Abstract
There are limited real-world mutational and virological outcomes data of treatment-experienced persons diagnosed with HIV-1 subtype C (HIV-1 C) who are failing Integrase Strand Transfer Inhibitor-based regimens. Requisition forms sent for HIV-1 genotypic resistance testing (GRT) between May 2015 and September 2019 were reviewed and participants experiencing virologic failure while on dolutegravir (DTG) or raltegravir (RAL) cART at sampling recruited. Sanger sequencing of the HIV-1 Pol gene was performed from residual plasma samples and drug resistance mutational (DRM) analysis performed using the Stanford University HIV drug resistance database. 40 HIV-1C integrase sequences were generated from 34 individuals, 24 of whom were on DTG cART, three on RAL cART and seven on an unknown (DTG or RAL)-anchored cART at time of GRT. 11/34 (32%) individuals had DRMs to DTG and other integrase inhibitors. 7/11 (64%) patients had exposure to a RAL-based cART at the time of sampling. Out of the 11 individuals with DRMs, one (9%) had 2-class, 6 (55%) had 3-class, and 4 (36%) had 4-class multidrug-resistant HIV-1C. 7/11 individuals (64%) are currently virologically suppressed. Of the four individuals not virologically suppressed, three had extensive DRMs involving 4-classes of ARV drugs and one individual has demised. Resistance to DTG occurs more often in patients exposed to RAL cART. Individuals with 4-class DRMs plus integrase T97 and E157Q mutations appear to have worse outcomes. There is a need for frequent VL monitoring and GRT amongst treatment-experienced HIV-1C diagnosed individuals.
Keywords: Botswana, dolutegravir, resistance, mutations, HIV-1C, integrase inhibitors
1. Introduction
Dolutegravir (DTG) is a second-generation integrase strand transfer inhibitor (INSTI) with superior efficacy, a higher genetic barrier to resistance, and a better safety profile than raltegravir (RAL), efavirenz, darunavir-ritonavir (DRV\r) combination antiretroviral therapy’s (cART) [1,2]. It is recommended as part of the first-line cART by multiple HIV treatment guidelines [3,4,5,6]. However, the emergence of antiretroviral (ARV) resistant HIV-1 is a rising global health threat [7] due to risk of onward transmission of drug-resistant HIV-1 variants [8,9,10] in addition to failure to achieve virological suppression with subsequent increase in morbidity and mortality [11,12]. Globally about 38 million people are living with HIV (PLWH) [13]. Botswana, with a population of about 2.2 million, has an adult HIV prevalence of 20.7% and about 310,000 PLWH on ART [14,15]. HIV-1 subtype C (HIV-1C) predominates in Botswana and the region. Botswana was amongst the first low/middle income country (LMIC) to adopt INSTIs such as RAL and DTG to its mature free ART programme for highly treatment-experienced individuals, and also adopted DTG in first line-therapy in 2016 [4]. However, there is limited ‘real-life’ clinical and virologic outcomes programmatic data on PLWH diagnosed with HIV-1C failing DTG/RAL based regimens.
Highly treatment-experienced PLWH previously failing a RAL-based cART regimen for prolonged periods are prone to develop virological failure (VF) when switched to DTG based cART due to cross-resistance and accumulation of more drug-resistant mutations (DRMs) that reduce DTG’s efficacy [16]. INSTI-naive highly treatment-experienced patients failing DTG cART have been found to have a virus with DRMs including G118R, D67N; H51H/Y, G118R, E138E/K, and less commonly R263R/K, V260I, R263R, N155H, G118R, and E138E [17]. These cases were mainly from clinical trials dominated by non-HIV-1C viruses [18,19,20,21]. However, there is limited information on the selection of INSTI DRMs and subsequent treatment outcomes on INSTI-based cART, especially amongst HIV-1C infected individuals.
We performed a comprehensive clinical and drug resistance genotypic characterization of HIV-1C from PLWH experiencing VF on DTG and/or RAL -based cART in Botswana.
2. Materials and Methods
2.1. Study Setting
HIV care, including cART, is provided free of charge to all citizens diagnosed with HIV in Botswana. RAL and DTG have been available in the Botswana national HIV treatment program cART since late 2008 and early 2016 respectively [2].
2.2. Selection of Study Population
We reviewed paper-based laboratory requisition forms sent for genotypic resistance testing on HIV-1 diagnosed adults (>18 years) who experienced virologic failure (generally two or more viral loads (VL) greater than 400 copies/mL while on cART, as per standard of care. These individuals were accessing public health facilities across Botswana as part of their routine HIV clinical care between May 2015 and September 2019.
We included participants whose laboratory requisition forms indicated that they were on DTG, RAL, or ‘INSTI’ cART at the time of the request. Laboratory requisition forms were accompanied by plasma biospecimens which were sent to a central laboratory, the Botswana Harvard HIV Reference Laboratory (BHHRL) which is SADCAS ISO 15189 accredited.
2.3. Clinical and Laboratory Methods Description
2.3.1. Clinical Data Extraction
We reviewed electronic and paper-based medical records to extract demographics, prior/current/subsequent ART regimens, HIV-1 RNA results, and documentation of poor adherence.
