Abstract
Plasmodesmata form intercellular channels which ensure the transport of various molecules during embryogenesis and postembryonic growth. However, high permeability of plasmodesmata may interfere with the establishment of auxin maxima, which are required for cellular patterning and the development of distinct tissues. Therefore, diffusion through plasmodesmata is not always desirable and the symplastic continuum must be broken up to induce or accomplish some developmental processes. Many data show the role of auxin maxima in the regulation of auxin-responsive genes and the establishment of various cellular patterns. However, still little is known whether and how these maxima are formed in the embryo proper before 16-cell stage, that is, when there is still a nonpolar distribution of auxin efflux carriers. In this work, we focused on auxin-dependent regulation of plasmodesmata function, which may provide rapid and transient changes of their permeability, and thus take part in the regulation of gene expression.
Keywords: auxin, plasmodesmata, calcium, embryogenesis, cellular patterning, callose, cell-to-cell communication, ARF, AUX/IAA, ABP1
1. Introduction
Expression of auxin-responsive genes seems to rely on a multilevel regulatory machinery, and it depends not only on sophisticated molecular mechanisms controlling transcription, but also on systems which underlie the establishment of auxin maxima. Auxin controls the expression of various genes in a threshold-dependent manner, and some of them were found to be upregulated while others repressed in response to a high auxin concentration [1,2,3]. Auxin synthesis starts in the apical region of the 16-cell embryos; however, a strong response to auxin was found in hypophysis. Auxin maxima are formed due to polar auxin transport (PAT), which relies on auxin influx and efflux transporters localized in the plasma membrane. The former group includes AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) transporters, the latter consists of ATP-BINDING CASSETTE subfamily B (ABCB) proteins and PIN-FORMED (PIN) carriers [4,5,6,7,8]. PIN polarization results from phosphorylation and dephosphorylation driven by PINOID protein kinase and PP2A phosphatase, respectively. Phosphorylated PIN proteins occupy the apical domain of the cell, and their dephosphorylation results in trafficking to the basal plasma membrane. However, PIN phosphorylation performed by D6 PROTEIN KINASE (D6PK) regulates auxin efflux activity without affecting protein location [9,10,11]. Mutations in genes responsible for PAT disrupt the formation of the apical-basal axis and may be lethal for the developing embryo [8,12,13]. Thus, the establishment of auxin maxima seems to be crucial during changes of the radial to bilateral symmetry.
On the contrary, before embryos reach the 16-cell stage, similar auxin concentrations are considered in all cells of the embryo proper due to nonpolar distribution of auxin efflux carriers [14,15]. Expression of the auxin-responsive DORNRÖSCHEN (DRN) gene [6,16] and activation of the DR5 promoter [8] in each cell of the embryo proper may support a homogenous auxin dispersion. However, cell-specific activation of auxin-dependent genes was found early during embryogenesis. After the first division of a zygote different genes are expressed in the apical cell rather than in the basal one, and this pattern is maintained during the following stages of embryo development. Auxin carriers and transcription factors belonging to WUSCHEL-related homeobox (WOX), homeodomain-leucine zipper class Ⅲ (HD-ZIP Ⅲ), or Apetala2 (AP2)-domain families are good examples of proteins being expressed differently in various regions of the developing embryo [12,17,18,19,20]. Thus, auxin threshold-dependent regulation of cell identity may occur after the first division of a zygote. However, one may ask whether various intracellular auxin concentrations may be established in an embryo proper before it reaches the 16-cell stage, that is, when there is still a nonpolar distribution of auxin efflux transporters.
A hypothesis can be put forward that the increase in the auxin level and the differences in their concentrations between the upper and lower tier of the 8-cell embryos may be transient, but long enough to initiate a distinct fate of these two embryonic regions. Based on the available data concerning the control of auxin-dependent genes during embryogenesis and post-embryonic growth, in this review we sketched possible molecular pathways which could regulate the expression of auxin-dependent genes in embryos before they reach the 16-cell stage. Furthermore, different regulatory mechanisms of plasmodesmata permeability were overviewed, pointing to these which possibly induce transient differences in auxin concentrations between the upper and lower tier during early stages of embryogenesis.
