Abstract
Stunting, decidedly prevalent in Ethiopia, is a reduction of linear growth associated with a series of adverse consequences. However, little is known about its determinants and factors associated in Ethiopia and elsewhere. Therefore, this study aimed to determine major undelying factors associated with risk of stunting among under-five children in Ethiopia. We used the 2016 Ethiopian Demographic and Heath Survey (EDHS) data and analysed a total of 11,023 children aged 0–59 months' data. Bivariate and multivariate logistic regression were fitted to identify key predictors and factors associated with stunting. Results show that, household and demographic factors such as maternal education (AOR: 0.67, 95% CI: 0.51, 0.89), wealth index (AOR: 0.65 (0.54, 0.78), sex of child (AOR: 0.78 (0.72, 0.85), possession of refrigerator (AOR: 0.57 (0.36, 0.89), possession of television and others like twin birth, house main floor material, types of cooking fuel were significantly association with stunting. Among dietary factors, early initiation of breast feeding; feeding powdered or fresh milk (AOR: 0.63 (0.52, 0.76); formula feeding (AOR: 0.41 (0.21, 0.81); consumption of organ meat(s) (AOR: 0.52 (0.32, 0.85) and beta-carotene rich fruits and vegetables were significantly associated lower odds of stunting. Antenatal care (ANC) follow-up, deworming during pregnancy (AOR : 0.11 (0.02, 0.74), institutional delivery (AOR : 0.64 (0.58, 0.71) and birth size (AOR: 5.1 (1.64, 15.88) were among the health care factors associated with stunting of under-five children. In conclusion, stunting is modulated by several household, dietary and healthcare factors, both at household and community-level. Likewise; improving household income, women empowerment, dietary diversity among mothers and children and improving maternal health care system are critical to mitigate under-five stunting more rapidly.
Keywords: Stunting, Undernutrition, Predicting variables, Children, Ethiopia
Stunting, Undernutrition, Predicting variables, Children, Ethiopia.
1. Introduction
Globally, 149 million and in Africa, 59 million children under-five years of age are stunted (UNICEF/WHO/WB, 2019). Unlike progressive improvement in many parts of the world, Africa has left behind where the number of stunted children is rising. More than a third of stunted children live in Africa (UNICEF/WHO/WB, 2019). In Ethiopia, four in every five (38%) children younger than five years are stunted, making the country to have the highest burden of under nutrition (CSA, 2016).
Stunting, a major predictor of impaired cognitive ability and reduced school and work performance, has been linked to multiple adverse health outcomes that extend beyond childhood into adult life (Black et al., 2008). The functional impairments associated with stunting are irreversible past the age of two years. Hence, timely and effective intervention needs to be in place to improve the situation.
Generally, unlike substantial burden of the problem, little is known about factors associated with stunting in many of the low-income settings including Ethiopia. According to few of the available studies in Ethiopia, stunting was associated with maternal education, short maternal stature, maternal body mass index (MBI), child birth weight, diet and diarrheal episodes (Berhe et al., 2019). A study in Afar, a pastoralist region in Ethiopia, reported that preceding birth interval (less than 24 months), antenatal care (ANC) follow up, access to latrine, colostrum feeding, and duration of breast feeding were key predictors of stunting (Kahssay et al., 2020). Other studies have also reported family size, maternal occupation, duration of exclusive breastfeeding, fruit and vegetable production, land ownership and complementary feeding practices predicting risk of stunting in the country (Fikadu et al., 2014).
However, none of these studies were nationally representative and used multi-dimensional data with large sample size. Likewise, more evidence is needed from a nationally representative data and rigorous analysis to inform policymakers and practitioners to implement more effective interventions and programs addressing the persistent challenge over long years. In this study, we conducted an in-depth analysis of the 2016 Ethiopian Demographic Health Survey (EDHS) data, to determine household, dietary and healthcare factors associated with stunting.
2. Methods
2.1. Data source
Data for this study was obtained from the EDHS 2016 up on permission from ICF-DHS program to use the EDHS data. The EDHS is a population-based household survey designed to provide representative data for the country as a whole; the nine regional states and the two city administrations. The study was conducted from January 18 to 27 June, 2016. Children age 0–59 months with anthropometry data (height-for-age-z-scores) were considered in the analyses of risk factors of stunting among under-five children. Missing values in the EDHS dataset were not included in the analyses.
2.2. Variables
2.2.1. Outcome variable
Stunting (height-for- age-z- scores), the most common anthropometric indicators used for monitoring undernutrition in children was used as an outcome variable. Stunting was defined as children with their length/height below 2 standard deviations from the WHO child growth standards median for same age and sex (WHO, 2006).
