Abstract
Sperm chemotaxis, which guide sperm toward oocyte, is tightly associated with sperm capacitation, motility, and fertility. However, the molecular mechanism of sperm chemotaxis is not known. Reproductive odorant and taste receptors, belong to G-protein-coupled receptors (GPCR) super-family, cause an increase in intracellular Ca2+ concentration which is pre-requisite for sperm capacitation and acrosomal reaction, and result in sperm hyperpolarization and increase motility through activation of Ca2+-dependent Cl¯ channels. Recently, odorant receptors (ORs) in olfactory transduction pathway were thought to be associated with post-thaw sperm motility, freeze tolerance or freezability and cryo-capacitation-like change during cryopreservation. Investigation of the roles of odorant and taste receptors (TRs) is important for our understanding of the freeze tolerance or freezability mechanism and improve the motility and fertility of post-thaw sperm. Here, we reviewed the roles, mode of action, impact of odorant and taste receptors on sperm chemotaxis and post-thaw sperm quality.
Keywords: sperm chemotaxis, odorant receptor, taste receptor, olfactory transduction, mammalian sperm, post-thaw sperm motility
1. Introduction
All organisms are dependent on odorant and taste receptors for survival as these are primarily involved in detecting diverse environmental cues like, finding and selection of food, avoiding life threatening predators and pollutants. The ORs and TRs are primarily expressed in nasal and oral sensory cells and respond only to their specific ligands [1,2] and convey electrical signals to higher perception centers.
These ORs and TRs are not only limited to nasal and oral tissues but are also expressed in ectopic organs which indicates their diverse biological functions. However, the concept of chemotaxis was originated from sea animals as they release their sperm and egg in the environment for fertilization, but the role of chemotaxis via ORs in mammals’ sperm and egg communication was established in late 1900s [3,4]. Moreover, discovery of ORs and TRs in sperm, prostate gland, and testes of human [5,6,7,8,9,10] have strengthen the belief about their role in sperm chemotaxis and reproduction success. It was reported that OR4C13, MOR23, OR1E2, and OD1 have been localized in testes of human [10], mouse [11], dog [12], and rat [13] respectively. Similarly, TAS2R3, TAS2R124, and α-gustducin have been localized in testes of human [14], mouse [15], and pig [16]. These ORs and TRs can modulate the motility, fertility, and survivability of sperm, and are even involved in gametogenesis at mitotic and meiotic levels [14]. Egg releases chemicals to attract sperm cell and existence of chemoattractant gradient give cues about interaction of sperm-egg through chemotaxis [4]. Additionally, chemotaxis in mammals is more complicated compared to sea animals as sperm movement in mammals is also dependent on thermal gradient along with chemicals released from the fallopian tube [17,18], oocyte, and cumulus oophorous cells [19]. Binding of these chemicals with their receptors present on sperm stimulate the voltage and ion gated channels, which induces capacitation like changes and cause hypermotility [20,21].
Recently, Ran and colleagues [22,23] reported that olfactory transduction pathway in pig and giant panda maybe associated with sperm freeze tolerance or freezability, and the mRNA expression level of some odorant receptors were dysregulated during cryopreservation. These findings indicated that ORs and TRs maybe associated with post-thaw sperm motility, acrosomal reaction, cryo-capacitation-like changes, and fertility. Although studies on ORs and TRs have already achieved some milestones, but only few manuscripts are available regarding their role in sperm chemotaxis and survival during and after cryopreservation. In one study, we have determined the differential expression of piRNA (related to olfactory transduction pathway) in fresh and frozen thawed pig and giant panda sperm (unpublished work) which indicate substantial future scope of this subject in improving artificial breeding. This review summarizes the classification and distribution of ORs and TRs, and regulating sperm chemotaxis, capacitation, motility, and fertility via ion and voltage gated channels in sperm. In addition, the challenges, limitations and future directions in sperm cryoinjury, freeze tolerance, or freezability during cryopreservation are also included.
2. Sperm Chemotaxis and Sperm–Egg Interaction
Currently, three guidance mechanisms are known for the sperm movement including thermotaxis (swimming due to temperature gradient), Rheotaxis (swimming due to fluid flow gradient), and chemotaxis (swimming due to concentration difference of chemoattractants) which have been demonstrated in human, rabbit, mice, bull and pig [3,17,24,25,26,27,28,29,30]. How chemotaxis occur in sperm through the involvement of ORs and TRs is the focus of this review as expression or suppression of these receptors effect motility, fertility, and viability of post-thaw sperm.
Mammalian sperm chemotaxis is a recent subject relative to other guidance mechanisms of sperm as it is based on the competitive nature of sperm in the female reproductive tract to reach the site of fertilization [20]. In fact, millions of sperm are ejaculated during natural mating but only fewer reach the egg to fertilize it [31]. Sperm maturation requires capacitation which leads to hyperactivation and enables them to penetrate viscoelastic environment in oviduct and finally cumulus oophorous cells surrounding the egg in mouse and pig [32,33]. This penetration results in acrosomal reaction in sperm and help in fusion with egg which results in fertilization [34]. Furthermore, chemo-attraction occurs only in capacitated sperm with unreacted acrosome and not in sperm with reacted acrosome as described in different mammalian species like human, mouse, bull, and rabbit [34,35,36,37,38] with the help of sperm selection assay (SSA) which suggest disorientation of sperm in reproductive tract due to premature acrosomal reaction [34].
Additionally, role of sperm chemotaxis in mammals is to select a group of capacitated sperm to guide toward egg but in case of non-mammalian species is to attract as much sperm as possible near the egg [39]. During copulation or artificial insemination a large number of sperm are ejaculated/deposited in the female reproductive tract and they get stored (in isthmus) in the tract to wait for the egg to be released [40,41]. At the time of ovulation only capacitated and hyperactivated sperm move toward the egg, possibly under some chemical stimulation released from the egg [39,42]. Moreover, observations in rats, mice, and pigs further strengthen the role of chemoattractants in sperm motility and fertilization capability as only single sperm is guided to one egg and after penetration of egg walls chemical signal stops and impedes the movement of other sperms toward egg [43].
Several studies have reported chemoattraction of human, mouse, pig, and rabbit sperms toward the gradient of progesterone (P4) secreted from cumulus oophorous cells of egg near ovulation [36,44,45,46,47]. This movement of sperm is due to mobilization of Ca2+ from cytoplasm preceded by membrane channels activation [48]. Membrane channels participating in regulation of Ca2+ movement in spermatic cell includes CatSper [49,50], cyclic nucleotide gated (CNG) [51,52], and calcium voltage-gated channels (CaV) [53,54]. These channels have been localized in head, mid piece, and more abundantly in tail region of human, pig, and other mammals’ sperm [55,56,57], which suggest capacitation and hypermotility are dependent on activation of these channels (Table 1). Moreover, the co-existence of ORs, TRs, and membrane channels indicate their coordinated functionality. The currently known molecular mechanism and role of ORs and TRs in chemotaxis of sperm are discussed in Section 4 and Section 5 of this review.
Table 1.
Ion and voltage-gated channels of mammalian sperm.
| Location | Specie | ||||
|---|---|---|---|---|---|
| Human | Bovine | Dog | Mouse | Rat | |
| Head | Cav2.1c, Cav3.1d, Cav3.3d, TRPC1a, TRPC3a, TRPC4a, TRPC6a, IP3R j | IP3R j | IP3R j | Cav1.2b, Cav2.1c, Cav2.2d, Cav2.3d, Cav3.1d, Cav3.2d, TRPC2a, IP3R j | IP3R j |
| Mid-piece | Cav1.2b, Cav2.3d, Cav3.1d, Cav3.2d, Cav3.3d, TRPC3a, TRPC4a, TRPC6a | CNGA3e | Catsper2h, Cav2.1c, Cav2.3d, Cav3.1d, Cav3.3d, TRPC1a, TRPC3a, TRPC6a | ||
| Upper tail | Cav1.2b, Cav3.1d, Cav3.2d, TRPC1a, TRPC4a, TRPC6a | CNGA3e, CNGB1f | Catsper1g, Catsper2h, Cav1.2b, Cav2.2d, Cav2.3d, Cav3.1k, Cav3.2d, Cav3.3d, TRPC1a, TRPC3a |
||
| Lower tail | Cav2.3d | CNGA3e | Cav3.2d, TRPC3a | ||
3. Odorant and Taste Receptors
Buck and Axel [74] discovered the genes of olfactory system in rat which is considered as the beginning of molecular research on olfactory transduction and ORs. It is assumed that the odorant receptors play their role in organ construction and cell–cell identification as ORs develop in spleen, heart, and chicken notochord [75,76]. The proposed function of these embryonic ORs is to position the somatic cells and neural tube. The ORs have been classified into two major classes based on their distinct amino acid arrangements and distribution. The class I ORs were first discovered in fish [77] and then in frog [78], and later it was determined that fishes including goldfish and teleost fish have only class I odorant receptor genes [79]. At the beginning, it was believed that only class II ORs exist in mammals and they lack class I receptors [77]. Moreover, scientists also believed that class II ORs are meant to detect volatile odorants and class I for water-soluble odorants. But, the recent genomic analysis contradicts this believe and proved that human and mouse genome have many class I OR genes [80,81,82,83,84]. Coelacanth, an evolutionary link between fish and tetrapod has many class I OR genes along with 7 class II OR genes [85]. The majority of ORs in mammals are class I receptors and it indicates that class I receptors play a crucial role in the olfaction [80,86]. The duplication and pseudogenization of genes were more in class II than class I genes as reported by Niimura et al., [87] by using reconciled-tree method.
Similar to ORs, TRs were first characterized in early 2000s [88] which resulted in discovery of six types of TRs till today. Out of these six types (salty, bitter, sweet, umami, sour, and fat) sour and salty taste sensations are carried out via membrane ion channels while the rest of them are governed by GPCRs like ORs [89]. Hofer et al., [90] first described the expression of α-gustducin protein in GIT of rats which suggested the taste perception-related activity in other bodily organs. Like ORs, no special expression of any type of TRs have been studied in aquatic or terrestrial animals. However, TRs have been categorized into six types for showing unique expression channels and mediators. Currently, sour, sweet, umami, salty, bitter, and fat-sensing receptors are known to exist [88]. However, salt and sour TRs are sensed by membrane ion channels whereas, remaining are sensed with GPCRs [91]. Umami and sweet GPCRs are heteromers detecting wide range of amino acids [92] and belongs to family 1 (TAS1Rs).
3.1. Distribution of Odorant and Taste Receptors
The genomic analysis revealed existence varying number of OR genes in different species [93] and specie’s environment also effect the number of functional genes [94]. Almost 4% of mammalian genes are devoted to encode ORs [95] and in human around 400 functional genes of ORs are expressed at mRNA level [5,80]. Moreover, all human chromosomes harbor clusters of ORs genes except the Y and 20 chromosomes [80]. Similarly, all the mouse chromosomes have loci for ORs except 18 and Y chromosomes [96]. About 1000 ORs in rodents are encoded by GPCR family for the olfaction [86,97,98]. The number and distribution of OR genes indicated that higher primates rely mostly on their vision instead of chemical identification of environment via olfaction [99]. During the evolution process the gene gain and loss occurred in several species according to their adaption of the new environment [100]. Today’s ORs gene family has evolved after a course of birth-and-death evolution during which many genes were duplicated, and others become pseudogenes [101]. The pseudogenes resulted from nonsense-mutation, deletion, frameshift, and or combination of these [87]. The number of intact and pseudogenes of OR and TR families are shown in Table 2. No doubt, the functional differences among these receptors do exist but they all belong to the same GPCR protein family.
Table 2.
