Abstract
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide. Early diagnosis of AF is crucial for preventing AF-related morbidity, mortality, and economic burden, yet the detection of the disease remains challenging. The 12-lead electrocardiogram (ECG) is the gold standard for the diagnosis of AF. Because of technological advances, ambulatory devices may serve as convenient screening tools for AF.
Objective
The objective of this review was to investigate the diagnostic accuracy of 2 relatively new technologies used in ambulatory devices, non-12-lead ECG and photoplethysmography (PPG), in detecting AF. We performed a meta-analysis to evaluate the diagnostic accuracy of non-12-lead ECG and PPG compared to the reference standard, 12-lead ECG. We also conducted a subgroup analysis to assess the impact of study design and participant recruitment on diagnostic accuracy.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. MEDLINE and EMBASE were systematically searched for articles published from January 1, 2015 to January 23, 2021. A bivariate model was used to pool estimates of sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and area under the summary receiver operating curve (SROC) as the main diagnostic measures. Study quality was evaluated using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool.
Results
Our search resulted in 16 studies using either non-12-lead ECG or PPG for detecting AF, comprising 3217 participants and 7623 assessments. The pooled estimates of sensitivity, specificity, PLR, NLR, and diagnostic odds ratio for the detection of AF were 89.7% (95% CI 83.2%-93.9%), 95.7% (95% CI 92.0%-97.7%), 20.64 (95% CI 10.10-42.15), 0.11 (95% CI 0.06-0.19), and 224.75 (95% CI 70.10-720.56), respectively, for the automatic interpretation of non-12-lead ECG measurements and 94.7% (95% CI 93.3%-95.8%), 97.6% (95% CI 94.5%-99.0%), 35.51 (95% CI 18.19-69.31), 0.05 (95% CI 0.04-0.07), and 730.79 (95% CI 309.33-1726.49), respectively, for the automatic interpretation of PPG measurements.
Conclusions
Both non-12-lead ECG and PPG offered high diagnostic accuracies for AF. Detection employing automatic analysis techniques may serve as a useful preliminary screening tool before administering a gold standard test, which generally requires competent physician analyses. Subgroup analysis indicated variations of sensitivity and specificity between studies that recruited low-risk and high-risk populations, warranting future validity tests in the general population.
Trial Registration
PROSPERO International Prospective Register of Systematic Reviews CRD42020179937; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=179937
Keywords: atrial fibrillation, ambulatory devices, electrocardiogram, photoplethysmography, diagnostic accuracy, ubiquitous health, mobile health, technology, ambulatory device
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, affecting approximately 33.5 million individuals. AF is more prevalent with increasing age, and its prevalence is expected to double by 2030 [1]. The disease can result in considerable morbidity and mortality by increasing the risk of heart failure, stroke, major cardiovascular events, sudden cardiac death, chronic kidney disease, peripheral arterial disease, and all-cause mortality despite often being asymptomatic [2]. A range of management choices, including anticoagulation, rate control, and rhythm control through medication or electrical cardioversion, can markedly reduce risks and relieve symptoms.
Although the treatment of AF is well established because of numerous guidelines and clinical trials, the detection of the disease remains challenging. The gold standard for AF detection is the 12-lead electrocardiogram (ECG) [3]. However, this method is not always available and can be unfeasible in certain groups of patients, such as individuals with paroxysmal AF who fail to undergo a 12-lead ECG in a timely manner or individuals with silent (subclinical) AF without any related symptoms. Therefore, devices that are convenient, inexpensive, and ambulatory are required to serve as preliminary screening tools; subsequently, initial diagnoses can be confirmed or excluded using the gold standard 12-lead ECG in hospital settings [4,5].
Several studies have investigated the accuracy and applicability of various ambulatory devices over the past few years. Rather than focusing on the devices themselves, this review targeted technologies used in ambulatory devices; thus, the summarized results are not limited to certain products. Among reviews of the use of ambulatory devices for AF detection, only one systematic review and meta-analysis focused on the accuracy of technologies compared with the gold standard [6]. That review concluded that blood pressure monitors and non-12-lead ECGs were most accurate; however, it failed to consider a newer technology, photoplethysmography (PPG). PPG is a new technology that has become ubiquitous in recent years, and one of its most widely known implementations is the Apple Watch [7]. Therefore, we conducted an updated systematic review focusing on 2 technologies that are used in ambulatory devices to detect AF: non-12-lead ECG and PPG. Additionally, the review focused on the automatic detection of AF utilizing built-in algorithms to validate their use as a convenient screening tool.
The aim of this paper was to provide a systematic overview of the accuracy of the 2 technologies compared with 12-lead ECG in the detection of AF as well as to describe their applicability, potential, and limitations.
Methods
Literature Search and Selection Criteria
We conducted a systematic search of MEDLINE (Ovid) and EMBASE for articles published from January 1, 2015 to January 23, 2021 using the search terms “mHealth,” “telemedicine,” “wearable,” “mobile health,” “mobile application,” and “digital treatment” in combination with the term “atrial fibrillation.” The search terms are presented in Multimedia Appendix 1. In addition, reference lists of relevant systematic reviews and included studies were hand-searched to identify additional articles. Only papers in English were included. All randomized trials, observational studies, and case series were included, whereas systematic reviews and case reports were excluded. Studies that recruited participants aged ≥18 years, investigated any method of identifying patients with suspected AF using an ambulatory device equipped with automatic interpretation by a mobile app or algorithm, provided a reference standard with 12-lead ECG interpreted by a competent professional, and reported sufficient data to enable the calculation of the diagnostic accuracy were included. Studies that investigated invasive methods of identifying AF, focused on the training of algorithms, validated the method and algorithm through a dataset, or failed to provide a timely reference standard for all participants were excluded. Two reviewers independently conducted the screening and reviewing of articles, and any disagreements were resolved by consensus with a third reviewer. The study strictly followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and the PRISMA search flow diagram is provided in Figure 1.
