Abstract
Squamous cell carcinomas (SCCs) are among the most frequent solid tumors in humans. SCCs, related or not to the human papillomavirus, share common molecular features. Immunotherapies, and specifically immune checkpoint inhibitors, have been shown to improve overall survival in multiple cancer types, including SCCs. However, only a minority of patients experience a durable response with immunotherapy. Epigenetic modulation plays a major role in escaping tumor immunosurveillance and confers resistance to immune checkpoint inhibitors. Preclinical evidence suggests that modulating the epigenome might improve the efficacy of immunotherapy. We herein review the preclinical and the clinical rationale for combining immunotherapy with an epidrug, and detail the design of PEVOsq, a basket clinical trial combining pembrolizumab with vorinostat, a histone deacetylase inhibitor, in patients with SCCs of different locations. Sequential blood and tumor sampling will be collected in order to identify predictive and pharmacodynamics biomarkers of efficacy of the combination. We also present how clinical and biological data will be managed with the aim to enable the development of a prospective integrative platform to allow secure and controlled access to the project data as well as further exploitations.
Key words: epigenetics, immunotherapy, squamous cell carcinoma, HPV, precision medicine clinical trial
Highlights
-
•
Squamous cell carcinomas (SCCs) of different locations share molecular features.
-
•
Immunotherapy improves overall survival in several cancers including SCCs.
-
•
Epigenetic modulation plays a major role in resistance to immunotherapy.
-
•
PEVOsq evaluates pembrolizumab/vorinostat combination in SCCs of several locations.
-
•
An integrative platform ensures secured optimal management of generated data.
Introduction
Oncological treatments have been tailored according to the cancer's histology and primary tumor location. A deeper understanding of the underlying molecular and genetic patterns involved in oncogenesis recently resulted in a paradigm change, where several drugs were approved based on a molecular alteration or a signature in a histology-independent way. A landmark within the tissue-agnostic drug assembly was the approval of larotrectinib and entrectinib by the Food and Drug Administration (FDA) for patients with any cancer harboring NTRK gene fusions.1 Pembrolizumab, an immune checkpoint inhibitor (ICI) targeting programmed cell death protein 1 (PD-1), which was also approved by the FDA in a histology-independent way, in patients with a high level of microsatellite instability (MSI-H), and in patients with a high tumor mutational burden.2 These approvals were mostly obtained based on small single-arm studies, with various tumor types and varied overall response rates (ORRs) per tumor types.
Several clinical trials have tested this precision medicine approach, including SHIVA01, MOSCATO 01, WINTHER, in which drugs were only given based on molecular alterations.3, 4, 5 None of these trials were able to demonstrate that drugs based on specific molecular alterations should be prescribed off-label outside their indications. The lack of clear demonstration of this precision medicine core concept raises the issue of whether an intermediate approach taking into account the histological subtype may be relevant in some instances.
Squamous cell carcinomas (SCCs) are among the most frequent solid tumors in humans and most frequently occur from the lung, cervix and the head and neck (HNSCC). SCCs can also originate from rarer locations, including the penile, vulva and anal regions. Some SCCs are consecutive to the human papillomavirus (HPV) infection. HPV is indeed the main cause of cervical and anal cancer and is often implicated in penile, vulvar and oropharyngeal SCC. Shared environmental factors including smoking, alcohol and other infections such as Mycobacterium tuberculosis are implicated in most other cases underlying common denominators in all SCC oncogenesis.6
The prognosis of SCC patients remains poor in the recurrent and/or metastatic setting and treatment options are limited in this situation. ICIs have been shown to produce antitumor activity in SCC patients, although ORRs remain low, mostly between 15% and 20%.7, 8, 9, 10 Remarkably, patients responding to ICIs often experience a durable benefit.11 It is therefore essential to find novel ways of improving the proportion of initial responders to ICIs. Priming the immune response to ICIs to increase the ORR, for instance by modulating the epigenome, is a promising approach with several clinical trials ongoing.12
Based on these observations, we set up a basket clinical trial combining pembrolizumab and vorinostat [a histone deacetylase inhibitor (HDACi)] in patients with recurrent and/or metastatic SCC of several primary tumor locations. We will review here the preclinical and clinical evidence supporting the combination of anti-PD-1 monoclonal antibodies with HDACi and present the PEVO data project including the PEVOsq trial (NCT04357873). An emphasis will be given to the integration of clinical and biological data obtained from sequential tumor and blood sampling in order to identify predictive and pharmacodynamics biomarkers of drug efficacy and resistance.
Molecular features of SCCS
SCC can derive from most squamous epitheliums. Integrated analysis of genomic, epigenetic and gene expression alterations in SCC all point towards significant similarities unrelated to their original location. For instance, almost all lung SCCs, and HPV-negative HNSCCs display somatic mutations of TP53 (60%-80%). Common alterations of cell cycle control (i.e. TP53, CDKN2A/RB1 mutations), oxidative stress (i.e. NFE2L2/KEAP1/CUL3), cell survival (i.e. genes in the Pi3K/Akt pathway), cell differentiation (i.e. SOX2/TP63/NOTCH1) and epigenetic (i.e. KMT2C, KMT2D) pathways are also broadly observed in all SCCs.13, 14, 15, 16, 17 Moreover, overlapping molecular features collectively setting SCC apart from other cancers, independently of their organ of origin, were reported in a study comparing the pan-SCC and panCan-33 datasets.18 There is therefore a strong rationale for tailoring treatment based on this histology itself and not the primary location of the SCC.
Additionally, six pan-SCC immune subtypes have been identified from The Cancer Genome Atlas data. Each subtype was associated with distinct molecular characteristics and clinical outcomes, consistently across all SCCs from different locations,19 supporting the rationale for a pan-SCC approach with immunotherapy-based strategies.
Efficacy of immunotherapy in SCC patients
Even if ICIs have revolutionized cancer treatment and have shown efficacy in most cancer types, ORR remains low with single-agent therapy.20 Available data for anti-programmed death-ligand 1 (anti-PD-L1) therapy in HNSCC and SCC of the anal, cervical, vulvar region and lung are summarized in Table 1. Interestingly, PD-L1 expression seems to be a predictive biomarker of response in HSNCC. In penile SCC, apart from a case report of a patient achieving a partial response with nivolumab, another anti-PD-1 agent, there are no available data yet of activity, but preclinical studies show encouraging results and clinical trials are ongoing.21
Table 1.
