Abstract
Serotype-specific surveillance for invasive pneumococcal disease (IPD) is essential for assessing the impact of 10- and 13-valent pneumococcal conjugate vaccines (PCV10/13). The Pneumococcal Serotype Replacement and Distribution Estimation (PSERENADE) project aimed to evaluate the global evidence to estimate the impact of PCV10/13 by age, product, schedule, and syndrome. Here we systematically characterize and summarize the global landscape of routine serotype-specific IPD surveillance in PCV10/13-using countries and describe the subset that are included in PSERENADE. Of 138 countries using PCV10/13 as of 2018, we identified 109 with IPD surveillance systems, 76 of which met PSERENADE data collection eligibility criteria. PSERENADE received data from most (n = 63, 82.9%), yielding 240,639 post-PCV10/13 introduction IPD cases. Pediatric and adult surveillance was represented from all geographic regions but was limited from lower income and high-burden countries. In PSERENADE, 18 sites evaluated PCV10, 42 PCV13, and 17 both; 17 sites used a 3 + 0 schedule, 38 used 2 + 1, 13 used 3 + 1, and 9 used mixed schedules. With such a sizeable and generally representative dataset, PSERENADE will be able to conduct robust analyses to estimate PCV impact and inform policy at national and global levels regarding adult immunization, schedule, and product choice, including for higher valency PCVs on the horizon.
Keywords: global, invasive pneumococcal disease, pneumococcal meningitis, surveillance, pneumococcal conjugate vaccines
1. Introduction
Streptococcus pneumoniae is an important cause of morbidity and mortality globally, in both children and adults [1,2]. In 2007, the World Health Organization (WHO) first recommended including pneumococcal conjugate vaccines (PCV) in childhood immunization programs worldwide to prevent pneumococcal disease. WHO encouraged countries to implement surveillance of invasive pneumococcal disease (IPD) to establish a baseline rate of disease for evaluating vaccine impact [3]. In 2019, WHO expanded IPD surveillance recommendations to encourage high-quality sentinel surveillance to monitor the distribution of serotypes causing IPD and ideally population-based surveillance for evaluating PCV impact on IPD incidence and serotype replacement disease [4]. By 2020, 145 countries, including countries from all regions of the world, had introduced PCV into infant immunization programs [5], many of which have IPD surveillance systems [6,7,8,9,10]. However, an individual country’s ability to assess vaccine impact and inform policy can be limited by small sample size, limited years of available data either pre- or post-vaccine introduction, limited serotyping capacity, lack of a population catchment area for estimating incidence rates, changes in surveillance systems over time that bias inferences on vaccine impact, or insufficient characterization of cases or evaluation of the detection system to enable assessment of potential bias [11]. Further, unrelated events and temporal changes that influence health or access to care and natural fluctuations in pneumococcal serotypes over time may obscure PCV impact. Even sites not affected by these issues cannot assess the long-term relative merits across PCV products or schedules among both vaccinated and unvaccinated individuals, and their results may not be generalizable to other settings without robust data. Multi-site analyses that include data from many surveillance sites representing a variety of settings and PCV regimens can overcome these limitations. Multisite analyses also lead to greater understanding of pneumococcal epidemiology and PCV impact around the world, and where there is heterogeneity, to greater understanding of the factors driving it, e.g., differences in local epidemiology versus PCV use.
WHO’s Strategic Advisory Group of Experts (SAGE) on Immunization previously commissioned an analysis of PCV7 (Prevenar/Prevnar, Pfizer) impact [11] and several global and regional systematic reviews of IPD serotype distribution have also been conducted [12,13,14,15]. However, these reviews do not reflect the current setting of PCV10 (Synflorix, GlaxoSmithKline) and PCV13 (Prevenar13/Prevnar13, Pfizer) use, evaluate only published data, do not evaluate effects of PCV10 and PCV13 separately, or do not account for duration of PCV use. An updated, more comprehensive global analysis of the long-term effects of PCV10/13 on serotype-specific IPD incidence and serotype distribution is needed to inform policy related to pneumococcal epidemiology in PCV10/13-using countries, the potential value of future higher-valency PCVs, and global and national vaccination policy around product choice and schedule for children and immunization recommendations for adults.
WHO commissioned the Pneumococcal Serotype Replacement and Distribution Estimation (PSERENADE) project to summarize and estimate the impact of PCV10/13 programs on IPD incidence and serotype distribution among children and adults. Here we aimed to describe the landscape of available published and unpublished serotype-specific IPD surveillance data globally that can be used for evaluating vaccine impact, to identify limitations and gaps in the availability of IPD surveillance data globally, and to describe the surveillance sites included in PSERENADE to provide greater clarity in how the data used in PSERENADE analyses were gathered and processed.
2. Materials and Methods
2.1. Identification of Surveillance Sites
We aimed to systematically identify sites conducting serotype-specific IPD surveillance in countries where PCV10 or PCV13 was universally recommended for all infants by 1 January 2017 to ensure at least one full year of post-PCV10/13 surveillance data. Countries using PCV10/13 and their year of introduction were identified using View-Hub, a publicly available database with current information on PCV use worldwide [5]. IPD surveillance sites were identified using multiple approaches. First, we contacted the following surveillance networks: WHO-coordinated Global Invasive Bacterial Vaccine Preventable Disease (IB-VPD) Surveillance Network, the Pan American Health Organization (PAHO) Sistema de Redes de Vigilancia de los Agentes responsables de Neumonias y Meningitis (SIREVA) Network, the European Centre for Disease Prevention and Control Streptococcus pneumoniae Invasive Disease Network (SpIDnet), The European Surveillance System (ECDC), and the U.S. Centers for Disease Control and Prevention (CDC) Active Bacterial Core Surveillance (ABCs) system. Second, we conducted a systematic literature review including articles published in any language with publication dates between 1 January 2011 and 20 December 2018 to identify additional sites where serotype-specific IPD surveillance was conducted for at least a full year following PCV10/13 introduction. Seven databases (Embase (with Medline), PubMed, Web of Science (all databases), Global Index Medicus (including regional databases), Africa Wide Information, Global Health Database, and PASCAL) were searched using search terms modified for each database that were reviewed by a specialist librarian (Supplementary Materials C). Third, results from the PCV Review of Impact Evidence (PRIME) literature review [16] and the View-Hub PCV10/13 impact study module database [5] were used to identify other sites and to validate the search terms to ensure relevant studies were captured. Two reviewers fluent in the language of the written report independently screened all studies and a third reviewer adjudicated disagreements. Fourth, we reviewed citations from a prior literature search on changes in IPD incidence after PCV7 introduction, which included studies published in 1994–2010 [11]. Fifth, International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD) abstracts from 2012–2018 were reviewed. Finally, experts on pneumococcal disease surveillance suggested additional countries or sites not yet identified.
2.2. Data Collection
Site investigators of identified surveillance sites and corresponding authors of studies identified in the literature review were contacted by email. Surveillance data were evaluated for suitability for inclusion in analyses of IPD serotype distribution and PCV impact on IPD incidence over time using standardized criteria intended to ensure comparability of methods and PCV uptake across sites (Table 1). Sites with suitable data were invited to participate in the PSERENADE project and contribute IPD surveillance data. IPD was defined as the isolation or detection of pneumococcus from a normally sterile site or detection of pneumococcus in cerebral spinal fluid (CSF) or pleural fluid using lytA-based PCR or antigen testing; pneumococcus detected in blood by PCR was not considered IPD given its low specificity [17]. Datasets provided by sites were preferentially used over data abstracted from literature in order to include the most up-to-date and comprehensive data available and to optimize the level of detail needed for planned analyses. Characterization of PSERENADE-eligible sites that chose not to participate in PSERENADE is based on descriptions in the published literature.
Table 1.
Data collection inclusion criteria.
| Data Collection Inclusion Criterion | Rationale |
|---|---|
| 1. Site reports annual serotype-specific and age-specific IPD case counts obtained from normally sterile sites | Data must meet a standardized case definition of IPD to ensure comparability across sites, be stratified by age in order to evaluate direct and indirect effects, and be characterized by serotype in order to estimate serotype distributions and evaluate vaccine-type and serotype-specific changes in disease rates over time. |
| 2. At least 50% of isolates serotyped per year in at least one age stratum | A minimum proportion of isolates must be serotyped to limit risks of non-representativeness of serotyped isolates, i.e., to ensure the serotype distribution based only on serotyped cases is not biased and to minimize chance from selective testing. |
| 3. At least one complete year of data post-PCV10/13, excluding the year of introduction | Twelve continuous months are required to ensure data are not limited to an outbreak period and to control for seasonal fluctuations in disease or serotype-specific distribution. |
| 4. At least 50% uptake for the primary PCV series at 12 months of age in at least one year post-PCV10/13 | The goal is to evaluate PCV, not the immunization program. Therefore, vaccine uptake must be high enough to be able to affect serotype distribution/IPD incidence rates at the population level [18,19] and to represent the experience of countries with high coverage. It also serves to help eliminate heterogeneous results due to coverage to enable focus on effects of product and schedule. |
| 5. Testing or reporting not limited to immunocompromised individuals or other specialized populations | PCV impact and serotype distribution may be different in specialized populations (e.g., HIV-positive populations) and may not be representative of the wider population [20]. |
| 6. No major changes or biases in surveillance that would affect estimates of serotype-specific proportions or rates | Changes in the surveillance system over the analysis period, such as a change in indication for blood culturing, introduction of new serotyping methods, or change in the population under surveillance, may bias interpretations of changes in incidence rates making it difficult to distinguish PCV effects from a change in the system. If changes are correlated with vaccine introduction, results may be incorrectly attributed to vaccine program impact. |
Surveillance sites shared annual serotype-specific IPD case data by age in either an individual case-level or aggregate format using a standardized template. Population-based denominators were provided where available. Prior to sharing, data were de-identified and anonymized per The US Health Insurance Portability and Accountability Act (HIPAA) and The European Union (EU) General Data Protection Regulation 2016/679 (GDPR). Data were stored on a secure database at Johns Hopkins University. Where possible, the following additional case characteristics were provided: hospitalized vs. outpatient status (for children under five years of age), HIV status, specimen type, and clinical syndrome (meningitis vs. pneumonia). For meningitis, two case definitions were used: confirmed positive CSF (CSF+) and site-defined clinical meningitis syndrome. Pneumonia cases were defined based on site-specific definitions. Characterization of non-pneumonia/non-meningitis IPD cases was not requested given limitations in data availability.
Site investigators also completed a questionnaire describing the site’s surveillance system and laboratory methods for detection of pneumococcus and serotyping of cases. The questionnaire requested information on the country’s pneumococcal immunization program, including annual immunization uptake estimates representative of the population under surveillance, PCV schedule, year of PCV introduction and product used (including use of PCV7 prior to introduction of PCV10/13), catch-up campaigns, and adult pneumococcal vaccination programs. We also abstracted WHO and UNICEF Estimates of National Immunization Coverage (WUENIC) for national uptake with three doses of PCV for all years of available surveillance data [21]. In the absence of evidence to the contrary, we assumed countries receiving funding from Gavi, the Vaccine Alliance to support PCV implementation did not have an adult pneumococcal vaccine program.
