Abstract
Many anthropogenic chemicals in general, and specifically aquatic herbicide formulations have the potential to modulate the thyroid pathways of the endocrine system of aquatic organisms, because they are normally applied directly into the aquatic system, to manage aquatic weeds. These thyroidal effects have been widely linked with disruption in developmental and reproductive processes. In fact, the exposure impacts of many of these substances on metamorphic organisms could produce a precocious metamorphosis. Using Xenopus Metamorphosis Assay (XEMA) protocol, this study assessed the thyroidal effects of environmentally relevant concentrations of Diquat dibromide at 0.05, 0.11, and 0.14 mg/L on Xenopus laevis metamorphosis. The formulation significantly reduced both the fore and hind limb lengths, and disrupted the developmental stage at concentrations of 0.11 and 0.14 mg/L, with a median at NF-stage 57, while median of NF-stage 60 was recorded in the control. Histopathologically, although there was no significant difference in thyroid gland area, the thyroid colloidal area was significantly reduced at 0.14 mg/L, while the mean height of the thyroid follicle increased at 0.05 mg/L The result indicates an extra-thyroidal pathway, due to the dissociation between stage developmental effects and thyroid histopathology. The role of stress pathway occasioned by oxidative mode of action, involving lipid peroxidation and cell damage observed in this study need further investigation, in order to further characterize the physiological and ecological effects on wildlife.
Keywords: Diquat, Amphibian, Herbicide, Oxidative-action, Reactive-oxygen-species
Diquat, Amphibian, herbicide, oxidative-action, reactive-oxygen-species
1. Introduction
1.1. Chemical factors in amphibian declines
Numerous anthropogenic substances originating from industrial, pharmaceutical, and agricultural activities (herbicides, fungicides, and insecticides etc) are found in the environment. These substances majorly end up in the aquatic ecosystem, which becomes the warehouse of their residues (Ortiz-Delgado et al., 2019). Many of these substances have the potential to modulate the thyroid functions through different pathways including hormone synthesis and release, hormone transportation through the blood, hormone metabolism as well as the final clearance (Diamanti-Kandarakis et al., 2009). Hence, the exposure impact of these anthropogenic substances often results in growth and physiological modulation, including disruption in metamorphosis of metamorphic organisms (Nugegoda and Kibria, 2017) and particularly amphibians (Kloas et al., 1999; Hayes et al., 2010). In these metamorphic organisms, the metamorphosis process is a developmental phase where numerous important physiological changes occurs, which are sensitive to sub-lethal effects of chemicals (Denver, 1997). The processes obligatorily depends on thyroid hormone (Organisation for Economic Co-operation and Development (OECD), 2007a, b; Awkerman and Raimondo, 2018), and this is best exemplified by the fact that surgical removal of the thyroid gland or chemical blockage of thyroid hormone synthesis often leads to the complete blockage of metamorphosis (Optiz et al., 2005). The pre-metamorphic amphibian larvae for example, are highly sensitive to exogenous thyroid hormone, producing precocious metamorphosis when exposed (Helbing et al., 2010).
This means that thyroid hormone receptors mediate both early and late developmental programs of metamorphosis, and that growing organisms, particularly during early development, are at risk from these substances (Kloas et al., 1999; van Wyk, 2013; Denver, 2013). Already, there is growing concern about the safety of larval stages of aquatic organisms (Nugegoda and Kibria, 2017) and amphibians in particular (Hayes et al., 2010), given the current global amphibians’ population decline (Wagner et al., 2013; Muñoz et al., 2015). This suggests that a better understanding of thyroid modulating activities of substances acting as agonists or antagonists is of great importance. In this regards, there is a dearth of information on the health and ecological impacts of several globally used herbicides (particularly the aquatic herbicides) on amphibian metamorphosis. Whereas the exposure impacts of a few herbicides and their formulations (e.g., glyphosate) are relatively well studied, the exposure impacts of most herbicides (e.g. Diquat dibromide, Paraquat, Glufosinate ammonium, Imazapyr etc.) are much less well known. This ecological gap makes the study of thyroidal activities of these herbicides very important, as part of their endocrine disruption indicators. These herbicides include glyphosate, diquat dibromide, Paraquat, glufosinate ammonium etc.
Diquat dibromide (9, 10-dihydro-8a, 10a-diazonia phenanthrene ion) is a post emergent herbicide. It is a non-selective contact herbicide and crop desiccant that is also used for aquatic weeds control (Emmett, 2002). Diquat has been registered with United States Environmental Protection Agency (USEPA) for terrestrial and aquatic ecosystem (Chung et al., 2008). Diquat is manufactured as bromide salt (C12H12Br2N2) (dibromide salt), and it can also exist as cation (C12H12N2) (Howard 1991; United State Environmental Protection Agency (USEPA), 1995b, United States Environmental Protection Agency, 1995a).The chemical name for the dibromide salt is 6,7-dihydrodipyrido(1,2-a:2,1-c)pyrazinedi-ium dibromide (United State Environmental Protection Agency (USEPA), 1995b, United States Environmental Protection Agency, 1995a). This herbicide formulation is widely used in the United States, North America, Europe, Australia, Japan and Africa (WHO, 2004). The expected environmental concentration of diquat dibromide is 0.073 mg/L (Peterson et al., 1994). This study was carried out in Canada, where they used an Expected Environmental Concentration (EEC) in evaluating the hazard of pesticides to non-target aquatic organisms. Test organisms were selected based on ecological relevance and present use in the test protocols. They calculated the EEC by assuming an overspray of a 15 cm deep fresh water body at the label application rate. The EEC of pesticides is then related to the EC50 for a given aquatic test organism. The phytotoxicity of the EEC of 23 different pesticides to ten algae (24 h inhibition of 14C uptake) and one vascular plant (7-day growth inhibition) was determined in an effort to examine the question of interspecific sensitivity and its relation to the development of pesticide registration guidelines. Diquat is not readily degraded by microbes, and exhibit low susceptibility to photodegradation (Howard, 1991; Paul et al., 1994; Chung et al., 2008; Siemering et al., 2008). It has high affinity for organic matter and water sediments, which normally decreases the concentration of free diquat in the water column (Ghalwa et al., 2012). In aquatic environment, diquat sorbs to suspended solid sediment and also to aquatic vegetation (Simsiman and Chesters, 1976; HSDB, 2003). Unfortunately, this adsorption to the organic matters and sediment is another important route of entry of toxicant in some amphibian species because of their feeding habit, whereby they nibble on organic matters in the water sediment. This study therefore assessed the exposure impacts of Diquat dibromide herbicide formulation on the thyroid system of developing Xenopus laevis.
