fasting: What questions should we be asking? Physiol. Behav. 2020;218:112827. doi: 10.1016/j.physbeh.2020.112827. [DOI] [PubMed] [Google Scholar]
- 77.Gabel K., Hoddy K.K., Haggerty N., Song J., Kroeger C.M., Trepanowski J.F., Panda S., Varady A.K. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging. 2018;4:345–353. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–1221.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tinsley G.M., Forsse J.S., Butler N.K., Paoli A., Bane A.A., La Bounty P.M., Morgan G.B., Grandjean P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017;17:200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 80.Gasmi M., Sellami M., Denham J., Padulo J., Kuvacic G., Selmi W., Khalifa R. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition. 2018;51–52:29–37. doi: 10.1016/j.nut.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 81.Moro T., Tinsley G., Bianco A., Marcolin G., Pacelli Q.F., Battaglia G., Palma A., Gentil P., Neri M., Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016;14:1–10. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 83.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017;76:361–368. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- 84.Lettieri-Barbato D., Cannata S.M., Casagrande V., Ciriolo M.R., Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS ONE. 2018;13:e0195912. doi: 10.1371/journal.pone.0195912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elamin M., Ruskin D.N., Masino S.A., Sacchetti P. Ketogenic Diet Modulates NAD+-Dependent Enzymes and Reduces DNA Damage in Hippocampus. Front. Cell. Neurosci. 2018;12:263. doi: 10.3389/fncel.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chapnik N., Genzer Y., Froy O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J. Nutr. Biochem. 2017;40:116–121. doi: 10.1016/j.jnutbio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 87.Mendoza J. Circadian Clocks: Setting Time By Food. J. Neuroendocr. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 88.Longo V.D., Mattson M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaix A., Zarrinpar A., Miu P., Panda S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luiten P., Ter Horst G., Steffens A. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog. Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 91.Kalsbeek A., Bruinstroop E., Yi C., Klieverik L., La Fleur S., Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 2010;1212:114–129. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- 92.Tsigos C., Chrousos G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 93.Gamble K.L., Berry R., Frank S.J., Young M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014;10:466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J. Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. [DOI] [PubMed] [Google Scholar]
- 95.Schally A.V., Arimura A., Kastin A.J. Hypothalamic Regulatory Hormones: At least nine substances from the hypothalamus control the secretion of pituitary hormones. Science. 1973;179:341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- 96.McMahon M., Gerich J., Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab. Rev. 1988;4:17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- 97.Gerich J.E., Charles M.A., Grodsky G.M. Regulation of Pancreatic Insulin and Glucagon Secretion. Annu. Rev. Physiol. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- 98.Elrick H., Stimmler L., Hlad C.J., Arai Y. Plasma Insulin Response to Oral and Intravenous Glucose Administration1. J. Clin. Endocrinol. Metab. 1964;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 99.Barthel A., Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Metab. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 100.Gerich J.E., Langlois M., Noacco C., Karam J.H., Forsham P.H. Lack of Glucagon Response to Hypoglycemia in Diabetes: Evidence for an Intrinsic Pancreatic Alpha Cell Defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 101.Jamshed H., Beyl R.A., Della Manna D.L., Yang E.S., Ravussin E., Peterson C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshino J., Almeda-Valdes P., Patterson B.W., Okunade A.L., Imai S.