For eligible PAHO countries participating in the SIREVA II surveillance network, the WHO-coordinated Global IB-VPD network facilitated data transfer for children under five years of age. For countries with additional data reported in SIREVA II reports beyond what was available in the WHO Global IB-VPD database, data for patients of all ages were abstracted from 2006–2016 (the last year of available data at the time of abstraction) by year, age group, and serotype [22]. Discrepancies in abstraction were adjudicated by a third reviewer (MGQ) fluent in Spanish. Colombia’s SIREVA II data were abstracted from a separate report published by the country, which included annual data through 2018 [23]. SIREVA II diagnostic and laboratory methods were abstracted from a standardized laboratory manual [24].
A standard data quality review was conducted independently for each site by two PSERENADE team members. Descriptive figures of the data with respect to each of the data quality check elements in Table 2 were shared with investigators with expertise in IPD surveillance at each site to assess the quality of the data. These characterizations and discussions with investigators at each site were used to define eligibility by year, age group, and syndrome for the various subsequent primary and secondary analyses of the study.
Table 2.
PSERENADE standard data quality review.
| Data Quality Check | Rationale |
|---|---|
| A. Are there dramatic changes in overall IPD incidence rates (IR) from year to year that might not be explained by vaccine introduction? | Stable surveillance system, population structure and clinical practices should not exhibit dramatic unexplained changes. |
| B. Are vaccine-serotype IRs decreasing in the target age groups after vaccine introduction as expected? | Vaccine-serotype IRs should be decreasing in target age groups after vaccine introduction, given sufficient vaccine uptake. |
| C. Are there dramatic changes in overall case counts from year to year that might not be explained by vaccine introduction? | Dramatic unexplained changes in case counts could indicate changes in the surveillance system or clinical practices. |
| D. Are vaccine-serotype case counts decreasing in the target age groups after vaccine introduction as expected? | Vaccine-serotype case counts should be decreasing in target age groups after vaccine introduction, given sufficient vaccine uptake. |
| E. Have the number of cases due to serotype 14 and 6B among children < 5 years been eliminated or greatly reduced in the post-PCV era? | Serotype 14 and 6B should be decreasing after vaccine introduction. Persistent serotype 14 or 6B cases may indicate low immunization coverage or surveillance system changes. |
| F. Do the denominators used to calculate IRs in each age group change over time? | Population-based denominators should vary slightly but not substantially over time. If annual population denominators are not available (i.e., denominator only available in some years) rates may be an under- or over-estimate. |
| G. Do the denominators in each age group make sense relative to each other? | Based on conventional population age structures, we expect the number of children aged < 5 years to be less than adults aged ≥ 18 years. The number of adults aged ≥ 65 years would be expected to be less than that of adults aged 18–64 years. |
| H. Do all IPD IRs in each age group make sense relative to each other and the setting? | Expect IPD IRs to be highest in young children and older adults who are most vulnerable, but there can be exceptions in some settings where other age groups have age-associated excess risk [28]. |
| I. Do at least 50% of cases for each age group/surveillance year stratum have a known serotype? | Ensures that the serotype distribution of serotyped cases is not biased or different from the serotype distribution of cases that were not serotyped or not fully serotyped. An exception can be made if the specimens were randomly selected for serotyping, when costs may prohibit all serotyped. |
| J. Does the site distinguish between: Serotype 6A and serotype 6C cases? Serotype 6B and serotype 6D cases? |
In 2007 researchers discovered that pneumococci classified as serotype 6A on the basis of phenotype could be further distinguished chemically, resulting in identification of a new serotype, 6C [29]. Similarly, in 2009 serotype 6D was discovered as a chemically distinct serotype from 6B [30]. Pneumococci previously classified as serotype 6A or 6B would have to be retrospectively reevaluated to distinguish serotypes 6C and 6D, respectively. |
| K. Are undistinguished PCV13-type serotypes identifiable (e.g., ‘6A/6C’ instead of ‘6A’)? | Because undistinguished PCV13-type cases (e.g., 6A/6C) will need to be reapportioned based on the distribution of fully serotyped PCV13-type cases, confirmed ‘6A’ cases need to be differentiated from unconfirmed (i.e., might be 6C). Dates of changes in serotyping methods or documentation of retrospective reclassification efforts are required. |
PCV-using countries that had IPD surveillance data were summarized by data collection eligibility criteria and participation in PSERENADE. Sites were characterized by UN region [25], World Bank income level [26], under five mortality rate [27], childhood pneumococcal disease burden prior to PCV introduction [5], Gavi-eligibility status, PCV product, and PCV schedule. The surveillance systems and PCV programs were also described and summarized for sites included in PSERENADE.
3. Results
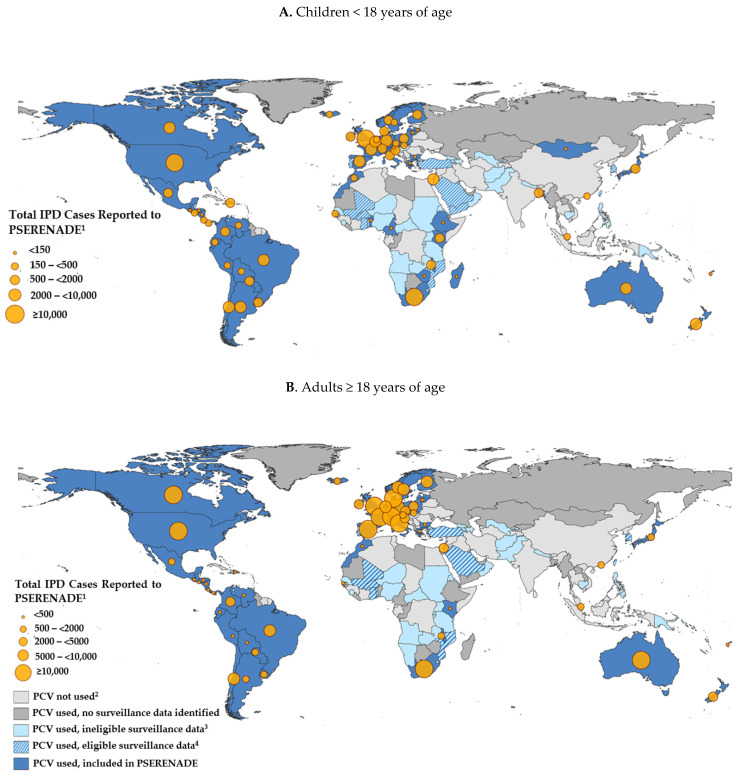
Pediatric and adult IPD surveillance data were available in every UN region of the world, representing countries from all World Bank income levels, under five mortality rate strata, levels of IPD disease burden, PCV products, and infant PCV schedules (Table 3). Of 138 countries with a universal infant PCV10/13 program operational for one or more years by January 1, 2018, we identified 109 conducting IPD surveillance (Table 3, Figure 1). Of these, 76 (69.7%) had surveillance that met PSERENADE eligibility criteria for data collection (Table 1) and 62 (81.6%) of those eligible participated. Surveillance sites in 14 countries that met data collection eligibility criteria did not contribute data to PSERENADE because they either did not respond or declined to participate. Characteristics associated with participation were not evaluated, but the proportion of participating eligible sites are detailed for each region (Table 3). The resulting dataset contained incidence rate data from 38 countries for evaluating PCV impact and case count data only from 24 additional countries for estimating serotype distribution.
Table 3.
Availability of invasive pneumococcal disease (IPD) surveillance data globally in PCV10/13-using countries.
| Strata | Category | All PCV-Using Countries 1 | Data in PSERENADE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. Countries Using PCV, N (% of Countries 2) |
B. PCV-Using Countries with IPD Surveillance, N (% of A 2) | C. Countries Eligible for PSERENADE, N (% of B 2,3) | D. Countries in PSERENADE, N (% of C 2) |
E. Countries with Incidence Data 4, N (% of D 2) | F. Number of Surveillance Sites | G. Total Number of Cases in Post-PCV10/13 Years 5 | H. Annual Number of Cases Averaged across Post-PCV10/13 Years, Median (IQR) 5,6 | I. Number of Years Post-PCV10/13 with Data, Median (IQR) 5 |
||
| Total | Total | 138 (70.4%) | 109 (79.0%) | 76 (69.7%) | 62 (81.6%) | 38 (61.3%) | 77 | 241,442 | 117 (26, 513) | 7 (5, 7) |
| Region 7 | North America | 2 (100.0%) | 2 (100.0%) | 2 (100.0%) | 2 (100.0%) | 2 (100.0%) | 10 | 37,187 | 124 (55, 269) | 7 (7, 8) |
| Latin America and the Caribbean | 22 (66.7%) | 19 (86.4%) | 19 (100.0%) | 18 (94.7%) | 2 (11.1%) | 19 | 20,609 | 50 (21, 227) | 5 (4, 6) | |
| Europe | 31 (73.8%) | 26 (83.9%) | 24 (92.3%) | 23 (95.8%) | 20 (87.0%) | 26 | 142,586 | 548 (115, 918) | 7 (6, 8) | |
| Sub-Saharan Africa | 39 (81.2%) | 31 (79.5%) | 14 (45.2%) | 9 (64.3%) | 4 (44.4%) | 11 | 19,734 | 10 (7, 23) | 6 (5, 6) | |
| Northern Africa and Western Asia | 17 (73.9%) | 14 (82.4%) | 7 (50.0%) | 2 (28.6%) | 2 (100.0%) | 2 | 4,380 | 313 (171, 454) | 7 (7, 7) | |
| Asia | 17 (53.1%) | 13 (76.5%) | 7 (53.8%) | 5 (71.4%) | 5 (100.0%) | 5 | 3,908 | 126 (77, 179) | 4 (3, 7) | |
| Oceania | 10 (62.5%) | 4 (40.0%) | 3 (75.0%) | 3 (100.0%) | 3 (100.0%) | 4 | 13,038 | 274 (48, 748) | 6 (6, 7) | |
| World Bank Income level 8 | High income | 52 (83.9%) | 45 (86.5%) | 40 (88.9%) | 33 (82.5%) | 29 (87.9%) | 46 | 206,562 | 377 (99, 824) | 7 (6, 8) |
| Upper middle income | 27 (50.0%) | 19 (70.4%) | 15 (78.9%) | 14 (93.3%) | 3 (21.4%) | 14 | 33,085 | 55 (21, 272) | 6 (4, 7) | |
| Lower middle income | 38 (74.5%) | 26 (68.4%) | 12 (46.2%) | 11 (91.7%) | 4 (36.4%) | 12 | 968 | 12 (8, 19) | 5 (4, 6) | |
