Abstract
Redox status is a key determinant in the fate of β-cell. These cells are not primarily detoxifying and thus do not possess extensive antioxidant defense machinery. However, they show a wide range of redox regulating proteins, such as peroxiredoxins, thioredoxins or thioredoxin reductases, etc., being functionally compartmentalized within the cells. They keep fragile redox homeostasis and serve as messengers and amplifiers of redox signaling. β-cells require proper redox signaling already in cell ontogenesis during the development of mature β-cells from their progenitors. We bring details about redox-regulated signaling pathways and transcription factors being essential for proper differentiation and maturation of functional β-cells and their proliferation and insulin expression/maturation. We briefly highlight the targets of redox signaling in the insulin secretory pathway and focus more on possible targets of extracellular redox signaling through secreted thioredoxin1 and thioredoxin reductase1. Tuned redox homeostasis can switch upon chronic pathological insults towards the dysfunction of β-cells and to glucose intolerance. These are characteristics of type 2 diabetes, which is often linked to chronic nutritional overload being nowadays a pandemic feature of lifestyle. Overcharged β-cell metabolism causes pressure on proteostasis in the endoplasmic reticulum, mainly due to increased demand on insulin synthesis, which establishes unfolded protein response and insulin misfolding along with excessive hydrogen peroxide production. This together with redox dysbalance in cytoplasm and mitochondria due to enhanced nutritional pressure impact β-cell redox homeostasis and establish prooxidative metabolism. This can further affect β-cell communication in pancreatic islets through gap junctions. In parallel, peripheral tissues losing insulin sensitivity and overall impairment of glucose tolerance and gut microbiota establish local proinflammatory signaling and later systemic metainflammation, i.e., low chronic inflammation prooxidative properties, which target β-cells leading to their dedifferentiation, dysfunction and eventually cell death.
Keywords: redox signaling, oxidative stress, redox homeostasis, pancreatic β-cells, de/differentiation, inflammation
1. Redox Homeostasis in β-Cell Development and Maturation
1.1. Sources of Reactive Oxygen Species and Antioxidants in β-Cells
Reactive oxygen species (ROS) are normally produced during the metabolism of β-cells and play an important role in cellular signaling. For instance, mitochondrial ROS are obligatory signals of glucose-induced insulin secretion (GSIS) [1,2]. However, excessive levels of ROS in both human and rodent β-cells cause oxidative stress, which is detrimental to the cells. Several such conditions leading to excessive ROS generation in β-cells have been proposed, such as hyperglycemia, hypoxia, hyperlipidemia and endoplasmic reticulum (ER) stress [3]. Pancreatic β-cells both of rodents and humans are reportedly determined to be especially vulnerable to oxidative damage [4] due to the low expression of classical antioxidant enzymes—catalases, glutathione peroxidases (GPX) and superoxide dismutases (SOD) when compared to other cell types [5,6,7]. The main antioxidant system in β-cells consists of peroxiredoxins (PRX), thioredoxins (TRX) and thioredoxin reductase (TRXR). Regeneration of PRX thiol groups is mediated by auxiliary enzymes TRX and glutaredoxins (GRX). The recycling of TRX is mediated by the activity of TRXR, which reduces TRX and allows the cycle to continue. NADPH serves as an electron donor for the reduction of TRXR [8]. GRX is reduced by glutathione, which is then regenerated by glutathione reductase with the concurrent utilization of NADPH [8]. This system was shown to be sufficient to protect β-cells against short-run oxidative burst while hypothetically also providing a signaling role necessary for GSIS in both rodent and human cells [9]. Nevertheless, long-term glucolipotoxic conditions, often caused by overnutrition leading to an increase in oxidative stress, can cause dysfunction of the β-cells and contribute to type 2 diabetes (see Section 3). The redox balance between ROS and the antioxidant system is, therefore, crucial for the proper physiological function of pancreatic β-cells, and thus body glucose homeostasis. Because of the internal redox compartmentalization in β-cells, we have focused on the local sources of ROS and types of antioxidant enzymes in individual cell parts (Figure 1). ROS are primarily produced during mitochondrial oxidative phosphorylation but also originate from other cell compartments and organelles, such as ER, peroxisomes or cytoplasm [10,11]. At a “redox triangle” called redoxosome, which is formed by closely attached membranes of the ER, mitochondria and peroxisomes, redox-regulatory enzymes are thought to assemble. These sense ROS accumulations and redox imbalances, and its enzymes use ROS to transmit intercompartmental signals via chemical modifications of downstream proteins and lipids [12]. Under physiological conditions, ROS production in individual compartments is well controlled by specific resident antioxidant enzymes with small differences between rodents and humans.
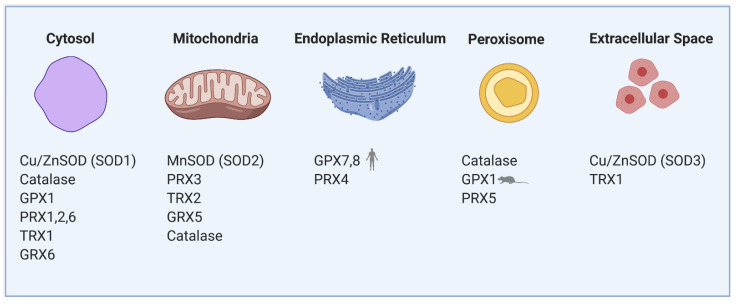
Figure 1.
Compartmentalization of redox regulating proteins in β-cells involves mitochondria, cytosol, endoplasmic reticulum (ER), peroxisomes and extracellular space. SOD—superoxide dismutase, GPX—glutathione peroxidase, PRX—peroxiredoxin, TRX—thioredoxin, GRX—glutaredoxin.
1.1.1. Mitochondria
Mitochondrial electron transport chain (ETC) complexes I and III produce superoxide (O2•−) into the mitochondrial matrix and complex III also into the cytosol (exactly to the intracristal space). Mitochondrial MnSOD (Cu/ZnSOD isoform in the cytosol) transforms O2•− into a more stable molecule H2O2, which is then reduced to water by an antioxidant enzyme GPX, mitochondrial PRX3 or catalase [13]. Under physiological conditions in the presence of glucose, mitochondrial production of ROS is not detrimental to the β-cells. GSIS does not increase mitochondrial ROS production in rodent β-cells [2,14,15]. However, elevated H2O2 derived from superoxide formed by fatty acid oxidation (specifically by electron transfer flavoprotein-ubiquinone oxidoreductase) activates mitochondrial phospholipase iPLA2γ. iPLA2γ-cleaved fatty acids are being utilized by UCP2 to induce mild uncoupling and subsequently reduce respiratory chain-mediated superoxide formation. Such an antioxidant feedback mechanism based on redox signaling initiated by fatty acid oxidation in rodent β-cells might suppress further ROS production and oxidative stress. Also, iPLA2γ-cleaved fatty acids were shown to amplify insulin secretion in mice through the GPR40 receptor [16,17]. This way, mitochondria may help the cells to cope with the nutrient overload.
1.1.2. Endoplasmic Reticulum
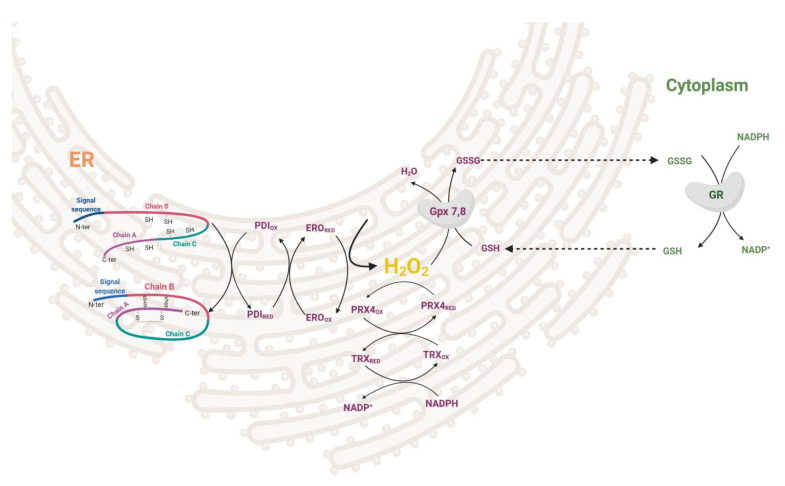
During the maturation of insulin in the ER, three disulfide bonds are formed by disulfide isomerase (PDI). After the formation of the S–S bond, PDI needs to be regenerated to its oxidized form. This is enabled either by ER oxidoreductin-1 (ERO1), which accepts electrons from PDI and donates them to oxygen resulting in the formation of H2O2 molecules or by GSSG-driven oxidation of substrate proteins through PDI (Figure 2). The interplay between the two oxidative pathways that produce or consume ER-luminal GSSG maintains ER redox status (manifested in human HEK293 cell line) [18]. H2O2 is then metabolized by PRX4 in both rodents and humans, together with GPX7/8 in humans [19,20,21,22]. Overexpression of PRX4 in rat INS-1E cells protects against ROS toxicity and increases insulin content by affecting ER protein folding capacity [19]. Redox balance on the ER level affects other membranes of the redoxosome. Long-term overproduction of ROS in ER leads to cell stress, activation of unfolded protein response, β-cell dedifferentiation and subsequently apoptosis (see Section 3.2). The state of protein folding in ER is controlled by chaperones of the Hsp70 and Hsp40 families, chaperone-like proteins, and lectins [23,24]. The key ER members of the Hsp70 family are represented by chaperone immunoglobulin heavy-chain-binding protein, BiP (also called GRP78) [25]. UPR signaling is mediated via three main transmembrane sensors: endoribonuclease inositol-requiring protein 1α (IRE1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6) [26]. When misfolded proteins accumulate in the ER, BiP dissociates from the UPR sensors and binds to the exposed hydrophobic domains of the unfolded proteins [27]. This induces oligomerization and auto-transphosphorylation of IRE1α and PERK, with their consequent activation [28].
Figure 2.
Redox regulation in ER during proinsulin folding. Disulfide bonds are formed by disulfide isomerase (PDI) together with ER oxidoreductin (ERO) oxidoreductases while giving rise to H2O2. This can be attenuated by PRX4/TRX or GPX7,8/GSH redox couples in conjunction with cytosolic redox status. PDI—disulfide isomerase, ERO—ER oxidoreductin, PRX—peroxiredoxin, TRX—thioredoxin, GPX—glutathione peroxidase, GSH—glutathione.
1.1.3. Peroxisomes
In peroxisomes, ROS are produced mainly during the β-oxidation of long-chain polyunsaturated fatty acids. Because of the weak antioxidant defense of peroxisomes in both rodents and humans, β-cells are unable to endure long-term peroxisomal stress. Catalase expression is rather suppressed to allow specific β-cell function [5,6]. Also, Prx5 is weakly expressed [20]. Thus, weak detoxifying machinery in peroxisomes leads to uncontrollable ROS production in lipotoxic conditions. However, the peroxisomal loss causes mitochondrial deterioration and cytoplasmic vacuolization in β-cells impairing glucose tolerance [29].
1.1.4. Cytoplasm and Membrane Rafts
In our previous research on mice [2], we have shown that after glucose uptake, the ROS production arises mainly in the cytoplasm, where specific H2O2 producers are localized: NADPH oxidases (NOX) and xanthine oxidases (XOD) [30,31,32]. These enzymes have an important role in the physiology but also the pathophysiology of pancreatic β-cells. Under physiological conditions, the cytoplasmic antioxidant system is fully sufficient to maintain the redox balance. During GSIS, the levels of NADPH and mitochondrial FADH2 are increased, NAD(P)H-dependent ROS production is stimulated, and detoxifying enzymes (NOX, XOD and PRX/TRX/TRXR system, respectively) are activated. The primary cytoplasmic antioxidants are PRX1, PRX2 [33] and, to a lower extent, also Cu/ZnSOD, catalase and GPX1 [5,6,11,20] (Figure 1). Thioredoxin interacting protein (TXNIP) was suggested to increase oxidative stress by binding TRX and inhibiting its reducing activity [34]. Upregulated by glucose, TXNIP regulates β-cell antioxidant defense and redox homeostasis. Carbohydrate response element-binding protein (ChREBP) participates in TXNIP expressional regulation, thus linking redox balance to glucose sensing through the ChREBP–TXNIP–TRX axis both in humans and rodent β-cells [35]. Besides TRX, many other proteins were suggested to be the binding targets to the TXNIP protein; thus, TXNIP may be a scaffold protein important in redox signaling [36]. As discovered on human cancer cell line MCF-7, one of TXNIP’s binding partners is importin-α, which allows the complex to directly enter the nucleus [37]. TXNIP overproduction during long-time hyperglycemia has a deleterious effect on β-cell function also through controlling microRNA (miR) expression. Through upregulation of miR-204, TXNIP downregulates expression of V-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA), one of the major insulin transcription factors [38]. Induction of another miR-124a expression elevates the production of islet amyloid polypeptide, further aggravating the Langerhans islets pathology [39].
1.2. ROS Signaling Pathways and Transcription Factors in β-Cell Physiology
The exact mechanisms by which ROS (mainly H2O2) serve as signaling molecules and metabolic coupling factors in well-functioning β-cells are still not fully understood. However, there is strong evidence of ROS importance in proper β-cell function [40]. It was found that administration of exogenous H2O2 and enhancing intracellular H2O2 levels increases insulin release in rat INS-1 (832/13) cells and both rat and mouse islets [41,42,43]. On the contrary, the application of exogenous antioxidants inhibits GSIS [43]. How could H2O2 (or other ROS) mediate its signaling effects with the antioxidant system present in the cell? In general, three main mechanisms of H2O2 function have been proposed. First, there may be strong localized intracellular H2O2 gradients that enable local H2O2 signaling despite the mean cytoplasmic concentration being low. Second, PRX may be locally transiently inactivated during signaling, allowing H2O2 levels to increase. Third, the action of H2O2 may be mediated by redox relays whereby PRX and/or glutathione peroxidases and catalase to less extent act as initial H2O2 sensors and then oxidize the end target proteins [44]. Redox sensing and signaling within the cells are mediated via reversible disulfide-dithiol exchange reactions and de-nitrosylation of Cys residues. The process is enzymatically controlled by members of the thioredoxin family comprising over 50 proteins, including TRX, PRX, PDI, glutathione-dependent glutaredoxins (GRX), GPX and glutathione-transferases [45]. The enzymes interact with many distinct proteins affecting cellular functions like gene expression [46], regulation of intracellular H2O2 levels [33], proliferation [47], apoptosis [48] and modulation of the immune response [49].
1.2.1. Redox Sensitive Signaling Pathways
Several cellular pathways were shown to be affected by redox status in β-cells. Signaling pathway KEAP1–NRF2–ARE, in general, maintains the cellular redox balance and induces adaptive response to oxidative stress [50]. This pathway consists of three main components: Kelch-like ECH-associated protein 1 (KEAP1), nuclear factor erythroid 2-related factor 2 (NRF2), and antioxidant response elements (ARE). Under physiological conditions, KEAP1 is associated with NRF2, and the ubiquitination of NRF2 is stimulated. However, under oxidizing conditions, ROS promote the dissociation of KEAP1 and NRF2, enabling NRF2 to transfer to the nucleus, where it binds to ARE and enhances the expression of detoxification enzymes, such as glutathione synthetase, glutathione reductase, GPX, TRX, TRXR and PRX to prevent oxidative stress [50]. Elevated NRF2 activity has a positive effect on β-cell function during glucolipotoxicity in mice [51]. However, under physiological conditions, insulin release is reduced after activation of NRF2 [51]. Chronic H2O2 treatment is known to activate KEAP1–NRF2–ARE signaling to promote the production of pro-apoptotic factors and leads to apoptosis of human pancreatic β-cells [52]. ROS were also shown to activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. NF-κB, when activated, translocates to the nucleus and works as a transcription factor that affects many cellular processes. This pathway can influence the redox status by increasing the expression of antioxidant proteins, such as Cu/ZnSOD, MnSOD, GPX, etc. [50]. However, NF-κB activation in pancreatic β-cells has mostly deleterious effects and contributes to the loss of differentiated β-cell functions and leads to cell death [53,54,55] (more in Section 3). Another major intracellular signal transduction pathway that plays an important role in various cellular processes is the mitogen-activated protein kinase (MAPK) cascades. This includes the extracellular signal-regulated kinase (ERK) pathway, the c-Jun N-terminal kinase (JNK) pathway, the p38 kinase pathway and the big MAP kinase 1 (BMK1/ERK5) pathway. ROS were reported to activate these pathways under physiological and pathophysiological conditions of β-cells [50,56].