The number of odorant and taste receptor genes in different species.
| Specie | Olfactory Genes Distribution | Reference | Taste Genes distribution | Reference | ||
|---|---|---|---|---|---|---|
| Intact genes | Pseudogenes | Intact genes | Pseudogenes | |||
| Human | 396 | 425 | [99] | 38 (T2R) | 16 (T2R) | [102,103] |
| Chimpanzee | 380 | 414 | [104] | 28 (T2R) | 10 (T2R) | [105,106] |
| Cow | 1186 | 1057 | [87] | 12 (T2R) | 15 (T2R) | [107] |
| Dog | 811 | 278 | [100] | 15 (T2R) | 5 (T2R) | [107] |
| Horse | 1066 | 1569 | [87] | 19 (T2R) | 36 (T2R) | [108] |
| Mouse | 1130 | 236 | [87] | 35 (T2R) | 5 (T2R) | [102] |
| Rat | 1207 | 508 | [100] | 36 (T2R) | 7 (T2R) | [109] |
| Rabbit | 768 | 256 | [87] | 28 (T2R) | 13 (T2R) | [110] |
| Pig | 1113 | 188 | [111] | 15 (T2R) | 7 (T2R) | [110] |
Many studies have reported the presence of odorant receptor transcripts in heart, brain, germ cells, sperms, and mammalian testes [9,11,13,86,112]. Their essential physiological roles include skin wound repair [113], inhibition of proliferation of oncogenic cells in liver, prostate and intestine [8,114,115], and modulation sperm motility [7]. Despite of all the efforts made to unveil the functional specificity of these receptors in the non-olfactory tissues, only few ORs could be aligned with their ligands. More strenuous work is demanded to complete the profiling of these receptors in the reproductive organs.
Similar to ORs, TRs discovery in endocrine cells of mouse [116] led to the subsequent findings in other organs of the body. Recent exploration for TRs in ectopic tissues have resulted in localization in brain [117,118], kidney [119], respiratory system [91,120], cardiovascular system [121], immune system [122,123,124], skin [125], thyroid gland [126], urethra [127], testis [15,128], prostate and ovary [129], and adipose tissues [130,131]. These organs harbor different types of TRs, i.e., sweet, free fatty acids, bitter, and sour taste receptors.
Although TRs have been extensively studied in regulation of appetite [132,133], diabetes [134], asthma [135], and other inflammatory processes [136,137] in the body but only little is known about reproductive TRs. TRs are being targeted by most of the available drugs used as therapeutic agents which indicates their potential role in the body. For example, TRs control the production of endocrine hormones from gut in response to volume and composition of food [138,139]. GI tract lining shows expression of carbohydrate, fatty acids, amino acids, and toxins sensing TRs (TAS1R1 to TAS1R3) if there are any in the feed [140,141,142]. Similarly, adipogenesis dependence on TRs have been identified through the expression of α-gustducin and TAS2Rs in pre-adipocytes and adipose tissues in mouse [91]. Similarly, free fatty acid TRs abundance in European people suggests their role in development of obesity due to mutations in taste responding genes [143]. Moreover, innate immunity and bronchodilation is governed by bitter taste receptors as they are expressed in immune cells, smooth muscles, and lung epithelial cells [144]. For instance, human nasal cilia express TAS2R38 which upon activation causes increase in Ca2+ that is responsible for increased beat frequency of cilia to clear mucous [145].
3.2. Reproductive Odorant and Taste Receptors
Testicular and sperm ORs of various species including dog, human, chimpanzee, mouse, and rat have been identified and some of them successfully related to their ligands. The ORs related to several mammalian species along with different techniques which were used to identify them are summarized in Table 3.
Table 3.
Mammalian reproductive odorant receptors/proteins.
| Species | Organ | Receptors | Analysis Method | Reference |
|---|---|---|---|---|
| Human | Sperm | OR7E24, OR4S1, OR4C13, OR1I1, HT2, OR1D4, OR51E1, OR51E2, OR6B2, OR10J1, OR2H1/2, OR2W3 | Confocal microscopy, WB, ICS | [10,167,168] |
| Prostate | OR51E2, o1r59, Olfr78, | RT-PCR, WB, NB | [8,169] | |
| Testis | OR4C13, OR7A5, OR4D1, OR1D2, OR1D2, OR4D1, OR1D2, OR1E1 hOR17-4, hOR17-2, Olfr16, OR4N4, OR3A2, OR10J1 |
RT-PCR, q-PCR, Ca-Imaging, RNA-seq | [6,9,10,170] | |
| Placenta | hOR-17 | RT-PCR | [171] | |
| Mouse | Sperm | MOR23 | RT-PCR, in situ hybridization | [11] |
| Prostate | N/A | N/A | N/A | |
| Testis | MOR23, MOR244-3, MOR139-3, MOR248-11, MOR267-13, MOR283-1, MOR8-1, MOR31-2, OR10J5, |
RT-PCR, NB | [11,12,172,173] | |
| Placenta | Olfr154, Olfr520, Olfr433, O1fr381 | Microarray | [174] | |
| Dog | Sperm | DTMT | RNase protection assay, WB | [44,148] |
| Prostate | N/A | N/A | N/A | |
| Testis | DTPCR64, HGMP07, DTMT, OR1E2, | RT-PCR | [5,12] | |
| Placenta | cOR2AV3 | Microarray, RT-PCR | [175] | |
| Rat | Sperm | Putative olfactory Proteins GRK3, beta-arrestin2, Golf | WB, ICC, IH | [13,176] |
| Prostate | N/A | N/A | N/A | |
| Testis | OD1, OD2, Olr825, Olr1696 | RT-PCR, WB, IH, ISH | [13,177] | |
| Placenta | O1r1767, Olr1513, Olr1687, Olr1571 | RT-PCR | [171] |
Note: WB, Western blotting; NB, Northern blotting; RT-PCR, reverse transcription polymerase chain reaction; q-PCR, quantitative polymerase chain reaction; RNA-seq, RNA sequencing; Ca-imaging, calcium imaging; IHC, immunohistochemistry; ICC, immunocytochemistry; ISH, in situ hybridization; ICS, immunocytochemical staining; N/A, data are not available.
To verify the role of ORs, studies are being conducted at transcript and protein levels. Parmentier et al., [5] discovered 3 and 21 mRNAs of ORs in dog and human testis, respectively. Within a decade, researchers belonging to different countries discovered the odorant receptor transcripts in testis of mouse, hamster and rat [12,13,146,147,148,149,150]. More than 80 cell types and tissues of different species have been explored by using next generation sequencing and at least one OR was expressed in every sample. However, the number of odorant receptors expressed differed in different studies with highest in testes, heart, and brain, respectively [9,112,151,152]. This may be due to multiple screening approaches and inflexibility of the analysis. The ordinary nasal olfactory receptors contain less intact sequences of amino acids than testicular ORs which indicate an important physiological function of these receptors in testes [153]. Furthermore, by using high throughput oligonucleotide microarray, 66 genes equipped with odorant receptors were discovered in mouse olfactory epithelium [154].
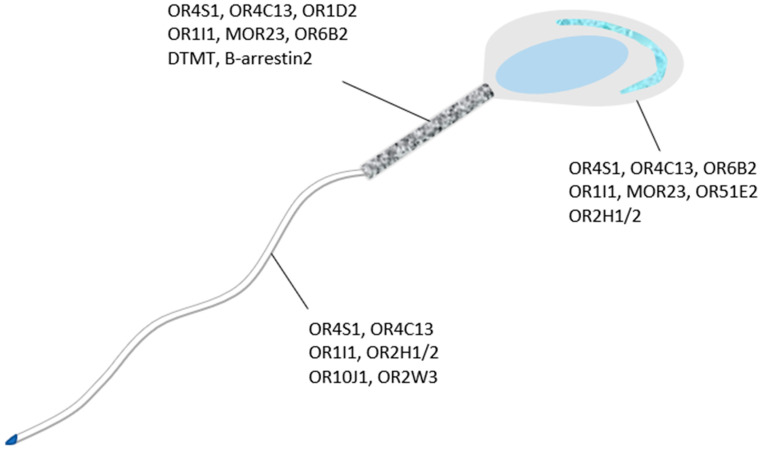
Oocyte and cumulus oophorous cells separately secrete odorants to attract the sperm [155]. The strong candidate for odorants of sperm like heparin, acetylcholine, and progesterone have been excluded from the list after experimental evaluation [39,156]. During in-vitro studies, it was proved that sperm cell demonstrate chemotaxis toward the chemicals withdrawn from follicular fluid (FF) [157]. The chemotaxis in human sperm was induced by the application of progesterone and RANTES (chemokines) which are the active ingredients of follicular fluid [158] which contradicts the aforementioned studies. Moreover, FF also contains heparin, Substance P, and β-endorphins which have proven to be substantial chemoattracts molecules for mouse, human, and pig sperm in vivo and in vitro [159,160,161]. Similarly, oocytes synthesizes more than ten types of prostaglandins which promote the sperm guidance and motility in the female reproductive tract [162,163]. Additionally, roles of estradiol, adrenaline, calcitonin, oxytocin, acetylcholine atrial natriuretic peptide, and nitric oxide have been verified up to some extent in sperm chemotaxis [39,164,165]. Furthermore, the sperm chemoattractant chemicals secreted by the oocytes and cumulus cells [155] are considered as a tool to attract only hyperactivated sperm toward the egg instead of attracting all the sperm in the female reproductive tract [166]. With the implementation of antibody staining techniques, odorant receptors were localized in head, tail, and mid-piece (Figure 1) of dog and other mammals’ spermatids [13,148]. However, due to unique amino acid sequence of testicular ORs they cannot be included in a specific odorant receptor subfamily [12].
Figure 1.
Different ORs/Protein localized on sperm head, mid-piece and tail. ORs in head of sperm cell are distributed at acrosomal, equatorial, and posterior head regions. Similarly, ORs of mid-piece and tail are also located along the entire lengths of these segments.
Many studies have confirmed the expression and localization of TRs in mammalian testes and spermatic cells. Mammalian sperm cells undergo around 200 genes mutations which at any stage can hinder the process of spermiogenesis [178] and lead to sub-fertility and infertility. Xu et al., [15] have identified 35 bitter sensing genes in mouse testes that expresses post-meiotic sperm cells. This finding indicates potential role of TRs in successful development of sperm cells in male reproductive system and suggestive of their critical role in female reproductive system. From vagina to the site of fertilization, sperm cell senses slight variations in the macro and micro-environment including carbohydrates [179], amino acids [180,181,182], and changes in composition and pH [183,184]. Sperm detects chemo-attractants secreted by the egg and its surrounding environments in order to successfully fertilize the egg [4,20]. These chemo-attractants and sperm interaction were further consolidated by the work of Meyer et al., [185] who discovered umami taste receptor family dimers (TAS1R1 and TAS1R3) in sperms of human and mouse. This study determined the rate of acrosomal reaction, intracellular Ca2+concentration, and cAMP level along with the expression level of TAS1R1 which indicates their role in maturation of sperm in the female reproductive tract.
Other similar studies have confirmed the expression of sweet, umami, bitter, and fatty acid sensing receptors in mammalian testis [186,187,188,189,190]. Furthermore, Fehr et al., [191] discovered the expression of α-gustducin protein during spermatogenesis and GPCRs distribution along the flagellum of human, rat, mouse, and bull sperm cells. Additionally, Governini et al., [14] analyzed the expression of TAS2Rs family bitter taste receptors in human testis and ejaculated sperm which also consolidate the idea of clinical significance of TRs in human reproduction. Only handful of studies available regarding expression of TRs and related proteins in reproductive organs of mammals (Table 4).
