Abstract
The global drive to vaccinate against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) began in December 2020 with countries in Europe, Middle East, and North America leading the roll out of a mass-vaccination program. This systematic review synthesised all available English-language guidelines and research regarding mass-vaccination for COVID-19 until 1 March 2021—the first three months of the global mass-vaccination effort. Data were extracted from national websites, PubMed, Embase, Medline and medRxiv, including peer and non-peer review research findings. A total of 15 national policy documents were included. Policies were summarised according to the World Health Organisation (WHO) framework for mass vaccination. All included policies prioritised front-line health care workers and the elderly. Limited information was available regarding staffing, cold chain, communication strategies and infrastructure requirements for effective vaccine delivery. A total of 26 research studies were identified, reporting roll-out strategies, vaccine uptake and reasons for refusal, adverse effects, and real-life estimates of efficacy. Early data showed a reduction in SARS-CoV-2 cases, hospitalisation and deaths in settings with good coverage. Very low rates of vaccine-related serious adverse events were observed. These findings provide an overview of current practice and early outcomes of COVID-19 mass-vaccination, guiding countries where roll-out is yet to commence.
Keywords: covid, vaccination, national policy data, implementation
1. Introduction
By the end of January 2021, the coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) was responsible for more than 100 million infections and 2.5 million deaths globally [1]. Vulnerable communities and ethnic minorities have shouldered particularly high physical, psychological, social, and economic burdens [2,3]. While the implementation of border restrictions, social distancing and infection control practices has curtailed the pandemic in some settings, such measures do not provide a feasible long-term solution, given that SARS CoV-2 has become an endemic virus [4].
Vaccination has the potential to substantially reduce the incidence of severe disease, morbidity, and mortality, especially if "herd immunity" can be attained. By January 2021, more than 10 vaccines were in production, utilising a range of established and new vaccine technologies, including novel mRNA approaches [5]. The variable efficacy reported with different vaccines has been the source of much scientific debate and media speculation. However, with regards to vaccine acceptance and uptake amongst the public, government trust and implementation strategies have been shown to be more important than objective measures of vaccine efficacy [6,7]. Despite the excellent progress made in some countries, equity of access to vaccines by vulnerable populations remains a challenge—particularly those in low- and middle-income countries. Without an effective global vaccination response, vulnerable populations will continue to experience preventable morbidity, economic recoveries are likely to stall, and border closures will remain.
The drive to vaccinate large populations began in earnest in mid-December 2020 in Europe, the Middle East, and North America [8]. By the end of February 2021, Israel had vaccinated over 80% of its population, presenting a model for rapid implementation [8]. Israel’s success has been attributed to high-level political commitment together with coordinated and well-resourced collaboration between the government and health care providers [9]. Despite this early progress, more than 100 countries are yet to commence vaccination [8]. The WHO has produced a framework for mass vaccination policies, including the following domains: coordination, planning, vaccination strategies, access, and community engagement [10]. However, few national plans for vaccine roll-out have been made publicly available—most of which were from high-income settings [11,12]. Dissemination of existing national guidelines for mass-vaccination will assist countries to develop their own local strategies.
This rapid review assessed the publicly-available policies and implementation strategies used for COVID-19 mass vaccination. It characterises differences in national vaccine policies and evaluates determinants of successful scale-up, so that countries currently developing mass-vaccination strategies can benefit from the experiences in countries where mass-vaccination has already begun.
2. Materials and Methods
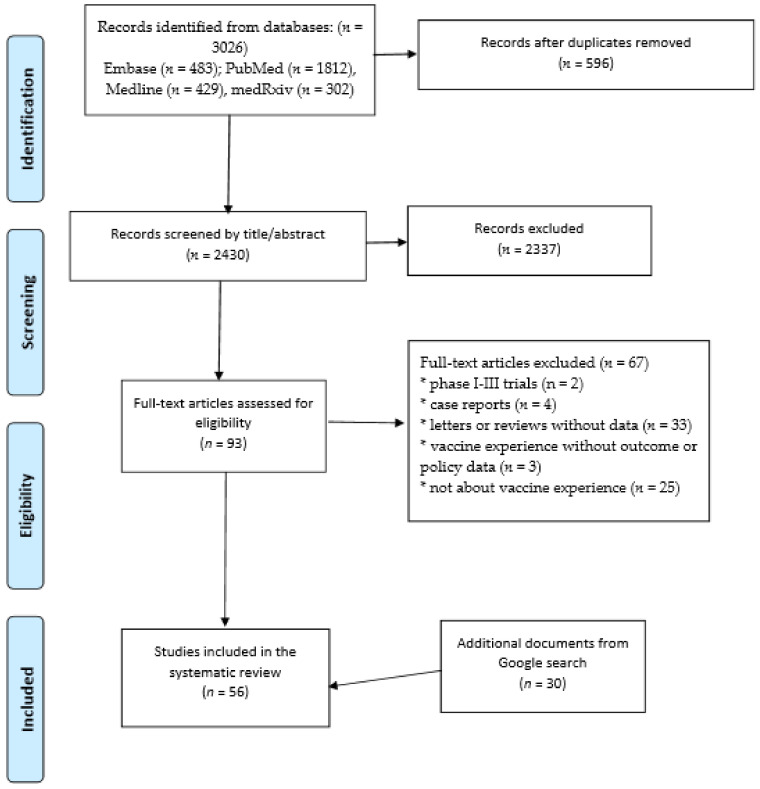
We reviewed all literature available until the 1st of March 2021. As this is a rapidly evolving area, articles from both peer-reviewed and selected non-peer reviewed online sources were included. The review was completed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines (Figure 1) [13].
Figure 1.