The Xenopus metamorphosis assay (XEMA) is an animal-based model designed to identify substances that may interfere with the normal functioning of the hypothalamic-pituitary thyroid (HPT) axis using X. laevis (Organisation for Economic Co-operation and Development (OECD), 2007b, Organisation for Economic Cooperation and Development (OECD), 2007a; Grim et al., 2009). The primary endpoints, including hind-limb length, developmental stage and thyroid gland histology, while snout vent length, wet weight, and mortality also help to distinguish between thyroid specific effect and generalized toxicity (Grim et al., 2009). The assay has several advantages over other animal-based in-vivo assays. It displays a temporal coupling of TH exposure to subsequent observable, and measurable morphological outcome to a degree unmatched by any other animal processes (Helbing et al., 2010). According to Helbing et al. (2010), the fact that this assay occurs entirely in aquatic medium, the ecological niche of the anuran larvae, also makes the protocol unique.
2. Materials and methods
2.1. The herbicide formulation
Midstream (Syngenta Ltd., South Africa) containing 373 g/L Diquat dibromide.
2.2. Xenopus laevis breeding and tadpole culture
Adult males and females X. laevis obtained from our in-house breeding stock were maintained separately in 15 L glass tanks containing buffered (2.5 g sea salt/10 L) reverse osmosis water (Kloas et al. 1999). The frogs were fed three times per week with fish pellets (Aqua-Nutro, South Africa). After each feeding, the tanks were cleaned and refilled with clean water. Gutter down-piping sections were placed in the holding tanks to create hide-outs for the frogs. The Breeding procedure was performed following protocol. Four days prior to the breeding, males and females were first primed with 100 IU human chorionic gonadotropin (hCG) (Merck Ltd Germany), injected into their dorsal lymph sac. This is followed by a second treatment just prior to the mating of 100 IU and 300 IU hCG to the males and females respectively. Single male and female were paired together in a 15L breeding tank, lined with plastic netting (to separate the eggs from the adults during oviposition), and placed in a well-ventilated dark place. The eggs were collected and spread out into several aerated 15 L tanks. Feeding of the tadpoles commenced at NF-stage 48, and they were fed with Sera Micron (Sera Heinsberg, Germany) twice daily until NF-stage 51. The tadpoles started on food ration of 30 mg/animal/day, which was later increased to 50 mg/animal/day to compensate for growth increase (Organisation for Economic Co-operation and Development (OECD), 2007b, Organisation for Economic Cooperation and Development (OECD), 2007a). Staging of the tadpoles was done using a normal developmental atlas described by Nieuwkoop and Faber (1994). Xenopus laevis used for this study were collected, cultured, cared for, and treated under strict compliance with all ethical practises and law and approved by the Animal Research Ethical Committee of the Stellenbosch University (Approval no- SU-ACUM 12-00015).
2.3. Test procedure
2.3.1. Exposure set-up
At the NF-stage 51, twenty (n = 20) premetamorphic tadpoles were carefully picked from the holding tanks and transferred to new 15 L tanks. Individual exposure tanks were replicated twice at each exposure concentrations and the control. Even though we did several pre exposure tests with four (4) replicates, our results didn't show much effects of these replicates. Secondly, because we worked on six herbicide formulations, requiring very large numbers of tadpoles, therefore, we did not consider replicate tanks as the experimental unit but individual tadpoles. We tested for a "tank effect". The exposures were done under controlled physical conditions following XEMA protocol: water temperature at 23 ± 1 °C, pH of 7.5–8.5, dissolved oxygen of >6.5 mg/L, 12 h of light and dark photoperiod (L12D12) regime (OECD, 2008).
2.3.2. Exposure concentrations
The selected exposure concentrations were centred on 96-h LC50 at NF-stage 48 of the X. laevis tadpoles (Babalola & van Wyk, 2018) (Table 1). The Mortality incidence was monitored daily while the exposure medium in the tanks was completely replaced at interval of three days. The three days was chosen as diquat has been noted to have 8 days half-life photo-degradation in distilled water with 210–260 nm light (Mackay et al., 1997), and is known to be stable to hydrolysis at pH 5-9 (United State Environmental Protection Agency (USEPA), 1995b, United States Environmental Protection Agency, 1995a), with no hydrolysis measured after 30 days at pH 5 or 7 (Ritter et al., 2000). Only mortality incidence less than 10% in the control group was accepted for the experiment (OECD, 2008).
Table 1.
The selected exposure concentrations (centred on the 15, 30 and 45% of 96-hour LC50 for NF-stage 48 of X. laevis tadpoles) of the Midstream formulation.
| Formulation | Exposure concentrations. |
|---|---|
| Midstream | 0, 0.05, 0.11 and 0.14 |
2.3.3. Nominal concentration test
To confirm the exposure concentrations in the study, 100 ml samples from the exposure water was taken from each of the exposure tanks, one hour after the introduction of the herbicide formulation. For the replicates, 100 ml from each of the tanks was taken and then mixed together, from where a single 100 ml was pooled to represent that concentration. The collected water samples from the exposure were immediately stored in icebox pack at a temperature range of -5 to -10 °C before being sent to Envirotech Laboratory, Lagos, Nigeria, for the determination of herbicide concentration, which was conducted within 10 h of the collection. The analysis of the water sample was done using the gas chromatography mass spectrometric (GC-MS) method with a detection limit of 0.05 μg∖L (De Almeida and Yonamine, 2007). The result of the detected concentrations showed low variations relative to the predicted nominal concentrations (Table 2).
Table 2.