Table 4.
Mammalian reproductive taste receptors/proteins.
| Specie | Location | Receptors/Protein | Analysis Method | Reference |
|---|---|---|---|---|
| Human | Sperm | Tas1r1, Tas1r3, α-gustducin, α-transducin | RT-PCR, WB | [14,185] |
| Testis | Tas1r14 | Droplets digital PCR | [14] | |
| Mouse | Testis | Tas2r102, Tas2r 105, Tas2r 106, Tas2r 113, Tas2r 114, Tas2r 116, Tas2r 124, Tas2r 125, Tas2r 134, Tas2r 135, Tas2r 136 |
RT-PCR | [15] |
| Boar | Sperm | α-gustducin, α-transducin |
WB, immunohistochemistry | [16] |
| Testis | T1R3 | RT-PCR, in situ hybridization | [192] |
4. Olfactory Transduction and Sperm Signaling Pathways
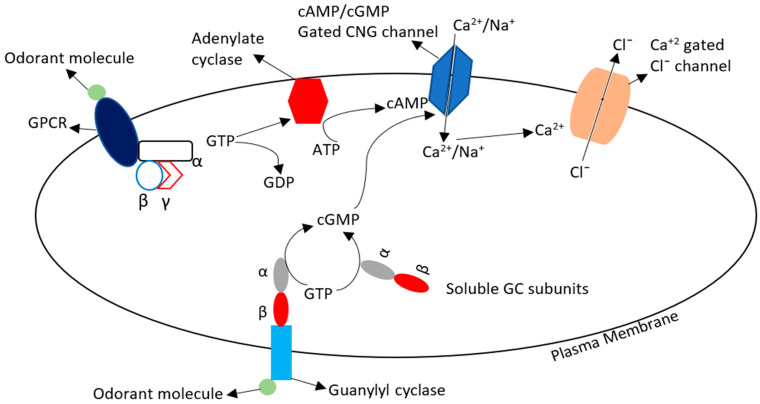
Binding of specific odorant molecule with its receptor initiates a cascade of reactions in the membrane and opens ion channels. The cyclic nucleotide gated Ca2+ ion channel is specific for mammals and is opened at higher concentration of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). The olfactory neurons contain olfactory specific G-protein (Golf) which on stimulation causes activation of adenylate cyclase which in turn increases the level of cyclic AMP. While, the membrane-bound guanylyl cyclase (GC) responds directly to the odorant molecule binding and cytosolic soluble guanylyl cyclase converts GTP into cGMP [193]. This increased cAMP and cGMP causes increases in oxygen consumption [194] and opening of CNG channels which results in the influx of Ca2+ and Na+ and thus causes depolarization of the membrane. The Ca2+-gated Cl− channels opening causes further amplification of depolarization and leads to generation of an action potential and hypermotility of sperm. This Cl− efflux causes 50–90% change in membrane potential of olfactory sensory neurons (Figure 2).
Figure 2.
General mechanism of odorant receptor activation and signal transduction pathway. Odorant molecules combined with membrane GPCR and their cytosolic subunit α activates the adenylate cyclase (AC) by converting GTP into GDP. This AC mediates conversion of ATP into cAMP which causes opening of CNG channels. Similarly, when Odorant molecule interacts with (guanylyl cyclase) GC their cytosolic subunits convert the GTP to cGMP which enhances the opening of CNG channels. Ca2+ enters the cell through these CNG channels and increased Ca2+ concentration influences the opening of Ca2+ gated chloride (Cl−) channels. So, the influx of positive ion (Ca2+) and efflux of negative ion (Cl−) changes the membrane potential causing waves of polarization and depolarization which helps in sperm motility. GTP, guanosine triphosphate; GDP, guanosine monophosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate.
In past studies, it was proposed that the two classes of odorant receptors initiate two different signaling pathways including cAMP and IP3 [195,196,197,198]. Further it was proved that, IP3 pathway may play just a modulatory role [199], and olfactory signaling is solely mediated by cAMP pathway. However, a cross-talk between cAMP and tyrosine kinase has been determined which resulted in Ca2+ regulation and capacitation of sperm [200]. This interaction between moieties of these signaling pathways requires the presence of Ca2+ and HCO3− and efflux of cholesterol from the cell [200,201]. Moreover, progesterone produced by the cumulus oophorous cells causes activation of CatSper channels which results in Ca2+ influx and hyperpolarization [202,203]. Bicarbonate (HCO3−) contents increases on mixing of epididymal and prostatic secretions which are essential for sperm hyperpolarization. The cAC, cAMP, and PKA pathways control the hyperactivity of sperm and are dependent on concentration of Ca2+ and HCO3−. The sAC is sperm specific and converts AMP into cAMP which results in the activation of serine/threonine kinase [204,205]. Other sperm signaling pathways include PIK3C-AKT and MAPK pathways of which the former act through the CatSper channels and causes influx of Ca2+. All these pathways result in phosphorylation of serine protein, a primary component of sperm motility [206]. cAMP appears to play a basic role in sperm motility via odorant receptors and activation and when mice were knocked out for cAMP pathway, they did not respond to any of the odorants [207,208,209].
5. Gustatory Transduction
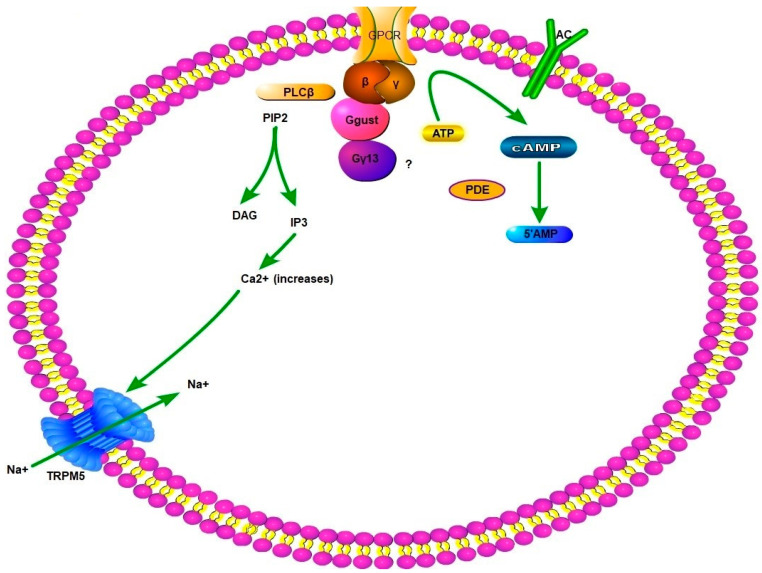
GPCRs involved in gustation possess many subunits including G-α transducing and gustducin, G-β3, G-γ13, and others. G-α and G-β mainly participate in taste perception and are closely linked in the cytosol. Upon interaction with a tastant molecule G-β subunit dissociates from G-α and initiate phospholipase-inositol activity which results in influx of Ca2+ in the cytosol [89]. This elevated Ca2+ results in the opening of TRPM5 (transient receptor potential cation channel subfamily M member 5) which depolarizes the taste perceiving cells [210]. Phosphodiesterase may be activated by G-α subunit, which leads to the breakdown of cAMP to avoid inhibition of phospholipase-inositol pathway [91,211]. The opened TRP channels lead to influx of Na+ and further depolarization due to opening of voltage-gated Na+ channels (Figure 3). These cellular processes cause release of ATP through Ca-homeostasis modulator 1 and 3 [212,213] which activates sensory neurons conveying messages to higher taste perception centers of brain. This taste perception or gustation has also been described in ectopic regions, but little is known about the expression of taste-related genes in reproductive system especially sperm.
Figure 3.
General mechanism of intracellular gustatory response. When tastant molecules combine with trans-membrane GPCR and the complex cytosolic subunit of βγ, Ggust, and Gγ13 cause conversion of ATP and PIP2 (with PLCβ, phospholipase Cβ) into cAMP and IP3, respectively. This IP3 mediated the increase of Ca2+ inside the cell through opening of channels in the Ca2+ storing organelles of cell, i.e., endoplasmic reticulum (not shown in the figure). This increased Ca2+ opens the TRPM5 channels which allow entry of Na+ ions. Both these positive ions change the membrane potential. Similarly, conversion of ATP into cAMP causes opening of AC channels which affects the downstream channels which may be similar to olfactory transduction signaling pathway. PLCβ, phospholipase Cβ; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-triphosphate; DAG, diacylglycerol; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate.
6. Cryopreservation and Olfactory Transduction
The cryopreservation elicits capacitation-like response in sperm i.e., increases intracellular Ca2+, pH, and cAMP along with plasma membrane and acrosomal membrane disintegration [214,215,216]. As we know, cryocapacitation-like changes after cryopreservation may largely contribute to lower sperm survival, motility, and fertility. It was reported that ORs, expressed in testes and mature sperm, are involved in sperm motility, sperm-egg recognition etc., [10,11,13,155]. Some specific odorant receptors in mid-piece and flagellum of sperm were found to be associated with spermatogenesis, epidydimal maturation, and sperm function [6,11]. Furthermore, some odorant-like molecules, including progesterone, heparin, ANP in the reproductive tract and oviduct can enhance or stimulate sperm chemotaxis or thermotaxis. These odorant-like molecules, probably combine with odorant receptors in sperm and increase intracellular Ca2+ level which results in rapid beat frequency of sperm flagellum and thus movement of sperm [19]. In frozen-thawed stallion sperm, the increased level of intracellular Na+ disturbed the membrane ion channels and mitochondrial membrane potential which indicate apoptotic-like changes [217]. Moreover, study showed lower cAMP concentration in frozen-thawed sperm as compared to fresh sperm in rat [218]. Cryopreservation negatively affects the cAMP-dependent PKA and AMPK activities in Atlantic salmon sperm [219]. These results indicate possible prior utilization of Ca2+ and Na+ due to activation of their membrane channels as a result of interaction with ligands during process of cryopreservation. In one study on pig sperm, when AMPK was inhibited, the sperm motility was also decreased [220]. Moreover, the human and boar frozen-thawed sperm have shown decreased response to progesterone compared to freshly ejaculated sperm, which lead to early depolarization and Ca2+ influx [221]. This Ca2+ influx is definitely related to activation of its voltage and ion gated channels which have not been proven yet. How these channels play role in Ca2+ transport and recognize chemical signals is an under-debate subject.
Recently, the report from high throughput sequencing in giant panda indicated that Ca2+-gated signaling, cAMP, cGMP, and neuroactive ligand receptor interaction pathways are enriched in post-thaw giant panda sperm [22]. It is demonstrated that giant panda sperm appears to have strong freeze tolerance capacity and is capable of surviving repeated freeze-thaw cycles [23]. These reports suggested that the cryo-resistance of sperm might be related to cAMP, cGMP, and Ca2+ abundance during cryopreservation and unique interaction between ligands and their receptors. More evidence showed that olfactory transduction pathway-related CNGB1 and CNGA3 channel proteins are enriched in post-thaw giant panda sperm. CNGA3 is a predicted target of three novel differentially expressed miRNAs (conservative_NW_003218630.1_359427, conservative_NW_003218630.1_359428, and unconservative_NW_003218488.1_343143). Therefore, the involvement of cAMP and Ca2+ may be responsible for regulating post-thaw quality of sperm [22]. Additionally, our ongoing studies have proved that cryopreservation causes m6A modifications in post-thaw sperm which are related to sperm cryo-injuries, freezability, and metabolic activities (unpublished work) along with altered expression of sperm olfactory genes.