PRISMA Flow diagram for study inclusion in the systematic review
2.1. Search Strategy—Research Articles
We performed a systematic search of the PubMed, Medline, Embase and medRxiv databases using the following terms for title/abstract: “SARS-CoV-2”, “covid*”, “coronavirus” and “vaccine”. The search strategy was restricted to articles published between the 10th of December (the approximate timing of the commencement of vaccination) and the 1st of March. Articles were imported into Endnote X9.3.2 (Clarivate Analytics), and duplicates were removed. All types of articles from all countries were considered acceptable for inclusion, provided they described real-life experience with vaccine rollout. Non-English language articles, case-reports, Phase 2 or Phase 3 clinical trials, and vaccine efficacy trials were excluded as they did not reflect large-scale vaccine deployment. Title and abstract screening for articles was performed to exclude articles not meeting the inclusion criteria. The remaining articles underwent full-text review for final inclusion. Articles in the reference lists of included papers were also screened for inclusion.
2.2. Search Strategy: Non-Peer Reviewed Literature
A search of "grey" (unpublished) literature was completed on 1 March 2021. National and health websites for United Kingdom (UK), European Union (EU), United States of America (USA), Israel, Canada, India, China, and Russia were searched. Additionally, data were sought from high-income, middle-income, and low-income countries [14], from each World Health Organization (WHO) region. Only English language sources were included. Documents that were not official government or related to national vaccine campaigns were excluded (e.g., sub-national policies). The most recent version of documents was included.
The Google.com search was used to identify publicly available national policy documents and reports of vaccine outcomes for all 85 countries that had commenced vaccinations by the March 1st, 2021 [8]. The following terms were used:
“[Country name]” COVID-19 vaccination policy
“[Country name]” COVID-19 vaccination delivery plan
“[Country name]” COVID-19 vaccination progress
“[Country name]” COVID-19 vaccination tracker
Country profiles in “Our World in Data” [8], a website which provides a live tracker of global vaccination status, were interrogated for articles which met inclusion criteria.
Data sources requiring clarification were reviewed by three different authors (TH, JB, GF) with a majority decision made about inclusion.
2.3. Data extraction and Analysis
Extracted data included population demographics, vaccination strategy, implementation challenges and vaccination outcomes. National policy documents were reviewed according to the WHO framework for vaccination [10]. Data was entered into Microsoft Excel. Findings were also synthesised narratively.
2.4. Ethical Issues
Ethical approval was not required for this search of publicly available documents.
3. Results
Our combined searches retrieved 3026 articles, of which 596 were duplicates and 2337 were excluded after title and abstract review (Figure 1). A total of 93 full-text articles were reviewed and 26 were included. Thirteen articles were from peer-reviewed sources and 13 were non-peer reviewed articles. An additional 30 online reports were also included. No additional data sources were identified by searching reference lists.
The review identified 15 national policy documents (Table 1) and 15 reports of vaccine outcomes summarising outcome data (Table S1). Findings from research article are summarised in Table 2.
Table 1.
Summary of publicly-available national policy documents for the implementation of mass-vaccination against COVID-19.
| Setting/Country | Australia | USA | Czech Republic | Qatar | Singapore | Monaco | Canada | Switzerland | EU | UK | South Africa | Lebanon | Cyprus | New Zealand | Ireland |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date of most recent update | 17 February 2021 | 29 October 2020 | 10 February 2021 | NR | 26 February 2021 | 23 February 2021 | 25 February 2021 | 26 February 2021 | 1 February 2021 | 11 January 2021 | NR | 28 January 2021 | 2020 | 22 December 202 | 24 February 2021 |
| Reference | [11] | [12] | [15] | [16] | [17] | [18] | [19] | [20] | [21] | [22] | [23] | [24] | [25] | [26] | [27] |
| Funding source | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public | Public |
| Pf/B Mod |
Pf/B | Private | AZ/Ox | COVAX | NR | ||||||||||
| Vaccine used | Pf/B | NR | NR | NR | Pf/B | No | Pf/B | Pf/B | Pf/B | Pf/B | NR | NR | NR | NR | Pf/B |
| NR | Mod | NR | Mod | Mod | NR | NR | Mod | ||||||||
| AZ/Ox | AZ/Ox | AZ/Ox | AZ/Ox | ||||||||||||
| Vaccination mandatory for target populations? | No | NR | NR | NR | No | No | No | No | No | No | NR | NR | No | ||
| Was an increased delay between the two doses recommended (compared to the frequency in published trials) | NR | NR | NR | NR | Priority 1 | NR | NR | In 2 countries | Yes | Priority 1 | NR | NR | NR | ||
| EQUITABLE ACCESS | Priority 1 | Priority 2 | |||||||||||||
| Priority criteria for vaccination | NR | Priority 1 | |||||||||||||
| Frontline HCW | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 2 | Priority 1 | Priority 2 | Priority 2 | Priority 1 | Priority 1 | Priority 2 | |||
