Abstract
Macrolides are a significant family of natural products with diverse structures and bioactivities. Considerable effort has been made in recent decades to isolate additional macrolides and characterize their chemical and bioactive properties. The majority of macrolides are obtained from marine organisms, including sponges, marine microorganisms and zooplankton, cnidarians, mollusks, red algae, bryozoans, and tunicates. Sponges, fungi and dinoflagellates are the main producers of macrolides. Marine macrolides possess a wide range of bioactive properties including cytotoxic, antibacterial, antifungal, antimitotic, antiviral, and other activities. Cytotoxicity is their most significant property, highlighting that marine macrolides still encompass many potential antitumor drug leads. This extensive review details the chemical and biological diversity of 505 macrolides derived from marine organisms which have been reported from 1990 to 2020.
Keywords: macrolides, marine organisms, chemical diversity, biological diversity, cytotoxicity
1. Introduction
The term “macrolide” was coined by Woodward in 1957 [1] to describe antibiotics which typically consist of 14-, 15- or 16-membered macrolactam rings and feature double bonds and different saccharide and aminosaccharide functional groups. The naturally occurring 14-membered lactones erythromycin and clarithromycin, 15-membered macrolides azithromycin and spiramycin, and the 16-membered avermectin B1a are typical macrolide antibiotics in clinical use [2,3,4]. The 26-membered macrolide oligomycin A (an inhibitor of ATP synthase) [5,6] and the 36-membered macrocyclic lactone amphotericin B (an antifungal agent) are also used clinically [7,8]. In the last thirty years, many studies have described the molecular features, structures, and bioactivities of the intriguing macrolides obtained from plants, animals, and microbes in terrestrial and marine ecosystems [9,10,11,12]. Macrolides with larger macrocyclic rings have been reported, exemplified by the cytotoxic swinholide H, with its 40-membered lactone ring, obtained from the New Zealand deep-water marine sponge Lamellomorpha strongylata (La. strongylata) [13], and the novel 62-membered polyol symbiodinolide from the symbiotic dinoflagellate Symbiodinium sp. [14]. Macrolides, therefore, can be considered more broadly as a class of uncorrelated compounds containing a ring of twelve or more members.
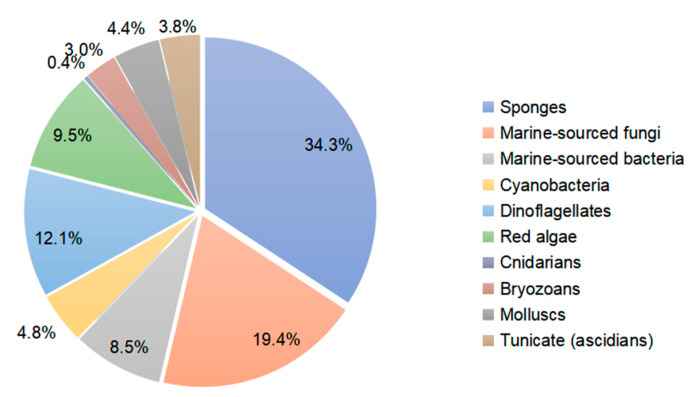
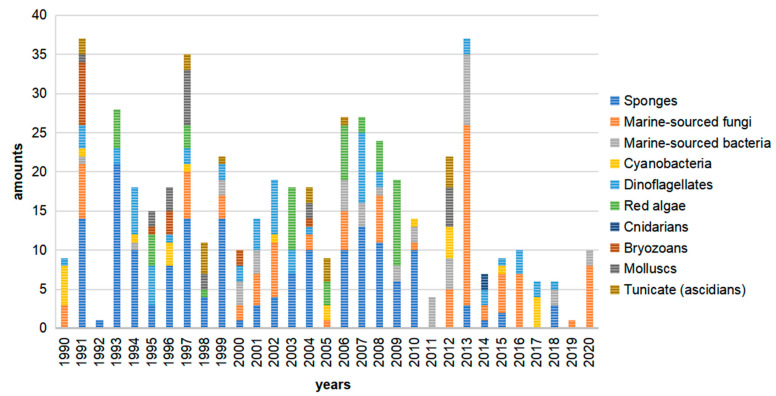
This literature review from 1990 to 2020 highlights 505 new macrolides derived from marine organisms (65.8% of which are from sponges, fungi, and dinoflagellates) (Figure 1). Compared with terrestrial environments, the oceans exhibit more wide-ranging hypersaline, hyperbaric, hypoxic, cryogenic, and oligotrophic conditions. Marine organisms must develop the capacity to produce diverse bioactive metabolites to survive in these complex and competitive ecosystems. Marine metabolites have huge potential as new drug leads, with nine approved pharmaceuticals and 31 compounds in clinical pharmaceutical trials [15]. Macrolides are a significant family of natural marine products (Figure 2). The marine macrolides reviewed herein display cytotoxic, antibacterial, antifungal, antimitotic, antiviral, antiplasmodial and other bioactivities, as listed in Table 1. This review discusses the isolation, structures, and chemical and bioactive diversity of marine macrolides from 309 publications.
Figure 1.
The percentage of macrolides from diverse marine organisms.
Figure 2.
All new macrolides by source/year, n = 505.
Table 1.
Biological activities of marine-derived macrolides.
| Drug Class | Compounds | Pharmacology | Activities | Ref. |
|---|---|---|---|---|
| Cytotoxic a | swinholides A–C (1–3) | KB cells | IC50: 0.041, 0,052, 1.1 μg/mL | [16] |
| miyakolide (13) | P388 cells | IC50: 17.5 μg/mL | [21] | |
| spongiastatin 1 (18) | HL-60, NCI-116, DMS 114 et al. | GI50: 2.5–3.5 × 10−11 M | [26] | |
| dictyostatin 1 (33) | P388 cells | undetermined | [32] | |
| superstolide B (37) | KB, P388, NSCLC-N6-L16 cells |
IC50: 0.005, 0.003, 0.039 μg/mL | [38] | |
| lasonolide A (38) | A-549, P388 cells | IC50: 40, 2 ng/mL | [40] | |
| latrunculin S (44), neolaulimalide (45) | P388, A549, HT29, MEL28 cells | IC50: 0.5–1.2 μg/mL, IC50: 0.01–0.05 μg/mL |
[46] | |
| leucascandrolide A (48) | KB, P388 cells | undetermined | [48] | |
| altohyrtins B–C (51–52) 5-desacetylaltohytrin A (53) | KB cell;L1210 cells | IC50: 0.02, 0.4; 0.3 ng/mL; IC50: 0.03, 1.3, 2.3 ng/mL |
[53] | |
| swinholide H (54) | P388 cells | undetermined | [13] | |
| neonorhalichondrin B (55), neohomohalichondrin B (56), 55-methoxyisohomohalichon-drin (57), 53-methoxyneoisohomohalichondrin B (58a) | P388 cells | IC50: 0.4, 0.8, 10, 0.1 ng/mL | [55] | |
| salicylihalamides A (59), B (60) | NCI 60 cells | GI50: 7 ± 2 nM; 60 ± 25 nM | [56] | |
| callipeltoside B (61), C (62) | NSCLC-N6 cells | IC50: 15.1, 30.0 μg/mL | [60] | |
| arenolide (67) | HCT-116, A2780 cells |
IC50: 21, 9.8 mM | [62] | |
| 30-hydroxymycalolide A (68), 32-hydroxymycalolide A (69), 38-hydroxymycalolide B (70) | L1210 cells | IC50: 0.019, 0.013, 0.015 μg/mL | [63] | |
| NA (76), NB (77), NC (78), ND (79) and NE (80) | P388, P388dox, KB tumor cells | undetermined | [66] | |
| spongidepsin (87) | J774.A1, HEK-392, WEHI-164 cells | IC50: 0.56, 0.66, 0.42 μg/mL | [71] | |
| dactylolide (88) | L1210,SK-OV-3 cells | IC50: 3.2 μg/mL | [72] | |
| neohalichondramide (101), (19Z)-halichondramide (102) | K562 cells | LC50: 4.9 μg/mL | [81] | |
| lasonolides C–E (106–108) | A-549,PANE-1 cells | IC50: 0.13, 4.5, 0.31 μM; 0.38. 4.89, 0.57, 15.6 μM | [83] | |
| leiodolides A (112) and B (113) | HCT-116 cells | IC50: 1.4, 3.8 μg/mL | [86,87] | |
| tedanolide C (114) | HCT-116 cells | IC50: 0.057 μg/mL | [88] | |
| kabiramide F–I (115–118) | NCI cells | undetermined | [89] | |
| swinholide I (120), hurghadolide A (121) |
HCT-116 cells | IC50: 5.6, 365 nM | [91] | |
| oxalatrunculin B (122) | HepG2, HCT-116,1301 cells | undetermined | [92] | |
| neopeltolide (123) | A-549, NCI-ADR-RES, P388 cell lines | IC50: 1.2, 5.1, 0.56 μg/mL | [93] | |
| phorbaside C (134) | HCT-116 cells | IC50: 2 μM | [97] | |
| tausalarin C (147) | K562 cells | IC50: 1 μg/mL | [102] | |
| enigmazole A (153) | IC-2 | IC50: 0.37 μg/mL | [105] | |
| callyspongiolide (168) | Jurkat J16 T, Ramos B lymphocytes | IC50: 70, 60 nM | [111] | |
| phormidolides B (169), C (170) | A-549, HT-29, MDA-MB-231 cells | undetermined | [112] | |
| poecillastrins E (171), F (172), G (173) | 3Y1 cells | IC50: 6.7, 1.2, 5.0 ng/mL | [113] | |
| macrosphelide M (180) | HL-60 cell | IC50: 33.2 μM | [120] | |
| 12,13-deoxyroridin E (191) | HL-60, L1210 cells | IC50: 25, 15 μg/mL | [126] | |
| myrothecines H, I (270–271) | HepG-2 cells | IC50: 8, 0.4 μM | [156] | |
| marinomycins A–D (283–286) | 60 cell line panel | LC50: 0.005–50 μM | [165] | |
| arenicolide A (287) | KB cells | IC50: 30 μg/mL | [166] | |
| halichoblelide B (293) | P388 cell line | ED50 0.63 | [169] | |
| juvenimicin C (303) | Hepa 1c1c7 cells | undetermined | [173] | |
| astolides A (311), B (312) | K-562, Pgp-positive MDR subline K-562/4 | IC50: 1.2–1.4 μM | [179] | |
| biselyngbyolide A (330), | HeLa S3, HL60 cells | IC50: 0.22, 0.027 μM | [190] | |
| biselyngbyolide B (331) | IC50: 3.5, 0.82 μM | [191] | ||
| amphidinolide E (339) | L1210, L5178Y cells | undetermined | [194] | |
| amphidinolide G,H (341–342) | L1210, KB cells | IC50: 0.0054, 0.00048 μg/mL; 0.0059, 0.00052 μg/mL | [197] | |
| amphidinolides O (351), P (352) |
L1210, KB cells | IC50: 1.7, 1.6 μg/mL; IC50: 3.6, 5.8 μg/mL. |
[208] | |
| amphidinolide Q (353) | L1210 cells | IC50: 6.4 μg/mL | [210] | |
| amphidinolides R (354), S (355) |
L1210, KB cells | IC50: 1.4, 4.0 μg/mL; IC50: 0.67, 6.5 μg/mL |
[213] | |
| amphidinolide C3 (357) | P388, L1210, KB cells | undetermined | [215] | |
| amphidinolide X (371) | L1210, KB cells | IC50: 0.6, 7.5 μg/mL | [226] | |
| amphidinolides B6 (374), B7 (375) |
DG-75 cells | IC50: 0.02, 0.4 μg/mL | [227] | |
| amphidinolide C2 (376) | L1210, KB cells | IC50: 0.8, 3 μg/mL | [228] | |
| caribenolide I (377) | HCT-116, HCT 116/VM 46,P388 | IC50: 1.6 nM, 1.6 nM, 0.03 mg/kg | [229] | |
| iriomoteolide-2a (387) | DG-75, cells | IC50: 0.006, 0.03 μg/mL | [238] | |
| iriomoteolide-3a (388) | DG-75 cells | IC50: 0.08 μg/mL | [239] | |
| iriomoteolide-4a (389), -5a (390) | DG-75 cells | IC50: 0.8, 1.0 μg/mL | [240] | |
| iriomoteolide-9a (391), -11a (392) | HeLa cells | IC50: 15, 2 μM | [241] | |
| iriomoteolide-10a (393) | HeLa, DG-75, MH134 cells | IC50: 1.5, 1.2, 3.3 μM | [242] | |
| iriomoteolide-12a (394) | DG-75 cells | IC50: 50 μM | [242] | |
| bromophycolide A (411) | A2780 cells | IC50: 6.7 μM | [258] | |
| bromophycolide H (419) | DU4475 cell line | IC50: 3.88 μM | [259] | |
| bromophycolides J–Q (421–428) | BT-549, DU4475, MDA-MD-468 et al. | IC50: 2.1–7.2 μM | [260] | |
| bromophycolide K (425) | DU4475 cell line | IC50: 1.5 μM | [260] | |
| bryostatin 10 (458) | P388 cell line | ED50: 0.33 μg/mL | [277] | |
| bryostatins 16 (459), 17 (460), 18 (461) |
P388 cell line | ED50: 0.0093, 0.019, 0.033 μg/mL | [282] | |
| aplyronines D–H (469–473) | HeLa S3 cells | IC50: 0.075, 0.18, 0.19, 0.12, 9.8 nM | [286] | |
| dolabelide A (474), dolabelide B (475) | HeLa S3 cells | IC50: 6.3, 1.3 μg/mL | [287] | |
| dolabelides C (476), D (477) | HeLa S3 cells | IC50: 1.9, 1.5 μg/mL | [288,289] | |
| iejimalides C (487) and D (488) | KB, L1210 cells | IC50: 4.7, 0.2 μg/mL; 10, 0.58 μg/mL | [298] | |
| lobatamides A–F (489–494) | NCT’S 60 cells | mean panel GI50’s 1.6 nM | [301,302] | |
| biselides A (496), C (497) | NCI-H460, MDA-MB-231 cells | IC50: 3.53, 3.72 μM; IC50: 18.0, 25.5 μM |
[303] | |
| palmerolide A (501) | HCC-2998, RXF 393 | LC50: 18, 6.5, 6.5 μM | [305] | |
| Antibacteria a | curvulone A (221) |
B. subtilis, Microbotryum, violaceum, Septoria tritici, Chlorella fusca |
undetermined | [139] |
| thiocladospolides F–J (264–268) | Edwardsiella tarda | MIC: 4 μg/mL | [154] | |
| marinomycins A–D (283–286) | MRSA, VREF | MIC: 0.1–0.6 μM | [165] | |
| 11′,12′-dehydroelaiophylin (305) | MRSA, vancomycin-resistant Enterococci pathogens | MIC: 1–4 μg/mL | [175] | |
| anthracimycin (308) | Bacillus anthracis (strain UM23C1–1) | MIC: 0.031 μg/mL | [177] | |
| bromophycolides A (411), B (412) | MRSA and VREF | MIC: 5.9, 5.9 μM; 5.9, 3.0 μM |
[258] | |
| bromophycolides P–Q (427–428) | MRSA and VREF | MIC: 1.4, 13 μM; 1.8, 5.8 μM |
[260] | |
| Antifugal a | leucascandrolide A (48) | C. albicans | undetermined | [48] |
| neohalichondramide (101), (19Z)-halichondramide (102) | C. albicans | 12.5 mm at 25 μg/disk | [81] | |
| neopeltolide (123) | C. albicans | MIC: 0.62 μg/mL | [93] | |
| BK223-A (181) BK223-B (182), BK223-C (183) |
Botrytis cinerea, Phoma lingam, Phoma bataem, Pyrenophora teres, Sclerotinia sclerotiorum, Moilinia fructigena, Ascochyta pisi and Alternaria alternata |
undetermined | [121] | |
| 15G256ɩ (197),15G256w; (198) | Neuropora crassa OS-1 | undetermined | [128] | |
| Astolides A (311), B (312) | C. albicans, A. niger 219, C. tropicales | MIC: 4, 8 μg/mL | [179] | |
| bromophycolides A (411), B (412) | C. albicans | MIC: 6.7, 27.7 μM | [258] | |
| bromophycolides F, I (417, 420) | amphotericin B-resistant C. albicans | undetermined | [259] | |
| Antimitotic | halistatin 1, 2 (15–16) | Inhibition of tubulin polymerization | undetermined | [23,24] |
| spirastrellolide A (94) | accelerating the entry of cells into mitosis | IC50: 100 ng/mL | [79] | |
| Antiviral | bromophycolides A (411) | HIV strains 96USHIPS7 and UG/92/029 inhibition | IC50: 9.1,9.8 μg/mL | [258] |
| Antiplasmodial | kabiramide L (119) | Against P. flaciparum K1 | IC50: 2.6 μM | [90] |
| Antiparasite | bromophycolides R–U (429–432) | Against Pla. falciparum. | IC50: 0.9–8.4 μM | [261] |
| VCAM b inhibition | halichlorine (47) | Inhibition to VCAM-1 | IC50: 7 μg/mL | [47] |
| Prevent fertilization | exiguolide (111) | Inhibited fertilization of sea urchin gametes | IC50: 21 μM | [84] |
| NFκB inhibition | fijiolides A (309) | Reducing TNF-α-inducing NFκB activation | IC50: 0.57 μM | [178] |
| Prevent fertilization | oscillariolide (321) | Inhibited fertilization of echinoderm eggs | IC50: 0.5 μg/mL | [182] |
| Molluscicidal activity | cyanolide A (329) | Against the snail vector B. glabrata | LC50: 1.2 μM | [189] |
| Vasoconstrict-ors | zooxanthellatoxins A (380), B (381) | undetermined | [232,233] | |
| Fast-acting toxin | prorocentrolide B (382) | Rapid toxic response in the mouse bioassay | undetermined | [234] |
| symbiodinolide (395) | Voltage-dependent N-type Ca2+ channel-opening activity | IC50: 7 nM | [14] | |
| acuminolide A (396) | IC50: 10−6 M | [246] | ||
| Prevent fertilizatoin | haterumalide B (495) | Inhibited fertilization of sea urchin eggs | IC50: 0.01 μg/mL | [302] |
a In the pharmacology column, cytotoxic, antibacteria and antifungal parts present species to which the compounds show inhibition bioactivities. b Vascular cell adhesion molecule.
2. Chemical and Biological Diversity of Marine-Derived Macrolides
2.1. Macrolides Extracted from Marine Organisms
2.1.1. Sponges
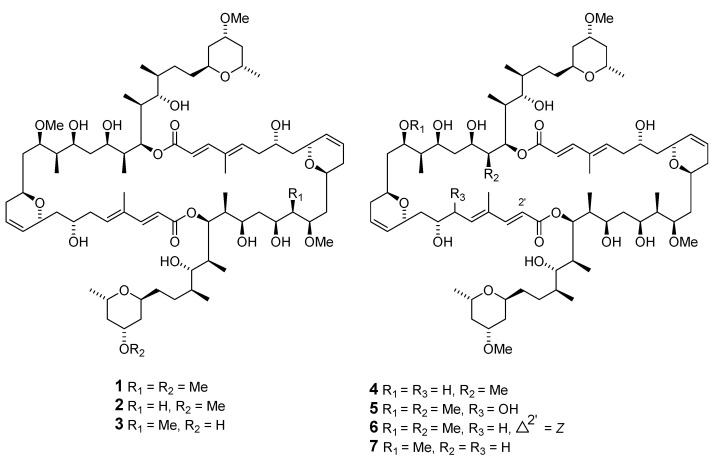
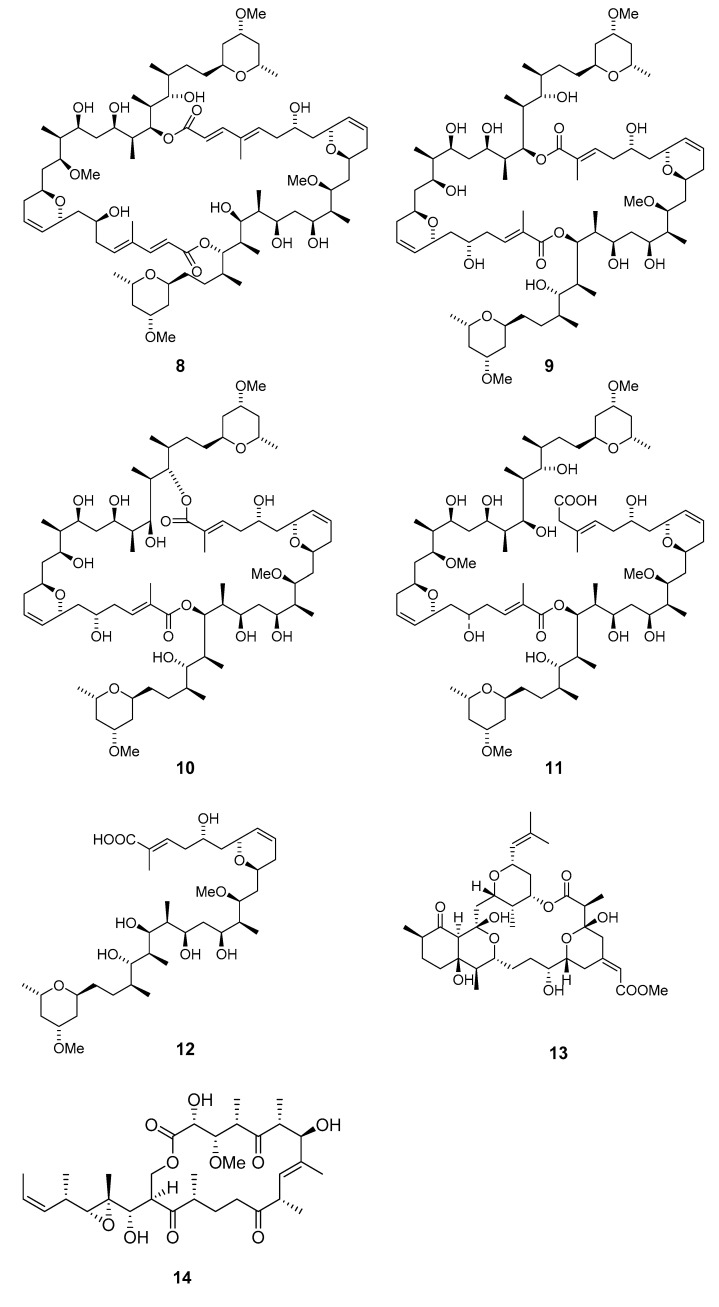
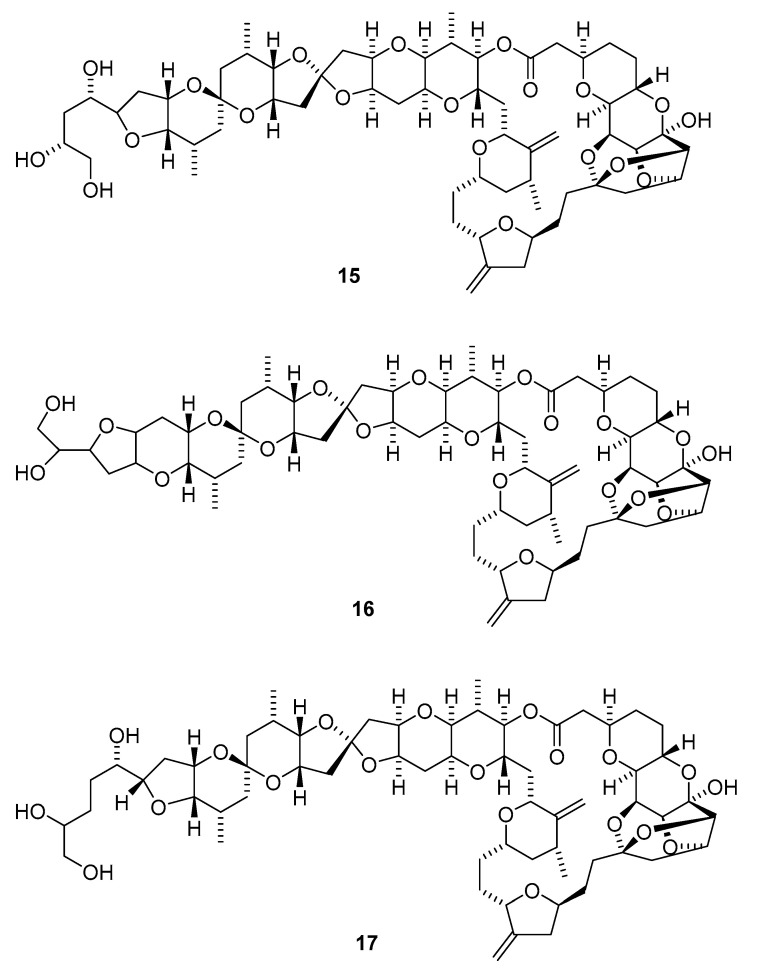
The Okinawan Theonella sp. (T. sp.) sponges produced a series of dimeric macrolides called swinholides A–G (1–7) and isoswinholide A (8) [16,17,18,19]. Four bistheonellide-related compounds—bistheonellide C (9), isobistheonellide A (10), and bistheonellic acids A (11) and B (12)—are also produced by Okinawan T. sp. sponges [20]. The structure of the macrolide miyakolide (13), which is weakly cytotoxic and obtained from Japanese sponge Polyfibrospongia sp., was elucidated by X-ray single crystal diffraction [21]. 13-Deoxytedanolide (14) was isolated from Mycale adhaerens (M. adhaerens) and identified by spectroscopic analysis [22].
The antimitotic macrolides halistatin 1 (15) and halistatin 2 (16) were isolated from Phakellia carteri from the Comoros Islands and Axinella cf. carteri (Dendy) from the Western Indian Ocean [23,24]. Halistatin 3 (17) was produced in extremely small quantities by Phakellia sponges collected at Chuuk [25].
Independent groups have reported the potent antitumor macrolides spongiastatins 1 (18), 2 (19), and 3 (20), which were isolated from Spongia sp. in the Republic of Maldives and identified via spectral data without stereochemistry [26,27]. Another group isolated spongiastatin congeners 4 (21), 5 (22), 6 (23), 7 (24), 8 (25), and 9 (26) from Spirastrella spinispirulfera (S. spinispirulfera) on the southeast coast of Africa [28,29].
Three macrolides sphinxolides B (27), C (28), and D (29) have been isolated from the Caledonian sponge Neosiphoniu superstes [30].
Three new trisoxazole macrolides, jaspisamides A (30), B (31), and C (32), were reported without stereochemical data in an Okinawan Juspis sponge [31].
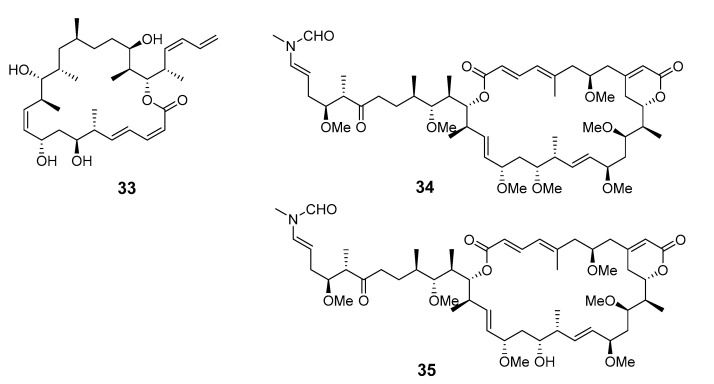
A new 22-membered macrocyclic lactone named dictyostatin 1 (33) was isolated from a Republic of Maldives Spongia sponge and exhibited significant cytotoxicity towards murine P388 lymphocytic leukemia [32]. The relative stereochemistry of dictyostatin 1 was determined by Murata’s method [33]. Two new 26-membered macrolides, reidispongiolides A (34) and B (35), have been produced by the marine sponge Reidispongia coerulea (R. coerulea) [34]. The relative and absolute stereochemistries of the C-23–C-35 portion of reidispongiolide A were determined by synthesis of an ozonolysis fragment of the natural product [35], which was later synthesized enantioselectively [36]. The relative stereochemistry of the C-7–C-15 fragment was reassigned through a series of diastereomers of a degradation fragment synthesis [37].
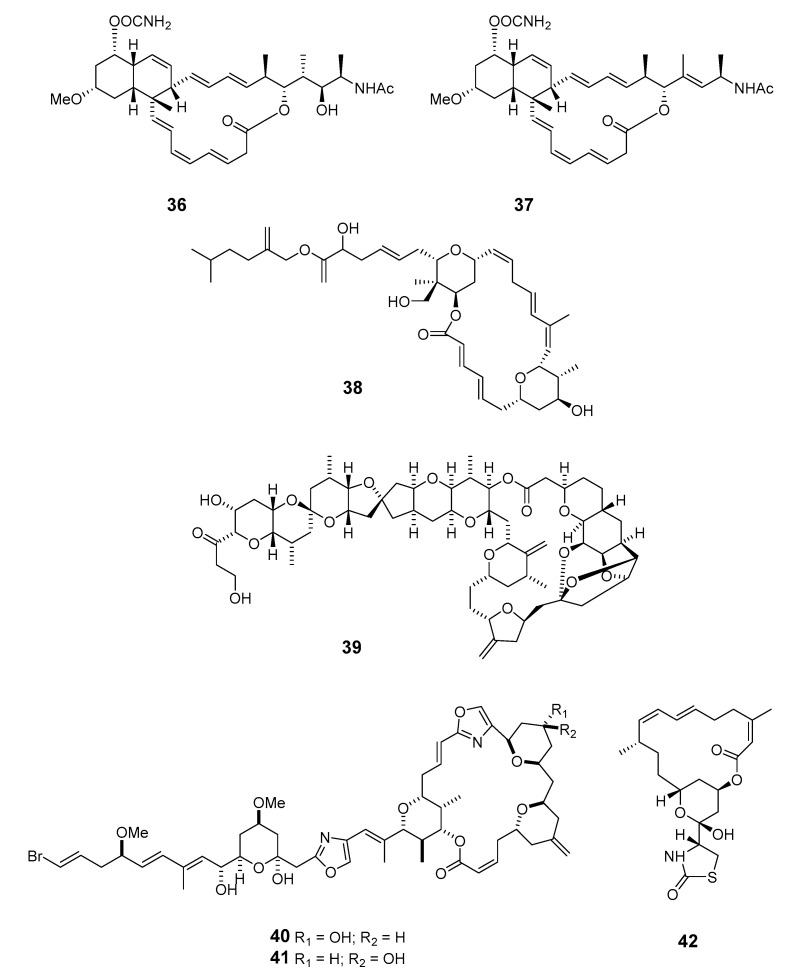
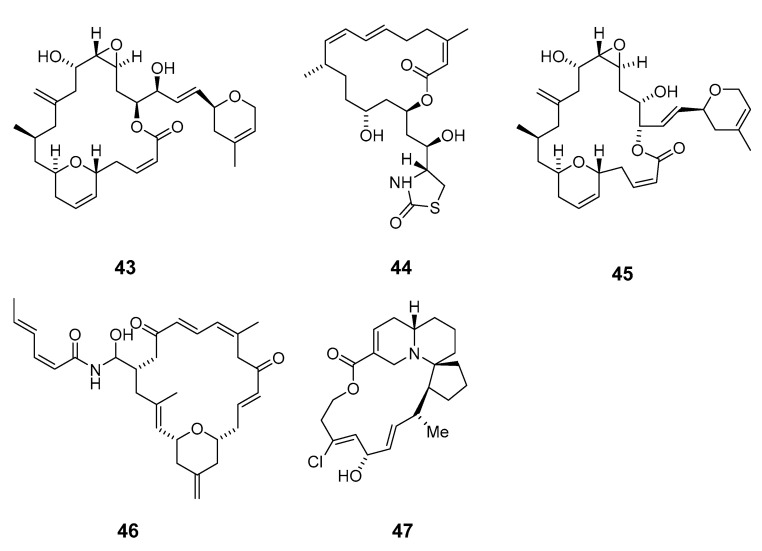
Cytotoxic superstolide A (36) and superstolide B (37) have been isolated from the deep-water marine sponge Neosiphonia superstes (N. superstes) [38,39]. Another cytotoxic macrolide, lasonolide A (38), was produced by the shallow-water Caribbean sponge Forcepia sp. [40]. Isohomohalichondrin B (39), belonging to the halichondrin family, was isolated from the New Zealand deep-water sponge Lissodendoryx sp. (Li. sp.) [41]. Phorboxazoles A (40) and B (41) have an unprecedented scaffold and were isolated from the Indian Ocean sponge Phorbas sp. (P. sp.), with complete stereochemistry and absolute configuration determined by spectroscopy and partial synthesis [42,43]. The structures and absolute configurations of latrunculin A (42) and laulimalide B (43) isolated from Okinawan sponge Fasciospongia rimosa were determined by X-ray analysis [44]. Other cytotoxic macrolides, latrunculin S (44), neolaulimalide (45) and zampanolide (46), have been produced by the F. rimosa genus [45,46]. Halichlorine (47), isolated from the marine sponge Halichodria okadai, exhibited significant inhibition of vascular cell adhesion molecule 1 (VCAM-1) [47].
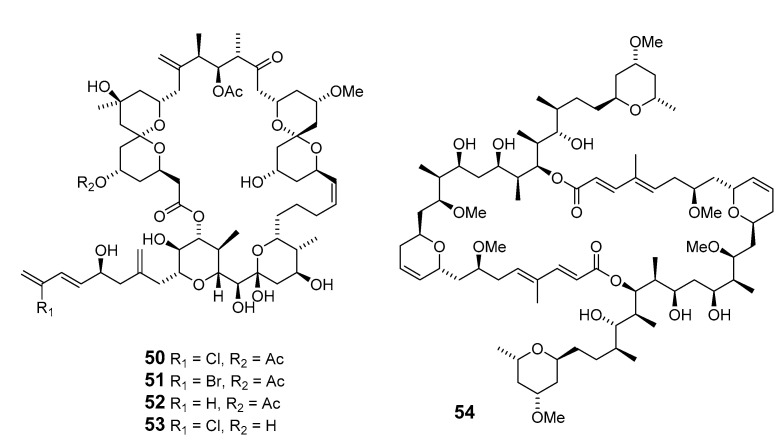
Leucascandrolide A (48), exhibiting antifungal and cytotoxic activities, was obtained from the sponge Leucascandra caveolata (Le. caveolata) [48]. The marine lithistida sponge Callipelta sp. (Cal. sp.) contains the first member of a new class of marine-derived macrolides, callipeltoside A (49), which incorporates an unusual chlorocyclopropyl group and an amino sugar [49]. The relative and absolute stereochemistry of the chlorocyclopropyl side chain of callipeltoside A was determined by stereoselective synthesis [50,51,52]. Cytotoxic macrolides altohyrtins A–C (50–52) and 5-desacetylaltohyrtin A (53) were isolated from the sponge Hyrtios altum and their absolute stereochemistries were determined by spectroscopy [53,54]. Screening of extracts from a New Zealand deep-water sponge La. strongylata for cytotoxicity towards the P388 cell line yielded swinholide H (54) [13].
