Abstract
Mycotic or fungal keratitis (FK) is a sight-threatening disease, caused by infection of the cornea by filamentous fungi or yeasts. In tropical, low and middle-income countries, it accounts for the majority of cases of microbial keratitis (MK). Filamentous fungi, in particular Fusarium spp., the aspergilli and dematiaceous fungi, are responsible for the greatest burden of disease. The predominant risk factor for filamentous fungal keratitis is trauma, typically with organic, plant-based material. In developed countries, contact lens wear and related products are frequently implicated as risk factors, and have been linked to global outbreaks of Fusarium keratitis in the recent past. In 2020, the incidence of FK was estimated to be over 1 million cases per year, and there is significant geographical variation; accounting for less than 1% of cases of MK in some European countries to over 80% in parts of south and south-east Asia. The proportion of MK cases is inversely correlated to distance from the equator and there is emerging evidence that the incidence of FK may be increasing. Diagnosing FK is challenging; accurate diagnosis relies on reliable microscopy and culture, aided by adjunctive tools such as in vivo confocal microscopy or PCR. Unfortunately, these facilities are infrequently available in areas most in need. Current topical antifungals are not very effective; infections can progress despite prompt treatment. Antifungal drops are often unavailable. When available, natamycin is usually first-line treatment. However, infections may progress to perforation in ~25% of cases. Future work needs to be directed at addressing these challenges and unmet needs. This review discusses the epidemiology, clinical features, diagnosis, management and aetiology of FK.
Keywords: microbial keratitis, fungal keratitis, microbiology, mycotic keratitis, epidemiology, Fusarium, Aspergillus, dematiaceous fungi, blindness
1. Introduction
Mycotic or fungal keratitis (FK) is a severe and potentially blinding infection of the cornea (Figure 1) and is considered an ophthalmic emergency [1,2]. It is one of the leading causes of microbial keratitis (MK) or corneal ulcer. The latest conservative estimates predict that there are close to 1.5 million new infections every year [3], which correlate with estimates published more than 20 years ago [4,5]. The burden of FK is greatest in tropical and subtropical countries, accounting for between 20 and 60% of MK cases presenting in tropical regions [6], likely a result of climate (higher temperatures and relative humidity) and frequent agriculture-related ocular trauma [7].
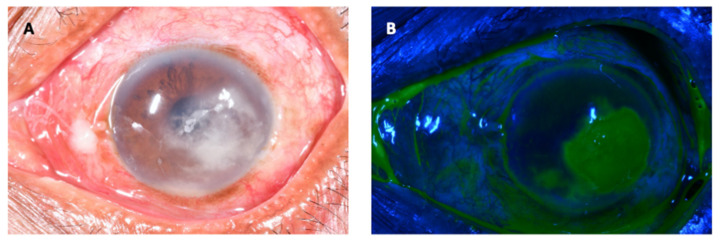
Figure 1.
Fungal keratitis in a patient presenting to an ophthalmic hospital in Nepal. The causative organism was confirmed to be Fusarium sp. on culture. (A): The conjunctiva is hyperaemic, causing the eye to be red. There is a white corneal infiltrate with feathery serrated margins and satellite lesions present. There is also a small hypopyon. (B): The same eye as viewed with a cobalt blue filter after instillation of topical fluorescein. The area staining in green represents a defect in the corneal epithelium.
Fungal keratitis is caused by yeasts and filamentous fungi but the pattern of infection varies globally with respect to aetiology and predisposing risk factors relating to geographical location and occupational exposure. Infections due to Candida spp. and other yeasts are typically associated with steroid use, ocular surface disorders, previous ocular surgery, contact lens wear and underlying illness resulting in immuno-incompetency [8], mostly occurring in temperate climes. However, the main burden of disease globally is attributable to the filamentous fungi and these infections predominantly affect the poorest patients in warm, humid, tropical climatic regions [7]. There have also been reports of an increase in Fusarium-related keratitis in contact lens wearers in temperate, industrialised regions [9,10,11]. Interestingly, even within developed countries fungal keratitis is a disease of poverty: infections are associated with contact lens wearers from deprived or low socioeconomic backgrounds [3,12].
Over one hundred different species of filamentous fungi isolated from infected corneas have been reported in the literature [13]. The most common genera isolated from filamentous fungal keratitis cases are Fusarium spp. and the aspergilli [14], followed by the dematiaceous fungi-a heterogenous group of fungi characterised by melanin-production and pigmentation-Curvularia spp. being the most commonly reported genus from this group [15,16,17].
Patients typically present with a red, painful eye, together with reduced vision. Clinical examination will demonstrate conjunctival hyperaemia, making the eye appear red, in conjunction with a corneal infiltrate-an area of corneal opacity, often white or cream in colour (Figure 1A). There will also usually be loss of the corneal epithelium overlying the infiltrate, which stains with topical fluorescein eye drops and fluoresces green under blue light (Figure 1B). These clinical signs can be observed without a slit-lamp, a simple torch with or without loupes will suffice, aided by a blue filter and fluorescein testing strips. More detailed examination using a slit-lamp biomicroscope yields more subtle signs that can help distinguish the different causative agents of MK to some extent; fungal keratitis is more likely if there are serrated margins, raised slough (dead epithelial tissue), and/or colour other than yellow [2].
Unfortunately, presentation to an appropriate eye care provider is usually delayed, with patients often taking a convoluted journey to reach an ophthalmic clinician [18]. Compounding this is the fact that patients often self-medicate with traditional eye medicine which commonly contains non-sterile plant matter, or inappropriate conventional medication (such as topical corticosteroids), exacerbating disease [1,19,20]. Primary health-care workers have little training in recognising, treating or referring MK [21]. This delay leads to advanced infections, which have poor outcomes [1,18]. Accurate diagnosis remains challenging as it is frequently not possible to clinically distinguish bacterial and fungal MK. Microbiology services are usually unavailable. Due to resource limitations in the populations most at risk of these infections microscopy and culture remain the mainstay for diagnosis-Gram, Potassium Hydroxide (KOH), calcofluor white (CFW). Microscopy is the gold standard with visualisation of fungal hyphae in corneal tissue specimens.
Added to this is the fact that FK is particularly challenging to treat. Current topical antifungals are not consistently effective and infections can progress despite prompt treatment, Figure 2 [1,22,23,24,25,26], with up to 30% of patients receiving current ‘gold-standard’ therapy progressing to corneal perforation and/or eye-loss, mediated by human and pathogen derived proteases [1,22,24]. Antifungal drops are rarely available in sub-Saharan Africa and often scarce elsewhere where the burden is greatest [1].
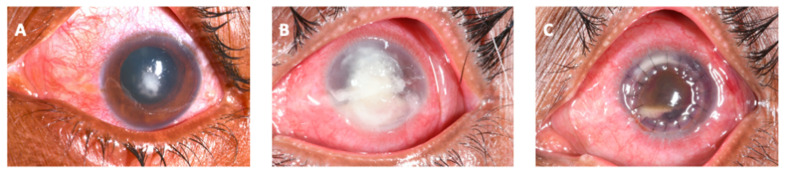
Figure 2.
The progression of a patient with fungal keratitis caused by Aspergillus sp. This patient presented early in the course of the disease with a relatively small corneal ulcer, with serrated feathery margins to the corneal infiltrate (A). Despite intense, appropriate, prompt treatment with topical natamycin 5%, the corneal infiltrate increased in size, ultimately perforating, and was temporarily treated with corneal gluing and bandage contact lens insertion (B). The patient ultimately underwent a therapeutic penetrating keratoplasty (C).
Treatment can be administered topically (first-line, with intensive hourly dosing for at least the first 48 h), orally or by intravenous, subconjunctival, corneal stromal or intracameral injection. The treatment of yeast infections is often different to filamentous fungi, with the former being more common in temperate climates and the latter in hot and humid locations [27,28]. Surgical therapy, typically with therapeutic penetrating keratoplasty (PK, corneal transplantation replacing the diseased cornea with donated corneal tissue), is generally reserved for cases of corneal perforation or progressive infection refractory to medical therapy. PK can also be performed for visual rehabilitation after the acute infection has resolved.
In this review, given the wide range of organisms implicated in fungal keratitis, we have classified the causative organisms into three main groups: hyaline fungi, dematiaceous fungi and yeasts; the focus of this review are the filamentous fungi. The epidemiology, clinical features, diagnosis and management will also be discussed for fungal keratitis as a distinct entity.
2. Epidemiology of Fungal Keratitis
2.1. Incidence
Until recently, the annual global incidence of fungal keratitis had never been estimated. In 2020, Brown et al. estimated the incidence of fungal keratitis to be 1,051,787 cases per annum, within a range of between 736,251 and 1,367,323 cases per annum [3]. The incidence may in fact be higher at 1,480,916 cases per annum (range 1,036,641–1,925,191) if it is assumed that all unconfirmed culture negative cases of microbial keratitis were in fact fungal in aetiology. The morbidity associated with FK is also important to note: approximately 10–25% of eyes with FK will perforate or need surgical removal, whilst at least 60% of patients, even if treated, are left monocularly blind, equating to approximately 800,000 people per year [1,22,24].
2.2. Geographical Distribution
The incidence of FK varies across regions, with the highest incidence in Asia and Africa and the lowest incidence in Europe [3]. Similarly, the proportion of fungal keratitis as a subset of microbial keratitis varies between geographical regions, within the range of 1% of MK cases in Spain to 60% in Vietnam (Table 1) [29,30]. There have been four large reviews that have considered the proportion of MK caused by filamentous fungi by geographical region [3,7,31,32]. The first study from 2002 plotted the proportion of FK as a subset of MK against latitude, and found the proportion of FK cases increases with decreasing latitude, i.e., increasing the closer one is to the equator [7]. The second review, from 2011, correlated the proportion of fungal cases of MK in a country with the country’s gross domestic product (GDP) [31]. This found the highest proportion of fungal infections within Asia, specifically in India and Nepal. The study found the lower the GDP per capita of a country, the higher the proportion of fungal MK. The most recent study looked at both GDP per capita and latitude as potential determinants of the proportion of fungal cases of all those with MK [3]. The findings here correlated to the previous two reviews, suggesting that both proximity to the equator and low GDP per capita are associated with a higher proportion of fungal MK cases [3,7,31]. However, it is important to note there was some considerable unexplained variability [3].
Table 1.
Global epidemiology of fungal keratitis (FK), most frequently isolated fungal organisms and summary results of select papers on risk factors for developing FK, grouped as per their geographical region (as defined by the UN).
| Country | Year | % FK # | % Culture Negative Cases ~ | Age (Mean) | % Male | % Trauma | % Steroid | % TEM | % CL | % OSD |
% HIV |
% DM |
Distance from Equator (km) | N (All MK Cases) |
Organism 1 (%) | Organism 2 (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFRICA | |||||||||||||||||
| Egypt (Zagazig) | 2012 | 55 | - | - | - | 63.6 | 6.1 | - | 12.1 | - | - | 18.2 | 3401 | 60 | Penicillium spp. (24.2) | Aspergillus spp. (21.2) | [37] |
| Egypt (Mansoura) | 2013–2015 | 65.5 | 55.5 | - | 66.1 | 51.4 | 5.3 | - | 2.4 | 4.5 | - | 15.1 | 3452 | 247 | Aspergillus (41.0) | - | [38] |
| Egypt (Tanta) | 2011–2013 | 43.3 | 15.5 | 49.2 | 65.9 | 58.4 | 32.7 | - | - | - | - | - | 3424 | 834 | Aspergillus flavus (29.1) | Aspergillus niger (16.1) | [39] |
| Ethiopia | 2014–2015 | 45.1 | - | - | 58.0 | 78.3 | 5.8 | - | - | - | - | 7.2 | 1003 | 153 | Fusarium spp. (27.6) | Aspergillus spp. (25.0) | [40] |
| Ghana | 1995 | 56.1 | 42.7 | 36.3 | 69.3 | - | - | - | - | - | - | - | 900 | 199 | Fusarium spp. (52.3) | Aspergillus spp. (15.3) | [41] |
| Ghana | 1999-2001 | 74.7 | 60.0 | - | - | - | - | - | - | - | - | - | 900 | 290 | Fusarium spp. (42.2) | Aspergillus spp. (17.4) | [7] |
| Libya | 2008–2010 | 32.9 | ^ | - | 60.7 | 78.5 | 3.6 | - | 21.4 | - | - | 17.8 | 3114 | 85 | Aspergillus spp. (50.0) | Fusarium spp. (39.3) | [42] |
| Sierra Leone | 2005–2006 | 37.7 | 5.5 | - | - | - | - | - | - | - | - | - | 941 | 73 | UFF (69.2) | Aspergillus spp. (15.4) | [35] |
| South Africa | 1982-1983 | 3.7 | - | - | - | 0 | 0 | - | 0 | - | 33.3 | 50.0 | 2913 | 164 | Curvularia spp. (33.3) | - | [43] |
| South Africa | 2013–2015 | 2.2 | - | - | - | - | - | - | - | - | - | - | 3399 | 46 | - | - | [44] |
| Tanzania | 2008–2010 | 51.6 | 45.6 | - | - | - | - | - | - | - | - | - | 667 | 170 | UFF (87.5) | Candida spp. (12.5) | [1] |
| Tanzania | 2013 | 40.2 | 8.9 | - | - | - | - | - | - | - | - | - | 755 | 202 | Candida spp. (60.8) | UFF (39.6) | [45] |
| Tunisia | 1995-2012 | 12.4 | 7 | 47.2 | 63.3 | 61.6 | 18.3 | 3.3 | 3.3 | 10.0 | - | 5 | 3862 | 483 | Fusarium spp. (49.0) | Aspergillus spp. (22.0) | [46] |
| Tunisia | 2010–2015 | 30.0 | 40 | 48.9 | 56.6 | 43.3 | 3.3 | - | 3.3 | - | - | 10 | 4124 | 30 | Fusarium spp. (50.0) | Aspergillus spp. (33.3) | [47] |
| Tunisia | 1996–2004 | 21.6 | 58 | - | - | 50.0 | - | - | - | 25 | - | - | 4094 | 100 | Fusarium spp. (87.5) | Acremonium spp. (12.5) | [48] |
| ASIA | |||||||||||||||||
| Bangladesh | 2008 | 39.2 | 5 | - | - | 49 | - | - | - | - | - | - | 2752 | 120 | - | - | [49] |
| Bangladesh | 1994 | 36 | 18.3 | - | - | - | - | - | - | - | - | - | 2483 | 142 | Aspergillus spp. (40.0) | Fusarium spp. (21.0) | [50] |
| China | 2009–2013 | 45.7 | 53.9 | - | - | - | - | - | - | - | - | - | 2504 | 2973 | Fusarium spp. (29.3) | Aspergillus spp. (24.1) | [51] |
| China | 1999-2004 | 61.9 | - | - | - | - | - | - | - | - | - | - | 4012 | 1054 | Fusarium spp. (73.3) | Aspergillus spp. (12.1) | [52] |
| China (Hong Kong) | 1997-1998 | 6.4 | 65.0 | - | - | 20 | 20 | - | - | - | - | - | 2491 | 223 | Fusarium spp. (60.0) | Penicillium spp. (20.0) | [53] |