-I., Mittendorfer B., Klein S. Diurnal Variation in Insulin Sensitivity of Glucose Metabolism Is Associated With Diurnal Variations in Whole-Body and Cellular Fatty Acid Metabolism in Metabolically Normal Women. J. Clin. Endocrinol. Metab. 2014;99:E1666–E1670. doi: 10.1210/jc.2014-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrasco-Benso M.P., Rivero-Gutierrez B., Lopez-Minguez J., Anzola A., Diez-Noguera A., Madrid J.A., Lujan J.A., Martínez-Augustin O., Scheer F.A.J.L., Garaulet M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016;30:3117–3123. doi: 10.1096/fj.201600269RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kalsbeek A., la Fleur S., Fliers E. Circadian control of glucose metabolism. Mol. Metab. 2014;3:372–383. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hara R., Wan K., Wakamatsu H., Aida R., Moriya T., Akiyama M., Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 107.Akhtar R.A., Reddy A.B., Maywood E.S., Clayton J.D., King V.M., Smith A.G., Gant T.W., Hastings M.H., Kyriacou C.P. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Curr. Biol. 2002;12:540–550. doi: 10.1016/S0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 108.Kornmann B., Schaad O., Bujard H., Takahashi J.S., Schibler U. System-Driven and Oscillator-Dependent Circadian Transcription in Mice with a Conditionally Active Liver Clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lamia K.A., Storch K.-F., Weitz C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang E.E., Liu Y., Dentin R., Pongsawakul P.Y., Liu A.C., Hirota T., Nusinow D.A., Sun X., Landais S., Kodama Y., et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woldt E., Sebti Y., Solt L.A., Duhem C., Lancel S., Eeckhoute J., Hesselink M.K., Paquet C., Delhaye S., Shin Y., et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schiaffino S., Blaauw B., Dyar K.A. The functional significance of the skeletal muscle clock: Lessons from Bmal1 knockout models. Skelet. Muscle. 2016;6:1–9. doi: 10.1186/s13395-016-0107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paschos G.K., Ibrahim S.A., Song W.-L., Kunieda T., Grant G.R., Reyes T.M., Bradfield C.A., Vaughan C.H., Eiden M., Masoodi M., et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Basse A.L., Dalbram E., Larsson L., Gerhart-Hines Z., Zierath J.R., Treebak J.T. Skeletal Muscle Insulin Sensitivity Show Circadian Rhythmicity Which Is Independent of Exercise Training Status. Front. Physiol. 2018;9:1–12. doi: 10.3389/fphys.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez-Abellan P., Gomez-Santos C., Madrid J.A., Milagro-Yoldi F.I., Campión-Zabalza J., Martinez J.A., Garaulet M. Circadian Expression of Adiponectin and Its Receptors in Human Adipose Tissue. Endocrinology. 2010;151:115–122. doi: 10.1210/en.2009-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shea S.A., Hilton M.F., Orlova C., Ayers R.T., Mantzoros C.S. Independent Circadian and Sleep/Wake Regulation of Adipokines and Glucose in Humans. J. Clin. Endocrinol. Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadacca L.A., Lamia K.A., Delemos A.S., Blum B., Weitz C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2010;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee J., Moulik M., Fang Z., Saha P., Zou F., Xu Y., Nelson D.L., Ma K., Moore D.D., Yechoor V.K. Bmal1 and -Cell Clock Are Required for Adaptation to Circadian Disruption, and Their Loss of Function Leads to Oxidative Stress-Induced -Cell Failure in Mice. Mol. Cell. Biol. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun X., Dang F., Zhang D., Yuan Y., Zhang C., Wu Y., Wang Y., Liu Y. Glucagon-CREB/CRTC2 Signaling Cascade Regulates Hepatic BMAL1 Protein. J. Biol. Chem. 2015;290:2189–2197. doi: 10.1074/jbc.M114.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawamoto T., Noshiro M., Furukawa M., Honda K.K., Nakashima A., Ueshima T., Usui E., Katsura Y., Fujimoto K., Honma S., et al. Effects of Fasting and Re-Feeding on the Expression of Dec1, Per1, and Other Clock-Related Genes. J. Biochem. 2006;140:401–408. doi: 10.1093/jb/mvj165. [DOI] [PubMed] [Google Scholar]
