Abstract
Background and objectives: Angiosarcomas are uncommon and extremely aggressive malignancies derived from vascular endothelial cells. Although they can occur anywhere in the body and at any age, they are more frequently found in the skin of the head and neck regions and in the elderly. Few cases have been recorded in deep soft tissues and in parenchymal organs. Angiosarcomas of the urinary bladder are exceedingly rare. They usually arise in adult patients with a history of radiation therapy, cigarette smoking, or exposure to chemical agents (e.g., vinyl chloride). Despite multidisciplinary treatment approaches combining surgery, radiotherapy, and chemotherapy, prognosis is dismal. Materials and Methods: We describe a case of a 78-year-old Caucasian man presenting with a vesical mass incidentally discovered with abdominal computerized tomography (CT). He underwent transurethral resection of the bladder (TURB), and histology was compatible with angiosarcoma. Results: The patient had been a heavy smoker and his medical history included therapeutic irradiation for prostate cancer eight years previously. Radical cystoprostatectomy was feasible, and pathologic examination of the surgical specimen confirmed angiosarcoma involving the urinary bladder, prostate, and seminal vesicles. Post-operative peritonitis resulted in progressive multi-organ failure and death. Conclusions: Angiosarcoma primary to the urinary bladder is seldom encountered, however, it should be considered in the differential diagnosis of vesical tumors, especially in elderly men with a history of pelvic radiotherapy.
Keywords: angiosarcoma, urinary bladder tumors, radiotherapy-associated tumors
1. Introduction
Sarcomas of the genitourinary tract are rare, accounting for 1–2% of all malignant genitourinary tumors [1,2]. Even within these entities, angiosarcoma of the urinary bladder is an uncommon diagnosis. First described in 1907 by Jungano [3], only a few cases of angiosarcoma primary to the bladder have been reported so far, most of which associated with previous pelvic radiotherapy (Table 1) [4,5,6,7,8,9,10,11,12,13,14,15]. Given its biologically aggressive phenotype, prognosis is dismal, with a five-year survival rate between 10% and 35% and common causes of death being local recurrence and distant metastases [16]. We present a case of vesical angiosarcoma in a patient who had undergone external radiotherapy for prostate cancer. A review of the related literature is also included.
Table 1.
Summary of reported bladder angiosarcomas associated with radiation-therapy.
| Year/Author | Sex | Age | Time from RT/RT Cause | Clinical Presentation | Treatment | Outcome/Months |
|---|---|---|---|---|---|---|
| 1989/ Morgan et al. |
F | 72 | 9 ys/ECar | Vaginal bleeding and hematuria | Chemotherapy (Doxorubicin) | DOD/7 |
| 1997/ Navon et al. |
M | 78 | 13 ys/PCa | Hematuria | RCP | NED/30 |
| 2006/ Seethala et al. |
M | 66 | 4 ys/PCa | Hematuria | RCP and chemotherapy (5 cycles, Gemcitabine plus Docetaxel) | NED/19 |
| 2007/ Kulaga et al. |
F | 83 | 14 ys/ECa | Hematuria | TURB | DOD/3 |
| 2008/ Tavora et al. |
M | 71 | 8 mos/PCa | Hematuria, voiding irritation | Biopsy (no treatment reported) | DOD/4 |
| 2008/ Tavora et al. |
F | 73 | 17 mos/ECa | Hematuria | Radical cystectomy | DOD/2 |
| 2008/ Williams et al. |
M | 71 | 10 ys/PCa | Hematuria | TURB followed by RCP and chemotherapy plus RT | DOD/3 |
| 2015/ Bahouth et al. |
M | 89 | 12 ys/PCa | Hematuria | TURB and palliative RT | DOD/3 |
| 2015/ Matoso et al. |
F | 73 | 10 ys/CCa | Hematuria | TURB followed by partial cystectomy | DOD/6 |
| 2015/ Matoso et al. |
M | 77 | 9 ys/PCa | Hematuria | TURB | DOD/14 |
| 2015/ Matoso et al. |
M | 71 | 10 ys/PCa | Hematuria | TURB followed by RCP | DOD/7 |
| 2015/ Matoso et al. |
M | 85 | 15 ys/PCa | Hematuria | TURB | DOD/6 |
| 2015/ Matoso et al. |
M | 64 | 6 ys/PCa | Hematuria | TURB followed by RCP | NED/12 |
| 2015/ Matoso et al. |
M | 64 | 15 ys/PCa | Hematuria | TURB | AWD/3 |
| 2015/ Ojerholm et al. |
M | 61 | 7 ys/PCa | Hematuria | RCP | NED/4 |
| 2016/ Wang et al. |
M | 79 | 6 ys/PCa | Hematuria | TURB followed by RCP | NED/20 |
| 2016/ Rallabandi et al. |
F | 65 | 22 ys/CCa | Hematuria | TURB | NA |
| 2017/ Tynski et al. |
M | 69 | 5 ys/PCa | Ascites, urinary retention | Palliative chemotherapy (docetaxel plus gemcitabine) | DOD/6 weeks |
| Current case | M | 78 | 8 ys/PCa | Incidental finding | TURB followed by RCP | Early post-operative death |
ECa: endometrial cancer; PCa: prostate cancer; CCa: cervical cancer; mos: months; RCP: radical cystoprostatectomy; RT: radiotherapy; TURB: transurethral resection of the bladder; ys: years; AWD: alive with disease; DOD: dead of disease; NA: not available; NED: no evidence of disease.