2.3.2. Viral Load Quantification
HIV-1 VL was quantified by either Abbott m2000sp/Abbott m2000rt platform (Wiesbaden, Germany), Cobas TaqMan/Cobas Ampliprep HIV-test (Roche Molecular Systems, Branchburg, NJ, USA) or Aptima HIV-1 Quant assay on Panther Systems (Hologic inc., San Diego, CA, USA) at BHHRL or district-based laboratories. VF was defined as two consecutive VL greater than 400 copies/mL and virologic suppression as a viral load < 400 copies/mL as per national ART guidelines. Because of the various VL detection platforms used and changes in the national VL reporting guidelines, some of the VL data was reported as <400, <50, <40 and <25 copies/mL.
2.3.3. Genotypic Resistance Testing (GRT)
Using residual plasma samples from individuals undergoing routine genotypic resistance testing, we amplified and Sanger sequenced the integrase (IN) region and/or reverse transcriptase (RT) and protease (PR) genes. We included testing of multiple plasma specimens that were identified to come from one individual over time and which were reported as ‘unique’.
Reverse transcription-polymerase chain reactions (RT-PCR), and sequencing reactions were performed using a commercial assay, ViroSeqTM HIV-1 Integrase RUO Genotyping kit (VS-Int) (Celera Corporation, New Jersey, USA) as per manufacturer’s instructions and an ‘inhouse’ integrase assay (IH-Int) as previously described elsewhere [22]. Capillary electrophoresis was performed on an ABI 3130XL Applied Biosystems TM Genetic Analyzer.
The raw sequence data from the sequencer were then assembled and edited using Sequencher® version 5.0 DNA sequence analysis software (Gene Codes Corporation, Ann Arbor, MI, USA) and manually edited using BioEdit version 7.2.0 software [23]. Unique sequences covering the IN-codon positions 51–263 were included in the analysis.
INSTI DRMs and accessory mutations were assessed using the Stanford University HIV drug resistance database algorithm version 8.7 (https://hivdb.stanford.edu/hivdb/by-sequences/ (accessed on 19 February 2021)). The mutations of interest included: T66A/I/K, E92Q, G118R, E138K/A/T, G140S/A/C, Y143R/C/H, S147G, Q148H/R/K, N155H and R263K [24]. Maximum-likelihood phylogenetic trees (bootstrap 1000) were generated using molecular evolutionary genetic analysis (MEGA) version X 10.1.6 [25] and included some duplicate sequences from the same individual collected over different time points (they cluster together with bootstrap values >95).
Determination of HIV-1 subtype was performed using Rega HIV subtyping tool v3.0 (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/ (accessed on 19 February 2021)) and the Stanford University HIV drug resistance database algorithm version 8.7 (https://hivdb.stanford.edu/hivdb/by-sequences/ (accessed on 19 February 2021)) [24,26].
We report reverse transcriptase (RT) and protease (PR) sequences for only the 11 patients found to have integrase DRMs. In instances where paired RT or PR sequences could not be performed, we looked for historical RT and PR results of the individuals and reported them. For one individual, we could not identify their paired or historic RT or PR sequences.
3. Results
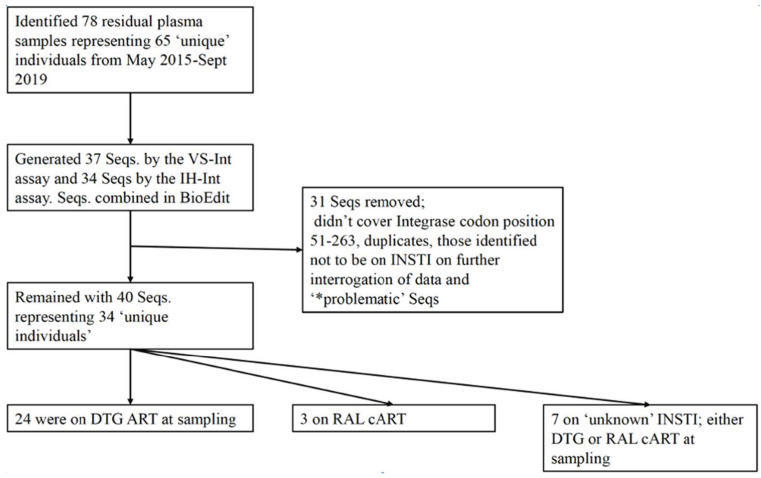
We retrieved 78 residual plasma samples from May 2015–September 2019, representing 65 unique individuals failing either a DTG or RAL-based cART regimen at the time of genotyping request. Of the 78 plasma samples, 40 (51%) (from 34 individuals) were successfully amplified and sequenced for HIV-1 Integrase by VS-Int and IH-Int assays Figure 1. Basic demographics and VL results of these individuals are shown in Table 1.
Figure 1.
Flow diagram revealing the number of plasma, sequences and patients on various cART regimens. Seqs, sequences; DTG, dolutegravir; RAL, raltegravir, cART, combination antiretroviral therapy; INSTI, integrase strand transfer inhibitors. VS-Int, ViroSeqTM HIV-1 Integrase RUO Genotyping kit Celera Corporation, USA; IH-Int, inhouse integrase assay. * Seqs clustering together with high bootstrap (>95) but being from different individuals.
Table 1.