The gas chromatography analytical result for Midstream (Diquat dibromide) with very low variations compared to the predicted nominal concentrations). The limit of detection was 0.05 μg/L.
| Midstream mg/L | |
|---|---|
| Nominal | Detected |
| 0 | 0 |
| 0.05 | 0.048 |
| 0.11 | 0.10 |
| 0.14 | 0.13 |
2.3.4. Autopsy procedure and morphometric measurements
At the 21-day termination of the exposure, the tadpoles were collected and euthanized in 0.1 % benzocaine. They were blotted dry and individually weighed (to nearest 0.01 g), and measured snout–vent length (to nearest 0.1 mm). They were then fixed in Davidson's solution for 72 h prior to being transferred to, and preserved in 4 % neutral buffered formalin (Shi et al., 2012). The fore and hind-limbs lengths were measured using Leica EZ4D stereo microscope (Leica Microscope Ltd, Germany). Limb lengths were determined on digital photographs of the tadpoles using the metric trace ruler software that has the capacity to measure both straight and curved lines using traced lines. The heads of tadpoles enclosing the thyroid glands were carefully severed transversely using a sharp blade, just posterior to the eye, and subjected to routine paraffin wax imbedding histological procedures (Bancroft and Stevens, 1977). Sectioning, mounting and staining then followed.
2.3.5. Developmental stage (NF-stage) determination
To measure the impacts on histology, histopathological variation in the thyroid gland was used. For this, five tadpoles per tank (at same stage) were randomly selected per concentration and compared to the matched five median developmental stage of the control group using the thyroid gland histopathological features. All tadpoles were first staged as described by Nieuwkoop and Faber (1994). This was then followed by matching five median developmental stage of the control with that of the exposure tanks at each of the concentrations, as recommended by the OECD, 2008 protocol.
2.3.6. Histological procedures
The lower jaw samples containing the thyroid glands were removed from formalin, washed in running tap water, and processed for routine paraffin wax-based histology (Bancroft and Stevens, 1977). The jaws were dehydrated in series of graded alcohol and embedded (in frontal plane to facilitate the caudal surface of the tissue first) in histowax (Histolab, Sweden). The tissues were sectioned at 7-8 μm using Reichert-Jung microtome (Cambridge Instrument, Germany), then the sections were mounted on clean albumin coated slides, and oven-dried (40 °C) overnight. The sections were then dewaxed, stained with haematoxylin and eosin (H & E) (Bancroft and Stevens, 1977), cleared in xylene before mounting with glass cover slips using a resin-based medium (DPX, Sigma Ltd).
2.3.7. Histological measurement of the thyroid gland
Using the right-side thyroid, the thyroid image, taken with Leica DMLB microscope equipped with digital camera (Leica Microscope Ltd, Germany) was used to measure the epithelia cell heights by taking measurement from the base to the apical edge of the cell. For each tadpole specimen, 15 epithelial cell height measurements were taken for four thyroid gland follicles, resulting in 60 epithelial cell height measurements per individual. A mean value was then calculated per individual and used with other individual group members to compute a group mean for follicle cell height. Follicular cross-sectional area (follicle lumen area), as well as thyroid gland cross-sectional area were also measured and calculated (using image analysis software (Sigmascan, Systat Software Inc.) using the right thyroid gland side for each individual specimen, 10 thyroid follicles in each section were measured, making 10 thyroid follicles per tadpole. The data was then combined for all the tadpoles in each exposure group. The average sum total of the thyroid gland cross-sectional areas gives the gland area for each exposure group.
2.3.8. Data analysis
The non-parametric Kruskal-Wallis test was used to assess variation in median NF-stage among exposure groups because stage is a categorical variable. The Dunn's multiple comparison test was subsequently used to identify significant pairwise differences in stages among treatments (Shi et al., 2012). Front limb length (FLL) and hind limb length (HLL) were normalized to snout vent length, to correct for the influence of developmental stage on limb size. Normality and homogeneity of variance in FLL, HLL, wet body mass (WBM), whole body length (WBL) and snout to vent length (SVL) data were assessed using Shapiro-Wilk's and Levene's tests respectively. One-Way ANOVA and Kruskal-Wallis ANOVA for parametric and non-parametric data respectively were used to analyse variation in morphometric endpoints (WBM, WBL and SVL) among exposure groups. The effect of treatment (i.e. specific pesticide concentration), developmental stage and the treatment-stage interaction on FLL and HLL was tested using mixed model ANOVA, with individual tadpole as a random factor. Pairwise differences in WBM, WBL, SVL and normalized FLL and HLL between treatments and the control groups were assessed using the Tukey HSD test with Spjotfoll/Stoline correction for parametric data or the Dunn's test for non-parametric data. Significant differences between treatments were taken at P < 0.05. All statistical analyses were performed using Statistica V12 (Statsoft Inc., USA).
3. Results
3.1. Mortality
No incidence of mortality (0 %) was observed in any of the exposure and the control tanks throughout the 21-day exposure period.
