Abstract
Despite their impressive diversity and already broad therapeutic applications, cone snail venoms have received less attention as a natural source in the investigation of antimicrobial peptides than other venomous animals such as scorpions, spiders, or snakes. Cone snails are among the largest genera (Conus sp.) of marine invertebrates, with more than seven hundred species described to date. These predatory mollusks use their sophisticated venom apparatus to capture prey or defend themselves. In-depth studies of these venoms have unraveled many biologically active peptides with pharmacological properties of interest in the field of pain management, the treatment of epilepsy, neurodegenerative diseases, and cardiac ischemia. Considering sequencing efficiency and affordability, cone snail venom gland transcriptome analyses could allow the discovery of new, promising antimicrobial peptides. We first present here the need for novel compounds like antimicrobial peptides as a viable alternative to conventional antibiotics. Secondly, we review the current knowledge on cone snails as a source of antimicrobial peptides. Then, we present the current state of the art in analytical methods applied to crude or milked venom followed by how antibacterial activity assay can be implemented for fostering cone snail antimicrobial peptides studies. We also propose a new innovative profile Hidden Markov model-based approach to annotate full venom gland transcriptomes and speed up the discovery of potentially active peptides from cone snails.
Keywords: cone snails, venom, conotoxins, antimicrobial peptides, antibacterial activity, proteotranscriptomic approach
1. Introduction
In 1928, Alexander Fleming discovered the now widely known Penicillin from a Penicillium mold, later identified as Penicillium rubens [1]. Sixteen years later, Selman Waksman isolated Streptomycin from Streptomyces griseus [2], and together with Penicillin, both these discoveries undoubtedly revolutionized modern medicine. Indeed, such antibiotics were produced in large quantities during the Second World War to successfully treat infectious diseases [3]. Since then, antibiotics have become an integral part of the lives of millions of people as a primary medication for multiple microbial infections. Unfortunately, the misuse and overuse of antibiotics led to the emergence of antimicrobial resistance, which has become one of the major threats to global human health, as pointed out by the World Health Organization [4]. In 2016, 700,000 deaths were reported to be directly imputable to resistant infections [5]. In the same way, a study on global antimicrobial resistance has estimated that by 2050, over 10 million deaths each year and a cumulative USD 100 trillion of economic losses will be directly imputable to drug resistance [6].
At the beginning of the 21st century, the pharmaceutical industry appeared to reach a critical juncture caused by the low number of novel drugs entering the clinical phases [7]. Nevertheless, in the same couple of years, the advent of new sequencing technologies, higher computational capacities, and advances in biological research led the industry and researchers to turn more deeply towards natural extracts like animal venoms as a source of novel drug leads [8]. Back in the 17th century, Francesco Redi led some studies on toxins, especially from the “poisonous” snakes. Some years later, the discovery of snake venom glands set the stage for the birth of modern toxicology [9]. Since then, the iconic scorpions, spiders, and snakes, but also more recently lesser-known invertebrates like cone snails, have been investigated for their venom toxins.
Conus sp. is a major group of marine invertebrates, with about 761 described species according to the world register of marine species (WoRMS) [10]. The major component of the venom gland of cone snails is made of small peptides known as conotoxins, which often have neurotoxic activities. It has been conservatively estimated that more than 80,000 biologically active conotoxins are yet to be discovered [11]. The studies of conotoxins have already led to the discovery of the well-known drug ziconotide (ω-MVIIA conotoxin) from Conus magus used to treat chronic pain [12,13]. Each species of cone snail produces hundreds of conotoxins, which are the result of years of evolution and coevolution in the same environment as their prey and predators [14]. Venom compounds can be classified based on their molecular targets and mode of action, like peptides with antimicrobial activity. Antimicrobial peptides (AMPs) are biomolecules that act on bacteria, viruses, or fungi. They are usually short polypeptides with a net positive charge that comes from their rich composition in lysine, arginine, and histidine [15].
Compared to other animals like scorpions or spiders, the antimicrobial peptides from cone snails have received little attention [16,17,18]. For example, the investigation of scorpion venoms led to the discovery of Hadrurin, Scorpine, Vejovine, StCT2, Pandinin 1, and Pandinin 2, which are effective antimicrobial peptides [19,20,21,22,23]. Similar studies also found antimicrobial peptides in snakes, spiders, bees, ants, centipedes, lugworms, wasps, and cuttlefish [15,24,25,26,27,28,29,30,31,32].
To fill this gap in cone snail venom studies, we show here the need for novel compounds in the battle against antimicrobial resistance, update the knowledge on cone snails as a source of antimicrobial peptides and present the analytical methods to decipher the venom of cone snail for antimicrobial peptides.
2. Global Antimicrobial Resistance and the Need for Novel Compounds
The word “antibiotic” was used by Selman Waksman, in 1941, to describe any small molecule made by a microorganism that antagonizes the growth of other microorganisms [33]. An antibiotic interferes with bacteria growth via a specific mode of action, at concentrations that present minimal toxicity and are sufficiently potent to be effective against the infection. Despite a 36% increase in human use of antibiotics from 2000 to 2010, approximately 20% of deaths worldwide remain related to infectious diseases because of antibiotic resistance [34]. This development of resistance is a normal evolutionary process for microorganisms. Nevertheless, it is worsened by the selective pressure exerted by the widespread use of antibacterial drugs, rendering them largely ineffective on bacterial infections.
In response to exposure to antibiotics, susceptible bacteria are killed. However, there is a resistant portion that recolonizes the infection site. Bacteria use two major mechanisms to “resist” antibiotics, which are mutational adaptation and the acquisition of genetic material through horizontal gene transfer. The latter is one of the most important drivers of bacterial evolution. In general, mutations alter antibiotic action via the structural modification of the antibiotic target site, preventing the molecule from reaching its target by decreasing penetration or actively extruding the antimicrobial compound [35]. Resistance to antibiotics can usually be achieved through multiple biochemical pathways. For example, resistant bacteria can produce enzymes like carbapenemase or beta-lactamases, which can degrade or destroy antibiotic molecules. An analysis of 83 studies conducted in Africa showed a prevalence of carbapenemase-producing bacteria isolated in hospitals ranging from 3% to 67.7%, with a mortality rate of up to 67% [36]. Beta-lactamases-producing bacteria and quinolone-resistant bacteria have been detected in humans, but also animals, and the environment [37,38]. Up to 35% of isolates from animals and 93.2% from humans have shown resistance to quinolones [37].
The increase in drug-resistant pathogens is the consequence of various factors. It is the result of biochemical and genetic modifications, as pointed out above, but also the result of the over-reliance on antibiotics. For example, antibiotics are largely used as growth promoters in livestock farming [39]. Resistant bacteria are, then, transmitted to humans through direct contact with animals [40,41,42], exposure to animal manure, and by the consumption of undercooked meat [43]. High rates of prescriptions of antibiotics are another critical factor in the evolution of resistance. In some countries, antibiotics are available without prescriptions. When they are prescribed, most prescriptions are unsuitable. Broad-spectrum antibiotics are favored to treat infections caused by several species of bacteria or those for which diagnosis is time-consuming. Additionally, some physicians are inclined to prescribe multiple antibiotics for the same condition [44]. Patients’ failure to comply with dosages is also a major contributor to the development of resistance. Indeed, patients miss doses, either by mistake or deliberately. Finally, exposure of surviving microorganisms to subtherapeutic concentrations of the drug also increases the chances of developing resistance [45]. Due to increasing resistance to antibiotics, a report has predicted that by 2050 10 million deaths will occur annually due to resistant pathogens. Without control action, by 2050 the cost of antimicrobial resistance will be approximately USD 100 trillion, with a 3.5% decline in global gross domestic product [6]. As the world is heading towards a postantibiotic era, there is an urgent need for novel compounds that are able to meet current needs, such as antimicrobial peptides.