2. Multi-Level Regulation of HD-ZIP Ⅲ and AP2-Domain Transcription Factors
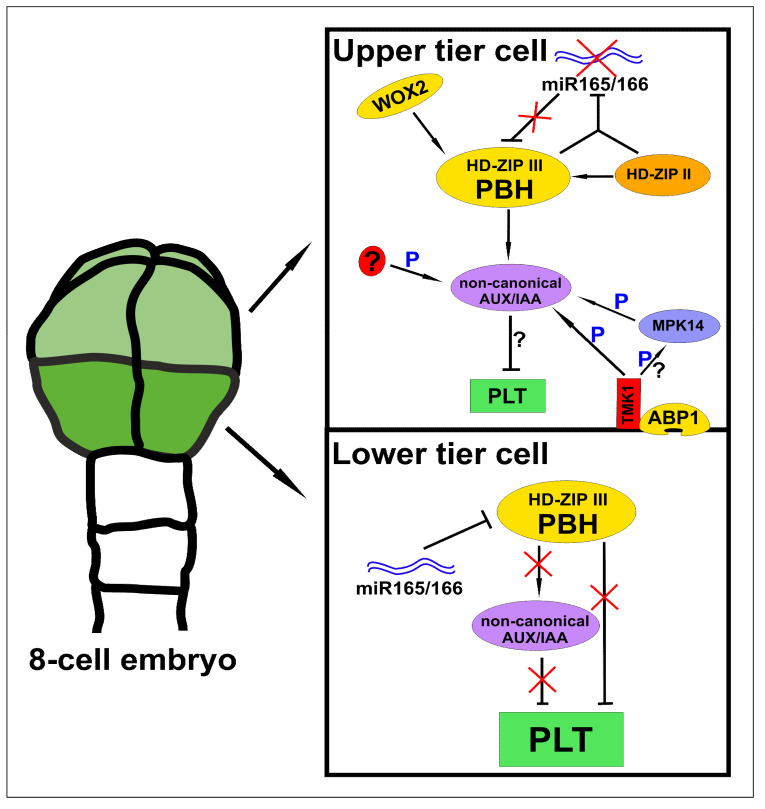
HD-ZIP Ⅲ and AP2-domain transcription factors are expressed in distinct cell populations during proembryo development. PLETHORA1 (PLT1) transcripts, coding one of the AP2-domain transcription factors, were found in the lower tier already at the 8-cell stage [21,22]. At the same time, antagonistically acting PHABULOSA (PHB), belonging to HD-ZIP Ⅲ transcription factors, appears in the upper tier [23]. WOX2, which was found in an apical cell after zygote division, seems to regulate PHB synthesis in this region. [24]. Lack of PLT transcription factors in the upper tier may result from AUXIN RESPONSE FACTOR (ARF) repression driven by non-canonical AUXIN/INDOLE-3-ACETIC ACID proteins (AUX/IAAs). This finding may be further supported by the fact that seedlings of Arabidopsis thaliana overexpressing IAA20 and IAA30 resemble PLT mutants [25]. Furthermore, the expression of these ARF repressors seems to be controlled by PHB protein [26]. Interactions between non-canonical AUX/IAAs and ARFs may also be regulated at the level of posttranscriptional modifications, and phosphorylation driven by TRANSMEMBRANE KINASE1 (TMK1) and MITOGEN-ACTIVATED PROTEIN KINASE14 (MPK14) was found to modulate the repressor function of non-canonical AUX/IAAs. Interestingly, AUXIN BINDING PROTEIN1 (ABP1), an apoplast-localized auxin receptor, may induce activation of the TMK1/MPK14-signaling pathway [27,28]. Although IAA20 and IAA30 were not revealed in the embryo proper at the 8-cell stage [29], Llavata–Peris and coworkers suggested that IAA33, another non-canonical AUX/IAA, was expressed in all cells until the late globular stage [30]. Thus, it is possible that the regulation of the expression of non-canonical AUX/IAAs and their phosphorylation take part in the repression of PLT genes in the upper tier at early stages of embryogenesis.
The activity of microRNAs (miRNAs) may be an additional yellow brick road which leads to better understanding how the expression of HD-ZIP Ⅲ and AP-2 domain transcription factors is established in various regions of developing embryos. Synthesis of HD-ZIP Ⅲ family proteins may be affected by miR165 and miR166, both expressed exclusively in suspensor cells at the 8-cell stage. Thus, lack of PHB transcript synthesis in the lower tier could result initially from transport of miRNAs to the embryo proper. Nevertheless, at the 16-cell stage, miR166 is also expressed in cells of the lower tier [23]. Significance of miRNAs to correct spatio-temporal expression of antagonistically acting HD-ZIP Ⅲ and AP-2 domain transcription factors was indicated in other studies as well. Embryos with the mutation in the ARABIDOPSIS SUPPRESSOR OF TY INSERTION 6-LIKE (SPT6L) gene, which encodes proteins necessary for miRNA-mediated gene silencing, show lack of PLT1 expression and ectopic synthesis of PHB transcripts in the basal region of an embryo. Interestingly, SPT6L/PHB/PHV mutants display partially restored expression of the PLT1 gene [31]. Thus, it is possible that lack of PLT proteins in the upper tier results either from blockage driven directly by HD-ZIP Ⅲ proteins or indirectly through non-canonical AUX/IAA repressors or other not yet revealed factors. Synergistic action of various pathways cannot also be excluded (Figure 1).
Figure 1.
Molecular pathways which may underlie the differential expression of homeodomain-leucine zipper class Ⅲ (HD-ZIP Ⅲ) and Apetala2 (AP2)-domain family transcription factors in the upper and lower tier of 8-cell embryos. In the upper tier, PHABULOSA (PHB), which belongs to HD-ZIP Ⅲ family transcription factors, may regulate the expression of non-canonical AUXIN/INDOLE-3-ACETIC ACID proteins (AUX/IAA) and thus support their high concentration. Phosphorylation of non-canonical AUX/IAAs (indicated as P), which underlies their stability, may be performed by TRANSMEMBRANE KINASE1 (TMK1), MITOGEN-ACTIVATED PROTEIN KINASE14 (MPK14), or other kinases. High level of phosphorylated AUX/IAAs blocks the expression of AP2-domain transcription factors. In the lower tier, miRNAs downregulate the expression of HD-ZIP Ⅲ transcription factors, and therefore do not support enhanced expression of non-canonical AUX/IAAs at this stage of embryogenesis. Absence of HD-ZIP Ⅲ proteins and the possible low concentration/phosphorylation of specific non-canonical AUX/IAAs may allow for the expression of AP2-domain transcription factors in the lower tier.
All of this indicates that the mechanisms underlying the expression of HD-ZIP Ⅲ and AP2-domain family proteins at the 8-cell stage still need elucidation. Since these antagonistically acting protein families are encoded by auxin-dependent genes, different auxin concentrations may underlie various expression patterns. Although PAT, supported by auxin efflux carriers, is not yet established at the 8-cell stage, different intracellular auxin concentrations could result from changes in the plasmodesmata (PD) permeability. The paragraphs below provide an overview of the functional connection between the establishment of auxin maxima and the auxin-dependent control of PD aperture.