Basic demographics and viral load characteristics of individuals failing DTG/RAL cART.
| Basic Characteristics | All Participants (n = 65) |
Participants with Sequences Generated (n = 34) | |
|---|---|---|---|
| * Age (years), median (Q1, Q3) | 40 (27, 49) | 41 (26, 45) | |
| Gender | Female n (%) | 37 (57%) | 15 (44%) |
| Male n (%) | 28 (43%) | 19 (56%) | |
| Median log 10 HIV-1 VL (Q1, Q3) copies/mL | † 3.78 (2.13, 4.49) | ± 4.53 (3.98, 5.10) | |
N.B; VL was measured from all plasma samples sent for GRT before commencing testing as per standard of care. † VL written as <20, <400 or <40 or TND, we used 20, 400, 40 and 20 respectively for analysis. In this analysis they were nine out of 65. * 6 individuals did not have age written on the requisition and could not calculate their age.; they are not included in the analysis of age. †± VL was from 35 of the 40 plasma samples at the time of GRT as 5 had UNK VLs. cART, combination antiretroviral therapy; DTG, dolutegravir; RAL, raltegravir; VL, viral load, UNK, unknown; TND, target not detected.
Amongst the 34 individuals, we obtained cART initiation dates of 24: their mean duration on ART was 11 years (Q1, Q3:8, 13) at the time of sampling for GRT.
All generated viral sequences were HIV-1C. Among the 34 individuals, 24 were on DTG ART, three on RAL cART and seven on ‘unknown’ INSTI (either DTG or RAL) at the time of collection of the sample for GRT. INSTI DRMs were found in 11(32%) of the study participants; their mean age (Q1, Q3) at GRT was 43 (40, 44) years, 7 (64%) of the 11 were male, and their mean duration on ART (Q1, Q3) was 11.5 (10, 13) years. Seven (64%) of the 11 individuals had documentation showing they were previously on a RAL-based regimen. The remaining four individuals were on a DTG regimen at sampling for resistance testing and had no known history of RAL cART exposure. The HIV-1 reverse transcriptase (RT) and protease (PR) region of the 11 individuals were sequenced and/or their historical RT and PR DRMs retrieved Table 2. 10 (91%), 8 (73%) and 4 (36%) of these 11 individuals had nucleoside/nucleotide reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI) DRMs respectively.
Table 2.
Demographics, viral loads, cART regimens and DRM’s of 11 individuals with multi-class drug resistance in Botswana.
| Patient # | ART Initiation Date | Date Sample Collected | Age at Sample Collection | Gender | VL of Sample Collected for GRT (cps/mL) | ART Regimen at Time of Sampling of GRT | Prior Exposure to RAL (Yes/No) | Major DRM’s Β | Current ART Regimen | Current VL (cps/mL) | Date of Current VL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT(NRTI; NNRTI) | PI | INSTI | |||||||||||
| 1 (139-0007-8) |
27-Apri-06 | 31-May-17 | 40 | M | 69,510 | TDF/FTC/DRV\r/DTG | NO | D67N, K70R, M184V, T215I, K219E; A98G, V106I, Y188L |
NONE | E138K, G140A, Q148K, (A128T) | TDF/FTC/DRV\r/DTG 200/300 mg od/600\100 mg bd/50 mg bd |
<400 | 10-Sep-20 |
| 2 (139-0006-2) |
25-Nov-03 | 21-Jun-17 | 38 | F | 826 | TDF/FTC/DTG | YES | M184V; K101E, G190S |
NONE | Q148R | TDF/FTC/DTG 200/300 mg OD/50 mg BD |
<50 | 22-Jul-20 |
| 3 (139-0008-6) |
21-Sept-11 | ± 10-Jan-18 | 43 | M | * <400 | AZT/3TC/DTG | NO | A62V, K65R, M184V; K103N, V106M |
NONE | G118R, E138K | 3TC/DRV\r/DTG (150 BD/600\100 BD/50 OD) |
<25 | 02-Sep-20 |
|
∞ 4a (139-0009-5a) |
14-May-07 | 19-Apr-17 | 41 | F | 119,563 | ABC/3TC/DTG | YES | M184V, T215Y; K103S, G190A |
NONE | S147G, N155H, D232N | TDF/FTC/DRV\r/DTG (300/200 mg od/600\100 mg bd/50 mg bd) |
<25 | 11-Jun-20 |
|
∞ 4b (139-0009-5b) |
± 18-May-18 | 42 | F | 79,028 | ABC/3TC/DTG | YES | M184V, T215Y; K103S, G190A |
NONE | S147G, N155H, D232N | TDF/FTC/DRV\r/DTG (300/200 mg od/600\100 mg bd/50 mg bd) |
<25 | 11-Jun-20 | |
| 5 (139-0147-0) |
27-Nov-06 | 28-Jun-17 | 21 | M | 815 | TDF/FTC/DTG | NO | ND | ND | N155ND | TDF/FTC/DTG 300/200/mg OD/50 mg BD |
12,304 | 24-Jul-19 |
| 6 (139-0002-8) |