Antimicrobial peptides (AMPs) are active molecules produced by a broad range of organisms ranging from microbes to mammals to ensure their self-defense against microbes [46]. AMPs are multifaceted molecules that kill microbes via a pleiotropic mechanisms of action, such as the destruction of the barrier function of the cellular membranes and the inhibition of macromolecule synthesis making them efficient even against multiresistant bacteria [47,48]. The efficiency of AMPs is due to rapid killing kinetics, reduced toxicity, and reduced microbial resistance [49] as demonstrated by many studies [50,51,52]. AMPs are therefore a promising alternative to conventional antibiotics.
3. Cone snails as a Source of Novel Antimicrobial Peptides
3.1. Cone Snails Diversity
The Conidae family is composed of 138 genera from which Conus sp. is the type taxon. Cone snails contain around 761 recognized species [53,54] from which approximately 118 have been studied to date (http://conoserver.org/?page=stats&tab=organisms (accessed on 28 January 2021)). However, it should be noted that although this is a rapidly evolving field, especially with the advent of transcriptomics, not all sequences deposited in Conoserver correspond to proteomic-verified conopeptides. The shell patterns of cone snails (Figure 1), clearly recognizable, have made them precious collection items through centuries [55].
Figure 1.
Shells of representative species of Conus sp., including fish hunters (C. magus and C. striatus), mollusk hunters (C. marmoreus and C. gloriamaris), and a worm hunter (C. ebraeus).
Cone snails inhabit tropical and subtropical coastal zones and oceans up to 1000 m of depth but are mostly found in coral reefs hiding in the sand, under the coral shelf, or in shallow waters [56]. The greatest diversity of cone snail species is found in the Indo-Pacific ocean, but they become rarer beyond the 40° N or S parallel [57,58]. Cone snails are active at night as they leave their hiding place to go hunting for prey. They are highly specialized predators and can be classified based on their feeding habits: worm hunters (vermivorous), mollusk hunters (molluscivorous), fish hunters (piscivorous), and generalist feeders. They first detect prey using chemosensors, then crawl softly towards the prey, and generally extend their proboscis and fire their venom-loaded harpoon upon contact. Venom injection into a prey animal induces rapid immobilization, which, in the case of fish hunters, can take less than a few hundred milliseconds [59].
3.2. Cone Snail’s Venom Composition and Conotoxins Classification
Conotoxins are the disulfide-rich peptides found in the venom of cone snails responsible for their toxicity [17,60]. They are produced in the venom gland as precursors, which generally contain a signal, a pro, and a mature sequence. The cone snail’s venom is a remarkably complex mixture of peptides that has drawn the attention of biomedical researchers for their high unprecedented potency and selectivity for their target. Conotoxins are classified following three different criteria: the precursor’s endoplasmic reticulum signal sequence, the cysteine pattern of the mature peptide region, and the pharmacological targets. Based on their signal peptide, conotoxins are further divided into 28 gene superfamilies: A, B1, B2, B3, C, D, E, F, G, I1, I2, I3, J, K, L, M, N, O1, O2, O3, P, Q, R, S, T, V, Y. The most expressed peptides from cone snail venom glands studied to date are from A, M, and O superfamilies [61].
Conotoxins can also be divided according to the cysteine pattern or the pharmacological target [62]. Conotoxins have been classified into cysteine frameworks (pattern of cysteine residues) following the arrangement of cysteine along the mature sequence or the disulfide connectivities. The updated cysteine arrangement and disulfide connectivities definition of known conotoxins is presented in Table 1.
Table 1.
Definition of conotoxins cysteine frameworks along with cysteine spacing and disulfide connectivity. Adapted from Kaas et al. [62].
| Name. | Number of Cysteines | Cysteine Pattern | Disulfide Connectivity |
|---|---|---|---|
| I | 4 | CC-C-C | I–III, II–IV |
| II | 6 | CCC-C-C-C | |
| III | 6 | CC-C-C-CC | (I–IV, II–V, III–VI), (I–VI, II–IV, III–V), (I–V, II–IV, III–VI) |
| IV | 6 | CC-C-C-C-C | I–V, II–III, IV–VI |
| V | 4 | CC-CC | I–III, II–IV |
| VI/VII | 6 | C-C-CC-C-C | I–IV, II–V, III–VI |
| VIII | 10 | C-C-C-C-C-C-C-C-C-C | |
| IX | 6 | C-C-C-C-C-C | I–IV, II–V, III–VI |
| X | 4 | CC-C-C | I–IV, II–III |
| XI | 8 | C-C-CC-CC-C-C | I–IV, II–VI, III–VII, V–VIII |
| XII | 8 | C-C-C-C-CC-C-C | |
| XIII | 8 | C-C-C-CC-C-C-C | |
| XIV | 4 | C-C-C-C | I–III, II–IV |
| XV | 8 | C-C-CC-C-C-C-C | |
| XVI | 4 | C-C-CC | |
| XVII | 8 | C-C-CC-C-CC-C | |
| XVIII | 6 | C-C-CC-CC | |
| XIX | 10 | C-C-C-CCC-C-C-C-C | |
| XX | 10 | C-CC-C-CC-C-C-C-C | |
| XXI | 10 | CC-C-C-C-CC-C-C-C | |
| XXII | 8 | C-C-C-C-C-C-C-C | |
| XXIII | 6 | C-C-C-CC-C | |
| XXIV | 4 | C-CC-C | |
| XXV | 6 | C-C-C-C-CC | |
| XXVI | 8 | C-C-C-C-CC-CC | |
| XXVII | 8 | C-C-C-CCC-C-C | |
| XXVIII | 10 | C-C-C-CC-C-C-C-C-C | |
| XXIX | 8 | CCC-C-CC-C-C | |
| XXX | 10 | C-C-CCC-C-C-C-CC | |
| XXXII | 6 | C-CC-C-C-C | |
| XXXIII | 12 | C-C-C-C-C-C-C-C-C-C-C-C |
Conotoxins exhibit a large range of pharmacological targets. Considering the receptor specificity, we can classify conotoxins into 12 families: α (alpha), γ (gamma), δ (delta), ε (epsilon), ι (iota), κ (kappa), µ (mu), ρ (rho), ς (sigma), τ (tau), χ (chi) and ω (omega) (Table 2). A more detailed description of pharmacological families is provided by Kaas et al. [62].
Table 2.
Pharmacological families of conotoxins. The UniProt version 2021_1 (https://uniprot.org) accession number of the representative conotoxins is indicated in the parenthesis. Adapted from Kaas et al. [62].
| Pharmacological Family | Definition | Conotoxin Representative |
|---|---|---|
| α (alpha) | Nicotinic acetylcholine receptors | Alpha-conotoxin GIA (P01519) |
| γ (gamma) | Neuronal pacemaker cation currents (inward cation current) | Gamma-conotoxin PnVIIA (P56711) |
| δ (delta) | Voltage-gated Na channels (agonist, delay inactivation) | Delta-conotoxin TxVIA (Q9U655) |
| ε (epsilon) | Presynaptic Ca channels or G protein-coupled presynaptic receptors | Epsilon-conotoxin TxVA (P81755) |
| ι (iota) | Voltage-gated Na channels (agonist, no delayed inactivation) | Iota-conotoxin RXIA (Q7Z094) |
| κ (kappa) | Voltage-gated K channels (blocker) | Kappa-conotoxin PVIIA (P56633) |
| µ (mu) | Voltage-gated Na channels (antagonist, blocker) | Mu-conotoxin GIIIA (P01523) |
| ρ (rho) | Alpha1-adrenoceptors (GPCR) | Rho-conotoxin TIA (P58811) |
| ς (sigma) | Serotonin-gated ion channels 5-HT3 | Sigma-conotoxin GVIIIA (P58924) |
| τ (tau) | Somatostatin receptor | Tau-conotoxin CnVA (P0DJL6) |
| χ (chi) | Neuronal noradrenaline transporter | Chi-conotoxin MrIA (P58808) |
| ω (omega) | Voltage-gated Ca channels (blocker) | Omega-conotoxin GVIA (P01522) |
3.3. Antimicrobial Activity of Conidae’s Conopeptides
Although conotoxins have a wide array of unique structures, the most common use of conotoxins is focused on pain management [63]. However, some conotoxins have proved to be highly effective against pathogens with little resistance due to their membrane-disruptive mechanisms [64]. The antimicrobial effects of conotoxins are seldomly documented and remain underexplored. An example of the in vitro antiparasitic activity of conotoxins was demonstrated on the tachyzoite form of Toxoplasma gondii [18]. The tachyzoite is the mobile, invasive, and intracellular obligate form of T. gondii. The synthetic conotoxin s-cal14.1a derived from Californiconus californicus, at micromolar concentration, lowers down to half the viability of extracellular tachyzoites and inhibits host cell invasion by 61%. In toxoplasma infections, no drugs have shown effectiveness when tachyzoites were localized in cytoplasmic parasitophorous vacuoles. However, s-cal14.1a inhibits not only the establishment of the infection but also the intracellular proliferation of T. gondii by 50%. This conotoxin potentially disrupts the replication machinery of the parasite by passing the membrane of the host cell, parasitophorous vacuoles, and parasite membranes without the host cell being affected.
Other studies have shown mitigated antibacterial effects of conotoxins. Conotoxin O1_cal29b, isolated from C. californicus, inhibited in vitro the growth of the multidrug-resistant Mycobacterium tuberculosis. O1_cal29b was effective at low concentration. The inhibition occurred at a minimal inhibitory concentration (MIC) of 0.22–3.52 µM [61]. In parallel, peptide Lo6/7a, a 24-residue conotoxin isolated from the venom of Conasprella longurionis, exhibited low and extremely specific activity against Bacillus megaterium at an exceedingly high concentration (1 mM) [65]. Additionally, the conolysin-Mt, a disulfide-poor conopeptide from Conus mustelinus, showed a low antimicrobial activity with a MIC greater than 50 µM against two Escherichia coli strains. Its MIC for the Gram-positive Staphylococcus aureus is in a range of 25–50 µM [66].