| China (Hong Kong) | 2004–2013 | 10.7 | 67.7 | - | - | - | - | - | - | - | - | - | 2491 | 260 | Fusarium spp. (33.3) | Candida spp. (25.0) | [54] |
| China (Taiwan) | 1992-2001 | 13.5 | 51.0 | - | - | - | - | - | - | - | - | - | 2758 | 453 | Fusarium spp. (29.4) | Candida spp. (29.4) | [55] |
| China (Taiwan) | 2012–2014 | 8.2 | 69 | - | - | - | - | - | - | - | - | - | 2669 | 233 | - | - | [56] |
| India (West Bengal) | 2007–2011 | 58.9 | 27.0 | - | 61.7 | 88.7 | 16.3 | - | - | 6.0 | - | 11.8 | 2510 | 928 | Aspergillus spp. (37.8) | Fusarium spp. (20.3) | [57] |
| India (West Bengal) | 2008 | 38.1 | 32.7 | 53 | 65.0 | 48.0 | 16.0 | 16.0 | - | - | - | - | 2510 | 289 | Aspergillus spp. (55.4) | Fusarium spp. (10.8) | [58] |
| India (Odisha) | 2006–2009 | 35.5 | 18.6 | - | 70.0 | 40.2 | - | - | - | - | - | 2.2 | 2253 | 997 | Aspergillus spp. (27.9) | Fusarium spp. (23.2) | [59] |
| India (Assam) | 2007–2009 | 60.6 | - | - | 68.8 | 76.40 | - | - | - | 1.5 | - | 2.5 | 3056 | 310 | Fusarium spp. (25.0) | Aspergillus spp. (19.0) | [60] |
| India (Delhi) | 2010–2015 | 68 | 65 | - | 76 | 89.4 | - | - | - | 5.3 | - | 1.3 | 3188 | 400 | Aspergillus spp. (30.8) | Fusarium spp. (27.6) | [61] |
| India (Chandigarh) | 2005–2011 | Ψ | 49 | - | - | 66.5 | 2 | - | - | 11.7 | - | - | 3418 | 765 | Aspergillus spp. (47.6) | Dematiaceous fungi (21.9) | [62] |
| India (Rajasthan) | 2005–2012 | 68.2 | 45 | - | 71.7 | 62.8 | - | - | 3.9 | - | 1.1 | 8.9 | 3116 | 480 | Aspergillus spp. (63.3) | Alternaria spp. (8.3) | [63] |
| India (Chandigarh) | 1999-2003 | 41.5 | 46.9 | - | 80.5 | 43.8 | 7.8 | - | - | - | - | - | 3418 | 64 | Aspergillus spp. (41.2) | Fusarium spp. (23.5) | [64] |
| India (Delhi) | 2007–2011 | 58.9 | 27 | - | 61.7 | 88.7 | 16.3 | - | - | 19 | - | 11.8 | 3188 | 928 | Aspergillus spp. (37.8) | Dematiaceous fungi (23.8) | [65] |
| India (Delhi) | 2000–2004 | 22.3 | - | - | 77.9 | 32.4 | 16.2 | 2.7 | 0 | - | - | - | 3188 | 346 | Aspergillus spp. (55.9) | Dematiaceous fungi (7.8) | [66] |
| India (Madurai) | 2012–2013 | 79 | 14.5 | 50 | 64 | 70 | 9 | 19 | 8 | 1103 | 252 | Fusarium spp. (39.0) | Aspergillus spp. (18.0) | [67] | |||
| India (Madurai) | 1999–2002 | 52.1 | 29.4 | - | 65 | 92.1 | 1.2 | - | 0 | 6.7 | - | 15.7 | 1103 | 3183 | Fusarium spp. (41.9) | Dematiaceous fungi (26.9) | [68] |
| India (Madurai) | 1994 | 51.8 | 31.6 | - | 61.3 | - | - | - | - | - | - | - | 1103 | 434 | Fusarium spp. (47.1) | Aspergillus spp. (16.1) | [4] |
| India (Hyderabad) | 1991–2000 | 39.8 | ^ | 40.4 | 71.2 | 54.4 | 5.9 | - | - | 11.7 | - | 6.4 | 1933 | 3399 | Fusarium spp. (37.2) | Aspergillus spp. (30.7) | [69] |
| India (Hyderabad) | 1991–2001 | 44.8 | 39.6 | 30.9 | - | 81.9 | 2.4 | - | 0.3 | 18.2 | - | - | 1933 | 5897 | Fusarium spp. (35.6) | Aspergillus spp. (26.8) | [70] |
| India (Madurai) | 2006–2009 | 63 | 42 | - | - | - | - | - | - | - | - | - | 1103 | 6967 | Fusarium spp. (42.3) | - | [71] |
| India (Bangalore) | 2012–2014 | 55.5 | 62.5 | - | - | - | - | - | - | - | - | 1442 | 312 | Fusarium spp. (31.0) | Aspergillus spp. (11.0) | [72] | |
| India (Tamil Nadu) | 1999–2001 | 44 | 31 | - | - | - | - | - | - | - | - | - | 1223 | 800 | Aspergillus spp. (39.9) | Fusarium spp. (21.5) | [7] |
| India (Maharashtra) | 2004–2009 | 57.9 | 37 | - | - | - | - | - | - | - | - | - | 2120 | 852 | Fusarium spp. (35.0) | Aspergillus spp. (18.0) | [12] |
| India (Gujarat) | 2006–2008 | 65 | 60 | 54 | 73 | - | - | - | - | - | - | 2498 | 100 | Aspergillus spp. (70.0) | Fusarium spp. (12.0) | [73] | |
| India (Gujarat) | 2003–2005 | 51.8 | 45 | - | - | - | - | - | - | - | - | - | 2498 | 200 | Fusarium spp. (29.8) | Aspergillus spp. (21.1) | [74] |
| India (Gujarat) | 2007–2008 | 34.9 | 40.7 | - | - | - | - | - | - | - | - | - | 2561 | 150 | Aspergillus spp. (35.4) | Fusarium spp. (22.5) | [75] |
| India (West Bengal) | 2001–2003 | 62.7 | 32 | - | - | - | - | - | - | - | - | - | 2669 | 1198 | Aspergillus spp. (59.9) | Fusarium spp. (21.2) | [76] |
| India (Delhi) | 2005 | 49.1 | 43.2 | - | - | - | - | - | - | - | - | - | 3183 | 1000 | Aspergillus spp. (41.6) | Fusarium spp. (19.8) | [77] |
| India (Hyderabad) | 2002 | 19.4 | 33.5 | - | - | - | - | - | - | - | - | - | 1933 | 170 | Fusarium spp. (72.7) | - | [78] |
| Iran (Tehran) | 2011–2013 | Ψ | 94.4 | 79.3 | - | - | - | - | - | - | - | 3969 | 2180 | Fusarium spp. (49.6) | Aspergillus spp. (26.4) | [79] | |
| Iran (Sari) | 2004–2005 | 77.8 | 59.1 | 61.5 | 71.4 | 28.6 | 0 | - | 0 | 14.3 | - | 14.3 | 4065 | 22 | Fusarium spp. (50.0) | Aspergillus spp. (50.0) | [80] |
| Iraq | 2002–2005 | 31.9 | 41.4 | - | - | 90 | - | - | 0 | 6.8 | - | - | 3707 | 396 | Aspergillus spp. (56.8) | Fusarium spp. (27.0) | [81] |
| Iraq | 2013–2014 | 6.8 | 30.5 | - | - | - | - | - | - | - | - | - | 3707 | 105 | Aspergillus spp. (60.0) | Alternaria spp. (40.0) | [82] |
| Iraq | 2017–2018 | 37 | ^ | 73 | 61 | - | - | - | - | - | - | 41 | 3707 | 234 | Aspergillus spp. (70.0) | Penicillium spp. (13.0) | [83] |
| Japan | 1999–2003 | 6.1 | 41.5 | - | - | - | - | - | - | - | - | - | 3991 | 122 | Candida spp. (83.3) | - | [84] |
| Japan | 2003 | 10.6 | 56.7 | - | - | - | - | - | - | - | - | - | 3969 | 261 | - | - | [85] |
| Korea (RO) | 2003–2008 | 26.9 | 37.3 | - | - | - | - | - | - | - | - | - | 4177 | 83 | Candida spp. (57.0) | Aspergillus spp. (28.6) | [86] |
| Malaysia | 2007–2011 | 25.3 | 12.8 | - | 61.7 | 48.9 | 17.0 | - | 4.3 | 10.6 | - | 10.6 | 371 | 186 | Fusarium spp. (46.0) | Aspergillus spp. (9.8) | [87] |
| Malaysia | 2017 | 36.4 | 59.9 | - | - | - | - | - | - | - | - | - | 367 | 137 | Fusarium spp. (60.0) | - | [88] |
| Nepal (Dharan) | 2004–2008 | 61.1 | 20.8 | - | - | - | - | - | - | 2980 | 351 | Aspergillus spp. (33.3) | Fusarium spp. (12.7) | [89] | |||
| Nepal (Dharan) | 1998–1999 | 65.5 | 32.6 | - | - | - | - | - | - | - | - | - | 2980 | 86 | Aspergillus spp. (60.5) | Fusarium spp. (13.2) | [90] |
| Nepal (Nepalgunj) | 2011–2012 | 36 | ^ | - | 59.3 | 58 | 12 | - | - | 6 | - | - | 3120 | 1880 | Fusarium spp. (31.9) | Curvularia spp. (17.7) | [91] |
| Nepal (Dharan) | 2007–2008 | 60 | 54.5 | - | - | - | - | - | - | - | - | - | 2980 | 44 | Aspergillus spp. (66.6) | - | [92] |
| Nepal (Kathmandu) | 2014 | 44 | 55.4 | - | - | - | - | - | - | - | - | - | 3080 | 101 | Fusarium spp. (24.0) | Aspergillus spp. (20.0) | [93] |
| Nepal (Kathmandu) | 1981 | 25 | 50 | - | - | - | - | - | - | - | - | - | 3080 | 133 | - | - | [94] |
| Nepal (Biratnagar) | 2011 | 70 | - | - | - | - | - | - | - | - | - | 2944 | 1644 | No culture performed, microscopy only | [95] | ||
| Oman | 2004–2007 | 31.3 | 57.9 | - | 59.4 | 25 | 31.3 | 15.6 | - | 18.8 | - | 9.4 | 2510 | 242 | Fusarium spp. (50.0) | Aspergillus spp. (34.4) | [96] |
| Oman | 2000–2006 | 11.8 | 56.9 | - | - | - | - | - | - | - | - | - | 2510 | 188 | - | - | [97] |
| Pakistan | 2010 | 64 | 32.3 | - | - | - | - | - | - | - | - | - | 2788 | 133 | - | - | [98] |
| Saudi Arabia | 1984–2004 | 10.3 | 69.4 | 55 | 79 | 20.9 | 16.9 | - | 0.8 | 8.87 | - | 12 | 2746 | 1200 | Aspergillus spp. (37.0) | Trichophyton spp. (20.0) | [99] |
| Singapore | 1991–2005 | Ψ | ^ | 40 | 79.3 | 55 | 24 | - | 7 | 14 | - | - | 143 | 29 | Fusarium spp. (52.0) | Aspergillus spp. (17.0) | [100] |
| Singapore | 2012–2014 | 0.7 | - | - | - | - | - | - | - | - | - | - | 143 | 531 | - | - | [56] |
| Sri Lanka | 1976–1981 | 81.5 | 59.1 | - | - | - | - | - | - | - | - | - | 811 | 66 | UFF (63.6) | Aspergillus spp. (18.0) | [36] |
| Thailand (Central) | 1988–2000 | 24.6 | 52.7 | - | - | - | - | - | - | - | - | - | 1529 | 292 | Fusarium spp. (34.3) | Aspergillus spp. (20.0) | [101] |
| Thailand (South) | 1982–2003 | 15.3 | - | 46.4 | 72.3 | 66 | - | - | - | - | - | - | 800 | 556 | Fusarium spp. (64.5) | Aspergillus spp. (10.5) | [102] |
| Thailand (North) | 2003–2006 | 50.8 | 74.4 | - | - | - | - | - | - | - | - | - | 2090 | 305 | Fusarium spp. (58.1) | Aspergillus spp. (12.9) | [103] |
| Thailand (Central) | 2001–2004 | 38 | ^ | - | 67.7 | 77.5 | - | - | 0 | 9.68 | - | - | 1529 | 127 | Fusarium spp. (26.0) | Dematiaceous fungi (20.0) | [104] |
| Turkey (Adana) | 2014–2015 | 9.4 | - | 39.3 | 50 | 50 | - | - | 25 | - | - | - | 4115 | 64 | Aspergillus spp. (66.7) | Fusarium spp. (33.3) | [105] |
| Turkey (West Anatolia) | 1990–2005 | 22.5 | 63.8 | - | - | - | - | - | - | - | - | - | 4278 | 620 | Fusarium spp. (50.0) | Aspergillus spp. (20.0) | [106] |
| Vietnam (North) | 2008 | 59.6 | 47.2 | - | 44.1 | 83.8 | 1.4 | 1.4 | - | - | - | - | 2338 | 1153 | Fusarium spp. (40.7) | Aspergillus spp. (25.9) | [30] |
| Vietnam | 1974–1982 | 23.6 | - | - | - | - | - | - | - | - | - | - | 2338 | 1219 | - | - | [107] |
| EUROPE | |||||||||||||||||
| Netherlands | 2002–2004 | 1.8 | 42.0 | - | - | - | - | - | - | 50 | 50 | - | 5823 | 156 | Candida albicans (100) | - | [108] |
| Netherlands | 2014–2017 | 14.0 | 50 | - | - | - | - | - | - | - | - | - | 5809 | 185 | - | - | [34] |
| UK (SW England) | 2006–2017 | 6.9 | 61.9 | - | - | - | - | - | - | - | - | - | 5721 | 2116 | UFF (54.2) | Candida spp. (45.8) | [109] |
| UK (London) | 2007–2014 | - | 34.8 | 47.2 | 41.4 | 11.6 | 32.1 | - | 57.1 | 22.3 | - | - | 5727 | 112 | Fusarium spp. (41.8) | Candida spp. (38.0) | [28] |
| UK (NE England) | 2008–2017 | 4.2 | 55.5 | 55.3 | 65 | - | - | - | - | - | - | - | 6113 | 407 | UFF (50.0) | Candida spp. (50.0) | [110] |
| UK – (NW England) | 2004–2015 | 7.1 | 67.4 | - | - | - | - | - | - | - | - | - | 5980 | 4229 | Candida spp. (53.2) | Fusarium spp. (25.7) | [111] |
| LATIN AMERICA AND THE CARRIBEAN | |||||||||||||||||
| Brazil (São Paulo) | 1975–2007 | 11 | 51.4 | - | - | - | - | - | - | - | - | - | 2547 | 6804 | Fusarium spp. (51.9) | Candida spp. (17.6) | [112] |
| Brazil (Uberlandia) | 2001–2004 | 56.3 | 50.8 | - | - | 55.6 | - | - | 0 | 0 | - | - | 2104 | 65 | Fusarium spp. (61.1) | Aspergillus spp. (16.7) | [113] |
| Brazil (São Paulo) | 2000–2004 | 13.8 | 63.4 | 40.7 | 80.3 | - | - | - | - | - | - | - | 2547 | 478 | Fusarium spp. (66.7) | Aspergillus spp. (10.6) | [114] |
| Brazil (São Paulo) | 2003–2010 | 25 | 82.4 | 43 | 74 | 49.3 | - | - | - | - | - | - | 2547 | 599 | Fusarium spp. (83.3) | Aspergillus spp. (16.7) | [115] |
| Mexico | 2013–2014 | 33.3 | 47.1 | - | - | - | - | - | - | - | - | - | 2161 | 51 | Fusarium spp. (44.4) | Aspergillus spp. (22.2) | [116] |
| Paraguay | 1988–2001 | 49 | 21 | - | - | - | - | - | - | - | - | - | 2814 | 660 | Acremonium spp. (40.0) | Fusarium spp. (15.0) | [117] |
| Paraguay | 2009–2011 | 72.1 | 10.4 | - | 71 | - | - | - | - | - | - | - | 2814 | 48 | Fusarium spp. (34.0) | Aspergillus spp. (16.1) | [118] |
| NORTH AMERICA | |||||||||||||||||
| USA (N California) | 1976–1999 | 8.4 | 62 | - | - | - | - | - | - | - | - | - | 4201 | 1121 | Candida spp. (30.5) | - | [119] |
| USA (Florida) | 1968–1977 | 35.8 | 44.0 | - | - | - | - | - | - | - | - | - | 2865 | 663 | Fusarium spp. (62.0) | Candida spp. (7.5%) | [120] |
| USA (Florida) | 1999–2006 | - | 29.8 | 48 | 75 | 43 | 29 | - | 44 | 8.3 | - | 7.1 | 3298 | 84 | Fusarium spp. (41.0) | Candida spp. (14.0) | [121] |
| USA (S California) | 1998–2008 | 1.4 | ^ | 56.1 | 54 | 14 | - | - | 24 | 12.7 | 1.6 | 16 | 3638 | 4651 | UFF (64.0) | Candida spp. (32.0) | [122] |
| USA (New York) | 1987–2003 | 1.2 | ^ | 47 | 35 | 11 | 7 | - | 10 | 23 | 25 | 7 | 4528 | 5083 | Candida spp. (66.0) | Aspergillus spp. (12.0) | [33] |
| OCEANIA | |||||||||||||||||
| Australia (Brisbane) | 1999–2004 | 8 | 35 | - | - | - | - | - | - | - | - | - | 3054 | 231 | Fusarium spp. most commonly isolated | - | [123] |
| Australia (Queensland) | 1996–2016 | - | ^ | 48 | 65 | - | - | - | - | - | - | - | 3054 | 215 | Fusarium spp. (33.3) | Aspergillus spp. (13.0) | [124] |
| Australia (Sydney) | 2009–2017 | - | 6 | 60 | 65 | 24 | 54 | - | 26 | 34 | - | - | 3764 | 51 | Candida spp. (33.0) | Fusarium spp. (28.0) | [125] |
| Australia (Queensland) | 2005–2015 | 6 | ^ | - | - | - | - | - | - | - | - | - | 3054 | 3182 | UFF (75.9) | Candida spp. (24.1) | [126] |
| Australia (Sydney) | 2012–2016 | 3.3 | 31 | 63.5 | 67 | 25 | 46 | - | 28 | 25 | - | 8 | 3764 | 1052 | Candida spp. (30.4) | Fusarium spp. (21.7) | [127] |
| New Zealand | 2003–2007 | 1.7 | 34.4 | - | - | - | - | - | - | - | - | - | 4097 | 265 | Fusarium spp. (66.7) | Candida spp. (33.3) | [128] |
# Confirmed fungal keratitis cases as a percentage of all culture positive microbial keratitis cases, including mixed bacterial-fungal infections. If diagnosis was based on microscopy (culture unavailable), this is a percent of all microbial keratitis cases examined by microscopy, and the results of these are given in italics. ~ Culture negative rate of all cultures taken within the study. - Data not presented. Ψ Studies that only included cases of FK and did not report the number of MK cases. ^ Studies that only included cases that were culture positive and did not report the overall culture negative rate. FK, fungal keratitis; TEM, traditional eye medication; CL, contact lens; OSD, ocular surface disease; HIV, Human Immunodeficiency Virus; DM, diabetes mellitus; MK, microbial keratitis; UFF, unspecified filamentous fungi.
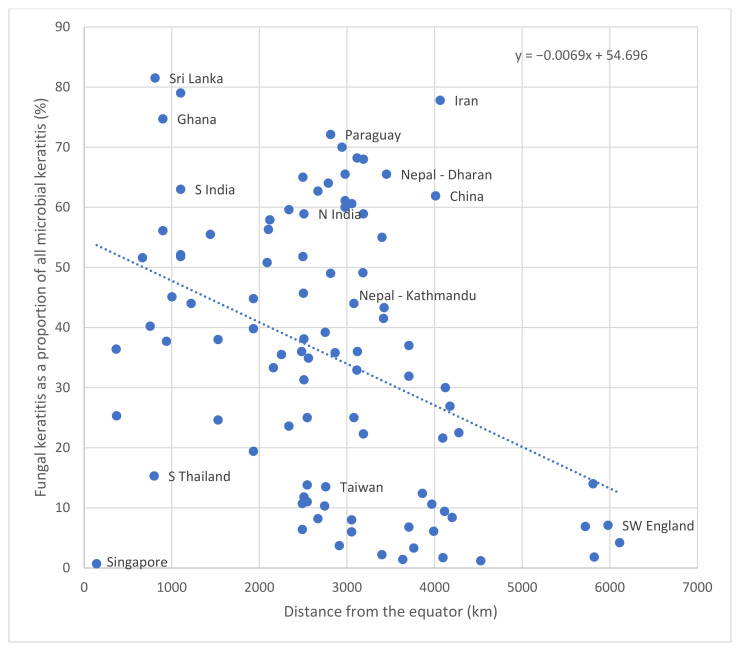
These epidemiological reviews have been updated in Table 1 and Figure 3, which plots the proportion of FK as a subset of MK against distance from the equator [3,32]. There remains a clear inverse correlation with the highest proportion of FK as a subset of MK close to the equator, with the proportion decreasing with increasing distance from the equator. There is, however, considerable variability, and a number of important outliers: Singapore for example is 143 km from the equator but FK accounts for 0.7% of MK cases suggesting that FK is not only climate dependent but also probably linked to rural and occupational risk factors.
Figure 3.
Fungal keratitis as a proportion of all culture positive microbial keratitis cases, by distance from the equator, with select locations shown, with calculated line of best fit given (dotted line, y = − 0.0069x + 54.696).
In general, filamentous fungal keratitis is a relatively rare cause of MK in temperate regions, where it is often associated with contact lens usage. Table 1 details the proportion of FK (as a subset of MK) in temperate regions such as Europe and temperate North America, which is in the range of 1.2–14.0% [33,34]. This contrasts to tropical regions, such as sub-Saharan Africa and South Asia, where the proportions are considerably higher, with rates reported as between 37.7% and 81.5%, Table 1 [35,36].