1.2.2. Redox Regulation of Transcription Factors Essential for β-Cell Maturation
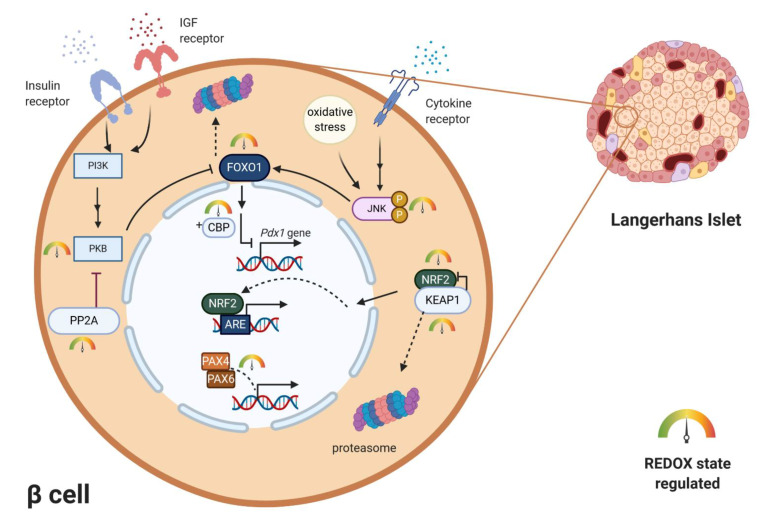
For a long time, activation of gene transcription has been considered to be primarily, if not exclusively, regulated by cascades of protein phosphorylation and dephosphorylation [57]. The concept that transcription might be controlled by redox reactions emerged when an important eukaryotic transcription factor–NF-κB–was found to be activated by oxidants and inhibited by antioxidants [57,58,59]. Several theories how oxidants could affect transcription were suggested—oxidative inactivation of phosphatases in signaling cascades, direct oxidation of transcription factors, activation of protein kinases, redox-dependent noncovalent binding of thioredoxin, thiol modifications of proteins that form cytosolic complexes with transcription factors, or heterodimer formation of GPX and PRX-type peroxidases with transcription factors. In the mature pancreas, transcription factors play a role in achieving glucose homeostasis by regulating the expression of key genes, most notably the insulin gene [60]. Mature β-cells of all species express high levels of transcriptional factors. The main ones include Pancreas/duodenum homeobox protein 1 (PDX1), MAFA, homeobox protein NKX6.1 and transcription factor neurogenic differentiation (NEUROD). All of these transcription factors were shown to be downregulated by persistent ROS in rodents and human β-cells [3]. However, these changes occur under pathophysiological conditions of oxidative stress and often lead to cell damage. PDX1 is essential in the development of β-cells and also binds to the regulatory elements and increases insulin gene transcription and several other islet-specific genes like glucose transporter2 (Glut2) or Nkx6.1 [60]. It contributes to β-cell mass expansion and glucose metabolism induced by activation of protein kinase B (PKB/Akt) signaling [61]. The activity of PDX1 was shown to be reduced by oxidative stress [62,63]. Another critically important activator of insulin gene transcription, MAFA, is sensitive to proteasomal degradation under conditions of oxidative stress in rodents [64,65]. The major regulator of MAFA degradation under oxidative stress is p38 MAPK, which directly binds to MAFA and triggers MAFA degradation via the ubiquitin proteasomal pathway. Inhibiting MAFA degradation under oxidative stress in rodents ameliorates β-cell dysfunction [66,67]. Reduction of intranuclear MAFA levels was observed in diabetic db/db mice. This adverse effect was prevented by β-cell-specific GPX1 overexpression, which induced β-cell antioxidant protection [68]. Both of the above-mentioned transcription factors are regulated by forkhead box O protein 1 (FOXO1) (). FOXO1 belongs to the FOXO family, which has an important role in many cellular processes, including resistance to cellular oxidative stress itself, in cellular metabolism and insulin signaling [69]. FOXO can sense ROS levels by oxidation of its cysteines and then regulates the activity of transcription factors. In humans, ROS induces the formation of cysteine-thiol disulfide-dependent complexes of FOXO and the p300/cAMP-responsive element-binding protein (CREB) binding protein (CBP) acetyltransferase, thus modulating FOXO biological activity [70]. Multiple FOXO connected pathways have been shown to be subject to regulatory cysteine oxidation [71]. The insulin and insulin-like growth factor (IGF) signaling pathways inhibit FOXO activity by activation of phosphoinositide 3-kinase (PI3K) and subsequently PKB [72] (Figure 3). Studies performed mainly on rodents showed that phosphorylation of FOXO by PKB causes FOXO inactivation and its export from the nucleus [69,71]. Under oxidative stress, H2O2-induced oxidation of PKB leads to the transformation of its Cys297 and Cys311 into intramolecular disulfide bonds [73,74], dephosphorylation of PKB and an increased association with protein phosphatase 2A (PP2A) (Figure 3). The higher affinity of PKB to PP2A leads to faster inactivation of this kinase, which enables nuclear localization of FOXO [71]. Interestingly, activated FOXO1 negatively regulates Pdx1 expression during oxidative stress in βTC-3 cell culture and mice [75]. It was also discovered that under oxidative burst, FOXO1 can bind to promyelocytic leukemia protein (PML) and NAD-dependent deacetylase sirtuin-1 (SIRT1) forming a single complex with a slightly inverse effect. This complex upregulates Ins2 gene transcription factors MAFA and NEUROD expression [76]. MAFA has an important role in β-cell development and replication. Shift from MAFB to MAFA expression during early development in β-cells has a significant role in β-cell maturity determination, replication and survival (reported on rodent model) [77]. This way FOXO1 in the short-term protects β-cells against oxidative stress and tries to preserve their proper function [3,76]. More investigations have to be made to clarify this dual effect of FOXO on β-cells. Apoptosis signal-regulating kinase 1 (ASK-1) is also activated in a redox-dependent manner. ASK-1 activates JNK, which leads to phosphorylation of FOXO and prevention of its export from nucleus [71]. Family of paired-homeobox genes (PAX) proteins (paired-homeobox genes) being crucial for islet development also occurs to be sensitive to redox regulations. PAX8 loses its ability to bind DNA molecules after oxidation of its cysteines Cys45 or Cys57. These cysteines are conserved in all Pax members, so the redox regulation occurs also in human and rodent β-cells, where PAX4 and PAX6 are present [78,79,80].
Figure 3.
Examples of redox regulation of specific transcription factors. Pdx1 gene is negatively regulated by redox-sensitive transcription factors FOXO and CBP. Localization and transcription activity of FOXO is negatively regulated by PI3K-PKB/AKT signaling and positively regulated by the JNK signaling pathway. Activated by oxidative stress/cytokine stimuli, JNK enables FOXO nuclear localization. Concurrently redox-sensitive PKB is inhibited by H2O2-induced intramolecular disulfide bond formation. Redox sensitive NRF2/KEAP1 system regulates expression of key antioxidant enzymes upon ARE region. Under oxidizing conditions, the cytoplasmic complex dissociates, NRF2 enters the nucleus and functions as a transcription factor. Redox-sensitive cysteines in PAX transcription factors directly regulate their DNA-binding ability. ARE—antioxidant response elements, CBP—p300/cAMP-responsive element-binding protein (CREB)-binding protein, FOXO—forkhead box O protein, IGF—insulin-like growth factor, JNK—c-Jun N-terminal kinase, KEAP1—Kelch-like ECH-associated protein 1, NRF2—nuclear factor erythroid 2-related factor 2, PAX4/6—paired-homeobox genes, PDX1—pancreas/duodenum homeobox protein 1, PI3K—phosphoinositide 3-kinase, PP2A—protein phosphatase 2A, PKB—protein kinase B.
1.3. Redox Regulation of β-Cell Differentiation and Proliferation
1.3.1. Redox Regulation of β-Cell Differentiation
During differentiation of β-cells from embryonic stem cells, strictly controlled repression or activation of specific transcription factors and genes that manage the transition occurs. During the embryonic days 8.5–12.5, expression of specific transcription factors is activated: Sox17, Pdx1, Hb9, Isl1, Hnf1β, Foxa2, Hnf6, Gata4 and Gata6. Between embryonic days 12.5–16.5, other transcription factors are activated: Ngn3, NeuroD1, Pax4, Pax6, Hes1, Hnf6, Sox9, Ptf1a, Rfx3, Rfx6, Isl1, Arx, Nkx2.2, Nkx6.1, Glis3, MafA and MafB [81,82,83,84]. Mutations of these genes and their deficiency during early embryonic days lead to reduced β-cell mass, hyperglycemia and development of diabetes mellitus in rodents [84]. Moreover, new approaches in the treatment of diabetes rely on the conversion of differentiated adult cells or progenitors into β-cells in a process called cellular reprogramming and the promotion of their further proliferation [85]. Identifying and understanding key regulators specific for the development and identity of β-cells is, therefore, necessary. Redox signaling was suggested to be one of these regulators as treatment of pancreatic progenitors by low ROS levels leads to β-cell formation and its viability increase [86,87]. In murine embryos, H2O2 production positively correlates with β-cell formation. Moreover, NOX4 production of H2O2 stimulates the differentiation of β-cells progenitors accompanied by positive effects on Sox9 and Ngn3 gene expression in vivo and in vitro. Low levels of H2O2 promote the expression of differentiation markers Ngn3 and Nkx6.1 and markers of maturation [88]. On the contrary, reducing H2O2 concentration leads to a decrease in β-cell development, viability and proliferation [86,89] and overexpression of catalase, specifically in mice mitochondria of β-cells leads to a significant reduction in β-cell proliferation and volume [87]. β-cell differentiation is mediated probably through modulation of the ERK1/2 signaling pathway, which controls proliferation, differentiation and also phosphorylation and protein levels of CREB [89,90]. CREB, together with its coactivator CBP participates in β-cell gene expression and proliferation. In mutated mice and isolated islet cultures, constitutively activated cAMP signaling and enhanced CREB–CBP binding leads to an increase in proliferation and doubling β-cell mass [91]. The human SOX9 proximal promoter is also regulated by the CREB in other cell types [92]; thus, the positive effect of H2O2 from NOX4 on Sox9 and Ngn3 genes could be connected to the activation of the ERK1/2 signaling. In other cell types, the influence of H2O2 on the activation of signaling pathways connected to β-cell proliferation was discovered: PI3K/PKB pathway [93] and WNT signaling pathway [94].
1.3.2. Adipokines Stimulate β-Cells Proliferation through Redox Signaling
Hormones leptin and adiponectin are secreted by adipose tissue and play a major role in the homeostasis of the organism and β-cell condition. They control body weight, food intake and overall metabolism [95,96]. Many studies have been performed to elucidate the effects of leptin and adiponectin on β-cells; however, results have been controversial [96]. There is a negative feedback between insulin and leptin secretion. Insulin promotes leptin release, and leptin then inhibits insulin production and secretion [97] by opening the KATP sensitive channels [98] and inhibiting protein phosphatase 1 [99], important in vesicle docking and exocytosis (well discussed in [100]). The effect of adiponectin on insulin secretion seems to rely on glucose concentration. Studies on rodent and human models showed that adiponectin does not affect basal insulin secretion; however, it stimulates insulin secretion at high glucose [96,101]. Adiponectin improves insulin sensitivity in target tissues due to the suppressive effect on gluconeogenesis and positive influence on glucose uptake and fatty acid oxidation in muscle and liver [96]. However, the exposition of β-cells to elevated levels of these adipokines leads to a significant increase in their proliferation and viability. This is due to the increase in NOX activity and thus an increase in ROS production, which was confirmed by using exogenous antioxidants. The positive effect of adipokines on ROS elevation is accompanied and supported by a decrease in the Cu/ZnSOD expression and Cu/ZnSOD, GPX and catalase activity in rodents [86].
1.3.3. ROS-Mediated Ca2+ Signaling in β-Cell Proliferation
Due to a direct effect of ROS on redox-sensitive Ca2+ channels [11,102], ROS-mediated intracellular rise in Ca2+ concentration can lead to activation of calcineurin, which potentiates both human and rodent β-cell proliferation [103,104]. Calcineurin phosphorylation of the Transducer of regulated CREB activity-2 (TORC2) leads to its translocation into the nucleus and association with other transcription factors (CREB, cAMP-responsive element modulator (CREM) and cyclic AMP-dependent transcription factor ATF-1 (ATF1)) leading to enhanced expression of cell-cycle-activating genes [103]. At low nutrient levels, when ROS production and Ca2+ signaling in β-cells are generally low, AMPK kinase member microtubule affinity regulating kinase 2 (MARK2) is activated. MARK2 has an opposite effect on TORC2 than calcineurin; thus, starvation blocks β-cell proliferation [103].