Interestingly, a study has shown that macrocyclization can convert a conotoxin into an effective antimicrobial peptide [67]. MVIIA is a linear cystine-knot peptide with multiple basic amino acids at both termini, but up to 500 µM, it was inactive against E. coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Meanwhile, it exhibited moderate antifungal activity against Candida kefyr and Candida tropicalis with MICs of 28.8 µM and 39.8 µM, respectively. Likewise, the linear analog MVIIA-GS, the MVIIA conotoxin with a Gly Ser linker, gave similar results, indicating that the linker did not affect the antimicrobial activity. The authors then considered ligating the two ends of MVIIA using a linker peptide. This ligation allowed the formation of epitopes that gain membranolytic activity on microbes. Indeed, all ten cyclic conotoxins derived from MVIIA were active against the selected three bacterial strains with MICs ranging from 3.3 to 90.2 µM. Most of these analogs exhibited improved antifungal activity with MICs, from up to 18.2 µM against C. kefyr and up to 11.4 µM against C. tropicalis. Additionally, the conversion of the disulfide bonds to aminobutyric acids improved the antimicrobial activity of the cyclic analogs. These results demonstrated that the end-to-end cyclization of a linear peptide improves its biological activity and confers antimicrobial properties that were not found in the linear form. The mimetics of conopeptides may serve as more promising candidates for the further development of therapeutically useful agents for the treatment of infections.
Although Conolysin-Mt and conotoxin MVIIA target the lipid bilayer of bacteria, in addition to the lipid bilayer, transpeptidase enzymes were explored as novel targets for antimicrobial conopeptides. For example, sortases that anchor surface proteins were investigated as attractive targets due to their prevalence in the cell wall of Gram-positive bacteria [16]. Their inhibition compromises the pathogenesis and the virulence of the bacteria. Based on structural studies, M2-conotoxin and contryphan-R were found to mediate the inhibition of SrtA and SrtB by obstructing the assembly of iron acquisition, immune evasion, complement pathway inhibition, clumps, biofilm formation, and host matrix attachment proteins within the cell wall of S. aureus. Therefore, more studies are required to validate the efficacy of M2-conotoxin and contryphan-R in bacterial cultures.
4. Bioinformatics-Aided Proteotranscriptomics
4.1. Cone Snail Venom Extraction
Cone snail venom can be obtained either by the dissection of the venom duct or by milking. Venom gland tissue extraction implies the availability of tens to hundreds of specimens to collect enough material for the discovery process. Besides the ethical concerns, this method has many disadvantages (cellular debris, unmatured and degraded products in the reconstituted venom) compared to venom “milking” [56]. Although dissection and tissue extraction has proven to be successful in many cases [68], venom milking should be considered whenever possible, as it provides a soluble fraction that contains fully mature conotoxins intended for a particular ecological role, as demonstrated by many studies [69,70,71,72,73,74,75,76]. Nevertheless, if possible, a combination of both strategies (milking and dissection) should be considered as all Conidae venoms tested to date for AMPs have not been milked but rather extracted. Indeed, it was found that cone snails can deploy different combinations of conotoxins depending on the stimulus (predatory vs. defense) [70].
To collect the predation-evoked venom, the procedure usually begins with a lure, which is a live prey according to the cone snail’s feeding habit [77]. The cone extends its proboscis toward the lure that is placed in front of a microtube. The venom is injected into the tube through a fine piece of prey tegument (fish fin for instance). Therefore, milking provides a more soluble venom, free of cellular debris and degraded products, that is ideally suited for biological and proteomic assays [56,78].
4.2. Next-Generation Transcriptomics Sequencing and Bioinformatics
Before the advent of next-generation sequencing, biologically active peptides from cone snails were discovered using “bioactivity-guided fractionation”. This method was limited because it required large amounts of crude venom and turned out to be time-consuming [79]. Next-generation sequencing revolutionized the field as the venom duct transcriptome can be completely sequenced and the conotoxin sequences recovered in one single experiment. From the next-generation sequencing data, bioinformatics tools are required to correctly identify conotoxin precursors from the raw reads and/or assembled contigs (Figure 2).
Figure 2.
Transcriptomic bioinformatic pipeline for putative conopeptide prediction. Both assembly free (1) and assembly (2) methods are possible. Sequencing: Blue, Preprocessing: Pale brown, Sequence annotation: Pale green, Result: Pink.
The raw reads obtained from Illumina or 454 sequencings are controlled for quality using FastQC v.0.11.9 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 24 March 2021)). FastQC helps researchers to be aware of some issues that can arise during sequencing by providing statistics on reads like k-mer content, GC content, or graph of quality per base. The validated reads are then trimmed for barcodes and primers using Trimmomatic version 0.39 [80] or Cutadapt version 3.3 [81]. Short Illumina reads obtained after RNA sequencing rarely contain the full-length conotoxin precursor, although some active peptides from cone snails can be as short as 10 amino acids [82]. Therefore, they need to be assembled in longer contigs. De novo assembly can be performed using software like Trinity version 2.12 [83], Velvet version 1.2.10 [84], and ABySS v.2.3.0 [85] to obtain the desired contigs. Another emerging method is the use of multiassembling tools like the Oyster river protocol [86] for a better recovery of contigs, as one method alone is not always able to properly identify all desired contigs.
The contigs are then translated in silico into amino acids. The tools used at this step should be well tested [87]. At this point, the traditional method consists of the homology search using BLAST [88] against a specialized database of conotoxins like ConoServer [89], followed by a second homology search against a larger database like the NCBI nonredundant (https://www.ncbi.nlm.nih.gov/refseq/about/nonredundantproteins accessed on 28 January 2021) [90] or the Uniprot/SwissProt [91] databases for validation of conopeptides and housekeeping genes identification.
4.3. Venom Gland Transcriptome Annotation Based on Profile Hidden Markov Models
A second way to elucidate transcriptome composition is by using ConoDictor version 2 [92] (new version in preparation), or ConoSorter [93] to predict putative conotoxins using methods such as hidden Markov models (HMMs). Using specialized predictive tools is helpful because they are exclusively designed to detect putative conopeptides from RNA-seq data and are way speedier compared to the traditional BLAST. Functional and structural annotations like superfamily belonging, signal peptide presence, or cysteine framework can then be predicted using signalP version 5 [94] or previously mentioned tools.
Nevertheless, currently, only a few HMMs are available on PFAM [95] to annotate conotoxins (Table 3).
Table 3.
Current conotoxins HMMs profiles available in PFAM.
| Accession | ID | Description |
|---|---|---|
| PF16981 | Chi-conotoxin | chi-Conotoxin or t superfamily |
| PF02950 | Conotoxin | Conotoxin |
| PF17557 | Conotoxin_I2 | I2-superfamily conotoxins |
| PF05374 | Mu-conotoxin | Mu-Conotoxin |
| PF07473 | Toxin_11 | Spasmodic peptide gm9a; conotoxin from Conus species |
| PF07829 | Toxin_14 | Alpha-A conotoxin PIVA-like protein |
| PF08087 | Toxin_18 | Conotoxin O-superfamily |
| PF08088 | Toxin_19 | Conotoxin I-superfamily |
| PF08094 | Toxin_24 | Conotoxin TVIIA/GS family |
| PF08097 | Toxin_26 | Conotoxin T-superfamily |
| PF07365 | Toxin_8 | Alpha conotoxin precursor |
To accelerate the annotation of cone snail transcriptome, we propose here an up-to-date exhaustive list of profile HMMs (pHMMs) that allow the annotation and classification of the major conotoxins superfamilies (available on our GitHub https://github.com/koualab/cono-amp-review/blob/main/conohmm.txt (accessed on 18 March 2021)). A sequence logo is associated with each of the newly built pHMM (Table 4).
Table 4.
Updated profile HMM (pHMM) of major conotoxins superfamily sequence signal and their associated sequence logo [96].
| Conotoxin Superfamily | pHMM | Cysteine Framework | Sequence Logo |
|---|---|---|---|
| A | CN_A | I, II, IV, VI/VII, XIV, XXII |