Figure 1.
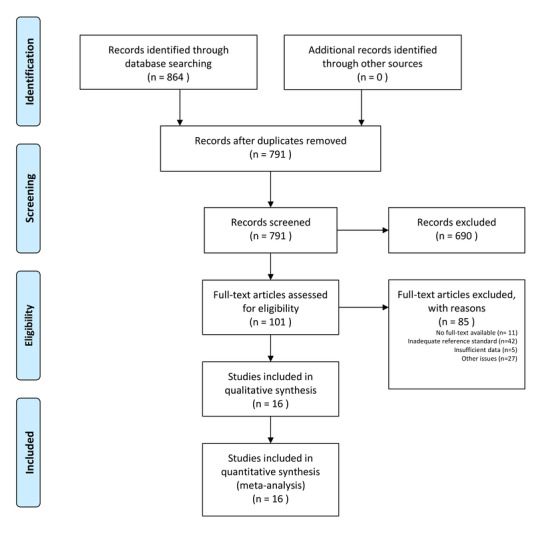
Summary of the study selection process using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Data Extraction and Quality Assessment
Two reviewers independently extracted the data for the selected articles, including the year of publication, country, study design, number of study participants, average age of the study population, characteristics of the study population, technology used for measurement, measurement time, and reference standard. The absolute numbers of true positive, false positive, false negative, and true negative were also extracted.
If a study provided data on both the measurement level and individual level, the data analysis performed on the measurement level was extracted first. If a study provided both training data and validation data, only validation data were extracted. If a study failed to provide sufficient data for the calculation of diagnostic accuracy, the lead authors of the studies were contacted to request the missing data. If the authors failed to reply, 2 reviewers calculated the incomplete information on the basis of available data. Studies were excluded only if both the aforementioned methods failed to identify any additional data.
Study quality was evaluated using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool, which is recommended for the evaluation of risk of bias and applicability in diagnostic accuracy studies [8,9]. The assessment tool focuses on 4 key domains: patient selection, index test, reference standard, and flow and timing. Risk of bias was evaluated in all 4 domains, and applicability concerns were evaluated in the first 3 domains. For each question, each study was graded as “low risk,” “high risk,” or “unclear risk.” A standardized table, recommended by the QUADAS-2 official website, was used to display the summarized results of the study quality appraisal.
Statistical Analysis
Diagnostic accuracy statistics were computed using R software version 4.0.0 (R Core Team). The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) — with their respective 95% CIs — were calculated using a bivariate diagnostic random-effects model in the mada package [10]. Tests for heterogeneity regarding the DOR were also performed and presented with Cochran Q and Higgins I2. Summary receiver operating characteristic (SROC) curves were used to summarize and visualize the diagnostic performance of each included study. The area under the summary receiver operating characteristic (AUSROC) curve was also calculated for both the non-12-lead ECG and PPG methods.
Publication bias was assessed using the Deek funnel plot asymmetry test, which is a scatterplot of (1/root [ESS]) against (ln[DOR]) [11]; a P value <.10 indicated publication bias. Fagan nomogram analysis was performed to determine the posttest probability of the disease based on the likelihood ratio of the diagnostic test. An age-adjusted prevalence of 0.5% was applied in the analysis to represent the general population worldwide [12], whereas a prevalence of 2.3% was adopted to represent a population with higher risks as targeted in a systematic review [13]. The left axis of a Fagan nomogram represents the pretest probability, the middle axis displays the likelihood ratio of the diagnostic test, and the right axis indicates the posttest probability.
Because the risk of patient selection bias among the studies was obvious, a subgroup analysis between low-risk groups (studies that recruited patients with and without AF) and high-risk groups (studies that recruited only patients with AF) was conducted to investigate the effect of the study design and population on diagnostic performance. Data synthesis and most statistical analyses were conducted using R version 4.0.0.
Study Registration
This systematic review and meta-analysis was registered in the PROSPERO International Prospective Register of Systematic Reviews (ID: CRD42020179937).
Results
Literature Search
Figure 1 illustrates the flowchart of study inclusion. The initial search yielded 864 publications from MEDLINE (Ovid) and EMBASE. Duplicates and irrelevant studies were removed, yielding 791 publications for title and abstract review. After exclusion of 690 publications for irrelevant study focus, the rest of the 101 publications were then assessed through full-text articles. In total, 85 publications were excluded for the following reasons: having insufficient context, being an inappropriate study type, having a study population aged <18 years, utilizing invasive or implantable devices to identify AF, or having an inadequate reference standard. The full-text evaluation yielded 16 publications that met the inclusion criteria; these papers were included in this systematic review and meta-analysis [14-29]. No additional studies were identified through the hand-searching process. The characteristics of the included studies are listed in Table 1. The details of the study population, the prevalence of AF, ambulatory devices, measuring time, and measurement data are presented in 2 respective tables for non-12-lead ECG and PPG in Multimedia Appendix 2.
Table 1.
