Abstract
Context:
Knowledge about dentin microstructure is essential for execution of clinical procedures which require adhesion of materials to dentin.
Aims:
To evaluate by scanning electron microscopy (SEM) the dentin ultrastructure after demineralization with 6 M and 12 M hydrochloric acid (HCl).
Subjects and Methods:
Twenty dentin segments were immersed in fixative solution and dehydrated in ethanol. After 24 h, segments were randomly divided into 2 groups (n = 10), demineralized with 6 M HCl (G6M) and 12 M HCl (G12M), and prepared for SEM analysis.
Statistical Analysis Used:
Based on photomicrographs and chemical composition (energy dispersive X-ray spectroscopy) of dentin, a descriptive analysis was conducted.
Results:
G6M samples revealed a demineralized surface with peritubular dentin exposure and small magnification of the dentinal tubules openings. The intertubular dentin was partially demineralized. Demineralization of G12M samples was more aggressive and at different depths, promoting erosion and “detachment” of dentin layers. Peritubular dentin was observed on the dentin surface. There was a large magnification of the dentinal tubules openings. In both groups, tubular structures showed a similar chemical composition to the intertubular dentin. Lamina limitans was not observed.
Conclusions:
Dentin demineralization is dependent on the HCl molarity and promotes exposure of peritubular dentin.
Keywords: Dentin demineralization, dentin ultrastructure, lamina limitans, scanning electron microscopy
INTRODUCTION
Dental procedures and studies about dentin morphology require some changes of the dentin ultrastructure. The composite adhesion to dentin depends on acids to promote its demineralization, thus increasing the permeability and altering the chemical composition on its surface.[1] Dentin demineralization is also indicated in endodontic treatments, by using a chelating solution (ethylenediamine tetra-acetic acid [EDTA] 17%) to remove smear layer[2] and unblock dentinal tubules, allowing better penetration of intracanal medicaments and sealing materials. Thus, knowledge about the dentin microstructure is essential for the execution of these procedures, and its peculiar morphology needs further investigation.
The dentin matrix consists of basic structural units named dentinal tubules, surrounded by a hypermineralized layer known as peritubular dentin, and an intertubular matrix in which most of the organic matter is concentrated.[3] The formation of peritubular dentin is still not thoroughly understood. Its development from the inner wall of the tubule, decreasing its space until it reaches a hypermineralization phase,[4] could be an explanation. Another possibility is the formation by continuous deposition of the pulp fluid, resulting in hypermineralization around the dentinal tubules.[5] Peritubular dentin could also be formed after complete mineralization of the intertubular dentin, by a slow and gradual deposition in centripetal way, being able to partially or totally obliterate dentinal tubules.[6]
Just as the formation, peritubular dentin composition is still not well defined. Collagen is practically not found. Therefore, the amount of organic matter is constituted by noncollagenous substances such as glycosaminoglycans, proteoglycans, and phosphorylated proteins.[3,7,8] The hydroxyapatite crystals are rich in magnesium, carbonate, and calcium phosphate.[6] Conversely, the intertubular dentine is composed of a complex collagen fibers network, in which some crystals of hydroxyapatite are deposited.[6] In addition to the structural units, there is also a sheet-like membrane composed of noncollagenous proteins responsible for coating the peritubular dentine along the entire length of the tubules, known as lamina limitans.[9,10]
Among techniques to investigate dentin microstructure and morphology, the scanning electron microscopy (SEM) allows the examination of materials at ×300,000 or more.[11] To perform SEM analysis, a slight demineralization of the dentin surface with acids at different concentrations and in diverse exposure times is required.[12] Besides these factors, other aspects, such as pH, pKa, molecular weight, molarity and viscosity, should also be considered during demineralization.[13] The most common acids used are hydrochloric acid (HCl),[12] maleic acid,[14] phosphoric acid,[15] and citric acid.[14]
The changes in dentin morphology caused by acid demineralization[16] include increased dentin superficial porosity[17] and the diameter of the dentinal tubules,[1] collapsed collagen fibers and altered ultrastructure of the intertubular and peritubular dentin.[18]
Considering that many details of the demineralization process are not well understood and often interpreted in different manners, this study analyzed the dentin morphology after superficial demineralization with HCl at two different concentrations by using SEM. Demineralization patterns, dentinal tubules openings, intertubular and peritubular dentin structure and chemical composition, and presence of lamina limitans were analyzed.