|
| B | CN_B | (conantokins, disulfide-poor) |

|
| D | CN_D | XXVIII, IV, XIV, XV, XX, XXIV |

|
| H | CN_H | VI/VII |

|
| I1 | CN_I1 | VI/VII, XI, XXII |
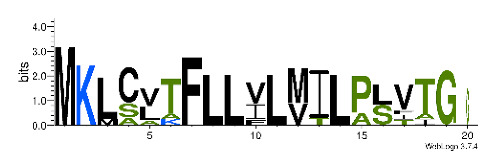
|
| I2 | CN_I2 | VI/VII, XI, XII, XIII, XIV |

|
| I3 | CN_I3 | VI/VII, XI |

|
| J | CN_J | XIV |

|
| K | CN_K | XXIII |

|
| L | CN_L | XIV, XXIV |

|
| M | CN_M | XXXII, I, II, III, IV, VI/VII, IX, XIV, XVI |

|
| N | CN_N | XV |

|
| O1 | CN_O1 | XXIX, I, VI/VII, IX, XII, XIV, XVI |

|
| O2 | CN_O2 | VI/VII, XIV, XV, XVI |
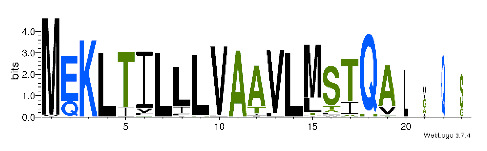
|
| O3 | CN_O3 | VI/VII, XVI |