Characteristics of the included studies and study population.
| Study authors | Year | Country | Study design | Index test | na | Population |
| Chen et al [14] | 2020 | China | Prospective, cross-sectional | Non-12-lead ECGb and PPGc | 401 | Inpatients and outpatients aged >18 years in a cardiovascular department |
| Lown et al [15] | 2020 | United Kingdom | Prospective, cross-sectional | Non-12-lead ECG | 415 | Participants aged >65 years (n=79 with AFd and n=336 without AF) |
| Wegner et al [16] | 2020 | Germany | Prospective, cross-sectional | Non-12-lead ECG | 92 | Inpatients with no predefined exclusion criteria |
| Yan et al [17] | 2020 | Hong Kong | Prospective, cross-sectional | PPG | 44 | 20 patients with permanent AF and 24 control individuals in sinus rhythm |
| Reverberi et al [18] | 2019 | Italy | Prospective, longitudinal | Non-12-lead ECG | 95 | Patients aged >18 years diagnosed with AF and scheduled for elective cardioversion |
| Proesmans et al [19] | 2019 | Belgium | Prospective, cross-sectional | Non-12-lead ECG & PPG | 223 | Patients aged ≥65 years, individuals with known AF and supplemented with individuals without AF |
| Himmelreich et al [20] | 2019 | Netherlands | Prospective, cross-sectional | Non-12-lead ECG | 214 | Patients aged ≥18 years assigned to 12-lead ECG for any nonacute indication |
| Haverkamp et al [21] | 2019 | Norway | Prospective, cross-sectional | Non-12-lead ECG | 94 | Patients admitted to the cardiology ward with ongoing 12-lead ECG surveillance |
| Fan et al [22] | 2019 | China | Prospective, cross-sectional | PPG | 108 | Patients aged ≥18 years admitted to the hospital |
| Yan et al [23] | 2018 | Hong Kong | Prospective, cross-sectional | PPG | 217 | Patients admitted to the cardiology ward |
| William et al [24] | 2018 | United States | Prospective, cross-sectional | Non-12-lead ECG | 52 | Patients aged 35-85 years diagnosed with AF and scheduled for anti-arrhythmic drug initiation |
| Rozen et al [25] | 2018 | United States | Prospective, longitudinal | PPG | 98 | Patients aged >18 years diagnosed with AF and scheduled for elective DCe cardioversion |
| Bumgarner et al [26] | 2018 | United States | Prospective, longitudinal | Non-12-lead ECG | 100 | Patients aged 18-90 years diagnosed with AF and scheduled for elective cardioversion |
| Lown et al [27] | 2018 | United Kingdom | Prospective, cross-sectional | Non-12-lead ECG | 418 | Patients aged >65 years (n=82 with AF and n=336 without AF) |
| Desteghe et al [28] | 2016 | Belgium | Prospective, cross-sectional | Non-12-lead ECG | 265 | Patients aged ≥18 years admitted to the cardiology ward |
| Haberman et al [29] | 2015 | United States | Prospective, cross-sectional | Non-12-lead ECG | 381 | Athletes, students, and patients of an ambulatory cardiology clinic |
an: number of participants.
bECG: electrocardiogram.
cPPG: photoplethysmography.
dAF: atrial fibrillation.
eDC: direct current.
Characteristics and Quality of Studies
All the included studies followed a prospective design and had a total of 3217 participants. Of the 16 publications, 13 were cross-sectional studies [14-17,19-24,27,28], where the measurement was conducted at a single point, and the other 3 were longitudinal [18,25,26], where the measurement was conducted more than once for each participant. The sample size of the studies ranged from 44 to 418 participants. Four studies excluded participants aged <65 years, 4 studies recruited only participants with a diagnosis or history of AF [18,24-26], and 4 studies identified patients with AF and supplemented the cohort with control participants [15,17,19,27]. The remaining 7 studies recruited in-hospital patients with various indications [14,16,20-23,28], and 1 study recruited athletes, students, and patients of an ambulatory cardiology clinic [29]. The AF prevalence among the participants in these studies ranged from 5% to 100%.
The 16 studies investigated 2 technologies: non-12-lead ECG and PPG. This review defines non-12-lead ECG as measurements recorded by any electrode-based device; participants simply placed their fingers on the electrodes, attached the electrodes to their chest, or held the electrodes in their hands. PPG is a technology that measures changes in tissue blood volume that enables the recording of each heartbeat; the signal can be detected by any device with a camera monitoring various body parts, including the fingertip, wrist, palm, and face. Among the publications included in this review, 10 studies focused on non-12-lead ECG equipped in 10 different electrode-based ambulatory devices [15,16,18,20,21,24,26-29]; 4 studies focused on PPG recorded by cameras [17,23], phone cameras [19,22,23,25], or wristbands [14,22]; and 2 studies investigated both technologies in the same cohort [14,19]. All measurements were processed automatically using algorithms or smartphone apps. The reference standard in all the studies was 12-lead-ECG, which was interpreted by competent physicians or cardiologists in a blinded manner. Data for the primary statistical analysis, including true positives, false positives, false negatives, and true negatives, are presented in Multimedia Appendix 2.
The quality of the included studies was evaluated according to the criteria of the QUADAS-2 tool. Regarding the risk of patient selection bias, case-control studies and participants from cardiology departments were labelled as unclear risk in yellow, and studies that included only patients with AF were labelled as high risk in red. To be included in this review, all studies must have used 12-lead-ECGs as the reference standard; however, if studies used other types of reference tests in a small proportion of participants, they were still included but marked as unclear risk in the reference standard column. In the flow and timing assessment, studies that sequentially performed the reference test right before or after the index test were labelled as unclear risk, whereas studies that performed both index and reference tests simultaneously on the participants were labelled as low risk. Other criteria were assessed as provided. Two independent reviewers performed the quality appraisal, and the results are presented in Figure 2.
Figure 2.
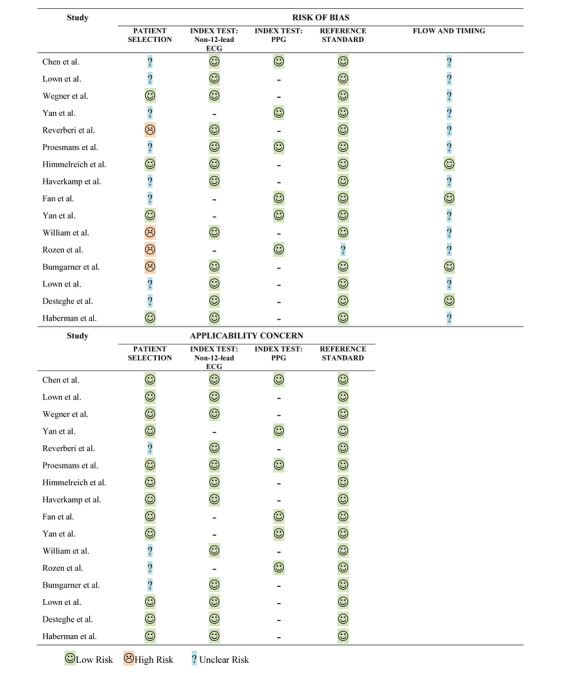
Summary of the QUADAS-2 quality appraisal of the included studies. ECG: electrocardiogram; PPG: photoplethysmography.