3. The Role of Plasmodesmata during Developmental Processes
Many developmental processes in plants rely on cell-to-cell communication, which is ensured by channels called plasmodesmata (PD). These intracellular pores show a far-reaching structural complexity, and many proteins responsible for their function have been described so far. The presence of a structural and functional symplastic continuum is crucial for the transport of signaling molecules, phytohormones, RNAs, and proteins [32,33,34,35]. Plants modify both the number of PD and the size of their aperture to regulate complicated developmental processes during ontogenesis and to provide a proper response to pathogens [36,37,38]. PD may already appear after the first division of a zygote [39]; thus, the regulation of their function plays a crucial role in the establishment of various cellular patterns during plant embryogenesis. In A. thaliana, the embryonic root patterning relies on TARGET OF MONOPTEROS 7 (TMO7) protein diffusion from cells of the lower tier of the embryo proper to the hypophysis, the uppermost suspensor cell. Interestingly, this protein does not appear in the upper tier of the embryo proper, indicating a directional hypophysis-oriented transport in this case [40,41]. The symplastic continuum is not always desirable for the correctness of the developmental processes and at the early heart stage the hypophysis was found to be isolated from other suspensor cells due to changes of the PD aperture. However, this isolation seems to be unidirectional, and although the proteins synthesized in the suspensor do not appear in the embryo proper, those expressed under the SHOOTMERISTEMLESS (STM) promoter are present in suspensor cells. A far-reaching isolation of embryo regions is established at the cotyledonary stage, and PD permeability is reduced for high molecular mass proteins in this case [42,43,44]. Although the symplastic continuum is beneficial under some circumstances, it seems that the cell-to-cell communication must be broken up not only at the heart stage, when different tissues and distinct regions of an embryo are formed, but also at early stages of embryogenesis when cellular patterning starts.
4. Do Callose-Dependent Changes of PD Permeability Regulate Transient Auxin Gradients?
Dysfunction of PD leads to severe developmental disorders during embryogenesis and postembryonic growth, indicating that the on-time regulation of PD permeability is crucial for various cellular processes [45,46,47]. During postembryonic growth, PD were proposed to provide auxin reflux from shootward transporting tissues, which helps in the establishment of an auxin sink in the root apical region [48]. Although it is possible that the reflux model plays a similar role during auxin accumulation in a radicle, auxin diffusion does not seem to be helpful during the establishment of various cellular patterns in proembryo cells. To counteract this and provide auxin maxima in particular cells during early stages of embryogenesis, dynamic and reversible changes of PD permeability are needed. Thus, it is reasonable to ask whether auxin may control PD permeability to support the establishment of an increased auxin concentration in various cells and tissues.
Several studies indicate that deposition of 1,3-β-glucan (callose) decreases the PD aperture during ontogenesis and in response to pathogens. The mutation in the GLUCAN SYNTHASE-LIKE 8 (GLS8) gene, which encodes a callose synthetase, leads to severe aberrations during embryo development. Callose-dependent changes of the PD aperture are responsible for the directional diffusion of auxin in midrib and petiole epidermis cells [49], and higher PD permeability for auxin was seen in gsl8 mutants, compared to wild-type plants [50]. Callose synthesis was also found to be indispensable during the formation of the apical-basal axis and the development of the embryonic root [45,46,51,52].
The establishment of auxin gradients during phototropic and gravitropic responses rely on callose-dependent PD closure, and high auxin levels were found to activate the gls8 gene. Furthermore, the expression of callose synthetase is regulated by ARF7 [50]. Auxin’s role in the regulation of PD aperture may be supported by the data which indicate that expression of PLASMODESMATA-LOCATED PROTEIN 5 (PDLP5) in cells surrounding the lateral root primordium requires derepression of ARF7/19 transcription factors. PDLP5 is a receptor-like transmembrane protein which stimulates PD closure in a callose-dependent manner [52]. All of this indicates that auxin may control the PD aperture due to callose deposition. However, does it ensure fast regulation of PD permeability which would be needed for dynamic processes during early stages of embryogenesis?
Although rapid callose deposition was found during a bacterial pathogen attack [53], returning to the initial state, which is necessary for transient changes of the PD aperture, requires the activity of glucanases, the enzymes responsible for the degradation of callose [54,55,56]. Glucanases, similarly to callose synthetase, were found to be regulated in an auxin-dependent manner [57,58]; however, callose synthesis and turnover do not seem to support dynamic changes of PD permeability. Thus, the auxin-dependent deposition of callose in PD may play a main role in slow developmental processes or plant responses which do not require fast and reversible modification of the PD aperture. It is reasonable to hypothesize that the regulation of PD permeability, which underlies the dynamic processes of cellular patterning during early stages of embryogenesis, might occur in a callose-independent manner.