2. Case Presentation
A 78-year-old Caucasian man presented with a highly vascular mass of the bladder, detected incidentally during abdominal computerized tomography (CT) at clinical follow-up for renal transplantation. The patient had a 50 pack-years of cigarette smoking but had no occupational exposure to carcinogens. His past medical history included renal transplantation for polycystic kidney disease 18 years previously, unstable angina and coronary artery occlusive disease treated with percutaneous transluminal coronary angioplasty (PTCA) 13 years before, and cT1b prostate cancer managed with radiotherapy eight years earlier. Daily medications were comprised of tacrolimus (1 mg b.i.d.) and prednisone (7.5 mg q.d.). The patient suffered no hematuria or pain.
Clinical examination demonstrated a supple abdomen with no palpable mass. Serum work-up assessment displayed creatinine value of 1.9 mg/dL, slight anemia with hemoglobin value of 10.3 g/dL, and prostate-specific antigen (PSA) concentration of 1.9 ng/dL. Transurethral resection of the bladder (TURB) was performed and histology was consistent with angiosarcoma. CT urography revealed diffuse thickening of the right bladder wall. The patient then underwent open radical cystoprostatectomy with urinary diversion and bilateral ureterocutaneous implantation.
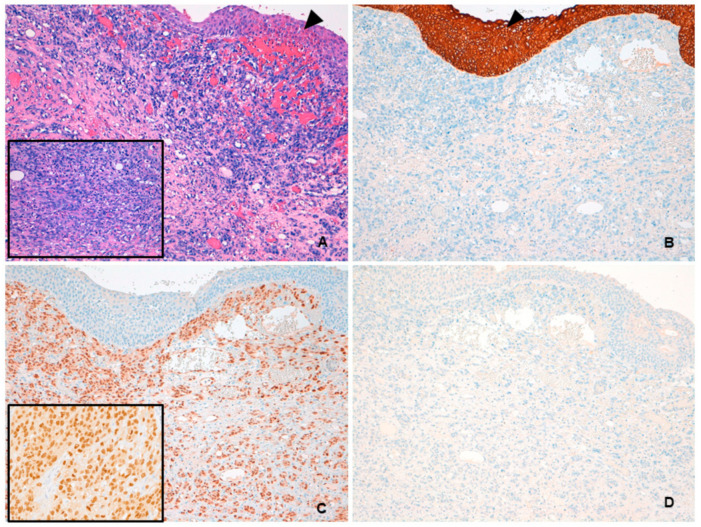
Pathologic examination of the surgical specimen showed angiosarcoma involving the entire bladder wall and extending directly to the prostate and seminal vesicles. The neoplasm exhibited a dissecting growth pattern with spindle cell areas composed of short fascicles with irregular vascular channels and sparse erythrocytes. Atypical endothelial cells were characterized by vesicular chromatin and prominent nucleoli. Large areas of tumor necrosis were observed. Immunohistochemically, neoplastic cells were positive for CD31 and ERG, and negative for AE1-AE3, CAM5.2, and HHV-8 (Figure 1).
Figure 1.
(A) Hematoxylin and eosin low-power image showing angiosarcoma with diffuse infiltration of the lamina propria. Overlying urothelium is uninvolved (arrowhead). At higher magnification, short fascicles of atypical spindle cells with slit-like vascular spaces were evident (inset). (B) PAN cytokeratin AE1-AE3 immunoreactivity was seen in the urothelium (arrowhead) but not in neoplastic cells. (C) Angiosarcoma stained positively for ERG, with nuclear localization (inset). (D) HHV-8 was not expressed by tumor cells.