Efficacy of anti-PD-1/PD-L1 single agents across squamous cell carcinomas of various origins
| Trial name | Study drug | Line of treatment | Patient selection | N | Phase | ORR (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Head and neck squamous cell carcinoma | |||||||
| CHECKMATE 141 | Nivolumab | Any | None - HPV+ - HPV− - PD-L1 TPS >1% - PD-L1 TPS <1% - PD-L1 TPS >5% - PD-L1 TPS <5% - PD-L1 TPS >10% - PD-L1 TPS <10% |
240 63 50 88 73 54 107 43 118 |
III | 13 16 8 17 12 22 11 28 10 |
Ferris et al.9 |
| KEYNOTE-048 | Pembrolizumab | 1 | None - PD-L1 CPS >1 - PD-L1 CPS >50 |
301 257 133 |
III | 17 19 23 |
Burtness et al.22 |
| KEYNOTE-040 | Pembrolizumab | 1-3 | None - PD-L1 CPS >1 - PD-L1 CPS <1 - PD-L1 TPS <1% - PD-L1 TPS >1% |
247 196 50 64 182 |
III | 15 17 4 27 10 |
Cohen et al.23 |
| KEYNOTE-055 | Pembrolizumab | Any | None - HPV+ - HPV− - PD-L1 CPS >1 - PD-L1 CPS <1 - PD-L1 CPS >50 - PD-L1 CPS <50 |
171 37 131 140 26 48 118 |
II | 16 16 15 18 12 27 13 |
Bauml et al.24 |
| KEYNOTE-012 | Pembrolizumab | Any | PD-L1 TPS >1% - HPV+ - HPV− |
45 16 29 |
I | 18 25 14 |
Seiwert et al.25 |
| KEYNOTE-012 | Pembrolizumab | Any | None - PD-L1 CPS >1 - PD-L1 CPS <1 - PD-L1 TPS >1% - PD-L1 TPS <1% - HPV+ - HPV− |
192 152 36 123 65 45 147 |
I | 18 21 6 18 19 24 16 |
Mehra et al.26 |
| PCD4989g | Atezolizumab | Any | None | 28 | I | Colevas et al.27 | |
| EAGLE | Durvalumab | 1+ | None - PD-L1 TPS >25% - PD-L1 TPS <25% |
240 68 172 |
III | 18 NR NR |
Ferris et al.28 |
| HAWK | Durvalumab | 2 | PD-L1 TPS >25% - HPV+ - HPV− |
111 34 65 |
II | 16 30 11 |
Zandberg et al.29 |
| CONDOR | Durvalumab | 2+ | TPS<25% | 65 | II | 9 | Siu et al.30 |
| 1108 | Durvalumab | Any | None - PD-L1 TPS >25% - PD-L1 TPS <25% - HPV+ - HPV− |
62 20 39 25 25 |
I/II | 6.5 15 3 0 8 |
Segal et al.31 |
| Anal squamous cell carcinoma | |||||||
| NCI 9673 | Nivolumab | 2+ | None | 37 | II | 24 | Morris et al.8 |
| KEYNOTE-028 | Pembrolizumab | Any | PD-L1 TPS >1% | 25 | I | 16 | Ott et al.20 |
| Cervical squamous cell carcinoma | |||||||
| CHECKMATE 358 | Nivolumab | 1-3 | HPV+ or unknown | 19 | I/II | 26 | Naumann et al.32 |
| KEYNOTE-028 | Pembrolizumab | Any | PD-L1 TPS >1% | 24 | I | 17 | Frenel et al.10 |
| Vulvar squamous cell carcinoma | |||||||
| CHECKMATE 358 | Nivolumab | 1-3 | HPV+ or unknown | 5 | I/II | 20 | Naumann et al.32 |
| KEYNOTE-028 | Pembrolizumab | Any | PD-L1 TPS >1% | 18 | I | 6 | Ott et al.20 |
| Lung squamous cell carcinoma | |||||||
| CHECKMATE 017 | Nivolumab | Any | None | 135 | III | 20 | Brahmer et al.7 |
| CHECKMATE 063 | Nivolumab | 2+ | None | 117 | II | 14.5 | Rizvi et al.33 |
CPS, combined positive score (number of PD-L1-positive tumor or immune cells divided by the total number of tumor cells and multiplied by 100); HPV, human papillomavirus; NR, not reported; NSCLC, non-small-cell lung cancer; ORR, overall response rate; PD-L1, programmed death-ligand 1; TPS, tumor proportion score (percentage of PD-L1-positive tumor cells among tumor cells).
Anti-PD-1 agents have become standards of care for lung SCC and HNSCC patients in the recurrent and/or metastatic setting. In lung SCC, nivolumab improved overall survival (OS) compared with docetaxel in patients who progressed during or after a platinum-based chemotherapy regimen. PD-L1 expression was neither predictive nor prognostic in this patient population.7 In the Checkmate 141 phase III study, HNSCC patients who had progressed on platinum-based therapy received nivolumab or standard, single-agent systemic therapy (methotrexate, docetaxel, or cetuximab).9 That study was positive independently of PD-L1 expression. Similar results were reported with pembrolizumab in the Keynote 040 phase III study, where the highest benefits were observed in patients whose tumors had a PD-L1 combined positive score >1 or a PD-L1 tumor proportion score (TPS) >50%.23
Despite outstanding outcomes in some patients, the majority of patients do not respond to single-agent ICI. Combinational approaches represent a logical strategy aiming to extend benefits to a greater proportion of patients. Effective biomarker identification is also of primary importance. Overall, PD-L1 expression using immunohistochemistry (IHC) helps in some instances, but is not a robust enough biomarker of ICI efficacy in SCC. Conflicting results have been reported regarding the impact of the HPV status on the efficacy of ICIs. A recent meta-analysis, however, suggested a higher efficacy in HPV+ versus HPV− HNSCC patients.34 This might be related to a more inflamed immune microenvironment.35 Establishing accurate ways to better select patients who will benefit from these drugs is crucial.