Data Synthesis of Diagnostic Accuracy
In total, 3217 participants with 7623 measurements were included in the data synthesis.
Automatic detection of AF based on non-12-lead ECG had a combined estimated sensitivity of 89.7% (95% CI 83.2%-93.9%), specificity of 95.7% (95% CI 92.0%-97.7%), PLR of 20.64 (95% CI 10.10-42.15), NLR of 0.11 (95% CI 0.06-0.19), and DOR of 224.75 (95% CI 70.10-720.56). A heterogeneity test among included studies was assessed with a Cochran Q of 13.99 (df=15, P=.526) and Higgins I2 of 0%. Automatic detection of AF based on recordings of PPG had a combined estimated sensitivity of 94.7% (95% CI 93.3%-95.8%), specificity of 97.6% (95% CI 94.5%-99.0%), PLR of 35.51 (95% CI 18.19-69.31), NLR of 0.05 (95% CI 0.04-0.07), and DOR of 730.79 (95% CI 309.33-1726.49). A test of heterogeneity for the PPG studies reported a Cochran Q of 8.78 (df=7, P=.269) and Higgins I2 of 20.26%. The forest plots of the pooled diagnostic accuracies are presented in Figure 3 and Figure 4. SROC curves for both the technologies in all the included studies are presented in Figure 5. The AUSROCs were 0.97 for non-12-lead ECG and 0.95 for PPG.
Figure 3.
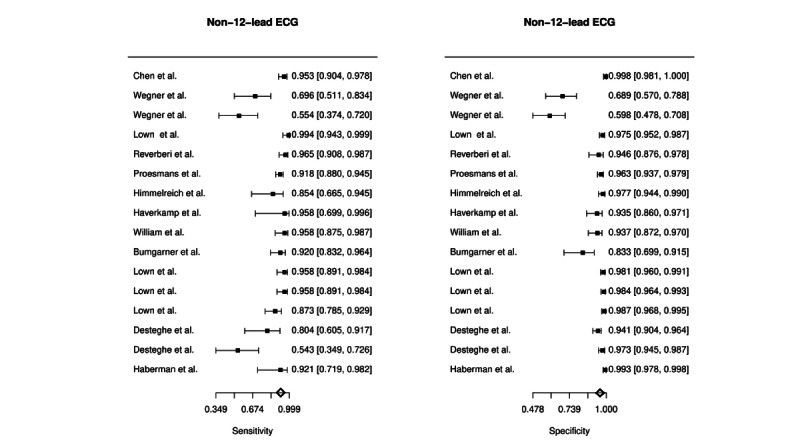
Forest plot of the combined diagnostic estimates of sensitivity and specificity of automatically interpreted non-12-lead electrocardiograms (ECGs).
Figure 4.
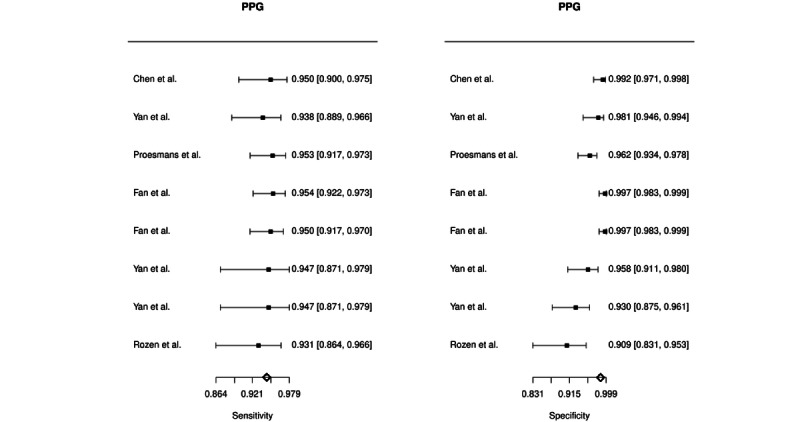
Forest plot of the combined diagnostic estimates of sensitivity and specificity of automatically interpreted photoplethymography (PPG).
Figure 5.
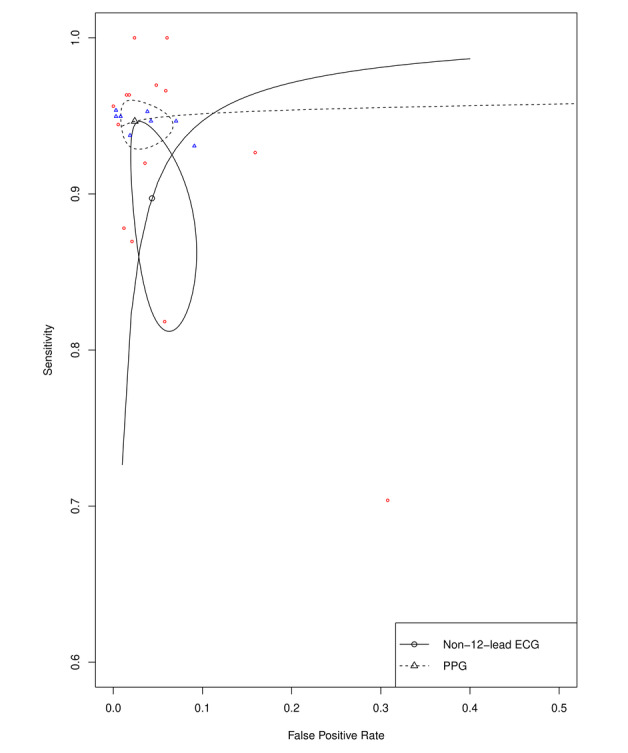
Summary receiver operating curves of the automatically interpreted non-12-lead electrocardiogram (ECG) and photoplethymography (PPG) in the diagnosis of atrial fibrillation.