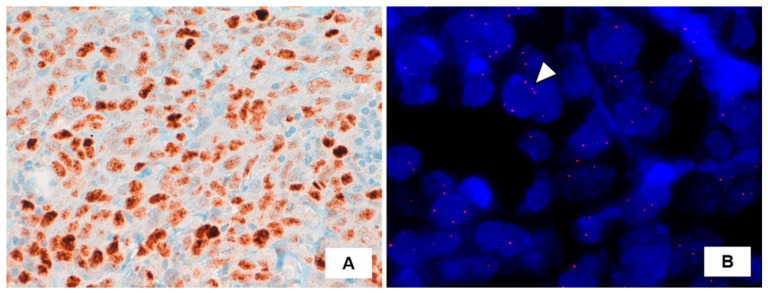
Strong nuclear c-MYC expression was seen, while MYC gene amplification was not detected by fluorescence in situ hybridization (FISH) analysis (Figure 2).
Figure 2.
(A) Immunohistochemistry for c-MYC protein displaying intense expression in angiosarcoma cells (brown precipitate indicates the presence of the target antigen; hematoxylin counterstaining). (B) Fluorescence in situ hybridization (FISH) results using a SpectrumOrange LSI c-MYC probe (Vysis, Abbott Park, IL, USA). Representative tumor cell (arrowhead) showing no increase in the number of signals, indicative for absence of MYC locus amplification. Nuclei are counterstained with 4–6-diamino-2phenylindole (DAPI, blue).
The patient died in the immediate post-operative period from peritonitis and septic shock.
3. Discussion
Angiosarcoma arising in the urinary bladder is an exceedingly rare neoplasm, with few cases reported in the literature to date. In 19 patients with primary bladder sarcomas reported by Spiess et al., leiomyosarcoma was the most frequent histology, whereas angiosarcoma was diagnosed in only three patients [17].
Angiosarcoma is more common in men (M:F = 5:1), with a median age of 63.5 years (range of 20–89 years) [16]. Several risk factors were identified, including a previous history of pelvic radiotherapy for prostate or gynecologic cancer, professional exposure to vinyl chlorides, and cigarette smoking [11]. However, bladder angiosarcoma was also recorded in younger patients without any known risk factors [18,19]. Our patient was a heavy smoker and had undergone pelvic radiotherapy for prostatic adenocarcinoma eight years prior.
The main presenting symptom is macroscopic or microscopic hematuria, although pelvic pain, painful voiding, and urinary obstruction may also occur [18,20,21]. At times, the tumor may be clinically silent and incidentally discovered, as in the current case.
Pathologic diagnosis can be challenging. The most useful morphologic feature to raise suspicion for angiosarcoma is the presence of anastomosing blood-filled spaces of variable shapes and sizes, lined by pleomorphic mitotically active cells. Solid areas composed of spindled and epithelioid cells are not infrequent and, along with the destructive invasive growth and significant cytologic atypia, help in distinguishing angiosarcoma from the more common hemangioma. Kaposi sarcoma may be seen in the urinary bladder, especially in immunocompromised patients. High-grade angiosarcomas may show spindled solid areas, resembling nodular Kaposi sarcoma. However, in angiosarcomas the nuclei of the spindle cells appear hyperchromatic, with coarse chromatin and occasional prominent nucleoli. In addition, angiosarcomas are consistently HHV-8 negative. The differential diagnosis also includes high-grade urothelial carcinomas and urothelial carcinomas with sarcomatoid differentiation [11]. Immunohistochemistry is an important tool for reaching the correct diagnosis, and consequently helps in guiding treatment decisions. Angiosarcoma is confirmed when at least one endothelial marker (e.g., CD31, CD34, ERG, or Factor VIII-related antigen) is positive and urothelial markers (e.g., p63 and GATA-3) are consistently negative [11].
MYC gene amplification is often observed in radiation-associated angiosarcomas and only rarely in de novo angiosarcomas [13,22]. Immunohistochemistry was shown to be a useful surrogate marker for MYC amplification in post-radiation angiosarcoma after treatment for breast carcinomas [23]. In our case, neoplastic cells showed strong and diffuse c-MYC nuclear staining, however, MYC gene amplification was not documented. Notably, concordance between MYC gene amplification and MYC expression was reported to be lower in angiosarcomas arising in non-mammary sites (65%) [24]. Discordant results between FISH and immunohistochemistry may be due to epigenetic alterations, including transcriptional, translational, and post-translational modifications [25].