Rationale for combining immunotherapy with an epidrug in SCC patients
Preclinical evidence
Epigenetics refers to chromatin modulations without any underlying change in the DNA sequence. It is a reversible adaptive phenomenon, persistent in time and through cell divisions. Epigenetic modifications include methylation/demethylation of the CpG islets of DNA, respectively, by the DNA methyltransferases (DNMTs) and the ten-eleven translocation family member proteins, and histone modifications, for instance through acetylation/deacetylation by histone acetyltransferases and HDACs. Global genome hypomethylation was the first epigenetic event linked to cancer and is associated with genome instability and aneuploidy. Alternatively, gene promoter hypermethylations often occur during oncogenesis and inactivate tumor suppressor genes. Histone modifications, including acetylations, alter the chromosomal structure and therefore regulate and modify DNA accessibility to the transcription machinery through the compaction/decompaction of chromatin.36
As previously stated, almost all SCCs harbor somatic inactivating mutations of TP53 or p53 inactivation by HPV proteins. Another member of the p53 family, p63, a crucial player in epithelial morphogenesis, is also frequently mutated in diverse SCCs and, contrary to p53, is rarely mutated in other kinds of human cancers.37
The mixed lineage leukemia (MLL) proteins and epigenetic regulators belong to the lysine (K) methyltransferase 2 (KMT2) family. These proteins control the methylation of histone 3 (H3), one of the most influential of the eight subunits composing each histone core. When methylated on its fourth lysine (H3K4), H3 recruits different transcription factors and enhances gene expression. Translocations of MLL1/KMT2A were first discovered in acute leukemia. Mutations of other members of the family have since been frequently identified in solid tumors and the MLL2/KMT2D mutations rate is high in lung SCC and HNSCC.38 In the BioRAIDs study in cervical cancer, we found mutations of MLL3/KMT2C and MLL2/KMT2D in 16% and 15% of patients tumors, respectively, and cumulative loss of function mutations in any epigenetic modulator gene alteration was as high as 47%.17 Unpublished data also showed mutations, frameshift deletions, insertions and/or stop codons in MLL2 and MLL3 genes in 17% and 19% patients, respectively. In HPV-negative HNSCC especially, inactivating mutations or deletions in KMT2C-D, NSD1 and ARID1A genes have been identified in >20% of tumors.14
Epigenetic processes allow dynamic, reversible and rapid tumor evolution and therefore a useful plasticity in order for the tumor to escape immunosurveillance and acquire drug resistance. Some processes have been shown to be involved in both primary and secondary resistance to ICI, notably by affecting the antigenic presentation machinery and/or expression of the tumor antigen recognized by the immune system.39 Epidrugs affect different oncogenic signaling and antitumor immunity pathways and can consequently help priming the immune response and potentially restore sensitivity to ICIs. We review here the main mechanism linked to HDACi, which belongs to the group of epigenetic erasers.
Regarding antigen presentation, TAP-1 and TAP-2 transporter associated with antigen processing 1 and 2 are molecules involved in the transport of peptides from the cytosol to the endoplasmic reticulum, which allow the formation of the major histocompatibility complex (MHC) I-peptide complex. A decrease in TAP-1 expression due to a loss of histone acetylation has been observed in mouse models, which can influence the recognition of the MHC I-peptide complex on the tumor cell surface and subsequent activation of antitumor T cells. Under treatments with HDACi, the expression of TAP 1 and 2 transporters is increased.40 In in vitro models, PD-L1 expression increased after exposure to HDACi.41 HDAC plays a crucial role in T cell differentiation and effector functions and may allow T cells to regain a functionality profile with reversion of exhaustion.42 Finally, some HDACs, in particular HDAC9, regulate the expression of FOXP3, the master transcription factor in regulatory T cells (Tregs).43 In mice, a decrease in Treg-mediated immunosuppression was observed in non-small-cell lung cancer (NSCLC) under treatment with HDACi and a bromodomain inhibitor, which belongs to another class of epidrugs.44 Epigenetic drugs may also impact dendritic cells maturation,45 and epigenetics can impact the immunometabolism through regulation of key metabolic actors such as indoleamine-2,3-dioxygenase 1.12
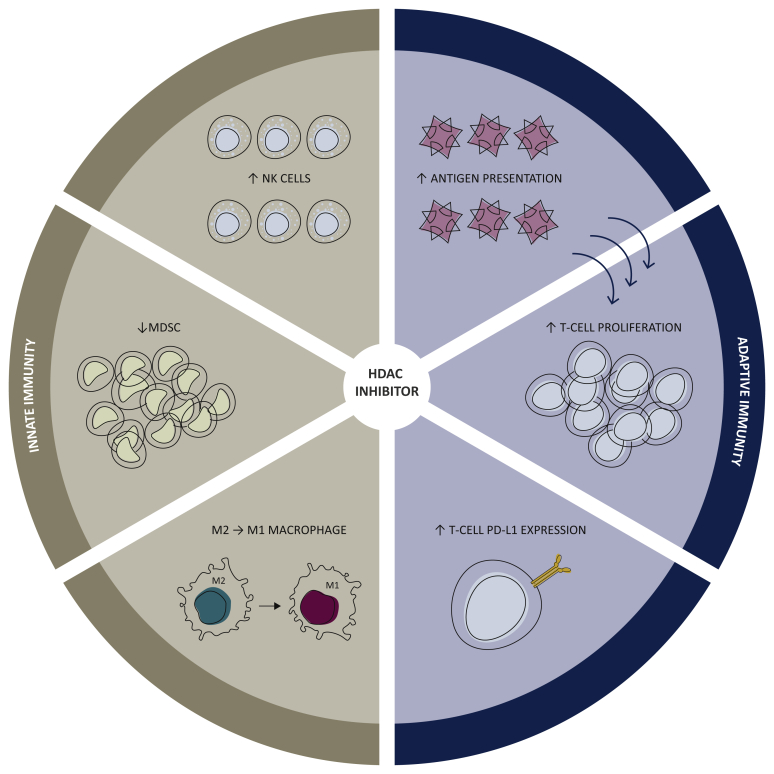
In a murine model of hepatocellular carcinoma, the association of belinostat, an HDACi, with dual cytotoxic T lymphocyte-associated 4 and PD-1 blockade improved antitumor activity, increased PD-L1 expression, and produced complete responses.46 The combination of panobinostat, another HDACi, and an anti-PD-1 agent in melanoma B16-F10 murine models also yielded better response rates than those obtained with either drug alone.41 Further amplifying an HDACi drug-class effect, entinostat increased the antitumor effect of anti-PD-1 inhibition in lung and renal cancer murine models, with functional inhibition of myeloid-derived suppressor cells (MDSC) and alteration of the cytokine/chemokine ratio towards a more pro-immune setting.47 Finally, HDACi have been shown to decrease MDSC, increase natural killer (NK) cell proliferation and reprogram macrophages toward an M1 antitumor phenotype.48 Effects of HDAC inhibition on innate and adaptive immune responses are summarized in Figure 1.