3.2. Variation in developmental stages
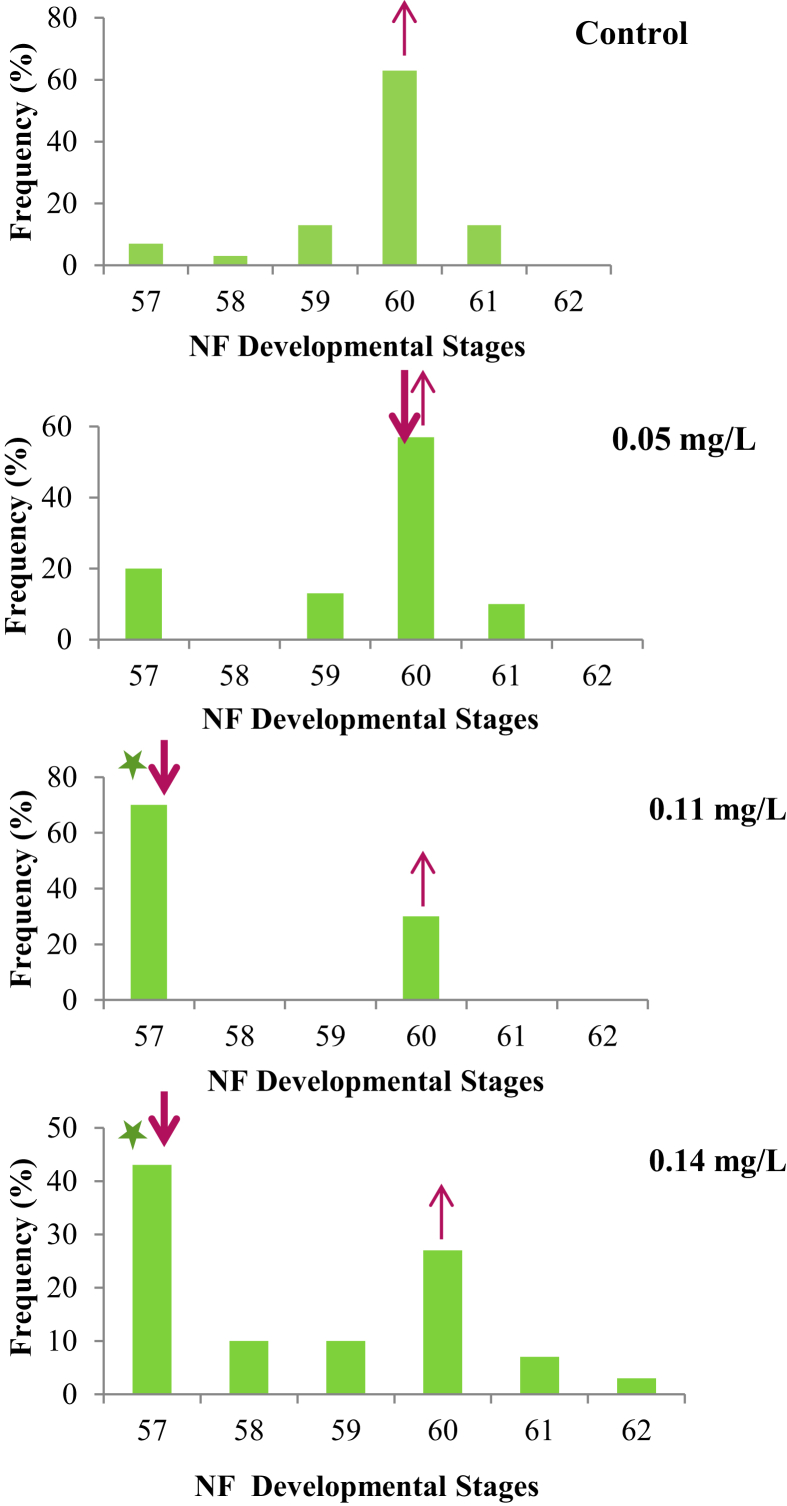
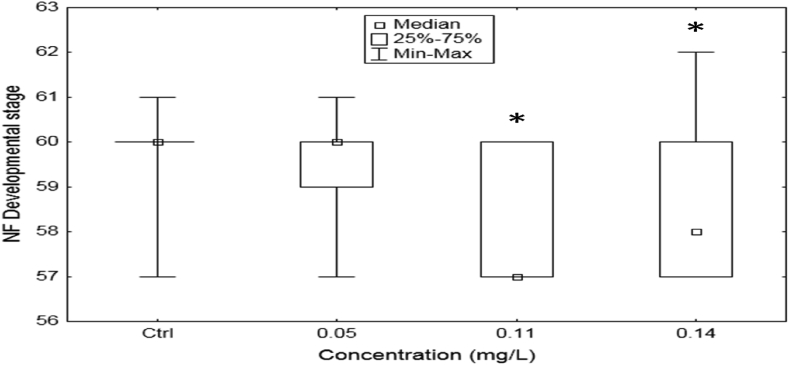
The exposure altered the frequency distribution pattern of the developmental stages. The median of the frequency distribution shifted to NF-stage 57 at the two highest exposure concentrations of 0.11 and 0.14 mg/L compared to NF-stage 60 at the control (Figure 1). Midstream treatment was a significant source of variation in developmental stage (H3,120 = 25.15, P < 0.001, Kruskal-Wallis ANOVA, followed by a significant variation (delay) in NF-stage development between the control and concentrations of 0.11 and 0.14 mg/L was confirmed (P = 0.0001, z = 4.25 and P = 0.03, z = 2.82 respectively, Dunn's test) (Figure 2).
Figure 1.
The frequency distributions (n = 20) of developmental stages attained by X. laevis tadpoles after the 21-day exposure to graduated concentrations of Midstream formulation relative to the control (Figure 1). The upward arrow showed the median NF-stage at the control relative to downward arrow that showed the median at the exposure concentrations. The asterisk showed significant difference (P < 0.05) relative to the control.
Figure 2.
Tadpoles stage differentiation after 21-day treatment with Midstream formulation relative to control (Ctrl). Asterisks indicate significant difference (P < 0.05) from control.
3.3. Morphometric analyses
The Midstream formulation resulted in slight reduction in mean whole body length (WBL) alongside slight increase in mean snout-vent length (SVL) (Table 2). The variation was, however not significantly different to that of the control group (WBL: 0.05 mg/L, P = 1, z = 0.23; 0.11 mg/L, P = 0.45, z = 1.78; 0.14 mg/L, P = 0.13, z = 2.29; SVL: 0.05 mg/L, P = 1, z = 1.18; 0.11 mg/L, P = 1, z = 0.34; 0.14 mg/L, P = 1, z = 0.42, Dunn's test). Similarly, variation in mean whole body mass (WBM) of the treated tadpoles (Table 3) also showed no significant difference when compared to the control (0.05 mg/L, P = 0.45, z = 1.78; 0.11 mg/L, P = 1, z = 1.13; 0.14 mg/L, P = 0.13, z = 2.29, Dunn's test). However, Midstream treatment was a significant source of variance in both normalised mean hind limb length (HLL) (F3,74 = 35.72, P < 0.0001, Mixed Model ANOVA) and front limb length (FLL) (F3,74 = 20.49, P < 0.0001, Mixed Model ANOVA) (Table 2). In particular, HLL and FLL was found to be significantly reduced in the 0.11 mg/L and 0.14 mg/L treatments relative to the control (HLL: P = 0.0001 and P = 0.0003 respectively, Tukey HSD test; FLL: P = 0.0001, z = 4.87 and P = 0.0005, z = 3.96 respectively, Dunn's test).