SUBJECTS AND METHODS
Specimens preparation
Five incisors extracted from bovine of approximately 30-month-old were used according to institution research guide. Immediately after extraction, teeth were individually inserted in plastic vials containing 10% formaldehyde and remained stored until use. After removal of the crown and apical third with a double-sided diamond disk (KG Sorensen, São Paulo, Brazil), the root canals of each tube were standardized with diamond burs 4083 (KG Microdont, São Paulo, Brazil). The dentin tubes were individually inserted in specific plastic packaging and then sterilized in an autoclave for 20 min at 121°C. After that, 2-mm-thick dentin disks (n = 10) were obtained using a water-cooled low-speed ISOMET diamond saw (Buehler, Lake Bluff, NY). Dentin disks were immersed in 17% EDTA solution for 3 min and 2% sodium hypochlorite (NaClO) also for 3 min to remove the smear layer. Afterward, the disks were sectioned in buccolingual direction to obtain hemi segments (n = 20).
The hemi segments were treated to be analyzed by SEM and energy-dispersive X-ray spectroscopy (EDX). Specimens were fixed on 2.5% glutaraldehyde buffered with 0.2 Molar (M) cacodylate solution for 12 h at 4°C, followed by washing in 0.2 M cacodylate buffer for 1 h with two exchanges and brief washing in distilled water. Dehydration was done at increasing concentrations of ethyl alcohol 50%, 75%, 90%, 95%, and 100% for 20 min in each solution and 1 h in absolute alcohol. The segments were kept in an oven at 37°C for 24 h. Thereafter, they were randomly divided into two experimental groups (n = 10) according to the demineralization process by immersion for 30 s in 6 M HCl (G6M) or 12 M HCl (G12M). After demineralization, the dentin segments were briefly washed in distilled water, immersed in 2% NaClO for 10 min and rinsed again in distilled water for the same period.
Scanning electron microscopy analysis
After drying at 37°C for 48 h, the hemi segments were fixed in stubs with the dentin walls upwards, and sputter-coated with two thin layers (≈10 nm) of gold (300 Å) using a metalizer (EM SCD 500, Leica). The ultrastructural analysis of dentin was performed by SEM (JEOL JSM-6390 LV), operating at 10 kV, and photomicrographs with magnification between ×25 and ×5000 were obtained.
Energy dispersive X-ray spectroscopy analysis
The EDX analytical technique was performed in conjunction with SEM (JEOL JSM-6390 LV), at an accelerating voltage <10 KeV. Spectra were obtained based on the analysis of the chemical composition of dentin. The main chemical elements were registered to allow, when necessary, the differentiation between observed structures.
Statistical analysis
Considering the present qualitative and descriptive investigation, based on observation of dentin ultrastucture in photomicrographs and its chemical composition, the analysis performed was descriptive and observational. It was conducted to provide and summarize information about the following criteria: demineralization patterns, dentinal tubules openings, intertubular and peritubular dentin structure, chemical elements, and the presence or absence of lamina limitans.
RESULTS
Dentin demineralization with 6 M hydrochloric acid
Superficial decalcification of dentin [Figure 1a and b] and peritubular dentin extensions with cracks along its length [Figure 1c and d] were observed in the majority of specimens. In some regions, there was a small enlargement of the dentinal tubule openings [Figure 1e and f]. The intertubular dentin was partially demineralized [Figure 1g and 1h-white arrow], and peritubular dentin was exposed [Figure 1i-white arrow]. In transverse sections, the presence of obstructed peritubular dentin projecting from the dentinal tubules was observed [Figure 1j-white arrow]. The lamina limitans was not identified in any specimen.
Figure 1.