Globally, Fusarium spp. and Aspergillus spp. are the most commonly isolated fungal causes of FK and are discussed in more detail below. Note, however, that non-filamentous FK is generally more common in temperate climates, where Candida spp. is most frequently implicated.
2.3. Changing Incidence over Time
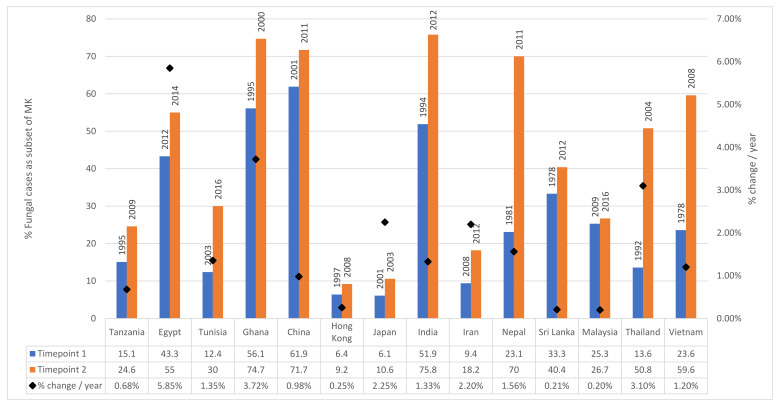
There is evidence that the proportion of MK attributable to fungi is increasing over time, particularly in low and middle-income countries (LMICs) [3]. For example, in Thailand between 1982 and 2003, the mean proportion of FK cases was 13.6% [102]. This increased to 50.8% between 2003 and 2006 [103]. Similar increases have been observed in other parts of Asia, including Nepal with an increase form 23.1% in 1981 to 70% in 2011 [94,95]. Increases have also been observed in Africa, for example in Ghana where the percentage of FK cases increased from 56.1% in 1995 to 74.7% between 1999 and 2001 [7,41]. For countries where there are multiple reports published at different time-points which we reviewed in Brown et al. [3], the relative proportion of FK is plotted against time in Figure 4.
Figure 4.
Percentage of fungal cases as a subset of MK plotted by country at two timepoints. Timepoint 1 represents the earliest year for which values were available, Timepoint 2 represents the latest year for which values are available. The years for the two studies are given as labels. The percentage change per year (calculated from the difference between the two timepoints) is plotted against the secondary y-axis.
The reason for the increase in LMICs is unclear and has not been formally studied. It could be attributable to the increased use of topical antibiotics as a primary prevention measure following corneal abrasions or as empirical treatment at a primary health level for microbial keratitis, resulting in only severe or resistant bacterial infections presenting to secondary or tertiary care along with all fungal cases. It may also be driven due to greater availability of topical antibiotics available without prescription from pharmacies. Another potential reason for this increase may include climate change: a study from Egypt in 2011 found a strong correlation between the increase in cases of fungal keratitis between 1997 and 2007 and the increase atmospheric temperature and humidity detected during the same period [129]. Other potential reasons include increased availability and use of topical steroids, increased prevalence of diabetes mellitus across the regions or simply due to improved culture and microbiology services in these countries, meaning that under-reported previous incidence is now being reported more accurately. Increased contact lens wear may also be a contributing factor, although on the whole contact-lens use remains infrequent in poorer countries across Asia and Africa.
In developed countries, there is also evidence of an increasing incidence over time, attributed to the widespread use of contact lenses, including bandage contact lenses, as well as topical steroid use [121,130]. For example, a study from a tertiary referral hospital in Florida showed an increase of over 100% in the number of cases of fungal keratitis between 1999 and 2005; contact lens wear was found to be the most common risk factor in this study [121]. A retrospective multi-centre case series from the US reported a significant increase in the incidence of non-Fusarium filamentous fungal keratitis cases between the period 2001–2004 and 2004–2007 (p < 0.0001) [130]. The number of Fusarium cases increased substantially between 2004 and 2006, when ReNu with MoistureLoc contact lens cleaning solution was on the market, and then returned to the pre-2004 incidence level for the remainder of the study [130]. For the increase described for non-Fusarium cases, the authors were unable to give a clear reason why this may have occurred; a contact lens-related product was unlikely to be responsible as the similar trends were seen for both contact lens wearers and non-contact lens wearers [130]. A more recent study from a tertiary eye hospital in the UK also reported a significant increase in the number of cases of filamentous fungal keratitis between 2007 and 2014 (p = 0.005), whilst there was no significant change in the incidence of yeast infections (p = 0.3) [28]. The same study also compared the incidence between data collected between 1994 and 2006 and data from 2007 to 2014, and found a significant increase in fungal keratitis cases (p = 0.03) [28]. All three of these studies report an increasing proportion of filamentous FK compared to yeast FK, with filamentous organisms (and in particular Fusarium spp.) now responsible for the majority of FK cases [28,121,130]. More research is required through case–control or national surveillance studies to explore reasons behind this apparent increase in incidence over time in both temperate and tropical locations.
2.4. Risk Factors
There are numerous risk factors for developing fungal keratitis, some attributable to the individual such as age, gender or pre-existing ophthalmic or systemic disease, with others dependent on extrinsic factors including the income status of the patient, occupation, contact-lens use, previous ocular surgery and region. Select risk factors from a number of epidemiological studies on fungal keratitis are presented in Table 1.
2.4.1. Age and Gender
Despite age and gender not being independent risk factors for fungal keratitis, they both affect other risk factors such as trauma, which is more common in younger men who tend to be agricultural labourers [12,131]. It is also important to note that older patients tend to have a more severe disease and worse outcome [108]. Furthermore, older patients are more likely to have predisposing systemic and ocular co-morbidities such as diabetes mellitus and ocular surface disease [108]. Patients between the ages of 20–40 make up the majority of cases [12,28,69,131]. In areas of high incidence of fungal keratitis such as south India, the majority of young patients (aged between 21 and 50) typically have fungal keratitis, compared to the majority of patients over 50 years old who typically have bacterial keratitis [68].
In SSA and India where the burden of FK is greatest, the majority of cases of fungal keratitis are reported in males [12,40,69]. Interestingly, one study from Nepal reports a higher proportion of females compared to males [93], whilst other studies from Nepal report male preponderance [91,95]. The reason for this difference is unclear; it may be due to different socioeconomic factors, health seeking behaviour or differing study methodology. In Europe and North America, there is considerable variation in the reported proportion of men with fungal keratitis [28,121].
2.4.2. Trauma
Preceding ocular trauma is a key predisposing risk factor for the development of fungal keratitis, regardless of geographical region. This is particularly true for trauma with vegetative material and trauma occurring during agricultural practices. Injury to the eye allows for a disruption to the corneal epithelium, permitting fungal pathogens to infiltrate the cornea [24,46,68,132,133,134]. Furthermore, injury with plant matter can lead to direct inoculation with fungal conidia. For regions where a fungal aetiology is the most common form of microbial keratitis such as South Asia and SSA, the reported rates of trauma range from 24 to 83% [1,76].
2.4.3. Occupation
Given the clear risk that trauma, particularly with organic material, poses to the cornea it is not surprising that occupations that carry a high risk of occupational ocular injury are associated with developing fungal keratitis. In particular, agricultural labourers and subsistence farmers are the most likely to develop fungal keratitis, reported to be between 56–74% of cases from studies in Nepal and India [12,91].
2.4.4. Diabetes Mellitus
Diabetes mellitus (DM) is of increasing public health concern globally, with the incidence increasing at an alarming rate in LMICs [135]. It is well-established that patients with DM are at an elevated risk of developing fungal infections [136], and DM is the most important systemic risk factor for developing fungal keratitis [60]. DM has also been shown to be an independent risk factor for the severity of fungal keratitis [137]. It is thought that hyperglycaemia can alter the ocular surface microenvironment including changes to the commensal organisms and enzyme action, allowing easier fungal adherence, proliferation and corneal penetration [137]. The associated reduced immune response seen in diabetes is also likely to be a significant factor in increasing host susceptibility to fungal infection [138].
2.4.5. HIV
There have been a number of studies from SSA that have suggested an association between HIV infection and fungal keratitis, following a number of case reports of fungal keratitis in AIDS patients at the start of the HIV/AIDS pandemic [139,140]. A prospective study from Tanzania in 1999 found that 81% of the patients with fungal keratitis were HIV positive, compared to only 33% in non-fungal cases (p < 0.001) [141]. Another study from Tanzania a few years later found the prevalence of HIV infection amongst MK cases to be double that of the wider population [1], although this did not directly compare the proportion of HIV positive fungal MK cases to bacterial MK cases. A more recent, nested case control study from Uganda where over 60% of MK cases were fungal, found a strong association between HIV infection and MK (OR 83.5, p = 0.02) [138,142]. There have been no studies to date looking at this association outside of SSA.
2.4.6. Traditional Eye Medicine
The use of traditional eye medicine (TEM) to treat a wide range of eye problems is commonplace in LMICs [143,144]. Most TEM contain non-sterile preparations comprising plant matter, often herbs or dried leaves, and are therefore a potential route for inoculating the cornea with microorganisms, particularly fungal pathogens [60]. Although there are no studies that have specifically looked at TEM as a risk factor for fungal keratitis, it has been found to be an independent risk factor in developing microbial keratitis in Tanzania and Uganda [20,138,142], where a fungal aetiology make up the majority of MK cases.
2.4.7. Topical Corticosteroids
It is well established that glucocorticoids are associated with an increased risk of invasive fungal infection due to the dysregulation of the patient’s immunity [145]. This holds true for prior topical steroid use, which is an independent risk factor for developing fungal keratitis [146]. Prior topical corticosteroid use is also associated with deeper corneal penetration and a worse clinical outcome [147]. Although topical corticosteroid use is associated with both yeast and filamentous fungal infections, it may be a stronger risk factor for yeast infection [28].
2.4.8. Ocular Surface Disease
Pre-existing ocular surface disease (OSD, a diverse range of disorders that lead to an abnormal ocular surface such as dry eye disease, corneal exposure, blepharitis, persistent epithelial defects or ocular surface inflammatory conditions) compromises the corneal epithelium and therefore allows fungal pathogens to invade the cornea. Furthermore, these conditions are often treated with topical corticosteroids or bandage contact lenses, which further increases the risk of developing fungal keratitis. Although OSD is more often associated with yeast infection [28], it remains a risk factor for filamentous fungal infection: a multi-centre study from the US found 29% of cases of fungal keratitis were associated with OSD, 42.6% of which were filamentous and 53.1% were yeast [148]. Cases of fungal keratitis with pre-existing OSD are less frequently reported in LMICs than in developed countries, other than in areas such as Tanzania, where OSD due to trachoma exists [149].
2.4.9. Contact Lens Usage
In industrialised countries, contact lens use constitutes the main predisposing factor for developing fungal keratitis, with studies showing between 37% and 67% of fungal cases were contact lens wearers [28,130,148]. It is important to consider, however, that it is not simply the contact lens wear itself that carries the risk-it is the type of lens used, the frequency of replacement and how the lenses are cleaned-and with what. For example, the global outbreak of Fusarium keratitis between 2005–2006 was caused by a specific contact lens cleaning solution [150]. The current proportion of patients with fungal keratitis in LMICs associated with contact lens usage is low, but this is likely to increase as these countries industrialise leading to an increased number of contact lens wearers and fewer people involved in manual agricultural labour.
2.4.10. Previous Ocular Surgery
A prior history of ocular surgery, including cataract, laser-refractive or corneal transplantation surgery, has been associated with the development of fungal keratitis in both developed and lower-middle income countries [151,152]. Yeasts are often the most commonly implicated pathogen following surgery [8]; for example, in a study from Boston, USA, yeasts accounted for 67% of post-surgical fungal infections. Of note, this group of patients had the worst outcome in terms of final visual acuity. In this study, all surgeries were a form of corneal transplantation [153]. However, it should be noted that prior ocular surgery is more likely to be a stronger risk factor for bacterial, rather than fungal, keratitis [61]; a study from Brazil found 32% of bacterial keratitis cases were associated with previous ocular surgery, compared to just 8% of fungal keratitis cases [154].
Despite intravitreal injections for retinal disease becoming the most commonly performed intraocular procedure globally [155], and corticosteroid periocular injections being used routinely for the treatment of diabetic macular oedema [156,157], there have been no cases of fungal keratitis associated with this treatment reported in the scientific literature to date. However, other complicating local fungal infections have been reported, including fungal endophthalmitis, fungal orbital abscesses and conjunctival mycetoma [158,159,160].
3. Clinical Features
It can be challenging to distinguish fungal keratitis from other forms of microbial keratitis, and even more difficult to distinguish different fungal aetiologies on clinical grounds. For example, a study whereby fifteen ophthalmologists had to predict the likely microbiological aetiology found that fungal keratitis was the most challenging to diagnose, with a sensitivity and specificity of 38% and 45%, respectively [161], whilst in a separate study using corneal photographs, corneal specialists were only able to correctly differentiate fungal and bacterial keratitis in 66% of cases [162].
There are, however, some clinical signs that have been shown to be useful predictors for filamentous fungal keratitis [2]. These are serrated margin, raised slough and colouration other than yellow. If one of these signs was present, the probability of fungal infection was 63%; if more than one of these were present the probability was 83% [2]. Without using colour as a discriminator, the probability increased to 89% [163]. Satellite lesions, which have historically been believed to be discriminatory for fungal keratitis, have been shown to occur in Acanthamoeba and fungal keratitis with the same frequency and are no more frequent in fungal than bacterial keratitis [164].
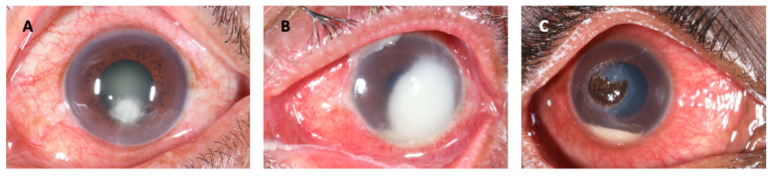
Some clinical features have been found to be more likely associated with Fusarium infection compared to Aspergillus infection. For example, Fusarium ulcers are more likely to have serrated (or “feathery”, indistinct) margins or edges and non-yellow infiltrate (Figure 5A), whilst cases of Aspergillus keratitis are more likely to have a raised surface or presence of hypopyon (Figure 5B) [67]. Another study agreed with these findings, with Aspergillus cases more likely to have a raised surface, but also presence of an endothelial plaque; these were less common in Fusarium cases [165]. Ring infiltrates were also predictive of Aspergillus. Pigmented corneal infiltrates are very likely to be caused by dematiaceous fungi; in the study by Oldenburg et al. all pigmented corneal ulcers were dematiaceous [165]. Presence of a raised profile is also associated with dematiaceous fungi such as Curvularia spp. (Figure 5C) [15,165,166].
Figure 5.
Differing clinical phenotypes of filamentous fungal keratitis depending on the fungal organism. (A): Fusarium sp. Note the serrated or feathery margins, satellite lesions, non-yellow infiltrate and lack of hypopyon. (B): Aspergillus sp. Note less obviously serrated margins compared to (A), raised profile, hypopyon. (C): Curvularia sp. Note the raised, pigmented infiltrate, in addition to the hypopyon.
Despite the above clinical signs being more frequently associated with fungal keratitis, other studies have shown a lack of statistical significance [161,164]. This adds to the challenge to accurately and confidently diagnose fungal keratitis on clinical grounds alone. Compounding this is the pleomorphic presentation as a result of late presentation, prior use of topical steroids or traditional eye remedies that unfortunately often occurs frequently in the regions where fungal keratitis is most prevalent [18].
Acutely, fungal keratitis typically leads to reduced vision due to the presence of the infection and inflammation in the cornea, blurring the vision. With treatment, the vision can improve, although often the patient is left with worse vision than they had previously due to the development of corneal scarring. At present there is no medical treatment to reverse this scarring process. Rigid contact lenses can help to a certain amount by improving the vision if there is scarring. Alternative options for severe scarring include corneal graft surgery, but this can be a technically challenging procedure and is often not available in places most in need. Fungal keratitis should therefore be considered a potentially blinding condition.
4. Making the Diagnosis
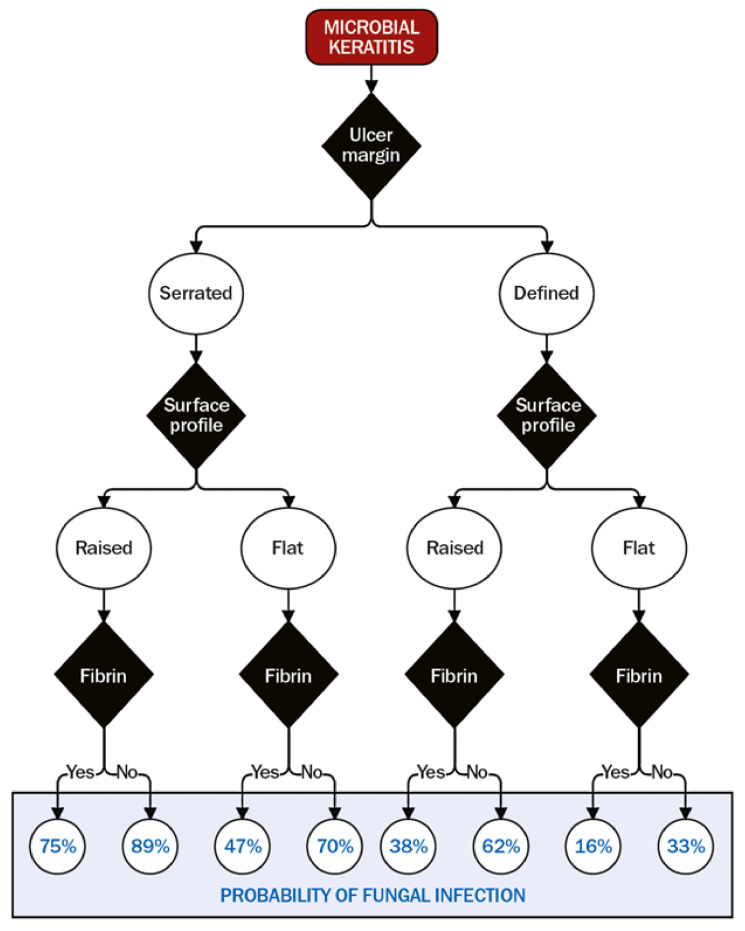
Even with all diagnostic modalities available, diagnosing fungal keratitis can be challenging. The burden of fungal keratitis globally is predominantly in low resource settings, where access to advanced diagnostic techniques is very limited. In these locations, diagnoses are still often made on clinical grounds alone (with the associated limitations as discussed above), sometimes supported by basic microscopy. However, an algorithm has been developed that uses the specific features that were systematically examined from a large case series from Ghana [163], and calculating a probability score that the microbial keratitis is fungal in aetiology, Figure 6 [2]. This can aid clinicians working in these locations and indicate the likelihood of fungal versus bacterial infection. Where diagnostic microbiology is available, however, it is best practice to rely on the results of this rather than these clinical signs, as the presence of fungal hyphae in corneal tissue is diagnostic [163].