5. Auxin May Regulate the Function of PD in a Callose-Independent Manner
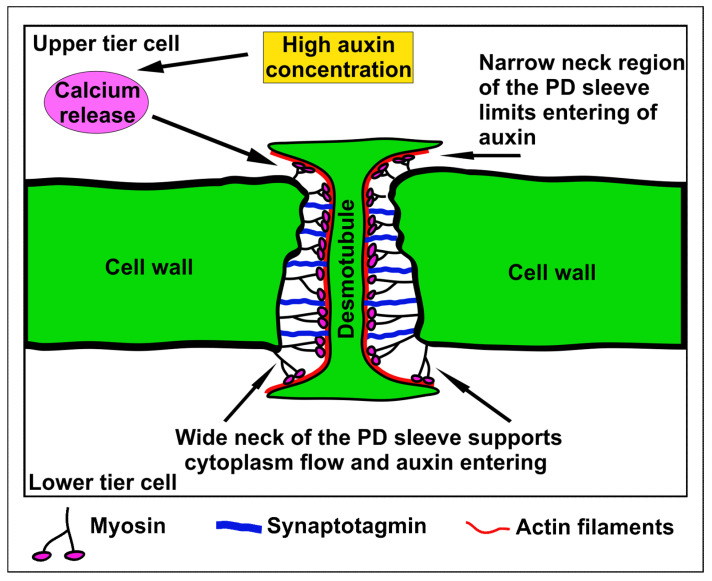
In the previous century, a calcium-dependent PD closure was observed in the staminal hairs of Setcreasea purpurea [59] and the role of calcium in this process was also revealed by other authors [60]. Since calmodulin, calreticulin, actin, and myosin Ⅷ were found in PD, calcium ions may regulate the organization of actin filaments and myosin motility properties which leads to changes in PD aperture. Actin–myosin structures may be localized in the neck region of PD; however, myosin Ⅷ was also postulated to form spoke-like structures which connect the plasma membrane and the desmotubule in the cytoplasmic sleeve of PD [61,62]. Additionally, other proteins such as plant synaptotagmins are suggested to form membrane contact sites (MCSs). Synaptotagmin-dependent tethering of the plasma membrane and desmotubule was reviewed by Tilsner and coworkers who proposed that calcium might reduce the distance between the plasma membrane and desmotubule similarly as it happens between the plasma membrane and endoplasmic reticulum in animal cells [63]. A high calcium concentration was found to shorten the neck domain of myosin Ⅺ in plants [64], which suggests that a similar effect of calcium could also concern myosin Ⅷ. Therefore, myosin localized in the cytoplasmic sleeve may take part in the reduction of the distance between the plasma membrane and the desmotubule. Since auxin was found to induce calcium release [65], calcium-dependent regulation of PD permeability at early stages of embryogenesis seems to be a very promising hypothesis, and this callose-independent pathway has been suggested in some review papers [38,66]. If calcium release occurs in only one of the adjacent cells, this mechanism could result in a wider opening of PD on one site of the cell wall, which helps to provide directional transport of molecules (Figure 2).
Figure 2.
A hypothetical model of rapid plasmodesmata aperture regulation. The auxin influx may induce calcium ion release in the upper tier cells. Next, a high calcium concentration in the cytoplasm triggers changes in the conformation of myosin and synaptotagmin proteins, which reduce the aperture of the neck region at one side of the plasmodesma. This could favor unidirectional auxin movement, which may underlie the establishment of different auxin maxima in adjacent cells. This mechanism could also induce a reduction of cell-to-cell communication if calcium release happens in both cells.
The most recent data indicate that the glucose-dependent activation of the plant TARGET OF RAPAMYCIN KINASE (TOR) restricts the transport through PD in leaf epidermal cells [67]. Interestingly, auxin was found to activate the TOR kinase via RHO of plants (ROPs), which are plasma membrane GTP-binding proteins. Another signaling pathway of TOR kinase activation is possible via phosphatidic acid (PA), which may also be formed in response to auxin. This auxin-dependent activation of TOR kinase was well reviewed by Schepetilnikov and Ryabova [68]. Since no connection between TOR activation and the deposition of callose has been shown so far, the TOR-dependent signaling pathway could function as an alternative mechanism of callose-independent regulation of PD permeability in response to auxin.
Another mechanism which could explain the transient and rapid changes in plasmodesmata permeability relies on the pressure forces generated inside the cell. In 1992, Oparka and Prior indicated that the increase in turgor pressure and differences in the pressure generated between the two cells stopped the intracellular transport [69]. Through the years, the pressure-dependent control of plasmodesmata permeability has been speculated and after over 20 years, Park and coworkers described a model explaining the regulation of PD function in a mechano-sensing manner. The authors proposed that the cytoplasmic part of desmotubules may work as a plug which closes the entrance to the cytoplasmic sleeve of PD when differences in pressure appear between cells [70]. Changes in turgor pressure may be induced by osmotic substances, and potassium ions are one of the main players which may regulate this process [71]. Auxin was found to activate channels which allow for the influx of potassium ion [72,73], and auxin-induced potassium channels seem to be indispensable for embryo development [74]. Thus, auxin-regulated variations in the turgor pressure may be responsible for rapid and transient changes of plasmodesmata permeability. Auxin may regulate this pathway already before high cellular concentration of auxin is achieved. Changes in potassium flux were indicated in cells overexpressing ABP1 [75] and after using antibodies against this protein [76]. Thus, signals which regulate the turgor pressure may appear already when auxin binds to ABP1 in the apoplast.