2.2.2. Predicting variables
To analyze the risk factors of stunting among under-five children, the study considered the following as predicting variables:
-
1)
Household factors: Mother's level of education (no education, primary education, secondary education and higher education); wealth index (poorest, poorer, middle, richer and richest); sex of child (male, female); child in twin (single birth, 1st of multiple and 2nd of multiple); house main floor material (cement/ceramic or wood, natural/earth/sand and polished with dung); types of cooking fuel (electricity, kerosene/biogas/charcoal, wood/straws/grass and animal dung); possession of household electricity (no, yes); possession of household refrigerator (no, yes); possession of household television (no, yes) and possession of mobile telephone (no, yes).
-
2)
Dietary factors: Initiation of breast feeding (immediately, after 1 h and hours missing); gave child tinned, powdered or fresh milk (no, yes); gave child baby formula (no, yes); gave child meat, organ meats and fish (no, yes); gave child beta-carotene rich vegetables (pumpkin, carrots, etc.) (no, yes); gave child mangoes, papayas, other beta-carotene rich fruits (no, yes) and gave child any dark green leafy vegetables (no, yes).
-
3)
Healthcare factors: Prenatal doctor or health officer (HO) (no, yes); prenatal nurse or midwife (no, yes); deworming during pregnancy (no, yes); place of delivery (home, health facility; delivery by caesarean section (no, yes); child size at birth (larger than average, average and smaller than average) and recent diarrhea (no, yes).
2.3. Ethical statement
The data were analyzed after the purpose of the analysis was communicated and approved by ICF-DHS program. Ethical clearance for the survey was provided by the Ethiopian Public Health Institute (EPHI) review board, the National Research Ethics Review Committee (NRERC) at the Ministry of Science and Technology, and the Institutional Review Board of ICF Macro International.
2.4. Statistical analysis
The data analysis was done using SPSS version 20. Both bivariate and multivariate logistic regression analysis was used to determine factors associated with understanding. For all analyses, p-values less than 0.05 (P < 0.05) were considered to be statistically significant. The resulting odds ratios with their corresponding 95% CI were presented in all the tables.
3. Results
3.1. Sociodemographic characteristics
A total of 11,023 children were included in the analysis, of which 9,494 (86%) were male and 1529 (14%) were female. The analysis showed that the majority (89%) of the study participants were from the rural setup, about two third (66%) of the mothers and nearly half of (46%) of their husbands had no education and almost all (94%) of the mothers were married. In terms of household index, about a quarter (24% and 23%) of the households were in the poorest and poorer wealth quintiles, respectively.
3.2. Magnitude and burden of stunting
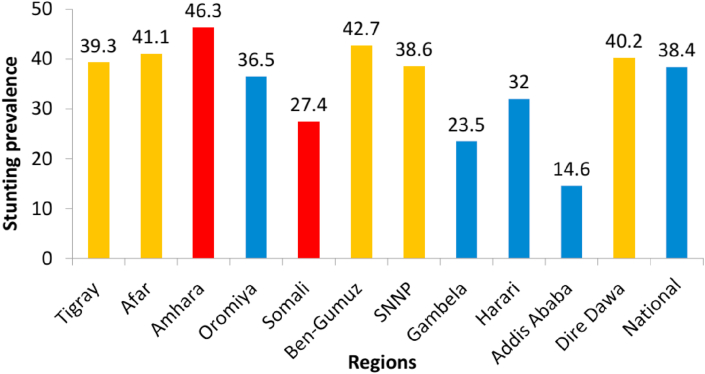
The overall prevalence of childhood stunting in Ethiopia was 38%. There were regional variations, with Amhara, Benishangul-Gumuz, Afar and Dire Dawa regions having a higher prevalence of stunting, while the lowest prevalence of stunting was found in Addis Ababa (Figure 1). Rural areas had a higher prevalence of stunting than urban areas.
Figure 1.
Prevalence (%) of under-five stunting across regions in Ethiopia (CSA, 2016).
3.3. Factors associated with stunting
3.3.1. Household factors
The bivariate and multivariate logistic regression analysis results for household factors associated with stunting are indicated in (Table 1). The risks of stunting were inversely related to mother's education level; the more schooling a mother had, the less likely her child was to be stunted (AOR: 0.67, 95% CI: 0.51, 0.89) than whose mothers who had never attended formal education. Lower economic status was associated with childhood stunting, the poorest households (lowest wealth index) had significantly higher odds of stunting relative to those in the richer (AOR: 0.65, 95% CI: 0.54, 0.78) households (Table 1). Male child, child in twin (both 1st and 2nd multiple), house main floor material made from natural/earth/sand or polished with dung, households that do not possess refrigerator and television were significantly associated with under-five stunting.