Figure 1.
Effects of HDAC inhibition on the tumor microenvironment immune cells.
HDAC, histone deacetylase; MDSC, myeloid-derived suppressor cell; NK, natural killer; PD-L1, programmed death-ligand 1; T cell, T lymphocyte.
Clinical evidence
The combination of an HDACi and an anti-PD-1 agent has been shown to be safe in early-phase clinical trials.49, 50, 51 Ongoing trials are summarized in Table 2.
Table 2.
Clinical trials evaluating the combination of an HDAC inhibitor with an anti-PD-1/PD-L1 agent
| HDAC inhibitor | Anti-PD-1/PD-L1 agent | Other drug | NCT Number | Cancer types | Line of therapy | Phase | Preliminary results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vorinostat | Pembrolizumab | 02638090 | NSCLC | 1 | I/Ib | ORR = 13% | Gray et al.49 | |
| II | ORR = 48% | Saltas et al.50 | ||||||
| Pembrolizumab | 02538510 | HNSCC, salivary gland tumors | Any | II | HNSCC: ORR = 32% |
Saltas et al.50 | ||
| Entinostat | Pembrolizumab | 02909452 | Advanced solid tumors | Any | I | Ongoing | Gandhi et al.52 | |
| Pembrolizumab | 02437136 | NSCLC | >1 | Ib/II | Prior progression on anti-PD-1/PD-L1 therapy ORR = 9% |
|||
| Pembrolizumab | Ipilimumab (anti-CTLA4) |
02453620 | Advanced solid tumors | Any | I | Ongoing | ||
| Nivolumab Pembrolizumab |
±Azacitidine (DNMTi) |
01928576 02437136 |
NSCLC Melanoma |
2 or 3 | II II |
Ongoing Prior progression on anti-PD-1/PD-L1 therapy ORR = 19% |
Rodriguez et al.51 | |
| >1 | ||||||||
| Belinostat | Nivolumab | ±Ipilimumab (anti-CTLA4) |
04315155 | Advanced solid tumors | Any | I | Ongoing | |
| Mocetinostat | Pembrolizumab | Guadecitabine (DNMTi) |
03220477 | NSCLC | >1 | I/Ib | Ongoing | |
| Pembrolizumab | 02954991 | NSCLC | >1 | II | Ongoing | |||
| Durvalumab | 02805660 | Advanced solid tumors | Any | I/II | Ongoing | |||
| Durvalumab | 02993991 | HNSCC | Any | I | Ongoing | |||
| Panobinostat | Spartalizumab | Everolimus | 02890069 | NSCLC | Any | Ib | Ongoing | |
| Citarinostat | Nivolumab | 02635061 | NSCLC | Any | Ib | Ongoing | ||
| Chidamide | Nivolumab | 02718066 | NSCLC, melanoma, RCC | Any | Ib/II | Ongoing |
CTLA4, cytotoxic T lymphocyte-associated 4; DNMTi, DNA methyltransferase inhibitor; HDAC, histone deacetylase; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small-cell lung cancer; ORR, overall response rate; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; RCC, renal cell carcinoma.
Vorinostat is a synthetic hydroxamic acid derivative and an HDACi. It was approved in 2006 by the FDA in third-line treatment of recurrent or progressive cutaneous T-cell lymphoma. Other FDA-approved HDACi are romidepsin, belinostat and panobinostat for specific hematological malignancies. Entinostat has been granted an FDA-approved drug designation for advanced stage breast cancer.53 Pembrolizumab is now FDA approved in a wide range of cancers including, NSCLC, small-cell lung cancer, HNSCC, melanoma, renal cell carcinoma and, as previously stated, MSI-H solid tumors.54 Other FDA-approved anti-PD-1 agents include nivolumab and cemiplimab.
The combination of vorinostat and pembrolizumab has been evaluated in a phase Ib study in NSCLC patients, the majority of them having been pretreated with an ICI. No dose-limiting toxicities or treatment-related deaths were reported. The most common side-effects were fatigue (33%), nausea (27%), vomiting (27%) and autoimmune hypothyroidism (15%).49
The adverse events reported with the combination of pembrolizumab and vorinostat are the ones observed with pembrolizumab and vorinostat as single agents. Pembrolizumab produces the usual immune-related adverse events, whereas the main adverse events reported with vorinostat include low-grade thrombocytopenia, creatinine increase, nausea, diarrhea and asthenia.
Among 30 assessable patients for efficacy, 4 (13%) experienced an objective response.49 Preliminary results from a randomized phase II trial comparing this combination with pembrolizumab alone in PD-L1 TPS > 1% metastatic NSCLC patients showed a higher ORR in the combination arm (48% versus 25%, P = 0.026).50 Of note, eight patients (35%) in the experimental arm experienced an increase in creatinine levels. No other safety alert was reported. A significant increase in tumor infiltrating lymphocytes (TILs) was observed after 15-21 days on-treatment in both arms. The ORR was significantly higher in the combination arm for patients with a low TIL level at baseline (70% versus 33%).50 An ORR of 32% was reported with this combination in 25 patients with recurrent and/or metastatic HNSCC,51 which is substantially higher than reported with single-agent pembrolizumab.
The ENCORE 601 phase II study evaluated the combination of entinostat and pembrolizumab in melanoma patients pretreated with anti-PD-1/PD-L1.55 The ORR was 19% with a median duration of response of 12.5 months.55
Altogether, these results, and especially the ones of the randomized trial in NSCLC patients,50 and the results obtained in anti-PD-1/PD-L1 agent pretreated patients,51,52 support further development of the combination of an anti-PD-1 agent with an HDACi.
The PEVOsq basket clinical trial
Study design
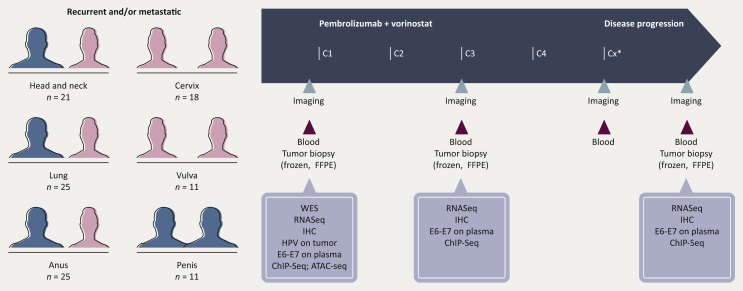
PEVOsq is a European open-label non-randomized multicenter phase II basket trial that will include patients with recurrent and/or metastatic SCC from the lung, head and neck, cervix, anus, vulva or penis. Patients should not have previously been exposed to ICI. Baseline biopsies are mandatory. Biopsies at first disease assessment and progression will be optional but encouraged. Patients will receive oral vorinostat 400 mg once daily and intravenous pembrolizumab 200 mg every 3 weeks (Figure 2).