In light of the 2020 European Society of Cardiology Clinical Practice Guidelines for the diagnosis and management of AF [30] that recommended a manually interpreted, single-lead ECG ≥30 seconds as the other option to establish a definitive diagnosis of AF, we also extracted relevant data from the included studies to perform a meta-analysis of such a method. Manual interpretation based on non-12-lead ECG had a combined sensitivity of 93.4% (95% CI 86.7%-96.8%), specificity of 96.3% (95% CI 92.9%-98.1%), PLR of 25.93 (95% CI 13.70-49.05), NLR of 0.07 (95% CI 0.04-0.14), and DOR of 439.64 (95% CI 202.89-952.65). All non-12-lead ECG segments included were recorded ≥30 seconds. The forest plots of the pooled diagnostic accuracies are shown in Multimedia Appendix 3. An SROC comparison curve between the automatic and manual interpretations of non-12-lead ECG are presented in Figure 6. No PPG recordings were examined manually among the included studies.
Figure 6.

Summary receiver operating curves of automatic and manual interpretations of non-12-lead electrocardiogram in the diagnosis of atrial fibrillation.
Subgroup Analysis: Study Population
A subgroup analysis (Figure 7) was performed to investigate the effect of the study design and population on the diagnostic accuracy. Studies were divided into 2 groups: low risk (group 1), including those that recruited participants with and without AF, and high risk (group 2), including those that only recruited participants with prediagnosed AF.
Figure 7.
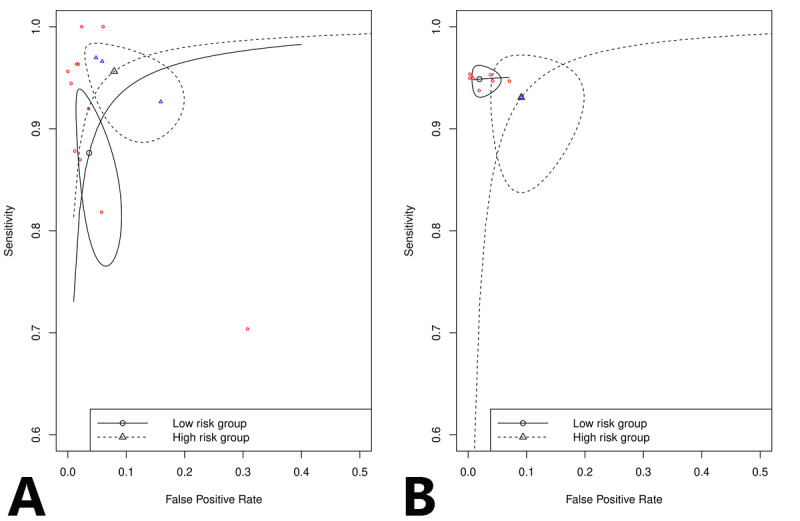
Subgroup analysis of the study population, including a comparison of summary receiver operating curves between the low-risk study population, which included patients with and without atrial fibrillation, and high-risk study population, which included only patients with atrial fibrillation, for (A) non-12-lead electrocardiogram and (B) photoplethysmography.
Non-12-lead ECG yielded a sensitivity of 87.6% and specificity of 96.4% in low-risk studies (n=13) and a sensitivity of 95.6% and specificity of 92.1% in high-risk studies (n=3). PPG yielded a sensitivity of 94.9% and specificity of 98.1% in low-risk studies (n=7) and a sensitivity of 93.1% and specificity of 90.9% in high-risk studies (n=1). The subgroup analysis of non-12-lead ECG indicated a higher sensitivity and lower specificity among the high-risk studies. On the other hand, only 1 study was included in the high-risk group for PPG; thus, no conclusion could be made regarding the subgroup analysis of the PPG technology.
Fagan Nomogram
The Fagan nomogram analysis (Multimedia Appendix 4 and Multimedia Appendix 5) demonstrated that, with an age-adjusted prevalence of 0.5% in the general population with pooled PLR of 20.64 and pooled NLR of 0.11, the posttest probabilities of non-12-lead ECG increased to 9.40% and decreased to 0.06%, respectively; with pooled PLR of 35.51 and pooled NLR of 0.05, the posttest probabilities of PPG increased to 15.14% and decreased to 0.03%, respectively. By contrast, in a high-risk population with a prevalence of 2.3%, the application of non-12-lead ECG had posttest probabilities of 32.70% and 0.26%, respectively, and PPG had posttest probabilities of 45.53% and 0.12%, respectively.
Publication Bias
Publication bias was assessed using Deek funnel plots (Multimedia Appendix 6 and Multimedia Appendix 7). For studies investigating the non-12-lead ECG method, visual inspection of the funnel plot indicated a likely absence of publication bias (P=.107). For studies investigating the PPG method, a visual evaluation of the funnel plot also provided no clear evidence of publication bias (P=.107).