2. Redox Homeostasis in Optimum β-Cell Function
2.1. Redox Signaling in Insulin Secretory Pathway
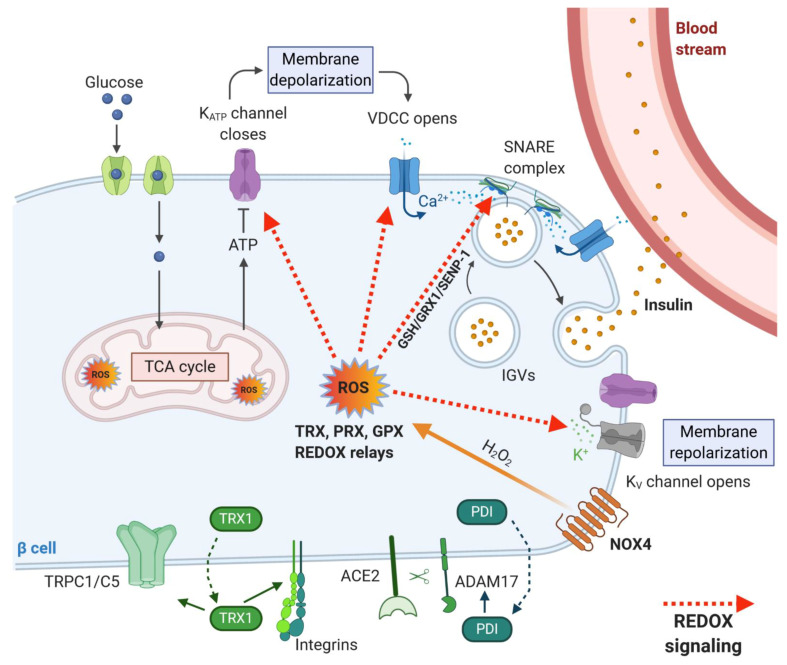
β-cells can adapt the rate of insulin secretion to the plasma concentration of glucose and other nutrients by a unique coupling system between nutrient metabolism and insulin production. The coupling system involves accelerating glycolysis, and mitochondrial metabolism with rapid changes in the oxidation state of several redox couples, e.g., NADH/NAD+ and NADPH/NADP+ and is also directly linked to ROS production as mentioned above. Upon higher substrate load, the demand for oxygen supply to the cells grows as well. At such a high metabolic activity, the oxygen demand exceeds oxygen supply, resulting in the establishment of intracellular transient hypoxia [105]. Such features have been described, for example, in neurosecretory cells, which require high mitochondrial activity and ATP production to restore resting membrane potential and to maintain intracellular Ca2+ levels [106] and also in working skeletal muscle cells, in which increased ATP demand is reflected by mitochondrial biogenesis and elevated oxygen consumption resulting in expression of hypoxia-inducible genes [107]. Hypoxia could serve as another coupling factor via enhanced ROS production for insulin secretion in β-cells. Moreover, the beneficial effect of hypoxia in healthy β-cells could be attributed to the biogenesis of the mitochondrial respiratory chain proteins [107] and effective calcium signaling [106], which are inevitable for the maintenance of mature β-cells. It was also suggested that the basal activity of hypoxia-induced pathways is necessary for normal β-cell function [108]. An interesting study by Olsson and Carlsson [109] has shown that oxygenation may differ widely between individual islets within the pancreas at a given time point. These differences may reflect a mechanism to recruit only a fraction of the available islets into an active (normoxic) β-cell mass. The remaining less well-oxygenated (hypoxic) islets may represent a dormant subpopulation, constituting a functional reserve of endocrine cells. According to this model, the reserve islet pool may be available for recruitment upon reduction of the total islet mass [109]. In any case, the importance of redox signaling in GSIS machinery was also documented by our research group, which showed recently that insulin secretion is inhibited upon deletion of constitutively active NOX4 enzyme in rodent β-cells in vivo. Our results demonstrated that the sole increase in ATP/ADP ratio is not sufficient to stimulate insulin secretion in vitro and in vivo. We thus confirmed that the presence of ROS, namely H2O2 as a metabolic coupling factor, is essential for insulin secretion [2]. Many targets of redox signaling in the insulin secretory pathway were suggested (Figure 4); however, a more detailed/complex view is still missing. KATP channel with its essential function in GSIS [110] mediating depolarization of plasma membrane was shown to be inhibited by H2O2 in smooth muscle cells [111], but no such regulation has been observed in β-cells. Also, another depolarizing transient receptor potential (TRP) channel subfamily M member (TRPM2) was reported to be directly redox activated [112]. The subsequent repolarizing events on the plasma membrane seem to be potentially redox-regulated as H2O2 could directly or indirectly inhibit repolarizing K+-channels, such as KV [113,114,115]. Besides redox targets of signaling pathways mentioned above, downstream aims in the secretory pathway were found to be, for example, redox-sensitive proteins of calcium signaling, e.g., ryanodine receptor 2 (RyR2), sarcoplasmic Ca2+ ATPase (SERCA) and inositol triphosphate receptor (IP3R) [11,102]. The glucose-induced changes in the redox environment affect these proteins, leading to increased exocytosis of insulin secretory vesicles. Further, proteins of the secretory machinery were also implicated to be redox-regulated. For example, redox-regulated posttranslational modification of exocytosis-regulating t-SNARE proteins has been proposed to result in an increased rate of maturation, or priming, of secretory vesicles in yeasts (for an overview, see [116]). mRNA/protein expression studies and electrophysiological analysis of exocytosis suggested that both the expression level of Grx1 and Trx1 in the β-cell as well as the NADPH/NADP+ redox status are important factors for the regulation of exocytosis [117]. Redox-dependent regulation was suggested for the NADPH–GSH–GRX1 signaling axis in rodent β-cells [118], and GRX1-GSH was shown to mediate deSUMOylation of sentrin/SUMO-specific protease 1 (SENP1) in humans [119] via modulation of its key thiol groups [120]. SUMOylation/deSUMOylation processes were previously reported to be redox-regulated and were implicated to be involved in response to oxidative stress [121]. Moreover, SUMOylation of insulin secretory pathway proteins had an inhibitory effect on insulin granule exocytosis, and several proteins involved in insulin granule exocytosis were shown to be modified by SUMOylation [121]. However, it is tempting to speculate that the redox signal mediated by the thiol exchange is transformed at the exocytotic site to signal mediated via SUMO posttranslational modification.
Figure 4.
Redox targets within insulin secretory pathway and proteins involved in extracellular redox signaling. Glucose entry through GLUT1 in humans or GLUT2 in rodents causes an increase in ATP/ADP ratio and elevation of ROS production in the cytoplasm (mediated by NOX4). Subsequently, the KATP channel closes, which causes depolarization of the plasma membrane, the opening of voltage-dependent calcium channels (VDCC) and Ca2+ entry into the cells. Ca2+ is required for insulin granule vesicles (IGV) trafficking, attachment and fusion with the plasma membrane (mediated by SNARE proteins) and finally, secretion of insulin from the cell. Repolarization of the membrane is mediated via voltage-gated K+ channels (KV). These repeating cycles lead to a high-frequency of action potential spikes and synchronized oscillations in the cytosolic Ca2+. ROS are utilized by redox-regulated proteins TRX, GPX and PRX, which can either neutralize the ROS action in the long-term or transform acute redox signal via redox relays. Targets of redox signaling involve KATP channels, VDCC, KV channels and proteins involved in granule exocytosis. TRX1 and PDIs are secreted from β-cells, and they interact with extracellular domains of transmembrane proteins. PDIs regulate the action of sheddase ADAM17, and TRX1 modifies the activity of integrins and TRPC1/5 channels.
2.2. Extracellular Redox Signaling in β-Cell Function
It was shown that many oxidoreductases could be secreted from the cells to the extracellular milieu as a response to changes in the cellular environment [122]. The involved secretory pathways differ based on secreted enzyme and cell type. GRX1 is secreted by human T-cells, B-cells and NK-cells of the immune system [123]. Different PDIs are secreted by platelets playing a crucial role in thrombosis [124]. PRXs were detected to be secreted by macrophages and HEK297 cells [125]. TRX1 is secreted by many different cell lines, including human fibroblasts, epithelial cells, cells of the immune system, hepatocytes and cancer cells [126]. Interestingly, current research revealed secretion of cytoplasmic isoforms of TRX1 and TRXR1 by murine and porcine pancreatic β-cells during hypoxia and inflammation [126]. Such phenomenon was originally described in immune cells and presence of TRX1 was detected in blood of patients suffering from rheumatoid arthritis [127] and diabetes [128]. Extracellular TRX1 seems to cause autocrine or paracrine regulation of the mouse β-cells as recombinant TRX1 injected to mice with transplanted pancreatic islets significantly improved cell viability and improved blood glucose control, while overexpression of TRX1 in grafted islet cells did not lead to such effect [129]. In other experiments extracellular TRX1 had beneficial effect on murine and porcine β-cells by preventing apoptosis and preserving insulin secretion [126]. It is also possible that binding of TXNIP to TRX1 could negatively affect its secretion and subsequent extracellular actions. It is highly likely that except from TRX1 β-cells secrete also other oxidoreductases and that all these enzymes contribute to extracellular redox signaling towards other endocrine cells. More experimental evidence in this field will definitely reveal remarkable findings in future.
Until now, there is only a very limited number of transmembrane proteins, which were described as targets of extracellular redox modifications by secreted oxidoreductases. PDIs were pointed out as inactivators of ADAM metallopeptidase domain 17 (ADAM17) shedding activity [130,131,132]. ADAM17 is a disintegrin and metalloproteinase that participates in the cleavage of surface proteins. An increase in ADAM17-mediated shedding activity and decrease in its substrate angiotensin-converting enzyme 2 (ACE2) have been observed with the progression of type 2 diabetes mellitus (T2D) [133], suggesting the importance of this mechanism in the disease. Indeed, restoration of ACE2 improved glycemia in db/db and Ang-II-infused mice. The beneficial effects of ACE2 could be attributed to reduced oxidative stress and ADAM17 expression in the islets of Langerhans, in addition to the improvement of blood flow to the β-cells [134]. It seems probable that the regulation mediated by the PDIs–ADAM17–ACE2 axis may play an important role in sensing the redox environment in the β-cells and their surrounding, resulting in signaling events leading to maintenance of the proper β-cell functionality.
Another group of surface proteins described as targets of TRX1 and PDIA6 are the integrins [135,136]. Integrins are transmembrane heterodimer receptors for extracellular matrix (ECM) components. They are expressed on all cell types and composed of one α and one β subunit. Integrins affect a variety of cell processes, including adhesion, migration, differentiation, and cell growth. In mammals, 24 α and 9 β subunits have been identified, which combine to create 24 unique heterodimers [137]. Both the α and β subunits are involved in the determination of ligand specificity; however, the β subunit facilitates adhesion and activates intracellular second messenger cascades [138]. Most notably, stimulation of the β1 integrin leads to activation of focal adhesion kinase (FAK), ERK, PKB, and the steroid receptor coactivator (SRC) family of kinases, all of which are involved in cell survival. Moreover, integrins also mediate crosstalk with growth factor receptors. The signals that are activated by the integrin–FAK–SRC complex can be integrated with signals from growth factor receptor binding to subsequently activate the RAS–MAPK kinase–MAPK pathway, which is canonically associated with cell proliferation and survival [139]. Ligands for integrins include fibronectin, laminin, collagen, fibrinogen, and thrombospondin [137]. Regulation of disulfide formation and cleavage by TRX1 and PDIs could, therefore, affect β-cell growth, proliferation and survival- processes mediated by integrins.
The last group of redox-regulated transmembrane proteins that must not be forgotten are TRP channels. They comprise six divergent subfamilies TRPV, TRPC, TRPM, TRPA1, TRPP and TRPML, with diverse biophysical properties and functions. The TRP channels have a common structure of homo- or heterotetramers with six transmembrane spanning regions and intracellular N- and C-termini. TRP channels are sensitive to changes in the microenvironment, including heat, osmotic pressure, mechanical force and changes in the redox environment of the cells. They were found to be sensitive to redox modifications (for an overview, see [112]). It has been reported that the extracellular reduced thioredoxin activates homomeric TRPC5 and heteromeric TRPC5–TRPC1 channels by breaking a disulfide bridge in the predicted extracellular loop next to the ion-selectivity filter of TRPC5 [140]. It has been shown that TRPC5 and TRPC1 are expressed in secretory fibroblast-like synoviocytes from patients with rheumatoid arthritis, whose extracellular concentration of thioredoxin is high. The endogenous TRPC5–TRPC1 channels of the cells are activated by reduced thioredoxin, and the blockade of the channels enhances the secretory activity and prevents the suppression of secretion by thioredoxin. As patients with diabetes also have high levels of extracellular TRX1, one can predict that a similar mechanism also exists in pancreatic β-cells. It was also documented that activation of multiple subtypes of TRP channels results in enhanced insulin production by β-cells, and redox signal causes activation of the channels [141].
3. Dysbalanced Redox Homeostasis Contributing to β-Cell Dysfunction under Metabolic Stress
3.1. Redox-Linked Transition to Dysfunctional β-Cell in T2D Pathology
Chronic overnutrition and a sedentary lifestyle are currently increasing the incidence of T2D. As previously stated, glucose metabolism transiently increases oxidative status in β-cells required for efficient insulin secretion; however, long-term substrate pressure modulates redox homeostasis towards persistent prooxidative as β-cells show low detoxifying capacity. Actually, classical antioxidant machinery (catalase, glutathione peroxidases) is compensated by peroxiredoxins, thioredoxins, haem oxygenases, metallothioneins, etc., also having a signaling role [142]. To emphasize the role of redox status in the physiology and pathology of β-cells, we searched the GEO Datasets database for potential redox genes that are dysregulated in patients with type 2 diabetes compared to normal healthy individuals. The gene expression datasets of GSE20966, GSE25724, GSE26168, GSE38642 were incorporated. These datasets include expression profiling from array data from type 2 diabetic and non-diabetic isolated human (both males and females) islets and β-cell-enriched tissue obtained by laser capture microdissection. We revealed nine “redox” genes that were either up-or downregulated in the patients suffering from T2D (Table 1), further validating the importance of the cell redox balance in maintaining the precise physiological function.
Table 1.
Dysregulated genes of redox-regulating proteins in type 2 diabetes mellitus (T2D) patients.
| Gene Symbol | Protein Name | Regulation | Dataset | Reference |
|---|---|---|---|---|
| TXN1 | Thioredoxin 1 | Up | GSE38642 | [237,238,239,240] |
| TXNR1 | Thioredoxin reductase 1 | Up | GSE26168 | [241] |
| TXNIP | Thioredoxin-interacting protein | Down | GSE26168 | [241] |
| PRX2 | Peroxiredoxin 2 | Up | GSE25724 | [242] |
| PRX4 | Peroxiredoxin 4 | Down | GSE25724 | [242] |
| PRX5 | Peroxiredoxin 5 | Up | GSE26168 | [241] |
| PRX6 | Peroxiredoxin 6 | Up | GSE38642 | [237,238,239,240] |
| GPX1 | Glutathione peroxidase 1 | Up | GSE26168 | [241] |
| SOD2 | Superoxide dismutase 2, mitochondrial | Up | GSE26168 | [241] |
Overnutrition leading to chronically overcharged metabolism impacts the insulin biosynthetic pathway, where ER overload and activation of the unfolded protein response pathway (UPR) are affected. Metabolism of β-cells adjusts to higher supply and fuel availability, increasing operating flux capacity and changing characterization of mitochondrial function and overall metabolism. Redox systems then adapt and respond to these changes by altered intracellular redox communication among pyridine nucleotides, ROS and thiols [143]. Rapid/chronic responses to nutrient intake can be observed further through the blood redox metabolome, thus exceeding intracellular compartments [143]. In parallel, a nutritional surplus is stored in peripheral tissues, mainly adipose tissue, which, when its capacity is exceeded, starts to be stored in other peripheral tissues, such as liver, muscle, etc., soon establishing insulin resistance. Precipitous increase of fat mass is accompanied by altered secretion of adipokines contributing to the reinforcement of systemic inflammation. The pro-inflammatory environment shows prooxidative behavior, further contributing to β-cell dysfunction. Information within cells is shared via the common metabolic cofactors, which are used by many pathways and enzymes [144]. Systemic inflammation/metainflammation, along with local proinflammatory activity in the pancreas, white adipose tissue, etc., is the key pathological determinant of T2D etiology. Here, otherwise, positive immunomodulators, such as macrophages, production and activity of IL1β cytokine and gut microbiota composition switch their activity under chronic nutrient conditions to proinflammatory, which further harmfully targets β-cells. The physiological activity of β-cells then starts to lose its identity, its function while compensating for insufficient insulin secretion by increasing β-cell mass. Later, β-cell metabolism trying to keep glucose homeostasis switches from hyperfunction to hypofunction. They continue to dedifferentiate into less mature β-cells, general endocrine cells or bi/multi-hormonal endocrine cells, thus reducing their β-cell mass where in late-stage of T2D or in parallel β-cells are significantly eliminated by cell death. Altered redox environment may affect not only individual β-cells, but also communication channels between individual β and other endocrine cells in pancreatic islets leading to desynchronization of β-cell signaling in islets and suppression of effective insulin secretion.