Figure 2.
PEVOsq clinical trial design.
ATAC-Seq, assay for transposase-accessible chromatin with sequencing; C, cycle; ChIP-Seq, chromatin immunoprecipitation with sequencing; FFPE, formalin fixed paraffin embedded; HPV, human papillomavirus; IHC, immunohistochemistry; pts, patients; RNAseq, RNA sequencing; WES, whole exome sequencing.
∗, duration of each treatment cycles is 3 weeks.
The primary endpoint is the ORR in each cohort, as defined by the ratio of patients experiencing a partial or a complete response within all assessable patients of the considered cohort and according to RECIST1.1. The secondary endpoints include immune overall response rate according to iRECIST, duration of response, progression-free survival (PFS), immune progression-free survival and OS. Safety will be evaluated in each cohort and in the overall study population according to National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
The required number of assessable patients for each cohort was determined using the A'Hern design in order to obtain a minimum power of 85% with a 5% or 10% alpha risk (Table 3). An additional 10% of patients have been included in each cohort in order to compensate for drop out. The total number of patients to be included is estimated to be 111. Assessments will be done in the per protocol population for safety and efficacy. The overall trial duration is estimated to be 54 months.
Table 3.
Statistical hypotheses and decision rules of the PEVOsq basket trial
| Cohorts | p0 (%) | p1 (%) | Alpha (%) | Power (%) | No. of assessable/included patients | No. of objective responses required |
|---|---|---|---|---|---|---|
| Head and neck | 10 | 35 | 5 | 85 | 19/21 | ≥5 CR/PR |
| Anus | 15 | 40 | 5 | 85 | 23/25 | ≥7 CR/PR |
| Cervix | 5 | 30 | 5 | 90 | 16/18 | ≥3 CR/PR |
| Vulva | 5 | 30 | 10 | 85 | 10/11 | ≥2 CR/PR |
| Lung | 15 | 40 | 5 | 85 | 23/25 | ≥7 CR/PR |
| Penis | 5 | 30 | 10 | 85 | 10/11 | ≥2 CR/PR |
CR, complete response; p0, maximal unacceptable rate of patient presenting an objective response for whom the experimental treatment will be considered as insufficiently active; p1, minimal acceptable rate of patient presenting an objective response for whom the experimental treatment will be considered as sufficiently active; PR, partial response.
Translational research
We will first assess the links between the tumor molecular profile, which includes epigenetic features, and immune parameters of both the tumor microenvironment (TME) on tumor samples and circulating immune cells via blood samples. Measures of efficacy will then be correlated to immune-related biomarkers in the TME and in blood samples. These biomarkers include (but are not limited to) tumor tissue PD-L1 expression by IHC, RNA gene expression profiling and DNA alteration analysis. More specifically, whole exome sequencing will assess the tumor mutational load, molecular signatures (such as homologous recombination deficiency and MSI) and gene alterations (mutations and copy number alterations). RNA sequencing will also be carried out to identify expression signatures and fusion transcripts. We will also monitor the modification of immune-related and molecular epigenetic biomarkers under treatment, the impact of TME and epigenetics parameters on sensitivity/response or resistance to treatment and, finally, the predictive value of circulating biomarkers on sensitivity or resistance to treatment.
Global and genome wide epigenetic analysis will be carried out to identify the role of key epigenetic modifications and potential biomarkers. A global methylation pattern of samples will be extracted and the methylation of promoters and genes of interest will be evaluated [chromatin immunoprecipitation with sequencing (ChIP-Seq) and assay for transposase-accessible chromatin with sequencing (ATAC-seq) on frozen tissue]. Immune parameters will be evaluated by IHC and via human leucocyte antigen (HLA) sequencing. Finally, circulating HPV DNA will be assessed by droplet digital polymerase chain reaction at different time points.
Our goal is also to understand whether the impact of the combination is linked to the effect of the epidrug on the tumor, its immune TME or both, and to identify genetic and epigenetic biomarkers of primary resistance, early response and secondary resistance.
Data generation and integration
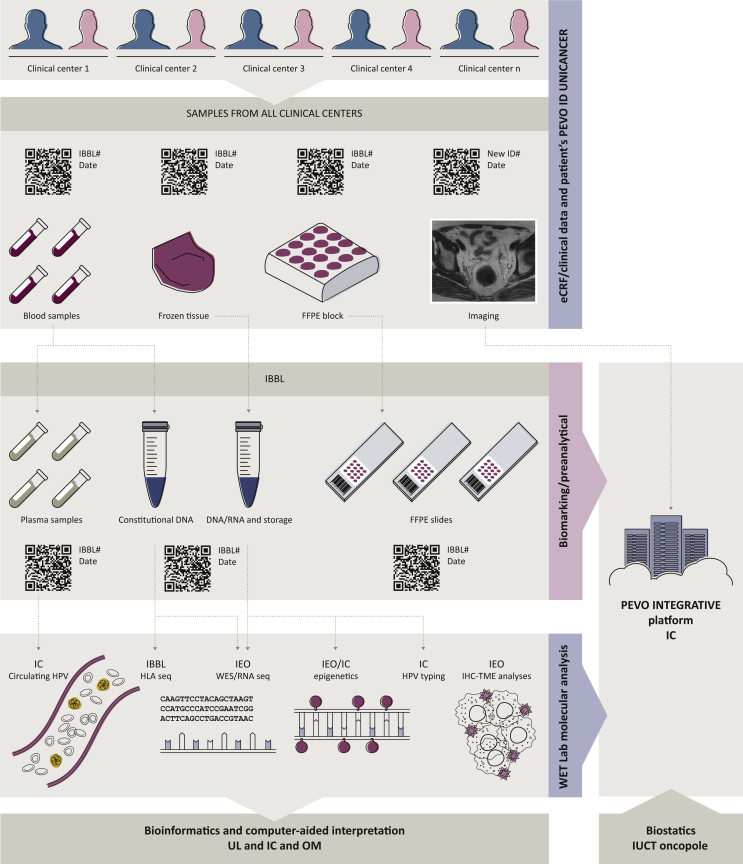
No biomarker has been identified yet to effectively select patients who will benefit from HDACi. PD-L1 expression is not a robust biomarker for ICI treatment in SCC either. The PEVOsq trial takes the opportunity to collect both blood and tissue samples at baseline, under treatment and at progression, in order to identify predictive biomarkers of response and/or resistance at baseline and observe treatment modifications to better identify short and long responders and mechanisms of both resistance and sensitivity. A challenging aspect is the prospective integration of different types of molecular and clinical data to allow secure and controlled access among the project stakeholders, as well as for future exploitations. In brief, the Integrated BioBank of Luxembourg (IBBL, Luxembourg) will act as the central repository for all sample collections and ensure logistics for storage, sample processing and redistribution to the relevant sites of downstream analysis. The centralized processing step includes DNA and RNA extraction from all frozen and/or formalin fixed paraffin embedded (FFPE) samples as well as circulating tumoral DNA extraction from plasma. Standard operating procedures will ensure standardization of procedures for all samples and thus ensure fitness-for-purpose for downstream analytical steps (Figure 3).