22-Sept-03 | 01-Nov-17 | 51 | M | 515 | TDF/FTC/DRV\r/DTG | YES | *+ K70R, M184V; K219N/Y181C 20APRIL2009) * ¥ D67N, K70R, M184V/ NONE (18AUG2016) |
*+ V32I, I47V, I54L, I84V (20 APRIL2009) * ¥ V32I, I47V, I54L, I84V (18AUG2016) |
E138K, G140A, S147G, Q148R, (T97A) | TDF/3TC/DRV\r/DTG (300/300 mg OD/600\100 BD/50 mg BD) |
22,690 | 31-Aug-20 |
| 7 (139-0004-6) |
19-May-04 | 06-Apr-18 | 45 | M | * 55,342 | AZT/3TC/DTG | NO | M41L, T69G, K70R, M184V, T215C, K219E; A98G, K101E |
M46I, I54V, L76V, V82A | T66A, G118R, E138EAKT | TDF/FTC/DRV\r/DTG 300/200 mg OD/600\100 mg BD/50 mg OD |
992 | 13-Oct-20 |
| 8 (139-0003-4) |
25-June-04 | 11-Apr-18 | 41 | F | 50,699 | TDF/FTC/RAL | YES | M184V, T215Y; NONE |
NONE | E138K, G140A, Q148R | TDF/3TC/DRV\r/DTG 300/300 mg OD/600\100 mg BD/50 mg BD |
<25 | 22-Jul-20 |
| 9 (139-0001-8) |
7-May-01 | 06-Dec-18 | 55 | M | 9775 | TDF/3TC/DTG | YES | M41L, D67N, K70KR, V75M, M184V, L210W, T215Y, K219E; A98G, Y181C, G190A |
M46I, I47V, I54L, L76V, I84V, Q58E, N83D | E138K, S147G, Q148R, N155H, (E157Q) | TDF/3TC/DTG | 177,268 | 13-Feb-20 |
| 10 (139-0011-3) |
6-Feb-04 | 13-May-15 | 50 | F | 1300 | TDF/FTC/DRV\r/RAL | YES | **+ D67N, K70R, K219E/K101Q, K103N (7DEC2009) ** ¥ NONE/P225H (10FEB2012) |
**+ NONE(7DEC2009) ** ¥ NONE (10FEB2012) |
N155H | TDF/FTC/DRV\r/DTG 300/200 mg OD/600\100 mg BD/50 mg BD |
<400 | 10 Jan 2020 |
| 11 (139-0012-9) |
3-Oct-02 | 18-May-15 | 44 | M | * 2300 | TDF/FTC/DRV\r/RAL | YES | Major; M184V, M41L, T215Y: NONE |
Major; M46I, V82A Accessory; L24I |
N155NH (D232DN) | AZT/3TC/DRV\r/DTG 450 mg BD/600\100 mg BD/50 mg BD |
<50 | 26-Aug-20 |
Β Major DRMs as determined by the Stanford HIV drug resistance database. ± Date sample collected not written on requisition forms, used date sample received in a testing laboratory and/or date samples testing started and/or results issued. * No VL results available for the sample, used last recorded VL before sampling for GRT obtained from IPMS. Historical DRMs denoted with a ’*’. Historical DRMs refers to GRT performed on plasma samples from the same individuals but at a different time point (date in appendix table). *+ from sample GRT performed on 20 April 2009. * ¥ from sample GRT performed on 18 Aug 2016. **+ from sample GRT performed on 7 DEC 2009. ** ¥ from sample GRT performed on 10 FEB 2012. ∞ 4a and 4b are the same individual but their specimens were collected at different time points. Major INSTI DRMS column, DRMs listed within brackets “()” are accessory INSTI resistance mutations. VL, viral load; ART, antiretroviral therapy; GRT, genotypic resistance testing; cps/ml, copies/mL; RT, reverse transcriptase; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PR, protease; PI, protease inhibitor; INSTI, integrase strand transfer inhibitors; DRMs, drug resistance mutations; VL, viral load; GRT, genotypic resistance test; RAL, raltegravir; 3TC, Lamivudine; DRV\r, darunavir\ritonavir; DTG, dolutegravir; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; BD, twice a day dosing; OD, once a day dosing; mg, milligrams; ND, not done; IPMS, integrated patient management software (a laboratory information systems software). Green colour depicts virological suppression and red colour non virological suppression.
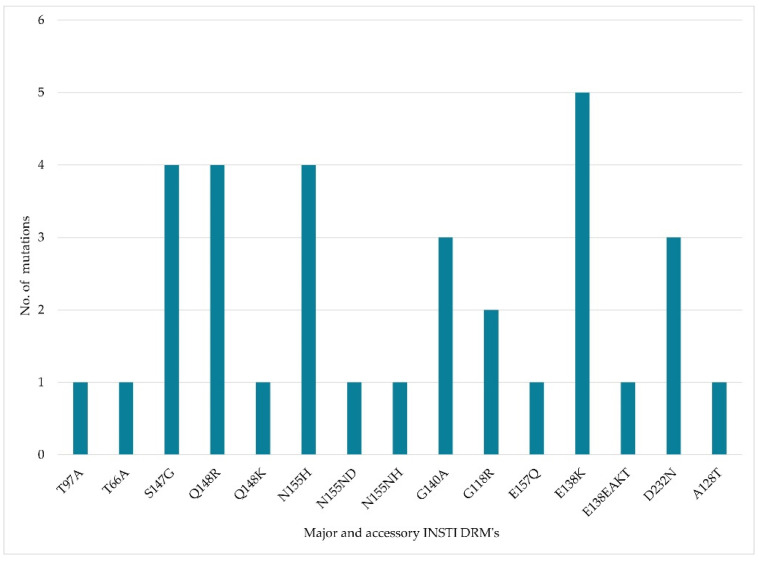
One (9%), 6 (55%) and 4 (36%) had 2-class, 3-class and 4-class multidrug-resistant HIV. 7 (64%) of the individuals are currently virologically suppressed and 4 (36%) are not suppressed. Of the suppressed individuals, 6 (86%) have no PI DRMs and 1 (14%) has major PI DRMs. Six out of the 7 currently suppressed individuals are on a salvage regimen anchored by DTG and darunavir boosted by ritonavir (DRV\r) Table 2. Three of the four individuals who were not suppressed had extensive DRMs involving 4-classes of ARV drugs. One of the individuals not suppressed has recently demised. The distribution of INSTI DRMs amongst the 11 individuals is shown in Figure 2 and Figure S1.