Table 1.
Household factors associated with stunting in Ethiopia.
| Predicting variables | N (%) | Outcome variable |
P-value | |
|---|---|---|---|---|
| Stunting | ||||
| COR (95%CI) | AOR (95% CI) | |||
| Mother's level of education | ||||
|
7284 (66.1) | 1 | ||
|
2951 (26.8) | 0.77 (0.69, 0.84) ∗∗ | 0.92 (0.83, 1.03) | 0.155 |
|
514 (4.7) | 0.38 (0.30, 0.48) ∗∗ | 0.67 (0.51, 0.89) ∗∗ | 0.006 |
|
274 (2.5) | 0.29 (0.21, 0.42) ∗∗ | 0.69 (0.42, 1.16) | 0.164 |
| Wealth index | ||||
|
2636 (23.9) | 1 | ||
|
2520 (22.9) | 0.92 (0.82, 1.04) | 0.91 (0.79, 1.04) | 0.181 |
|
2280 (20.7) | 0.74 (0.65, 0.84) ∗∗ | 0.73 (0.63, 0.86) ∗∗ | 0.00 |
|
1999 (18.1) | 0.65 (0.57, 0.74) ∗∗ | 0.65 (0.54, 0.78) ∗∗ | 0.00 |
|
1588 (14.4) | 0.42 (0.36, 0.48) ∗∗ | 0.82 (0.62, 1.08) | 0.155 |
| Sex of child | ||||
|
5725 (51.9) | 1 | ||
|
5298 (48.1) | 0.78 (0.72. 0.85) ∗∗ | 0.78 (0.72, 0.85) ∗∗ | 0.00 |
| Child in twin | ||||
|
10730 (97.3) | 1 | ||
|
146 (1.3) | 1.80 (1.26, 2.58) ∗∗ | 2.16 (1.47, 3.19) ∗∗ | 0.00 |
|
146 (1.3) | 2.34 (1.58, 3.46) ∗∗ | 2.43 (1.61, 3.65) ∗∗ | 0.00 |
| House main floor material | ||||
|
1466 (13.3) | 1 | ||
|
5975 (54.2) | 2.09 (1.81, 2.41) ∗∗ | 1.33 (1.08, 1.64) ∗∗ | 0.007 |
|
3582 (32.5) | 2.37 (2.05, 2.75) ∗∗ | 1.57 (1.27, 1.93) ∗∗ | 0.00 |
| Types of cooking fuel | ||||
|
305 (2.8) | 1 | ||
|
542 (4.9) | 1.06 (0.74, 1.52) | 0.62 (0.41, 0.93) ∗∗ | 0.019 |
|
9372 (85.0) | 2.22 (1.65, 2.99) ∗∗ | 0.64 (0.43, 0.93) ∗∗ | 0.02 |
|
804 (7.3) | 3.03 (2.18, 4.21) ∗∗ | 1.04 (0.68, 1.57) | 0.87 |
| Possession of household electricity | ||||
|
9316 (84.5) | 1 | ||
|
1706 (15.5) | 0.53 (0.47, 0.60) ∗∗ | 0.93 (0.75, 1.15) | 0.489 |
| Possession of household refrigerator | ||||
|
10682 (96.9) | 1 | ||
|
340 (3.1) | 0.22 (0.15, 0.31) ∗∗ | 0.57 (0.36, 0.89) ∗∗ | 0.014 |
| Possession of household television (TV) | ||||
|
10072 (91.4) | 1 | ||
|
950 (8.6) | 0.34 (0.28, 0.41) ∗∗ | 0.59 (0.44, 0.80) ∗∗ | 0.001 |
| Possession of mobile telephone | ||||
|
9227 (83.7) | 1 | ||
|
1795 (16.3) | 0.51 (0.45, 0.58) ∗∗ | 0.87 (0.74, 1.02) | 0.075 |
∗∗P < 0.05, COR: Crude odd ratio, AOR: Adjusted odd ratio, CI: Confidence interval.
3.3.2. Dietary factors
The results of our bivariate and multivariate logistic regression analysis to examine dietary factors associated with under-five stunting are presented in (Table 2). Children who had delayed (by 1 h) initiation of breast feeding had a 56%, (AOR: 1.56, 95% CI: 1.14, 2.14) more risk of stunting than those who had immediately breastfeeding after birth. Under-five children who were fed with tinned, powdered, or fresh milk had a 37% (AOR: 0.63, 95% CI: 0.52, 0.76) less risk of stunting compared to those who didn't. Similarly, under-five children who were fed with baby formula had 59% (AOR: 0.41, 95% CI: 0.21, 0.81) less risk of stunting compared to those children who did not had baby formula.