Figure 6.
Algorithm for determining the probability of fungal keratitis [163]. The black diamonds are decision points about three clinical features: ulcer/infiltrate margin, surface profile, and anterior chamber fibrin. These probabilities are based on data presented in Thomas et al. [2]. This is reproduced here from [163] with permission under a CC BY-NC 4.0 license (https://creativecommons.org/licenses/by-nc/4.0/, accessed on 16 March 2021).
4.1. Laboratory
4.1.1. Microscopy and Culture
Infected corneal tissue/material is gently removed from the surface of the anaesthetised cornea using a sterile needle or scalpel blade and transferred to microscope slides and a range of solid and liquid phase culture media, including blood agars and Sabouraud dextrose agar [13].
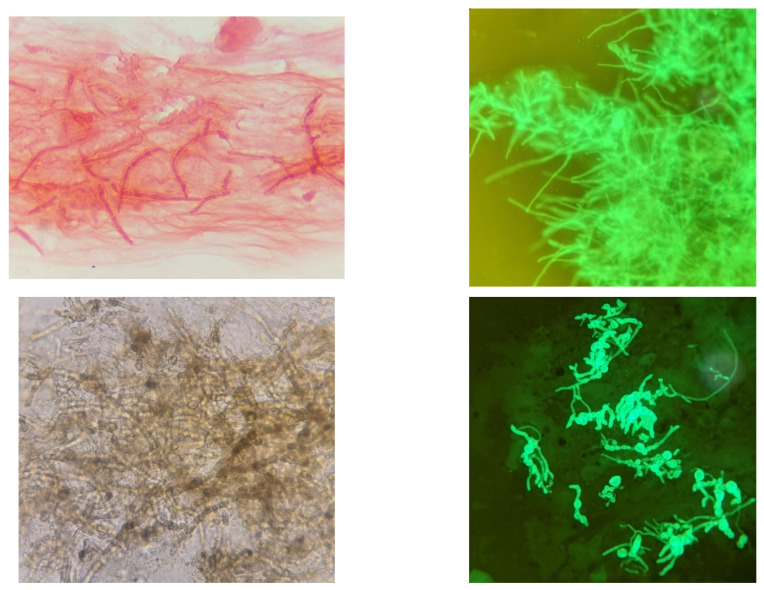
Microscopy is still regarded as the gold standard in laboratory diagnosis of fungal keratitis and is often the only diagnostic tool available in settings where the incidence of FK is highest. The presence of fungal hyphae in corneal scrape preparations is always significant and are clearly visible using Gram stain, KOH, CFW or lactophenol cotton blue (LPCB, Figure 7) [13,167]. The ubiquitous distribution and environmental reservoirs of fungal ocular pathogens mean that positive microscopy is critical to exclude contaminants.
Figure 7.
Microscopic appearance of filamentous fungal hyphae in corneal tissue (corneal scrape specimens) using different staining techniques. Clockwise from top-left: Fungal hyphae in Gram-stained corneal smear (magnification 1000x, oil immersion); fungal hyphae visible with CFW, Curvularia sp. stained with CFW, pigmented hyphae (Curvularia sp.) in a KOH preparation (magnification 400x). These images were taken using an afocal photography technique; the camera zoom was used for additional magnification.
Culture positivity rates reported vary greatly between institutions and settings [32]. Low culture positivity is attributable to the very small size of the specimen, use of antimicrobial agents by the patient prior to presentation, the quality of the corneal scrape and incorrect inoculation of media, in addition to laboratory factors [168,169]. Subculture for identification to species level may require the use of plant-based agars, most commonly examples are potato dextrose and cornmeal agars, in addition to diurnal culture methods to induce sporulation for the purpose of identification.
4.1.2. Molecular Techniques
Rapid diagnosis to inform prompt and appropriate treatment is critical to the successful clinical management of fungal keratitis. Development of molecular techniques, such as pan-fungal 16S rRNA PCR, have been favoured due to the very small size of specimen. PCR has emerged as both sensitive and specific test for the diagnosing fungal keratitis, benefiting from a high positive detection rate [14,170,171,172,173,174], with some evidence that it may be more sensitive than the traditional microbiological techniques of microscopy and culture [175]. However, the accuracy of PCR to diagnose fungal keratitis is dependent on adequate sampling and the primers used. Recent promising developments include evaluation of ITS primers and multiplex PCR for direct identification of fungal species from corneal tissue demonstrating high sensitivity and specificity [14].
An area currently under research that could have important therapeutic and prognostic implications is the development of genotyping methods for rapid species identification. This has shown promise for the rapid detection of Fusarium solani using a specific restriction site in the EF-1a gene [176]. Fusarium solani has been shown to have a worse prognosis, including higher voriconazole resistance, compared to other Fusarium species [177]. If rapid species identification using molecular methods were readily available, tailored treatment could be started earlier, thereby potentially improving the overall prognosis. However, the expense of molecular diagnostic methods precludes their use in many settings where FK is prevalent and further highlights the need for low-cost, point of care diagnostic tests which could be made more widely available.
Matrix-assisted laser desorption/ionization time of flight-mass spectrometry (MALDI-ToF MS) is a relatively novel rapid and reliable, high-throughput tool for the identification of microorganisms, allowing the identification of fungal isolates within minutes [178]. It also benefits from a fast turnaround time and low cost for consumables, making it potentially relevant to tertiary referral centres in LMICs where the burden of fungal keratitis is greatest. However, there are no published studies comparing MALDI-ToF MS to conventional methods for diagnosing fungal keratitis. One study has compared MALDI-ToF MS to conventional morphology and PCR sequencing which included one sample of Aspergillus keratitis which showed a good level of agreement between the different modalities [179]. There are a number of case reports and case series that explain how it is a useful tool in rapidly diagnosing FK, particularly for rare or unusual organisms [180,181,182,183,184].
4.1.3. In Vivo Confocal Microscopy
Fungal culture can have a relatively low yield-studies report a sensitivity of up to 50% [185,186]. Growth may be slow; several days, even weeks; and identification complicated due to poor sporulation in vitro. Microscopy is very helpful but can have its limitations, particularly given the infection is often deep within the stroma making yield from corneal scrapings poor [167]. Early treatment (and therefore diagnosis) is crucial in treating FK appropriately and preventing the blinding complications associated with it [1]. A potential answer to these challenges comes in the form of in vivo confocal microscopy (IVCM), which allows for real-time imaging of the cornea down to the cellular and micro-structural level. It is able to detect the presence of fungal hyphae, Figure 8 [185,186].
Figure 8.
In vivo confocal microscopy of fungal keratitis (A) clinical image; (B) In vivo confocal microscopy scan of the same cornea showing extensive, branching fungal hyphae. Scale bar 100 μm. (C) Light microscopy demonstrated septate fungal hyphae, visible on Gram staining (magnification 1000x, oil immersion); (D) and KOH preparation (magnification 400x). Images (C,D) were taken using an afocal photography technique; the camera zoom was used for additional magnification.
IVCM can be used in the diagnosis of FK as well as in monitoring the response to treatment [185,186,187,188,189]. Chidambaram et al. reported a sensitivity of 79.1–86.8% and specificity of 73.7–85.9%, whilst Hau et al. correctly identified fungal infection 8.3–41.2% of the time [185,190]. However, it cannot reliably differentiate the organism causing the infection, meaning culture remains the gold standard for identification.
4.1.4. Systematic Approach to Making a Diagnosis
With numerous tools available to aid in the diagnosis of fungal keratitis, it is useful to have a systemic approach. This will depend on what tools are available; as mentioned above, there are unfortunately many locations globally where access to these investigations are unavailable. In these locations, the algorithm in Figure 6 should be used. If all tests are available, we recommend following the algorithm given in Figure 9. A high index of suspicion is an important first step to diagnosing fungal infections: if a patient presents with a history of vegetative trauma, particularly if they are in a subtropical or tropical location, then fungal keratitis needs to be ruled out on the outset. As described above, if clinical signs including feathery or serrated margins, a raised profile or satellite lesions are present, then this should raise the probability of fungal keratitis. At this point a baseline corneal photograph is useful for future reference to guide future response, although staining with fluorescein should be delayed until after the PCR sample is taken to avoid theoretical interference with primers.
Figure 9.
Algorithm for diagnosing fungal keratitis.
In these cases, the first investigation to be performed is IVCM. This should be performed before taking a corneal scrape, as taking a corneal scrape can reduce the image quality obtained by IVCM and therefore the sensitivity. Evidence of fungal hyphae are diagnostic. Ideally, the cornea should be anaesthetised with preservative free topical 0.5% proxymetacaine hydrochloride, as this is less likely to interfere with culture or PCR results. The subsequent step would be to take corneal scrapes for microscopy and culture, as described in detail in Section 4.1.1. It should be noted that a fresh sterile needle should be used for each slide or culture media being inoculated. Finally, a sample for PCR should be taken as a corneal swab. At this point, a second corneal photograph could be taken using a blue filter and topical fluorescein staining to demonstrate the size of the epithelial defect.
5. Management
Most cases of filamentous fungal keratitis are challenging to treat, requiring long-term therapy with topical, and occasionally systemic, antifungal agents. However, even when intensive appropriate topical therapy is initiated, infections frequently progress relentlessly to perforation and loss of the eye in ~25% of cases [1,22,24]. Surgery in the form of therapeutic penetrating keratoplasty (TPK) is often required. There are a limited number of antifungals available with action against fungal keratitis, of which there are four main groups: imidazoles, triazoles, polyenes and fluorinated pyrimidines. These may be available topically, orally or by intravenous injection. Subconjunctival injection or corneal stromal injection may also be given [27,28]. The current gold standard treatment for filamentous fungal keratitis is topical natamycin 5%.
There have been a number of clinical trials comparing various treatment options for fungal keratitis over the last few decades, which have been reviewed systematically [191,192]. Natamycin (NATA), which was approved in the 1960s by the FDA for FK, has been compared to a number of newer agents. In a randomised controlled superiority trial of 116 patients from India, there was no statistical difference found between econazole 2% or natamycin 5% [26]. Voriconazole, a newer generation triazole agent, was subsequently introduced to the market and an initial prospective RCT showed no significant difference between the groups in terms of primary outcome measure (time to healing of epithelial defect). The authors therefore concluded that voriconazole was “an effective and well-tolerated drug” and larger trials were warranted to demonstrate superiority [193]. Meanwhile, Prajna et al. also compared topical natamycin to voriconazole in a therapeutic exploratory randomised clinical trial; 120 patients were randomised to either natamycin or voriconazole and either had repeated corneal epithelial scraping or not. The study also concluded that there was no significant difference between groups for the primary outcome of visual acuity at three months, with a non-significant trend favouring voriconazole. Incidentally, repeated scraping was associated with a worse outcome, although again this was non-significant (p = 0.06) [194]. To investigate this, the Mycotic Ulcer Treatment Trials (MUTT) were developed [22,24]. In MUTT1, topical natamycin 5% was compared to topical voriconazole 1% in a trial that was due to recruit 368 patients but was terminated earlier on recommendation by the trial Data Safety and Monitoring Committee, as the number of perforations in the voriconazole group were significantly higher than in the natamycin group (34 vs. 18 perforations, p = 0.02; 323 recruited). Vision was −0.18 logMAR better at three months in the natamycin group compared to the voriconazole group (p = 0.006) [24]. Sharma et al. also found natamycin to be superior to voriconazole in a more recent randomised controlled trial [133].
MUTT 2 compared oral voriconazole with placebo with all patients receiving both natamycin and topical voriconazole. There was no difference in primary outcome (perforation rate or corneal graft) within three months between groups, with more side effects reported in the voriconazole group (p < 0.001). The study therefore concluded that there was no benefit in adding oral voriconazole in the treatment of severe filamentous fungal corneal infections [22]. As a result of these studies, topical natamycin 5% without oral voriconazole remains the recommended first-line agent for filamentous FK. MUTT also investigated the susceptibility of different fungal species to either medication, and found that Aspergillus spp. were least susceptible to natamycin, whilst Fusarium spp. were least susceptible to voriconazole. In the study population where MUTT was conducted, Fusarium spp. was the most commonly isolated organism. However, many patients continue to progress despite treatment with natamycin 5%, meaning that alternative treatment strategies are required. In addition, natamycin 5% is difficult to formulate, expensive and often unavailable in countries where it is required, despite being on the WHO Essential Medicines List. Chlorhexidine (CHX) is an antiseptic agent, with both antibacterial and antifungal properties. It is a widely used broad-spectrum biocide, killing microorganisms through cell membrane disruption. Pilot studies from the 1990s have suggested it as a potential alternative to natamycin 5% [23,25,191,195], and a randomised controlled trial comparing natamycin 5% to chlorhexidine 0.2% for fungal keratitis is currently underway [196].
Despite MUTT 2 showing no benefit for adjunctive oral voriconazole, some ophthalmologists recommend systemic, oral therapy in severe cases of fungal keratitis, particularly if the infiltrate is larger than 5 mm or deeper than 50% corneal thickness [197]. If oral voriconazole is not available, alternative options include ketoconazole or itraconazole. A randomised controlled trial comparing oral ketoconazole with oral voriconazole found similar healing times between groups, although patients treated with voriconazole achieved a significantly smaller scar size and better final vision [198]. It is, however, important to remember that these oral anti-fungal agents can have serious adverse effects, particularly in terms of hepatotoxicity; they should be used cautiously with correct dosing depending on the patient’s weight, together with liver function monitoring. Oral voriconazole has also been associated with treatment-related visual adverse events including blurred vision and colour vision changes [199], although these have been found to be non-progressive and reversible [199].
In addition to topical treatment, injections of antifungals into the corneal stroma have also been performed in severe disease [200,201]. This was investigated in a randomised controlled trial of 40 patients who were not responding to natamycin 5%, and compared topical voriconazole 1% to intrastromal injections of voriconazole 50 μg/0.1 mL. The authors found that patients receiving topical voriconazole had a mean BSCVA of −0.397 better than the intrastromal injection group (p = 0.008). Additionally, 19/20 patients receiving topical voriconazole healed with therapy. The authors concluded that topical, as opposed to intrastromal, voriconazole may be beneficial in addition to natamycin in recalcitrant disease not-responding to natamycin 5% monotherapy [202]. There is therefore no evidence indicating a benefit from intrastromal injections of voriconazole.
More recently, corneal collagen cross-linking (CXL) has been considered for the treatment of FK [203]. However, the evidence for this is limited with heterogenous protocols and conflicting results [204]. Indeed, three prospective randomised controlled trials have found no benefit of CXL over standard-of-care and, of concern, potentially worse outcomes in the CXL group [205,206].
The last intervention option for treating FK is surgical in the form of corneal transplantation or TPK. For large corneal perforations, TPK is the only option left to salvage the eye by restoring normal anatomy, with the added advantage of removing the site of the infection [207]. Unfortunately, however, recurrence of fungal infection in the graft often occurs, particularly in the presence of a hypopyon, corneal perforation, larger infiltrates and limbal involvement [208,209]. There is therefore a degree of debate around whether to perform TPK earlier in the course of the disease, rather than waiting for the eye to perforate, when future graft failure becomes more likely [207]. A recent retrospective study from India suggests that surgical intervention should be considered early in recalcitrant cases to improve the chances of graft survival [209]. However, TPK is a relatively technical procedure requiring an appropriately trained and experienced surgeon. Lack of donor graft availability is a significant challenge in large parts of the world where the need is greatest, in part due to legal and cultural barriers.
6. Ocular Mycology
6.1. Fusarium spp.
Fusarium keratitis is a sight-threatening condition that often affects otherwise healthy individuals during their most economically active years of life [1,210]. The infection is very challenging to treat due to resistance of Fusarium spp. to many antifungals. Without adequate treatment, infection progresses relentlessly to perforation [1,22,24], endophthalmitis [211], and ultimately loss of the eye in the form of enucleation [151,212].
Epidemiology
Fusarium keratitis is most common in tropical and sub-tropical locations [13]. The main risk factor for developing infection in this setting, in common with fungal keratitis with filamentous fungal aetiology, is trauma, typically with vegetative matter, resulting in a defect in the corneal epithelium [24,46,68,132,133,134]. This either directly inoculates the cornea with fungal conidia or allows subsequent fungal entry to the corneal stroma. There is a history of preceding trauma in 40–60% of cases [60,70]. Other risk factors include previous ocular surgery [151,152], ocular surface disease, previous use of corticosteroids [146], contact lens use [213], immunosuppression [146], or use of traditional eye medicines [142]. Fungal keratitis caused by Fusarium spp. accounts for between 42% and 52.5% of all cases of FK, depending on geographical location [14,28]. It typically occurs in young healthy males who are undertaking agricultural work [13].
However, Fusarium keratitis is not confined to the tropics. In tandem with the increased use of disposable planned-replacement contact lenses, the numbers of Fusarium keratitis reported in temperate countries with developed economies has also risen. As discussed above, between 2005 and 2006 there was an outbreak of contact lens-related Fusarium keratitis due to the contact lens cleaning solution “ReNu with MoisutureLoc” (Bausch & Lomb, Bridgewater, New Jersey, USA) [150]. The highest number of cases was seen in the Far East, with Hong Kong reporting 33 cases between January 2005 and May 2006 [214], and Singapore reporting Fusarium keratitis in 68 eyes of 66 patients between March 2005 and May 2006 [215]. Given the high prevalence of myopia in these industrialised locations and widespread, increasing use of soft contact lenses [216], it is not unsurprising that these countries saw the highest incidence during this outbreak. However, other countries including the USA (164 cases 2005–2006), [217] and European Nations reported a similar peak between 2005 and 2006 [27,218,219,220].
Irrespective of the ReNu outbreak, there appears to be an increasing incidence in Fusarium keratitis in temperate climates. In the UK, a London tertiary ophthalmic hospital reported an increase in the proportion of Fusarium spp. isolates of all fungal keratitis cases from 18% between 1994 and 2006 to 42% between 2007 and 2014 [28]. Contact-lens use was found to be a significant risk factor (OR 4.35, 95% CI 1.50–12.7). In Germany, the national reference laboratory have reported 15 cases of Fusarium keratitis over 2 years between January 2014 and December 2015 [10]. The majority of these were contact lens wearers (73.3%) with no cases reporting preceding trauma or immunosuppression. However, as the reference laboratory only commenced operations in 2014, comparisons to previous results was not possible. Similar reports of a rising incidence of Fusarium keratitis have been described from the Netherlands [11], which also finds contact lens use as a significant risk factor in this setting, as well as in Denmark where 9/10 cases were attributable to filamentous fungi between 2010–13, of which 6/9 were confirmed as Fusarium spp. [146]. Unlike Fusarium keratitis seen in tropical countries, in temperate climates it is more common in females, likely reflecting the demographics of contact lens use [10,11,28].