Figure 2.
Prevalence of INSTI DRM in the 11 HIV-1C Integrase sequences from treatment-experienced individuals with virological failure whilst on dolutegravir/INSTI based therapy. INSTI, integrase strand transfer inhibitor, DRMs drug resistance mutations.
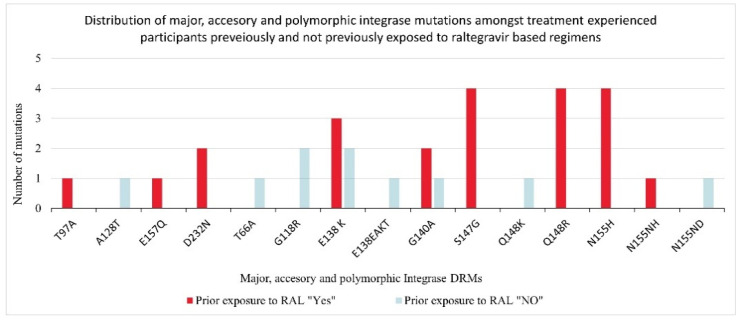
Amongst the individuals previously exposed to RAL ART (seven out of eleven), DRMs selected whilst failing their current DTG based regimens were E138K, G140A, Q148R; and N155H Figure 3. The four individuals failing DTG cART but with no documented prior exposure to RAL, their selected DRMs were E138K, G140A, Q148K, A128T; G118R, E138K; N155ND and T66A, G118R, E138EAKT Figure 3.
Figure 3.
Distribution of integrase drug resistance mutations according to prior exposure to raltegravir. DRM, drug resistance mutations, RAL, raltegravir.
4. Discussion
We evaluated virological, clinical, and HIVDR mutational pathways from a national programmatic cohort of highly treatment-experienced PLWH failing a DTG/RAL based regimen in Botswana. We identified 11 (32%) individuals with INSTI DRMs (eight being on DTG and three on RAL based regimens at HIVDR testing). They had a longer mean duration of prior cART at the time of GRT (11.5 years), and seven of them were previously exposed to RAL based regimens. Longer duration of ART has been previously associated with the development of HIV-DR [27]. Despite having INSTI DRMs, two out of the 11 individuals were on DTG once a day dosing; the Viking trials demonstrated twice a day dosing to be effective amongst such individuals [28].
A notable finding from our study was the identification of multi-drug resistance (MDR) HIV-1C variants; 2-class MDR 9.1%, 3-class MDR 54.5% and 4 class MDR 36.4%. We detected high rates of NRTI (90%), NNRTI (72.7%), and PI (36.4%) DRM’s. Another remarkable finding from our study was that despite this extensive MDR HIV-1C, nearly two-thirds of individuals (64%) are currently virologically suppressed. The Viking trial also showed 69% VL suppression despite extensive DRMs at 24 weeks [28]. The high VL suppression numbers despite multiclass DRMs could be explained by the inclusion of a potent PI-DRV\r and lack of PI DRMs in the suppressed individuals (six out of seven). It could also not be ruled out that DTG retains some suppressive activity against variants with some INSTI resistance mutations. It was recently shown that it is only upon the development of the T97A mutation that variants harboring Q148H and G140S Integrase mutations started to have increased VLs [22,27].
In our study, E138K (n = 5), S147G (n = 4), Q148R (n = 4) and N155H (n = 4) where the most frequent INSTI DRMs identified. This is in contrast to Y143C/R/H (n = 12), N155H (n = 9) and T97A(n = 13) from a similar study in the region where HIV-1C also predominates [29]. The longer duration of cART treatment of our participants with subsequent accumulation of mutations could explain these differences. Comparable with others, amongst individuals with prior exposure to RAL, the most common mutations selected were N155H (n = 4), Q148R (n = 4), S147G (n = 4) and E138K (n = 3) as others have similarly found [30,31].
Another unique finding of our study was the lack of selection of the integrase codon 263 substitution amongst the four individuals with virological failure who had no previous exposure to RAL cART. Compared with a similar cohort of patients from clinical trials, the selection of major integrase DRM- R263K was common [17]. Perhaps, this could be explained by the different HIV-1 subtypes; our cohort was all subtype C viruses whilst the other one was predominantly subtype B viruses.
Limitations of our study include the small sample size of participants; we managed to recruit 65 individuals and successfully genotyped the virus from 34 individuals. Although the study was heavily reliant on existing routine national programmatic clinical data, which often contains missing or unknown results, the results presented here represent ‘real-life’ events in HIV-1C diagnosed patients with prolonged cART treatment and DRMs.
For the 68% of participants with VF while on DTG/INSTI based regimens but devoid of INSTI DRMs, we did not assess for other mutations in nef, reverse transcriptase and protease genes that could contribute to VF [32,33].