| Chronic comorbidities | Priority 2 | Priority 2 | Priority 2 | Priority 1 | Priority 1 | Priority 1 | Priority 4 | Priority 4 | |||||||
| Elderly in aged care | Priority 1 | Priority 2 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | ||||||
| Other elderly | Priority 2 | Priority 2 | Priority 1 | Priority 1 | Priority 1 | Priority 1 | Priority 2 | Priority 2 | Priority 1 | Priority 3 | |||||
| Institutionalised | Priority 3 | Priority 2 | Priority 4 | Priority 2 | Priority 5 | ||||||||||
| Indigenous population | Priority 2 | Priority 3 | Priority 1 | ||||||||||||
| Other HCW | Priority 2 | Priority 2 | NR | NR | Priority 1 | NR | Priority 2 | NR | |||||||
| Essential public services | Priority 3 | Priority 2 | NR | NR | NR | Yes | |||||||||
| Children | |||||||||||||||
| Expected date when whole adult population vaccinated | NR | NR | NR | NR | Sep 2021 | NR | NR | NR | NR | NR | Mid 2022 | NR | NR | ||
| Will extra doses be donated to COVAX | Yes | NR | NR | NR | Yes | NR | Yes | NR | Yes | NR | NR | NR | |||
| VACCINATION STRATEGIES | |||||||||||||||
| Location of vaccination | NR | NR | NR | NR | |||||||||||
| Large public venues | Yes | Yes | Drive through | Care home | Yes | Yes | Yes | Yes | Yes | ||||||
| Hospitals | Yes | NR | NR | Yes | NR | Electronic | |||||||||
| Clinics and pharmacies Other |
Yes | Yes | NR | Self-reported to clinic, hotline, or email | Yes | Yes | NR | Yes | NR | Yes | |||||
| Vaccination record (electronic/paper) | NR | NR | NR | NR | NR | Paper | Electronic | Electronic | Electronic | Electronic | NR | ||||
| Adverse-effect reporting (automated electronic, electronic self-report, GP self-report) | Active surveillance via electronic prompts | NR | NR | NR | Self-reported to local doctor | NR | NR | NR | 5 countries with self-reported electronic system | Review of electronic health records | NR | Self-reported electronic system | NR | 9 extra freezer procured | NR |
| COORDINATION | |||||||||||||||
| Cold chain infrastructure | NR | Extra freezer procured | NR | NR | NR | NR | NR | NR | Extra freezer procured | NR | NR | 1 freezer procured | Freezer available | NR | |
| PLANNING | Yes | ||||||||||||||
| Increased labour requirements | NR | NR | NR | NR | Yes | NR | |||||||||
| Non-medical staff employed for vaccination | Yes | Yes | Yes | Yes | Yes | Yes | |||||||||
| Training provided to staff | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | NR | ||||||
| COMMUNITY ENGAGEMENT | |||||||||||||||
| Are the following employed? | NR | NR | NR | NR | NR | NR | |||||||||
| Community strategies | Yes | Yes | Yes | ||||||||||||
| Social media | Yes | Yes | Yes | ||||||||||||
| Media campaigns | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | NR | ||||||
| Other | Qatar | Monaco | Celebrities | South Africa | New Zealand | ||||||||||
| Strategies to reduce misinformation | NR | NR | NR | NR | NR | 23 February 2021 | NR | NR | Monitor media, online fact checker | NR | NR | Rumour tracking team | NR | 22 December 202 | NR |
AZ/Ox AstraZenca/Oxford, EU European Union, GP general practitioner, HCW health care worker, Mod Moderna, NR not reported, Pf/B Pfizer BioNTech, UK United Kingdom, USA United States of America.
Table 2.
Published peer-reviewed and non-peer reviewed articles summarising real-life experience with vaccination against SARS-CoV-2.
| Country/Reference | Peer Reviewed (Yes/No) | Number Vaccinated in Study | Age Groups Immunised | Female (%) | Vaccines Used (% *) | Outcomes |
|---|---|---|---|---|---|---|
| Studies reporting demographic details | ||||||
| USA Gharpure et al. [40] |
Yes | 713,909 (LTCF residents) 582,104 (HCW) |
NR | NR | NR | Estimated 77.8% residents and 37.5% staff in 11,460 care facilities, vaccinated by Jan 17 (at least one dose) |
| USA Painter et al. [41] |
Yes | 12,928,749 | <0.1% <18 70.9% 18–64 29% ≥65 |
63 | Pf/B Mod |
|
| Studies reporting adverse events | ||||||
| Brazil Pagotta et al. [53] |
No | 683 (HCW) | 99.3% 18–60 0.7% >60 |
68 | Gamaleya | Adverse effects reported by 71.3% in a survey. Most common—injection site pain, myalgia, fever |
| India Jayadevan et al. [49] |
No | 5396 | NR | NR | BB (95) AZ/Ox (3.3) Pf/B (0.8) Sinopharm (0.8) |
Adverse effects reported by 65.9% in a survey, highest in those 20–39 years of age and females. Most common adverse effects—lethargy, myalgia, fever |
| USA CDC [42] |
Yes | 4,041,396 | NR | NR | Mod | Anaphylaxis reported in 0.0002% (n = 10), serious adverse effects in 0.03% (n = 1266) |
| USA CDC [43] |
Yes | 1,893,360 | NR | NR | Pf/B | Anaphylaxis reported in 0.001% (n = 21), serious adverse effects in 0.2% (n = 4393) |
| USA Gee et al. [44] |
Yes | 13,794,904 | 0.2% 0–17 90.3% 18–64 6.2% ≥65 |
61 | Pf/B Mod |
Adverse effects reported in 0.05% (n = 6994) through a passive national surveillance system |
| USA McMurray et al. [45] |
No | 31,029 | NR | NR | Pf/B Mod |
Review of electronic medical records found a 2.1 to 1500 times reduced frequency of adverse events reported when events were obtained from self-reported health interactions compared to active solicitation in trials or in post-marketing surveillance |
| Studies reporting vaccine acceptance | ||||||
| Saudi Arabia Barry et al. [50] |
No | 352 (HCW) | 91.9% 20–50 8.1% >50 |
57 | Pf/B | Factors associated with not enrolling for vaccine: female, younger age, use of social media, foreign national. Percentage not yet registered for vaccine: 66.7% (n = 706) |