|
| P | CN_P | IX, XIV |

|
| R | CN_R | XIV |

|
| S | CN_S | XXXIII, VIII |

|
| T | CN_T | I, V, X, XVI |
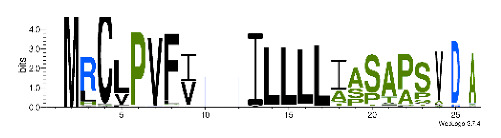
|
4.4. Venom Proteomics
The complex cone snail venom mixture made of polypeptides, peptides, inorganic salt, and amines accounts for most of the molecular and functional diversity as well as the observed behavioral and biochemical modulations [70,76,97]. For the high propensity of post-translational modifications (PTMs) alone, the conopeptides cannot be simply deciphered from transcriptomic data without proteomic evidence. The introduction in the field of mass spectrometry-based techniques has drastically changed the landscape of conotoxin studies [98]. This technique allowed us not only to correctly estimate the number of conopeptides expressed by a cone snail but also improved the resolution of the identified peptides. For instance, LC-MS of milked (injected) venom can help to reveal dramatic intraspecific variation like in some recent studies [97,99,100]. Furthermore, using deep venomics, Dutertre et al., were able to increase the estimation of the number of peptides expressed by Conus marmoreus (around 8000 peptides) originated from only 105 conotoxins peptide precursors [69].
4.5. Proteotranscriptomics
Proteotranscriptomic studies combine the best of both worlds: de novo deduced sequences of the digested peptides are directly mapped to the RNAseq generated peptide database using dedicated tools. The data obtained through bottom-up proteomics are generally compared to a sequence database using tools like ConoMass [89], MASCOT (Matrix Science, Boston, MA, USA; www.matrixscience.com version 2.5 ProteinPilot (SCIEX, Framingham, MA, USA), v.4.4 or PEAKS studio (Bioinformatics Solutions, Waterloo, ON, Canada) version 8.5. The top-down bioinformatic analyses are more difficult due to the nature of the obtained data. Such data often leads to false-positive identification due to the newness of the sequence variants and PTMs that they represent. To overcome this issue, new and less stringent algorithms have been developed, such as Byonic [101] and ProSight version 2 [102]. Both can be used to identify sequences and PTMs from top-down proteomic data.
4.6. In Silico AMPs Structure Determination
Structure determination of AMPs is usually conducted by NMR. However, the potentially large number of sequences identified by transcriptomics, bioinformatics, and proteomics prevent the generalized use of NMR, as it will be too expensive and time-consuming. A better approach involves the in silico determination of the predicted AMPs. With the development of new technologies and algorithms in biophysics, in silico prediction of the structure is becoming more and more precise. Rosetta has proven to be successful in predicting the three-dimensional structure of proteins ab initio from their amino acid sequence. By incorporating new energy functions, Rosetta has been able to predict a completely new protein fold [72,73]. Following a similar strategy, QUARK [103] has shown the highest scores in CASP9 (http://predictioncenter.org/casp9/CD/data/html/groups.server.fm.html (accessed on 28 January 2021)) and CASP10 (http://predictioncenter.org/casp10/groups_analysis.cgi?type=server&tbm=on&tbm_hard=on&tbmfm=on&fm=on&submit=Filter (accessed on 28 January 2021)) challenges. Additionally, the 14th CASP experiment has crowned the i-TASSER web portal (https://zhanglab.ccmb.med.umich.edu/I-TASSER (accessed on 28 January 2021)) as the first protein structure prediction. AlphaFold in CASP13 has shown the greatest advance in the resolution of the protein folding challenges using machine learning methods and, once available, could be used for AMPs structure prediction [104,105].
5. Antibacterial Activity Assays
Microbiological bioassays involve the detection and characterization of the antibacterial activity of newly discovered AMPs. Usually, microdilution [66], radial diffusion assay [67], and spot diffusion [65] were used to determine the minimal inhibitory concentration of the tested conopeptide. Quantitative susceptibility testing is performed by making 2-fold dilutions of the tested AMP in a liquid culture medium inoculating it with a standard number of microorganisms (10.5 to 10.6 colony-forming unit (cfu)/mL) and incubating it at 35–37 °C for 18–24 h. Mueller–Hinton Broth is the recommended medium for the susceptibility testing of commonly isolated, rapidly growing aerobic or aeroanaerobic organisms because it supports the satisfactory growth of most pathogens [106]. However, LB medium and Tryptic Soy Broth can also be used [66,67]. The amount of AMP that inhibits the visible growth of the microorganism is called the minimal inhibitory concentration [107]. Beyond MIC, minimal bactericidal concentration (MBC) can be determined to characterize the type of activity performed by the conopeptide. Subcultures of the samples obtained from the clear tubes or wells are plated on a solid medium and reincubated for an additional 18–24 h. The MBC represents the lowest concentration that either revealed no visible bacterial growth after subculturing or resulted in a 3-log10 reduction in colony-forming units (cfu) per mL on subculture. Briefly, a 3-log10 (or 99.9%) reduction in viable bacterial count in an 18–24 h period is the accepted definition of bactericidal activity [108]. The bacteriostatic activity has been defined as a ratio of MBC to MIC > 4 [81].
In case of low activity, different peptide mixtures can be tested in combination to increase the bactericidal activity and the synergistic antimicrobial effect evaluated. The fractional inhibitory concentration (FIC) index is defined as the inhibitory concentration of the antimicrobial combination divided by that of the single antimicrobial component [109]. The following equation (Equation (1)) represents the method for determining the FIC index:
| (1) |
The FIC indices are interpreted as follows: ≤0.5 = synergism; 0.5–1 = additivity; 1–4 = indifference; and >4 = antagonism.
Lastly, to distinguish if the antibacterial activity is concentration and/or time-dependent, time-kill curves can be performed. Viable colony counts are determined at different time points up to 24 h. The cfu of the organisms is to be determined and a graph of the log cfu/mL is plotted against time. This kinetics can also be related to the stages of the growth of the bacteria (lag, exponential, stationary phase). Concentration-dependent bactericidal activity occurs when the rate of killed-bacteria increases with progressively higher AMP concentrations corresponding to multiples of the MIC (i.e., 1×, 5×, 10×). Time-dependent bactericidal activity occurs when bacterial killing does not change with increasing AMPs concentrations to more than the MIC.
6. Conclusions
Cone snail venom has already been the source of approved toxin-based drugs, and these mixtures are also potential reservoirs for antimicrobial peptides. We first reported the standard methods in venom drug-based research for transcriptome sequencing and proteomics. We further showed a new strategy based on a dedicated predictive approach focused on up-to-date profile Hidden Markov models that should then make it easy to quickly identify potentially interesting putative peptides from cone snail venom gland transcriptomes. We also pointed out the proteomics and proteotranscriptomics methods to confirm conotoxin transcriptome-based prediction. Furthermore, a point on the in silico prediction of conotoxins’ three-dimensional structure has been made. We conclude our review by mentioning the antibacterial activity assay methods for the proper assay of conotoxins AMPs. We hope that this review will help researchers and industry to lead projects on cone snail antimicrobial peptides to tackle the increasing global antimicrobial resistance.
Author Contributions
Conceptualization, A.E., D.K. and A.A.; writing—original draft preparation, A.E., A.A.; writing—review and editing, D.K., S.K.-N., S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaynes R. The Discovery of Penicillin—New Insights After More than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017;23:849–853. doi: 10.3201/eid2305.161556. [DOI] [Google Scholar]