| Low income | 21 (72.4%) | 19 (90.5%) | 9 (47.4%) | 4 (44.4%) | 2 (50.0%) | 5 | 827 | 10 (9, 29) | 6 (5, 6) | |
| Under 5 mortality rate (2018) 9 | Low | 87 (66.4%) | 71 (81.6%) | 60 (84.5%) | 52 (86.7%) | 33 (63.5%) | 65 | 221,478 | 179 (42, 587) | 7 (5, 7) |
| Medium | 35 (79.5%) | 25 (71.4%) | 11 (44.0%) | 8 (72.7%) | 5 (62.5%) | 10 | 19,948 | 14 (9, 65) | 6 (5, 7) | |
| High | 16 (76.2%) | 13 (81.2%) | 5 (38.5%) | 2 (40.0%) | 0 (0.0%) | 2 | 16 | 3 (3, 3) | 6 (6, 6) | |
| Pre-PCV under 5 Spn disease burden (2000) 10 | Low burden | 42 (77.8%) | 38 (90.5%) | 33 (86.8%) | 29 (87.9%) | 27 (93.1%) | 41 | 200,066 | 469 (117, 878) | 7 (7, 8) |
| Medium burden | 34 (60.7%) | 23 (67.6%) | 20 (87.0%) | 17 (85.0%) | 3 (17.6%) | 18 | 20,356 | 64 (24, 275) | 6 (5, 7) | |
| High burden | 62 (74.7%) | 48 (77.4%) | 23 (47.9%) | 16 (69.6%) | 8 (50.0%) | 18 | 21,020 | 17 (9, 30) | 5 (4, 6) | |
| Gavi status 11 | Gavi | 57 (78.1%) | 44 (77.2%) | 19 (43.2%) | 13 (68.4%) | 5 (38.5%) | 15 | 1455 | 10 (7, 15) | 5 (4, 6) |
| Non-Gavi | 81 (65.9%) | 65 (80.2%) | 57 (87.7%) | 49 (86.0%) | 33 (67.3%) | 62 | 239,987 | 262 (57, 625) | 7 (6, 8) | |
| Product | PCV10 | 22 (15.9%) 15 | 21 (95.5%) | 15 (71.4%) | 14 (93.3%) | 8 (57.1%) | 18 | 23,967 | 49 (14, 416) | 6 (5, 7) |
| PCV13 | 93 (67.4%) 15 | 68 (73.1%) | 44 (64.7%) | 34 (77.3%) | 19 (55.9%) | 42 | 183,610 | 123 (31, 594) | 7 (6, 7) | |
| PCV10 and PCV13 12 | 23 (16.7%) 15 | 20 (87.0%) | 17 (85.0%) | 14 (82.4%) | 11 (78.6%) | 17 | 33,865 | 209 (56, 386) | 7 (6, 7) | |
| Schedule 13 | 3 + 0 | 58 (42.0%) 16 | 44 (75.9%) | 20 (45.5%) | 14 (70.0%) | 5 (35.7%) | 17 | 10,825 | 12 (8, 29) | 6 (5, 6) |
| 2 + 1 | 48 (34.8%) 16 | 40 (83.3%) | 36 (90.0%) | 33 (91.7%) | 20 (60.6%) | 38 | 151,942 | 308 (70, 594) | 7 (5, 7) | |
| 3 + 1 | 19 (13.8%) 16 | 13 (68.4%) | 10 (76.9%) | 6 (60.0%) | 5 (83.3%) | 13 | 32,716 | 92 (42, 247) | 7 (7, 8) | |
| 3 + 0 and 2 + 1/3 + 1 14 |
3 (2.2%) 16 | 2 (66.7%) | 2 (100.0%) | 1 (50.0%) | 1 (100.0%) | 014 | 0 | 0 (0, 0) | 0 (0, 0) | |
| 3 + 1 and 2 + 1 15 | 10 (7.2%) 16 | 10 (100.0%) | 8 (80.0%) | 8 (100.0%) | 7 (87.5%) | 9 | 45,959 | 634 (276, 932) | 7 (7, 8) | |
1 Countries with a full year of a PCV10/13 immunization program for infants by 2018 (i.e., introduced by 1 January 2017). Countries with only a risk immunization program rather than universal are also not counted as PCV-using countries. Data from View-Hub [5]. Taiwan and Hong Kong are not merged with China in this table given differences in PCV use and availability of IPD surveillance data compared to the rest of China. 2 Percentage by category unless otherwise specified. 3 To be eligible for PSERENADE, a surveillance site must have had at least one full year of post-PCV10/13 IPD incidence or four years of post-PCV10/13 IPD case counts, over 50% vaccination uptake, and over 50% of cases serotyped by age/year group (Table 1). 4 Incidence data are only available for pneumococcal meningitis cases in Brazil and Greece, as opposed to all IPD in all other countries. 5 Post-PCV10/13 years exclude the year of introduction. 6 The average number of cases in post-PCV10/13 years was calculated for each surveillance site and used to estimate the median (IQR) across strata categories. 7 United Nations (UN) regions adapted from UN Statistics Division [25]. 8 World Bank Income level as of November 2020 [26]. 9 Under 5-year mortality rate data from United Nations Interagency Group for Child Mortality Estimation (2020), 2018 estimate by country. Low: <30 deaths per 1000 livebirths, medium: 30 to <75 deaths, high: 75 to <150 deaths [27]. 10 Pre-PCV pneumococcal disease burden estimates for children <5 years calculated as the sum of estimated pneumonia, meningitis, and invasive non-pneumonia, non-meningitis incidence rates in 2000 [5]. Strata were defined as fewer than 300 cases per 100,000 children (low burden), 300 to fewer than 2000 cases per 100,000 children (medium burden), or 2000 or more cases per 100,000 children (high burden). Countries missing any or all incidence rates were categorized as “Unknown”. 11 Gavi countries are those that are eligible or have graduated. 12 Countries that either used both products concurrently or switched between PCV10 and PCV13. 13 3 + 0: three primary doses and no booster; 2 + 1: two primary doses and a booster; 3 + 1: three primary doses and a booster. 14 Countries that used PCV10/13 schedules with and without a booster dose. Australia, included in PSERENADE, uses 3 + 1 among indigenous populations and used 3 + 0 among non-indigenous populations until 2018, when non-indigenous changed to 2 + 1. Because Australia (non-indigenous) predominantly used 3 + 0 during the years described here, that surveillance site was categorized as 3 + 0 in columns FI, and Australia (indigenous) was categorized as a 3 + 1 surveillance site in columns F–I. Not included in PSERENADE were Trinidad and Tobago (switched from 3 + 0 to 3 + 1) and Libyan Arab Jamahiriya (switched from 3 + 0 to 2 + 1). 15 Countries that used 3 + 1 and 2 + 1 PCV10/13 schedules. All switched from 3 + 1 to 2 + 1 except for Poland, which uses 2 + 1 in the National Immunization Program (NIP) and 3 + 1 in the private market, and Canada, which uses 3 + 1 and/or 2 + 1 in different provinces. Canadian surveillance sites for individual provinces are categorized accordingly in columns F-I. 16 Percentage is of the 138 PCV-using countries.
Figure 1.
Availability of IPD surveillance data for countries with universal recommendations for PCV in the infant immunization program. 1 Cases from multiple surveillance sites within the same country were aggregated.2 PCV not universally introduced into the routine infant immunization program by 2018 (includes India which began sub-national introduction in 2017). 3 IPD surveillance data did not meet PSERENADE data collection eligibility criteria (Box 1). 4 IPD surveillance data met data collection eligibility criteria but did not participate in PSERENADE.
Eligibility of IPD surveillance data varied by region, income level, and epidemiological setting (Table 3). In Asia and Africa, where most pneumococcal deaths occur, fewer than half (48.3%) of the 58 countries conducting IPD surveillance met PSERENADE inclusion criteria, compared to 75.0–100% of countries elsewhere, and only 57.1% of the 28 that were eligible participated in PSERENADE. Although most (90.5%) PCV-using low-income countries (LICs) had IPD surveillance, the surveillance was less likely to meet eligibility criteria than that in upper-middle-(UMICs) or high-income countries (HICs) (47.4% for LICs vs. 78.9 for UMICs and 88.9% for HICs). Among those countries with surveillance meeting eligibility criteria, LICs were also less likely to contribute to PSERENADE (44.4% vs. 82.5–93.3%). Similarly, countries with high or medium under-5 mortality rates were less likely to have surveillance systems meeting eligibility criteria (38.5% and 44.0%, respectively) than low mortality countries (84.5%), and of the 13 high-mortality countries with IPD surveillance, only 5 (38.5%) were eligible for PSERENADE and only 2 participated, neither of which had population-based denominators to estimate incidence rates. There were 19 Gavi-eligible PCV-using countries with IPD surveillance eligible for PSERENADE, 13 (68.4%) of which participated, including 5 with incidence data. Of the 61 countries using a schedule with three primary doses and no booster (3 + 0), only 22 (36.1%) had eligible data, compared to 56 (70.0%) of 80 countries using an infant PCV schedule with a booster dose (3 + 1 or 2 + 1). Although the proportion of countries with surveillance systems meeting eligibility criteria was similar by PCV product (PCV13: 64.7%; PCV10: 71.4%), there were more PCV13-using countries eligible for PSERENADE analyses (n = 44 vs. 15).
Seventy-seven sites from 62 countries participated in PSERENADE (Table 3 and Table 4). All surveillance sites contributing data to PSERENADE collected pediatric data; although 88.0% overall also collected adult IPD data, those that did not were disproportionately from Sub-Saharan Africa and Asia where only 54.5% and 60.0% of sites, respectively, collected adult IPD data (Table 4). Data from the period prior to PCV introduction was available from 58 (77.3%) of surveillance sites. Although 51 (68.0%) sites conducted population-based surveillance with population denominators enabling incidence estimation, few of these were from the regions of Latin America and the Caribbean (three sites from two countries), Sub-Saharan Africa (six sites from four countries), and Northwestern Africa and Western Asia (two sites from two countries) (Table 4 and Supplementary Table S2).
Table 4.
Summary of PSERENADE surveillance sites by region 1,2.