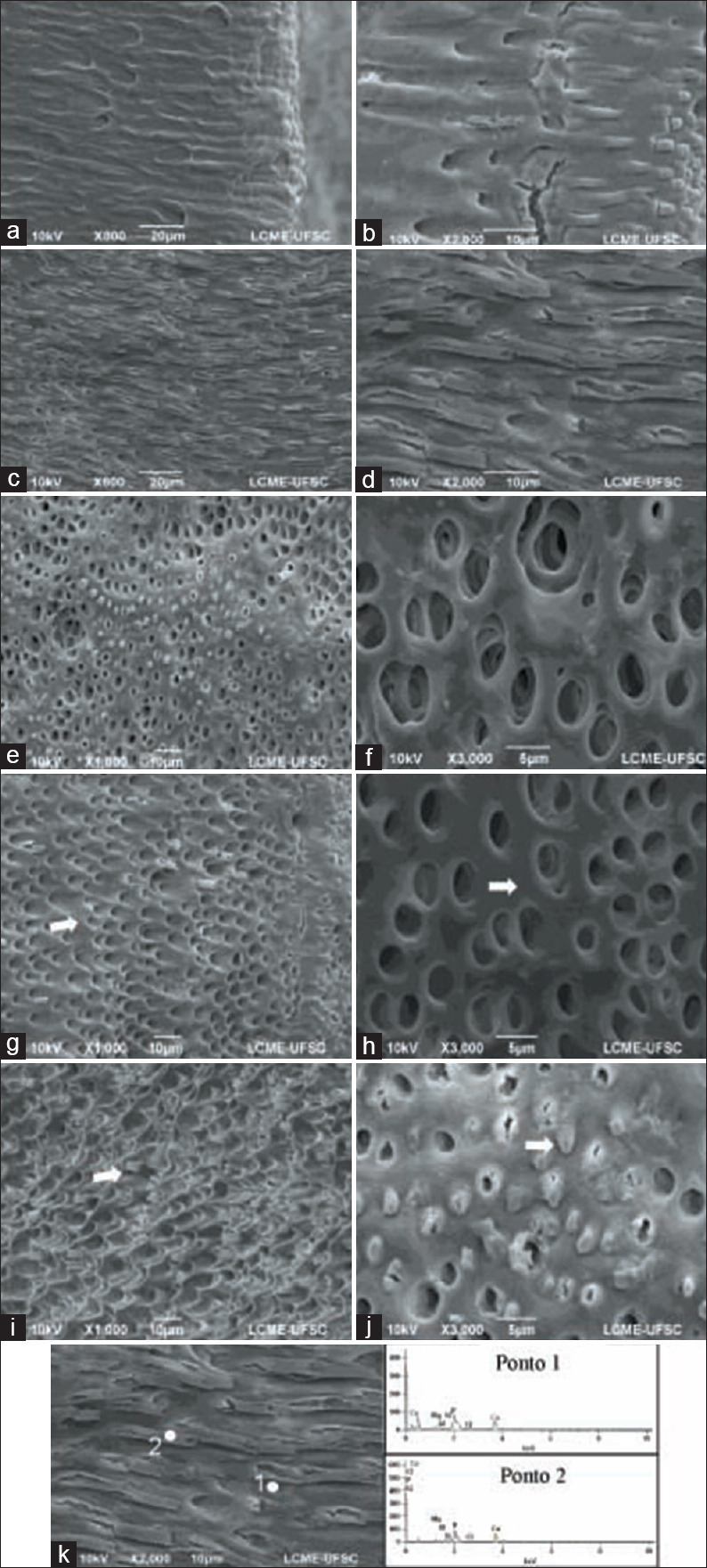
Superficially demineralized dentin (a and b). Peritubular dentin extensions with cracks on the dentin surface (c and d). Opening dentinal tubules with slight magnification (e and f). Partially demineralized intertubular dentin (g and h-white arrow). Exposed peritubular dentin (i-white arrow). Obstructed peritubular dentin (j-white arrow). Energy dispersive X-ray spectroscopy spectrum of dentin (points 1 and 2) demineralized with 6 M hydrochloric acid (k)
Dentin demineralization with 12 M hydrochloric acid
A more aggressive demineralization was observed at different depths, causing areas of erosion and “detachment” of dentin layers [Figure 2a and b]. Extensions of peritubular dentin were also visualized in few specimens [Figure 2c and d]. In some regions, there was the great enlargement of the dentinal tubule openings, promoting the connection between them [Figure 2e and f]. The intertubular dentin was demineralized to a greater extent and depth [Figure 2g], and the peritubular dentin was partially decalcified [Figure 2h]. The lamina limitans was not identified in any specimen.
Figure 2.