3.2. Affected Redox Homeostasis Impacts Insulin Biosynthesis in β-Cells
Extensive insulin biosynthesis requires robust ER, which produces more than 1 million molecules of insulin per minute in a single β-cell [145]. Upon overnutrition, ER is overloaded, and induced stress activates UPR. UPR then restores folding homeostasis/proteostasis in β-cell experiencing manageable, often temporary, levels of ER stress. Once the threshold is exceeded, e.g., under chronic nutritional conditions, UPR triggers cell degeneration, alters β-cell differentiation and induces apoptosis through inositol-requiring enzyme 1α (IRE1α) kinase/endoribonuclease (RNase) accompanied by ROS production [146,147]. Affected maturation of insulin can reflect the failure of redox homeostasis within the cytoplasm of β-cell being set towards prooxidation. A recent in vitro study showed that mitochondrial antioxidant MitoQ efficiently reduced ER stress (documented by GRP78 and P-eIF2α markers) and proinflammatory NFκB-p65 signaling under hyperglycemic conditions [148].-folding of proinsulin in ER involves the establishment of three disulfide bonds (B7-A7, B19-A20, A6-A11) within the proinsulin chain. Substantially oxidizing equivalents from cytosol are transferred to the ER lumen mediated through ERO1 to PDI and proinsulin, as stated above. Affected cysteine pairing or altered redox ER status can thus cause misfolding and accumulation of toxic aggregation of proinsulin in ER. Proinsulin folding is facilitated by 15–20 PDIs in conjunction with ERO1α/β. The later one then regenerates PDIs for subsequent rounds of disulfide bond generation (more in Section 1.1.2). Disulfide formation in the ER is dependent on the cytosolic NADPH–thioredoxin redox system, which is tightly reflected by changes in glucose metabolism. Furthermore, extensive oxidative folding machinery produces a large amount of ROS and depletes the GSH pool, thus further contributing to redox disbalance towards prooxidation [149]. This is evident from using Akita mouse islets, where expression of the mutant form of proinsulin, which is prone to misfolding, further increase oxidative stress in β-cells [150]. ER stress is also able to induce the activity of TXNIP through the PERK and IRE1 pathway, leading to NLR family pyrin domain containing 3 (NLRP3) protein inflammasome formation and induction of cytokine secretion, thus promoting inflammation [151]. Such a vicious cycle amplifies the disruption of ER function, thus proper insulin maturation and redox homeostasis. It is known that early T2D and prediabetes are accompanied by an accumulation of misfolded proinsulin. Thus, imbalanced redox homeostasis, which otherwise relies on the continuous supply of reducing equivalent, is crucial for the proper function of the β-cell, especially insulin secretion [152]. Another evidence supporting the role of redox status in ER biosynthetic machinery showed that chronic overnutrition and obesity increasing blood pro-atherogenic oxidized low-density lipoproteins (LDL) cholesterol, then initiates oxidatively induced ER stress. H2O2 mimicked oxidized LDL induction of Chop/CHOP and p58IPK/P58IPK (ER stress markers) and was blocked by co-treatment with N-acetyl cysteine (NAC) [153]. Thus, redox homeostasis in β-cells has an impact on insulin biosynthesis, and its disbalance upon chronic nutrient overload accelerates β-cell dysfunction. As a part of the adaptive phase of UPR, crosstalk between ER and mitochondria by calcium fluxes through mitochondria-associated membranes (MAM) sites has been shown to modulate energy consumption, increasing ATP synthesis in mitochondria [154]. Thus, permanent ER stress also affects calcium homeostasis, which can further have an effect on mitochondrial function and derived redox status eventually alters insulin secretion machinery. Moreover, sustained calcium uptake to mitochondria can sensitize β-cells to cell death.
3.3. Adipose Tissue Hypertrophy-Induced β-Cell Dysfunction
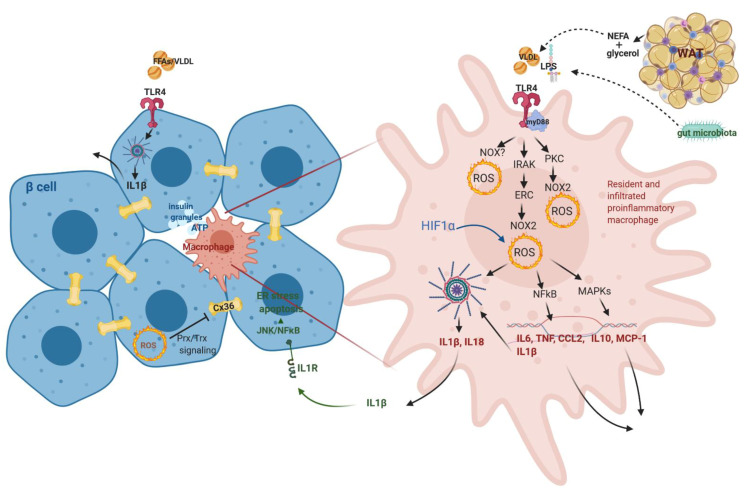
Chronic nutritional overload causes adipocyte hypertrophy and hyperplasia, mainly in white adipose tissue (WAT). Hypertrophic adipocytes have a blunted response to insulin and progressively become more lipolytic, thereby releasing an excess of non-esterified fatty acids (NEFA) to circulation [155]. These compounds together with lipoproteins then reach β-cells and induce their inflammation mostly through toll-like receptor 4 (TLR4) on islet macrophages and β-cells further involving myD88-NF-κB/MAPK signaling pathway along with induction of prooxidative signaling inducing expression and release of TNFα, IL6, IL1β, etc. [156] (Figure 5). The establishment of prooxidative status can be suggested to be regulated through the TLR4-PKC-NOX pathway or TLR4-IRAK-ERC-NOX2 or TLR4-NOX directly [157,158,159] (Figure 5). Moreover, it was suggested that oxidative stress established in adipose tissue in the early to middle stages of obesity is induced mainly by NOX recruiting macrophages to inflamed tissue, while the later stages involve mitochondrial ROS production maintaining the proinflammatory environment and contributing to insulin resistance [160,161]. The inflammatory cellular state and redox homeostasis is also guarded by antioxidant induction through NRF2/KEAP1/ARE pathway [162]. The rapid increase of WAT mass establishes hypoxia, further inducing the production of cytokines, chemokines and other proinflammatory mediators, which are secreted to circulation, maintain a proinflammatory systemic metabolic state characterized by increased C-reactive protein and aberrant cytokine generation. For example, adipose tissue macrophages are the main source of IL6, with an estimated contribution of 15–35% of the total circulating IL6 [163]. Hypertrophic WAT, its chronic inflammation, and ER stress further accelerate the recruitment of immune cells and the production of proinflammatory metabolites through 12/15-lipoxygenases (more in [164]). Altered adipokines production upon chronic overnutrition modulates the levels of leptin and adiponectin, which have an impact on insulin sensitivity, content, secretion and vascular function (for an overview, see [165]). When the storage capacity of WAT exceeds the threshold, ectopic fat deposition takes place. Those can be the liver, pancreas, muscles, etc. Under these conditions, the pancreas was shown to increase triglyceride content in T2D pathology, where lipotoxic pancreatic lipids impair insulin secretion and induce insulin resistance [166].
Figure 5.
Activation of proinflammatory macrophages involves prooxidative signaling. Altered insulin secretion from β-cells signals to activation and differentiation of resident and infiltrating macrophages in islets, the latter one being also activated through TLR4 by lipoproteins from WAT and microbiota-derived metabolites. Activation induces prooxidative metabolism required for inflammasome formation and production of proinflammatory cytokines/chemokines, which target β-cells towards dysfunction. Upon chronic nutritional overload, also β-cells can form inflammasome and secrete IL1β. Gap junctions (composed of CX36) between β-cells of the pancreatic islet are suggested to reflect β-cell redox status and thus regulate islet insulin secretion. TLR4—Toll-like receptor 4, WAT—white adipose tissue, IL1β—interleukin 1 β, CX36—connexin 36.
3.4. Impaired Redox Homeostasis of the Immune System Leading to Β-Cell Dysfunction
3.4.1. Macrophage Role in Langerhans Islets- from Guarding Β-Cell Activity to Its Dysfunction
Resident macrophages (CD11b+Ly6C− or F4/80highCD11clow) in pancreatic islets guard the activity of β-cells [167]. Their depletion in healthy islets from lean chow-fed mice was shown to decrease GSIS [167]. The authors monitor β-cell function through the content of secreted insulin granules containing insulin, ATP, C-peptide and serotonin, being a potent stimulator of immune cells and proliferation and/or regeneration of β-cells [168,169,170]. In the case of acute injury of β-cells or under higher insulin demand, they slowly proliferate and release connective tissue growth factor as a chemoattractant to recruit further macrophages, which then facilitate the recovery/increase of β-cell mass [171]. These are bone marrow-derived macrophages recruited to the site of injury. They, together with resident macrophages, provide signals (e.g., PDGF) driving β-cell hyperplasia to overcome insulin insufficiency adapting to early weight gain and the development of insulin resistance [167]. The main communication molecule was suggested to be ATP as ATP released from over-functional β-cells induces anti-inflammatory cytokine IL10 and matrix metallopeptidase 9 (MMP-9) production from resident/perivascular macrophages in islets through purinergic signaling (Figure 5). Matrix metalloproteinase MMP-9 activity is important for β-cell function, islet vascularization, reduction of cellular inflammation and degradation of islet amyloid. Weitz et al. showed that in obese and diabetic states, purinergic receptor expression in macrophages, thus the response to ATP, is downregulated [168]. Under the pathological condition of T2D, the number of macrophages is increasing where mainly circulating macrophages (CD11b+Ly6C+ recruited from circulating monocytes) are infiltrating into islets, but also F4/80lowCD11chigh proliferating macrophages in situ [156,167,172,173]. CD3+ cells (T lymphocytes) were not found to be significantly increased. Thus obesity-associated islet inflammation is dominated by macrophages, with negligible involvement of adaptive immune cells [167,174]. Their depletion improves GSIS in HFD fed obese mice [156,167,173]. They can be activated through TLR4 by nutritional triglyceride-rich lipids (palmitate, VLDL, etc.), which upregulates intracellular levels of ceramides and potentiate the pro-inflammatory response [175]. Inflammatory macrophages are characterized by significant prooxidative behavior and metabolic adjustment (increased production of citrate, succinate, etc.), leading to the production of many cytokines (TNFα, CCL2, IL6, etc.) where IL1β deserves special interest in respect to β-cell dysfunction since β-cells show very high expression of its receptor in the membrane [176] (Figure 5). Production of mature IL1β involves activation of inflammasome through the AMPK–autophagy–mitochondrial/cytosolic ROS signaling axis [177]. β-cells can also produce IL1β, probably initiating the inflammatory process (see in Section 3.4.2); however, macrophages are seemingly the major source of islet IL1β production in obesity [172,178]. Intracellular impairment of β-cell function by inflammation involves JNK and NF-κB signaling pathways. NF-κB activation in β-cell can, for example, reduce expression of calcium pumps of ER, leading to ER stress and decreased GSIS [179]. JNK activation can lead to suppression of insulin receptor substrate/IRS-PI3K-PKB signaling, which further increases nuclear localization of FOXO1, which decreases binding to PDX1 and thus reduces insulin production and secretion [180,181]. IL1β contributes to insulin resistance through TNFα-dependent and -independent mechanisms [177].
3.4.2. IL1β Dual Role in β-Cell Function/Dysfunction
IL1β signaling is associated with proinflammation in most tissues; however, β-cells show dual effects being integral for cell physiology and inflammation. IL1β is crucial for β-cell activity, as knockout for the receptor for IL1β in vivo cause β-cell dedifferentiation [182], while chronic accumulation or elevated production of IL1β is rather proinflammatory and detrimental to β-cell GSIS. High IL1β concentrations suppress the expression of genes associated with fully differentiated β-cell phenotype and increase the number of bi-hormonal cells in islets [183]. IL1β β-cell dysfunction depends on ER stress (PERK activation), calcium release and JNK signaling [184]. This, together with reduced expression of IL1 receptor antagonist, accompanies T2D pathology [176]. The physiological effect of IL1β on β-cells is evident. The number of peritoneal macrophages increases postprandially, and secreted IL1β induces insulin secretion. This effect depends on the bacterial product (translocation of an ingested or intestinal bacterial product rather than by microbiota itself) and, together with enhanced glucose metabolism and derived prooxidative intracellular status (through TXNIP or PI3K), enable IL1β maturation. IL1β then activates PKB/PI(3)K pathway, glucose uptake via glucose transporter 1 (GLUT1) and insulin secretion [185,186]. Insulin also increases glucose uptake by macrophages, making loopback [187]. However, under chronic overnutrition, long-term upregulation of IL1β leads to elevated insulin levels, which keeps macrophages in the inflammatory state. Early-stage increased insulin plasma concentration might also contribute to low chronic grade inflammation. Insulin preferentially acts on inflammatory macrophages having more expressed insulin receptors, enhanced phosphorylation of PKB and glycolytic activity [187]. In parallel, chronic glucose/nutrient overload of β-cells affecting redox homeostasis leads to activation of inflammasome assembly and further IL1β processing towards mature protein, which is then released (Figure 5). This can recruit further macrophages from circulation and propagate inflammation [167,188]. Inflammasome thus serves as a critical sensor of nutrient overload. The deletion of the NLRP3 inflammasome improves β-cell physiology and viability during oxidative stress and hypoxia that may be associated with anti-inflammatory effects, such as attenuated macrophage islet infiltration [189], however, show probably deleterious effect in physiological conditions.
3.5. Gut Microbiota Supervision of the Immune System
The intestinal microbiota is recognized as a strong immunomodulator. Under the development of the metabolic disease, obesity or T2D microbial dysbiosis is established, associated with the expansion of underrepresented microorganisms (usually opportunistic pathogens) and lower phylogenetic alpha diversity. Such bacteria express proinflammatory behavior (e.g., gram-negative E. coli) at the expense of anti-inflammatory, e.g., Faecalibacterium prausnitzii, Roseburia, etc. [190,191,192]. In obese individuals, proinflammatory bacteria can extract more energy from the diet compared to healthy lean controls [193]. Reduced anti-inflammatory bacteria are linked to disturbed short-chain fatty acid (SCFA) production (such as propionate, butyrate, acetate as the results of microbial degradation of fibers) having an otherwise beneficial effect on host metabolism, especially insulin sensitivity through regulating mitochondrial metabolism of peripheral tissues and thus keeping redox homeostasis [194,195]. The effect of SCFA, which enters the cells through FFA2/GPR43 and FFA3/GPR41 receptors, on β-cells is rather controversial [196]. Nevertheless, SCFA can mimic histone deacetylase (HDAC) inhibitors in β-cells, which promotes their proliferation, differentiation and function [197]. Proinflammatory microbes are then able to get through an intestinal wall as its permeability is increasing based on evidence of suppressed expression of tight junction genes in the intestine under HFD [198]. Proinflammatory bacteria show moreover increased adherence to the epithelium, thus enabling local inflammation [199]. Microbiota regulates metabolic inflammation directly through TLR receptors (mainly TLR4/2) present on cells of the immune system (Figure 5). TLR induced NFκB expression along with induced ROS production activates inflammasome, which induces metainflammation.