7. Perspective
Odorant and taste receptors are extensively expressed in ectopic organs and their role in wound healing, cancer cells division inhibition, and obesity have been identified (19, 10, 26, 29). Targeting of TAS2R family and OLFR544 from ORs and TRs, respectively, shows promising future in controlling obesity and asthma [188,189,222]. However, mysteries regarding testicular and sperm odorant and taste receptors interaction and functionality still need to be revealed. Researchers are trying to determine the role of ORs and TRs in sperm-egg communication and in pre-fusion processes like capacitation induction, acrosomal reaction, and movement from uterine and oviductal storage. The role of these receptors in sperm chemotaxis and the role of egg, fallopian tube, uterus, and cervix in producing odorant [223] and tastant molecules need to be deciphered to maximize the success of artificial breeding.
Similarly, it is hypothesized that the components of cryoprotectants may act as ligands for the sperm ORs and elicit early capacitation which result in decreased motility, viability, and acrosomal integrity. Currently, no data are available on the topic of cryopreservation effects on modulation of gustation in sperm physiological parameters. But, the low survivability of pig’s sperm in comparison to other mammalian species indicate involvement of some hidden entities including ORs [22,224] and TRs.
Researchers opine that individual sperm may express distinct set of ORs and TRs and respond differently as speculated for odorant sensory neuron’s “one neuron-one receptor” model [225,226]. If this is true for sperm than the complexity of behavioral cues will require further exploration. Moreover, the opening of ion channels which are involved in olfactory and gustatory transductions i.e., CatSper1, CatSper2, and CNGA3 (Ca2+ channels) require different concentration of cAMP and cGMP [68,70,71,227] which has not been determined yet for all the mammalian sperm.
In conclusion, ORs and TRs are associated with spermatogenesis, maturation, sperm-egg recognition, sperm chemotaxis, capacitation, motility, and fertility. In addition, ORs and olfactory transduction pathway were thought to be responsible for sperm freeze tolerance and post-thaw sperm quality. However, what and how many odorant-like molecules in the extenders and reproductive tracts interact with ORs to facilitate sperm chemotaxis, capacitation, hyperactivation, and fertility, are still unknown and worth further studying.
Acknowledgments
We offer special thanks to Izhar Hyder Qazi for his kindly review and revise this manuscript.
Author Contributions
Conceptualization, C.Z.; validation, M.A.A. and Y.W.; Investigation, C.Z.; data curation, M.A.A., Y.W., Z.Q., and X.Y.; writing—original draft preparation, M.A.A. and Y.W.; writing—review and editing, Y.Z., C.Z.; visualization, M.A.A. and Y.W.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China grant number [31872356 and 31570533].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ihara S., Yoshikawa K., Touhara K. Chemosensory signals and their receptors in the olfactory neural system. Neuroscience. 2013;254:45–60. doi: 10.1016/j.neuroscience.2013.08.063. [DOI] [PubMed] [Google Scholar]
- 2.Dalton R.P., Lomvardas S.J.A. Chemosensory receptor specificity and regulation. Annu. Rev. Neurosci. 2015;38:331–349. doi: 10.1146/annurev-neuro-071714-034145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralt D., Manor M., Cohen-Dayag A., Tur-Kaspa I., Ben-Shlomo I., Makler A., Yuli I., Dor J., Blumberg S., Mashiach S., et al. Chemotaxis and Chemokinesis of Human Spermatozoa to Follicular Factors1. Biol. Reprod. 1994;50:774–785. doi: 10.1095/biolreprod50.4.774. [DOI] [PubMed] [Google Scholar]
- 4.Kaupp U.B., Kashikar N.D., Weyand I. Mechanisms of Sperm Chemotaxis. Annu. Rev. Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- 5.Parmentier M., Libert F., Schurmans S., Schiffmann S., Lefort A., Eggerickx D., Ledent C., Mollereau C., Gérard C., Perret J., et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nat. Cell Biol. 1992;355:453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- 6.Spehr M., Gisselmann G., Poplawski A., Riffell J.A., Wetzel C.H., Zimmer R.K., Hatt H. Identification of a Testicular Odorant Receptor Mediating Human Sperm Chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 7.Braun T., Voland P., Kunz L., Prinz C., Gratzl M. Enterochromaffin Cells of the Human Gut: Sensors for Spices and Odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Neuhaus E.M., Zhang W., Gelis L., Deng Y., Noldus J., Hatt H. Activation of an Olfactory Receptor Inhibits Proliferation of Prostate Cancer Cells. J. Biol. Chem. 2009;284:16218–16225. doi: 10.1074/jbc.M109.012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegel C., Manteniotis S., Osthold S., Hatt H., Gisselmann G. Expression Profile of Ectopic Olfactory Receptors Determined by Deep Sequencing. PLoS ONE. 2013;8:e55368. doi: 10.1371/journal.pone.0055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milardi D., Colussi C., Grande G., Vincenzoni F., Pierconti F., Mancini F., Baroni S., Castagnola M., Marana R., Pontecorvi A. Olfactory Receptors in Semen and in the Male Tract: From Proteome to Proteins. Front. Endocrinol. 2018;8:379. doi: 10.3389/fendo.2017.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda N., Yomogida K., Okabe M., Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004;117:5835–5845. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- 12.Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. Specific Repertoire of Olfactory Receptor Genes in the Male Germ Cells of Several Mammalian Species. Genomics. 1997;39:239–246. doi: 10.1006/geno.1996.4490. [DOI] [PubMed] [Google Scholar]
- 13.Walensky L.D., Roskams A.J., Lefkowitz R.J., Snyder S.H., Ronnett G.V. Odorant Receptors and Desensitization Proteins Colocalize in Mammalian Sperm. Mol. Med. 1995;1:130–141. doi: 10.1007/BF03401561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Governini L., Semplici B., Pavone V., Crifasi L., Marrocco C., de Leo V., Arlt E., Gudermann T., Boekhoff I., Luddi A., et al. Expression of Taste Receptor 2 Subtypes in Human Testis and Sperm. J. Clin. Med. 2020;9:264. doi: 10.3390/jcm9010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J., Cao J., Iguchi N., Riethmacher D., Huang L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2012;19:17–28. doi: 10.1093/molehr/gas040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinaci M., Bucci D., Mazzoni M., Giaretta E., Bernardini C., Vallorani C., Tamanini C., Clavenzani P., Galeati G.J.T. Expression of α-gustducin and α-transducin, G proteins coupled with taste receptors, in boar sperm. Theriogenology. 2014;82:144–151. doi: 10.1016/j.theriogenology.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Cerezales S., Laguna-Barraza R., de Castro A.C., Sánchez-Calabuig M.J., Cano-Oliva E., de Castro-Pita F.J., Montoro-Buils L., Pericuesta E., Fernández-González R., Gutiérrez-Adán A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-21335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan Y.-G., Wehry U.P., Buhren B.A., Schrumpf H., Oláh P., Bünemann E., Yu C.-F., Chen S.-J., Müller A., Hirchenhain J., et al. CCL20-CCR6 axis directs sperm–oocyte interaction and its dysregulation correlates/associates with male infertility. Biol. Reprod. 2020;103:630–642. doi: 10.1093/biolre/ioaa072. [DOI] [PubMed] [Google Scholar]
- 19.Olaniyan O.T., Dare A., Okotie G.E., Adetunji C.O., Ibitoye B.O., Eweoya O., Dare J.B., Okoli B.J. Ovarian odorant-like biomolecules in promoting chemotaxis behavior of spermatozoa olfactory receptors during migration, maturation, and fertilization. Middle East. Fertil. Soc. J. 2021;26:1–14. doi: 10.1186/s43043-020-00049-w. [DOI] [Google Scholar]
- 20.Eisenbach M., Giojalas L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 21.Kaupp U. 100 years of sperm chemotaxis. J. Gen. Physiol. 2012;140:583–586. doi: 10.1085/jgp.201210902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ran M.-X., Zhou L.K., Wang Z., Yang J.-D., Wu K., Huang Y., Luo B., Qazi I.H. Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca) Biomolecules. 2019;9:432. doi: 10.3390/biom9090432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago-Moreno J., Esteso M.C., Pradieé J., Castano C., Toledano-Díaz A., O’Brien E., López-Sebastián A., Martinez-Nevado E., Delclaux M., Fernandez-Morán J., et al. Giant panda (Ailuropoda melanoleuca) sperm morphometry and function after repeated freezing and thawing. Andrologia. 2015;48:470–474. doi: 10.1111/and.12468. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira R.G., Tomasi L., Rovasio R.A., Giojalas L.C. Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. Reproduction. 1999;115:23–27. doi: 10.1530/jrf.0.1150023. [DOI] [PubMed] [Google Scholar]
- 25.Giojalas L., Fabro G., Eisenbach M., Rovasio R.J.J.A. Capacitated and chemotactic rabbit spermatozoa appear to be shortly available around ovulation. J. Androl. 2001;22:92. [Google Scholar]
- 26.Bahat A., Tur-Kaspa I., Gakamsky A., Giojalas L.C., Breitbart H., Eisenbach M. Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract. Nat. Med. 2003;9:149–150. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- 27.Miki K., Clapham D.E. Rheotaxis Guides Mammalian Sperm. Curr. Biol. 2013;23:443–452. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondal A., Takagi Y., Baba S.A., Hamano K.-I. Involvement of calcium channels and intracellular calcium in bull sperm thermotaxis. J. Reprod. Dev. 2017;63:143–148. doi: 10.1262/jrd.2016-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S.W., Ki M.S., Kim C.-L., Hwang I.-S., Jeon I.S. A Simple Confocal Microscopy-based Method for Assessing Sperm Movement. Dev. Reprod. 2017;21:229–235. doi: 10.12717/DR.2017.21.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata M.B., Egashira J., Katafuchi N., Endo K., Ogata K., Yamanaka K., Yamanouchi T., Matsuda H., Hashiyada Y., Yamashita K. Bovine sperm selection procedure prior to cryopreservation for improvement of post-thawed semen quality and fertility. J. Anim. Sci. Biotechnol. 2019;10:1–14. doi: 10.1186/s40104-019-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams M., Hill C., Scudamore I., Dunphy B., Cooke I., Barratt C. Physiology: Sperm numbers and distribution within the human Fallopian tube around ovulation. Hum. Reprod. 1993;8:2019–2026. doi: 10.1093/oxfordjournals.humrep.a137975. [DOI] [PubMed] [Google Scholar]
- 32.Suarez S., Dai X.J.B. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol. Reprod. 1992;46:686–691. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- 33.Suarez S.S., Dai X.B., de Mott R.P., Redfern K., Mirando M.A. Movement characteristics of boar sperm obtained from the oviduct or hyperactivated in vitro. J. Androl. 1992;13:75–80. [PubMed] [Google Scholar]