6.2. Aspergillus sp.
Aspergillus spp. are the second most frequently reported causative organisms of fungal keratitis globally. Several species have been associated with corneal infection, the commonest being A. flavus, A. fumigatus, A. niger and A. terreus [7,14,221]. Corneal trauma with vegetative or organic matter is the predominant risk factor reported [76]. The pattern of disease is similar to that seen with Fusarium keratitis, but in vitro susceptibility data for ocular isolates of Aspergillus spp. demonstrates lower MICs compared to antifungal susceptibility profiles for Fusarium spp. [221] although visual outcome is also determined by other factors such as the severity of the infection on presentation in clinic; deep lesions have a poorer prognosis [13].
Epidemiology
Mycotic keratitis due to Aspergillus spp. also predominates in tropical and sub-tropical latitudes [222]. However, within these regions and within countries there is climatic variation-wet, dry and semi-arid climes. Aspergillus corneal infections predominate in drier environments in sub-tropical latitudes, for example, in northern Ghana, where the environment is dry, with seasonal harmattan winds facilitating dispersal of airborne conidia; the more temperate areas of West Bengal and in northern India where the number of infections due to aspergilli eclipsed those caused by Fusarium spp., including in fungal keratitis in children [7,62,65,76,223].
6.3. Dematiaceous Fungi
The most commonly reported ocular pathogens after Fusarium spp. and Aspergillus spp. are representatives from the dematiaceous moulds, a diverse group of fungi characterised by their ability to produce melanin, which has long been regarded as a unique pathogenic advantage [224]. Although ubiquitous, this group of moulds are not common causes of disease in humans, but many species are plant pathogens of agricultural importance, colonising spoil and vegetation. The link with occupational risk factors and ocular trauma is as described for other types of mycotic keratitis.
Melanin pigmentation of hyphae and conidia within this heterogenous group may be useful in rapid diagnosis in this form of phaeohyphomycosis. Darkly pigmented infected corneal tissue may be obvious on direct observation of the eye, but this is not a common clinical presentation. There are few instances where morphological appearance of fungi in vivo are specific, however, direct microscopy of corneal tissue infected with some dematiaceous species may reveal pigmented fungal elements, including swollen, irregular hyphae and yeast-like structures, which are characteristic in appearance. Some species are weakly pigmented and may appear hyaline [225,226].
Curvularia spp. are the most commonly reported of the dematiaceous fungi globally. Many other genera have also been reported to cause keratitis including Bipolaris spp., Exserohilum spp., Alternaria spp., Ulocladium spp., Lasidoplodia theobromae and Colletotrichum spp. (Figure 10) [7,15,17,32,227].
Figure 10.
Examples of dematiaceous fungal genera isolated from cases of fungal keratitis stained with LPCB. Clockwise from top-left: Curvularia sp., Bipolaris sp. (magnification 400x); Alternaria sp., Exserohilum sp. (magnification x1000, oil immersion). These images were taken using an afocal photography technique; the camera zoom was used for additional magnification.
Epidemiology
Ocular infections due to the dematiaceous fungi have been reported from every continent. Although more commonly reported from regions with warmer, humid seasonality members of this heterogenous group have also been reported from semi-arid regions [17,228,229,230,231,232]. In the terai of Nepal, the country with the highest documented incidence of fungal keratitis in the world, dematiaceous fungi such as Curvularia spp. are more frequently isolated than Fusarium spp. and Aspergillus spp., (personal experience & comms). Curvularia spp. were the most common filamentous fungi in a ten-year review of mycotic keratitis at a tertiary referral centre in North Carolina, South-eastern USA [122]. In order to understand regional patterns of causality it is important to reflect on the environmental reservoirs of many of these species, for example, Curvularia spp., which are pathogens of rice, maize, wheat, cassava, sorghum and grasses; common cash and subsistence crops in regions with a high incidence of fungal keratitis. To date there have been no phylogenetic studies comparing clinical (ocular) and environmental dematiaceous fungal isolates.
6.4. Other Filamentous Fungi
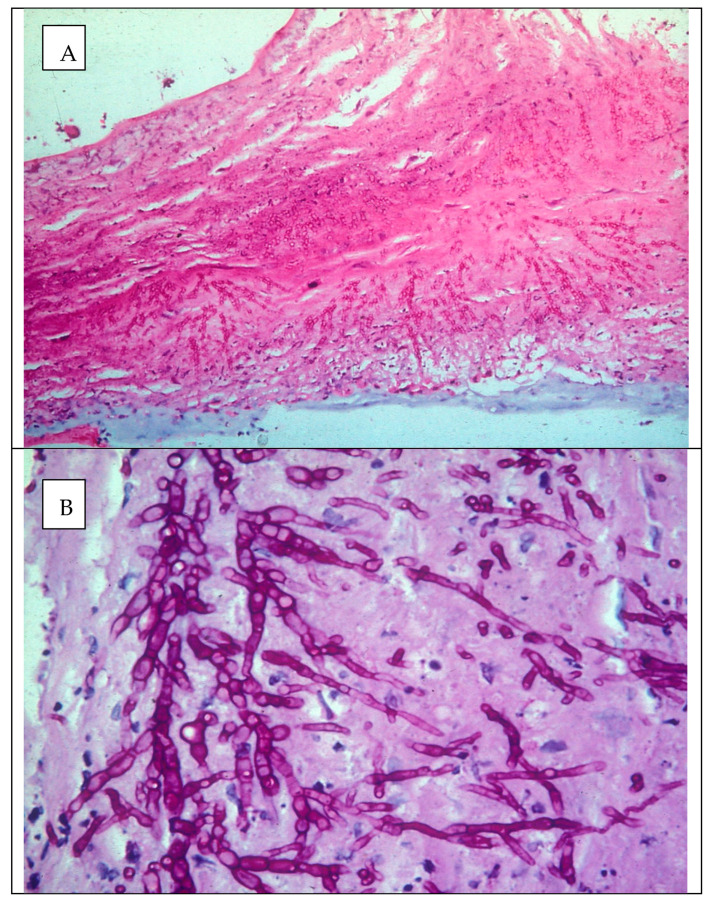
As previously mentioned there are more than 100 species of fungi reported as causing mycotic keratitis [13]. Other filamentous fungi less frequently reported include: Sarocladium spp., Penicillium spp., Paecilomyces spp., Scedosporium spp. and Purepureocillum lilacinum. Some of the least favourable therapeutic outcomes documented are mycotic keratitis cases due to Scedosporium spp., well characterised for their resistance to antifungal agents (Figure 11).
Figure 11.
Histology section of a corneal button infected with Scedosporium apiospermum stained with H&E/PAS (A—magnification ×100, B—magnification ×1000, oil immersion).
7. Unsolved Problems and Future Work
Fungal keratitis is a disease that disproportionately affects poor people living in some of the world’s poorest countries. There is evidence to suggest that the incidence of fungal keratitis is increasing globally. Unfortunately, for most people who have FK, access to appropriate diagnosis and treatment is very limited. To help address this apparent “neglect”, there has been a recent push for fungal keratitis, as part of microbial keratitis, to be included in the World Health Organization’s list of neglected tropical diseases (NTDs), which would help focus global attention and funding [233]. As it stands, there are a number of key areas where there are challenges and significant unmet needs, where addressing these may greatly reduce the morbidity associated with FK:
Use of traditional eye medicine and inappropriate use of conventional medicines [1,19,20]
Limited relevant ophthalmic formal training of front-line health workers [1]
Limited or no access to appropriate diagnostic investigations
Topical antifungals are frequently unavailable [1]
FK is challenging to treat, and treatment failure is common [1,22,23,24,25,26]
Sight-loss from severe microbial keratitis (MK) in LMIC results from a combination of these factors. In response, current and future work is focused on addressing these areas. Research projects are underway to improve the understanding of patients’ health-seeking behaviour, such as that recently completed in Uganda [18,138,142]. Linked to this is implementation research into primary preventative measures, specifically how to prevent ocular injuries from occurring in the first place. Secondary preventative measures, for example antibiotic or antiseptic prophylaxis following corneal trauma, need to be enhanced. Several studies from South Asia found early antibiotic prophylaxis of uninfected corneal abrasions with chloramphenicol ointment reduced risk of MK developing [210,234,235,236]. However, these did not address early management of established MK presenting in the community, which still occurred in considerable numbers [210]. A suitable alternative to prevent fungal as opposed to bacterial keratitis also needs to be considered. Enhanced training of primary health workers, in addition with early referral, could potentially improve outcome.
To enhance the ability to accurately diagnose MK, microbiology laboratory capacity must be improved. This can be aided by the development of affordable point of care tests. As discussed above, the fungal species responsible (and therefore treatment susceptibility) varies with geographical location-and time-so it is essential for clinicians to be aware of the local aetiology to adjust treatment strategies. Continued microbiological surveillance is required to ensure that a change in aetiology is detected in good time.
Given that a large proportion of FK is attributed to trauma with vegetative material, and many fungal species causing FK are in fact plant pathogens, phylogenetic studies should be used to determine which plant pathogens are causing disease, specifically assessing their virulence and pathogenicity.
Regarding treatment, despite natamycin being added to the WHO Essential Medicines List in 2017 which was a huge step forward, there are still frequent shortages and in many countries is still not licensed or available [1]. When it is available, it is often too expensive for most people. Accessibility to the current gold standard treatment needs to be improved and the evidence-base into alternative treatments, such as chlorhexidine 0.2%, needs to be widened. Randomised controlled trials are currently underway to assess its efficacy [196].
8. Conclusions
Mycotic keratitis, particularly when caused by filamentous fungi, is a global problem. The incidence and main risk factors vary with geographical location and level of economic development; in tropical LMICs, trauma with organic material is the main risk factor whilst in wealthier, temperate countries contact lens use or ocular surface disease are the predominant associations. There is emerging evidence that the incidence is increasing worldwide, possibly linked in part to climate change, with other factors at play; further research is required to explore this in detail. Unfortunately, mycotic keratitis remains a severe, sight-threatening condition for millions.
Acknowledgments
The authors would like to thank the staff of Sagarmatha Choudhary Eye Hospital (SCEH), Nepal for their assistance with providing the clinical and microbiological photos, as well as those of confocal microscopy: in particular we would like to thank Sandip Das, Reena Yadav, Pankaj Choudhary, Rabi Sah and Kamlesh Yadav. Histology images (Figure 11) were reproduced with kind permission from Melville Matheson (Moorfields Eye Hospital).
Author Contributions
Searched the literature: J.J.H. and A.L.; Drafted the manuscript: J.J.H. and A.L.; Critically revised the manuscript: J.J.H., A.L. and M.J.B.; Conceptualization: J.J.H., A.L. and M.J.B.; Funding acquisition: M.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through a Senior Research Fellowship to M.J.B. from the Wellcome Trust (207472/Z/17/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burton M.J., Pithuwa J., Okello E., Afwamba I., Onyango J.J., Oates F., Chevallier C., Hall A.B. Microbial Keratitis in East Africa: Why are the Outcomes so Poor? Ophthalmic Epidemiol. 2011;18:158–163. doi: 10.3109/09286586.2011.595041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas P.A., Leck A.K., Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br. J. Ophthalmol. 2005;89:1554–1558. doi: 10.1136/bjo.2005.076315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown L., Leck A.K., Gichangi M., Burton M.J., Denning D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30448-5. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan M., Gonzales C.A., George C., Cevallos V., Mascarenhas J.M., Asokan B., Wilkins J., Smolin G., Whitcher J.P. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br. J. Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcher J.P., Srinivasan M., Upadhyay M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 6.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leck A.K., Thomas P.A., Hagan M., Kaliamurthy J., Ackuaku E., John M., Newman M.J., Codjoe F.S., Opintan J.A., Kalavathy C.M., et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br. J. Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao G.L., Ling J., Wong T., Yeung S.N., Iovieno A. Candida Keratitis: Epidemiology, Management, and Clinical Outcomes. Cornea. 2020;39:801–805. doi: 10.1097/ICO.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 9.Ahearn D.G., Zhang S., Stulting R.D., Schwam B.L., Simmons R.B., Ward M.A., Pierce G.E., Crow S.A., Jr. Fusarium keratitis and contact lens wear: Facts and speculations. Med. Mycol. 2008;46:397–410. doi: 10.1080/13693780801961352. [DOI] [PubMed] [Google Scholar]
- 10.Walther G., Stasch S., Kaerger K., Hamprecht A., Roth M., Cornely O.A., Geerling G., Mackenzie C.R., Kurzai O., von Lilienfeld-Toal M. Fusarium Keratitis in Germany. J. Clin. Microbiol. 2017;55:2983–2995. doi: 10.1128/JCM.00649-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira Dos Santos C., Kolwijck E., van Rooij J., Stoutenbeek R., Visser N., Cheng Y.Y., Santana N.T.Y., Verweij P.E., Eggink C.A. Epidemiology and Clinical Management of Fusarium keratitis in the Netherlands, 2005–2016. Front. Cell Infect. Microbiol. 2020;10:133. doi: 10.3389/fcimb.2020.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deorukhkar S., Katiyar R., Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: A five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singap. Med. J. 2012;53:264–267. [PubMed] [Google Scholar]
- 13.Thomas P.A., Kaliamurthy J. Mycotic keratitis: Epidemiology, diagnosis and management. Clin. Microbiol. Infect. 2013;19:210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 14.Manikandan P., Abdel-Hadi A., Randhir Babu Singh Y., Revathi R., Anita R., Banawas S., Bin Dukhyil A.A., Alshehri B., Shobana C.S., Panneer Selvam K., et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. Biomed. Res. Int. 2019;2019:6395840. doi: 10.1155/2019/6395840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg P., Gopinathan U., Choudhary K., Rao G.N. Keratomycosis: Clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107:574–580. doi: 10.1016/S0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A., Kaur H., Gupta A., Singh S., Rudramurthy S.M., Gupta S., Chakrabarti A. Emerging Dematiaceous and Hyaline Fungi Causing Keratitis in a Tertiary Care Centre From North India. Cornea. 2020;39:868–876. doi: 10.1097/ICO.0000000000002295. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A., Khurana A., Sharma M., Chauhan L. Causative fungi and treatment outcome of dematiaceous fungal keratitis in North India. Indian J. Ophthalmol. 2019;67:1048–1053. doi: 10.4103/ijo.IJO_1612_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arunga S., Kintoki G.M., Gichuhi S., Onyango J., Newton R., Leck A., Macleod D., Hu V.H., Burton M.J. Delay Along the Care Seeking Journey of Patients with Microbial Keratitis in Uganda. Ophthalmic Epidemiol. 2019;26:311–320. doi: 10.1080/09286586.2019.1616775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courtright P., Lewallen S., Kanjaloti S., Divala D.J. Traditional eye medicine use among patients with corneal disease in rural Malawi. Br. J. Ophthalmol. 1994;78:810–812. doi: 10.1136/bjo.78.11.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yorston D., Foster A. Traditional eye medicines and corneal ulceration in Tanzania. J. Trop. Med. Hyg. 1994;97:211–214. [PubMed] [Google Scholar]
- 21.Burn H., Puri L., Roshan A., Singh S.K., Burton M.J. Primary Eye Care in Eastern Nepal. Ophthalmic Epidemiol. 2019;22:1–12. doi: 10.1080/09286586.2019.1702217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prajna N.V., Krishnan T., Rajaraman R., Patel S., Srinivasan M., Das M., Ray K.J., O’Brien K.S., Oldenburg C.E., McLeod S.D., et al. Effect of Oral Voriconazole on Fungal Keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): A Randomized Clinical Trial. JAMA Ophthalmol. 2016;134:1365–1372. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman M.R., Johnson G.J., Husain R., Howlader S.A., Minassian D.C. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br. J. Ophthalmol. 1998;82:919–925. doi: 10.1136/bjo.82.8.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prajna N.V., Krishnan T., Mascarenhas J., Rajaraman R., Prajna L., Srinivasan M., Raghavan A., Oldenburg C.E., Ray K.J., Zegans M.E., et al. The mycotic ulcer treatment trial: A randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131:422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M.R., Minassian D.C., Srinivasan M., Martin M.J., Johnson G.J. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiol. 1997;4:141–149. doi: 10.3109/09286589709115721. [DOI] [PubMed] [Google Scholar]
- 26.Prajna N.V., John R.K., Nirmalan P.K., Lalitha P., Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br. J. Ophthalmol. 2003;87:1235–1237. doi: 10.1136/bjo.87.10.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galarreta D.J., Tuft S.J., Ramsay A., Dart J.K.G. Fungal keratitis in London: Microbiological and clinical evaluation. Cornea. 2007;26:1082–1086. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 28.Ong H.S., Fung S.S.M., Macleod D., Dart J.K.G., Tuft S.J., Burton M.J. Altered Patterns of Fungal Keratitis at a London Ophthalmic Referral Hospital: An Eight-Year Retrospective Observational Study. Am. J. Ophthalmol. 2016;168:227–236. doi: 10.1016/j.ajo.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordillo M.A., Cano N., Luquin A., Gutiérrez C., Verdú E., Cabrera G., Fierro J.M., Lamarca-Mateu J., España J., Marticorena-Álvarez P., et al. Queratitis secundaria a Fusarium spp. en España 2012–2014. Arch. Soc. Esp. Oftalmol. 2017;93 doi: 10.1016/j.oftal.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Nhung P.T., Thu T.A., Ngoc L.H., Ohkusu K., Ezaki T. Epidemiology of Fungal Keratitis in North Vietnam. J. Clin. Exp. Ophthalmol. 2012;3:328. doi: 10.4172/2155-9570.1000238. [DOI] [Google Scholar]