- 2.Woodruff H.B., Selman A. Waksman, Winner of the 1952 Nobel Prize for Physiology or Medicine. Appl. Environ. Microbiol. 2014;80:2–8. doi: 10.1128/AEM.01143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penicillin S. CDC Control of Infectious Diseases, 1900–1999. JAMA. 1999;282:1029. doi: 10.1001/jama.282.11.1029. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Gallo R.L. Antimicrobial Peptides. Curr. Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 5.O’Neil J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations 2016. Government of the United Kingdom; London, UK: 2016. [Google Scholar]
- 6.Humphreys G., Fleck F. United Nations Meeting on Antimicrobial Resistance. Bull. World Health Organ. 2016;94:638–639. doi: 10.2471/BLT.16.020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prashanth J.R., Brust A., Jin A.-H., Alewood P.F., Dutertre S., Lewis R.J. Cone Snail Venomics: From Novel Biology to Novel Therapeutics. Future Med. Chem. 2014;6:1659–1675. doi: 10.4155/fmc.14.99. [DOI] [PubMed] [Google Scholar]
- 8.Bordon K.D.C.F., Cologna C.T., Fornari-Baldo E.C., Pinheiro-Júnior E.L., Cerni F.A., Amorim F.G., Anjolette F.A.P., Cordeiro F.A., Wiezel G.A., Cardoso I.A., et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utkin Y.N. Animal Venom Studies: Current Benefits and Future Developments. World J. Biol. Chem. 2015;6:28–33. doi: 10.4331/wjbc.v6.i2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worms E.B. World Register of Marine Species. [(accessed on 28 January 2021)]; Available online: http://www.marinespecies.org7813.
- 11.Gao B., Peng C., Yang J., Yi Y., Zhang J., Shi Q. Cone Snails: A Big Store of Conotoxins for Novel Drug Discovery. Toxins. 2017;9:397. doi: 10.3390/toxins9120397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisapoor S.S., Jamili S., Shahbazzadeh D., Mostafavi P.G., Bagheri K.P. A New, High Yield, Rapid, and Cost-Effective Protocol to Deprotection of Cysteine-Rich Conopeptide, Omega-Conotoxin MVIIA. Chem. Biol. Drug Des. 2016;87:687–693. doi: 10.1111/cbdd.12702. [DOI] [PubMed] [Google Scholar]
- 13.Rigo F.K., Dalmolin G.D., Trevisan G., Tonello R., Silva M.A., Rossato M.F., Klafke J.Z., Cordeiro M.D.N., Junior C.J.C., Montijo D., et al. Effect of ω-Conotoxin MVIIA and Phα1β on Paclitaxel-Induced Acute and Chronic Pain. Pharmacol. Biochem. Behav. 2013;114:16–22. doi: 10.1016/j.pbb.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Herzig V., Cristofori-Armstrong B., Israel M.R., Nixon S.A., Vetter I., King G.F. Animal Toxins—Nature’s Evolutionary-Refined Toolkit for Basic Research and Drug Discovery. Biochem. Pharmacol. 2020;181:114096. doi: 10.1016/j.bcp.2020.114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fratini F., Cilia G., Turchi B., Felicioli A. Insects, Arachnids and Centipedes Venom: A Powerful Weapon against Bacteria. A Literature Review. Toxicon. 2017;130:91–103. doi: 10.1016/j.toxicon.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Younis S., Taj S., Rashid S. Structural Studies of Staphylococcus Aureus Sortase Inhibiton via Conus Venom Peptides. Arch. Biochem. Biophys. 2019;671:87–102. doi: 10.1016/j.abb.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Bernáldez-Sarabia J., Figueroa-Montiel A., Dueñas S., Cervantes-Luévano K., Beltrán J., Ortiz E., Jiménez S., Possani L., Paniagua-Solís J., Gonzalez-Canudas J., et al. The Diversified O-Superfamily in Californiconus Californicus Presents a Conotoxin with Antimycobacterial Activity. Toxins. 2019;11:128. doi: 10.3390/toxins11020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de León-Nava M.A., Romero-Núñez E., Luna-Nophal A., Bernáldez-Sarabia J., Sánchez-Campos L.N., Licea-Navarro A.F., Morales-Montor J., Muñiz-Hernández S. In Vitro Effect of the Synthetic Cal14.1a Conotoxin, Derived from Conus Californicus, on the Human Parasite Toxoplasma Gondii. Mar. Drugs. 2016;14:66. doi: 10.3390/md14040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed U., Malik Mujaddad-ur-Rehman N.K., Fawad S.A., Fatima A. Antibacterial Activity of the Venom of Heterometrus Xanthopus. Indian J. Pharmacol. 2012;44:509. doi: 10.4103/0253-7613.99332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao L., Li Z., Zhang R., Wu Y., Li W., Cao Z. StCT2, a New Antibacterial Peptide Characterized from the Venom of the Scorpion Scorpiops Tibetanus. Peptides. 2012;36:213–220. doi: 10.1016/j.peptides.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Conde R., Zamudio F.Z., Rodríguez M.H., Possani L.D. Scorpine, an Anti-Malaria and Anti-Bacterial Agent Purified from Scorpion Venom. FEBS Lett. 2000;471:165–168. doi: 10.1016/S0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- 22.Corzo G., Escoubas P., Villegas E., Barnham K.J., He W., Norton R.S., Nakajima T. Characterization of Unique Amphipathic Antimicrobial Peptides from Venom of the Scorpion Pandinus Imperator. Biochem. J. 2001;359:35–45. doi: 10.1042/bj3590035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Aponte C.A., Silva-Sanchez J., Quintero-Hernández V., Rodríguez-Romero A., Balderas C., Possani L.D., Gurrola G.B. Vejovine, a New Antibiotic from the Scorpion Venom of Vaejovis Mexicanus. Toxicon. 2011;57:84–92. doi: 10.1016/j.toxicon.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Benoist L., Houyvet B., Henry J., Corre E., Zanuttini B., Zatylny-Gaudin C. In-Depth In Silico Search for Cuttlefish (Sepia Officinalis) Antimicrobial Peptides Following Bacterial Challenge of Haemocytes. Mar. Drugs. 2020;18:439. doi: 10.3390/md18090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canelli A.P., Dos Santos Rodrigues T.F., de Goes V.F.F., Caetano G.F., Mazzi M.V. Evaluation of the Effectiveness of Crotoxin as an Antiseptic against Candida spp. Biofilms. Toxins. 2020;12:532. doi: 10.3390/toxins12090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leandro L.F., Mendes C.A., Casemiro L.A., Vinholis A.H., Cunha W.R., de Almeida R., Martins C.H. Antimicrobial Activity of Apitoxin, Melittin and Phospholipase A2 of Honey Bee (Apis Mellifera) Venom against Oral Pathogens. An. Acad. Bras. Ciênc. 2015;87:147–155. doi: 10.1590/0001-3765201520130511. [DOI] [PubMed] [Google Scholar]
- 27.Melo-Braga M.N., Almeida F.D.M., Dos Santos D.M., de Avelar Júnior J.T., Dos Reis P.V.M., de Lima M.E. Antimicrobial Peptides from Lycosidae (Sundevall, 1833) Spiders. Curr. Protein Pept. Sci. 2020;21:527–541. doi: 10.2174/1389203721666200116091911. [DOI] [PubMed] [Google Scholar]
- 28.Nie Y., Zeng X.-C., Yang Y., Luo F., Luo X., Wu S., Zhang L., Zhou J. A Novel Class of Antimicrobial Peptides from the Scorpion Heterometrus Spinifer. Peptides. 2012;38:389–394. doi: 10.1016/j.peptides.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Primon-Barros M., Macedo A.J. Animal Venom Peptides: Potential for New Antimicrobial Agents. Curr. Top. Med. Chem. 2017;17:1119–1156. doi: 10.2174/1568026616666160930151242. [DOI] [PubMed] [Google Scholar]
- 30.Santos D.M., Verly R.M., Piló-Veloso D., de Maria M., de Carvalho M.A.R., Cisalpino P.S., Soares B.M., Diniz C.G., Farias L.M., Moreira D.F.F., et al. LyeTx I, a Potent Antimicrobial Peptide from the Venom of the Spider Lycosa Erythrognatha. Amino Acids. 2010;39:135–144. doi: 10.1007/s00726-009-0385-x. [DOI] [PubMed] [Google Scholar]
- 31.Schneider R., Primon-Barros M., von Borowski R.G., Chat S., Nonin-Lecomte S., Gillet R., Macedo A.J. Pseudonajide Peptide Derived from Snake Venom Alters Cell Envelope Integrity Interfering on Biofilm Formation in Staphylococcus Epidermidis. BMC Microbiol. 2020;20:237. doi: 10.1186/s12866-020-01921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan L., Adams M.E. Lycotoxins, Antimicrobial Peptides from Venom of the Wolf SpiderLycosa Carolinensis. J. Biol. Chem. 1998;273:2059–2066. doi: 10.1074/jbc.273.4.2059. [DOI] [PubMed] [Google Scholar]
- 33.Clardy J., Fischbach M., Currie C. The Natural History of Antibiotics. Curr. Biol. 2009;19:R437–R441. doi: 10.1016/j.cub.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da Cunha B.R., Fonseca L.P., Calado C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics. 2019;8:45. doi: 10.3390/antibiotics8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4:481–511. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manenzhe R.I., Zar H.J., Nicol M.P., Kaba M. The Spread of Carbapenemase-Producing Bacteria in Africa: A Systematic Review. J. Antimicrob. Chemother. 2015;70:23–40. doi: 10.1093/jac/dku356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baguy O.M., Nathalie G.K., David C.N.G., Daniel S.N.G., Julien C.K., Rose K.N., Djénéba O.G., Valérie G., Bertin T.K., Mireille D. First Report of Qnr Genes in Multidrugs Resistant (ESBL) Enterobacteria Isolated from Different Ecosystems in Abidjan, Ivory Coast. Int. J. Biol. Sci. Appl. 2014;21:170–175. [Google Scholar]