- 34.Guidobaldi H.A., Hirohashi N., Cubilla M., Buffone M.G., Giojalas L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017;84:310–315. doi: 10.1002/mrd.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabro G., Rovasio R.A., Civalero S., Frenkel A., Caplan S.R., Eisenbach M., Giojalas L.C. Chemotaxis of Capacitated Rabbit Spermatozoa to Follicular Fluid Revealed by a Novel Directionality-Based Assay1. Biol. Reprod. 2002;67:1565–1571. doi: 10.1095/biolreprod.102.006395. [DOI] [PubMed] [Google Scholar]
- 36.Gatica L.V., Guidobaldi H.A., Montesinos M.M., Teves M.E., Moreno A.I., Unates D.R., Molina R.I., Giojalas L.C. Picomolar gradients of progesterone select functional human sperm even in subfertile samples. Mol. Hum. Reprod. 2013;19:559–569. doi: 10.1093/molehr/gat037. [DOI] [PubMed] [Google Scholar]
- 37.Uñates D.R., Guidobaldi H.A., Gatica L.V., Cubilla M.A., Teves M.E., Moreno A., Giojalas L.C. Versatile Action of Picomolar Gradients of Progesterone on Different Sperm Subpopulations. PLoS ONE. 2014;9:e91181. doi: 10.1371/journal.pone.0091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez E.M., Moreno-Irusta A., Guidobaldi H.A., Tribulo H., Giojalas L.C. Improved bovine in vitro embryo production with sexed and unsexed sperm selected by chemotaxis. Theriogenology. 2018;122:1–8. doi: 10.1016/j.theriogenology.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Eisenbach M. Sperm chemotaxis. Rev. Reprod. 1999;4:56–66. doi: 10.1530/ror.0.0040056. [DOI] [PubMed] [Google Scholar]
- 40.Suarez S.S.J.B. The oviductal sperm reservoir in mammals: Mechanisms of formation. Biol. Reprod. 1998;58:1105–1107. doi: 10.1095/biolreprod58.5.1105. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre R., Suarez S.S.J.B. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol. Reprod. 1996;54:575–582. doi: 10.1095/biolreprod54.3.575. [DOI] [PubMed] [Google Scholar]
- 42.Yanagimachi R. Fertility of mammalian spermatozoa: Its development and relativity. Zygote. 1994;2:371–372. doi: 10.1017/S0967199400002240. [DOI] [PubMed] [Google Scholar]
- 43.Hunter R.H.F. Sperm: Egg ratios and putative molecular signals to modulate gamete interactions in polytocous mammals. Mol. Reprod. Dev. 1993;35:324–327. doi: 10.1002/mrd.1080350315. [DOI] [PubMed] [Google Scholar]
- 44.Vanderhyden B.C., Tonary A.M.J.B. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor (s) secreted by the oocyte. Biol. Reprod. 1995;53:1243–1250. doi: 10.1095/biolreprod53.6.1243. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita Y., Shimada M., Okazaki T., Maeda T., Terada T. Production of Progesterone from De Novo-Synthesized Cholesterol in Cumulus Cells and Its Physiological Role During Meiotic Resumption of Porcine Oocytes1. Biol. Reprod. 2003;68:1193–1198. doi: 10.1095/biolreprod.102.010934. [DOI] [PubMed] [Google Scholar]
- 46.Guidobaldi H.A., Teves M.E., Uñates D.R., Anastasía A., Giojalas L.C.J. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS ONE. 2008;3:e3040. doi: 10.1371/journal.pone.0003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giojalas L.C., Guidobaldi H.A., Gatica L.V., Teves M.E., del Mar Montesinos M., Uñates D.R. Device for Diagnosis of Physiologic Status and/or Selection of the Best Spermatozoa of a Semen Sample Based on Chemotaxis, and Procedure of use thereof. No. 8,993,310. U.S. Patent. 2015 Mar 31;
- 48.Teves M.E., Guidobaldi H.A., Uñates D.R., Sanchez R., Miska W., Giojalas L.C. Progesterone sperm chemoattraction may be modulated by its corticosteroid-binding globulin carrier protein. Fertil. Steril. 2010;93:2450–2452. doi: 10.1016/j.fertnstert.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Martinez C.A., Alvarez-Rodriguez M., Wright D., Rodriguez-Martinez H. Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels? Int. J. Mol. Sci. 2020;21:1840. doi: 10.3390/ijms21051840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lissabet J.F.B., Belén L.H., Lee-Estevez M., Risopatrón J., Valdebenito I., Figueroa E., Farías J.G. The CatSper channel is present and plays a key role in sperm motility of the Atlantic salmon (Salmo salar) Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2020;241:110634. doi: 10.1016/j.cbpa.2019.110634. [DOI] [PubMed] [Google Scholar]
- 51.Raju D.N., Hansen J.N., Rassmann S., Stüven B., Jikeli J.F., Strünker T., Körschen H.G., Möglich A., Wachten D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells. 2019;8:648. doi: 10.3390/cells8070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darszon A., Nishigaki T., López-González I., Visconti P.E., Treviño C.L. Differences and Similarities: The Richness of Comparative Sperm Physiology. Physiology. 2020;35:196–208. doi: 10.1152/physiol.00033.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott I., Reveco A.R. The Restraint of Bovine Sperm Cell Motility Increases Survival: Role of Extracellular Calcium in the Phenomena. J. Veter. Sci. Technol. 2016;7:2. doi: 10.4172/2157-7579.1000359. [DOI] [Google Scholar]
- 54.Priego-Espinosa D.A., Darszon A., Guerrero A., González-Cota A.L., Nishigaki T., Martínez-Mekler G., Carneiro J. Modular analysis of the control of flagellar Ca2+-spike trains produced by CatSper and CaV channels in sea urchin sperm. PLoS Comput. Biol. 2020;16:e1007605. doi: 10.1371/journal.pcbi.1007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson G.P., English A.-M., Cronin S., Hoey D.A., Meade K.G., Fair S. Genomic identification, expression profiling, and functional characterization of CatSper channels in the bovine. Biol. Reprod. 2017;97:302–312. doi: 10.1093/biolre/iox082. [DOI] [PubMed] [Google Scholar]
- 56.Lehti M.S., Sironen A.J.B. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017;97:522–536. doi: 10.1093/biolre/iox096. [DOI] [PubMed] [Google Scholar]
- 57.Yeste M., Llavanera M., Mateo-Otero Y., Catalán J., Bonet S., Pinart E. HVCN1 Channels Are Relevant for the Maintenance of Sperm Motility During In Vitro Capacitation of Pig Spermatozoa. Int. J. Mol. Sci. 2020;21:3255. doi: 10.3390/ijms21093255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castellano L.E., Treviño C.L., Rodríguez D., Serrano C.J., Pacheco J., Tsutsumi V., Felix R., Darszon A. Transient receptor potential (TRPC) channels in human sperm: Expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Lett. 2003;541:69–74. doi: 10.1016/S0014-5793(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 59.Minke B. Drosophila mutant with a transducer defect. Eur. Biophys. J. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- 60.Montell C., Jones K., Hafen E., Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 61.Wissenbach U., Niemeyer B.A., Flockerzi V. TRP channels as potential drug targets. Biol. Cell. 2004;96:47–54. doi: 10.1016/j.biolcel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Goodwin L.O., Karabinus D.S., Pergolizzi R.G., Benoff S. L-type voltage-dependent calcium channel alpha-1C subunit mRNA is present in ejaculated human spermatozoa. Mol. Hum. Reprod. 2000;6:127–136. doi: 10.1093/molehr/6.2.127. [DOI] [PubMed] [Google Scholar]
- 63.Serrano C.J., Treviño C.L., Felix R., Darszon A. Voltage-dependent Ca(2+) channel subunit expression and immunolocalization in mouse spermatogenic cells and sperm. FEBS Lett. 1999;462:171–176. doi: 10.1016/S0014-5793(99)01518-5. [DOI] [PubMed] [Google Scholar]
- 64.Espinosa F., López-González I., Serrano C., Gasque G., de la Vega-Beltrán J., Treviño C., Darszon A. Anion channel blockers differentially affect t-type Ca2 currents of mouse spermatogenic cells, α1E currents expressed in Xenopus oocytes and the sperm acrosome reaction. Dev. Genet. 1999;25:103–114. doi: 10.1002/(SICI)1520-6408(1999)25:2<103::AID-DVG4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 65.Liévano A., Santi C.M., Serrano C., Treviño C.L., Bellvé A.R., Hernández-Cruz A., Darszon A. T-type Ca2+ channels and α 1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 1996;388:150–154. doi: 10.1016/0014-5793(96)00515-7. [DOI] [PubMed] [Google Scholar]
- 66.Park J.-Y., Ahn H.-J., Gu J.-G., Lee K.-H., Kim J.-S., Kang H.-W., Lee J.-H. Molecular identification of Ca(2+)channels in human sperm. Exp. Mol. Med. 2003;35:285–292. doi: 10.1038/emm.2003.39. [DOI] [PubMed] [Google Scholar]
- 67.Wiesner B., Weiner J., Middendorff R., Hagen V., Kaupp U.B., Weyand I. Cyclic Nucleotide-gated Channels on the Flagellum Control Ca2+ Entry into Sperm. J. Cell Biol. 1998;142:473–484. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weyand I., Godde M., Frings S., Weiner J., Müller F., Altenhofen W., Hatt H., Kaupp U.B., Mueller F. Cloning and functional expression of a cyclic-nucleotide-gated channel from mammalian sperm. Nat. Cell Biol. 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- 69.Gerstner A., Zong X., Hofmann F., Biel M. Molecular Cloning and Functional Characterization of a New Modulatory Cyclic Nucleotide-Gated Channel Subunit from Mouse Retina. J. Neurosci. 2000;20:1324–1332. doi: 10.1523/JNEUROSCI.20-04-01324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren D., Navarro B., Perez G.I., Jackson A.C., Hsu S., Shi Q., Tilly J.L., Clapham D.E. A sperm ion channel required for sperm motility and male fertility. Nat. Cell Biol. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quill T.A., Ren D., Clapham D.E., Garbers D.L. A voltage-gated ion channel expressed specifically in spermatozoa. Proc. Natl. Acad. Sci. USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zapata O., Ralston J., Beltraán C., Parys J.B., Chen J.L., Longo F.J., Darszon A. Inositol triphosphate receptors in sea urchin sperm. Zygote. 1997;5:355–364. doi: 10.1017/S0967199400003932. [DOI] [PubMed] [Google Scholar]
- 73.Treviño C.L., Felix R., Castellano L.E., Gutierrez C., Rodríguez D., Pacheco J., López-González I., Gomora J.C., Tsutsumi V., Hernández-Cruz A., et al. Expression and differential cell distribution of low-threshold Ca2+ channels in mammalian male germ cells and sperm. FEBS Lett. 2004;563:87–92. doi: 10.1016/S0014-5793(04)00257-1. [DOI] [PubMed] [Google Scholar]
- 74.Buck L., Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- 75.Blache P., Gros L., Salazar G., Bataille D. Cloning and Tissue Distribution of a New Rat Olfactory Receptor-like (OL2) Biochem. Biophys. Res. Commun. 1998;242:669–672. doi: 10.1006/bbrc.1997.8041. [DOI] [PubMed] [Google Scholar]
- 76.Drutel G., Arrang J.M., Diaz J., Wisnewsky C., Schwartz K., Schwartz J.C. Cloning of OL1, a putative olfactory receptor and its expression in the developing rat heart. Recept. Channels. 1995;3:33–40. [PubMed] [Google Scholar]
- 77.Ngal J. The family of genes encoding odorant receptors in the channel catfish. Cell. 1993;72:657–666. doi: 10.1016/0092-8674(93)90395-7. [DOI] [PubMed] [Google Scholar]
- 78.Freitag J., Krieger J., Strotmann J., Breer H. Two classes of olfactory receptors in xenopus laevis. Neuron. 1995;15:1383–1392. doi: 10.1016/0896-6273(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 79.Freitag J., Ludwig G., Andreini I., Rossler P., Breer H. Olfactory receptors in aquatic and terrestrial vertebrates. J. Comp. Physiol. A. 1998;183:635–650. doi: 10.1007/s003590050287. [DOI] [PubMed] [Google Scholar]