| North America N = 9 |
Latin America and the Caribbean N = 19 |
Europe N = 26 |
Sub-Saharan Africa N = 11 |
N. Africa and W. Asia N = 2 |
Asia N = 5 |
Oceania N = 3 |
Total N = 75 |
|
|---|---|---|---|---|---|---|---|---|
| Availability of data, N (%) | ||||||||
| 0–17 years | 9 (100%) | 19 (100%) | 26 (100%) | 11 (100%) | 2 (100%) | 5 (100%) | 3 (100%) | 75 (100%) |
| ≥18 years | 7 (77.8%) | 19 (100%) | 26 (100%) | 6 (54.5%) | 2 (100%) | 3 (60.0%) | 3 (100%) | 66 (88.0%) |
| Pre-PCV period | 7 (77.8%) | 19 (100%) | 17 (65.4%) | 6 (54.5%) | 2 (100%) | 4 (80.0%) | 3 (100%) | 58 (77.3%) |
| PCV7 period 3 | 8 (88.9%) | 8 (100%) | 16 (84.2%) | 2 (100%) | 1 (100%) | 3 (75.0%) | 2 (100%) | 40 (88.9%) |
| PCV10/13 period | 9 (100%) | 19 (100%) | 26 (100%) | 11 (100%) | 2 (100%) | 5 (100%) | 3 (100%) | 75 (100%) |
| Incidence data 4 | 9 (100%) | 3 (15.8%) | 23 (88.5%) | 6 (54.5%) | 2 (100%) | 5 (100%) | 3 (100%) | 51 (68.0%) |
| Clinical syndrome data | 8 (88.9%) | 11 (57.9%) | 20 (76.9%) | 10 (90.9%) | 1 (50.0%) | 5 (100%) | 3 (100%) | 58 (77.3%) |
| Specimens collected, N (%) 5 | ||||||||
| Blood | 9 (100%) | 19 (100%) | 25 (100%) | 11 (100%) | 2 (100%) | 5 (100%) | 3 (100%) | 74 (100%) |
| CSF | 9 (100%) | 19 (100%) | 25 (100%) | 9 (81.8%) | 2 (100%) | 5 (100%) | 3 (100%) | 72 (97.3%) |
| Pleural fluid | 7 (77.8%) | 18 (94.7%) | 17 (68.0%) | 2 (18.2%) | 1 (50.0%) | 4 (80.0%) | 2 (66.7%) | 51 (68.9%) |
| Additional detection methods, N (%) | ||||||||
| Nucleic acid | 2 (22.2%) | 16 (84.2%) | 23 (88.5%) | 6 (54.5%) | 1 (50.0%) | 4 (80.0%) | 2 (66.7%) | 54 (72.0%) |
| Antigen detection | 0 (0.0%) | 13 (68.4%) | 14 (53.8%) | 0 (0.0%) | 0 (0.0%) | 3 (60.0%) | 2 (66.7%) | 32 (42.7%) |
| Serotyping methods, N (%) 5 | ||||||||
| Quellung | 9 (100%) | 19 (100%) | 23 (92.0%) | 4 (36.4%) | 2 (100%) | 3 (60.0%) | 3 (100%) | 63 (85.1%) |
| Non-Quellung | 2 (22.2%) | 12 (63.2%) | 22 (88.0%) | 11 (100%) | 1 (50.0%) | 4 (80.0%) | 2 (66.7%) | 54 (73.0%) |
| Latex agglutination | 1 (11.1%) | 2 (10.5%) | 15 (60.0%) | 3 (27.3%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 22 (29.7%) |
| Any PCR method 6 | 2 (22.2%) | 12 (63.2%) | 14 (56.0%) | 11 (100%) | 1 (50.0%) | 4 (80.0%) | 2 (66.7%) | 46 (62.2%) |
| PCR35/37/38 7,8 | 0 (0.0%) | 10 (52.6%) | 2 (8.0%) | 2 (18.2%) | 0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 15 (20.3%) |
| PCR70/76 7,8 | 2 (22.2%) | 5 (26.3%) | 8 (32.0%) | 0 (0.0%) | 1 (50.0%) | 3 (60.0%) | 1 (33.3%) | 20 (27.0%) |
| Other method 9 | 1 (11.1%) | 0 (0.0%) | 6 (24.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (9.5%) |
1 Subpopulations (e.g., indigenous and non-indigenous) from the same surveillance system were presented as one site. Countries with more than one surveillance site are represented more than once. Data for individual surveillance sites are in Supplementary Table S3. 2 United Nations (UN) regions adapted from UN Statistics Division [25]. N. Africa and W. Asia: Northern Africa and Western Asia. 3 Sites that did not use PCV7 were excluded from calculations of PCV7 period data availability (not applicable). Total calculations are out of the 45 sites that used PCV7. 4 Incidence data from Brazil and Greece are for pneumococcal meningitis only. 5 One site (Lithuania) with unknown specimen type and serotyping data was excluded from calculations. Total calculations are out of 74 sites. 6 Comprised of sites that use PCR at any capacity—including those with unknown or custom PCR schemes that do not fall into PCR35/37/38 or PCR70/76 categories. 7 The number following “PCR” indicates the number of serotypes able to be identified by PCR. Similar serotyping capacities were grouped together. 8 Argentina, Mexico, and Paraguay use both PCR37 and PCR70 and are counted in both of those categories. 9 Includes sites that reported other serotyping methods: Whole genome sequencing (WGS), Next generation sequencing (NGS), Capsular sequence typing (CST), or Gel diffusion (GD).
All surveillance sites collected both blood and CSF except those in Sub-Saharan Africa, of which two (18.2%) collected blood only (Table 4); 68.9% of surveillance sites collected pleural fluid, with this proportion also lowest in Sub-Saharan Africa (2/11; 18.2%). Cases were characterized by clinical syndrome at 77.3% of sites overall, but those that did not characterize cases by clinical syndrome were disproportionately from the Latin America and the Caribbean region (11 of 19). Most surveillance sites (77.3%) used detection methods on CSF beyond culture (42.7% used antigen detection and 72.0% used nucleic acid detection). To identify the serotype, most (85.1%) sites used Quellung reaction and 73.0% used another method, primarily PCR (62.2%) and latex agglutination (29.7%) (Table 4 and Supplementary Table S3).
In total, PSERENADE collected data on over 240,000 post-PCV10/13 IPD cases, with the majority from Europe (n = 142,586) and North America (n = 37,187), but with a substantial number also from Latin America and the Caribbean (n = 20,609), Sub-Saharan Africa (n = 19,734), and Oceania (n = 13,038) (Table 3). The average number of annual cases post-PCV10/13 was lowest among Sub-Saharan Africa (median across sites = 10) and Latin America and the Caribbean (median = 50) compared to other regions (median range: 124–548). The number of cases per site in total was generally lower for sites without surveillance among all ages, those with smaller population catchment areas, and those with fewer years since PCV10/13 introduction (data not shown). The median number of surveillance years post-PCV10/13 across regions ranged from 4 (Asia) to 7 (North America, Europe and Northern Africa/Western Asia; Table 3).
Most (54.5%) PSERENADE sites used PCV13, 23.4% used PCV10 and 22.1% used both products concurrently or switched between products (Table 3 and Table 5). The majority of sites introduced PCV10/13 without a catch-up program (69.9%) and have a booster dose schedule (77.9%). PCV10/13 immunization coverage across the post-PCV10/13 period was high in most sites (mean uptake 87.9%, range 55–98%). The majority of sites have an adult pneumococcal vaccine program for polysaccharide vaccine (PPV23) and/or PCV13. Among these, 62.3% and 63.6% of sites recommend PPV23 for older adults and individuals at high risk for IPD, respectively, and 35.1% and 55.8%, respectively, recommend PCV13. Data on adult PPV23 and PCV13 uptake were available from 24 sites; 45.8% had >50% uptake (data not shown).
Table 5.
Description of infant and adult pneumococcal immunization use for PSERENADE sites.
| Infant PCV Product 1 | PCV10/13 Schedule 1 | Region 2 | Site 3 | PCV7 Period | PCV10 Period | PCV13 Period | PCV10/13 Catch-Up | Mean PCV10/13 Uptake (%) | Other PCV/PPV Recommendations 7 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Series 5 | WUENIC PCV3 6 | Adult | High Risk | ||||||||
| PCV10 | 3 + 0 | LA and C | Ecuador (SIREVA, WHO) | 2010–2011 | 2010– | -- | N | -- | 85 | -- | -- |
| Sub-Saharan Africa | Ethiopia (WHO) | -- 4 | 2011– | -- | N | -- | 58 | -- | -- | ||
| Kenya, Asembo | -- | 2011– | -- | Y | 86 | 81 | -- | -- | |||
| Kenya, Kibera | -- | 2011– | -- | N | 87 | 81 | -- | -- | |||
| Kenya, Kilifi | -- | 2011– | -- | Y | 83 | 78 | -- | -- | |||
| Madagascar (WHO) | -- | 2012– | -- | N | -- | 74 | -- | -- | |||
| Asia | Bangladesh | -- | 2015– | -- | N | -- | 97 | -- | -- | ||
| Oceania | Fiji | -- | 2012– | -- | N | 90 | 99 | -- | -- | ||
| 2 + 1 | LA and C | Colombia (SIREVA) | -- | 2011– | -- | N | -- | 82 | -- | PPV | |
| Europe | Austria (ECDC)10 | -- | 2012– | -- | N | -- | -- | PPV, PCV | PPV | ||
| Finland | -- | 2010– | -- | N | 95 | 90 | PPV, PCV | PPV, PCV | |||
| Iceland | -- | 2011– | -- | N | -- | 89 | PPV | PPV, PCV | |||
| Latvia | 2010–2011 | 2012– | -- | N | 91 | 85 | -- | -- | |||
| Lithuania (ECDC) | -- | 2014– | -- | Unk | -- | 82 | -- | -- | |||
| Slovenia | -- | 2015–2019 | 2019– | N | 55 12 | 55 | PPV, PCV | PPV, PCV | |||
| 3 + 1 | Europe | Bulgaria | -- | 2010– | -- | N | -- | 91 | PPV, PCV | -- | |
| 3 + 1/2 + 1 | LA and C | Brazil | -- | 2010– | -- | Y | 91 8 | 88 | -- | PPV, PCV | |
| Europe | Netherlands | 2006–2011 | 2011– | -- | N | 95 | 94 | -- | PPV, PCV | ||
| PCV13 | 3 + 0 | LA and C | Bolivia (SIREVA) | -- | -- | 2014– | N | -- | -- | -- | -- |
| Honduras (SIREVA, WHO) | -- | -- | 2011– | N | -- | 99 | -- | -- | |||
| Nicaragua (SIREVA, WHO) | -- | -- | 2010– | N | -- | 98 | PPV | PPV | |||
| Sub-Saharan Africa | Benin (WHO) | -- | -- | 2011– | N | -- | 73 | -- | -- | ||
| Cameroon (WHO) | -- | -- | 2011– | N | -- | 72 | -- | -- | |||
| Malawi, Blantyre District | -- | -- | 2011– | Y | 92 | 88 | -- | -- | |||
| The Gambia, Basse | 2009–2011 | -- | 2011– | N | 77 | 95 | -- | -- | |||
| Zimbabwe (WHO) | -- | -- | 2012– | N | -- | 90 | -- | -- | |||
| 2 + 1 | N. Am. | Canada, Alberta | 2002–2010 | -- | 2010– | N | 88 8 | 77 | PPV | PPV, PCV | |
| LA and C | Argentina | -- | -- | 2012– | Y | -- | 84 | PPV, PCV | PPV, PCV | ||
| Costa Rica | -- | -- | 2011– | N | 94 | 92 | -- | PPV, PCV | |||
| Dominican Republic (SIREVA) | -- | -- | 2013– | N | -- | -- | -- | -- | |||
| Guatemala (SIREVA) | -- | -- | 2012– | N | -- | 81 | -- | -- | |||
| Mexico (SIREVA) | 2009–2013 | -- | 2011– | Y | -- | 90 | PPV | PPV | |||
| Panama (SIREVA) | 2010–2011 | -- | 2011– | Unk | -- | 93 | PPV, PCV | PPV, PCV | |||
| Uruguay (SIREVA) | 2008–2010 | -- | 2010– | Y | -- | 94 | PPV | -- | |||
| Europe | Denmark | 2007–2010 | -- | 2010– | N | 91 8 | 93 | PPV, PCV | PPV, PCV | ||
| France | 2006–2010 | -- | 2010– | N | 93 | 90 | -- | PPV, PCV | |||
| Ireland | 2008–2010 | -- | 2010– | N | 91 | 91 | PPV | PPV, PCV | |||
| Italy | 2006–2009 | -- | 2010– | N | 86 8 | 87 | PPV, PCV | -- | |||
| Norway | 2006–2011 | -- | 2011– | N | 93 | 93 | PPV | PPV, PCV | |||
| Spain, Madrid | 2006–2010 | -- | 2010– | N | 98 | 93 | PPV, PCV | PPV, PCV | |||
| Switzerland | 2005–2010 | -- | 2010– | Y | 79 8 | 77 | -- | PCV | |||
| UK, England | 2006–2009 | -- | 2010– | N | 94 | 92 | PPV | PPV | |||
| UK, Scotland | 2006–2010 | -- | 2010– | N | 97 | 92 | PPV | PPV, PCV | |||
| N. Africa and W. Asia | Israel | 2009–2010 | -- | 2010– | N | 95 | 93 | PPV, PCV | PPV, PCV | ||
| Sub-Saharan Africa | South Africa | 2009–2011 | -- | 2011– | Y | 77 8 | 77 | PPV, PCV | PPV, PCV | ||
| Asia | Mongolia | -- | -- | 2016– | Y | 93 | 20 | -- | -- | ||
| Singapore | 2009–2011 | -- | 2011– | Y | 83 | 74 | PPV, PCV | PPV, PCV | |||
| 3 + 1 | N. Am. | USA, ABCs | 2000–2009 | -- | 2010– | Y | 88 | 93 | PPV, PCV | PPV, PCV | |
| USA, Alaska | 2001–2009 | -- | 2010– | Y | 83 | 93 | PPV, PCV | PPV, PCV | |||
| USA, California | 2000–2009 | -- | 2010– | Y | 96 | 93 | PPV, PCV | PPV, PCV | |||