| UK Martin et al. [51] |
No | 12,278 (HCW) | 18.7% <30 72% 30–60 9.3% >60 |
76 | Pf/B AZ/Ox |
Factors associated with lower vaccine uptake: ethnic minority, younger age, female, lower socio-economic status |
| UK Kim [52] |
No | 66,994 | NR | NR | NR | Factors associated with vaccine uptake—pre-pandemic income, education. Least likely to take up vaccination—Black Hispanics |
| USA Pamplona, Sullivan, Kotanko [46] |
Yes | 115 (HCW) | NR | NR | NR | Factors associated with not being vaccinated in a dialysis ward (26.8%, n = 42)—past COVID-19, pregnancy, absence. 3.8% (n = 6) declined vaccine |
| USA Schradering et al. [47] |
Yes | 1136 (HCW) | 98.6% 22–64 1.4% ≥65 |
59 | NR | 14% (n = 195) HCWs refused vaccination—usually for concern about adverse effects. Ethnic group most likely to decline vaccine: with non-Hispanic black HCWs |
| Studies reporting efficacy (excluding Phase 2 and 3 trials of vaccine efficacy) | ||||||
| Israel Abu Jabal et al. [30] |
Yes | 514 | 2.1% <30 79.4% 30–59 18.5% ≥60 |
63 | Pf/B | Immunogenicity post vaccination similar by ethnicity and sex, but decrease with age. Increased immunogenicity in previous COVID-19 cases |
| Israel Amit et al. [31] |
Yes | 4081 | NR | NR | Pf/B | In vaccinated HCW, 0.54% (n = 22) developed COVID-19 within 10 days of vaccination |
| Israel Amit et al. [32] |
Yes | NR | NR | NR | NR | 30% and 75% reduction in SARS-CoV-2 cases in vaccinated HCW vs. unvaccinated HCW 14 and 28 days, respectively after vaccination |
| Israel Aran [33] |
No | NR | NR | NR | Pf/B | 72% reduction in cases and 83% reduction in hospitalisation by modelling |
| Israel Chodick et al. [34] |
No | 503,875 | Mean 59.7 | 52 | Pf/B | Cases of COVID-19 infection 24 days post vaccination: 0.84% (n = 3098) 51% vaccine effectiveness calculated after 1st dose |
| Israel Dagan et al. [35] |
Yes | 596,618 | 72% <60 28% ≥60 |
50 | Pf/B | 92% reduction in COVID-19 cases, 87% reduction in hospitalisation, 72% reduction in deaths: at 7 days after second dose |
| Israel De Leon et al. [36] |
No | NR | NR | NR | >50% estimated vaccine effectiveness by modelling | |
| Israel Levine et al. [37] |
No | 1755 COVID-19 cases after vaccination | NR | NR | NR | Four-fold reduction in SARS-CoV-2 viral load for people developing infections 12–28 days after first dose |
| Israel Petter et al. [38] |
No | NR | NR | NR | NR | 1.6 to 20x reduction in overall SARS-CoV-2 viral load by vaccinating the community |
| Israel Rossman et al. [39] |
No | NR | NR | NR | Pf/B | 49% drop in cases, 36% drop in hospitalisations: 1.5 months after vaccine initiation |
| Scotland Vasileious et al. [54] |
Yes | 1,137,775 | 34.8% 18–64 65.2% ≥65 |
61 | Pf/B | Estimated efficacy of single dose 85% (Pfizer) and 94% (AstraZeneca) at 28–34 days post vaccination 81% reduction in hospitalisation in those over 80 |
| UK Hall et al. [55] |
Yes | 20,641 | 16.1% <25 76% 25–64 7.9% ≥65 |
85 | Pf/B (94) AZ/Ox (6) |
Vaccine effectiveness at 21 days: 72% after 1 dose, 86% after 2 doses 67% receiving vaccine had previous COVID-19 |
| USA Bradley, Grundberg, Selvarangan [48] |
No | 188 (HCW) | NR | NR | NR | HCW with previous documented SARS-COV-2 infection have higher IgG titres after COVID-19 vaccination |
AZ/Ox AstraZenca/Oxford, BB Bharat Biotech, HCW Health care worker, LTCF Long-term care facility, Mod Moderna, NR not reported, Pf/B Pfizer BioNTech, SARS-CoV-2 severe acute respiratory syndrome-coronavirus-2, UK United Kingdom, USA United States of America. * Where data was available for percentage vaccinated by each vaccine type for the study group included.
3.1. National Policy Documents
Out of 85 countries searched using Google.com, 71 did not have English language documents available. One document described policy for the European Union as a whole, with additional national policy documents located from different EU countries not in English. Of nine countries in Africa where vaccination had commenced, only two had English language documents (South Africa and Seychelles). Data from Seychelles was available through a government Facebook page. Two countries had twitter information in non-English language formats (Senegal and Zimbabwe). One other document was available in a non-English format (Morocco). Four English-language documents detailed policy information and vaccination priority groups via a question-and-answer format on national websites (Czech Republic, Qatar, Singapore, Monaco) [15,16,17,18].
Fifteen national policy documents were included. Fourteen came from high-income settings, one came from an upper-middle income setting (Lebanon), and none were identified from lower-middle, or lower income settings [11,12,15,16,17,18,19,20,21,22,23,24,25,26,27] (Table 1). One document was published from the European Union (EU) and encompassed a description of policies for 26 EU member countries [21]. Vaccination was not mandated in any setting. All documents stipulated that COVID-19 vaccination programs were publicly-funded. In Switzerland, mandatory individual private health insurance provided funding in the first instance, before public funding was used [20]. In Lebanon, it was anticipated that COVAX, vaccines would be supplied by the GAVI, the Vaccine Alliance (GAVI), and WHO [24].