Discussion
Principal Findings
This systematic review and meta-analysis on the diagnostic accuracy of 16 studies published between 2015 and 2021 confirmed that both non-12-lead ECG and PPG are highly accurate technologies in detecting AF. Automatically interpreted PPG provided the highest sensitivity and specificity for the diagnosis of AF, immediately followed by the manual interpretation of non-12-lead ECG, whereas automatically diagnosed AF based on non-12-lead ECG performed relatively weaker than the first 2 methods. However, they all demonstrated outstanding diagnostic accuracy. The differences among these approaches may be attributed to measuring techniques: PPG optically records volumetric changes in local arterioles and, in this manner, measures pulse variability (or R-R interval) and heart rhythm variability, while non-12-lead ECG assesses the electrical activity of the heart using electrodes. Other possible explanations for the variability include different classification thresholds and different methods used in the algorithm design.
Early diagnosis of AF is crucial for preventing AF-related morbidity, mortality, and economic burden. The prevalence of undiagnosed AF in the United States is estimated to be 1%-2%, and the incremental cost burden could amount to US $3.1 billion per year [31]. In comparison, smart wearable devices and mobile phones are almost ubiquitous worldwide, and the incremental cost for their application is relatively low. Our summarized findings suggest that the appropriate application of these technologies enables early diagnosis and treatment, which might possibly contribute to reducing the cost of illness. The current guidance suggests pulse palpation and 12-lead ECG in AF screening. However, in a meta-analysis by Taggar et al [6], pulse palpation was proved inferior to 4 other methods because of lower specificity; additionally, the use of 12-lead ECG is limited by its inconvenience and the fact that it captures an ECG at only 1 time point. In clinical practice, continuous Holter monitoring is one of the most commonly used methods to detect AF. However, this examination requires patients to carry the machine with them for an extended period, which results in inconvenience; thus, it is also not a suitable tool for general screening.
The 2 main technologies reviewed in this meta-analysis, non-12-lead ECG and PPG, have several advantages as screening tools for AF. First, they involve lightweight, low-cost, and ambulatory devices. In this review, non-12-lead ECGs were employed in devices such as handheld tablets [16,19-21,24,27,28], watchbands [14,26], handheld rod-like sensors [28], and electrodes attached to the chest [15,16,18,19,27]. By contrast, PPG signals can be assessed on the fingertips [19,22,23,25], wrist [14,22], earlobe, and face [17,23] by any device with simple optoelectronic components, such as a smartphone. Second, these technologies require a relatively short monitoring time, which enables fast and timely screening; the monitoring time most commonly varied from 30 seconds to 1 minute. Third, measurements obtained by these devices were automatically interpreted by built-in algorithms, which means that the tests can be conducted without the presence of a health care professional. Several studies [16,20,24,26,28] included in this review conducted additional analyses comparing algorithms’ and physicians’ interpretations of the same non-12-lead ECG segments and concluded that automated algorithm performance is not inferior to competent professional interpretation. Finally, as demonstrated in the results, the automatically generated diagnoses established with both technologies yielded outstanding diagnostic accuracy. Automatically interpreted PPG had a sensitivity and specificity of 94.7% and 97.6%, respectively. Automatically interpreted non-12-lead ECG had a sensitivity and specificity of 89.7% and 95.7%, respectively. As for the interpretation of non-12-lead ECG by competent physicians, an established method to diagnose AF according to the 2020 European Society of Cardiology Clinical Guidelines had a sensitivity and specificity of 93.4% and 96.3%, respectively. Demonstrating effectiveness, convenience, and time savings with high diagnostic accuracies, we suggest using these technologies with built-in automatic interpretation as preliminary screening tools for the detection of AF when the gold standard method, which generally requires a physician’s interpretation, is not feasible. Notably, the screening program should target high-risk populations (eg, elderly) to avoid false positives.
We performed a subgroup analysis of both technologies on account of the apparent patient selection bias among included studies. The sensitivity and specificity of a test are generally believed to not vary with the disease prevalence of a population; however, variations of these diagnostic parameters were often spotted. According to a previous article that summarized the phenomenon and proposed several possible causes [32], the specificity of a test tended to be lower with high disease prevalence, and although not significant, the sensitivity appeared higher with high prevalence of some diseases. This review also observed instability of the sensitivity and specificity between low-risk groups (studies that recruited patients with and without AF) and high-risk groups (studies that recruited only patients with AF). Higher sensitivity and lower specificity were generated in the high-risk groups using the non-12-lead ECG tests; however, no conclusion could be made for the PPG method because only 1 study included a high-risk group. The results indicate that the performance of these technologies was affected by the recruited population and design of the included studies. Future validation conducted in a more general population is warranted to investigate such an occurrence.
Strengths and Limitations
To our knowledge, this is the first systematic review and meta-analysis to compare the diagnostic accuracy of 2 common technologies, namely non-12-lead ECG and PPG, used in ambulatory devices for detecting AF. This review followed PRISMA guidelines, implemented a comprehensive search strategy, applied strict inclusion and exclusion criteria, and employed 2 independent reviewers to assess all the included studies. For instance, one of the criteria was to exclude studies that failed to provide a timely reference standard; this resulted in the exclusion of multiple large-scale screening studies but ensured time consistency between the index test and reference standard. Moreover, we investigated the heterogeneity and publication bias of included studies, as well as the posttest probability of AF using these ambulatory device technologies. Finally, an additional subgroup analysis was performed to investigate the effect of the study population on diagnostic performance. The result identified sensitivity and specificity variations between low-risk and high-risk populations, indicating future validation of the diagnostic accuracy of these tests is needed in a more general population.
This review of studies on AF detection using ambulatory devices has several limitations. The most noteworthy concern is the study population investigated. Except for the 4 studies that recruited only patients with AF for their assessment, other studies were mostly conducted in a case-control style or recruited inpatients from hospitals. This problem was reflected in the QUADAS-2 quality assessment and possibly contributed to the instability of diagnostic accuracy in the subgroup analysis. Additionally, the heterogeneity of devices and algorithms used should be considered. Although we explored the accuracy of ambulatory devices from a technology perspective, these technologies were applied in diverse devices, and the measurements were automatically interpreted by their respective algorithms. Furthermore, measurements of insufficient quality or unclassified by algorithms tended to be excluded in the calculation of the diagnostic accuracy in some of the studies [14,19,22,24,26]; the proportion of insufficient or unclassified recordings ranged from 0.5% to 33.8%. Finally, some studies regarded atrial flutter as the same disease state as AF, viewing the incident as a positive AF result [16,19,20,24,26-29].