3.6. β-Cell Dedifferentiation as the Adaptation to Metabolic and Redox Changes
There is still a dispute whether and at which stage of diabetes pathogenesis insulin insufficiency is the result of the compromised β-cell function (i.e., the amount of released insulin by individual β-cell) and reduced β-cell mass (i.e., the number of β-cell per individual pancreas) and/or combination of both. A recent study on human pancreas tissue slices of control, prediabetic and T2D individuals by Cohrs. et al. proved evidence that the functional deficit of persistent β-cells is more dominant and an early feature of T2D pathogenesis [200]. They showed that early stages of T2D pathogenesis at which subjects exhibited impaired glucose tolerance and a loss of first-phase insulin release but are not insulin resistant and are not yet diabetic exhibit significant functional deterioration and exhaustion, while their β-cell volume is maintained at this stage of disease progression. β-cells within islets were shown not to be uniformly based on their functional activities. Such heterogeneity may be relevant for T2D pathogenesis [201]. Altered redox status was found to contribute to β-cell dedifferentiation upon chronic nutrient overload, mainly through alteration in insulin gene expression [202] through major transcriptional factors PDX1 and MAFA. Transgenic expression of GPX1 restored MAFA expression in db/db mice [68,203] while ebselen (GPX mimetic) established the nuclear expression of PDX1, MAFA in diabetic ZDF rats [204]. Redox-regulated nuclear localization and DNA-binding activity of PDX1 involves JNK signaling and FOXO1 [180,205]. FOXO1 seems to play complex adaptive/deleterious function under stress conditions [83]. Under oxidative stress induced by chronic nutrient overload, it translocates to the nucleus and promotes the expression of MafA and NeuroD1 expression [76], while its somatic deletion in β-cells induces ER stress and dedifferentiation with upregulation of progenitor markers [206]. In severely hyperglycemic db/db mice and insulin-resistant GIRKO mice, FOXO1 is downregulated, and this further affects the expression of MAFA and NKX6.1 [206,207,208]. On the other hand, FOXO1 can stimulate antioxidant protection in the various cellular compartment by expressional induction of antioxidant genes (more in [209]). Thus, FOXO1 is the regulator of cellular redox status and its posttranslational modifications; localization reflects intracellular redox status where detailed induction and signaling in β-cells require further studies [209]. Various subpopulations of β-cells exist differing in responsiveness to glucose, in diverse expression profiles associated with β-cell maturation, glucose metabolism, insulin secretion and pathology of T2D [210]. These subpopulations exist in adult islets and their distribution alters in the process of T2D development. In T2D and long exposure of β-cells to hyperglycemia and metabolic stress, cells tend to differentiate into immature, developmental expression program, which is characterized by low insulin content, poor insulin secretion, gene markers indicating a potential for proliferation [211]. Dedifferentiation is connected to the appearance of forbidden genes, where expressional induction of some of them, such as lactate dehydrogenase (LDH-A) was initiated by oxidative status, either hypoxia-inducible factor (HIF-1) signaling or others [212,213]. Long-term exposure to high glucose due to peripheral glucose intolerance and increased insulin demand induces hypoxia and hypoxia-induced pathways both in cell lines and isolated islets [214]. An increase in hypoxia-related genes, including HIF-1α, vascular endothelial growth factor A (VEGF-A), PAI-1 and LDH-A, was detected in islets of prediabetic Zucker diabetic fatty rats [215], indicating that these pathways are activated during the development of T2D. At normoxia, HIF-1α protein is constantly hydroxylated by prolyl hydroxylases on conserved proline residues [216] and subsequently recognized and degraded by ubiquitination [216]. However, at hypoxia, HIF-1α is no more degraded and dimerizes with the HIF-1β subunit to create a functional dimer, which is transported to the nucleus to execute its transcriptional functions [217]. HIF-1 transcriptional activity regulates a plethora of genes connected with angiogenesis, cell proliferation/survival, and glucose/iron metabolism [218]. One of the main cellular metabolism changes mediated by HIFs is a switch from aerobic to anaerobic energy production. An increase in expression of GLUT1, several glycolytic enzymes, LDH-A and pyruvate dehydrogenase kinase (PDK1) and a parallel decrease in mitochondrial oxygen consumption strengthens the ATP production through the glycolytic pathway [219]. HIF-1α was shown in a recent study to mediate expression of β-cell disallowed genes like Ldha/LDH-A, Glut1/GLUT1, a marker of dedifferentiation aldehyde dehydrogenase 1 family, member A3 (Aldh1a3/ALDH1A3/ALDH1A3) and endocrine progenitor cell marker NGN3 and to suppress the expression of β-cell functional genes like Glut2/GLUT2, Neurod1/NEUROD1, Mafa/MAFA, Nkx6.1 and Pdx1 suggesting that hypoxia-induced pathways are involved in β-cell dedifferentiation [220]. Long-term hypoxia in β-cells results in cell-death, typically by necrosis [221] and also induction of apoptosis-related pathways upon hypoxia exposure has been described in β-cells [222,223]. Recently, a new group of transcription factors called inhibitor of differentiation protein (IDs) was found to be a novel class of oxidative stress-responsive genes in β-cells enhancing antioxidant pathway through NFE2L2-small MAFs pathway [224]. Dedifferentiation and possible transdifferentiation of endocrine cell types were described as the reason for the presence of polyhormonal cells in islets of T2D subjects [225]. Thus, various subgroups of β-cells undergo gradual cell fate transition while adapting the metabolic gene expression profile to function given a particular metabolic status in response to the glycemic and metabolic situation. They adapt to discrete roles within functional islet syncytium [201]. For example, increased substrate pressure and metabolic stress on β-cells caused by overnutrition and low-energy expenditure might stimulate some β-cell subpopulations towards proliferation through FOXO1 signaling in order to adjust to enhanced insulin demand [226]. When the pressure on β-cells becomes chronic, other factors like inflammation are induced, there is no way back, and exhaustion of β-cells leads to their functional deterioration and death evidenced in late-stages of T2D pathology. β-cells seem to be metabolically flexible, and thus, the identification and characterization of β-cell subpopulations in islets represents a powerful tool for targeting treatment of T2D into earlier phases of its development, which might then enhance the success of T2D therapy.
3.7. β-Cell Communication in Islets under Redox Regulation
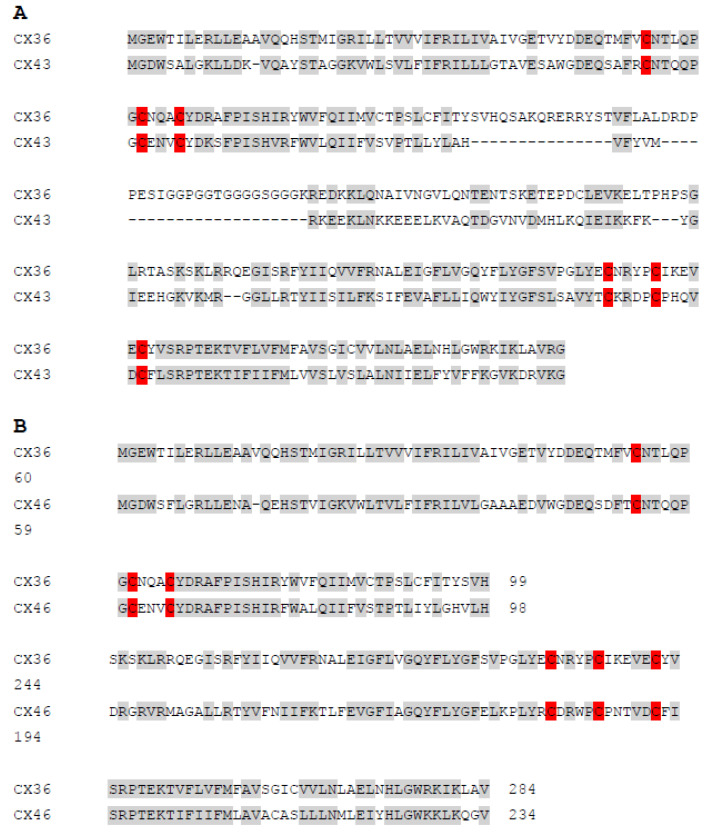
Each β-cell responds to glucose or other stimuli autonomously; however, the interaction among groups of β-cells was shown to be tightly controlled and contributes to efficient insulin secretion upon stimuli induction. Involvement of interaction with other endocrine cells will further orchestrate tight control of insulin secretion. Although the spatiotemporal distribution of β-cells with other endocrine cells in pancreatic islets differs between rodents and humans, β-cells always assemble to groups of various numbers, and their proper communication is a prerequisite for insulin release [227]. β-cells exhibit heterogeneous electrical activity, which needs to be synchronized with the related intracellular calcium oscillations all over the islet to achieve pulsatile insulin release [228]. Gap junctions between adjacent cells play a fundamental role. Recent studies applying optogenetic and photopharmacological methods enriched the knowledge about the dynamics of the β-cell network in humans and rodents [229,230]. They uncovered the existence of few subpopulations of β-cells with reduced β-cell identity (lower levels of PDX1, INS1) but more sensitive to glucose (increased expression of glucokinase) called hubs, which drive the response of the remaining β-cells called followers through cellular coupling [231,232]. This was found to be conserved across species (including mice, humans and fish) [201]. However, consensus needs to be reached about the role of hubs as pacemakers for the islet activity. Those hub β-cells showing enhanced sensitivity to glucose might be the reason why these cells are more susceptible to diabetic insults and their loss of function might be critical for T2D development. In any case, functional gap junction coupling is essential for this process. β-cells are linked through gap junctions, which are composed of connexin36 (CX36) [233] (Figure 5). It provides electrical coupling between β-cells and thus promotes the regulation of electrical activity and insulin secretion. CX36 gap junctions enable Ca2+ oscillations to be coordinated across the islets. Its deletion significantly affects the synchronization of calcium oscillations, and CX36-deficient mice show disrupted first-phase and pulsatile second phase of insulin release [234,235]. CX36-dependent signaling may be thus disrupted under prediabetic conditions. In general, connexin-based channels comprise hemichannels and gap junction channels. The opening of gap junction channels permits the diffusional exchange of ions and molecules between the cytoplasm and contacting cells. Recently, it was hypothesized that connexin composed gap junction and hemichannels properties might be also redox-regulated through extracellular and intracellular cysteines based on the evidence that reduced intracellular free radical production along with high reduced glutathione decrease opening probability of hemichannel [236]. Although this was the case of CX43 and CX46 of blood vessel wall and oocytes, respectively, with possible implication for other cell types., i.e., astrocytes, retina and kidney, we might suggest that possible redox regulation might be valid also in the case of β-cell gap junctions. This might further be involved in insulin secretion efficiency response as suggested above. This assumption is based on amino-acid sequence alignments of CX36/CX43 and CX36/CX46, which shows high similarity in cysteine positions (in red) and the sequence especially between CX36 and CX43 (Figure 6). The sequence of CX36 shares 56% sequence similarity with CX43. The sequence of CX36 shares up to 74% of sequence similarity with CX46, however, the central part of CX36 ( position of residues 109–138, 145–154 and 167–175) is missing in CX46 and CX46 contains a much longer C-terminus, which decrease the sequence similarity of CX36/CX46 to 33%.
Figure 6.
Human CX36 amino-acid sequence alignment with CX43 (A) and CX46 (B); identical and similar amino acids are depicted in grey, Cys residues-potential targets to redox modifications are highlighted in red; the primary amino-acid sequences were aligned using protein blast tool available at https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 23 January 2021.
4. Conclusions
Redox homeostasis and signaling is essential in the physiology and pathophysiology of β-cells. β-cell redox homeostasis is the equilibrium of a highly compartmentalized, however interconnected, local redox environment. While imbalanced, T2D is quickly developing, as apparent from expressional dysregulation of redox regulating genes in human patients with T2D. Localized redox milieu involves mainly ER required especially for proper insulin folding and calcium handling, mitochondria required preferentially for bioenergetic supply, peroxisomes being important mainly upon conditions of increased lipids in circulation and cytoplasm connecting all compartments. Redox status impacts three major signaling pathways in β-cells. The KEAP1–NRF2–ARE pathway provides major antioxidant protection. NFκB also participates in antioxidant balance but is active mainly upon deleterious insults. MAPK cascades (ERK, JNK, p38, MAPK1, etc.) signal under physiological and also pathophysiological conditions. Downstream targets of signaling pathways are often transcription factors represented by MAFA, PDX1, NKX6.1, NEUROD, PAX, etc. and other proteins (e.g., FOXO1, PPAs), which have the essential role in insulin expression, β-cell differentiation into the mature functional cell and its proliferation. Redox signaling also targets many components of insulin secretory machinery, such as ion channels, calcium homeostasis and vesicle exocytosis, thus being essential for the proper function of β-cells. Intercommunication of β-cells through gap junctions in pancreatic islets strongly reflects redox homeostasis inside β-cells. This is under the supervision of extracellular redox signaling, which affects the activity of integrins and metalloproteinases, being essential for β-cell differentiation, proliferation and sufficient blood supply. Transmembrane TRP channels also serve as signal transducers of the extracellular redox environment towards β-cells. Thus, redox signaling is essential already for β-cells ontogenesis into mature β-cell, for its main function of insulin secretion upon any insult and communication between β-cell syncytium, perhaps also with other endocrine and exocrine cells in pancreatic islets. The above-mentioned physiological signaling utilizes transient alternation in redox status, briefly disturbing redox homeostasis. When an insult altering redox homeostasis toward prooxidative status exceeds the capacity of antioxidant defense in β-cells and becomes permanent, signaling switches to pathological. This can happen by chronic overnutrition when high supply and fuel availability permanently increase metabolic flux establishing prooxidative status. ER stress and activation of UPR then set β-cells towards metabolic stress, inflammasome formation, inflammatory metabolism, impaired insulin secretion, establishing a vicious cycle of oxidative stress. Chronic overnutrition also affects peripheral tissues, first white adipose tissue responding by altered secretion of adipokines, hypoxia, activation of inflammatory macrophages through TLR4, further supporting ectopic lipid storage in liver and muscles and increasing the release of lipids to circulation. This, together with activation of proinflammatory macrophages by gut microbiota, establishes low chronic inflammation, metainflammation, targeting β-cells towards dedifferentiation, pathological signaling and eventually cell death, the markers of T2D.
Acknowledgments
All figures were created in Biorender.com (Toronto, Canada), accessed 20210115; thus, we kindly acknowledge the software. The authors also acknowledge Petr Ježek, the Institute of Physiology, Czech Academy of Sciences for financial support and comments to the manuscript.