Figure 3.
PEVOsq translational data management and integration.
#, number; C, cycle of treatment; constit. DNA, constitutional DNA; eCRF, electronic case report form; FFPE, formalin fixed paraffin embedded; HLA, human leucocyte antigen; HPV, human papillomavirus; IBBL, Integrated BioBank of Luxembourg; IC, Institut Curie; ID, identification; IEO, Instituto Europeo di Oncolegia; IHC, immunohistochemistry; IUCT, Institut Universitaire du Cancer de Toulouse; OM, Oncompass Medicine; RNAseq, RNA sequencing; TME, tumor microenvironment; UL, University of Leipzig; WES, whole exome sequencing.
Each partner within the consortium will handle and generate specific sets of data: (i) HLA sequencing will be done on site at IBBL; (ii) molecular analyses will be carried out by the Instituto Europeo di Oncolegia (IEO, Milan, Italy) and Institut Curie (IC, Paris, France); (iii) bioinformatics analyses will be centralized by University of Leipzig (Leipzig, Germany) and IC; (iv) the clinical database will be set up and data managed by Unicancer, the sponsor of the trial; (v) biostatistical analyses will be carried out by Institut Claudius Regaud (Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France) in collaboration with all partners and (vi) computer-aided interpretation of molecular data will be carried out by Oncompass Medicine. Finally, data will be integrated, and afterwards correlated, with clinical data on outcome to allow molecular biomarkers identification at the IC (Figure 3).
Perspectives
The PEVOsq trial has several different aims and distinctive features we wish to highlight. It is a basket trial based on a sole histological subtype and therefore a bridge between the histological approach, which drove clinical trials in the last decades but was restricted to a specific organ, and the new paradigm of treating cancer based on a molecular alteration independently from the organ of origin. Here, we focus on shared molecular similarities within a specific histological subtype, but across multiple locations.
Promising results have been shown with the combination of an HDACi and an anti-PD-1 agent, and there is a strong biological rationale for this combination. Of note, the effect of an epidrug is often histotype-specific,39 which further rationalized the idea of a basket trial independent of location, but with a unique histological type. Apart from the strong preclinical rationale, this combination is promising when considering the safety profile of each drug, the minimal overlapping in their toxicities and their wide therapeutic index.
It was hypothesized that HPV-induced SCC should be more sensitive to immunotherapy thanks to foreign viral antigens in the TME. However, the HPV status has not been correlated to anti-PD-1 therapy efficacy in previous studies.
We are addressing an unmet medical need for our patients, since recurrent and metastatic SCCs are on the rise and associated with a poor prognosis. Despite the fact that ICIs have become a standard of care even in the first-line recurrent and/or metastatic setting in SCC, less than half of patients benefit from immunotherapy, as previously stated. Combinational approaches are undeniably required.
A comprehensive analysis of potential biomarkers will be carried out with a comparative analysis of the epigenome, the immune TME and tumor genome, as well as its evolution under treatment. This will be a major opportunity to better understand how each of these actors impacts the others and their dynamic partnership, to improve patient selection and design in subsequent trials. Our wide and integrated analysis of both tissue and blood samples at different time points is the ideal setting to discover predictive biomarkers and candidates for effectiveness under treatment monitoring. Precision medicine trials are also a unique opportunity to test those potential biomarkers. In the future, a two-step design could be foreseen with first, broad analysis of treatment activity while monitoring patients' samples, and then expansion cohorts based on a potential biomarker instead of a specific location.
Epigenetics are a growing field in oncology and other epidrugs have been incorporated in our daily practice. Two DNMT inhibitors, decitabine and azacitidine (AZA) are already FDA-approved for myelodysplastic syndromes and AZA is also approved for acute myeloid leukemia and chronic myelomonocytic leukemia.56 They are especially interesting when investigating immune priming, considering what is called the viral mimicry phenomenon, which reflects transcriptional repression of endogenous retroviruses (ERVs). ERVs account for nearly 8% of the human genome. They have been incorporated into it over millennia and are generally silenced in somatic cells by epigenetic processes, such as DNA methylation. DNMT inhibitors will therefore re-enable their transcription, which will lead to an antiviral immune response and induction of proimmune interferon 1 signaling.57
Most third generation epidrugs are more target-specific and linked in some settings to predictive biomarkers. Ivosidenib, an isocitrate dehydrogenase 1 (IDH1) inhibitor, is approved for IDH1-mutant leukemia.56 Tazemetostat, an enhancer of zeste homologue 2 (EZH2) inhibitor, is approved for patients with either EZH2 mutant hematological malignancies or highly aggressive tumors with genetic abnormalities in the SWI/SNF chromatin-remodeling complex.58 Bromodomain and extra-terminal inhibitors (BETi) target members of the bromodomain-containing protein (BRD) family and responses to BETi have been observed in BRD4-rearranged NUT midline carcinoma.59 Combinatorial approaches of one or two epidrugs and immunotherapy are under evaluation with strong preclinical rationales.53
Most epigenetic analyses are still limited to research and are not carried out through routine procedures. Current resources to study reversible epigenetics events focus on DNA methylation and chromatin modifications through histones state assessment. Measurements of genome wide-DNA methylation were first developed as microarrays and then adapted to next-generation sequencing use. Analyses are highly dependent on sample preservation methods, which greatly impact both chromatin integrity and DNA-protein binding strength. Most of these procedures are consequently still limited to fresh tissue analysis, and also require a larger quantity of tissue than human biopsies can provide. Finally, chromatin digestion is a pre-analytical crucial step, which can deeply impact results; digestion susceptibility is also subject to very high interindividual variability. Those techniques are therefore harder to apply to biopsy samples at the present time. However, evolution toward single cell analysis has been an important step since it allows low cell count analysis and the use of smaller samples. Recent techniques using ChIP-Seq, such as pathology tissue-chromatin immunoprecipitation (PAT-ChIP), which has been selected for this trial, have been optimized for the analysis of chromatin derived from FFPE samples.39 Finally, epigenetic events are dynamic, so the timing of biomarkers evaluation will probably play a bigger role in their predictive strength for epidrugs than any other treatment. Ideally, sequential evaluation should be favored with eagerly awaited liquid biopsy approaches.