- 121.Mukherji A., Dachraoui M., Baumert T.F. Perturbation of the circadian clock and pathogenesis of NAFLD. Metabolism. 2020;111:154337. doi: 10.1016/j.metabol.2020.154337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holst J.J., Christensen M., Lund A., De Heer J., Svendsen B., Kielgast U., Knop F.K. Regulation of glucagon secretion by incretins. Diabetes Obes. Metab. 2011;13:89–94. doi: 10.1111/j.1463-1326.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 123.Macdonald P.E., El-Kholy W., Riedel M.J., Salapatek A.M.F., Light P.E., Wheeler M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes. 2002;51:S434–S442. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 124.Jakubowicz D., Wainstein J., Landau Z., Raz I., Ahren B., Chapnik N., Ganz T., Menaged M., Barnea M., Bar-Dayan Y., et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals With Diabetes: A Randomized Clinical Trial. Diabetes Care. 2017;40:1573–1579. doi: 10.2337/dc16-2753. [DOI] [PubMed] [Google Scholar]
- 125.Chaix A., Manoogian E.N., Melkani G.C., Panda S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019;39:291–315. doi: 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adamovich Y., Rousso-Noori L., Zwighaft Z., Neufeld-Cohen A., Golik M., Kraut-Cohen J., Wang M., Han X., Asher G. Circadian Clocks and Feeding Time Regulate the Oscillations and Levels of Hepatic Triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J.A., et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Behre C. Adiponectin: Saving the starved and the overfed. Med. Hypotheses. 2007;69:1290–1292. doi: 10.1016/j.mehy.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 130.Jung S.H., Park H.S., Kim K.-S., Choi W.H., Ahn C.W., Kim B.T., Kim S.M., Lee S.Y., Ahn S.M., Kim Y.K., et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008;19:371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 131.Anderlová K., Kremen J., Dolezalová R., Housová J., Haluzíková D., Kunesová M., Haluzík M. The influence of very-low-calorie-diet on serum leptin, soluble leptin receptor, adiponectin and resistin levels in obese women. Physiol. Res. 2006;55:277–283. doi: 10.33549/physiolres.930779. [DOI] [PubMed] [Google Scholar]
- 132.Cho Y., Hong N., Kim K.-W., Cho S.J., Lee M., Lee Y.-H., Lee Y.-H., Kang E.S., Cha B.-S., Lee B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019;8:1645. doi: 10.3390/jcm8101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M.-Y., Chen L., Clark G.O., Lee Y., Stevens R.D., Ilkayeva O.R., Wenner B.R., Bain J.R., Charron M.J., Newgard C.B., et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl. Acad. Sci. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pedersen B.K., Saltin B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25(Suppl. S3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 135.Blair S.N. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 136.Battaglini C.L., Hackney A.C., Garcia R., Groff D., Evans E., Shea T. The Effects of an Exercise Program in Leukemia Patients. Integr. Cancer Ther. 2009;8:130–138. doi: 10.1177/1534735409334266. [DOI] [PubMed] [Google Scholar]
- 137.Stults-Kolehmainen M.A., Sinha R. The Effects of Stress on Physical Activity and Exercise. Sports Med. 2014;44:81–121. doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Booth F.W., Roberts C.K., Laye M.J. Lack of Exercise Is a Major Cause of Chronic Diseases. Compr. Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moser O., Eckstein M.L., West D.J., Goswami N., Sourij H., Hofmann P. Type 1 Diabetes and Physical Exercise: Moving (forward) as an Adjuvant Therapy. Curr. Pharm. Des. 2020;26:946–957. doi: 10.2174/1381612826666200108113002. [DOI] [PubMed] [Google Scholar]