Author Contributions
Conceptualization, L.P.-H., writing, review—Š.B., B.H., L.P.-H., funding acquisition, Š.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant Agency of the Czech Republic, grant No. 20-00408S (to PJ) and Charles University Grant Agency, grant No. 110120 (to ŠB).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L., Colombani A.L., Ktorza A., Casteilla L., Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plecita-Hlavata L., Jaburek M., Holendova B., Tauber J., Pavluch V., Berkova Z., Cahova M., Schroeder K., Brandes R.P., Siemen D., et al. Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes. 2020 doi: 10.2337/db19-1130. [DOI] [PubMed] [Google Scholar]
- 3.Wang J., Wang H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid. Med. Cell. Longev. 2017;2017:1930261. doi: 10.1155/2017/1930261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grankvist K., Marklund S.L., Taljedal I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 6.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 7.Miki A., Ricordi C., Sakuma Y., Yamamoto T., Misawa R., Mita A., Molano R.D., Vaziri N.D., Pileggi A., Ichii H. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS ONE. 2018;13:e0196570. doi: 10.1371/journal.pone.0196570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalinina E.V., Chernov N.N., Saprin A.N. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry. 2008;73:1493–1510. doi: 10.1134/S0006297908130099. [DOI] [PubMed] [Google Scholar]
- 9.Stancill J.S., Broniowska K.A., Oleson B.J., Naatz A., Corbett J.A. Pancreatic beta-cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J. Biol. Chem. 2019;294:4843–4853. doi: 10.1074/jbc.RA118.006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro D., Treberg J.R. A radical shift in perspective: Mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 2017;220:1170–1180. doi: 10.1242/jeb.132142. [DOI] [PubMed] [Google Scholar]
- 11.Roma L.P., Jonas J.C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and beta-Cells. J. Mol. Biol. 2019 doi: 10.1016/j.jmb.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Yoboue E.D., Sitia R., Simmen T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018;9:331. doi: 10.1038/s41419-017-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurgul E., Lortz S., Tiedge M., Jorns A., Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004;53:2271–2280. doi: 10.2337/diabetes.53.9.2271. [DOI] [PubMed] [Google Scholar]
- 14.Plecita-Hlavata L., Engstova H., Jezek J., Holendova B., Tauber J., Petraskova L., Kren V., Jezek P. Potential of Mitochondria-Targeted Antioxidants to Prevent Oxidative Stress in Pancreatic beta-cells. Oxid. Med. Cell. Longev. 2019;2019:1826303. doi: 10.1155/2019/1826303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plecita-Hlavata L., Engstova H., Holendova B., Tauber J., Spacek T., Petraskova L., Kren V., Spackova J., Gotvaldova K., Jezek J., et al. Mitochondrial Superoxide Production Decreases on Glucose-Stimulated Insulin Secretion in Pancreatic beta Cells Due to Decreasing Mitochondrial Matrix NADH/NAD(+) Ratio. Antioxid. Redox Signal. 2020;33:789–815. doi: 10.1089/ars.2019.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jezek J., Dlaskova A., Zelenka J., Jaburek M., Jezek P. H(2)O(2)-Activated Mitochondrial Phospholipase iPLA(2)gamma Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein-Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic beta-Cells. Antioxid. Redox Signal. 2015;23:958–972. doi: 10.1089/ars.2014.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jezek P., Holendova B., Garlid K.D., Jaburek M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling. Antioxid. Redox Signal. 2018;29:667–714. doi: 10.1089/ars.2017.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appenzeller-Herzog C., Riemer J., Zito E., Chin K.T., Ron D., Spiess M., Ellgaard L. Disulphide production by Ero1alpha-PDI relay is rapid and effectively regulated. EMBO J. 2010;29:3318–3329. doi: 10.1038/emboj.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehmeti I., Lortz S., Elsner M., Lenzen S. Peroxiredoxin 4 improves insulin biosynthesis and glucose-induced insulin secretion in insulin-secreting INS-1E cells. J. Biol. Chem. 2014;289:26904–26913. doi: 10.1074/jbc.M114.568329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenzen S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic beta-cells. Biochim Biophys Acta Gen. Subj. 2017;1861:1929–1942. doi: 10.1016/j.bbagen.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Tavender T.J., Sheppard A.M., Bulleid N.J. Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem. J. 2008;411:191–199. doi: 10.1042/BJ20071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen V.D., Saaranen M.J., Karala A.R., Lappi A.K., Wang L., Raykhel I.B., Alanen H.I., Salo K.E., Wang C.C., Ruddock L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011;406:503–515. doi: 10.1016/j.jmb.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Hassler J.R., Scheuner D.L., Wang S., Han J., Kodali V.K., Li P., Nguyen J., George J.S., Davis C., Wu S.P., et al. The IRE1alpha/XBP1s Pathway Is Essential for the Glucose Response and Protection of beta Cells. PLoS Biol. 2015;13:e1002277. doi: 10.1371/journal.pbio.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearse B.R., Hebert D.N. Lectin chaperones help direct the maturation of glycoproteins in the endoplasmic reticulum. Biochim. Biophys. Acta. 2010;1803:684–693. doi: 10.1016/j.bbamcr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plemper R.K., Bohmler S., Bordallo J., Sommer T., Wolf D.H. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 26.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 27.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 28.Brozzi F., Eizirik D.L. ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups J. Med. Sci. 2016;121:133–139. doi: 10.3109/03009734.2015.1135217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baboota R.K., Shinde A.B., Lemaire K., Fransen M., Vinckier S., Van Veldhoven P.P., Schuit F., Baes M. Functional peroxisomes are required for beta-cell integrity in mice. Mol. Metab. 2019;22:71–83. doi: 10.1016/j.molmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira H.R., Verlengia R., Carvalho C.R., Britto L.R., Curi R., Carpinelli A.R. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52:1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 31.Uchizono Y., Takeya R., Iwase M., Sasaki N., Oku M., Imoto H., Iida M., Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80:133–139. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z., Li J., Yang L., Chen R., Yang R., Zhang H., Cai D., Chen H. The cytotoxic role of intermittent high glucose on apoptosis and cell viability in pancreatic beta cells. J. Diabetes Res. 2014;2014:712781. doi: 10.1155/2014/712781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stancill J.S., Happ J.T., Broniowska K.A., Hogg N., Corbett J.A. Peroxiredoxin 1 plays a primary role in protecting pancreatic β-cells from hydrogen peroxide and peroxynitrite. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R1004–R1013. doi: 10.1152/ajpregu.00011.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 35.Wondafrash D.Z., Nire’a A.T., Tafere G.G., Desta D.M., Berhe D.A., Zewdie K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. 2020;13:43–51. doi: 10.2147/DMSO.S232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshihara E., Masaki S., Matsuo Y., Chen Z., Tian H., Yodoi J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishinaka Y., Masutani H., Oka S., Matsuo Y., Yamaguchi Y., Nishio K., Ishii Y., Yodoi J. Importin alpha1 (Rch1) mediates nuclear translocation of thioredoxin-binding protein-2/vitamin D(3)-up-regulated protein 1. J. Biol. Chem. 2004;279:37559–37565. doi: 10.1074/jbc.M405473200. [DOI] [PubMed] [Google Scholar]
- 38.Xu G., Chen J., Jing G., Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013;19:1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing G., Westwell-Roper C., Chen J., Xu G., Verchere C.B., Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J. Biol. Chem. 2014;289:11807–11815. doi: 10.1074/jbc.M113.525022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pi J., Zhang Q., Fu J., Woods C.G., Hou Y., Corkey B.E., Collins S., Andersen M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharm. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janjic D., Maechler P., Sekine N., Bartley C., Annen A.S., Wolheim C.B. Free radical modulation of insulin release in INS-1 cells exposed to alloxan. Biochem. Pharm. 1999;57:639–648. doi: 10.1016/S0006-2952(98)00346-3. [DOI] [PubMed] [Google Scholar]
- 42.Maechler P., Jornot L., Wollheim C.B. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J. Biol. Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 43.Pi J., Bai Y., Zhang Q., Wong V., Floering L.M., Daniel K., Reece J.M., Deeney J.T., Andersen M.E., Corkey B.E., et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 44.Travasso R.D.M., Sampaio Dos Aidos F., Bayani A., Abranches P., Salvador A. Localized redox relays as a privileged mode of cytoplasmic hydrogen peroxide signaling. Redox Biol. 2017;12:233–245. doi: 10.1016/j.redox.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kontou M., Will R.D., Adelfalk C., Wittig R., Poustka A., Hirsch-Kauffmann M., Schweiger M. Thioredoxin, a regulator of gene expression. Oncogene. 2004;23:2146–2152. doi: 10.1038/sj.onc.1207334. [DOI] [PubMed] [Google Scholar]
- 47.Bian M., Fan R., Zhao S., Liu W. Targeting the Thioredoxin System as a Strategy for Cancer Therapy. J. Med. Chem. 2019;62:7309–7321. doi: 10.1021/acs.jmedchem.8b01595. [DOI] [PubMed] [Google Scholar]
- 48.Jastrząb A., Skrzydlewska E. Thioredoxin-dependent system. Application of inhibitors. J. Enzym. Inhib. Med. Chem. 2021;36:362–371. doi: 10.1080/14756366.2020.1867121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muri J., Kopf M. Redox regulation of immunometabolism. Nat. Reviews. Immunol. 2020 doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultheis J., Beckmann D., Mulac D., Muller L., Esselen M., Dufer M. Nrf2 Activation Protects Mouse Beta Cells from Glucolipotoxicity by Restoring Mitochondrial Function and Physiological Redox Balance. Oxid. Med. Cell. Longev. 2019;2019:7518510. doi: 10.1155/2019/7518510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J., Zhang X., Lian C., Wu J., Fang Y., Ye X. KEAP1/NRF2 axis regulates H2O2-induced apoptosis of pancreatic beta-cells. Gene. 2019;691:8–17. doi: 10.1016/j.gene.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 53.Cardozo A.K., Heimberg H., Heremans Y., Leeman R., Kutlu B., Kruhoffer M., Orntoft T., Eizirik D.L. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J. Biol. Chem. 2001;276:48879–48886. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- 54.Meyerovich K., Fukaya M., Terra L.F., Ortis F., Eizirik D.L., Cardozo A.K. The non-canonical NF-kappaB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia. 2016;59:512–521. doi: 10.1007/s00125-015-3817-z. [DOI] [PubMed] [Google Scholar]
- 55.Meyerovich K., Ortis F., Cardozo A.K. The non-canonical NF-kappaB pathway and its contribution to beta-cell failure in diabetes. J. Mol. Endocrinol. 2018;61:F1–F6. doi: 10.1530/JME-16-0183. [DOI] [PubMed] [Google Scholar]
- 56.Lee K., Esselman W.J. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic. Biol. Med. 2002;33:1121–1132. doi: 10.1016/S0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- 57.Brigelius-Flohe R., Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baeuerle P.A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 60.Cerf M.E. Transcription factors regulating beta-cell function. Eur. J. Endocrinol. 2006;155:671–679. doi: 10.1530/eje.1.02277. [DOI] [PubMed] [Google Scholar]
- 61.Jara M.A., Werneck-De-Castro J.P., Lubaczeuski C., Johnson J.D., Bernal-Mizrachi E. Pancreatic and duodenal homeobox-1 (PDX1) contributes to beta-cell mass expansion and proliferation induced by Akt/PKB pathway. Islets. 2020;12:32–40. doi: 10.1080/19382014.2020.1762471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneto H., Kajimoto Y., Miyagawa J., Matsuoka T., Fujitani Y., Umayahara Y., Hanafusa T., Matsuzawa Y., Yamasaki Y., Hori M. Beneficial effects of antioxidants in diabetes: Possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y., Gleason C.E., Tran P.O., Harmon J.S., Robertson R.P. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc. Natl. Acad. Sci. USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuoka T.A., Kaneto H., Stein R., Miyatsuka T., Kawamori D., Henderson E., Kojima I., Matsuhisa M., Hori M., Yamasaki Y. MafA regulates expression of genes important to islet beta-cell function. Mol. Endocrinol. 2007;21:2764–2774. doi: 10.1210/me.2007-0028. [DOI] [PubMed] [Google Scholar]
- 65.Harmon J.S., Stein R., Robertson R.P. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J. Biol. Chem. 2005;280:11107–11113. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- 66.Kondo T., El Khattabi I., Nishimura W., Laybutt D.R., Geraldes P., Shah S., King G., Bonner-Weir S., Weir G., Sharma A. p38 MAPK is a major regulator of MafA protein stability under oxidative stress. Mol. Endocrinol. 2009;23:1281–1290. doi: 10.1210/me.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Khattabi I., Sharma A. Preventing p38 MAPK-mediated MafA degradation ameliorates beta-cell dysfunction under oxidative stress. Mol. Endocrinol. 2013;27:1078–1090. doi: 10.1210/me.2012-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harmon J.S., Bogdani M., Parazzoli S.D., Mak S.S., Oseid E.A., Berghmans M., Leboeuf R.C., Robertson R.P. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barthel A., Schmoll D., Unterman T.G. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Dansen T.B., Smits L.M., van Triest M.H., de Keizer P.L., van Leenen D., Koerkamp M.G., Szypowska A., Meppelink A., Brenkman A.B., Yodoi J., et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat. Chem. Biol. 2009;5:664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- 71.De Keizer P.L., Burgering B.M., Dansen T.B. Forkhead box o as a sensor, mediator, and regulator of redox signaling. Antioxid. Redox Signal. 2011;14:1093–1106. doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- 72.Burgering B.M., Coffer P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 73.Huang X., Begley M., Morgenstern K.A., Gu Y., Rose P., Zhao H., Zhu X. Crystal structure of an inactive Akt2 kinase domain. Structure. 2003;11:21–30. doi: 10.1016/S0969-2126(02)00937-1. [DOI] [PubMed] [Google Scholar]
- 74.Murata H., Ihara Y., Nakamura H., Yodoi J., Sumikawa K., Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J. Biol. Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W.H., 3rd, Wright C.V., White M.F., Arden K.C., Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Investig. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitamura Y.I., Kitamura T., Kruse J.P., Raum J.C., Stein R., Gu W., Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura W., Kondo T., Salameh T., El Khattabi I., Dodge R., Bonner-Weir S., Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao X., Kambe F., Ohmori S., Seo H. Oxidoreductive modification of two cysteine residues in paired domain by Ref-1 regulates DNA-binding activity of Pax-8. Biochem. Biophys Res. Commun. 2002;297:288–293. doi: 10.1016/S0006-291X(02)02196-4. [DOI] [PubMed] [Google Scholar]
- 79.Walther C., Guenet J.L., Simon D., Deutsch U., Jostes B., Goulding M.D., Plachov D., Balling R., Gruss P. Pax: A murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- 80.Swisa A., Avrahami D., Eden N., Zhang J., Feleke E., Dahan T., Cohen-Tayar Y., Stolovich-Rain M., Kaestner K.H., Glaser B., et al. PAX6 maintains beta cell identity by repressing genes of alternative islet cell types. J. Clin. Investig. 2017;127:230–243. doi: 10.1172/JCI88015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rieck S., Bankaitis E.D., Wright C.V. Seminars in Cell & Developmental Biology. Vol. 23. Academic Press; Cambridge, MA, USA: 2012. Lineage determinants in early endocrine development; pp. 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bastidas-Ponce A., Roscioni S.S., Burtscher I., Bader E., Sterr M., Bakhti M., Lickert H. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic beta-cells. Mol. Metab. 2017;6:524–534. doi: 10.1016/j.molmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bensellam M., Jonas J.C., Laybutt D.R. Mechanisms of beta-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 2018;236:R109–R143. doi: 10.1530/JOE-17-0516. [DOI] [PubMed] [Google Scholar]
- 84.Balakrishnan S., Dhavamani S., Prahalathan C. beta-Cell specific transcription factors in the context of diabetes mellitus and beta-cell regeneration. Mech. Dev. 2020;163:103634. doi: 10.1016/j.mod.2020.103634. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chetboun M., Abitbol G., Rozenberg K., Rozenfeld H., Deutsch A., Sampson S.R., Rosenzweig T. Maintenance of redox state and pancreatic beta-cell function: Role of leptin and adiponectin. J. Cell. Biochem. 2012;113:1966–1976. doi: 10.1002/jcb.24065. [DOI] [PubMed] [Google Scholar]