A question that will not be addressed in the PEVOsq trial is the timing of this association. There is a strong rationale for immune priming and the use of an immune-inducing drug to boost the ICI subsequent response, especially in cold tumors. As a proof of concept, the TONIC trial is an adaptive randomized phase II trial investigating the impact of an induction treatment with radiotherapy, doxorubicin, cyclophosphamide or cisplatin for 2 weeks before nivolumab in metastatic TNBC. The cohort of patients who received immune induction with doxorubicin has been chosen for expansion in stage II of the trial, according to the ‘pick the winner’ concept, with best ORR, up-regulation of a gene signature associated with response to anti-PD-1 (IO 360TM), and increase in T cells proportion and clonality.60 One of the first observations that led to combining epidrugs and ICI was the promising disease control rate under anti-PD-1 treatment in five patients with NSCLC,53 who were previously enrolled in a trial evaluating the combination of entinostat and azacitidine.61 To that effect, an ongoing trial evaluates a 2 weeks priming approach with either azacitidine, romidepsin or both, and then a combination of the epidrug and pembrolizumab in advanced colorectal cancer (NCT01252172).
Lastly, PEVOsq is testing an innovative approach in big data management and international collaborations, in order to better apprehend and exploit the overwhelming load of data produced and the drastic underuse of information. Developing technologies and methods to allow both analysis and sharing of international data is paramount in the era of big data and precision medicine. Those trials generate prospective data with a wide spectrum of molecular characterization and a temporally dynamic analysis, which aim to facilitate our constant research for efficient biomarkers. But without supervised analysis and a comprehensive integration with clinical data and treatment outcomes, it can result in the search for a needle in a haystack. Data integration and management must be done efficiently to allow a global and dynamic analysis.
The PEVOsq trial will address these pitfalls and help moving the field of precision medicine from a proven concept to standard of care.
Acknowledgments
Funding
This work was supported by: EraPerMed [grant number ERAPERMED2018-078-PEVOdata] (ANR-18-PERM-0010); The Luxembourg National Research Fund [grant number INTER/ERAPerMed/18/13061865]; Association pour la Recherche contre le Cancer [grant number PGA1RC20190208493]; Nemzeti Kutatási, Fejlesztési és Innovációs Hivatal (NKFI Hivatal) (National Research, Development and Innovation Office, NRDI Office) [grant number ED_18-2-2019-0001]; Hungarian Innovation Agency [grant numbers ED_18-2-2019-0001, NVKP_16-1-2016-0005, 2019-1.1.1-PIACI-KFI-2019-00367]; Diese Massnahme wird mitfinanziert mit Steuermitteln auf Grundlage des von den Abgeordneten des Sächsischen Landtags beschlossenen Haushalts, Germany. Free State of Saxony, Sächsische Aufbaubank (SAB), application no. 100371715; and Italian Ministry of Health [grant number ERAPerMed 2018_PEVODATA_MAZZARELLA].
Disclosure
GC: Roche, Seattle Genetics, Novartis, Lilly, Pfizer, Foundation Medicine, Nanostring, Samsung, Celltrion, Ellipsis, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Mylan. CLT: Roche, Seattle Genetics, Rakuten, Nanobiotix, MSD, BMS, Merck Serono, AstraZeneca, GlaxoSmithKline, Novartis, Celgene. XF: Roche, Janssen, BMS, Leo Pharma, Amgen Research. LC: Leo Pharma, Amgen. JG: Roche, Leo Pharma. IP: employee and equity holder in Oncompass Medicine Ltd. RD, PF, and AD are employees of Oncompass Medicine Ltd. All other authors have declared no conflicts of interest.