- 38.Carole G.M.V., Kouadio G.N., Baguy O.M., Djeneba O.G., Ayayi A., Bertin T.K., Anatole T.A., Innocent K.K., Kpoda D.S., Eric T., et al. Antimicrobial Resistance Profile and Molecular Characterization of Extended-Spectrum Beta-Lactamase Genes in Enterobacteria Isolated from Human, Animal and Environment. J. Adv. Microbiol. 2018;10:1–9. doi: 10.9734/JAMB/2018/39955. [DOI] [Google Scholar]
- 39.Collignon P., Wegener H.C., Braam P., Butler C.D. The Routine Use of Antibiotics to Promote Animal Growth Does Little to Benefit Protein Undernutrition in the Developing World. Clin. Infect. Dis. 2005;41:1007–1013. doi: 10.1086/433191. [DOI] [PubMed] [Google Scholar]
- 40.Addis Z., Kebede N., Sisay Z., Alemayehu H., Wubetie A., Kassa T. Prevalence and Antimicrobial Resistance of Salmonella Isolated from Lactating Cows and in Contact Humans in Dairy Farms of Addis Ababa: A Cross Sectional Study. BMC Infect. Dis. 2011;11:222. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortini D., Fashae K., Garcia-Fernandez A., Villa L., Carattoli A. Plasmid-Mediated Quinolone Resistance and -Lactamases in Escherichia Coli from Healthy Animals from Nigeria. J. Antimicrob. Chemother. 2011;66:1269–1272. doi: 10.1093/jac/dkr085. [DOI] [PubMed] [Google Scholar]
- 42.Kikuvi G.M., Ombui J.N., Mitema E.S. Serotypes and Antimicrobial Resistance Profiles of Salmonella Isolates from Pigs at Slaughter in Kenya. J. Infect. Dev. Ctries. 2010;4:243–248. doi: 10.3855/jidc.446. [DOI] [PubMed] [Google Scholar]
- 43.Martin M.J., Thottathil S.E., Newman T.B. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am. J. Public Health. 2015;105:2409–2410. doi: 10.2105/AJPH.2015.302870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds L., McKee M. Factors Influencing Antibiotic Prescribing in China: An Exploratory Analysis. Health Policy. 2009;90:32–36. doi: 10.1016/j.healthpol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob. Resist. Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zasloff M. Antimicrobial Peptides of Multicellular Organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 47.Haney E.F., Petersen A.P., Lau C.K., Jing W., Storey D.G., Vogel H.J. Mechanism of Action of Puroindoline Derived Tryptophan-Rich Antimicrobial Peptides. Biochim. Biophys. Acta Biomembr. 2013;1828:1802–1813. doi: 10.1016/j.bbamem.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Roy R.N. The Mechanism of Inhibition of Protein Synthesis by the Proline-Rich Peptide Oncocin. Mol. Biol. 2015;22:466–469. doi: 10.1038/nsmb.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S., Thacker P.A., Watford M., Qiao S. Functions of Antimicrobial Peptides in Gut Homeostasis. Curr. Protein Pept. Sci. 2015;16:582–591. doi: 10.2174/1389203716666150630135847. [DOI] [PubMed] [Google Scholar]
- 50.Magana M., Pushpanathan M., Santos A.L., Leanse L., Fernandez M., Ioannidis A., Giulianotti M.A., Apidianakis Y., Bradfute S., Ferguson A.L., et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020;20:e216–e230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 51.Tan X.W., Goh T.W., Saraswathi P., Nyein C.L., Setiawan M., Riau A., Lakshminarayanan R., Liu S., Tan D., Beuerman R.W., et al. Effectiveness of Antimicrobial Peptide Immobilization for Preventing Perioperative Cornea Implant-Associated Bacterial Infection. Antimicrob. Agents Chemother. 2014;58:5229–5238. doi: 10.1128/AAC.02859-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang K., Zhang H., Gao C., Chen R., Li C. Antimicrobial Mechanism of PBD2 against Staphylococcus Aureus. Molecules. 2020;25:3513. doi: 10.3390/molecules25153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar P.S. A Perspective on Toxicology of Conus Venom Peptides. Asian Pac. J. Trop. Med. 2015;8:337–351. doi: 10.1016/S1995-7645(14)60342-4. [DOI] [PubMed] [Google Scholar]
- 54.Eric M., Loïc L., Christophe R., Alain R. A Taxonomic Iconography of Living Conidae. Volume 1. ConchBooks; Harxheim, Germany: 2018. [Google Scholar]
- 55.Olivera B.M., Corneli P.S., Watkins M., Fedosov A. Biodiversity of Cone Snails and Other Venomous Marine Gastropods: Evolutionary Success Through Neuropharmacology. Annu. Rev. Anim. Biosci. 2014;2:487–513. doi: 10.1146/annurev-animal-022513-114124. [DOI] [PubMed] [Google Scholar]
- 56.Dutertre S., Lewis R. Snails: Biology, Ecology and Conservation. Nova Science Publishers, Inc.; New York, NY, USA: 2013. Cone Snail Biology, Bioprospecting and Conservation; pp. 85–105. [Google Scholar]
- 57.Gao B., Peng C., Lin B., Chen Q., Zhang J., Shi Q. Screening and Validation of Highly-Efficient Insecticidal Conotoxins from a Transcriptome-Based Dataset of Chinese Tubular Cone Snail. Toxins. 2017;9:214. doi: 10.3390/toxins9070214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz J.R., Norton A.G., Gilly W.F. The Projectile Tooth of a Fish-Hunting Cone Snail: Conus Catus Injects Venom into Fish Prey Using a High-Speed Ballistic Mechanism. Biol. Bull. 2004;207:77–79. doi: 10.2307/1543581. [DOI] [PubMed] [Google Scholar]
- 59.Duque H.M., Dias S.C., Franco O.L. Structural and Functional Analyses of Cone Snail Toxins. Mar. Drugs. 2019;17:370. doi: 10.3390/md17060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson S.D., Norton R.S. Conotoxin Gene Superfamilies. Mar. Drugs. 2014;12:6058–6101. doi: 10.3390/md12126058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akondi K.B., Muttenthaler M., Dutertre S., Kaas Q., Craik D.J., Lewis R.J., Alewood P.F. Discovery, Synthesis, and Structure—Activity Relationships of Conotoxins. Chem. Rev. 2014;114:5815–5847. doi: 10.1021/cr400401e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaas Q., Westermann J.-C., Craik D.J. Conopeptide Characterization and Classifications: An Analysis Using ConoServer. Toxicon. 2010;55:1491–1509. doi: 10.1016/j.toxicon.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Lewis R.J., Dutertre S., Vetter I., Christie M.J. Conus Venom Peptide Pharmacology. Pharmacol. Rev. 2012;64:259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- 64.Yacoub T., Rima M., Karam M., Sabatier J.-M., Fajloun Z. Antimicrobials from Venomous Animals: An Overview. Molecules. 2020;25:2402. doi: 10.3390/molecules25102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebbe E.K.M., Ghequire M.G.K., Peigneur S., Mille B.G., Devi P., Ravichandran S., Waelkens E., D’Souza L., de Mot R., Tytgat J. Novel Conopeptides of Largely Unexplored Indo Pacific Conus sp. Mar. Drugs. 2016;14:199. doi: 10.3390/md14110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biggs J.S., Rosenfeld Y., Shai Y., Olivera B.M. Conolysin-Mt: A Conus Peptide That Disrupts Cellular Membranes. Biochemistry. 2007;46:12586–12593. doi: 10.1021/bi700775p. [DOI] [PubMed] [Google Scholar]
- 67.Hemu X., Tam J.P. Macrocyclic Antimicrobial Peptides Engineered from ω-Conotoxin. Curr. Pharm. Des. 2017;23:2131–2138. doi: 10.2174/1381612822666161027120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivera B.M. Conus Peptides: Biodiversity-Based Discovery and Exogenomics. J. Biol. Chem. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- 69.Dutertre S., Jin A., Kaas Q., Jones A., Alewood P.F., Lewis R.J. Deep Venomics Reveals the Mechanism for Expanded Peptide Diversity in Cone Snail Venom. Mol. Cell. Proteom. 2013;12:312–329. doi: 10.1074/mcp.M112.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutertre S., Jin A.-H., Vetter I., Hamilton B., Sunagar K., Lavergne V., Dutertre V., Fry B.G., Antunes A., Venter D.J., et al. Evolution of Separate Predation- and Defence-Evoked Venoms in Carnivorous Cone Snails. Nat. Commun. 2014;5:3521. doi: 10.1038/ncomms4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson S.D., Safavi-Hemami H., Raghuraman S., Imperial J.S., Papenfuss A.T., Teichert R.W., Purcell A.W., Olivera B.M., Norton R.S. Discovery by Proteogenomics and Characterization of an RF-Amide Neuropeptide from Cone Snail Venom. J. Proteom. 2015;114:38–47. doi: 10.1016/j.jprot.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergeron Z.L., Chun J.B., Baker M.R., Sandall D.W., Peigneur S., Yu P.Y.C., Thapa P., Milisen J.W., Tytgat J., Livett B.G., et al. A ‘Conovenomic’ Analysis of the Milked Venom from the Mollusk-Hunting Cone Snail Conus Textile—The Pharmacological Importance of Post-Translational Modifications. Peptides. 2013;49:145–158. doi: 10.1016/j.peptides.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin A.-H., Vetter I., Himaya S.W.A., Alewood P.F., Lewis R.J., Dutertre S. Transcriptome and Proteome of Conus Planorbis Identify the Nicotinic Receptors as Primary Target for the Defensive Venom. Proteomics. 2015;15:4030–4040. doi: 10.1002/pmic.201500220. [DOI] [PubMed] [Google Scholar]