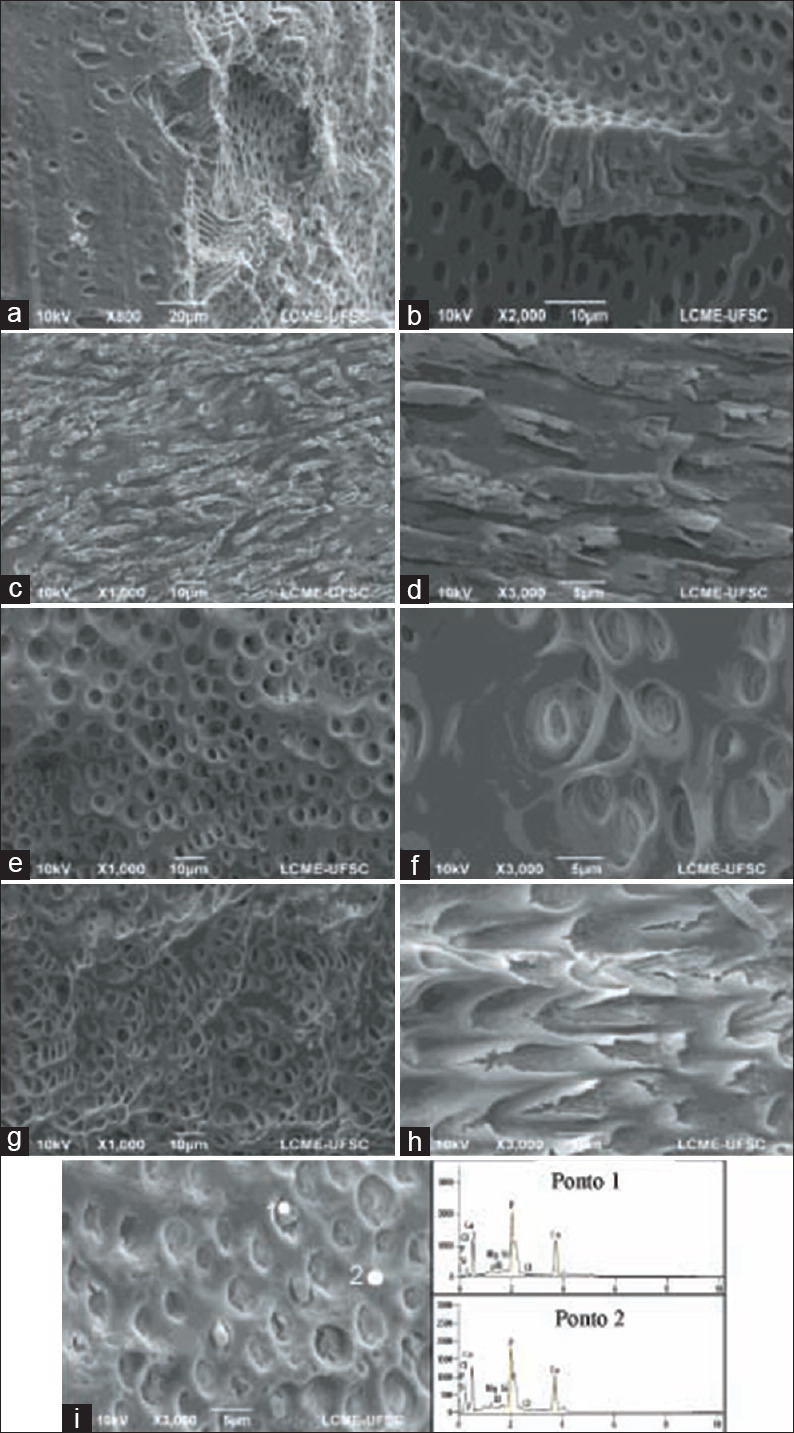
More aggressive demineralization with erosion areas (a) and “detachment” of dentin layers (b). Segments of peritubular dentin on the surface (c and d). Great enlargement of the dentinal tubules openings with connections between them (e and f). Demineralized intertubular dentin in greater extent and depth (g). Partially decalcified peritubular dentin (h). Energy dispersive X-ray spectroscopy spectrum of peritubular dentin (points 1 and 2) of samples demineralized with 12 M hydrochloric acid (i)
Energy dispersive X-ray spectroscopy analysis
Data on the chemical composition of dentin obtained by EDX analysis showing main elements of the observed structures after demineralization with 6 M and 12 M HCl are shown in Tables 1 and 2, and Figures 1k and 2i. The longitudinal tubular projections on the dentin surface, which were projected to the interior of the dentinal tubules in the transverse direction, were recognized as peritubular dentin.
Table 1.