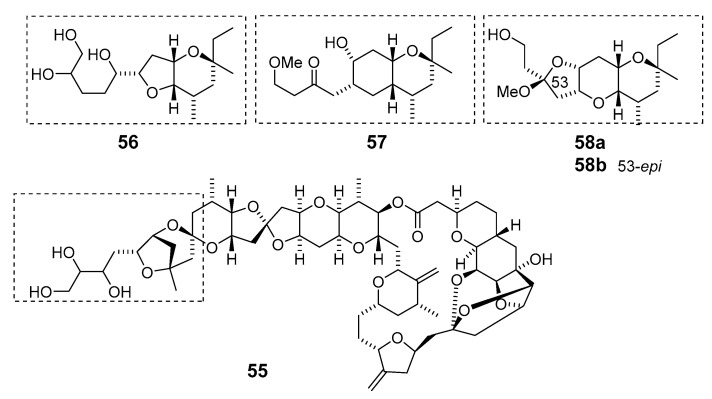
Another deep-water (> 100 m) sponge of the genus Li. produced the antitumor macrolides neonorhalichondrin B (55), neohomohalichondrin B (56), 55-methoxyisohomohalichondrin (57), 53-methoxyneoisohomohalichondrin B (58a) and 53-epi-53-methoxyneoisohomohalichondrin B (58b) [55].
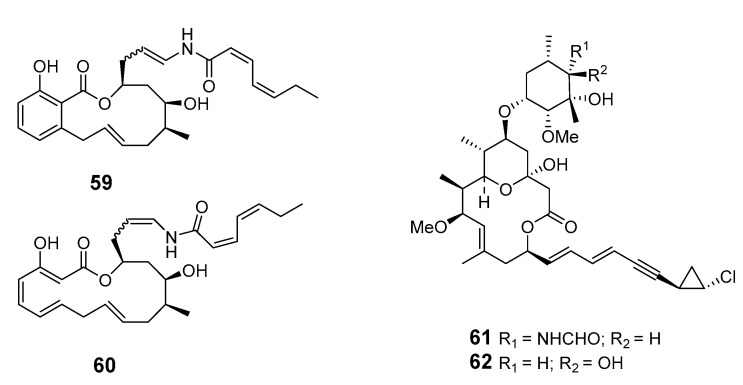
Macrolide salicylihalamides A (59) and B (60) were isolated from the Haliclona sponge, representing a potentially important new class of antitumor leads [56]. The absolute configurations of salicylihalamides A and B have been revised by a reinterpretation of Mosher ester derivatives and enantioselective syntheses of both enantiomers [57,58,59]. Cytotoxic callipeltoside B (61) and C (62), two members of a novel class of marine glycoside macrolides, were isolated from the sponge Cal. sp. [60].
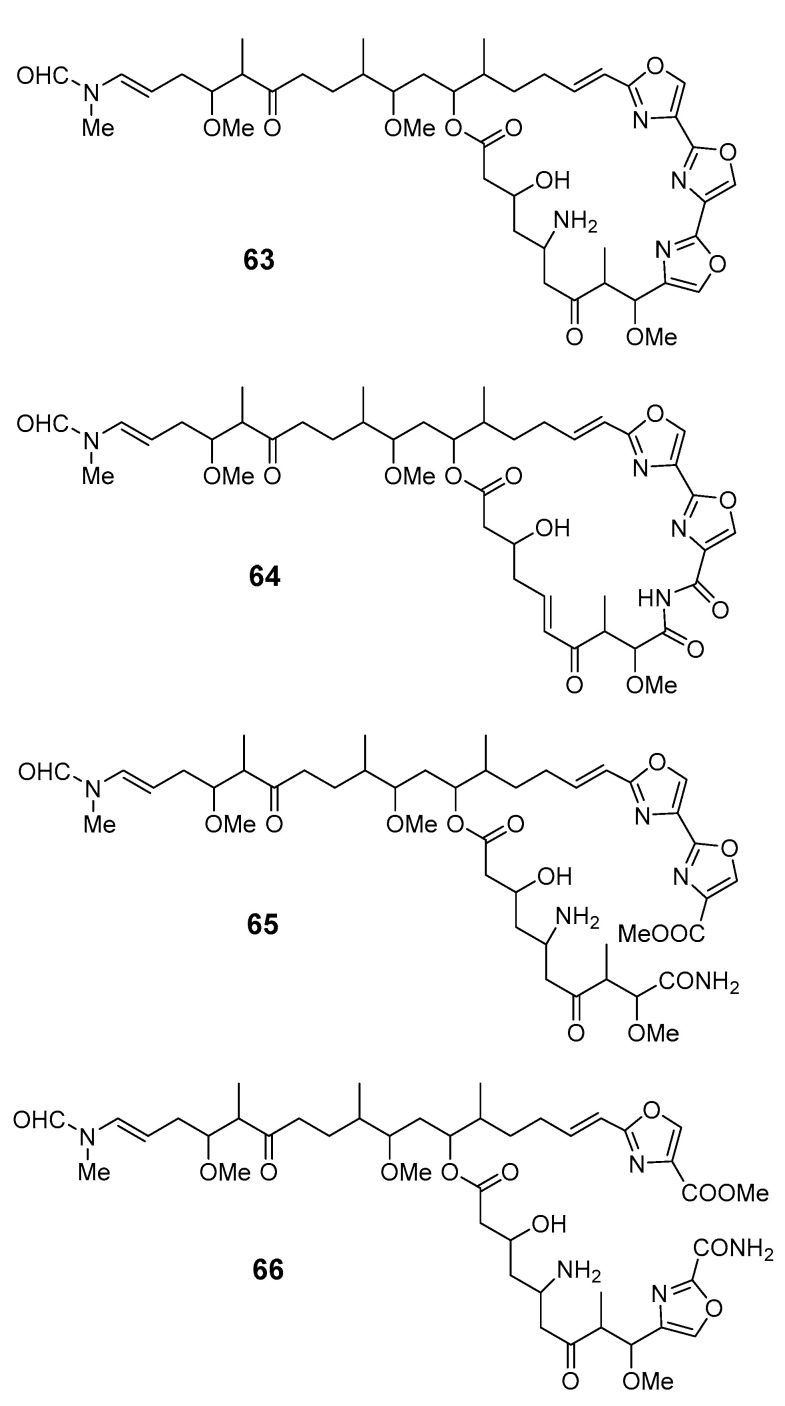
Four new oxazole-containing compounds, halishigamides A–D (63–66), were isolated from an Okinawan marine sponge, Halichondria sp. [61].
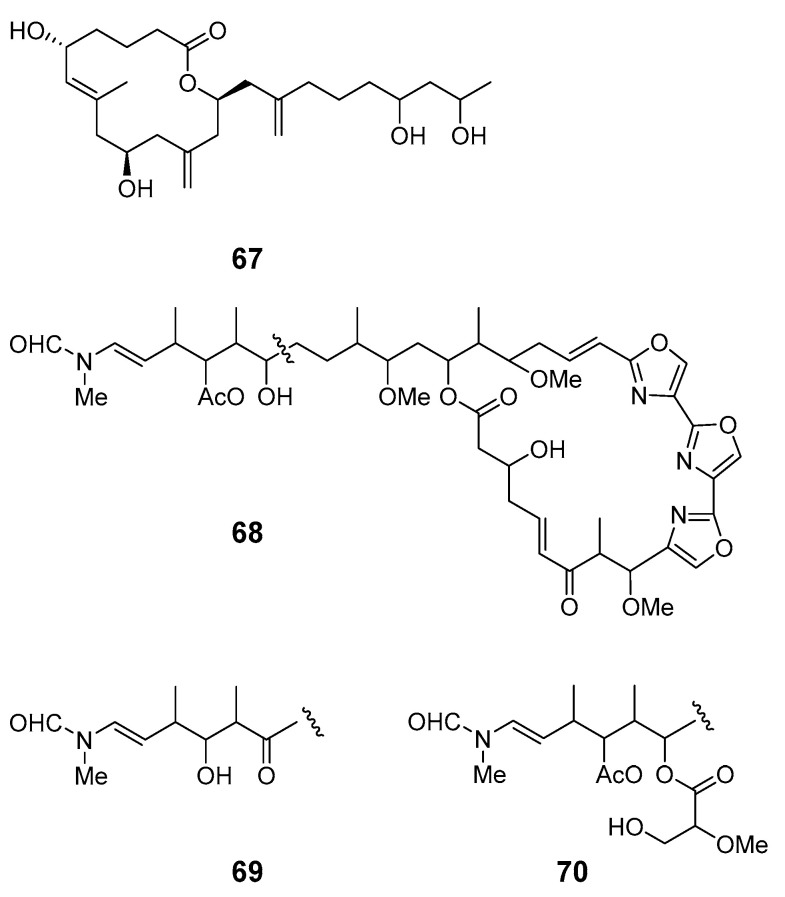
A Palau Dysidea sp. sponge contained a 14-membered macrolide, arenolide (67), showing modest cytotoxicity [62]. Three macrolides, 30-hydroxymycalolide A (68), 32-hydroxymycalolide A (69), and 38-hydroxymycalolide B (70), were isolated from the marine sponge M. magellanica and showed cytotoxicity towards L1210 cells [63].
Pateamine 1 (71), a thiazole-containing macrolide with an unique dilactone functionality, was isolated from M. sp. sponge [64]. Four new macrocyclic lactones/lactams, amphilactams A–D (72–75), were produced by the marine sponge Amphimedon spp. collected in the Great Australian Bight [65].
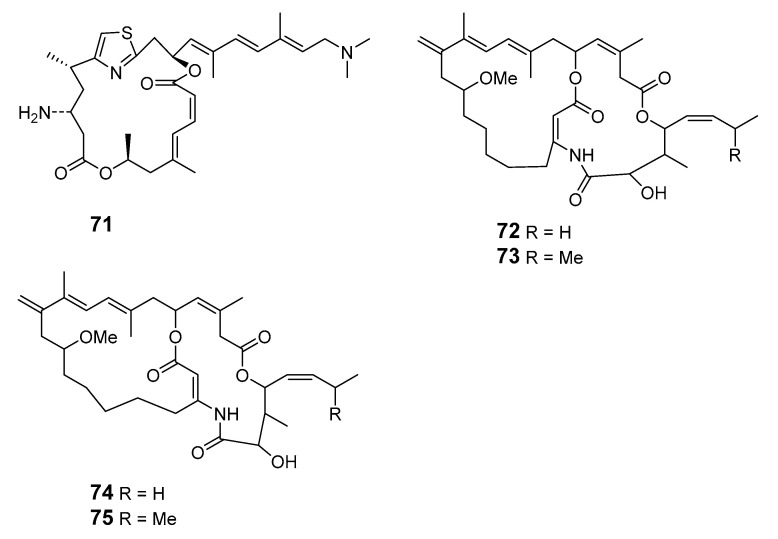
Cytotoxic macrolides haterumalides NA (76), NB (77), NC (78), ND (79) and NE (80) were isolated from the New Caledonian Litbistida sponges N. superstes and R. Coerulea [66]. Sphinxolides E–G (81–83) and reidispongiolide C (84) are new cytotoxic macrolides from Okinawan species of Ircinia [67]. Leucascandrolide B (85) is a 16-membered macrolide from the calcareous sponge Le. caveolata from the northeastern waters of New Caledonia [68]. The New Zealand marine sponge M. sp. contained the polyoxygenated, pyranose ring-containing, 16-membered macrolide peloruside A (86) [69], which was synthesized via a Mitsunobu-type lactonization [70].
Cytotoxic spongidepsin (87) has been isolated from the Vanuatu marine sponge Spongia sp. [71]. A new cytotoxic 20-membered macrolide, dactylolide (88), was isolated from a marine sponge of the genus Dactylospongia. This has been synthesized and the relative stereochemistry of the acyloxymethine and the absolute configuration of the whole molecule have been determined [72]. The Vanuatu marine sponge Ha. sp. was found to contain the cyclic metabolite haliclamide (89) [73].
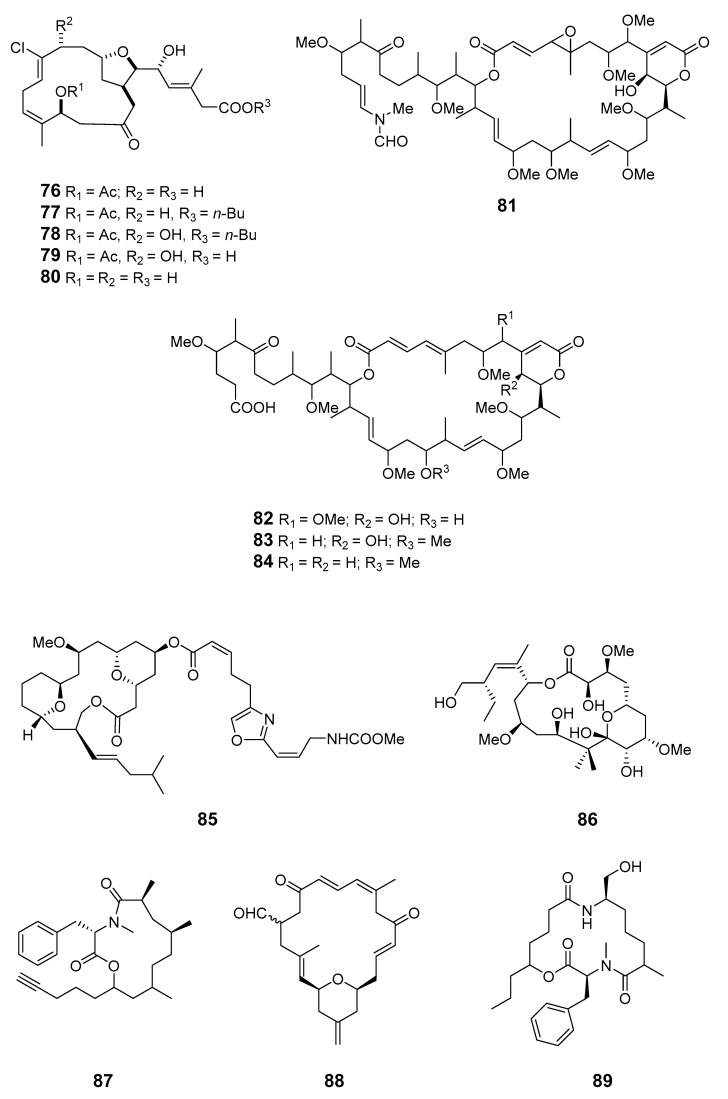
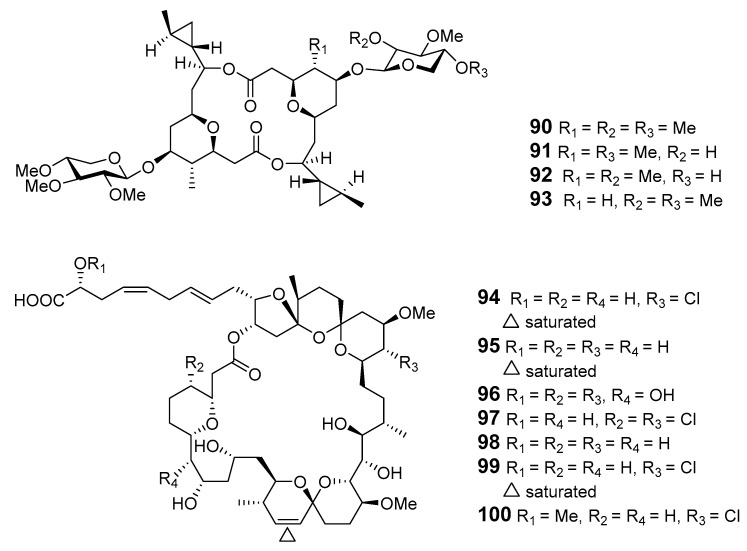
Clavosolides A–D (90–93) have been found in sponge Myriastra clavosa [74,75]. The absolute configurations of clavosides A and B were determined by total synthesis [74,75,76]. Spirastrellolides A–G (94–100) are antimitotic macrolides isolated from the Caribbean marine sponge S. coccinea [77,78,79,80]. Spirastrellolide A exhibited selective inhibition of protein phosphatase 2A [80].
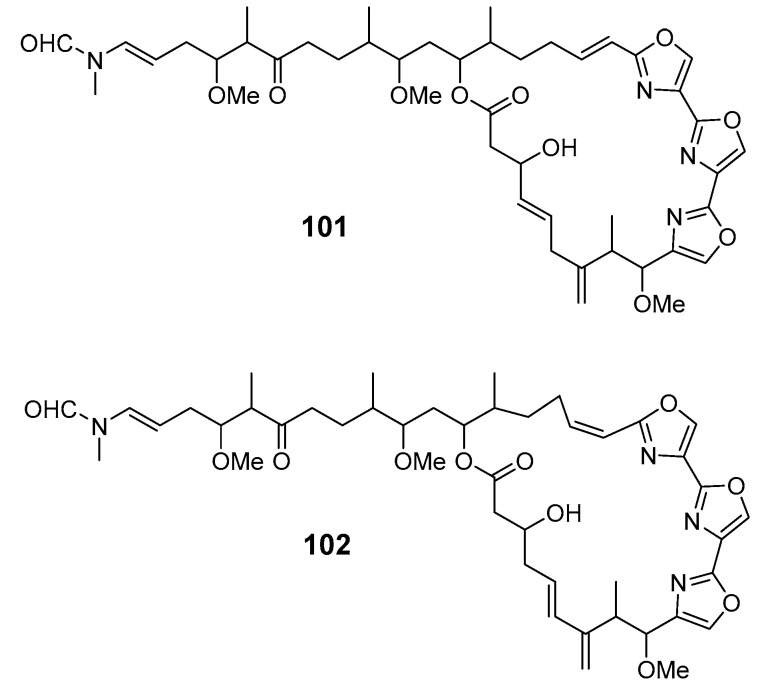
The sponge Chondrosia corticata produced two oxazole-containing macrolides, neohalichondramide (101) and (19Z)-halichondramide (102), and the open ringed secohalichondramide. Neohalichondramide and (19Z)-halichondramide exhibited significant cytotoxicity and antifungal activity toward the human leukemia cell-line K562 and Candida albicans (C. albicans) [81].
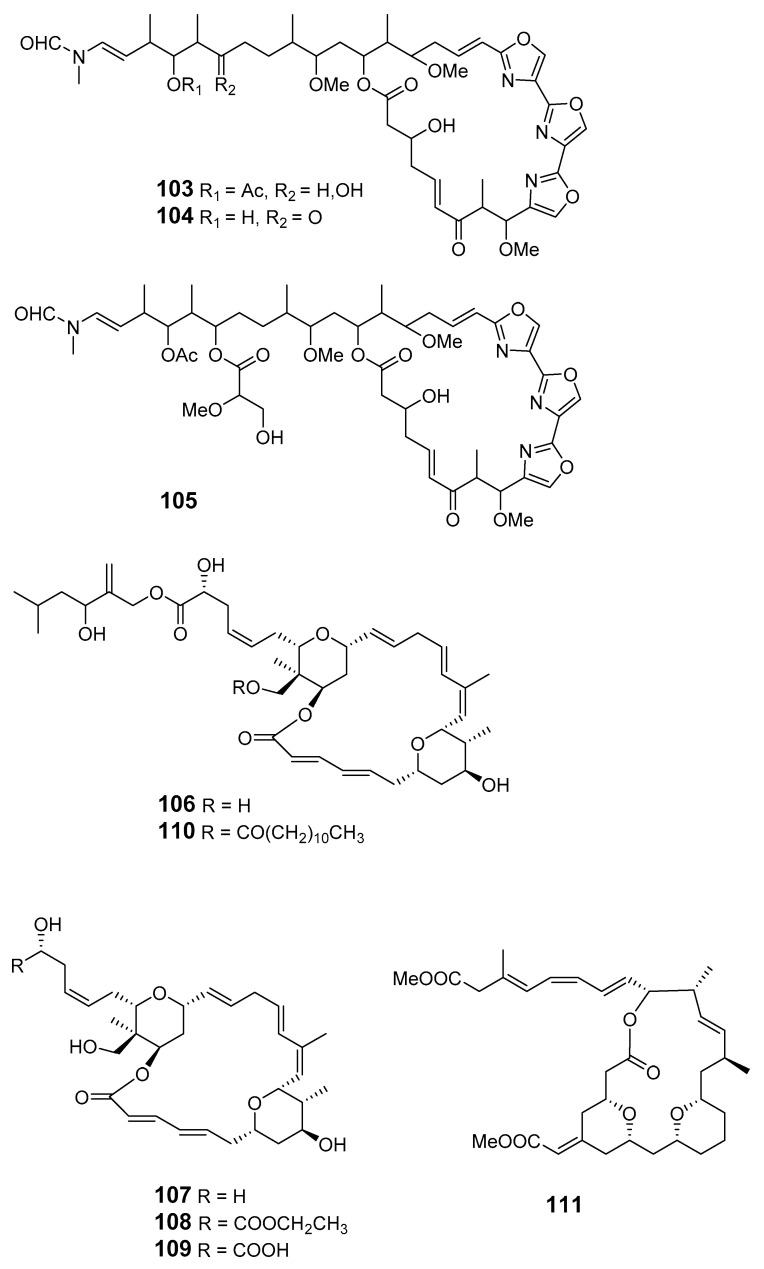
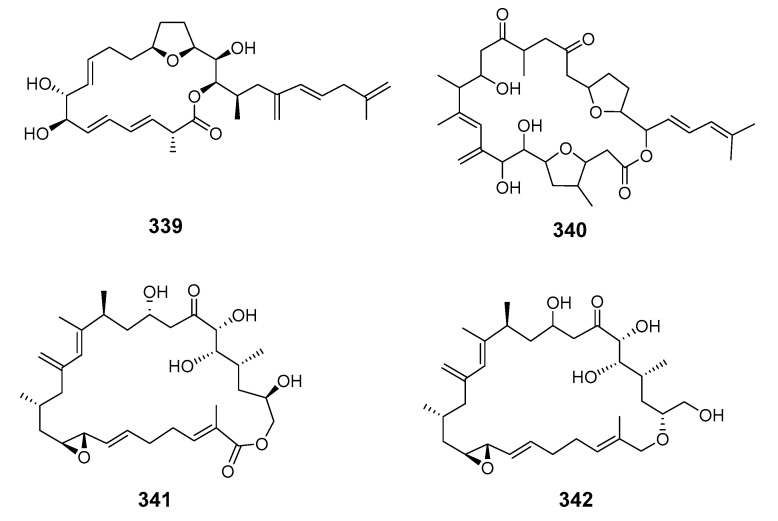
Three cytotoxic mycalolides, 30-hydroxymycalolide A (103), 32-hydroxymycalolide A (104) and 38-hydroxymycalolide B (105), have been isolated from a Japanese M. magellanica [82]. The five antiproliferative lasonolide congeners C–G (106–110) were isolated from Forcepia sponge collected in the U.S. Gulf of Mexico [83]. Exiguolide (111), isolated from the marine sponge Geodia exigua, was reported to inhibit fertilization of sea urchin (Hemicentrotus pulcherrimus) gametes but not embryogenesis [84]. The absolute configuration of exiguolide was determined by total synthesis of the enantiomer [85].
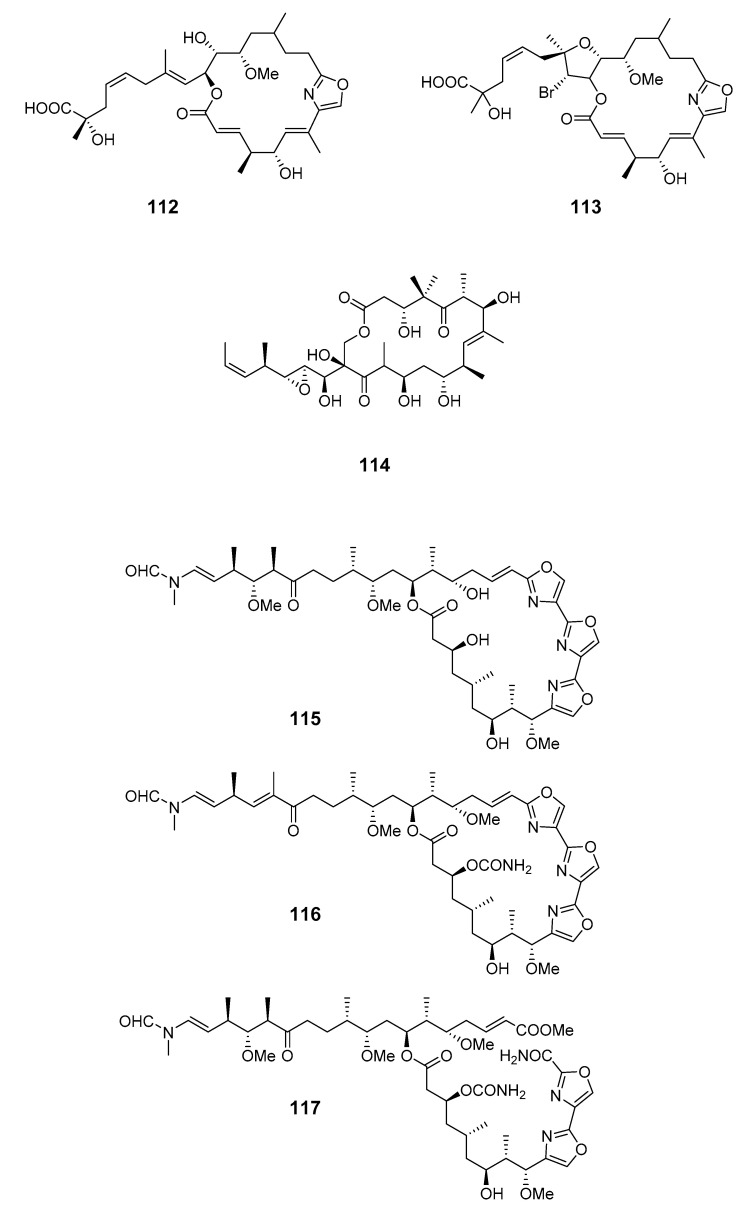
Cytotoxic macrolides leiodolides A (112) and B (113) were obtained from a deep-water (>200 m) Leiodermatium sponge [86,87]. Tedanolide C (114), isolated from Ircinia sp. (Papua New Guinea), was found to be potently cytotoxic, causing S-phase arrest, suggestive of protein synthesis inhibition [88]. Cytotoxic kabiramides F–I (115–118) were produced by Pachastrissa nux (P. nux) (Gulf of Thailand) [89].
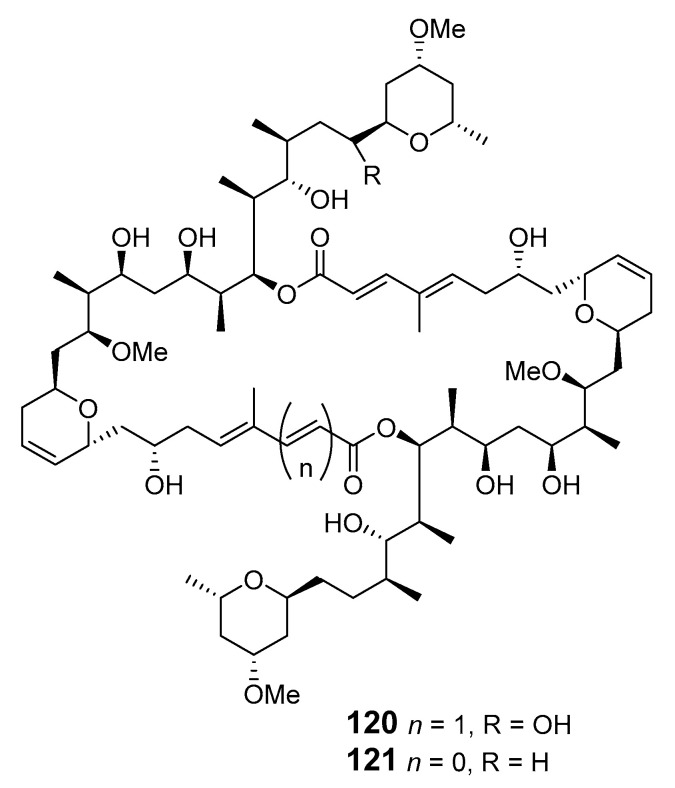
An antiplasmodial macrolide, kabiramide L (119), was isolated from P. nux sponge [90]. Swinholide I (120) and the related hurghadolide A (121), with cytotoxicity towards human colon cancer cells, were produced by T. swinhoei (Hurghada, Egypt) [91].
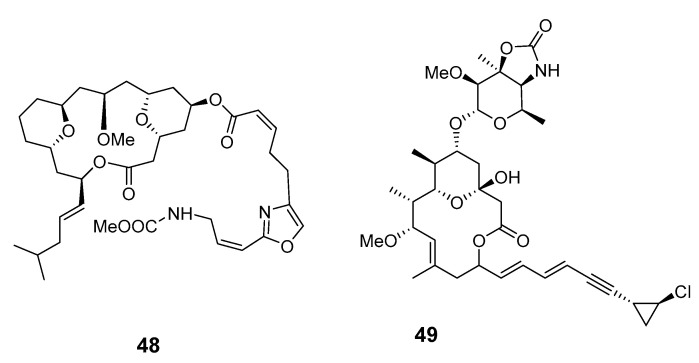
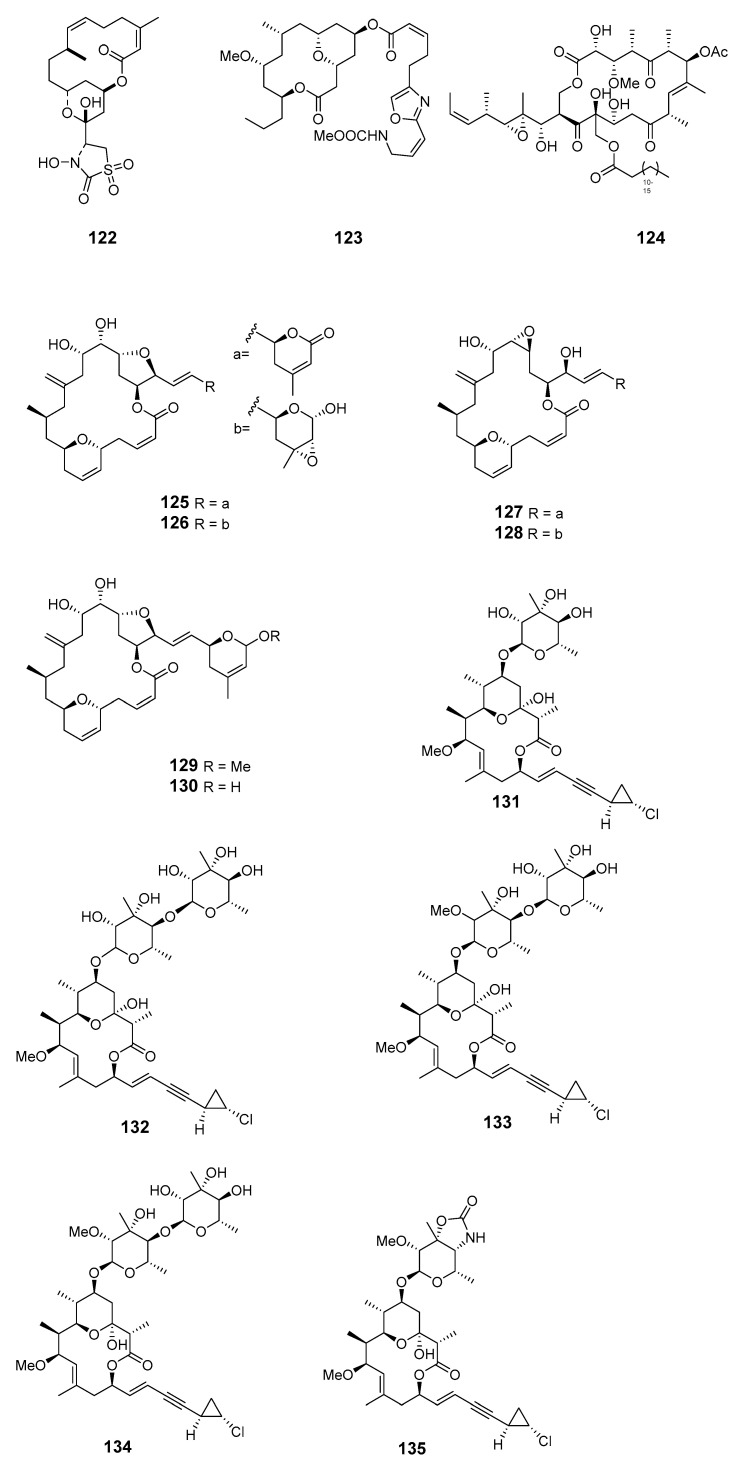
Oxalatrunculin B (122) was isolated from Red Sea sponge Negombata corticata and showed significant antifungal and anticancer activities, suggesting it as a potential member of the bioactive latrunculin family [92]. A Lithistid sponge of the family neopeltidae contained the macrolide neopeltolide (123) with potential cytotoxic and antifungal activities. This compound was synthesized to determine its absolute configuration and the relative stereochemistry of C-13 [93]. Candidaspongiolides (124), a complex mixture of acyl esters of a macrolide related to tedanolide, was isolated from Candidaspongia sp. (Can. sp.) (Papua New Guinea) and Can. flabellata (Great Barrier Reef, Australia) [94]. Fijianolides D–I (125–130) were produced by sponge Cacospongia mycofijiensis (Cac. mycofijiensis) (Mele Bay, Vanuatu) [95]. Phorbasides A–E (131–135) are chlorocyclopropane macrolides isolated from marine sponge P. sp. [96,97].
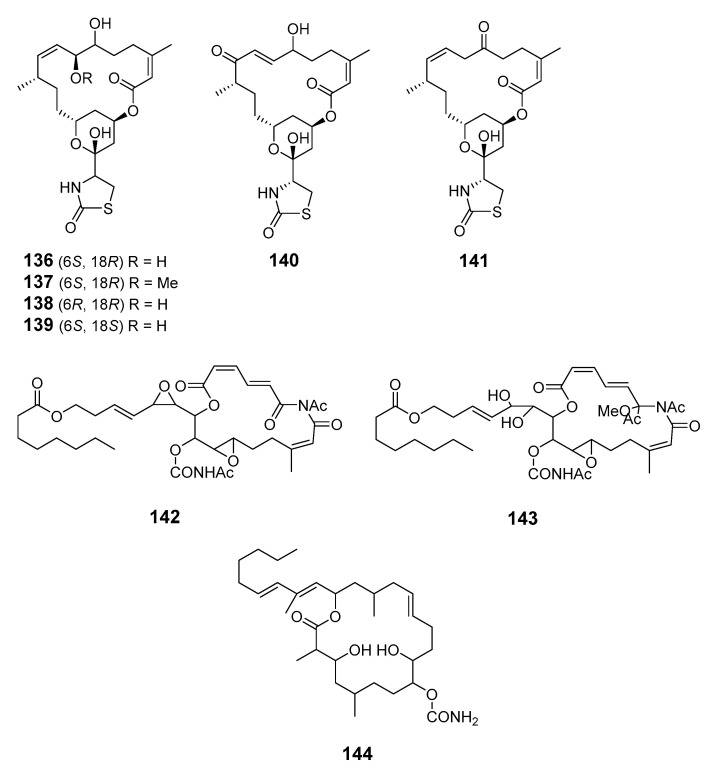
Latrunculin analogs, latrunculol A–C (136–138), 18-epi-latrunculol (139) and latrunculones A (140) and B (141), were obtained from Cac. mycofijiensis [98]. Salarin A (142), salarin B (143) and tulearin A (144) were obtained from repeated collections of the Madagascan sponge Fascaplysinopsis sp. (F. sp.) [99].