- 140.Buford T.W., Roberts M.D., Church T.S. Toward Exercise as Personalized Medicine. Sports Med. 2013;43:157–165. doi: 10.1007/s40279-013-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moser O., Riddell M.C., Eckstein M.L., Adolfsson P., Rabasa-Lhoret R., Boom L.V.D., Gillard P., Nørgaard K., Oliver N.S., Zaharieva D.P., et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA) Pediatr. Diabetes. 2020;21:1375–1393. doi: 10.1111/pedi.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohta M., Okufuji T., Matsushima Y., Ikeda M. The Effect of Lifestyle Modification on Physical Fitness and Work Ability in Different Workstyles. J. UOEH. 2004;26:411–421. doi: 10.7888/juoeh.26.411. [DOI] [PubMed] [Google Scholar]
- 143.Ortlepp J.R., Metrikat J., Albrecht M., Maya-Pelzer P. Relationship between physical fitness and lifestyle behaviour in healthy young men. Eur. J. Cardiovasc. Prev. Rehabilitation. 2004;11:192–200. doi: 10.1097/01.hjr.0000131578.48136.85. [DOI] [PubMed] [Google Scholar]
- 144.World Health Organization . Global Recommendations on Physical Activity for Health. WHO Press; Geneva, Switzerland: 2015. [Google Scholar]
- 145.Wilson K. Mobile cell phone technology puts the future of health care in our hands. Can. Med. Assoc. J. 2018;190:E378–E379. doi: 10.1503/cmaj.180269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Direito A., Jiang Y., Whittaker R., Maddison R. Smartphone apps to improve fitness and increase physical activity among young people: Protocol of the Apps for IMproving FITness (AIMFIT) randomized controlled trial. BMC Public Health. 2015;15:635. doi: 10.1186/s12889-015-1968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parasuraman S., Sam A.T., Yee S.W.K., Chuon B.L.C., Ren L.Y. Smartphone usage and increased risk of mobile phone addiction: A concurrent study. Int. J. Pharm. Investig. 2017;7:125–131. doi: 10.4103/jphi.JPHI_56_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maher F., Vannucci S.J., Simpson I.A. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- 149.Mul J.D., Stanford K.I., Hirshman M.F., Goodyear L.J. Exercise and Regulation of Carbohydrate Metabolism. Prog. Mol. Biol. Transl. Sci. 2015;135:17–37. doi: 10.1016/bs.pmbts.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnston J.D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 2014;27:107–118. doi: 10.1017/S0954422414000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kräuchi K., Cajochen C., Werth E., Wirz-Justice A. Alteration of Internal Circadian Phase Relationships after Morning versus Evening Carbohydrate-Rich Meals in Humans. J. Biol. Rhythm. 2002;17:364–376. doi: 10.1177/074873040201700409. [DOI] [PubMed] [Google Scholar]
- 152.Burke L.M., Hawley J.A., Wong S.H.S., Jeukendrup A.E. Carbohydrates for training and competition. J. Sports Sci. 2011;29:S17–S27. doi: 10.1080/02640414.2011.585473. [DOI] [PubMed] [Google Scholar]
- 153.Burke L.M., Kiens B., Ivy J.L. Carbohydrates and fat for training and recovery. J. Sports Sci. 2004;22:15–30. doi: 10.1080/0264041031000140527. [DOI] [PubMed] [Google Scholar]
- 154.Sopowski M.J., Hampton S.M., Ribeiro D.C.O., Morgan L., Arendt J. Postprandial Triacylglycerol Responses in Simulated Night and Day Shift: Gender Differences. J. Biol. Rhythm. 2001;16:272–276. doi: 10.1177/074873001129001881. [DOI] [PubMed] [Google Scholar]
- 155.Burdge G.C., Jones A.E., Frye S.M., Goodson L., Wootton S.A. Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. Eur. J. Clin. Nutr. 2003;57:1536–1544. doi: 10.1038/sj.ejcn.1601722. [DOI] [PubMed] [Google Scholar]
- 156.Moran-Ramos S., Guerrero-Vargas N.N., Mendez-Hernandez R., Basualdo M.D.C., Escobar C., Buijs R.M. The suprachiasmatic nucleus drives day-night variations in postprandial triglyceride uptake into skeletal muscle and brown adipose tissue. Exp. Physiol. 2017;102:1584–1595. doi: 10.1113/EP086026. [DOI] [PubMed] [Google Scholar]
- 157.Daniel H. Molecular and Integrative Physiology of Intestinal Peptide Transport. Annu. Rev. Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 158.Pan X., Terada T., Irie M., Saito H., Inui K.-I. Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am. J. Physiol. Liver Physiol. 2002;283:G57–G64. doi: 10.1152/ajpgi.00545.2001. [DOI] [PubMed] [Google Scholar]