Moles percentage in atoms - G6M
| Mg | Al | Si | P | Cl | Ca | |
|---|---|---|---|---|---|---|
| Point 1 | 3.59 | 0.28 | 0.38 | 33.87 | 1.00 | 60.88 |
| Point 2 | 3.92 | 0.00 | 0.00 | 26.44 | 0.00 | 69.64 |
Table 2.
Moles percentage in atoms - G12M
| Mg | Al | Si | P | Cl | Ca | |
|---|---|---|---|---|---|---|
| Point 1 | 4.48 | 0.00 | 0.74 | 31.08 | 0.00 | 63.70 |
| Ponto 2 | 3.00 | 0.00 | 0.89 | 27.15 | 0.00 | 68.95 |
DISCUSSION
In the present study, although bovine teeth have been used due to ethical issues limiting research with human teeth, the results obtained were not impaired. Similarities regarding morphological,[19] mechanical,[20] and biochemical parameters[21] allow comparison between human and bovine dentin.
SEM is a versatile observational method. It can be used for the analysis of the microstructure of solid material, such as dentin,[22,23] and visualization of the interface of several materials to dental tissues.[24] The images generated by SEM are easily interpreted, despite the complexity of the mechanisms to obtain them.[24] Several studies used SEM and EDX analysis to evaluate dentin ultrastructure after different protocols for surface treatment,[22,23] and consistent findings were demonstrated, proving the efficacy of these methods.
The ultrastructural analysis showed that both concentrations of HCl were able to demineralize dentin, as previously demonstrated by Perdigão et al.,[12] when 6 M HCl was used. The dentin demineralization promoted by 6 M HCl was milder and superficial compared to that of 12 M. The intertubular dentin was partially demineralized, and the peritubular dentin was exposed with cracks along its length. These cracks were probably caused by the dehydration process with increased concentrations of ethyl alcohol and by drying specimens at 37°C. In some regions, there was a slight enlargement of the dentinal tubule openings, and most of them were unobstructed. A possible explanation to that pattern may be the composition of structures. Most organic matter of the intertubular dentin is concentrated as collagen fibers.[3] In contrast, the peritubular dentin is hypermineralized, with hydroxyapatite crystals immersed in an organic matrix consisting of glycosaminoglycans, proteoglycans, and phosphorylated proteins.[3,7] Consequently, it is more resistant to acid action.
The EDX analysis confirmed as peritubular dentin the longitudinal tubular projections on the dentin surface, which were projected to the interior of the dentinal tubules in the transverse direction. It can be justified by the percentage in moles of magnesium atoms,[8] phosphorus, and calcium,[25] recognized as chemical elements of peritubular dentin [Figures 1k, 2i and Tables 1, 2].[6]
When specimens were observed transversely, some of them showed the presence of an obstructed peritubular dentin, possibly by the presence of smear layer in the tubule openings or as a consequence of some artifacts produced during dentin cutting.[10]
The demineralization pattern caused by 12 M HCl was more aggressive, and deeper decalcification occurred due to the molar concentration or molarity of the acids. Molarity is the most commonly used concentration measure to provide quantitative data of the amount of solute, in mol, dissolved in a certain amount of solvent.[26] It is widely used to provide evidence of the acidity or basicity of solutions since the pH is directly linked to the molar concentration of hydronium ions (H3O+) from the ionization of acids, and hydroxyl ions (OH−) arising from the dissociation of bases. In acid solutions, the higher its molar concentration, the more H3O+ ions exist in the solution; consequently, the acid is stronger, and the pH of the solution is lower.[27]
Due to the aggressiveness of the 12 M HCl solution, erosion areas and “detachment” of dentin layers were observed. Erosion may have been a consequence of using a double-sided diamond disc, which probably caused defects in dentin during cutting, promoting the accumulation of acid in some regions. On the other hand, the “detachment” of dentin layers can be explained by the process of dentin formation, which occurs by overlapping layers, and is characterized by the regular and rhythmic deposition of dentin by odontoblasts.[6]
Peritubular dentin was partially decalcified, and extensions were also observed on the dentin surface. There was a significant enlargement of the dentinal tubule openings, connecting them when they were close. Superficially, the intertubular dentin was demineralized, corroborating the findings of Marshal et al.[28]
Acid concentration and time of action on the dentin structure can significantly alter its histological characteristics,[3] thus influencing dental therapies. The enlargement of dentinal tubules with EDTA, for example, allows penetration of intracanal dressing during endodontic treatment, improving dentin disinfection.[2] It also allows endodontic cements penetration, improving its sealing ability, push-out bond strength to dentin, and marginal adaptation.[2] On the other hand, when overused, EDTA can weaken the root dentin, increasing the chances of root fracture.[2] The use of acids also has a positive effect on the bonding of restorative materials to dentin. The adhesion mechanism of adhesive systems is based on resin monomer infiltration into the demineralized dentin surface. After polymerization, this creates a mixed substrate composed of collagen fibers and a resin material, called hybrid layer.[29] For an adequate bonding, the smear layers need to be removed using acid etching to remove mineral content from superficial dentin zone, reducing the hydroxyapatite concentration and increasing the diameter of the dentinal tubules and dentin permeability.[30] However, excessive dentin decalcification can cause loss of important organic structures that may negatively influence such procedure.[1,31] Based on the arguments reported above, knowledge about the morphology of demineralized dentin is essential to identify the efficacy and duration of clinical procedures.