Angiosarcomas display a distinct tendency towards local recurrence and distant metastases, although early hematogenous spread to the lungs, liver, and bone are also typical [2]. The scientific literature on sarcomas of the urinary bladder have underlined the poor prognosis associated with these tumors, regardless of histologic subtype. The largest review of genitourinary sarcomas to date involves 131 patients with disease-specific survival of 56% at five years and 42% at 10 years, and a median survival of 7.6 years [2]. In the series reported by Spiess et al., of ten patients undergoing salvage therapy, nine died with a median survival of 20 months, emphasizing the need for local control as the single most significant factor in the management of bladder sarcomas [17]. Owing to the rarity of these tumors, no consensus on optimal treatment has been reached. Sarcomas are usually treated with a multimodal approach combining surgery, radiotherapy, and chemotherapy, although no significant superiority of any one strategy has been demonstrated. Adequate tumor resection with wide margins is an important determinant of survival [21]. Radiotherapy and chemotherapeutic regimens, including ifosfamide, epirubicin, or docetaxel plus gemcitabine, may be recommended as adjuvant therapy or in case of metastatic disease [15,19].
4. Conclusions
We report a case of bladder angiosarcoma, a very rare, aggressive neoplasm with poor prognosis. Pathologic features may overlap with high-grade (poorly differentiated/sarcomatoid) urothelial carcinoma, therefore careful morphologic and immunohistochemical evaluation is recommended since the therapeutic approaches involved are as different as the tumors are.
Author Contributions
G.C., writing—original draft preparation; R.S., writing—review and editing; L.G. and I.C.G., investigation; V.L.M. and S.S., resources—investigation; G.N., supervision. Authorship is limited to those who have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Article type is Case report−the study did not require ethical approval.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mondaini N., Palli D., Saieva C., Nesi G., Franchi A., Ponchietti R., Tripodi S., Miracco C., Meliani E., Carini M., et al. Clinical Characteristics and Overall Survival in Genitourinary Sarcomas Treated with Curative Intent: A Multicenter Study. Eur. Urol. 2005;47:468–473. doi: 10.1016/j.eururo.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Dotan Z.A., Tal R., Golijanin D., Snyder M.E., Antonescu C., Brennan M.F., Russo P. Adult Genitourinary Sarcoma: The 25-Year Memorial Sloan-Kettering Experience. J. Urol. 2006;176:2033–2039. doi: 10.1016/j.juro.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Jungano F. Sur un cas d’angio-sarcome de la vessie. Ann. Mal. Org. Genitourin. 1907;25:1451–1461. [Google Scholar]
- 4.Morgan M.A., Moutos D.M., Pippitt C.H., Jr., Suda R.R., Smith J.J., Thurnau G.R. Vaginal and bladder angiosarcoma after therapeutic irradiation. South. Med. J. 1989;82:1434–1436. doi: 10.1097/00007611-198911000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Navon J.D., Rahimzadeh M., Wong A.K., Carpenter P.M., Ahlering T.E. Angiosarcoma of the bladder after therapeutic irradiation for prostate cancer. J. Urol. 1997;157:1359–1360. doi: 10.1016/S0022-5347(01)64980-2. [DOI] [PubMed] [Google Scholar]
- 6.Seethala R.R., Gomez J.A., Vakar-Lopez F. Primary Angiosarcoma of the Bladder. Arch. Pathol. Lab. Med. 2006;130:1543–1547. doi: 10.5858/2006-130-1543-PAOTB. [DOI] [PubMed] [Google Scholar]
- 7.Kulaga A., Yilmaz A., Wilkin R.P., Trpkov K. Epithelioid angiosarcoma of the bladder after irradiation for endometrioid adenocarcinoma. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 2006;450:245–246. doi: 10.1007/s00428-006-0336-9. [DOI] [PubMed] [Google Scholar]
- 8.Tavora F., Montgomery E., Epstein J.I. A Series of Vascular Tumors and Tumorlike Lesions of the Bladder. Am. J. Surg. Pathol. 2008;32:1213–1219. doi: 10.1097/PAS.0b013e31816293c5. [DOI] [PubMed] [Google Scholar]