- 159.Winget C.M., Deroshia C.W., Holley D.C. Circadian rhythms and athletic performance. Med. Sci. Sports Exerc. 1985;17:498–516. doi: 10.1249/00005768-198510000-00002. [DOI] [PubMed] [Google Scholar]
- 160.Thun E., Bjorvatn B., Flo E., Harris A., Pallesen S. Sleep, circadian rhythms, and athletic performance. Sleep Med. Rev. 2015;23:1–9. doi: 10.1016/j.smrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 161.Drust B., Waterhouse J., Atkinson G., Edwards B., Reilly T. Circadian Rhythms in Sports Performance—An Update. Chronobiol. Int. 2005;22:21–44. doi: 10.1081/CBI-200041039. [DOI] [PubMed] [Google Scholar]
- 162.Smolander J., Lindgvist A., Kolari P., Laitinen L.A. Circadian variation in peripheral blood flow in relation to core temperature at rest. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993;67:192–196. doi: 10.1007/BF00376666. [DOI] [PubMed] [Google Scholar]
- 163.Waterhouse J., Edwards B., Bedford P., Hughes A., Robinson K., Nevill A., Weinert D., Reilly T. Thermoregulation during mild exercise at different circadian times. Chronobiol. Int. 2004;21:253–275. doi: 10.1081/CBI-120037799. [DOI] [PubMed] [Google Scholar]
- 164.Weinert D., Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol. Behav. 1998;63:837–843. doi: 10.1016/S0031-9384(97)00546-5. [DOI] [PubMed] [Google Scholar]
- 165.Weinert D., Waterhouse J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 2007;90:246–256. doi: 10.1016/j.physbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 166.Bessot N., Nicolas A., Moussay S., Gauthier A., Sesboüé B., Davenne D. The Effect of Pedal Rate and Time of Day on the Time to Exhaustion from High-Intensity Exercise. Chronobiol. Int. 2006;23:1009–1024. doi: 10.1080/07420520600920726. [DOI] [PubMed] [Google Scholar]
- 167.Hill D.W. Morning–evening differences in response to exhaustive severe-intensity exercise. Appl. Physiol. Nutr. Metab. 2014;39:248–254. doi: 10.1139/apnm-2013-0140. [DOI] [PubMed] [Google Scholar]
- 168.Hill D.W. Effect of time of day on aerobic power in exhaustive high-intensity exercise. J. Sports Med. Phys. Fit. 1996;36:155–160. [PubMed] [Google Scholar]
- 169.Reilly T., Baxter C. Influence of time of day on reactions to cycling at a fixed high intensity. Br. J. Sports Med. 1983;17:128–130. doi: 10.1136/bjsm.17.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vitale J.A., Weydahl A. Chronotype, Physical Activity, and Sport Performance: A Systematic Review. Sports Med. 2017;47:1859–1868. doi: 10.1007/s40279-017-0741-z. [DOI] [PubMed] [Google Scholar]
- 171.Facer-Childs E., Brandstaetter R. The Impact of Circadian Phenotype and Time since Awakening on Diurnal Performance in Athletes. Curr. Biol. 2015;25:518–522. doi: 10.1016/j.cub.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 172.Brown F.M., Neft E.E., LaJambe C.M. Collegiate Rowing Crew Performance Varies by Morningness-Eveningness. J. Strength Cond. Res. 2008;22:1894–1900. doi: 10.1519/JSC.0b013e318187534c. [DOI] [PubMed] [Google Scholar]
- 173.Thomas J.M., Kern P.A., Bush H.M., McQuerry K.J., Black W.S., Clasey J.L., Pendergast J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight. 2020;5:12–14. doi: 10.1172/jci.insight.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chtourou H., Souissi N. The Effect of Training at a Specific Time of Day. J. Strength Cond. Res. 2012;26:1984–2005. doi: 10.1519/JSC.0b013e31825770a7. [DOI] [PubMed] [Google Scholar]
- 175.Bernard T., Giacomoni M., Gavarry O., Seymat M., Falgairette G. Time-of-day effects in maximal anaerobic leg exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997;77:133–138. doi: 10.1007/s004210050311. [DOI] [PubMed] [Google Scholar]
- 176.Souissi N., Gauthier A., Sesboüé B., LaRue J., Davenne D. Circadian Rhythms in Two Types of Anaerobic Cycle Leg Exercise: Force-Velocity and 30-s Wingate Tests. Int. J. Sports Med. 2004;25:14–19. doi: 10.1055/s-2003-45226. [DOI] [PubMed] [Google Scholar]