- 31.Shah A., Sachdev A., Coggon D., Hossain P. Geographic variations in microbial keratitis: An analysis of the peer-reviewed literature. Br. J. Ophthalmol. 2011;95:762–767. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ung L., Bispo P.J.M., Shanbhag S.S., Gilmore M.S., Chodosh J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019;64:255–271. doi: 10.1016/j.survophthal.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritterband D.C., Seedor J.A., Shah M.K., Koplin R.S., McCormick S.A. Fungal keratitis at the new york eye and ear infirmary. Cornea. 2006;25:264–267. doi: 10.1097/01.ico.0000177423.77648.8d. [DOI] [PubMed] [Google Scholar]
- 34.Birker I.C.Y.Y., Luyten G.P. Infectious corneal ulcers: A rising incidence in a Dutch referral centre. Acta Ophthalmol. 2018;96:3–49. doi: 10.1111/aos.13736. [DOI] [PubMed] [Google Scholar]
- 35.Capriotti J.A., Pelletier J.S., Shah M., Caivano D.M., Turay P., Ritterband D.C. The etiology of infectious corneal ulceration in Sierra Leone. Int. Ophthalmol. 2010;30:637–640. doi: 10.1007/s10792-010-9348-1. [DOI] [PubMed] [Google Scholar]
- 36.Gonawardena S.A., Ranasinghe K.P., Arseculeratne S.N., Seimon C.R., Ajello L. Survey of mycotic and bacterial keratitis in Sri Lanka. Mycopathologia. 1994;127:77–81. doi: 10.1007/BF01103062. [DOI] [PubMed] [Google Scholar]
- 37.Shabrawy R., El Badawy N., Harb A. The incidence of fungal keratitis in Zagazig University Hospitals, Egypt and the value of direct microscopy and PCR technique in rapid diagnosis. J. Microbiol. Infect. Dis. 2013;3:186–191. doi: 10.5799/ahinjs.02.2013.04.0106. [DOI] [Google Scholar]
- 38.Badawi A.E., Moemen D., El-Tantawy N.L. Epidemiological, clinical and laboratory findings of infectious keratitis at Mansoura Ophthalmic Center, Egypt. Int. J. Ophthalmol. 2017;10:61–67. doi: 10.18240/ijo.2017.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khater M.M., Shehab N.S., El-Badry A.S. Comparison of mycotic keratitis with nonmycotic keratitis: An epidemiological study. J. Ophthalmol. 2014;2014:254302. doi: 10.1155/2014/254302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kibret T., Bitew A. Fungal keratitis in patients with corneal ulcer attending Minilik II Memorial Hospital, Addis Ababa, Ethiopia. BMC Ophthalmol. 2016;16:148. doi: 10.1186/s12886-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagan M., Wright E., Newman M., Dolin P., Johnson G. Causes of suppurative keratitis in Ghana. Br. J. Ophthalmol. 1995;79:1024–1028. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta R., Mehta P., Rao M., Acharya Y., Arja S.B., Sowmya K. A Study of Fungal Keratitis in North Africa: Exploring Risk Factors and Microbiological Features. Int. J. Life-Sci. Sci. Res. 2016;2:579–582. doi: 10.21276/ijlssr.2016.2.5.11. [DOI] [Google Scholar]
- 43.Carmichael T.R., Wolpert M., Koornhof H.J. Corneal ulceration at an urban African hospital. Br. J. Ophthalmol. 1985;69:920–926. doi: 10.1136/bjo.69.12.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaftenaar E., Peters R.P., Baarsma G.S., Meenken C., Khosa N.S., Getu S., McIntyre J.A., Osterhaus A.D., Verjans G.M. Clinical and corneal microbial profile of infectious keratitis in a high HIV prevalence setting in rural South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1403–1409. doi: 10.1007/s10096-016-2677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mafwiri M.M., Kanyaro N.D., Padhan D.H., Sanyiwa A., Sangawe J.L.F., Kinabo N.N. The microbial aetiology of corneal ulceration among patients attending a tertiary referral centre in Dar es Salaam. J. Ophthalmol. East. Cent. South. Afr. 2013;16:25–28. [Google Scholar]
- 46.Cheikhrouhou F., Makni F., Neji S., Trigui A., Sellami H., Trabelsi H., Guidara R., Fki J., Ayadi A. Epidemiological profile of fungal keratitis in Sfax (Tunisia) J. Mycol. Med. 2014;24:308–312. doi: 10.1016/j.mycmed.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 47.Zbiba W., Baba A., Bouayed E., Abdessalem N., Daldoul A. A 5-year retrospective review of fungal keratitis in the region of Cap Bon. J. Fr. Ophtalmol. 2016;39:843–848. doi: 10.1016/j.jfo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Limaiem R., Mghaieth F., Merdassi A., Mghaieth K., Aissaoui A., El Matri L. Severe microbial keratitis: Report of 100 cases. J. Fr. Ophtalmol. 2007;30:374–379. doi: 10.1016/S0181-5512(07)89607-0. [DOI] [PubMed] [Google Scholar]
- 49.Talukder A.K., Halder S.K., Sultana Z., Bhuiyan S.I. Epidemiology and outcome of non viral keratitis. Mymensingh Med. J. 2011;20:356–361. [PubMed] [Google Scholar]
- 50.Dunlop A.A., Wright E.D., Howlader S.A., Nazrul I., Husain R., McClellan K., Billson F.A. Suppurative corneal ulceration in Bangladesh. Aust. N. Z. J. Ophthalmol. 1994;22:105–110. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 51.Lin L., Lan W., Lou B., Ke H., Yang Y., Lin X., Liang L. Genus Distribution of Bacteria and Fungi Associated with Keratitis in a Large Eye Center Located in Southern China. Ophthalmic Epidemiol. 2017;24:90–96. doi: 10.1080/09286586.2016.1254250. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W.X., Sun S.Y., Zhao J., Shi W.Y., Xie L.X. Retrospective study of suppurative keratitis in 1054 patients. Zhonghua Yan Ke Za Zhi. 2007;43:245–250. [PubMed] [Google Scholar]
- 53.Houang E., Lam D., Fan D., Seal D. Microbial keratitis in Hong Kong: Relationship to climate, environment and contact-lens disinfection. Trans. R Soc. Trop. Med. Hyg. 2001;95:361–367. doi: 10.1016/S0035-9203(01)90180-4. [DOI] [PubMed] [Google Scholar]
- 54.Ng A.L., To K.K., Choi C.C., Yuen L.H., Yim S.M., Chan K.S., Lai J.S., Wong I.Y. Predisposing Factors, Microbial Characteristics, and Clinical Outcome of Microbial Keratitis in a Tertiary Centre in Hong Kong: A 10-Year Experience. J. Ophthalmol. 2015;2015:769436. doi: 10.1155/2015/769436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fong C.F., Tseng C.H., Hu F.R., Wang I.J., Chen W.L., Hou Y.C. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am. J. Ophthalmol. 2004;137:329–336. doi: 10.1016/j.ajo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Khor W.-B., Prajna V.N., Garg P., Mehta J.S., Xie L., Liu Z., Padilla M.D.B., Joo C.-K., Inoue Y., Goseyarakwong P., et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am. J. Ophthalmol. 2018;195:161–170. doi: 10.1016/j.ajo.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 57.Bandyopadhyay S., Das D., Mondal K.K., Ghanta A.K., Purkrit S.K., Bhasrar R. Epidemiology and laboratory diagnosis of fungal corneal ulcer in the Sundarban Region of West Bengal, eastern India. Nepal. J. Ophthalmol. 2012;4:29–36. doi: 10.3126/nepjoph.v4i1.5847. [DOI] [PubMed] [Google Scholar]
- 58.Saha S., Banerjee D., Khetan A., Sengupta J. Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman. J. Ophthalmol. 2009;2:114–118. doi: 10.4103/0974-620x.57310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rautaraya B., Sharma S., Kar S., Das S., Sahu S.K. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol. 2011;11:39. doi: 10.1186/1471-2415-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nath R., Baruah S., Saikia L., Devi B., Borthakur A.K., Mahanta J. Mycotic corneal ulcers in upper Assam. Indian J. Ophthalmol. 2011;59:367–371. doi: 10.4103/0301-4738.83613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy P., Das S., Singh N.P., Saha R., Kajla G., Snehaa K., Gupta V.P. Changing trends in fungal and bacterial profile of infectious keratitis at a tertiary care hospital: A six-year study. Clin. Epidemiol. Glob. Health. 2017;5:40–45. doi: 10.1016/j.cegh.2016.02.001. [DOI] [Google Scholar]
- 62.Ghosh A.K., Gupta A., Rudramurthy S.M., Paul S., Hallur V.K., Chakrabarti A. Fungal Keratitis in North India: Spectrum of Agents, Risk Factors and Treatment. Mycopathologia. 2016;181:843–850. doi: 10.1007/s11046-016-0042-3. [DOI] [PubMed] [Google Scholar]
- 63.Binnani A., Gupta P.S., Gupta A. Epidemio-Clinico-Microbiological Study of Mycotic Keratitis in North-West Region of Rajasthan. Mycopathologia. 2018;183:717–722. doi: 10.1007/s11046-016-0102-8. [DOI] [PubMed] [Google Scholar]
- 64.Chander J., Singla N., Agnihotri N., Arya S.K., Deep A. Keratomycosis in and around Chandigarh: A five-year study from a north Indian tertiary care hospital. Indian J. Pathol. Microbiol. 2008;51:304–306. doi: 10.4103/0377-4929.41700. [DOI] [PubMed] [Google Scholar]
- 65.Gupta A., Capoor M.R., Gupta S., Kochhar S., Tomer A., Gupta V. Clinico-demographical profile of keratomycosis in Delhi, North India. Indian J. Med. Microbiol. 2014;32:310–314. doi: 10.4103/0255-0857.136582. [DOI] [PubMed] [Google Scholar]
- 66.Saha R., Das S. Mycological profile of infectious Keratitis from Delhi. Indian J. Med. Res. 2006;123:159–164. [PubMed] [Google Scholar]
- 67.Chidambaram J.D., Venkatesh Prajna N., Srikanthi P., Lanjewar S., Shah M., Elakkiya S., Lalitha P., Burton M.J. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018;25:297–305. doi: 10.1080/09286586.2018.1454964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bharathi M.J., Ramakrishnan R., Meenakshi R., Padmavathy S., Shivakumar C., Srinivasan M. Microbial Keratitis in South India: Influence of Risk Factors, Climate, and Geographical Variation. Ophthalmic Epidemiol. 2007;14:61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 69.Gopinathan U., Garg P., Fernandes M., Sharma S., Athmanathan S., Rao G.N. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South India. Cornea. 2002;21:555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Gopinathan U., Sharma S., Garg P., Rao G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthalmol. 2009;57:273–279. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin C.C., Lalitha P., Srinivasan M., Prajna N.V., McLeod S.D., Acharya N.R., Lietman T.M., Porco T.C. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123–1127. doi: 10.1097/ICO.0b013e31825694d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranjini C.Y., Waddepally V.V. Microbial Profile of Corneal Ulcers in a Tertiary Care Hospital in South India. J. Ophthalmic Vis. Res. 2016;11:363–367. doi: 10.4103/2008-322X.194071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajesh Somabhai K., Nilesh Dhanjibhai P., Mala S. A Clinical Microbiological Study of Corneal Ulcer Patients at Western Gujarat, India. Acta Med. Iran. 2013;51:399–403. [PubMed] [Google Scholar]
- 74.Kumar A., Pandya S., Kavathia G., Antala S., Madan M., Javdekar T. Microbial keratitis in Gujarat, Western India: Findings from 200 cases. Pan. Afr. Med. J. 2011;10:48. [PMC free article] [PubMed] [Google Scholar]
- 75.Tewari A., Sood N., Vegad M.M., Mehta D.C. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian J. Ophthalmol. 2012;60:267–272. doi: 10.4103/0301-4738.98702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basak S.K., Basak S., Mohanta A., Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, eastern India. Indian J. Ophthalmol. 2005;53:17–22. doi: 10.4103/0301-4738.15280. [DOI] [PubMed] [Google Scholar]
- 77.Panda A., Satpathy G., Nayak N., Kumar S., Kumar A. Demographic pattern, predisposing factors and management of ulcerative keratitis: Evaluation of one thousand unilateral cases at a tertiary care centre. Clin. Exp. Ophthalmol. 2007;35:44–50. doi: 10.1111/j.1442-9071.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S., Taneja M., Gupta R., Upponi A., Gopinathan U., Nutheti R., Garg P. Comparison of clinical and microbiological profiles in smear-positive and smear-negative cases of suspected microbial keratitis. Indian J. Ophthalmol. 2007;55:21–25. doi: 10.4103/0301-4738.29490. [DOI] [PubMed] [Google Scholar]
- 79.Ebadollahi-Natanzi A., Arab-Rahmatipour G., Tabatabaei S.A. Prevalence of Fungal Keratitis (FK) in Patients with Corneal Ulcers in Tehran, Iran. Asia Pac. J. Med. Toxicol. 2016;5:94–97. doi: 10.22038/apjmt.2016.7675. [DOI] [Google Scholar]
- 80.Shokohi T., Nowroozpoor-Dailami K., Moaddel-Haghighi T. Fungal Keratitis in Patients with Corneal Ulcer, in Sari, Northern Iran. Arch. Iran. Med. 2006;9:222–227. [PubMed] [Google Scholar]
- 81.Al-Shakarchi F. Initial therapy for suppurative microbial keratitis in Iraq. Br. J. Ophthalmol. 2007;91:1583–1587. doi: 10.1136/bjo.2007.123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shakarchi F., Hussein M., Al-Shaibani A. Profile of Microbial Keratitis at a Referral Center in Iraq. J. Al-Nahrain Univ. Sci. 2015;18:141–147. doi: 10.22401/JNUS.18.1.21. [DOI] [Google Scholar]
- 83.Khalil Z., Hadi A., Kamil S. Determination and Prevalence of Bacterial and Fungal Keratitis among Patients in Baghdad City. J. Pure Appl. Microbiol. 2018;12:1455–1463. doi: 10.22207/JPAM.12.3.49. [DOI] [Google Scholar]
- 84.Toshida H., Kogure N., Inoue N., Murakami A. Trends in microbial keratitis in Japan. Eye Contact Lens. 2007;33:70–73. doi: 10.1097/01.icl.0000237825.98225.ca. [DOI] [PubMed] [Google Scholar]
- 85.National Surveillance of Infectious Keratitis in Japan National Surveillance of Infectious Keratitis in Japan—Current status of isolates, patient background, and treatment. Nippon Ganka Gakkai Zasshi. 2006;110:961–972. [PubMed] [Google Scholar]
- 86.Han S.B., Lim T.H., Wee W.R., Lee J.H., Kim M.K. Current characteristics of infectious keratitis at a tertiary referral center in South Korea. Jpn. J. Ophthalmol. 2009;53:549–551. doi: 10.1007/s10384-009-0705-4. [DOI] [PubMed] [Google Scholar]
- 87.Mohd-Tahir F., Norhayati A., Siti-Raihan I., Ibrahim M. A 5-Year Retrospective Review of Fungal Keratitis at Hospital Universiti Sains Malaysia. Interdiscip. Perspect. Infect. Dis. 2012;2012:851563. doi: 10.1155/2012/851563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ratnalingam V., Umapathy T., Sumugam K., Hanafi H., Retnasabapathy S. Microbial keratitis in West and East Malaysia. Int. Eye Sci. 2017;17:1989–1992. doi: 10.3980/j.issn.1672-5123.2017.11.01. [DOI] [Google Scholar]
- 89.Amatya R., Shrestha S., Khanal B., Gurung R., Poudel N., Bhattacharya S., Badu P. Etiological agents of corneal ulcer: Five years prospective study in eastern Nepal. Nepal Med. Coll. J. NMCJ. 2012;14:219–222. [PubMed] [Google Scholar]
- 90.Khanal B., Kaini K.R., Deb M., Badhu B., Thakur S.K. Microbial keratitis in eastern Nepal. Trop. Dr. 2001;31:168–169. doi: 10.1177/004947550103100319. [DOI] [PubMed] [Google Scholar]
- 91.Ganguly S., Kansakar I., Sharma M., Bastola P., Pradhan R. Pattern of fungal isolates in cases of corneal ulcer in the western periphery of Nepal. Nepal. J. Ophthalmol. 2011;3:118–122. doi: 10.3126/nepjoph.v3i2.5262. [DOI] [PubMed] [Google Scholar]
- 92.Lavaju P., Arya S., Khanal B., Amatya R., Patel S. Demograhic pattern, clinical features and treatment outcome of patients with infective keratitis in the eastern region of Nepal. Nepal. J. Ophthalmol. 2010;1:101–106. doi: 10.3126/nepjoph.v1i2.3683. [DOI] [PubMed] [Google Scholar]
- 93.Suwal S., Bhandari D., Thapa P., Shrestha M., Amatya J. Microbiological profile of corneal ulcer cases diagnosed in a tertiary care ophthalmological institute in Nepal. BMC Ophthalmol. 2016;16 doi: 10.1186/s12886-016-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Upadhyay M., Rai N., Brandt F., Shrestha R. Corneal ulcers in Nepal. Graefe’s Arch. Clin. Exp. Ophthalmol. 1982;219:55–59. doi: 10.1007/BF02173440. [DOI] [PubMed] [Google Scholar]
- 95.Sitoula R.P., Singh S., Mahaseth V., Sharma A., Labh R. Epidemiology and etiological diagnosis of infective keratitis in eastern region of Nepal. Nepal. J. Ophthalmol. 2015;7:10–15. doi: 10.3126/nepjoph.v7i1.13146. [DOI] [PubMed] [Google Scholar]
- 96.Idiculla T., Zachariah G., Br K., Basu S. A Retrospective Study of Fungal Corneal Ulcers in the South Sharqiyah Region in Oman. Sultan Qaboos Univ. Med. J. 2009;9:59–62. [PMC free article] [PubMed] [Google Scholar]
- 97.Keshav B.Z.G., Ideculla T., Bhat V., Joseph M. Epidemiological characteristics of corneal ulcers in South sharqiya region. Oman Med. J. 2008;23:34–39. [PMC free article] [PubMed] [Google Scholar]
- 98.Riaz Q., Fawwad U., Bhatti N., Rehman A.U., Hasan M.U. Epidemiology of Microbial Keratitis in a Tertiary Care Center in Karachi. Pak. J. Ophthalmol. 2013;29:94–99. [Google Scholar]
- 99.Jastaneiah S., Al-Rajhi A., Abbott D. Ocular mycosis at a referral center in Saudi Arabia: A 20-year study. Saudi J. Ophthalmol. 2011;25:231–238. doi: 10.1016/j.sjopt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong T.Y., Fong K.S., Tan D.T. Clinical and microbial spectrum of fungal keratitis in Singapore: A 5-year retrospective study. Int. Ophthalmol. 1997;21:127–130. doi: 10.1023/A:1026462631716. [DOI] [PubMed] [Google Scholar]