| USA, Massachusetts | 2000–2009 | -- | 2010– | Y | 94 | 93 | PPV, PCV | PPV, PCV | |||
| USA, Southwest (Indigenous) | 2000–2009 | -- | 2010– | Y | 82 | 93 | PPV, PCV | PPV, PCV | |||
| USA, Utah | 2000–2009 | -- | 2010– | Y | 88 | 93 | PPV, PCV | PPV, PCV | |||
| Europe | Greece 10 | 2006–2009 | -- | 2010– | N | 82 | 75 | PPV, PCV | PPV, PCV | ||
| Asia | Japan | 2010–2013 | -- | 2013– | N | 94 8 | 98 | PPV | PPV | ||
| 3 + 0/2 + 1 | Oceania | Australia (Non-Indigenous) 13 | 2005–2011 | -- | 2011– | Y | 92 | 92 | PPV, PCV | PPV, PCV | |
| 3 + 1/2 + 1 | LA and C | Venezuela (SIREVA) | -- | -- | 2014– | Unk | -- | 7 | -- | PPV, PCV | |
| Europe | Germany 10 | 2006–2009 | -- | 2009– | N | 85 | 84 | PPV | PPV, PCV | ||
| Spain, Catalonia | 2001–2010 9 | -- | 2010–2015 9 2016– |
N | 70 9 | 93 | PPV | PPV, PCV | |||
| Spain, Navarra | 2004–2009 9 | -- | 2010–2015 9
2016– |
N | 719 | 93 | PPV | PPV, PCV | |||
| PCV10/13 | 2 + 1 | N. Am. | Canada, Quebec (excluding Nunavik) | 2004–2009 | 2009–2010 | 2011–2018 | N | 97 | 75 | PPV | PPV, PCV |
| 2018– | |||||||||||
| LA and C | Chile, Metropolitan Region | 2009–2010 | 2011–2015 | 2016– | N | 97 | 89 | PPV | PPV | ||
| Chile, Non-Metropolitan Regions | -- | 2011–2017 | 2017– | N | 97 | 89 | PPV | PPV | |||
| El Salvador (SIREVA, WHO) | 2010–2011 | 2018– | 2011–2018 | Unk | -- | 87 | PPV | PPV, PCV | |||
| Paraguay | -- | 2012–2017 | 2017– | Y | 78 | 91 | PPV | -- | |||
| Peru (SIREVA, WHO) | 2009–2011 | 2011–2015 | 2015– | N | -- | -- | PPV | PPV | |||
| Europe | Belgium | 2007–2011 | 2015–2019 | 2011–2015 | N | 938 | 94 | PPV, PCV | PPV, PCV | ||
| 2019– | |||||||||||
| Slovakia | 2009–2010 | 2011– | 2011– | Y | 97 | 97 | PCV | PCV | |||
| Sweden | 2009–2010 | 2010– | 2010–2019 | N | 97 8 | 97 | PPV | PPV, PCV | |||
| N. Africa and W. Asia | Morocco, Grand Casablanca | -- | 2012– | 2010–2012 | N | 91 | 90 | -- | -- | ||
| 3 + 1 | N. Am. | Canada, Quebec-Nunavik | 2002–2009 | 2009–2010 | 2011– | N | 97 | 75 | PPV | PPV, PCV | |
| Canada, Ontario | 2005–2009 | 2009–2010 | 2010– | Y | 72 8 | 77 | PPV | PPV, PCV | |||
| Asia | Hong Kong | 2009–2010 | 2010–2011 | 2011– | N | 98 | -- | PPV, PCV | PPV, PCV | ||
| Oceania | New Zealand | 2008–2011 | 2011–2014, 2017– |
2014-2017 | N | 93 | 93 | PPV, PCV | PPV, PCV | ||
| Australia, Northern Territory | 2001–2009 | 2009–2011 | 2011– | Y | 88 | 92 | PPV, PCV | PPV, PCV | |||
| 3 + 1/2 + 1 | Europe | Czech Republic | -- | 2010– | 2010– | N | 74 8 | -- | PPV, PCV | PPV, PCV | |
| Poland 11 | -- | 2017– | 2017– | N | 94 | 60 | PPV, PCV | PPV, PCV | |||
1 Product and schedule classifications intend to represent what was widely used in the population as of 2018, which occasionally differ from the national universal recommendation. 2 United Nations (UN) regions adapted from UN Statistics Division [25]. 3 (WHO): WHO Global Invasive Bacterial Vaccine-Preventable Diseases (IB-VPD) Surveillance Network; (SIREVA): Pan American Health Organization Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonias y Meningitis Bacterianas (SIREVA); (ECDC): The European Surveillance System (ECDC). 4 “—” represents PCV not universally used. 5 Annual PCV uptake estimates provided by the surveillance site for the primary series of PCV by 12 months of age (if available, for some sites up to 15 months of age), excluding year of vaccine rollout; ‘—’ represents no coverage information provided to PSERENADE project. 6 WUENIC PCV3 uptake, excluding the year of vaccine rollout (PCV3 represents the third dose whether given before 12 months or at or after 12 months, but in some cases uptake estimates may reflect the percentage of surviving infants who received two doses of PCV prior to the 1st birthday); ‘—’ represents no WUENIC coverage information available. 7 Pneumococcal vaccine recommendation for other age groups or risk conditions. Adult recommendations are for all adults aged 50 years and above, aged 60 years and above, or aged 65 years and above. High-risk population age recommendations and populations included varies across sites. ‘--’ represents no product is recommended. 8 Annual PCV uptake estimates provided by the surveillance site for the primary series plus the booster dose by 23 months of age, excluding year of vaccine rollout. 9 Recommended for high-risk populations only but had substantial (≥50% annually) private market uptake among the general population. 10 Although both PCV10 and PCV13 were recommended in the guidelines, the country was classified according to the product that was in substantially wide use. 11 PCV10 and PCV13 became available in the private market in 2009 and 2010, respectively. Widespread use began in 2017 when PCV was introduced into the Polish National Immunization Program (NIP). Private market use of PCV uses a different schedule (3 + 1) than the NIP (2 + 1). To date, PCV10 has been chosen for the NIP and private market PCV13 use is approximately 30%. 12 Range of vaccine uptake is 49–60%. 13 Australia (non-indigenous) switched from 3 + 0 to 2 + 1 in 2018. Abbreviations: N. Am.: North America; LA and C: Latin America and the Caribbean; N. Africa and W. Asia: Northern Africa and Western Asia; Y: Yes; N: No; Unk: unknown; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine; 3 + 1: 3 primary doses plus booster; 2 + 1: 2 primary doses plus booster; 3 + 0: 3 primary doses and no booster.
4. Discussion
As part of the PSERENADE project, the largest and most comprehensive global serotype-specific IPD database was compiled though a comprehensive and systematic search. All available serotype-specific IPD surveillance data in countries using PCV10/13 were identified and characterized to evaluate the global evidence to estimate the impact of PCV10/13 by age, product, schedule, and syndrome. IPD surveillance is recommended by the WHO [4] and nearly 80% of countries using PCV in 2018 had an active IPD surveillance system. Seventy percent of these met PSERENADE eligibility criteria for potential to evaluate PCV impact or post-PCV-era serotype distribution, and over half of the eligible countries had annual IPD incidence rate data. Eligible IPD surveillance data existed for both children and adults, in all regions of the world, from both PCV10- and PCV13-using countries, from countries with and without a booster dose schedule, and from all income and infant mortality rate strata. The majority of countries that met PSERENADE eligibility criteria were from HICs and used a booster dose. Although there were eligible data from at least 15 countries representing low- or lower-middle income countries (LMICs), 3 + 0 schedules, and the African or Asian regions, when restricted to analyses of incidence or stratified by product, data become very sparse for addressing some questions. While there are challenges in drawing inferences from observational surveillance data and some gaps remain, the breadth and depth of the data compiled by PSERENADE increase our capacity to address many questions.
This multisite database overcomes common IPD data limitations, including having too few cases or years of data available, temporal confounding, and changes of surveillance systems over time. It can facilitate more robust results with greater accuracy by observing trends at many sites, thus increasing confidence in interpretation and improving PCV policy relevance at both national and global levels. The PSERENADE collaboration will enable robust analyses to answer policy-relevant epidemiologic questions. These questions relate to how well PCVs performed in reducing vaccine-type disease, the magnitude of indirect effects of PCVs on vaccine-type disease in unimmunized older children and adults, the degree and heterogeneity of serotype replacement (i.e., where non-vaccine type disease increases as a result of reduction of vaccine-types) in all age groups, how these may differ by product or schedule, what optimizes the impact of available pneumococcal vaccines, and what the potential impacts of future higher valency PCVs in addressing residual IPD will be. Furthermore, serotype-specific IPD incidence rate data will allow for estimation of the effectiveness of PCV10/13 against important vaccine-type or -related serotypes, in particular serotypes 3, 6A, 6C, and 19A, and the magnitude of replacement disease due to specific non-vaccine serotypes.
Although there was representativeness across a wide array of settings, the quantity and depth of data at some sites were limited, reducing its usefulness for analyses. Resource-poor settings, which are often those with the highest disease burden, face many challenges. As a result, these were more likely to have the smallest IPD sample sizes and less well characterized cases. For example, resource-poor settings are more likely to have frequent use of antibiotics that limit detection of S. pneumoniae by bacterial culture, an inability to identify cases meeting the IPD case definition, a lack of capacity to perform more sensitive PCR-based tests of the CSF or using PCR serotyping methods that are limited in the number of serotypes they can identify (both of which may bias the serotype distribution [31,32]), or an inability to link laboratory results with clinical data. In addition, outbreak-prone settings can be overwhelmed during peak seasons with case management, and may not be able to keep up with specimen collection, testing, and reporting to national surveillance systems [33]. These challenges are reflected by a greater proportion of surveillance sites from LMICs not meeting PSERENADE data collection eligibility criteria. Further, some sites with eligible data were unable to contribute to the project due to a lack of data management resources. However, many of these surveillance sites are still able to serve local purposes, such as serving as sentinels to identify pockets of vaccine-type disease where immunization uptake may be suboptimal. Eligible sites from LMICs that participated in PSERENADE were also more likely to have small sample size compared to those from UMICs or HICs. Further, although most (88%) surveillance included adults in addition to children, surveillance of adult disease was less common in Africa (54%) and Asia (60%). Pneumococcal pneumonia and meningitis are common in adults and surveillance in this age group is important for assessing indirect effects of infant immunization, including replacement disease, particularly in the meningitis belt of Africa, where serotype 1 IPD outbreaks are common [33,34,35,36,37,38,39]. Improving the quality of existing systems in key high-burden settings could help address remaining questions and increase representation from all settings in global analyses.