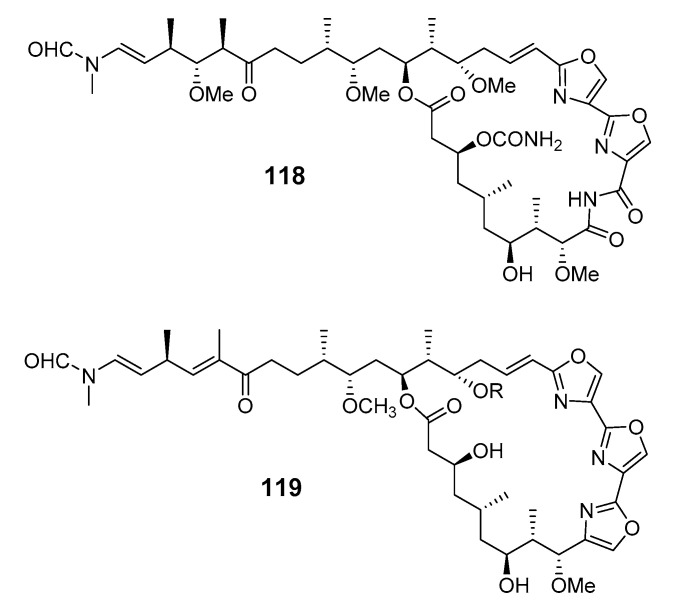
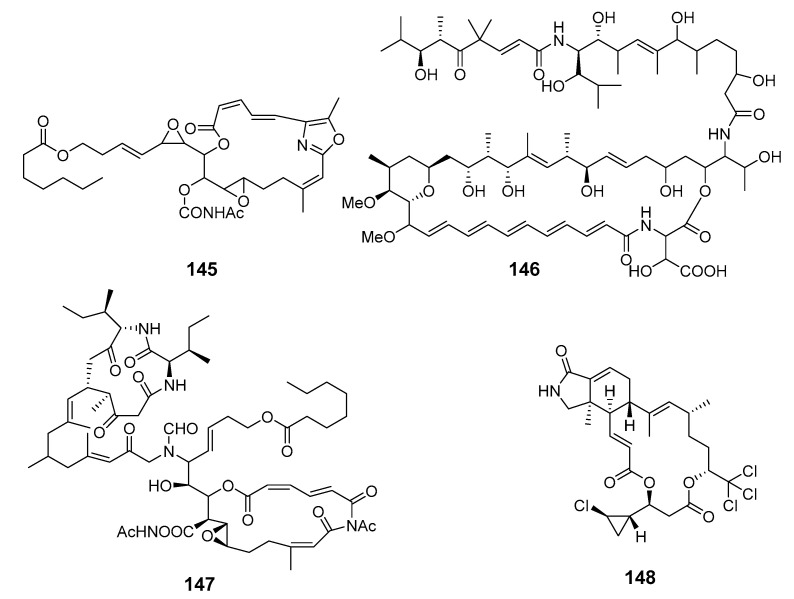
A further collection led to the isolation of salarin C (145), which was considered to be the precursor of salarins A and B [100]. Marine sponge Siliquariaspongia mirabilis contained an antitumor macrolide lactam named mirabilin (146) [101]. The nitrogenous bismacrolide tausalarin C (147) was isolated from the Madagascar sponge F. sp. and was found to inhibit proliferation of K562 leukemia cells [102]. Muironolide A (148), containing a rare hexahydro-1H-isoindolone and trichlorocarbinol ester, was isolated from marine sponge of the genus Phorbas [103].
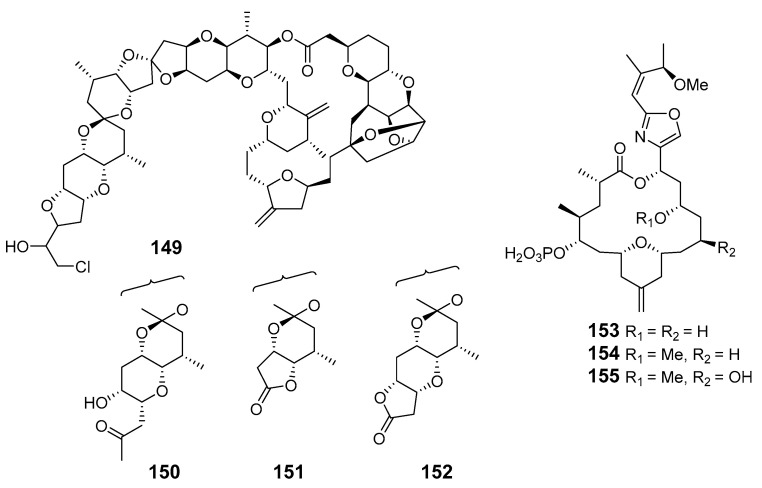
Four variants of halichondrin B, B-1140 (149), B-1092 (150), B-1020 (151) and B-1076 (152), were extracted from the Poecilosclerid sponge Li. sp. in microgram quantities and their structures were elucidated by capillary NMR spectroscopy [104].
Cytotoxic phosphate-containing macrolide enigmazole A (153) and two analogs, 15-O-methylenigmazole A (154) and 13-hydroxy-15-O-methylenigmazole A (155), were extracted from the marine sponge Cinachyrella enigmatica collected in Papua New Guinea [105] and their structures were confirmed by total synthesis [106].
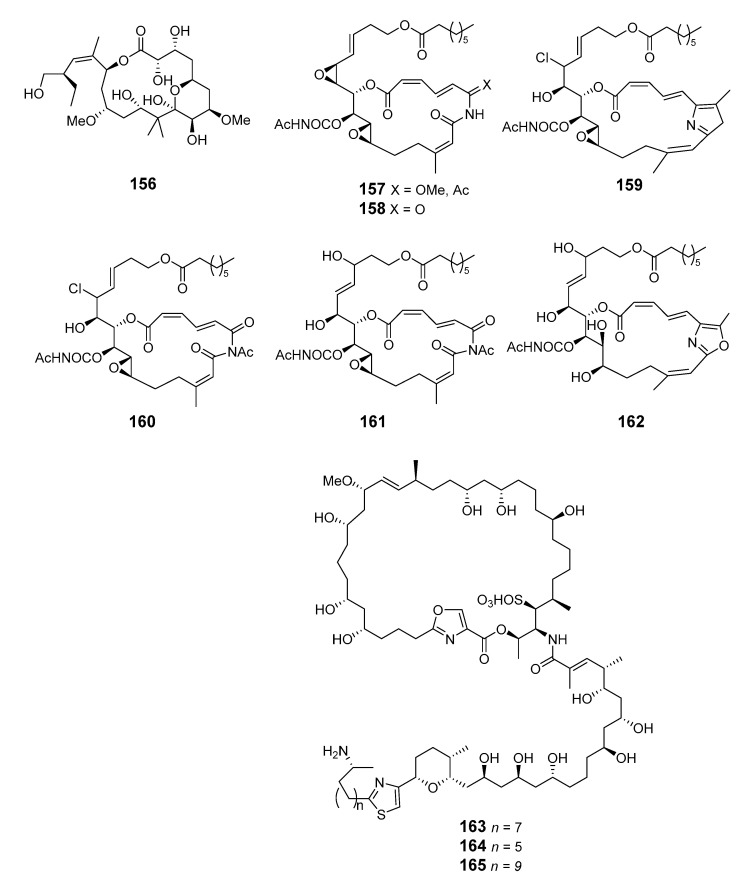
Seven scalarin analogs D–J (156–162) were obtained from the Madagascan F. sp. sponge. Scalarins D, E, H, and J inhibited cell proliferation in a dose- and time-dependent manner [107]. Theonezolides A–C (163–165) were obtained from the Okinawan marine sponge T. sp. and absolute configurations were determined by combining a JBCA method, a universal NMR database, and a 13C-acetonide method [108,109].
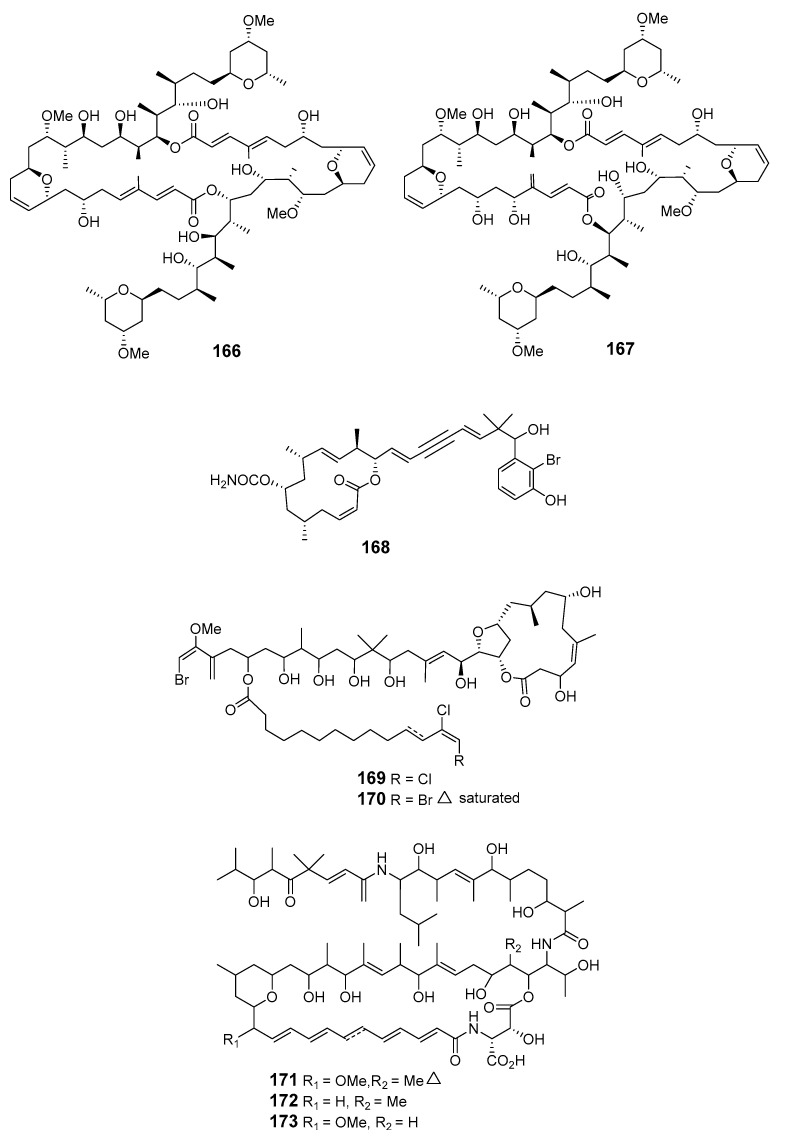
The Indonesian sponge T. swinhoei yielded the dimeric macrolides isoswinholide B (166) and swinholide K (167) [110]. An unusual carbamate, callyspongiolide (168), with strong cytotoxicity towards human Jurkat J16 T and Ramos B lymphocytes, was isolated from marine sponge Cal. sp. [111]. Cytotoxic polyketide macrolides phormidolides B (169) and C (170) were isolated from Petrosiidae sponge with stereochemical assignment via enantioselective synthesis of the macrocyclic core [112]. Cytotoxic chondropsin-type macrolides poecillastrins E (171), F (172), and G (173) were isolated from the marine sponge Poecillastra sp. [113].
2.1.2. Microorganisms and Zooplankton
Fungi
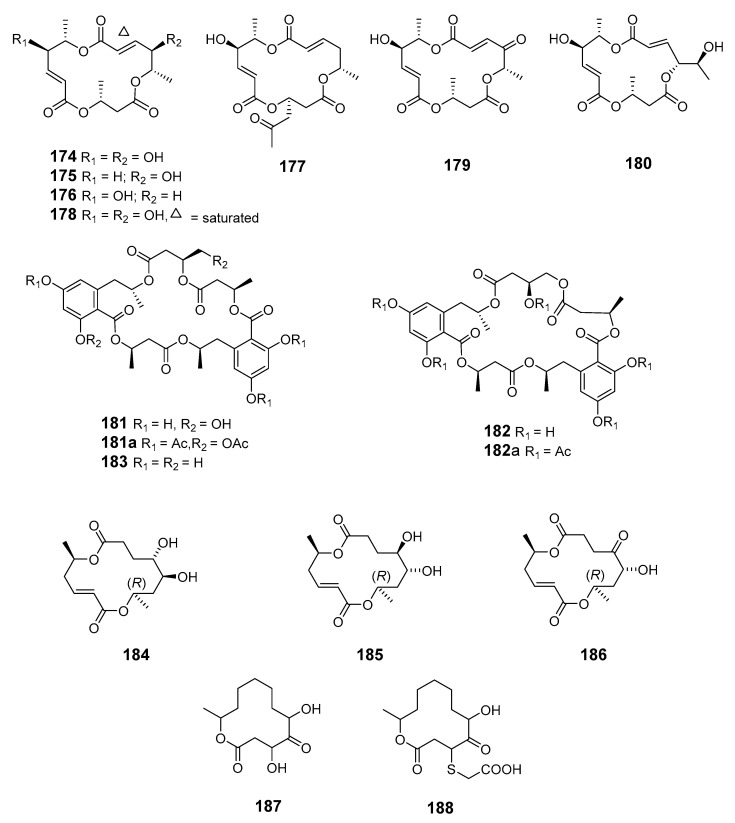
The fungus Periconia byssoides (Per. byssoides), obtained from the sea hare Aplysia kurodai (Ap. sp.), was reported to produce the cytotoxic triols pericosides A and B, and four new macrolides, macrosphelides E–H (174–177) [114]. Macrosphelide I (178) and macrosphelides E–H from Per. byssoides isolated from Ap. kurodai were also reported elsewhere [115]. Macrosphelide E was synthesized at a high yield via a key chemoenzymatic reaction [116]. The synthesis of macrosphelides H and G has also been described [117,118]. Absolute configurations determined by spectroscopy and chemical transformation have been reported for macrosphelides L (179) and H produced by Per. byssoides from Ap. kurodai, and the cytotoxic macrosphelide M (180) [114,119,120]. Penicillum verruculosum (IMI352119) was reported to produce three macrolides with antifungal activity: BK223-A (181), BK223-B (182) and BK223-C (183) [121]. The mitosporic fungus Varicosporina ramulosa has been reported to produce (6R,11S,12S,14R)-colletodiol (184), (6R,11R,12R,14R)-colletodiol (185) and colletoketol (186) [122,123]. The 12-membered macrolides pandangolide 1 (187) and pandangolide 2 (188) were extracted from an unidentified fungus isolated from marine sponge collected in Indonesia [124].
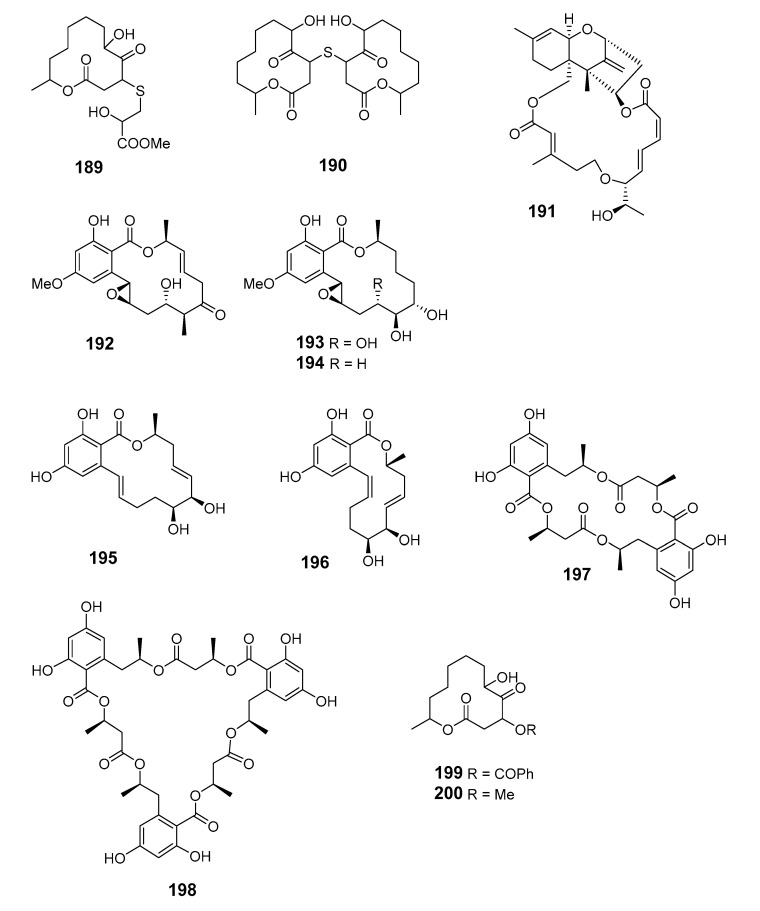
Pandangolide 3 (189), macrolide dimer pandangolide 4 (190), and a new acetyl derivative of 5-hydroxymethylfuran-2-carboxylic acid were produced by the fungus Cladosporium herbarum (Cla. herbarum), associated with the sponge Callyspongia aerizusa (Cal. aerizusa) and collected in Bali [125]. The cytotoxic macrocyclic trichothecene 12,13-deoxyroridin E (191) was obtained from an extract of the marine fungus Myrothecium roridum (M. roridum) [126]. The 14-membered resorcylic macrolides aigialomycins A–E (192–196) were isolated from the mangrove fungus Aigialus parvus BCC 5311 [127]. Potential antifungal macrocyclic polyesters 15G256ɩ (197) and 15G256ѡ (198) were obtained from the marine fungus Hypoxylon oceanicum LL-15G256 [128]. Two cytotoxic macrolides, sporiolides A (199) and B (200), were produced by the fungus Cladosporium isolated from the brown alga Actinotrichia fragilis (Okinawa, Japan) [129].
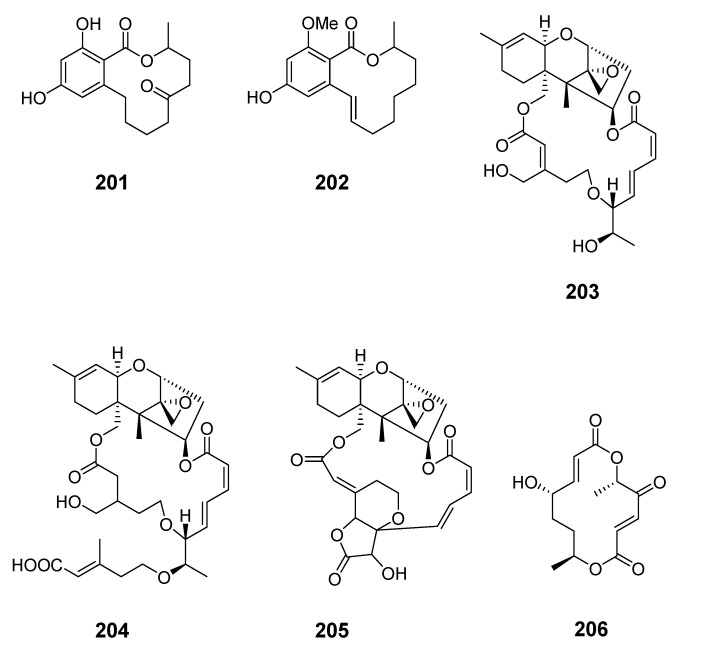
An unidentified endophytic fungus from the brown alga Sargassum sp. (Zhanjiang Sea, China) was the source of two 12-membered ring lactones (201–202) [130]. 12-Hydroxyroridin E (203), roridin Q (204) and 2,3-deoxyroritoxin D (205) were obtained from M. roridum on submerged wood in Palau [131]. Gliocladium sp. isolated from the alga Durvillaea antarctica (Tauranga Bay, New Zealand) yielded 4-ketoclonostachydiol (206) [132].
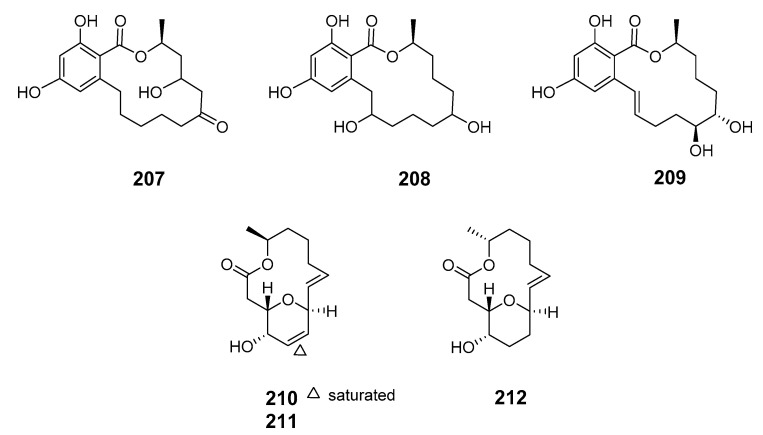
The 14-membered resorcylic acid lactone derivatives 8′-hydroxyzearalanone (207) and 2′-hydroxyzearalanol (208) were isolated from the marine-derived fungus Penicillium sp. (Pen. sp.) [133]. β-resorcylic macrolide 5′-hydroxyzearalenol (209) was obtained from the culture broth of the fungus Fusarium sp. 05ABR26 [134]. The cytotoxic 14-membered macrolides aspergillide A–C (210–212) were isolated from the culture broth of the marine sponge-derived fungus Aspergillus ostianus (As. ostianus) (Pohnpei, Micronesia) [135].
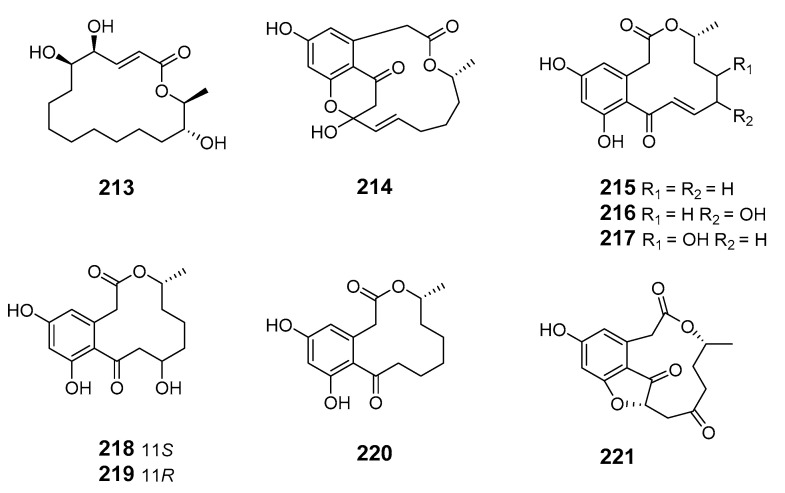
The marine-derived fungus As. sp. SCSGAF 0076 was reported to produce the 16-membered macrolide aspergillide D (213) [136]. Apralactone A (214) and enantiomers of curvularin (215–220) were isolated from Curvularia sp. (Cur. sp.) [137,138]. The macrolide curvulone A (221) was produced by Cur. sp. isolated from the marine alga Gracilaria folifera and inhibited the growth of B. subtilis, Microbotryum violaceum, Septoria tritici, and Chlorella fusca [139].
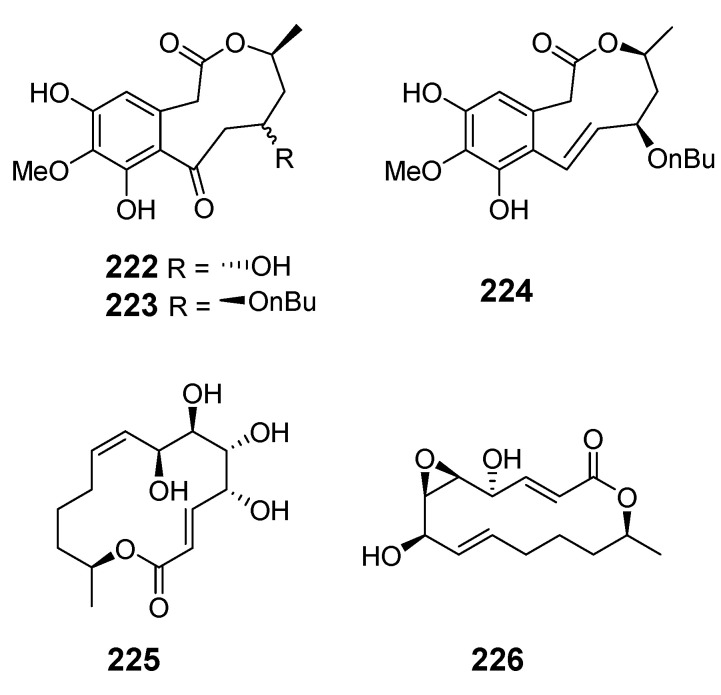
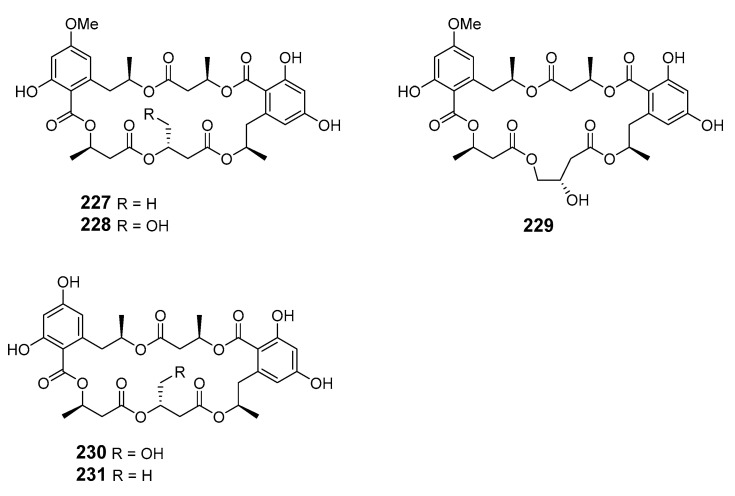
Three decalactones, xestodecalactones D–F (222–224), were purified from an ethyl acetate extract of Corynespora cassiicola isolated from leaf tissues of the Chinese mangrove medicinal plant Laguncularia racemose [140]. Seiricuprolide pestalotioprolides A (225) and B (226) (as the diacetate) were isolated from the fungus Pestalotiopsis spp., which is associated with mangrove twigs of Rhizophora mucronata [141]. Calcarides A–C (227–229), 15G256α (230), and 15G256β (231) were obtained from crude extracts of the fungus Calcarisporium sp. KF525 isolated from German Wadden Sea water samples [142].
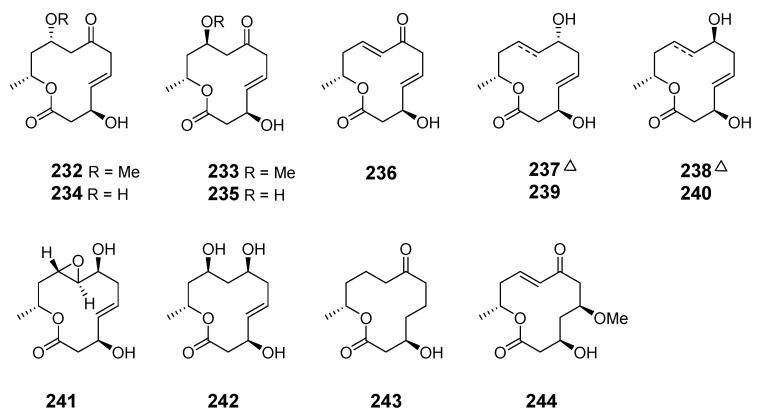
Thirteen new 12-membered macrolides, dendrodolides A–M (232–244), were obtained from the fungus Dendrodochium sp. derived from sea cucumber Holothuria nobilis Selenka in the South China Sea [143]. Dendrodolide K was obtained from a commercially available substrate by a convergent strategy, and the dendrolides F, G, I, J, and L were synthesized via a unified strategy employing ring-closing metathesis [144,145].
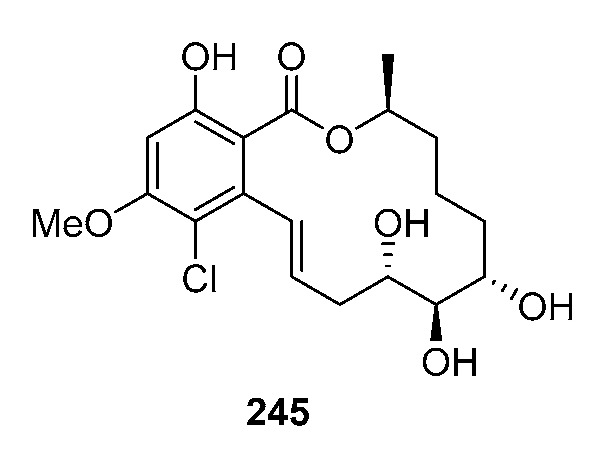
Cochliomycin C (245) was produced by the gorgonian-derived fungus Cochliobolus lunatus (Coc. lunatus) [146], its absolute configuration was corrected in a later study [147].
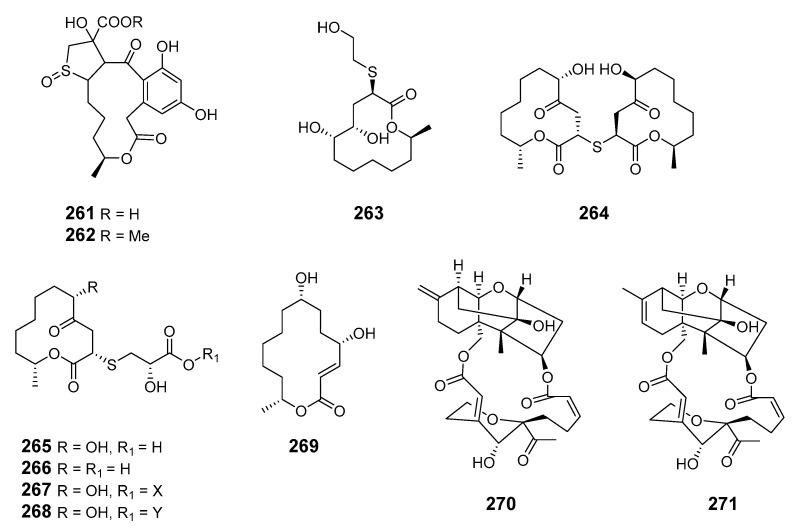
The fungus Pen. sumatrense MA-92, associated with the mangrove Lumnitzera racemose, yielded the sulfur-containing curvularin derivatives sumalarins A−C (246–248) [148]. Chemical epigenetic manipulation of the marine-derived fungus Coc. lunatus (TA26-46) with histone deacetylase inhibitors led to the elucidation of two 14-membered resorcylic acid lactones: 5-bromozeaenol (249) and 3,5-dibromozeaenol (250) [149]. Gliomasolides A–E (251–255) were obtained from a sponge-derived fungus Gliomastix sp. ZSDS1-F7-2, their structures being determined by spectroscopy and single crystal X-ray diffraction [150]. Two 13-membered macrolides (256–257) were isolated from the marine-derived fungus Pen. meleagrinum var. viridiflavum [151]. Application of published procedures for experimental design and chemometric analysis to enhance the production of curvularin-related compounds by marine-derived Penicillium sp. DRF2 led to the isolation of cyclothiocurvularins (258–260) and cyclosulfoxicurvularins (261–262) [152]. Thiocladospolide E (263) was produced by the mangrove endophytic fungus Cladosporium sp. (Cla. sp.) SCNU-F0001 and its absolute configuration was determined by X-ray diffraction [153]. Thiocladospolides F–J (264–268) were isolated from another mangrove-derived endophytic fungus species in the same Cla. genus [154]. The macrolide 6,7,9,10-tetrahydromutolide (269) was isolated from endophytic fungus Aplosporella javeedii [155]. Two trichothecene macrolides, myrothecines H and I (270–271), were obtained from the endophytic fungus Paramyrothecium roridum isolated from the medicinal plant Morinda officinalis [156].
Bacteria
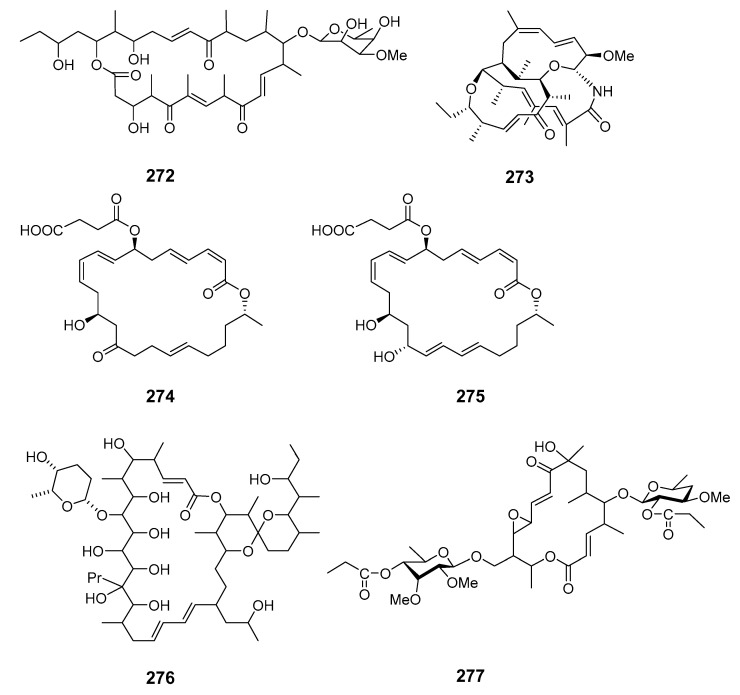
The 24-membered macrolide maduralide (272) was isolated from a marine bacterium in the order Actinomycetales [157]. Halichomycin (273) was produced by Streptomyces hygroscopicus (S. hygroscopicus) isolated from the marine fish Halichoeres bleekeri [158]. 7-O-Succinyl macrolactin F (274) and 7-O-succinyl macrolactin A (275) were isolated from a culture of marine Bacillus sp. (B. sp.) Sc026 [159].
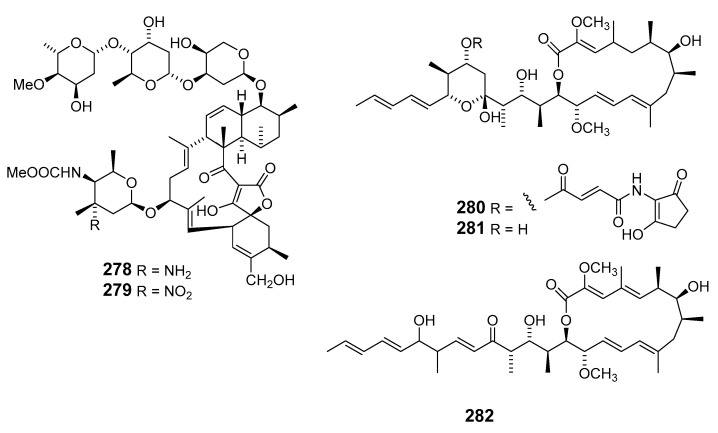
Cytotoxic macrolide IB-96212 (276) was obtained from marine actinomycete L-25-ES25-008 [160]. Chalcomycin B (277) was isolated from marine Streptomycete isolate B7064 and was bioactive in both microorganisms and microalgae [161]. Lobophorins A and B (278–279) have been extracted from culture broths of bacteria isolated from the surface of the Caribbean brown alga Lobophora variegata (Dictyotales) [162]. Micromonospolides A–C (280–282) were produced by Micromonospora sp. (M. sp.) and demonstrated inhibition of gastrulation in starfish embryos [163,164].