- 101.Boonpasart S., Kasetsuwan N., Puangsricharern V., Pariyakanok L., Jittpoonkusol T. Infectious keratitis at King Chulalongkorn Memorial Hospital: A 12-year retrospective study of 391 cases. J. Med. Assoc. Thai. 2002;85(Suppl. 1):S217–S230. [PubMed] [Google Scholar]
- 102.Hirunpat C., Masae N. Fungal keratitis in Songklanagarind Hospital. Songklanagarind Med. J. 2005;23:429–434. [Google Scholar]
- 103.Tananuvat N., Punyakhum O., Ausayakhun S., Chaidaroon W. Etiology and clinical outcomes of microbial keratitis at a tertiary eye-care center in northern Thailand. J. Med. Assoc. Thai. 2012;95:S8–S17. [PubMed] [Google Scholar]
- 104.Sirikul T., Prabriputaloong T., Smathivat A., Chuck R.S., Vongthongsri A. Predisposing Factors and Etiologic Diagnosis of Ulcerative Keratitis. Cornea. 2008;27:283–287. doi: 10.1097/ICO.0b013e31815ca0bb. [DOI] [PubMed] [Google Scholar]
- 105.Erdem E., Yagmur M., Boral H., Ilkit M., Ersoz R., Seyedmousavi S. Aspergillus flavus Keratitis: Experience of a Tertiary Eye Clinic in Turkey. Mycopathologia. 2017;182:379–385. doi: 10.1007/s11046-016-0089-1. [DOI] [PubMed] [Google Scholar]
- 106.Yilmaz S., Ozturk I., Maden A. Microbial keratitis in West Anatolia, Turkey: A retrospective review. Int. Ophthalmol. 2007;27:261–268. doi: 10.1007/s10792-007-9069-2. [DOI] [PubMed] [Google Scholar]
- 107.Nguyên D.T., Nguyên H. Keratomycoses in Viet-Nam. Rev. Int. Trach. Pathol. Ocul. Trop. Subtrop. Sante Publique. 1990;67:203–206. [PubMed] [Google Scholar]
- 108.Van der Meulen I.J., van Rooij J., Nieuwendaal C.P., Van Cleijnenbreugel H., Geerards A.J., Remeijer L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea. 2008;27:539–544. doi: 10.1097/ICO.0b013e318165b200. [DOI] [PubMed] [Google Scholar]
- 109.Tavassoli S., Nayar G., Darcy K., Grzeda M., Luck J., Williams O.M., Tole D. An 11-year analysis of microbial keratitis in the South West of England using brain–heart infusion broth. Eye. 2019;33:1619–1625. doi: 10.1038/s41433-019-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ting D.S.J., Settle C., Morgan S.J., Baylis O., Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England Study. Eye. 2018;32:1416–1417. doi: 10.1038/s41433-018-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tan S.Z., Walkden A., Au L., Fullwood C., Hamilton A., Qamruddin A., Armstrong M., Brahma A.K., Carley F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye. 2017;31:1229–1236. doi: 10.1038/eye.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cariello A., Passos R., Yu M., Hofling-lima A.L. Microbial keratitis at a referral center in Brazil. Int. Ophthalmol. 2011;31:197–204. doi: 10.1007/s10792-011-9441-0. [DOI] [PubMed] [Google Scholar]
- 113.Furlanetto R.L., Andreo E.G.V., Finotti L.G.A., Arcieri E.S., Ferreira M.A., Rocha F.J. Epidemiology and Etiologic Diagnosis of Infectious Keratitis in Uberlandia, Brazil. Eur. J. Ophthalmol. 2010;20:498–503. doi: 10.1177/112067211002000312. [DOI] [PubMed] [Google Scholar]
- 114.Ibrahim M.M., Vanini R., Ibrahim F.M., Fioriti L.S., Furlan E.M.R., Provinzano L.M.A., De Castro R.S., De Faria Esousa S.J., Rocha E.M. Epidemiologic Aspects and Clinical Outcome of Fungal Keratitis in Southeastern Brazil. Eur. J. Ophthalmol. 2009;19:355–361. doi: 10.1177/112067210901900305. [DOI] [PubMed] [Google Scholar]
- 115.Müller G.G., Kara-José N., Castro R.S. Epidemiological profile of keratomycosis at the HC-UNICAMP. Arq. Bras. Oftalmol. 2012;75:247–250. doi: 10.1590/S0004-27492012000400005. [DOI] [PubMed] [Google Scholar]
- 116.Parra-Rodríguez D.S., García-Carmona K.P., Vázquez-Maya L., Bonifaz A. Incidencia de úlceras corneales microbianas en el Servicio de Oftalmología del Hospital General de México Dr. Eduardo Liceaga. Rev. Mex. Oftalmol. 2016;90:209–214. doi: 10.1016/j.mexoft.2015.10.010. [DOI] [Google Scholar]
- 117.Laspina F., Samudio M., Cibils D., Ta C.N., Fariña N., Sanabria R., Klauß V., Miño de Kaspar H. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: A 13-year survey in Paraguay. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004;242:204–209. doi: 10.1007/s00417-003-0808-4. [DOI] [PubMed] [Google Scholar]
- 118.Nentwich M.M., Bordón M., di Martino D.S., Campuzano A.R., Torres W.M., Laspina F., Lichi S., Samudio M., Farina N., Sanabria R.R., et al. Clinical and epidemiological characteristics of infectious keratitis in Paraguay. Int. Ophthalmol. 2015;35:341–346. doi: 10.1007/s10792-014-9951-7. [DOI] [PubMed] [Google Scholar]
- 119.Varaprasathan G., Miller K., Lietman T., Whitcher J.P., Cevallos V., Okumoto M., Margolis T.P., Yinghui M., Cunningham E.T.J. Trends in the Etiology of Infectious Corneal Ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–364. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Liesegang T.J., Forster R.K. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 1980;90:38–47. doi: 10.1016/S0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 121.Iyer S.A., Tuli S.S., Wagoner R.C. Fungal Keratitis: Emerging Trends and Treatment Outcomes. Eye Contact Lens. 2006;32:267–271. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- 122.Ho J.W., Fernandez M.M., Rebong R.A., Carlson A.N., Kim T., Afshari N.A. Microbiological profiles of fungal keratitis: A 10-year study at a tertiary referral center. J. Ophthalmic Inflamm. Infect. 2016;6:5. doi: 10.1186/s12348-016-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Green M., Apel A., Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–39. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 124.Chew R., Woods M.L. Epidemiology of fungal keratitis in Queensland, Australia. Clin. Exp. Ophthalmol. 2019;47:26–32. doi: 10.1111/ceo.13346. [DOI] [PubMed] [Google Scholar]
- 125.Watson S.L., Cabrera-Aguas M., Keay L., Khoo P., McCall D., Lahra M.M. The clinical and microbiological features and outcomes of fungal keratitis over 9 years in Sydney, Australia. Mycoses. 2020;63:43–51. doi: 10.1111/myc.13009. [DOI] [PubMed] [Google Scholar]
- 126.Green M., Carnt N., Apel A., Stapleton F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019;103:1481–1486. doi: 10.1136/bjophthalmol-2018-312881. [DOI] [PubMed] [Google Scholar]
- 127.Khoo P., Cabrera-Aguas M.P., Nguyen V., Lahra M.M., Watson S.L. Microbial keratitis in Sydney, Australia: Risk factors, patient outcomes, and seasonal variation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020;258:1745–1755. doi: 10.1007/s00417-020-04681-0. [DOI] [PubMed] [Google Scholar]
- 128.Pandita A., Murphy C. Microbial keratitis in Waikato, New Zealand. Clin. Exp. Ophthalmol. 2011;39:393–397. doi: 10.1111/j.1442-9071.2010.02480.x. [DOI] [PubMed] [Google Scholar]
- 129.Saad-Hussein A., El-Mofty H.M., Hassanien M.A. Climate change and predicted trend of fungal keratitis in Egypt. East Mediterr. Health J. 2011;17:468–473. doi: 10.26719/2011.17.6.468. [DOI] [PubMed] [Google Scholar]
- 130.Gower E.W., Keay L.J., Oechsler R.A., Iovieno A., Alfonso E.C., Jones D.B., Colby K., Tuli S.S., Patel S.R., Lee S.M., et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117:2263–2267. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 131.Bharathi M.J., Ramakrishnan R., Vasu S., Meenakshi R., Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J. Ophthalmol. 2003;51:315–321. [PubMed] [Google Scholar]
- 132.Bharathi M.J., Ramakrishnan R., Meenakshi R., Shivakumar C., Raj D.L. Analysis of the risk factors predisposing to fungal, bacterial & Acanthamoeba keratitis in south India. Indian J. Med. Res. 2009;130:749–757. [PubMed] [Google Scholar]
- 133.Sharma S., Das S., Virdi A., Fernandes M., Sahu S.K., Kumar Koday N., Ali M.H., Garg P., Motukupally S.R. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br. J. Ophthalmol. 2015;99:1190–1195. doi: 10.1136/bjophthalmol-2014-306485. [DOI] [PubMed] [Google Scholar]
- 134.Das S., Sharma S., Mahapatra S., Sahu S.K. Fusarium keratitis at a tertiary eye care centre in India. Int. Ophthalmol. 2015;35:387–393. doi: 10.1007/s10792-014-9961-5. [DOI] [PubMed] [Google Scholar]
- 135.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 136.Hine J.L., de Lusignan S., Burleigh D., Pathirannehelage S., McGovern A., Gatenby P., Jones S., Jiang D., Williams J., Elliot A.J., et al. Association between glycaemic control and common infections in people with Type 2 diabetes: A cohort study. Diabet. Med. 2017;34:551–557. doi: 10.1111/dme.13205. [DOI] [PubMed] [Google Scholar]
- 137.Dan J., Zhou Q., Zhai H., Cheng J., Wan L., Ge C., Xie L. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE. 2018;13:e0196741. doi: 10.1371/journal.pone.0196741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arunga S., Kintoki G.M., Gichuhi S., Onyango J., Ayebazibwe B., Newton R., Leck A., Macleod D., Hu V.H., Burton M.J. Risk Factors of Microbial Keratitis in Uganda: A Case Control Study. Ophthalmic Epidemiol. 2020;27:98–104. doi: 10.1080/09286586.2019.1682619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Santos C., Parker J., Dawson C., Ostler B. Bilateral fungal corneal ulcers in a patient with AIDS-related complex. Am. J. Ophthalmol. 1986;102:118–119. doi: 10.1016/0002-9394(86)90221-7. [DOI] [PubMed] [Google Scholar]
- 140.Parrish C.M., O’Day D.M., Hoyle T.C. Spontaneous fungal corneal ulcer as an ocular manifestation of AIDS. Am. J. Ophthalmol. 1987;104:302–303. doi: 10.1016/0002-9394(87)90423-5. [DOI] [PubMed] [Google Scholar]
- 141.Mselle J. Fungal keratitis as an indicator of HIV infection in Africa. Trop. Dr. 1999;29:133–135. doi: 10.1177/004947559902900303. [DOI] [PubMed] [Google Scholar]
- 142.Arunga S., Kintoki G.M., Mwesigye J., Ayebazibwe B., Onyango J., Bazira J., Newton R., Gichuhi S., Leck A., Macleod D., et al. Epidemiology of Microbial Keratitis in Uganda: A Cohort Study. Ophthalmic Epidemiol. 2020;27:121–131. doi: 10.1080/09286586.2019.1700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Anguria P., Ntuli S., Interewicz B., Carmichael T. Traditional eye medication and pterygium occurrence in Limpopo Province. South Afr. Med. J. 2012;102:687–690. doi: 10.7196/SAMJ.5930. [DOI] [PubMed] [Google Scholar]
- 144.Bisika T., Courtright P., Geneau R., Kasote A., Chimombo L., Chirambo M. Self treatment of eye diseases in Malawi. Afr. J. Tradit. Complement Altern. Med. 2008;6:23–29. doi: 10.4314/ajtcam.v6i1.57070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lionakis M.S., Kontoyiannis D.P. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 146.Nielsen S.E., Nielsen E., Julian H.O., Lindegaard J., Højgaard K., Ivarsen A., Hjortdal J., Heegaard S. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015;93:54–58. doi: 10.1111/aos.12440. [DOI] [PubMed] [Google Scholar]
- 147.Cho C.H., Lee S.B. Clinical analysis of microbiologically proven fungal keratitis according to prior topical steroid use: A retrospective study in South Korea. BMC Ophthalmol. 2019;19:207. doi: 10.1186/s12886-019-1212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keay L.J., Gower E.W., Iovieno A., Oechsler R.A., Alfonso E.C., Matoba A., Colby K., Tuli S.S., Hammersmith K., Cavanagh D., et al. Clinical and Microbiological Characteristics of Fungal Keratitis in the United States, 2001–2007: A Multicenter Study. Ophthalmology. 2011;118:920–926. doi: 10.1016/j.ophtha.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Poole T.R.G., Hunter D.L., Maliwa E.M.K., Ramsay A.R.C. Aetiology of microbial keratitis in northern Tanzania. Br. J. Ophthalmol. 2002;86:941–942. doi: 10.1136/bjo.86.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bullock J.D., Warwar R.E., Elder B.L., Khamis H.J. Microbiological Investigations of ReNu Plastic Bottles and the 2004 to 2006 ReNu With MoistureLoc-Related Worldwide Fusarium Keratitis Event. Eye Contact Lens. 2016;42:147–152. doi: 10.1097/ICL.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 151.Buchta V., Feuermannová A., Váša M., Bašková L., Kutová R., Kubátová A., Vejsová M. Outbreak of fungal endophthalmitis due to Fusarium oxysporum following cataract surgery. Mycopathologia. 2014;177:115–121. doi: 10.1007/s11046-013-9721-5. [DOI] [PubMed] [Google Scholar]
- 152.Labiris G., Troeber L., Gatzioufas Z., Stavridis E., Seitz B. Bilateral Fusarium oxysporum keratitis after laser in situ keratomileusis. J. Cataract Refract. Surg. 2012;38:2040–2044. doi: 10.1016/j.jcrs.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 153.Jurkunas U., Behlau I., Colby K. Fungal keratitis: Changing pathogens and risk factors. Cornea. 2009;28:638–643. doi: 10.1097/ICO.0b013e318191695b. [DOI] [PubMed] [Google Scholar]
- 154.Ibrahim M.M., Vanini R., Ibrahim F.M., Martins W.d.P., Carvalho R.T.d.C., Castro R.S.d., Rocha E.M. Epidemiology and medical prediction of microbial keratitis in southeast Brazil. Arq. Bras. Oftalmol. 2011;74:7–12. doi: 10.1590/S0004-27492011000100002. [DOI] [PubMed] [Google Scholar]
- 155.Grzybowski A., Told R., Sacu S., Bandello F., Moisseiev E., Loewenstein A., Schmidt-Erfurth U. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica. 2018;239:181–193. doi: 10.1159/000486145. [DOI] [PubMed] [Google Scholar]
- 156.Luo D., Zhu B., Zheng Z., Zhou H., Sun X., Xu X. Subtenon Vs Intravitreal Triamcinolone injection in Diabetic Macular Edema, A prospective study in Chinese population. Pak. J. Med. Sci. 2014;30:749–754. doi: 10.12669/pjms.304.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weinberg T., Loewenstein A. The role of steroids in treating diabetic macular oedema in the era of anti-VEGF. Eye. 2020;34:1003–1005. doi: 10.1038/s41433-019-0739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Durand M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017;30:597–613. doi: 10.1128/CMR.00113-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Galor A., Karp C.L., Forster R.K., Dubovy S.R., Gaunt M.L., Miller D. Subconjunctival mycetoma after sub-Tenon’s corticosteroid injection. Cornea. 2009;28:933–935. doi: 10.1097/ICO.0b013e3181930bb7. [DOI] [PubMed] [Google Scholar]
- 160.Iwahashi C., Eguchi H., Hotta F., Uezumi M., Sawa M., Kimura M., Yaguchi T., Kusaka S. Orbital abscess caused by Exophiala dermatitidis following posterior subtenon injection of triamcinolone acetonide: A case report and a review of literature related to Exophiala eye infections. BMC Infect. Dis. 2020;20:566. doi: 10.1186/s12879-020-05294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dahlgren M.A., Lingappan A., Wilhelmus K.R. The Clinical Diagnosis of Microbial Keratitis. Am. J. Ophthalmol. 2007;143:940–944.e1. doi: 10.1016/j.ajo.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dalmon C., Porco T.C., Lietman T.M., Prajna N.V., Prajna L., Das M.R., Kumar J.A., Mascarenhas J., Margolis T.P., Whitcher J.P., et al. The Clinical Differentiation of Bacterial and Fungal Keratitis: A Photographic Survey. Investig. Opthalmol. Vis. Sci. 2012;53:1787–1791. doi: 10.1167/iovs.11-8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leck A., Burton M. Distinguishing fungal and bacterial keratitis on clinical signs. Community Eye Health. 2015;28:6–7. [PMC free article] [PubMed] [Google Scholar]
- 164.Mascarenhas J., Lalitha P., Prajna N.V., Srinivasan M., Das M., D’Silva S.S., Oldenburg C.E., Borkar D.S., Esterberg E.J., Lietman T.M., et al. Acanthamoeba, Fungal, and Bacterial Keratitis: A Comparison of Risk Factors and Clinical Features. Am. J. Ophthalmol. 2014;157:56–62. doi: 10.1016/j.ajo.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oldenburg C.E., Prajna V.N., Prajna L., Krishnan T., Mascarenhas J., Vaitilingam C.M., Srinivasan M., See C.W., Cevallos V., Zegans M.E., et al. Clinical signs in dematiaceous and hyaline fungal keratitis. Br. J. Ophthalmol. 2011;95:750–751. doi: 10.1136/bjo.2010.198648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barron B.A., Gee L., Hauck W.W., Kurinij N., Dawson C.R., Jones D.B., Wilhelmus K.R., Kaufman H.E., Sugar J., Hyndiuk R.A. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–1882. doi: 10.1016/S0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- 167.Mahmoudi S., Masoomi A., Ahmadikia K., Tabatabaei S.A., Soleimani M., Rezaie S., Ghahvechian H., Banafsheafshan A. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses. 2018;61:916–930. doi: 10.1111/myc.12822. [DOI] [PubMed] [Google Scholar]
- 168.Bhadange Y., Das S., Kasav M.K., Sahu S.K., Sharma S. Comparison of culture-negative and culture-positive microbial keratitis: Cause of culture negativity, clinical features and final outcome. Br. J. Ophthalmol. 2015;99:1498–1502. doi: 10.1136/bjophthalmol-2014-306414. [DOI] [PubMed] [Google Scholar]
- 169.Ngo J., Khoo P., Watson S. Improving the Efficiency and the Technique of the Corneal Scrape Procedure via an Evidence Based Instructional Video at a Quaternary Referral Eye Hospital. Curr. Eye Res. 2020;45 doi: 10.1080/02713683.2019.1676910. [DOI] [PubMed] [Google Scholar]