6. Unidirectional Transport through PD—Science or Fiction
In the cytoplasm, small molecules such auxin move generally via diffusion; thus, the regulation of PD permeability seems to be necessary to generate transient intracellular auxin maxima when the PAT is not yet established. It could be achieved by either PD closure in a mechano-sensing manner or by directional auxin transport resulting from changes in the structure of PD channels. Christensen and coworkers [77] observed diffusion of fluorescent probes from an epidermal cell to a trichome basal cell, but not in the opposite direction. This unidirectional transport did not depend directly on actin filaments and it was blocked by treatment with sodium azide, which is a metabolic inhibitor. Unidirectional transport was also indicated in elegant studies which showed the transfer of photoactivatable green fluorescent protein (PA-GFP) from basal to apical cell in embryos of Nicotiana tabacum, but not in the opposite direction [78]. One-way transport through PD may be indirectly supported by other data. GFP expressed in the base of a hypocotyl was found to diffuse into the root apical meristem (RAM) and the entire hypocotyl, while GFP synthesized in RAM was unable to cross the boundary between the root and hypocotyl [43]. All of this shows that PD by unidirectional transport could support the establishment of auxin maxima even if the system based on auxin influx and efflux transporters does not allow for this. However, the exact molecular mechanism of unidirectional transport remains to be discovered.
Another pathway which could participate in the generation of auxin maxima may be based on vesicle-dependent auxin transport [79,80,81]. Although the presence of auxin secretory vesicles was questioned by some authors [82], Hille and coworkers suggested that this vesicular trafficking was possible; however, they concluded at the same time that its role during directional transport is negligible [83]. Studies which showed no directional aggregation of endosomes until embryos reach the 16-cell stage [84] cast doubt on the importance of secretory vesicle-dependent auxin transport at early stages of embryogenesis. However, it cannot be excluded that very small secretory vesicles still function as a platform of directional auxin transport during embryo development.
7. Conclusions
Many developmental processes during early stages of embryogenesis may depend on the rapid and transient establishment of auxin maxima; thus, cells must counteract auxin diffusion to provide sufficient auxin concentrations. Current data indicate that auxin may regulate dynamic changes of PD permeability during various cellular processes, and the control of PD function seems to take advantage of their complex structure. Mechano-sensing and myosin-based regulation of PD permeability could play a prominent role during the establishment of auxin maxima in situations when auxin efflux transporters do not show a polar distribution.
Although the basis of callose-dependent changes of the PD aperture seems to be well understood, the dynamic regulation of their permeability in a callose-independent manner still needs elucidation. Thus, detailed studies on the regulation of auxin-responsive genes in the context of cytophysiological mechanisms responsible for the establishment of auxin gradients should be continued. The existence of yet unknown regulating systems is very plausible, and they are still waiting to be discovered.
Acknowledgments
We thank M. Fronczak for English language corrections.
Author Contributions
K.W. conceptualized and wrote most of the manuscript with the support of J.T.P., A.Ż. and J.M. J.T.P. prepared the figures. J.T.P. and A.Ż. consulted the content of the manuscript and J.M. provided critical revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jenik P.D., Barton M.K. Surge and destroy: The role of auxin in plant embryogenesis. Development. 2005;132:3577–3585. doi: 10.1242/dev.01952. [DOI] [PubMed] [Google Scholar]
- 2.Lee D.J., Park J.W., Lee H.W., Kim J. Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J. Exp. Bot. 2009;60:3935–3957. doi: 10.1093/jxb/erp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paponov I.A., Paponov M., Teale W., Menges M., Chakrabortee S., Murray J.A., Palme K. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol. Plant. 2008;1:321–337. doi: 10.1093/mp/ssm021. [DOI] [PubMed] [Google Scholar]
- 4.Mohanta T.K., Bashir T., Hashem A., Abd_Allah E.F., Khan A.L., Al-Harrasi A.S. Molecular players of auxin transport systems: Advances in genomic and molecular events. J. Plant. Interact. 2018;13:483–495. doi: 10.1080/17429145.2018.1523476. [DOI] [Google Scholar]
- 5.Zazimalova E., Murphy A.S., Yang H., Hoyerova K., Hosek P. Auxin transporters—why so many? Cold Spring Harb Perspect Biol. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole M., Chandler J., Weijers D., Jacobs B., Comelli P., Werr W. DORNROSCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development. 2009;136:1643–1651. doi: 10.1242/dev.032177. [DOI] [PubMed] [Google Scholar]