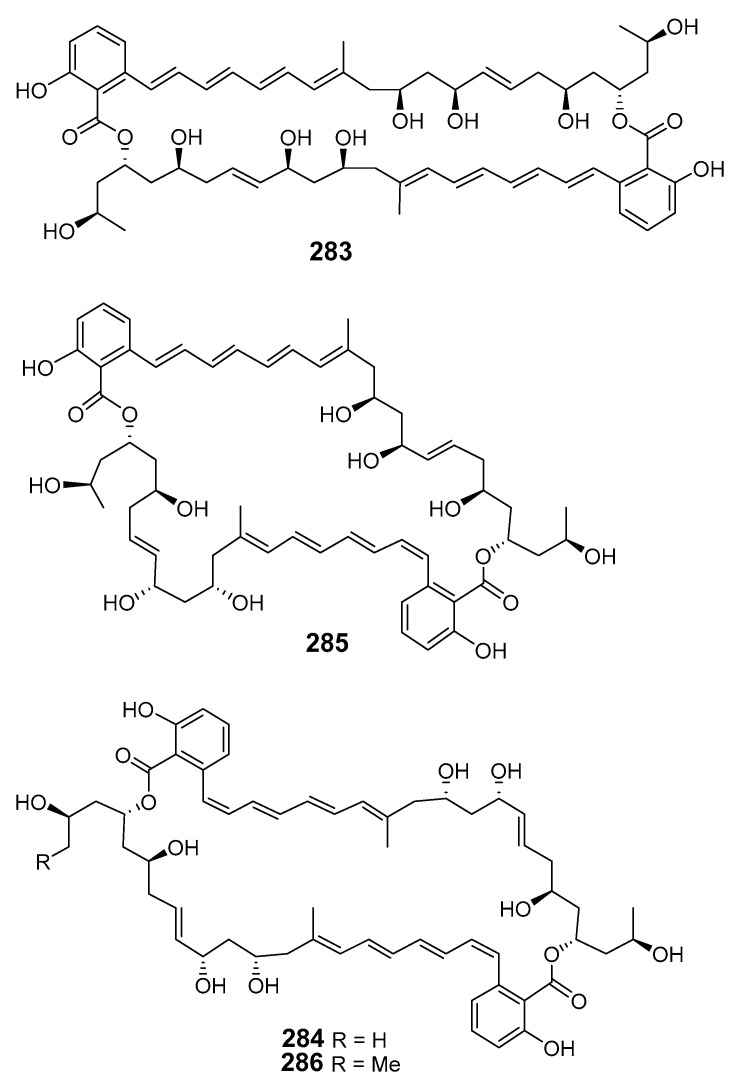
Marinomycins A–D (283–286) were isolated from actinomycete “Marinispora”. These marinomycins showed antibacterial activity towards methicillin-resistant S. aureus (MRSA), while marinomycin A inhibited vancomycin-resistant S. faecium (VREF) and C. albicans (weakly). Marinomycins A–C demonstrated cytotoxic activity against a panel of 60 tumor cell lines, including six of the eight melanoma cell lines [165].
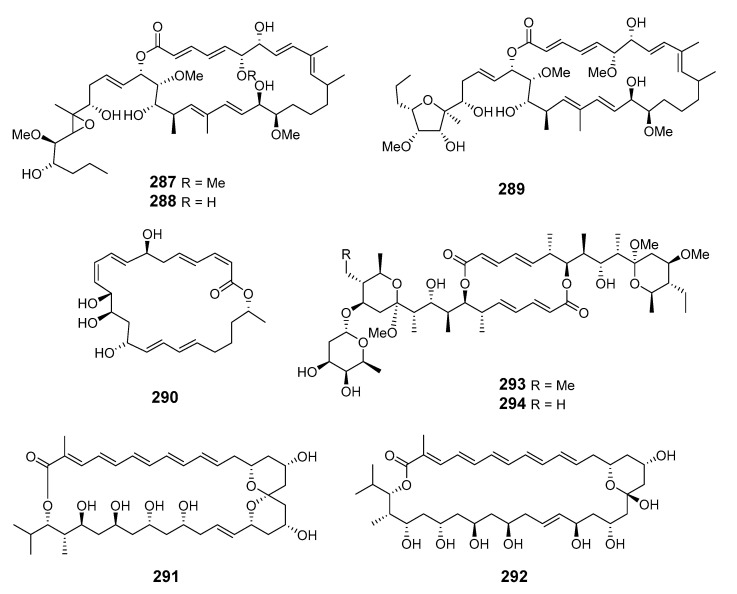
Marine actinomycete Salinispora arenicola yielded the three macrolide polyketides arenicolides A–C (287–289), with arenicolides A showing moderate cytotoxicity [166]. Macrolactin S (290) has been reported in a culture of marine Bacillus sp. [167]. The actinomycete strain CNQ-140 in the genus “Marinispora” yielded polyene macrolides marinisporolides A (291) and B (292), which photoisomerized to the geometric isomers marinisporolides C–E, suggesting that they may be artefacts [168]. S. hygroscopicus (associated with the marine fish Halichoeres bleekeri) produced halichoblelides B (293) and C (294), which are cytotoxic to tumor cells [169].
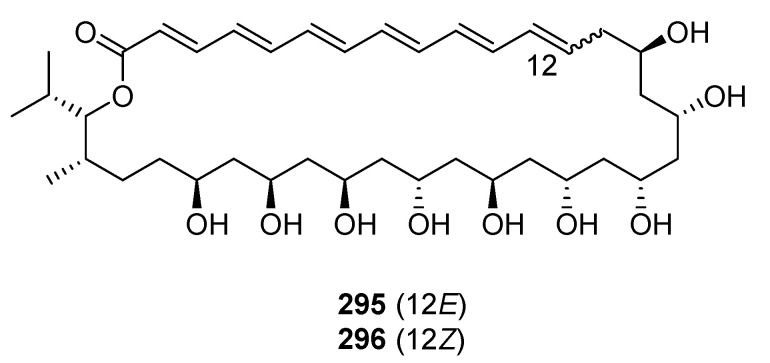
Two 36-membered macrolides, bahamaolides A and B (295–296), were obtained from the culture of a marine actinomycete S. sp. isolated from a sediment sample collected at North Cat Cay in the Bahamas [170].
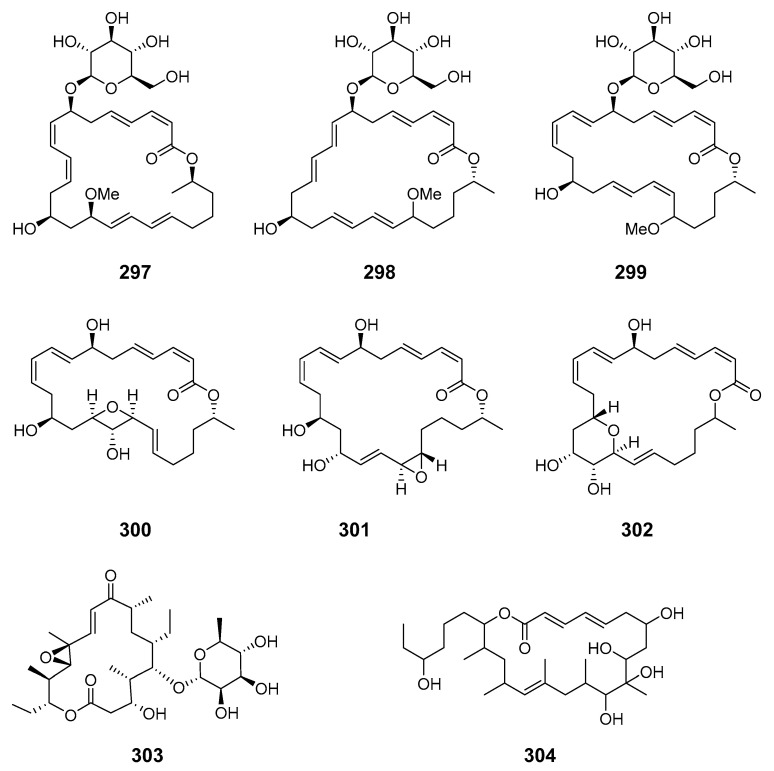
B. subtilis isolated from marine sediment collected at Gageocho (Republic of Korea) was a source of three new glycosylated methoxy-macrolactins (297–299) [171]. Three new 24-membered macrolactones, macrolactins X–Z (300–302), featuring an oxetane, an epoxide, and a tetrahydropyran ring, were isolated from an ethyl acetate extract of a marine B. sp. [172]. Cytotoxic juvenimicin C (303) was produced by a marine-derived actinomycete strain (CNJ-878) [173]. The M. strain FIM07-0019 isolated from shallow coastal waters near the island of Chiloe (Chile) produced a 20-membered macrolide, levantilide C (304) [174].
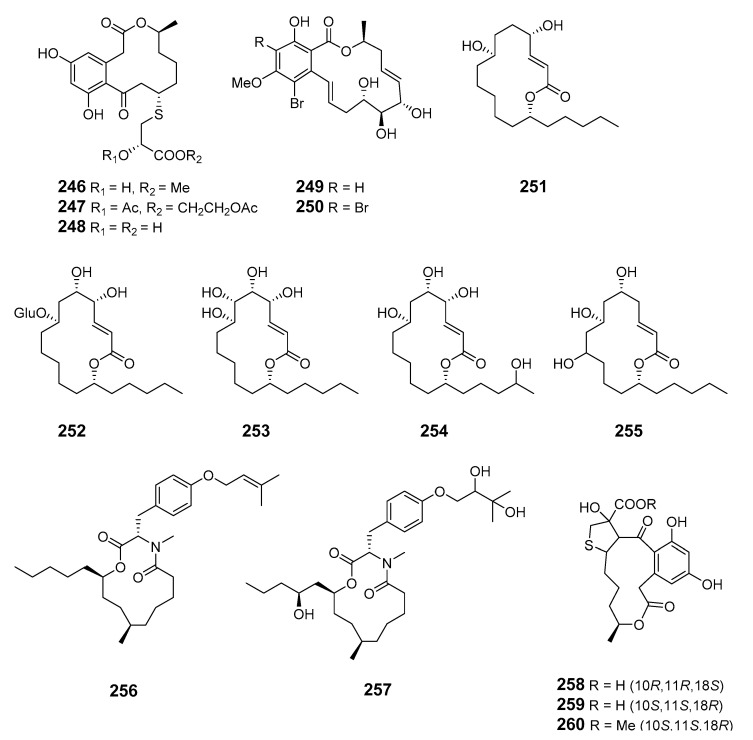
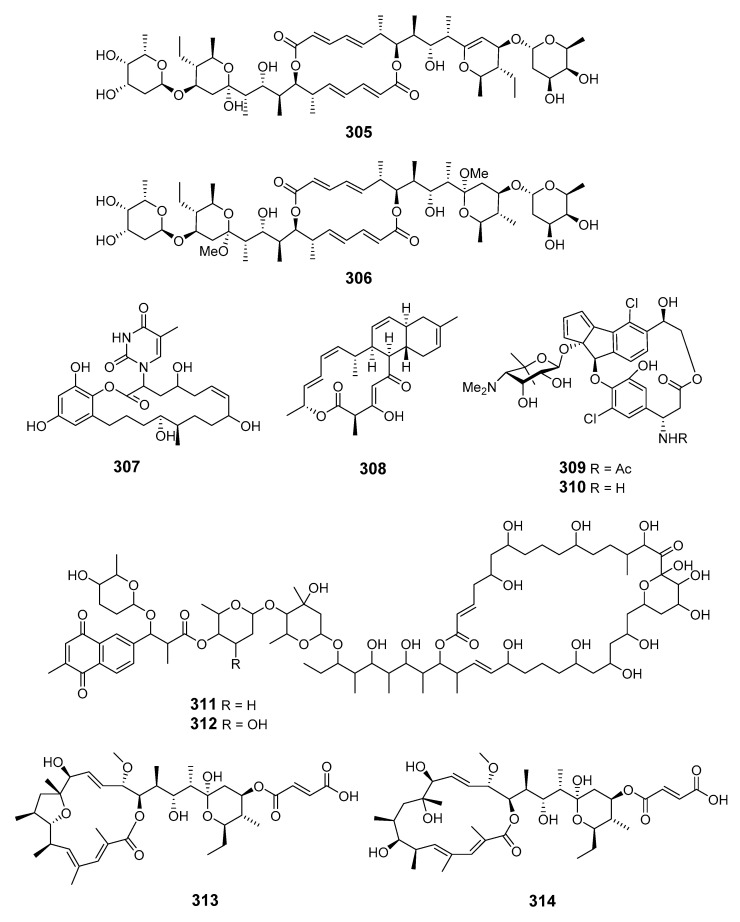
Investigation of a S. sp. in sediment from Heishijiao Bay (Dalian, China) yielded 11′,12′-dehydroelaiophylin (305) and 11,11′-O-dimethyl-14′-deethyl-14′-methylelaiophylin (306)—both 6-deoxyhexose-containing antibiotics—with the former exhibiting inhibition of MRSA and vancomycin-resistant Enterococci pathogens [175]. A rare 18-membered macrolide, macplocimine A (307), was produced by a marine-derived filamentous sulfur bacteria, Thioploca sp. [176]. A potent anthrax antibiotic, anthracimycin (308), was isolated from marine sediment-derived actinomycete S. sp. (Santa Barbara, California, U.S.A.) [177]. Fijiolides A (309) and B (310) were identified in marine-derived bacteria of the genus Nocardiopsis and demonstrated inhibition towards TNF-α-induced NFκB activation (fijiolide A to a greater extent than fijiolide B) [178]. Astolides A (311) and B (312) were obtained from S. hygroscopicus in the alkaline soil of the Saratov region of Russia. They exhibited significant cytotoxicity towards doxorubicin-resistant human leukemia cells [179]. Two hygrolidin macrolides, catenulisporidins A (313) and B (314), were isolated from the actinobacterium Catenulispora sp. KCB13F192 [180].
Cyanobacteria
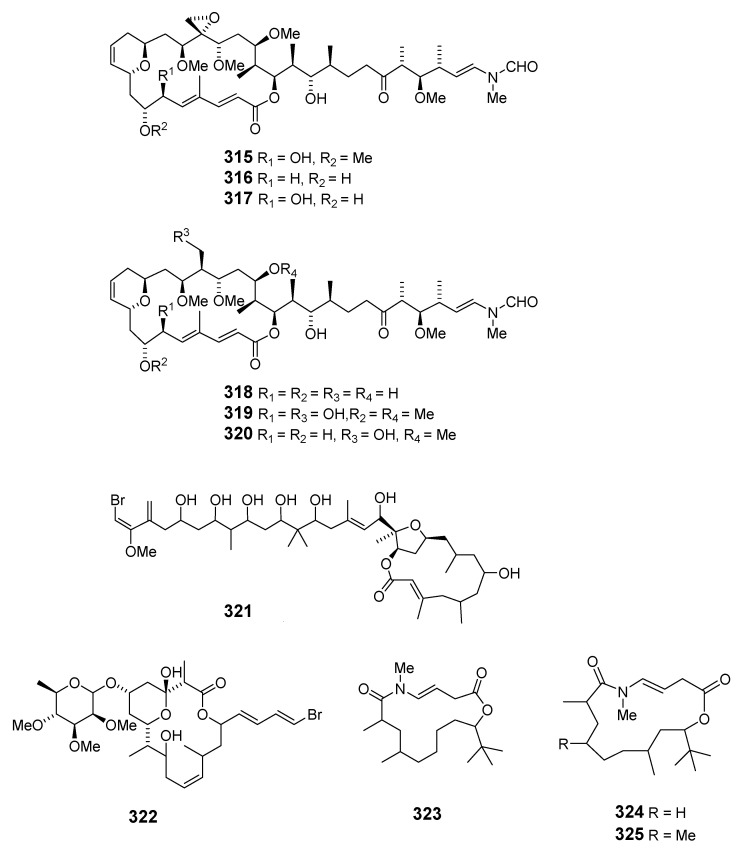
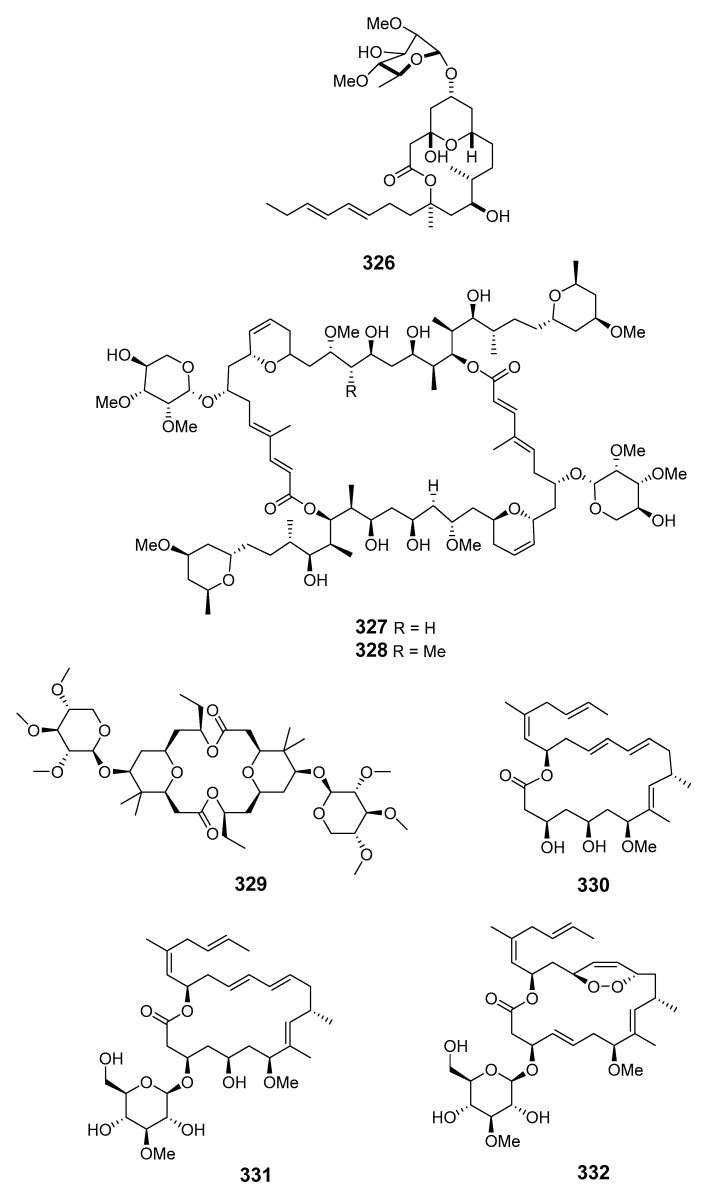
Cyanobacteria Scytonema mirabile BY-8-1, S. burmanicum DO-4-1, and S. ocellatum DD-8-1, FF-65-1 and FF-66-3 have been reported to produce tolytoxin (315). S. burmanicum DO-4-1 also yielded scytophycin B (316), 6-hydroxyscytophycin B (317), 19-O-demethylscytophycin C (318), 6-hydroxy-7-O-methylscytophycin E (319), and scytophycin E (320) [181]. A macrolide, oscillatoriolide (321), was isolated from Japanese Oscillatoria sp. and demonstrated inhibition towards fertilized echinoderm eggs [182]. The marine cyanobacterium Lyngbya bouillonii (L. bouillonii) collected on Laing Island (Papua New Guinea) produced lyngbyaloside (322) [183] in addition to the macrolides laingolide (323), madangolide (324), and laingolide A (325) [184,185], and the glycosidic macrolide lyngbouilloside (326), for which the configuration of C-11 was later revised [186,187].
Two glycosylated swinholides, ankaraholides A (327) and B (328), together with swinholide A previously obtained from the marine sponge T. swinhoei [91], were isolated from cyanobacterium Geitlerinema sp. collected in Madagascar [188]. Cyanolide A (329), demonstrating significant molluscicidal activity towards the snail vector Biomphalaria glabrata, was also isolated from L. bouillonii from Papua New Guinea [189]. Biselyngbyolide A (330) was isolated from L. sp. and showed strong apoptosis-inducing activity in HeLa S3 and HL60 cells [190], while its analogs, biselyngbyolide B–D (331–333), were produced by another L. cyanobacterium sampled on Tokunoshima Island (Japan) [191]. Biselyngbyolide B exhibited inhibition and apoptosis-inducing activity in HeLa S3 and HL60 cells and increased the cytosolic Ca2+ concentration in HeLa S3 cells [191].
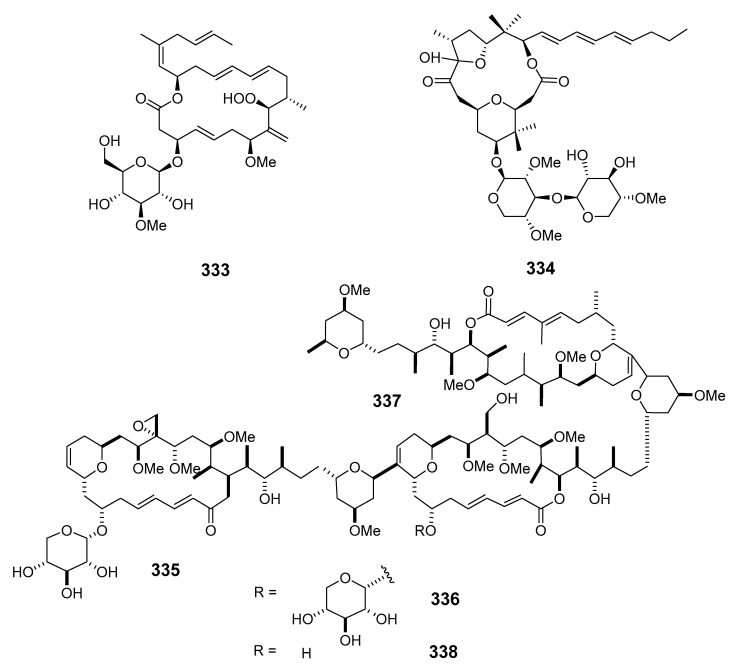
The Caribbean Okeania cyanobacterium VQR28MAR11-2 has been reported to produce polycavernoside D (334) [192], while four cytotoxic macrolides, leptolyngbyolides A–D (335–338), have been isolated from Leptolyngbya sp. collected in Okinawa [193].
Dinoflagellates
Amphidinolide E (339) was isolated from the Okinawan flatworm Amphiscolops sp. (Amphis. sp.) and exhibited cytotoxicity towards murine leukemia cells L1210 and L5178Y [194]. The absolute stereochemistry of amphidinolide E was determined by NMR spectroscopy, modified Mosher’s method and the exciton chirality method [195]. The potent cytotoxic macrolides amphidinolides F (340), G (341) and H (342) were produced by dinoflagellate Amphidinium sp. (Amphid. sp.) associated with the Okinawan flatworm Amphis. breviviridis [196,197].
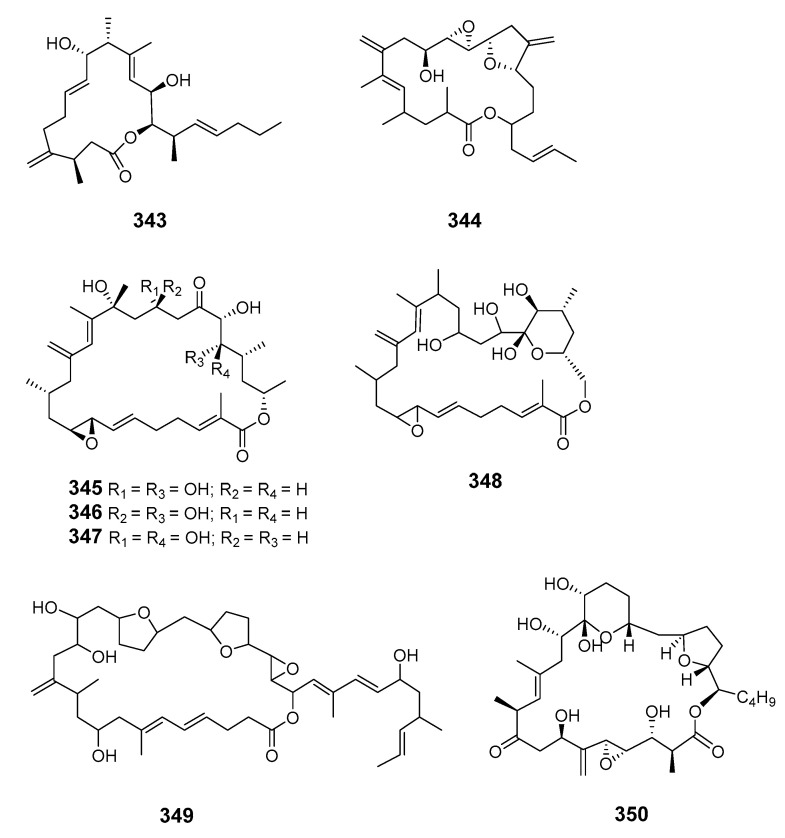
Amphidinolides G and H were elucidated by X-ray diffraction analysis and interconversion [198]. Amphidinolides J (343) and K (344) were isolated from symbiotic dinoflagellate Amphid. sp. and later synthesized [199,200]. Amphidinolides B1 (345), B2 (346) and B3 (347) were also isolated from Amphid. sp. [201,202,203], as were amphidinolides L (348), M (349) and N (350) [204,205,206].
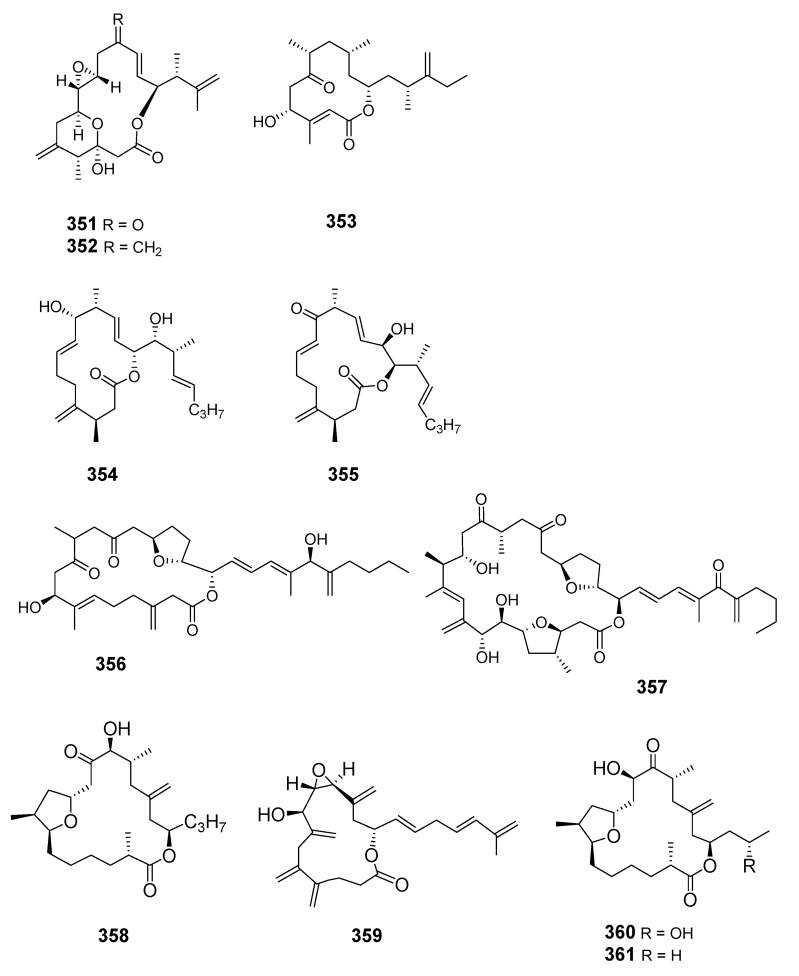
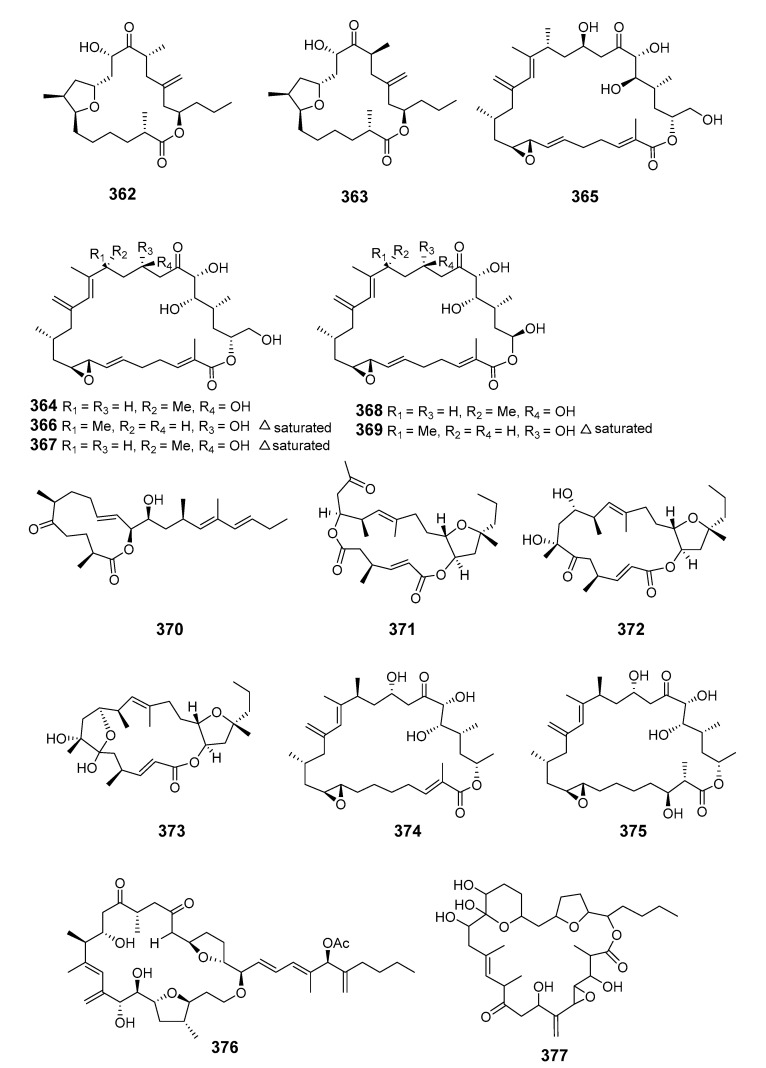
The structure of amphidinolide N was later revised and stereochemistry assigned [207]. Cytotoxic 15-membered macrolides, amphidinolides O (351) and P (352), were also isolated from Amphis. sp. [208]. The absolute stereochemistry of amphidinolide P was confirmed by convergent total synthesis [209]. The 12-membered macrolide amphidinolide Q (353), showing moderate cytotoxicity towards murine lymphoma L1210 cells in vitro (IC50 6.4 μg/mL), was obtained from the symbiotic flatworm Amphis. sp. of dinoflagellate Amphid. sp. [210]. Amphidinolide Q was synthesized stereoselectively by combined Julia coupling, Myers alkylation, and Yamaguchi lactonization [211]. The absolute configurations at five chiral centers in amphidinolide Q were determined as 4R, 7R, 9S, 11R, and 13R on the basis of NMR analysis and a modified Mosher’s method [212]. Cytotoxic macrolides amphidinolides R (354) and S (355) were also isolated from Amphid. sp. [213]. The 20-membered macrolide amphidinolide U (356) was obtained from a cultured Amphid. sp. Y-56 isolated from the flatworm Amphis. sp. in Okinawa [214]. A 25-membered macrolide, amphidinolide C3 (357), was also obtained from the Y-56 dinoflagellate strain and exhibited cytotoxicity towards P388, L1210 and KB cells [215]. Y-56 has also been reported to yield the 19-membered macrolide amphidinolide T (T1) (358) [216], while the A. sp. Y-5 produced the 14-membered polyene amphidinolide V (359) [217]. Total synthesis of amphidinolide V was accomplished and the absolute stereochemistry assigned [218]. Analogs of amphidinolides T2 (360), T3 (361), T4 (362), and T5 (363) were produced by Amphid. sp. [219,220]. Amphidinolides H2 (364), H3 (365), H4 (366), H5 (367), G2 (368), and G3 (369) were produced by Amphid. sp. strain Y-42 isolated from marine acoel flatworms Amphis. sp. The absolute configurations of these compounds were determined by coupling constant data, distance geometry calculations, and chemical means [221]. Amphidinolide T2 was synthesized using methyl (S)-lactate via a 16-step linear sequence [222]. Amphidinolide W (370) was isolated from an Amphid. sp. and the absolute stereochemistry determined by a combination of J-based configuration analysis and modified Mosher’s method [223]. Total synthesis was later achieved and its C-6 stereochemistry revised [224]. Amphidinolides X (371) and Y (372) were produced by symbiotic dinoflagellate Amphid. sp. strain Y-42 from Okinawan Amphis. species. Amphidinolide Y exists as a 9:1 equilibrium mixture of the 6-keto- and 6(9)-hemiacetal forms (373). Both amphidinolides X and Y showed significant cytotoxicity against murine lymphoma L1210 and human epidermoid carcinoma KB cells in vitro [225,226]. Two 26-membered macrolides, amphidinolides B6 (374) and B7 (375), were isolated from a culture of a symbiotic dinoflagellate Amphid. sp. from Amphis. sp. and demonstrated cytotoxicity against human B lymphocyte DG-75 cells [227]. Amphidinolide C2 (376) was isolated from dinoflagellate Amphid. sp. (Y-71 strain) [228].
The Amphid. strain S1-36-5 yielded the highly cytotoxic 26-membered caribenolide I (377) [229].
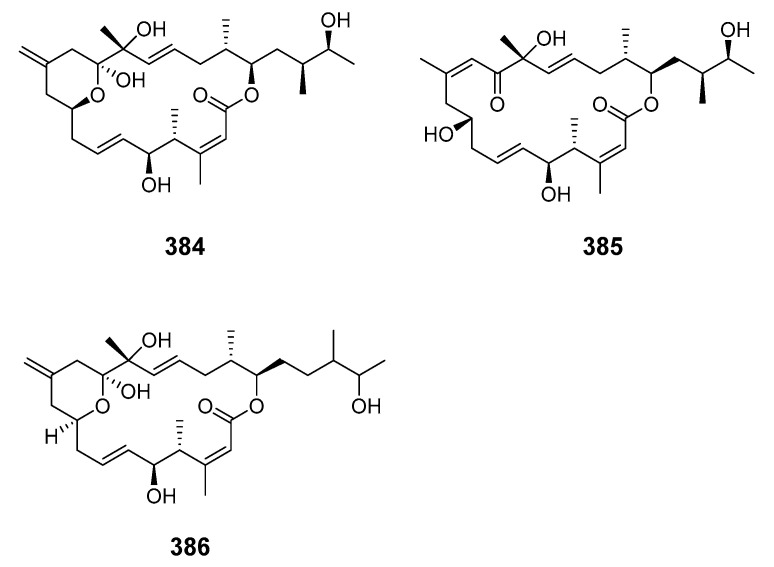
The 13-membered macrolide amphidinolactone A (378) and a 26-membered macrolide amphidinolactone B (379) have been isolated from cultures of Amphid. sp. Amphidinolactone A was synthesized totally via a ring-closing metathesis reaction and the absolute configuration was elucidated [8,230,231]. The vasoconstrictors zooxanthellatoxins A (380) and B (381) were isolated from a symbiotic dinoflagellate Symbiodinium sp. (Y-6 strain), which was associated with Amphis. sp. [232,233]. Bioassay-guided fractionation of a butanol extract of the tropical dinoflagellate Prorocentrum maculosum Faust yielded the fast-acting toxin prorocentrolide B (382) [234]. Hoffmanniolide (383) was identified in the marine dinoflagellate P. hoffmannianum [235]. The 20-membered iriomoteolide-1a (384), -1b (385) and -1c (386) were isolated from a marine benthic dinoflagellate Amphid. sp. (strain HYA024) [236,237].