The majority of PSERENADE sites were able to classify IPD cases by specimen type, thus enabling identification of pneumococcal meningitis cases (cases with detection of pneumococcus in CSF), which is important for understanding syndrome-specific vaccine impact. This is important in regions with a history of meningitis outbreaks, such as the African meningitis belt. However, only a small proportion of surveillance sites have laboratory data systematically linked to clinical data to allow characterization of cases by clinical diagnoses. Therefore, few PSERENADE sites were able to identify bacteremic pneumonia cases because blood cultures are also obtained for other non-pneumonia IPD syndromes. As a result, few surveillance sites can directly assess the relative PCV impact on meningitis versus bacteremic pneumonia. Countries within the African meningitis belt also tended to have less comprehensive surveillance systems, resulting in IPD being reported predominantly from meningitis cases [36,40,41] or having incomplete data on age or serotype [42]. Improving the availability and characterization of cases has direct relevance to WHO’s global roadmap for defeating meningitis by 2030, which set targets for vaccine-preventable meningitis surveillance, including for pneumococcal meningitis. In particular, the roadmap calls for strengthening of surveillance systems where a lack of laboratory capacity and resources for conducting surveillance hinder meningitis outbreak responses and provide data of limited quality to inform vaccine use and evaluate vaccine impact [43].
Despite investment in IPD surveillance globally, important gaps remain in the availability of IPD data needed for some assessments of PCV impact across diverse settings. The vast majority of IPD surveillance data are from HICs in Europe and North America. In these settings, pneumococcal epidemiology, serotype distribution, and disease burden differ from LMICs, which makes it difficult to be confident that global analyses are fully representative. Africa and Asia are the most under-represented. Most sites in these regions report lower case counts despite having a higher disease burden. Furthermore, stable population-based surveillance over time, important for estimating incidence rates, is particularly sparse in LMICs using a 3 + 0 schedule; only 5 countries (Bangladesh, Fiji, The Gambia, Kenya, and Malawi) provided data, with Bangladesh providing data for children only. A limitation of PSERENADE was not being able to get all key data that are available, including from two important countries using 3 + 0 schedules and conducting surveillance in the meningitis belt, Ghana and Burkina Faso. Population-based surveillance data over time for estimating impact were particularly limited from Latin America and the Caribbean, where only Chile had population-based surveillance data for all IPD, and Brazil had population-based surveillance data for pneumococcal meningitis only. Currently, the ability to answer important sub-group questions, including the indirect effects and some serotype-specific effects of 3 + 0 schedules in high-burden settings (particularly in meningitis outbreak prone settings), is limited. Additional high-quality and well-characterized data for all ages in these settings will be needed to answer these questions.
5. Conclusions
A large amount of IPD data is available globally for both children and adults. PSERENADE’s systematic assessment and combined database of the available serotype-specific IPD surveillance data in countries using PCV10/13 will facilitate answering important questions as well as highlight the gaps needed to be filled to address remaining questions. These data have the potential to inform policy around pneumococcal vaccine use, for both PCV and PPV, at national and global levels, including recommendations concerning product choice, schedule, and adult immunization. The PSERENADE project has informed WHO SAGE recommendations around pneumococcal vaccine use in adults and in community outbreak settings [33] and will contribute important evidence for other pneumococcal vaccine policy decisions. The ongoing collection of serotype-specific IPD surveillance data in countries that have introduced or plan to introduce PCV, as recommended by WHO, will provide data needed to understand PCV impact and inform pneumococcal vaccine policy decisions, particularly if efforts are made to support and enhance surveillance capacity in key areas underrepresented in global analyses.
Acknowledgments
This work was only possible through the contributions of many other individuals and organizations. The list of names of key individuals is provided in the Supplementary Acknowledgement List. We thank all individuals involved in the surveillance activities at the sites contributing to the PSERENADE project for trusting us with their data and for the assistance along the way to ensure this work provides value. This includes the surveillance network coordinators at EpiConcept, European Centre for Disease Prevention and Control, Pan American Health Organization and WHO who facilitated access to and understanding of the data. We thank the Johns Hopkins University librarians and research assistants for providing literature review and data collection support. We thank the technical and strategic advisors from the WHO for providing guidance on the objectives and for facilitating introductions to the many surveillance sites globally. Finally, we are greatly indebted to the members of our PSERENADE Technical Advisory Group for providing us with expert technical review and strategic advice on all aspects of the methods, presentation and interpretation throughout the project. The PSERENADE project is funded by the Bill and Melinda Gates Foundation as part of the World Health Organization Pneumococcal Vaccines Technical Coordination Project.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9040742/s1, Search Terms, Table S1: Surveillance data sources for sites in PSERENADE, Table S2: PCV-using countries with IPD surveillance, whether included in PSERENADE and if so whether incidence data were provided, Table S3: Characteristics of data in PSERENADE by surveillance site.
Appendix A
Table A1.
PSERENADE team.
| Name | Affiliation |
|---|---|
| Pedro Alarcon | Instituto de Salud Pública de Chile, 7780050 Santiago, Santiago Metropolitan, Chile |
| Samanta C. G. Almeida | National Laboratory for Meningitis and Pneumococcal Infections, Center of Bacteriology, Institute Adolfo Lutz (IAL), São Paulo, 01246-902, Brazil |
| Zahin Amin-Chowdhury | Immunisation and Countermeasures Division, Public Health England, NW9 5EQ, London, UK |
| Michelle Ang | National Public Health Laboratory, National Centre for Infectious Diseases, Singapore 308442, Singapore |
| Mária Avdicová | National Reference Centre for Pneumococcal and Haemophilus Diseases, Regional Authority of Public Health, 975 56 Banská Bystrica, Slovak Republic |
| Sithembile Bilima | Malawi-Liverpool-Wellcome Trust Clinical Research Programme, P.O. Box 30096, Chichiri, Blantyre 3, Malawi |
| Rita Born | Federal Office of Public Health, 3097 Liebefeld, Switzerland |
| Dana Bruden | Arctic Investigations Program, Division of Preparedness and Emerging Infections, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Anchorage, AK 99508, USA |
| Carrie L. Byington | University of Utah Department of Pediatrics (emeritus), Salt Lake City, UT 84108, United States; University of California Health System, Oakland, CA 94607, USA |
| Gustavo Chamorro | National Program on Immunopreventable Diseases and Expanded Program on Immunizations, Asunción, Paraguay |
| Kin-Hung Chow | Department of Microbiology and Carol Yu Centre for Infection, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China |
| Urtnasan Chuluunbat | National Center of Communicable Diseases (NCCD), Ministry of Health, Bayanzurkh district, 13336 Ulaanbaatar, Mongolia |
| Cheryl Cohen | Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Sandringham, 2192, Johannesburg, South Africa; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, 2000, Johannesburg, South Africa |
| Edoardo Colzani | European Centre for Disease Prevention and Control, 169 73 Solna, Sweden |
| Tine Dalby | Bacteria, Parasites and Fungi, Statens Serum Institut, DK-2300 Copenhagen S, Denmark |
| Geneviève Deceuninck | Quebec University Hospital Research Centre, Québec, QC G1V 4G2, Canada |
| Elina Dimina | Centre for disease prevention and control of Latvia, Ziemelu District, Riga, LV-1005, Latvia |
| Mignon du Plessis | Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases of the National Health Laboratory Service, Sandringham, 2192, Johannesburg, South Africa |
| Helga Erlendsdottir | Department of Clinical Microbiology, Landspitali-The National University Hospital, Hringbraut, 101 Reykjavik, Iceland |
| Ryan Gierke | National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30333, USA |
| Charlotte Gilkison | Epidemiology Team, Institute of Environmental Science and Research, Porirua, 5022 Wellington, New Zealand |
| Noga Givon-Lavi | Pediatric Infectious Disease Unit and Clinical Microbiology Laboratory, Soroka University Medical Center, Ben-Gurion University of the Negev, Beer-Sheva, 8410501, Israel |
| Marcela Guevara | Instituto de Salud Pública de Navarra-IdiSNA, 31003 Pamplona, Navarra, Spain; CIBER Epidemiología y Salud Pública, (CIBERESP), 28029 Madrid, Spain |
| Md. Hasanuzzaman | Child Health Research Foundation, Dhaka 1207, Bangladesh |
| Ilias Hossain | Medical Research Council Unit The Gambia at London School of Hygiene & Tropical Medicine, P.O. Box 273, Banjul, The Gambia |
| Aníbal Kawabata | Laboratorio Central de Salud Pública, Asunción, Paraguay (Central Laboratory of Public Health, Asunción, Paraguay), Asunción, Paraguay |
| Nicola P Klein | Vaccine Study Center, Kaiser Permanente, Oakland, CA 94612, USA |
| Vicki Krause | Centre for Disease Control, Department of Health and Community Services, Darwin City NT 8000, Australia |
| Pavla Krizova | National Institute of Public Health (NIPH), 100 42, Praha, Czech Republic |
| Alicja Kuch | National Reference Centre for Bacterial Meningitis, National Medicines Institute, 00-725 Warsaw, Poland |
| Brigitte Lefebvre | Laboratoire de Santé Publique du Québec, Sainte-Anne-de-Bellevue, Quebec H9X 3R5, Canada |
| Laura MacDonald | Public Health Scotland, G2 6QE Glasgow, UK |
| Ioanna Magaziotou | National Public Health Organisation, 15123 Athens, Greece |
| Jolita Mereckiene | HSE Health Protection Surveillance Centre, Mountjoy, Dublin, D01 A4A3, Ireland |
| Eva Morfeldt | Department of Microbiology Public Health Agency of Sweden, 171 82 Solna, Sweden |
| Carmen Muñoz-Almagro | Molecular Microbiology Department, Hospital Sant Joan de Déu Research Institute, 08950 Esplugues de Llobregat, Barcelona, Spain; Medicine Department, Universitat Internacional de Catalunya, 08017 Barcelona, Spain; CIBER Epidemiología y Salud Pública, (CIBERESP), 28029 Madrid, Spain |
| Tomoka Nakamura | World Health Organization, 1202 Geneva, Switzerland |
| Stephen I. Pelton | Boston University Schools of Medicine and Public Health, Boston, MA 02118, USA |
| Kate Pennington | Communicable Disease Epidemiology and Surveillance Section, Office of Health Protection, Australian Government Department of Health, 2606 Canberra ACT, Australia |
| Marie-Cecile Ploy | University Hospital Centre Limoges, Regional Observatories for Pneumococci, 87000 Limoges, France |
| Gloria Rey-Benito | Pan American Health Organization, World Health Organization, Washington, DC 20037, United States |
| Flavia Riccardo | Department of Infectious Diseases, Italian National Institute of Health (Istituto Superiore di Sanità, ISS), 00161 Rome, Italy |
| Leah J. Ricketson | Department of Pediatrics, University of Calgary, Calgary AB T3B 6A8, Canada |
| Fiona Russell | Murdoch Children’s Research Institute, Parkville VIC 3052, Australia |
| Nahuel M. Sánchez Eluchans | Servicio de Bacteriología Clínica, Departamento de Bacteriología, INEI-ANLIS “Dr. Carlos G. Malbrán”, C1282 AFF, Buenos Aires, Argentina |
| Juan Carlos Sanz | Laboratorio Regional de Salud Pública, Dirección General de Salud Pública, Comunidad de Madrid, 28053 Madrid, Spain |
| Shigeru Suga | Infectious Disease Center and Department of Clinical Research, National Hospital Organization Mie Hospital, Tsu, Mie 514-0125, Japan |
| Catherine G. Sutcliffe | Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, United States |
| Koh Cheng Thoon | KK Women’s and Children’s Hospital, 229899, Singapore |
| Maija Toropainen | Department of Health Security, Finnish Institute for Health and Welfare, 00271 Helsinki, Finland |
| Georgina Tzanakaki | National Meningitis Reference Laboratory, National School of Public Health Athens, Athens, Greece |
| Maria Teresa Valenzuela | Universidad de los Andes, Santiago, Las Condes, Metropolitan Region, Chile |
| Nina M. van Sorge | Medical Microbiology and Infection Prevention, Netherlands Reference Laboratory for Bacterial Meningitis, Amsterdam University Medical Centers, location AMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands |
| Emmanuelle Varon | National Reference Centre for Pneumococci, Centre Hospitalier Intercommunal de Créteil, 94000 Créteil, France |
| Jennifer R. Verani | National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA 30333, USA; Centers for Disease Control and Prevention (CDC), Center for Global Health (CGH), Division of Global Health Protection (DGHP), Nairobi, Kenya |
| Brita A. Winje | Department of Infection Control and Vaccine, Norwegian Institute of Public Health, 0456 Oslo, Norway |
| Khalid Zerouali | Department of Microbiology, Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Casablanca 20000, Morocco; Bacteriology-Virology and Hospital Hygiene Laboratory, Ibn Rochd University Hospital Centre, Casablanca 20250, Morocco |
| Toronto Invasive Bacterial Diseases Network | |
| WHO Invasive Bacterial Vaccine-Preventable Diseases (IB-VPD) Network | |
Table A2.