Only three national policy documents reported policies in all domains included in the WHO framework for mass-vaccination [12,21,24]. Communication strategies were only listed in six documents [10,11,12,21,22,24], strategies included a "rumour tracking system" in Lebanon [24] and an online fact checker in some European settings [21].
3.2. Vaccine Deployment
The most common vaccines deployed were the vaccines developed by Pfizer, Moderna and AstraZeneca. Only five national policy documents [12,21,24,25,26] specified how they would manage the cold-chain requirements for the vaccines—in particular, Pfizer BioNTech and Moderna, which must be maintained at -80 degrees Celsius [12]. The policy document from Lebanon, stipulated a central storage site for the vaccine with cold-chain courier capacity [24]. New Zealand have procured nine central freezers with courier capacity [26] and the USA have multiple fridges in various locations [12].
Most national policy documents have not yet detailed problems with the vaccination drive. The EU policy document reported problems with extracting the final dose out of six-dose Pfizer vaccine vials, including a shortage of "dead space" syringes [21]. Belgium reported the total number of “wasted doses” on their website and at the end of February this was approximately equivalent to 16,000 doses (2% of total doses) [28].
The UK and two countries in the EU specified an acceptance of increased dosing interval for multi-dose vaccines [21,22]. In the UK despite having almost 30% vaccinated with the first dose of a COVID-19 vaccine, only 1.1% have received their second dose [29].
3.3. Vaccination of Priority Groups
All but three national policy documents provided a prioritisation strategy for the vaccination of vulnerable groups [10,11,12,15,18,19,20,21,22,23,24,25,27]. Six countries simultaneously prioritised both health care workers (HCW) at risk of exposure to the virus, as well as elderly individuals in care facilities [11,15,18,19,21,24]. Three countries prioritised frontline HCWs over elderly individuals in facilities [10,12,23] and three prioritised the elderly in facilities over frontline HCWs [20,22,27]. Three national policy documents discussed the prioritisation of those in institutional care, or younger people with chronic conditions [19,20,21]. Canada and Australia have indicated a priority strategy for Indigenous communities [11,19]. No national policy document recommended the vaccination of children under 16 years of age.
3.4. Infrastructure and Staffing for Vaccination
Vaccination was described to be mostly taking place in large vaccination hubs, such as sporting venues and other entertainment venues. Only six guidelines discuss the additional staffing, training, and recruitment requirements to achieve mass vaccination delivery [12,21,22,24,25,26].
3.5. Peer-Reviewed Research of Vaccination Outcomes
Of the 26 included peer-reviewed articles, ten were from Israel [30,31,32,33,34,35,36,37,38,39] and eight from the USA [40,41,42,43,44,45,46,47,48]. Six articles looked at adverse event data [42,43,44,45,49] and five looked at vaccine acceptance [46,47,50,51,52].
Several studies, mostly set in Israel look at vaccination outcomes on COVID-19 diagnosis, hospitalisation and mortality. All these studies confirm a >50% reduction in all of these values, with higher rates of reduction for those studies which look at outcomes after second dose [35]. Two Israeli studies report cases of incident COVID-19 after vaccination [31,37]. One of these demonstrated an average SARS-CoV-2 viral load in these individuals to be lower compared to the unvaccinated populations [37]. Two studies suggest that the immunological response is greater after vaccination, in those who have previously had COVID-19 [30,48].
4. Discussion
This rapid systematic review evaluated publicly-available policies and reports for mass-vaccination against COVID-19. Included national policy documents in this study provide an insight into the prioritisation and infrastructure requirements of a COVID-19 mass-vaccination system. These early published findings provide promising evidence of decreases in COVID-19 cases, hospitalisations, and death following vaccine scale-up.
The synthesis of the early national policy documents which are available in the English language provides a framework for the development of future policy documents. One of the most important aspects of vaccine delivery is the equitable access of vaccines to the entire population and vaccinating vulnerable populations to reduce morbidity and mortality [56]. Although Israel has been successful in delivering vaccines to the vast majority of its population, the country is wealthy, well-resourced, and has a small population [9]. The ability to extend this to the Palestinian population remains a challenge [9]. Similarly, vaccinating in settings with larger or vulnerable populations will be challenging. Furthermore, the possibility of shortages to vaccine supply remain an ongoing reality [56]. Although priority groups are clearly stipulated in the policy documents, only two policy documents acknowledge that vaccine shortages may compromise scale-up [21,24].
The national policy documents included in this study illustrate the way in which mass-vaccination campaigns can be implemented in a range of settings. Most countries have been able to rapidly convert large public spaces into vaccination hubs. Other suggested venues for vaccination include hospitals, clinics, places of worship, and nursing homes. Advantages of large venues include the availability of parking spaces and the ability to accommodate large populations [22]. Another option discussed, includes a drive-through parking facility which is used by Qatar, for the second dose, with the first dose administered at a clinic to better facilitate adverse event monitoring [16]. Policies from the UK and New Zealand also provide a framework for recruitment of the increased staffing needs for vaccine delivery via an online volunteer registry [22,26]. The UK policy also details various roles volunteers can have including the military volunteers for logistics and non-trained civilian volunteers as guides and assistance, such as in the car-park [22].