Table 2.
Dietary factors associated with stunting in Ethiopia.
| Predicting variables | N (%) | Outcome variable |
P-value | |
|---|---|---|---|---|
| Stunting | ||||
| COR (95%CI) | AOR (95% CI) | |||
| Initiation of breast feeding | ||||
|
7703 (84.9) | 1 | ||
|
305 (3.4) | 1.12 (0.88, 1.44) | 1.56 (1.14, 2.14) ∗∗ | 0.005 |
|
1060 (11.7) | 1.08 (0.94, 1.24) | 1.00 (0.84, 1.20) | 0.984 |
| Gave child tinned, powdered or fresh milk | ||||
|
5914 (86.2) | 1 | ||
|
950 (13.8) | 0.63 (0.54, 0.75) ∗∗ | 0.63 (0.52, 0.76) ∗∗ | 0.00 |
| Gave child baby formula | ||||
|
6772 (98.7) | 1 | ||
|
93 (1.3) | 0.29 (0.15, 0.55) ∗∗ | 0.41 (0.21, 0.81) ∗∗ | 0.011 |
| Gave child meat (beef, pork, lamb, chicken, etc.) | ||||
|
6590 (96.0) | 1 | ||
|
274 (4.0) | 0.59 (0.44, 0.81) ∗∗ | 0.79 (0.55, 1.13) | 0.191 |
| Gave child liver, heart, other organs meat | ||||
|
6695 (97.5) | 1 | ||
|
169 (2.5) | 0.41 (0.27, 0.63) ∗∗ | 0.52 (0.32, 0.85) ∗∗ | 0.008 |
| Gave child fish or shellfish | ||||
|
6803 (99.1) | 1 | ||
|
61 (0.9) | 0.44 (0.23, 0.84) ∗∗ | 0.69 (0.34, 1.43) | 0.327 |
| Gave child eggs | ||||
|
6056 (88.2) | 1 | ||
|
808 (11.8) | 0.86 (0.73, 1.02) | 1.05 (0.86, 1.28) | 0.66 |
| Gave child beta carotene rich vegetables like pumpkin, carrots, etc. | ||||
|
6292 (91.7) | 1 | ||
|
572 (8.3) | 0.68 (0.56, 0.84) ∗∗ | 0.72 (0.56, 0.91) ∗∗ | 0.006 |
| Gave child mangoes, papayas, other beta carotene rich fruits | ||||
|
6248 (91.0) | 1 | ||
|
616 (9.0) | 0.65 (0.54, 0.79) ∗∗ | 0.77 (0.61, 0.98) ∗∗ | 0.031 |
| Gave child any dark green leafy vegetables | ||||
|
6207 (90.4) | 1 | ||
|
657 (9.6) | 1.09 (0.92, 1.30) | 1.28 (1.03, 1.60) ∗∗ | 0.03 |
∗∗P < 0.05, COR: Crude odd ratio, AOR: Adjusted odd ratio, CI: Confidence interval.
With regards to the consumption of meat and other meat related items, our study showed that, under-five children who were fed with flesh foods (meat, fish, poultry, organ meats) had close to half (52%) (AOR: 0.52, 95% CI: 0.32, 0.85) lower risk of stunting compared to those who were not fed with either. Similarly, children who were fed with beta-carotene rich vegetables such as pumpkin, carrots and mangoes, papayas, other beta carotene rich fruits had a 28% and 23% (AOR: 0.72, 95% CI: 0.56, 0.91) and (AOR: 0.77, 95% CI: 0.61, 0.98) lower risk of stunting respectively.
3.3.3. Healthcare factors
The findings from the analysis of logistic regression of healthcare factors associated with under-five stunting is presented in (Table 3). Women who obtained deworming services during pregnancy had an 89% (AOR: 0.11, 95% CI: 0.02, 0.74) lower risk of stunting than those who didn't get deworming. In the same way, children born at health facility had a 36% (AOR: 0.64, 95% CI: 0.58, 0.71) less risk of stunting compared to those who were born at home. Child birth size was also the other health related predictor of child stunting; as such babies who had small (less than average) birth size had a fivefold (AOR: 5.1, 95% CI: 1.64, 15.88) increased risk of stunting (Table 3).
Table 3.