Contributor Information
M. Kamal, Email: maud.kamal@curie.fr.
C. Le Tourneau, Email: Christophe.letourneau@curie.fr.
References
- 1.Doebele R.C., Drilon A., Paz-Ares L. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marabelle A., Fakih M., Lopez J. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 3.Le Tourneau C., Delord J.-P., Gonçalves A. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 4.Massard C., Michiels S., Ferté C. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7:586–595. doi: 10.1158/2159-8290.CD-16-1396. [DOI] [PubMed] [Google Scholar]
- 5.Rodon J., Soria J.-C., Berger R. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25:751–758. doi: 10.1038/s41591-019-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dotto G.P., Rustgi A.K. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell. 2016;29:622–637. doi: 10.1016/j.ccell.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris V.K., Salem M.E., Nimeiri H. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:446–453. doi: 10.1016/S1470-2045(17)30104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris R.L., Blumenschein G., Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frenel J.-S., Le Tourneau C., O'Neil B. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35:4035–4041. doi: 10.1200/JCO.2017.74.5471. [DOI] [PubMed] [Google Scholar]
- 11.Pons-Tostivint E., Latouche A., Vaflard P. Comparative analysis of durable responses on immune checkpoint inhibitors versus other systemic therapies: a pooled analysis of phase III trials. JCO Precis Oncol. 2019;3:1–10. doi: 10.1200/PO.18.00114. [DOI] [PubMed] [Google Scholar]
- 12.Aspeslagh S., Morel D., Soria J.-C. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol. 2018;29:812–824. doi: 10.1093/annonc/mdy050. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Research Network Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoadley K.A., Yau C., Hinoue T. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholl S., Popovic M., de la Rochefordiere A. Clinical and genetic landscape of treatment naive cervical cancer: alterations in PIK3CA and in epigenetic modulators associated with sub-optimal outcome. EbioMedicine. 2019;43:253–260. doi: 10.1016/j.ebiom.2019.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell J.D., Yau C., Bowlby R. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep. 2018;23:194–212.e6. doi: 10.1016/j.celrep.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B., Cui Y., Nambiar D.K., Sunwoo J.B., Li R. The immune subtypes and landscape of squamous cell carcinoma. Clin Cancer Res. 2019;25:3528–3537. doi: 10.1158/1078-0432.CCR-18-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott P.A., Bang Y.-J., Piha-Paul S.A. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37:318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 21.Trafalis D.T., Alifieris C.E., Kalantzis A., Verigos K.E., Vergadis C., Sauvage S. Evidence for efficacy of treatment with the anti-PD-1 mab nivolumab in radiation and multichemorefractory advanced penile squamous cell carcinoma. J Immunother. 2018;41:300–305. doi: 10.1097/CJI.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 22.Burtness B., Harrington K.J., Greil R. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 23.Cohen E.E.W., Soulières D., Le Tourneau C. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 24.Bauml J., Seiwert T.Y., Pfister D.G. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seiwert T.Y., Burtness B., Mehra R. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 26.Mehra R., Seiwert T.Y., Gupta S. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colevas A.D., Bahleda R., Braiteh F. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29:2247–2253. doi: 10.1093/annonc/mdy411. [DOI] [PubMed] [Google Scholar]
- 28.Ferris R.L., Haddad R., Even C. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942–950. doi: 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Zandberg D.P., Algazi A.P., Jimeno A. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–152. doi: 10.1016/j.ejca.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Siu L.L., Even C., Mesía R. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5:195–203. doi: 10.1001/jamaoncol.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal N.H., Ou S.-H.I., Balmanoukian A. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer. 2019;109:154–161. doi: 10.1016/j.ejca.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Naumann R.W., Hollebecque A., Meyer T. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J Clin Oncol. 2019;37:2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi N.A., Hellmann M.D., Snyder A. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galvis M.M., Borges G.A., Oliveira TB de. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;150:102966. doi: 10.1016/j.critrevonc.2020.102966. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Sun H., Zeng Q. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep. 2019;9:13404. doi: 10.1038/s41598-019-49771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy D.M., Walsh L.A., Chan T.A. Driver mutations of cancer epigenomes. Protein Cell. 2014;5:265–296. doi: 10.1007/s13238-014-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatti V., Fierro C., Annicchiarico-Petruzzelli M., Melino G., Peschiaroli A. ΔNp63 in squamous cell carcinoma: defining the oncogenic routes affecting epigenetic landscape and tumour microenvironment. Mol Oncol. 2019;13:981–1001. doi: 10.1002/1878-0261.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford D.J., Dingwall A.K. The cancer COMPASS: navigating the functions of MLL complexes in cancer. Cancer Genet. 2015;208:178–191. doi: 10.1016/j.cancergen.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Morel D., Jeffery D., Aspeslagh S., Almouzni G., Postel-Vinay S. Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise. Nat Rev Clin Oncol. 2020;17:91–107. doi: 10.1038/s41571-019-0267-4. [DOI] [PubMed] [Google Scholar]
- 40.Arenas-Ramirez N., Sahin D., Boyman O. Epigenetic mechanisms of tumor resistance to immunotherapy. Cell Mol Life Sci. 2018;75:4163–4176. doi: 10.1007/s00018-018-2908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods D.M., Sodré A.L., Villagra A., Sarnaik A., Sotomayor E.M., Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauken K.E., Sammons M.A., Odorizzi P.M. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falkenberg K.J., Johnstone R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 44.Adeegbe D.O., Liu Y., Lizotte P.H. Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov. 2017;7:852–867. doi: 10.1158/2159-8290.CD-16-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tesone A.J., Rutkowski M.R., Brencicova E. Satb1 overexpression drives tumor-promoting activities in cancer-associated dendritic cells. Cell Rep. 2016;14:1774–1786. doi: 10.1016/j.celrep.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Llopiz D., Ruiz M., Villanueva L. The HDAC inhibitor belinostat enhances the anti-tumor efficacy of immune checkpoint inhibitors in a murine hepatocellular carcinoma model. J Hepatol. 2018;68:S677. doi: 10.1007/s00262-018-2283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orillion A., Hashimoto A., Damayanti N. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–5201. doi: 10.1158/1078-0432.CCR-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerriero J.L., Sotayo A., Ponichtera H.E. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray J.E., Saltos A., Tanvetyanon T. Phase I/Ib study of pembrolizumab plus vorinostat in advanced/metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25:6623–6632. doi: 10.1158/1078-0432.CCR-19-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saltos A.N., Tanvetyanon T., Creelan B.C. Phase II randomized trial of first-line pembrolizumab and vorinostat in patients with metastatic NSCLC (mNSCLC) J Clin Oncol. 2020;38:9567. [Google Scholar]
- 51.Rodriguez C.P., Wu Q.V., Voutsinas J. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. 2020;26:837–845. doi: 10.1158/1078-0432.CCR-19-2214. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi L., Janne P.A., Opyrchal M. Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in patients with non-small cell lung cancer (NSCLC) previously treated with anti-PD-(L)1 therapy. J Clin Oncol. 2018;36:9036. [Google Scholar]
- 53.Topper M.J., Vaz M., Marrone K.A., Brahmer J.R., Baylin S.B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol. 2020;17:75–90. doi: 10.1038/s41571-019-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Vugt M.J.H., Stone J.A., De Greef R.H.J.M.M. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer. 2019;7:212. doi: 10.1186/s40425-019-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan R.J., Moschos S.J., Johnson M.L. Abstract CT072: Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in patients with melanoma previously treated with anti-PD1 therapy. Cancer Res. 2019;79:CT072. [Google Scholar]
- 56.DiNardo C.D., Pratz K., Pullarkat V. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn J., Rao S. Epigenetics and immunotherapy: the current state of play. Mol Immunol. 2017;87:227–239. doi: 10.1016/j.molimm.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Italiano A., Soria J.-C., Toulmonde M. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19:649–659. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 59.Stathis A., Zucca E., Bekradda M. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voorwerk L., Slagter M., Horlings H.M. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 61.Juergens R.A., Wrangle J., Vendetti F.P. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]