- 80.Glusman G., Yanai I., Rubin I., Lancet D. The Complete Human Olfactory Subgenome. Genome Res. 2001;11:685–702. doi: 10.1101/gr.171001. [DOI] [PubMed] [Google Scholar]
- 81.Godfrey P.A., Malnic B., Buck L.B. The mouse olfactory receptor gene family. Proc. Natl. Acad. Sci. USA. 2004;101:2156–2161. doi: 10.1073/pnas.0308051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malnic B., Godfrey P.A., Buck L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA. 2004;101:2584–2589. doi: 10.1073/pnas.0307882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young J.M., Friedman C., Williams E.M., Ross J.A., Tonnes-Priddy L., Trask B.J. Different evolutionary processes shaped the mouse and human olfactory receptor gene families. Hum. Mol. Genet. 2002;11:535–546. doi: 10.1093/hmg/11.5.535. [DOI] [PubMed] [Google Scholar]
- 84.Zozulya S., Echeverri F., Nguyen T. The human olfactory receptor repertoire. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-6-research0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoover K.C. Evolution of Olfactory Receptors. Adv. Struct. Saf. Stud. 2013;1003:241–249. doi: 10.1007/978-1-62703-377-0_18. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X., Firestein S. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- 87.Niimura Y., Matsui A., Touhara K. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 2014;24:1485–1496. doi: 10.1101/gr.169532.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adler E., Hoon M.A., Mueller K.L., Chandrashekar J., Ryba N.J., Zuker C.S. A Novel Family of Mammalian Taste Receptors. Cell. 2000;100:693–702. doi: 10.1016/S0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 89.Roper S.D., Chaudhari N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017;18:485–497. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hofer D., Puschel B., Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc. Natl. Acad. Sci. USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S.-J., Depoortere I., Hatt H. Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 2019;18:116–138. doi: 10.1038/s41573-018-0002-3. [DOI] [PubMed] [Google Scholar]
- 92.Nelson G., Chandrashekar J., Hoon M.A., Feng L., Zhao G., Ryba N.J.P., Zuker C.S. An amino-acid taste receptor. Nat. Cell Biol. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 93.Niimura Y. Olfactory Receptor Multigene Family in Vertebrates: From the Viewpoint of Evolutionary Genomics. Curr. Genom. 2012;13:103–114. doi: 10.2174/138920212799860706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayden S., Bekaert M., Crider T.A., Mariani S., Murphy W.J., Teeling E.C. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2009;20:1–9. doi: 10.1101/gr.099416.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 96.Niimura Y., Nei M. Comparative evolutionary analysis of olfactory receptor gene clusters between humans and mice. Gene. 2005;346:13–21. doi: 10.1016/j.gene.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 97.Axel R. The Molecular Logic of Smell. Sci. Am. 1995;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- 98.Mombaerts P. Molecular biology of odorant receptors in vertebrates. Annu. Rev. Neurosci. 1999;22:487–509. doi: 10.1146/annurev.neuro.22.1.487. [DOI] [PubMed] [Google Scholar]
- 99.Matsui A., Go Y., Niimura Y. Degeneration of Olfactory Receptor Gene Repertories in Primates: No Direct Link to Full Trichromatic Vision. Mol. Biol. Evol. 2010;27:1192–1200. doi: 10.1093/molbev/msq003. [DOI] [PubMed] [Google Scholar]
- 100.Niimura Y., Nei M. Extensive Gains and Losses of Olfactory Receptor Genes in Mammalian Evolution. PLoS ONE. 2007;2:e708. doi: 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nei M., Rooney A.P. Concerted and Birth-and-Death Evolution of Multigene Families. Annu. Rev. Genet. 2005;39:121–152. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bachmanov A.A., Beauchamp G.K. Taste Receptor Genes. Annu. Rev. Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conte C., Ebeling M., Marcuz A., Nef P., Andres-Barquin P. Identification and characterization of human taste receptor genes belonging to the TAS2R family. Cytogenet. Genome Res. 2002;98:45–53. doi: 10.1159/000068546. [DOI] [PubMed] [Google Scholar]
- 104.Go Y., Niimura Y. Similar Numbers but Different Repertoires of Olfactory Receptor Genes in Humans and Chimpanzees. Mol. Biol. Evol. 2008;25:1897–1907. doi: 10.1093/molbev/msn135. [DOI] [PubMed] [Google Scholar]
- 105.Sugawara T., Go Y., Udono T., Morimura N., Tomonaga M., Hirai H., Imai H. Diversification of Bitter Taste Receptor Gene Family in Western Chimpanzees. Mol. Biol. Evol. 2010;28:921–931. doi: 10.1093/molbev/msq279. [DOI] [PubMed] [Google Scholar]
- 106.Fischer A., Gilad Y., Man O., Pääbo S. Evolution of Bitter Taste Receptors in Humans and Apes. Mol. Biol. Evol. 2004;22:432–436. doi: 10.1093/molbev/msi027. [DOI] [PubMed] [Google Scholar]
- 107.Shi P., Shi P., Zhang J., Zhang J. Extraordinary Diversity of Chemosensory Receptor Gene Repertoires Among Vertebrates. Chem. Biol. Pteridine Folates. 2009;47:57–75. doi: 10.1007/400_2008_4. [DOI] [PubMed] [Google Scholar]
- 108.Dong N., Jones G., Zhang S. Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evol. Biol. 2009;9:12. doi: 10.1186/1471-2148-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu S.V., Chen M.C., Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol. Genom. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 110.Li D., Zhang J. Diet Shapes the Evolution of the Vertebrate Bitter Taste Receptor Gene Repertoire. Mol. Biol. Evol. 2014;31:303–309. doi: 10.1093/molbev/mst219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nguyen D.T., Lee K., Choi H., Choi M.-K., Le M.T., Song N., Kim J.-H., Seo H.G., Oh J.-W., Lee K., et al. The complete swine olfactory subgenome: Expansion of the olfactory gene repertoire in the pig genome. BMC Genom. 2012;13:584. doi: 10.1186/1471-2164-13-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X., de la Cruz O., Pinto J.M., Nicolae D., Firestein S., Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol. 2007;8:R86. doi: 10.1186/gb-2007-8-5-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Busse D., Kudella P., Grüning N.-M., Gisselmann G., Ständer S., Luger T., Jacobsen F., Steinsträßer L., Paus R., Gkogkolou P., et al. A Synthetic Sandalwood Odorant Induces Wound-Healing Processes in Human Keratinocytes via the Olfactory Receptor OR2AT4. J. Investig. Dermatol. 2014;134:2823–2832. doi: 10.1038/jid.2014.273. [DOI] [PubMed] [Google Scholar]
- 114.Manteniotis S., Wojcik S., Göthert J.R., Dürig J., Dührsen U., Gisselmann G., Hatt H. Deorphanization and characterization of the ectopically expressed olfactory receptor OR51B5 in myelogenous leukemia cells. Cell Death Discov. 2016;2:16010. doi: 10.1038/cddiscovery.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maßberg D., Simon A., Häussinger D., Keitel V., Gisselmann G., Conrad H., Hatt H. Monoterpene (−)-citronellal affects hepatocarcinoma cell signaling via an olfactory receptor. Arch. Biochem. Biophys. 2015;566:100–109. doi: 10.1016/j.abb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 116.Wu S.V., Rozengurt N., Yang M., Young S.H., Sinnett-Smith J., Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren X., Zhou L., Terwilliger R., Newton S.S., de Araujo I.E. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin C.R., Mayer E.A. Gut-Brain Axis and Behavior. Issues Complem. Feed. 2017;88:45–53. doi: 10.1159/000461732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X., Gu F., Jiang L., Chen F., Li F., Xin L., Li J., Fuxue C. Expression of bitter taste receptor Tas2r105 in mouse kidney. Biochem. Biophys. Res. Commun. 2015;458:733–738. doi: 10.1016/j.bbrc.2015.01.089. [DOI] [PubMed] [Google Scholar]
- 120.Hariri B.M., Payne S.J., Chen B., Mansfield C., Doghramji L.J., Adappa N.D., Palmer J.N., Kennedy D.W., Niv M.Y., Lee R.J. In vitro effects of anthocyanidins on sinonasal epithelial nitric oxide production and bacterial physiology. Am. J. Rhinol. Allergy. 2016;30:261–268. doi: 10.2500/ajra.2016.30.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foster S.R., Roura E., Thomas W.G. Extrasensory perception: Odorant and taste receptors beyond the nose and mouth. Pharmacol. Ther. 2014;142:41–61. doi: 10.1016/j.pharmthera.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Oh D.Y., Walenta E. Omega-3 Fatty Acids and FFAR4. Volume 5. NCBI; Bethesda, MA, USA: 2014. p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ekoff M., Choi J.-H., James A., Dahlén B., Nilsson G., Dahlén S.-E., Immunology C. Bitter taste receptor (TAS2R) agonists inhibit IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014;134:475–478. doi: 10.1016/j.jaci.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 124.Alvarez-Curto E., Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 125.Wölfle U., Elsholz F.A., Kersten A., Haarhaus B., Müller W.E., Schempp C.M. Expression and Functional Activity of the Bitter Taste Receptors TAS2R1 and TAS2R38 in Human Keratinocytes. Ski. Pharmacol. Physiol. 2015;28:137–146. doi: 10.1159/000367631. [DOI] [PubMed] [Google Scholar]
- 126.Clark A.A., Dotson C.D., Elson A.E.T., Voigt A., Boehm U., Meyerhof W., Steinle N.I., Munger S.D. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29:164–172. doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deckmann K., Filipski K., Krasteva-Christ G., Fronius M., Althaus M., Rafiq A., Papadakis T., Renno L., Jurastow I., Wessels L., et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc. Natl. Acad. Sci. USA. 2014;111:8287–8292. doi: 10.1073/pnas.1402436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mosinger B., Redding K.M., Parker M.R., Yevshayeva V., Yee K.K., Dyomina K., Li Y., Margolskee R.F. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc. Natl. Acad. Sci. USA. 2013;110:12319–12324. doi: 10.1073/pnas.1302827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin L.T.P., Nachtigal M.W., Selman T., Nguyen E., Salsman J., Dellaire G., Dupré D.J. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol. Cell. Biochem. 2018;454:203–214. doi: 10.1007/s11010-018-3464-z. [DOI] [PubMed] [Google Scholar]
- 130.Simon B.R., Learman B.S., Parlee S.D., Scheller E.L., Mori H., Cawthorn W.P., Ning X., Krishnan V., Ma Y.L., Tyrberg B., et al. Sweet Taste Receptor Deficient Mice Have Decreased Adiposity and Increased Bone Mass. PLoS ONE. 2014;9:e86454. doi: 10.1371/journal.pone.0086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quesada-López T.T., Cereijo R.R., Turatsinze J.-V., Planavila A.A., Cairó M.M., Gavaldà-Navarro A.A., Peyrou M.M., Moure R.R., Iglesias R.R., Giralt M.M., et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016;7:13479. doi: 10.1038/ncomms13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Camilleri M. Peripheral Mechanisms in Appetite Regulation. Gastroenterol. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Augustine V., Gokce S.K., Oka Y. Peripheral and Central Nutrient Sensing Underlying Appetite Regulation. Trends Neurosci. 2018;41:526–539. doi: 10.1016/j.tins.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis T.M.J.T.A. Fenofibrate and Impaired Taste Perception in Type 2 Diabetes. Am. J. Case Rep. 2020;21:e927647-1. doi: 10.12659/AJCR.927647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nayak A.P., Shah S.D., Michael J.V., Deshpande D.A. Bitter Taste Receptors for Asthma Therapeutics. Front. Physiol. 2019;10:884. doi: 10.3389/fphys.2019.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee R.J., Cohen N.A. Taste receptors in innate immunity. Cell. Mol. Life Sci. 2015;72:217–236. doi: 10.1007/s00018-014-1736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Turner A., Chijoff E., Veysey M., Keely S., Scarlett C.J., Lucock M., Beckett E.L. Interactions between taste receptors and the gastrointestinal microbiome in inflammatory bowel disease. J. Nutr. Intermed. Metab. 2019;18:100106. doi: 10.1016/j.jnim.2019.100106. [DOI] [Google Scholar]