Conclusions
Both non-12-lead ECG and PPG technologies offered high diagnostic accuracies for AF. Automatically interpreted PPG recordings generated the highest sensitivity and specificity compared to both the manual and automatic interpretations of non-12-lead ECG. Detection of AF employing automatic analysis techniques may serve as a useful preliminary screening tool before administering a gold standard test, which generally requires analyses by competent physicians. Subgroup analysis indicated variations of sensitivity and specificity between studies that recruited low-risk and high-risk populations, and future validation of these diagnostic tests in the general population is warranted.
Acknowledgments
The authors thank Mark Lown, Felix K Wegner, and Aviram Hochstadt for sharing their original data and contributing to the analytic calculations. The authors also thank Wallace Academic Editing for manuscript editing. We also thank the Higher Education Sprout Project of the Ministry of Education (MOE) in Taiwan for financially supporting this work [DP2-110-21121-01-A-04].
Abbreviations
- AF
atrial fibrillation
- AUSROC
area under the summary receiver operating curve
- DOR
diagnostic odds ratio
- ECG
electrocardiogram
- NLR
negative likelihood ratio
- PLR
positive likelihood ratio
- PPG
photoplethysmography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS
quality assessment of diagnostic accuracy studies
- SROC
summary receiver operating characteristic
Appendix
The literature search strategy for the systematic review and meta-analysis in Medline (Ovid) and Embase databases.
Details of the study population, ambulatory devices, measuring time, reference standard, prevalence, and measurement data of the included studies.
Forest plot of the combined diagnostic estimates of sensitivity and specificity of manually interpreted non-12-lead electrocardiogram.
Fagan nomogram for the evaluation of the clinical utility of automatically interpreted non-12-lead electrocardiogram (ECG).
Fagan nomogram for the evaluation of the clinical utility of automatically interpreted photoplethysmography (PPG).
Deek funnel plots for the assessment of publication bias of non-12-lead electrocardiogram (ECG) studies.
Deek funnel plots for the assessment of publication bias of photoplethysmography (PPG) studies.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: a major public health concern. Heart. 2018 Dec;104(24):1989–1990. doi: 10.1136/heartjnl-2018-313350. [DOI] [PubMed] [Google Scholar]
- 2.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016 Sep 06;354:i4482. doi: 10.1136/bmj.i4482. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=27599725. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, ESC Scientific Document Group 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct 07;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Jones N, Taylor C, Hobbs F, Bowman L, Casadei B. Screening for atrial fibrillation: a call for evidence. Eur Heart J. 2020 Mar 07;41(10):1075–1085. doi: 10.1093/eurheartj/ehz834. http://europepmc.org/abstract/MED/31811716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence Atrial fibrillation: management (Clinical guideline [CG180]) National Institute for Health and Care Excellence. [2021-01-25]. https://www.nice.org.uk/guidance/cg180. [DOI] [PubMed]
- 6.Taggar JS, Coleman T, Lewis S, Heneghan C, Jones M. Accuracy of methods for detecting an irregular pulse and suspected atrial fibrillation: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016 Aug;23(12):1330–8. doi: 10.1177/2047487315611347. https://journals.sagepub.com/doi/10.1177/2047487315611347?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019 Nov 14;381(20):1909–1917. doi: 10.1056/nejmoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration. London, United Kingdom: The Cochrane Collaboration, 2009; 2009. Oct 27, Assessing methodological quality. [Google Scholar]
- 9.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM, QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. https://www.acpjournals.org/doi/10.7326/0003-4819-155-8-201110180-00009?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PubMed] [Google Scholar]
- 10.Philipp D, Heinz H. Meta-Analysis of Diagnostic Accuracy with mada. cran.r-project.org. [2021-01-25]. https://cranr-projectorg/web/packages/mada/vignettes/madapdf.%202012.