Healthcare factors associated with stunting in Ethiopia.
| Predicting variables | N (%) | Outcome variable |
P-value | |
|---|---|---|---|---|
| Stunting | ||||
| COR (95% CI) | AOR (95% CI) | |||
| Prenatal doctor or health officer (HO) follow-ups | ||||
|
7007 (92.3) | 1 | ||
|
583 (7.7) | 0.65 (0.53, 0.79) ∗∗ | 0.39 (0.06, 2.35) | 0.302 |
| Prenatal nurse or midwife follow-ups | ||||
|
4281 (54.7) | 1 | ||
|
3308 (43.6) | 0.85 (0.76, 0.93) ∗∗ | 1.76 (0.43, 7.14) | 0.432 |
| Deworming during pregnancy | ||||
|
7158 (94.3) | 1 | ||
|
432 (5.7) | 0.86 (0.69, 1.06) | 0.11 (0.02, 0.74) ∗∗ | 0.023 |
| Place of delivery | ||||
|
7997 (72.6) | 1 | ||
|
3026 (27.4) | 0.64 (0.58, 0.71) ∗∗ | 0.64 (0.58, 0.71) ∗∗ | 0.009 |
| Delivery by caesarean section | ||||
|
10810 (98.1) | 1 | ||
|
213 (1.9) | 0.46 (0.32, 0.65) ∗∗ | 1.14 (0.10, 12.93) | 0.914 |
| Child size at birth | ||||
|
3485 (31.6) | 1 | ||
|
4580 (41.6) | 1.11 (1.01, 1.23) ∗∗ | 0.51 (0.16, 1.59) | 0.247 |
|
2958 (26.8) | 1.49 (1.34, 1.66) ∗∗ | 5.1 (1.64, 15.88) ∗∗ | 0.005 |
| Recent diarrhea | ||||
|
9190 (88.2) | 1 | ||
|
1227 (11.8) | 1.12 (0.99, 1.27) | 0.09 (0.01, 0.63) ∗∗ | 0.015 |
∗∗P < 0.05, COR: Crude odd ratio, AOR: Adjusted odd ratio, CI: Confidence interval.
4. Discussion
This study, aimed to determine household, dietary and healthcare factors associated with stunting in Ethiopia. Among household factors, mother's education was identified as an important predictor of stunting. As the mother is more educated, the odds of stunting reduced. Educated mothers are believed to provide better childcare, nutritional and health issues for their children. This is consisitent with previous studies from Bangladesh (Mistry et al., 2019), Democratic Republic of Congo (Kismul et al., 2018), Ethiopia (Berhe et al., 2019; Kahssay et al., 2020), Kenya (Abuya et al., 2012), and Rwanda (Binagwaho et al., 2020). Another significant factor attributed to stunting was wealth index. Children born from poor family were at higher risk of stunting. Our findings are in lined with previous studies (Apio et al., 2019; Beal et al., 2018; Binagwaho et al., 2020; Bogale et al., 2020; Kismul et al., 2018; Karlsson et al., 2020; Tusting et al., 2020).
In this study male children were more likely to be stunted. This is inline with the findings of previous studies (Beal et al., 2018; Binagwaho et al., 2020; Haile et al., 2016; Mistry et al., 2019; Wamani et al., 2007). Gender variation in stunting is still unclear, although some researchers have argued that this disparity is more prevalent among poor households. However, the biological factors, living conditions, and differences in maternal feeding patterns that likely cause growth differences among sex needs further investigation. Similarly, our study showed that twin children were more stunted than a single child. This result is in agreement with previous study by Yaya et al. (2020) who reported, multiple-birth children were 2.09 times more likely to be stunted compared with a singleton. This is due to the fact that, managing twins is more difficult than managing single child, there by it requires twofold the resources and efforts.
Our study also shows that house main floor material, types of cooking fuel, possession of refrigerator, and television were a significant impact on under-five stunting. This is in line with a previous study by Tusting et al. (2020) who reported that, improved housing made from cement/ceramic was strongly associated with reductions in stunting. This might be explained that improved housing is associated with lower chances of infectious and diarrhoeal diseases as a result, stunting is reduced.
Interestingly, our study also shows that households with refrigerator reduces stunting significantly. This result is inline with a previous study (Karlsson et al., 2020). This might be, refrigerators can reduce food spoilage and extend shelf life, there by improving food and nutrition security. Owning television also reduces the odds of stunting, which supports the importance of mass media for nutrition and health interventions. A previous study in Tanzania by Alexander et al. (2019) also reported, media access is associated with improved knowledge towards hygienic practices, which has a positive health outcome. Another study by LeFevre et al. (2019) reported that having mobile phone may affect child health positively by providing information through mhealth applications.