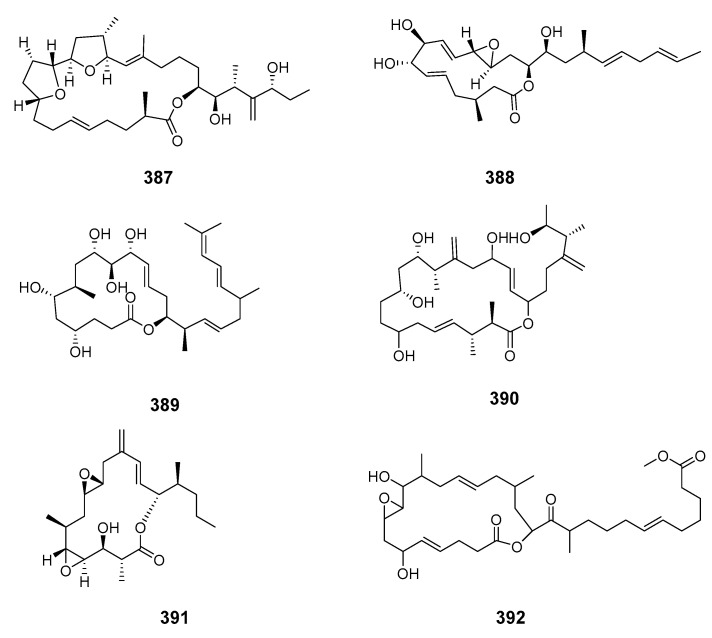
The cytotoxic 23-membered iriomoteolide-2a (387) was also obtained from Amphid. sp. [238]. The 15-membered macrolide iriomoteolide-3a (388) containing an allyl epoxide was obtained from Amphid. sp. strain HYA024 and was potently cytotoxic to human B lymphocyte DG-75 cells and Epstein–Barr virus (EBV)-infected Raji cells [239]. Iriomoteolide-4a (389) and -5a (390) were isolated from a benthic dinoflagellate Amphid. sp. (strain HYA024) and showed moderate cytotoxicity towards human B lymphocytes DG-75 [240]. The 15- and 19-membered iriomoteolide-9a (391) and -11a (392) were cytotoxic towards human cervix adenocarcinoma HeLa and murine hepatocellular carcinoma MH134 cells [241].
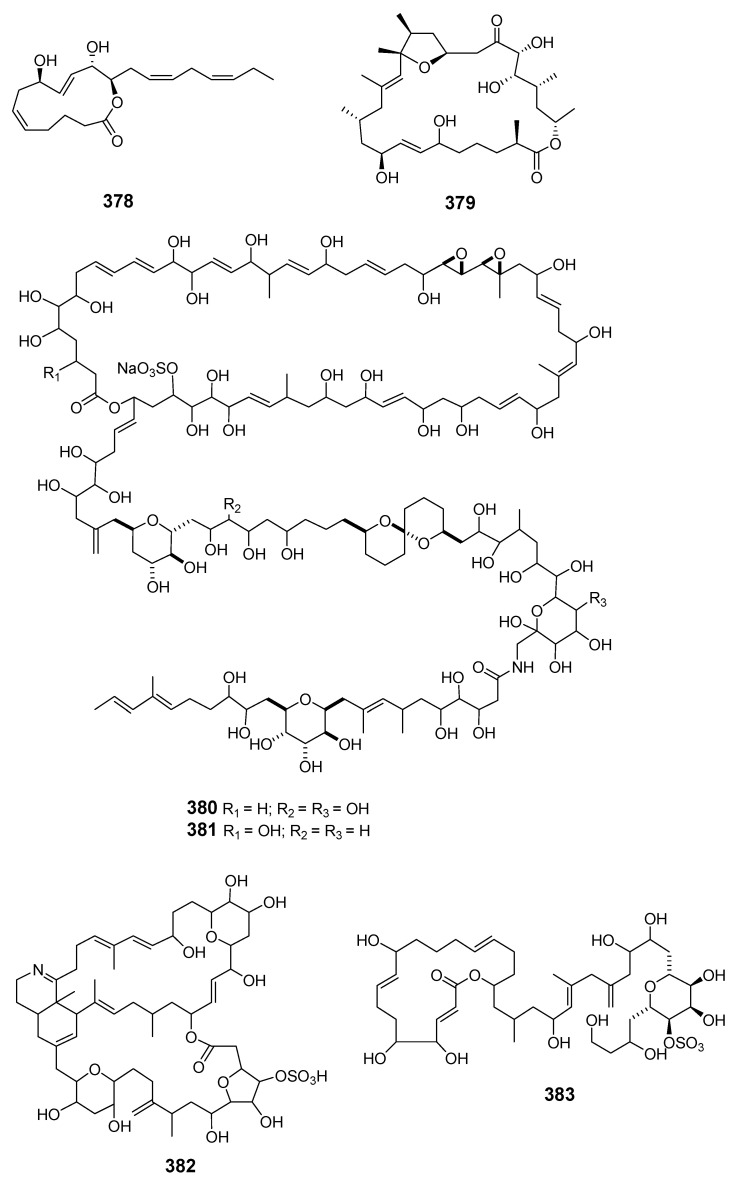
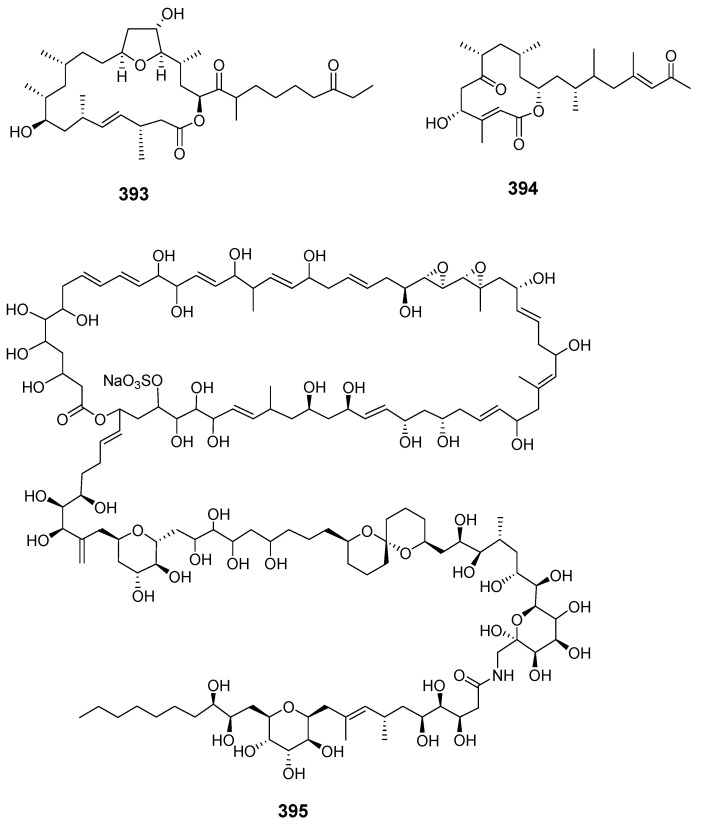
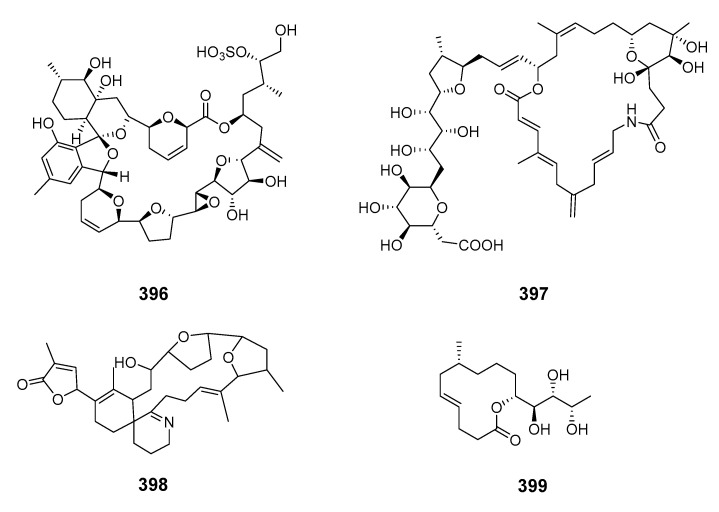
Iriomoteolide-10a (393) and -12a (394) were isolated from a marine dinoflagellate Amphid. sp. (KCA09053 strain) with iriomoteolide-10a being cytotoxic to human cervix adenocarcinoma HeLa and murine hepatocellular carcinoma MH134 cells [242]. The 62-membered novel polyol macrolide symbiodinolide (395) was isolated from the symbiotic dinoflagellate Symbiodinium sp. (S. sp.) and showed significant voltage-dependent N-type Ca2+ channel-opening activity at 7 nM and immediately ruptured the surface tissue of the acoel flatworm Amphis. sp. at 2.5 mM [14]. The stereochemistries of C-23–C-34 were revised by stereoselective synthesis and the (17S,18R,21R) configurations were determined by synthesis [243,244]. The synthesis of the C-33–C-42 fragment elucidated (36S,40S) and (C-1′–C-25′) [243,244,245]. The dinoflagellate-derived macrolide acuminolide A (396) caused potent stimulation of actomyosin ATPase activity [246]. The 25-membered polyketide-derived macrocycle belizentrin (397) was isolated from cultures of the marine dinoflagellate Prorocentrum belizeanum [247]. Gymnodimine D (398) was extracted and purified from a culture of dinoflagellate Alexandrium ostenfeldii from the Baltic Sea [248]. Symbiodinolactone A (399) was isolated from a culture of the symbiotic marine dinoflagellate S. sp. [249].
2.1.3. Red algae
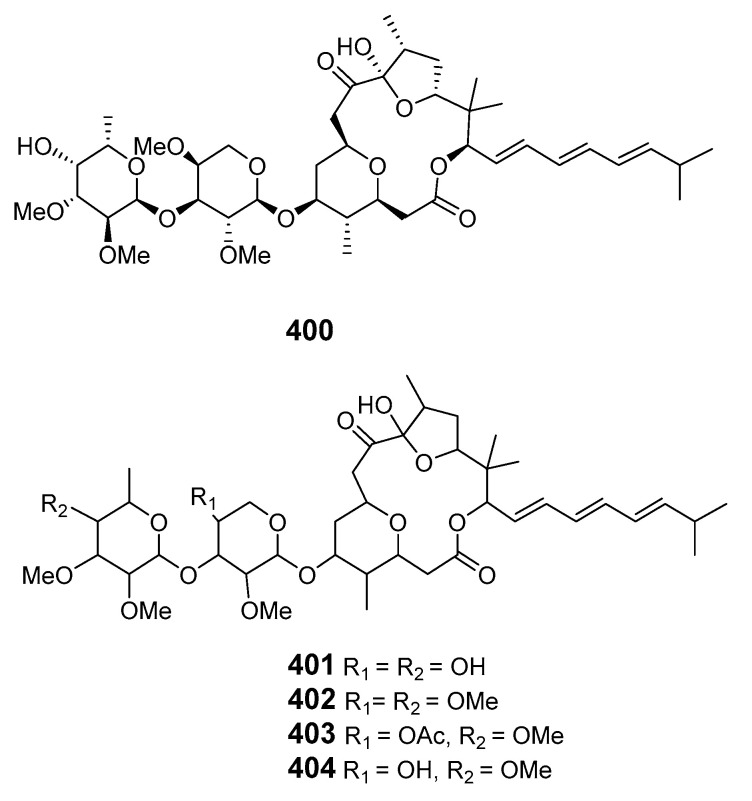
Polycavernosa tsudai (Gracilaria edulis) contained the macrolide polycavernoside A (400), which led to human illness and death in Guam [250]. The relative configuration of polycavernosolide A was assigned and the sugar substructure was synthesized [251,252]. Its structure was confirmed by total synthesis in a stereocontrolled manner [253]. Polycavernosides A2 (401), A3 (402), B (403) and B2 (404) were also obtained from Polycavernosa red algae [254].
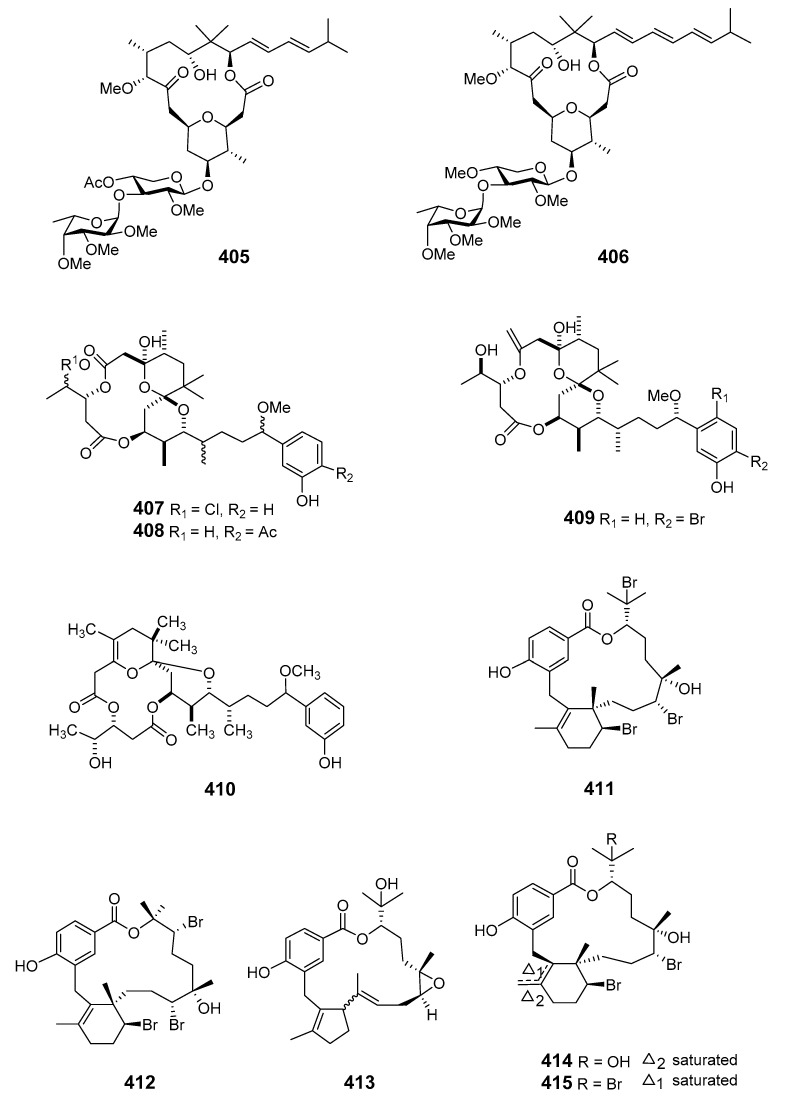
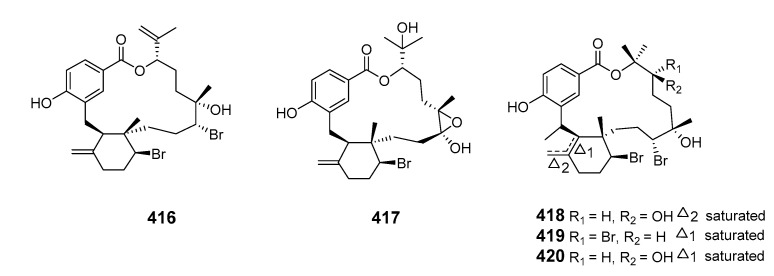
Two analogs of polycavernosolide A, polycavernosides C (405) and C2 (406), were isolated from the red alga Gracilaria edulis (G. edulis) [255]. Manauealides A–C (407–409) were isolated from extracts of red alga G. coronopifolia [256]. Anhydrodebromoaplysiatoxin (410) and manauealide C were extracted from Hawaiian G. coronopifolia [257]. Investigation of Fijian red alga Callophycus serratus (C. serratus) led to the isolation of three diterpene-benzoate natural products: bromophycolides A (411) and B (412), and a nonhalogenated compound (413). Bromophycolides A and B exhibited moderate antibacterial and antifungal properties while bromophycolides A demonstrated potent anti-HIV and moderate cytotoxic activities [258]. Bromophycolides C–I (414–420) were also isolated from extracts of C. serratus. All the bromophycolides exhibited modest antineoplastic activity towards a range of human tumor cell lines while bromophycolides F and I showed weak antifungal activity [259].
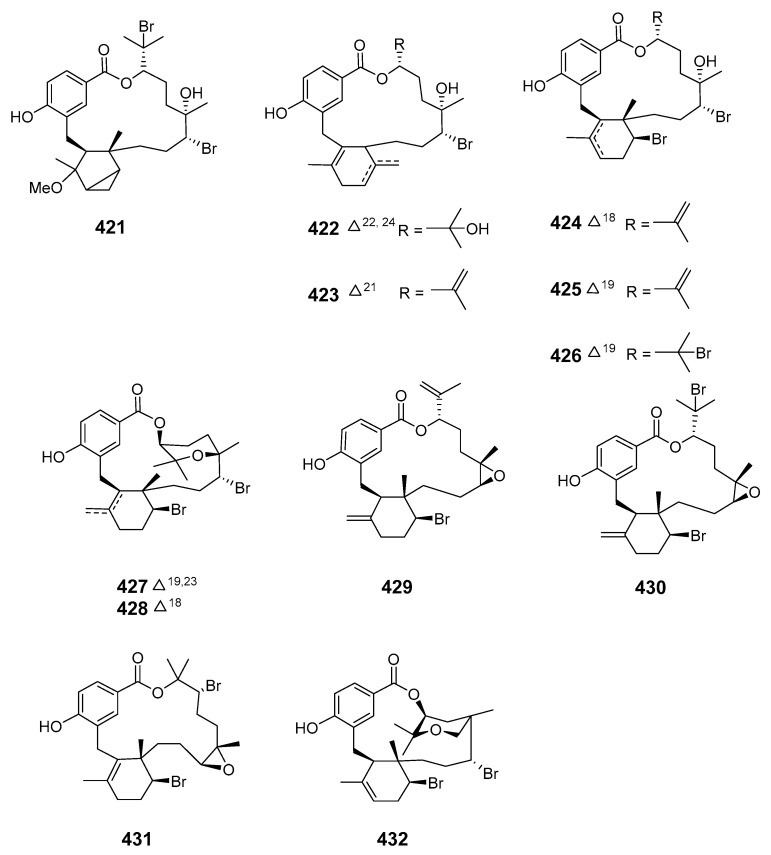
Further investigation of the C. serratus extract yielded a series of unusual antimalarial diterpene-benzoate macrolides, bromophycolides J–Q (421–428), with a range of moderate to strong antimicrobial and anticancer properties [260]. C. serratus was also a source of the diterpene-benzoate macrolides bromophycolides R–U (429–432). These demonstrated modest cytotoxicity toward selected human cancer cell lines while bromophycolide S was active (at submicromolar concentrations) against the human malaria parasite Plasmodium falciparum (Pla. falciparum) [261].
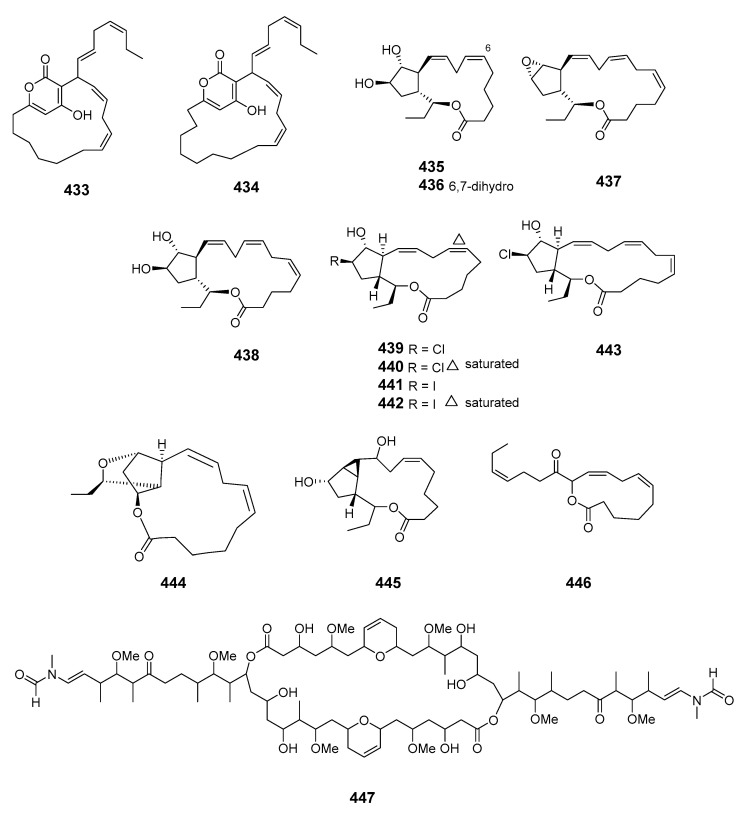
The α-pyrone macrolides neurymenolides A (433) and B (434) were obtained from the Fijian red alga Neurymenia fraxinifolia [262]. The brown alga Ecklonia stolonifera produced ecklonialactones C (435) and D (436) containing a 14-membered lactone moiety, and ecklonialactones E (437) and F (438), with a 16-membered moiety [263]. The absolute configurations of ecklonialactones A, B and E were determined from chiroptical data [264]. Eight oxylipins (439–446) with a macrolide scaffold and one cymathere-type oxylipin with an open ring were isolated from the brown alga Eisenia bicyclis. The absolute configurations of compounds 439–443 and 446 were determined by NMR spectroscopy with the relative stereochemistry at C-9 in 446 remaining unassigned [265]. The metamorphosis-enhancing macrodiolide luminaolide (447) was isolated from the crustose coralline alga Hydrolithon reinboldii and its absolute relative configuration was determined by NMR spectroscopy with the relationships of the two side chains to the macrolide ring remaining unassigned [266,267].
2.1.4. Cnidarians
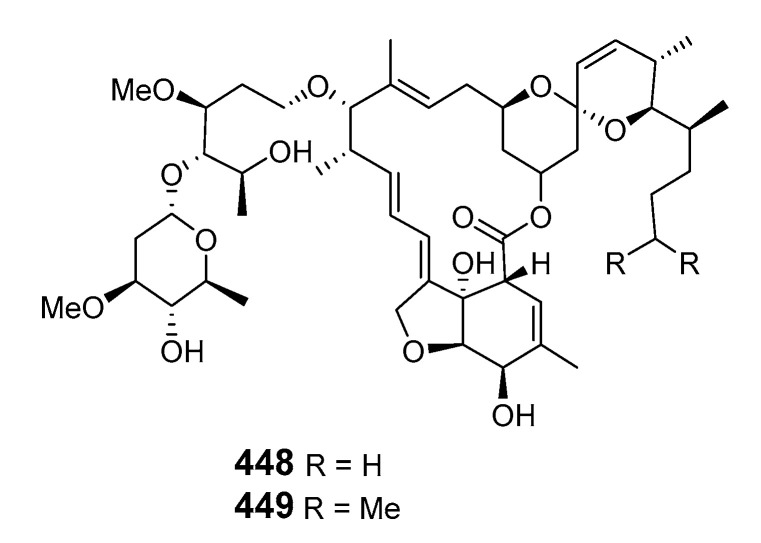
Two avermectin congeners, avermectins B1c (448) and B1e (449), exhibiting moderate antifouling activity were obtained from Anthrogorgia caerulea collected in the South China Sea [268].
2.1.5. Bryozoans
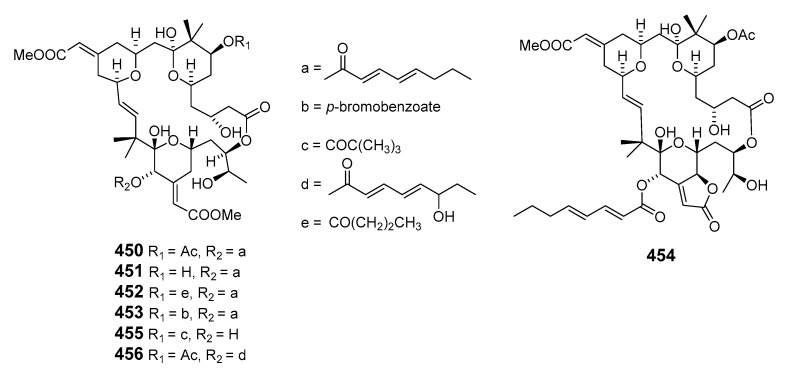
Large-scale isolation of bryostatin 1 (450) from the marine bryozoan Bugula neritina (L.) was carried out to provide material for clinical study [269]. Bryostatin 2 (451) has been converted to bryostatin 1 and bryostatin 12 (452) by selective protection and deprotection involving the C-26 hydroxyl group [270]. The stereochemistries of bryostatins 1 and 2 were assigned by X-ray analysis of p-bromobenzoate (453) [271], while the assignments of bryostatin 1 from 1H- and 13C-NMR were later revised [272]. Bryostatin 3 was isolated from B. neritina and reinvestigation of 2D NMR spectroscopic data revised the structure of bryostatin 3 to structure 454 [273].
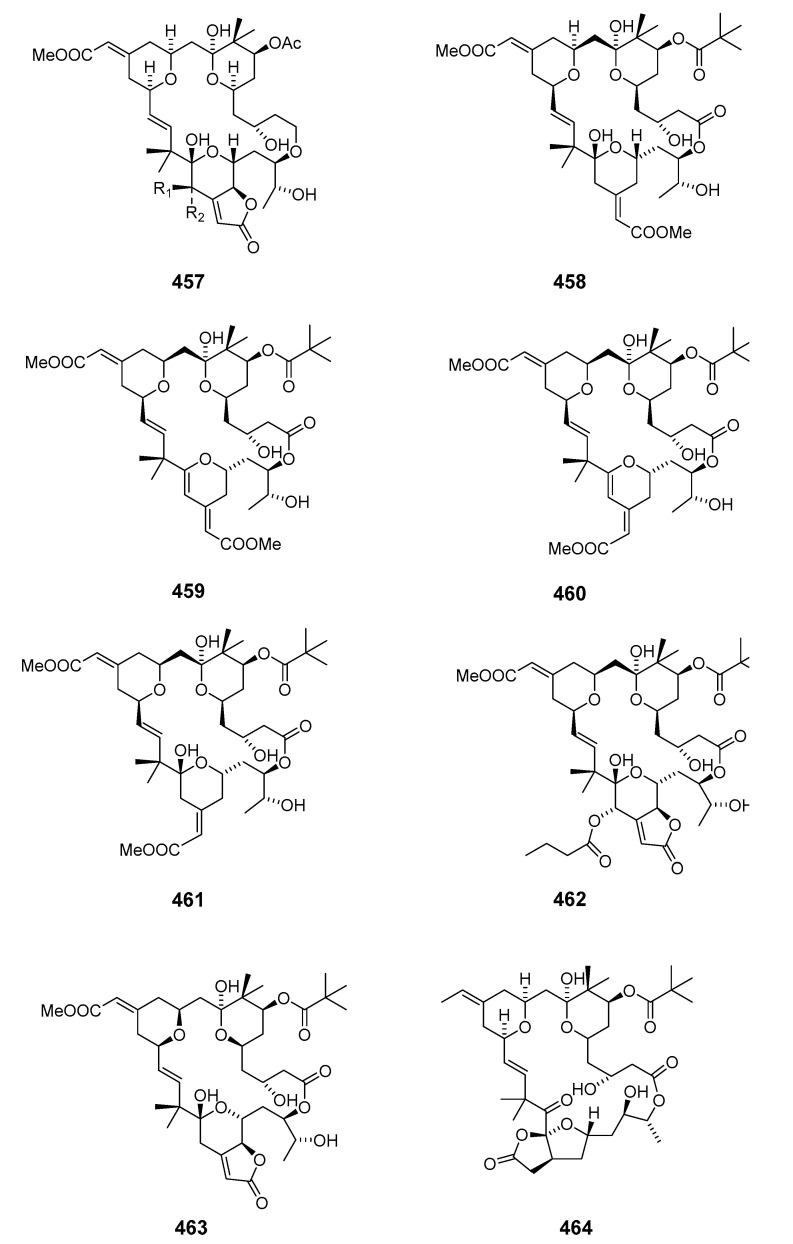
Further investigation of B. neritina led to the identification of bryostatins 14 (455) and 15 (456) [274]. The structures of bryostatin 3 and 20-epi-bryostatin 3 (457) have been elucidated by NMR spectroscopy [271,273,275]. Bryostatin 3 was then synthesized in an enantioselective manner [276]. Bryostatin 10 (458) was determined to be the major cytotoxic component of B. neritina [277]. Three additional antileukemic macrolides, bryostatins 16 (459), 17 (460), and 18 (461), were isolated in trace amounts from B. neritina from the Gulf of Mexico [278]. Antineoplastic bryostatin 19 (462) was isolated from B. neritina collected from the South China Sea [279]. A further member of the bryostatins, bryostatin 20 (463), was produced by the larvae of B. neritina and its structure determined by spectral comparison with previously described bryostatins [280]. Bioassay-guided isolation elucidated the first member of a new family of macrocycles, neristatin 1 (464), which was cytotoxic towards the P388 lymphocytic leukemia cell line [281].
2.1.6. Mollusks
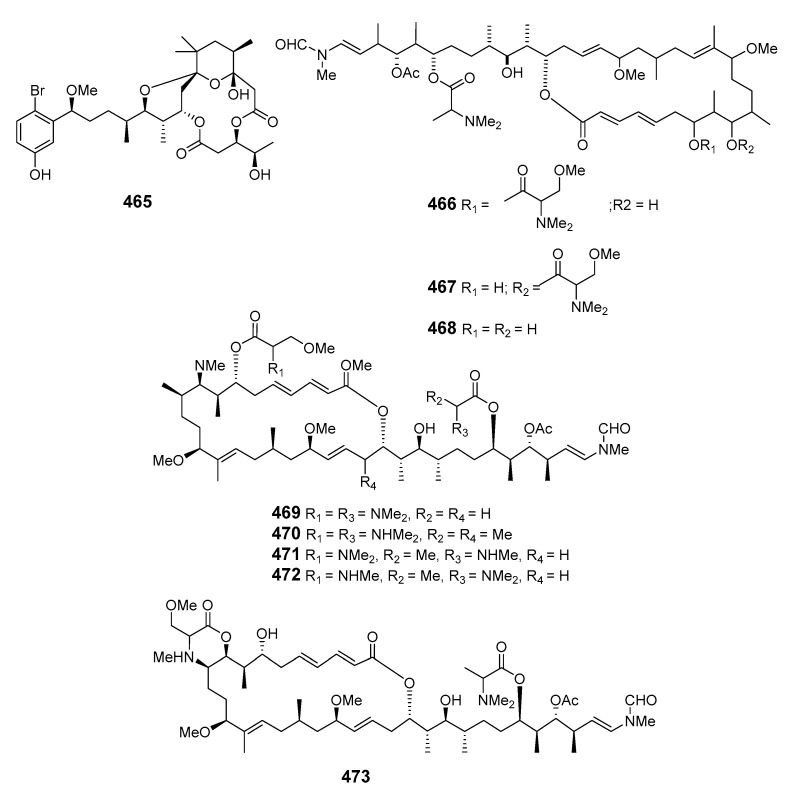
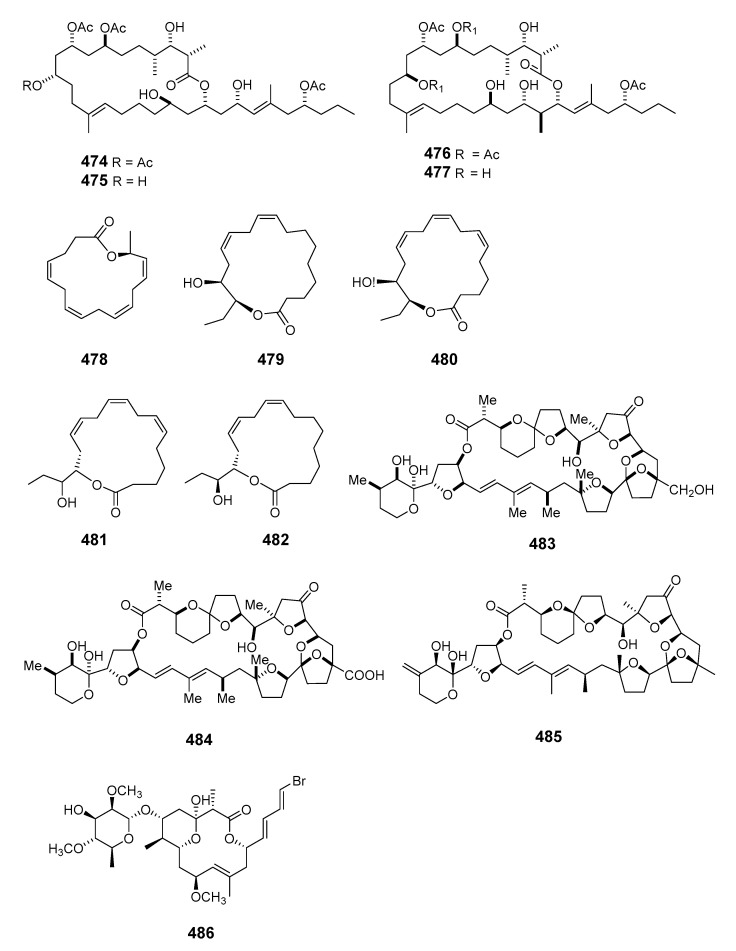
Aplysiatoxin (465) was isolated from an extract of the sea hare Stylocheilus longicauda and synthesized [282,283]. The sea hare Aplysia kurodai Baba contained the novel and potently cytotoxic macrolides aplyronines A (466), B (467) and C (468). The absolute configuration of aplyronine A was assigned following enantioselective synthesis of its degradation products and total synthesis was also reported [284,285]. Five cytotoxic macrolides, aplyronines D–H (469–473), were also isolated from the Japanese sea hare Aplysia kurodai [286]. The 22-membered macrolide dolabelide A (474) and the diacetyl derivative dolabelide B (475), both cytotoxins, were obtained from the Japanese sea hare Dolobella auricularia [287]. Cytotoxic 24-membered macrolides dolabelides C (476) and D (477) were also isolated from Dolabella auricularia, the originally assigned structure of dolabelide D being confirmed by total synthesis [288,289]. Five unprecedented C-16 and C-18 fatty acid lactones named aplyolides A–E (478–482) were found in the skin of the marine mollusk Aplysia depilans, and were ichthyotoxic to the mosquito fish Gambusia affinis [290]. The stereochemistry of (-)-aplyolide A was confirmed by synthesis [291] and the absolute stereochemistries of aplyolides B–E were confirmed by total synthesis [292,293]. Pectenotoxins 4 (483) and 7 (484) were isolated from Patinopecten yessoensis scallops [294]. LC–MS analysis of shellfish extracts identified PTX-12 (485) as a pectenotoxin accumulating in Norwegian blue mussels (Mytilus edulis) and cockles (Cerastoderma edule) [295]. Dolastatin 19 (486), containing a 14-membered macrocyclic lactone linked to a 2,4-di-O-methyl-L-R-rhamnopyranoside, was found in the Gulf of California in the shell-less mollusk Dolabella auricularia [296]. The stereochemistry of (+)-dolastatin 19 was confirmed by total synthesis [297].