Acknowledgement list.
| PSERENADE Technical Advisory Group |
|---|
| Thomas Cherian |
| William P. Hausdorff |
| Marc Lipsitch |
| Shabir A. Madhi |
| Elizabeth Miller |
| Catherine Satzke |
| Cynthia G. Whitney |
| World Health Organization |
| Katherine L. O’Brien |
| Jenny A. Walldorf |
| Johns Hopkins University |
| Yunfeng Cao |
| Peggy Gross |
| Donna Hesson |
| Ananya Kumar |
| Kate Perepezko |
| Francesca Schiaffino Salazar |
| Daniel Stephens |
| Epidemiology Department, EpiConcept |
| Germaine Hanquet |
| Dirección General de Salud Pública, Comunidad de Madrid, Spain |
| Luis García Comas |
| Maria Ordobás Gavín |
| Department of Infectious Diseases, Italian National Institute of Health (Istituto Superiore di Sanità, ISS), Rome, Italy |
| Martina Del Manso |
| Faculty of Sciences and Health Techniques, Mohammed VI University of Health Sciences (UM6SS) of Casablanca, Casablanca, Morocco; National Reference Laboratory, Mohammed VI University of Health Sciences (UM6SS), Casablanca, Morocco |
| Idrissa Diawara |
| Surveillance and Public Health Emergency Response, Public Health Agency of Catalonia, Barcelona, Spain |
| Sonia Broner |
| Conchita Izquierdo |
| Australian National Notifiable Diseases Surveillance data were provided by the Office of Health Protection, Australian Government Department of Health, on behalf of the Communicable Diseases Network Australia and the Enhanced Invasive Pneumococcal Disease Surveillance Working Group. |
Author Contributions
Conceptualization, M.D.K., K.H., J.C.B., M.G.Q., E.W.K., M.E.P.; Methodology, M.D.K., K.H., J.C.B., M.G.Q., M.E.P., E.W.K.; Software and Analysis, J.C.B., M.E.P., M.G.Q.; Writing—Original Draft Preparation, M.D.K., J.C.B., K.H., M.E.P.; Writing—Review & Editing, M.D.K., K.H., M.G.Q., E.W.K., D.R.F., M.K.H., Y.Y., J.N.S.; Visualization, J.N.S., M.G.Q., M.K.H.; Supervision, K.H., M.D.K。, A.L.C.; Project Administration, J.C.B., M.G.Q.; Funding Acquisition, A.L.C., M.D.K., K.H. All other authors contributed data and critically reviewed results. All authors have read and agreed to the published version of the manuscript.
Funding
The PSERENADE project is funded by the Bill and Melinda Gates Foundation as part of the World Health Organization Pneumococcal Vaccines Technical Coordination Project, grant number INV-010429/OPP1189065.
Institutional Review Board Statement
This study was determined to not quality as human subjects research as defined by DHHS regulations 45 CFR 46.102 by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, due to the use of existing, de-identified data. Therefore, Institutional Review Board oversight was not required, and ethical review and approval were waived for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained under data sharing agreements from contributing surveillance sites and can only be shared by contributing organizations with their permission.
Conflicts of Interest
KH conducted the study and analyses while working at the Johns Hopkins School of Public Health but is an employee at Pfizer, Inc. as of 26 October 2020. JCB reports funding from Pfizer in the past year, unrelated to the submitted work. MDK reports grants from Merck, personal fees from Merck, and grants from Pfizer, outside the submitted work. JAS reports grants from the Bill & Melinda Gates Foundation, the Wellcome Trust, the UK MRC, National Institute of Health Research, outside the submitted work. MCB reports lectures fee from MSD outside from submitted work. AS reports grants and personal fees from Pfizer and personal fees from MSD and Sanofi Pasteur, outside the submitted work. ML has been a member of advisory boards and has received speakers’ honoraria from Pfizer and Merck. German pneumococcal surveillance has been supported by Pfizer and Merck. SD reports grant from Pfizer, outside the submitted work. KA reports a grant from Merck, outside the submitted work. MC has previously received a professional fee from Pfizer (Ireland), an unrestricted research grant from Pfizer Ireland (2007–2016) and an Investigator Initiated Reward from Pfizer Ireland in 2018 (W1243730). AvG has received researching funding from Pfizer (last year 2017, Pfizer Investigator-Initiated Research [IIR] Program IIR WI 194379); attended advisory board meetings for Pfizer and Merck. AM received research support to her institution from Pfizer and Merck; honoraria for advisory board membership from GlaxoSmithKline, Merck and Pfizer. SNL performs contract research for GSK, Pfizer, Sanofi Pasteur on behalf of St. George’s University of London, but receives no personal remuneration. IY stated she was a member of mRNA-1273 study group and has received funding to her institution to conduct clinical research from BioFire, MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Novavax, Sanofi-Pasteur, and Micron. RD has received grants/research support from Pfizer, Merck Sharp & Dohme and Medimmune; has been a scientific consultant for Pfizer, MeMed, Merck Sharp & Dohme, and Biondvax; had served on advisory boards of Pfizer, Merck Sharp & Dohme and Biondvax and has been a speaker for Pfizer. MH received an educational grant from Pfizer AG for partial support of this project. However, Pfizer AG had no role in the data analysis and content of the manuscript. CLB, MD has intellectual property in BioFire Diagnostics and receives royalties through the University of Utah. CLB is an advisor to IDbyDNA. AK reports personal fees from Pfizer, outside the submitted work. MT reports grants from GlaxoSmithKline and grants from Pfizer Inc. to the Finnish Institute for Health and Welfare for research projects outside the submitted work, in which she has been a co-investigator. JCS reports had received assistance from Pfizer for attending scientific meetings outside the submitted work. NPK has received research support from Pfizer for studies on pneumococcal serotyping and pneumococcal conjugate vaccine effectiveness. NPK has also received research support for unrelated studies from GlaxoSmithKline, Sanofi Pasteur, Merck, and Protein Sciences (now Sanofi Pasteur). SCGA received a travel grant from Pfizer. CMA reports grants and personal fees from Pfizer, Qiagen, and BioMerieux and grants from Genomica SAU, outside the submitted work. BL had two research grants from Pfizer on Streptococcus pneumoniae. EV reports grants from the French public health agency, during the conduct of the study; grants from Pfizer, grants from Merck, outside the submitted work. LLH reports research grants to her institution from GSK, Pfizer, and Merck. JDK has received an unrestricted grant-in-aid from Pfizer Canada that supports, in part, the CASPER invasive pneumococcal disease surveillance project. CGS reports grant funding from Pfizer, Merck, and AstraZeneca in the past three years. NMvS reports grants and fee for service from Pfizer, fee for service from MSD and GSK, outside the submitted work; In addition, NMvS has a patent WO 2013/020090 A3 with royalties paid to University of California San Diego (inventors: Nina van Sorge/Victor Nizet). All other authors did not declare any conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization (WHO) or of the Centers for Disease Control and Prevention (CDC). The authors Lucia Helena de Oliveira and Gloria Rey-Benito are staff members of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wahl B., O’Brien K.L., Greenbaum A., Majumder A., Liu L., Chu Y., Lukšić I., Nair H., McAllister D.A., Campbell H., et al. Burden of Streptococcus Pneumoniae and Haemophilus Influenzae Type b Disease in Children in the Era of Conjugate Vaccines: Global, Regional, and National Estimates for 2000–15. Lancet Glob. Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Pneumococcal Disease. [(accessed on 4 January 2021)]; Available online: https://www.who.int/ith/diseases/pneumococcal/en/
- 3.World Health Organization Pneumococcal Conjugate Vaccine for Childhood Immunization—WHO Position Paper. Wkly. Epidemiol. Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 4.World Health Organization Pneumococcal Conjugate Vaccines in Infants and Children under 5 Years of Age: WHO Position Paper. Wkly. Epidemiol. Rec. 2019;94:85–104. [Google Scholar]
- 5.International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health VIEW-Hub. [(accessed on 29 December 2020)]; Available online: https://view-hub.org.