However, having refrigerator, television and improved housing are deepened on the household income. But, having good income is not a grantee to have refrigerator, television and built improved houses in rural areas in Ethiopia. Most people are resistant to change, preferred to lead traditional life.
Concerning the dietary factors, early initiation of breast feeding had lower odds of stunting. This is in agreement with a previous study by Muldiasman et al. (2018), who reported that early initiation of breastfeeding reduces the odds of stunting among young children. Early initiation to breastfeeding ensures that the baby gets colostrum that increases the child's immunity to infection. Further, early breastfeeding initiation is one of the entrances to successful breastfeeding at a later time and ensures children receive appropriate nutritional intake, that can reduce the risk of stunting (Black et al., 2008; WHO, 2014).
Generally, consumption of animal source foods (especially milk and milk products and flesh foods (organ meats) appeared to be associated with a decreased risk of stunting in our study. This finding is inline with previous studies by (Darapheak et al., 2013; Herber et al., 2020; Krasevec et al., 2017; Krebs et al., 2011). The possible explanation might be, milk contains calcium and insulin like growth factor-1, in addition to the macronutrients, that are of major relevance for children's development and growth. Similarly, meat contains better amino acid and iron profiles which is very important in child growth and development (Esfarjani et al., 2013). Similarly, consumption of baby formula is associated with a decreased risk of stunting. Baby formula is given to infants (up to the age of six months) if the mother does not produce enough breast milk, that is the breast milk is unable to satisfy the infants requirements. Baby formula is also given to infants if the breast milk is assumed to be low quality, the mother has low economic status (Bosch et al., 2009). However, this does not mean it is better than breast milk.
Our study also shows that consuming beta-carotene rich fruits and vegetables are significantly reduces the risks of stunting. This is inline with a previous study in Ethiopia by Mohammed et al. (2019) who reported that, consumption of beta-carotene rich fruits and vegetables were associated with lower chances of co-occurrence of anemia and stunting. This might be due to the role of vitamin A in promoting growth, thereby reducing stunting (Semba et al., 2010).
The healthcare factors, such as ANC follow-ups, especially with doctors/HO (bivariate analysis result) were associated with under-five stunting. A previous study in Ethiopia by (Kahssay et al., 2020) also reported that, children whose mother had no ANC follow-up were 2.81 times more stunted than those mothers that had ANC follow up. This is might be, access to ANC visit during pregnancy is important for the mother to be aware of healthy eating during pregnancy and the importance of breastfeeding as well as complementary feeding practices. However, in this study the multivariate analysis was not significant. This might be, the ANC would not be able to enhance mothers' knowledge and attitude about healthy eating during pregnancy and proper breastfeeding and complementary feeding practices after delivery. Lack of proper monitoring by health extension workers (HEWs) and the economical status of the mothers’ might also be the reason why ANC follow ups was not decreased the odds of stunting.
Deworming was a risk factor for under-five stunting in this study. However, a previous study by Thayer et al. (2017) reported that deworming did not show consistent benefits for pregnant women and growth in children. Another risk factor attributed to stunting was place of delivery. Our results show that babies born at health facilities had less risk of stunting than those born at home. This is in line with a previous study (Budhathoki et al., 2020; Mtoi and Nyaruhucha, 2019). This might be due to the fact that, delivery at health facilities is a good opportunity for the mothers as well as the child, for early initiation of breast feeding and to get access to vaccinations. Child birth size was also found to be associated with stunting. Our result is in agreement with previous studies (Berhe et al., 2019; Binagwaho et al., 2020; Budhathoki et al., 2020; McGovern, 2019; Mistry et al., 2019; Singh et al., 2017; Woldeamanuel and Tesfaye, 2019), who reported that low birth weight babies were at higher risk of stunting.
Overall, this study has several strengths. One of its strengths is that, nationally representative data collected by the CSA, MOH, and EPHI with a large sample size was used, that is adequate to analyze the risk factors for under-five stunting. However, the study has some limitations. The study was cross-sectional, it was unable to examine causation or seasonal variation of nutritional outcomes. Further, the information examined in this study depended on the mother's recall ability.
5. Conclusion
Our study identified a series of risk factors, such as no formal education in mother, wealth index, sex of child, child in twin, house main floor material, absence of refrigerator and television, consumption of (baby formula, flesh foods, beta-carotene rich fruits and vegetables), ANC follow-ups, deworming, place of delivery and size of child at birth were the factors associated with stunting. Therefore, the influence of these factors should be considered to tailor strategies for reducing under-five stunting in Ethiopia.