Table 3.
Morphometric of tadpoles exposed to Midstream formulation (±SD) including whole body mass (WBM), whole body length (WBL), hind limb length (HLL), front limb length (FLL) and snout vent length (SVL) after 21-day exposure. Asterisks indicate significant different (P < 0.05).
| Herbicide | Conc. (mg/L) | WBM (g) | WBL (mm) | HLL (mm) | FLL (mm) | SVL (mm) |
|---|---|---|---|---|---|---|
| Midstream | 0 | 0.84 ± 0.11 | 61.23 ± 2.81 | 6.67 ± 1.22 | 2.54 ± 0.34 | 21.12 ± 1.39 |
| 0.05 | 0.89 ± 0.11 | 61.23 ± 2.98 | 6.54 ± 1.28 | 2.39 ± 0.40 | 21.42 ± 1.12 | |
| 0.11 | 0.78 ± 0.15 | 59.57 ± 3.88 | 4.89 ± 1.48∗ | 2.0 ± 0.40∗ | 20.95 ± 1.27 | |
| 0.14 | 0.74 ± 0.13 | 58.83 ± 4.58 | 5.12 ± 1.59∗ | 2.11 ± 0.48∗ | 21.02 ± 1.64 |
3.4. Histopathological endpoints
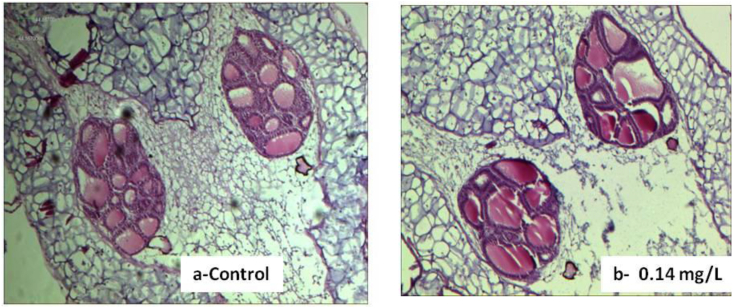
The thyroid follicular lumen area in the treated tadpoles showed evidence of atrophy, as it significantly reduced at the highest exposure concentration of 0.14 mg/L compared to the control (P < 0.0001, z = 5.05, Dunn's test) (Table 4). The mean height of the thyroid follicle epithelium also showed hypertrophy with a significant increase at only the lowest exposure concentration of 0.05 mg/L, compared to the control (P < 0.0001, z = 5.57, Dunn's test). The mean of thyroid gland area (Figure 3), on the other hand, showed no significant difference at all the exposure concentrations compared to the control (P = 0.58, z = 1.66; P = 1, z = 0.48; P = 0.193, 2.14 respectively, Dunn's test).
Table 4.
Histo-morphometric data following a 21-day XEMA exposure to graded concentrations of Midstream formulation. Values represent the mean ± SD. Asterisks represent significant difference relative to the control.
| Treatment Mg/L |
Mean Follicle Epithelium ∖(μm ± SD) |
Mean Follicular lumen Area (μm ± SD) |
Mean Gland Area (μm ± SD) |
|---|---|---|---|
| 0 | 7.82 (1.65) | 3410.64 (2299.2) | 53903.80 (27630.7) |
| 0.05 | 8.92 (1.68)∗ | 3146.04 (1793.6) | 69585.69 (12328.7) |
| 0.11 | 8.27 (1.22) | 2413.78 (1195.1) | 59964.97 (15335.3) |
| 0.14 | 8.28 (1.33) | 1832.86 (999.8)∗ | 39340.26 (10961.2) |
Figure 3.
The gland size area of tadpoles exposed to Midstream formulation at 0.14 mg/L compared to the control (Mag X 100).
4. Discussion
The global widespread malformation and decline in amphibian population is already established, but what remain unknown are some specific causes driving this decline, sometimes in relation to some species. The increasing use of pesticides (including aquatic herbicides) has been variously suggested as one of the possible factors (Grain and Guillette, 1997; Stuart et al., 2004; Khan and Law, 2005; Egea-Serrano et al., 2012). There is increasing concern about the health impacts of these pesticides on human and wildlife, particularly on thyroid system (Miyata and Ose, 2012). The significant function of these thyroid hormones makes it important to identify toxicants that could disrupt their functions. This study therefore assessed the thyroidal impacts of Diquat dibromide herbicide (Midstream formulation), using X. laevis as sentinel organism.
The control tadpoles from this study were healthy and went through the processes and stages of metamorphosis at the expected rate. At test termination, the control tadpoles were between NF-stage 57-61 (NF 60 median stages), which is consistent with OECD pre-validation guidelines, according to which control tadpoles should minimally be at median developmental stage of NF 57 at the test termination (Organisation for Economic Co-operation and Development (OECD), 2007b, Organisation for Economic Cooperation and Development (OECD), 2007a).
The exposure concentrations in this study were environmentally relevant and showed no toxicity on the survival of the X. laevis, as none of the exposed tadpoles died in the course of the exposure. Compared with the OECD's phase 1 pre-validation studies at stage 51 tadpoles, which has 0.944 g and 19.5 mm as the mean weight and mean snout-vent length respectively (Organisation for Economic Co-operation and Development (OECD), 2007b, Organisation for Economic Cooperation and Development (OECD), 2007a; Coady et al., 2010), the overall mean wet weight and snout-vent length (±SD) of the control tadpoles in this study after 21 days were 0.84 ± 0.11 g and 21.12 ± 1.39 mm for this formulation. These values compared favourably with the OECD pre-validation tadpoles, as the tadpoles in this study were even longer. As noted by Coady et al. (2010), the difference in length could be due to the current Sera micron food administered compared to combination of Tetrafin, spirulina algae, Silver Cup Trout Starter and live brine shrimp that was administered in the OECD test studies.