- 87.Ahmed Alfar E., Kirova D., Konantz J., Birke S., Mansfeld J., Ninov N. Distinct Levels of Reactive Oxygen Species Coordinate Metabolic Activity with Beta-cell Mass Plasticity. Sci. Rep. 2017;7:3994. doi: 10.1038/s41598-017-03873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang J., Wu S.Y., Zhang D., Wang L., Leung K.K., Leung P.S. NADPH Oxidase-Dependent Reactive Oxygen Species Stimulate beta-Cell Regeneration Through Differentiation of Endocrine Progenitors in Murine Pancreas. Antioxid. Redox Signal. 2016;24:419–433. doi: 10.1089/ars.2014.6135. [DOI] [PubMed] [Google Scholar]
- 89.Hoarau E., Chandra V., Rustin P., Scharfmann R., Duvillie B. Pro-oxidant/antioxidant balance controls pancreatic beta-cell differentiation through the ERK1/2 pathway. Cell Death Dis. 2014;5:e1487. doi: 10.1038/cddis.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costes S., Broca C., Bertrand G., Lajoix A.D., Bataille D., Bockaert J., Dalle S. ERK1/2 control phosphorylation and protein level of cAMP-responsive element-binding protein: A key role in glucose-mediated pancreatic beta-cell survival. Diabetes. 2006;55:2220–2230. doi: 10.2337/db05-1618. [DOI] [PubMed] [Google Scholar]
- 91.Hussain M.A., Porras D.L., Rowe M.H., West J.R., Song W.J., Schreiber W.E., Wondisford F.E. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol. Cell. Biol. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piera-Velazquez S., Hawkins D.F., Whitecavage M.K., Colter D.C., Stokes D.G., Jimenez S.A. Regulation of the human SOX9 promoter by Sp1 and CREB. Exp. Cell Res. 2007;313:1069–1079. doi: 10.1016/j.yexcr.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D., Wu H., Kornblum H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Funato Y., Michiue T., Asashima M., Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat. Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 95.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y.H., Magkos F., Mantzoros C.S., Kang E.S. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism. 2011;60:1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Kulkarni R.N., Wang Z.L., Wang R.M., Hurley J.D., Smith D.M., Ghatei M.A., Withers D.J., Gardiner J.V., Bailey C.J., Bloom S.R. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Investig. 1997;100:2729–2736. doi: 10.1172/JCI119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kieffer T.J., Heller R.S., Leech C.A., Holz G.G., Habener J.F. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes. 1997;46:1087–1093. doi: 10.2337/diab.46.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuehnen P., Laubner K., Raile K., Schofl C., Jakob F., Pilz I., Path G., Seufert J. Protein phosphatase 1 (PP-1)-dependent inhibition of insulin secretion by leptin in INS-1 pancreatic beta-cells and human pancreatic islets. Endocrinology. 2011;152:1800–1808. doi: 10.1210/en.2010-1094. [DOI] [PubMed] [Google Scholar]
- 100.Sim A.T., Baldwin M.L., Rostas J.A., Holst J., Ludowyke R.I. The role of serine/threonine protein phosphatases in exocytosis. Biochem. J. 2003;373:641–659. doi: 10.1042/bj20030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Staiger K., Stefan N., Staiger H., Brendel M.D., Brandhorst D., Bretzel R.G., Machicao F., Kellerer M., Stumvoll M., Fritsche A., et al. Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J. Clin. Endocrinol. Metab. 2005;90:6707–6713. doi: 10.1210/jc.2005-0467. [DOI] [PubMed] [Google Scholar]
- 102.Llanos P., Contreras-Ferrat A., Barrientos G., Valencia M., Mears D., Hidalgo C. Glucose-Dependent Insulin Secretion in Pancreatic beta-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors. PLoS ONE. 2015;10:e0129238. doi: 10.1371/journal.pone.0129238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jansson D., Ng A.C., Fu A., Depatie C., Al Azzabi M., Screaton R.A. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl. Acad. Sci. USA. 2008;105:10161–10166. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bernal-Mizrachi E., Kulkarni R.N., Scott D.K., Mauvais-Jarvis F., Stewart A.F., Garcia-Ocana A. Human beta-cell proliferation and intracellular signaling part 2: Still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sato Y., Endo H., Okuyama H., Takeda T., Iwahashi H., Imagawa A., Yamagata K., Shimomura I., Inoue M. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem. 2011;286:12524–12532. doi: 10.1074/jbc.M110.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhdanov A.V., Ward M.W., Prehn J.H., Papkovsky D.B. Dynamics of intracellular oxygen in PC12 Cells upon stimulation of neurotransmission. J. Biol. Chem. 2008;283:5650–5661. doi: 10.1074/jbc.M706439200. [DOI] [PubMed] [Google Scholar]
- 107.O’Hagan K.A., Cocchiglia S., Zhdanov A.V., Tambuwala M.M., Cummins E.P., Monfared M., Agbor T.A., Garvey J.F., Papkovsky D.B., Taylor C.T., et al. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc. Natl. Acad. Sci. USA. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerber P.A., Rutter G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017;26:501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olsson R., Carlsson P.O. A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes. 2011;60:2068–2075. doi: 10.2337/db09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashcroft F.M., Harrison D.E., Ashcroft S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 111.Yasui S., Mawatari K., Morizumi R., Furukawa H., Shimohata T., Harada N., Takahashi A., Nakaya Y. Hydrogen peroxide inhibits insulin-induced ATP-sensitive potassium channel activation independent of insulin signaling pathway in cultured vascular smooth muscle cells. J. Med. Investig. 2012;59:36–44. doi: 10.2152/jmi.59.36. [DOI] [PubMed] [Google Scholar]
- 112.Sakaguchi R., Mori Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020;146:36–44. doi: 10.1016/j.freeradbiomed.2019.10.415. [DOI] [PubMed] [Google Scholar]
- 113.Finol-Urdaneta R.K., Remedi M.S., Raasch W., Becker S., Clark R.B., Struver N., Pavlov E., Nichols C.G., French R.J., Terlau H. Block of Kv1.7 potassium currents increases glucose-stimulated insulin secretion. EMBO Mol. Med. 2012;4:424–434. doi: 10.1002/emmm.201200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MacDonald P.E., Salapatek A.M., Wheeler M.B. Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells. J. Physiol. 2003;546:647–653. doi: 10.1113/jphysiol.2002.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mittal M., Gu X.Q., Pak O., Pamenter M.E., Haag D., Fuchs D.B., Schermuly R.T., Ghofrani H.A., Brandes R.P., Seeger W., et al. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic. Biol. Med. 2012;52:1033–1042. doi: 10.1016/j.freeradbiomed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 116.Gerst J.E. SNARE regulators: Matchmakers and matchbreakers. Biochim Biophys Acta. 2003;1641:99–110. doi: 10.1016/S0167-4889(03)00096-X. [DOI] [PubMed] [Google Scholar]
- 117.Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., Renstrom E., Schuit F.C. Redox control of exocytosis: Regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 118.Reinbothe T.M., Ivarsson R., Li D.Q., Niazi O., Jing X., Zhang E., Stenson L., Bryborn U., Renstrom E. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol. Endocrinol. 2009;23:893–900. doi: 10.1210/me.2008-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferdaoussi M., Dai X., Jensen M.V., Wang R., Peterson B.S., Huang C., Ilkayeva O., Smith N., Miller N., Hajmrle C., et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J. Clin. Investig. 2015;125:3847–3860. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Z., Lam L.S., Lam L.H., Chau S.F., Ng T.B., Au S.W. Molecular basis of the redox regulation of SUMO proteases: A protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J. 2008;22:127–137. doi: 10.1096/fj.06-7871com. [DOI] [PubMed] [Google Scholar]
- 121.Ferdaoussi M., MacDonald P.E. Toward Connecting Metabolism to the Exocytotic Site. Trends Cell Biol. 2017;27:163–171. doi: 10.1016/j.tcb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 122.Lorenzen I., Eble J.A., Hanschmann E.M. Thiol switches in membrane proteins - Extracellular redox regulation in cell biology. Biol. Chem. 2020 doi: 10.1515/hsz-2020-0266. [DOI] [PubMed] [Google Scholar]
- 123.Lundberg M., Fernandes A.P., Kumar S., Holmgren A. Cellular and plasma levels of human glutaredoxin 1 and 2 detected by sensitive ELISA systems. Biochem. Biophys. Res. Commun. 2004;319:801–809. doi: 10.1016/j.bbrc.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 124.Xiong B., Jha V., Min J.K., Cho J. Protein disulfide isomerase in cardiovascular disease. Exp. Mol. Med. 2020;52:390–399. doi: 10.1038/s12276-020-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mullen L., Hanschmann E.M., Lillig C.H., Herzenberg L.A., Ghezzi P. Cysteine Oxidation Targets Peroxiredoxins 1 and 2 for Exosomal Release through a Novel Mechanism of Redox-Dependent Secretion. Mol. Med. 2015;21:98–108. doi: 10.2119/molmed.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanschmann E.M., Petry S.F., Eitner S., Maresch C.C., Lingwal N., Lillig C.H., Linn T. Paracrine regulation and improvement of β-cell function by thioredoxin. Redox Biol. 2020;34:101570. doi: 10.1016/j.redox.2020.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jikimoto T., Nishikubo Y., Koshiba M., Kanagawa S., Morinobu S., Morinobu A., Saura R., Mizuno K., Kondo S., Toyokuni S., et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol. Immunol. 2002;38:765–772. doi: 10.1016/S0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 128.Kakisaka Y., Nakashima T., Sumida Y., Yoh T., Nakamura H., Yodoi J., Senmaru H. Elevation of serum thioredoxin levels in patients with type 2 diabetes. Horm. Metab. Res. 2002;34:160–164. doi: 10.1055/s-2002-23201. [DOI] [PubMed] [Google Scholar]
- 129.Asami K., Inagaki A., Imura T., Sekiguchi S., Fujimori K., Masutani H., Yodoi J., Satomi S., Ohuchi N., Goto M. Thioredoxin-1 attenuates early graft loss after intraportal islet transplantation in mice. PLoS ONE. 2013;8:e70259. doi: 10.1371/journal.pone.0070259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Willems S.H., Tape C.J., Stanley P.L., Taylor N.A., Mills I.G., Neal D.E., McCafferty J., Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem. J. 2010;428:439–450. doi: 10.1042/BJ20100179. [DOI] [PubMed] [Google Scholar]
- 131.Bass R., Edwards D.R. ADAMs and protein disulfide isomerase: The key to regulated cell-surface protein ectodomain shedding? Biochem. J. 2010;428:e3–e5. doi: 10.1042/BJ20100568. [DOI] [PubMed] [Google Scholar]
- 132.Düsterhöft S., Jung S., Hung C.W., Tholey A., Sönnichsen F.D., Grötzinger J., Lorenzen I. Membrane-proximal domain of a disintegrin and metalloprotease-17 represents the putative molecular switch of its shedding activity operated by protein-disulfide isomerase. J. Am. Chem. Soc. 2013;135:5776–5781. doi: 10.1021/ja400340u. [DOI] [PubMed] [Google Scholar]
- 133.Pedersen K.B., Chodavarapu H., Porretta C., Robinson L.K., Lazartigues E. Dynamics of ADAM17-Mediated Shedding of ACE2 Applied to Pancreatic Islets of Male db/db Mice. Endocrinology. 2015;156:4411–4425. doi: 10.1210/en.2015-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chhabra K.H., Chodavarapu H., Lazartigues E. Angiotensin converting enzyme 2: A new important player in the regulation of glycemia. IUBMB Life. 2013;65:731–738. doi: 10.1002/iub.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bergerhausen L., Grosche J., Meißner J., Hecker C., Caliandro M.F., Westerhausen C., Kamenac A., Rezaei M., Mörgelin M., Poschmann G., et al. Extracellular Redox Regulation of α7β Integrin-Mediated Cell Migration Is Signaled via a Dominant Thiol-Switch. Antioxidants. 2020;9:227. doi: 10.3390/antiox9030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Passam F., Chiu J., Ju L., Pijning A., Jahan Z., Mor-Cohen R., Yeheskel A., Kolšek K., Thärichen L., Aponte-Santamaría C., et al. Mechano-redox control of integrin de-adhesion. eLife. 2018;7 doi: 10.7554/eLife.34843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Townsend S.E., Gannon M. Extracellular Matrix-Associated Factors Play Critical Roles in Regulating Pancreatic β-Cell Proliferation and Survival. Endocrinology. 2019;160:1885–1894. doi: 10.1210/en.2019-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hynes R.O. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 139.Alam N., Goel H.L., Zarif M.J., Butterfield J.E., Perkins H.M., Sansoucy B.G., Sawyer T.K., Languino L.R. The integrin-growth factor receptor duet. J. Cell. Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 140.Xu S.Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C.J., et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Islam M.S. Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells. 2020;9:685. doi: 10.3390/cells9030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bensellam M., Van Lommel L., Overbergh L., Schuit F.C., Jonas J.C. Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia. 2009;52:463–476. doi: 10.1007/s00125-008-1245-z. [DOI] [PubMed] [Google Scholar]
- 143.Corkey B.E., Deeney J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020;11:567796. doi: 10.3389/fphys.2020.567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corkey B.E., Shirihai O. Metabolic master regulators: Sharing information among multiple systems. Trends Endocrinol. Metab. 2012;23:594–601. doi: 10.1016/j.tem.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scheuner D., Kaufman R.J. The unfolded protein response: A pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghosh R., Colon-Negron K., Papa F.R. Endoplasmic reticulum stress, degeneration of pancreatic islet beta-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol. Metab. 2019;27S:S60–S68. doi: 10.1016/j.molmet.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Herbert T.P., Laybutt D.R. A Reevaluation of the Role of the Unfolded Protein Response in Islet Dysfunction: Maladaptation or a Failure to Adapt? Diabetes. 2016;65:1472–1480. doi: 10.2337/db15-1633. [DOI] [PubMed] [Google Scholar]
- 148.Escribano-Lopez I., Banuls C., Diaz-Morales N., Iannantuoni F., Rovira-Llopis S., Gomis R., Rocha M., Hernandez-Mijares A., Murphy M.P., Victor V.M. The Mitochondria-Targeted Antioxidant MitoQ Modulates Mitochondrial Function and Endoplasmic Reticulum Stress in Pancreatic beta Cells Exposed to Hyperglycaemia. Cell Physiol. Biochem. 2019;52:186–197. doi: 10.33594/000000013. [DOI] [PubMed] [Google Scholar]
- 149.Zeeshan H.M., Lee G.H., Kim H.R., Chae H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan Q., Tang W., Zhang X., Hinson J.A., Liu C., Osei K., Wang J. Proinsulin atypical maturation and disposal induces extensive defects in mouse Ins2+/Akita beta-cells. PLoS ONE. 2012;7:e35098. doi: 10.1371/journal.pone.0035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oslowski C.M., Hara T., O’Sullivan-Murphy B., Kanekura K., Lu S., Hara M., Ishigaki S., Zhu L.J., Hayashi E., Hui S.T., et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vasiljevic J., Torkko J.M., Knoch K.P., Solimena M. The making of insulin in health and disease. Diabetologia. 2020;63:1981–1989. doi: 10.1007/s00125-020-05192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plaisance V., Brajkovic S., Tenenbaum M., Favre D., Ezanno H., Bonnefond A., Bonner C., Gmyr V., Kerr-Conte J., Gauthier B.R., et al. Endoplasmic Reticulum Stress Links Oxidative Stress to Impaired Pancreatic Beta-Cell Function Caused by Human Oxidized LDL. PLoS ONE. 2016;11:e0163046. doi: 10.1371/journal.pone.0163046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bravo R., Gutierrez T., Paredes F., Gatica D., Rodriguez A.E., Pedrozo Z., Chiong M., Parra V., Quest A.F., Rothermel B.A., et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int. J. Biochem. Cell Biol. 2012;44:16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imai Y., Dobrian A.D., Morris M.A., Nadler J.L. Islet inflammation: A unifying target for diabetes treatment? Trends Endocrinol. Metab. 2013;24:351–360. doi: 10.1016/j.tem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F., Yagi N., Ohto U., Kimoto M., Miyake K., et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 157.Kim J.J., Sears D.D. TLR4 and Insulin Resistance. Gastroenterol. Res. Pract. 2010;2010 doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh A., Singh V., Tiwari R.L., Chandra T., Kumar A., Dikshit M., Barthwal M.K. The IRAK-ERK-p67phox-Nox-2 axis mediates TLR4, 2-induced ROS production for IL-1beta transcription and processing in monocytes. Cell. Mol. Immunol. 2016;13:745–763. doi: 10.1038/cmi.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park H.S., Jung H.Y., Park E.Y., Kim J., Lee W.J., Bae Y.S. Cutting edge: Direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 160.Marseglia L., Manti S., D’Angelo G., Nicotera A., Parisi E., Di Rosa G., Gitto E., Arrigo T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016;40:272–279. doi: 10.4093/dmj.2016.40.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.David J.A., Rifkin W.J., Rabbani P.S., Ceradini D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017;2017:4826724. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lieb D.C., Brotman J.J., Hatcher M.A., Aye M.S., Cole B.K., Haynes B.A., Wohlgemuth S.D., Fontana M.A., Beydoun H., Nadler J.L., et al. Adipose tissue 12/15 lipoxygenase pathway in human obesity and diabetes. J. Clin. Endocrinol. Metab. 2014;99:E1713–E1720. doi: 10.1210/jc.2013-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dunmore S.J., Brown J.E. The role of adipokines in beta-cell failure of type 2 diabetes. J. Endocrinol. 2013;216:T37–T45. doi: 10.1530/JOE-12-0278. [DOI] [PubMed] [Google Scholar]