Declarations
Author contribution statement
Abebe Ayelign, Taddese Zerfu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abuya B.A., Ciera J., Kimani-Murage E. Effect of mother’s education on child’s nutritional status in the slums of Nairobi. BMC Pediatr. 2012;12(1):80. doi: 10.1186/1471-2431-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C.C., Shrestha S., Tounkara M.D., Cooper S., Hunt L., Hoj T.H.…Crookston B. Media access is associated with knowledge of optimal water, sanitation and hygiene practices in Tanzania. Int. J. Environ. Res. Publ. Health. 2019;16(11):1963. doi: 10.3390/ijerph16111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apio B.R.S., Mawa R., Lawoko S., Sharma K.N. Socio-economic inequality in stunting among children aged 6-59 Months in a Ugandan population based cross-sectional study. American J. Pediatri. 2019;5(3):125–132. [Google Scholar]
- Beal T., Tumilowicz A., Sutrisna A., Izwardy D., Neufeld L.M. A review of child stunting determinants in Indonesia. Matern. Child Nutr. 2018;14(4) doi: 10.1111/mcn.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe K., Seid O., Gebremariam Y., Berhe A., Etsay N. Risk factors of stunting (chronic undernutrition) of children aged 6 to 24 months in Mekelle City, Tigray Region, North Ethiopia: an unmatched case-control study. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0217736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binagwaho A., Rukundo A., Powers S., Donahoe K.B., Agbonyitor M., Ngabo F., Karema C., Scott K.W., Fawzi M., Central Statistical Agency.S. Trends in burden and risk factors associated with childhood stunting in Rwanda from 2000 to 2015: policy and program implications. BMC Publ Health. 2020;20(1):1–9. doi: 10.1186/s12889-020-8164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., De Onis M., Ezzati M.…Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Bogale B., Gutema B.T., Chisha Y. Prevalence of stunting and its associated factors among children of 6–59 Months in arba minch health and demographic surveillance site (HDSS), southern Ethiopia: a community-based cross-sectional study. J. Environ. Publ. Health. 2020;2020 doi: 10.1155/2020/9520973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhathoki S.S., Bhandari A., Gurung R., Gurung A., Ashish K.C. Stunting among under 5-year-olds in Nepal: trends and risk factors. Matern. Child Health J. 2020;24(1):39–47. doi: 10.1007/s10995-019-02817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch V., Golfetto I., Alonso H., Laurentin Z., Materan M., Garcia N. Fatty acids in mature breast milk from low socioeconomic levels of Venezuelan women: influence of temperature and time of storage. Arch. Latinoam. Nutr. 2009;59(1):61–65. [PubMed] [Google Scholar]
- Central Statistical Agency (CSA) CSA and ICF; Maryland, USA: 2016. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville. [Google Scholar]
- Darapheak C., Takano T., Kizuki M., Nakamura K., Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int. Arch. Med. 2013;6(1):29. doi: 10.1186/1755-7682-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfarjani F., Roustaee R., Mohammadi-Nasrabadi F., Esmaillzadeh A. Major dietary patterns in relation to stunting among children in Tehran, Iran. J. Health Popul. Nutr. 2013;31(2):202. doi: 10.3329/jhpn.v31i2.16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikadu T., Assegid S., Dube L. Factors associated with stunting among children of age 24 to 59 months in Meskan district, Gurage Zone, South Ethiopia: a case-control study. BMC Publ. Health. 2014;14(1):800. doi: 10.1186/1471-2458-14-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D., Azage M., Mola T., Rainey R. Exploring spatial variations and factors associated with childhood stunting in Ethiopia: spatial and multilevel analysis. BMC Pediatr. 2016;16(1):49. doi: 10.1186/s12887-016-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber C., Bogler L., Subramanian S.V., Vollmer S. Association between milk consumption and child growth for children aged 6–59 months. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-63647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahssay M., Woldu E., Gebre A., Reddy S. Determinants of stunting among children aged 6 to 59 months in pastoral community, Afar region, North East Ethiopia: unmatched case control study. BMC Nutr. 2020;6(1):1–8. doi: 10.1186/s40795-020-00332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Kim R., Joe W., Subramanian S.V. The relationship of household assets and amenities with child health outcomes: an exploratory cross-sectional study in India 2015–2016. SSM-Popul. Health. 2020;10:100513. doi: 10.1016/j.ssmph.2019.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs N.F., Mazariegos M., Tshefu A., Bose C., Sami N., Chomba E.