- 7.Wabnik K., Robert H.S., Smith R.S., Friml J. Modeling framework for the establishment of the apical-basal embryonic axis in plants. Curr. Biol. 2013;23:2513–2518. doi: 10.1016/j.cub.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jurgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 9.Armengot L., Marques-Bueno M.M., Jaillais Y. Regulation of polar auxin transport by protein and lipid kinases. J. Exp. Bot. 2016;67:4015–4037. doi: 10.1093/jxb/erw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willige B.C., Chory J. A current perspective on the role of AGCVIII kinases in PIN-mediated apical hook development. Front. Plant. Sci. 2015;6:767. doi: 10.3389/fpls.2015.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamowski M., Friml J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell. 2015;27:20–32. doi: 10.1105/tpc.114.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller B., Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. 2009;1:a001545. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert H.S., Grunewald W., Sauer M., Cannoot B., Soriano M., Swarup R., Weijers D., Bennett M., Boutilier K., Friml J. Plant embryogenesis requires AUX/LAX-mediated auxin influx. Development. 2015;142:702–711. doi: 10.1242/dev.115832. [DOI] [PubMed] [Google Scholar]
- 14.Robert H.S., Friml J. Auxin and other signals on the move in plants. Nat. Chem. Biol. 2009;5:325–332. doi: 10.1038/nchembio.170. [DOI] [PubMed] [Google Scholar]
- 15.Baba A.I., Valkai I., Labhane N.M., Koczka L., Andrasi N., Klement E., Darula Z., Medzihradszky K.F., Szabados L., Feher A., et al. CRK5 Protein Kinase Contributes to the Progression of Embryogenesis of Arabidopsis thaliana. Int. J. Mol. Sci. 2019;20:6120. doi: 10.3390/ijms20246120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slane D., Kong J., Berendzen K.W., Kilian J., Henschen A., Kolb M., Schmid M., Harter K., Mayer U., De Smet I., et al. Cell type-specific transcriptome analysis in the early Arabidopsis thaliana embryo. Development. 2014;141:4831–4840. doi: 10.1242/dev.116459. [DOI] [PubMed] [Google Scholar]
- 17.Zhao P., Shi D.-Q., Yang W.-C. Patterning the embryo in higher plants: Emerging pathways and challenges. Front. Biol. 2011;6:3–11. doi: 10.1007/s11515-011-1119-5. [DOI] [Google Scholar]
- 18.Zhang Z., Laux T. The asymmetric division of the Arabidopsis zygote: From cell polarity to an embryo axis. Sex. Plant Reprod. 2011;24:161–169. doi: 10.1007/s00497-010-0160-x. [DOI] [PubMed] [Google Scholar]
- 19.Emery J.F., Floyd S.K., Alvarez J., Eshed Y., Hawker N.P., Izhaki A., Baum S.F., Bowman J.L. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Smith Z.R., Long J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature. 2010;464:423–426. doi: 10.1038/nature08843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aida M., Beis D., Heidstra R., Willemsen V., Blilou I., Galinha C., Nussaume L., Noh Y.S., Amasino R., Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 23.Miyashima S., Honda M., Hashimoto K., Tatematsu K., Hashimoto T., Sato-Nara K., Okada K., Nakajima K. A comprehensive expression analysis of the Arabidopsis MICRORNA165/6 gene family during embryogenesis reveals a conserved role in meristem specification and a non-cell-autonomous function. Plant Cell Physiol. 2013;54:375–384. doi: 10.1093/pcp/pcs188. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Tucker E., Hermann M., Laux T. A Molecular Framework for the Embryonic Initiation of Shoot Meristem Stem Cells. Dev. Cell. 2017;40:264–277 e264. doi: 10.1016/j.devcel.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Sato A., Yamamoto K.T. Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant. 2008;133:397–405. doi: 10.1111/j.1399-3054.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 26.Muller C.J., Valdes A.E., Wang G., Ramachandran P., Beste L., Uddenberg D., Carlsbecker A. PHABULOSA Mediates an Auxin Signaling Loop to Regulate Vascular Patterning in Arabidopsis. Plant Physiol. 2016;170:956–970. doi: 10.1104/pp.15.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv B., Yu Q., Liu J., Wen X., Yan Z., Hu K., Li H., Kong X., Li C., Tian H., et al. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J. 2020;39:e101515. doi: 10.15252/embj.2019101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S., Luschnig C., Friml J. Pho-view of Auxin: Reversible Protein Phosphorylation in Auxin Biosynthesis, Transport and Signaling. Mol. Plant. 2021;14:151–165. doi: 10.1016/j.molp.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Rademacher E.H., Lokerse A.S., Schlereth A., Llavata-Peris C.I., Bayer M., Kientz M., Freire Rios A., Borst J.W., Lukowitz W., Jurgens G., et al. Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell. 2012;22:211–222. doi: 10.1016/j.devcel.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Llavata-Peris C.I. PhD Thesis. Wageningen University; Wageningen, The Netherlands: 2013. Proteomic and mechanistic analysis of Auxin Response Factors in the Arabidopsis embryo. [Google Scholar]
- 31.Gu X.L., Wang H., Huang H., Cui X.F. SPT6L encoding a putative WG/GW-repeat protein regulates apical-basal polarity of embryo in Arabidopsis. Mol. Plant. 2012;5:249–259. doi: 10.1093/mp/ssr073. [DOI] [PubMed] [Google Scholar]
- 32.Roberts A.G., Oparka K.J. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 2003;26:103–124. doi: 10.1046/j.1365-3040.2003.00950.x. [DOI] [Google Scholar]
- 33.White R.G., Barton D.A. The cytoskeleton in plasmodesmata: A role in intercellular transport? J. Exp. Bot. 2011;62:5249–5266. doi: 10.1093/jxb/err227. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa M., Jackson D. Plasmodesmata-Mediated Cell-to-Cell Communication in the Shoot Apical Meristem: How Stem Cells Talk. Plants. 2017;6:12. doi: 10.3390/plants6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., Huang D., Chen X. Dynamic regulation of plasmodesmatal permeability and its application to horticultural research. Hortic. Res. 2019;6:47. doi: 10.1038/s41438-019-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sager R.E., Lee J.Y. Plasmodesmata at a glance. J. Cell Sci. 2018:131. doi: 10.1242/jcs.209346. [DOI] [PubMed] [Google Scholar]