Exposure to the Diquat formulation resulted in a significant reduction in mean developmental stage at concentrations of 0.11 and 0.14 mg/L, which suggests potential inhibitory tendency in this formulation. However, histological observation showed that the gland area was not significantly different when compared to the control tadpoles, although there was occurrence of atrophy in follicular lumen area (at 0.14 mg/L) alongside a slight hypertrophy in follicular epithelium (significant at 0.05 mg/L). The dissociation of developmental effects and thyroid histopathology could imply a target tissue effect (extra-thyroidal pathways) (OECD 2008; Shi et al., 2012) or the inhibition of T4 to T3 transformation (Miyata and Ose, 2012), rather than HPT regulatory effects. Saka et al. (2013) reported a similar result for Simetryn herbicide, and concluded that the delay in development may be due to non-thyroidal effects. As also pointed out by Fort et al. (2011), when chemical affect the whole body length, snout vent length, and whole body mass, but not hind limb length and thyroid gland, (it indicates non-endocrine effects, but extra-thyroidal pathways.
Several studies have linked diquat to the formation of toxic radicals in the cell, which leads to oxidative stress (Sewalk et al., 2001; Hook et al., 2006; Higuchi et al., 2011; Bouetard et al., 2013), and severe stress condition (Yadav et al., 2013). The radicals from the diquat and the hydroxyl, which form from the oxygen radicals, absorbs electrons from cell membrane lipids, leading to lipid peroxidation, cell damage and cell death (Jones and Vale, 2000; Chung et al., 2008). According to Lushchak (2014), oxidative stress is usually the product of imbalance between reactive oxygen species (ROS) generation and the elimination, leading to certain consequences for cell physiology. Paraquat, a similarly quaternary herbicide, has been noted to exert its toxicity by production of the superoxides anion, which leads to the formation of more toxic reactive oxygen species (ROS), such as hydrogen peroxide and hydroxyl radicals.
The observed growth inhibition of the tadpoles in this study could be a short term physiological response to the toxicity of the herbicide formulation. The stress response physiology can also cause negative impacts on growth and reproduction in the chronically stressed organisms, as the body respond to protecting the organism (Cribb, 2018). When organisms are under mild or intermediate oxidative stress, their system usually block the general programs of their life cycle such as immune system, reproduction or extensive biosynthesis (which could cause growth inhibition) to develop responses towards preventing or neutralizing the negative effects of reactive oxygen species (ROS) (Lushchak, 2014). It could also be a long term response due to severity of the toxic exposure.
For this test formulation to have severely impacted the developmental stages of the treated tadpoles, the observed toxicity may be directed against the antioxidant defence systems, that operate to provide optimum reactive oxygen species homeostasis, or by increasing the production of ROS, leading to severe or high intensity oxidative stress in the tadpoles (Hurd and Murphy, 2009; Gomez-Mestre et al., 2013; Lushchak, 2014).
Another possible pathway is that the disruption of ROS homeostasis could suppress their roles as secondary messengers in cell signalling and developmental functions, leading to growth inhibition as witness in this study (Mittler, 2002; Hurd and Murphy, 2009; Gomez-Mestre et al., 2013). Although the function of normalized reactive oxygen species as secondary messenger in the cell signalling and developmental function is not yet full characterized, studies have shown their essential contributions in diverse physiological processes and many normal functions of living organisms (Gomez-Mestre et al., 2013; Lushchak, 2014).
In this redox homeostasis disruption, several pathways are possible. The introduction of certain oxidant(s) that could be beyond the capacity of the tadpoles antioxidants; the increasing production of reactive oxygen species (ROS) beyond the normal; depletion of reserves of low molecular mass antioxidants (ascorbic acids, tocophenol, carotenoids, polyphenols etc); decreased production of antioxidant enzymes and low molecular mass antioxidants as well as certain combination of two or more of all these pathways could lead to serious impacts on cellular homeostasis and cause severe oxidative damage in both short and long term (Costantini, 2008; Metcalfe and Alonso-Alvarez, 2010; Lushchak, 2014).
As noted by Cribb (2018), while the short term effect of the increased oxidative stress may include general metabolic processes. The long term effects may result in numerous serious physiological effects including the immune system, the reproductive system and even reduced longevity of the organisms in general that may not be reversible for the tadpoles (Hurd and Murphy, 2009; Gomez-Mestre et al., 2013; Lushchak, 2014). In all, the inhibition of developmental stages and other thyroid controlled endpoint such as hind limb length by the Diquat formulation at concentration below 0.733 mg/L which is the expected environmental concentration of this formulation (Peterson et al., 1994) is a serious ecological highpoint that deserves further attention.
5. Conclusion
An assessment of the chronic exposure impact of Diquat dibromide formulation (Midstream) based on both morphometric and histopathological endpoints, following the XEMA protocol revealed that this formulation disrupted the developmental stage of the treated tadpoles, and reduced the mean hind limb length, but without significant changes in thyroid gland histology. This disconnection between the morphometric endpoints and the thyroid gland histology showed that the exposure impacts of this Diquat formulation is through extra-thyroidal pathway and not thyroidal effects. Therefore, further study on this formulation will be necessary to characterise how it affect some critical thyroid dependent endpoints like hind limb length and mean colloidal area in thyroid gland, even without the thyroid gland effects. The roles and effects of oxidative stress due to reactive oxygen species from the exposure impacts of the Diquat formulation should also be further investigated. The result of this study also showed that many salient properties of the test formulation that could cause critical ecological impacts on aquatic organisms, particularly through the stress pathway, still exist. Therefore, exposure impacts of other similar aquatic herbicides need further attention, particularly on amphibian populations.
Declarations
Author contribution statement
Babalola, O. Oluwaseun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Van Wyk, Hannes Johannes: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Water Research Commission (Grant No K5/ 1952), South Africa. The grant was awarded to Prof Hannes van Wyk.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank the Working for Water Department, Ministry of Water Affairs, South Africa, for the supply of the herbicide formulation for this study.