- 138.Calvo S.S.-C., Egan J.M. The endocrinology of taste receptors. Nat. Rev. Endocrinol. 2015;11:213–227. doi: 10.1038/nrendo.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kok B.P., Galmozzi A., Littlejohn N.K., Albert V., Godio C., Kim W., Kim S.M., Bland J.S., Grayson N., Fang M., et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018;16:76–87. doi: 10.1016/j.molmet.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim J., Pullicin A.J. Oral carbohydrate sensing: Beyond sweet taste. Physiol. Behav. 2019;202:14–25. doi: 10.1016/j.physbeh.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 141.Bachmanov A., Bosak N.P., Lin C., Matsumoto I., Ohmoto M., Reed D.R., Nelson T.J.C.M. Genetics of taste receptors. Curr. Pharm. Des. 2014;20:2669–2683. doi: 10.2174/13816128113199990566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vegezzi G., Anselmi L., Huynh J., Barocelli E., Rozengurt E., Raybould H., Sternini C. Diet-Induced Regulation of Bitter Taste Receptor Subtypes in the Mouse Gastrointestinal Tract. PLoS ONE. 2014;9:e107732. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nat. Cell Biol. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 144.Devillier P., Naline E., Grassin-Delyle S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther. 2015;155:11–21. doi: 10.1016/j.pharmthera.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 145.Lee R.J., Xiong G., Kofonow J.M., Chen B., Lysenko A., Jiang P., Abraham V., Doghramji L., Adappa N.D., Palmer J.N., et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Linardopoulou E., Mefford H.C., Nguyen O., Friedman C., Engh G.V.D., Farwell D.G., Coltrera M., Trask B.J. Transcriptional activity of multiple copies of a subtelomerically located olfactory receptor gene that is polymorphic in number and location. Hum. Mol. Genet. 2001;10:2373–2383. doi: 10.1093/hmg/10.21.2373. [DOI] [PubMed] [Google Scholar]
- 147.Thomas M.B., Haines S.L., Akeson R.A. Chemoreceptors expressed in taste, olfactory and male reproductive tissues. Gene. 1996;178:1–5. doi: 10.1016/0378-1119(96)00311-3. [DOI] [PubMed] [Google Scholar]
- 148.Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J. Cell Biol. 1993;123:1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Volz A., Ehlers A., Younger R., Forbes S., Trowsdale J., Schnorr D., Beck S., Ziegler A. Complex Transcription and Splicing of Odorant Receptor Genes. J. Biol. Chem. 2003;278:19691–19701. doi: 10.1074/jbc.M212424200. [DOI] [PubMed] [Google Scholar]
- 150.Ziegler A., Dohr G., Uchanska-Ziegler B. Possible Roles for Products of Polymorphic MHC and Linked Olfactory Receptor Genes during Selection Processes in Reproduction*. Am. J. Reprod. Immunol. 2002;48:34–42. doi: 10.1034/j.1600-0897.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- 151.Feldmesser E., Olender T., Khen M., Yanai I., Ophir R., Lancet D. Widespread ectopic expression of olfactory receptor genes. BMC Genom. 2006;7:121. doi: 10.1186/1471-2164-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ichimura A., Kadowaki T., Narukawa K., Togiya K., Hirasawa A., Tsujimoto G. In silico approach to identify the expression of the undiscovered molecules from microarray public database: Identification of odorant receptors expressed in non-olfactory tissues. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008;377:159–165. doi: 10.1007/s00210-007-0255-6. [DOI] [PubMed] [Google Scholar]
- 153.Branscomb A., Seger J., White R.L. Evolution of odorant receptors expressed in mammalian testes. Genetics. 2000;156:785–797. doi: 10.1093/genetics/156.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang X., Rogers M., Tian H., Zou D.-J., Liu J., Ma M., Shepherd G.M., Firestein S.J., Zhang X., Zhang X. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proc. Natl. Acad. Sci. USA. 2004;101:14168–14173. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun F., Bahat A., Gakamsky A., Girsh E., Katz N., Giojalas L.C., Tur-Kaspa I., Eisenbach M. Human sperm chemotaxis: Both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum. Reprod. 2005;20:761–767. doi: 10.1093/humrep/deh657. [DOI] [PubMed] [Google Scholar]
- 156.Jaiswal B.S., Tur-Kaspa I., Dor J., Mashiach S., Eisenbach M. Human Sperm Chemotaxis: Is Progesterone a Chemoattractant?1. Biol. Reprod. 1999;60:1314–1319. doi: 10.1095/biolreprod60.6.1314. [DOI] [PubMed] [Google Scholar]
- 157.Villanueva-Diaz C., Vadillo-Ortega F., Kably-Ambe A., Diaz-Pérez M.D.L.A., Krivitzky S.K. Evidence that human follicular fluid contains a chemoattractant for spermatozoa. Fertil. Steril. 1990;54:1180–1182. doi: 10.1016/S0015-0282(16)54027-8. [DOI] [PubMed] [Google Scholar]
- 158.Isobe T., Minoura H., Tanaka K., Shibahara T., Hayashi N., Toyoda N. The effect of RANTES on human sperm chemotaxis. Hum. Reprod. 2002;17:1441–1446. doi: 10.1093/humrep/17.6.1441. [DOI] [PubMed] [Google Scholar]
- 159.Śliwa L. Heparin as a Chemoattractant for Mouse Spermatozoa. Arch. Androl. 1993;31:149–152. doi: 10.3109/01485019308988393. [DOI] [PubMed] [Google Scholar]
- 160.Śliwa L. Effect of Heparin on Human Spermatozoa Migration in Vitro. Arch. Androl. 1993;30:177–181. doi: 10.3109/01485019308987754. [DOI] [PubMed] [Google Scholar]
- 161.Śliwa L.J.A. Substance and ß-Endorphin Act as Possible Chemoattractants of mouse sperm. Arch. Androl. 2001;46:135–140. doi: 10.1080/01485010151094056. [DOI] [PubMed] [Google Scholar]
- 162.Hoang H.D., Prasain J.K., Dorand D., Miller M.A. A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Caenorhabditis elegans Reproductive Tract. PLoS Genet. 2013;9:e1003271. doi: 10.1371/journal.pgen.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Edmonds J.W., Prasain J.K., Dorand D., Yang Y., Hoang H.D., Vibbert J., Kubagawa H.M., Miller M.A. Insulin/FOXO Signaling Regulates Ovarian Prostaglandins Critical for Reproduction. Dev. Cell. 2010;19:858–871. doi: 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Machado-Oliveira G., Lefièvre L., Ford C., Herrero M.B., Barratt C., Connolly T.J., Nash K., Morales-Garcia A., Kirkman-Brown J., Publicover S. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: A role for NO synthesised in the female reproductive tract. Development. 2008;135:3677–3686. doi: 10.1242/dev.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gimeno S., Alquezar-Baeta C., Peinado J., Nadal P., Casao A., Cebrián-Pérez J.A., Muiño-Blanco T., Pérez-Pé R. Progesterone and estradiol influence on spermatic orientation in ram sperm. Zaragoza. 2017;2017:395–397. [Google Scholar]
- 166.Cohen-Dayag A., Tur-Kaspa I., Dor J., Mashiach S., Eisenbach M. Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. Proc. Natl. Acad. Sci. USA. 1995;92:11039–11043. doi: 10.1073/pnas.92.24.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goto T., Salpekar A., Monk M. Expression of a testis-specific member of the olfactory receptor gene family in human primordial germ cells. Mol. Hum. Reprod. 2001;7:553–558. doi: 10.1093/molehr/7.6.553. [DOI] [PubMed] [Google Scholar]
- 168.Eflegel C., Evogel F., Hofreuter A.E., Schreiner B.S.P., Eosthold S., Eveitinger S., Ebecker C., Brockmeyer N.H., Emuschol M., Ewennemuth G., et al. Characterization of the Olfactory Receptors Expressed in Human Spermatozoa. Front. Mol. Biosci. 2016;2:73. doi: 10.3389/fmolb.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yuan T.T.-T., Toy P., McClary J.A., Lin R.J., Miyamoto N.G., Kretschmer P.J. Cloning and genetic characterization of an evolutionarily conserved human olfactory receptor that is differentially expressed across species. Gene. 2001;278:41–51. doi: 10.1016/S0378-1119(01)00709-0. [DOI] [PubMed] [Google Scholar]
- 170.Veitinger T., Riffell J.R., Veitinger S., Nascimento J.M., Triller A., Chandsawangbhuwana C., Schwane K., Geerts A., Wunder F., Berns M.W., et al. Chemosensory Ca2+ Dynamics Correlate with Diverse Behavioral Phenotypes in Human Sperm. J. Biol. Chem. 2011;286:17311–17325. doi: 10.1074/jbc.M110.211524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Itakura S., Ohno K., Ueki T., Sato K., Kanayama N. Expression of Golf in the rat placenta: Possible implication in olfactory receptor transduction. Placenta. 2006;27:103–108. doi: 10.1016/j.placenta.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 172.Asai H., Kasai H., Matsuda Y., Yamazaki N., Nagawa F., Sakano H., Tsuboi A. Genomic Structure and Transcription of a Murine Odorant Receptor Gene: Differential Initiation of Transcription in the Olfactory and Testicular Cells. Biochem. Biophys. Res. Commun. 1996;221:240–247. doi: 10.1006/bbrc.1996.0580. [DOI] [PubMed] [Google Scholar]
- 173.Fukuda N., Touhara K. Developmental expression patterns of testicular olfactory receptor genes during mouse spermatogenesis. Genes Cells. 2005;11:71–81. doi: 10.1111/j.1365-2443.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 174.Mao J., Zhang X., Sieli P.T., Falduto M.T., Torres K.E., Rosenfeld C.S. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc. Natl. Acad. Sci. USA. 2010;107:5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fellows E., Hazzard T., Kutzler M. Gene Expression in Pre-Term, Pre-Labour and Parturient Canine Placenta. Reprod. Domest. Anim. 2012;47:182–185. doi: 10.1111/rda.12021. [DOI] [PubMed] [Google Scholar]
- 176.Makeyeva Y., Nicol C., Ledger W.L., Ryugo D.K. Immunocytochemical Localization of Olfactory-signaling Molecules in Human and Rat Spermatozoa. J. Histochem. Cytochem. 2020;68:491–513. doi: 10.1369/0022155420939833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walensky L.D., Ruat M., Bakin R.E., Blackshaw S., Ronnett G.V., Snyder S.H. Two Novel Odorant Receptor Families Expressed in Spermatids Undergo 5′-Splicing. J. Biol. Chem. 1998;273:9378–9387. doi: 10.1074/jbc.273.16.9378. [DOI] [PubMed] [Google Scholar]
- 178.Schultz N., Hamra F.K., Garbers D.L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl. Acad. Sci. USA. 2003;100:12201–12206. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leese H.J., Astley N.R., Lambert D. Glucose and fructose utilization by rat spermatozoa within the uterine lumen. Reprod. 1981;61:435–437. doi: 10.1530/jrf.0.0610435. [DOI] [PubMed] [Google Scholar]