Although HCl has been used in the present study, in routine clinical conditions, the professional must be aware of the use of acids on dentin, such as the use of EDTA during root canal preparation or the dentin conditioning with phosphoric acid, before the restorative procedure. It is essential that manufacturers' recommendations with regard to the different products are observed and followed, as well as protocols proposed by international academies.
CONCLUSIONS
Based on the results, it was possible to conclude that dentin demineralization was dependent on acid molarity, which promoted peritubular dentin exposure. While HCl 6M promoted superficial demineralization, HCl 12M was more intense, demineralizing dentin at different depths and enlarging dentinal tubule openings. Intertubular and peritubular dentin presented similar chemical composition. The tubular structure observed in both groups is peritubular dentin. Lamina limitans was not identified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Central Electron Microscopy Laboratory of the Federal University of Santa Catarina for kindly provided schedules in the scanning electron microscope.
REFERENCES
- 1.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–31. [PubMed] [Google Scholar]
- 2.Teixeira CS, Felippe MC, Felippe WT. The effect of application time of EDTA and NaClO on intracanal smear layer removal: An SEM analysis. Int Endod J. 2005;38:285–90. doi: 10.1111/j.1365-2591.2005.00930.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix-limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8:2419–33. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takuma S, Eda S. Structure and development of peritubular matrix in dentin. J Dent Res. 1966;45:683–92. [Google Scholar]
- 5.Dai XF, Ten Cate AR, Limeback H. The extent and distribution of intratubular collagen fibrils in human dentine. Arch Oral Biol. 1991;36:775–8. doi: 10.1016/0003-9969(91)90045-v. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris ME, Muños AC. Histology and oral embryology. Ed Guanabara: Koogan; 2006. pp. 218–28. [Google Scholar]
- 7.Gotliv BA, Veis A. Peritubular dentin, a vertebrate apatite mineralized tissue without collagen: Role of a phospholipid–proteolipid complex. Calcif Tissue Int. 2007;81:191–205. doi: 10.1007/s00223-007-9053-x. [DOI] [PubMed] [Google Scholar]
- 8.Gotliv BA, Veis A. The composition of bovine peritubular dentin: Matching TOF-SIMS, scanning electron microscopy and biochemical component distributions. New light on peritubular dentin function. Cells Tissues Organs. 2009;189:12–9. doi: 10.1159/000151726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isokawa S, Toda Y, Kubota K. A scanning electron microscopic observation of etched human peritubular dentin. Arch Oral Biol. 1970;15:1303–6. doi: 10.1016/0003-9969(70)90018-x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas HF, Carella P. Correlation of scanning and transmission electron microscopy of human dentinal tubules. Arch Oral Biol. 1984;29:641–6. doi: 10.1016/0003-9969(84)90135-3. [DOI] [PubMed] [Google Scholar]
- 11.Dedavid BA, Gomes CI, Machado G. Scanning Electron Microscopy: Applications and Preparations of Samples: Polymeric, Metallic and Semiconductor Materials. Ed. Porto Alegre EDIPUCRS. 2007 [Google Scholar]
- 12.Perdigão J, van Meerbeek B, Lopes MM, Ambrose WW. The effect of a re-weting agent on dentin bonding. Dent Mater. 1999;15:282–95. doi: 10.1016/s0109-5641(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 13.Pashley DH. The effects of acid etching on the pulpodentin complex. Oper Dent. 1992;17:229–42. [PubMed] [Google Scholar]