Another challenge with COVID-19 vaccination will be the cost of procurement and delivery of the vaccination process. Only two national policy documents estimate a cost associated with vaccinating the population. The estimated total cost of the vaccination drive in New Zealand is $66 million (US$48million) [26], while in Lebanon the cost of deploying vaccines is budgeted at US$16 million [24]. The estimated cost in Lebanon includes an estimate of supply, training, cold-chain, and other infrastructure requirements for vaccination. It does not include the cost of the vaccines itself; a large portion of which Lebanon plans to procure its vaccines from WHO through the COVAX facility. This is an indication of the expenses associated with an effective vaccination policy. Furthermore, Lebanon has a very small population of 6.8 million, and larger countries will have much greater costs. While Israel has provided a model for vaccination success with the USA, UK, and EU all progressing slowly towards achieving successes with their vaccination drive, middle- and low-income countries will remain vulnerable due to lack of vaccine supply and high infrastructure costs. Another enduring challenge will be the vaccination of refugees and minority groups with uncertain legal status. Although the Lebanese policy, a country where one-third of the population are refugees, discusses the vaccination of refugees [24], in settings where such groups are marginalised or ostracised, reaching out to this population will remain a challenge. Only three national policy documents have stipulated a dedication to providing excess vaccines through COVAX [11,20,22].
The included research articles in this systematic review, confirms the overall safety of vaccines available. Anaphylaxis is rare, indicated by an incidence of <0.001% in large, vaccinated populations in the United States [49,53]. However, when reviewing electronic records of reports of adverse events by individuals presenting to clinicians post-vaccination, the frequency of adverse events was found to be much lower compared to systems that use active surveillance, such as in clinical trials [45]. Furthermore, in the USA, a national passive surveillance system for adverse events in over 13 million vaccinated individuals found the incidence of adverse events to be a 0.05% [44]. The true incidence of serious adverse-effects however need to be determined by studies that are appropriately statistically powered and through large meta-analysis. This can assist in planning scale-up of vaccination, to reassure the populations of the safety of vaccinations and to plan extra health care staff required for adverse event management.
There were five studies which evaluated vaccine acceptance [46,47,50,51,52]. All studies involved HCW. Factors associated with low vaccine uptake include younger age [50], female sex [51] and black populations [47,52]. Higher rates of acceptance was associated with higher income and education levels in one setting [52]. Effective health marketing, which is tailored to and reaches younger people, females, as well as ethnically/linguistically diverse populations should be incorporated into national vaccination strategies.
This study has several limitations. The restriction to English language sources resulted in a high proportion of included reports and policies being from high-income countries. Only one policy was identified from an upper middle-income country. Furthermore, vaccination outcomes in countries with large, COVID-19 affected populations such as China, India, Russia, and Brazil were not included due to the language restriction. Vaccines against COVID-19 only became commercially available three months prior to this review; hence, this study reports only the early experiences with mass-vaccination. Further updated reviews should be undertaken as scale-up proceeds.
Based on the findings in this study we recommend the following strategies for countries up-scaling or commencing the vaccination process:
-
-
Vaccinate health care workers, elderly, and those with chronic comorbidities as a priority.
-
-
Utilise mass vaccination hubs such as sporting venues, for maximal scale and efficiency.
-
-
Consider novel delivery techniques such as drive-through clinics for the second dose, where this is feasible and culturally acceptable.
-
-
Consider use of automated electronic surveys for monitoring side-effects among all recipients.
-
-Engage the community to increase vaccination awareness and acceptance including by
-
○Including and training volunteers in the vaccination effort.
-
○Using social media and media campaigns to raise awareness.
-
○Having a means to monitor mis-information.
-
○Utilising high profile "champions", such as political leaders and social celebrities. Support COVAX and global commitments to deliver vaccines to marginalised and vulnerable populations, because everyone deserves to be protected and the global population cannot be safe until we are all vaccinated.
-
○
Despite these recommendations, it is important to remember that a vaccination strategy alone may not curb the COVID-19 pandemic. Ongoing infection control and social distancing must remain in place until long term solutions are established. Furthermore, although the perceived risk is low, with no evidence of long-term adverse effects in animal models and limited biological plausibility in humans, prior to the current pandemic, no mRNA or DNA based vaccines authorised for use and long-term effects of use of novel vaccination techniques remain unknown. Research into drug therapy remains important including new and repurposed therapy active against SARS-CoV-2 [57,58].
5. Conclusions
This study summarises data from early mass-vaccination programmes against COVID-19 and offers insights into those initial efforts as a contribution to important debates about strategy and approach globally. It highlights strategies that can be used to improve scale-up, equitable delivery, and vaccine acceptance in support of ongoing vaccination effort by governments and public health bodies around the world.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9040326/s1, Table S1: Overview of COVID-19 vaccination data available on government websites in English.
Author Contributions
T.H.: conceptualization, systematic review, preparation of manuscript; J.B.: conceptualization, editing of manuscript; B.J.M.: conceptualization, editing of manuscript; T.A.N.: conceptualization, editing of manuscript; G.J.F.: conceptualization, supervision, editing of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant funded by the Australian Department of Foreign Affairs and Trade (APP1153346).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.John Hopkins University . COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. John Hopkins University; Baltimore, MD, USA: 2021. [(accessed on 1 March 2021)]. Available online: https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 2.Pierce M., Hope H., Ford T., Hatch S., Hotopf M., Kontopantelis E., John A., Webb R.T., Wessely S., McManus S., et al. Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7:883–892. doi: 10.1016/S2215-0366(20)30308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhera R., Wadhera P., Gaba P., Figueroa J.F., Maddox K.E.J., Yeh R.W., Shen C. Variation in COVID-19 Hospitalizations and Deaths Across New York City Boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covid-19-will-become-endemic-but-with-decreased-potency-over-time,-scientists-believe. BMJ. 2021;372:n492. doi: 10.1136/bmj.n494. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation . The COVID-19 Candidate Vaccine Landscape and Tracke. World Health Organisation; Geneva, Switzerland: 2021. [Google Scholar]
- 6.Paltiel A., Schwartz J., Zheng A., Walensky R.P. Clinical outcomes of A COVID-19 vaccine: Implementation over efficacy. Health Aff. 2020;40:42–52. doi: 10.1377/hlthaff.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong M., Wong E., Huang J., Cheung A., Law K., Chong M., Ng R.W.Y., Lai C.K.C., Boon S.S., Lau J.T.F., et al. Acceptance of the COVID-19 vaccine based on the health belief model: A population-based survey in Hong Kong. Vaccine. 2021;39:1148–1156. doi: 10.1016/j.vaccine.2020.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Our World in Data Coronavirus (COVID-19) Vaccinations. [(accessed on 1 March 2021)];University of Oxford. 2021 Available online: https://ourworldindata.org/covid-vaccinations.