- 37.Faulkner C. Plasmodesmata and the symplast. Curr. Biol. 2018;28:R1374–R1378. doi: 10.1016/j.cub.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Sager R., Lee J.Y. Plasmodesmata in integrated cell signalling: Insights from development and environmental signals and stresses. J. Exp. Bot. 2014;65:6337–6358. doi: 10.1093/jxb/eru365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz R., Jensen W.A. Capsella embryogenesis: The egg, zygote, and young embryo. Am. J. Bot. 1968;55:807–819. doi: 10.1002/j.1537-2197.1968.tb07438.x. [DOI] [Google Scholar]
- 40.Schlereth A., Moller B., Liu W., Kientz M., Flipse J., Rademacher E.H., Schmid M., Jurgens G., Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464:913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- 41.Llavata-Peris C., Lokerse A., Moller B., De Rybel B., Weijers D. Imaging of phenotypes, gene expression, and protein localization during embryonic root formation in Arabidopsis. Methods Mol. Biol. 2013;959:137–148. doi: 10.1007/978-1-62703-221-6_8. [DOI] [PubMed] [Google Scholar]
- 42.Kim I., Zambryski P.C. Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr. Opin. Plant Biol. 2005;8:593–599. doi: 10.1016/j.pbi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Kim I., Kobayashi K., Cho E., Zambryski P.C. Subdomains for transport via plasmodesmata corresponding to the apical–basal axis are established during Arabidopsis embryogenesis. PNAS. 2005;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stadler R., Lauterbach C., Sauer N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 2005;139:701–712. doi: 10.1104/pp.105.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saatian B., Austin R.S., Tian G., Chen C., Nguyen V., Kohalmi S.E., Geelen D., Cui Y. Analysis of a novel mutant allele of GSL8 reveals its key roles in cytokinesis and symplastic trafficking in Arabidopsis. BMC Plant Biol. 2018;18:295. doi: 10.1186/s12870-018-1515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X.Y., Liu L., Lee E., Han X., Rim Y., Chu H., Kim S.W., Sack F., Kim J.Y. The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol. 2009;150:105–113. doi: 10.1104/pp.108.133918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillmor C.S., Lukowitz W., Brininstool G., Sedbrook J.C., Hamann T., Poindexter P., Somerville C. Glycosylphosphatidylinositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell. 2005;17:1128–1140. doi: 10.1105/tpc.105.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellor N.L., Voss U., Janes G., Bennett M.J., Wells D.M., Band L.R. Auxin fluxes through plasmodesmata modify root-tip auxin distribution. Development. 2020:147. doi: 10.1242/dev.181669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao C., Liu X., De Storme N., Jensen K.H., Xu Q., Yang J., Liu X., Chen S., Martens H.J., Schulz A., et al. Directionality of Plasmodesmata-Mediated Transport in Arabidopsis Leaves Supports Auxin Channeling. Curr. Biol. 2020;30:1970–1977 e1974. doi: 10.1016/j.cub.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Han X., Hyun T.K., Zhang M., Kumar R., Koh E.J., Kang B.H., Lucas W.J., Kim J.Y. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev. Cell. 2014;28:132–146. doi: 10.1016/j.devcel.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Jackson D. Plasmodesmata spread their influence. F1000Prime Rep. 2015;7:25. doi: 10.12703/P7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sager R., Wang X., Hill K., Yoo B.C., Caplan J., Nedo A., Tran T., Bennett M.J., Lee J.Y. Auxin-dependent control of a plasmodesmal regulator creates a negative feedback loop modulating lateral root emergence. Nat. Commun. 2020;11:364. doi: 10.1038/s41467-019-14226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu B., Cheval C., Laohavisit A., Hocking B., Chiasson D., Olsson T.S.G., Shirasu K., Faulkner C., Gilliham M. A calmodulin-like protein regulates plasmodesmal closure during bacterial immune responses. New Phytol. 2017;215:77–84. doi: 10.1111/nph.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Storme N., Geelen D. Callose homeostasis at plasmodesmata: Molecular regulators and developmental relevance. Front. Plant Sci. 2014;5:138. doi: 10.3389/fpls.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavaliev R., Ueki S., Epel B.L., Citovsky V. Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma. 2011;248:117–130. doi: 10.1007/s00709-010-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balasubramanian V., Vashisht D., Cletus J., Sakthivel N. Plant beta-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012;34:1983–1990. doi: 10.1007/s10529-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 57.Benitez-Alfonso Y., Faulkner C., Pendle A., Miyashima S., Helariutta Y., Maule A. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell. 2013;26:136–147. doi: 10.1016/j.devcel.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Kotake T., Nakagawa N., Takeda K., Sakurai N. Auxin-Induced Elongation Growth and Expressions of Cell Wall-Bound Exoand Endo-b-Glucanases in Barley Coleoptiles. Plant Cell Physiol. 2000;41:1272–1278. doi: 10.1093/pcp/pcd056. [DOI] [PubMed] [Google Scholar]
- 59.Tucker E.B., Boss W.F. Mastoparan-lnduced lntracellular Ca2+ Fluxes May Regulate CeII-to-CeII communication in PIants. Plant Physiol. 1996;11:459–467. doi: 10.1104/pp.111.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holdaway-Clarke T.L., Walker N.A., Heple P.K., Overall R.L. Physiological elevations in cytoplasmic free calcium by cold or ion injection result in transient closure of higher plant plasmodesmata. Planta. 2000;210:329–335. doi: 10.1007/PL00008141. [DOI] [PubMed] [Google Scholar]
- 61.Overall R.L., Blackman L.M. A model of the macromolecular structure of plasmodesmata. Trends Plant Sci. 1996;9:307–311. doi: 10.1016/S1360-1385(96)88177-0. [DOI] [Google Scholar]