In summary, there is a need for frequent VL testing and/or genotypic resistance testing amongst treatment-experienced PLWH experiencing VF while on DTG-based regimens. In Botswana and most low/middle-income countries, DTG-based regimens are preferred as first and second-line anchor drugs amongst PLWH initiating and failing non-DTG based cART respectively [4,6]. Constructing effective ART regimens amongst treatment-experienced individuals with over 3-class DRMs in resource-limited settings can be daunting considering the limited drug therapeutic options, need for regular training to ensure correct dosing of ARVs and lack of genotypic and phenotypic resistance testing. Similarly, to what others have found [34,35,36], we recommend that boosted darunavir should be included as part of a salvage regimen amongst treatment-experienced individuals identified to have multiclass HIV DRMs. For individuals with multi-class DRM who are not virologically suppressed, optimizing ART regimens to include newer ARVs such as the attachment inhibitor-fostemsavir and/or anti-CD-4 antibody-ibalizumab might suffice and avert death [37,38]. In the era of mass roll-out of DTG based regimens, there is a need for continued surveillance for INSTI DRMs and determining the clinical significance of these mutations in HIV-1C infections.
Acknowledgments
We thank our patients and staff of all Infectious Disease Care Clinics (IDCC) that we visited throughout Botswana in particular; Phase II clinic-Kgomotso Sanani, Fortunate Puso; BTA clinic-Thato Moreri, Thomo Kgosithebe, Setshego Baakile, Israel Motsomi, Onneile Bakwena, Mosarwa Lentswe, Nelly Goitsemodimo, Refilwe Kelentse; Palapye Primary Hospital-Juliet Seaeye, Gracious Mhaka, Gasegamotho Sennamose, Tebogo Nthubatsang, Thato Maikano, Lorna Matengu, Onkemetse Rasesigo, Oratile Sekopane, Malebogo Basaakane, Tlholego Lesedi, G. Venson, Panko L. Franchising, Diana Keeenetswe, Emannuel Limbo; Nyangabwe Referral Hospital -Lebani Dema, Taita Morapedi, Nancy Modisaotsile, Vincent Santsoma, Pilot Samson, Seitlamo Kgalalelo, Sharon Mudonyo, Themba Bangu, Catherine Thapelo, Tyreman Moira; Sekgoma Memorial Hospital-Botho Keangobota, Poloko Kgari, Boitumelo Seane, Kefilwe Moloi, Gobuamang Lenkwetse, Annah Wale, Joyce Kaphepa, Leano Keobotse, Botho Keangobota; Masunga Hospital-Gaipone Bengani, Imil Muchapa, Balisi Tshuma, Gloria Makombo, Naledi Manase, Boikhutso Chaba, Mpho Mikoto, Mbati Basupi, Bethea Khumalo, Joyce Motlamaloba; Julia Molefhe clinic-Pretty Ntsheme; Extension 14 clinic-Joyce Khumanego, Neo Gareomane, Princes Marina Hospital- Dithapelo Medupe, Kelapile Dineo, Annah B. Pile, Tebogo Ngwanawamonno. We thank the Botswana Harvard HIV Reference Laboratory staff and Botswana Ministry of Health and Wellness in particular, the Department of HIV/AIDS Prevention for their collaboration. This study would not have been possible without the support of the leadership of the Botswana-Harvard AIDS Institute Partnership, including Ria Madison, Bernadette Kgake, Data management office, IT department, Regulatory Office in particular Tumalano Sekoto, Ngozana Seonyatseng and colleagues.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13040594/s1, Figure S1: Phylogenetic tree of 40 HIV-1C Integrase sequences from treatment-experienced individuals whose plasma samples were sent for GRT while on DTG or INSTI based regimens. Analysis was performed using the Maximum Likelihood method and General Time Reversible model bootstrapped at 1000 replicates using MEGA X [25]. Diamond tagged sequences are those identified to have major INSTI DRMs. Letters, ‘a’, ‘b’, and ‘c’ at the end of some participants IDs indicate that the samples were collected over different time points but were from the same individual. GRT, genotypic resistance testing; DTG, dolutegravir; INSTI, integrase strand transfer inhibitors; DRM, drug resistance mutations.
Author Contributions
Writing—original draft preparation, K.K.S.; writing—Review & Editing, K.K.S., D.M., W.T.C., T.N., N.M., M.M. (Mompati Mogwele), M.K., B.N., D.R., J.M., M.M. (Mompati Mmalane), M.M. (Madisa Mine), I.K., S.L., S.M., S.G.; Conceptualization, K.K.S., S.G.; Methodology, K.S., S.G., S.M.; Software, K.K.S., W.T.C., S.G.; Validation, K.K.S., S.G., S.M., W.T.C.; Investigation, K.K.S., D.M., W.T.C., T.N., N.M., M.M. (Mompati Mogwele); data curation, K.K.S., S.G., S.M.; formal analysis, K.K.S., W.T.C., S.G.,T.N., S.M.; Visualization, K.K.S., S.G.; Project administration, K.K.S., N.M., S.G.,S.M., J.M., M.M. (Madisa Mine); Supervision, M.M. (Madisa Mine), I.K., S.L., S.M., S.G.; funding acquisition, K.K.S., S.L., S.M., S.G.; Resources, K.K.S., S.L., S.M., S.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, National Institute on Aging, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of General Medical Sciences, National Institute on Minority Health and Health Disparities, National Institute of Dental and Craniofacial Research, Office of AIDS Research, and Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant # 107752/Z/15/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The research reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43 TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SL was supported by the National Institutes of Health NIH/ National Institute of Allergy and Infectious Diseases K24 mentoring grant - NIH K24 AI131928. All funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Botswana (UBR/RES/IRB/BIO/157) and the health research and development division (HPDME: 13/18/1) which serves as the Botswana Ministry of Health and Wellness Institutional Review Board (IRB). A waiver of informed consent to use existing stored routine clinical plasma samples and routine health data was granted.