- 180.Harris S.E., Gopichandran N., Picton H.M., Leese H.J., Orsi N.M. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology. 2005;64:992–1006. doi: 10.1016/j.theriogenology.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 181.Józwik M., Józwik M., Teng C., Battaglia F.C.J.H.R. Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid. Hum. Reprod. 2006;21:2776–2782. doi: 10.1093/humrep/del038. [DOI] [PubMed] [Google Scholar]
- 182.Jóźwik M., Jóźwik M., Milewska A.J., Battaglia F.C., Jóźwik M.J.S. Competitive inhibition of amino acid transport in human preovulatory ovarian follicles. Biomed. Res. Int. 2017;63:311–317. doi: 10.1080/19396368.2017.1341962. [DOI] [PubMed] [Google Scholar]
- 183.Suarez S., Pacey A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Updat. 2005;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 184.De Blas G.A., Darszon A., Ocampo A.Y., Serrano C.J., Castellano L.E., Hernández-González E.O., Chirinos M., Larrea F., Beltran C., Treviño C.L. TRPM8, a Versatile Channel in Human Sperm. PLoS ONE. 2009;4:e6095. doi: 10.1371/journal.pone.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Meyer D., Voigt A., Widmayer P., Borth H., Huebner S., Breit A., Marschall S., de Angelis M.H., Boehm U., Meyerhof W., et al. Expression of Tas1 Taste Receptors in Mammalian Spermatozoa: Functional Role of Tas1r1 in Regulating Basal Ca2+ and cAMP Concentrations in Spermatozoa. PLoS ONE. 2012;7:e32354. doi: 10.1371/journal.pone.0032354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Parks J.E., Arion J.W., Foote R.H. Lipids of Plasma Membrane and Outer Acrosomal Membrane from Bovine Spermatozoa. Biol. Reprod. 1987;37:1249–1258. doi: 10.1095/biolreprod37.5.1249. [DOI] [PubMed] [Google Scholar]
- 187.Bischof J.C., Wolkers W.F., Tsvetkova N.M., Oliver A.E., Crowe J.H. Lipid and protein changes due to freezing in Dunning AT-1 cells. Cryobiology. 2002;45:22–32. doi: 10.1016/S0011-2240(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 188.Vadnais M.L., Althouse G.C. Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology. 2011;76:1508–1516. doi: 10.1016/j.theriogenology.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 189.Yeste M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology. 2016;85:47–64. doi: 10.1016/j.theriogenology.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 190.Yeste M., Bonet S., Rodríguez-Gil J.E. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017;84:802–813. doi: 10.1002/mrd.22840. [DOI] [PubMed] [Google Scholar]
- 191.Fehr J., Meyer D., Widmayer P., Borth H.C., Ackermann F., Wilhelm B., Gudermann T., Boekhoff I. Expression of the G-protein α-subunit gustducin in mammalian spermatozoa. J. Comp. Physiol. A. 2006;193:21–34. doi: 10.1007/s00359-006-0168-8. [DOI] [PubMed] [Google Scholar]
- 192.Kiuchi S., Yamada T., Kiyokawa N., Saito T., Fujimoto J., Yasue H. Genomic structure of swine taste receptor family 1 member 3, TAS1R3, and its expression in tissues. Cytogenet. Genome Res. 2006;115:51–61. doi: 10.1159/000094801. [DOI] [PubMed] [Google Scholar]
- 193.Gibson A.D., Garbers D.L. Guanylyl Cyclases as a Family of Putative Odorant Receptors. Annu. Rev. Neurosci. 2000;23:417–439. doi: 10.1146/annurev.neuro.23.1.417. [DOI] [PubMed] [Google Scholar]
- 194.Luddi A., Governini L., Wilmskötter D., Gudermann T., Boekhoff I., Piomboni P. Taste Receptors: New Players in Sperm Biology. Int. J. Mol. Sci. 2019;20:967. doi: 10.3390/ijms20040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Boekhoff I., Tareilus E., Strotmann J., Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990;9:2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Huque T., Bruch R.C. Odorant- and guanine nucleotide-stimulated phosphoinositide turnover in olfactory cilia. Biochem. Biophys. Res. Commun. 1986;137:36–42. doi: 10.1016/0006-291X(86)91172-1. [DOI] [PubMed] [Google Scholar]
- 197.Ronnett G.V., Cho H., Hester L.D., Wood S.F., Snyder S.H. Odorants differentially enhance phosphoinositide turnover and adenylyl cyclase in olfactory receptor neuronal cultures. J. Neurosci. 1993;13:1751–1758. doi: 10.1523/JNEUROSCI.13-04-01751.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sklar P.B., Anholt R.R., Snyder S.H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J. Biol. Chem. 1986;261:15538–15543. doi: 10.1016/S0021-9258(18)66747-X. [DOI] [PubMed] [Google Scholar]
- 199.Spehr M., Wetzel C.H., Hatt H., Ache B.W. 3-Phosphoinositides Modulate Cyclic Nucleotide Signaling in Olfactory Receptor Neurons. Neuron. 2002;33:731–739. doi: 10.1016/S0896-6273(02)00610-4. [DOI] [PubMed] [Google Scholar]
- 200.Visconti P.E., Galantino-Homer H., Ning X., Moore G.D., Valenzuela J.P., Jorgez C.J., Alvarez J.G., Kopf G.S.J.J. Cholesterol efflux-mediated signal transduction in mammalian sperm: β-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- 201.Visconti P.E., Moore G.D., Bailey J.L., Leclerc P., Connors S.A., Pan D., Olds-Clarke P., Kopf G.S.J.D. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 202.Lishko P.V., Botchkina I.L., Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nat. Cell Biol. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 203.Strünker T., Goodwin N., Brenker C., Kashikar N.D., Weyand I., Seifert R., Kaupp U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nat. Cell Biol. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 204.Visconti P., Westbrook V., Chertihin O., Demarco I., Sleight S., Diekman A. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002;53:133–150. doi: 10.1016/S0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 205.Signorelli J., Diaz E.S., Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012;349:765–782. doi: 10.1007/s00441-012-1370-3. [DOI] [PubMed] [Google Scholar]
- 206.Sagare-Patil V., Vernekar M., Galvankar M., Modi D. Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol. Cell. Endocrinol. 2013;374:82–91. doi: 10.1016/j.mce.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 207.Belluscio L., Gold G.H., Nemes A., Axel R. Mice Deficient in Golf Are Anosmic. Neuron. 1998;20:69–81. doi: 10.1016/S0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 208.Brunet L.J., Gold G.H., Ngai J. General Anosmia Caused by a Targeted Disruption of the Mouse Olfactory Cyclic Nucleotide–Gated Cation Channel. Neuron. 1996;17:681–693. doi: 10.1016/S0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 209.Wong S.T., Trinh K., Hacker B., Chan G.C., Lowe G., Gaggar A., Xia Z., Gold G.H., Storm D.R. Disruption of the Type III Adenylyl Cyclase Gene Leads to Peripheral and Behavioral Anosmia in Transgenic Mice. Neuron. 2000;27:487–497. doi: 10.1016/S0896-6273(00)00060-X. [DOI] [PubMed] [Google Scholar]
- 210.Pérez C.A., Huang L., Rong M., Kozak J.A., Preuss A.K., Zhang H., Max M., Margolskee R.F. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 211.Clapp T.R., Trubey K.R., Vandenbeuch A., Stone L.M., Margolskee R.F., Chaudhari N., Kinnamon S.C. Tonic activity of Gα-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Taruno A., Vingtdeux V., Ohmoto M., Ma Z., Dvoryanchikov G., Li A., Adrien L., Zhao H., Leung S., Abernethy M., et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nat. Cell Biol. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ma Z., Taruno A., Ohmoto M., Jyotaki M., Lim J.C., Miyazaki H., Niisato N., Marunaka Y., Lee R.J., Hoff H., et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron. 2018;98:547–561.e10. doi: 10.1016/j.neuron.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Holt W. Fundamental aspects of sperm cryobiology: The importance of species and individual differences. Theriogenology. 2000;53:47–58. doi: 10.1016/S0093-691X(99)00239-3. [DOI] [PubMed] [Google Scholar]
- 215.Medeiros C., Forell F., Oliveira A., Rodrigues J. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology. 2002;57:327–344. doi: 10.1016/S0093-691X(01)00674-4. [DOI] [PubMed] [Google Scholar]
- 216.Susko-Parrish J.L., Uguz C., First N.L. Differences in the Role of Cyclic Adenosine 3′,5′-Monophosphate during Capacitation of Bovine Sperm by Heparin or Oviduct Fluid1. Biol. Reprod. 1994;51:1099–1108. doi: 10.1095/biolreprod51.6.1099. [DOI] [PubMed] [Google Scholar]
- 217.Leemans B., Stout T.A.E., de Schauwer C., Heras S., Nelis H., Hoogewijs M., van Soom A., Gadella B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction. 2019;157:R181–R197. doi: 10.1530/REP-18-0541. [DOI] [PubMed] [Google Scholar]
- 218.Yamashiro H., Toyomizu M., Toyama N., Aono N., Sakurai M., Hiradate Y., Yokoo M., Moisyadi S., Sato E. Extracellular ATP and Dibutyryl cAMP Enhance the Freezability of Rat Epididymal Sperm. J. Am. Assoc. Lab. Anim. Sci. 2010;49:167–172. [PMC free article] [PubMed] [Google Scholar]
- 219.Lee-Estevez M., Herrera L., Díaz R., Beltrán J., Figueroa E., Dumorné K., Ulloa-Rodríguez P., Short S., Risopatrón J., Valdebenito I., et al. Effects of cryopreservation on cAMP-dependent protein kinase and AMP-activated protein kinase in Atlantic salmon (Salmo salar) spermatozoa: Relation with post-thaw motility. Anim. Reprod. Sci. 2019;209:106133. doi: 10.1016/j.anireprosci.2019.106133. [DOI] [PubMed] [Google Scholar]
- 220.De Llera A.H., Martin-Hidalgo D., Gil M.C., Garcia-Marin L.J., Bragado M.J. AMP-Activated Kinase AMPK Is Expressed in Boar Spermatozoa and Regulates Motility. PLoS ONE. 2012;7:e38840. doi: 10.1371/journal.pone.0038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fraser L.R. The “switching on” of mammalian spermatozoa: Molecular events involved in promotion and regulation of capacitation. Mol. Reprod. Dev. 2009;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- 222.Wang Q., Liszt K.I., Depoortere I. Extra-oral bitter taste receptors: New targets against obesity? Peptide. 2020;127:170284. doi: 10.1016/j.peptides.2020.170284. [DOI] [PubMed] [Google Scholar]
- 223.Spehr M., Schwane K., Riffell J.A., Zimmer R.K., Hatt H. Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm. Mol. Cell. Endocrinol. 2006;250:128–136. doi: 10.1016/j.mce.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 224.Ran M.-X., Li Y., Zhang Y., Liang K., Ren Y.-N., Zhang M., Zhou G.-B., Zhou Y.-M., Wu K., Wang C.-D., et al. Transcriptome Sequencing Reveals the Differentially Expressed lncRNAs and mRNAs Involved in Cryoinjuries in Frozen-Thawed Giant Panda (Ailuropoda melanoleuca) Sperm. Int. J. Mol. Sci. 2018;19:3066. doi: 10.3390/ijms19103066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lewcock J.W., Reed R.R. A feedback mechanism regulates monoallelic odorant receptor expression. Proc. Natl. Acad. Sci. USA. 2004;101:1069–1074. doi: 10.1073/pnas.0307986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Serizawa S., Miyamichi K., Sakano H. Negative Feedback Regulation Ensures the One Neuron-One Receptor Rule in the Mouse Olfactory System. Chem. Senses. 2005;30:i99–i100. doi: 10.1093/chemse/bjh133. [DOI] [PubMed] [Google Scholar]
- 227.Carlson A.E., Westenbroek R.E., Quill T., Ren D., Clapham D.E., Hille B., Garbers D.L., Babcock D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.