- 74.Prashanth J.R., Dutertre S., Jin A.H., Lavergne V., Hamilton B., Cardoso F.C., Griffin J., Venter D.J., Alewood P.F., Lewis R.J. The Role of Defensive Ecological Interactions in the Evolution of Conotoxins. Mol. Ecol. 2016;25:598–615. doi: 10.1111/mec.13504. [DOI] [PubMed] [Google Scholar]
- 75.Jin A.-H., Dutertre S., Dutt M., Lavergne V., Jones A., Lewis R.J., Alewood P.F. Transcriptomic-Proteomic Correlation in the Predation-Evoked Venom of the Cone Snail, Conus Imperialis. Mar. Drugs. 2019;17:177. doi: 10.3390/md17030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prator C.A., Murayama K.M., Schulz J.R. Venom Variation during Prey Capture by the Cone Snail, Conus Textile. PLoS ONE. 2014;9:e98991. doi: 10.1371/journal.pone.0098991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hopkins C., Grilley M., Miller C., Shon K.-J., Cruz L.J., Gray W.R., Dykert J., Rivier J., Yoshikami D., Olivera B.M. A New Family of Conus Peptides Targeted to the Nicotinic Acetylcholine Receptor. J. Biol. Chem. 1995;270:22361–22367. doi: 10.1074/jbc.270.38.22361. [DOI] [PubMed] [Google Scholar]
- 78.Bingham J.-P., Mitsunaga E., Bergeron Z.L. Drugs from Slugs—Past, Present and Future Perspectives of ω-Conotoxin Research. Chem. Biol. Interact. 2010;183:1–18. doi: 10.1016/j.cbi.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 79.Prashanth J.R., Lewis R.J., Dutertre S. Towards an Integrated Venomics Approach for Accelerated Conopeptide Discovery. Toxicon. 2012;60:470–477. doi: 10.1016/j.toxicon.2012.04.340. [DOI] [PubMed] [Google Scholar]
- 80.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 82.Hu H., Bandyopadhyay P.K., Olivera B.M., Yandell M. Characterization of the Conus Bullatus Genome and Its Venom-Duct Transcriptome. BMC Genom. 2011;12:60. doi: 10.1186/1471-2164-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zerbino D.R. Using the Velvet de Novo Assembler for Short-Read Sequencing Technologies. Curr. Protoc. Bioinform. 2010;31:11–15. doi: 10.1002/0471250953.bi1105s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simpson J.T., Wong K., Jackman S.D., Schein J.E., Jones S.J.M., Birol İ. ABySS: A Parallel Assembler for Short Read Sequence Data. Genome Res. 2009;19:1117–1123. doi: 10.1101/gr.089532.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacManes M.D. The Oyster River Protocol: A Multi-Assembler and Kmer Approach for de Novo Transcriptome Assembly. PeerJ. 2018;6:e5428. doi: 10.7717/peerj.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubio A., Mier P., Andrade-Navarro M.A., Garzón A., Jiménez J., Pérez-Pulido A.J. CRISPR Sequences Are Sometimes Erroneously Translated and Can Contaminate Public Databases with Spurious Proteins Containing Spaced Repeats. Database. 2020;2020:baaa088. doi: 10.1093/database/baaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaas Q., Yu R., Jin A.-H., Dutertre S., Craik D.J. ConoServer: Updated Content, Knowledge, and Discovery Tools in the Conopeptide Database. Nucleic Acids Res. 2012;40:D325–D330. doi: 10.1093/nar/gkr886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acland A., Agarwala R., Barrett T., Beck J., Benson D.A., Bollin C., Bolton E., Bryant S.H., Canese K., Church D.M., et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2014;42:D7–D17. doi: 10.1093/nar/gkt1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Consortium T.U. UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koua D., Brauer A., Laht S., Kaplinski L., Favreau P., Remm M., Lisacek F., Stöcklin R. ConoDictor: A Tool for Prediction of Conopeptide Superfamilies. Nucleic Acids Res. 2012;40:W238–W241. doi: 10.1093/nar/gks337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lavergne V., Dutertre S., Jin A., Lewis R.J., Taft R.J., Alewood P.F. Systematic Interrogation of the Conus Marmoreus Venom Duct Transcriptome with ConoSorter Reveals 158 Novel Conotoxins and 13 New Gene Superfamilies. BMC Genom. 2013;14:708. doi: 10.1186/1471-2164-14-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Armenteros J.J.A., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 95.Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J., et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crooks G.E., Hon G., Chandonia J.-M., Brenner S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodriguez A.M., Dutertre S., Lewis R.J., Marí F. Intraspecific Variations in Conus Purpurascens Injected Venom Using LC/MALDI-TOF-MS and LC-ESI-TripleTOF-MS. Anal. Bioanal. Chem. 2015;407:6105–6116. doi: 10.1007/s00216-015-8787-y. [DOI] [PubMed] [Google Scholar]
- 98.Jones A., Bingham J.-P., Gehrmann J., Bond T., Loughnan M., Atkins A., Lewis R.J., Alewood P.F. Isolation and Characterization of Conopeptides by High-Performance Liquid Chromatography Combined with Mass Spectrometry and Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996;10:138–143. doi: 10.1002/(SICI)1097-0231(19960115)10:1<138::AID-RCM442>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 99.Romeo C., Francesco L.D., Oliverio M., Palazzo P., Massilia G.R., Ascenzi P., Polticelli F., Schininà M.E. Conus Ventricosus Venom Peptides Profiling by HPLC-MS: A New Insight in the Intraspecific Variation. J. Sep. Sci. 2008;31:488–498. doi: 10.1002/jssc.200700448. [DOI] [PubMed] [Google Scholar]
- 100.Abdel-Rahman M.A., Abdel-Nabi I.M., El-Naggar M.S., Abbas O.A., Strong P.N. Intraspecific Variation in the Venom of the Vermivorous Cone Snail Conus Vexillum. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011;154:318–325. doi: 10.1016/j.cbpc.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Bern M., Cai Y., Goldberg D. Lookup Peaks: A Hybrid of de Novo Sequencing and Database Search for Protein Identification by Tandem Mass Spectrometry. Anal. Chem. 2007;79:1393–1400. doi: 10.1021/ac0617013. [DOI] [PubMed] [Google Scholar]
- 102.Zamdborg L., LeDuc R.D., Glowacz K.J., Kim Y.-B., Viswanathan V., Spaulding I.T., Early B.P., Bluhm E.J., Babai S., Kelleher N.L. ProSight PTM 2.0: Improved Protein Identification and Characterization for Top down Mass Spectrometry. Nucleic Acids Res. 2007;35:W701–W706. doi: 10.1093/nar/gkm371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu D., Zhang Y. Toward Optimal Fragment Generations for Ab Initio Protein Structure Assembly: Ab Initio Fragment Generation. Proteins Struct. Funct. Bioinform. 2013;81:229–239. doi: 10.1002/prot.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Senior A.W., Evans R., Jumper J., Kirkpatrick J., Sifre L., Green T., Qin C., Žídek A., Nelson A.W.R., Bridgland A., et al. Protein Structure Prediction Using Multiple Deep Neural Networks in the 13th Critical Assessment of Protein Structure Prediction (CASP13) Proteins Struct. Funct. Bioinform. 2019;87:1141–1148. doi: 10.1002/prot.25834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Senior A.W., Evans R., Jumper J., Kirkpatrick J., Sifre L., Green T., Qin C., Žídek A., Nelson A.W.R., Bridgland A., et al. Improved Protein Structure Prediction Using Potentials from Deep Learning. Nature. 2020;577:706–710. doi: 10.1038/s41586-019-1923-7. [DOI] [PubMed] [Google Scholar]
- 106.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard. 10th ed. Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2015. [Google Scholar]
- 107.Luna-Ramirez K., Tonk M., Rahnamaeian M., Vilcinskas A. Bioactivity of Natural and Engineered Antimicrobial Peptides from Venom of the Scorpions Urodacus Yaschenkoi and U. Manicatus. Toxins. 2017;9:22. doi: 10.3390/toxins9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gumerova N.I., Al-Sayed E., Krivosudský L., Čipčić-Paljetak H., Verbanac D., Rompel A. Antibacterial Activity of Polyoxometalates Against Moraxella Catarrhalis. Front. Chem. 2018;6:336. doi: 10.3389/fchem.2018.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Almaaytah A., Qaoud M.T., Abualhaijaa A., Al-Balas Q., Alzoubi K.H. Hybridization and Antibiotic Synergism as a Tool for Reducing the Cytotoxicity of Antimicrobial Peptides. Infect. Drug Resist. 2018;11:835–847. doi: 10.2147/IDR.S166236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.