- 6.World Health Organization Invasive Bacterial Vaccine Preventable Diseases Laboratory Network. [(accessed on 5 January 2021)]; Available online: http://www.who.int/immunization/monitoring_surveillance/burden/laboratory/IBVPD/en/
- 7.Johnson H.L., Deloria-Knoll M., Levine O.S., Stoszek S.K., Hance L.F., Reithinger R., Muenz L.R., O’Brien K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausdorff W.P., Bryant J., Paradiso P.R., Siber G.R. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part I. Clin. Infect. Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 9.Deloria-Knoll M., Nonyane B.A., Garcia C., Levine O.S., O’Brien K.L., Johnson H.L., AGEDD Project Team Global Serotype Distribution of Invasive Pneumococcal Disease (IPD) in Older Children and Adults: AGEDD Study; Proceedings of the 9th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); Hyderabad, India. 9–13 March 2014. [Google Scholar]
- 10.World Health Organization Meeting of the Strategic Advisory Group of Experts on Immunization, November 2013—Conclusions and Recommendations. Wkly. Epidemiol. Rep. 2014;89:1–20. [PubMed] [Google Scholar]
- 11.Feikin D.R., Kagucia E.W., Loo J.D., Link-Gelles R., Puhan M.A., Cherian T., Levine O.S., Whitney C.G., O’Brien K.L., Moore M.R., et al. Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites. PLoS Med. 2013;10:e1001517. doi: 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savulescu C., Krizova P., Lepoutre A., Mereckiene J., Vestrheim D.F., Ciruela P., Ordobas M., Guevara M., McDonald E., Morfeldt E., et al. Effect of High-Valency Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Children in SpIDnet Countries: An Observational Multicentre Study. Lancet Respir. Med. 2017;5:648–656. doi: 10.1016/S2213-2600(17)30110-8. [DOI] [PubMed] [Google Scholar]
- 13.Iroh Tam P.-Y., Thielen B.K., Obaro S.K., Brearley A.M., Kaizer A.M., Chu H., Janoff E.N. Childhood Pneumococcal Disease in Africa—A Systematic Review and Meta-Analysis of Incidence, Serotype Distribution, and Antimicrobial Susceptibility. Vaccine. 2017;35:1817–1827. doi: 10.1016/j.vaccine.2017.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y.A., Patel H., O’Neil W.M., Li S., Saddier P. Pneumococcal Serotype Distribution: A Snapshot of Recent Data in Pediatric and Adult Populations around the World. Hum. Vaccines Immunother. 2017;13:1229–1241. doi: 10.1080/21645515.2016.1277300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsells E., Guillot L., Nair H., Kyaw M.H. Serotype Distribution of Streptococcus Pneumoniae Causing Invasive Disease in Children in the Post-PCV Era: A Systematic Review and Meta-Analysis. PLoS ONE. 2017;12:e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherian T., Cohen M., de Oliveira L., Farrar J.L., Goldblatt D., Knoll M., Moisi J.C., O’Brien K.L., Pilishvili T., Ramakrishnan M., et al. Pneumococcal Conjugate Vaccine (PCV) Review of Impact Evidence (PRIME): Summary of Findings from Systematic Review. Volume 1. WHO; Geneva, Switzerland: Oct, 2017. pp. 1–215. Report to Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization. [Google Scholar]
- 17.Morpeth S.C., Deloria Knoll M., Scott J.A.G., Park D.E., Watson N.L., Baggett H.C., Brooks W.A., Feikin D.R., Hammitt L.L., Howie S.R.C., et al. Detection of Pneumococcal DNA in Blood by Polymerase Chain Reaction for Diagnosing Pneumococcal Pneumonia in Young Children From Low- and Middle-Income Countries. Clin. Infect. Dis. 2017;64:S347–S356. doi: 10.1093/cid/cix145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant L.R., Hammitt L.L., O’Brien S.E., Jacobs M.R., Donaldson C., Weatherholtz R.C., Reid R., Santosham M., O’Brien K.L. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Carriage Among American Indians. Pediatr. Infect. Dis. J. 2016;35:907–914. doi: 10.1097/INF.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loughlin A.M., Hsu K., Silverio A.L., Marchant C.D., Pelton S.I. Direct and Indirect Effects of PCV13 on Nasopharyngeal Carriage of PCV13 Unique Pneumococcal Serotypes in Massachusetts’ Children. Pediatr. Infect. Dis. J. 2014;33:504–510. doi: 10.1097/INF.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 20.Von Gottberg A., de Gouveia L., Tempia S., Quan V., Meiring S., von Mollendorf C., Madhi S.A., Zell E.R., Verani J.R., O’Brien K.L., et al. Effects of Vaccination on Invasive Pneumococcal Disease in South Africa. N. Engl. J. Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. UNICEF WHO-UNICEF Estimates of PCV3 Coverage. [(accessed on 30 December 2020)]; Available online: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepcv3.html.
- 22.Pan American Health Organization. World Health Organization Sistema Regional de Vacunas (SIREVA) II. [(accessed on 31 August 2020)]; Available online: https://www.paho.org/es/sireva.
- 23.Dirección Redes en Salud Pública. Subdirección Laboratorio Nacional De Referencia. Grupo de Microbiología . Vigilancia por Laboratorio de aislamientos invasores de Streptococcus pneumoniae Colombia 2006-2018: SIREVA II. Instituto Nacional de Salud; Bogotá, Colombia: 2019. pp. 1–16. [Google Scholar]
- 24.Organización Panamericana de la Salud. Instituto Nacional de Salud Colombia . Procedimientos Para El Diagnóstico de Neumonías y Meningitis Bacterianas y La Caracterización de Cepas de Streptococcus Pneumoniae y Haemophilus Influenzae, SIREVA II. Grupo Microbiología, Instituto Nacional de Salud Bogotá—Colombia, Organización Panamericana dela Salud; Colombia: 2012. [(accessed on 29 March 2021)]. pp. 1–121. Available online: http://antimicrobianos.com.ar/ATB/wp-content/uploads/2013/01/PAHO-Manual-Neumo-Haemophilus-SIREVA-2012.pdf. [Google Scholar]
- 25.United Nations Methodology: Geographic Regions. [(accessed on 22 December 2020)]; Available online: https://unstats.un.org/unsd/methodology/m49/#geo-regions.
- 26.The World Bank World Bank Country and Lending Groups. [(accessed on 5 November 2020)]; Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 27.United Nations Levels and Trends in Child Mortality: 2020 Report. [(accessed on 30 January 2021)]; Available online: https://www.un.org/development/desa/pd/news/levels-and-trends-child-mortality-2020-report.
- 28.Cohen C., Walaza S., Moyes J., Groome M., Tempia S., Pretorius M., Hellferscee O., Dawood H., Haffejee S., Variava E., et al. Epidemiology of Severe Acute Respiratory Illness (SARI) among Adults and Children Aged ≥ 5 Years in a High HIV-Prevalence Setting, 2009–2012. PLoS ONE. 2015;10:e0117716. doi: 10.1371/journal.pone.0117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park I.H., Pritchard D.G., Cartee R., Brandao A., Brandileone M.C.C., Nahm M.H. Discovery of a New Capsular Serotype (6C) within Serogroup 6 of Streptococcus Pneumoniae. J. Clin. Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P., Kong F., Xiao M., Oftadeh S., Zhou F., Liu C., Russell F., Gilbert G.L. First Report of Putative Streptococcus Pneumoniae Serotype 6D among Nasopharyngeal Isolates from Fijian Children. J. Infect. Dis. 2009;200:1375–1380. doi: 10.1086/606118. [DOI] [PubMed] [Google Scholar]
- 31.Swarthout T.D., Gori A., Bar-Zeev N., Kamng’ona A.W., Mwalukomo T.S., Bonomali F., Nyirenda R., Brown C., Msefula J., Everett D., et al. Evaluation of Pneumococcal Serotyping of Nasopharyngeal-Carriage Isolates by Latex Agglutination, Whole-Genome Sequencing (PneumoCaT), and DNA Microarray in a High-Pneumococcal-Carriage-Prevalence Population in Malawi. J. Clin. Microbiol. 2020;59:e02103-20. doi: 10.1128/JCM.02103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satzke C., Dunne E.M., Porter B.D., Klugman K.P., Mulholland E.K. The PneuCarriage Project: A Multi-Centre Comparative Study to Identify the Best Serotyping Methods for Examining Pneumococcal Carriage in Vaccine Evaluation Studies. PLoS Med. 2015;12:e1001903. doi: 10.1371/journal.pmed.1001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . Strategic Advisory Group of Experts on Immunization Yellow Book. World Health Organization Department of Immunization, Vaccines and Biologicals (IVB); Geneva, Switzerland: 2020. p. 8. [Google Scholar]
- 34.Moïsi J.C., Makawa M.-S., Tall H., Agbenoko K., Njanpop-Lafourcade B.-M., Tamekloe S., Amidou M., Mueller J.E., Gessner B.D. Burden of Pneumococcal Disease in Northern Togo before the Introduction of Pneumococcal Conjugate Vaccine. PLoS ONE. 2017;12:e0170412. doi: 10.1371/journal.pone.0170412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tornheim J.A., Manya A.S., Oyando N., Kabaka S., Breiman R.F., Feikin D.R. The Epidemiology of Hospitalized Pneumonia in Rural Kenya: The Potential of Surveillance Data in Setting Public Health Priorities. Int. J. Infect. Dis. 2007;11:536–543. doi: 10.1016/j.ijid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Bozio C.H., Abdul-Karim A., Abenyeri J., Abubakari B., Ofosu W., Zoya J., Ouattara M., Srinivasan V., Vuong J.T., Opare D., et al. Continued Occurrence of Serotype 1 Pneumococcal Meningitis in Two Regions Located in the Meningitis Belt in Ghana Five Years after Introduction of 13-Valent Pneumococcal Conjugate Vaccine. PLoS ONE. 2018;13:e0203205. doi: 10.1371/journal.pone.0203205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campagne G., Schuchat A., Djibo S., Ousséini A., Cissé L., Chippaux J.P. Epidemiology of Bacterial Meningitis in Niamey, Niger, 1981-96. Bull. World Health Organ. 1999;77:499–508. [PMC free article] [PubMed] [Google Scholar]
- 38.Aku F.Y. Meningitis Outbreak Caused by Vaccine-Preventable Bacterial Pathogens—Northern Ghana, 2016. MMWR. 2017;66:806–810. doi: 10.15585/mmwr.mm6630a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soeters H.M., Diallo A.O., Bicaba B.W., Kadadé G., Dembélé A.Y., Acyl M.A., Nikiema C., Sadji A.Y., Poy A.N., Lingani C., et al. Bacterial Meningitis Epidemiology in Five Countries in the Meningitis Belt of Sub-Saharan Africa, 2015–2017. J. Infect. Dis. 2019;220:S165–S174. doi: 10.1093/infdis/jiz358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soeters H.M., Kambiré D., Sawadogo G., Ouédraogo-Traoré R., Bicaba B., Medah I., Sangaré L., Ouédraogo A.-S., Ouangraoua S., Yaméogo I., et al. Impact of 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis, Burkina Faso, 2016–2017. J. Infect. Dis. 2019;220:S253–S262. doi: 10.1093/infdis/jiz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak R.T., Ronveaux O., Bita A.F., Aké H.F., Lessa F.C., Wang X., Bwaka A.M., Fox L.M. Future Directions for Meningitis Surveillance and Vaccine Evaluation in the Meningitis Belt of Sub-Saharan Africa. J. Infect. Dis. 2019;220:S279–S285. doi: 10.1093/infdis/jiz421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel J.C., Soeters H.M., Diallo A.O., Bicaba B.W., Kadadé G., Dembélé A.Y., Acyl M.A., Nikiema C., Lingani C., Hatcher C., et al. MenAfriNet: A Network Supporting Case-Based Meningitis Surveillance and Vaccine Evaluation in the Meningitis Belt of Africa. J. Infect. Dis. 2019;220:S148–S154. doi: 10.1093/infdis/jiz308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization . Global Vaccine Action Plan: Defeating Meningitis by 2030 Meningitis Prevention and Control. WHO; Geneva, Switzerland: 2020. pp. 1–4. World Health Organization Seventy-third World Health Assembly. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained under data sharing agreements from contributing surveillance sites and can only be shared by contributing organizations with their permission.