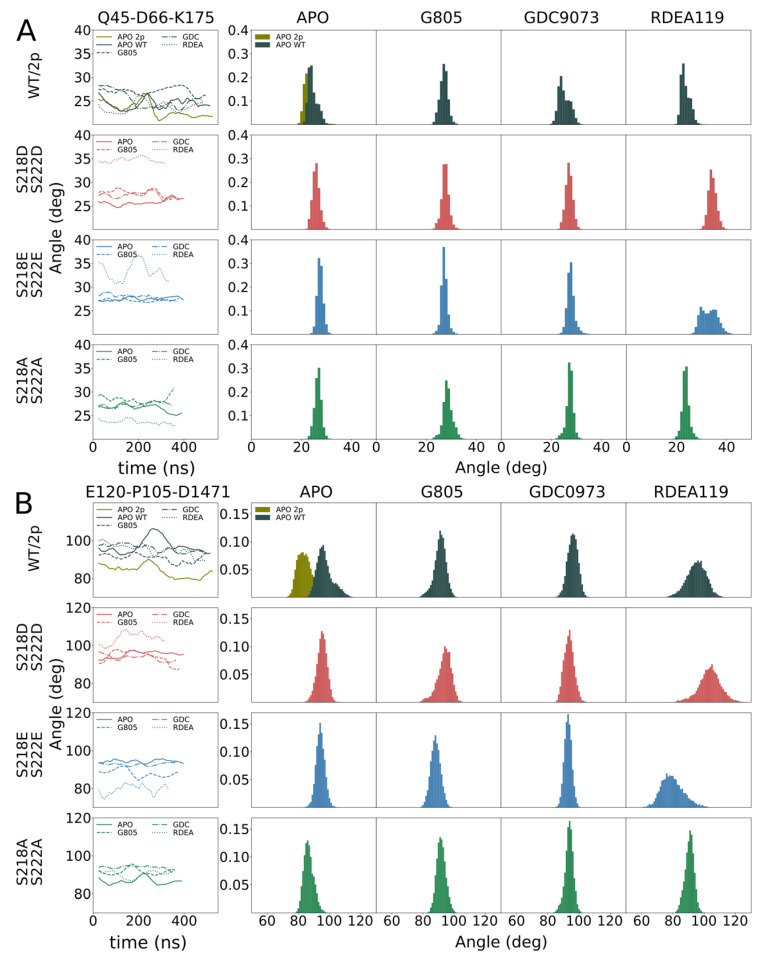
Figure 4.
Structural dynamics of phosphorylation-mimetic MEK1 mutants: (A) MEK1 A-helix motion as characterized by a change in angle with respect to time where the observed angle is defined by Q45, D66, and K175 Cα atoms. The average angle over time for three systems (APO, G805 bound, and GDC0973 bound) suggests that Glu/Glu and Ala/Ala mutants adopt a narrower range of conformations compared to both Asp/Asp mutants and WT MEK1. (B) Similarly, MEK1 αC-helix motion is tracked by measuring the angle defined by αC-helix residues E120, P105, and hinge residue D147. Signature structural rearrangements that occur during kinase activation as expressed through αC-helix in/out conformations appear greatest between unphosphorylated and phosphorylated WT APO MEK1.