- 170.Vengayil S., Panda A., Satpathy G., Nayak N., Ghose S., Patanaik D., Khokhar S. Polymerase chain reaction-guided diagnosis of mycotic keratitis: A prospective evaluation of its efficacy and limitations. Investig. Ophthalmol. Vis. Sci. 2009;50:152–156. doi: 10.1167/iovs.07-1283. [DOI] [PubMed] [Google Scholar]
- 171.Thomas P.A., Theresa P.A., Theodore J., Geraldine P. PCR for the molecular diagnosis of mycotic keratitis. Expert Rev. Mol. Diagn. 2012;12:703–718. doi: 10.1586/erm.12.65. [DOI] [PubMed] [Google Scholar]
- 172.Tananuvat N., Salakthuantee K., Vanittanakom N., Pongpom M., Ausayakhun S. Prospective comparison between conventional microbial work-up vs PCR in the diagnosis of fungal keratitis. Eye. 2012;26:1337–1343. doi: 10.1038/eye.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim E., Chidambaram J.D., Srinivasan M., Lalitha P., Wee D., Lietman T.M., Whitcher J.P., Van Gelder R.N. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am. J. Ophthalmol. 2008;146:714–723.e1. doi: 10.1016/j.ajo.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 174.Ghosh A., Basu S., Datta H., Chattopadhyay D. Evaluation of polymerase chain reaction-based ribosomal DNA sequencing technique for the diagnosis of mycotic keratitis. Am. J. Ophthalmol. 2007;144:396–403. doi: 10.1016/j.ajo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 175.Ferrer C., Alio J.L. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J. Ophthalmic Inflamm. Infect. 2011;1:15–22. doi: 10.1007/s12348-011-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Homa M., Shobana C.S., Singh Y.R.B., Manikandan P., Selvam K.P., Kredics L., Narendran V., Vágvölgyi C., Galgóczy L. Fusarium keratitis in South India: Causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses. 2013;56:501–511. doi: 10.1111/myc.12062. [DOI] [PubMed] [Google Scholar]
- 177.Oechsler R.A., Feilmeier M.R., Miller D., Shi W., Hofling-Lima A.L., Alfonso E.C. Fusarium Keratitis: Genotyping, In Vitro Susceptibility and Clinical Outcomes. Cornea. 2013;32:667–673. doi: 10.1097/ICO.0b013e318277ac74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dingle T.C., Butler-Wu S.M. Maldi-tof mass spectrometry for microorganism identification. Clin. Lab. Med. 2013;33:589–609. doi: 10.1016/j.cll.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 179.Atalay A., Koc A.N., Suel A., Sav H., Demir G., Elmali F., Cakir N., Seyedmousavi S. Conventional Morphology Versus PCR Sequencing, rep-PCR, and MALDI-TOF-MS for Identification of Clinical Aspergillus Isolates Collected Over a 2-Year Period in a University Hospital at Kayseri, Turkey. J. Clin. Lab. Anal. 2016;30:745–750. doi: 10.1002/jcla.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang L., Yu H., Jiang L., Wu J., Yi M. Fungal keratitis caused by a rare pathogen, Colletotrichum gloeosporioides, in an east coast city of China. J. Mycol. Med. 2020;30:100922. doi: 10.1016/j.mycmed.2019.100922. [DOI] [PubMed] [Google Scholar]
- 181.Ting D.S.J., McKenna M., Sadiq S.N., Martin J., Mudhar H.S., Meeney A., Patel T. Arthrographis kalrae Keratitis Complicated by Endophthalmitis: A Case Report With Literature Review. Eye Contact Lens. 2020;46:e59–e65. doi: 10.1097/ICL.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 182.Rohilla R., Meena S., Mohanty A., Gupta N., Kaistha N., Gupta P., Mangla A., Singh A. Etiological spectrum of infectious keratitis in the era of MALDI-TOF-MS at a tertiary care hospital. J. Fam. Med. Prim. Care. 2020;9:4576–4581. doi: 10.4103/jfmpc.jfmpc_630_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miqueleiz Zapatero A., Hernando C., Barba J., Buendía B. First report of a case of fungal keratitis due to Curvularia hominis in Spain. Rev. Iberoam. Micol. 2018;35:155–158. doi: 10.1016/j.riam.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 184.Cavallini G.M., Ducange P., Volante V., Benatti C. Successful treatment of Fusarium keratitis after photo refractive keratectomy. Indian J. Ophthalmol. 2013;61:669–671. doi: 10.4103/0301-4738.120213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chidambaram J.D., Prajna N.V., Larke N.L., Palepu S., Lanjewar S., Shah M., Elakkiya S., Lalitha P., Carnt N., Vesaluoma M.H., et al. Prospective Study of the Diagnostic Accuracy of the In Vivo Laser Scanning Confocal Microscope for Severe Microbial Keratitis. Ophthalmology. 2016;123:2285–2293. doi: 10.1016/j.ophtha.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chidambaram J.D., Prajna N.V., Larke N., Macleod D., Srikanthi P., Lanjewar S., Shah M., Lalitha P., Elakkiya S., Burton M.J. In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br. J. Ophthalmol. 2017;101:1119–1123. doi: 10.1136/bjophthalmol-2016-309656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Labbé A., Khammari C., Dupas B., Gabison E., Brasnu E., Labetoulle M., Baudouin C. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul. Surf. 2009;7:41–52. doi: 10.1016/S1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- 188.Chidambaram J.D. Recent advances in the diagnosis and management of bacterial keratitis. Int. Ophthalmol. Clin. 2007;47:1–6. doi: 10.1097/IIO.0b013e318074e797. [DOI] [PubMed] [Google Scholar]
- 189.Shi W., Li S., Liu M., Jin H., Xie L. Antifungal chemotherapy for fungal keratitis guided by in vivo confocal microscopy. Graefe Arch. Clin. Exp. Ophthalmol. 2008;246:581–586. doi: 10.1007/s00417-007-0719-x. [DOI] [PubMed] [Google Scholar]
- 190.Hau S.C., Dart J.K.G., Vesaluoma M., Parmar D.N., Claerhout I., Bibi K., Larkin D.F.P. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br. J. Ophthalmol. 2010;94:982–987. doi: 10.1136/bjo.2009.175083. [DOI] [PubMed] [Google Scholar]
- 191.FlorCruz N.V., Evans J.R. Medical interventions for fungal keratitis. Cochrane Database Syst. Rev. 2015;4:CD004241. doi: 10.1002/14651858.CD004241.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schein O.D. Evidence-Based Treatment of Fungal Keratitis. JAMA Ophthalmol. 2016;134:1372–1373. doi: 10.1001/jamaophthalmol.2016.4167. [DOI] [PubMed] [Google Scholar]
- 193.Parchand S., Gupta A., Ram J., Gupta N., Chakrabarty A. Voriconazole for fungal corneal ulcers. Ophthalmology. 2012;119:1083. doi: 10.1016/j.ophtha.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 194.Prajna N.V., Mascarenhas J., Krishnan T., Reddy P.R., Prajna L., Srinivasan M., Vaitilingam C.M., Hong K.C., Lee S.M., McLeod S.D., et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch. Ophthalmol. 2010;128:672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martin M.J., Rahman M.R., Johnson G.J., Srinivasan M., Clayton Y.M. Mycotic keratitis: Susceptibility to antiseptic agents. Int. Ophthalmol. 1995;19:299–302. doi: 10.1007/BF00130925. [DOI] [PubMed] [Google Scholar]
- 196.Hoffman J.J., Yadav R., Das Sanyam S., Chaudhary P., Roshan A., Singh S.K., Arunga S., Matayan E., Macleod D., Weiss H.A., et al. Topical chlorhexidine 0.2% versus topical natamycin 5% for fungal keratitis in Nepal: Rationale and design of a randomised controlled non-inferiority trial. BMJ Open. 2020;10:e038066. doi: 10.1136/bmjopen-2020-038066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma N., Sahay P., Maharana P.K., Singhal D., Saluja G., Bandivadekar P., Chako J., Agarwal T., Sinha R., Titiyal J.S., et al. Management Algorithm for Fungal Keratitis: The TST (Topical, Systemic, and Targeted Therapy) Protocol. Cornea. 2019;38:141–145. doi: 10.1097/ICO.0000000000001781. [DOI] [PubMed] [Google Scholar]
- 198.Sharma N., Singhal D., Maharana P.K., Sinha R., Agarwal T., Upadhyay A.D., Velpandian T., Satpathy G., Titiyal J.S. Comparison of Oral Voriconazole Versus Oral Ketoconazole as an Adjunct to Topical Natamycin in Severe Fungal Keratitis: A Randomized Controlled Trial. Cornea. 2017;36:1521–1527. doi: 10.1097/ICO.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 199.Zrenner E., Tomaszewski K., Hamlin J., Layton G., Wood N. Effects of multiple doses of voriconazole on the vision of healthy volunteers: A double-blind, placebo-controlled study. Ophthalmic Res. 2014;52:43–52. doi: 10.1159/000359952. [DOI] [PubMed] [Google Scholar]
- 200.Prakash G., Sharma N., Goel M., Titiyal J.S., Vajpayee R.B. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am. J. Ophthalmol. 2008;146:56–59. doi: 10.1016/j.ajo.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 201.Sharma N., Agarwal P., Sinha R., Titiyal J.S., Velpandian T., Vajpayee R.B. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: Case series. Br. J. Ophthalmol. 2011;95:1735–1737. doi: 10.1136/bjo.2010.192815. [DOI] [PubMed] [Google Scholar]
- 202.Sharma N., Chacko J., Velpandian T., Titiyal J.S., Sinha R., Satpathy G., Tandon R., Vajpayee R.B. Comparative evaluation of topical versus intrastromal voriconazole as an adjunct to natamycin in recalcitrant fungal keratitis. Ophthalmology. 2013;120:677–681. doi: 10.1016/j.ophtha.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 203.Garg P., Das S., Roy A. Collagen Cross-linking for Microbial Keratitis. Middle East Afr. J. Ophthalmol. 2017;24:18–23. doi: 10.4103/meajo.MEAJO_305_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ting D.S.J., Henein C., Said D.G., Dua H.S. Photoactivated chromophore for infectious keratitis—Corneal cross-linking (PACK-CXL): A systematic review and meta-analysis. Ocul. Surf. 2019;17:624–634. doi: 10.1016/j.jtos.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 205.Uddaraju M., Mascarenhas J., Das M.R., Radhakrishnan N., Keenan J.D., Prajna L., Prajna V.N. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. Am. J. Ophthalmol. 2015;160:131–134.e5. doi: 10.1016/j.ajo.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 206.Prajna N.V., Radhakrishnan N., Lalitha P., Austin A., Ray K.J., Keenan J.D., Porco T.C., Lietman T.M., Rose-Nussbaumer J. Cross-Linking-Assisted Infection Reduction: A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology. 2020;127:159–166. doi: 10.1016/j.ophtha.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Prajna N.V., Sangoi K. Commentary: Timing of therapeutic keratoplasty. Indian J. Ophthalmol. 2019;67:1606. doi: 10.4103/ijo.IJO_793_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shi W., Wang T., Xie L., Li S., Gao H., Liu J., Li H. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology. 2010;117:890–896. doi: 10.1016/j.ophtha.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 209.Mundra J., Dhakal R., Mohamed A., Jha G., Joseph J., Chaurasia S., Murthy S. Outcomes of therapeutic penetrating keratoplasty in 198 eyes with fungal keratitis. Indian J. Ophthalmol. 2019;67:1599–1605. doi: 10.4103/ijo.IJO_1952_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Upadhyay M.P., Karmacharya P.C., Koirala S., Shah D.N., Shakya S., Shrestha J.K., Bajracharya H., Gurung C.K., Whitcher J.P. The Bhaktapur eye study: Ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br. J. Ophthalmol. 2001;85:388–392. doi: 10.1136/bjo.85.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pflugfelder S.C., Flynn H.W., Jr., Zwickey T.A., Forster R.K., Tsiligianni A., Culbertson W.W., Mandelbaum S. Exogenous fungal endophthalmitis. Ophthalmology. 1988;95:19–30. doi: 10.1016/S0161-6420(88)33229-X. [DOI] [PubMed] [Google Scholar]
- 212.Lübke J., Auw-Hädrich C., Meyer-Ter-Vehn T., Emrani E., Reinhard T. Fusarium keratitis with dramatic outcome. Ophthalmologe. 2017;114:462–465. doi: 10.1007/s00347-016-0303-z. [DOI] [PubMed] [Google Scholar]
- 213.Hu S., Fan V.C., Koonapareddy C., Du T.T., Asbell P.A. Contact lens-related Fusarium infection: Case series experience in New York City and review of fungal keratitis. Eye Contact Lens. 2007;33:322–328. doi: 10.1097/ICL.0b013e3180645d17. [DOI] [PubMed] [Google Scholar]
- 214.Ma S.K., So K., Chung P.H., Tsang H.F., Chuang S.K. A multi-country outbreak of fungal keratitis associated with a brand of contact lens solution: The Hong Kong experience. Int. J. Infect. Dis. 2009;13:443–448. doi: 10.1016/j.ijid.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 215.Khor W.B., Aung T., Saw S.M., Wong T.Y., Tambyah P.A., Tan A.L., Beuerman R., Lim L., Chan W.K., Heng W.J., et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–2873. doi: 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- 216.Charm J., Cheung S.W., Cho P. Practitioners’ analysis of contact lens practice in Hong Kong. Contact Lens Anterior Eye. 2010;33:104–111. doi: 10.1016/j.clae.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 217.Au L., Saha K., Fernando B., Ataullah S., Spencer F. ‘Fast-track’ cataract services and diagnostic and treatment centre: Impact on surgical training. Eye. 2008;22:55–59. doi: 10.1038/sj.eye.6702512. [DOI] [PubMed] [Google Scholar]
- 218.Gaujoux T., Chatel M.A., Chaumeil C., Laroche L., Borderie V.M. Outbreak of contact lens-related Fusarium keratitis in France. Cornea. 2008;27:1018–1021. doi: 10.1097/ICO.0b013e318173144d. [DOI] [PubMed] [Google Scholar]
- 219.Kaufmann C., Frueh B.E., Messerli J., Bernauer W., Thiel M.A. Contact lens-associated fusarium keratitis in Switzerland. Klin. Monbl. Augenheilkd. 2008;225:418–421. doi: 10.1055/s-2008-1027357. [DOI] [PubMed] [Google Scholar]
- 220.Daniel C.S., Rajan M.S., Saw V.P., Claerhout I., Kestelyn P., Dart J.K. Contact lens-related Fusarium keratitis in London and Ghent. Eye. 2009;23:484–485. doi: 10.1038/eye.2008.188. [DOI] [PubMed] [Google Scholar]
- 221.Al-Hatmi A.M.S., Castro M.A., de Hoog G.S., Badali H., Alvarado V.F., Verweij P.E., Meis J.F., Zago V.V. Epidemiology of Aspergillus species causing keratitis in Mexico. Mycoses. 2019;62:144–151. doi: 10.1111/myc.12855. [DOI] [PubMed] [Google Scholar]
- 222.Venugopal P.L., Venugopal T.L., Gomathi A., Ramakrishna E.S., Ilavarasi S. Mycotic keratitis in Madras. Indian J. Pathol. Microbiol. 1989;32:190–197. [PubMed] [Google Scholar]
- 223.Panda A., Sharma N., Das G., Kumar N., Satpathy G. Mycotic keratitis in children: Epidemiologic and microbiologic evaluation. Cornea. 1997;16:295–299. doi: 10.1097/00003226-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 224.Chongkae S., Nosanchuk J.D., Pruksaphon K., Laliam A., Pornsuwan S., Youngchim S. Production of melanin pigments in saprophytic fungi in vitro and during infection. J. Basic Microbiol. 2019;59:1092–1104. doi: 10.1002/jobm.201900295. [DOI] [PubMed] [Google Scholar]
- 225.Revankar S.G., Sutton D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guarner J., Brandt M.E. Histopathologic Diagnosis of Fungal Infections in the 21st Century. Clin. Microbiol. Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sahay P., Goel S., Nagpal R., Maharana P.K., Sinha R., Agarwal T., Sharma N., Titiyal J.S. Infectious Keratitis Caused by Rare and Emerging Micro-Organisms. Curr. Eye Res. 2020;45:761–773. doi: 10.1080/02713683.2019.1708407. [DOI] [PubMed] [Google Scholar]
- 228.Yew S.M., Chan C.L., Lee K.W., Na S.L., Tan R., Hoh C.C., Yee W.Y., Ngeow Y.F., Ng K.P. A five-year survey of dematiaceous fungi in a tropical hospital reveals potential opportunistic species. PLoS ONE. 2014;9:e104352. doi: 10.1371/journal.pone.0104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wilhelmus K.R., Jones D.B. Curvularia keratitis. Trans. Am. Ophthalmol. Soc. 2001;99:111–130. [PMC free article] [PubMed] [Google Scholar]
- 230.Wilhelmus K.R. Climatology of dematiaceous fungal keratitis. Am. J. Ophthalmol. 2005;140:1156–1157. doi: 10.1016/j.ajo.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 231.Satpathy G., Ahmed N.H., Nayak N., Tandon R., Sharma N., Agarwal T., Vanathi M., Titiyal J.S. Spectrum of mycotic keratitis in north India: Sixteen years study from a tertiary care ophthalmic centre. J. Infect. Public Health. 2019;12:367–371. doi: 10.1016/j.jiph.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 232.Chowdhary A., Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 233.Ung L., Acharya N.R., Agarwal T., Alfonso E.C., Bagga B., Bispo P.J., Burton M.J., Dart J.K., Doan T., Fleiszig S.M., et al. Infectious corneal ulceration: A proposal for neglected tropical disease status. Bull. World Health Organ. 2019;97:854–856. doi: 10.2471/BLT.19.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Srinivasan M., Upadhyay M.P., Priyadarsini B., Mahalakshmi R., Whitcher J.P. Corneal ulceration in south-east Asia III: Prevention of fungal keratitis at the village level in south India using topical antibiotics. Br. J. Ophthalmol. 2006;90:1472–1475. doi: 10.1136/bjo.2006.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Maung N., Thant C.C., Srinivasan M., Upadhyay M.P., Priyadarsini B., Mahalakshmi R., Whitcher J.P. Corneal ulceration in South East Asia. II: A strategy for the prevention of fungal keratitis at the village level in Burma. Br. J. Ophthalmol. 2006;90:968–970. doi: 10.1136/bjo.2006.094706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Getshen K., Srinivasan M., Upadhyay M., Priyadarsini B., Mahalaksmi R., Whitcher J. Corneal ulceration in South East Asia. I: A model for the prevention of bacterial ulcers at the village level in rural Bhutan. Br. J. Ophthalmol. 2006;90:276–278. doi: 10.1136/bjo.2005.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]