Informed Consent Statement
Patient consent was waived as the blood samples were previously collected as part of routine patient care. Additional genotyping (for integrase inhibitor resistance) was performed on stored leftover plasma and combined with routinely collected demographic/clinical data.
Data Availability Statement
IN sequences have been deposited into the national centre for biotechnology information (NCBI) GenBank and their accession numbers are MW690052-MW690089, MG989443.1, MG989444.1.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kandel C.E., Walmsley S.L. Dolutegravir—A review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des. Devel. Ther. 2015;9:3547–3555. doi: 10.2147/DDDT.S84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina J.M., Clotet B., Van Lunzen J., Lazzarin A., Cavassini M., Henry K., Kulagin V., Givens N., De Oliveira C.F., Brennan C., et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2:e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 3.EACS European AIDS Clinical Society (EACS) Guidelines Version 10.0. [(accessed on 22 April 2020)];2019 Nov; English. Available online: http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf.
- 4.Botswana Ministry of Health Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. [(accessed on 15 April 2020)]; Available online: https://www.moh.gov.bw/Publications/Handbook_HIV_treatment_guidelines.pdf.
- 5.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. [(accessed on 15 February 2020)]; Available online: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 6.WHO . Update of Recommendations on First- and Second-Line Antiretroviral Regimens. (WHO/CDS/HIV/19.15) World Health Organization; Geneva, Switzerland: 2019. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 7.WHO . HIV Drug Resistance Report. (WHO/CDS/HIV/19.21) World Health Organization; Geneva, Switzerland: 2019. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 8.McGee K.S., Okeke N.L., Hurt C.B., McKellar M.S. Open Forum Infectious Diseases. Oxford University Press; Oxford, UK: 2018. Canary in the Coal Mine? Transmitted Mutations Conferring Resistance to All Integrase Strand Transfer Inhibitors in a Treatment-Naive Patient. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurt C.B., Sebastian J., Hicks C.B., Eron J.J. Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009–2012. Clin. Infect. Dis. 2014;58:423–431. doi: 10.1093/cid/cit697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd S.D., Maldarelli F., Sereti I., Ouedraogo G.L., Rehm C.A., Boltz V., Shoemaker D., Pau A.K. Transmitted raltegravir resistance in an HIV-1 CRF_AG-infected patient. Antivir. Ther. 2011;16:257–261. doi: 10.3851/IMP1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brien W.A., Hartigan P.M., Martin D., Esinhart J., Hill A., Benoit S., Rubin M., Simberkoff M.S., Hamilton J.D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N. Engl. J. Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 12.Mellors J.W., Rinaldo C.R., Gupta P., White R.M., Todd J.A., Kingsley L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS Global HIV & AIDS statistics—2020 Fact Sheet. [(accessed on 23 December 2020)]; Available online: https://www.unaids.org/en/resources/fact-sheet.
- 14.Hemelaar J., Elangovan R., Yun J., Dickson-Tetteh L., Fleminger I., Kirtley S., Williams B., Gouws-Williams E., Ghys P.D., Alash’le G A., et al. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019;19:143–155. doi: 10.1016/S1473-3099(18)30647-9. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS Country Factsheets Botswana. [(accessed on 23 December 2020)];2019 Available online: https://www.unaids.org/en/regionscountries/countries/botswana.