- 9.Williams S.K., Romaguera R.L., Kava B. Angiosarcoma of the Bladder: Case Report and Review of the Literature. Sci. World J. 2008;8:508–511. doi: 10.1100/tsw.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahouth Z., Masarwa I., Halachmi S., Nativ O. Primary Angiosarcoma of Urinary Bladder: 13th Reported Patient. Case Rep. Oncol. Med. 2015;2015:1–4. doi: 10.1155/2015/652870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matoso A., Epstein J.I. Epithelioid angiosarcoma of the bladder: A series of 9 cases. Am. J. Surg. Pathol. 2015;39:1377–1382. doi: 10.1097/PAS.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 12.Ojerholm E., Stripp D., Mamtani R., Van Arsdalen K., Tripp P. Angiosarcoma of the Bladder Following Prostate Radiotherapy. Am. J. Med. 2015;128:e11–e12. doi: 10.1016/j.amjmed.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Wang G., Black P.C., Skinnider B.F., Hayes M.M., Jones E.C. Post-radiation epithelioid angiosarcoma of the urinary bladder and prostate. Can. Urol. Assoc. J. 2016;10:E197–E200. doi: 10.5489/cuaj.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rallabandi H.B., Swain M., Gowrishankar S., Sinha S. Postradiation angiosarcoma of bladder with extensive osseous metaplasia. Indian J. Pathol. Microbiol. 2016;59:78–80. doi: 10.4103/0377-4929.178234. [DOI] [PubMed] [Google Scholar]
- 15.Tynski Z., Barrett A.J., Bastacky S.I. Primary urinary bladder angiosarcoma with ascites. Hum. Pathol. Case Rep. 2017;10:5–9. doi: 10.1016/j.ehpc.2017.02.003. [DOI] [Google Scholar]
- 16.Gerbaud F., Ingels A., Ferlicot S., Irani J. Angiosarcoma of the Bladder: Review of the Literature and Discussion About a Clinical Case. Urol. Case Rep. 2017;13:97–100. doi: 10.1016/j.eucr.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiess P.E., Kassouf W., Steinberg J.R., Tuziak T., Hernandez M., Tibbs R.F., Czerniak B., Kamat A.M., Dinney C.P., Grossman H.B. Review of the M.D. Anderson experience in the treatment of bladder sarcoma. Urol. Oncol. Semin. Orig. Investig. 2007;25:38–45. doi: 10.1016/j.urolonc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Beyazal M., Pirinççi N., Yavuz A., Özkaçmaz S., Bulut G. Computed tomography and magnetic resonance imaging findings of primary bladder angiosarcoma: A case report. Clin. Imaging. 2014;38:212–214. doi: 10.1016/j.clinimag.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Warne R.R., Ong J.S.L., Snowball B., Vivian J.B. Primary angiosarcoma of the bladder in a young female. BMJ Case Rep. 2011;2011:1120103484. doi: 10.1136/bcr.11.2010.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nawar N.A., Olsen J., Jelic T.M., He C. Primary Urinary Bladder Angiosarcoma with Osteoclast-Like Multinucleated Giant Cells: A Case Report and Literature Review. Am. J. Case Rep. 2016;17:143–149. doi: 10.12659/AJCR.896266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazona J.F., Gupta R., Wysock J., Schaeffer A.J., Smith N.D. Angiosarcoma of Bladder: Long-Term Survival After Multimodal Therapy. Urology. 2007;69:575.e9–575.e10. doi: 10.1016/j.urology.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Mito J.K., Qian X., Jo V.Y., Doyle L.A. MYC expression has limited utility in the distinction of undifferentiated radiation-associated sarcomas from sporadic sarcomas and sarcomatoid carcinoma. Histopathology. 2020;77:667–672. doi: 10.1111/his.14168. [DOI] [PubMed] [Google Scholar]
- 23.Mentzel T., Schildhaus H.U., Palmedo G., Buttner R., Kutzner H.J. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: Clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod. Pathol. 2011;25:75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 24.Ginter P.S., Mosquera J.M., MacDonald T.Y., D’Alfonso T.M., Rubin M.A., Shin S.J. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum. Pathol. 2014;45:709–716. doi: 10.1016/j.humpath.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Motaparthi K., Lauer S.R., Patel R.M., Vidal C.I., Linos K. MYC gene amplification by fluorescence in situ hybridization and MYC protein expression by immunohistochemistry in the diagnosis of cutaneous angiosarcoma: Systematic review and appropriate use criteria. J. Cutan. Pathol. 2020 doi: 10.1111/cup.13912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.