2.1.7. Tunicates
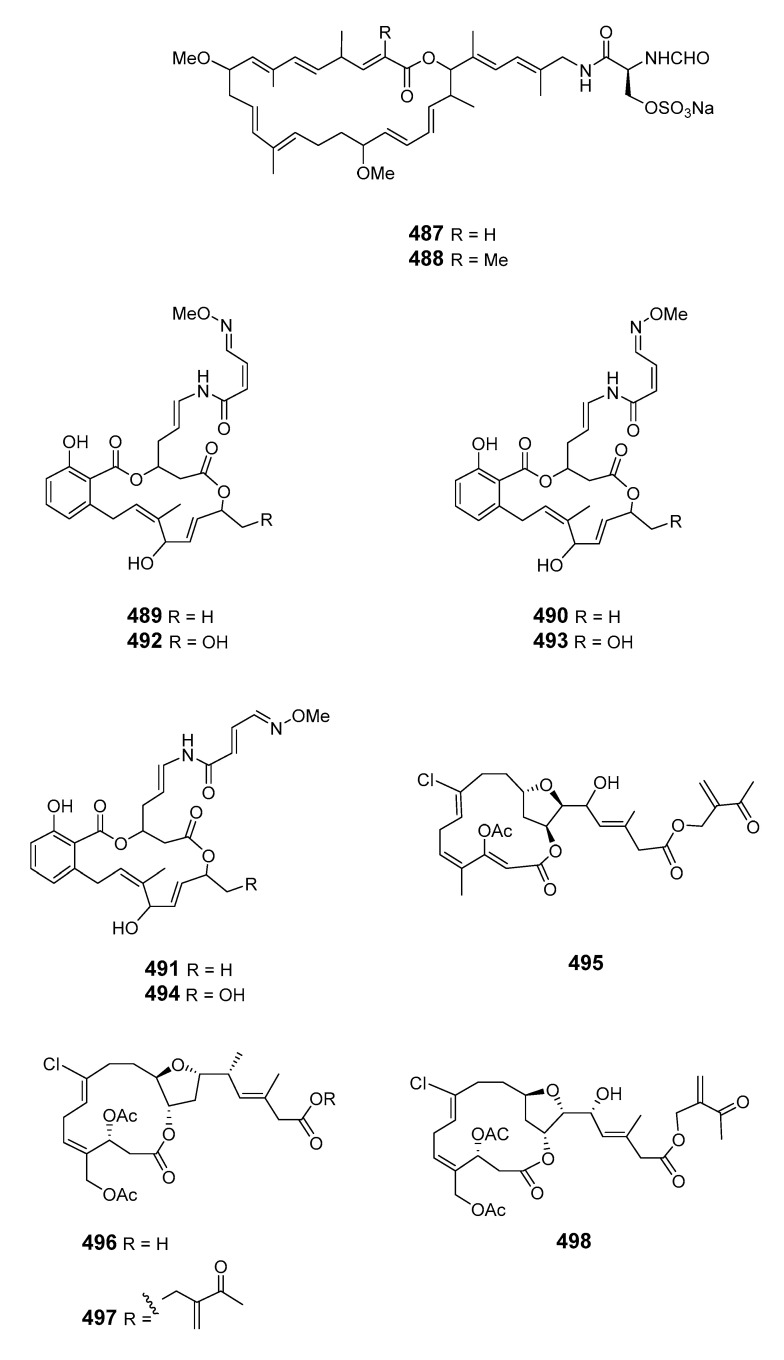
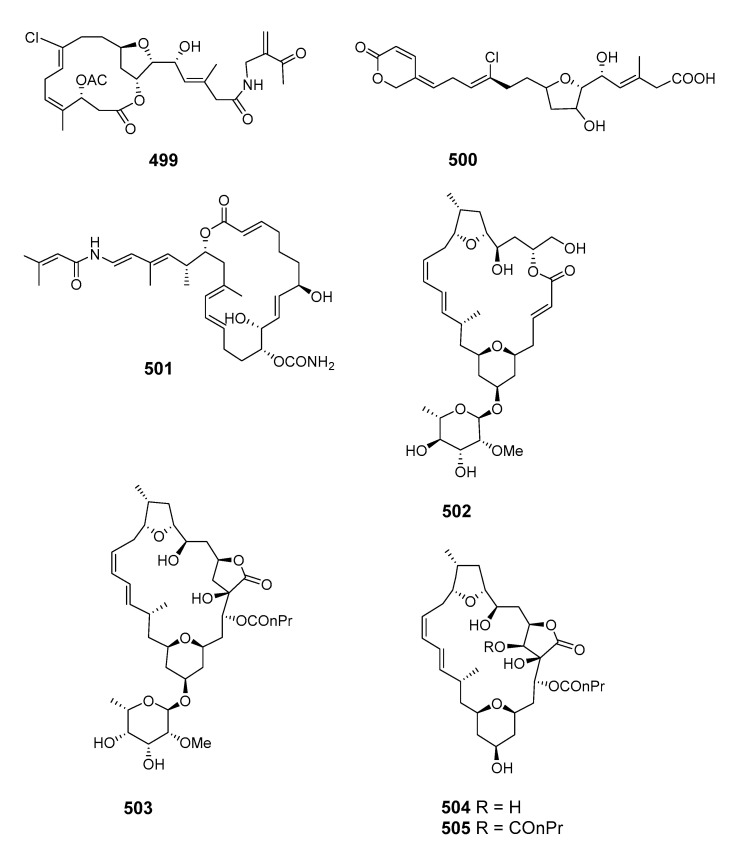
Two 24-membered macrolide sulfates showing antineoplastic activity, iejimalides C (487) and D (488), were isolated from the Okinawan tunicate Eudistoma cf. rigida [298]. Two cytotoxic macrolides, lobatamides A (489) and B (490), were reported in the tunicate Aplidium lobatum [299]. A. lobatum from shallow waters in Australia, A. sp. from deep water, and an unidentified Philippine ascidian have been reported as sources of a series of macrolides, lobatamides C–F (491–494), demonstrating cytotoxicity towards human tumor cell lines [300]. The absolute stereochemistry of lobatamide C was determined by stereospecific synthesis [301]. The chlorinated macrolide haterumalide B (495) was obtained from an Okinawan ascidian L. sp. by bioassay-guided isolation and was shown to inhibit the first cleavage of fertilized sea urchin eggs at 0.01 μg/mL [302]. The Okinawan ascidian Didemnidae sp. was the source of the macrolides biselides A (496) and B (497) [303]. Further investigation of the D. sp. led to the isolation of biselides C (498), D (499) and E (500) which exhibited cytotoxicity against human cancer cells NCI-H460 and MDA-MB-231 [304]. Cytotoxic palmerolide A (501) was obtained from the Antarctic tunicate Synoicum adareanum [305] and its stereochemistry was revised and confirmed by synthesis [306,307].
Glycosylated macrolides mandelalides A−D (502–505) were isolated from Lissoclinum ascidian collected in Algoa Bay near Port Elizabeth and the surrounding Nelson Mandela Metropole in South Africa [308].
2.2. Bioactivities of Marine-Derived Macrolides
The biological activities of marine-derived macrolides have been studied extensively. As listed in Table 1, marine macrolides harbor a broad range of bioactive properties including cytotoxicity, antibacteria, antifungi, antimitotic, antiviral, and other activities, with cytotoxicity being their most significant bioactivity.
3. Conclusions and Outlook
This review presents a summary of 505 marine-derived macrolides reported from 1990 to 2020 and highlights their chemical and biological diversity. As shown in Figure 1, sponges are the dominant producer of marine macrolides, yielding 173 of these 505 compounds (34.3%). Fungi and dinoflagellates are also important sources, producing 19.4% and 12.1%, respectively, of the macrolides reviewed. Marine animals (cnidarians, bryozoans, tunicates, and mollusks) produced significantly fewer macrolides with a combined percentage of 11.6%, while marine plants (red algae) yielded 9.5%. Marine microbes (including fungi, bacteria, cyanobacteria) produced 32.7% of 505 macrolides. Notably, macrolides obtained from sponges have fallen since 2010, while microbes, especially fungi, have grown to be important producers (Figure 2). This phenomenon suggests that biochemists are acknowledging that sampling slow-growing sessile organisms to identify natural products is not an eco-friendly practice. More attention is now being given to microbes due to their capacity for unlimited reproduction and the ease with which their genome can be mined for targeted metabolites. Marine macrolides have a broad range of properties, including cytotoxic, antifungal, antimitotic, and some other activities (Table 1). Cytotoxicity is their most significant bioactivity, highlighting that marine macrolides include many potential antitumor drug leads.
For macrolides with larger macrocyclic rings, such as reidispongiolides A and B [34], symbiodinolide [14] and zooxanthellatoxins A and B [232,233], the flexible ring structures make stereochemistry identification more difficult. Novel configuration determination technologies, such as sponge crystals [309], are needed to solve this problem. Although they possess diverse bioactivities, few marine macrolides have been developed into approved antitumor drugs or even for clinical trials during the last thirty years. Limited production from natural biomaterials and difficulties in synthesis may be hindering new drug discovery. High throughput screening and investigation of target prediction and additional bioactivity mechanisms must be employed to increase the successful discovery of lead compounds from marine macrolides. This should include mining for more structurally unusual macrolides with broader bioactivities.
Author Contributions
J.Z., X.Y., J.C., X.C., J.W. and Y.L. searched and collected literature; T.W. and H.Z. carried out the writing work. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China (NSFC; grant 41906093 to T.W.), the Natural Science Foundation of Zhejiang Province (grant LGF21D060003 to T.W.), Research Fund for Science in Ningbo University (XYL20021 to T.W.), the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund of Ningbo University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Woodward R.B. Struktur und biogenese der makrolide. Angew. Chem. 1957;69:50–58. doi: 10.1002/ange.19570690109. [DOI] [Google Scholar]
- 2.Oliynyk M., Samborskyy M., Lester J.B., Mironenko T., Scott N., Dickens S., Haydock S.F., Leadlay P.F. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat. Biotechnol. 2007;25:447–453. doi: 10.1038/nbt1297. [DOI] [PubMed] [Google Scholar]
- 3.Arsic B., Barber J., Čikoš A., Mladenovic M., Stankovic N., Novak P. 16-Membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents. 2018;51:283–298. doi: 10.1016/j.ijantimicag.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Butler M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 5.Lysenkova L.N., Turchin K.F., Korolev A.M., Dezhenkova L.G., Bekker O.B., Shtil A.A., Danilenko V.N., Preobrazhenskaya M.N. Synthesis and cytotoxicity of oligomycin a derivatives modified in the side chain. Bioorgan. Med. Chem. 2013;21:2918–2924. doi: 10.1016/j.bmc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 6.Salim A.A., Tan L., Huang X.C., Cho K.J., Lacey E., Hancock J.F., Capon R.J. Oligomycins as inhibitors of K-Ras plasma membrane localisation. Org. Biomol. Chem. 2016;14:711–715. doi: 10.1039/C5OB02020D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeed A.F.U.H., Su J., Ouyang S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021;134:111091. doi: 10.1016/j.biopha.2020.111091. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y., Kubota T., Kobayashi J. Amphidinolactone B, a new 26-membered macrolide from dinoflagellate Amphidinium sp. J. Antibiot. 2007;60:376–379. doi: 10.1038/ja.2007.51. [DOI] [PubMed] [Google Scholar]
- 9.Janas A., Przybylski P. 14- and 15-membered lactone macrolides and their analogues and hybrids: Structure, molecular mechanism of action and biological activity. Eur. J. Med. Chem. 2019;182:111662. doi: 10.1016/j.ejmech.2019.111662. [DOI] [PubMed] [Google Scholar]
- 10.Hu C., Zhang Y., Zhou Y., Liu Z.F., Meng Q., Feng X.S. A review of pretreatment and analysis of macrolides in food (update since 2010) J. Chromatogr. A. 2020;1634:461662. doi: 10.1016/j.chroma.2020.461662. [DOI] [PubMed] [Google Scholar]
- 11.Olano C., Mendez C., Salas J.A. Antitumor compounds from marine actionmycetes. Mar. Drugs. 2019;7:210. doi: 10.3390/md7020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpiński T.M. Marine macrolides with antibacterial and/or antifungal activity. Mar. Drugs. 2019;17:241. doi: 10.3390/md17040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumdei E.J., Blunt J.W., Munro M.H.G., Pannell L.K. Isolation of calyculins, calyculinamides, and swinholide H from the new zealand deep-water marine sponge Lamellomorpha strongylata. J. Org. Chem. 1997;62:2636–2639. doi: 10.1021/jo961745j. [DOI] [PubMed] [Google Scholar]
- 14.Kita M., Ohishi N., Konishi K., Kondo M., Koyama T., Kitamura M., Yamada K., Uemura D. Symbiodinolide, a novel polyol macrolide that activates N-type Ca2+ channel, from the symbiotic marine dinoflagellate Symbiodinium sp. Tetrahedron. 2007;63:6241–6251. doi: 10.1016/j.tet.2007.02.093. [DOI] [Google Scholar]
- 15.Alejandro M.S., Aimee J.G., Abimael D.R., Orazio T.S., Fumiaki N., Nobuhiro F. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-Inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs. 2020;18:5. doi: 10.3390/md18010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi M., Tanaka J., Katori T., Matsuura M., Yamashita M., Kitagawa I. Marine natural products. XXII. the absolute sereostructure of swinholide A, a potent cytotoxic dimeric macrolide from the okinawan marine sponge Theonella swinhoei. Chem. Pharm. Bull. 1990;38:2409–2418. doi: 10.1248/cpb.38.2409. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa I., Kobayashi M., Katori T., Yamashita M. Absolute stereostructure of swinholide A, a potent cytotoxic macrolide from the okinawan marine sponge Theonella swinhoei. J. Am. Chem. Soc. 1990;112:3710–3712. doi: 10.1021/ja00165a094. [DOI] [Google Scholar]
- 18.Kobayashi M., Tanaka J., Katori T., Kitagawa I. Marine natural products. XXIII. three new cytotoxic dimeric macrolides, swinholides B and C and isoswinholide A, congeners of swinholide A, from the okinawan marine sponge Theonella swinhoei. Chem. Pharm. Bull. 1990;38:2960–2966. doi: 10.1248/cpb.38.2960. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto S., Ishibashi M., Sasaki T., Kobayashi J. New congeners of swinholides from the okinawan marine sponge Theonella sp. J. Chem. Soc. 1991;23:3185–3188. doi: 10.1039/p19910003185. [DOI] [Google Scholar]
- 20.Kobayashi J., Tsukamoto S., Island I., Island Z. New congeners of bistheonellides from okinawan marine sponges of the genus Theonella. J. Chem. Soc. Chem. 1991:2379–2383. doi: 10.1039/p19910002379. [DOI] [Google Scholar]
- 21.Higa T., Tanaka J., Komesu M. Miyakolide: A bryostatin-like macrolide from a spongge Polyfibrospongia sp. J. Am. Chem. Soc. 1992;114:7587–7588. doi: 10.1021/ja00045a055. [DOI] [Google Scholar]
- 22.Fusetani N., Sugawara T., Matsunaga S., Hirota H. Cytotoxic metabolites of the marine sponge Mycale adhaerens lambe. J. Org. Chem. 1991;56:4971–4974. doi: 10.1021/jo00016a031. [DOI] [Google Scholar]
- 23.Pettit G.R., Gao F., Doubek D.L., Boyd M.R., Hamel E., Bai R., Schmide J.M., Tackett L.P., Ruetzier K. ChemInform abstract: Antineoplastic agents. part 252. isolation and structure of halistatin 2 from the comoros marine sponge Axinella carteri. ChemInform. 2010;24:371–377. doi: 10.1002/chin.199346264. [DOI] [Google Scholar]
- 24.Pettit G.R., Tan R., Gao F., Williams M.D., Doubek D.L., Boyd M.R., Schmidt J.M., Chapuis J., Hamel E., Bai R., et al. Isolation and structure of halistatin 1 from the eastern indian ocean marine sponge phakellia carteri. J. Org. Chem. 1993;58:2538–2543. doi: 10.1021/jo00061a030. [DOI] [Google Scholar]
- 25.Pettit G.R., Ichihara Y., Wurzel G., Williams M.D., Schmidt J.M. Isolation and structure of halistatin 3 from the western pacific (chuuk) marine sponge Phakellia sp. J. Chem. Soc. Chem. Commun. 1995;3:383–385. doi: 10.1039/c39950000383. [DOI] [Google Scholar]
- 26.Pettit G.R., Chicacz Z.A., Gao F., Herald C.L., Boyd M.R., Schmidt J.M., Hooper J.N.A. Antineoplastic agents. 257. isolation and structure of spongistatin 1. J. Org. Chem. 1993;58:1302–1304. doi: 10.1021/jo00058a004. [DOI] [Google Scholar]
- 27.Pettit G.R., Cichacz Z.A., Gao F., Herald C.L., Boyd M.R. Antineoplastic agents. part 282. isolation and structure of the remarkable human cancer cell growth iinhibitors spongistatins 2 (Ia) and 3 (Ib) from an eastern Indian ocean Spongia sp. J. Chem. Soc. 1993;14:1166–1168. [Google Scholar]
- 28.Pettit G.R., Herald C.L., Cichacz Z.A., Gao F., Schmidt J.M., Boyd M.R., Christie N.D., Boettner F.E. Isolation and structure of the powerful human cancer cell growth inhibitors spongistatins 4 and 5 from an African Spirastrella spinispirulifera (Porifera) J. Chem. Soc. Chem. Commun. 1993;3:1805–1807. doi: 10.1039/c39930001805. [DOI] [Google Scholar]
- 29.Pettit G., Herald C., Cichacz Z., Gao F., Boyd M., Christie N., Schmidt J. Antineoplastic agents 293. the exceptional human cancer cell growth inhibitors spongistatins 6 and 7. Nat. Prod. Lett. 1993;3:239–244. doi: 10.1080/10575639308043871. [DOI] [Google Scholar]
- 30.D’Auria M.V., Paloma L.G., Minale L., Zampella A., Verbist J.F., Roussakis C., Debitus C. Three new potent cytotoxic macrolides closely related to sphinxolide from the new caledonian sponge Neosiphonia superstes. Tetrahedron. 1993;49:8657–8664. doi: 10.1016/S0040-4020(01)96271-4. [DOI] [Google Scholar]
- 31.Kobayashi J., Murata O., Shigemori H., Sasaki T. Jaspisamides A–C, new cytotoxic macrolides from the okinawan sponge Jaspis sp. J. Nat. Prod. 1993;56:787–791. doi: 10.1021/np50095a021. [DOI] [PubMed] [Google Scholar]
- 32.Pettit G.R., Cichacz Z.A., Gao F., Boyd M.R., Schmidt J.M. Isolation and structure of the cancer cell growth inhibitor dictyostatin 1. J. Chem. Soc. Chem. Commun. 1994;9:1111–1112. doi: 10.1039/c39940001111. [DOI] [Google Scholar]
- 33.Paterson I., Britton R., Delgado O., Wright A.E. Stereochemical determination of dictyostatin, a novel microtubule-stabilizing macrolide from the marine sponge Corallistidae sp. Chem. Commun. 2004;6:632–633. doi: 10.1039/b316390c. [DOI] [PubMed] [Google Scholar]
- 34.D’Auria M.V., Paloma L.G., Minale L., Zampella A., Verbist J.F., Roussakis C., Debitus C., Patissou J. Reidispongiolide A and B, two new potent cytotoxic macrolides from the new caledonian sponge Reidispongia coerulea. Tetrahedron. 1994;50:4829–4834. doi: 10.1016/S0040-4020(01)85019-5. [DOI] [Google Scholar]
- 35.Zampella A., Sepe V., D’Orsi R., Bifulco G., Bassarello C., D’Auria M.V. Stereochemical assignment of the C23–C35 portion of sphinxolide/reidispongiolide class of natural products by asymmetric synthesis. Tetrahedron Asymmetry. 2003;14:1787–1798. doi: 10.1016/S0957-4166(03)00372-0. [DOI] [Google Scholar]
- 36.Paterson I., Ashton K., Britton R., Cecere G., Chouraqui G., Florence G.J., Stafford J. Total synthesis of (-)-reidispongiolide A, an actin-targeting marine macrolide. Angew. Chem. Int. Ed. 2007;46:6167–6171. doi: 10.1002/anie.200702178. [DOI] [PubMed] [Google Scholar]
- 37.Paterson I., Britton R., Ashton K., Knust H., Stafford J. Synthesis of antimicrofilament marine macrolides: Synthesis and configurational assignment of a C5–C16 degradation fragment of reidispongiolide A. Proc. Natl. Acad. Sci. USA. 2004;101:11986–11991. doi: 10.1073/pnas.0401548101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valeria M., A D., Gomez L., Minu L., Zampella A. A novel cytotoxic macrolide, superstolide B, related to superstolide A, from the new caledonian marine sponge Neosiphonia superstes. J. Nat. Prod. 1994;57:1595–1597. doi: 10.1021/np50113a024. [DOI] [PubMed] [Google Scholar]
- 39.D’Auria M.V., Paloma L.G., Zampella A., Debiti C., Minale L. Superstolide A: A potent cytotoxic macrolide of a new type from the new caledonian deep water marine sponge Neosiphonia superstes. J. Am. Chem. Soc. 1994;116:6658–6663. doi: 10.1021/ja00094a022. [DOI] [Google Scholar]
- 40.Horton P.A., Koehn F.E., Longley R.E., McConnell O.J. Lasonolide A, a new cytotoxic macrolide from the marine sponge Forcepia sp. J. Am. Chem. Soc. 1994;116:6015–6016. doi: 10.1021/ja00092a081. [DOI] [Google Scholar]
- 41.Litaudon M., Hart J.B., Blunt J.W., Lake R.J., Munro M. Isohomohalichondrin B, a new antitumour polyether macrolide from the new zealand deep-water sponge Lissodendoryx sp. Tetrahedron Lett. 1994;35:9435–9438. doi: 10.1016/S0040-4039(00)78563-7. [DOI] [Google Scholar]
- 42.Searle P.A., Molinski T.F. Phorboxazoles A and B: Potent cytostatic macrolides from marine sponge Phorbas sp. J. Am. Chem. Soc. 1995;117:8126–8131. doi: 10.1021/ja00136a009. [DOI] [Google Scholar]
- 43.Molinski T.F. Absolute configuration of phorboxazoles A and B from the marine sponge, Phorbas sp. 2. C43 and complete stereochemistry. Tetrahedron Lett. 1996;37:7879–7880. doi: 10.1016/0040-4039(96)01804-7. [DOI] [Google Scholar]
- 44.Jefford C.W., Bernardinelli G., Tanaka J.I., Higa T. Structures and absolute configurations of the marine toxins, latrunculin A and laulimalide. Tetrahedron Lett. 1996;37:159–162. doi: 10.1016/0040-4039(95)02113-2. [DOI] [Google Scholar]
- 45.Tanaka J.I., Higa T. Zampanolide, a new cytotoxic macrolide from a marine sponge. Tetrahedron Lett. 1996;37:5535–5538. doi: 10.1016/0040-4039(96)01149-5. [DOI] [Google Scholar]
- 46.Tanaka J., Higa T., Bernardinelli G., Jefford C.W. New cytotoxic macrolides from the sponge Fasciospongia rimosa. Chem. Lett. 1995;25:255–256. doi: 10.1246/cl.1996.255. [DOI] [Google Scholar]
- 47.Kuramoto M., Tong C., Yamada K., Chiba T., Hayashi Y., Uemura D. Halichlorine, an inhibitor of VCAM-1 induction from the marine sponge Halichondria okadai kadota. Tetrahedron Lett. 1996;37:3867–3870. doi: 10.1016/0040-4039(96)00703-4. [DOI] [Google Scholar]
- 48.D’Ambrosio M., Guerriero A., Debitus C., Pietra F. Leucascandrolide A, a new type of macrolide: The first powerfully bioactive metabolite of calcareous sponges (Leucascandra caveolata, a new genus from the coral sea) Helv. Chim. Acta. 1996;79:51–60. doi: 10.1002/hlca.19960790107. [DOI] [Google Scholar]
- 49.Zampella A., D’Auria M.V., Minale L., Debitus C., Roussakis C. Callipeltoside A: A cytotoxic aminodeoxy sugar-containing macrolide of a new type from the marine lithistida sponge Callipelta sp. J. Am. Chem. Soc. 1996;118:11085–11088. doi: 10.1021/ja9621004. [DOI] [Google Scholar]
- 50.Trost B.M., Dirat O., Gunzner J.L. Callipeltoside A: Assignment of absolute and relative configuration by total synthesis (p 841–843) Angew. Chem. Int. Ed. 2002;41:841–843. doi: 10.1002/1521-3773(20020301)41:5<841::AID-ANIE841>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Evans D.A., Hu E., Burch J.D., Jaeschke G. Enantioselective total synthesis of callipeltoside A. J. Am. Chem. Soc. 2002;124:5654–5655. doi: 10.1021/ja026235n. [DOI] [PubMed] [Google Scholar]
- 52.Trost B.M., Gunzner J.L., Dirat O., Rhee Y.H. Callipeltoside A: Total synthesis, assignment of the absolute and relative configuration, and evaluation of synthetic analogues. J. Am. Chem. Soc. 2002;124:10396–10415. doi: 10.1021/ja0205232. [DOI] [PubMed] [Google Scholar]
- 53.Kobayash M., Sasaki T., Aok S., Saka H., Kihara N., Kitagawa I. Altohyrtins B and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolide congeners of altohyrtin A, from the okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1993;41:989–991. doi: 10.1248/cpb.41.989. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi M., Aoki S., Gato K., Kitagawa I. Marine natural products. XXXVIII. absolute stereostructures of altohyrtins A, B, and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolides, from the okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1996;44:2142–2149. doi: 10.1248/cpb.44.2142. [DOI] [Google Scholar]
- 55.Litaudon M., Hickford S.J.H., Lill R.E., Lake R.J., Blunt J.W., Munro M.H.G. Antitumor polyether macrolides: New and hemisynthetic halichondrins from the new zealand deep-water sponge Lissodendoryx sp. J. Org. Chem. 1997;62:1868–1871. doi: 10.1021/jo962231n. [DOI] [Google Scholar]
- 56.Erickson K.L., Beutler J.A., Ii J.H.C., Boyd M.R. Salicylihalamides A and B, novel cytotoxic macrolides from the marine sponge Haliclona sp. Clin. Trials. 1997;3263:8188–8192. doi: 10.1021/jo971556g. [DOI] [PubMed] [Google Scholar]
- 57.Labrecque D., Charron S., Rej R., Blais C., Lamothe S. Enantioselective total synthesis of salicylihalamides A and B. Tetrahedron Lett. 2001;42:2645–2648. doi: 10.1016/S0040-4039(01)00278-7. [DOI] [Google Scholar]
- 58.Snider B.B., Song F. Total synthesis of (−) -salicylihalamide A. Org. Lett. 2001;3:1817–1820. doi: 10.1021/ol015822v. [DOI] [PubMed] [Google Scholar]
- 59.Alois F., Dierkes T., Thiel O.R., Blanda G. Total synthesis of (–) -salicylihalamide. Chem. Eur. J. 2001;24:5286–5298. doi: 10.1002/1521-3765(20011217)7:24<5286::aid-chem5286>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 60.Zampella A., D’Auria M.V., Minale L., Debitus C. Callipeltosides B and C, two novel cyotoxic glycoside macrolides from a marine lithistida sponge Callipelta sp. Tetrahedron. 1997;53:3243–3248. doi: 10.1016/S0040-4020(97)00035-5. [DOI] [Google Scholar]
- 61.Kobayashi J., Tsuda M., Fuse H., Sasaki T., Mikami Y. Halishigamides A–D, new cytotoxic oxazole-containing metabolites from okinawan sponge Halichondria sp. J. Nat. Prod. 1997;60:150–154. doi: 10.1021/np960558d. [DOI] [Google Scholar]
- 62.Lu Q., Faulkner D.J. Three dolabellanes and a macrolide from the sponge Dysidea sp. from Palau. J. Nat. Prod. 1998;61:1096–1100. doi: 10.1021/np980134e. [DOI] [PubMed] [Google Scholar]
- 63.Matsunaga S., Sugawara T., Fusetani N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J. Nat. Prod. 1998;61:1164–1167. doi: 10.1021/np980102r. [DOI] [PubMed] [Google Scholar]
- 64.Northcote P.T., Blunt J.W., Munro M.H.G. Pateamine: A potent cytotoxin from the new zealand marine sponge, Mycale sp. Tetrahedron Lett. 1991;32:6411–6414. doi: 10.1016/0040-4039(91)80182-6. [DOI] [Google Scholar]
- 65.Ovenden S.P.B., Capon R.J., Lacey E., Gill J.H., Friedel T., Wadsworth D. Amphilactams A–D: Novel nematocides from southern australian marine sponges of the genus Amphimedon. J. Org. Chem. 1999;64:1140–1144. doi: 10.1021/jo981377e. [DOI] [Google Scholar]
- 66.Takada N., Sato H., Suenaga K., Arimoto H., Yamada K., Ueda K., Uemura D. Isolation and structures of haterumalides NA, NB, NC, ND, and NE, novel macrolides from an okinawan sponge Ircinia sp. Tetrahedron Lett. 1999;40:6309–6312. doi: 10.1016/S0040-4039(99)01291-5. [DOI] [Google Scholar]
- 67.Carbonelli S., Zampella A., Randazzo A., Debitus C., Gomez-Paloma L. Sphinxolides E–G and reidispongiolide C: Four new cytotoxic macrolides from the new caledonian lithistida sponges N. superstes and R. coerulea. Tetrahedron. 1999;55:14665–14674. doi: 10.1016/S0040-4020(99)00912-6. [DOI] [Google Scholar]
- 68.D’Ambrosio M., Tatò M., Pocsfalvi G., Debitus C., Pietra F. Leucascandrolide B, a new 16-membered, extensively methyl-branched polyoxygenated macrolide from the calcareous sponge Leucascandra caveolata from northeastern waters of new caledonia. Helv. Chim. Acta. 1999;82:347–353. doi: 10.1002/(SICI)1522-2675(19990310)82:3<347::AID-HLCA347>3.0.CO;2-9. [DOI] [Google Scholar]
- 69.West L.M., Northcote P.T., Battershill C.N. Peloruside A: A potent cytotoxic macrolide isolated from the new zealand marine sponge Mycale sp. J. Org. Chem. 2000;65:445–449. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]
- 70.Liao X., Wu Y., Brabander J. Total synthesis and absolute configuration of the novel microtubule-stabilizing agent peloruside A**. Angew. Chem. Int. Ed. 2003;42:1648–1652. doi: 10.1002/anie.200351145. [DOI] [PubMed] [Google Scholar]
- 71.Grassia A., Bruno I., Debitus C., Marzocco S., Pinto A., Gomez-Paloma L., Riccio R. Spongidepsin, a new cytotoxic macrolide from Spongia sp. Tetrahedron. 2001;57:6257–6260. doi: 10.1016/S0040-4020(01)00587-7. [DOI] [Google Scholar]
- 72.Cutignano A., Bruno I., Bifulco G., Casapullo A., Debitus C., Gomez-Paloma L., Riccio R. Dactylolide, a new cytotoxic macrolide from the Vanuatu sponge Dactylospongia sp. European J. Org. Chem. 2001:775–778. doi: 10.1002/1099-0690(200102)2001:4<775::AID-EJOC775>3.0.CO;2-Z. [DOI] [Google Scholar]
- 73.Randazzo A., Debitus C., Gomez-Paloma L. Haliclamide, a novel cyclic metabolite from the Vanuatu marine sponge Haliclona sp. Tetrahedron. 2001;57:4443–4446. doi: 10.1016/S0040-4020(01)00335-0. [DOI] [Google Scholar]
- 74.Rao M.R., Faulkner D.J. Clavosolides A and B, dimeric macrolides from the philippines sponge Myriastra clavosa. J. Nat. Prod. 2002;65:386–388. doi: 10.1021/np010495l. [DOI] [PubMed] [Google Scholar]
- 75.Erickson K.L., Gustafson K.R., Pannell L.K., Beutler J.A., Boyd M.R. New dimeric macrolide glycosides from the marine sponge Myriastra clavosa. J. Nat. Prod. 2002;65:1303–1306. doi: 10.1021/np020193z. [DOI] [PubMed] [Google Scholar]
- 76.Son J.B., Hwang M., Lee W., Lee D. Enantioselective total synthesis of (−) -clavosolide B. Org. Lett. 2007;9:3898–3900. doi: 10.1021/ol7015115. [DOI] [PubMed] [Google Scholar]
- 77.Williams D.E., Lapawa M., Feng X., Tarling T., Roberge M., Andersen R.J. Spirastrellolide A: Revised structure, progress toward the relative configuration, and inhibition of protein phosphatase 2A. Org. Lett. 2004;6:2607–2610. doi: 10.1021/ol0490983. [DOI] [PubMed] [Google Scholar]
- 78.Warabi K., Williams D.E., Patrick B.O., Roberge M., Andersen R.J. Spirastrellolide B reveals the absolute configuration of the spirastrellolide macrolide core. J. Am. Chem. Soc. 2007;129:508–509. doi: 10.1021/ja068271i. [DOI] [PubMed] [Google Scholar]
- 79.Williams D.E., Roberge M., Van Soest R., Andersen R.J. Spirastrellolide A, an antimitotic macrolide isolated from the caribbean marine sponge Spirastrella coccinea. J. Am. Chem. Soc. 2003;125:5296–5297. doi: 10.1021/ja0348602. [DOI] [PubMed] [Google Scholar]
- 80.Williams D.E., Keyzers R.A., Warabi K., Desjardine K., Riffell J.L., Roberge M., Andersen R.J. Spirastrellolides C to G: Macrolides obtained from the marine sponge Spirastrella coccinea. J. Org. Chem. 2007;72:9842–9845. doi: 10.1021/jo7018174. [DOI] [PubMed] [Google Scholar]
- 81.Shin J., Lee H.S., Kim J.Y., Hee J.S., Ahn J.W., Paul V.J. New macrolides from the sponge Chondrosia corticata. J. Nat. Prod. 2004;67:1889–1892. doi: 10.1021/np040124f. [DOI] [PubMed] [Google Scholar]
- 82.Matsunaga S., Fusetani N. Utilization of marine invertebrates as resource for bioactive metabolites: Isolation of new mycalolides and calyculins. Dev. Food Sci. 2004:131–138. [Google Scholar]
- 83.Wright A.E., Chen Y., Winder P.L., Pitts T.P., Pomponi S.A., Longley R.E. Lasonolides C-G, five new lasonolide compounds from the sponge Forcepia sp. J. Nat. Prod. 2004;67:1351–1355. doi: 10.1021/np040028e. [DOI] [PubMed] [Google Scholar]
- 84.Ohta S., Uy M.M., Yanai M., Ohta E., Hirata T., Ikegami S. Exiguolide, a new macrolide from the marine sponge Geodia exigua. Tetrahedron Lett. 2006;47:1957–1960. doi: 10.1016/j.tetlet.2006.01.062. [DOI] [Google Scholar]
- 85.Fuwa H., Suzuki T., Kubo H., Yamori T., Sasaki M. Total synthesis and biological assessment of (−)-exiguolide and analogues. Chem. Eur. J. 2011;17:2678–2688. doi: 10.1002/chem.201003135. [DOI] [PubMed] [Google Scholar]
- 86.Sandler J.S., Colin P.L., Kelly M. Cytotoxic macrolides from a new spexies of the deep-water marine sponge Leiodermatium. J. Org. Chem. 2006;71:8684. doi: 10.1021/jo061926d. [DOI] [PubMed] [Google Scholar]
- 87.Sandler J.S., Colin P.L., Kelly M., Fenical W. Cytotoxic macrolides from a new species of the deep-water marine sponge Leiodermatium. J. Org. Chem. 2006;71:7245–7251. doi: 10.1021/jo060958y. [DOI] [PubMed] [Google Scholar]
- 88.Chevallier C., Bugni T.S., Feng X., Harper M.K., Orendt A.M., Ireland C.M. Tedanolide C: A potent new 18-membered ring cytotoxic macrolide isolated from the Papua new guinea marine sponge Ircinia sp. ChemInform. 2006;37:2510–2513. doi: 10.1002/chin.200633237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petchprayoon C., Asato Y., Higa T., Garcia-Fernandez L.F., Pedpradab S., Marriott G., Suwanborirux K., Tanaka J. Four new kabiramides from the thai sponge Pachastrissa nux. Heterocycles. 2006;69:447–456. doi: 10.1002/chin.200714225. [DOI] [Google Scholar]
- 90.Sirirak T., Brecker L., Plubrukarn A. Kabiramide L, a new antiplasmodial trisoxazole macrolide from the sponge Pachastrissa nux. Nat. Prod. Res. 2013;27:1213–1219. doi: 10.1080/14786419.2012.724410. [DOI] [PubMed] [Google Scholar]
- 91.Youssef D.T.A., Mooberry S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the red sea sponge Theonella swinhoei. J. Nat. Prod. 2006;69:154–157. doi: 10.1021/np050404a. [DOI] [PubMed] [Google Scholar]