- 14.Breschi L, Gobbi P, Mazzotti G, Falconi M, Ellis TH, Stangel I. High resolution SEM evaluation of dentin etched with maleic and citric acid. Dent Mater. 2002;18:26–35. doi: 10.1016/s0109-5641(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 15.Caiado AC, de Goes MF, de Souza-Filho FJ, Rueggeberg FA. The effect of acid etchant type and dentin location on tubular density and dimension. J Prosthet Dent. 2010;103:352–61. doi: 10.1016/S0022-3913(10)60076-5. [DOI] [PubMed] [Google Scholar]
- 16.Marshall GW, Jr, Balooch M, Tench RJ, Kinney JH, Marshall SJ. Atomic force microscopy of acid effects on dentin. Dent Mater. 1993;9:265–8. doi: 10.1016/0109-5641(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 17.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 18.Inokoshi S, Hosoda H, Harnirattisai C, Shimida Y, Tatsumi T. A study on the resin-impregnated layer of dentin. Part I: A comparative study on the decalcified and undecalcified sections and the application of argon ion bea, etching to disclose the resin-impregnated layer of dentin. J Conservat Dent. 1990;33:427–42. [Google Scholar]
- 19.Costa BM, Iwamoto AS, Puppin-Rontani RM, Pascon FM. Comparative analysis of root dentin morphology and structure of human versus bovine primary teeth. Microsc Microanal. 2015;21:689–94. doi: 10.1017/S1431927615000434. [DOI] [PubMed] [Google Scholar]
- 20.Wegehaupt F, Gries D, Wiegand A, Attin T. Is bovine dentine an appropriate substitute for human dentine in erosion/abrasion tests? J Oral Rehabil. 2008;35:390–4. doi: 10.1111/j.1365-2842.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- 21.Kato MT, Hannas AR, Leite AL, Bolanho A, Zarella BL, Santos J, et al. Activity of matrix metalloproteinases in bovine versus human dentine. Caries Res. 2011;45:429–34. doi: 10.1159/000330525. [DOI] [PubMed] [Google Scholar]
- 22.Wagner MH, da Rosa RA, de Figueiredo JA, Duarte MA, Pereira JR, Só MV. Final irrigation protocols may affect intrarradicular dentin ultrastructre. Oral Investig. 2017;21:2173–82. doi: 10.1007/s00784-016-2006-x. [DOI] [PubMed] [Google Scholar]
- 23.Lopes FC, Roperto R, Akkus A, Silva Souza YT, Sousa-Neto MD. Evaluation of chemical and morphological changes in radicular dentin after diferente final surfasse treatments. Microsc Res Technol. 2018;81:973–9. doi: 10.1002/jemt.23060. [DOI] [PubMed] [Google Scholar]
- 24.Tedesco M, Chain MC, Bortoluzzi EA, da Fonseca Roberti Garcia L, Alves AM, Teixeira CS. Comparison of two observational methods, scanning electron and confocal laser scanning microscopies, in the adhesive interface analysis of endodontic sealers to root dentine. Clin Oral Investig. 2018;22:2353–61. doi: 10.1007/s00784-018-2336-y. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. J Endod. 2009;35:731–6. doi: 10.1016/j.joen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Russel JB. General Chemistry. Ed. São Paulo: Pearson Education of Brazil, Makron Books; 1994. [Google Scholar]
- 27.Sardella A, Mateus E. Chemistry Course: General Chemistry. Ed. Ática, São Paulo/SP. 1995 [Google Scholar]
- 28.Marshall GW, Jr, Inai N, Wu-Magidi IC, Balooch M, Kinney JH, Tagami J, et al. Dentin demineralization: Effects of dentin depth, pH and different acids. Dent Mater. 1997;13:338–43. doi: 10.1016/s0109-5641(97)80104-2. [DOI] [PubMed] [Google Scholar]
- 29.Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991;3:133–8. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosales-Leal JI, Osorio R, Holgado-Terriza JA, Cabrerizo-Vílchez MA, Toledano M. Dentin wetting by four adhesive systems. Dent Mater. 2001;17:526–32. doi: 10.1016/s0109-5641(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. The effect of hybrid layer thickness on bond strenght: Demineralized dentin zone of the hybrid layer. Dent Mater. 2000;16:406–11. doi: 10.1016/s0109-5641(00)00035-x. [DOI] [PubMed] [Google Scholar]