- 9.Rosen B., Waitzberg R., Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr. J. Health Policy Res. 2021;10:6. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation . Framework for Decision-Making: Implementation of Mass Vaccination Campaigns in the Context of COVID-19. World Health Organisation; Geneva, Switzerland: 2020. [Google Scholar]
- 11.Australian Government . Australian COVID-19 Vaccination Policy. Australian Government; Canberra, Australia: 2021. [Google Scholar]
- 12.Centers for Disease Control and Prevention . COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2021. [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.The World Bank . Countries and Economies. The World Bank; Washington, DC, USA: 2021. [(accessed on 1 March 2021)]. Available online: https://data.worldbank.org/country. [Google Scholar]
- 15.Vnitra M. How Does the Prioritization System Works? Ministerstvo vnitra; Prague, Czech Republic: 2021. [(accessed on 1 March 2021)]. Available online: https://covid.gov.cz/en/situations/register-vaccination/how-does-prioritization-system-works. [Google Scholar]
- 16.Ministry of Public Health . Coronavirus 2019 (COVID-19) Ministry of Public Health; Doha, Qatar: 2021. [(accessed on 1 March 2021)]. Available online: https://covid19.moph.gov.qa/EN/Covid19-Vaccine/Pages/FAQ.aspx. [Google Scholar]
- 17.Government of Singapore . COVID-19 Vaccination. Government of Singapore; Singapore: 2021. [(accessed on 1 March 2021)]. Available online: https://www.gov.sg/faq. [Google Scholar]
- 18.Gouvernement Princier . Vaccination COVID-19. Gouvernement Princier; Monaco: 2021. [(accessed on 1 March 2021)]. Available online: https://covid19.mc/en/mesures/tout-sur-les-vaccins/ [Google Scholar]
- 19.Government of Canada Vaccines and Treatments for COVID-19: Vaccine Rollout. [(accessed on 1 March 2021)];2021 Available online: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks/covid-19-vaccine-treatment/vaccine-rollout.html.
- 20.FOPH Federal Office of Public Health Coronavirus: Vaccination. [(accessed on 1 March 2021)];2021 Available online: https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/impfen.html.
- 21.European Centre for Disease Prevention and Control . Overview of the Implementation of COVID-19 Vaccination Strategies and Vaccine Deployment Plans in the EU/EEA. European Centre for Disease Prevention and Control; Stockholm, Sweden: 2021. [Google Scholar]
- 22.Department of Health and Social Care . UK COVID-19 Vaccines Delivery Plan. Department of Health and Social Care; London, UK: 2021. [Google Scholar]
- 23.South African Government . South African Government; 2021. [(accessed on 1 March 2021)]. COVID-19/Novel Coronavirus. Available online: https://www.gov.za/covid-19/vaccine/vaccine#. [Google Scholar]
- 24.Ministry of Public Health . Lebanon National Deployment and Vaccination Plan for COVID-19 Vaccines. Ministry of Public Health; Beirut, Lebanon: 2021. [Google Scholar]
- 25.Ministry of Health . National Vaccination Plan for COVID 19. Minstry of Health; Nicosia, Cyprus: 2021. [Google Scholar]
- 26.Ministry of Health . COVID-19: Vaccine Strategy and Planning. Ministry of Health; Auckland, New Zealand: 2021. [(accessed on 1 March 2021)]. Available online: https://www.health.govt.nz/our-work/diseases-and-conditions/covid-19-novel-coronavirus/covid-19-vaccines/covid-19-vaccine-strategy-and-planning. [Google Scholar]
- 27.Health Service Executive . Rollout of COVID-19 Vaccines in Ireland. Health Service Executive; Dublin, Ireland: 2021. [(accessed on 1 March 2021)]. Available online: https://www2.hse.ie/screening-and-vaccinations/covid-19-vaccine/rollout-covid-19-vaccines-ireland.html. [Google Scholar]
- 28.datrix.be Covid Vaccinations Belgium. [(accessed on 1 March 2021)];2021 Available online: https://covid-vaccinatie.be/en.
- 29.Gov.UK Coronavirus (COVID-19) in the UK. [(accessed on 1 March 2021)];2021 Available online: https://coronavirus.data.gov.uk/details/vaccinations.
- 30.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amit S., Beni S.A., Biber A., Grinberg A., Leshem E., Regev-Yochay G. Post-Vaccination COVID-19 among Healthcare Workers, Israel. Emerg. Infect. Dis. 2021;27 doi: 10.3201/eid2704.210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aran D. Estimating real-world COVID-19 vaccine effectiveness in Israel using aggregated counts. medRxiv. 2021 doi: 10.1101/2021.02.05.21251139. [DOI] [Google Scholar]
- 34.Chodick G., Tene L., Patalon T., Gazit S., Tov A.B., Cohen D., Muhsen K. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13–24 days after immunization: Real-world evidence. medRxiv. 2021 doi: 10.1101/2021.01.27.21250612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De-Leon H., Calderon-Margalit R., Pederiva F., Ashkenazy Y., Gazit D. First indication of the effect of COVID-19 vaccinations on the course of the outbreak in Israel. medRxiv. 2021 doi: 10.1101/2021.02.02.21250630. [DOI] [Google Scholar]
- 37.Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., Wolf T., Nadler V., Ben-Tov A., Kuint J., et al. Decreased SARS-CoV-2 viral load following vaccination. medRxiv. 2021 doi: 10.1101/2021.02.06.21251283. [DOI] [PubMed] [Google Scholar]
- 38.Petter E., Mor O., Zuckerman N., Oz-Levi D., Younger A., Aran D., Erlich Y. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. medRxiv. 2021 doi: 10.1101/2021.02.08.21251329. [DOI] [Google Scholar]
- 39.Rossman H., Shilo S., Meir T., Gorfine M., Shalit U., Segal E., Patterns of COVID-19 pandemic dynamics following deployment of a broad national immunization program medRxiv 2021. [(accessed on 2 March 2021)]; Available online: http://medrxiv.org/content/early/2021/02/09/2021.02.08.21251325.abstract.