References
- Bancroft J.D., Stevens A. Churchill Livingstone; Edinburg: 1977. Theory & Practice of Histological Techniques. [Google Scholar]
- Babalola O.O., van Wyk J.H. Comparative early life stage toxicity of African clawed frog, X. laevis following exposure to selected herbicide formulations applied to eradicate alien plants in S/Africa. Arch. Environ. Contam. Toxicol. 2018 doi: 10.1007/s00244-017-0463-0. [DOI] [PubMed] [Google Scholar]
- Bouetard A., Besnard A.L., Vassaux D., Lagadic L., Coutellec M.A. Impact of the redox-cycling herbicide diquat on transcript expression and antioxidant enzymatic activities of the freshwater snail Lymnaea stagnalis. Aquat. Toxicol. 2013;126:256–265. doi: 10.1016/j.aquatox.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Chung K.W., Chandler A.R., Key P.B. Toxicity of carbaryl, diquat dibromide, and fluoranthene, individually and in mixture, to larval grass shrimp, Palaemonetes pugio. J. Environ. Sci. Health Part B. 2008;43:293–299. doi: 10.1080/03601230801941600. [DOI] [PubMed] [Google Scholar]
- Coady k., Marino T., Thomas J., Currie R., Hancock G., Crofoot J., Mcnalley L., Mcfadden L., Geter D., Klecka G. Evaluation of amphibian metamorphosis assay: exposure to the goitrogen methimazole and the endogenous thyroid hormone-thyroxine. Environ. Toxicol. Chem. 2010;29(4):869–880. doi: 10.1002/etc.74. [DOI] [PubMed] [Google Scholar]
- Costantini D. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 2008;11:1238–1251. doi: 10.1111/j.1461-0248.2008.01246.x. PubMed: 18803642. [DOI] [PubMed] [Google Scholar]
- Cribb E. University of Calgary; Calgary, AB: 2018. “Effects of Diquat Dibromide Herbicide Exposure on Biomarkers of Stress and Chemical Exposure in Two Non-target Fish Species” (Unpublished Master's Thesis)http://hdl.handle.net/1880/109402 master thesis [Google Scholar]
- Denver R.J. Proximate mechanisms of phenotypic plasticity in amphibian metamorphosis. Am. Zool. 1997;37:172–184. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- De Almeida R.M., Yonamine M. Gas chromatographic-mass spectrometric method for the determination of the herbicide Paraquat and Diquat in Plasma and Urine samples. J. Chromatogr., B. 2007;855:260–264. doi: 10.1016/j.jchromb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Denver R.J. Neuroendocrinology of amphibian metamorphosis. Curr. Top. Dev. Biol. 2013;103:195–227. doi: 10.1016/B978-0-12-385979-2.00007-1. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine disrupting chemicals. An endocrine society scientific statement. Endocr. Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Serrano A., Relyea R.A., Tejedo M., Torralva M. Understanding of the impact of chemicals on amphibians: a meta-analytic review. Ecol. Evol. 2012;2:1382–1397. doi: 10.1002/ece3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett K. 2002. Final Risk Assessment for Diquat Bromide. The Water Quality Program of the Washington State Department of Ecology. 2-10-0046.www.wsde.org/document 12-3-2019. [Google Scholar]
- Ghalwa N.A., Abu-shawish H.M., Hamada M., Hartani K., Basaheer A.A.H. Studies on degradation of diquat pesticide in aqueous solutions using electrochemical method. Am. J. Anal. Chem. 2012;3:99–105. [Google Scholar]
- Gomez-Mestre I., Kulkarni S., Buchholz D.R. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0084266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grain D.A., Guillette L.J., Jr. Endocrine disrupting contaminants and reproduction in vertebrate wildlife. Rev. Toxicol. 1997;1:47–70. [Google Scholar]
- Grim K.C., Wolfe M., Braunbeck T., Iguchi T., Ohta Y., Tool O., Touart L., Wolf D.C., Tietge J. Thyroid histopathology assessments for the Amphibian metamorphosis assay to detect thyroid-active substances. Toxicol. Pathol. 2009;37:415–424. doi: 10.1177/0192623309335063. [DOI] [PubMed] [Google Scholar]
- Hayes T.B., Khoury V., Narayan A., Nazir M., Park A., Brown T., Adame L., Chan E., Bucholz D., Stueve T., Gallipeau S. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:4612–4617. doi: 10.1073/pnas.0909519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbing C.C., Maher S.K., Han J., Gunderson M.P., Borchers C. Peering into molecular mechanisms of action with FrogSCOPE. Gen. Comp. Endocrinol. 2010;168:190–198. doi: 10.1016/j.ygcen.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Yoshikawa Y., Orino K., Watanabe K. Effect of diquat-induced oxidative stress on iron metabolism in male Fischer-344 rats. Biometals. 2011;24:1123–1131. doi: 10.1007/s10534-011-9471-0. [DOI] [PubMed] [Google Scholar]
- Howard P., editor. Handbook of Physical Properties of Organic Chemicals. CRC Lewis Publishers; Boca Raton. Florida: 1991. [Google Scholar]
- Hook S.E., Skillman A.D., Small J.A., Schultz I.R. Gene expression patterns in rainbow trout, O. mykiss, exposed to suite of model toxicants. Aquat. Toxicol. 2006;77:372–385. doi: 10.1016/j.aquatox.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd T.R., Murphy M.P. C Jacob and PG Winyard. Redox Signalling and Regulation in Biology and Medicine. Wiley-VCH; Weinheim: 2009. Biological systems relevant for redox signalling and control; pp. 13–45pp. [Google Scholar]
- Hazardous Substance Data Bank HSDB . National Institute of Health; 2003. Database Prepared by the U.S. National Library of Medicine, Office of Health & Human Services.http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?hsdbb.htm Available at website: [Google Scholar]
- Jones G.M., Vale J.A. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J. Toxicol. Clin. Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- Khan M.Z., Law F.C.P. Adverse effects of pesticides and related chemicals on enzymes and hormone systems of fish, amphibians and reptiles: a review. Proc. Pakistan Acad. Sci. 2005;42(4):315–323. [Google Scholar]