- 166.Tushuizen M.E., Bunck M.C., Pouwels P.J., Bontemps S., van Waesberghe J.H., Schindhelm R.K., Mari A., Heine R.J., Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- 167.Ying W., Lee Y.S., Dong Y., Seidman J.S., Yang M., Isaac R., Seo J.B., Yang B.H., Wollam J., Riopel M., et al. Expansion of Islet-Resident Macrophages Leads to Inflammation Affecting beta Cell Proliferation and Function in Obesity. Cell Metab. 2019;29:457–474.e455. doi: 10.1016/j.cmet.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weitz J.R., Makhmutova M., Almaca J., Stertmann J., Aamodt K., Brissova M., Speier S., Rodriguez-Diaz R., Caicedo A. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia. 2018;61:182–192. doi: 10.1007/s00125-017-4416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jacques-Silva M.C., Correa-Medina M., Cabrera O., Rodriguez-Diaz R., Makeeva N., Fachado A., Diez J., Berman D.M., Kenyon N.S., Ricordi C., et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. USA. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Almaca J., Molina J., Menegaz D., Pronin A.N., Tamayo A., Slepak V., Berggren P.O., Caicedo A. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell Rep. 2016;17:3281–3291. doi: 10.1016/j.celrep.2016.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Riley K.G., Pasek R.C., Maulis M.F., Dunn J.C., Bolus W.R., Kendall P.L., Hasty A.H., Gannon M. Macrophages are essential for CTGF-mediated adult beta-cell proliferation after injury. Mol. Metab. 2015;4:584–591. doi: 10.1016/j.molmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boni-Schnetzler M., Meier D.T. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41:501–513. doi: 10.1007/s00281-019-00745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ying W., Fu W., Lee Y.S., Olefsky J.M. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat. Rev. Endocrinol. 2020;16:81–90. doi: 10.1038/s41574-019-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M.K., Biollaz G., et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 175.Shin K.C., Hwang I., Choe S.S., Park J., Ji Y., Kim J.I., Lee G.Y., Choi S.H., Ching J., Kovalik J.P., et al. Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat. Commun. 2017;8:1087. doi: 10.1038/s41467-017-01232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J.A., Kerr-Conte J., Pattou F., Halban P.A., Weir G.C., Donath M.Y. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Westwell-Roper C., Denroche H.C., Ehses J.A., Verchere C.B. Differential Activation of Innate Immune Pathways by Distinct Islet Amyloid Polypeptide (IAPP) Aggregates. J. Biol. Chem. 2016;291:8908–8917. doi: 10.1074/jbc.M115.712455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cardozo A.K., Ortis F., Storling J., Feng Y.M., Rasschaert J., Tonnesen M., Van Eylen F., Mandrup-Poulsen T., Herchuelz A., Eizirik D.L. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 180.Kawamori D., Kaneto H., Nakatani Y., Matsuoka T.A., Matsuhisa M., Hori M., Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 181.Kaneto H., Xu G., Fujii N., Kim S., Bonner-Weir S., Weir G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 182.Burke S.J., Batdorf H.M., Burk D.H., Martin T.M., Mendoza T., Stadler K., Alami W., Karlstad M.D., Robson M.J., Blakely R.D., et al. Pancreatic deletion of the interleukin-1 receptor disrupts whole body glucose homeostasis and promotes islet beta-cell de-differentiation. Mol. Metab. 2018;14:95–107. doi: 10.1016/j.molmet.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nordmann T.M., Dror E., Schulze F., Traub S., Berishvili E., Barbieux C., Boni-Schnetzler M., Donath M.Y. The Role of Inflammation in beta-cell Dedifferentiation. Sci. Rep. 2017;7:6285. doi: 10.1038/s41598-017-06731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Q., Zhang H., Zhao B., Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol. Cell. Biochem. 2009;324:183–190. doi: 10.1007/s11010-008-9997-9. [DOI] [PubMed] [Google Scholar]
- 185.Cruz C.M., Rinna A., Forman H.J., Ventura A.L., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 187.Dror E., Dalmas E., Meier D.T., Wueest S., Thevenet J., Thienel C., Timper K., Nordmann T.M., Traub S., Schulze F., et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017;18:283–292. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 188.Herder C., Dalmas E., Boni-Schnetzler M., Donath M.Y. The IL-1 Pathway in Type 2 Diabetes and Cardiovascular Complications. Trends Endocrinol. Metab. 2015;26:551–563. doi: 10.1016/j.tem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 189.Sokolova M., Sahraoui A., Hoyem M., Ogaard J., Lien E., Aukrust P., Yndestad A., Ranheim T., Scholz H. NLRP3 inflammasome mediates oxidative stress-induced pancreatic islet dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018;315:E912–E923. doi: 10.1152/ajpendo.00461.2017. [DOI] [PubMed] [Google Scholar]
- 190.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 191.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 192.Fei N., Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 194.Mollica M.P., Mattace Raso G., Cavaliere G., Trinchese G., De Filippo C., Aceto S., Prisco M., Pirozzi C., Di Guida F., Lama A., et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes. 2017;66:1405–1418. doi: 10.2337/db16-0924. [DOI] [PubMed] [Google Scholar]
- 195.Vezza T., Abad-Jimenez Z., Marti-Cabrera M., Rocha M., Victor V.M. Microbiota-Mitochondria Inter-Talk: A Potential Therapeutic Strategy in Obesity and Type 2 Diabetes. Antioxidants. 2020;9:848. doi: 10.3390/antiox9090848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu J.L., Segovia I., Yuan X.L., Gao Z.H. Controversial Roles of Gut Microbiota-Derived Short-Chain Fatty Acids (SCFAs) on Pancreatic beta-Cell Growth and Insulin Secretion. Int. J. Mol. Sci. 2020;21:910. doi: 10.3390/ijms21030910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Christensen D.P., Dahllof M., Lundh M., Rasmussen D.N., Nielsen M.D., Billestrup N., Grunnet L.G., Mandrup-Poulsen T. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol. Med. 2011;17:378–390. doi: 10.2119/molmed.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rohr M.W., Narasimhulu C.A., Rudeski-Rohr T.A., Parthasarathy S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020;11:77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermudez-Humaran L.G., Smirnova N., Berge M., Sulpice T., Lahtinen S., et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cohrs C.M., Panzer J.K., Drotar D.M., Enos S.J., Kipke N., Chen C., Bozsak R., Schoniger E., Ehehalt F., Distler M., et al. Dysfunction of Persisting beta Cells Is a Key Feature of Early Type 2 Diabetes Pathogenesis. Cell Rep. 2020;31:107469. doi: 10.1016/j.celrep.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 201.Da Silva Xavier G., Rutter G.A. Metabolic and Functional Heterogeneity in Pancreatic beta Cells. J. Mol. Biol. 2020;432:1395–1406. doi: 10.1016/j.jmb.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 202.Robertson R.P., Harmon J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic. Biol. Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 203.Guo S., Dai C., Guo M., Taylor B., Harmon J.S., Sander M., Robertson R.P., Powers A.C., Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mahadevan J., Parazzoli S., Oseid E., Hertzel A.V., Bernlohr D.A., Vallerie S.N., Liu C.Q., Lopez M., Harmon J.S., Robertson R.P. Ebselen treatment prevents islet apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves beta-cell mass and function in ZDF rats. Diabetes. 2013;62:3582–3588. doi: 10.2337/db13-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kawamori D., Kajimoto Y., Kaneto H., Umayahara Y., Fujitani Y., Miyatsuka T., Watada H., Leibiger I.B., Yamasaki Y., Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes. 2003;52:2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 206.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim-Muller J.Y., Zhao S., Srivastava S., Mugabo Y., Noh H.L., Kim Y.R., Madiraju S.R., Ferrante A.W., Skolnik E.Y., Prentki M., et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20:593–602. doi: 10.1016/j.cmet.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cinti F., Bouchi R., Kim-Muller J.Y., Ohmura Y., Sandoval P.R., Masini M., Marselli L., Suleiman M., Ratner L.E., Marchetti P., et al. Evidence of beta-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016;101:1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Klotz L.O., Sanchez-Ramos C., Prieto-Arroyo I., Urbanek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T., Horton T., Hebrok M., Streeter P.R., Kaestner K.H., et al. Human islets contain four distinct subtypes of beta cells. Nat. Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rutter G.A., Georgiadou E., Martinez-Sanchez A., Pullen T.J. Metabolic and functional specialisations of the pancreatic beta cell: Gene disallowance, mitochondrial metabolism and intercellular connectivity. Diabetologia. 2020;63:1990–1998. doi: 10.1007/s00125-020-05205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sasaki M., Fujimoto S., Sato Y., Nishi Y., Mukai E., Yamano G., Sato H., Tahara Y., Ogura K., Nagashima K., et al. Reduction of reactive oxygen species ameliorates metabolism-secretion coupling in islets of diabetic GK rats by suppressing lactate overproduction. Diabetes. 2013;62:1996–2003. doi: 10.2337/db12-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bensellam M., Duvillie B., Rybachuk G., Laybutt D.R., Magnan C., Guiot Y., Pouyssegur J., Jonas J.C. Glucose-induced O(2) consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic beta-cells. PLoS ONE. 2012;7:e29807. doi: 10.1371/journal.pone.0029807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sato Y., Inoue M., Yoshizawa T., Yamagata K. Moderate hypoxia induces β-cell dysfunction with HIF-1-independent gene expression changes. PLoS ONE. 2014;9:e114868. doi: 10.1371/journal.pone.0114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li X., Zhang L., Meshinchi S., Dias-Leme C., Raffin D., Johnson J.D., Treutelaar M.K., Burant C.F. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- 216.Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Science’s STKE: Signal transduction knowledge environment. Sci. Stke. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 217.Jiang B.H., Rue E., Wang G.L., Roe R., Semenza G.L. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 218.Lee J.W., Bae S.H., Jeong J.W., Kim S.H., Kim K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 219.Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 220.Liu N., Cai X., Liu T., Zou J., Wang L., Wang G., Liu Y., Ding X., Zhang B., Sun P., et al. Hypoxia-inducible factor-1α mediates the expression of mature β cell-disallowed genes in hypoxia-induced β cell dedifferentiation. Biochem. Biophys. Res. Commun. 2020;523:382–388. doi: 10.1016/j.bbrc.2019.12.063. [DOI] [PubMed] [Google Scholar]
- 221.Giuliani M., Moritz W., Bodmer E., Dindo D., Kugelmeier P., Lehmann R., Gassmann M., Groscurth P., Weber M. Central necrosis in isolated hypoxic human pancreatic islets: Evidence for postisolation ischemia. Cell Transplant. 2005;14:67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 222.Fang Y., Zhang Q., Tan J., Li L., An X., Lei P. Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and protective effects of antioxidant intervention. Nutr. Diabetes. 2014;4:e131. doi: 10.1038/nutd.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zheng X., Zheng X., Wang X., Ma Z., Gupta Sunkari V., Botusan I., Takeda T., Björklund A., Inoue M., Catrina S.B., et al. Acute hypoxia induces apoptosis of pancreatic β-cell by activation of the unfolded protein response and upregulation of CHOP. Cell Death Dis. 2012;3:e322. doi: 10.1038/cddis.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bensellam M., Montgomery M.K., Luzuriaga J., Chan J.Y., Laybutt D.R. Inhibitor of differentiation proteins protect against oxidative stress by regulating the antioxidant-mitochondrial response in mouse beta cells. Diabetologia. 2015;58:758–770. doi: 10.1007/s00125-015-3503-1. [DOI] [PubMed] [Google Scholar]
- 225.Wang Y.J., Schug J., Won K.J., Liu C., Naji A., Avrahami D., Golson M.L., Kaestner K.H. Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes. 2016;65:3028–3038. doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang T., Kim D.H., Xiao X., Lee S., Gong Z., Muzumdar R., Calabuig-Navarro V., Yamauchi J., Harashima H., Wang R., et al. FoxO1 Plays an Important Role in Regulating beta-Cell Compensation for Insulin Resistance in Male Mice. Endocrinology. 2016;157:1055–1070. doi: 10.1210/en.2015-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gilon P., Shepherd R.M., Henquin J.C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J. Biol. Chem. 1993;268:22265–22268. doi: 10.1016/S0021-9258(18)41522-0. [DOI] [PubMed] [Google Scholar]
- 229.Jacob S., Kohler M., Troster P., Visa M., Garcia-Prieto C.F., Alanentalo T., Moede T., Leibiger B., Leibiger I.B., Berggren P.O. In vivo Ca(2+) dynamics in single pancreatic beta cells. FASEB J. 2020;34:945–959. doi: 10.1096/fj.201901302RR. [DOI] [PubMed] [Google Scholar]
- 230.Frank J.A., Broichhagen J., Yushchenko D.A., Trauner D., Schultz C., Hodson D.J. Optical tools for understanding the complexity of beta-cell signalling and insulin release. Nat. Rev. Endocrinol. 2018;14:721–737. doi: 10.1038/s41574-018-0105-2. [DOI] [PubMed] [Google Scholar]
- 231.Johnston N.R., Mitchell R.K., Haythorne E., Pessoa M.P., Semplici F., Ferrer J., Piemonti L., Marchetti P., Bugliani M., Bosco D., et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016;24:389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salem V., Silva L.D., Suba K., Georgiadou E., Neda Mousavy Gharavy S., Akhtar N., Martin-Alonso A., Gaboriau D.C.A., Rothery S.M., Stylianides T., et al. Leader beta-cells coordinate Ca(2+) dynamics across pancreatic islets in vivo. Nat. Metab. 2019;1:615–629. doi: 10.1038/s42255-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Benninger R.K., Zhang M., Head W.S., Satin L.S., Piston D.W. Gap junction coupling and calcium waves in the pancreatic islet. Biophys. J. 2008;95:5048–5061. doi: 10.1529/biophysj.108.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Head W.S., Orseth M.L., Nunemaker C.S., Satin L.S., Piston D.W., Benninger R.K. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61:1700–1707. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Corezola do Amaral M.E., Kravets V., Dwulet J.M., Farnsworth N.L., Piscopio R., Schleicher W.E., Miranda J.G., Benninger R.K.P. Caloric restriction recovers impaired beta-cell-beta-cell gap junction coupling, calcium oscillation coordination, and insulin secretion in prediabetic mice. Am. J. Physiol. Endocrinol. Metab. 2020;319:E709–E720. doi: 10.1152/ajpendo.00132.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Retamal M.A., Garcia I.E., Pinto B.I., Pupo A., Baez D., Stehberg J., Del Rio R., Gonzalez C. Extracellular Cysteine in Connexins: Role as Redox Sensors. Front. Physiol. 2016;7:1. doi: 10.3389/fphys.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Taneera J., Lang S., Sharma A., Fadista J., Zhou Y., Ahlqvist E., Jonsson A., Lyssenko V., Vikman P., Hansson O., et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16:122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 238.Taneera J., Fadista J., Ahlqvist E., Zhang M., Wierup N., Renström E., Groop L. Expression profiling of cell cycle genes in human pancreatic islets with and without type 2 diabetes. Mol. Cell. Endocrinol. 2013;375:35–42. doi: 10.1016/j.mce.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 239.Kanatsuna N., Taneera J., Vaziri-Sani F., Wierup N., Larsson H.E., Delli A., Skärstrand H., Balhuizen A., Bennet H., Steiner D.F., et al. Autoimmunity against INS-IGF2 protein expressed in human pancreatic islets. J. Biol. Chem. 2013;288:29013–29023. doi: 10.1074/jbc.M113.478222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hänzelmann S., Wang J., Güney E., Tang Y., Zhang E., Axelsson A.S., Nenonen H., Salehi A.S., Wollheim C.B., Zetterberg E., et al. Thrombin stimulates insulin secretion via protease-activated receptor-3. Islets. 2015;7:e1118195. doi: 10.1080/19382014.2015.1118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Karolina D.S., Armugam A., Tavintharan S., Wong M.T., Lim S.C., Sum C.F., Jeyaseelan K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS ONE. 2011;6:e22839. doi: 10.1371/annotation/698b7123-174f-4a09-95c9-fd6f5017d622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dominguez V., Raimondi C., Somanath S., Bugliani M., Loder M.K., Edling C.E., Divecha N., da Silva-Xavier G., Marselli L., Persaud S.J., et al. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J. Biol. Chem. 2011;286:4216–4225. doi: 10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]