…Hambidge K.M. Meat consumption is associated with less stunting among toddlers in four diverse low-income settings. Food Nutr. Bull. 2011;32(3):185–191. doi: 10.1177/156482651103200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kismul H., Acharya P., Mapatano M.A., Hatløy A. Determinants of childhood stunting in the democratic Republic of Congo: further analysis of demographic and health survey 2013–14. BMC Publ. Health. 2018;18(1):74. doi: 10.1186/s12889-017-4621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasevec J., An X., Kumapley R., Bégin F., Frongillo E.A. Diet quality and risk of stunting among infants and young children in low-and middle-income countries. Matern. Child Nutr. 2017;13 doi: 10.1111/mcn.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre A., Agarwal S., Chamberlain S., Scott K., Godfrey A., Chandra R.…Bhatnagar A. Are stage-based health information messages effective and good value for money in improving maternal newborn and child health outcomes in India? Protocol for an individually randomized controlled trial. Trials. 2019;20(1):272. doi: 10.1186/s13063-019-3369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern M.E. How much does birth weight matter for child health in developing countries? Estimates from siblings and twins. Health Econ. 2019;28(1):3–22. doi: 10.1002/hec.3823. [DOI] [PubMed] [Google Scholar]
- Mistry S.K., Hossain M.B., Khanam F., Akter F., Parvez M., Yunus F.M.…Rahman M. Individual-, maternal-and household-level factors associated with stunting among children aged 0–23 months in Bangladesh. Publ. Health Nutr. 2019;22(1):85–94. doi: 10.1017/S1368980018002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S.H., Larijani B., Esmaillzadeh A. Concurrent anemia and stunting in young children: prevalence, dietary and non-dietary associated factors. Nutr. J. 2019;18(1):10. doi: 10.1186/s12937-019-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldiasman M., Kusharisupeni K., Laksminingsih E., Besral B. Can early initiation to breastfeeding prevent stunting in 6–59 months old children? J. Health. Res. 2018 [Google Scholar]
- Mtoi E.H., Nyaruhucha C.N. Child care practices and nutritional status of under-five children in Tanzania: evidence from fishing communities in Pangani District. Int. J. Asian Soc. Sci. 2019;9(7):390–405. [Google Scholar]
- Semba R.D., de Pee S., Sun K., Campbell A.A., Bloem M.W., Raju V.K. Low intake of vitamin A–rich foods among children, aged 12–35 months, in India: association with malnutrition, anemia, and missed child survival interventions. Nutrition. 2010;26(10):958–962. doi: 10.1016/j.nut.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Singh A., Upadhyay A.K., Kumar K. Birth size, stunting and recovery from stunting in Andhra Pradesh, India: evidence from the Young Lives Study. Matern. Child Health J. 2017;21(3):492–508. doi: 10.1007/s10995-016-2132-8. [DOI] [PubMed] [Google Scholar]
- Thayer W.M., Clermont A., Walker N. Effects of deworming on child and maternal health: a literature review and meta-analysis. BMC Publ. Health. 2017;17(4):830. doi: 10.1186/s12889-017-4747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusting L.S., Gething P.W., Gibson H.S., Greenwood B., Knudsen J., Lindsay S.W., Bhatt S. Housing and child health in sub-Saharan Africa: a cross-sectional analysis. PLoS Med. 2020;17(3) doi: 10.1371/journal.pmed.1003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF/WHO/WB . 2019. Levels and Trends in Child Malnutrition. UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates. Key Findings of the 2019 Edition. [Google Scholar]
- Wamani H., Åstrøm A.N., Peterson S., Tumwine J.K., Tylleskär T. Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007;7(1):17. doi: 10.1186/1471-2431-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldeamanuel B.T., Tesfaye T.T. Risk factors associated with under-five stunting, wasting, and underweight based on Ethiopian demographic health survey datasets in tigray region, Ethiopia. J. Nutr. Metab. 2019;2019 doi: 10.1155/2019/6967170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) World Health Organization; 2014. Global Nutrition Targets 2025: Stunting Policy Brief. (No. WHO/NMH/NHD/14.3) [Google Scholar]
- World Health Organization (WHO) Methods and development department; Geneva, Switzerland: 2006. WHO Child Growth Standards: Length/height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass index-forage. [Google Scholar]
- Yaya S., Oladimeji O., Odusina E.K., Bishwajit G. Household structure, maternal characteristics and children’s stunting in sub-Saharan Africa: evidence from 35 countries. Int. Health. 2020 doi: 10.1093/inthealth/ihz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.