- 62.Baluska F., Cvrckova F., Kendrick-Jones J., Volkmann D. Sink Plasmodesmata as Gateways for Phloem Unloading. Myosin VIII and Calreticulin as Molecular Determinants of Sink Strength. Plant Physiol. 2001;126:39–46. doi: 10.1104/pp.126.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilsner J., Nicolas W., Rosado A., Bayer E.M. Staying Tight: Plasmodesmal Membrane Contact Sites and the Control of Cell-to-Cell Connectivity in Plants. Annu. Rev. Plant Biol. 2016;67:337–364. doi: 10.1146/annurev-arplant-043015-111840. [DOI] [PubMed] [Google Scholar]
- 64.Tominaga M., Kojima H., Yokota E., Nakamori R., Anson M., Shimmen T., Oiwa K. Calcium-induced Mechanical Change in the Neck Domain Alters the Activity of Plant Myosin XI. J. Biol. Chem. 2012;287:30711–30718. doi: 10.1074/jbc.M112.346668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanneste S., Friml J. Calcium: The Missing Link in Auxin Action. Plants. 2013;2:650–675. doi: 10.3390/plants2040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez-Hernandez V., Benitez M., Boudaoud A. Interplay between turgor pressure and plasmodesmata during plant development. J. Exp. Bot. 2020;71:768–777. doi: 10.1093/jxb/erz434. [DOI] [PubMed] [Google Scholar]
- 67.Brunkard J.O., Xu M., Scarpin M.R., Chatterjee S., Shemyakina E.A., Goodman H.M., Zambryski P. TOR dynamically regulates plant cell-cell transport. Proc. Natl. Acad. Sci. USA. 2020;117:5049–5058. doi: 10.1073/pnas.1919196117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schepetilnikov M., Ryabova L.A. Auxin Signaling in Regulation of Plant Translation Reinitiation. Front. Plant Sci. 2017;8:1014. doi: 10.3389/fpls.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oparka K.J., Prior D.A.M. Direct evidence for pressure-generated closure of plasmodesmata. Plant J. 1992;2:741–750. doi: 10.1111/j.1365-313X.1992.tb00143.x. [DOI] [Google Scholar]
- 70.Park K., Knoblauch J., Oparka K., Jensen K.H. Controlling intercellular flow through mechanosensitive plasmodesmata nanopores. Nat. Commun. 2019;10:3564. doi: 10.1038/s41467-019-11201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dreyer I., Uozumi N. Potassium channels in plant cells. FEBS J. 2011;278:4293–4303. doi: 10.1111/j.1742-4658.2011.08371.x. [DOI] [PubMed] [Google Scholar]
- 72.Jahn L., Mucha S., Bergmann S., Horn C., Staswick P., Steffens B., Siemens J., Ludwig-Muller J. The Clubroot Pathogen (Plasmodiophora brassicae) Influences Auxin Signaling to Regulate Auxin Homeostasis in Arabidopsis. Plants. 2013;2:726–749. doi: 10.3390/plants2040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philippar K., Ivashikina N., Ache P., Christian M., Luthen H., Palme K., Hedrich R. Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana. Plant J. 2004;37:815–827. doi: 10.1111/j.1365-313X.2003.02006.x. [DOI] [PubMed] [Google Scholar]
- 74.Philippar K., Buchsenschutz K., Edwards D., Loffler J., Luthen H., Kranz E., Edwards K.J., Hedrich R. The auxin-induced K(+) channel gene Zmk1 in maize functions in coleoptile growth and is required for embryo development. Plant Mol. Biol. 2006;61:757–768. doi: 10.1007/s11103-006-0047-2. [DOI] [PubMed] [Google Scholar]
- 75.Bauly J.M., Sealy I.M., Macdonald H., Brearley J., Droge S., Hillmer S., Robinson D.G., Venis M.A., Blatt M.R., Lazarus C.M., et al. Overexpression of Auxin-Binding Protein Enhances the Sensitivity of Guard Cells to Auxin. Plant Physiol. 2000;124:1229–1238. doi: 10.1104/pp.124.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thiel G., Blatt M.R., Fricker M.D., White I.R., Millner P. Modulation of K+ channels in Vicia stomatal guard cells by peptide homologs to the auxin-binding protein C terminus. Proc. Natl. Acad. Sci. USA. 1993;90:11493–11497. doi: 10.1073/pnas.90.24.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christensen N.M., Faulkner C., Oparka K. Evidence for unidirectional flow through plasmodesmata. Plant Physiol. 2009;150:96–104. doi: 10.1104/pp.109.137083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S., He Y., Zhao J., Zhang L., Sun M.X. Polar protein transport between apical and basal cells during tobacco early embryogenesis. Plant Cell Rep. 2013;32:285–291. doi: 10.1007/s00299-012-1362-5. [DOI] [PubMed] [Google Scholar]
- 79.Mettbach U., Strnad M., Mancuso S., Baluska F. Immunogold-EM analysis reveal brefeldin a-sensitive clusters of auxin in Arabidopsis root apex cells. Commun. Integr. Biol. 2017;10:e1327105. doi: 10.1080/19420889.2017.1327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlicht M., Strnad M., Scanlon M.J., Mancuso S., Hochholdinger F., Palme K., Volkmann D., Menzel D., Baluska F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal. Behav. 2006;1:122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baluska F., Strnad M., Mancuso S. Substantial Evidence for Auxin Secretory Vesicles. Plant Physiol. 2018;176:2586–2587. doi: 10.1104/pp.18.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson D.G., Hawes C., Hillmer S., Jurgens G., Schwechheimer C., Stierhof Y.D., Viotti C. Auxin and Vesicle Traffic. Plant Physiol. 2018;176:1884–1888. doi: 10.1104/pp.17.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hille S., Akhmanova M., Glanc M., Johnson A., Friml J. Relative Contribution of PIN-Containing Secretory Vesicles and Plasma Membrane PINs to the Directed Auxin Transport: Theoretical Estimation. Int. J. Mol. Sci. 2018;19:3566. doi: 10.3390/ijms19113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao C.Y., Weijers D. A toolkit for studying cellular reorganization during early embryogenesis in Arabidopsis thaliana. Plant J. 2018;93:963–976. doi: 10.1111/tpj.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]