- 177.Racinais S., Connes P., Bishop D., Blonc S., Hue O. Morning Versus Evening Power Output and Repeated-Sprint Ability. Chronobiol. Int. 2005;22:1029–1039. doi: 10.1080/07420520500397918. [DOI] [PubMed] [Google Scholar]
- 178.Racinais S., Perrey S., Denis R., Bishop D. Maximal power, but not fatigability, is greater during repeated sprints performed in the afternoon. Chronobiol. Int. 2010;27:855–864. doi: 10.3109/07420521003668412. [DOI] [PubMed] [Google Scholar]
- 179.Melhim A.F. Investigation of Circadian Rhythms in Peak Power and Mean Power of Female Physical Education Students. Int. J. Sports Med. 1993;14:303–306. doi: 10.1055/s-2007-1021182. [DOI] [PubMed] [Google Scholar]
- 180.Douglas C.M., Hesketh S.J., Esser K.A. Time of Day and Muscle Strength: A Circadian Output? Physiology. 2021;36:44–51. doi: 10.1152/physiol.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Waterhouse P.J., Drust B., Weinert D., Edwards B., Gregson W., Atkinson G., Kao S., Aizawa S., Reilly T. The Circadian Rhythm of Core Temperature: Origin and some Implications for Exercise Performance. Chronobiol. Int. 2005;22:207–225. doi: 10.1081/CBI-200053477. [DOI] [PubMed] [Google Scholar]
- 182.Sato S., Basse A.L., Schönke M., Chen S., Samad M., Altıntaş A., Laker R.C., Dalbram E., Barrès R., Baldi P., et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019;30:92–110.e4. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 183.Mason S.D., Howlett R.A., Kim M.J., Olfert I.M., Hogan M.C., McNulty W., Hickey R.P., Wagner P.D., Kahn C.R., Giordano F.J., et al. Loss of Skeletal Muscle HIF-1α Results in Altered Exercise Endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Smilios I., Pilianidis T., Karamouzis M., Tokmakidis S.P. Hormonal Responses after Various Resistance Exercise Protocols. Med. Sci. Sports Exerc. 2003;35:644–654. doi: 10.1249/01.MSS.0000058366.04460.5F. [DOI] [PubMed] [Google Scholar]
- 185.Kiyonaga A., Arakawa K., Tanaka H., Shindo M. Blood pressure and hormonal responses to aerobic exercise. Hypertension. 1985;7:125–131. doi: 10.1161/01.HYP.7.1.125. [DOI] [PubMed] [Google Scholar]
- 186.Raastad T., Bjøro T., Hallén J. Hormonal responses to high- and moderate-intensity strength exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000;82:121–128. doi: 10.1007/s004210050661. [DOI] [PubMed] [Google Scholar]
- 187.Kraemer W.J., Ratamess N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005;35:339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 188.Kennedy J.W., Hirshman M.F., Gervino E.V., Ocel J.V., Forse R.A., Hoenig S.J., Aronson D., Goodyear L.J., Horton E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- 189.Richter E.A., Hargreaves M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 190.Russell E., Koren G., Rieder M., Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 191.Duclos M., Tabarin A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front. Horm. Res. 2016;47:12–26. doi: 10.1159/000445149. [DOI] [PubMed] [Google Scholar]
- 192.Maresh C.M., Whittlesey M.J., Armstrong L.E., Yamamoto L.M., Judelson D.A., Fish K.E., Casa D.J., Kavouras S.A., Castracane V.D. Effect of Hydration State on Testosterone and Cortisol Responses to Training-Intensity Exercise in Collegiate Runners. Int. J. Sports Med. 2006;27:765–770. doi: 10.1055/s-2005-872932. [DOI] [PubMed] [Google Scholar]
- 193.Khani S., Tayek J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001;101:739–747. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- 194.Djurhuus C.B., Gravholt C.H., Nielsen S., Mengel A., Christiansen J.S., Schmitz O.E., Møller N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. Metab. 2002;283:E172–E177. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 195.McEwen B.S. Stress and the Individual. Arch. Intern. Med. 1993;153:2093–2101. doi: 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- 196.Hackney A.C., Viru A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin. Physiol. 1999;19:178–182. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 197.Chen C., Nakagawa S., An Y., Ito K., Kitaichi Y., Kusumi I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocr. 2017;44:83–102. doi: 10.1016/j.yfrne.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 198.Kanaley J.A., Weltman J.Y., Pieper K.S., Weltman A., Hartman M.L. Cortisol and Growth Hormone Responses to Exercise at Different Times of Day 1. J. Clin. Endocrinol. Metab. 2001;86:2881–2889. doi: 10.1210/jc.86.6.2881. [DOI] [PubMed] [Google Scholar]