- 11.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005 Sep;58(9):882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y, McAnulty JH, Zheng Z, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014 Feb 25;129(8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. http://europepmc.org/abstract/MED/24345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. Thromb Haemost. 2017 Dec 04;110(08):213–222. doi: 10.1160/th13-02-0165. [DOI] [PubMed] [Google Scholar]
- 14.Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou J, Huo Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm. 2020 May;17(5 Pt B):847–853. doi: 10.1016/j.hrthm.2020.01.034. https://linkinghub.elsevier.com/retrieve/pii/S1547-5271(20)30089-8. [DOI] [PubMed] [Google Scholar]
- 15.Lown M, Brown M, Brown C, Yue AM, Shah BN, Corbett SJ, Lewith G, Stuart B, Moore M, Little P. Machine learning detection of Atrial Fibrillation using wearable technology. PLoS One. 2020 Jan 24;15(1):e0227401. doi: 10.1371/journal.pone.0227401. https://dx.plos.org/10.1371/journal.pone.0227401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegner FK, Kochhäuser S, Ellermann C, Lange PS, Frommeyer G, Leitz P, Eckardt L, Dechering DG. Prospective blinded Evaluation of the smartphone-based AliveCor Kardia ECG monitor for Atrial Fibrillation detection: The PEAK-AF study. Eur J Intern Med. 2020 Mar;73:72–75. doi: 10.1016/j.ejim.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Yan BP, Lai WHS, Chan CKY, Au ACK, Freedman B, Poh YC, Poh M. High-Throughput, Contact-Free Detection of Atrial Fibrillation From Video With Deep Learning. JAMA Cardiol. 2020 Jan 01;5(1):105–107. doi: 10.1001/jamacardio.2019.4004. http://europepmc.org/abstract/MED/31774461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reverberi C, Rabia G, De Rosa F, Bosi D, Botti A, Benatti G. The RITMIA™ Smartphone App for Automated Detection of Atrial Fibrillation: Accuracy in Consecutive Patients Undergoing Elective Electrical Cardioversion. Biomed Res Int. 2019;2019:4861951. doi: 10.1155/2019/4861951. doi: 10.1155/2019/4861951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B. Mobile Phone-Based Use of the Photoplethysmography Technique to Detect Atrial Fibrillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR Mhealth Uhealth. 2019 Mar 27;7(3):e12284. doi: 10.2196/12284. https://mhealth.jmir.org/2019/3/e12284/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himmelreich JC, Karregat EP, Lucassen WA, van Weert HC, de Groot JR, Handoko ML, Nijveldt R, Harskamp RE. Diagnostic Accuracy of a Smartphone-Operated, Single-Lead Electrocardiography Device for Detection of Rhythm and Conduction Abnormalities in Primary Care. Ann Fam Med. 2019 Sep 09;17(5):403–411. doi: 10.1370/afm.2438. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=31501201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haverkamp HT, Fosse SO, Schuster P. Accuracy and usability of single-lead ECG from smartphones - A clinical study. Indian Pacing Electrophysiol J. 2019;19(4):145–149. doi: 10.1016/j.ipej.2019.02.006. https://linkinghub.elsevier.com/retrieve/pii/S0972-6292(19)30033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Li Y, Li J, Cheng W, Shan Z, Wang Y, Guo Y. Diagnostic Performance of a Smart Device With Photoplethysmography Technology for Atrial Fibrillation Detection: Pilot Study (Pre-mAFA II Registry) JMIR Mhealth Uhealth. 2019 Mar 05;7(3):e11437. doi: 10.2196/11437. https://mhealth.jmir.org/2019/3/e11437/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan BP, Lai WHS, Chan CKY, Chan SC, Chan L, Lam K, Lau H, Ng C, Tai L, Yip K, To OTL, Freedman B, Poh YC, Poh M. Contact-Free Screening of Atrial Fibrillation by a Smartphone Using Facial Pulsatile Photoplethysmographic Signals. J Am Heart Assoc. 2018 Apr 05;7(8):1. doi: 10.1161/JAHA.118.008585. https://www.ahajournals.org/doi/10.1161/JAHA.118.008585?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, Saliba W, Wolski K, Hussein A, Lindsay BD, Wazni OM, Tarakji KG. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm. 2018 Oct;15(10):1561–1565. doi: 10.1016/j.hrthm.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Rozen G, Vaid J, Hosseini SM, Kaadan MI, Rafael A, Roka A, Poh YC, Poh M, Heist EK, Ruskin JN. Diagnostic Accuracy of a Novel Mobile Phone Application for the Detection and Monitoring of Atrial Fibrillation. Am J Cardiol. 2018 May 15;121(10):1187–1191. doi: 10.1016/j.amjcard.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 26.Bumgarner JM, Lambert CT, Hussein AA, Cantillon DJ, Baranowski B, Wolski K, Lindsay BD, Wazni OM, Tarakji KG. Smartwatch Algorithm for Automated Detection of Atrial Fibrillation. J Am Coll Cardiol. 2018 May 29;71(21):2381–2388. doi: 10.1016/j.jacc.2018.03.003. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(18)33486-7. [DOI] [PubMed] [Google Scholar]
- 27.Lown M, Yue AM, Shah BN, Corbett SJ, Lewith G, Stuart B, Garrard J, Brown M, Little P, Moore M. Screening for Atrial Fibrillation Using Economical and Accurate Technology (From the SAFETY Study) Am J Cardiol. 2018 Oct 15;122(8):1339–1344. doi: 10.1016/j.amjcard.2018.07.003. doi: 10.1016/j.amjcard.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, Schurmans J, Vanduynhoven P, Dendale P, Heidbuchel H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2017 Jan;19(1):29–39. doi: 10.1093/europace/euw025. [DOI] [PubMed] [Google Scholar]
- 29.Haberman ZC, Jahn RT, Bose R, Tun H, Shinbane JS, Doshi RN, Chang PM, Saxon LA. Wireless Smartphone ECG Enables Large-Scale Screening in Diverse Populations. J Cardiovasc Electrophysiol. 2015 May 19;26(5):520–6. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 30.Hindricks G, Potpara T, Dagres N. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021 Feb 01;42(5):507. doi: 10.1093/eurheartj/ehaa798. [DOI] [PubMed] [Google Scholar]
- 31.Turakhia M, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman D. Economic Burden of Undiagnosed Nonvalvular Atrial Fibrillation in the United States. Journal of the American College of Cardiology. 2015 Mar;65(10):A475. doi: 10.1016/s0735-1097(15)60475-2. [DOI] [PubMed] [Google Scholar]
- 32.Leeflang MMG, Rutjes AWS, Reitsma JB, Hooft L, Bossuyt PMM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ. 2013 Aug 06;185(11):E537–44. doi: 10.1503/cmaj.121286. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=23798453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The literature search strategy for the systematic review and meta-analysis in Medline (Ovid) and Embase databases.
Details of the study population, ambulatory devices, measuring time, reference standard, prevalence, and measurement data of the included studies.
Forest plot of the combined diagnostic estimates of sensitivity and specificity of manually interpreted non-12-lead electrocardiogram.
Fagan nomogram for the evaluation of the clinical utility of automatically interpreted non-12-lead electrocardiogram (ECG).
Fagan nomogram for the evaluation of the clinical utility of automatically interpreted photoplethysmography (PPG).
Deek funnel plots for the assessment of publication bias of non-12-lead electrocardiogram (ECG) studies.
Deek funnel plots for the assessment of publication bias of photoplethysmography (PPG) studies.