- 92.Ahmed S.A., Odde S., Daga P.R., Bowling J.J., Mesbah M.K., Youssef D.T., Khalifa S.I., Doerksen R.J., Hamann M.T. Latrunculin with a highly oxidized thiazolidinone ring: Structure assignment and actin docking. Org. Lett. 2007;9:4773–4776. doi: 10.1021/ol7020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wright A.E., Botelho J.C., Guzmán E., Harmody D., Linley P., McCarthy P.J., Pitts T.P., Pomponi S.A., Reed J.K. Neopeltolide, a macrolide from a lithistid sponge of the family neopeltidae. J. Nat. Prod. 2007;70:412–416. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- 94.Meragelman T.L., Willis R.H., Woldemichael G.M., Heaton A., Murphy P.T., Snader K.M., Newman D.J., Van Soest R., Boyd M.R., Cardellina J.H., et al. Candidaspongiolides, distinctive analogues of tedanolide from sponges of the genus Candidaspongia. J. Nat. Prod. 2007;70:1133–1138. doi: 10.1021/np0700974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson T.A., Tenney K., Cichewicz R.H., Morinaka B.I., White K.N., Amagata T., Subramanian B., Media J., Mooberry S.L., Valeriote F.A., et al. Sponge-derived fijianolide polyketide class: Further evaluation of their structural and cytotoxicity properties. J. Med. Chem. 2007;50:3795–3803. doi: 10.1021/jm070410z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skepper C.K., MacMillan J.B., Zhou G.X., Masuno M.N., Molinski T.F. Chlorocyclopropane macrolides from the marine sponge Phorbas sp. assignment of the configurations of phorbasides A and B by quantitative CD. J. Am. Chem. Soc. 2007;129:4150–4151. doi: 10.1021/ja0703978. [DOI] [PubMed] [Google Scholar]
- 97.MacMillan J.B., Guang X.Z., Skepper C.K., Molinski T.F. Phorbasides A-E, cytotoxic chlorocyclopropane macrolide glycosides from the marine sponge Phorbas sp. CD determination of C-methyl sugar configurations. J. Org. Chem. 2008;73:3699–3706. doi: 10.1021/jo702307t. [DOI] [PubMed] [Google Scholar]
- 98.Amagata T., Johnson T.A., Cichewicz R.H., Tenney K., Mooberry S.L., Media J., Edelstein M., Valeriote F.A., Crews P. Interrogating the bioactive pharmacophore of the latrunculin chemotype by investigating the metabolites of two taxonomically unrelated sponges. J. Med. Chem. 2008;51:7234–7242. doi: 10.1021/jm8008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bishara A., Rudi A., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Salarins A and B and tulearin A: New cytotoxic sponge-derived macrolides. Org. Lett. 2008;10:153–156. doi: 10.1021/ol702221v. [DOI] [PubMed] [Google Scholar]
- 100.Bishara A., Rudi A., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Salarin C, a new cytotoxic sponge-derived nitrogenous macrolide. Tetrahedron Lett. 2008;49:4355–4358. doi: 10.1016/j.tetlet.2008.05.038. [DOI] [Google Scholar]
- 101.Plaza A., Baker H.L., Bewley C.A. Mirabilin, an antitumor macrolide lactam from the marine sponge Siliquariaspongia mirabilis. J. Nat. Prod. 2008;71:473–477. doi: 10.1021/np070603p. [DOI] [PubMed] [Google Scholar]
- 102.Bishara A., Rudi A., Goldberg I., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Tausalarin C: A new bioactive marine sponge-derived nitrogenous bismacrolide. Org. Lett. 2009;11:3538–3541. doi: 10.1021/ol9011019. [DOI] [PubMed] [Google Scholar]
- 103.Dalisay D.S., Morinaka B.I., Skepper C.K., Molinski T.F. A tetrachloro polyketide hexahydro-1H-isoindolone, muironolide A, from the marine sponge Phorbas sp. natural products at the nanomole scale. J. Am. Chem. Soc. 2009;131:7552–7553. doi: 10.1021/ja9024929. [DOI] [PubMed] [Google Scholar]
- 104.Hickford S.J.H., Blunt J.W., Munro M.H.G. Antitumour polyether macrolides: Four new halichondrins from the new zealand deep-water marine sponge Lissodendoryx sp. Bioorganic Med. Chem. 2009;17:2199–2203. doi: 10.1016/j.bmc.2008.10.093. [DOI] [PubMed] [Google Scholar]
- 105.Oku N., Takada K., Fuller R.W., Wilson J.A., Peach M.L., Pannell L.K., McMahon J.B., Gustafson K.R. Isolation, structural elucidation, and absolute stereochemistry of enigmazole A, a cytotoxic phosphomacrolide from the Papua new guinea marine sponge Cinachyrella enigmatica. J. Am. Chem. Soc. 2010;132:10278–10285. doi: 10.1021/ja1016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skepper C.K., Quach T., Molinski T.F. Total synthesis of enigmazole a from Cinachyrella enigmatica. bidirectional bond constructions with an ambident 2,4-disubstituted oxazole synthon. J. Am. Chem. Soc. 2010;132:10286–10292. doi: 10.1021/ja1016975. [DOI] [PubMed] [Google Scholar]
- 107.Bishara A., Rudi A., Aknin M., Neumann D., Ben-Califa N., Kashman Y. Salarins D-J, seven new nitrogenous macrolides from the madagascar sponge Fascaplysinopsis sp. Tetrahedron. 2010;66:4339–4345. doi: 10.1016/j.tet.2010.04.035. [DOI] [Google Scholar]
- 108.Kondo K., Ishibashi M., Kobayashi J. Isolation and structures of theonezolides B and C from the okinawan marine sponge Theonella sp. Tetrahedron. 1994;50:8355–8362. doi: 10.1016/S0040-4020(01)85558-7. [DOI] [Google Scholar]
- 109.Nozawa K., Tsuda M., Tanaka N., Kubota T., Fukushi E., Kawabata J., Kobayashi J. Stereochemistry of theonezolides A-C. Tetrahedron Lett. 2013;54:783–787. doi: 10.1016/j.tetlet.2012.11.120. [DOI] [Google Scholar]
- 110.Sinisi A., Calcinai B., Cerrano C., Dien H.A., Zampella A., D’Amore C., Renga B., Fiorucci S., Taglialatela-Scafati O. Isoswinholide B and swinholide K, potently cytotoxic dimeric macrolides from Theonella swinhoei. Bioorganic Med. Chem. 2013;21:5332–5338. doi: 10.1016/j.bmc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Pham C.D., Hartmann R., Böhler P., Stork B., Wesselborg S., Lin W., Lai D., Proksch P. Callyspongiolide, a cytotoxic macrolide from the marine sponge Callyspongia sp. Org. Lett. 2014;16:266–269. doi: 10.1021/ol403241v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lorente A., Gil A., Fernández R., Cuevas C., Albericio F., Álvarez M. Phormidolides B and C, cytotoxic agents from the sea: Enantioselective synthesis of the macrocyclic core. Chem. Eur. J. 2015;21:150–156. doi: 10.1002/chem.201404341. [DOI] [PubMed] [Google Scholar]
- 113.Irie R., Hitora Y., Ise Y., Okada S., Takada K., Matsunaga S. Poecillastrin E, F, and G, cytotoxic chondropsin-type macrolides from a marine sponge Poecillastra sp. Tetrahedron. 2018;74:1430–1434. doi: 10.1016/j.tet.2018.01.037. [DOI] [Google Scholar]
- 114.Numata A., Iritani M., Yamada T., Minoura K., Matsumura E., Yamori T., Tsuruo T. Novel antitumour metabolites produced by a fungal strain from a sea hare. Tetrahedron Lett. 1997;38:8215–8218. doi: 10.1016/S0040-4039(97)10198-8. [DOI] [Google Scholar]
- 115.Yamada T., Iritani M., Doi M., Minoura K., Ito T., Numata A. Absolute stereostructures of cell-adhesion inhibitors, macrosphelides C, E–G and I, produced by a periconia species separated from an Aplysia sea hare. J. Chem. Soc. 2001;1:3046–3053. doi: 10.1039/b104337b. [DOI] [Google Scholar]
- 116.Nakamura H., Ono M., Yamada T., Numata A., Akita H. Determination of the absolute stereostructure of seco-macrosphelide E produced by a fungal stain from a sea hare. Chem. Pharm. Bull. 2002;50:303–306. doi: 10.1248/cpb.50.303. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi Y., Wang Y. Synthesis of macrosphelides H and G. Tetrahedron Lett. 2002;43:4381–4384. doi: 10.1016/S0040-4039(02)00781-5. [DOI] [Google Scholar]
- 118.Nakamura H., Ono M., Shida Y., Akita H. New total syntheses of (+)-macrosphelides C, F and G. Tetrahedron Asymmetry. 2002;13:705–713. doi: 10.1016/S0957-4166(02)00180-5. [DOI] [Google Scholar]
- 119.Yamada T., Iritani M., Minoura K., Numata A. Absolute stereostructures of cell adhension inhibitors, macrosphelides H and L, from Periconia byssoides OUPS-N133. J. Antibiot. 2002;55:147–154. doi: 10.7164/antibiotics.55.147. [DOI] [PubMed] [Google Scholar]
- 120.Yamada T., Minoura K., Tanaka R., Numata A. Cell-adhesion inhibitors produced by a sea hare-derived Periconia sp.: III absolute stereostructures of peribysin J and macrosphelide M. J. Antibiot. (Tokyo) 2007;60:370–375. doi: 10.1038/ja.2007.50. [DOI] [PubMed] [Google Scholar]
- 121.Breinholt J., Jensen G.W., Nielsen R.I., Olsen C.E., Frisvad J.C. Antifungal macrocyclic polylactones from Penicillium verruculosum. J. Antibiot. 1993;46:1101–1108. doi: 10.7164/antibiotics.46.1101. [DOI] [PubMed] [Google Scholar]
- 122.Höller U., König G.M., Wright A.D. A new tyrosine kinase inhibitor from a marine isolate of ulocladium botrytis and new metabolites from the marine fungi Asteromyces cruciatus and Varicosporina ramulosa. European J. Org. Chem. 1999;49:2949–2955. doi: 10.1002/(SICI)1099-0690(199911)1999:11<2949::AID-EJOC2949>3.0.CO;2-Y. [DOI] [Google Scholar]
- 123.Ohta K., Miyagawa O., Tsutsui H., Mitsunobu O. Total synthesis of grahamimycin A1. Bull. Chem. Soc. Jpn. 1993;66:523–535. doi: 10.1246/bcsj.66.523. [DOI] [Google Scholar]
- 124.Smith C.J., Abbanat D., Bernan V.S., Maiese W.M., Greenstein M., Jompa J., Tahir A., Ireland C.M. Novel polyketide metabolites from a species of marine fungi. J. Nat. Prod. 2000;63:142–145. doi: 10.1021/np990361w. [DOI] [PubMed] [Google Scholar]
- 125.Jadulco R., Proksch P., Wray V., Sudarsono, Berg A., Gräfe U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J. Nat. Prod. 2001;64:527–530. doi: 10.1021/np000401s. [DOI] [PubMed] [Google Scholar]
- 126.Namikoshi M., Akano K., Meguro S., Kasuga I., Mine Y., Takahashi T., Kobayashi H. A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine-derived fungus Myrothecium roridum collected in palau. J. Nat. Prod. 2001;64:396–398. doi: 10.1021/np000443g. [DOI] [PubMed] [Google Scholar]
- 127.Isaka M., Suyarnsestakorn C., Tanticharoen M., Kongsaeree P., Thebtaranonth Y. Aigialomycins A-E, new resorcylic macrolides from the marine mangrove fungus Aigialus parvus. J. Org. Chem. 2002;67:1561–1566. doi: 10.1021/jo010930g. [DOI] [PubMed] [Google Scholar]
- 128.Schlingmann G., Milne L., Carter G.T. Isolation and identification of antifungal polyesters from the marine fungus Hypoxylon oceanicum LL-15G256. Tetrahedron. 2002;58:6825–6835. doi: 10.1016/S0040-4020(02)00746-9. [DOI] [Google Scholar]
- 129.Shigemori H., Kasai Y., Komatsu K., Tsuda M., Mikami Y., Kobayashi J. Sporiolides A and B, new cytotoxic twelve-membered macrolides from a marine-derived fungus Cladosporium species. Mar. Drugs. 2004;2:164–169. doi: 10.3390/md204164. [DOI] [Google Scholar]
- 130.Yang R.Y., Li C.Y., Lin Y.C., Peng G.T., She Z.G., Zhou S.N. Lactones from a brown alga endophytic fungus (No. ZZF36) from the south China sea and their antimicrobial activities. Bioorg. Med. Chem. Lett. 2006;16:4205–4208. doi: 10.1016/j.bmcl.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 131.Xu J., Takasaki A., Kobayashi H., Oda T., Yamada J., Mangindaan R.E.P., Ukai K., Nagai H., Namikoshi M. Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J. Antibiot. (Tokyo) 2006;59:451–455. doi: 10.1038/ja.2006.63. [DOI] [PubMed] [Google Scholar]
- 132.Han J., Su Y., Jiang T., Xu Y., Huo X., She X., Pan X. Asymmetric total synthesis and revision of the absolute configuration of 4-keto-clonostachydiol. J. Org. Chem. 2009;74:3930–3932. doi: 10.1021/jo900370a. [DOI] [PubMed] [Google Scholar]
- 133.Yang X., Khong T.T., Chen L., Choi H.D., Kang J.S., Son B.W. 8′-Hydroxyzearalanone and 2′-hydroxyzearalanol: Resorcyclic acid lactone derivatives from the marine-derived fungus Penicillium sp. Chem. Pharm. Bull. 2008;56:1355–1356. doi: 10.1248/cpb.56.1355. [DOI] [PubMed] [Google Scholar]
- 134.Zhao L.L., Gai Y., Kobayashi H., Hu C.Q., Zhang H.P. 5′-Hydroxyzearalenol, a new β-resorcylic macrolide from Fusarium sp. 05ABR26. Chinese Chem. Lett. 2008;19:1089–1092. doi: 10.1016/j.cclet.2008.06.025. [DOI] [Google Scholar]
- 135.Kito K., Ookura R., Yoshida S., Namikoshi M., Ooi T., Kusumi T. New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Org. Lett. 2008;10:225–228. doi: 10.1021/ol702598q. [DOI] [PubMed] [Google Scholar]
- 136.Bao J., Xu X.Y., Zhang X.Y., Qi S.H. A new macrolide from a marine-derived fungus Aspergillus sp. Nat. Prod. Commun. 2013;8:1127–1128. doi: 10.1177/1934578X1300800825. [DOI] [PubMed] [Google Scholar]
- 137.Munro H.D., Musgrave O.C., Templeton R. Curvularin. part V. the compound C16H18O5, αβ-dehydrocurvularin. Org. Lett. 1964:947–948. doi: 10.1039/J39670000947. [DOI] [Google Scholar]
- 138.Lai S., Shizuri Y., Yamamura S., Kawai L., Furukawa H. New curvalarin-type metabolites from the hybrid strain ME 0005 derived from Penicillium citreo-viride B. IFP 4692 and 6200. Chem. Soc. 1991;3:1048–1050. [Google Scholar]
- 139.Dai J., Krohn K., Flörke U., Pescitelli G., Kerti G., Papp T., Kövér K.E., Bényei A.C., Draeger S., Schulz B., et al. Curvularin-type metabolites from the fungus Curvularia sp. isolated from a marine alga. European J. Org. Chem. 2010:6928–6937. doi: 10.1002/ejoc.201001237. [DOI] [Google Scholar]
- 140.Ebrahim W., Aly A.H., Mándi A., Totzke F., Kubbutat M.H.G., Wray V., Lin W.H., Dai H., Proksch P., Kurtán T., et al. Decalactone derivatives from corynespora cassiicola, an endophytic fungus of the mangrove plant Laguncularia racemosa. European J. Org. Chem. 2012:3476–3484. doi: 10.1002/ejoc.201200245. [DOI] [Google Scholar]
- 141.Rukachaisirikul V., Rodglin A., Phongpaichit S., Buatong J., Sakayaroj J. α-Pyrone and seiricuprolide derivatives from the mangrove-derived fungi Pestalotiopsis spp. PSU-MA92 and PSU-MA119. Phytochem. Lett. 2012;5:13–17. doi: 10.1016/j.phytol.2011.08.008. [DOI] [Google Scholar]
- 142.Silber J., Ohlendorf B., Labes A., Erhard A., Imhoff J.F. Calcarides A-E, antibacterial macrocyclic and linear polyesters from a Calcarisporium strain. Mar. Drugs. 2013;11:3309–3323. doi: 10.3390/md11093309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun P., Xu D.X., Mándi A., Kurtán T., Li T.J., Schulz B., Zhang W. Structure, absolute configuration, and conformational study of 12-membered macrolides from the fungus Dendrodochium sp. associated with the sea cucumber holothuria nobilis selenka. J. Org. Chem. 2013;78:7030–7047. doi: 10.1021/jo400861j. [DOI] [PubMed] [Google Scholar]
- 144.Mohapatra D.K., Pulluri K., Gajula S., Yadav J.S. 13-Step total synthesis of dendrodolide K following iterative bartlett-smith iodocarbonate cyclization. Tetrahedron Lett. 2015;56:6377–6380. doi: 10.1016/j.tetlet.2015.09.126. [DOI] [Google Scholar]
- 145.Mohapatra D.K., Reddy D.P., Gajula S., Pulluri K., Yadav J.S. A unified synthetic strategy for dendrodolides E, F, G, I, J, and L. Asian J. Org. Chem. 2015;4:452–461. doi: 10.1002/ajoc.201500037. [DOI] [Google Scholar]
- 146.Shao C.L., Wu H.X., Wang C.Y., Liu Q.A., Xu Y., Wei M.Y., Qian P.Y., Gu Y.C., Zheng C.J., She Z.G., et al. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J. Nat. Prod. 2011;74:629–633. doi: 10.1021/np100641b. [DOI] [PubMed] [Google Scholar]
- 147.Shao C.L., Wu H.X., Wang C.Y., Liu Q.A., Xu Y., Wei M.Y., Qian P.Y., Gu Y.C., Zheng C.J., She Z.G., et al. Correction to potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus Lunatus. J. Nat. Prod. 2013;76:302. doi: 10.1021/np400079t. [DOI] [PubMed] [Google Scholar]
- 148.Meng L.H., Li X.M., Lv C.T., Li C.S., Xu G.M., Huang C.G., Wang B.G. Sulfur-containing cytotoxic curvularin macrolides from Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera racemosa. J. Nat. Prod. 2013;76:2145–2149. doi: 10.1021/np400614f. [DOI] [PubMed] [Google Scholar]
- 149.Zhang W., Shao C.L., Chen M., Liu Q.A., Wang C.Y. Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 2014;55:4888–4891. doi: 10.1016/j.tetlet.2014.06.096. [DOI] [Google Scholar]
- 150.Zhang J., Lin X.P., Li L.C., Zhong B.L., Liao X.J., Liu Y.H., Xu S.H. Gliomasolides A-E, unusual macrolides from a sponge-derived fungus Gliomastix sp. ZSDS1-F7-2. RSC Adv. 2015;5:54645–54648. doi: 10.1039/C5RA08559D. [DOI] [Google Scholar]
- 151.Okabe M., Sugita T., Kinoshita K., Koyama K. Macrolides from a marine-derived fungus, Penicillium meleagrinum var. viridiflavum, showing synergistic effects with fluconazole against azole-resistant candida albicans. J. Nat. Prod. 2016;79:1208–1212. doi: 10.1021/acs.jnatprod.6b00019. [DOI] [PubMed] [Google Scholar]
- 152.De Castro M.V., Ióca L.P., Williams D.E., Costa B.Z., Mizuno C.M., Santos M.F.C., De Jesus K., Ferreira É.L.F., Seleghim M.H.R., Sette L.D., et al. Condensation of macrocyclic polyketides produced by Penicillium sp. DRF2 with mercaptopyruvate represents a new fungal detoxification pathway. J. Nat. Prod. 2016;79:1668–1678. doi: 10.1021/acs.jnatprod.6b00295. [DOI] [PubMed] [Google Scholar]
- 153.Huang C., Chen T., Yan Z., Guo H., Hou X., Jiang L., Long Y. Thiocladospolide E and cladospamide A, novel 12-membered macrolide and macrolide lactam from mangrove endophytic fungus Cladosporium sp. SCNU-F0001. Fitoterapia. 2019;137:104246. doi: 10.1016/j.fitote.2019.104246. [DOI] [PubMed] [Google Scholar]
- 154.Wang W., Feng H., Sun C., Che Q., Zhang G., Zhu T., Li D. Thiocladospolides F-J, antibacterial sulfur containing 12-membered macrolides from the mangrove endophytic fungus Cladosporium oxysporum HDN13-314. Phytochemistry. 2020;178:112462. doi: 10.1016/j.phytochem.2020.112462. [DOI] [PubMed] [Google Scholar]
- 155.Gao Y., Stuhldreier F., Schmitt L., Wesselborg S., Wang L., Müller W.E.G., Kalscheuer R., Guo Z., Zou K., Liu Z., et al. Sesterterpenes and macrolide derivatives from the endophytic fungus Aplosporella javeedii. Fitoterapia. 2020;146:6–11. doi: 10.1016/j.fitote.2020.104652. [DOI] [PubMed] [Google Scholar]
- 156.Liu H., Chen Y., Li S., Zhang W., Liu Z., Tan H., Zhang W. Trichothecene macrolides from the endophytic fungus Paramyrothecium roridum and their cytotoxic activity. Fitoterapia. 2020;147:104768. doi: 10.1016/j.fitote.2020.104768. [DOI] [PubMed] [Google Scholar]
- 157.Pathirana C., Tapiolas D., Jensen P.R., Dwight R., Fenical W. Structure determination of maduralide: A new 24-membered ring macrolide glycoside produced by a marine bacterium (actinomycetales) Tetrahedron Lett. 1991;32:2323–2326. doi: 10.1016/S0040-4039(00)79914-X. [DOI] [Google Scholar]
- 158.Takahaahi C., Takada T., Yamada T., Mlnoura K. Halichomycin, a new class of potent cytotoxic macrolide produced by an actionmycete from a marine fish. Tetrahedron Lett. 1994;35:5013–5014. doi: 10.1016/S0040-4039(00)73307-7. [DOI] [Google Scholar]
- 159.Jaruchoktaweechai C., Suwanborirux K., Tanasupawatt S., Kittakoop P., Menasveta P. New macrolactins from a marine Bacillus sp. Sc026. J. Nat. Prod. 2000;63:984–986. doi: 10.1021/np990605c. [DOI] [PubMed] [Google Scholar]
- 160.Fernández-Chimeno R.I., Cañedo L., Espliego F., Grávalos D., De la Calle F., Fernández-Puentes J.L., Romero F. IB-96212, a novel cytotoxic macrolide produced by a marine Micromonospora. I. taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 2000;53:474–478. doi: 10.7164/antibiotics.53.474. [DOI] [PubMed] [Google Scholar]
- 161.Asolkar R.N., Maskey R.P., Helmke E., Laatsch H. Chalcomycin B, a new macrolide antibiotic from the marine isolate strptomtces sp. B7064. J. Antibiot. 2002;55:893–898. doi: 10.7164/antibiotics.55.893. [DOI] [PubMed] [Google Scholar]
- 162.Jiang Z.D., Jensen P.R., Fenical W. Lobophorins A and B, new antiinflammatory macrolides produced by a tropical marine bacterium. Bioorganic Med. Chem. Lett. 1999;9:2003–2006. doi: 10.1016/S0960-894X(99)00337-6. [DOI] [PubMed] [Google Scholar]
- 163.Ohta E., Kubota N.K., Ohta S., Suzuki M., Ogawa T., Yamasaki A., Ikegami S. Micromonospolides A-C, new macrolides from Micromonospora sp. Tetrahedron. 2001;57:8463–8467. doi: 10.1016/S0040-4020(01)00843-2. [DOI] [Google Scholar]
- 164.Ohta E., Ohta S., Kubota N.K., Suzuki M., Ogawa T., Yamasaki A., Ikegami S. Micromonospolide A, a new macrolide from Micromonospora sp. Tetrahedron Lett. 2001;25:4179–4181. doi: 10.1016/S0040-4039(01)00683-9. [DOI] [Google Scholar]
- 165.Kwon H.C., Kauffman C.A., Jensen P.R., Fenical W. Erratum: Marinomycins A-D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “ Marinispora”. J. Am. Chem. Soc. 2006;128:16410. doi: 10.1021/ja0666497. [DOI] [PubMed] [Google Scholar]
- 166.Williams P.G., Miller E.D., Asolkar R.N., Jensen P.R., Fenical W. Arenicolides A-C, 26-membered ring macrolides from the marine actinomycete Salinispora arenicola. J. Org. Chem. 2007;72:5025–5034. doi: 10.1021/jo061878x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu X.L., Xu Q.Z., Shen Y.H., Liu X.Y., Jiao B.H., Zhang W.D., Ni K.Y. Macrolactin S, a novel macrolactin antibiotic from marine Bacillus sp. Nat. Prod. Res. 2008;22:342–347. doi: 10.1080/14786410701768162. [DOI] [PubMed] [Google Scholar]
- 168.Kwon H.C., Kauffman C.A., Jensen P.R., Fenical W. Marinisporolides, polyene-polyol macrolides from a marine actinomycete of the new genus Marinispora. J. Org. Chem. 2009;74:675–684. doi: 10.1021/jo801944d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yamada T., Kikuchi T., Tanaka R., Numata A. Halichoblelides B and C, potent cytotoxic macrolides from a Streptomyces species separated from a marine fish. Tetrahedron Lett. 2012;53:2842–2846. doi: 10.1016/j.tetlet.2012.03.114. [DOI] [Google Scholar]
- 170.Kim D.G., Moon K., Kim S.H., Park S.H., Park S., Lee S.K., Oh K.B., Shin J., Oh D.C. Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. J. Nat. Prod. 2012;75:959–967. doi: 10.1021/np3001915. [DOI] [PubMed] [Google Scholar]
- 171.Shin H.J., Tareq F.S., Kim J.H., Lee M.A., Lee H.S., Lee Y.J., Lee J.S. Glycosylated methoxy-macrolactins from a marine sediment bacterium Bacillus subtilis. Heterocycles. 2013;87:307–318. doi: 10.3987/COM-12-12594. [DOI] [Google Scholar]
- 172.Mondol M.A.M., Tareq F.S., Kim J.H., Lee M.A., Lee H.S., Lee Y.J., Lee J.S., Shin H.J. Cyclic ether-containing macrolactins, antimicrobial 24-membered isomeric macrolactones from a marine Bacillus sp. J. Nat. Prod. 2011;74:2582–2587. doi: 10.1021/np200487k. [DOI] [PubMed] [Google Scholar]
- 173.Carlson S., Marler L., Nam S.J., Santarsiero B.D., Pezzuto J.M., Murphy B.T. Potential chemopreventive activity of a new macrolide antibiotic from a marine-derived Micromonospora sp. Mar. Drugs. 2013;11:1152–1161. doi: 10.3390/md11041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fei P., Wang C.X., Yang X., Jiang H.L., Chen L.J., Uribe P., Bull A.T., Goodfellow M., Hong J., Lian Y.Y. A new 20-membered macrolide produced by a marine-derived Micromonospora strain. Nat. Prod. Res. 2013;27:1366–1371. doi: 10.1080/14786419.2012.740038. [DOI] [PubMed] [Google Scholar]
- 175.Wu C., Tan Y., Gan M., Wang Y., Guan Y., Hu X., Zhou H., Shang X., You X., Yang Z., et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013;76:2153–2157. doi: 10.1021/np4006794. [DOI] [PubMed] [Google Scholar]
- 176.Li X., Vanner S., Wang W., Li Y., Gallardo V.A., Magarvey N.A. Macplocimine A, a new 18-membered macrolide isolated from the filamentous sulfur bacteria Thioploca sp. J. Antibiot. (Tokyo) 2013;66:443–446. doi: 10.1038/ja.2013.52. [DOI] [PubMed] [Google Scholar]
- 177.Jang K.H., Nam S.J., Locke J.B., Kauffman C.A., Beatty D.S., Paul L.A., Fenical W. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew. Chem. Int. Ed. 2013;52:7822–7824. doi: 10.1002/anie.201302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nam S.J., Gaudêncio S.P., Kauffman C.A., Jensen P.R., Kondratyuk T.P., Marler L.E., Pezzuto J.M., Fenical W. Fijiolides A and B, inhibitors of TNF-α-induced NFκB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J. Nat. Prod. 2010;73:1080–1086. doi: 10.1021/np100087c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alferova V.A., Novikov R.A., Bychkova O.P., Rogozhin E.A., Shuvalov M.V., Prokhorenko I.A., Sadykova V.S., Kulko A.B., Dezhenkova L.G., Stepashkina E.A., et al. Astolides A and B, antifungal and cytotoxic naphthoquinone-derived polyol macrolactones from Streptomyces hygroscopicus. Tetrahedron. 2018;74:7442–7449. doi: 10.1016/j.tet.2018.11.015. [DOI] [Google Scholar]
- 180.Son S., Jang M., Lee B., Lee J.S., Hong Y.S., Kim B.Y., Ko S.K., Jang J.H., Ahn J.S. Catenulisporidins A and B, 16-membered macrolides of the hygrolidin family produced by the chemically underexplored actinobacterium Catenulispora species. Bioorganic Med. Chem. Lett. 2020;30:127005. doi: 10.1016/j.bmcl.2020.127005. [DOI] [PubMed] [Google Scholar]
- 181.Carmeli S., Moore R.E., Patterson G.M.L. Tolytoxin and new scytophycins from three species of scytonema. J. Nat. Prod. 1990;53:1533–1542. doi: 10.1021/np50072a021. [DOI] [PubMed] [Google Scholar]
- 182.Murakami M., Matsuda H., Makabe K., Yamaguchi K. Oscillariolide, a nocel macrolide from a blue-green alga Oscillatoria sp. Tetrahedron Lett. 1991;32:2391–2394. doi: 10.1016/S0040-4039(00)79931-X. [DOI] [Google Scholar]
- 183.Klein D., Braekman J.C., Daloze D., Hoffmann L., Demoulin V. Lyngbyaloside, a novel 2,3,4-tri-O-methyl-6-deoxy-α-mannopyranoside macrolide from Lyngbya bouillonii (Cyanobacteria) J. Nat. Prod. 1997;60:1057–1059. doi: 10.1021/np9702751. [DOI] [PubMed] [Google Scholar]
- 184.Klein D., Braekman J.C., Daloze D., Hoffmann L., Demoulin V. Laingolide, a novel 15-membered macrolide from Lyngbya bouillonii (Cyanophyceae) Tetrahedron Lett. 1996;37:7519–7520. doi: 10.1016/0040-4039(96)01728-5. [DOI] [Google Scholar]
- 185.Klein D., Braekman J.C., Daloze D., Hoffmann L., Castillo G., Demoulin V. Madangolide and laingolide A, two novel macrolides from Lyngbya bouillonii (Cyanobacteria) J. Nat. Prod. 1999;62:934–936. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]
- 186.Elmarrouni A., Lebeuf R., Gebauer J., Heras M., Arseniyadis S., Cossy J. Total synthesis of nominal lyngbouilloside aglycon. Org. Lett. 2012;14:314–317. doi: 10.1021/ol203064r. [DOI] [PubMed] [Google Scholar]
- 187.Tan L.T., Márquez B.L., Gerwick W.H. Lyngbouilloside, a novel glycosidic macrolide from the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2002;65:925–928. doi: 10.1021/np010526c. [DOI] [PubMed] [Google Scholar]
- 188.Andrianasolo E.H., Gross H., Goeger D., Musafija-Girt M., McPhail K., Leal R.M., Mooberry S.L., Gerwick W.H. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org. Lett. 2005;7:1375–1378. doi: 10.1021/ol050188x. [DOI] [PubMed] [Google Scholar]
- 189.Pereira A.R., McCue C.F., Gerwick W.H. Cyanolide A, a glycosidic macrolide with potent molluscicidal activity from the papua new guinea cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2010;73:217–220. doi: 10.1021/np9008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morita M., Ohno O., Suenaga K. Biselyngbyolide A, a novel cytotoxic macrolide from the marine cyanobacterium Lyngbya sp. Chem. Lett. 2012;41:165–167. doi: 10.1246/cl.2012.165. [DOI] [Google Scholar]
- 191.Morita M., Ohno O., Teruya T., Yamori T., Inuzuka T., Suenaga K. Isolation and structures of biselyngbyasides B, C, and D from the marine cyanobacterium Lyngbya sp., and the biological activities of biselyngbyasides. Tetrahedron. 2012;68:5984–5990. doi: 10.1016/j.tet.2012.05.038. [DOI] [Google Scholar]
- 192.Navarro G., Cummings S., Lee J., Moss N., Glukhov E., Valeriote F.A., Gerwick L., Gerwick W.H. Isolation of polycavernoside D from a marine cyanobacterium. Environ. Sci. Technol. Lett. 2015;2:166–170. doi: 10.1021/acs.estlett.5b00116. [DOI] [Google Scholar]
- 193.Cui J., Morita M., Ohno O., Kimura T., Teruya T., Watanabe T., Suenaga K., Shibasaki M. Leptolyngbyolides, cytotoxic macrolides from the marine cyanobacterium Leptolyngbya sp.: Isolation, biological activity, and catalytic asymmetric total synthesis. Chem. Eur. J. 2017;23:8500–8509. doi: 10.1002/chem.201701183. [DOI] [PubMed] [Google Scholar]
- 194.Kobayashi J., Ishibashi M., Murayama T., Takamatsu M., Iwamura M., Ohizumi Y., Sasaki T. Amphidinolide E, a novel antileukemic 19-membered macrolide from the cultured symbiotic dinoflagellate Amohidinium sp. J. Org. Chem. 1990;55:3421–3423. doi: 10.1021/jo00297a089. [DOI] [Google Scholar]
- 195.Kubota T., Tsuda M., Kobayashi J. Absolute stereochemistry of amphidinolide E. J. Org. Chem. 2002;67:1651–1656. doi: 10.1021/jo016326n. [DOI] [PubMed] [Google Scholar]
- 196.Tsuda M., Ishibashi M., Shigemori H., Yamasua T., Hohaha T.H. Amphidinolide F, a new cytotoxic macrolide from the marine dinoflagellate Amphidinium sp. J. Antibiot. 1991;44:1259–1261. doi: 10.7164/antibiotics.44.1259. [DOI] [PubMed] [Google Scholar]