- 199.Rodenbeck A., Huether G., Rüther E., Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci. Lett. 2002;324:159–163. doi: 10.1016/S0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 200.Buman M.P., Phillips B.A., Youngstedt S.D., Kline C.E., Hirshkowitz M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America. Poll. Sleep Med. 2014;15:755–761. doi: 10.1016/j.sleep.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 201.Florini J.R. Hormonal control of muscle growth. Muscle Nerve. 1987;10:577–598. doi: 10.1002/mus.880100702. [DOI] [PubMed] [Google Scholar]
- 202.Gore D.C., Jahoor F., Wolfe R.R., Herndon D.N. Acute Response of Human Muscle Protein to Catabolic Hormones. Ann. Surg. 1993;218:679–684. doi: 10.1097/00000658-199321850-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bird S.P., Tarpenning K.M. Influence of Circadian Time Structure on Acute Hormonal Responses to a Single Bout of Heavy-Resistance Exercise in Weight-Trained Men. Chronobiol. Int. 2004;21:131–146. doi: 10.1081/CBI-120027987. [DOI] [PubMed] [Google Scholar]
- 204.Bell-Pedersen D., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Davidson A.J. Search for the feeding-entrainable circadian oscillator: A complex proposition. Am. J. Physiol. Integr. Comp. Physiol. 2006;290:R1524–R1526. doi: 10.1152/ajpregu.00073.2006. [DOI] [PubMed] [Google Scholar]
- 206.Borer K.T., Wuorinen E.C., Lukos J.R., Denver J.W., Porges S.W., Burant C.F. Two Bouts of Exercise before Meals, but Not after Meals, Lower Fasting Blood Glucose. Med. Sci. Sports Exerc. 2009;41:1606–1614. doi: 10.1249/MSS.0b013e31819dfe14. [DOI] [PubMed] [Google Scholar]
- 207.Edinburgh R.M., Bradley H.E., Abdullah N.-F., Robinson S.L., Chrzanowski-Smith O.J., Walhin J.-P., Joanisse S., Manolopoulos K.N., Philp A., Hengist A., et al. Lipid Metabolism Links Nutrient-Exercise Timing to Insulin Sensitivity in Men Classified as Overweight or Obese. J. Clin. Endocrinol. Metab. 2019;105:660–676. doi: 10.1210/clinem/dgz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Toghi-Eshghi S.R., Yardley J.E. Morning (Fasting) vs Afternoon Resistance Exercise in Individuals With Type 1 Diabetes: A Randomized Crossover Study. J. Clin. Endocrinol. Metab. 2019;104:5217–5224. doi: 10.1210/jc.2018-02384. [DOI] [PubMed] [Google Scholar]
- 209.Coggan A.R., Coyle E.F. Carbohydrate ingestion during prolonged exercise: Effects on metabolism and performance. Exerc. Sport Sci. Rev. 1991;19:1–40. doi: 10.1249/00003677-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 210.Jeukendrup A., Brouns F., Wagenmakers A.J.M., Saris W.H.M. Carbohydrate-Electrolyte Feedings Improve 1 h Time Trial Cycling Performance. Int. J. Sports Med. 1997;18:125–129. doi: 10.1055/s-2007-972607. [DOI] [PubMed] [Google Scholar]
- 211.Coyle E.F. Timing and method of increased carbohydrate intake to cope with heavy training, competition and recovery. J. Sports Sci. 1991;9:29–52. doi: 10.1080/02640419108729865. [DOI] [PubMed] [Google Scholar]
- 212.Cajochen C., Kräuchi K., Wirz-Justice A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 213.Middleton B., Arendt J., Stone B. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J. Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- 214.Khalsa S.B.S., Jewett M.E., Cajochen C., Czeisler C.A. A Phase Response Curve to Single Bright Light Pulses in Human Subjects. J. Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wittmann M., Dinich J., Merrow M., Roenneberg T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 216.Maury E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019;20:1597. doi: 10.3390/ijms20071597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.