- Kloas W., Lutz I., Einspanier R. Amphibians as a model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo. Sci. Total Environ. 1999;225:59–68. doi: 10.1016/s0048-9697(99)80017-5. [DOI] [PubMed] [Google Scholar]
- Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Mackay D., Wan-Ying S., Kuo-ching M. Lewis Publishers; Chelsea, Michigan: 1997. Handbook of Environmental Fate and Exposure Data for Organic Chemicals. Volume III. Pesticides. [Google Scholar]
- Metcalfe N.B., Alonso-Alvarez C. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 2010;24:984–996. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. PubMed: 12234732. [DOI] [PubMed] [Google Scholar]
- Miyata K., Ose K. Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J. Toxicol. Pathol. 2012;25:1–9. doi: 10.1293/tox.25.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz L.M.H., Rojas C.M.M., Bautista M.H.B. Acute toxicity and sublethal effects of the mixture glyphosate (Roundup® Active) and Cosmo-Flux®411F to anuran embryos and tadpoles of four Colombian species. Rev. Biol. Trop. (Int. J. Trop. Biol. 2015;63(1):223–233. doi: 10.15517/rbt.v63i1.12893. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D., Faber J. North-Holland Publishing Co; Amsterdam: 1994. Normal Table of Xenopus laevis. [Google Scholar]
- Nugegoda D., Kibria G. Effects of environmental chemicals on fish thyroid function: implications for fisheries and aquaculture in Australia. Gen. Comp. Endocrinol. 2017;244:40–53. doi: 10.1016/j.ygcen.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Opitz R., Braunbeck T., Bögi C., Pickford D.B., Nentwig G., Oehlmann J., Tooi O., Lutz I., Kloas W. Description and initial evaluation of a Xenopus metamorphosis assay for detection of thyroid system-disrupting activities of environmental compounds. Environ. Toxicol. Chem. 2005;24:653–664. doi: 10.1897/04-214r.1. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD) 2007. Validation of the Amphibian Metamorphosis Assay as a Screen for Thyroid-Active Chemicals: AMA Report.www.oecd.org/documents (Accessed July, 2018) [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) 2007. Guidance Document on Amphibian Thyroid Histology. Environment Directorate Joint Meeting of the Chemical Committee and the Working Party on Chemical, Pesticides and Biotechnology. Series on Testing and Assessment Number 82. ENV/JM/MONO; p. 31. [Google Scholar]
- Ortiz-Delgado J.B., Funes v., Sarasquete C. The organophosphate pesticide –Opmalathion inducing thyroidal disruptions and failures in the metamorphosis of the Senegalese sole, Solea senegalensis. BMC Vet. Res. 2019;15:57. doi: 10.1186/s12917-019-1786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul E., Simonin H., Symula J., Bauer R. The toxicity of diquat, endothall, and fluridone to the early life stages of fish. J. Freshw. Ecol. 1994;9:229–239. [Google Scholar]
- Peterson H.G., Boutin G., Martin P.A., Freemark K.E., Ruecker N.J., Moody M.J. Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquat. Toxicol. 1994;28:275–292. [Google Scholar]
- Ritter A.M., Shaw J.L., Williams W.M., Travis K.Z. Characterizing aquatic ecological risks from pesticides using a diquat dibromide case study. 1. Probabilistic exposure estimates. Environ. Toxicol. Chem. 2000;19(3):749–759. [Google Scholar]
- Saka M., Tada N., Kamata Y. Application of an amphibian metamorphosis assay to the testing of the chronic toxicity of three rice paddy herbicides: Simetryn, mefenacet and thiobencarb. Ecotoxicol. Environ. Saf. 2013;92:135–143. doi: 10.1016/j.ecoenv.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Sewalk C.J., Brewer G.L., Hoffman D.J. Effects of diquat, an aquatic herbicide, on the development of mallard embryos. J. Toxicol. Environ. Health,Part A. 2001;62:33–45. doi: 10.1080/00984100050201659. [DOI] [PubMed] [Google Scholar]
- Shi H., Zhu P., Guo S. Effects of tributyltin on metamorphosis and gonadal differentiation of X. laevis at environmentally relevant concentrations. Toxicol. Ind. Health. 2012:1–7. doi: 10.1177/0748233712457440. [DOI] [PubMed] [Google Scholar]
- Siemering G.S., Hayworth J.D., Greenfield B.K. Assessment of potential aquatic herbicide impacts to California aquatic ecosystems. Arch. Environ. Contam. Toxicol. 2008;55:415–431. doi: 10.1007/s00244-008-9137-2. [DOI] [PubMed] [Google Scholar]
- Simsiman G.V., Chesters G. Persistence of diquat in the aquatic environment. Water Res. 1976;10:105–112. [Google Scholar]
- Stuart S.N., Chanson J.S., Cox N.A., Young B.E., Rodrigues A.S.L., Fischman D.L., Waller R.W. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency . 1995. Reregistration Eligibility Decision (RED) Diquat Dibromide. Washington, D.C. [Google Scholar]
- United State Environmental Protection Agency (USEPA) U.S. Environmental Protection Agency; Washington D.C: 1995. Great Lakes Water Quality Initiative Criteria Documents for the Protection of Wildlife: DDT, Mercury, 2, 3, 7, 8-TCDD, and PCBs. EPA 820/B95/008. March, 1995. Office of Water. [Google Scholar]
- World Health Organisation . WHO; 2004. Diquat in Drinking Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality.www.who.com/publications (Assessed on 16th, May, 2019) [Google Scholar]
- Yadav S.S., Giri S., Singha U., Boro F., Giri A. Toxic and genotoxic effects of Roundup on tadpoles of the Indian skittering frog (E. cyanophlyctis) in the presence and absence of predator stress. Aquat. Toxicol. 2013;132–133:1–8. doi: 10.1016/j.aquatox.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Wagner N., Wolfram R., Hanka T., Beatrix T., Stefan L. Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ. Toxicol. Chem. 2013;32:1688–1700. doi: 10.1002/etc.2268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.