- 16.Lombardi F., Giacomelli A., Armenia D., Lai A., Dusina A., Bezenchek A., Timelli L., Saladini F., Vichi F., Corsi P., et al. Prevalence and factors associated with HIV-1 multi-drug resistance over the past two decades in the Italian ARCA database. Int. J. Antimicrob. Agents. 2021;57:106252. doi: 10.1016/j.ijantimicag.2020.106252. [DOI] [PubMed] [Google Scholar]
- 17.Cevik M., Orkin C., Sax P.E. Emergent Resistance to Dolutegravir Among INSTI-Naive Patients on First-line or Second-line Antiretroviral Therapy: A Review of Published Cases. Open Forum Infect. Dis. 2020;7:ofaa202. doi: 10.1093/ofid/ofaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aboud M., Kaplan R., Lombaard J., Zhang F., Hidalgo J.A., Mamedova E., Losso M.H., Chetchotisakd P., Brites C., Sievers J., et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): An open-label, non-inferiority, phase 3b trial. Lancet Infect. Dis. 2019;19:253–264. doi: 10.1016/S1473-3099(19)30036-2. [DOI] [PubMed] [Google Scholar]
- 19.Cahn P., Pozniak A.L., Mingrone H., Shuldyakov A., Brites C., Andrade-Villanueva J.F., Richmond G., Buendia C.B., Fourie J., Ramgopal M., et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 20.Lepik K.J., Harrigan P.R., Yip B., Wang L., Robbins M.A., Zhang W.W., Toy J., Akagi L., Lima V.D., Guillemi S., et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS. 2017;31:1425–1434. doi: 10.1097/QAD.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 21.Viani R.M., Ruel T., Alvero C., Fenton T., Acosta E.P., Hazra R., Townley E., Palumbo P., Buchanan A.M., Vavro C., et al. Long-Term Safety and Efficacy of Dolutegravir in Treatment-Experienced Adolescents With Human Immunodeficiency Virus Infection: Results of the IMPAACT P1093 Study. J. Pediatric. Infect. Dis. Soc. 2020;9:159–165. doi: 10.1093/jpids/piy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seatla K.K., Avalos A., Moyo S., Mine M., Diphoko T., Mosepele M., Gaolatlhe T., Rowley C.F., Ramaabya D., Jarvis J.N., et al. Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana. AIDS. 2018;32:1899–1902. doi: 10.1097/QAD.0000000000001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall T.A. Nucleic Acids Symposium Series. Information Retrieval Ltd.; London, UK: 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT; pp. c1979–c2000. [Google Scholar]
- 24.Liu T.F., Shafer R.W. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin. Infect. Dis. 2006;42:1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pineda-Peña A.C., Faria N.R., Imbrechts S., Libin P., Abecasis A.B., Deforche K., Gómez-López A., Camacho R.J., De Oliveira T., Vandamme A.M. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infect. Genet. Evol. 2013;19:337–348. doi: 10.1016/j.meegid.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 27.George J.M., Kuriakose S.S., Dee N., Stoll P., Lalani T., Dewar R., Khan M.A., Rehman M.T., Grossman Z., Maldarelli F., et al. Rapid development of high-level resistance to dolutegravir with emergence of T97A mutation in 2 treatment-experienced individuals with baseline partial sensitivity to dolutegravir. Open Forum Infect. Dis. 2018 doi: 10.1093/ofid/ofy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castagna A., Maggiolo F., Penco G., Wright D., Mills A., Grossberg R., Molina J.M., Chas J., Durant J., Moreno S., et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J. Infect. Dis. 2014;210:354–362. doi: 10.1093/infdis/jiu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steegen K., Van Zyl G., Letsoalo E., Claassen M., Hans L., Carmona S. Resistance in patients failing integrase strand transfer inhibitors: A call to replace raltegravir with dolutegravir in third-line treatment in South Africa. Open Forum Infect. Dis. 2019;6:ofz377. doi: 10.1093/ofid/ofz377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallien S., Delaugerre C., Charreau I., Braun J., Boulet T., Barrail-Tran A., De Castro N., Molina J.M., Kuritzkes D.R. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS. 2011;25:665–669. doi: 10.1097/QAD.0b013e3283445834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang S.Y., Lin P.H., Cheng C.L., Chen M.Y., Sun H.Y., Hsieh S.M., Sheng W.H., Su Y.C., Su L.H., Chang S.F., et al. Prevalence of Integrase Strand Transfer Inhibitors (INSTI) Resistance Mutations in Taiwan. Sci. Rep. 2016;6:35779. doi: 10.1038/srep35779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siedner M.J., Moorhouse M.A., Simmons B., De Oliveira T., Lessells R., Giandhari J., Kemp S.A., Chimukangara B., Akpomiemie G., Serenata C.M., et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-19801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malet I., Subra F., Charpentier C., Collin G., Descamps D., Calvez V., Marcelin A.G., Delelis O. Mutations Located outside the Integrase Gene Can. Confer Resistance to HIV-1 Integrase Strand Transfer Inhibitors. mBio. 2017 doi: 10.1128/mBio.00922-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huhn G.D., Tebas P., Gallant J., Wilkin T., Cheng A., Yan M., Zhong L., Callebaut C., Custodio J.M., Fordyce M.W., et al. A Randomized, Open-Label Trial to Evaluate Switching to Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Plus Darunavir in Treatment-Experienced HIV-1-Infected Adults. J. Acquir. Immune Defic. Syndr. 2017;74:193–200. doi: 10.1097/QAI.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madruga J.V., Berger D., McMurchie M., Suter F., Banhegyi D., Ruxrungtham K., Norris D., Lefebvre E., De Béthune M.-P., Tomaka F., et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: A randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 36.Armenia D., Bouba Y., Gagliardini R., Fabeni L., Borghi V., Berno G., Vergori A., Cicalini S., Mussini C., Antinori A., et al. Virological response and resistance profile in highly treatment-experienced HIV-1-infected patients switching to dolutegravir plus boosted darunavir in clinical practice. HIV Med. 2021 doi: 10.1111/hiv.13062. [DOI] [PubMed] [Google Scholar]
- 37.Seval N., Frank C., Kozal M. Fostemsavir for the treatment of HIV. Expert Rev. Anti Infect. Ther. 2021 doi: 10.1080/14787210.2021.1865801. [DOI] [PubMed] [Google Scholar]
- 38.Chahine E.B., Durham S.H. Ibalizumab: The First Monoclonal Antibody for the Treatment of HIV-1 Infection. Ann. Pharmacother. 2021;55:230–239. doi: 10.1177/1060028020942218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
IN sequences have been deposited into the national centre for biotechnology information (NCBI) GenBank and their accession numbers are MW690052-MW690089, MG989443.1, MG989444.1.