- 40.Gharpure R., Guo A., Bishnoi C.K., Patel U., Gifford D., Tippins A., Jaffe A., Shulman E., Stone N., Mungai E., et al. Early COVID-19 First-Dose Vaccination Coverage Among Residents and Staff Members of Skilled Nursing Facilities Participating in the Pharmacy Partnership for Long-Term Care Program—United States, December 2020–January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:178–182. doi: 10.15585/mmwr.mm7005e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Painter E.M., Ussery E.N., Patel A., Hughes M.M., Zell E.R., Moulia D.L., Scharf L.G., Lynch M., Ritchey M.D., Toblin R.L., et al. Demographic Characteristics of Persons Vaccinated During the First Month of the COVID-19 Vaccination Program—United States, December 14, 2020–January 14, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:174–177. doi: 10.15585/mmwr.mm7005e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC Covid-Response Team, Food Drug Administration Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021 Dec 21;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC Covid-Response Team, Food Drug Administration Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine— United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021 Dec 14;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., Nair N., Martin S., Clark T., Markowitz L., et al. First Month of COVID-19 Vaccine Safety Monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurry R., Lenehan P.J., Awasthi S., Silvert E., Puranik A., Pawlowski C., Venkatakrishnan A.J., Anand P., Agarwal V., O’Horo J.C., et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA vaccines for COVID-19 from Moderna and Pfizer/BioNtech. medRxiv. 2021 doi: 10.1101/2021.02.20.21252134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pamplona G., Sullivan T., Kotanko P. COVID-19 vaccination acceptance and hesitancy in dialysis staff: First results from New York City. Kidney Int. Rep. 2021 doi: 10.1016/j.ekir.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrading W.A., Trent S.A., Paxton J.H., Rodriguez R.M., Swanson M.B., Mohr N.M., Talan D.A., Project COVERED Emergency Department Network Vaccination Rates and Acceptance of SARS-CoV-2 Vaccination among US Emergency Department Health Care Personnel. Acad. Emerg. Med. 2021 doi: 10.1111/acem.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley T., Grundberg E., Selvarangan R. Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. medRxiv. 2021 doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayadevan R., Shenoy R., Ts A. Survey of symptoms following COVID-19 vaccination in India. medRxiv. 2021 doi: 10.1101/2021.02.08.21251366. [DOI] [Google Scholar]
- 50.Barry M., Temsah M.-H., Aljamaan F., Saddik B., Al-Eyadhy A., Alenezi S., Alamro N., Alhuzaimi A.N., Alhaboob A., Alhasan K., et al. COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout. medRxiv. 2021 doi: 10.1101/2021.01.29.21250749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin C.A., Marshall C., Patel P., Goss C., Jenkins D.R., Ellwood C., Barton L., Price A., Brunskill N.J., Khunti K., et al. Association of demographic and occupational factors with SARS-CoV-2 vaccine uptake in a multi-ethnic UK healthcare workforce: A rapid real-world analysis. medRxiv. 2021 doi: 10.1101/2021.02.11.21251548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D. Associations of Race/Ethnicity and Other Demographic and Socioeconomic Factors with Vaccination during the COVID-19 Pandemic in the United States. medRxiv. 2021 doi: 10.1101/2021.02.16.21251769. [DOI] [Google Scholar]
- 53.Pagotto V., Ferloni A., Soriano M.M., Díaz M., Golde M.B., González M.I., Asprea V., Staneloni I., Vidal G., Silveira M., et al. Active surveillance of the SPUTNIK V vaccine in health workers. medRxiv. 2021 doi: 10.1101/2021.02.03.21251071. [DOI] [Google Scholar]
- 54.Vasileiou E., Simpson C.R., Robertson C., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., et al. Effectiveness of First Dose of COVID-19 Vaccines Against Hospital Admissions in Scotland: National Prospective Cohort Study of 5.4 Million People. SSRN Electron. J. 2021 doi: 10.2139/ssrn.3789264. [DOI] [Google Scholar]
- 55.Effectiveness of BNT162b2 mRNA vaccine against infection and COVID-19 vaccine coverage in healthcare workers in England, multicentre prospective cohort study (the SIREN study) Lancet Prepr. [(accessed on 2 March 2021)];2021 doi: 10.1016/S0140-6736(21)00790-X. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3790399. [DOI] [PMC free article] [PubMed]
- 56.World Health Organisation . WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply. World Health Organisation; Geneva, Switzerland: 2021. [Google Scholar]
- 57.Borbone N., Piccialli G., Roviello G.N., Oliviero G. Nucleoside analogs and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules. 2021;26:986. doi: 10.3390/molecules26040986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan S., Yin X., Meng X., Chan J., Ye Z., Riva L., Pache L., Chan C.C.-Y., Lai P.-M., Chan C.C.-S., et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.