- 197.Kobayashi J., Shigemori H., Ishibashi M., Yamasu T., Hirota H., Sasaki T. Amphidinolides G and H: New potent cytotoxic macrolides from the cultured symbiotic dinoflagellate Amphidinium sp. J. Org. Chem. 1991;56:5221–5224. doi: 10.1021/jo00017a044. [DOI] [Google Scholar]
- 198.Kobayashi J., Shimbo K., Sato M., Shiro M., Tsuda M. Absolute stereochemistry of amphidinolides G and H. Org. Lett. 2000;2:2805–2807. doi: 10.1021/ol006223b. [DOI] [PubMed] [Google Scholar]
- 199.Ishibashi M., Sato M., Kobayashi J. Amphidinolide K, a new 19-membered macrolide from the cultured symbiotic dinoflagellate Amphidinium sp. J. Org. Chem. 1993;1:6928–6929. doi: 10.1021/jo00076a074. [DOI] [Google Scholar]
- 200.Kobayashi J., Sato M., Ishibashi M. Amphidinolide J: A cytotoxic macrolide from the marine dinoflagellate Amphidinium sp. determination of the absolute stereochemistry. J. Org. Chem. 1993;58:2645. doi: 10.1021/jo00062a001. [DOI] [Google Scholar]
- 201.Kobayashi J., Ishibashi M., Nakamura H., Ohizumi Y., Yamasu T., Hirata Y., Sasaki T., Ohta T., Nozoe S. Cytotoxic macrolides from a cultured marine dinoflagellate of the genus Amphidinium. J. Nat. Prod. 1989;52:1036–1041. doi: 10.1021/np50065a021. [DOI] [PubMed] [Google Scholar]
- 202.Bauer I., Maranda L., Shimizu Y., Peterson R.W., Cornell L., Steiner J.R., Clardy J. The Structures of amphidinolide B isomers: Strongly cytotoxic macrolides produced by a free-swimming dinoflagellate, Amphidinium sp. J. Am. Chem. Soc. 1994;116:2657–2658. doi: 10.1021/ja00085a071. [DOI] [Google Scholar]
- 203.Ishibashi M., Ishiyama H., Kobayashi J. Absolute stereochemistry of amphidinolide B. Tetrahedron Lett. 1994;35:8241–8242. doi: 10.1016/0040-4039(94)88292-4. [DOI] [Google Scholar]
- 204.Tsuda M., Sasaki T., Kobayashi J. Amphidinolide L, a new cytotoxic 27-membered macrolide from the cultured dinoflagellate Amphidinium sp. J. Org. Chem. 1994;59:3734–3737. doi: 10.1021/jo00092a045. [DOI] [Google Scholar]
- 205.Kobayashi J., Yamaguchi N., Ishibashi M. Amphidinolide M, a novel 29-menberd macrolide from the cultured marine dinoflagellate Amphidinium sp. J. Org. Chem. 1994;59:4698–4700. doi: 10.1021/jo00095a058. [DOI] [Google Scholar]
- 206.Ishibashi M., Yamaguchi N., Sasaki T., Kobayashi J. Amphidinolide N, a novel 26-membered macrolide with remarkably potent cytotoxicity from the cultured marine dinoflagellate Amphidinium sp. J. Chem. Soc. Chem. Commun. 1994:1455–1456. doi: 10.1039/c39940001455. [DOI] [Google Scholar]
- 207.Tsuda M., Akakabe M., Minamida M., Kumagai K., Tsuda M., Konishi Y., Tominaga A., Fukushi E., Kawabata J. Structure and stereochemistry of amphidinolide N congeners from marine dinoflagellate Amphidinium species. Chem. Pharm. Bull. 2021;69:141–149. doi: 10.1248/cpb.c20-00745. [DOI] [PubMed] [Google Scholar]
- 208.Ishibashi M., Takahashi M., Kobayashi J. Amphidinolides O and P, novel 15-membered macrolides from the dinoflagellate Amphidinium sp.: Analysis of the relative stereochemistry and stable solution conformation. J. Org. Chem. 1995;60:6062–6066. doi: 10.1021/jo00124a015. [DOI] [Google Scholar]
- 209.Williams D.R., Myers B.J., Mi L. Total synthesis of (−) -amphidinolide P. Org. Lett. 2000;2:945–948. doi: 10.1021/ol0000197. [DOI] [PubMed] [Google Scholar]
- 210.Kobayashi J., Takahshi M., Ishibashi M. Amphidinolide Q, a novel 12-membered macrolide from the cultured marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 1996;37:1449–1450. doi: 10.1016/0040-4039(96)00029-9. [DOI] [Google Scholar]
- 211.Hangyou M., Ishiyama H., Takahashi Y., Kobayashi J. Total synthesis of amphidinolide Q. Org. Lett. 2009;11:5046–5049. doi: 10.1021/ol902026f. [DOI] [PubMed] [Google Scholar]
- 212.Takahashi Y., Kubota T., Fukushi E., Kawabata J. Absolute stereochemistry of amphidinolide Q. Org. Lett. 2008;10:3709–3711. doi: 10.1021/ol8013123. [DOI] [PubMed] [Google Scholar]
- 213.Feeding S., Ishibashi M., Takahashi M., Kobayashi J. Studies on the macrolides from marine dinoflagellate Amphidinium sp.:structure of amphidinolides R and S and a succinate feeding experiment. Tetrahedron. 1997;53:7827–7832. [Google Scholar]
- 214.Tsuda M., Endo T., Kobayashi J. Amphidinolide U, novel 20-membered macrolide from marine dinoflagellate Amphidinium sp. Tetrahedron. 1999;55:14565–14570. doi: 10.1016/S0040-4020(99)00932-1. [DOI] [Google Scholar]
- 215.Kubota T., Suzuki A., Yamada M., Baba S., Kobayashi J. Amphidinolide C3, a new cytotoxic 25-menbered macrolide from marine dinofagellate Amphidinium sp. Heterocycles. 2010;82:333–338. [Google Scholar]
- 216.Tsuda M., Endo T., Kobayashi J. Amphidinolide T, novel 19-membered macrolide from marine dinoflagellate Amphidinium sp., Amphiscolops sp., and isolated a series of cytotoxic. J. Org. Chem. 2000;65:1349–1352. doi: 10.1021/jo991393r. [DOI] [PubMed] [Google Scholar]
- 217.Kubota T., Tsuda M., Kobayashi J. Amphidinolide V, novel 14-membered macrolide from marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2000;41:713–716. doi: 10.1016/S0040-4039(99)02170-X. [DOI] [Google Scholar]
- 218.Furstner A., Flugge S., Larionov O., Takahashi Y., Kubota T., Kobayashi J. Total synthesis and biological ecaluation of amphidinolide V and analogues. Chem. Eur. J. 2009:4011–4029. doi: 10.1002/chem.200802068. [DOI] [PubMed] [Google Scholar]
- 219.Kobayashi J., Kubota T., Endo T., Tsuda M. Amphidinolides T2, T3, and T4, new 19-membered macrolides from the dinoflagellate Amphidinium sp. and the biosynthesis of amphidinolide T1. J. Org. Chem. 2001;66:134–142. doi: 10.1021/jo005607c. [DOI] [PubMed] [Google Scholar]
- 220.Kubota T., Endo T., Tsuda M., Shiro M., Kobayashi J. Amphidinolide T5, a new 19-membered macrolide from a dinoflagellate and X-ray structure of amphidinolide T1. Tetrahedron. 2001;57:6175–6179. doi: 10.1016/S0040-4020(01)00591-9. [DOI] [Google Scholar]
- 221.Kobayashi J., Shimbo K., Sato M., Tsuda M. Amphidinolides H2-H5, G2, and G3, new cytotoxic 26- and 27-membered macrolides from dinoflagellate Amphidinium sp. J. Org. Chem. 2002;67:6585–6592. doi: 10.1021/jo016222c. [DOI] [PubMed] [Google Scholar]
- 222.Li H., Wu J., Luo J., Dai W.M. A concise total synthesis of amphidinolide T2. Chem. Eur. J. 2010;16:11530–11534. doi: 10.1002/chem.201001794. [DOI] [PubMed] [Google Scholar]
- 223.Shimbo K., Tsuda M., Izui N., Kobayashi J. Amphidinolide W, a new 12-membered macrolide from dinoflagellate Amphidinium sp. J. Org. Chem. 2002;67:1020–1023. doi: 10.1021/jo016089o. [DOI] [PubMed] [Google Scholar]
- 224.Ghosh A.K., Gong G. Total synthesis and structural revision of (+) -amphidinolide W. J. Am. Chem. Soc. 2004;126:3704–3705. doi: 10.1021/ja049754u. [DOI] [PubMed] [Google Scholar]
- 225.Tsuda M., Izui N., Shimbo K., Sato M., Fukushi E., Kawabata J., Kobayashi J. Amphidinolide Y, a novel 17-membered macrolide from dinoflagellate Amphidinium sp.: Plausible biogenetic precursor of amphidinolide X. J. Org. Chem. 2003;68:9109–9112. doi: 10.1021/jo035278z. [DOI] [PubMed] [Google Scholar]
- 226.Tsuda M., Izui N., Shimbo K., Sato M., Fukushi E., Kawabata J., Katsumata K., Horiguchi T., Kobayashi J. Amphidinolide X, a novel 16-membered macrodiolide from dinoflagellate Amphidinium sp. J. Org. Chem. 2003;68:5339–5345. doi: 10.1021/jo0343634. [DOI] [PubMed] [Google Scholar]
- 227.Oguchi K., Tsuda M., Iwamoto R., Okamoto Y., Endo T., Kobayashi J., Ozawa T., Masuda A. Amphidinolides B6 and B7, cytotoxic macrolides from a symbiotic dinoflagellate Amphidinium species. J. Nat. Prod. 2007;70:1676–1679. doi: 10.1021/np0703085. [DOI] [PubMed] [Google Scholar]
- 228.Kubota T., Sakuma Y., Tsuda M., Kobayashi J. Amphidinolide C2, new macrolide from marine dinoflagellate Amphidinium species. Mar. Drugs. 2004;2:83–87. doi: 10.3390/md203083. [DOI] [Google Scholar]
- 229.Bauer I., Maranda L., Young K.A., Shimizu Y., Fairchild C., Cornell L., Macbeth J., Huang S. Isolation and structure of caribenolide I, a highly potent antitumrr macrolide from a cultured free-swimming caribbean dinoflagellate, Amphidinium sp. S1-36-5. J. Org. Chem. 1995;60:1084–1086. doi: 10.1021/jo00109a050. [DOI] [Google Scholar]
- 230.Takahashi Y., Kubota T., Kobayashi J. Amphidinolactone A, a new 13-menbered macrolide from dinoflagellate Amphidinium sp. Grad. Sch. Pharm. Sci. 2007;1:567–572. doi: 10.1038/ja.2007.51. [DOI] [PubMed] [Google Scholar]
- 231.Hangyou M., Ishiyama H., Takahashi Y., Kubota T., Kobayashi J. Total synthesis of amphidinolactone A and its absolute configuration. Tetrahedron Lett. 2009;50:1475–1477. doi: 10.1016/j.tetlet.2009.01.065. [DOI] [Google Scholar]
- 232.Nakamura H., Asari T., Fujimaki K., Maruyama K., Murai A., Ohizumi Y., Kan Y. Zooxanthellatoxin B, vasoconstrictive congener of zooxanthellatoxin-A from a symbiotic dinoflagellate Symbiodinium sp. Tetrahedron Lett. 1995;36:7255–7258. doi: 10.1016/0040-4039(95)01503-A. [DOI] [Google Scholar]
- 233.Nakamura H., Asari T., Murai A., Kan Y., Kondo T., Yoshida K., Ohizumi Y. Zooxanthellatoxin A, a potent vasoconstrictive 62-membered lactone from a symbiotic dinoflagellate. J. Am. Chem. Soc. 1995;117:550–551. doi: 10.1021/ja00106a071. [DOI] [Google Scholar]
- 234.Hu T., DeFreitas A.S.W., Curtis J.M., Oshima Y., Walter J.A., Wright J.L.C. Isolation and structure of prorocentrolide B, a fast-acting toxin from Prorocentrum maculosum. J. Nat. Prod. 1996;59:1010–1014. doi: 10.1021/np960439y. [DOI] [PubMed] [Google Scholar]
- 235.Hu T., Curtis J.M., Walter J.A., Wright J.L.C. Hoffmanniolide: A novel macrolide from Prorocentrum hoffmannianum. Tetrahedron Lett. 1999;40:3977–3980. doi: 10.1016/S0040-4039(99)00513-4. [DOI] [Google Scholar]
- 236.Tsuda M., Oguchi K., Iwamoto R., Okamoto Y., Fukushi E., Kawabata J., Ozawa T., Masuda A. Iriomoteolides-1b and -1c, 20-membered macrolides from a marine dinoflagellate Amphidinium species. J. Nat. Prod. 2007;70:1661–1663. doi: 10.1021/np0702537. [DOI] [PubMed] [Google Scholar]
- 237.Tsuda M., Oguchi K., Iwamoto R., Okamoto Y., Kobayashi J., Fukushi E., Kawabata J., Ozawa T., Masuda A., Kitaya Y., et al. Iriomoteolide-1a, a potent cytotoxic 20-membered macrolide from a benthic dinoflagellate Amphidinium species. J. Org. Chem. 2007;72:4469–4474. doi: 10.1021/jo070414b. [DOI] [PubMed] [Google Scholar]
- 238.Kumagai K., Tsuda M., Masuda A., Fukushi E., Kawabata J. Iriomoteolide-2a, a cytotoxic 23-membered macrolide from marine benthic dinoflagellate Amphidinium species. Heterocycles. 2015;91:265–274. [Google Scholar]
- 239.Oguchi K., Tsuda M., Iwamoto R., Okamoto Y., Kobayashi J., Fukushi E., Kawabata J., Ozawa T., Masuda A., Kitaya Y., et al. Iriomoteolide-3a, a cytotoxic 15-membered macrolide from a marine dinoflagellate Amphidinium species. J. Org. Chem. 2008;73:1567–1570. doi: 10.1021/jo702440s. [DOI] [PubMed] [Google Scholar]
- 240.Kumagai K., Tsuda M., Fukushi E., Kawabata J. Iriomoteolides-4A and -5A, hydrophilic macrolides from marine dinoflagellate Amphidinium species. Heterocycles. 2013;87:2615–2623. doi: 10.3987/COM-13-12841. [DOI] [Google Scholar]
- 241.Kumagai K., Tsuda M., Fukushi E., Kawabata J., Masuda A., Tsuda M. Iriomoteolides-9a and 11a: Two new odd-numbered macrolides from the marine dinoflagellate Amphidinium species. J. Nat. Med. 2017;71:506–512. doi: 10.1007/s11418-017-1080-y. [DOI] [PubMed] [Google Scholar]
- 242.Akakabe M., Kumagai K., Tsuda M., Konishi Y., Tominaga A., Kaneno D., Fukushi E., Kawabata J., Masuda A., Tsuda M. Iriomoteolides-10a and 12a, cytotoxic macrolides from marine dinoflagellate Amphidinium species. Chem. Pharm. Bull. 2016;64:1019–1023. doi: 10.1248/cpb.c16-00026. [DOI] [PubMed] [Google Scholar]
- 243.Takamura H., Kadonaga Y., Yamano Y., Han C., Aoyama Y., Kadota I., Uemura D. Synthesis and structural determination of the C33–C42 fragment of symbiodinolide. Tetrahedron Lett. 2009;50:863–866. doi: 10.1016/j.tetlet.2008.11.056. [DOI] [Google Scholar]
- 244.Takamura H., Murata T., Asai T., Kadota I., Uemura D. Stereoselective synthesis and absolute configuration of the C1’–C25’ fragment of symbiodinolide. J. Org. Chem. 2009;74:6658–6666. doi: 10.1021/jo901162v. [DOI] [PubMed] [Google Scholar]
- 245.Takamura H., Kadonaga Y., Yamano Y., Han C., Kadota I., Uemura D. Stereoselective synthesis and absolute configuration of the C33–C42 fragment of symbiodinolide. Tetrahedron. 2009;65:7449–7456. doi: 10.1016/j.tet.2009.07.019. [DOI] [Google Scholar]
- 246.Hwang B.S., Kim H.S., Yih W., Jeong E.J., Rho J.R. Acuminolide a: Structure and bioactivity of a new polyether macrolide from dinoflagellate Dinophysis acuminata. Org. Lett. 2014;16:5362–5365. doi: 10.1021/ol502567g. [DOI] [PubMed] [Google Scholar]
- 247.Domínguez H.J., Napolitano J.G., Fernández-Sánchez M.T., Cabrera-García D., Novelli A., Norte M., Fernández J.J., Daranas A.H. Belizentrin, a highly bioactive macrocycle from the dinoflagellate Prorocentrum belizeanum. Org. Lett. 2014;16:4546–4549. doi: 10.1021/ol502102f. [DOI] [PubMed] [Google Scholar]
- 248.Harju K., Koskela H., Kremp A., Suikkanen S., De La Iglesia P., Miles C.O., Krock B., Vanninen P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic sea. Toxicon. 2016;112:68–76. doi: 10.1016/j.toxicon.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 249.Kurimoto S.I., Iinuma Y., Kobayashi J., Kubota T. Symbiodinolactone A, a new 12-membered macrolide from symbiotic marine dinoflagellate Symbiodinium sp. Tetrahedron Lett. 2018;59:4496–4499. doi: 10.1016/j.tetlet.2018.11.018. [DOI] [Google Scholar]
- 250.Yotsu-Yamashita M., Haddock R.L., Yasumoto T. Polycavernoside A: A novel glycosidic macrolide from the red alga Polycavernosa tsudai (Gtacilaria edulis) J. Am. Chem. Soc. 1993;3:1147–1148. doi: 10.1021/ja00056a048. [DOI] [Google Scholar]
- 251.Fujiwara K., Amano S., Oka T., Murai A. Synthesis of the tetrahydropyran ring part of a marine toxin polycavernoside A. Chem. Lett. 1994;11:2147–2150. doi: 10.1246/cl.1994.2147. [DOI] [Google Scholar]
- 252.Fujiwara K., Amano S., Oka T., Murai A. Relative configuration of a marine toxin polycavernoside A. Chem. Lett. 1995:855–856. doi: 10.1246/cl.1995.855. [DOI] [Google Scholar]
- 253.Paquette L.A., Barriault L., Pissarnitski D., Johnston J.N. Stereocontrolled elaboration of natural (-) -polycavernoside A, a powerfully toxic metabolite of the red alga Polycavernosa tsudai. J. Am. Chem. Soc. 2000;122:619–631. doi: 10.1021/ja993487o. [DOI] [Google Scholar]
- 254.Yotsu-Yamashita M., Seki T., Paul V.J., Naoki H., Yasumoto T. Four new analogs of polycavernoside A. Tetrahedron Lett. 1995;36:5563–5566. doi: 10.1016/00404-0399(50)1052J-. [DOI] [Google Scholar]
- 255.Yotsu-Yamashita M., Abe K., Seki T., Fujiwara K., Yasumoto T. Polycavernoside C and C2, the new analogs of the human lethal toxin polycavernoside A, from the red alga, Gracilaria edulis. Tetrahedron Lett. 2007;48:2255–2259. doi: 10.1016/j.tetlet.2007.02.003. [DOI] [Google Scholar]
- 256.Nagai H., Yasumoto T., Hokama Y. Manauealides, some of the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. J. Nat. Prod. 1997;60:925–928. doi: 10.1021/np970193c. [DOI] [PubMed] [Google Scholar]
- 257.Nagai H., Kan Y., Fujita T., Sakamoto B., Hokama Y. Manauealide C and anhydrodebromoaplysiatoxin, toxic constituents of the hawaiian red alga, Gracilaria coronopifolia. Biosci. Biotechnol. Biochem. 1998;62:1011–1013. doi: 10.1271/bbb.62.1011. [DOI] [PubMed] [Google Scholar]
- 258.Kubanek J., Prusak A.C., Snell T.W., Giese R.A., Hardcastle K.I., Fairchild C.R., Aalbersberg W., Raventos-Suarez C., Hay M.E. Antineoplastic diterpene-benzoate macrolides from the fijian red alga Callophycus serratus. Org. Lett. 2005;7:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kubanek J., Prusak A.C., Snell T.W., Giese R.A., Fairchild C.R., Aalbersberg W., Hay M.E. Bromophycolides C-I from the fijian red alga Callophycus serratus. J. Nat. Prod. 2006;69:731–735. doi: 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lane A.L., Stout E.P., Lin A.S., Prudhomme J., Le Roch K., Fairchild C.R., Franzblau S.G., Hay M.E., Aalbersberg W., Kubanek J. Antimalarial bromophyeolides J-Q from the fijian red alga Callophycus serratus. J. Org. Chem. 2009;74:2736–2742. doi: 10.1021/jo900008w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lin A.S., Stout E.P., Prudhomme J., Roch K.L., Fairchild C.R., Franzblau S.C., Aalbersberg W., Hay M.E., Kubanek J. Bioactive bromophycolides R-U from the fijian red alga Callophycus serratus. J. Nat. Prod. 2010;73:275–278. doi: 10.1021/np900686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Stout E.P., Hasemeyer A.P., Lane A.L., Davenport T.M., Engel S., Hay M.E., Fairchild C.R., Prudhomme J., Roch K.L., Aalbersberg W., et al. Antibacterial neurymenolides from the fijian red alga Neurymenia fraxinifolia. Org. Lett. 2009;11:225–228. doi: 10.1021/ol8024814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.National T., Word K. Ecklonilactoness C-F from the brown alga Ecklonia stolonifera. Phytochemistry. 1993;33:155–159. [Google Scholar]
- 264.Todd J.S., Proteau P.J., Gerwick W.H. The absolute configuration of ecklonialactones A, B, and E, novel oxylipins from brown algae of the genera Ecklonia and Egregia. J. Nat. Prod. 1994;57:171–174. doi: 10.1021/np50103a028. [DOI] [PubMed] [Google Scholar]
- 265.Kousaka K., Ogi N., Akazawa Y., Fujieda M., Yamamoto Y., Takada Y., Kimura J. Novel oxylipin metabolites from the brown alga Eisenia bicyclis. J. Nat. Prod. 2003;66:1318–1323. doi: 10.1021/np030049t. [DOI] [PubMed] [Google Scholar]
- 266.Kitamura M., Schupp P.J., Nakano Y., Uemura D. Luminaolide, a novel metamorphosis-enhancing macrodiolide for scleractinian coral larvae from crustose coralline algae. Tetrahedron Lett. 2009;50:6606–6609. doi: 10.1016/j.tetlet.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Maru N., Inuzuka T., Yamamoto K., Kitamura M., Schupp P.J., Yamada K., Uemura D. Relative configuration of luminaolide. Tetrahedron Lett. 2013;54:4385–4387. doi: 10.1016/j.tetlet.2013.05.143. [DOI] [Google Scholar]
- 268.Wang Y., Chen Y., He B., Zhang R., Xu M. Two new avermectin derivatives from the beibu gulf gorgonian Anthogorgia caerulea. Chem. Biodivers. 2014;11:812–818. doi: 10.1002/cbdv.201300265. [DOI] [PubMed] [Google Scholar]
- 269.Koleck M.P., Vatakis A.M., Belinda Alvarado A., Andrews P., Marzo L.V., Muschik G.M., Roach J., Ross J.T., Lebherz W.B., Reeves M.P., et al. The large-scale isolation of bryostatin 1 from Bugula neritina following current good manufacturing practices. J. Nat. Prod. 1991;54:1265–1270. doi: 10.1021/np50077a004. [DOI] [PubMed] [Google Scholar]
- 270.Pettit G.R., Sengupta D., Herald C.L., Sharkey N.A., Blumberg P.M. Synthetic conversion of bryostatin 2 to bryostatin 1 and related bryopyrans. Can. J. Chem. 1991;69:856–860. doi: 10.1139/v91-126. [DOI] [Google Scholar]
- 271.Pettit G.R., Herald D.L., Gao F., Sengupta D., Herald C.L. Antineoplastic agents. 200. absolute configuration of the bryostatins. J. Org. Chem. 1991;56:1337–1340. doi: 10.1021/jo00003a086. [DOI] [Google Scholar]
- 272.Daniel E.S., Gwendolyn N.C., Mary P.K. 1H and 13C NMR assignments of the antitumor macrolide bryostatin 1. Magn. Reson. Chem. 1991;29:366–374. [Google Scholar]
- 273.Schaufelberger D.E., John N.C., Mary A.B., Alvarado A.B., Schaufelberger B.W., Muschikt G.M. Revised structure of bryostatin 3 and isolation of the bryostatin 3 26-ketone from Bugula neritina. J. Org. Chem. 1991;56:2895–2900. doi: 10.1021/jo00008a054. [DOI] [Google Scholar]
- 274.Pettit G.R., Sengupta D., Coll J.C., Schmidt J.H., Van Camp J.R., Rudloe J.J., Nieman R.A. Isolation and structure of bryostatins 14 and 15. Tetrahedron. 1991;47:3601–3610. doi: 10.1016/S0040-4020(01)80873-5. [DOI] [Google Scholar]
- 275.Pettit G.R., Herald C.L., Kamano Y. Structure of the Bugula neritina (marine bryozoa) antineoplastic component bryostatin 3. J. Org. Chem. 1983;48:5354–5356. doi: 10.1021/jo00174a037. [DOI] [Google Scholar]
- 276.Sprinz J., Steinhagen H., Wiese B., Helmchen G., Ohmori K., Ogawa Y., Obitsu T., Ishikawa Y., Nishiyama S., Yamamura S. Total synthesis of bryostatin 3. Science. 2000:2290–2294. [PubMed] [Google Scholar]
- 277.Kamano Y., Zhang H.P., Yoshida M., Kawamura M., Koyano T., Takahashi H., Itokawa H., Pettit G.R. An improved source of bryostatin 10, Bugula nertina from the gulf of aomori, Japan. J. Nat. Prod. 1995;58:1868–1875. doi: 10.1021/np50126a009. [DOI] [PubMed] [Google Scholar]
- 278.Pettit G.R., Gao F., Blumberg P.M., Herald C.L., Coll J.C., Kamano Y., Lewin N.E., Schmidt J.M., Chapuis J. Antineoplastic agents. 340. isolation and structural elucidation of bryostatins 16–18. J. Nat. Prod. 1996;59:286–289. doi: 10.1021/np960100b. [DOI] [PubMed] [Google Scholar]
- 279.Lin H.W., Yi Y.H., Li W.L. Bryostatin19: A new antineoplastic component from Bugula neritina in the south China sea. Chin. J. Mar. Drugs. 1998;17:1. [Google Scholar]
- 280.Lopanik N., Gustafson K.R., Lindquist N. Structure of bryostatin 20: A symbiont-produced chemical defense for larvae of the host bryozoan, Bugula neritina. J. Nat. Prod. 2004;67:1412–1414. doi: 10.1021/np040007k. [DOI] [PubMed] [Google Scholar]
- 281.Pettit G.R., Gao F., Herald D.L., Blumberg P.M., Lewin N.E., Nieman R.A. Antineoplastic agents. 224. isolation and structure of neristatin 1. J. Am. Chem. Soc. 1991;113:6693–6695. doi: 10.1021/ja00017a062. [DOI] [Google Scholar]
- 282.Kato Y., Scheuer P.J. Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk Styloheilus longicauda. J. Am. Chem. Soc. 1973:2245–2246. doi: 10.1021/ja00814a041. [DOI] [PubMed] [Google Scholar]
- 283.Okamura H., Kuroda S., Tomita K., Ikegami S., Sugimoto Y., Sakaguchi S., Katsuki T., Yamaguchi M. Synthesis of aplysiatoxin: Stereoselective synthesis of key fragments. Tetrahedron Lett. 1991;32:5137–5140. doi: 10.1016/S0040-4039(00)93448-8. [DOI] [Google Scholar]
- 284.Ojika M., Kigoshi H., Ishigaki T., Tsukada I., Tsuboi T., Ogawa T., Yamada K. Absolute stereochemistry of aplyronine A, a potent antitumor substance of marine origin. J. Am. Chem. Soc. 1994;116:7441–7442. doi: 10.1021/ja00095a071. [DOI] [Google Scholar]
- 285.Kigoshi H., Ojika M., Ishigaki T., Suenaga K., Mutou T., Sakakura A., Ogawa T., Yamada K. Total synthesis of aplyronine A, a potent antitumor substance of marine origin. J. Am. Chem. Soc. 1994;116:7443–7444. doi: 10.1021/ja00095a072. [DOI] [Google Scholar]
- 286.Ojika M., Kigoshi H., Suenaga K., Imamura Y., Yoshikawa K., Ishigaki T., Sakakura A., Mutou T., Yamada K. Aplyronines D-H from the sea hare Aplysia kurodai: Isolation, structures, and cytotoxicity. Tetrahedron. 2012;68:982–987. doi: 10.1016/j.tet.2011.11.095. [DOI] [Google Scholar]
- 287.Ojika M., Nagoya T., Yamada K. Dolabelides A and B, cytotoxic 22-membered macrolides isolated from the sea hare Dolabella auricularia. Tetrahedron Lett. 1995;36:7491–7494. doi: 10.1016/0040-4039(95)01605-8. [DOI] [Google Scholar]
- 288.Suenaga K., Nagoya T., Shibata T., Kigoshi H., Yamada K. Dolabelides C and D, cytotoxic macrolides isolated from the sea hare Dolabella auricularia. J. Nat. Prod. 1997;60:155–157. doi: 10.1021/np960612q. [DOI] [Google Scholar]
- 289.Park P.K., O’Malley S.J., Schmidt D.R., Leighton J.L. Total synthesis of dolabelide D. J. Am. Chem. Soc. 2006;128:2796–2797. doi: 10.1021/ja058692k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Spinella A., Zubía E., Martînez E., Ortea J., Cimino G. Structure and stereochemistry of aplyolides A-E, lactonized dihydroxy fatty acids from the skin of the marine mollusk Aplysia depilans. J. Org. Chem. 1997;62:5471–5475. doi: 10.1021/jo961805l. [DOI] [Google Scholar]
- 291.Hansen T.V., Stenstrøm Y. First total synthesis of (−) -aplyolide A. Tetrahedron. 2001;12:1407–1409. doi: 10.1016/S0957-4166(01)00250-6. [DOI] [Google Scholar]
- 292.Caruso T., Spinella A. First total synthesis of natural aplyolides C and E, ichthyotoxic macrolides isolated from the skin of the marine mollusc Aplysia. Tetrahedron. 2002;13:2071–2073. doi: 10.1016/S0957-4166(02)00575-X. [DOI] [Google Scholar]
- 293.Spinella A., Caruso T., Coluccini C. First total synthesis of natural aplyolides B and D, ichthyotoxic macrolides isolated from the skin of the marine mollusk Aplysia depilans. Tetrahedron Lett. 2002;43:1681–1683. doi: 10.1016/S0040-4039(02)00117-X. [DOI] [Google Scholar]
- 294.Sasaki K., Wright J.L.C., Yasumoto T. Identification and characterization of pectenotoxin (PTX) 4 and PTX7 as spiroketal stereoisomers of two previously reported pectenotoxins. J. Org. Chem. 1998;63:2475–2480. doi: 10.1021/jo971310b. [DOI] [PubMed] [Google Scholar]
- 295.Miles C.O., Wilkins A.L., Samdal I.A., Sandvik M., Petersen D., Quilliam M.A., Naustvoll L.J., Rundberget T., Torgersen T., Hovgaard P., et al. A novel pectenotoxin, PTX-12, in Dinophysis spp. and shellfish from Norway. Chem. Res. Toxicol. 2004;17:1423–1433. doi: 10.1021/tx049870a. [DOI] [PubMed] [Google Scholar]
- 296.Pettit G.R., Xu J., Doubek D.L., Chapuis J.-C., Schmidt J.M. Isolation and structure of dolastatin 19 from the gulf of caifornia seahare Dolabella auricularia. J. Nat. Prod. 2004;67:1252–1255. doi: 10.1021/np030198b. [DOI] [PubMed] [Google Scholar]
- 297.Paterson I., Findlay A.D., Florence G.J. Total synthesis and stereochemical reassignment of (+)-dolastatin 19. Org. Lett. 2006;8:2131–2134. doi: 10.1021/ol060609q. [DOI] [PubMed] [Google Scholar]
- 298.Kikuchi Y., Ishibashi M., Sasaki T., Kobayashi J. Iejimalides C and D, new antineoplastic 24-membered macrolide sulfates from the okinawan marine tunicate Eudistoma cf. rigida. Tetrahedron Lett. 1991;32:797–798. doi: 10.1016/S0040-4039(00)74889-1. [DOI] [Google Scholar]
- 299.Galinis D.L., Mckee T.C., Pannell L.K., Ii J.H.C., Boyd M.R. Lobatamides A and B, novel cytotoxic macrolides from the tunicate Aplidium Lobtum. Mar. Technol. 1997;3263:8968–8969. [Google Scholar]
- 300.McKee T.C., Galinis D.L., Pannell L.K., Cardellina J.H., Laakso J., Ireland C.M., Murray L., Capon R.J., Boyd M.R. The lobatamides, novel cytotoxic macrolides from southwestern pacific tunicates. J. Org. Chem. 1998;63:7805–7810. doi: 10.1021/jo980939r. [DOI] [Google Scholar]
- 301.Shen R., Lin C.T., Porco J.A. Total synthesis and stereochemical assignment of the salicylate antitumor macrolide lobatamide C1. Cheminform. 2002;23:5650–5651. doi: 10.1021/ja026025a. [DOI] [PubMed] [Google Scholar]
- 302.Ueda K., Hu Y. Haterumalide B: A new cytotoxic macrolide from an okinawan ascidian Lissoclinum sp. Tetrahedron Lett. 1999;40:6305–6308. doi: 10.1016/S0040-4039(99)01290-3. [DOI] [Google Scholar]
- 303.Teruya T., Shimogawa H., Suenaga K., Kigoshi H. Biselides A and B, novel macrolides from the okinawan ascidian Didemnidae sp. Chem. Lett. 2004;33:1184–1185. doi: 10.1246/cl.2004.1184. [DOI] [Google Scholar]
- 304.Teruya T., Suenaga K., Maruyama S., Kurotaki M., Kigoshi H. Biselides A-E: Novel polyketides from the okinawan ascidian Didemnidae sp. Tetrahedron. 2005;61:6561–6567. doi: 10.1016/j.tet.2005.04.052. [DOI] [Google Scholar]
- 305.Diyabalanage T., Amsler C.D., McClintock J.B., Baker B.J. Palmerolide A, a cytotoxic macrolide from the antarctic tunicate Synoicum adareanum. J. Am. Chem. Soc. 2006;128:5630–5631. doi: 10.1021/ja0588508. [DOI] [PubMed] [Google Scholar]
- 306.Nicolaou K.C., Guduru R., Sun Y.P., Banerji B., Chen D.Y.K. Total synthesis of the originally proposed and revised structures of palmerolide A. Angew. Chem. Int. Ed. 2007;46:5896–5900. doi: 10.1002/anie.200702243. [DOI] [PubMed] [Google Scholar]
- 307.Jiang X., Liu B., Lebreton S., De Brabander J.K. Total synthesis and structure revision of the marine metabolite palmerolide A. J. Am. Chem. Soc. 2007;129:6386–6387. doi: 10.1021/ja0715142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sikorska J., Hau A.M., Anklin C., Parker-Nance S., Davies-Coleman M.T., Ishmael J.E., McPhail K.L. Mandelalides A-D, cytotoxic macrolides from a new Lissoclinum species of south african tunicate. J. Org. Chem. 2012;77:6066–6075. doi: 10.1021/jo3008622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Inokuma Y., Yoshioka S., Ariyoshi J., Aria T., Hitora Y., Takada K., Matsunaga S., Rissanen K., Fujita M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature. 2013;495:461–466. doi: 10.1038/nature11990. [DOI] [PubMed] [Google Scholar]